Abstract
There is mounting evidence in support of a significant role for influenza infection in the development of atherosclerosis and the triggering of its complications. Here we review the biologic basis of this relationship, with special emphasis on the pro-inflammatory and pro-thrombotic effects of influenza infection. We also discuss the related epidemiologic findings and discuss in detail the possible causal relationship between influenza and cardiovascular disease.
We appraise the relationship between influenza and coronary heart disease, on the basis of Bradford Hill's criteria of causality. We show that our proposed relationship meets the following criteria: strength of association, consistency, temporal sequence, coherence, biologic plausibility, experimental evidence, and analogy. Further studies are needed to assess whether it meets the criterion of biologic gradient. Specificity is not met, but meeting that criterion is of least importance in the study of multifactorial chronic diseases such as coronary heart disease. These criteria do not yield indisputable evidence for or against cause-and-effect, but they can help researchers appraise available evidence and determine the areas that need further research. The case for expanding the research on the effect of influenza on cardiovascular disease is a strong one, for most of Hill's criteria are met.
Key words: Arteriosclerosis, atherosclerosis, blood coagulation factors/antagonists & inhibitors, Chlamydia pneumoniae, immunization, inflammation/virology, epidemiology, influenza/complications/prevention & control, influenza vaccine, myocardial infarction/etiology, risk factors, vaccines
Although rarely a cause of death until the early 20th century, coronary heart disease (CHD) assumed epidemic proportions by the mid 1950s. Concurrently, a decrease in deaths related to communicable diseases established CHD as the chief cause of death in the Western world. Today, coronary disease is also on the rise in developing countries. 1 This transition in the pattern of CHD has led to a shift in epidemiologic approaches to studying the causes of disease in general. Whereas the 19th century was marked by recognition of the role of infection (a single causative factor), scientists were faced by the beginning of the 20th century with noninfectious diseases, which had multiple predisposing factors. In 1961, the Framingham Heart Study 2 introduced the term “risk factor.” Extensive research is now under way to identify and reduce CHD risk factors, thereby decreasing the risk of cardiovascular events.
Infection, Inflammation, and Atherosclerosis
The role of infectious agents in atherosclerosis has been recognized for more than a century. William Osler 3 was one of the first to propose a major role for acute infection in the pathogenesis of atherosclerosis. In the early 20th century, a few pioneer scientists used several infectious agents (Salmonella typhi, streptococci, etc.) to induce atherosclerosis in animal models. By the late 1970s, scientists began to study the role of herpesviruses and Chlamydia pneumoniae and, later, of Helicobacter pylori, Mycoplasma pneumoniae, Porphyromonas gingivalis, enterovirus, and a growing list of other agents in atherogenesis (Table I). 4–11 This effort coincided with the emergence of new evidence pointing to atherosclerosis as an inflammatory disease. 12 The role of infection in endothelial injury and vascular wall inflammation came under scrutiny. 13 Many infectious agents have been investigated in this regard, but none of them has proved to play a causative and specific role. Chlamydia pneumoniae has been studied the most extensively, but the results of large clinical trials of antibiotics against this disease have been largely disappointing. 14,15
TABLE I. Infectious Agents Implicated in Atherosclerosis

Atherosclerosis, Vulnerable Plaques, and Triggers
Atherosclerosis is a highly prevalent disease, having been present from antiquity and having been observed in ancient mummies. 16 In 1909, Osler 17 observed that “it is exceptional to find no patches of arterial degeneration in any body postmortem, and even children might show some slight foci of fatty degeneration.” More systematic data emerged from studies conducted by Enos, 18 MacNamara, 19 and colleagues, who observed CHD in young soldiers killed in action in Korea and Vietnam. Major studies have been done to elucidate the factors that convert established, stable atherosclerotic plaques into unstable, life-threatening plaques. 20 Acute coronary syndromes involve the rupture of vulnerable plaques, which are usually nonobstructive and which have inflammatory cell infiltrates, a thin fibrous cap, and a large lipid core. 21 When the surfaces of these plaques rupture or erode, a thrombotic event will ensue. The factors that lead to plaque inflammation followed by a myocardial infarction (MI) are not fully understood. Our group has hypothesized that influenza may play a role in some patients. Whereas most other infectious agents result in a chronic, indolent infection, which presumably increases chronic inflammation of the arterial walls, influenza induces notable acute arterial-wall inflammation and may trigger plaque destabilization that leads to acute coronary syndrome.
Epidemiologic Studies
Influenza epidemics are associated with a significant increase in cardiovascular deaths. Selwyn Collins, in 1932, 22 was one of the first researchers to report that for nearly every influenza epidemic there is a peak in deaths related to organic heart disease, which corresponds chronologically to the influenza peak.
Acute MI shows a seasonal variation, having its highest incidence in the winter months. 23–25 Influenza activity has been suggested as a reason for this winter peak in the MI rate. 26–28 Glezen and colleagues showed that peaks in influenza activity are followed, 2 weeks later, by a peak in deaths related to ischemic heart disease, hypertension, and cerebrovascular disease. 29 This finding is supported by clinical studies showing that many acute MIs are preceded by an upper respiratory tract infection. 30–36
Effect on Excess Death
Influenza is a major cause of morbidity and death. Each year, in the United States alone, “the flu” accounts for 110,000 hospitalizations, 1 to 3 billion dollars in direct costs, and 10 to 15 billion dollars in indirect costs. 37 Although earlier estimates cited 22,000 excess influenza-related deaths each year, 38 newer estimates—derived from epidemiologic data—cite 36,000 deaths involving the respiratory and circulatory systems and 51,000 all-cause deaths related to influenza each year in the United States. 39 This increased death is partly due to the aging of the population and to the advent of more virulent viral strains. However, the influenza-related death toll may be even higher: because influenza is not a recognized trigger of MI, it is very unlikely to be recorded on the death certificates of patients who die of MI, stroke, heart failure, or cardiac arrest. Consequently, the number of deaths triggered by influenza is under-recorded. In fact, our estimates—derived from clinical trials and case-control studies—show that, by triggering cardiovascular events, influenza may cause up to 90,000 deaths per year.
Influenza Vaccination Prevents Cardiovascular Events
In 2000, Naghavi and associates 40 reported that influenza vaccination has a protective effect in a high-risk population (patients with known CHD). In this case-control study, vaccination against influenza was associated with a 67% reduction in the risk of MI during the subsequent influenza season (odds ratio [OR], 0.33; 95% confidence interval [CI], 0.13–0.82; P = 0.017), but it did not reduce deaths. In a case-control study, Siscovick and coworkers 41 found that influenza vaccination was associated with a 49% reduction (OR, 0.51; 95% CI, 0.33–0.79) in the risk of an out-of-hospital primary cardiac arrest. Lavallée and colleagues 42 found a 50% decrease (OR, 0.50; 95% CI, 0.26–0.94) in the stroke rate for patients vaccinated against influenza, including a 48% risk reduction (OR, 0.42; 95% CI, 0.21–0.81) in those vaccinated during the preceding 5 years. Conversely, in studying survivors of a 1st MI, Jackson and coworkers 43 failed to show that influenza vaccination had any protective effect against recurrent coronary events (adjusted hazard ratio, 1.18; 95% CI, 0.79–1.75). However, this study may have been hampered by a misclassification of the subjects, and exclusion of the early (90-day) period after an acute MI (when complications are most likely to develop) may have limited the researchers' ability to detect a potential benefit.
During 2 influenza seasons, Nichol and associates 44 studied community-dwelling elderly subjects (288,238 persons ≥65 years old), in whom vaccination against influenza was associated with a reduced risk of hospitalization for heart disease (reduction, 19%; P < 0.001) and cerebrovascular disease (reduction, 16%–23%; P < 0.018) and a reduced all-cause mortality rate (reduction, 48%–50%; P < 0.001) during influenza seasons. 44
In a small, randomized, pilot clinical trial conducted by Gurfinkel and colleagues, 45 the mortality rate was 2% for the vaccinated group versus 8% for the control group (relative risk [RR], 0.25; 95% CI, 0.07–0.86; P = 0.01). 45 The triple composite endpoint occurred in 11% of the vaccinated patients versus 23% of the control patients (P = 0.009). At 1 year, the incidence of cardiovascular death was significantly lower in the vaccine recipients (6%) than in the control group (17%) (RR, 0.34; 95% CI, 0.17–0.71). 46 The triple composite endpoint occurred in 22% of the vaccinated group versus 37% of the control group (P = 0.004). 46 The two-year follow-up evaluation of patients who were revaccinated showed a decreased incidence of the combined endpoint of death and MI after revaccination (3.4% vs 9.7%; P = 0.05). 47
Mechanisms
Influenza affects the vascular system in multiple ways. We have shown that inoculation of atherosclerotic apolipoprotein-E (apoE)–deficient mice with influenza A results in heavy infiltration of atherosclerotic plaques by inflammatory cells (macrophages and T cells), as well as smooth muscle cell proliferation, fibrin deposition, platelet aggregation, and thrombosis. 48 These profound inflammatory and prothrombotic changes mimic those seen in coronary plaques after a fatal MI. 49
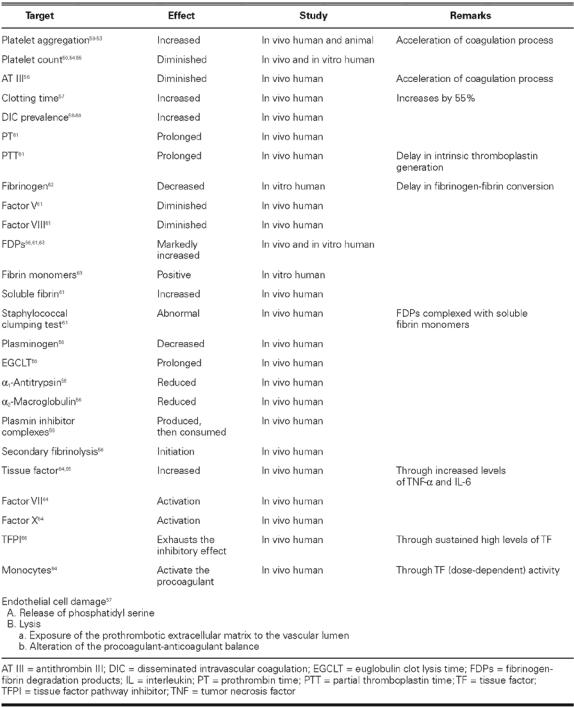
Influenza is associated with a greatly increased number of proinflammatory, prothrombotic cytokines, and it causes endothelial dysfunction, increased plasma viscosity, tachycardia, and release of endogenous catecholamines. Clinical flu is also associated with psychological distress, dehydration leading to hypotension, and hemoconcentration, hypoxemia, and demand ischemia. Van Lenten and coworkers have shown that influenza decreases the anti-inflammatory properties of high-density lipoprotein cholesterol particles and increases entry of macrophages into the arterial wall. Influenza infection also has extensive and profound procoagulant effects (Table II). 67,68
TABLE II. Effects of Influenza on the Coagulation System
Viremia after influenza and extrapulmonary seeding of the virus are considered to be rare; 69 however, a direct infection of the atherosclerotic plaques and chronic plaque infection cannot be ruled out. Whether or not the virus infects the plaque, influenza may induce an antigenic cross-reactivity, which may result in enhanced immune response against plaque antigens.
In our mice experiments, only the plaques, not the normal arterial segments, were inflamed. In an ecological study, Azambuja and Duncan demonstrated an association between the distribution of the ages at which patients died of influenza and pneumonia during the 1918–19 United States influenza pandemic and the distribution of CHD death from 1920 to 1985 in survivors from the corresponding birth cohorts. 70 These researchers suggest that the 1918 influenza pandemic (and probably subsequent flu epidemics) played a role in the CHD epidemic of the 20th century, probably by initiating immune responses related to potential antigenic mimicry.
Is There a Causal Relationship?
Three case-control studies, 40–42 1 large cohort study, 44 and 1 pilot, randomized clinical trial 45 have suggested that influenza vaccination has a protective role against CHD (Fig. 1). It is important to clarify, however, whether a true cause-and-effect relationship exists or whether these results were due to chance, a bias, or a simple noncausal association.
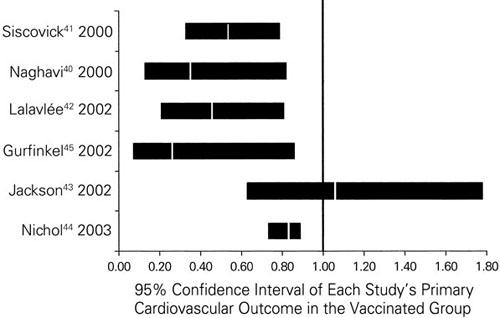
Fig. 1 Studies of influenza vaccination and cardiovascular outcomes.
Treacherous philosophical issues regarding the nature of causality have long been recognized and were detailed by Hume in the 18th century. 71 Various “criteria” have been proposed to help assess possible causal relationships in epidemiologic studies. Although these criteria cannot be considered decisive in differentiating between causal and noncausal associations, they can help support the evidence for a causal relationship instead of one based on chance, bias, or confounding factors. 72 These criteria were published in the United States Surgeon General's 1st report on smoking and health (in 1964) and were later improved by Sir Bradford Hill (Table III) and Mervyn Susser. 73–75 In the following paragraphs, we briefly appraise the relationship between influenza and CHD, on the basis of Hill's criteria of causality.
TABLE III. Hill's Criteria for Causality 74
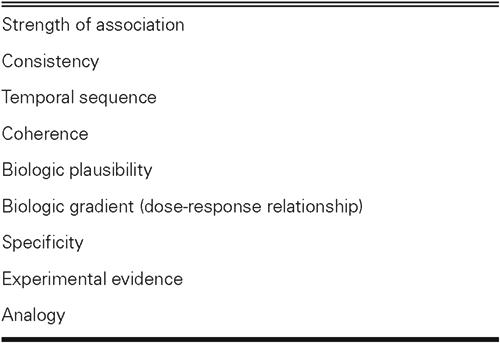
Strength of Association. Strong associations provide better evidence of causality than do weak ones. Influenza is associated with a 2- to 3-fold increase in the risk of an MI. Influenza vaccination is associated with an unusually marked reduction in the cardiovascular outcomes, which ranges from 20% to 70% (50% on average, Fig. 1).
Consistency. Consistency refers to the finding of similar or consistent results by different investigators, using a variety of methods, in diverse studies involving different populations and circumstances. The greater the consistency among the diverse findings, the stronger the evidence for causality. Influenza has been associated with an increased risk of CHD events in multiple previous studies, 23-25,30–36 in addition to 3 case-control studies, 40–42 1 cohort study, 44 and 1 clinical trial, 45 which showed that influenza vaccination had a beneficial effect in primary and secondary prevention settings with different outcomes.
Temporal Sequence. This criterion requires that exposure to a disease precede the onset of the disease by a reasonable amount of time. Epidemiologic studies have shown a 7- to 10-day lag in the development of CHD outcomes after influenza in individual patients and a similar pattern in the peaking of CHD after influenza seasonal activity. Similarly, our animal studies have shown that the most significant pathologic changes happen 7 to 10 days after influenza infection in apoE–deficient mice.
Coherence. Coherence suggests that the available information regarding the disease forms a cohesive entity and that the proposed relationship does not contradict or conflict with existing theory and knowledge. Reports concerning an increased incidence of CHD events tend to parallel reports concerning an increase in all-cause deaths associated with influenza. In studies of the effect of vaccination on all-cause mortality, the magnitude of the observed effect fully supports the protection seen in cardiovascular studies. In a meta-analysis of 20 cohort studies, influenza vaccination reduced deaths by 68%; in case-control studies, it reduced deaths from all causes by 30%. 76,77
Biologic Plausibility. This criterion requires the association in question to be plausible and explainable on the basis of known biologic facts. We have previously described the growing body of evidence of a procoagulant effect of influenza infection.
Biologic Gradient (Dose-Response Relationship). An increase in the level, intensity, duration, or total amount of exposure is associated with a progressive increase in the risk of disease. So far, there has been no evidence to support the theory that multiple bouts or more severe cases of influenza produce more deleterious cardiovascular effects. This question has not been adequately answered, and it merits further research. Indirect supporting evidence may be inferred from the studies of excess death in milder epidemics of influenza. During such epidemics, Simonsen and colleagues documented a lower excess death: an average of 3,200 deaths in 9 seasons of influenza B and A (H1N1). In contrast, during more severe epidemics, the excess death was higher: an average of 7,600 deaths in 11 seasons of influenza A (H3N2). 38 Re-evaluation of the existing data would show whether there was a similar difference in the cardiovascular deaths during those same years. Researchers also need to determine whether multiple infections or more virulent viral strains result in worse cardiovascular outcomes in experimental and clinical cases.
Specificity. This criterion maintains that exposure leads to only 1 disease and that the disease results from only 1 exposure. However, in chronic diseases (especially coronary artery disease, which is multifactorial), this is rarely true. Therefore, an absence of specificity does not rule out a causal relationship.
Experimental Evidence. This criterion holds that a disease may be altered (prevented or ameliorated) by an appropriate experimental regimen (in vitro tests, animal experiments, or clinical trials). Satisfaction of this criterion is not a necessity; in most cases, such proof is elusive and is not even sought. However, in multiple studies showing that influenza vaccination protects against CHD events, this criterion has been met (Fig. 1).
Analogy. Analogy implies a similarity in some respects among things that are otherwise different. According to Hill, this is among the least important of the criteria. The influenza-atherosclerosis relationship is analogous to some other relationships between CHD risk factors and atherosclerosis.
Although these criteria can help researchers better appraise the available evidence, none of them, per se, can yield indisputable evidence for or against the cause-and-effect hypothesis, and none of them (except perhaps temporal sequence) is a sine qua non. Causation should be thought of as a multifactorial web. Interaction among several factors (obesity, smoking, hypertension, dyslipidemia, male sex, etc.) causes coronary artery disease, and each of these factors has its own causal web. Also, many such webs may exist for a given disease. 72
Public Health Aspects
Recognition of the effects of influenza on CHD provides the medical community with a valuable opportunity to further reduce cardiovascular death and morbidity. Influenza vaccination is a safe, inexpensive, and effective method for reducing morbidity in high-risk patients with cardiovascular disease. However, this method is extremely under-utilized.
The influenza vaccine is very safe and well tolerated in all age groups. 78 Randomized, double-blind clinical trials have documented a scarcity of systemic symptoms after vaccination. 79,80 In 1976, after a universal vaccination program was implemented in response to a swine flu scare, a controversial increase in the incidence of Guillain-Barré syndrome (GBS) was reported. 81,82 However, methodologic questions arose concerning these reports, and GBS was not observed in subsequent studies of other vaccination programs, including the United States Army's mass influenza vaccination program (1980–1988). 83–85 The Advisory Committee on Immunization Practices 78 found no indication that current influenza vaccines are associated with a substantial increase in GBS and stated that, even if the influenza vaccine does pose a risk, it probably involves slightly more than 1 additional case per million persons vaccinated. The Committee concluded that the potential benefits of influenza vaccination outweigh the possible risk of developing vaccine-associated GBS.
Multiple studies have shown that vaccination can reduce both direct medical costs and indirect costs related to absenteeism from work. 86–88 Influenza vaccination is one of the most cost-effective interventions available and, in selected groups, it may even be cost saving. In fact, vaccination has a far more favorable cost–benefit profile than most cardiovascular prevention methods, such as statins. For this reason, the under-use of influenza vaccination is unacceptable.
Current national guidelines advise that high-risk CHD patients need to be vaccinated against influenza. Also included are children and household contacts who live with patients who have heart disease. However, the main best-practice cardiology guidelines from the American Heart Association, European Society of Cardiology, and American College of Cardiology fail to specify the need for vaccinating CHD patients. 89–93 In general, cardiologists ignore the impact of influenza on the cardiovascular system and consider immunization a responsibility of primary care physicians. This lack of knowledge and failure to recommend or practice vaccination has led to a very low rate of immunization in cardiovascular patients. The national objectives for influenza vaccination, as stated by “Healthy People 2000” and “Healthy People 2010,” are 60% for adults 18 to 64 years old with high-risk conditions and 90% for persons 65 or older. 94 However, according to the 1997–2001 National Health Interview Survey, the prevalence of self-reported influenza vaccination was only 22.7%, 49.2%, and 76.7% in CHD patients aged 18 to 49, 50 to 64, and ≥65 years, respectively. 95 This significant shortcoming needs urgent and focused corrective action.
Although extremely important, education of healthcare professionals at the primary and specialty levels is not enough. Community leaders, teachers, churches, and the mass media have an equal responsibility to spread the right message, correct myths, and motivate people to get vaccinated. New research is needed to identify the obstacles to vaccination of CHD patients and to develop programs that are designed to overcome those obstacles. Public health officials must recognize prevailing myths and misinformation and must develop programs to counteract them. The prophylactic use of new neuraminidase inhibitors appears promising for preventing influenza; if this benefit is verified in clinical trials, these agents may find a place in preventing CHD. Besides offering additional protection to elderly persons and those with a weak immune response to vaccine, neuraminidase inhibitors can be especially important in vaccine-virus mis-matches. 96
Future Research
The effect of influenza on CHD is just beginning to be recognized, and it warrants extensive research, which may pave the way for new, more efficient methods for preventing and controlling CHD. We have recommended a series of actions that can improve influenza control in CHD patients (Table IV). Basic studies are needed to elucidate the mechanism by which influenza affects the vascular system. It is essential to determine whether influenza directly affects the vascular walls and directly damages the arterial walls. Different anti-inflammatory, anticoagulant, antioxidant, lipid-lowering, immunomodulating, antimacrophage, and antiviral drugs must be tested, in animal studies, for their impact on influenza-induced vascular effects. If effective, these agents must be tested later in human clinical trials. Because of the antigenic similarity of influenza antigens and oxidized low-density-lipoprotein cholesterol epitopes, studies are needed to evaluate potential cross-activation of the immune system against plaques after influenza infections. Various viral strains may behave differently in this regard.
TABLE IV. Recommendations for Improving Influenza Control in Cardiovascular Patients

Clinical trials will determine whether vaccination against influenza can protect against cardiovascular events in high-risk subjects. Ethical issues may preclude the use of a placebo in patients with known CHD and in persons older than 50 years, because these groups are urged to receive the vaccine. However, they may be given prophylactic oseltamivir in randomized, placebo-controlled trials, since this approach would provide sufficient and additional protection against influenza. Younger subjects (< 50 years of age) with a high risk of CHD, on the basis of multiple risk factors or of subclinical CHD, are a large portion of the population and should be studied in clinical trials of influenza vaccine and oseltamivir to reduce their risk of CHD events.
Another interesting approach is to vaccinate the children and household contacts of persons who are at high risk for CHD. Reichert and associates showed that vaccination of Japanese schoolchildren against influenza decreased the incidence and mortality rate of that disease among older persons; moreover, this method attenuated the seasonal variability in mortality rate related to cardiovascular and respiratory disease and also to all causes. 97,98
Conclusion
Growing evidence suggests that influenza may play a causal role in the development of atherosclerosis and its complications. Further basic, animal, and clinical studies are needed to elaborate the mechanism by which influenza increases the risk of CHD and to determine how vaccines and antiviral agents may protect CHD patients. To increase influenza vaccination of patients at high risk for CHD, an intense public health effort is needed. Moreover, clinical trials are necessary to identify which groups may benefit most from influenza prevention in terms of cardiovascular prevention.
Acknowledgments
The authors wish to thank Drs. Deborah Vela and Navid Sadeghi for their assistance in preparing this manuscript.
Footnotes
Address for reprints: Mohammad Madjid, MD, University of Texas–Houston Health Science Center, 6431 Fannin, MSB 1.246, Houston, TX 77030
E-mail: Mohammad.Madjid@uth.tmc.edu
This paper has its basis in a presentation made at the First Symposium on Influenza and Cardiovascular Disease: Science, Practice, and Policy, held on 26 April 2003, at the Texas Heart Institute, Houston, Texas.
This work was supported in part by U.S. Department of Defense Grant #DAMD17-01-2-0047.
References
- 1.American Heart Association. Heart Disease and Stroke Statistics—2003 Update. Dallas: American Heart Association; 2002.
- 2.Kannel WB, Dawber TR, Kagan A, Revotskie N, Stokes J 3rd. Factors of risk in the development of coronary heart disease—six year follow-up experience. The Framingham Study. Ann Intern Med 1961;55:33–50. [DOI] [PubMed]
- 3.Osler W. Lectures on angina pectoris and allied states (1897). In: Fye BF, editor. William Osler's collected papers on the cardiovascular system. New York: Adams, LB; 1985. p. 239-57.
- 4.Higuchi ML, Sambiase N, Palomino S, Gutierrez P, Demarchi LM, Aiello VD, Ramires JA. Detection of Mycoplasma pneumoniae and Chlamydia pneumoniae in ruptured atherosclerotic plaques. Braz J Med Biol Res 2000; 33:1023–6. [DOI] [PubMed]
- 5.Haraszthy VI, Zambon JJ, Trevisan M, Zeid M, Genco RJ. Identification of periodontal pathogens in atheromatous plaques. J Periodontol 2000;71:1554–60. [DOI] [PubMed]
- 6.Alber DG, Powell KL, Vallance P, Goodwin DA, Grahame-Clarke C. Herpesvirus infection accelerates atherosclerosis in the apolipoprotein E-deficient mouse. Circulation 2000; 102:779–85. [DOI] [PubMed]
- 7.Epstein SE, Zhou YF, Zhu J. Infection and atherosclerosis: emerging mechanistic paradigms. Circulation 1999;100: e20-8. [DOI] [PubMed]
- 8.Burch GE, Harb JM, Hiramoto Y, Shewey L. Viral infection of the aorta of man associated with early atherosclerotic changes. Am Heart J 1973;86:523–34. [DOI] [PubMed]
- 9.Li L, Messas E, Batista EL Jr, Levine RA, Amar S. Porphyromonas gingivalis infection accelerates the progression of atherosclerosis in a heterozygous apolipoprotein E-deficient murine model [published erratum appears in Circulation 2002;105:1617]. Circulation 2002;105:861–7. [DOI] [PubMed]
- 10.Fabricant CG, Fabricant J, Litrenta MM, Minick CR. Virus-induced atherosclerosis. J Exp Med 1978;148:335–40. [DOI] [PMC free article] [PubMed]
- 11.Muhlestein JB, Anderson JL, Hammond EH, Zhao L, Trehan S, Schwobe EP, Carlquist JF. Infection with Chlamydia pneumoniae accelerates the development of atherosclerosis and treatment with azithromycin prevents it in a rabbit model. Circulation 1998;97:633–6. [DOI] [PubMed]
- 12.Ross R, Glomset JA. The pathogenesis of atherosclerosis (first of two parts). N Engl J Med 1976;295:369–77. [DOI] [PubMed]
- 13.Libby P, Egan D, Skarlatos S. Roles of infectious agents in atherosclerosis and restenosis: an assessment of the evidence and need for future research. Circulation 1997;96:4095–103. [DOI] [PubMed]
- 14.Muhlestein JB, Anderson JL, Carlquist JF, Salunkhe K, Horne BD, Pearson RR, et al. Randomized secondary prevention trial of azithromycin in patients with coronary artery disease: primary clinical results of the ACADEMIC study. Circulation 2000;102:1755–60. [DOI] [PubMed]
- 15.O'Connor CM, Dunne MW, Pfeffer MA, Muhlestein JB, Yao L, Gupta S, et al. Azithromycin for the secondary prevention of coronary heart disease events: the WIZARD study: a randomized controlled trial. JAMA 2003;290:1459–66. [DOI] [PubMed]
- 16.Moodie RL. Paleopathology: an introduction to the study of ancient evidences of disease. Urbana (IL): University of Illinois Press; 1923.
- 17.Woodstock C. Arteriosclerosis. Br Med J 1909;2:1800.
- 18.Enos WF, Holmes RH, Beyer J. Landmark article, July 18, 1953: Coronary disease among United States soldiers killed in action in Korea. Preliminary report. By William F. Enos, Robert H. Holmes and James Beyer. JAMA 1986;256:2859–62. [DOI] [PubMed]
- 19.McNamara JJ, Molot MA, Stremple JF, Cutting RT. Coronary artery disease in combat casualties in Vietnam. JAMA 1971;216:1185–7. [PubMed]
- 20.Willerson JT. Conversion from chronic to acute coronary heart disease syndromes. Role of platelets and platelet products. Tex Heart Inst J 1995;22:13–9. [PMC free article] [PubMed]
- 21.Shah PK. Mechanisms of plaque vulnerability and rupture. J Am Coll Cardiol 2003;41(4 Suppl S):15S-22S. [DOI] [PubMed]
- 22.Collins S. Excess mortality from causes other than influenza and pneumonia during influenza epidemics. Pub Health Rep 1932;47:2159–79.
- 23.Spencer FA, Goldberg RJ, Becker RC, Gore JM. Seasonal distribution of acute myocardial infarction in the second National Registry of Myocardial Infarction. J Am Coll Cardiol 1998;31:1226–33. [DOI] [PubMed]
- 24.Sheth T, Nair C, Muller J, Yusuf S. Increased winter mortality from acute myocardial infarction and stroke: the effect of age. J Am Coll Cardiol 1999;33:1916–9. [DOI] [PubMed]
- 25.Jakovljevic D, Salomaa V, Sivenius J, Tamminen M, Sarti C, Salmi K, et al. Seasonal variation in the occurrence of stroke in a Finnish adult population. The FINMONICA Stroke Register. Finnish Monitoring Trends and Determinants in Cardiovascular Disease. Stroke 1996;27:1774–9. [DOI] [PubMed]
- 26.Bainton D, Jones GR, Hole D. Influenza and ischaemic heart disease—a possible trigger for acute myocardial infarction? Int J Epidemiol 1978;7:231–9. [DOI] [PubMed]
- 27.Cold exposure and winter mortality from ischaemic heart disease, cerebrovascular disease, respiratory disease, and all causes in warm and cold regions of Europe. The Eurowinter Group. Lancet 1997;349:1341–6. [PubMed]
- 28.Gordon T, Thom T. The recent decrease in CHD mortality. Prev Med 1975;4:115–25. [DOI] [PubMed]
- 29.Glezen WP, Payne AA, Snyder DN, Downs TD. Mortality and influenza. J Infect Dis 1982;146:313–21. [DOI] [PubMed]
- 30.Spodick DH, Flessas AP, Johnson MM. Association of acute respiratory symptoms with onset of acute myocardial infarction: prospective investigation of 150 consecutive patients and matched control patients. Am J Cardiol 1984;53:481–2. [DOI] [PubMed]
- 31.Pesonen E, Siitonen O. Acute myocardial infarction precipitated by infectious disease [letter]. Am Heart J 1981;101:512–3. [DOI] [PubMed]
- 32.Abinader EG, Sharif DS, Omary M. Inferior wall myocardial infarction preceded by acute exudative pharyngitis in young males. Isr J Med Sci 1993;29:764–9. [PubMed]
- 33.Bourne G, Wedgwood J. Heart-disease and influenza. Lancet 1959;1:1226–8. [DOI] [PubMed]
- 34.Penttinen J, Valonen P. The risk of myocardial infarction among Finnish farmers seeking medical care for an infection. Am J Public Health 1996;86:1440–2. [DOI] [PMC free article] [PubMed]
- 35.Zheng ZJ, Mittleman MA, Tofler GH, Pomeroy C, Dampier C, Wides B, Muller JE. Infections prior to acute myocardial infarction onset [abstract]. J Am Coll Cardiol 1998; 31(2 Suppl A):132A.
- 36.Meier CR, Jick SS, Derby LE, Vasilakis C, Jick H. Acute respiratory-tract infections and risk of first-time acute myocardial infarction. Lancet 1998;351:1467–71. [DOI] [PubMed]
- 37.Office of Technology Assessment US Congress. Cost-effectiveness of influenza vaccination. No. 052-003-00855-6. Washington, DC: Government Printing Office; 1981.
- 38.Simonsen L, Clarke MJ, Williamson GD, Stroup DF, Arden NH, Schonberger LB. The impact of influenza epidemics on mortality: introducing a severity index. Am J Public Health 1997;87:1944–50. [DOI] [PMC free article] [PubMed]
- 39.Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, Fukuda K. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA 2003;289:179–86. [DOI] [PubMed]
- 40.Naghavi M, Barlas Z, Siadaty S, Naguib S, Madjid M, Casscells W. Association of influenza vaccination and reduced risk of recurrent myocardial infarction. Circulation 2000;102:3039–45. [DOI] [PubMed]
- 41.Siscovick DS, Raghunathan TE, Lin D, Weinmann S, Arbogast P, Lemaitre RN, et al. Influenza vaccination and the risk of primary cardiac arrest. Am J Epidemiol 2000;152:674–7. [DOI] [PubMed]
- 42.Lavallee P, Perchaud V, Gautier-Bertrand M, Grabli D, Amarenco P. Association between influenza vaccination and reduced risk of brain infarction [published erratum appears in Stroke 2002;33:1171]. Stroke 2002;33:513–8. [DOI] [PubMed]
- 43.Jackson LA, Yu O, Heckbert SR, Psaty BM, Malais D, Barlow WE, et al. Influenza vaccination is not associated with a reduction in the risk of recurrent coronary events. Am J Epidemiol 2002;156:634–40. [DOI] [PubMed]
- 44.Nichol KL, Nordin J, Mullooly J, Lask R, Fillbrandt K, Iwane M. Influenza vaccination and reduction in hospitalizations for cardiac disease and stroke among the elderly. N Engl J Med 2003;348:1322–32. [DOI] [PubMed]
- 45.Gurfinkel EP, de la Fuente RL, Mendiz O, Mautner B. Influenza vaccine pilot study in acute coronary syndromes and planned percutaneous coronary interventions: the FLU Vaccination Acute Coronary Syndromes (FLUVACS) Study. Circulation 2002;105:2143–7. [DOI] [PubMed]
- 46.Gurfinkel EP, Leon de la Fuente R, Mendiz O, Mautner B. Flu vaccination in acute coronary syndromes and planned percutaneous coronary interventions (FLUVACS) Study. One-year follow-up. Eur Heart J 2004;25:25–31. [DOI] [PubMed]
- 47.Gurfinkel EP, de la Fuente RL. Flu vaccination in acute coronary syndromes and planned percutaneous coronary interventions (FLUVACS) registry. Two-year follow-up in the southern hemisphere. Tex Heart Inst J 2004;31:28–32. [PMC free article] [PubMed]
- 48.Naghavi M, Wyde P, Litovsky S, Madjid M, Akhtar A, Naguib S, et al. Influenza infection exerts prominent inflammatory and thrombotic effects on the atherosclerotic plaques of apolipoprotein E-deficient mice. Circulation 2003;107:762–8. [DOI] [PubMed]
- 49.Virmani R, Kolodgie FD, Burke AP, Farb A, Schwartz SM. Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol 2000;20:1262–75. [DOI] [PubMed]
- 50.Terada H, Baldini M, Ebbe S, Madoff MA. Interaction of influenza virus with blood platelets. Blood 1966;28:213–28. [PubMed]
- 51.Terada H. Studies on platelets and interaction with particular reference to the interaction of influenza virus with blood platelets [in Japanese]. Nippon Ketsueki Gakkai Zasshi 1969; 32:45–51. [PubMed]
- 52.Barshtein IuA, Frolov AF, Persidskii IuV, Gavrilov SV. Characteristics of the action of the influenza virus on the microcirculatory vessels in an experiment [in Russian]. Mikrobiol Zh 1989;51:44–50. [PubMed]
- 53.Bogomolov BP, Barinov VG, Deviatkin AV, Shvedova LP, Arkharova MN, Frolova IN, et al. Hemostasis in influenza and acute respiratory viral infections in the middle-aged and elderly [in Russian]. Ter Arkh 1990;62:98–102. [PubMed]
- 54.Bouwman JJ, Visseren FL, Bosch MC, Bouter KP, Diepersloot RJ. Procoagulant and inflammatory response of virus-infected monocytes. Eur J Clin Invest 2002;32:759–66. [DOI] [PubMed]
- 55.Cotran RS. American Association of Pathologists president's address. New roles for the endothelium in inflammation and immunity. Am J Pathol 1987;129:407–13. [PMC free article] [PubMed]
- 56.Settle H, Glueck HI. Disseminated intravascular coagulation associated with influenza. Ohio State Med J 1975;71:541–3,547. [PubMed]
- 57.Whitaker AN, Bunce I, Graeme ER. Disseminated intravascular coagulation and acute renal failure in influenza A2 infection. Med J Aust 1974;2:196–201. [DOI] [PubMed]
- 58.Danon D, Jerushalmy Z, De Vries A. Incorporation of influenza virus in human blood platelets in vitro. Electron microscopical observation. Virology 1959;9:719–22. [DOI] [PubMed]
- 59.Talley NA, Assumpcao CA. Disseminated intravascular clotting complicating viral pneumonia due to influenza. Med J Aust 1971;2:763–6. [DOI] [PubMed]
- 60.McKay DG, Margaretten W. Disseminated intravascular coagulation in virus diseases. Arch Intern Med 1967;120:129–52. [PubMed]
- 61.Davison AM, Thomson D, Robson JS. Intravascular coagulation complicating influenza A virus infection. Br Med J 1973;1:654–5. [DOI] [PMC free article] [PubMed]
- 62.Anisimova IuN, Trushnikova GV. Infectious-toxic shock and disseminated intravascular coagulation in a pregnant woman with influenza [in Russian]. Arkh Patol 1994;56:79–82. [PubMed]
- 63.Visseren FL, Bouwman JJ, Bouter KP, Diepersloot RJ, de Groot PH, Erkelens DW. Procoagulant activity of endothelial cells after infection with respiratory viruses. Thromb Haemost 2000;84:319–24. [PubMed]
- 64.Jerushalmy Z, Adler A, Rechnic J, Kohn A, de Vries. Effect of myxoviruses on the clotting and clotretracting activities of human blood platelets “in vitro”. Pathol Biol (Paris) 1962; 10:41–8. [PubMed]
- 65.Kisker CT, Rush R. Detection of intravascular coagulation. J Clin Invest 1971;50:2235–41. [DOI] [PMC free article] [PubMed]
- 66.van Gorp EC, Suharti C, ten Cate H, Dolmans WM, van der Meer JW, ten Cate JW, Brandjes DP. Review: infectious diseases and coagulation disorders. J Infect Dis 1999;180:176–86. [DOI] [PubMed]
- 67.Van Lenten BJ, Wagner AC, Nayak DP, Hama S, Navab M, Fogelman AM. High-density lipoprotein loses its anti-inflammatory properties during acute influenza A infection. Circulation 2001;103:2283–8. [DOI] [PubMed]
- 68.Van Lenten BJ, Wagner AC, Anantharamaiah GM, Garber DW, Fishbein MC, Adhikary L, et al. Influenza infection promotes macrophage traffic into arteries of mice that is prevented by D-4F, an apolipoprotein A-I mimetic peptide. Circulation 2002;106:1127–32. [DOI] [PubMed]
- 69.Murphy BR, Webster RG. Viremia: an extrapulmonary manifestation of influenza. In: Fields BN, Knipe DM, Howley PM, editors. Fields virology. Vol 1. Philadelphia: Lippincott-Raven; 1996. p. 1407.
- 70.Azambuja MI, Duncan BB. Similarities in mortality patterns from influenza in the first half of the 20th century and the rise and fall of ischemic heart disease in the United States: a new hypothesis concerning the coronary heart disease epidemic. Cad Saude Publica 2002;18:557–77. [DOI] [PubMed]
- 71.Hume D. A treatise of human nature. Book 1: Of the understanding. Harmondsworth (UK): Penguin; 1984. (First published, 1739).
- 72.Labarthe DR. The casual complex. In: Colilla J, editor. Epidemiology and prevention of cardiovascular diseases: a global challenge. Gaithersburg (MD): Aspen publishers, Inc.; 1998. p. 449-64.
- 73.Advisory Committee to the Surgeon General of the Public Health Services. Smoking and Health: Report of the Advisory Committee to the Surgeon General. Atlanta: Public Health Service, US Dept of Health, Education and Welfare; 1964.
- 74.Hill AB. The environment and disease: association or causation? Proc R Soc Med 1965;58:295–300. [DOI] [PMC free article] [PubMed]
- 75.Susser M. What is a cause and how do we know one? A grammar for pragmatic epidemiology. Am J Epidemiol 1991;133:635–48. [DOI] [PubMed]
- 76.Gross PA, Hermogenes AW, Sacks HS, Lau J, Levandowski RA. The efficacy of influenza vaccine in elderly persons. A meta-analysis and review of the literature. Ann Intern Med 1995;123:518–27. [DOI] [PubMed]
- 77.Nichol KL, Wuorenma J, von Sternberg T. Benefits of influenza vaccination for low-, intermediate-, and high-risk senior citizens. Arch Intern Med 1998;158:1769–76. [DOI] [PubMed]
- 78.Bridges CB, Fukuda K, Cox NJ, Singleton JA; Advisory Committee on Immunization Practices. Prevention and control of influenza. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2001;50(RR-4):1–44. [PubMed]
- 79.Margolis KL, Nichol KL, Poland GA, Pluhar RE. Frequency of adverse reactions to influenza vaccine in the elderly. A randomized, placebo-controlled trial [published erratum appears in JAMA 1991;265:2810]. JAMA 1990;264:1139–41. [PubMed]
- 80.Govaert TM, Dinant GJ, Aretz K, Masurel N, Sprenger MJ, Knottnerus JA. Adverse reactions to influenza vaccine in elderly people: randomised double blind placebo controlled trial. BMJ 1993;307:988–90. [DOI] [PMC free article] [PubMed]
- 81.Ropper AH, Victor M. Influenza vaccination and the Guillain-Barre syndrome [comment]. N Engl J Med 1998;339:1845–6. [DOI] [PubMed]
- 82.Schonberger LB, Bregman DJ, Sullivan-Bolyai JZ, Keenlyside RA, Ziegler DW, Retailliau HF, et al. Guillain-Barre syndrome following vaccination in the National Influenza Immunization Program, United States, 1976–1977. Am J Epidemiol 1979;110:105–23. [DOI] [PubMed]
- 83.Kaplan JE, Katona P, Hurwitz ES, Schonberger LB. Guillain-Barre syndrome in the United States, 1979-1980 and 1980-1981. Lack of an association with influenza vaccination. JAMA 1982;248:698–700. [PubMed]
- 84.Hurwitz ES, Schonberger LB, Nelson DB, Holman RC. Guillain-Barre syndrome and the 1978-1979 influenza vaccine. N Engl J Med 1981;304:1557–61. [DOI] [PubMed]
- 85.Roscelli JD, Bass JW, Pang L. Guillain-Barre syndrome and influenza vaccination in the US Army, 1980-1988. Am J Epidemiol 1991;133:952–5. [DOI] [PubMed]
- 86.Nichol KL, Lind A, Margolis KL, Murdoch M, McFadden R, Hauge M, et al. The effectiveness of vaccination against influenza in healthy, working adults. N Engl J Med 1995; 333:889–93. [DOI] [PubMed]
- 87.Campbell DS, Rumley MH. Cost-effectiveness of the influenza vaccine in a healthy, working-age population. J Occup Environ Med 1997;39:408–14. [DOI] [PubMed]
- 88.Bridges CB, Thompson WW, Meltzer MI, Reeve GR, Talamonti WJ, Cox NJ, et al. Effectiveness and cost-benefit of influenza vaccination of healthy working adults: A randomized controlled trial. JAMA 2000;284:1655–63. [DOI] [PubMed]
- 89.Smith SC Jr, Blair SN, Criqui MH, Fletcher GF, Fuster V, Gersh BJ, et al. Preventing heart attack and death in patients with coronary disease. Circulation 1995;92:2–4. [PubMed]
- 90.Grundy SM, Balady GJ, Criqui MH, Fletcher G, Greenland P, Hiratzka LF, et al. Guide to primary prevention of cardiovascular diseases. A statement for healthcare professionals from the Task Force on Risk Reduction. American Heart Association Science Advisory and Coordinating Committee. Circulation 1997;95:2329–31. [DOI] [PubMed]
- 91.Prevention of coronary heart disease in clinical practice. Recommendations of the Second Joint Task Force of European and other Societies on coronary prevention. Eur Heart J 1998;19:1434–503. [DOI] [PubMed]
- 92.Preventive cardiology and atherosclerotic disease. American College of Cardiology Prevention and Cardiovascular Disease Committee. J Am Coll Cardiol 1994;24:838. [PubMed]
- 93.Mosca L, Grundy SM, Judelson D, King K, Limacher M, Oparil S, et al. AHA/ACC scientific statement: consensus panel statement. Guide to preventive cardiology for women. American Heart Association/American College of Cardiology. J Am Coll Cardiol 1999;33:1751–5. [DOI] [PubMed]
- 94.U.S. Department of Health and Human Services. Healthy People 2010: Understanding and improving health. Washington, DC: U.S. Government Printing Office; November 2000.
- 95.Singleton JA, Wortley P, Lu PJ. Influenza vaccination of persons with cardiovascular disease in the United States. Tex Heart Inst J 2004;31:22–7. [PMC free article] [PubMed]
- 96.Peters PH Jr, Gravenstein S, Norwood P, De Bock V, Van Couter A, Gibbens M, et al. Long-term use of oseltamivir for the prophylaxis of influenza in a vaccinated frail older population. J Am Geriatr Soc 2001;49:1025–31. [DOI] [PubMed]
- 97.Reichert TA, Sharma A. The seasonability of human mortality: the role of influenza. Int Congr Ser 2001;1219:95–101.
- 98.Reichert TA, Sugaya N, Fedson DS, Glezen WP, Simonsen L, Tashiro M. The Japanese experience with vaccinating schoolchildren against influenza. N Engl J Med 2001;344:889–96. [DOI] [PubMed]


