Abstract
Given the CYP3A4 and CYP3A5's impact on the efficacy of drugs, the genetic backgrounds of individuals and populations are regarded as an important factor to be considered in the prescription of personalized medicine. However, genetic studies with Korean population are relatively scarce compared to those with other populations. In this study, we aimed to identify CYP3A4/5 polymorphisms and compare the genotype distributions among five ethnicities. To identify CYP3A4/5 SNPs, we first performed direct sequencing with 288 DNA samples which consisted of 96 Koreans, 48 European-Americans, 48 African-Americans, 48 Han Chinese, and 48 Japanese. The direct sequencing identified 15 novel SNPs, as well as 42 known polymorphisms. We defined the genotype distributions, and compared the allele frequencies among five ethnicities. The results showed that minor allele frequencies of Korean population were similar with those of the Japanese and Han Chinese populations, whereas there were distinct differences from European-Americans or African-Americans. Among the pharmacogenetic markers, frequencies of CYP3A4*1B (rs2740574) and CYP3A5*3C (rs776742) in Asian groups were different from those in other populations. In addition, minor allele frequency of CYP3A4*18 (rs28371759) was the highest in Korean population. Additional in silico analysis predicted that two novel non-synonymous SNPs in CYP3A5 (+27256C>T, P389S and +31546T>G, I488S) could alter protein structure. The frequency distributions of the identified polymorphisms in the present study may contribute to the expansion of pharmacogenetic knowledge.
Keywords: CYP3A4, CYP3A5, Cytochrome P450, Pharmacogenetics, SNP
INTRODUCTION
Given that genetic differences between individuals or populations can impact the efficacy of drugs, defining pharmacogenetic differences is regarded as an important factor to consider in the treatment of diseases and conditions with personalized medicine. Therefore, to enhance the prediction of efficacy and toxicity of drugs in individuals, recent pharmacogenetic studies have focused on phase I and phase II drug-metabolism related genes such as the N-acetyltransferase (NAT) family, the Cytochrome P450 (CYP) family, and the Uridine diphosphate glucuronosyl transferase (UGT) family [1-3].
The CYP3A family is a well-known phase I metabolism-related gene family and consists of four genes, CYP3A4, CYP3A5, CYP3A7, and CYP3A43, all of which are located in the 231-kb region of chromosome 7q21.1 [4]. It has been demonstrated that the CYP enzymes account for approximately 75% of metabolic reactions [5]. The CYP3A4 and CYP3A5 genes are known to perform a mono-oxygenase reaction, which is involved in several drug-related reactions such as bio-activation of medicines, excretion of drug compounds, and deactivation of drug compounds [6]. According to previous reports, approximately 30% of CYP enzymes showed a high expression level in the liver and intestine, and activities of CYP3A4 and CYP3A5 constituted approximately 36% of all CYP3A activity [7-9]. It was also reported that CYP3A4 and CYP3A5 polymorphisms affected the treatment of various diseases by changing the balance of drug metabolism [10-12]. In addition, it was demonstrated that the CYP enzymes showed genetic variation across individuals, with deficiencies occurring in 1 to 30% of populations, depending on ethnicity [13]. Therefore, a large number of studies were conducted to validate the effect of single-nucleotide polymorphisms (SNPs) of CYP3A4 and CYP3A5 on these polymorphic expressions and the risk of various diseases [14-16].
Previously, 22 and 11 types of pharmacogenetic markers were identified in CYP3A4 and CYP3A5, respectively (reviewed in [17]). Also, it is well-known that frequency differences of the genetic polymorphisms are responsible for diverse gene expressions which are related with various drug responses. For example, the high frequency of CYP3A5*3 allele in Caucasian led to a high area under curve value for cyclosporine metabolism [18]. Moreover, it was also demonstrated that the CYP3A5*6 and *7 alleles, which were responsible for loss of the protein synthesis, showed frequencies of 10 to 20% in African but were not found in other ethnicities [19].
A number of previous studies showed that the frequencies of the CYP3A4 and CYP3A5 polymorphisms were different based on ethnicities. However, genetic studies of the two genes with Korean population are insufficient. Therefore, we performed direct sequencing of CYP3A4 and CYP3A5 to define the genotype frequencies for known genetic polymorphisms and identify novel polymorphisms in a Korean population. Following this, we compared allele distributions in five different ethnic groups comprising 96 Koreans, 48 Japanese, 48 Han Chinese, 48 African-Americans, and 48 European-Americans.
METHODS
Study subjects
DNA samples were obtained from a total of 288 subjects consisting of 96 Koreans, 48 African-Americans, 48 European-Americans, 48 Japanese, and 48 Han Chinese. DNA samples from 96 unrelated Korean individuals were provided by the Center for Genome Science, Korea Centers for Disease Control and Prevention. DNA samples from other ethnic groups were obtained from a large panel of anonymous, unrelated DNA samples from the Human Variation Panels available at the Coriell Institute (Camden, NJ, USA).
Sequencing analysis of CYP3A4/5 genes
The promoter, all exons, and exon-intron boundaries (±50 bp) were PCR-amplified and directly sequenced using the ABI PRISM 3730 genetic analyzer (Applied Biosystems, Foster City, CA, USA). Primers for the amplification and sequencing analysis were designed using Primer3 software [20] based on the GenBank sequence of respective genes (Ref. genome seq.: NG_008421.1 and NG_007938.1 for CYP3A4 and CYP3A5, respectively). The sequences of primers are displayed in Supplementary Table 1. Sequence variants were verified by chromatograms using SeqMan software (Supplementary Fig. 1).
Statistical analysis
The χ2 tests were used to determine whether individual variants were in Hardy-Weinberg equilibrium (HWE) at each locus in each population. HaploView software was used for obtaining linkage disequilibrium (LD) blocks of each gene [21,22]. The Helical Wheel project, web-based software (http://cti.itc.virginia.edu/~cmg/Demo/wheel/wheelApp.html), was used to predict the functional role of novel SNPs.
RESULTS
In the present study, we identified the CYP3A4/5 polymorphisms in five ethnicities using direct sequencing, and compared the genotype distributions among ethnicities. The direct sequencing of CYP3A4/5 was performed in a total of 288 healthy subjects consisting of 96 Koreans, 48 European-Americans, 48 African-Americans, 48 Han Chinese, and 48 Japanese.
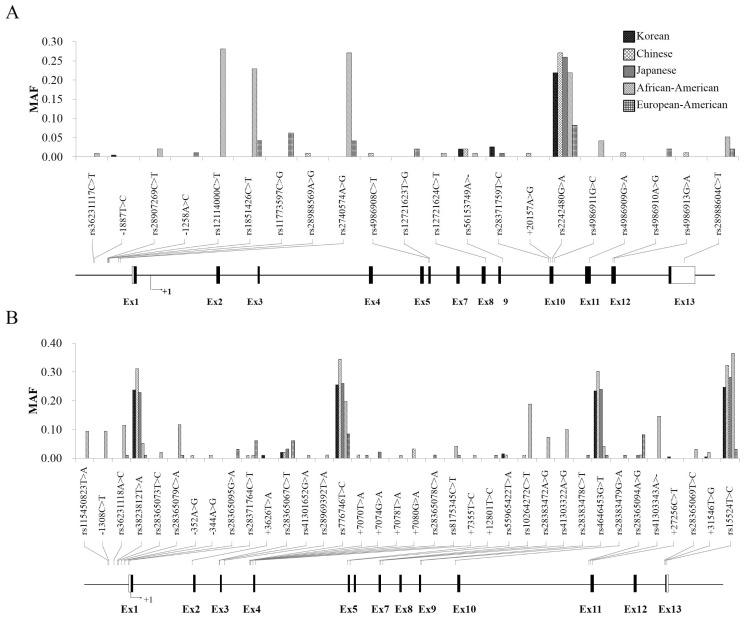
From the direct sequencing, we obtained a total of 15 novel polymorphisms which consist of 3 CYP3A4 SNPs (-1887T>C, -1258A>C, and +20157A>G (V318V) and 12 CYP3A5 variants (-1308C>T, -352A>G, -344A>G, +3626T>A, +7070T>A, +7074G>A, +7078T>A, +7080G >A, +7355T>C, +12801T>C, +27256C>T (P389S), and +31546T>G (I488S) (Table 1). Also, we observed 18 and 24 previously reported SNPs in CYP3A4/5 genes, respectively (Table 1). Locations of the polymorphisms are shown in each physical gene map along with their minor allele frequencies (MAFs) (Fig. 1).
Table 1.
Results from direct sequencing of CYP3A4 and CYP3A5 with five different ethnic groups
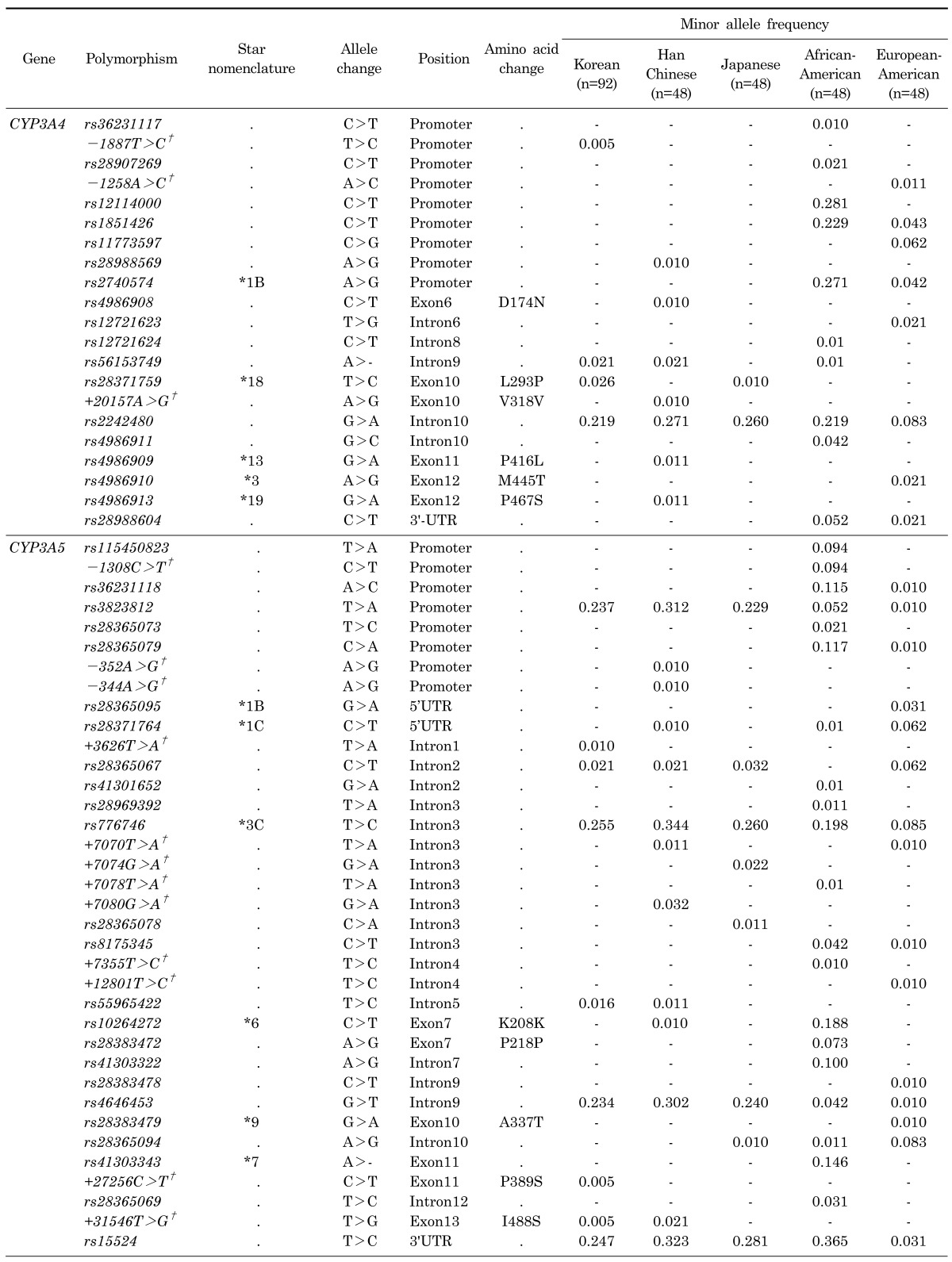
Variants which are monomorphic in all ethnicities are not shown in the Table. A hyphen (-) indicates that the variant was monomorphic in the particular ethnicity. Data not applicable are marked with a dot (.).
†These polymorphisms were newly identified in this study.
Fig. 1.
(A) A physical map of CYP3A4 with minor allele frequencies using results from Korean, African-American, European-American, Han Chinese, and Japanese populations. Novel SNPs are labeled with their locations and allele changes. (B) A physical map of CYP3A5 with minor allele frequencies using results from Korean, African-American, European-American, Han Chinese, and Japanese populations. Novel SNPs are labeled with their locations and allele changes.
Most of the CYP3A4 and CYP3A5 polymorphisms showed low frequencies or monomorphic genotypes. In general, the MAFs of CYP3A4 polymorphisms were similar across the Asian populations, whereas MAFs of African-American and European-American populations differed from those of Asians. Among the pharmacogenetic markers, MAFs of CYP3A4*1B (rs2740574) were detected in European-Americans (0.042) and African-Americans (0.271), whereas the polymorphism was not detected in any Asian populations. On the other hand, rs2242480 was identified with a low frequency in the European-American population (0.083) compared to other ethnicities (>0.200), and three SNPs (rs12114000, rs1851426, and rs2740574) were identified as having high frequencies among African-Americans (0.281, 0.229, and 0.271, respectively), but was almost monomorphic in other populations. Detailed information regarding SNPs in CYP3A4 is displayed in Table 1.
In CYP3A5, CYP3A5*3C (rs776746) showed higher MAFs in Asian populations (0.255, Korean; 0.344, Han Chinese; 0.260, Japanese) than in other ethnic groups (0.198, African-American; 0.085, European-American). Other two polymorphisms (rs3823812 and rs4646453) were also detected that had higher MAFs among Asians (>0.200) compared to other populations (<0.060). On the other hand, the MAF of rs15524 was lowest in European-Americans (0.031), while the frequencies in other populations were higher than 0.200. CYP3A5*6 (rs10264272) and CYP3A5*7 (rs41303343) were detected with high as having MAFs in African-Americans (0.188 and 0.146, respectively), but was almost monomorphic in other populations. Detailed information regarding SNPs in CYP3A5 is displayed in Table 1.
p-values for Hardy-Weinberg equilibrium of each polymorphism were calculated for the five ethnic groups (Supplementary Table 2). All of CYP3A4 alleles were in Hardy-Weinberg equilibrium. However, in CYP3A5, p-values of rs28365067 in Japanese and +7080G>A in Han Chinese were not in Hardy-Weinberg equilibrium.
LD structures of CYP3A4 and CYP4A5 in five ethnicities were calculated by using SNPs which were identified in more than two ethnicities, and the results were displayed in Supplementary Fig. 2. However, LD structures were not clearly constructed due to the SNPs with low or monomorphic frequencies.
DISCUSSION
CYP3A4 and CYP3A5 enzymes are regarded as important markers in the development of the personalized medicine due to the enzymes' impact on efficacy of drugs based on genetic background of individuals or populations. Therefore, we conducted the present study to compare genetic differences in the CYP3A4 and CYP3A5 genes among five ethnicities. The sequencing results showed that many pharmacogenetic markers in CYP3A4 and CYP3A5 were either monomorphic or had low frequencies. This trend was consistent with previous observations in which a large number of CYP3A polymorphisms exhibited low frequencies (reviewed in [23]). This indicates that a larger sample size may be needed to detect the polymorphisms.
Among the pharmacogenetic markers in CYP3A4, CYP3A4*1B (rs2740574) is known to be the polymorphism that increases expression by changing the transcription factor binding affinity [24]. Recently, it was demonstrated that CYP3A4*1B carriers showed higher drug clearance for anti-cancer agents, such as docetaxel and cyclophosphamide, than wild type subjects [25-27]. However, although CYP3A4*1B plays an important role in the enzyme activity, the marker has not been detected in Asian populations in previous studies [28-30]. Our results also showed that CYP3A4*1B was not detected in Asians, including a Korean population. These observations suggest that the alteration of metabolism of docetaxel and cyclophosphamide by CYP3A4*1B might be difficult to find in Asian populations.
The other pharmacogenetic marker, CYP3A4*18 (rs28371759) has been reported as the polymorphism that accounts for bidirectional enzyme activity. Previous studies showed that the polymorphism increased the turnover rate of testosterone and chlorpyrifos, but decreased the metabolic turnover rate of midazolam and nifedipine [31-35]. In addition, previous studies reported that CYP3A4*18 was frequently identified in Asian populations such as Chinese (frequency, 0.008~0.01), Japanese (frequency, 0.013), Koreans (frequency, 0.012~0.017) and Malaysians (frequency, 0.021) [33,36-40]. The result of the present study also showed that the polymorphism was detected in two Asian populations (Korean, 0.021 and Japanese, 0.010), while other populations showed monomorphic genotypes. Therefore, Asian populations may have more genetic protection against toxicity of chlorpyrifos than other populations. Moreover, Asian populations tend to experience an effective dose with lower amounts of midazolam and nifedipine for treatment of seizure and cardiac/circulatory disorders.
A recent study reported that the CYP3A4*22 (rs35599367) allele played an important role in the hepatic CYP3A4 expression and CYP3A4 activity, as well as alteration of statin, tacrolimus and cyclosporine metabolism [17]. This SNP was not found in our subjects. According to the NCBI database, the polymorphism had a frequency of around 0.025 in only Caucasian population. Therefore, no detection of the polymorphisms in the present study may occur due to the low frequency of the allele.
In CYP3A5, CYP3A5*3C (rs776746) is well known as the polymorphism that causes severe decrease of enzyme activity by a splicing defect [41]. It has been reported that individuals with CYP3A5*3C show a lower clearance rate of drugs such as carbamazepine, vincristine, and ifosfamide, which are used for treatment using anticonvulsants, moodstabilizers, and anti-cancer agents [42-45]. In the present study, we observed that the frequency of the CYP3A5*3C polymorphism was relatively higher in Asian populations than in other populations (Korean, 0.255; Han Chinese, 0.344; Japanese, 0.260 vs. African-American, 0.198; European-American, 0.085). Therefore, identifying the CYP3A5*3C genotype could be important for application of carbamazepine, vincristine, and ifosfamide in treating Asian epilepsy, bipolar disorder, trigeminal neuralgia, and cancer patients.
Due to the important roles of non-synonymous SNPs in protein functions, we selected exonic variants that cause amino acid change (+27256C>T, P389S and +31546T>G, I488S in CYP3A5) so as to predict the functional role of the SNPs using web-based software. Results from the analysis showed that the amino acid substitutions by the polymorphisms could change the charge of residues from nonpolar to polar. These alterations of amino acid properties can cause a change in protein structure [46]. Therefore, the two polymorphisms may affect enzyme activity through the modification of protein structure, although further functional studies would be required to confirm the result.
Conclusively, we performed direct sequencing of the CYP3A4/5 in five ethnicities to identify SNPs, and compared the frequency differences of the polymorphisms among ethnicities. From the analysis, we obtained a total of 57 SNPs composed of 15 novel polymorphisms and 42 known variants. Our results indicated that genotype frequencies of Asian populations were different from those of other ethnic groups. Additional in silico analysis revealed that two novel non-synonymous SNPs could cause alteration of protein folding. Although our LD structures were not accurately calculated due to the low frequencies of the SNPs, there appears to be no linkage between novel polymorphisms and known pharmacogenetic marker. Further studies with large scale sample may be required to obtain reliable results, as well as exact p-values for Hardy-Weinberg equilibrium. The results of the present study may be helpful for further understanding of pharmacogenetics.
ACKNOWLEDGEMENT
This work was supported by a grant from the Korean National Institute of Food and Drug Safety Evaluation (NIFDS) funded by the Korea Food and Drug Administration (KFDA).
ABBREVIATIONS
- NAT
N-acetyltransferase
- CYP
cytochrome P450
- UGT
uridine diphosphate glucuronosyl transferase
- SNP
single-nucleotide polymorphism
- HWE
Hardy-Weinberg equilibrium
- LD
linkage disequilibrium
SUPPLEMENTARY MATERIALS
Supplementary data including one figure can be found with this article online at http://pdf.medrang.co.kr/paper/pdf/Kjpp/Kjpp017-06-01-s001.pdf.
(A) Chromatograms of novel polymorphisms in CYP3A4. (B) Chromatograms of novel polymorphisms in CYP3A5.
LD blocks of CYP3A4 (upper panel) and CYP3A5 (lower panel) with five ethnic groups which are comprised of Korean, Japanese, Han Chinese, European-American, and African-American. The LD structures were calculated using SNPs which showed frequencies in more than two populations.
Notch1 silencing. MDA-MB-231 cells were transfected with siRNAs to inhibit the expression of Notch1 or with a non-targeting control for 48 h. (A) The mRNA levels of human Notch1 and β-actin were detected by RT-PCR analysis. (B) The protein levels of human t-Notch1, c-Notch1, and β-actin were detected by Western blot analysis.
Primer information of CYP3A4 and CYP3A5
P-values for Hardy-Weinberg equilibrium of CYP3A4 and CYP3A5 polymorphisms in five ethnicities
References
- 1.Sim SC, Ingelman-Sundberg M. Pharmacogenomic biomarkers: new tools in current and future drug therapy. Trends Pharmacol Sci. 2011;32:72–81. doi: 10.1016/j.tips.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 2.Savonarola A, Palmirotta R, Guadagni F, Silvestris F. Pharmacogenetics and pharmacogenomics: role of mutational analysis in anti-cancer targeted therapy. Pharmacogenomics J. 2012;12:277–286. doi: 10.1038/tpj.2012.28. [DOI] [PubMed] [Google Scholar]
- 3.Kristyanto H, Utomo AR. Pharmacogenetic application in personalized cancer treatment. Acta Med Indones. 2010;42:109–115. [PubMed] [Google Scholar]
- 4.Gellner K, Eiselt R, Hustert E, Arnold H, Koch I, Haberl M, Deglmann CJ, Burk O, Buntefuss D, Escher S, Bishop C, Koebe HG, Brinkmann U, Klenk HP, Kleine K, Meyer UA, Wojnowski L. Genomic organization of the human CYP3A locus: identification of a new, inducible CYP3A gene. Pharmacogenetics. 2001;11:111–121. doi: 10.1097/00008571-200103000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Guengerich FP. Cytochrome p450 and chemical toxicology. Chem Res Toxicol. 2008;21:70–83. doi: 10.1021/tx700079z. [DOI] [PubMed] [Google Scholar]
- 6.Sun H, Bessire AJ, Vaz A. Dirlotapide as a model substrate to refine structure-based drug design strategies on CYP3A4-catalyzed metabolism. Bioorg Med Chem Lett. 2012;22:371–376. doi: 10.1016/j.bmcl.2011.10.121. [DOI] [PubMed] [Google Scholar]
- 7.Wrighton SA, Stevens JC. The human hepatic cytochromes P450 involved in drug metabolism. Crit Rev Toxicol. 1992;22:1–21. doi: 10.3109/10408449209145319. [DOI] [PubMed] [Google Scholar]
- 8.Hall SD, Thummel KE, Watkins PB, Lown KS, Benet LZ, Paine MF, Mayo RR, Turgeon DK, Bailey DG, Fontana RJ, Wrighton SA. Molecular and physical mechanisms of first-pass extraction. Drug Metab Dispos. 1999;27:161–166. [PubMed] [Google Scholar]
- 9.Wacher VJ, Wu CY, Benet LZ. Overlapping substrate specificities and tissue distribution of cytochrome P450 3A and P-glycoprotein: implications for drug delivery and activity in cancer chemotherapy. Mol Carcinog. 1995;13:129–134. doi: 10.1002/mc.2940130302. [DOI] [PubMed] [Google Scholar]
- 10.Ozdemir V, Kalow W, Tang BK, Paterson AD, Walker SE, Endrenyi L, Kashuba AD. Evaluation of the genetic component of variability in CYP3A4 activity: a repeated drug administration method. Pharmacogenetics. 2000;10:373–388. doi: 10.1097/00008571-200007000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Wrighton SA, Brian WR, Sari MA, Iwasaki M, Guengerich FP, Raucy JL, Molowa DT, Vandenbranden M. Studies on the expression and metabolic capabilities of human liver cytochrome P450IIIA5 (HLp3) Mol Pharmacol. 1990;38:207–213. [PubMed] [Google Scholar]
- 12.Hustert E, Haberl M, Burk O, Wolbold R, He YQ, Klein K, Nuessler AC, Neuhaus P, Klattig J, Eiselt R, Koch I, Zibat A, Brockmöller J, Halpert JR, Zanger UM, Wojnowski L. The genetic determinants of the CYP3A5 polymorphism. Pharmacogenetics. 2001;11:773–779. doi: 10.1097/00008571-200112000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Rostami-Hodjegan A, Tucker GT. Simulation and prediction of in vivo drug metabolism in human populations from in vitro data. Nat Rev Drug Discov. 2007;6:140–148. doi: 10.1038/nrd2173. [DOI] [PubMed] [Google Scholar]
- 14.Lee AJ, Mills LH, Kosh JW, Conney AH, Zhu BT. NADPH-dependent metabolism of estrone by human liver microsomes. J Pharmacol Exp Ther. 2002;300:838–849. doi: 10.1124/jpet.300.3.838. [DOI] [PubMed] [Google Scholar]
- 15.Lee AJ, Kosh JW, Conney AH, Zhu BT. Characterization of the NADPH-dependent metabolism of 17beta-estradiol to multiple metabolites by human liver microsomes and selectively expressed human cytochrome P450 3A4 and 3A5. J Pharmacol Exp Ther. 2001;298:420–432. [PubMed] [Google Scholar]
- 16.Huang Z, Guengerich FP, Kaminsky LS. 16Alpha-hydroxylation of estrone by human cytochrome P4503A4/5. Carcinogenesis. 1998;19:867–872. doi: 10.1093/carcin/19.5.867. [DOI] [PubMed] [Google Scholar]
- 17.Elens L, van Gelder T, Hesselink DA, Haufroid V, van Schaik RH. CYP3A4*22: promising newly identified CYP3A4 variant allele for personalizing pharmacotherapy. Pharmacogenomics. 2013;14:47–62. doi: 10.2217/pgs.12.187. [DOI] [PubMed] [Google Scholar]
- 18.Min DI, Ellingrod VL, Marsh S, McLeod H. CYP3A5 polymorphism and the ethnic differences in cyclosporine pharmacokinetics in healthy subjects. Ther Drug Monit. 2004;26:524–528. doi: 10.1097/00007691-200410000-00010. [DOI] [PubMed] [Google Scholar]
- 19.MacPhee IA. Pharmacogenetic biomarkers: cytochrome P450 3A5. Clin Chim Acta. 2012;413:1312–1317. doi: 10.1016/j.cca.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 20.Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol. 2000;132:365–386. doi: 10.1385/1-59259-192-2:365. [DOI] [PubMed] [Google Scholar]
- 21.Devlin B, Risch N. A comparison of linkage disequilibrium measures for fine-scale mapping. Genomics. 1995;29:311–322. doi: 10.1006/geno.1995.9003. [DOI] [PubMed] [Google Scholar]
- 22.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 23.Lamba JK, Lin YS, Schuetz EG, Thummel KE. Genetic contribution to variable human CYP3A-mediated metabolism. Adv Drug Deliv Rev. 2012;64:256–269. doi: 10.1016/s0169-409x(02)00066-2. [DOI] [PubMed] [Google Scholar]
- 24.Amirimani B, Ning B, Deitz AC, Weber BL, Kadlubar FF, Rebbeck TR. Increased transcriptional activity of the CYP3A4*1B promoter variant. Environ Mol Mutagen. 2003;42:299–305. doi: 10.1002/em.10199. [DOI] [PubMed] [Google Scholar]
- 25.Hesselink DA, van Gelder T, van Schaik RH, Balk AH, van der Heiden IP, van Dam T, van der Werf M, Weimar W, Mathot RA. Population pharmacokinetics of cyclosporine in kidney and heart transplant recipients and the influence of ethnicity and genetic polymorphisms in the MDR-1, CYP3A4, and CYP3A5 genes. Clin Pharmacol Ther. 2004;76:545–556. doi: 10.1016/j.clpt.2004.08.022. [DOI] [PubMed] [Google Scholar]
- 26.Tran A, Jullien V, Alexandre J, Rey E, Rabillon F, Girre V, Dieras V, Pons G, Goldwasser F, Tréluyer JM. Pharmacokinetics and toxicity of docetaxel: role of CYP3A, MDR1, and GST polymorphisms. Clin Pharmacol Ther. 2006;79:570–580. doi: 10.1016/j.clpt.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 27.Petros WP, Hopkins PJ, Spruill S, Broadwater G, Vredenburgh JJ, Colvin OM, Peters WP, Jones RB, Hall J, Marks JR. Associations between drug metabolism genotype, chemotherapy pharmacokinetics, and overall survival in patients with breast cancer. J Clin Oncol. 2005;23:6117–6125. doi: 10.1200/JCO.2005.06.075. [DOI] [PubMed] [Google Scholar]
- 28.Walker AH, Jaffe JM, Gunasegaram S, Cummings SA, Huang CS, Chern HD, Olopade OI, Weber BL, Rebbeck TR. Characterization of an allelic variant in the nifedipine-specific element of CYP3A4: ethnic distribution and implications for prostate cancer risk. Mutations in brief no. 191. Online. Hum Mutat. 1998;12:289. [PubMed] [Google Scholar]
- 29.Hsieh KP, Lin YY, Cheng CL, Lai ML, Lin MS, Siest JP, Huang JD. Novel mutations of CYP3A4 in Chinese. Drug Metab Dispos. 2001;29:268–273. [PubMed] [Google Scholar]
- 30.Ball SE, Scatina J, Kao J, Ferron GM, Fruncillo R, Mayer P, Weinryb I, Guida M, Hopkins PJ, Warner N, Hall J. Population distribution and effects on drug metabolism of a genetic variant in the 5' promoter region of CYP3A4. Clin Pharmacol Ther. 1999;66:288–294. doi: 10.1016/S0009-9236(99)70037-8. [DOI] [PubMed] [Google Scholar]
- 31.Watkins PB. Noninvasive tests of CYP3A enzymes. Pharmacogenetics. 1994;4:171–184. doi: 10.1097/00008571-199408000-00001. [DOI] [PubMed] [Google Scholar]
- 32.Streetman DS, Bertino JS, Jr, Nafziger AN. Phenotyping of drug-metabolizing enzymes in adults: a review of in-vivo cytochrome P450 phenotyping probes. Pharmacogenetics. 2000;10:187–216. doi: 10.1097/00008571-200004000-00001. [DOI] [PubMed] [Google Scholar]
- 33.Kang YS, Park SY, Yim CH, Kwak HS, Gajendrarao P, Krishnamoorthy N, Yun SC, Lee KW, Han KO. The CYP3A4*18 genotype in the cytochrome P450 3A4 gene, a rapid metabolizer of sex steroids, is associated with low bone mineral density. Clin Pharmacol Ther. 2009;85:312–318. doi: 10.1038/clpt.2008.215. [DOI] [PubMed] [Google Scholar]
- 34.Dai D, Tang J, Rose R, Hodgson E, Bienstock RJ, Mohrenweiser HW, Goldstein JA. Identification of variants of CYP3A4 and characterization of their abilities to metabolize testosterone and chlorpyrifos. J Pharmacol Exp Ther. 2001;299:825–831. [PubMed] [Google Scholar]
- 35.Lee SJ, Bell DA, Coulter SJ, Ghanayem B, Goldstein JA. Recombinant CYP3A4*17 is defective in metabolizing the hypertensive drug nifedipine, and the CYP3A4*17 allele may occur on the same chromosome as CYP3A5*3, representing a new putative defective CYP3A haplotype. J Pharmacol Exp Ther. 2005;313:302–309. doi: 10.1124/jpet.104.078758. [DOI] [PubMed] [Google Scholar]
- 36.Lee SJ, Lee SS, Jeong HE, Shon JH, Ryu JY, Sunwoo YE, Liu KH, Kang W, Park YJ, Shin CM, Shin JG. The CYP3A4*18 allele, the most frequent coding variant in asian populations, does not significantly affect the midazolam disposition in heterozygous individuals. Drug Metab Dispos. 2007;35:2095–2101. doi: 10.1124/dmd.107.016733. [DOI] [PubMed] [Google Scholar]
- 37.Wen S, Wang H, Ding Y, Liang H, Wang S. Screening of 12 SNPs of CYP3A4 in a Chinese population using oligonucleotide microarray. Genet Test. 2004;8:411–416. doi: 10.1089/gte.2004.8.411. [DOI] [PubMed] [Google Scholar]
- 38.Hu YF, He J, Chen GL, Wang D, Liu ZQ, Zhang C, Duan LF, Zhou HH. CYP3A5*3 and CYP3A4*18 single nucleotide polymorphisms in a Chinese population. Clin Chim Acta. 2005;353:187–192. doi: 10.1016/j.cccn.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 39.Ruzilawati AB, Suhaimi AW, Gan SH. Genetic polymorphisms of CYP3A4: CYP3A4*18 allele is found in five healthy Malaysian subjects. Clin Chim Acta. 2007;383:158–162. doi: 10.1016/j.cca.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 40.Yamamoto T, Nagafuchi N, Ozeki T, Kubota T, Ishikawa H, Ogawa S, Yamada Y, Hirai H, Iga T. CYP3A4*18: it is not rare allele in Japanese population. Drug Metab Pharmacokinet. 2003;18:267–268. doi: 10.2133/dmpk.18.267. [DOI] [PubMed] [Google Scholar]
- 41.Kuehl P, Zhang J, Lin Y, Lamba J, Assem M, Schuetz J, Watkins PB, Daly A, Wrighton SA, Hall SD, Maurel P, Relling M, Brimer C, Yasuda K, Venkataramanan R, Strom S, Thummel K, Boguski MS, Schuetz E. Sequence diversity in CYP3A promoters and characterization of the genetic basis of polymorphic CYP3A5 expression. Nat Genet. 2001;27:383–391. doi: 10.1038/86882. [DOI] [PubMed] [Google Scholar]
- 42.Park PW, Seo YH, Ahn JY, Kim KA, Park JY. Effect of CYP3A5*3 genotype on serum carbamazepine concentrations at steady-state in Korean epileptic patients. J Clin Pharm Ther. 2009;34:569–574. doi: 10.1111/j.1365-2710.2009.01057.x. [DOI] [PubMed] [Google Scholar]
- 43.Seo T, Nakada N, Ueda N, Hagiwara T, Hashimoto N, Nakagawa K, Ishitsu T. Effect of CYP3A5*3 on carbamazepine pharmacokinetics in Japanese patients with epilepsy. Clin Pharmacol Ther. 2006;79:509–510. doi: 10.1016/j.clpt.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 44.Dennison JB, Kulanthaivel P, Barbuch RJ, Renbarger JL, Ehlhardt WJ, Hall SD. Selective metabolism of vincristine in vitro by CYP3A5. Drug Metab Dispos. 2006;34:1317–1327. doi: 10.1124/dmd.106.009902. [DOI] [PubMed] [Google Scholar]
- 45.McCune JS, Risler LJ, Phillips BR, Thummel KE, Blough D, Shen DD. Contribution of CYP3A5 to hepatic and renal ifosfamide N-dechloroethylation. Drug Metab Dispos. 2005;33:1074–1081. doi: 10.1124/dmd.104.002279. [DOI] [PubMed] [Google Scholar]
- 46.Weinshilboum R, Wang L. Pharmacogenomics: bench to bedside. Nat Rev Drug Discov. 2004;3:739–748. doi: 10.1038/nrd1497. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Chromatograms of novel polymorphisms in CYP3A4. (B) Chromatograms of novel polymorphisms in CYP3A5.
LD blocks of CYP3A4 (upper panel) and CYP3A5 (lower panel) with five ethnic groups which are comprised of Korean, Japanese, Han Chinese, European-American, and African-American. The LD structures were calculated using SNPs which showed frequencies in more than two populations.
Notch1 silencing. MDA-MB-231 cells were transfected with siRNAs to inhibit the expression of Notch1 or with a non-targeting control for 48 h. (A) The mRNA levels of human Notch1 and β-actin were detected by RT-PCR analysis. (B) The protein levels of human t-Notch1, c-Notch1, and β-actin were detected by Western blot analysis.
Primer information of CYP3A4 and CYP3A5
P-values for Hardy-Weinberg equilibrium of CYP3A4 and CYP3A5 polymorphisms in five ethnicities