Abstract
Purpose
To estimate prevalence and severity of undetected glaucoma in the population.
Design
Cross-sectional study.
Participants
32,918 subjects from 55 to 79 years of age from Malmö, Sweden, who were screened between 1992 and 1997. All subjects in the screened age groups living in the catchment area, and for whom there were no recent records at the Malmö University Hospital Ophthalmology department, had been invited. The main purpose of the screening was to recruit subjects for the Early Manifest Glaucoma Trial.
Methods
We registered age, gender and amount of visual field loss in subjects with previously undiagnosed glaucoma identified at the screening. The disease was categorized into five stages based on perimetric mean deviation (MD) values.
Main Outcome Measures
Prevalence of undetected glaucoma at various disease stages in different age groups expressed as percentages.
Results
Among the screened subjects, who were 77.5% of all invited subjects, a total of 406 subjects (1.23%) were identified with previously undetected glaucoma. Prevalence increased with age, from 0.55% at 55–59 years to 2.73% at age 75–79 years. Unilateral disease accounted for 66% of all cases. Extent of visual field loss was similar in all age groups from 60 years and up. Most eyes had early (35%) or moderate (31%) glaucomatous visual field defects, but 134 subjects (33%) had advanced visual field loss in at least one eye. No subject was blind in both eyes, but 3.4% of the newly diagnosed patients were unilaterally blind due to glaucoma.
Conclusions
Prevalence of undetected glaucoma increased with age, while disease severity did not increase in subjects older than 60 years. One third of subjects with previously undetected glaucoma had advanced or later stage disease in at least one eye. Unilaterally blind subjects were seen in all age groups.
INTRODUCTION
Population-based studies have shown that in developed countries about half of all persons with manifest glaucoma are unaware of their disease.1–6 Because of its asymptomatic initial phases, glaucoma is often detected late, when patients have extensive and irreversible damage, or by chance.7 Early detection and subsequent management are important to prevent visual impairment, and population screening for glaucoma has been discussed. There are, however, many important considerations when determining whether some form of screening is worthwhile8; these include age-specific prevalence and the severity of undetected disease.
The aim of the present paper was to determine the prevalence of undetected glaucoma in different age groups, and the magnitude of visual field loss of the subjects identified with previously undetected glaucoma.
METHODS
A population based screening of 32,918 residents of Malmö was performed during 1991 to 1997 in order to identify subjects with previously undetected glaucoma for recruitment to the Early Manifest Glaucoma Trial (EMGT),9 (National Institutes of Health Clinical Trials gov identifier NCT00000132; registered September 23, 1999).
Residents of Malmö, Sweden, were invited to a free eye health examination at the Department of Ophthalmology of Malmö University Hospital. All men in ages ranging from 60 to 79 years and women from 55 to 79 years, were invited to the screening with the exception of those who had visited the Malmö University Hospital Department of Ophthalmology at Malmö University Hospital within the previous year. Screened subjects found to have been diagnosed with glaucoma before the screening were removed from the analysis. EMGT and the screening were approved by the Ethics Committee of the University of Lund.
The screening examination has been previously described in detail.7,9 Briefly we measured visual acuity and refractive error, and intraocular pressure was measured using Goldmann tonometry. Monoscopic fundus color photographs were obtained after dilation using Topcon non-mydriatic TRC-NW3 fundus cameras (Topcon, Tokyo, Japan) and Kodachrome 64 ASA film. All subjects completed a questionnaire about their ophthalmic history, and listed all current medications.
Positive screening criteria were intraocular pressure (IOP) >25 mmHg, suspect glaucomatous optic nerve head, e.g., localized narrowing of the rim or vertically extended cupping, optic disc hemorrhages or retinal nerve fiber layer defects, as assessed by at least one glaucoma specialist on a fundus photograph. Another positive screening criterion was first-degree relative with glaucoma. Subjects who screened positively were invited to a post-screening examination.
Post-screening examinations were intended to establish or reject a diagnosis of glaucoma and ascertain whether the patient met the inclusion criteria of the EMGT. A complete ophthalmic history was obtained and a standard ophthalmic examination was performed including visual acuity, tonometry and fundus examination. Visual field testing with the 24-2 Full-Threshold program of the Humphrey Field Analyzer (Carl Zeiss Meditec, Dublin, CA) was also performed in both eyes.
A glaucoma diagnosis was made when subjects fulfilled one of the following criteria:
Repeatable visual field defects compatible with glaucoma and not explained by other causes.
In subjects for whom only one visual field test was available, visual field defects compatible with glaucoma and corresponding structural damage of the optic nerve head and/or retinal nerve fiber layer were required.
In subjects where no visual field tests were available, obvious glaucoma damage at the optic nerve head was sufficient.
Humphrey Full Threshold visual fields had been obtained at the post-screening visit in 91.7% of the newly diagnosed glaucoma eyes, and in 0.7% already at the screening. In another 3.5% of the eyes visual fields had been obtained within a year from the screening examination. In 4% of glaucomatous eyes no visual fields had been obtained, or the first fields had been obtained more than one year after the screening. Such late fields were not used. Reliability indices of visual field tests were not considered. Thus, no visual fields were excluded unless they were obviously erratic, e.g. a clover-leaf field10, c.f. below in the results section.
The magnitude of visual field damage was defined by the mean deviation (MD) value from the first visual field test. We used a simplified version of the Glaucoma Staging System described by Mills and co-workers in 200611, to categorize glaucomatous eyes in to five glaucoma stages (1–5), Table 1.
Table 1.
Perimetric glaucoma staging
| Glaucoma Stage | Mean Devation (MD) |
Severity of Disease |
|---|---|---|
| 1 | Better than −6.00 dB | Early |
| 2 | −6.01 to −12.00 dB | Moderate |
| 3 | −12.01 to −20.00 dB | Advanced |
| 4 | −20.01 dB or worse | Severe |
| 5 | Not applicable | End-Stage/Blind |
Blindness was defined according to World Health Organization (WHO) criteria12: visual acuity less than 3/60 with best possible correction and/or constriction of the central visual field to less than 10° in its widest diameter.
Results were calculated for 5 year age groups, from 55 –60 and up to 75–79 years. Only women were screened in the youngest age group 55–60 years.
RESULTS
Out of 46, 614 citizens in the age group of interest 4,117 were not invited since they had visited the Department of Ophthalmology of Malmö University Hospital within one year prior to the time for the screening. Thus, 42,497 citizens were invited. A total of 32,918 subjects (77.5%), 21,218 women and 11,700 men, attended the screening program. Our department delivers about 75% of all primary glaucoma care in the catchment area.
Five hundred and forty-five eyes of 406 subjects, who were previously unaware of their condition, were diagnosed with glaucoma. In 86% of eyes the diagnosis was based on presence of repeatable visual field loss compatible with glaucoma, in 9% diagnosis was based on visual field loss compatible with glaucoma at a single field test plus corresponding optic nerve changes, and in 5% (29 eyes in 24 patients) the diagnosis was based upon disc appearance only, since useful visual fields were not available. Reasons for missing fields were inability to undergo perimetry (n=10), e.g., due to blindness, lost to follow up (n=8), or that perimetry had not been performed for unknown reasons (n=3). There were also three patients with grossly artifactual visual fields that could not be used, e.g., fields with cloverleaf patterns.10
The total prevalence of undetected glaucoma in the screened population was 1.23% (Table 2). Prevalence increased with increasing age, and was approximately five times higher in the oldest age group than in the youngest one. Gender distribution of undetected glaucoma was equal between men and women, after correcting for the fact that more women than men were screened.
Table 2.
No. of screened subjects and glaucoma prevalences in different age groups.
| Age cohorts (years) |
Women | Men | Total | Prevalence change between each age group |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Number screened |
Number diagnosed |
Prevalence (%) | Number screened |
Number diagnosed |
Prevalence (%) | Prevalence increase |
% | ||
| 55–59 | 2,704 | 15 | 0.55 | 0 | 0 | N/A | 0.55 | N/A | N/A |
| 60–64 | 6,391 | 49 | 0.77 | 2,388 | 15 | 0.63 | 0.73 | 0.18 | 33 |
| 65–69 | 6,266 | 84 | 1.34 | 4,960 | 61 | 1.23 | 1.29 | 0.56 | 77 |
| 70–74 | 4,208 | 57 | 1.35 | 3,215 | 49 | 1.52 | 1.43 | 0.14 | 11 |
| 75–79 | 1,649 | 49 | 2.97 | 1,137 | 27 | 2.37 | 2.73 | 1.3 | 91 |
| Total | 21,218 | 254 | 1.2 | 11,700 | 152 | 1.3 | 1.23 | N/A | N/A |
N/A = not available
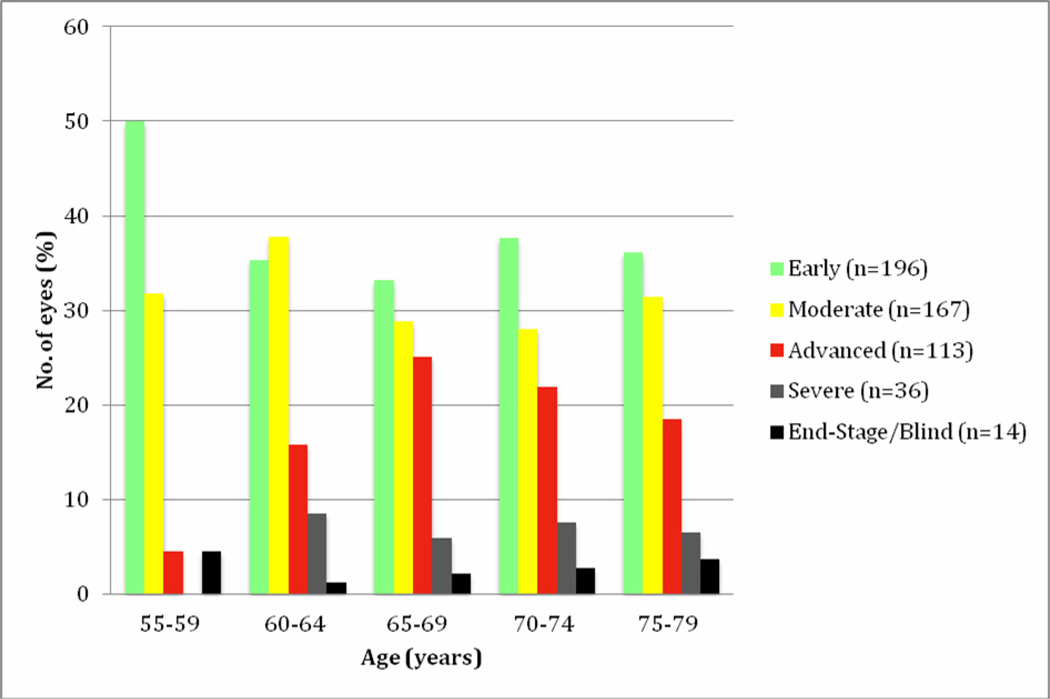
Most of the glaucoma eyes in each age cohort had early disease (Stage 1). The extent of field loss was similar in all age groups from 60–64 and older (Fig. 1), while early stage glaucoma was more frequent in the youngest group, aged 55 – 59 years. The mean age of patients in each glaucoma stage was similar, between 69 and 70 years.
Fig 1.
Stages of glaucomatous visual field loss in eyes with newly detected disease. The severity of field loss was similarly distributed from age 60 and up, with 30–40% at the early stage and more than ≥25% at advanced, severe or end stages.
A total of 267 patients (66%) had unilateral disease. The proportion of patients with bilateral disease increased with age in age groups greater than 60 years, ranging from 28% in ages 60–64 to 42% in the oldest age group.
The distribution of glaucoma severity in patients with unilateral disease was of similar magnitude to that of the better eye in patients with bilateral disease. Thirty-seven percent of patients with bilateral disease were at the same glaucoma stage in both eyes.
No subjects were bilaterally blind, but 14 (3.4%) were blind in one eye due to glaucoma, Table 3. Seven eyes were blind according to the WHO visual field criterion. Another seven eyes were blind according to the WHO visual acuity criterion, but no visual fields were available in these eyes. Half of the patients with a blind eye had unilateral glaucoma.
Table 3.
Unilateral blindness (no. of subjects and prevalence) in different age groups.
| Age group (years) |
Subjects with glaucoma n |
Unilaterally blind subjects, n (%) |
Prevalence (%) |
|---|---|---|---|
| 55–59 | 15 | 1 (7) | 0.04 |
| 60–64 | 64 | 1 (2) | 0.01 |
| 65–69 | 145 | 4 (3) | 0.04 |
| 70–74 | 106 | 4 (4) | 0.05 |
| 75–79 | 76 | 4 (5) | 0.14 |
| All | 406 | 14 (3.4) | 0.04 |
DISCUSSION
The primary aim of the current study was to assess age-specific prevalences and severity of visual field loss in previously undetected glaucoma. The total prevalence of undetected glaucoma in the surveyed population was 1.23% and increased with increasing age. These findings are consistent with previously published findings if we consider that undetected glaucoma often represents about 50% of all glaucoma cases in a population.1–6 Disease severity was similar in all age groups, except for more frequent early stage glaucoma among the youngest (55 –59 years of age). The sample size is probably large enough to motivate the latter negative statement, that disease severity does not increase substantially with age, since the power of detecting a worsening corresponding to one stage in Mill’s staging system11 (= 6dB) in the 75–79 year-old group as compared with the 60–64 year-old group was 99%. The power to detect a worsening of 3 dB was 83%. One third of newly diagnosed subjects had advanced, severe or end-stage disease, a seemingly high proportion of subjects with serious visual field loss being unaware of their disease.
With knowledge of typical rates of progression in untreated glaucoma13 we can conclude that the average undiagnosed subject must have had detectable disease present for several or many years. The difference in levels of glaucoma damage in patients detected at this screening and in routine clinical practice is large7, with an average MD of −8.0 dB in the worse eye in patients who were detected at the screening versus −16.2dB in clinically diagnosed patients in the same age groups in Malmö. This indicates that in the average patient detected at the screening, a number of years would have passed before a clinical diagnosis would have been made.
One strength of the current study is the fact that it is based on a very large population based screening of 32,918 subjects. This made it possible to estimate prevalences of undetected glaucoma and amount of visual field damage for different age groups. No other population survey to date has examined as many subjects. The second largest was carried out in Japan and included 8,126 screened subjects.14 Other major population studies typically screened around 3,000 to 6,000 individuals2,15–25, 28, or fewer.6,26,27 Another strength is that the great majority of patients with newly detected glaucoma underwent visual field testing with a commonly used standard test.
One limitation of the study is that patients were only invited to a post-screening visit including a full ophthalmic examination and visual field testing if they screened positive according to our criteria, c.f. above. It is likely that a number of glaucoma patients who had normal or almost normal intraocular pressures and small optic nerve heads may have been missed. Thus, in the population-based Visual Impairment Project in Melbourne 6 of 26 patients with previously undetected definite glaucoma had visual field defects, but normal IOP values and cup-disc ratios <0.718 Therefore, our estimates of undetected manifest glaucoma with field loss in the population are minimum estimates and somewhat lower than the true rates. It is re-assuring, however, that in the Malmö screening very few patients who underwent visual field testing because they screened positive, e.g., because of elevated IOP, had normal optic discs (unpublished data).
Another limitation is that the population screened in this study was mainly Caucasian. Our results, therefore, cannot be used to draw conclusions about prevalence and magnitude of undetected glaucoma in other racial groups. Whether our results can be generalized to other Caucasian populations depends, among other things, on the availability of eye care to the populations. The number of ophthalmologists in Sweden is similar to that of many other countries, approximately one ophthalmologist per 12,000 inhabitants. There are relatively few optometrists in Sweden, but a large number of opticians. Many opticians routinely measure intraocular pressure in older customers, and refer persons with elevated pressure. One reason to believe that the current results may be representative for many Western countries is that we know that this screening approximately doubled the number of subjects with known glaucoma in the population in the screened age groups. Thus, 354 clinically diagnosed patients of the same age cohorts as those screened were followed at the Department of Ophthalmology at Malmö University Hospital as compared to 402 patients detected in the screening.7 The majority of glaucoma patients in the catchment area were followed at the university Eye Department, but a smaller proportion were seen in a few private practices. Thus, the proportion of undetected glaucoma patients in Malmö was similar to that reported in other Western countries, where population studies have been performed.1, 6,20,27,28
The screening was conducted between 1992 and 1997. This might raise concerns of whether the results are still valid today. There have been no major changes in glaucoma practice or case finding, and the number of eye care professionals in the catchment area is still very similar. Therefore, we believe that the results would have been very similar also if the population based screening had been conducted during the last years.
In summary, the prevalence of undetected glaucoma increased with age, while disease severity did not in subjects over 60 – 65 years. One third of patients with undetected glaucoma had advanced disease. Unilaterally blind subjects were found even in the youngest age group.
We believe that knowledge about age-specific prevalence and of disease severity is important for health service decisions. It is clear that data of this type will be useful in assessing the value of population based screening for glaucoma (Wilson R, Leske C, Lee P, et al. Screening for open-angle glaucoma: Where are we now and where to from here? Report from the Global AIGS Committee on screening for open angle glaucoma. International Glaucoma Review 2006;7-3:350–354).
Acknowledgments
FINANCIAL SUPPORT:
Swedish Research Council grant K2011-63X-10426-19-3, National Eye Institute, Bethesda, Maryland grant no U10EY10260, The Herman Järnhardt Foundation, the Foundation for Visually Impaired in Former Malmöhus County, and Crown Princess Margareta's Foundation. The National Eye Institute participated in the design of the study. Other sponsors or funding organizations had no role in the design or conduct of this research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: None of the authors have had received any support or honoraria specifically related to this work. AH is a consultant of Carl Zeiss Meditec, Allergan and Alcon, and has received research grants and patent royalties from Carl Zeiss Meditec. BB is a consultant of Carl Zeiss Meditec. No conflicting relationships exist for SEO.
REFERENCES
- 1.Tielsch JM, Sommer A, Katz J, et al. Racial variations in the prevalence of primary open angle variations in the prevalence of primary open angle glaucoma: the Baltimore Eye Survey. JAMA. 1991;266:369–374. [PubMed] [Google Scholar]
- 2.Leske MC, Connell AM, Schachat AP, Hyman L Barbados Eye Study Group. The Barbados Eye Study: prevalence of open angle glaucoma. Arch Ophthalmol. 1994;112:821–829. doi: 10.1001/archopht.1994.01090180121046. [DOI] [PubMed] [Google Scholar]
- 3.Rudnicka AR, Mt-Isa S, Owen CG, et al. Variations in primary open-angle glaucoma prevalence by age, gender and race: a Bayesian meta-analysis. Invest Ophthalmol Vis Sci. 2006;47:4254–4261. doi: 10.1167/iovs.06-0299. [DOI] [PubMed] [Google Scholar]
- 4.Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90:262–267. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leske MC. Open-angle glaucoma--an epidemiologic overview. Ophthalmic Epidemiol. 2007;14:166–172. doi: 10.1080/09286580701501931. [DOI] [PubMed] [Google Scholar]
- 6.Topouzis F, Coleman AL, Harris A, et al. Factors associated with undiagnosed open-angel glaucoma: the Thessaloniki Eye Study. Am J Ophthalmol. 2008;145:327–335. doi: 10.1016/j.ajo.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 7.Grødum K, Heijl A, Bengtsson B. A comparison of glaucoma patients identified through mass screening and in routine clinical practice. Acta Ophthalmol Scand. 2002;80:627–631. doi: 10.1034/j.1600-0420.2002.800613.x. [DOI] [PubMed] [Google Scholar]
- 8.Wilson JM, Jungner G. Principles and Practice of Screening for Disease. Geneva: WHO; 1968. [Accessed November 22, 2012]. pp. 26–27. Available from: http://whqlibdoc.who.int/php/WHO_PHP_34.pdf. [Google Scholar]
- 9.Leske MC, Heijl A, Hyman L, Bengtsson B Early Manifest Glaucoma Trial Group. Early Manifest Glaucoma Trial: design and baseline data. Ophthalmology. 1999;106:2144–2153. doi: 10.1016/s0161-6420(99)90497-9. [DOI] [PubMed] [Google Scholar]
- 10.Cioffi GA, editor. Basic and Clinical Science Course 2010–2011, Section 10 Glaucoma. San Francisco, CA: American Academy of Ophthalmology; 2011. p. 74. [Google Scholar]
- 11.Mills RP, Budenz DL, Lee PP, et al. Categorizing the stage of glaucoma from pre-diagnosis to end-stage disease. Am J Ophthalmol. 2006;141:24–30. doi: 10.1016/j.ajo.2005.07.044. [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization. ICD 10 definition H 54.4 och H54.9. [accessed Jan 14,2013];Recommendations of the WHO Consultation on “Development of Standards for Characterization of Vision Loss and Visual Functioning". 2003 Sep; http://apps.who.int/classifications/icd10/browse/2010/en#/H53-H54.
- 13.Heijl A, Bengtsson B, Hyman K, Leske MC Early Manifest Glaucoma Trial Group. Natural history of open-angle glaucoma. Ophthalmology. 2009;116:2271–2276. doi: 10.1016/j.ophtha.2009.06.042. [DOI] [PubMed] [Google Scholar]
- 14.Shiose Y, Kitazawa Y, Tsukahara S, et al. Epidemiology of glaucoma in Japan--a nationwide glaucoma survey. Jpn J Ophthalmol. 1991;35:133–155. [PubMed] [Google Scholar]
- 15.Hollows FS, Graham PA. Intraocular pressure, glaucoma, and glaucoma suspects in a defined population. Br J Ophthalmol. 1966;50:570–586. doi: 10.1136/bjo.50.10.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bankes JL, Perkins ES, Tsolakis S, Wright JE. Bedford glaucoma survey. Br Med J. 1968;1:791–796. doi: 10.1136/bmj.1.5595.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klein BE, Klein R, Sponsel WE, et al. Prevalence of glaucoma: the Beaver Dam Eye Study. Ophthalmology. 1992;99:1499–1504. doi: 10.1016/s0161-6420(92)31774-9. [DOI] [PubMed] [Google Scholar]
- 18.Wensor MD, McCarty CA, Stanislavsky YL, et al. The prevalence of glaucoma in the Melbourne Visual Impairment Project. Ophthalmology. 1998;105:733–739. doi: 10.1016/S0161-6420(98)94031-3. [DOI] [PubMed] [Google Scholar]
- 19.Bonomi L, Marchini G, Marraffa M, et al. Prevalence of glaucoma and intraocular pressure distribution in a defined population: the Egna-Neumarkt Study. Ophthalmology. 1998;105:209–215. doi: 10.1016/s0161-6420(98)92665-3. [DOI] [PubMed] [Google Scholar]
- 20.Mitchell P, Smith W, Attebo K, Healey PR. Prevalence of open-angle glaucoma in Australia: the Blue Mountains Eye Study. Ophthalmology. 1996;103:1661–1669. doi: 10.1016/s0161-6420(96)30449-1. [DOI] [PubMed] [Google Scholar]
- 21.Quigley HA, West SK, Rodrigues J, et al. The prevalence of glaucoma in a population-based study of Hispanic subjects: Proyecto VER. Arch Ophthalmol. 2001;119:1819–1826. doi: 10.1001/archopht.119.12.1819. [DOI] [PubMed] [Google Scholar]
- 22.Ramakrishnan R, Nirmalan PK, Krishnadas R, et al. Glaucoma in a rural population of southern India: the Aravind Comprehensive Eye Survey. Ophthalmology. 2003;110:1484–1490. doi: 10.1016/S0161-6420(03)00564-5. [DOI] [PubMed] [Google Scholar]
- 23.Varma R, Ying-Lai M, Francis BA, et al. Los Angeles Latino Eye Study Group. Prevalence of open-angle glaucoma and ocular hypertension in Latinos: the Los Angeles Latino Eye Study. Ophthalmology. 2004;111:1439–1448. doi: 10.1016/j.ophtha.2004.01.025. [DOI] [PubMed] [Google Scholar]
- 24.Wang YX, Xu L, Yang H, Jonas JB. Prevalence of glaucoma in North China: the Beijing Eye Study. Am J Ophthalmol. 2010;150:917–924. doi: 10.1016/j.ajo.2010.06.037. [DOI] [PubMed] [Google Scholar]
- 25.Wong EY, Keeffe JE, Rait JL, et al. Detection of undiagnosed glaucoma by eye health professionals. Ophthalmology. 2004;111:1508–1514. doi: 10.1016/j.ophtha.2004.01.029. [DOI] [PubMed] [Google Scholar]
- 26.Bengtsson B. The prevalence of glaucoma. Br J Ophthalmol. 1981;65:46–49. doi: 10.1136/bjo.65.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coffey M, Reidy A, Wormald R, et al. Prevalence of glaucoma in the west of Ireland. Br J Ophthalmol. 1993;77:17–21. doi: 10.1136/bjo.77.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dielemans I, Vingerling JR, Wolfs RC, et al. The prevalence of primary open-angle glaucoma in a population-based study in The Netherlands: the Rotterdam Study. Ophthalmology. 1994;101:1851–1855. doi: 10.1016/s0161-6420(94)31090-6. [DOI] [PubMed] [Google Scholar]