Abstract
Gestational exposures to estrogenic compounds, both endogenous hormones and exogenous endocrine-disrupting chemicals (EDCs), have long-term effects on reproductive physiology and behavior. We tested the hypothesis that prenatal treatment of rats with low doses of Aroclor 1221 (A1221), a weakly estrogenic polychlorinated biphenyl mix previously used in industry, or estradiol benzoate (EB), alters development of the hypothalamus in a sexually dimorphic manner and subsequently perturbs reproductive function. Pregnant Sprague-Dawley rats were injected on embryonic days 16 and 18 with vehicle (dimethylsulfoxide), A1221 (1 mg/kg), or EB (50 μg/kg). Developmental milestones were monitored, and on postnatal days 15, 30, 45, and 90, 1 male and 1 female per litter were euthanized. Because of their key roles in the mediation of steroid actions on reproductive function, the anteroventral periventricular nucleus (AVPV) and the arcuate nucleus (ARC) were punched for a low-density quantitative PCR array of 48 neuroendocrine genes and analysis of DNA methylation of a subset of genes. Gestational exposure to A1221 or EB delayed the timing of puberty in males and disrupted estrous cyclicity in females. In the AVPV, 28 genes were affected by treatment in a developmental stage–specific manner, mostly in females, which exhibited a masculinized expression profile. This included 2 clock genes, Per2 and Arntl, implicating circadian circuits as being vulnerable to endocrine disruption. DNA methylation analysis of 2 genes, Per2 and Ar, showed no effect of EDCs and suggested alternative mechanisms for the altered mRNA levels. In the ARC, 12 genes were affected by treatment, mostly in males, again with dynamic developmental changes. Bionetwork analysis of relationships among genes, hormones, and physiological markers showed sexually dimorphic effects of estrogenic EDC exposures, with the female AVPV and the male ARC being most vulnerable, and provided novel relationships among hypothalamic genes and postnatal reproductive maturation.
For an individual to attain reproductive competency, a myriad of tightly coordinated molecular and physiological processes must occur with the proper timing and sequence, beginning in embryonic development and continuing throughout adulthood. Although the entire hypothalamic-pituitary-gonadal axis undergoes dynamic changes, the hypothalamus ultimately integrates the internal and external stimuli, including metabolic status, serum hormone concentrations, stressors, circadian indicators, and immune function, leading to and optimizing reproductive capacity in adulthood (1). The maturation of the hypothalamic GnRH neural population and its regulatory inputs from other neurons and glia is responsible for the acquisition of reproductive function postnatally (2). To understand the hypothalamic control of reproductive development, numerous studies have shown changes in gene expression (3–10), protein expression (7, 11–13), epigenetic processes (14, 15), Land morphology (16–18) of this neuroendocrine network in developing rodents.
The hypothalamic control of reproduction is also sexually dimorphic (19), with substantial differences in male- and female-typical reproductive function, physiology and developmental processes (20–22). Sexual differentiation of the brain begins during a critical period of gestation/early postnatal development in which the brain is permanently masculinized or feminized through exposures to sex-typical levels of steroid hormones (17, 18, 23–25). Mounting evidence suggests that sexual differentiation of the brain involves epigenetic mechanisms, including DNA methylation and chromatin remodeling, which “program” hypothalamic gene expression (for reviews, see Refs. 26, 27). Perturbations of this process by developmental exposure to estrogenic compounds, including environmental endocrine-disrupting chemicals (EDCs), can result in lifelong effects on reproductive physiology and behavior by “programming” the expression of sexually dimorphic genes.
An EDC is defined as “an exogenous chemical, or mixture of chemicals, that interferes with any aspect of hormone action” (28); EDCs include a variety of plastics, plasticizers (phthalates and bisphenol A), pharmaceuticals (eg, diethylstilbestrol), pesticides (DDT and methoxychlor), and industrial contaminants (polychlorinated biphenyls [PCBs] and dioxins), among others. Here, we investigated whether prenatal exposure to low doses of estrogenic compounds, estradiol benzoate (EB), or a mixture of PCBs with weak estrogenic activity, Aroclor 1221 (A1221) (29, 30) alters the developmental profiles of gene expression in two nuclei in the hypothalamus known to regulate reproductive function: the anteroventral periventricular (AVPV) and arcuate (ARC) nucleus. We sought to identify relationships between gene expression in the AVPV and ARC with peripheral hormones, to explore potential changes to DNA methylation for identified target genes, and to assess EDC effects on reproductive physiology.
Materials and Methods
Animals
All animal protocols were conducted in accordance with the Guide for the Care and Use of Laboratory Animals and approved by The University of Texas at Austin Institutional Animal Care and Use Committee. Animals were part of a larger study and were siblings of those discussed in a recent publication in which detailed methods on animal husbandry and the generation of individuals in this study can be found (31). In brief, Sprague-Dawley rats were purchased from Harlan Laboratories, switched to low phytoestrogen Harlan-Teklad 2019 Global Diet ad libitum and impregnated in-house. On embryonic day (E) 16 and E18 (E1 = day after successful mating), dams were weighed and randomly assigned to 1 of 3 treatment groups and injected with 0.1 mL of vehicle (dimethylsulfoxide [DMSO] 99.5%, catalog no. D4540; Sigma-Aldrich), 50 μg/kg EB as a positive estrogenic control (catalog no. E8515; Sigma-Aldrich), or 1 mg/kg A1221 (catalog no., AccuStandard). Doses, routes, and timing of exposure were chosen based on previous work in our laboratory (32–35) and that of others (36–40), together with literature articles suggesting that the exposure to the fetus is in the human-relevant range (in humans, approximately 1–9 ppb, depending on tissue, geographical region, and time when the study was conducted) (41–46). Animals were housed under constant humidity and temperature (21–22°C) with a partially reversed 12:12 light cycle (lights on at 11:00 pm). On the day after birth (postnatal day [P] 1), litter composition, birth weights, and anogenital distance were recorded, and the litters were culled to equal sex ratios with a goal of 6 males and 6 females per litter. Body weights were monitored weekly throughout the life cycle, and anogenital distance was measured weekly until weaning. Pups were weaned on P21 and housed with same sex littermates (2–3 per cage). Rats were monitored for a secondary sex characteristic of the onset of puberty daily (preputial separation in males and vaginal opening in females). After the onset of puberty, estrous cycles were monitored by daily vaginal smears. Investigators were blind to treatment throughout the experiment. Because of the large number of animals necessary for both studies, animals were raised in 5 cohorts with treatments and assignment of littermates to different timepoints equally distributed across each cohort.
Tissue collection and storage
On P15, 30, 45, and 90, male and female littermates were euthanized approximately 2 to 3 hours before lights out via rapid decapitation, trunk blood samples were collected, and endocrine tissues were removed and weighed. Rats were distributed such that 1 male and 1 female per litter were euthanized at different ages to avoid litter bias. Postpubertal females were euthanized on proestrus based on vaginal smears. Not every litter had 6 males and 6 females, and, therefore, final sample sizes were as follows: DMSO, 14 to 20; EB, 15 to 21; and A1221, 18 to 25, depending on the age and/or sex of each group. A subset of 8 animals per group was used for gene expression and methylation assays. Brains were removed and sectioned, and punches of AVPV and ARC were collected using methods reported previously (31). Trunk blood samples were allowed to clot, and serum was separated via centrifugation (1500 × g for 5 minutes). Tissues and serum were stored at −80°C until use.
Extraction of nucleic acids and preparation for PCR and pyrosequencing
DNA and RNA were extracted from frozen AVPV and ARC punches of male and female rats using an Allprep DNA/RNA mini kit according to the manufacturer's protocols (QIAGEN). RNA samples were isolated as reported previously (31). DNA samples were diluted to 20 ng/μL and 500 ng of DNA was shipped to EpigenDx for bisulfite conversion and pyrosequencing of Per2 (2 assays: ADS3539, −597 to −550 bp from transcription start site [TSS]; and ADS872, −111 to +173 bp from TSS) and Ar (1 assay: ADS070F, −70 to +39 bp from TSS) regulatory regions.
TaqMan microfluidic real-time PCR cards
RNA samples (200 ng) were converted to cDNA using a high-capacity cDNA reverse transcription kit (Applied Biosystems) according to the manufacturer's protocol and run on custom-designed microfluidic 48-gene quantitative PCR cards (Applied Biosystems). Specific gene assays were chosen based on a priori hypotheses and published reports on their importance in neuroendocrine function and sensitivity to disruption by estrogenic compounds (46 genes of interest and 2 housekeeping genes; see Supplemental Tables 1 and 2 published on The Endocrine Society's Journals Online web site at http://mend.endojournals.org).
Real-time PCR was performed using the same protocols as those reported previously (31). Relative expression was determined for each sample using the comparative Ct method (47–49). Samples were normalized to 18s (the other housekeeping gene, Gapdh is sexually dimorphic in the AVPV) and calibrated to the median δ − Ct of the group with the lowest expression to determine fold change in expression for each individual.
Serum hormone assays
Serum hormone concentrations were measured in the same radioimmunoassays as those presented by Walker et al (31). In brief, serum LH was measured in duplicate 50-μL samples in the laboratory of Dr Michael Woller (University of Wisconsin-Whitewater) by a double-antibody competitive binding RIA, and intra-assay variability was 3.85%. Serum concentrations of testosterone (MP Biomedicals) and estradiol (Beckman Coulter) were measured in 1 (intra-assay coefficient of variation [CV] = 1.397%) and 2 (intra-assay CVs = 6.48% and 1.64%; interassay CV = 4.55%) assays, respectively. The sensitivity of the testosterone assay was 0.3 ng/mL and that for estradiol was 2.2 pg/mL.
Statistics
Multiple regression analysis was conducted using PASW software (IBM) to compare each endpoint (genes, hormones, DNA methylation, and endocrine tissues) using age, sex, and treatment as independent variables. For gene expression data, statistical analyses were performed using the same techniques and assumptions as those described in detail previously (31). If data did not meet the assumptions for multiple regression analysis, they were transformed (natural log or square root) and reanalyzed. In a few cases, transformed data did not meet the assumptions for statistical analysis by multiple regression. In those cases, data were analyzed using a Kruskal-Wallis test followed by a Mann-Whitney test between each group. For hormone concentrations, an effect was considered significant at a value of P < .05. For gene expression data, a Benjamini and Hochberg false discovery rate correction (50, 51) was used to correct our P values to account for the large number of variables measured. Parametric data were tested for outliers using the z score of the residuals from the initial regression. A data point was considered an outlier if the residual was >2.5 SDs from the initial line of best fit. Nonparametric data were tested for outliers using the Grubbs outlier test. Confirmed outliers were excluded from the final analysis.
Vaginal smear data were analyzed with the same rule-based pattern matching techniques as used previously (31) to determine number and length of each cycle for each animal. Cycles were identified for each animal by scanning the smear data from the day of vaginal opening until euthanasia for the diestrus 2 to proestrus transition. The makeup and length of each cycle were recorded throughout the life cycle. A repeated-measures ANOVA was conducted using the moving average of the cycle length (using 3 cycle lengths) for each animal to account for any missing data. The output of this analysis was also used to investigate subtle differences in the cycles of each animal. This was accomplished by categorizing every unique cycle observed in each animal and comparing the categories of smears across different animals. This enabled us to look for series that were common to all animals and provided us with information regarding composition of regular/irregular smears and to determine whether specific irregular series were more likely to occur in treated vs control females. Differences were analyzed using a χ2 test and compared using the frequencies in control animals as our expected values.
Hierarchical cluster analysis was performed and heatmaps were generated using Multiple Experiment Viewer V4.8.1 (TM4.org). Clusters were validated using R statistical packages.
To determine whether developmental gene expression profiles in females and males from each treatment were similar to those of control animals, gene expression data (sex × treatment) were compared using two methods, both implemented as Python scripts. The first method used Bayesian analysis to compare 2 developmental profiles (control vs treatment), and Bayes factors were computed as an indicator of the probability that gene expression data for the 2 groups is generated by the same normal distribution (marginalized over mean and variance) vs the probability that the gene expression data are generated by two different normal distributions (marginalized over their parameters). Equations are presented in the Supplemental Material. A low (0–5) or negative Bayes factor, expressed in decibans (dB), indicates no meaningful difference between the groups, whereas a high Bayes factor (>20) indicates a high probability that each group's expression data were generated by a different distribution.
The second comparison method used an ad hoc measure of “distance” between the gene expression curves. A piecewise-linear curve for each group was generated using the average expression value at each age by connecting each data point with a line. We then connected the expression profiles of each group on P15 and P90 to generate a polygon. As a measure of distance between curves, the area between the curves was calculated using the geometric formulas for the area of a polygon (equations presented in Supplemental Material). Note that in the extreme case of the 2 curves being exactly the same, the area between the curves would be 0. However, any deviation of one curve from the other, regardless of which curve is above or below, creates a positive area between the curves, which is reflected in our distance measure.
Taken together, the 2 methods allowed us to compare the developmental profiles between the sexes. Whereas Bayes factors measure the probability that gene expression profiles are generated by different distributions, this method of analysis is exceptionally sensitive to any one age group being different from another. Therefore, to reduce the number of false-positive results, we used the area between curves as a way to confirm that the 2 sexes were similar or different because it is a measure of the actual difference between average gene expression levels. We assumed that a group was different from another if the area between the curves was altered by more than 50% compared to the control counterparts.
Finally, a bootstrapping technique used previously by our laboratory (10) was used to identify relationships between genes, hormones, and endocrine tissues within the sexes from different treatment groups.
Results
Littermates exposed to EB and A1221 in utero were monitored from birth through 90 days of age to determine whether exposure to estrogenic and environmental EDCs alters hypothalamic development and reproductive function. Changes in endocrine tissues, female estrous cycles, and timing of developmental transitions were monitored. To ascertain potential molecular mechanisms underlying the developmental effects of EDCs, expression levels of 48 genes were measured in AVPV and ARC. We also investigated whether developmental changes in DNA methylation of the Ar and Per2 promoter regions were altered after exposure to EDCs in utero. In the AVPV and ARC, 28 and 12 genes, respectively, were changed by treatment (main effect or interaction, P < .05). Of those affected by treatment, 18 genes in the AVPV and 2 genes in the ARC survived a Benjamini and Hochberg false discovery rate correction (50, 52) and are indicated in bold in Supplemental Tables 1 and 2. Because genes were specifically chosen based on an a priori hypothesis, we report significant effects as P < .05 and trends as P < .1.
Effects of EDCs on somatic and reproductive development
Significant main effects of treatment were found for the timing of eye opening (female) and puberty (male) (Table 1). In males, treatment significantly delayed the timing of puberty as measured by preputial separation (P = .017), and post hoc analysis revealed that the effect was specific to males exposed to A1221 compared with controls. In females, the timing of eye opening was delayed by treatment (P = .027). Post hoc analysis revealed that the effects were specific to females exposed to A1221. Treatment effects were also observed on estrous cycles (Table 2). Compared with DMSO controls, females exposed to EB were less likely to be classified as having regular cycles (>30% of total cycles were irregular, P = .008), more likely to have an abnormally long period of diestrus (≥6 days of leukocytic [L] cells, P = .021), and more likely to transition from a leukocytic to cornified (C) smear (P = .003) and have at least 2 elongated series of C and/or L smears (at least 5 days of C and/or L, P = .023). Whereas the estrous cycles of females exposed to A1221 were not greatly affected by treatment, rats in this group were slightly more likely to have at least 2 elongated series of C and/or L smears (at least 5 days of C and/or L, P = .096) compared with DMSO controls.
Table 1.
Summary of EDC Effects on Developmental Endpoints
| Developmental Measures | P Values (Female/Male) | Females |
Males |
||||
|---|---|---|---|---|---|---|---|
| DMSO | EB | A1221 | DMSO | EB | A1221 | ||
| P1 body weight | .401/.293 | 6.08 (0.08) | 6.12 (0.08) | 6.17 (0.06) | 6.33 (0.09) | 6.45 (0.08) | 6.45 (0.06) |
| P1 AGD ratio | .124/.524 | 0.86 (0.01) | 0.90 (0.01) | 0.90 (0.02) | 1.89 (0.01) | 1.90 (0.02) | 1.91 (0.01) |
| P7 AGD ratio | .976/.275 | 1.20 (0.02) | 1.20 (0.01) | 1.20 (0.02) | 2.28 (0.02) | 2.33 (0.03) | 2.33 (0.02) |
| P14 AGD ratio | .287/.070 | 1.58 (0.01) | 1.55 (0.01) | 1.57 (0.01) | 2.73 (0.03) | 2.67 (0.02) | 2.77 (0.03) |
| Age at eye opening, d | .027a/.321 | 15.19 (0.08) | 15.32 (0.08) | 15.48 (0.07)a | 15.36 (0.09) | 15.48 (0.08) | 15.54 (0.08) |
| Age at puberty, d | .470/.017a | 34.08 (0.24) | 34.11 (0.25) | 34.45 (0.23) | 42.12 (0.2) | 42.51 (0.21) | 42.94 (0.2)a |
Abbreviations: AGD, anogenital distance; C, cornified smear; L, leukocytic smear; PD, persistent diestrus; d, days of age. Data shown are means (± SEM).
Significant effect (P < .05).
Table 2.
Summary of EDC Effects on Estrous Cycle Characteristics
| Estrous Cycle Characteristics, % | Females |
||
|---|---|---|---|
| DMSO | EB | A1221 | |
| Regular cycles (P90 females only) | 85.0 | 63.2a | 85.0 |
| Animals with PD (all cycling females) | 5.1 | 13.5a | 6.7 |
| Cycles that skip proestrus (all cycling females) | 20.5 | 40.5a | 28.9 |
| First 3 cycles regular (all cycling females) | 89.7 | 83.8a | 84.4 |
| At least 2 strings of >5 days of C/L vaginal smears | 10.3 | 21.6a | 17.8b |
Abbreviations: AGD, anogenital distance; C, cornified smear; L, leukocytic smear; PD, persistent diestrus; d, days of age. Data shown are means (± SEM).
Significant effect (P < .05).
Trend (.05 < P < .1) compared to corresponding DMSO group.
Treatment had sex-specific effects on endocrine tissues and serum hormone concentrations that were age dependent (Table 3). Males exposed to A1221 had lower serum concentrations of LH on P15 (P = .05) and increased adrenal weights on P30 (P = .037). Males exposed to A1221 showed a trend for a decrease in the gonadal somatic index (gonad weight/body weight) (P = .079). Females exposed to EB showed a trend for lower serum estradiol on P15 (P = .07) and significantly higher adrenal weights on P30 (P = .025).
Table 3.
Summary of EDC Effects on Serum Hormone Concentrations and Endocrine Tissues Throughout Development
| Males |
Females |
|||||
|---|---|---|---|---|---|---|
| DMSO | EB | A1221 | DMSO | EB | A1221 | |
| P15 | ||||||
| T, ng/mL | 0.23 (0.1) | 0.02 (0.0) | 0.12 (0.05) | 0.02 (0.003) | 0.11 (0.09) | 0.03 (0.01) |
| LH, ng/mL | 2.27 (0.46) | 1.10 (0.16)a | 1.08 (0.09)a | 1.93 (0.6) | 1.67 (0.6) | 2.85 (0.71) |
| E2, pg/mL | 95.83 (15.32) | 102.01 (19.02) | 102.06 (20.62) | 93.43 (13.97) | 54.43 (8.53)a | 98.29 (16.1) |
| Body weight, g | 31.52 (1.07) | 34.21 (1.07) | 31.78 (1.01) | 30.99 (1.11) | 33.44 (1.06) | 30.88 (0.76) |
| GSI | 3.14 (0.09) | 3.10 (0.08) | 3.25 (0.08) | 0.25 (0.01) | 0.24 (0.01) | 0.26 (0.01) |
| Uterus weight, mg | NA | NA | NA | 17.20 (0.8) | 18.01 (0.72) | 16.75 (0.73) |
| Adrenal weight, mg | 0.72 (0.08) | 0.80 (0.05) | 0.75 (0.04) | 0.78 (0.05) | 0.78 (0.04) | 0.70 (0.05) |
| Pituitary weight, mg | 1.86 (0.23) | 2.26 (0.23) | 1.78 (0.1) | 2.19 (0.22) | 2.18 (0.1) | 2.02 (0.11) |
| P30 | ||||||
| T, ng/mL | 0.03 (0.01) | 0.02 (0.01) | 0.05 (0.02) | ND | 0.11 (0.02) | ND |
| LH, ng/mL | 1.35 (0.16) | 1.36 (0.13) | 1.79 (0.26) | 1.33 (0.16) | 1.35 (0.16) | 1.41 (0.14) |
| E2, pg/mL | 8.50 (1.68) | 8.25 (1.08) | 9.96 (2.68) | 7.09 (0.8) | 7.72 (1.63) | 7.06 (0.98) |
| Body weight, g | 91.97 (1.82) | 96.13 (3.04) | 93.08 (1.7) | 82.64 (1.35) | 86.69 (1.86) | 80.74 (2.18) |
| GSI | 9.54 (0.15) | 9.23 (0.14) | 9.09 (0.13)b | 0.39 (0.01) | 0.42 (0.03) | 0.42 (0.03) |
| Uterus weight, mg | NA | NA | NA | 62.13 (5.07) | 80.20 (17.72) | 62.14 (5.46) |
| Adrenal weight, mg | 1.97 (0.15) | 2.35 (0.07)a | 2.34 (0.21)a | 1.93 (0.1) | 2.34 (0.11)a | 2.19 (0.1) |
| Pituitary weight, mg | 4.43 (0.24) | 4.54 (0.25) | 4.35 (0.23) | 4.12 (0.19) | 4.45 (0.15) | 4.11 (0.22) |
| P45 | ||||||
| T, ng/mL | 0.20 (0.05) | 0.20 (0.04) | 0.34 (0.07) | 0.02 (0.00) | 0.03 (0.01) | 0.03 (0.01) |
| LH, ng/mL | 1.29 (0.14) | 1.14 (0.1) | 1.36 (0.11) | 4.90 (2.61) | 1.55 (0.3) | 8.69 (5.29) |
| E2, pg/mL | 7.20 (0.6) | 7.84 (1.54) | 8.77 (0.75) | 21.94 (2.41) | 20.36 (3.41) | 28.78 (4.09) |
| Body weight, g | 195.59 (3.98) | 197.52 (2.37) | 200.19 (3.39) | 157.79 (2.23) | 156.91 (2.12) | 161.52 (3.01) |
| GSI | 11.55 (0.62) | 12.03 (0.13) | 12.16 (0.14) | 0.53 (0.01) | 0.54 (0.02) | 0.50 (0.02) |
| Uterus weight, mg | NA | NA | NA | 510.51 (33.97) | 469.03 (21.85) | 526.55 (31.09) |
| Adrenal weight, mg | 3.14 (0.2) | 3.28 (0.08) | 3.18 (0.11) | 3.64 (0.15) | 3.89 (0.23) | 3.96 (0.13) |
| Pituitary weight, mg | 8.30 (0.23) | 8.69 (0.32) | 9.24 (0.38) | 9.29 (0.32) | 8.53 (0.21) | 8.91 (0.36) |
| P90 | ||||||
| T, ng/mL | 0.63 (0.12) | 0.57 (0.1) | 0.68 (0.16) | 0.02 (0.01) | 0.02 (0.00) | 0.02 (0.01) |
| LH, ng/mL | 0.72 (0.05) | 0.72 (0.07) | 0.87 (0.08) | 4.17 (2.11) | 2.85 (1.65) | 4.61 (2.11) |
| E2, pg/mL | 7.86 (1.46) | 6.44 (0.78) | 8.11 (0.89) | 20.15 (2.8) | 23.04 (4.55) | 20.16 (2.12) |
| Body weight, g | 384.60 (7.3) | 390.13 (8.35) | 383.46 (6.7) | 262.95 (7.23) | 257.30 (5.19) | 249.41 (4.26) |
| GSI | 9.62 (0.5) | 10.04 (0.19) | 10.18 (0.2) | 0.46 (0.02) | 0.47 (0.01) | 0.46 (0.01) |
| Uterus weight, mg | NA | NA | NA | 666.70 (31.06) | 734.32 (52.13) | 727.10 (45.37) |
| Adrenal weight, mg | 4.57 (0.17) | 4.83 (0.1) | 4.89 (0.12) | 5.72 (0.14) | 5.20 (0.43) | 5.85 (0.18) |
| Pituitary weight, mg | 12.56 (0.64) | 12.79 (0.27) | 12.40 (0.47) | 13.84 (0.7) | 13.56 (0.44) | 13.42 (0.61) |
Abbreviations: E2, estradiol; GSI, gonadal somatic index, calculated as gonad weight/body weight; NA, not applicable; ND, not determined; T, testosterone. Data shown are means (± SEM).
Significant effect (P < .05) compared to corresponding DMSO group.
Trend (.05 < P < .1) compared with the corresponding DMSO group.
To summarize, perinatal exposure to estrogenic compounds had sex-specific effects on reproductive physiology. Specifically, A1221 delayed the timing of puberty in males, decreased serum LH on P15, and increased adrenal weights on P30. In females, the timing of eye opening was delayed by A1221, whereas exposure to EB increased the number of irregular estrous cycles and increased the weight of the adrenal glands on P30 only.
Gestational exposure to estrogenic compounds results in sexually dimorphic expression of numerous genes in the AVPV
In the AVPV, 28 genes were altered by gestational exposure to estrogenic compounds. Further analysis revealed that 21 of those genes were specifically affected in the female AVPV but not the male (Pdyn was the only gene affected in both males and females), of which 9 displayed a sexually dimorphic expression pattern throughout development (Figure 1) based on the following statistical criteria: (1) ANOVA or nonparametric statistics indicated an interaction of sex × treatment or sex × treatment × age; (2) male and female profiles in EB or A1221 animals have different distributions based on Bayesian analysis (Supplemental Material); and (3) the area between the EB or A1221 male and female curves was 50% greater than the difference between those for the DMSO males and females. Nine and 4 genes, respectively, met these criteria in the EB- and A1221-treated animals. Identified genes (Figure 1) fell into 4 functional groups: steroid hormone receptors (Esr2), hypothalamic neuropeptides and their receptors (Avpr1a, Kiss1, Mc3r, Oxt, Pdyn, and Tac2), and growth factors and their receptors (Bdnf and Igf1).
Figure 1.
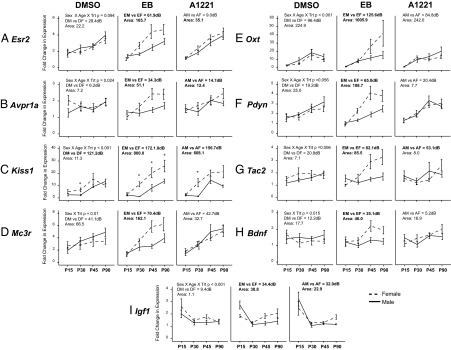
Developmental profiles of genes affected by treatment in the AVPV that are sexually dimorphic in the EB and/or A1221 but not DMSO rats. Significant treatment effects were specific to females for all genes except Pdyn (significant in both males and females). A, Esr2; B, Avpr1a; C., Kiss1; D, Mc3r; E, Oxt; F, Pdyn; G, Tac2; H, Bdnf; I, Igf1. Bayes analysis results are presented in decibans (dB); a high value (>20) indicates a high probability of a different distribution. Bold text indicates that the requirements of each statistical method were met (see text). DM, DMSO males; DF, DMSO females; EM, EB males; EF, EB females; AM, A1221 males; AF, A1221 females; Trt, treatment; Area, area between the curves.
Gene expression profiles in the female AVPV are masculinized after gestational exposure to EDCs
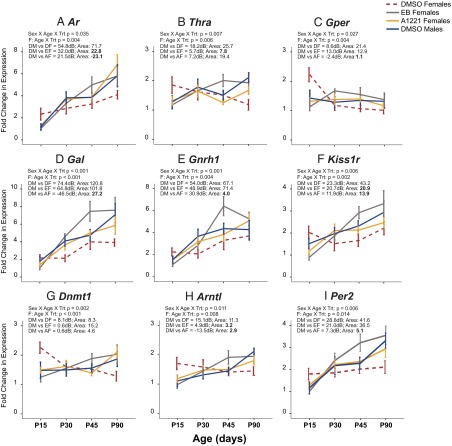
We noticed that profiles of genes in the AVPV of treated females appeared to be similar to those of control (DMSO) males. To test this statistically, we applied the following criteria: (1) genes must display an age × treatment effect in the females; (2) gene expression in the treated females, based on Bayesian analysis (Supplemental Material), was similar to that for DMSO males; and (3) the area between the DMSO male and treated female curves (Supplemental Material) was at least 50% lower than the difference between the control males and females. Nine of the 21 genes altered in the female AVPV displayed a male-typical expression pattern throughout development (Figure 2). These genes fell into 4 functional groups: steroid or nuclear hormone receptors (Ar, Thra, and Gper), neuropeptides, and their receptors (Gal, Gnrh1, and Kiss1r), epigenetic processes (Dnmt1), and circadian clock genes (Arntl and Per2).
Figure 2.
Expression profiles of 9 genes affected by treatment in the female AVPV that had a masculinized pattern in EB and/or A1221 rats. A, Ar; B, Thra; C, Gper; D, Gal; E, Gnrh1; F, Kiss1r; G, Dnmt1; H, Arntl; I, Per2. Data are also shown for DMSO males (DM; blue) to enable comparison with the three female groups: DMSO females (DF; red dashed), EB females (EF; gray) and A1221 females (AF; yellow). Trt, treatment; Area, area between the curves.
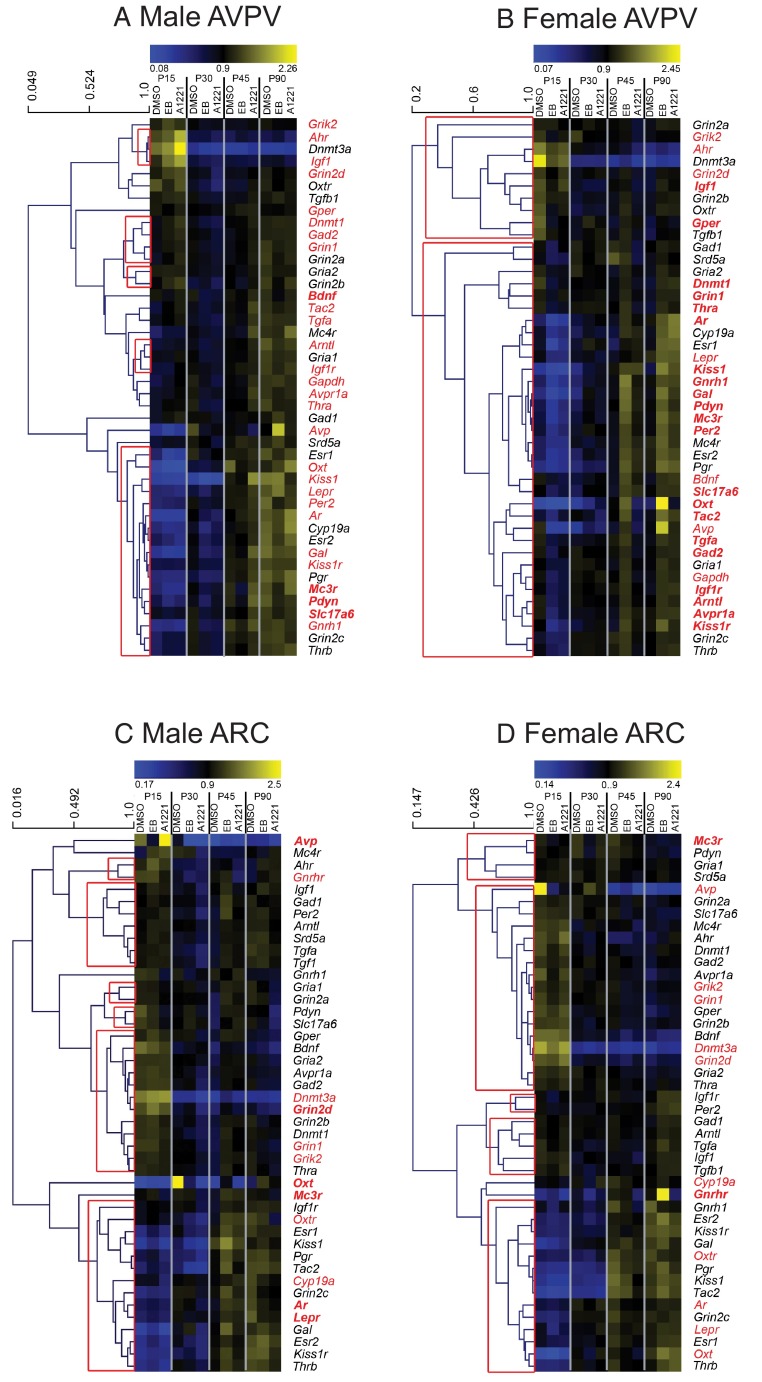
Hierarchical cluster analysis was conducted using the average linking method and correlation coefficients to determine which genes shared similar expression patterns across development in each sex (Figure 3). In the female AVPV, 2 large clusters were validated (red box in Figure 3B). The top cluster includes genes that decrease from P15, and the bottom cluster is composed of genes that increase from P15 to P90. In males (Figure 3A), there were very few validated clusters, but, in general, gene expression in the top clusters decreased from P15 to P90 or underwent very little change, whereas the genes in the bottom cluster increased from P15 to P90. The genes significantly altered by treatment are indicated in red, and those with specific effects in each sex are highlighted in bold.
Figure 3.
Clustergrams of gene expression in the AVPV and ARC of males and females. A, Male AVPV. B, Female AVPV. C, Male ARC. D, Female ARC. Genes with a significant main treatment effect or interaction are in red text. Those with sex-specific effects are indicated in bold text. Validated gene clusters are indicated by red boxes.
In summary, treatment with estrogenic compounds altered developmental profiles of gene expression in the female AVPV. We noted 2 general alterations: those genes that were sexually dimorphic after treatment (Figure 1) and those that were masculinized (Figure 2). Genes clustered according to developmental profiles: those that increased from P15 to P90 and those that decreased from P15 to P90 (Figure 3). Genes affected by treatment did not appear to cluster in either males or females.
DNA methylation is not altered in the Ar or Per2 promoters throughout development
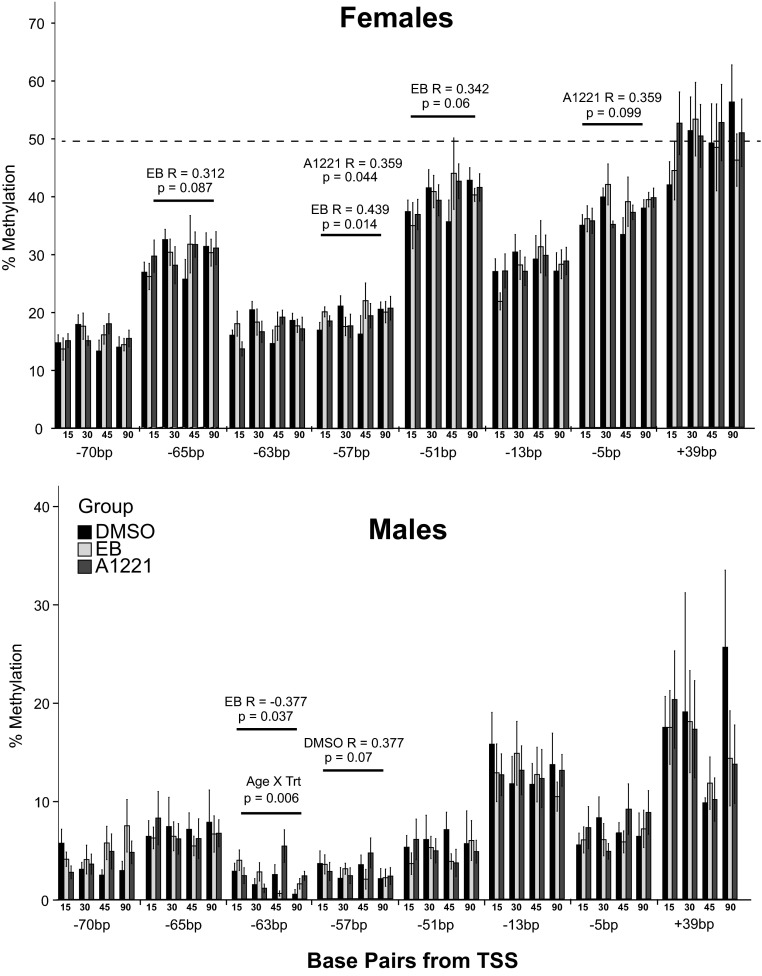
To determine whether epigenetic mechanisms were altered by exposure to EDCs we used pyrosequencing to measure DNA methylation on promoter regions of 2 genes that were affected by treatment (Ar and Per2), chosen because each displayed a significant sex × age × treatment effect and had large CpG islands surrounding the transcription start sites. With the exception of 2 CpGs, the Per2 promoter was almost completely demethylated by P15 and remained so throughout development (data not shown). The Ar promoter region (Figure 4) displayed sexually dimorphic methylation at each CpG investigated (−70 to +39 bp from the transcription start site) with females (Figure 4A) having higher methylation compared with males (Figure 4B), a sex difference that was anticipated based on the expression of Ar on the X chromosome. Further analysis revealed that 1 CpG site at −63 displayed a significant interaction of age and treatment in males (P = .006). Although there were few changes in methylation, we investigated whether methylation correlated with expression of Ar mRNA in individuals across all ages. Significant differences (P < .05) and trends (0.05 < P < .1) are shown (Figure 4). In females exposed to EB and/or A1221, but not DMSO, mRNA expression was positively correlated (significant or trends) with methylation at CpGs −65, −57, −51, and −5 bp, and average methylation across the region also tended to be positively correlated (EB, P = .064; A1221, P = .085). In males exposed to DMSO, expression tended to be positively correlated with methylation at −57 bp. In males exposed to EB, expression was negatively correlated with methylation at CpG −63 (P = .037; R = −0.377). Although treatment did not affect DNA methylation of either promoter region, we did identify differences between treatment groups at specific CpGs where DNA methylation was correlated with expression of Ar.
Figure 4.
Methylation of individual CpG sites from −70 to +39 bp from the transcription start site in the promoter region of Ar is shown for each age in females (top) and males (bottom) for each treatment group. The one significant age × treatment interaction is shown (−63 bp in males). Methylation was positively correlated with expression of Ar mRNA at the following CpGs: in females, −65 bp (EB), −57 bp (EB and A1221), −51 bp (EB), and −5 bp (A1221); and in males, −57 bp (DMSO). Methylation was negatively correlated with Ar mRNA levels at −63 bp (EB) in males. R values and P values for correlation results are shown.
Sex differences and treatment effects in the ARC
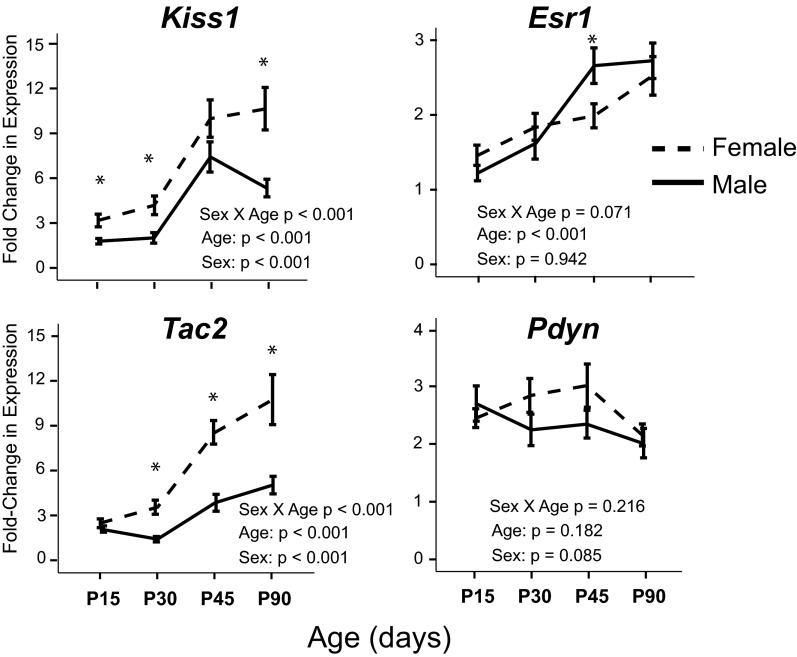
In the ARC, a subpopulation of neurons involved in the control of reproduction coexpress Kiss1, Tac2 (encoding neurokinin B protein), and Pdyn (encoding the precursor to the dynorphin peptide) and are referred to as KNDy neurons (53). KNDy neurons also coexpress Esr1 (54, 55). Three of these 4 genes had sexually dimorphic expression in the ARC independent of treatment (Figure 5).
Figure 5.
Gene expression of KNDy neurons (Kiss1, Tac2, and Pdyn) and Esr1 is shown for the developing ARC, collapsed across treatment groups. Kiss1 and Tac2 expression levels were greater in the female ARC than in the male ARC, independent of treatment group. Esr1 expression was greater in the male ARC only on P45. Pdyn expression was not sexually dimorphic, nor did it change with age. –––, females; ——, males. *, P < .05 vs corresponding male at the same age.
In addition, 12 genes in the ARC were altered by gestational exposure to EDCs (main effect or interaction of treatment P < .05) and are indicated in red on Figure 3, C and D. Further analysis revealed that 6 of those genes were specifically affected in the male ARC (Figure 6), by category: sex steroid hormone receptor (Ar), neuropeptides and their receptors (Lepr, Mc3r, Avp, and Oxt), and neurotransmitter receptor subunit (Grin2d). Each gene had a unique expression pattern and differential effects of treatment in a developmental age–specific manner. In addition to these effects in males, there were also significant interactions of sex × age × treatment for Ar (P = .033), Lepr (P = .03), and Avp (P < .001) and interactions of sex × treatment for Mc3r (P = .006), Oxt (P = .046), and Grin2d (P = .004).
Figure 6.
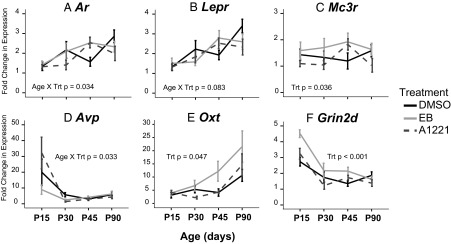
Expression of genes with a significant main effect of treatment or interaction of treatment with another variable in the male ARC. A. Ar; B. Lepr; C, Mc3r; D, Avp; E, Oxt; F, Grin2d. Three genes (Mc3r, Oxt, and Grin2d) had significantly greater expression in the EB-treated than DMSO-treated males. Trt, treatment.
Cluster analysis of the ARC (Figure 3, C and D) demonstrated 5 (female) and 6 (male) validated clusters, although many contained only a few genes (2 to 5). In general, genes were grouped into those that decreased from P15 to P90 (top) or increased from P15 to P90. When comparing validated clusters in the male ARC with those in the female, we noted that the bottom cluster in the males consisted of 14 genes and closely resembled the lower cluster in the female ARC. These clusters shared 11 of 14 genes, 5 of which had a significant treatment effect.
To summarize, most of the effects of treatment in the ARC were observed in the males. Cluster analysis revealed that there were similarities between males and females; however, the genes altered by treatment did not cluster together in males or females.
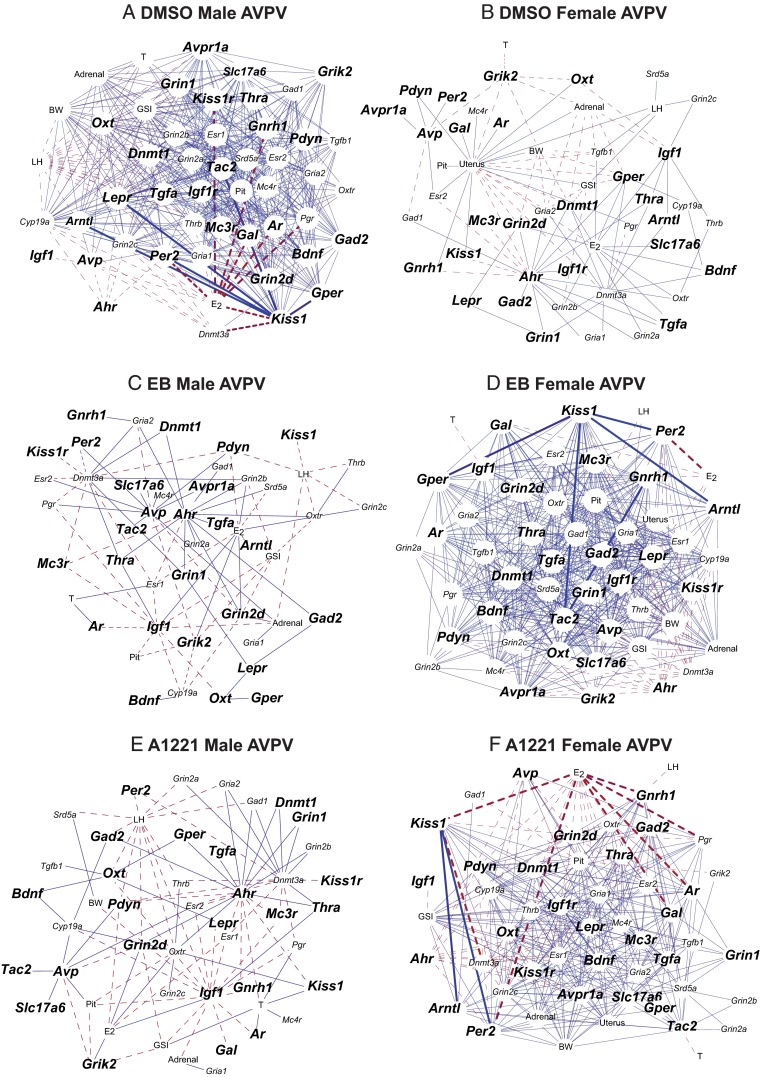
Bionetwork analysis revealed male-typical relationships in the female AVPV after EDC exposure
Bionetwork analysis was conducted using Pearson correlation coefficients to enable investigation into relationships among genes, hormones, and somatic markers and to determine effects of prenatal EDCs and sex differences. Although these interactions are not specific to individual cells, they still provide important information on how genes may be changing throughout a specific neuronal population and highlight important changes involved in reproductive function and physiology. In the AVPV (Figure 7), we observed a greater number of unique relationships in the EB and A1221 female AVPV networks compared with those in the DMSO females; many of the former were similar to the control male AVPV. In addition, although the number of correlations in the EB and A1221 females was similar, the relationships that overlapped with the males differed. While there are too many correlations to discuss here, we will provide two examples to illustrate this point, one for serum estradiol and the other for the Kiss1 gene. In the DMSO males, estradiol and Kiss1 had a number of significant correlations (16 and 21, respectively) not observed in the DMSO females. In the A1221 females, estradiol had a number of negative correlations (15 genes), 12 of which overlapped with correlations with estradiol in the DMSO males. The EB females only had 5 correlations with estradiol, but 4 of these 5 overlapped with correlations in DMSO males. For Kiss1, EB females had 23 interactions, 17 of which overlapped with those of the DMSO males, whereas in A1221 females there were 13 significant correlations, 9 of which overlapped with the those of the DMSO males. Although there was substantial overlap between hubs in EDC-treated females and DMSO males, implying that females are masculinized, there was little overlap between EDC-treated males and DMSO females, suggesting that males are not feminized. The only hub in common among DMSO females, EB males, and A1221 males was Ahr. Such results provide interesting candidates for follow-up studies investigating sexual differentiation of the brain and identifying differing pathways by which hormones or EDCs act during the perinatal period.
Figure 7.
Cytoscape networks of genes, hormones, and somatic changes in the AVPV across development in treated animals. A, DMSO male AVPV. B, DMSO female AVPV. C, EB Male AVPV, D, EB female AVPV. E, A1221 male AVPV. F, A1221 female AVPV. Genes with a significant interaction of age × treatment × sex are indicated by larger font and bold (P < .05). Networks are presented as follows: DMSO males were compared directly with DMSO females, and vice versa; EDC-treated females were compared with the DMSO females and EDC-treated males with the DMSO males. GSI, gonadal somatic index; E2, estradiol; T, testosterone; Pit, pituitary weight; BW, body weight; Adrenal, adrenal weight; Uterus, uterus weight.
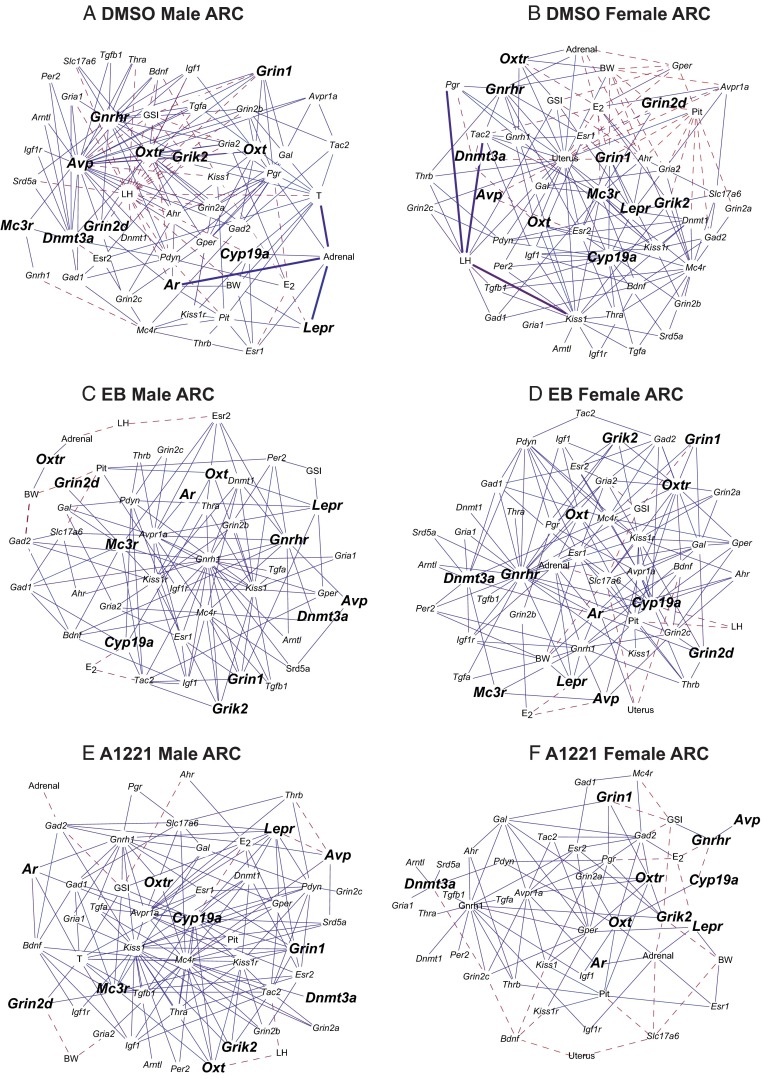
Networks were also generated for the ARC (Figure 8). Unlike in the AVPV, there were few differences in the number of significant correlations between males and females nor were there many differences between the treatment groups.
Figure 8.
Cytoscape networks of genes, hormones, and somatic changes in the ARC across development in treated animals. A, DMSO male ARC. B, DMSO female ARC. C, EB Male ARC. D, EB female ARC. E, A1221 male ARC. F, A1221 female ARC. Genes with a significant interaction of age × treatment × sex are indicated by larger font and bold (P < .05). Networks are presented, and abbreviations are the same as in the legend to Figure 7.
Discussion
EDCs have become a major focus of investigation into the fetal basis of adult disease. With the exception of a few studies (56–58), there is a dearth of evidence for the effects of EDCs in a developmental context, including those in the hypothalamus, something we addressed here in our model of prenatal EB or A1221 exposure. A1221 has been shown previously to perturb estrous cycles (31, 33), reproductive behavior (34, 36), timing of puberty (33), and reproductive senescence (59). Our current study differs from studies reported in the literature in several fundamental ways. First, we provide a comprehensive developmental profile of 48 neuroendocrine genes beginning in the juvenile period (P15), through puberty (P30 and P45), and continuing into adulthood (P90). Second, we have also refined previous work (10, 33) by focusing on 2 of the key subnuclei, the AVPV and ARC, in the current analysis. Third, we related changes in reproductive physiology and function back to gene expression changes in the hypothalamus. Fourth, we investigated whether epigenetic mechanisms are disrupted by characterizing changes in DNA methylation of 2 of the genes that were significantly disrupted in a sex- and age-specific manner. Finally, analyzing a large number of genes allowed us to identify novel gene targets and molecular mechanisms of endocrine disruption and to conduct a bionetwork analysis.
Gestational exposure to EDCs disrupt gene expression in the AVPV of females, together with effects on reproductive function
After prenatal EDC treatment, some AVPV genes that were not dimorphic in control animals showed sex differences, whereas a different subset of genes were masculinized in the females. In reference to the former point, Kiss1, Tac2, and Pdyn were not dimorphic in control AVPVs but were dimorphic in the EDC-treated group. Although this result is contrary to literature reports showing sex differences in these genes (Kiss1 [60] and Pdyn [61]), differences are probably due to the timing of when females were euthanized in their estrous cycles. Our laboratory reported previously that Kiss1 is not sexually dimorphic in the rostral hypothalamus in adulthood (P60) (10), a result that we replicate here in our more refined AVPV punches. We also believe the difference may be due to effects of EDCs on biological rhythms, discussed below.
Concomitant with these gene expression changes were differences in the physiology of EDC-treated females. Our goal was to euthanize our postpubertal females during their LH surge (1–3 hours before lights out on proestrus) (62, 63). Prenatal EB treatment resulted in significantly fewer animals with regular cycles. More subtle but potentially biologically relevant changes to estrous cycles were also seen in the A1221 group. Both of these EDC-treated groups of females had increased expression of Kiss1, Tac2, Esr1, and Pdyn on P90 in the AVPV than their control counterparts, consistent with a relationship between expression of these genes and the regulation of reproductive cycles, together with vulnerability of these pathways to endocrine disruptors.
We also noticed that some gene expression profiles of EDC-treated females more closely resembled those in control males. Nine genes that were much more similar in expression to those in the DMSO male than the DMSO female were identified in the EDC-treated female AVPV. We interpret this result as a masculinization of these genes by estrogenic EDC treatment. Furthermore, the developmental profiles of many of these genes underwent dynamic changes in both a treatment- and age-specific manner from prepuberty through adulthood.
Disruption of circadian gene expression in the female AVPV
One novel finding of our study was that the 2 clock genes (Arntl and Per2) included on our array exhibited developmental sex differences in control animals. Furthermore, gestational exposure to EDCs resulted in a male-typical expression profile throughout development in the female AVPV. There are sex differences in clock genes in the suprachiasmatic nucleus (SCN) (64, 65) and other tissues (66, 67), and EDCs (notably dioxin) affect clock gene expression and function throughout the body (68–70). To our knowledge, we are the first to show that clock genes in the AVPV are sexually dimorphic, a target of EDCs, and that expression can be programmed by gestational exposure to estrogenic compounds in a rodent model. However, circadian gene expression (Per1 and RevErbβ2) was decreased in the hypothalamus of female fish exposed to effluents from paper mills. Interestingly, this effect was associated with an inhibition of egg production and spawning events, suggesting that ovulation was also disrupted (71). A recent study focusing on clock genes in the SCN reported that rhythmic expression of Arntl and Per2, among others, was activated between E19 and P3 with the rhythms becoming more pronounced by P10 in juvenile rats (7). Although sex differences were not investigated, those data (7) suggest that the rhythmic expression of clock genes is developing during the critical period of sexual differentiation of the brain, a time period that may be vulnerable to estrogenic EDCs. Finally, recent evidence suggests that the AVPV plays an important role in circadian regulation of GnRH synthesis and release. Although not investigated in males, Kiss1 displays rhythmic expression in the female AVPV (72–74) and is directly regulated by the circadian transcription factor DBP. As a whole, our results on Per2 and Arntl expression and regulation by EDCs in the developing AVPV suggest that not only is this region part of the central circadian network, but it also undergoes developmental programming. Although further investigation is necessary, we interpret the masculinized expression pattern as a possible mechanism for the alterations in estrous cyclicity observed in our treated females, a hypothesis supported by the lack of ovulation observed in the fathead minnow populations with similar disruptions in clock gene expression (71).
DNA methylation effects of gestational exposure to EDC
We measured DNA methylation of promoter regions of 2 genes (Per2 and Ar) that were masculinized in the AVPV of treated females. The Per2 promoter region was completely demethylated by P15, an effect that has been reported in the SCN (75). There were a few significant effects of treatment (data not shown); however, the level of methylation was very low (<5% at most sites), suggesting that changes, if any, may be cell type specific. For Ar, a significant treatment effect was observed for 1 CpG in the male AVPV (−63), but this finding should be interpreted with caution because this site was almost completely demethylated and the change was less than 5%. Ar is expressed on the X chromosome, allowing us to deduce that EDCs are probably not altering global methylation because there was no evidence that any CpGs had escaped X inactivation in females. Finally, we noted small but significant differences in correlations of specific CpGs with Ar gene expression. More specifically, in the treated females, methylation was positively correlated with mean methylation of several CpGs investigated, an effect that was not observed in the DMSO females. Interestingly, 2 of these sites (−57 and −51) are a putative binding site for Rev-erb, a transcriptional repressor of circadian gene expression (76, 77), and the gene altered by effluents in the fathead minnow (71), providing support for our hypothesis that the AVPV is a regulator of circadian signaling and is vulnerable to endocrine disruption. Interestingly, we found that methylation is positively correlated with expression of Ar mRNA at many of the CpG sites in the female, suggesting potential sex differences in epigenetic regulation of expression. This needs further testing in future experiments.
Gene expression changes in the ARC are specific to males
The ARC regulates negative feedback to the GnRH system and is not sexually dimorphic in terms of overall morphology (for a review, see Ref. 78). Therefore, we were not expecting gestational exposure to EDCs to result in sex-specific effects in the ARC. Contrary to this prediction, 12 of the 48 genes measured were altered by gestational exposure to EDCs, and of those, 6 (Ar, Lepr, Mc3r, Avp, Oxt, and Grin2d) were affected specifically in the male ARC. In addition, males exposed to A1221 went through puberty later than their DMSO counterparts. Although we cannot determine a causal relationship, it is possible that there is a link between genes altered in the male ARC by A1221 and the hypothalamic control of the timing of puberty. Consistent with this idea, there is some limited evidence that the ARC is important for regulating the timing of reproductive transitions (79). Two of our identified genes, Ar and Lepr, were significantly altered by A1221 exposure. These genes are critical for the hypothalamus to sense and respond to peripheral changes in metabolism (Lepr) and gonadal status (Ar), both permissive cues that are known to drive the onset of puberty (1). The expression profiles of both genes were similar but were “out of phase” with those of the DMSO males: when expression was up in the DMSO animals it was suppressed in the A1221 animals and vice versa. Our data identify Ar and Lepr as candidate genes, which may be related to the later onset of puberty observed in the males exposed to A1221, for follow-up in the ARC.
In a companion article (31), we found that 5 of these 6 genes (all but Avp) overlapped with genes identified in the ARC of female littermates treated with A1221 or EB that had been allowed to age to 9 months. Those EDC-exposed females underwent age-related changes in estrous cyclicity at a different pace from that for the control rats and had a number of gene expression changes in the ARC, but few in the AVPV. When that study is compared with this one, it is notable that the AVPV of females is most affected early in life (from P15 to P90), whereas later in life, at 9 months (31), genes in the female ARC have a differential expression pattern. Although it is difficult to interpret the functional role of these gene expression changes during reproductive transitions of puberty and senescence, our data identify potential candidate genes in the ARC that may be important for regulating reproductive transitions in males and females. They also underscore how expression patterns of specific genes in specific brain regions undergo dynamic changes from birth through aging.
In conclusion, most previous studies on effects of gestational exposure to EDCs on the hypothalamus have focused on a single adult age or one of the sexes (for a review, see Ref. 80). Our present study extended all past work by considering EDC effects on brain sexual differentiation in the context of dynamic postnatal development change from infancy to adulthood and by relating results back to altered reproductive physiology and development. This approach has enabled us to identify novel targets of EDCs, notably the clock genes Per2 and Arntl, and to develop new hypotheses regarding the functional outcomes of sexual differentiation of the brain. Our results suggesting that gene expression in the female AVPV is masculinized by prenatal EDCs also provides insight into how we might define a masculinized phenotype. Last, the results underscore the importance of considering developmental age and cycle stage when the effects of gestational exposure to EDCs is examined. The postnatal profiles of genes in the hypothalamus underwent dynamic developmental changes in control animals, and effects of EDCs were expressed differentially in male and female rats as they matured into adulthood. Thus, taking a snapshot of the effects of EDCs at a single age may not fully represent the dynamic interplay of hormones, gene expression, and functional outcomes.
Additional material
Supplementary data supplied by authors.
Acknowledgments
We thank Ross Gillette for technical assistance, Dr Michael Woller for performing the LH RIA, Dean Kirson for help with bionetwork analysis, and David Barrett for help with analyzing estrous cycle data.
This work was supported by the National Institutes of Health (Grants 1RC1 ES018139 and 1R01 ES020662 (to A.C.G.) and 1F31 AG034813 (to D.M.W.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- A1221
- Aroclor 1221
- ARC
- arcuate nucleus
- AVPV
- anteroventral periventricular nucleus
- CV
- coefficient of variation
- DMSO
- dimethylsulfoxide
- E
- embryonic day
- EDC
- endocrine-disrupting chemical
- P
- postnatal day
- PCB
- polychlorinated biphenyl
- SCN
- suprachiasmatic nucleus
- TSS
- transcription start site.
References
- 1. Ebling FJ. The neuroendocrine timing of puberty. Reproduction. 2005;129:675–683. [DOI] [PubMed] [Google Scholar]
- 2. Terasawa E, Fernandez DL. Neurobiological mechanisms of the onset of puberty in primates. Endocr Rev. 2001;22:111–151. [DOI] [PubMed] [Google Scholar]
- 3. Cao J, Patisaul HB. Sexually dimorphic expression of hypothalamic estrogen receptors α and β and Kiss1 in neonatal male and female rats. J Comp Neurol. 2011;519:2954–2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McAbee MD, DonCarlos LL. Ontogeny of region-specific sex differences in androgen receptor messenger ribonucleic acid expression in the rat forebrain. Endocrinology. 1998;139:1738–1745. [DOI] [PubMed] [Google Scholar]
- 5. Navarro VM, Castellano JM, Fernández-Fernández R, et al. Developmental and hormonally regulated messenger ribonucleic acid expression of KiSS-1 and its putative receptor, GPR54, in rat hypothalamus and potent luteinizing hormone-releasing activity of KiSS-1 peptide. Endocrinology. 2004;145:4565–4574. [DOI] [PubMed] [Google Scholar]
- 6. Navarro VM, Ruiz-Pino F, Sánchez-Garrido MA, et al. Role of neurokinin B in the control of female puberty and its modulation by metabolic status. J Neurosci. 2012;32:2388–2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sládek M, Sumová A, Koáciková Z, Bendová Z, Laurinová K, Illnerová H. Insight into molecular core clock mechanism of embryonic and early postnatal rat suprachiasmatic nucleus. Proc Natl Acad Sci USA. 2004;101:6231–6236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tena-Sempere M, González LC, Pinilla L, Huhtaniemi I, Aguilar E. Neonatal imprinting and regulation of estrogen receptor α and β mRNA expression by estrogen in the pituitary and hypothalamus of the male rat. Neuroendocrinology. 2001;73:12–25. [DOI] [PubMed] [Google Scholar]
- 9. Walker DM, Juenger TE, Gore AC. Developmental profiles of neuroendocrine gene expression in the preoptic area of male rats. Endocrinology. 2009;150:2308–2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Walker DM, Kirson D, Perez LF, Gore AC. Molecular profiling of postnatal development of the hypothalamus in female and male rats. Biol Reprod. 2012;87:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Davis AM, Seney ML, Walker HJ, Tobet SA. Differential colocalization of Islet-1 and estrogen receptor α in the murine preoptic area and hypothalamus during development. Endocrinology. 2004;145:360–366. [DOI] [PubMed] [Google Scholar]
- 12. Quadros PS, Goldstein AY, De Vries GJ, Wagner CK. Regulation of sex differences in progesterone receptor expression in the medial preoptic nucleus of postnatal rats. J Neuroendocrinol. 2002;14:761–767. [DOI] [PubMed] [Google Scholar]
- 13. Yokosuka M, Okamura H, Hayashi S. Postnatal development and sex difference in neurons containing estrogen receptor-alpha immunoreactivity in the preoptic brain, the diencephalon, and the amygdala in the rat. J Comp Neurol. 1997;389:81–93. [DOI] [PubMed] [Google Scholar]
- 14. Nugent BM, Schwarz JM, McCarthy MM. Hormonally mediated epigenetic changes to steroid receptors in the developing brain: implications for sexual differentiation. Horm Behav. 2011;59:338–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schwarz JM, Nugent BM, McCarthy MM. Developmental and hormone-induced epigenetic changes to estrogen and progesterone receptor genes in brain are dynamic across the life span. Endocrinology. 2010;151:4871–4881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Abeliovich A, Beal MF. Parkinsonism genes: culprits and clues. J Neurochem. 2006. 99:1062–1072. [DOI] [PubMed] [Google Scholar]
- 17. Davis EC, Popper P, Gorski RA. The role of apoptosis in sexual differentiation of the rat sexually dimorphic nucleus of the preoptic area. Brain Res. 1996;734:10–18. [PubMed] [Google Scholar]
- 18. Davis EC, Shryne JE, Gorski RA. Structural sexual dimorphisms in the anteroventral periventricular nucleus of the rat hypothalamus are sensitive to gonadal steroids perinatally, but develop peripubertally. Neuroendocrinology. 1996;63:142–148. [DOI] [PubMed] [Google Scholar]
- 19. Wilson CA, Davies DC. The control of sexual differentiation of the reproductive system and brain. Reproduction. 2007;133:331–359. [DOI] [PubMed] [Google Scholar]
- 20. Clark MM, Robertson RK, Galef BG., Jr Intrauterine position effects on sexually dimorphic asymmetries of Mongolian gerbils: testosterone, eye opening, and paw preference. Dev Psychobiol. 1993;26:185–194. [DOI] [PubMed] [Google Scholar]
- 21. Pignatelli D, Xiao F, Gouveia AM, Ferreira JG, Vinson GP. Adrenarche in the rat. J Endocrinol. 2006;191:301–308. [DOI] [PubMed] [Google Scholar]
- 22. Sisk CL, Zehr JL. Pubertal hormones organize the adolescent brain and behavior. Front Neuroendocrinol. 2005;26:163–174. [DOI] [PubMed] [Google Scholar]
- 23. Jacobson CD, Shryne JE, Shapiro F, Gorski RA. Ontogeny of the sexually dimorphic nucleus of the preoptic area. J Comp Neurol. 1980;193:541–548. [DOI] [PubMed] [Google Scholar]
- 24. Rhees RW, Shryne JE, Gorski RA. Onset of the hormone-sensitive perinatal period for sexual differentiation of the sexually dimorphic nucleus of the preoptic area in female rats. J Neurobiol. 1990;21:781–786. [DOI] [PubMed] [Google Scholar]
- 25. Rhees RW, Shryne JE, Gorski RA. Termination of the hormone-sensitive period for differentiation of the sexually dimorphic nucleus of the preoptic area in male and female rats. Brain Res Dev Brain Res. 1990;52:17–23. [DOI] [PubMed] [Google Scholar]
- 26. Matsuda KI, Mori H, Kawata M. Epigenetic mechanisms are involved in sexual differentiation of the brain. Rev Endocr Metab Disord. 2012;13:163–171. [DOI] [PubMed] [Google Scholar]
- 27. McCarthy MM, Auger AP, Bale TL, De Vries GJ, Dunn GA, Forger NG, Murray EK, Nugent BM, Schwarz JM, Wilson ME. The epigenetics of sex differences in the brain. J Neurosci. 2009;29:12815–12823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zoeller RT, Brown TR, Doan LL, et al. Endocrine-disrupting chemicals and public health protection: a statement of principles from The Endocrine Society. Endocrinology. 2012;153:4097–4110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Layton AC, Sanseverino J, Gregory BW, Easter JP, Sayler GS, Schultz TW. In vitro estrogen receptor binding of PCBs: measured activity and detection of hydroxylated metabolites in a recombinant yeast assay. Toxicol Appl Pharmacol. 2002;180:157–163. [DOI] [PubMed] [Google Scholar]
- 30. Shekhar PV, Werdell J, Basrur VS. Environmental estrogen stimulation of growth and estrogen receptor function in preneoplastic and cancerous human breast cell lines. J Natl Cancer Inst. 1997;89:1774–1782. [DOI] [PubMed] [Google Scholar]
- 31. Walker DM, Kermath BA, Woller MJ, Gore AC. Disruption of reproductive aging in female and male rats by gestational exposure to estrogenic endocrine disruptors. Endocrinology. 2013;154:2129–2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dickerson SM, Cunningham SL, Gore AC. Prenatal PCBs disrupt early neuroendocrine development of the rat hypothalamus. Toxicol Appl Pharmacol. 2011;252:36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dickerson SM, Cunningham SL, Patisaul HB, Woller MJ, Gore AC. Endocrine disruption of brain sexual differentiation by developmental PCB exposure. Endocrinology. 2011;152:581–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Steinberg RM, Juenger TE, Gore AC. The effects of prenatal PCBs on adult female paced mating reproductive behaviors in rats. Horm Behav. 2007;51:364–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Steinberg RM, Walker DM, Juenger TE, Woller MJ, Gore AC. Effects of perinatal polychlorinated biphenyls on adult female rat reproduction: development, reproductive physiology, and second generational effects. Biol Reprod. 2008;78:1091–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chung YW, Clemens LG. Effects of perinatal exposure to polychlorinated biphenyls on development of female sexual behavior. Bull Environ Contam Toxicol. 1999;62:664–670. [DOI] [PubMed] [Google Scholar]
- 37. Gillette DM, Corey RD, Helferich WG, et al. Comparative toxicology of tetrachlorobiphenyls in mink and rats. I. Changes in hepatic enzyme activity and smooth endoplasmic reticulum volume. Fundam Appl Toxicol. 1987;8:5–14. [DOI] [PubMed] [Google Scholar]
- 38. Gillette DM, Corey RD, Lowenstine LJ, Shull LR. Comparative toxicology of tetrachlorobiphenyls in mink and rats. II. Pathology. Fundam Appl Toxicol. 1987;8:15–22. [DOI] [PubMed] [Google Scholar]
- 39. Murugesan P, Kanagaraj P, Yuvaraj S, Balasubramanian K, Aruldhas MM, Arunakaran J. The inhibitory effects of polychlorinated biphenyl Aroclor 1254 on Leydig cell LH receptors, steroidogenic enzymes and antioxidant enzymes in adult rats. Reprod Toxicol. 2005;20:117–126. [DOI] [PubMed] [Google Scholar]
- 40. Murugesan P, Senthilkumar J, Balasubramanian K, Aruldhas MM, Arunakaran J. Impact of polychlorinated biphenyl Aroclor 1254 on testicular antioxidant system in adult rats. Hum Exp Toxicol. 2005;24:61–66. [DOI] [PubMed] [Google Scholar]
- 41. Takagi Y, Aburada S, Hashimoto K, Kitaura T. Transfer and distribution of accumulated [14C]polychlorinated biphenyls from maternal to fetal and suckling rats. Arch Environ Contam Toxicol. 1986;15:709–715. [DOI] [PubMed] [Google Scholar]
- 42. Karmaus W, Zhu X. Maternal concentration of polychlorinated biphenyls and dichlorodiphenyl dichlorethylene and birth weight in Michigan fish eaters: a cohort study. Environ Health. 2004;3:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Law DC, Klebanoff MA, Brock JW, Dunson DB, Longnecker MP. Maternal serum levels of polychlorinated biphenyls and 1,1-dichloro-2,2-bis(p-chlorophenyl)ethylene (DDE) and time to pregnancy. Am J Epidemiol. 2005;162:523–532. [DOI] [PubMed] [Google Scholar]
- 44. Longnecker MP, Klebanoff MA, Brock JW, Guo X. Maternal levels of polychlorinated biphenyls in relation to preterm and small-for-gestational-age birth. Epidemiology. 2005;16:641–647. [DOI] [PubMed] [Google Scholar]
- 45. Schantz SL. Developmental neurotoxicity of PCBs in humans: what do we know and where do we go from here? Neurotoxicol Teratol. 1996;18:217–227; discussion 229–276. [DOI] [PubMed] [Google Scholar]
- 46. Lackmann GM. Polychlorinated biphenyls and hexachlorobenzene in full-term neonates. Reference values updated. Biol Neonate. 2002;81:82–85. [DOI] [PubMed] [Google Scholar]
- 47. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. [DOI] [PubMed] [Google Scholar]
- 48. Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. [DOI] [PubMed] [Google Scholar]
- 50. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B (Methodol). 1995;57:289–300. [Google Scholar]
- 51. Hochberg Y, Benjamini Y. More powerful procedures for multiple significance testing. Stat Med. 1990;9:811–818. [DOI] [PubMed] [Google Scholar]
- 52. Benjamini Y, Hochberg Y. On the adaptive control of the false discovery rate in multiple testing with independent statistics. J Educ Behav Stat. 2000;25:60–83. [Google Scholar]
- 53. Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA. Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J Neurosci. 2009;29:11859–11866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology. 2005;146:3686–3692. [DOI] [PubMed] [Google Scholar]
- 55. Smith JT, Dungan HM, Stoll EA, et al. Differential regulation of KiSS-1 mRNA expression by sex steroids in the brain of the male mouse. Endocrinology. 2005;146:2976–2984. [DOI] [PubMed] [Google Scholar]
- 56. Cao J, Mickens JA, McCaffrey KA, Leyrer SM, Patisaul HB. Neonatal bisphenol A exposure alters sexually dimorphic gene expression in the postnatal rat hypothalamus. Neurotoxicology 2012;33:23–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ho SM, Tang WY, Belmonte de Frausto J, Prins GS. Developmental exposure to estradiol and bisphenol A increases susceptibility to prostate carcinogenesis and epigenetically regulates phosphodiesterase type 4 variant 4. Cancer Res. 2006;66:5624–5632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Prins GS, Birch L, Couse JF, Choi I, Katzenellenbogen B, Korach KS. Estrogen imprinting of the developing prostate gland is mediated through stromal estrogen receptor α: studies with αERKO and βERKO mice. Cancer Res. 2001;61:6089–6097. [PubMed] [Google Scholar]
- 59. Gellert RJ. Uterotrophic activity of polychlorinated biphenyls (PCB) and induction of precocious reproductive aging in neonatally treated female rats. Environ Res. 1978;16:123–130. [DOI] [PubMed] [Google Scholar]
- 60. Oakley AE, Clifton DK, Steiner RA. Kisspeptin signaling in the brain. Endocr Rev. 2009;30:713–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Simerly RB, Young BJ, Carr AM. Co-expression of steroid hormone receptors in opioid peptide-containing neurons correlates with patterns of gene expression during the estrous cycle. Brain Res Mol Brain Res. 1996;40:275–284. [DOI] [PubMed] [Google Scholar]
- 62. Urbanski HF, Ojeda SR. The development of afternoon minisurges of luteinizing hormone secretion in prepubertal female rats is ovary dependent. Endocrinology. 1986;118:1187–1193. [DOI] [PubMed] [Google Scholar]
- 63. Scarbrough K, Wise PM. Age-related changes in pulsatile luteinizing hormone release precede the transition to estrous acyclicity and depend upon estrous cycle history. Endocrinology. 1990;126:884–890. [DOI] [PubMed] [Google Scholar]
- 64. Iwahana E, Karatsoreos I, Shibata S, Silver R. Gonadectomy reveals sex differences in circadian rhythms and suprachiasmatic nucleus androgen receptors in mice. Horm Behav. 2008;53:422–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Karatsoreos IN, Butler MP, Lesauter J, Silver R. Androgens modulate structure and function of the suprachiasmatic nucleus brain clock. Endocrinology. 2011;152:1970–1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Hirao J, Nishimura M, Arakawa S, et al. Sex and circadian modulatory effects on rat liver as assessed by transcriptome analyses. J Toxicol Sci. 2011;36:9–22. [DOI] [PubMed] [Google Scholar]
- 67. Textoris J, Ban LH, Capo C, Raoult D, Leone M, Mege JL. Sex-related differences in gene expression following Coxiella burnetii infection in mice: potential role of circadian rhythm. PloS One. 2010;5:e12190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Mukai M, Lin TM, Peterson RE, Cooke PS, Tischkau SA. Behavioral rhythmicity of mice lacking AhR and attenuation of light-induced phase shift by 2,3,7,8-tetrachlorodibenzo-p-dioxin. J Biol Rhythms. 2008;23:200–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Mukai M, Tischkau SA. Effects of tryptophan photoproducts in the circadian timing system: searching for a physiological role for aryl hydrocarbon receptor. Toxicol Sci. 2007;95:172–181. [DOI] [PubMed] [Google Scholar]
- 70. Tischkau SA, Jaeger CD, Krager SL. Circadian clock disruption in the mouse ovary in response to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol Lett. 2011;201:116–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Popesku JT, Tan EY, Martel PH, et al. Gene expression profiling of the fathead minnow (Pimephales promelas) neuroendocrine brain in response to pulp and paper mill effluents. Aquat Toxicol. 2010;99:379–388. [DOI] [PubMed] [Google Scholar]
- 72. Robertson JL, Clifton DK, de la Iglesia HO, Steiner RA, Kauffman AS. Circadian regulation of Kiss1 neurons: implications for timing the preovulatory gonadotropin-releasing hormone/luteinizing hormone surge. Endocrinology. 2009;150:3664–3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Smarr BL, Morris E, de la Iglesia HO. The dorsomedial suprachiasmatic nucleus times circadian expression of kiss1 and the luteinizing hormone surge. Endocrinology. 2012;153:2839–2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Xu Z, Kaga S, Tsubomizu J, et al. Circadian transcriptional factor DBP regulates expression of Kiss1 in the anteroventral periventricular nucleus. Mol Cell Endocrinol. 2011;339:90–97. [DOI] [PubMed] [Google Scholar]
- 75. Ji Y, Qin Y, Shu H, Li X. Methylation analyses on promoters of mPer1, mPer2, and mCry1 during perinatal development. Biochem Biophys Res Commun. 2010;391:1742–1747. [DOI] [PubMed] [Google Scholar]
- 76. Guillaumond F, Dardente H, Giguère V, Cermakian N. Differential control of Bmal1 circadian transcription by REV-ERB and ROR nuclear receptors. J Biol Rhythms. 2005;20:391–403. [DOI] [PubMed] [Google Scholar]
- 77. Preitner N, Damiola F, Lopez-Molina L, et al. The orphan nuclear receptor REV-ERBα controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell. 2002;110:251–260. [DOI] [PubMed] [Google Scholar]
- 78. Navarro VM. New insights into the control of pulsatile GnRH release: the role of Kiss1/neurokinin B neurons. Front Endocrinol (Lausanne). 2012;3:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Navarro VM, Gottsch ML, Wu M, et al. Regulation of NKB pathways and their roles in the control of Kiss1 neurons in the arcuate nucleus of the male mouse. Endocrinology. 2011;152:4265–4275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Dickerson SM, Gore AC. Estrogenic environmental endocrine-disrupting chemical effects on reproductive neuroendocrine function and dysfunction across the life cycle. Rev Endocr Metab Disord. 2007;8:143–159. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.