Abstract
Signal recognition particle (SRP)-dependent protein targeting to membranes is a multistep quality control process. Ribosomes translating weakly hydrophobic signal sequences can be rejected after SRP binding from the targeting reaction. Here, we show that the early targeting complex, formed by Escherichia coli SRP and its receptor FtsY with ribosomes translating the incorrect cargo EspP, is unstable and rearranges inefficiently into subsequent conformational states, such that FtsY dissociation is favoured over successful targeting. The N-terminal extension of EspP is responsible for these defects in the early targeting complex. The cryo-electron microscopic structure of this ‘false’ early complex with EspP reveals an ordered M-domain of Ffh (SRP protein) making two ribosomal contacts, and the NG-domains of Ffh and FtsY forming a distorted, flexible heterodimer. Our results provide a structural basis for SRP-mediated signal sequence selection during the recruitment of the SRP receptor.
INTRODUCTION
The universally conserved signal recognition particle (SRP) targets nascent proteins with hydrophobic signal sequences to translocation machineries at the target membrane1–4. Escherichia coli contains a minimal SRP consisting of the protein, Ffh (SRP54 homologue), and the 4.5S RNA which forms a stable hairpin structure with an evolutionary conserved tetraloop5. Ffh is composed of three domains: the N-terminal four-helix bundle and the GTPase domain that together form the functional NG-domain6, as well as the M-domain which binds the 4.5S RNA and the hydrophobic signal sequence7–9. FtsY, the bacterial SRP receptor, also contains a NG-domain10 preceded by an A-domain implicated in membrane and translocon (SecYEG in bacteria) binding11,12. The Ffh and FtsY NG-domains form a heterodimeric complex with a composite active site13,14, in which GTP hydrolysis is activated without requiring an external GTPase activating protein.
During co-translational targeting, both the SRP and FtsY undergo sequential and discrete conformational states in the SRP-FtsY heterodimer, which have been characterized by fluorescence spectroscopy, mutational and structural analyses. First, SRP binds with high affinity and is retained longer on ribosomes with a nascent chain in the exit tunnel or exposing a hydrophobic signal sequence (RNC, cargo)15,16. In these cargo-SRP complexes, the Ffh NG-domain is positioned close to the SRP RNA tetraloop17 which accelerates FtsY docking18 and stabilizes the early SRP-FtsY targeting complex19,20. Subsequently, phospholipids and SecYEG drive GTP-dependent rearrangement from the transient early state, which lacks tight interaction between the Ffh-FtsY NG-domains, into the closed state21,22. Rearrangement into the closed state involves formation of a stable NG-domain complex with a continuous interface around the GTP molecules13,14. Subsequent GTPase activation involves optimization of the GTPase active site and relocation of the entire NG-domain complex to the opposite end of the SRP RNA (activated state)22,23. This drives the delivery of the cargo onto the SecYEG protein-conducting channel and the disassembly of the SRP-FtsY complex after GTP hydrolysis24. Throughout the targeting cycle, these GTPase rearrangements allow the SRP and FtsY to actively sense and respond to the presence of the cargo to achieve accurate temporal and spatial control15,16,19.
In E. coli, the co-translational SRP pathway is mostly used for the integration of inner membrane proteins25,26. The hydrophobicity of the nascent chain is the main criterion for whether a nascent polypeptide is targeted co-translationally by the SRP pathway or post-translationally via SecA-SecB26,27. A threshold level of hydrophobicity appears to exist for SRP targeting because overexpression of the SRP cannot reroute a model substrate (maltose binding protein) from post- to co-translational targeting27, indicating a high degree of specificity of the SRP pathway. More recent work suggests that the selection of cargos by the SRP is a multistep quality control process15. If “incorrect cargos” containing weak or no signal sequences are bound to the SRP, they can be rejected after FtsY docking as the SRP-FtsY complex undergoes sequential conformational changes during the delivery and unloading of cargo. Amongst them, a critical checkpoint is the early RNC-SRP-FtsY targeting complex, which is stabilized by at least a factor of 50 by a correct cargo compared to incorrect cargos or non-translating ribosomes16,19.
A striking example for an “incorrect cargo” is the bacterial autotransporter EspP. The N-terminus of EspP comprises an unusual 55 amino acid signal sequence composed of a classical signal sequence and a N-terminal extension conserved among autotransporters 28,29 (Fig. 1a). In vivo, EspP is recognized by SRP, but translocated post-translationally using the SecA-B pathway28. In vitro, the RNCEspP (RNC displaying the EspP signal sequence) is bound by the SRP with high affinity (13.6 nM)15. However, the early SRP-FtsY targeting complex formed in the presence of RNCEspP yields a lower fluorescence resonance energy transfer (FRET) signal between donor-labeled Ffh and acceptor-labeled FtsY as compared to RNCs carrying strong signal sequences from bona-fide SRP substrates15. This indicates that the early targeting complex formed with RNCEspP adopts a different structure than that formed with a strong SRP cargo, such as FtsQ (RNCFtsQ)20.
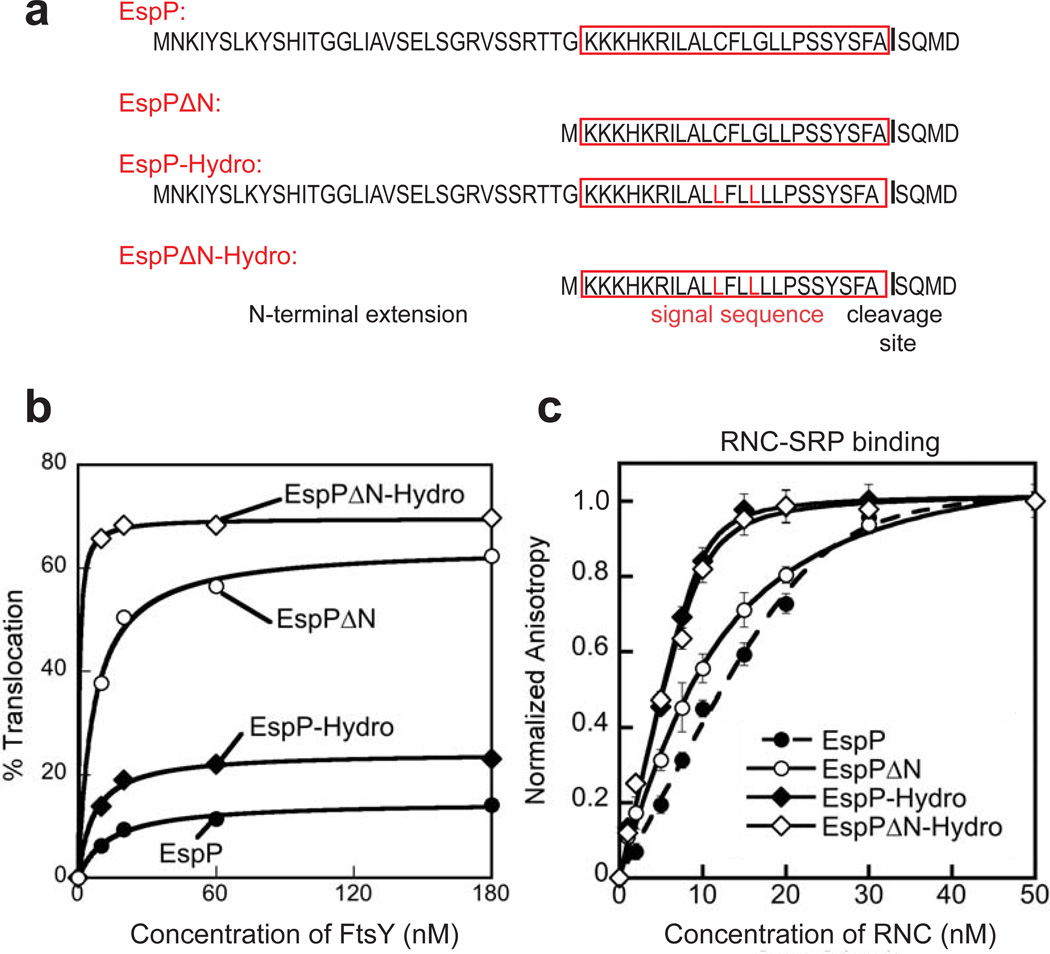
Figure 1. The N-terminal extension of EspP inhibits co-translational protein targeting but does not affect RNC-SRP binding.
(a) Signal sequences of EspP and its variants used in this study. The N-terminal extension of the signal sequence and the signal peptide cleavage site are indicated. The classical signal sequence is marked by a red box, and mutations are highlighted by red letters. (b)In vitro targeting and translocation efficiency of EspP signal sequence variants fused to Prolactin using microsomal membranes. (c) Equilibrium titration of RNC-SRP binding. Error bars represent standard deviation, with n = 3..
To provide insights into the molecular mechanism of signal sequence selection by the SRP, we have determined the structure of the RNCEspP-SRP-FtsY complex by single particle cryo-electron microscopy. By fitting the available high-resolution structures of the E. coli ribosome30, the SRP6–8,23,31 and FtsY10 into the EM density, we generated a quasi-atomic model of the RNCEspP-SRP-FtsY complex. This structure represents an unstable, ‘false’ early targeting complex, which is destined to be rejected from the SRP pathway. We identify functionally important differences in the conformation of the Ffh M- and NG-domains in the EM structure of this ‘false’ early targeting complex with RNCEspP as compared to the RNC-SRP complex17,32 and the early state complex formed with RNCFtsQ 20. Our structural data, underpinned by quantitative thermodynamic and kinetic analyses, provide a rationale for the rejection of this substrate from the SRP targeting pathway.
RESULTS
RNC-SRP binding alone cannot account for EspP rejection
We first asked whether deleting the N-terminal extension and increasing the hydrophobicity of the signal sequence may lead to a higher efficiency in the SRP-dependent targeting of EspP. To this end, the wildtype and three variants of the EspP signal sequence were fused to Prolactin as a model substrate, which is efficiently translocated across the membrane. One EspP signal sequence variant had the N-terminal extension of EspP deleted (EspPΔN), the second variant contained two leucine mutations (C42L G45L; EspP-Hydro), and the third variant comprised both of these alterations (EspPΔN-Hydro) (Fig. 1a). We measured the efficiency of SRP and FtsY to target these cargos in a heterologous in vitro protein targeting and translocation assay using microsomal membranes33,34. EspP was poorly translocated in vitro (Fig. 1b, Table 1 and Supplementary Fig. 1a), thus reproducing in vivo experiments28. Interestingly, increasing the hydrophobicity of the EspP signal sequence (EspP-Hydro) improved the translocation efficiency by less than a factor of two, whereas deletion of the N-terminal extension (EspPΔN and EspPΔN-Hydro) led to efficient targeting and translocation (Fig. 1b, Table 1), in agreement with previous observations in vivo28. These results strongly suggest that the N-terminal extension is the primary cause of the inefficient targeting of EspP.
Table 1. Interaction parameters of complexes formed by RNC, SRP and FtsY, and resulting translocation efficiency.
This table summarizes the results derived from experiments shown in Figure 1b,c, in Figure 2a and in Figure 5a,b. Values represent mean ±SD.
| RNC-SRP Kd (nM) |
Early complex Kd (nM) |
Early complex FRET |
Early-to-closed rearrangement ke→c (s−1) |
Closed complex assembly rate kon×103 (M−1s−1) |
% Translocation | |
|---|---|---|---|---|---|---|
| EspP | 13.6 ± 1.2 | 311 ± 11 | 0.39 ± 0.02 | 0.04 ± 0.01 | 9.2 ± 1.1 | 15.8 ± 1.2 |
| EspPΔN | 13.4 ± 0.78 | 58 ± 10 | 0.55 ± 0.01 | 0.13 ± 0.01 | 91.2 ± 7.8 | 66.7 ± 5.3 |
| EspP Hydro | 1.0 ± 0.05 | 300 ± 4 | 0.51 ± 0.01 | 0.10 ± 0.01 | 15.2 ± 1.2 | 22.8 ± 2.0 |
| EspPΔN Hydro | 1.0 ± 0.3 | 42 ± 5 | 0.61 ± 0.02 | 0.18 ± 0.02 | 112 ± 5.3 | 68.3 ± 1.9 |
We then asked whether deletion of the N-terminal extension improves the binding affinity of SRP for EspP cargos. As described earlier, RNCEspP bound to SRP with moderate affinity (Kd ~13.6 nM; Fig. 1c, Table 1), comparable to that of another SRP-dependent substrate, 3A7L (LALLLLLALA), which is efficiently targeted15. Deletion of the N-terminal extension (RNCEspPΔN) did not alter this binding affinity (Fig. 1c, Table 1). As expected, RNCEspP-Hydro, which contains a highly hydrophobic signal sequence, bound to SRP strongly (Kd ~1.0 nM; Fig. 1c). Deletion of the N-terminal extension (RNCEspPΔ–Hydro) did not affect the SRP binding affinity either (Fig. 1c, Table 1). These results show that the N-terminal extension did not exert its inhibitory role on the SRP pathway by reducing the affinity between the SRP and the RNCs (Table 1). In light of these results and recent work15, we hypothesize that the N-terminal extension of the EspP signal sequence interferes with subsequent steps of the SRP pathway, such as SRP-FtsY assembly.
EspP N-terminal extension leads to a distorted early complex
The SRP loaded with a correct cargo forms a stabilized RNC-SRP-FtsY early targeting complex, whereas incorrect cargos fail to provide this stabilization15,16. To test whether this were the case with EspP and its variants, we measured the stability of the early complex using FRET between DACM (donor)-labeled Ffh(C235) and BODIPY-FL (acceptor)-labeled FtsY(C487)35. The early targeting complexes formed with RNCEspPΔN and RNCEspPΔN–Hydro were highly stable (Fig. 2a and Table 1), whereas inclusion of the N-terminal extension in the EspP signal sequence caused a reduction in the stability of the early targeting complexes formed with RNCEspP and RNCEspP-Hydro (Fig. 2a, Table 1). In addition, the maximal FRET efficiencies of the early targeting complexes are 0.10–0.16 units lower with RNCs containing nascent chains with the N-terminal extension (Fig. 2a, Table 1), suggesting that the early targeting complex formed with RNCEspP positions the SRP and FtsY GTPase domains differently from that with RNCEspPΔN or a correct SRP cargo.
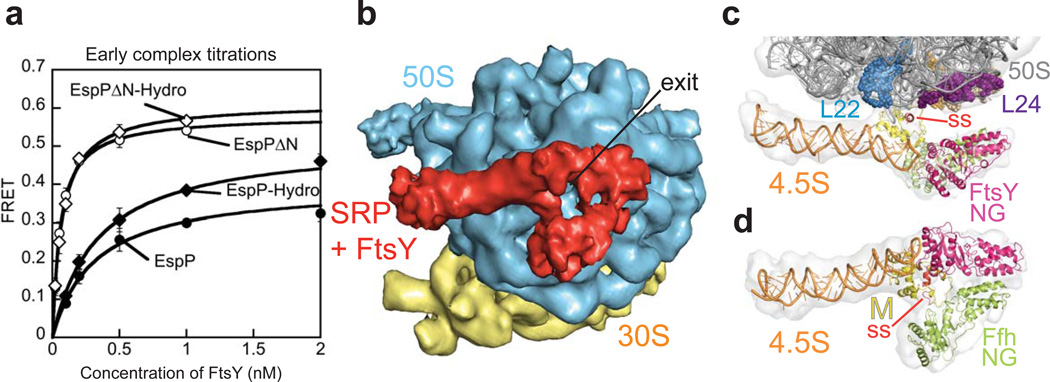
Figure 2. The EspPN-terminal extension leads to a weaker and distorted early targeting complex.
(a) Equilibrium titration of the SRP•FtsY early complex in the presence of RNCs bearing EspP signal sequence variants. 300–500 nM RNCs were used to ensure that most of the SRP is ribosome-bound. Error bars represent standard deviation, with n = 3. (b) Cryo-EM structure of RNCEspP-SRP-FtsY shown with the view into the polypeptide exit tunnel. The large ribosomal subunit (50S) is depicted in blue, the small ribosomal subunit (30S) in yellow and the single chain SRP-FtsY in red. (c,d) EM reconstruction and quasi-atomic model of the RNCEspP-SRP-FtsY early complex (c) in a close-up view from the back of the 50S subunit and (d) in a view as in (b). The experimental density is shown in light grey, 4.5S RNA in orange, the EspP signal sequence (ss) in red, the Ffh M-domain in yellow, the Ffh NG-domain in greenyellow, the FtsY NG-domain in magenta, the 50S rRNA in dark gray, ribosomal proteins L24 in purple and L22 in skyblue. The density of the ribosome is not shown in (d) for clarity.
Cryo-EM structure of the RNCEspP-SRP-FtsY early complex
To gain insight into the nature of these differences, we determined the structure of the RNCEspP-SRP-FtsY early targeting complex by single particle cryo-EM. To efficiently assemble the EspP early complex, we fused the C-terminus of FtsY to the N-terminus of Ffh via a 31-amino acid, glycine- and serine-rich linker (~117 Å). The resulting single-chain construct behaved similarly to the unlinked SRP and FtsY in ribosome binding and GTP hydrolysis experiments 20. Importantly, a similar FtsY-SRP fusion was completely functional in vivo 36. In co-sedimentation experiments, this single-chain SRP construct bound RNCEspP equally well as RNCFtsQ (Supplementary Fig. 1b). For cryo-EM, RNCEspP complexes were incubated with a ten-fold excess of single-chain SRP construct in the absence of GTP to prevent subsequent rearrangements of the targeting complex, which may lead to additional conformational heterogeneity. After computational sorting and refinement (online Methods, Supplementary Fig. 2), the RNCEspP-SRP-FtsY structure was reconstructed at 12 Å resolution (FSC 0.5 criterion) (Supplementary Fig. 3).
At the exit of the ribosomal tunnel, distinct elongated density was observed accounting for the SRP-FtsY complex (red in Fig. 2b), which had two connections to the large ribosomal subunit (50S; blue in Fig. 2b). A two-lobed density is positioned directly above the tunnel exit where the EspP nascent chain emerged. To generate a quasi-atomic model of the EspP early state, the crystal structures of the E. coli 70S ribosome 30, the E. coli SRP 6,8,23 and FtsY 10 were placed into the experimental density (Fig. 2c,d). The NG-domains of Ffh and FtsY were placed into the two-lobed density above the tunnel exit (Fig. 2c,d). The quasi-atomic model indicates that FtsY forms a contact with the 4.5S RNA tetraloop, which has been shown to stabilize FtsY binding to the SRP in the early complex (Fig. 2c,d)20,37. Although our single-chain construct of Ffh and FtsY contained the complete N-terminal A-domain of FtsY, this domain is not visible in our structure and thus is likely disordered. Notably, the complete M-domain of Ffh could be located in the density (Fig. 2c,d; see more discussions below). Two conformations of the signal sequence bound to the M-domain were reported8,9. In the quasi-atomic model, we included the structure fitting with the higher correlation coefficient8. We cannot exclude that the EspP signal sequence is bound slightly differently to the Ffh M-domain, as it is 35 residues longer than the 20 amino acid yeast dipeptyl aminopeptidase B signal sequence visible in the crystal structure8. Upon FtsY docking, the largest conformational change in the current structure when compared to the RNCFtsQ-SRP complex17 is the detachment of the Ffh N-domain from ribosomal protein L23 to contact the FtsY NG-domain (Supplementary Fig. 4).
M-domain is ribosome-bound in the RNCEspP-SRP-FtsY complex
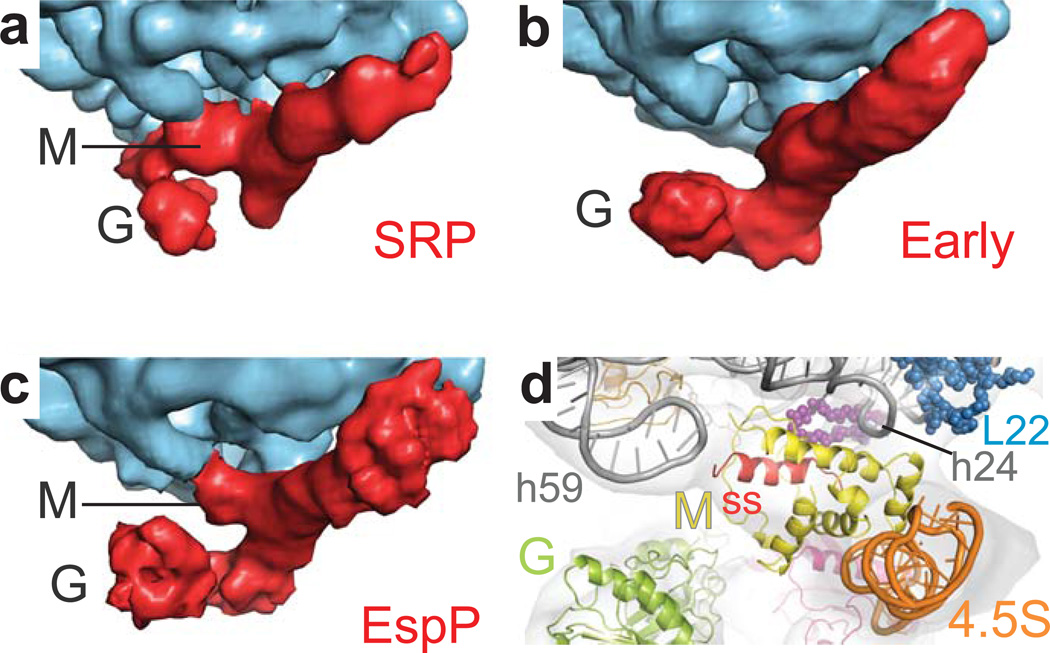
SRP recognition of a signal sequence leads to tight binding of the SRP to the RNC. In the RNC-SRP complex, the signal sequence is buried between the M-domain and the ribosome17,32, and the M-domain is well-resolved forming contacts with rRNA helices h24 and h59 (Fig. 3a). In contrast, in the early targeting complex formed by RNCFtsQ 20, the signal sequence-binding part of the M-domain detaches from h59, presumably without releasing the signal sequence, and is not visible in the EM reconstruction (Fig. 3b, Supplementary Fig. 5). Only a single ribosomal contact remains involving the 4.5S–binding part of the M-domain and h2420. In the RNCEspP-SRP-FtsY ‘false’ early complex, however, we found that the M-domain is ordered (Fig. 3c, Supplementary Fig. 5) and forms contacts to h24 and h59. This M-domain geometry resembles the M-domain conformation observed in the RNCFtsQ-SRP complex devoid of FtsY (Fig. 3a,c,d). Thus, important rearrangements induced by the correct cargo that lead to a flexible M-domain20 are not observed in the ‘false’ early complex.
Figure 3. M-domain arrangement during co-translational targeting.
(a) Close-up view on the exit of the ribosomal tunnel and the M-domain of the RNCFtsQ-SRP cryo-EM structure17. Color coding is the same as in Figure 2c. (b) Same view as in (a) for the RNCFtsQ-SRP-FtsY early complex20. (c) Cryo-EM structure of the RNCEspP-SRP-FtsY early complex shown in the same view as (a,b) for comparison. (d) Close-up on the M-domain with the signal sequence in the EM reconstruction and quasi-atomic model of the RNCEspP-SRP-FtsY complex. Color coding is the same as in Figure 2d,e. The positions of the Ffh M- and G-domains are indicated.
The FtsY NG-domain interacts weakly with RNCEspP-SRP
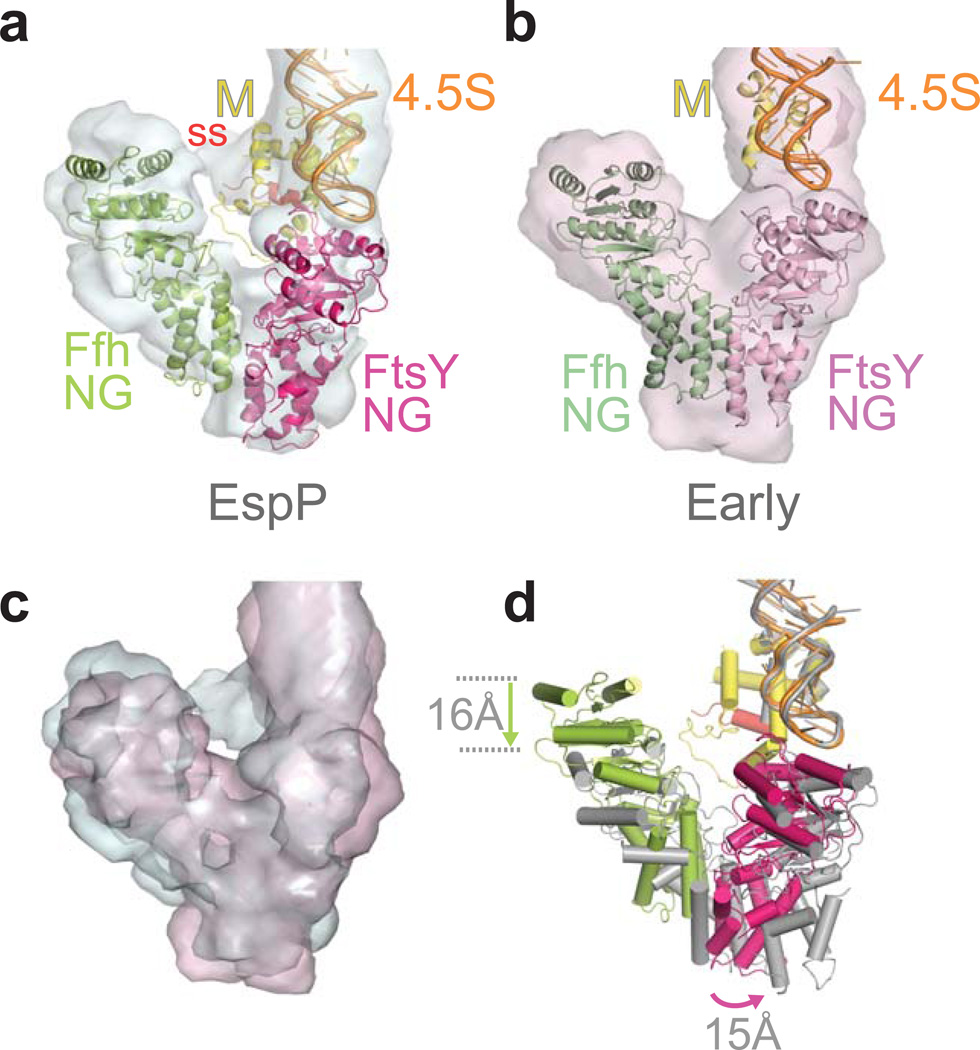
In the early targeting complex formed with RNCEspP, the NG-domains of Ffh and FtsY are weakly associated (Fig. 4a) compared to the NG-domain arrangement observed in the crystal structures in which extensive contacts are formed between the two NG-domains13,14. Compared to the FtsQ early state (Fig. 4b), the Ffh NG-domain is closer to the M-domain (~16 Å) in the ‘false’ early complex. In our structure, the N-domain of Ffh contacts the N-domain and the NG-domain interface of FtsY. Overall, the NG-domain interaction is not very well defined (see below). In contrast, in the early complex formed with RNCFtsQ, the Ffh-FtsY N-domains interact to form a pseudo-symmetric V-shaped complex (Fig. 4b,c)20. In both complexes, the G-domains are not involved in the interaction, and both GTPase active sites are accessible, consistent with the fact that the early complexes can form with or without nucleotides (Supplementary Fig. 1). In agreement with the FRET measurements (Fig. 2a), we observe a larger distance of the Ffh-FtsY G-domains (~68 Å) as compared to the FtsQ early complex (~60 Å)20 and to the closed complex in which the G-domains interact tightly (31 Å)13,14.
Figure 4. Ffh-FtsY NG-domain arrangement in the ‘false’ early complex formed with EspP compared to the productive early complex formed with a correct cargo.
(a) In the RNCEspP•SRP•FtsY ‘false’ early complex, the Ffh-FtsY NG-domains have a weak interface involving the N-domain of Ffh and the NG-domain of FtsY. The experimental density is depicted in pale cyan; unfilled density indicates flexibility in this part. (b) Pseudo-symmetric V-shaped NG-domain arrangement in the early targeting complex with RNCFtsQ 20. The experimental density of this complex is shown in light pink. (c,d) Overlays of (c) the EM densities of the early targeting complexes from (a) & (b), and (d) the corresponding quasi-atomic models. Arrows indicate positional differences of the NG-domains. For the overlays (c,d), the RNA tetraloops of the structures (c) and models (d) have been aligned. Color codings of the quasi-atomic models are as in Figure 2 except in (d), where the NG-domain complex of the early targeting complex with RNCFtsQ is depicted in grey for clarity.
The FtsY NG-domain is tilted in the ‘false’ early complex, and the tip of the N-domain is displaced ~15 Å towards the Ffh NG-domain (Fig. 4c,d). The interaction between the FtsY G-domain and the RNA tetraloop is a major stabilizing interaction of the early targeting complex (Fig. 4b)20,37. The tilted conformation of the FtsY NG-domain in the EspP ‘false’ early complex likely weakens its interaction with the RNA tetraloop. Together with the weak interaction of the Ffh-FtsY NG-domains, this likely explains the low affinity of the early targeting complex formed with RNCEspP (Fig. 2a, Table 1). In our EM structure, FtsY is covalently linked to Ffh by a flexible linker and therefore, this weak interaction is stabilized to prevent the disassembly of the complex.
We analyzed the structural heterogeneity of the SRP-FtsY in the ‘false’ early complex. The 3D variance map of the RNCEspP-SRP-FtsY reconstruction indicates that the flexibility of the complex is highest in the Ffh-FtsY NG-domains (Supplementary Fig. 6). In comparison, the M-domain and the rRNA h59 are more rigid in this structure. We sorted for the SRP-FtsY heterogeneity in the ‘false’ early complex using 3D–classification by maximum-likelihood and multi-reference 3D angular refinement38. Maps showing differences in the SRP-FtsY part of the structure were used as input maps for supervised classification using the software Spider39 resulting in two maps with clearly distinct SRP-FtsY conformations (Supplementary Fig. 7). Both structures contain the density for the M-domain, and a connecting density is visible between the Ffh NG-domain and the M-domain. The position of the Ffh NG-domain is different in the two structures. In one structure, the Ffh NG-domain is closer to the M-domain. In the second, the Ffh NG-domain moved away from the M-domain and is tilted such that the G-domain has a larger distance to the ribosome (Supplementary Fig. 7). Both conformations differ substantially from the pseudo-symmetric NG-domain arrangement observed in the early state (Fig. 4b)20, underscoring multiple possibilities of how the NG-domains interact in the early state40 depending on the nature of the nascent chain signal sequence.
Slow rearrangement of RNCEspP to the closed/activated state
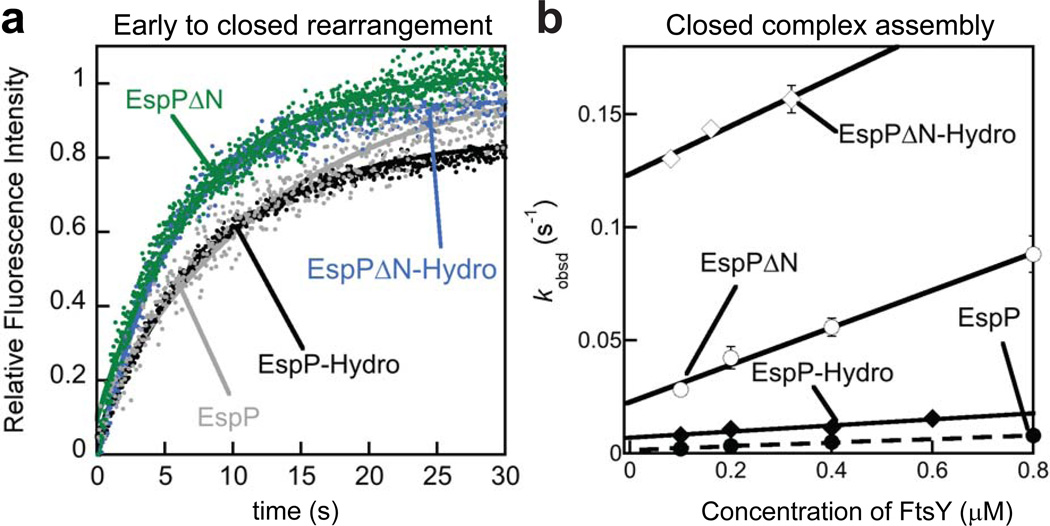
The less favorable Ffh-FtsY NG-domain arrangement observed in the RNCEspP early complex likely impedes the formation of the stable closed complex, which is the subsequent step in the SRP pathway and required to deliver the RNC to the membrane21. Consistent with this hypothesis, RNCEspP and RNCEspP-Hydro mediated the rearrangement from the early to closed complex at rate constants of 0.04 s−1 and 0.10 s−1, respectively, which are slower than that previously observed with bona-fide SRP cargos by a factor of 3–7 (Fig. 5a, Table 1, Supplementary Table 1)15. When the N-terminal extension is deleted from EspP or EspP-hydro, this rearrangement occurred at faster rates, 0.13 s−1 and 0.18 s−1 respectively. Collectively, the lower stability of the early targeting complex (Fig. 2a, Table 1) and the slower early-to-close rearrangement (Fig. 5a) would lead to less efficient assembly of the closed complex, which is evidenced by direct measurements using a FRET assay: RNCEspP and RNCEspP-Hydro mediated the assembly of the closed complex an order of magnitude slower than RNCEspPΔN and RNCEspPΔN-Hydro (Fig. 5b, Table 1).
Figure 5. The N-terminal extension leads to a less productive early complex and slower assembly of the closed SRP-FtsY complex.
(a) Kinetics for rearrangement of the early to the closed complex for EspP 15 and variants. (b) Assembly rates of the closed SRP-FtsY complex mediated by RNCs displaying EspP signal sequence variants. Error bars represent standard deviation, with n = 4.
DISCUSSION
Genetic, biochemical and structural analyses of the SRP and its receptor have led to considerable insight into the co-translational targeting cycle2–4,41,42. However, the molecular mechanism and structural details by which substrate proteins are surveyed and directed into the co- or post-translational targeting pathways have remained elusive to date. Here, we address this question using EspP, a secreted serine protease autotransporter with an unusually long, less hydrophobic signal sequence containing a basic N-terminal extension (Fig. 1a)28,29,43, as a model substrate. EspP was chosen because RNCs translating EspP have been shown to interact well with the SRP in vitro15, but EspP is targeted post-translationally in vivo 28. We show that the N-terminal extension preceding the signal sequence causes EspP to be rejected from the SRP pathway after SRP binding. Our cryo-EM analysis of the RNCEspP-SRP-FtsY ‘false’ early complex provides a structural basis for rejection of RNCEspP from the SRP pathway.
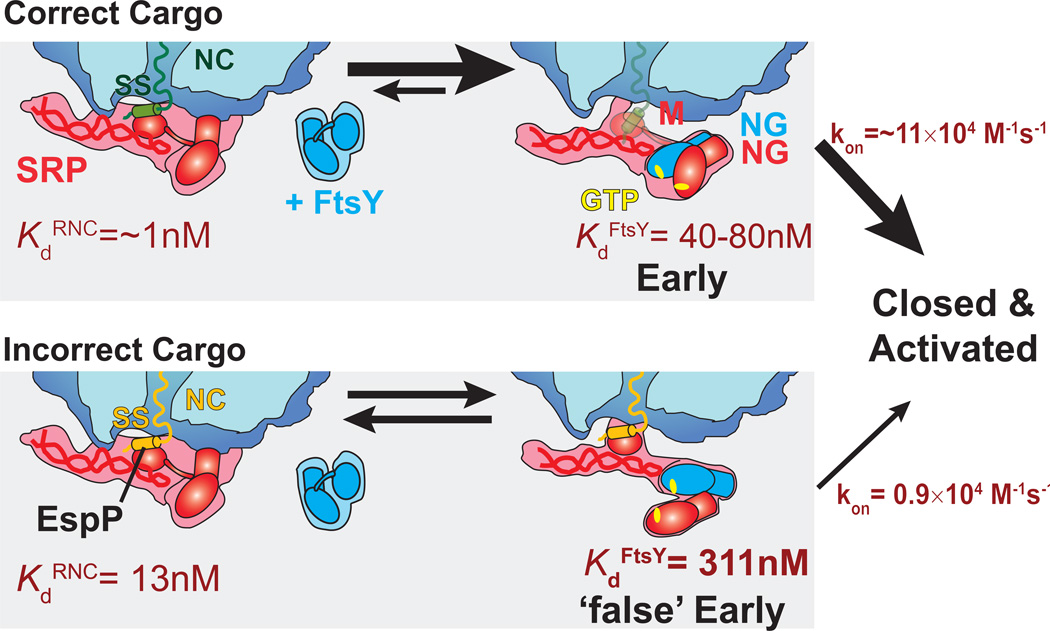
Our results show that the basic N-terminal extension of the EspP signal sequence provides a strong ‘SRP-avoidance’ sequence that rejects substrate proteins from the SRP pathway. Even the EspP variant bearing a highly hydrophobic signal sequence is targeted poorly by the SRP in the presence of this extension. Interestingly, the N-terminal extension does not exert its inhibitory effect by disrupting high affinity binding of SRP to the RNC, as both RNCEspP-Hydro and RNCEspPΔN-Hydro bound the SRP with 1 nM affinity but were targeted with significantly different efficiency. Rather, this extension compromises the ability of RNCs to stimulate subsequent SRP-FtsY interactions, which are critical for completing the targeting cycle. In particular, SRP loaded with RNCEspP or RNCEspP-Hydro forms a less stable and less productive early targeting complex with FtsY, leading to two consequences that disfavour the targeting reaction. First, FtsY dissociates more easily from a labile early targeting complex, requiring additional rounds of assembly and disassembly for the targeting reaction to proceed. Second, a distorted early targeting complex renders subsequent formation of a closed/activated RNCEspP-SRP-FtsY complex slower. For these reasons, we term the early complex formed with RNCEspP a ‘false’ early complex that is less conducive to complete a successful protein targeting reaction.
The cryo-EM structure of the RNCEspP–SRP-FtsY complex reveals the structural origin of the unstable and non-productive ‘false’ early complex. Compared to the early complex with RNCFtsQ, which adopts a pseudo-symmetric V-shaped SRP-FtsY NG-domain orientation, we observe in the RNCEspP ‘false’ early complex a flexible, asymmetric heterodimer structure arrangement in which the N-domain of the SRP protein primarily interacts with the NG-domain interface of FtsY (Fig. 4a,c,d; Supplementary Fig. 7). Furthermore, the Ffh NG-domain is displaced towards the M-domain, and the FtsY NG-domain is tilted towards Ffh. This likely results in a weaker interaction with the SRP RNA tetraloop, which is crucial for formation and stabilization of the early Ffh-FtsY complex37,43. Both effects may contribute to the lower affinity of the early targeting complex formed with RNCEspP (Kd = 311 nM) as compared to those complexes formed with a strong SRP cargo (Kd ~40 nM) (Fig. 6). The conformational heterogeneity of the Ffh-FtsY NG-domain complex provides additional evidence for the lack of stable molecular interactions in the ‘false’ early complex. Moreover, it indicates that the incorrect cargo interferes with the formation of a pseudo-symmetric V-shaped NG-domain arrangement (Supplementary Fig. 7). Rearrangement into the stable, quasi-symmetric closed complex from this unfavourable and rather unstable NG-domain arrangement(s) would be more difficult, compared to a complex with properly prepositioned NG-domains.
Figure 6. Model of signal sequence surveillance by the SRP and FtsY.
The SRP tightly binds to correct cargos. FtsY binding leads to detachment of SRP from the ribosome, and a pseudo-symmetric NG-domain arrangement. EspP has a less hydrophobic signal sequence, leading to a moderate affinity of SRP (13 nM). FtsY has a lower affinity (311 nM) for this SRP-RNCEspP complex and forms a less favourable, distorted and flexible Ffh-FtsY NG-domain heterodimer. The EspP ‘false’ early targeting complex NG-domains that are loosely associated and rearrange inefficiently into the closed/activated state leading to premature FtsY dissociation rather than successful completion of the targeting reaction.
An intriguing observation in this ‘false’ early complex is an ordered and visible Ffh M-domain forming two ribosomal contacts to rRNA helices 24 and 59. This arrangement resembles the ribosome-bound M-domain in the RNC-SRP structures in the absence of FtsY17,32 where the signal sequence is buried between the M-domain and the ribosome. In contrast, in the early complex formed with a strong SRP cargo, the M-domain with the bound signal sequence became flexible20, which likely represents a FtsY-induced rearrangement that ultimately leads to the release of the signal sequence from SRP and successful handover of the cargo to the translocon. It remains to be clarified whether the detachment of the M-domain is a prerequisite for a productive early state complex. The precise structural details of how the signal sequence bound M-domain communicates with the Ffh-FtsY NG-domains are still not understood. In principle, information could be transmitted from SRP to FtsY via the structurally flexible linker connecting the Ffh NG-domain with the M-domain or via the SRP RNA. Biochemical and structural data show that binding of the signal sequence to SRP induces an extensive conformational change that arranges the SRP RNA tetraloop to favorable positions for FtsY docking 17,20,37,43. Concomitantly, this event requires a major reorientation of the linker to bring the Ffh NG-domain close to the tetraloop, which would be driven by productive interaction of the M-domain with a correct signal sequence on the ribosome, but not with an incorrect signal sequence. Our structure offers a first view of cargo-induced differences.
How does the N-terminal extension of EspP disrupt the early targeting complex? The N-terminal extension (EspP 1–25; Fig. 1) has a high propensity to form beta strand structures44 One hypothesis is that this presents a steric block that prevents productive interaction of the SRP M-domain with the signal sequence. This could disrupt the communication between the M- and NG-domains of Ffh and thus lead to less productive interaction of Ffh with FtsY. Alternatively, the steric block from the EspP N-terminal extension could directly interfere with the formation of the NG-domain complex between Ffh and FtsY. The latter possibility is less likely, given that the NG-domain interface is at least 40 Å away from the hydrophobic core of the M-domain with the signal sequence.
The less favourable, non-symmetric NG-domain arrangement observed in the ‘false’ early state is likely responsible for its lower stability and slower rearrangement to the closed state observed biochemically. Thus, dissociation of the ‘false’ early complex will likely dominate over the delivery of RNC to the target membrane (Fig. 6). Moreover, translation of EspP continues during SRP targeting, and ribosomes with long nascent chains cannot be targeted efficiently via the SRP pathway to the membrane45, imposing a limited time window for successful SRP-dependent targeting45,46. Incorrect signal sequences like EspP interfere with efficient SRP-FtsY early complex formation, and thereby render the targeting reaction to proceed too slowly and unlikely to be completed within this critical time window, thus resulting in their rejection from the co-translational targeting pathway. Similar observations have been made with RNCs bearing other incorrect signal sequences, which are rejected at steps after the initial SRP binding, in part due to the unstable and unproductive early complex15.
The SRP is present in all kingdoms of life. The mechanisms described here are obtained with prokaryotic SRP. Similar mechanisms are likely used by the eukaryotic SRP to reject incorrect substrate proteins. N-terminal extensions that act as ‘SRP-avoidance’ sequences are not unique to bacterial autotransporters like EspP. Several mitochondrial proteins contain extensions N-terminal to their first hydrophobic transmembrane domain 47. These extensions effectively inhibit the nascent polypeptide from engaging the SRP pathway, and their deletion leads to efficient SRP-dependent targeting to the ER 47. Presumably, these N-terminal extensions are important for directing the proteins to the mitochondria. Moreover, in a systematic screen of Saccharomyces cerevisiae SRP substrates, substantial binding of the SRP to nascent chains with neither transmembrane nor signal sequences was detected48. Nascent polypeptide-associated complex (NAC), which has overlapping substrate specificity with SRP in vivo, was suggested to improve the specificity of SRP in binding the correct cargos49; however, yeast cells lacking NAC do not suffer from increased protein mistargeting48. Given the evolutionary conservation of the SRP and its receptor, it is likely that highly similar surveillance mechanisms using induced fit and proofreading mechanisms exist also in eukaryotes to reject incorrect substrate proteins after initial SRP-RNC recognition.
ONLINE METHODS
Preparation of RNCEspP-SRP-FtsY complexes
The plasmid pUC19StrepEspPSecM encodes for a N-terminal triple StrepII-tag, 88 amino acids of the EspP N-terminus with the signal sequence and 33 amino acids of the SecM C-terminus including the stalling sequence15. pUC19StrepEspPSecM was transcribed and translated in vitro using membrane-free cell extract as previously described50. Briefly, the ribosome-nascent chain complexes (RNCs) were purified by sucrose gradient centrifugation and affinity chromatography50. After centrifugation, the ribosomal pellet was dissolved in buffer A (50 mM Hepes-KOH, 100 mM KOAc, 8 mM Mg(OAc)2, pH 7.5). To stabilize the SRP-FtsY complex, we used a construct in which full-size FtsY is linked to full-size Ffh via a 31 amino acid linker (corresponding to ~ 117 Å linker)20. The single-chain SRP-FtsY construct (scSRP) was purified by affinity purification via the hexahistidine-tag and anion exchange chromatography (MonoQ) as described before20. The complexes were reconstituted by incubation of 200 nM RNCs displaying the EspP nascent chain (RNCEspP) with a 10-fold molar excess of scSRP for at least 60 min on ice. The binding of scSRP to RNCEspP under these conditions was confirmed by co-sedimentation experiments through a 0.5 M sucrose cushion in buffer A (Supplementary Fig. 1).
Electron microscopy and image processing of RNCEspP-SRP-FtsY complexes
Lacey carbon grids (Cu 300 mesh, Agar scientific) were glow discharged on both sides for 30 s, and 3 µl sample (200 nM RNCs) was applied on the carbon side. The grids were plunge frozen in liquid ethane using a Mark IV vitrification robot (FEI) after blotting for 1 s at 20 °C and 100% relative humidity. CCD frames were recorded under low-dose conditions on a Tecnai G2 Polara (FEI) operating at 300 kV and a specimen level magnification of 76,000x with a Gatan 4k x 4k CCD camera in a defocus range between −0.7 µm and −5.7 µm with an initial pixel size of 1.875 Å on the object scale.
The contrast transfer function (CTF) was determined and corrected with bctf (Bsoft package 51). The CCD frames were re-sampled to 3.75 Å/pixel. A total number of 165,820 particles was selected semi-automatically from 1,974 CCD frames using e2boxer (EMAN2)52. The data were classified into four subsets according to ribosomal conformations, using low pass filtered ratcheted and not-ratcheted vacant ribosomes as initial references (EMDB IDs: 1363, 105653,54), and SRP-FtsY complex presence in SPIDER (Supplementary Fig. 2)39. We also used maximum likelihood 3D refinement by XMIPP 38 on the complete dataset using a band-pass filtered 50S structure as initial reference to avoid bias in the sorting procedure55. However, the resulting three structures containing density corresponding to SRP-FtsY ultimately did not refine to the same resolution as with the approach described above. The pool of not-ratcheted RNCEspP–SRP-FtsY complex contained 52,020 particles. Special care was taken not to have an overrepresentation of raw images in some of the class averages used for the reconstruction. Limiting the population of each class to the same number resulted in using 46,945 images for the final reconstruction. Full-size images were used for the last round of refinement. At the end of refinement, the data were split randomly to generate two reconstructions. These two independent reconstructions were then used for calculation of the FSC curve, and the resolution was assessed to be 12 Å by the Fourier shell correlation with a 0.5 threshold, and 8.3 Å according to the FSC 0.143 criterion56 (Supplementary Fig. 3).
Analysis of Conformational Heterogeneity
The conformational heterogeneity of the RNCEspP-SRP-FtsY complex was analyzed by maximum likelihood 3D refinement using mlf_refine3d (XMIPP57) and by hypergeometrically stratified resampling (HGSR) using SPARX58.
For the 3D variance map of RNCEspP-scSRP (Supplementary Fig. 6), HGSR in SPARX was applied with an angular step size of 7.5 degree to assign the data set into 377 angular regions. This resulted in 23 2D–projection images per stratum. With 90% of data retained in least populated strata, each resampled volume was generated from a set of 8,671 projections (21.4% of total data set) and a total of 9,990 re-sampled volumes were generated with these settings.
The average and variance (sxvar.py of SPARX) were obtained for the 15 Å low-pass-filtered, re-sampled volumes for the data set. Supplementary Figure 6 shows the 3D average map of all re-sampled volumes for RNCEspP-scSRP coloured by their variance. The color code is based on the voxel values of the variance map.
In Xmipp, the data was split randomly into 10 parts with which 10 initial volumes were calculated using the angles from an alignment against a vacant, low pass filtered not-ratcheted ribosome53. The resulting volumes were compared, and based on differences in the SRP-FtsY density, three volumes were chosen for simultaneous 3D multi-reference refinement and classification in mlf_refine3d. The multi-reference refinement was converged after 20 iterations. Visual inspection of these volumes indicated the existence of at least two clearly distinct SRP-FtsY conformations showing differences in the Ffh M-domain and in the Ffh-FtsY NG-domain arrangement. Furthermore, these three volumes were used as initial models for supervised classification using SPIDER resulting in two very similar volumes and a third markedly different volume. The two sub-pools after 3 rounds of supervised classification contained 19,292 and 21,282 particles showing clear differences in the SRP-FtsY conformation (Supplementary Fig. 7).
Generation of the Quasi-Atomic Model
The crystal structure of the E. coli 70S ribosome30 was fitted into the EM map with UCSF Chimera59. The atomic model of the E. coli SRP-FtsY complex was generated using the crystal structures of (1) the E. coli 4.5S RNA23, of (2) the Sulfolobus solfataricus Ffh M-domain with the yeast dipeptidyl aminopeptidase signal sequence8 which fitted our density better (correlation coefficient 0.152) as compared to the Methanococcus jannaschii M-domain with an idealized signal sequence (cc of 0.148)9, and of (3,4) the Ffh and FtsY NG-domains6,10. The domains were placed as rigid bodies into the EM density using UCSF Chimera 59. The resulting model was energy minimized in CNS Version 1.060. The figures were generated with PyMOL (DeLano Scientific).
Protein Targeting and Translocation Assay
The signal sequences of EspP and variants (Fig. 1a) were fused N-terminally to the signal peptidase cleavage site and to the mature region of pre-Prolactin. Their respective targeting efficiencies were determined by a co-translational protein targeting and translocation assay in the presence of SRP, FtsY and endoplasmatic reticulum microsomes, as described previously34. To accurately determine targeting efficiency and to avoid a bottleneck in the translocation step, Prolactin was chosen as a model substrate rather than the EspP protein because its translocation is highly efficient across the membrane and thus is not rate-limiting for the observed reaction. For the same reason, microsomal membranes were used for this assay because they are more active in in vitro translocation compared to E. coli membranes. Endogenous SRP and SRP receptor from microsomal membranes have been removed by high salt wash and trypsin digestion. Importantly, E. coli SRP and FtsY can mediate protein targeting in this assay as efficiently as the mammalian SRP and SRP receptor33.
Fluorescence Measurements
Fluorescence measurements were carried out on a FluoroLog-3–22 spectrofluorometer (Jobin-Yvon) or an SF-2004 stopped-flow apparatus (KinTek). In experiments involving SRP-RNC complexes, saturating concentrations of RNCs (a factor of 50 or 100 above the respective Kd value) were used to ensure that >90% of SRP was bound with cargo. All reactions were carried out at 25 °C in assay buffer (50 mM KHEPES pH 7.5, 150 mM KOAc, 10 mM Mg(OAc)2, 2 mM DTT and 10% glycerol).
The binding affinities of SRP for RNCs were determined using fluorescence anisotropy as described 15. Equilibrium titrations were carried out with 5–10 nM of fluorescein-labeled Ffh(C421) and varying concentrations of RNC. Observed anisotropy values (A) are fit to equation 1,
| (1) |
in which A0 is the anisotropy value of free SRP, A1 is the anisotropy value when SRP is bound to cargo, and Kd is the equilibrium dissociation constant of SRP for the RNC.
Equilibrium titrations of the early intermediate were carried out using FRET between donor (DACM)-labeled SRP(C235) and acceptor (BODIPY-FL)-labeled FtsY(C487), respectively, as described previously35. Rate constants for rearrangement of the early intermediate to the closed complex were measured using SRP-C235 labeled with acrylodan. An RNC-SRP-FtsY early intermediate was preformed in the presence of saturating SRP and FtsY with respect to the Kd value of the early intermediate. The reaction was initiated by mixing 500 mM GppNHp with the early intermediate. The time course of fluorescence change was fit to single-exponential functions to give the rearrangement rate constants.
Association rate constants for SRP–FtsY closed complex formation were determined using FRET as described previously19. Complex assembly was initiated by mixing SRP with varying amounts of FtsY in the presence of 100 µM GppNHp, and the time course of fluorescence change was monitored, giving the observed rate constants for SRP-FtsY binding (kobsd). Linear fits (eq 2) of the observed rate constants were plotted as a function of FtsY concentration to give the second-order association rate constant, kon.
| (2) |
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. T. Shaikh for advice with Spider refinement and Dr. P. Penczek for assistance with SPARX. We thank Dr. M. Bacia for excellent technical assistance and the protein expression facility at EMBL Heidelberg as well as the Partnership for Structural Biology in Grenoble for support. The Polara microscope is part of the IBS Structural Biology and Dynamics GIS-IBISA-labeled platform. C.S. acknowledges support by the Agence Nationale de la Recherche (JC09_471873), the region Rhône-Alpes (CIBLE_1976) and the European Research Council by an ERC Starting grant (project 281331). K.K. was supported by a postdoctoral EMBO fellowship. We thank I. Saraogi in the Shan lab for sharing unpublished results and for critical reading of the manuscript. S.S. is supported by US National Institutes of Health (NIH) grant R01 GM078024, and the Fellowship for science and engineering from the David and Lucile Packard foundation. A.A. was supported by the US National Institute of General Medical Sciences (NIGMS) Ruth L. Kirschstein National Research Service Award (F31GM095294) and the NIH/ National Research Service Award (NRSA) Training Grant 5T32GM07616. X.Z. is a Howard Hughes Medical Institute Fellow of the Helen Hay Whitney Foundation.
Footnotes
ACCESSION CODES
Protein Data Bank: The atomic model of SRP-FtsY in the ‘false’ early conformation has been deposited with accession code 3ZN8. The cryo-EM map has been deposited in the 3D–EM database under accession number EMDB-2316.
AUTHOR CONTRIBUTIONS
C.S., I.B, X.Z., and S.S designed experiments; C.S., K.H., O.V.L., A.A. and X.Z. performed sample preparations; A.A. and X.Z. carried out biochemical experiments; K.K., G.S. and M.K. performed the electron microscopy; O.V.L., M.K. and C.S. performed image analysis and model building; C.S., O.V.L., A.A., X.Z., and S.S. prepared the manuscript.
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
MAIN TEXT REFERENCES
- 1.Gilmore R, Walter P, Blobel G. Protein translocation across the endoplasmic reticulum. II. Isolation and characterization of the signal recognition particle receptor. J. Cell Biol. 1982;95:470–477. doi: 10.1083/jcb.95.2.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Keenan RJ, Freymann DM, Stroud RM, Walter P. The signal recognition particle. Annu. Rev. Biochem. 2001;70:755–775. doi: 10.1146/annurev.biochem.70.1.755. [DOI] [PubMed] [Google Scholar]
- 3.Nagai K, et al. Structure, function and evolution of the signal recognition particle. EMBO J. 2003;22:3479–3485. doi: 10.1093/emboj/cdg337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doudna JA, Batey RT. Structural insights into the signal recognition particle. Annu. Rev. Biochem. 2004;73:539–557. doi: 10.1146/annurev.biochem.73.011303.074048. [DOI] [PubMed] [Google Scholar]
- 5.Poritz MA, et al. An E. coli ribonucleoprotein containing 4.5S RNA resembles mammalian signal recognition particle. Science. 1990;250:1111–1117. doi: 10.1126/science.1701272. [DOI] [PubMed] [Google Scholar]
- 6.Freymann DM, Keenan RJ, Stroud RM, Walter P. Structure of the conserved GTPase domain of the signal recognition particle. Nature. 1997;385:361–364. doi: 10.1038/385361a0. [DOI] [PubMed] [Google Scholar]
- 7.Batey RT, Rambo RP, Lucast L, Rha B, Doudna JA. Crystal structure of the ribonucleoprotein core of the signal recognition particle. Science. 2000;287:1232–1239. doi: 10.1126/science.287.5456.1232. [DOI] [PubMed] [Google Scholar]
- 8.Janda CY, et al. Recognition of a signal peptide by the signal recognition particle. Nature. 2010;465:507–510. doi: 10.1038/nature08870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hainzl T, Huang S, Merilainen G, Brannstrom K, Sauer-Eriksson AE. Structural basis of signal-sequence recognition by the signal recognition particle. Nat. Struct. Mol. Biol. 2011;18:389–391. doi: 10.1038/nsmb.1994. [DOI] [PubMed] [Google Scholar]
- 10.Montoya G, Svensson C, Luirink J, Sinning I. Crystal structure of the NG domain from the signal-recognition particle receptor FtsY. Nature. 1997;385:365–368. doi: 10.1038/385365a0. [DOI] [PubMed] [Google Scholar]
- 11.Angelini S, Deitermann S, Koch HG. FtsY, the bacterial signal-recognition particle receptor, interacts functionally and physically with the SecYEG translocon. EMBO Rep. 2005;6:476–481. doi: 10.1038/sj.embor.7400385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weiche B, et al. A cleavable N-terminal membrane anchor is involved in membrane binding of the Escherichia coli SRP receptor. J. Mol. Biol. 2008;377:761–773. doi: 10.1016/j.jmb.2008.01.040. [DOI] [PubMed] [Google Scholar]
- 13.Egea PF, et al. Substrate twinning activates the signal recognition particle and its receptor. Nature. 2004;427:215–221. doi: 10.1038/nature02250. [DOI] [PubMed] [Google Scholar]
- 14.Focia PJ, Shepotinovskaya IV, Seidler JA, Freymann DM. Heterodimeric GTPase core of the SRP targeting complex. Science. 2004;303:373–377. doi: 10.1126/science.1090827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang X, Rashid R, Wang K, Shan SO. Sequential checkpoints govern substrate selection during cotranslational protein targeting. Science. 2010;328:757–760. doi: 10.1126/science.1186743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holtkamp W, et al. Dynamic switch of the signal recognition particle from scanning to targeting. Nat. Struct. Mol. Biol. 2012;19:1332–1337. doi: 10.1038/nsmb.2421. [DOI] [PubMed] [Google Scholar]
- 17.Halic M, et al. Following the signal sequence from ribosomal tunnel exit to signal recognition particle. Nature. 2006;444:507–511. doi: 10.1038/nature05326. [DOI] [PubMed] [Google Scholar]
- 18.Peluso P, Shan SO, Nock S, Herschlag D, Walter P. Role of SRP RNA in the GTPase cycles of Ffh and FtsY. Biochemistry. 2001;40:15224–15233. doi: 10.1021/bi011639y. [DOI] [PubMed] [Google Scholar]
- 19.Zhang X, Schaffitzel C, Ban N, Shan SO. Multiple conformational switches in a GTPase complex control co-translational protein targeting. Proc. Natl. Acad. Sci. USA. 2009;106:1754–1759. doi: 10.1073/pnas.0808573106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Estrozi LF, Boehringer D, Shan SO, Ban N, Schaffitzel C. Structure of the E. coli Co-translational Targeting Complex in the Stable Early Conformation. Nat. Struct. Mol. Biol. 2011;18:88–90. doi: 10.1038/nsmb.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lam VQ, Akopian D, Rome M, Henningsen D, Shan SO. Lipid activation of the signal recognition particle receptor provides spatial coordination of protein targeting. J. Cell Biol. 2010;190:623–635. doi: 10.1083/jcb.201004129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shen K, Arslan S, Akopian D, Ha T, Shan SO. Activated GTPase movement on an RNA scaffold drives co-translational protein targeting. Nature. 2012;492:271–275. doi: 10.1038/nature11726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ataide SF, et al. The crystal structure of the signal recognition particle in complex with its receptor. Science. 2011;331:881–886. doi: 10.1126/science.1196473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Connolly T, Rapiejko P, Gilmore R. Requirement of GTP hydrolysis for dissociation of the signal recognition particle from its receptor. Science. 1991;252:1171–1173. doi: 10.1126/science.252.5009.1171. [DOI] [PubMed] [Google Scholar]
- 25.Tian H, Boyd D, Beckwith J. A mutant hunt for defects in membrane protein assembly yields mutations affecting the bacterial signal recognition particle and Sec machinery. Proc. Natl. Acad. Sci. USA. 2000;97:4730–4735. doi: 10.1073/pnas.090087297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Valent QA, et al. Early events in preprotein recognition in E. coli: interaction of SRP and trigger factor with nascent polypeptides. EMBO J. 1995;14:5494–5505. doi: 10.1002/j.1460-2075.1995.tb00236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee HC, Bernstein HD. The targeting pathway of Escherichia coli presecretory and integral membrane proteins is specified by the hydrophobicity of the targeting signal. Proc. Natl. Acad. Sci. USA. 2001;98:3471–3476. doi: 10.1073/pnas.051484198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peterson JH, Szabady RL, Bernstein HD. An unusual signal peptide extension inhibits the binding of bacterial presecretory proteins to the signal recognition particle, trigger factor, and the SecYEG complex. J. Biol. Chem. 2006;281:9038–9048. doi: 10.1074/jbc.M508681200. [DOI] [PubMed] [Google Scholar]
- 29.Szabady RL, Peterson JH, Skillman KM, Bernstein HD. An unusual signal peptide facilitates late steps in the biogenesis of a bacterial autotransporter. Proc. Natl. Acad. Sci. USA. 2005;102:221–226. doi: 10.1073/pnas.0406055102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schuwirth BS, et al. Structures of the bacterial ribosome at 3.5 A resolution. Science. 2005;310:827–834. doi: 10.1126/science.1117230. [DOI] [PubMed] [Google Scholar]
- 31.Rosendal KR, Wild K, Montoya G, Sinning I. Crystal structure of the complete core of archaeal signal recognition particle and implications for interdomain communication. Proc. Natl. Acad. Sci. USA. 2003;100:14701–14706. doi: 10.1073/pnas.2436132100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schaffitzel C, et al. Structure of the E. coli signal recognition particle bound to a translating ribosome. Nature. 2006;444:503–506. doi: 10.1038/nature05182. [DOI] [PubMed] [Google Scholar]
- 33.Powers T, Walter P. Co-translational protein targeting catalyzed by the Escherichia coli signal recognition particle and its receptor. EMBO J. 1997;16:4880–4886. doi: 10.1093/emboj/16.16.4880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shan SO, Chandrasekar S, Walter P. Conformational changes in the GTPase modules of the signal reception particle and its receptor drive initiation of protein translocation. J. Cell Biol. 2007;178:611–620. doi: 10.1083/jcb.200702018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang X, Kung S, Shan SO. Demonstration of a multistep mechanism for assembly of the SRP x SRP receptor complex: implications for the catalytic role of SRP RNA. J. Mol. Biol. 2008;381:581–593. doi: 10.1016/j.jmb.2008.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Braig D, et al. Signal sequence-independent SRP-SR complex formation at the membrane suggests an alternative targeting pathway within the SRP cycle. Mol. Biol. Cell. 2011;22:2309–2323. doi: 10.1091/mbc.E11-02-0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shen K, Shan SO. Transient tether between the SRP RNA and SRP receptor ensures efficient cargo delivery during cotranslational protein targeting. Proc. Natl. Acad. Sci. USA. 2010;107:7698–7703. doi: 10.1073/pnas.1002968107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scheres SH, et al. Disentangling conformational states of macromolecules in 3D–EM through likelihood optimization. Nat. Methods. 2007;4:27–29. doi: 10.1038/nmeth992. [DOI] [PubMed] [Google Scholar]
- 39.Shaikh TR, et al. SPIDER image processing for single-particle reconstruction of biological macromolecules from electron micrographs. Nat. Protoc. 2008;3:1941–1974. doi: 10.1038/nprot.2008.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang X, et al. Direct visualization reveals dynamics of a transient intermediate during protein assembly. Proc. Natl. Acad. Sci. USA. 2011;108:6450–6455. doi: 10.1073/pnas.1019051108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Knoops K, Schoehn G, Schaffitzel C. Cryo-electron microscopy of ribosomal complexes in cotranslational folding, targeting, and translocation. Wiley Interdiscip. Rev. RNA. 2011;3:429–441. doi: 10.1002/wrna.119. [DOI] [PubMed] [Google Scholar]
- 42.Cross BC, Sinning I, Luirink J, High S. Delivering proteins for export from the cytosol. Nat. Rev. Mol. Cell Biol. 2009;10:255–264. doi: 10.1038/nrm2657. [DOI] [PubMed] [Google Scholar]
- 43.Bradshaw N, Neher SB, Booth DS, Walter P. Signal sequences activate the catalytic switch of SRP RNA. Science. 2009;323:127–130. doi: 10.1126/science.1165971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peterson JH, Woolhead CA, Bernstein HD. The conformation of a nascent polypeptide inside the ribosome tunnel affects protein targeting and protein folding. Mol. Microbiol. 2010;78:203–217. doi: 10.1111/j.1365-2958.2010.07325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Raine A, et al. Targeting and insertion of heterologous membrane proteins in. E. coli. Biochimie. 2003;85:659–668. doi: 10.1016/s0300-9084(03)00130-5. [DOI] [PubMed] [Google Scholar]
- 46.Flanagan JJ, et al. Signal recognition particle binds to ribosome-bound signal sequences with fluorescence-detected subnanomolar affinity that does not diminish as the nascent chain lengthens. J. Biol. Chem. 2003;278:18628–18637. doi: 10.1074/jbc.M300173200. [DOI] [PubMed] [Google Scholar]
- 47.Miyazaki E, Kida Y, Mihara K, Sakaguchi M. Switching the sorting mode of membrane proteins from cotranslational endoplasmic reticulum targeting to posttranslational mitochondrial import. Mol. Biol. Cell. 2005;16:1788–1799. doi: 10.1091/mbc.E04-08-0707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.del Alamo M, et al. Defining the specificity of cotranslationally acting chaperones by systematic analysis of mRNAs associated with ribosome-nascent chain complexes. PLoS Biol. 2011;9:e1001100. doi: 10.1371/journal.pbio.1001100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lauring B, Sakai H, Kreibich G, Wiedmann M. Nascent polypeptide-associated complex protein prevents mistargeting of nascent chains to the endoplasmic reticulum. Proc. Natl. Acad. Sci. USA. 1995;92:5411–5415. doi: 10.1073/pnas.92.12.5411. [DOI] [PMC free article] [PubMed] [Google Scholar]
ONLINE METHODS REFERENCES
- 50.Schaffitzel C, Ban N. Generation of ribosome nascent chain complexes for structural and functional studies. J. Struct. Biol. 2007;158:463–471. doi: 10.1016/j.jsb.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 51.Heymann JB, Belnap DM. Bsoft: image processing and molecular modeling for electron microscopy. J. Struct. Biol. 2007;157:3–18. doi: 10.1016/j.jsb.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 52.Tang G, et al. EMAN2: an extensible image processing suite for electron microscopy. J. Struct. Biol. 2007;157:38–46. doi: 10.1016/j.jsb.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 53.Valle M, et al. Incorporation of aminoacyl-tRNA into the ribosome as seen by cryo-electron microscopy. Nat. Struct. Biol. 2003;10:899–906. doi: 10.1038/nsb1003. [DOI] [PubMed] [Google Scholar]
- 54.Valle M, et al. Locking and unlocking of ribosomal motions. Cell. 2003;114:123–134. doi: 10.1016/s0092-8674(03)00476-8. [DOI] [PubMed] [Google Scholar]
- 55.Fischer N, Konevega AL, Wintermeyer W, Rodnina MV, Stark H. Ribosome dynamics and tRNA movement by time-resolved electron cryomicroscopy. Nature. 2010;466:329–333. doi: 10.1038/nature09206. [DOI] [PubMed] [Google Scholar]
- 56.Rosenthal PB, Henderson R. Optimal determination of particle orientation, absolute hand, and contrast loss in single-particle electron cryomicroscopy. J. Mol. Biol. 2003;333:721–745. doi: 10.1016/j.jmb.2003.07.013. [DOI] [PubMed] [Google Scholar]
- 57.Scheres SH, Nunez-Ramirez R, Sorzano CO, Carazo JM, Marabini R. Image processing for electron microscopy single-particle analysis using XMIPP. Nat. Protoc. 2008;3:977–990. doi: 10.1038/nprot.2008.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Penczek PA, Kimmel M, Spahn CM. Identifying conformational states of macromolecules by eigen-analysis of resampled cryo-EM images. Structure. 2011;19:1582–1590. doi: 10.1016/j.str.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pettersen EF, et al. UCSF Chimera--a visualization system for exploratory research and analysis. J. Comput. Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 60.Brunger AT, et al. Crystallography & NMR system: A new software suite for macromolecular structure determination. Acta Crystallogr. D Biol. Crystallogr. 1998;54:905–921. doi: 10.1107/s0907444998003254. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.