Abstract
Background
More than 2.9 million serum vitamin B12 tests were performed in 2010 in Ontario at a cost of $40 million. Vitamin B12 deficiency has been associated with a few neurocognitive disorders.
Objective
To determine the clinical utility of B12 testing in patients with suspected dementia or cognitive decline.
Methods
Three questions were addressed:
Is there an association between vitamin B12 deficiency and the onset of dementia or cognitive decline?
Does treatment with vitamin B12 supplementation improve cognitive function in patients with dementia or cognitive decline and vitamin B12 deficiency?
What is the effectiveness of oral versus parenteral vitamin B12 supplementation in those with confirmed vitamin B12 deficiency?
A literature search was performed using MEDLINE, Embase, EBSCO Cumulative Index to Nursing & Allied Health Literature (CINAHL), the Cochrane Library, and the Centre for Reviews and Dissemination database, from January 2002 until August 2012.
Results
Eighteen studies (7 systematic reviews and 11 observational studies) were identified to address the question of the association between B12 and the onset of dementia. Four systematic reviews were identified to address the question of the treatment of B12 on cognitive function. Finally, 3 randomized controlled trials were identified that compared oral B12 to intramuscular B12.
Conclusions
Based on very low quality evidence, there does appear to be an association between elevated plasma homocysteine levels (a by-product of B vitamins) and the onset of dementia.
Based on moderate quality evidence, but with less than optimal duration of follow-up, treatment with B12 supplementation does not appreciably change cognitive function.
Based on low to moderate quality of evidence, treatment with vitamin B12 and folate in patients with mild cognitive impairment seems to slow the rate of brain atrophy.
Based on moderate quality evidence, oral vitamin B12 is as effective as parenteral vitamin B12 in patients with confirmed B12 deficiency.
Plain Language Summary
Low levels of vitamin B12 have been associated with neurocognitive disorders. This evidence-based analysis assessed the usefulness of serum vitamin B12 testing as it relates to brain function. This review found very low quality evidence that suggests a connection between high plasma homocysteine levels (a by-product of B vitamin metabolism in the body) and the onset of dementia. Moderate quality of evidence indicates treatment with vitamin B12 does not improve brain function. Moderate quality of evidence also indicates treatment using oral vitamin B12 supplements is as effective as injections of vitamin B12.
Background
Objective of Analysis
This evidence-based analysis (EBA) aims to establish the clinical utility of testing serum vitamin B12 in patients with suspected dementia or cognitive decline. This EBA attempts to answer the following 3 questions:
Is there an association between vitamin B12 deficiency and the onset of dementia or cognitive decline?
Does treatment with vitamin B12 supplementation improve cognitive function in patients with dementia or cognitive decline and vitamin B12 deficiency?
What is the effectiveness of oral versus parenteral vitamin B12 supplementation in those with confirmed vitamin B12 deficiency?
Clinical Need and Target Population
Vitamin B12 is a water-soluble, essential vitamin. A deficiency in vitamin B12 can lead to a specific set of neurologic disorders (subacute combined degeneration of the spinal cord, cognitive impairment) and one hematologic disorder (megaloblastic anemia) disorders.
There are 4 main reasons a person becomes vitamin B12 deficient: (1)
Inadequate dietary intake of vitamin B12, as in strict vegetarianism (over the long term)
-
Malabsorption of vitamin B12
– Autoimmune pernicious anemia
– Age-related atrophic gastritis
– Gastrectomy or gastric bypass
Ileal disease (e.g., Crohn disease) or ileal resection
Drug use (e.g., metformin and, possibly, proton pump inhibitors)
On the basis of results from a 5-year observational study of Australians in general practice, the rate of macrocytosis (mean corpuscular volume [MCV] > 100 fL) is about 2% to 3%. (2;3) Important causes of macrocytosis include alcohol overuse, B vitamin deficiency, medications, and bone marrow disorders. (2;4) Based on a summary of studies, Kaferle and Strzoda (4) estimated that vitamin B12 deficiency was the cause of macrocytosis in 6% to 28% of the cases. However, not all cases of vitamin B12 deficiency are associated with macrocytosis or anemia. The 1988 studies by Carmel (5) and by Lindenbaum et al (6) noted that about 15% of patients can have low vitamin B12 levels without laboratory findings consistent with anemia or macrocytosis: so-called subclinical B12 (cobalamin) deficiency.
Prevalence of Vitamin B12 Deficiency
In 1996, Carmel (7) reported that the prevalence of undiagnosed pernicious anemia among 729 older adults (age ≥ 60 years) was 1.9%. Then in 2004, Guralnik et al (8) reported that 10.6% of the population age 65 years or older in the National Health and Nutritional Examination Survey (NHANES) had anemia and that 11.3% of the anemia cases were B12-related. In Ontario, this translates to between 22,668 and 50,473 people older than 60 years who might have B12-related anemia. In fiscal year 2010/2011, there were 1.2 million B12 tests in people aged 60 years or older in Ontario.
Vitamin B12 and Cognitive Function
The Third Canadian Consensus Conference on the Diagnosis and Treatment of Dementia (CCCDTD) was held in 2007. Delegates at that meeting recommended that serum cobalamin (vitamin B12) levels be determined for all older adults suspected of dementia or cognitive decline (Grade B, Level 2). (9) “Grade B” implies that there was fair evidence to support vitamin B12 testing, and ”Level 2” suggests that the evidence was obtained from observational studies. (10) This recommendation was added to the consensus guidelines in 2007. (11)
The process for developing CCCDTD recommendations is quite rigorous. (10) Vitamin B12 testing was just one of several domains that were part of the consensus conference. Each domain was assigned a working group with representation from the College of Family Physicians of Canada, in addition to other experts with particular interests in certain domains. Each working group performed literature searches, summarized the data, and drafted recommendations. The summary reports and recommendations were posted on a website for feedback from all conference participants. All recommendations achieved consensus of 80% or greater.
In 2007 and 2008, 2 articles were published providing more background on the vitamin B12 recommendation for diagnosis of suspected dementia or cognitive decline in older adults. (11;12) Neither article clearly identified the studies on which the CCCDTD recommendation was based, nor how the recommendation was determined to be Grade B, Level 2. Feldman et al stated that “the primary role of laboratory investigations ... is to rule out the rare presence of a treatable disorder presenting as memory loss.” (11)
Feldman et al (11) could have been referring to reversible dementia. Given that vitamin B12 deficiency can cause neurologic deficits, a dementia caused by B12 deficiency is thought to be reversible. Other causes of possibly reversible dementias include normal-pressure hydroencephalus and brain tumours. In 2003, Clarfield (13) published a systematic review of 39 studies (N = 7,042) examining reversible dementia. He reported that approximately 9% of the cases were potentially reversible dementia and that 0.6% of cases actually reversed.
In 2007, the Guidelines and Protocols Advisory Committee of British Columbia released guidelines on the diagnosis of cognitive impairment in the elderly. (14) The committee recommended that vitamin B12 be performed in the initial work-up for those with suspected mild cognitive impairment or dementia, but added the caveat, “Current data from systematic reviews of randomized double blind trials, however, do not provide evidence of improvement in cognition or dementia with B12 treatment.”
Ontario Context
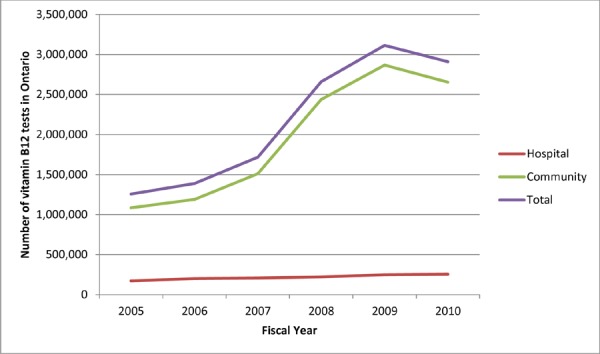
In fiscal year 2010/2011, more than 2.9 million serum vitamin B12 laboratory tests were billed to the province at a cost of approximately $40 million. The number of vitamin B12 tests performed has increased since fiscal year 2005/2006, particularly in the community setting (Table 1, Figure 1). In 2007 the vitamin B12 test was added to the laboratory requisition form that physicians use to request lab tests; as noted previously, the recommendations of the CCCDTD published in 2007 stated that older adults with suspected dementia or cognitive decline should have their vitamin B12 levels assessed. (9) Laboratory tests for vitamin B12 increased by nearly 1 million between 2007/2008 and 2008/2009. The serum vitamin B12 test was removed from the laboratory requisition form in November 2012.
Table 1. Volume of Vitamin B12 Laboratory Tests in Ontario for Fiscal Years 2005/2006 to 2010/2011.
| Fiscal Year | Hospital | Community | Total |
|---|---|---|---|
| 2005/2006 | 173,284 | 1,083,219 | 1,256,503 |
| 2006/2007 | 199,412 | 1,188,066 | 1,387,478 |
| 2007/2008 | 206,917 | 1,509,800 | 1,716,717 |
| 2008/2009 | 222,136 | 2,436,765 | 2,658,901 |
| 2009/2010 | 247,746 | 2,865,684 | 3,113,430 |
| 2010/2011 | 255,620 | 2,651,992 | 2,907,612 |
Figure 1. Number of Vitamin B12 Tests in Ontario for Fiscal Years 2005/2006 to 2010/2011.
Diagnostic Accuracy of Serum Vitamin B12 Testing
In 2011, Willis et al (15) published a systematic review and meta-analysis of the diagnostic accuracy of serum tests for assessing vitamin B12 (or cobalamin). They searched the literature from 1990 to 2009 and identified 54 studies for inclusion. They reported that no consistent reference standard is used to measure the accuracy of the serum vitamin B12 test, thus making it difficult to establish the accuracy of the test. They reported a range of variability for sensitivity and specificity across the studies. Sensitivity ranged from 13% to 75%, and specificity ranged from 45% to 100%. Willis et al (15) attributed the wide ranges of sensitivity and specificity to the inconsistent use of a reference standard.
Hvas and Nexo (16) also published an article regarding the diagnostic accuracy for serum vitamin B12 testing. Although their review was not systematic, they described the strengths and weaknesses of each of the serum tests for assessing vitamin B12. A summary of the tests based on the review of Hvas and Nexo (16) is listed in Table 2.
Table 2. Summary of Laboratory Tests to Assess Vitamin B12 Deficiency.
| Laboratory Test | Rationale for Test | Advantages | Disadvantages |
|---|---|---|---|
| Cobalamin | Decreases in vitamin B12 deficiency | Easily accessible test $10–$15 per test (in Ontario) Most commonly used test with the most literature about abnormal cut-offs | Sensitivity and specificity is unclear |
| Methymalonic acida | Increases with vitamin B12 deficiency | High sensitivity | Questionable specificity ~$105 per test in Ontario (uninsured) |
| Total homocysteinea | Increases with vitamin B12 deficiency | High sensitivity | Low specificity influenced by lifestyle factors (smoking, alcohol consumption, coffee consumption) ~$65 per test in Ontario (uninsured) |
| Holotranscobalamina | Decreases with vitamin B12 deficiency Newer test, clinical utility unclear |
High sensitivity | Specificity unclear |
These laboratory tests are uninsured in community laboratories in Ontario.
Source: Hvas and Nexo. (16)
The National Health and Nutrition Examination Survey measures the health status of Americans. Part of the survey includes assessments of vitamin B12 biomarkers including cobalamin, methylmalonic acid (MMA), and total homocysteine (Hcy). They established that, because of the challenges in sensitivity and specificity of tests, 2 tests (preferably cobalamin and MMA) should be performed when assessing vitamin B12 levels. They recommended MMA over total Hcy because Hcy also increases in the absence of other vitamins (folate and B6). (17;18)
Existing Guidelines
In addition to the British Columbia recommendations mentioned above (14), two other guidelines on the diagnosis of vitamin B12 deficiency were identified. The 3 guidelines (19-21) were assessed using the Appraisal of Guidelines for Research and Evaluation tool. (22) All 3 guidelines scored poorly on the linkages provided between the evidence and recommendations.
The recommendations from each of the guidelines are listed in Table 3. The systematic review by Andres et al (21) used a flow chart or care pathway to describe their recommendations, rather than text. A notable difference in the guideline by Andres et al (21) was that they recommended screening all patients in institutions or psychiatric hospitals for vitamin B12 deficiency. This was not a factor in either of the other guidelines. Andres et al (21) reported that the prevalence of vitamin B12 deficiency was much higher (30% to 40%) in patients who were sick or institutionalized. As mentioned previously, in all guidelines the relationship between the recommendations and the evidence presented was very weak.
Table 3. Guidelines for Assessment of Vitamin B12 Levels.
| Guideline | Whom to Test? | How Frequently? | Overall Recommendation |
|---|---|---|---|
| BC Guidelines and Protocols Advisory Committee, 2012 (19) | Patients with unexplained neurologic symptoms (paresthesia, numbness, poor motor coordination, memory lapses) Patients with acrocytic anemia or macrocytosis |
Not reported | Routine screening for vitamin B12 deficiency is not recommended |
| Smellie et al, 2005 (20) | Patients with acrocytic anemia Patients with acrocytosis Patients with specific neurocognitive abnormalities |
”There is no obvious merit in repeating vitamin B12 measurements unless lack of compliance is suspected or anemia recurs” | Not reported |
| Andres et al, 2004 (21) | Elderly patients with malnutrition All patients in institutions and psychiatric hospitals All patients with hematologic or neurocognitive manifestations of vitamin B12 deficiency |
Not reported | Not reported |
The Scottish Intercollegiate Guidelines Network (SIGN) also issued guidelines in 2006 on the management of patients with dementia. On the basis of their evidence review, they stated that “[t]here is no evidence that routine batteries of laboratory tests improve the accuracy of the clinical diagnosis of dementia.” (23) According to SIGN, this statement was based on high-quality case-control or cohort studies.
Evidence-Based Analysis
Research Questions
Is there an association between vitamin B12 deficiency and the onset of dementia or cognitive decline?
Does treatment with vitamin B12 supplementation improve cognitive function in patients with dementia or cognitive decline and vitamin B12 deficiency?
What is the effectiveness of oral versus parenteral vitamin B12 supplementation in those with confirmed vitamin B12 deficiency?
Research Methods
Literature Search
Search Strategy
A literature search was performed on August 26, 2012, using Ovid MEDLINE, Ovid MEDLINE In-Process and Other Non-Indexed Citations, Ovid Embase, EBSCO Cumulative Index to Nursing & Allied Health Literature (CINAHL), the Wiley Cochrane Library, and the Centre for Reviews and Dissemination database, for studies published from January 1, 2002, until August 26, 2012. Abstracts were reviewed by a single reviewer and, for those studies meeting the eligibility criteria, full-text articles were obtained. Reference lists were also examined for any additional relevant studies not identified through the search.
Inclusion and Exclusion Criteria and Outcomes of Interest
Question 1. Is there an association between vitamin B12 deficiency and the onset of dementia or cognitive decline?
Inclusion criteria
Systematic reviews, meta-analyses, randomized controlled trials (RCTs), longitudinal observational studies
No clinical signs or symptoms of dementia at onset of study
Study must report onset of dementia, Alzheimer disease (AD), or cognitive decline as an outcome measure
N ≥ 100
Observational period 2 years or longer
Exclusion criteria
Cross-sectional studies, case series, case reports
Non-English studies
Institutionalized subjects (inpatients)
Outcomes of interest
Onset of dementia or Alzheimer disease
Change in cognitive function
Question 2. Does treatment with vitamin B12 supplementation improve cognitive function in patients with dementia or cognitive decline and vitamin B12 deficiency?
Inclusion criteria
Systematic reviews, meta-analyses, RCTs
Supplementation with vitamin B12 alone
Any dosage or method (oral or parenteral) of vitamin B12 included
12 months or more of follow-up
Patients with confirmed vitamin B12 deficiency (for the purpose of this review B12 < 200 pmol/L)
Results reported for patients with cognitive impairment, any type of dementia, or AD separately from the results of healthy subjects
Exclusion criteria
Observational studies, case series, case reports
Non-English studies
Studies looking at multivitamin supplementation
Outcomes of interest
Change in cognitive function
Change in vitamin B12 levels
Question 3. What is the effectiveness of oral versus parenteral vitamin B12 supplementation in those with confirmed vitamin B12 deficiency?
Inclusion criteria
Systematic reviews, meta-analyses, RCTs
Studies comparing oral versus parenteral vitamin B12 of any dose
No limitation on duration of supplementation or follow-up
Patients with demonstrated vitamin B12 deficiency (as defined by the study)
Exclusion criteria
Observational studies, case series, case reports
Non-English studies
Outcomes of interest
Serum vitamin B12 measurements
Adverse events
Acceptability and compliance
Statistical Analysis
Because the outcomes reported were heterogeneous, the studies were not pooled.
Expert Panel
In the fall of 2012, a Clinical Expert Advisory Panel on Appropriate Utilization of Vitamin B12 Testing for Neurocognitive-Based Indications was appointed to place the evidence produced by HQO in context and to provide advice on the clinical utility of vitamin B12 in older adults with suspected dementia or cognitive impairment within the Ontario health care system. The panel was comprised of physicians, personnel from the Ministry of Health and Long-Term Care, and representatives from the community.
The statements, conclusions, and views expressed in this report do not necessarily represent the views of Advisory Panel members.
Quality of Evidence
The quality of the body of evidence for each outcome was examined according to the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) Working Group criteria. (24) The overall quality was determined to be high, moderate, low, or very low using a step-wise, structural methodology.
Study design was the first consideration; the starting assumption was that randomized controlled trials (RCTs) are high quality, whereas observational studies are low quality. Five additional factors—risk of bias, inconsistency, indirectness, imprecision, and publication bias—were then taken into account. Limitations in these areas resulted in downgrading the quality of evidence. Finally, 3 main factors that may raise the quality of evidence were considered: large magnitude of effect, dose response gradient, and accounting for all residual confounding factors. (24) For more detailed information, please refer to the latest series of GRADE articles. (24)
As stated by the GRADE Working Group, the final quality score can be interpreted using the following definitions:
| High | High confidence in the effect estimate—the true effect lies close to the estimate of the effect |
| Moderate | Moderate confidence in the effect estimate—the true effect is likely to be close to the estimate of the effect, but may be substantially different |
| Low | Low confidence in the effect estimate—the true effect may be substantially different from the estimate of the effect |
| Very Low | Very low confidence in the effect estimate—the true effect is likely to be substantially different from the estimate of effect |
Results of Evidence-Based Analysis
The database search yielded 1,360 citations published between January 1, 2002, and August 26, 2012 (with duplicates removed). Articles were excluded on the basis of information in the title and abstract. The full texts of potentially relevant articles were obtained for further assessment. (Figure 2)
Figure 2. Citation Flow Chart.
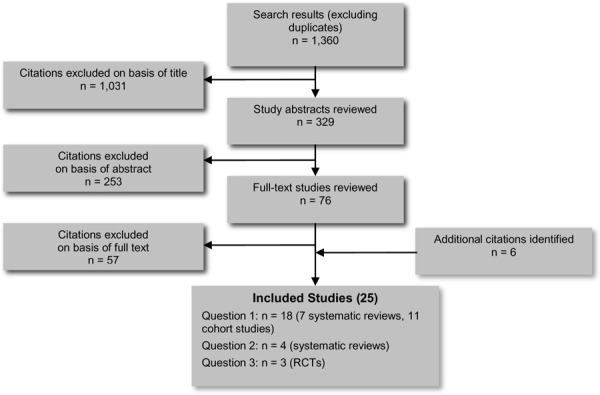
Abbreviation: RCT, randomized controlled trial.
For each included study, the study design was identified and is summarized in Table 4, which is a modified version of a hierarchy of study design by Goodman. (25)
Table 4. Body of Evidence Examined According to Study Design.
| Study Design | Number of Eligible Studies | ||||
|---|---|---|---|---|---|
| Question 1 | Question 2 | Question 3 | |||
| RCT studies | |||||
| Systematic review of RCTs | - | 4 | - | ||
| Large RCT (N > 100) | - | - | - | ||
| Small RCT (N < 100) | - | - | 3 | ||
| Observational studies | |||||
| Systematic review of non-RCTs with contemporaneous controls | 7 | - | - | ||
| Non-RCT with non-contemporaneous controls | - | - | - | ||
| Systematic review of non-RCTs with historical controls | - | - | - | ||
| Non-RCT with historical controls | - | - | - | ||
| Longitudinal cohort study | 11 | ||||
| Database, registry, or cross-sectional study | - | - | - | ||
| Case series | - | - | - | ||
| Retrospective review, modelling | - | - | - | ||
| Studies presented at an international conference | - | - | - | ||
| Expert opinion | - | - | - | ||
| Total | 18 | 4 | 3 | ||
Abbreviation: RCT, randomized controlled trial.
Question 1: Is there an association between vitamin B12 deficiency and the onset of dementia or cognitive decline?
Seven systematic reviews examined the relationship between vitamin B12 and cognitive function. (26-32) Table 5 summarizes the systematic reviews.
Table 5. Summary of Systematic Reviews Examining the Association Between Vitamin B12 (or Hyperhomocysteinemia) and the Onset of Dementia.
| Author, Year | Question | Sources/Dates Searched | No. of Studies Included | Conclusion |
|---|---|---|---|---|
| Moore et al, 2012 (26) | What is the association between low vitamin B12 levels, neurodegenerative disease, and cognitive impairment? | MEDLINE, PsychINFO, PubMed/to 2011 | Unclear | Low serum vitamin B12 levels are associated with neurodegenerative disease and cognitive impairment. |
| Ho et al, 2011 (27) | What is the role of Hcy as a risk factor for dementia, cognitive decline, and cognitive impairment? | PubMed, Embase, PsychINFO, Biosis, Cochrane Library/to 2008 | 17 | Individuals with AD have higher Hcy levels than controls; however, a causal relationship between high Hcy level and risk of developing dementia is not supported. |
| Wald et al, 2011 (28) | What is the relationship between serum Hcy and dementia? | MEDLINE, PsychINFO/to 2009 | 8 (cohort) | There is a positive association between serum Hcy and dementia. |
| Dangour et al, 2010 (29) | What is the strength of the available evidence that serum nutrient levels, dietary consumption, or nutrient supplementation were associated with primary prevention or treatment of dementia? | PubMed, Embase, Cochrane Library/to 2007 | 33 | Available evidence is insufficient to draw definitive conclusions on the association of B vitamins with cognitive decline or dementia. |
| Van Dam and Van Gool, 2009 (30) | What is the association between Hcy levels and AD? | PubMed, Embase, PsychINFO/to 2007 | 18 | Increased serum levels of Hcy predispose to AD; more studies are needed. |
| Raman et al, 2007 (31) | What is the association between blood levels or dietary intake of B vitamins and the risk and progression of neurocognitive deficit? | MEDLINE, Commonwealth Agricultural Bureau/to 2006 | 24 (cohort) | Data supporting the association (of B vitamins and cognitive function) were limited because of the heterogeneity in cognition-assessment methodology and due to scarcity of good quality studies and standardized threshold levels for categorizing low B-vitamin status. |
| Ellinson et al, 2004 (32) | What is the evidence of an association between low serum vitamin B12 and cognitive impairment in subjects older than 60 years? | Medline, PsychINFO, CINAHL, Embase, Food and Nutrition in AGRIS, Biosis/to 2003 | 6 | The evidence does not support a correlation between serum vitamin B12 and cognitive impairment in people older than 60 years. |
Abbreviations: AD, Alzheimer disease, AGRIS, International System for Agricultural Science and Technology; CINAHL, EBSCO Cumulative Index to Nursing & Allied Health Literature; Hcy, homocysteine; No., number.
Some of the systematic reviews were searching for studies of B vitamins; others were looking for the relationship between cognitive function and Hcy levels. B vitamins (folate, B6, and B12) convert Hcy into methionine (an amino acid) into protein. High levels of Hcy are associated with low levels of B vitamins. Thus, levels of serum Hcy are an indirect measure of serum vitamin B12 levels, but Hcy is also elevated in the presence of renal impairment, a common abnormality in older adults, whose decline in renal function worsens with advancing age.
Overall, the systematic reviews concluded that there may be an association between high Hcy levels and the onset of dementia, but that, at this time, there was insufficient evidence to confidently make this statement. Moreover, the relation between elevated plasma Hcy and cognitive impairment can be confounded by the presence of renal impairment, possibly caused by a combination of chronic hypertension and diabetes mellitus, compounded by increasing age.
These systematic reviews included a series of different studies. For this EBA, all of the studies in each of the systematic reviews were reviewed, in addition to searching for additional studies through the aforementioned literature search.
Overall, 11 longitudinal observational studies were included in this study. (33-43) The studies are described in Table 6. The most recent study by Zylberstein et al (33) reported the longest follow-up at 30 years. This Swedish study recruited women in 1968 and 1969 who were between the ages of 38 and 60 years at the time. Researchers measured their Hcy levels at baseline, and then followed the women for 30 years in the longitudinal study. The researchers found that higher levels of Hcy in mid-life were significantly associated with the onset of dementia and AD in later life.
Table 6. Studies Included in Highest-Scoring Systematic Reviews, Sorted by Length of Observation Period.
| Author, Year (Location) | No. of Subjects (Dementia Cases) | Mean Age (Years) at Baseline (SD) | Mean Baseline B12 (SD) | Mean Baseline Hcy (SD) | Hcy as a Predictor for Dementia (Adjusted) (95% CI) | Hcy as a Predictor for AD (Adjusted) (95% CI) | Adjustment Factors | Obs. Period (Years) |
|---|---|---|---|---|---|---|---|---|
| Zylberstein et al, 2011 (33) (Sweden) | 1,368 (151) | 46.8 (no SD) (all women) | 397 pmol/L (138) | 11.8 μmol/L (4.6) | Highest vs lowest Hcy quintile: HR, 1.7 (1.1–2.6) |
Highest vs lowest Hcy quintile: HR, 2.13 (1.22–3.73) |
Age, creatinine, vitamin B12, education, and other risk factors | 30 |
| Seshadri et al, 2002 (34) (USA) | 1,092 (111) | Men 76 (5) Women 77 (6) |
416 pg/mL (209) | 13.1 μmol/L (6.3) | Hcy as a continuous variable: RR, 1.3 (1.1–1.5) |
Hcy as a continuous variable: RR 1.4 (1.1–1.7) |
Age, sex | 8 |
| Kivipelto et al, 2009 (35) (Sweden) | 228 (83) | 81.0 (4.6) | 317 pmol/L (138) | 18.6 μmol/L (10.7) | Hcy as a continuous variable: RR, 1.01 (1.00–1.02) |
Hcy as a continuous variable: RR, 1.01 (1.00–1.03) |
Age, sex, education | 6.7 |
| Nurk et al, 2005 (36) (Norway) | 2,189 (235) | 72.0 (no SD) at follow-up, all patients were eligible between 65–67 years | 347 pmol/L (341–354) | 11.5 μmol/L (11.3–11.6) | Highest vs lowest Hcy quintile: OR, 2.34 (1.39–3.91) |
NR | Sex, APOE genotype, education, CVD, HBP, depression score | 6 |
| Luchsinger et al, 2004 (37) (USA) | 679 (109) | 76.2 (5.7) | NR | Cut-off value: > 15.6 μmol/L | NR | Highest vs lowest Hcy quartile: HR, 1.4 (0.8–2.4) |
Age, sex, education, APOE genotype | 4.7 |
| Haan et al, 2007 (38) (USA) | 1,779 (62) | 60–101 years (mean NR) | 334 pmol/L (150) | 10.8 μmol/L (6.5) | Highest vs lowest Hcy tertile: HR, 2.39 (1.11–5.16) |
NR | Vitamin B12, education | 4.5 |
| Ravaglia et al, 2005 (39) (Italy) | 816 (112) | 73.6 (6.3) | NR | 259 pmol/L (95% CI, 94–708) | Hcy >15 μmol/L: HR, 2.08 (1.31–3.30) |
Hcy >15 μmol/L: HR, 2.11 (1.19–3.76) |
Age, sex, education, APOE genotype, stroke, creatinine, folate, B12 | 4 |
| Wang et al, 2001 (40) (Sweden) | 370 (78) | >75 (mean NR) | Cut-off value: B12 < 150 pmol/L |
NR | Vitamin B12: RR, 1.3 (0.7–2.3) |
Vitamin B12: RR, 1.6 (0.9–2.8) |
Age, sex, education | 3 |
| Kalmijn et al, 1999 (41) (Nether-lands) | 702 (110) | 67.7 (7.1) | NR | 15.6 μmol/L (SE, 0.35) | Highest vs lowest Hcy tertile: OR, 1.30 (0.50–3.38) |
NR | Age, sex, education | 2.6 |
| Kim et al, 2008 (42) (South Korea) | 518 (45) | 71.6 (4.9) | 382 pmol/L (148) | 12.3 μmol/L (5.2) | Highest vs lowest Hcy quintile: OR, 1.10 (0.86–1.40) |
NR | Age, sex, education | 2.4 |
| Rowan et al, 2007 (43) (UK) | 126 (26) | 79.4 (IQR, 77.3–82.8) post-stroke | 264 pmol/L (169) | 16.0 μmol/L (5.1) | Unclear if it was adjusted Hcy as a continuous variable: OR, 1.04 (0.96–1.14) |
NR | N/A | 2 |
Abbreviations: APOE, apolipoprotein E; CI, confidence interval; CVD, cardiovascular disease; Hcy, homocysteine; HBP, high blood pressure; HR, hazard ratio; IQR, interquartiile range;
N/A, not applicable; No., number; NR, not reported; Obs, observation; OR, odds ratio; RR, relative risk; SD, standard deviation; SE, standard error; vs, versus.
An interesting trend was observed among the studies assessing the association between Hcy levels and the onset of dementia. The studies that followed patients for less than 3 years were much less likely to report a significant association between high Hcy levels and the onset of dementia than studies with longer follow-up.
All studies assessed Hcy levels as a surrogate for vitamin B12 levels, except for the study by Wang et al, (40) which measured vitamin B12 levels. After 3 years of follow-up, Wang et al did not report a significant difference in the onset of dementia or AD between those with low B12 levels versus those with normal B12 levels. Again, these results could be misleading, in that elevated Hcy and the onset of dementia can be confounded by renal impairment, chronic hypertension, and diabetes mellitus, all of which are known risk factors for vascular dementia.
The observational studies were assessed for quality using GRADE. There was risk of bias in some studies due to incomplete follow-up and lack of adjustment for confounding. Also, because Hcy is a surrogate measure of vitamin B12, directness in these studies was limited, as the intent was to find the association between vitamin B12 and the onset of dementia, not the association of Hcy and the onset of dementia. More details on the assessment of quality can be found in Appendix 3. Therefore, very low quality evidence indicates an association between Hcy levels and the onset of dementia, but it is impossible to make a conclusion about the specific association between vitamin B12 and the onset of dementia.
Question 2: Does treatment with vitamin B12 supplementation improve cognitive function in patients with dementia or cognitive decline and vitamin B12 deficiency?
Four systematic reviews investigated the effect of treatment of vitamin B12 in patients with cognitive impairment or dementia. (44-47) No additional RCTs were identified that were not included in at least one of the systematic reviews. All 4 systematic reviews came to the same conclusion, which was that treatment with vitamin B12 supplementation did not improve cognitive function. With the exception of the systematic review by the Cochrane Collaboration (47), systematic reviews included heterogeneous study populations. The systematic reviews included studies of patients with varying degrees of cognitive impairment, and vitamin B12 deficiency was not part of the eligibility criteria for any of the systematic reviews (including the Cochrane review).
None of the 17 RCTs included in the systematic reviews met the inclusion criteria for this evidence-based analysis. (48-64) (Table 7) The criteria for this analysis state that studies needed to include patients with vitamin B12 deficiency and with cognitive impairment or dementia. Also, the minimum follow-up duration was 12 months. None of the 17 RCTs identified were able to meet all 3 of these criteria. The RCTs all used various measures for assessing cognitive function, and none of the studies demonstrated a change in cognitive function between the vitamin B12 treatment and placebo arms regardless of what combination of cognitive function tests were used.
Table 7. Studies Included in Systematic Reviews of the Effectiveness of Vitamin B12 on Cognitive Function.
| Author, Yeara | Vitamin B12 Deficient? | Cognitive Impairment or Dementia? | Duration of Followup (months) |
|---|---|---|---|
| De Jager et al, 2012 (48) | No | Yes (mild cognitive impairment) | 24 |
| Kwok et al, 2011 (49) | No | Yes (mild to moderate AD or vascular dementia) | 24 |
| Ford et al, 2010 (50) | Not reported | Unclear | 24 |
| Aisen et al, 2008 (51) | No | Yes (mild to moderate AD) | 18 |
| Kang et al, 2008 (52) | Not reported | Not reported at baseline | 60 |
| Eussen et al, 2006 (53) | Yes (100–200 pmol/L) | Mixed (40% mild to moderate cognitive impairment); results not stratified for patients with cognitive impairment | 6 |
| McMahon et al, 2006 (54) | Likely (high Hcy implies low B12) | No | 24 |
| Lewerin et al, 2005 (55) | No | Unclear | 4 |
| Stott et al, 2005 (56) | No | Mixed (mild or moderate cognitive impairment included) | 12 |
| Garcia et al, 2004 (57) | No | No | 6 |
| Hvas et al, 2004 (58) | Likely (increased MMA implies low B12) | Mixed (1/3 cognitive impairment) | 3 |
| Clarke et al, 2003 (59) | No | Yes (mild to moderate dementia) | 3 |
| Bryan et al, 2002 (60) | Not reported | No | 1 |
| Seal et al, 2002 (61) | Yes (100–150 pmol/L) | Mixed (1/3 cognitive impairment); results not stratified for patients with cognitive impairment | 1 |
| Kwok et al, 1998 (62) | Yes (< 120 pmol/L) | Mixed (20% with dementia); results not stratified for patients with dementia | 4 |
| De la Fourniere et al, 1997 (63) | Yes (< 178 pmol/L) | Yes (all AD) | Not reported |
| Kral et al, 1970 (64) | N/A | N/A | N/A |
Studies where B12 was part of the treatment arm.
Abbreviations: AD, Alzheimer disease; Hcy, homocysteine; MMA, methylmaloninc acid; N/A, not applicable.
The studies assessing cognitive function had to be at least 12 months in duration. Experts believe it can take up to 18 months for cognitive function assessments to measure a change. According to experts, results from cognitive tests can vary from day to day in the same person, and the overall variance is large in populations. (Confidential personal communication, October 30, 2012) Most studies that measured the effect of vitamin B12 on cognitive function were less than 6 months in duration.
One small RCT aimed to answer whether treatment with vitamin B12 in patients with dementia (or cognitive impairment) and vitamin B12 deficiency improved cognitive function. (63) Only 11 patients were enrolled in the study, and the duration of follow-up was not reported. Researchers did not find any improvement in cognitive function with vitamin B12 treatment, but the study was not powered to detect a difference. All of the other studies either included a combination of “healthy” and cognitively impaired older adults with vitamin B12 deficiency, or cognitively impaired adults without vitamin B12 deficiency.
One study did not use cognitive function as the primary outcome; rather, researchers looked at the effect of B vitamins on the rate of brain atrophy, as a surrogate measure of functioning. The article by Smith et al (65) described the results of the VITACOG study. Patients (N = 168) were randomized to receive supplementation with vitamins B12, B6, and folate or placebo for 2 years. All patients underwent magnetic resonance imaging to assess brain atrophy at the start of the trial and again at 2 years. The results of the intent-to-treat analysis indicated a significant difference in the rate of atrophy per year in the group receiving the B vitamin supplements (0.76%; 95% CI, 0.63%–0.90%) versus the group receiving the placebo (1.08%; 95% CI, 0.94%–1.22%; P = 0.001). According to the authors of this RCT, this is also a clinically significant difference. They stated that, in normal aging, one expects 0.5% atrophy per year and 2.5% atrophy per year in AD. (66)
Thus, none of the studies assessing cognitive function were able to identify a difference in cognitive performance between vitamin B12 supplementation versus placebo; however, a study of the rate of brain atrophy did demonstrate that B vitamins slowed the rate of brain atrophy in patients with mild cognitive impairment. The quality of the evidence was assessed using GRADE. For the outcome of cognitive function, the GRADE is moderate. This is based on RCTs with the primary limitation being that none of these trials were performed in the population of interest (i.e., patients with cognitive impairment or dementia and vitamin B12 deficiency). Evidence for the outcome of rate of brain atrophy per year was graded to be low to moderate because only 1 study assessed brain atrophy, which is an indirect measure of disease progression and cognitive function. More details on the quality assessment of the evidence using GRADE can be found in Appendix 3.
Therefore, moderate quality evidence indicates treatment with vitamin B12 supplementation does not change cognitive function in patients with or without dementia or cognitive impairment and with or without vitamin B12 deficiency. Low to moderate quality of evidence indicates treatment with vitamin B12 and folate for patients with mild cognitive impairment slows the rate of brain atrophy compared with those who receive a placebo.
Question 3: What is the effectiveness of oral versus parenteral vitamin B12 supplementation in those with confirmed vitamin B12 deficiency?
In 2005, Vidal-Alaball et al published a Cochrane systematic review comparing oral vitamin B12 to intramuscular vitamin B12 for patients with vitamin B12 deficiency. (67) They identified 2 RCTs that met their inclusion criteria. (68;69) They found that the limited evidence provided in the 2 RCTs indicated oral vitamin B12 was as effective as intramuscular vitamin B12.
The Canadian Agency for Drugs and Technologies in Health (CADTH) published a health technology assessment in 2007 comparing oral versus injectable vitamin B12. (70) They used the Vidal-Alaball et al (67) review as the basis for their rapid review and concluded that oral supplementation was as effective as parenteral vitamin B12, but more research was needed to confirm long-term efficacy.
In addition to the 2 RCTs identified in the review by Vidal-Alaball et al, Castelli et al published a study in 2011, which was funded by the manufacturer of an oral B12 supplement. The study compared oral B12 to intramuscular B12 in patients with mild B12 deficiency (< 258 pmol/L). (71) The authors state that none of the patients had symptoms of vitamin B12 deficiency. The cut-off for vitamin B12 deficiency of 258 pmol/L was higher than most other studies (< 150–200 pmol/L). However, there is some evidence to support increasing the threshold for deficiency to 300 pmol/L. (72) The authors randomized 50 patients to receive either oral B12 or intramuscular B12 for 90 days. They included patients who were older than 60 years of age and also patients who were younger than 60 years of age who had gastrointestinal abnormalities or were on a restricted diet (vegan). After 90 days, all of the patients had vitamin B12 levels higher than 258 pmol/L. It was unclear why the study was designed as a superiority trial and not a noninferiority trial, given that the hypothesis was to establish whether oral B12 is an appropriate alternative to intramuscular B12.
Bolaman et al conducted their RCT in Turkey. (69) At the time their study was being conducted, oral B12 in tablet form was unavailable, so they suspended the 1,000 μg of vitamin B12 in 20 mL of fruit juice for participants to consume. The primary end points for this study were change in hemoglobin levels from baseline to 90 days and signs and symptoms of anemia. The authors did not describe specifically which signs and symptoms of anemia they were measuring. Unlike the Castelli et al RCT, this study does not appear to have been funded by the manufacturer of the B12 supplement.
The study by Kuzminski et al randomized 38 patients to receive either oral or intramuscular vitamin B12. (68) Unlike the other studies, participants receiving oral B12 had 2,000 μg daily, instead of 1,000 μg daily. The group receiving intramuscular B12 in this study received 1,000 μg, like the intramuscular group in other studies. It is unsurprising that, at the final measurement of B12 levels on day 120, participants in the oral B12 group had substantially higher B12 values than participants in the intramuscular B12 group (742 pmol/L versus 240 pmol/L), given that the oral group was receiving twice the amount of B12 that the intramuscular group was receiving and that the oral group was taking the vitamin B12 supplement daily, while the intramuscular group had not had an injection since day 90. Nonetheless, both groups reported mean values well above the cut-off threshold for vitamin B12 deficiency defined in this study (118 pmol/L).
Patients with pernicious anemia are characterized by a lack of intrinsic factor in the gastrointestinal tract, limiting the ability to absorb vitamin B12. One of the reasons for injecting vitamin B12 intramuscularly is to avoid relying on the absorption of vitamin B12 through the gastrointestinal tract. The study by Kuzminski et al reported results for each patient and listed which patients had pernicious anemia. All 5 patients in the oral B12 group with pernicious anemia achieved serum B12 levels greater than 232 pmol/L (range, 232 pmol/L to 1,550 pmol/L) after 4 months of oral therapy.
None of the RCTs reported any severe adverse events associated with taking oral or intramuscular B12 supplementation. The RCTs are summarized in Table 8.
Table 8. Randomized Controlled Trials Comparing Oral Vitamin B12 to Intramuscular Vitamin B12 in Patients with Vitamin B12 Deficiency.
| Author, Year | Number of Patients | Population | Cut-off for B12 Deficiency | Interventions | Primary Outcome | Intent-to-Treat Analysis | Outcomes |
|---|---|---|---|---|---|---|---|
| Castelli et al, 2011 (71) | 50 (48 completed the study, both drop-outs from the oral group) | Vitamin B12-deficient patients ≥60 years or ≥18 years with GI abnormalities or receiving a restricted diet |
< 258 pmol/L | Oral (n = 22): 1,000 μg taken daily for 90 days IM (n = 26): 1,000 μg given on days 1, 3, 7, 10, 14, 21, 30, 60, and 90 |
Proportion of patients in each treatment arm with normalized vitamin B12 levels (≥ 258 pmol/L) | Yes | All patients had vitamin B12 levels > 258 pmol/L on days 61 and 91 |
| Bolaman et al, 2003a (69) | 70 (10 dropped out after < 10 days of treatment) | Patients with megaloblastic anemia (including 11 patients with suspected pernicious anemia) ≥16 years |
< 118 pmol/L | Oral (n = 26): 1,000 μg taken daily for 10 days, then weekly for 4 weeks, then monthly IM (n = 34): 1,000 μg given daily for 10 days, then weekly for 4 weeks, then monthly |
Hemoglobin level and signs or symptoms of anemia | No; drop-outs excluded from analysis | Significant improvement in hemoglobin levels in both groups from baseline to 90 days, approaching normal value (P < .01) |
| Kuzminski et al, 1998a (68) | 38 (5 excluded from analysis because of folate deficiency, not B12 deficiency) | Vitamin B12 deficient (including 7 with confirmed pernicious anemia) | < 118 pmol/L | Oral (n = 18): 2,000 μg daily for 120 days IM (n = 15): 1,000 μg given on days 1, 3, 7, 10, 14, 21, 30, 60, and 90 |
Not specified | No; 5 patients with folate deficiency excluded from analysis | The mean vitamin B12 level in both the oral and intramuscular group was > 240 pmol/L |
Abbreviations: GI, gastrointestinal; IM, intramuscular.
These randomized controlled trials were included in the Cochrane systematic review by Vidal-Alaball et al, 2005. (67)
In January 2012, the British Columbia Guidelines and Protocols Advisory Committee released guidelines on the diagnosis and management of vitamin B12 deficiency. The Committee recommended oral vitamin B12 supplementation because it is as effective as parenteral vitamin B12. (19)
The GRADE quality of evidence for these 3 RCTs was assessed as moderate. The quality was downgraded because of the risk of bias in each of the RCTs. Some limitations include the impossibility of blinding in any of the studies, the failure to report a primary outcome in the study by Bolaman et al, (69) the funding and conduct of the study by Castelli et al (71) by the manufacturer of the oral vitamin B12 supplement, and the imbalance between the treatment courses received in the study by Kuzminski et al (68). More details of the GRADE assessment are listed in Appendix 3.
It is also important to note that these studies were all short term; the long-term effects of taking oral B12 instead of intramuscular injections are unknown.
Discussion
The clinical utility of serum vitamin B12 testing is a complicated issue. To begin with, the accuracy of the serum B12 test is not great. (15) In 2011, Willis et al conducted a systematic review and meta-analysis of serum B12 testing and found that the sensitivity ranged from 13% to 75%, and the specificity ranged from 45% to 100%. (15) This variation in sensitivity and specificity is likely due to the lack of a consistent reference standard to assess serum B12 levels. Thus, widespread screening using the serum B12 test would be ill-advised because many inaccurate results would lead to inappropriate treatment and unnecessary repeat testing.
The cut-off point to establish vitamin B12 deficiency also varies across studies and across community laboratories in Ontario. One laboratory in Ontario sets the cut-off for B12 deficiency at 107 pmol/L, while another sets the cut-off at 148 pmol/L. This variation is likely due to the methods of analysis used by each of the laboratories. In the literature, thresholds range from 150 to 350 pmol/L. Without a consistent definition of B12 deficiency, it is challenging to even comment on the prevalence of the condition. Studies estimate the rate of deficiency from less than 2% to more than 30%. (21;73-76) This variation is likely due to the differences in cut-off values for deficiency and the inaccuracy of the B12 test. Probably a cut-off of 150 pmol/L is the preferred value to define vitamin B12 deficiency.
Given the limitations of the diagnostic accuracy of serum B12 testing and the inconsistency of cut-off values, one could conclude that the clinical utility of vitamin B12 testing is limited because the test itself is unreliable. However, the widely cited study by Lindenbaum et al from 1988 (6) stated that “neuropsychiatric disorders due to cobalamin deficiency occur commonly in the absence of anemia,” thus creating utility in measuring serum B12 levels in addition to complete blood count, because vitamin B12 deficiency could exist without anemia. There were some limitations of this study, including some inconsistencies in the results reported. For instance, Lindenbaum et al list the laboratory findings of patients with neurocognitive abnormalities but without anemia. Even though they state these patients do not have anemia, 15 of 40 patients have abnormally high mean corpuscular volumes (based on the cut-off criteria Lindenbaum et al used), and 6 additional patients have abnormal hematocrit levels. These results lead to a suspicion of anemia. This evidence suggests that some patients have vitamin B12 deficiency without anemia, but the prevalence is lower than reported. Dr Carmel reported similar findings in 1988. (5)
Another possible reason raised for assessing serum vitamin B12 levels was to identify cases of reversible dementia. Because vitamin B12 deficiency can cause neurologic deficits, there is some thought that a dementia caused by vitamin B12 deficiency can be reversed. However, in 2003, Clarfield (13) published a systematic review of 39 studies (N = 7042) examining reversible dementia. He reported that approximately 9% of the cases were potentially reversible and that 0.6% of cases actually reversed. Thus, he demonstrated that truly reversible dementias are quite rare.
It is important to note that the Clinical Expert Advisory Panel on Appropriate Utilization of Vitamin B12 Testing for Neurocognitive-Based Indications, convened by Health Quality Ontario to place the evidence for this analysis into context, believed strongly that testing serum B12 levels has a place in clinical practice. Panel members were also certain that oral B12 supplementation should not be recommended for a general population of older adults because it is unclear what the risks of taking vitamin B12 supplements might be. In 2012 CADTH reported in a rapid review that “[v]itamin B12 supplementation was not found to slow cognitive decline or improve cognitive function in participants with or without cognitive impairment.” (77)
Another challenge with the body of evidence on the association between vitamin B12 deficiency and cognitive function is the measure of cognitive function. Several tests are designed to assess cognitive function, and all of the studies in this evidence-based analysis used a different battery of tests to measure cognitive function, limiting the possibility of pooling the results. There is no standard for assessing change in cognitive function. (78-80) Changes in cognitive function can occur slowly over time, so studies of short duration (less than 6 months) are unlikely to be able to demonstrate a change in cognitive function. As it turned out, none of the 17 RCTs identified that compared treatment with B vitamins to placebo demonstrated a difference in cognitive function, regardless of the duration of follow-up or the battery of cognitive function tests used.
Finally, a recent commentary by Dr Stabler in the New England Journal of Medicine (81) reiterated some of the findings of this evidence based analysis: 1) The sensitivity and specificity of the vitamin B12 test is questionable, and 2) high-dose oral vitamin B12 supplementation is as effective as intramuscular B12. Dr Stabler stated that vitamin B12 deficiency should be determined on the basis of both vitamin B12 test results and clinical symptoms of deficiency (including paresthesias). She also endorsed the vitamin B12 supplementation in adults older than 50 years recommended by the Institute of Medicine’s Food and Nutrition Board, which states that “It is advisable for most of [the recommended daily intake of vitamin B12 to] be obtained by consuming foods fortified with B12 or a B12-containing supplement.” (82)
Conclusions
Question 1: Is there an association between vitamin B12 deficiency and the onset of dementia or cognitive decline?
Based on very low quality evidence, there does appear to be an association between homocysteine levels (a by-product of B vitamins) and the onset of dementia.
Question 2: Does treatment with vitamin B12 supplementation improve cognitive function in patients with dementia or cognitive decline and vitamin B12 deficiency?
Based on moderate quality evidence, treatment with vitamin B12 supplementation does not change cognitive function in patients with or without dementia or cognitive impairment and with or without vitamin B12 deficiency.
Based on low to moderate quality of evidence, treatment with vitamin B12 and folate in patients who have mild cognitive impairment seems to slow the rate of brain atrophy compared with patients who have mild cognitive impairment receiving a placebo. Whether this translates into clinical benefit is unknown.
Question 3: What is the effectiveness of oral versus parenteral vitamin B12 supplementation in those with confirmed vitamin B12 deficiency?
Based on moderate quality evidence, oral vitamin B12 is as effective as parenteral vitamin B12 in patients with confirmed B12 deficiency in the short term.
Acknowledgements
Editorial Staff
Elizabeth Jean Betsch, ELS
Amy Zierler, BA
Medical Information Services
Corinne Holubowich, BEd, MLIS
Kellee Kaulback, BA(H), MISt
Clinical Expert Advisory Panel: Appropriate Utilization of Vitamin B12 Testing for Neurocognitive-Based Indications
| Panel Members | Affiliation(s) | Appointment(s) |
|---|---|---|
| Chair | ||
| Dr. Joel Ray | St. Michael’s Hospital/University of Toronto | Scientist/Assistant Professor, Department of Medicine |
| Family Medicine | ||
| Dr. Andrea Moser | Baycrest Health Services | Associate Medical Director |
| Geriatric Medicine | ||
| Dr. Angeles Garcia | Queen’s University | Professor, Department of Medicine |
| Neurology | ||
| Dr. Stephen H. Pasternak | University of Western Ontario | Director, Cognitive Neurology & Alzheimer’s Disease Research |
| Elizabeth Finger | University of Western Ontario | Assistant Professor, Clinical Neurological Sciences |
| Medial Biochemistry & Medical Genetics | ||
| Dr. David E. C. Cole | University of Toronto | Professor, Laboratory Medicine & Pathobiology |
| Health Care System Representation | ||
| Laurie Sweeting | Ministry of Health & Long Term Care | Senior Program Consultant |
Appendices
Appendix 1: Literature Search Strategy
|
Search date: August 26, 2012 Databases searched: Ovid MEDLINE, MEDLINE In-Process and Other Non-Indexed Citations, Embase; Wiley; Cochrane; Centre for Reviews and Dissemination (CRD) database Limits: 2002-present; Human; English; NOT case reports, comments, editorials, letters Filters: RCTS & MAs/SRs/HTAs for administration route question (#3) | |
| 1 | exp Vitamin B 12 Deficiency/ use mesz |
| 2 | exp Vitamin B 12/df use mesz |
| 3 | Transcobalamins/df use mesz |
| 4 | exp Cyanocobalamin Deficiency/ use emez |
| 5 | ((b12 or b 12 or cyanocobalamin or cobalamin* or transcobalamin* or cobamide? or hydroxocobalamin or hydroxo-cobalamin or hydroxycobalamin) adj3 (deficien* or inadequa* or insufficien* or low blood level* or low serum level* or low plasma level* or suboptimal or sub-optimal or subnormal or sub-normal)).ti,ab. |
| 6 | (an?emia* adj2 (addison* or pernicious* or megaloblastic)).ti,ab. |
| 7 | or/1-6 |
| 8 | exp Vitamin B 12/ use mesz |
| 9 | Transcobalamins/ use mesz |
| 10 | Transcobalamin/ use emez |
| 11 | Cyanocobalamin/ use emez |
| 12 | (b12 or b 12 or cyanocobalamin or cobalamin* or transcobalamin* or cobamide? or hydroxocobalamin or hydroxo-cobalamin or hydroxycobalamin).ti,ab. |
| 13 | or/8-12 |
| 14 | exp Dementia/ |
| 15 | exp Cognition Disorders/ use mesz |
| 16 | (alzheimer* or amentia? or dementia* or demention or senile or senility or (cognit* adj (decline or disorder? or impair*))).ti,ab. |
| 17 | or/14-16 |
| 18 | (7 or 13) and 17 |
| 19 | Case Reports/ or Comment.pt. or Editorial.pt. or Letter.pt. |
| 20 | Case Report/ or Comment/ or Editorial/ or Letter/ |
| 21 | or/19-20 |
| 22 | 18 not 21 |
| 23 | limit 22 to english language |
| 24 | limit 23 to yr="2002 -Current" |
| 25 | exp animals/ |
| 26 | exp animal experimentation/ |
| 27 | exp models animal/ |
| 28 | exp animal experiment/ |
| 29 | nonhuman/ |
| 30 | exp vertebrate/ |
| 31 | or/25-30 |
| 32 | exp humans/ |
| 33 | exp human experiment/ |
| 34 | or/32-33 |
| 35 | 31 not 34 |
| 36 | 24 not 35 |
| 37 | remove duplicates from 36 |
| 38 | 7 and 13 |
| 39 | 38 not 21 |
| 40 | limit 39 to (controlled clinical trial or meta analysis or randomized controlled trial) |
| 41 | exp Technology Assessment, Biomedical/ or exp Evidence-based Medicine/ use mesz |
| 42 | exp Biomedical Technology Assessment/ or exp Evidence Based Medicine/ use emez |
| 43 | (health technology adj2 assess$).ti,ab. |
| 44 | exp Random Allocation/ or exp Double-Blind Method/ or exp Control Groups/ or exp Placebos/ use mesz |
| 45 | Randomized Controlled Trial/ or exp Randomization/ or exp RANDOM SAMPLE/ or Double Blind Procedure/ or exp Triple Blind Procedure/ or exp Control Group/ or exp PLACEBO/ use emez |
| 46 | (random* or RCT).ti,ab. |
| 47 | (placebo* or sham*).ti,ab. |
| 48 | (control* adj2 clinical trial*).ti,ab. |
| 49 | meta analysis/ use emez |
| 50 | (meta analy* or metaanaly* or pooled analysis or (systematic* adj2 review*) or published studies or published literature or medline or embase or data synthesis or data extraction or cochrane).ti,ab. |
| 51 | or/41-50 |
| 52 | (39 and 51) or 40 |
| 53 | limit 52 to english language |
| 54 | limit 53 to yr="2002 -Current" |
| 55 | remove duplicates from 54 |
Appendix 2: Systematic Reviews of Treatment with B12 for Cognitive Function
Table A1. Systematic Reviews of Treatment with B12 for Cognitive Function.
| Author, Year | Question (Sources Searched/Dates) | Studies Where B12 was Part of Treatment Arm | Study Included in this EBA? | Vitamin B12 Deficient? | Cognitive Impairment or Dementia? | Duration of Study (Months) | Overall Conclusion |
|---|---|---|---|---|---|---|---|
| Ford and Almeida, 2012 (44) | What is the efficacy of treatment with vitamins B12, B6, or folate in slowing cognitive decline among older adults with and without cognitive impairment? (PubMed, PsychINFO, Embase, Cochrane/to 2011) | Aisen et al, 2008 (51) | No | No | Yes (mild to moderate AD) | 18 | No difference in cognitive function whether receiving vitamin B12 supplementation or not |
| Clarke et al, 2003 (59) | No | No | Yes (mild to moderate dementia) | 3 | |||
| De Jager et al, 2012 (48) | No | No | Yes (mild cognitive impairment) | 24 | |||
| Kwok et al, 2011 (49) | No | No | Yes (mild to moderate AD or vascular dementia) | 24 | |||
| Garcia et al, 2004 (57) | No | No | No | 6 | |||
| Hvas et al, 2004 (58) | No | Likely (increased MMA implies low B12) | Mixed (1/3 cognitive impairment) | 3 | |||
| Eussen et al, 2006 (53) | No | Yes (100–200 pmol/L) | Mixed (40% mild-moderate cognitive impairment); results not stratified for patients with cognitive impairment | 6 | |||
| Ford et al, 2010 (50) | No | Not reported | Unclear | 24 | |||
| Kang et al, 2008 (52) | No | Not reported | Not reported at baseline | 60 | |||
| Seal et al, 2002 (61) | No | Yes (100–150 pmol/L) | Mixed (1/3 cognitive impairment); results not stratified for patients with cognitive impairment | 1 | |||
| Lewerin et al, 2005 (55) | No | No | Unclear | 4 | |||
| McMahon et al, 2006 (54) | No | Likely (high Hcy implies low B12) | No | 24 | |||
| Stott et al, 2005 (56) | No | No | Mixed (mild or moderate cognitive impairment included) | 12 | |||
| Jia et al, 2008 (45) | What is effect of nutrient supplementation on cognitive function in people aged ≥ 65 years? (MEDLINE, Embase/ to 2006) | De la Fourniere et al, 1997 (63) | No (full study in French, N = 11) | Yes (< 178 pmol/L) | Yes (all AD) | Not reported | No difference in cognitive function whether receiving vitamin B12 supplementation or not |
| Kwok et al, 1998 (62) | No | Yes (< 120 pmol/L) | Mixed (20% with dementia); results not stratified for patients with dementia | 4 | |||
| Bryan et al, 2002 (60) | No | Not reported | No | 1 | |||
| Seal et al, 2002 (61) | No | Yes (100–150 pmol/L) | Mixed (1/3 cognitive impairment); results not stratified for patients with cognitive impairment | 1 | |||
| Clarke et al, 2003 (59) | No | No | Yes (mild to moderate dementia) | 3 | |||
| Lewerin et al, 2005 (55) | No | No | Unclear | 4 | |||
| Stott et al, 2005 (56) | No | No | Mixed (mild or moderate cognitive impairment included) | 12 | |||
| Eussen et al, 2006 (53) | No | Yes (100–200 pmol/L) | Mixed (40% mild to moderate cognitive impairment); results not stratified for patients with cognitive impairment | 6 | |||
| McMahon et al, 2006 (54) | No | Likely (high Hcy implies low B12) | No | 24 | |||
| Balk et al, 2007 (46) | Does supplementation with vitamins B6, B12, and folic acid prevent or decrease progression of neurologic changes associated with dementia? (MEDLINE, Commonwealth Agricultural Bureau/to 2005) | Hvas et al, 2004 (58) | No | Likely (increased MMA implies low B12) | Mixed (1/3 cognitive impairment) | 3 | No difference in cognitive function whether receiving vitamin B12 supplementation or not |
| Eussen et al, 2006 (53) | No | Yes (100–200 pmol/L) | Mixed (40% mild-moderate cognitive impairment); results not stratified for patients with cognitive impairment | 6 | |||
| Bryan et al, 2002 (60) | No | Not reported | No | 1 | |||
| Seal et al, 2002 (61) | No | Yes (100–150 pmol/L) | Mixed (1/3 cognitive impairment); results not stratified for patients with cognitive impairment | 1 | |||
| Kwok et al, 1998 (62) | No | Yes (< 120 pmol/L) | Mixed (20% with dementia); results not stratified for patients with dementia | 3 | |||
| Kral et al, 1970 (64) | No (outdated) | N/A | N/A | N/A | |||
| Malouf and Areosa, 2003 (Cochrane Review) (47) | What is effect of vitamin B12 supplementation on cognitive function of demented and elderly healthy people in terms of preventing the onset or progression of cognitive impairment? (MEDLINE, Embase, Cochrane/to 2006) | Hvas et al, 2004 (58) | No | Likely (increased MMA implies low B12) | Mixed (1/3 cognitive impairment) | 3 | No difference in cognitive function whether receiving vitamin B12 supplementation or not |
| Seal et al, 2002 (61) | No | Yes (100–150 pmol/L) | Mixed (1/3 cognitive impairment); results not stratified for patients with cognitive impairment | 1 | |||
| De la Fourniere et al, 1997 (63) | No (full study in French, N = 11) | Yes (< 178 pmol/L) | Yes (all AD) | Not reported |
Abbreviations: AD, Alzheimer disease; EBA, evidence-based analysis; Hcy, homocysteine; MMA, methylmalonic acid.
Appendix 3: Evidence Quality Assessment
Table A2. GRADE Evidence Profile for the Association Between Homocysteine and the Onset of Dementia.
| No. of Studies (Design) | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Upgrade Considerations | Quality |
|---|---|---|---|---|---|---|---|
| Homocysteine as a predictor of dementia | |||||||
| 11 longitudinal observational studies | Some limitationsa | No serious limitationsb | Serious limitations (-1)c | No serious limitations | Undetected | None | ⊕⊕ Low |
There were some limitations in studies used to answer this question. Limitations included incomplete follow-up, insufficient control for confounding, and inappropriate measure of outcomes—but overall studies were well reported.
When studies are stratified by years of follow-up, there is consistency in the outcomes—the longer the follow-up period, the more likely an association between homocysteine levels and the onset of dementia.
Homocysteine is an indirect measure of serum vitamin B12.
Table A3. Risk of Bias Among Observational Trials for the Association Between Homocysteine and the Onset of Dementia.
| Author, Year | Appropriate Eligibility Criteria | Appropriate Measurement of Exposure | Appropriate Measurement of Outcome | Adequate Control for Confounding | Complete Follow-Up |
|---|---|---|---|---|---|
| Zylberstein et al, 2011 (33) | No limitations | No limitations | No limitations | No limitations | No limitations |
| Seshadri et al, 2002 (34) | No limitations | No limitations | No limitations | No limitations | No limitations |
| Kivipelto et al, 2009 (35) | No limitations | No limitations | No limitations | No limitations | No limitations |
| Nurk et al, 2005 (36) | No limitations | No limitations | No limitations | No limitations | Limitationsa |
| Luchsinger et al, 2004 (37) | No limitations | No limitations | No limitations | No limitations | No limitations |
| Haan et al, 2007 (38) | No limitations | No limitations | Limitationsb | Limitationsc | No limitations |
| Ravaglia et al, 2005 (39) | No limitations | No limitations | No limitations | No limitations | No limitations |
| Wang et al, 2001 (40) | No limitations | No limitations | Limitationsd | No limitations | No limitations |
| Kalmijn et al, 1999 (41) | No limitations | No limitations | Limitationsb | No limitations | No limitations |
| Kim et al, 2008 (42) | No limitations | No limitations | No limitations | No limitations | No limitations |
| Rowan et al, 2007 (43) | No limitations | No limitations | No limitations | Limitationse | No limitations |
It is unclear how many patients completed full 6 years of follow-up.
Studies reported wide confidence intervals.
This analysis was not adjusted for age or sex.
This study measured vitamin B12 levels, while all other studies measured homocysteine.
It is unclear whether the analysis was adjusted.
Table A4. GRADE Evidence Profile for Comparison of B Vitamins Versus Placebo.
| No. of Studies (Design) | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Upgrade Considerations | Quality |
|---|---|---|---|---|---|---|---|
| Cognitive function | |||||||
| 4 systematic reviews (including 17 RCTs) | No serious limitations | No serious limitations | Serious limitations (-1)a | No serious limitations | Undetected | None | ⊕⊕⊕ Moderate |
| Rate of brain atrophy per year | |||||||
| 1 RCT (VITACOG) | No serious limitations | Consistency unknown (single study) | Serious limitations (-1)b | No serious limitations | Undetected | None | ⊕⊕ Low-Moderate |
None of the RCTs identified included the population of interest (i.e., patients with dementia or cognitive impairment and vitamin B12 deficiency).
Rate of brain atrophy is an indirect measure of disease progression and cognitive function. Abbreviation: RCT, randomized controlled trial.
Table A5. Risk of Bias Among Randomized Controlled Trials for Comparison of B Vitamins Versus Placebo.
| Author, Year | Allocation Concealment | Blinding | Complete Accounting of Patients and Outcome Events | Selective Reporting Bias | Other Limitations |
|---|---|---|---|---|---|
| Smith et al VITACOG, 2010 (65) | No limitations | No limitations | No limitations | No limitations | No limitations |
Table A6. GRADE Evidence Profile for Comparison of Oral Vitamin B12 and Parenteral Vitamin B12.
| No. of Studies (Design) | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Upgrade Considerations | Quality |
|---|---|---|---|---|---|---|---|
| Mean vitamin B12 level above cut-off at end of study | |||||||
| 3 (RCTs) | Serious limitations (-1)a | No serious limitations | No serious limitations | No serious limitations | Undetected | None | ⊕⊕⊕ Moderate |
The risk of bias assessment identified limitations with each of the randomized controlled trials (see Table A7).
Abbreviation: RCT, randomized controlled trial.
Table A7. Risk of Bias Among Randomized Controlled Trials for Comparison of Oral Vitamin B12 and Parenteral Vitamin B12.
| Author, Year | Allocation Concealment | Blinding | Complete Accounting of Patients and Outcome Events | Selective Reporting Bias | Other Limitations |
|---|---|---|---|---|---|
| Castelli et al, 2011 (71) | No limitations | Limitationsa | No limitations | No limitations | Limitationsb |
| Bolaman et al, 2003 (69) | No limitations | Limitationsa | Limitationsc | Limitationsd | No limitations |
| Kuzminski et al, 1998 (68) | Limitationse | Limitationsa | Limitationsc | No limitations | No limitations |
It is impossible to blind this randomized controlled trial because participants were randomized to oral or injected vitamin B12; thus it is impossible to disguise or hide the intervention.
This study was funded and conducted by the manufacturer of the oral vitamin B12 supplement.
An intent-to-treat analysis was not reported.
The primary end point was reported to be signs and symptoms of anemia; however, no detail was given to describe the signs and symptoms of anemia in the results.
The randomization process was not described.
Suggested Citation
This report should be cited as follows:
Health Quality Ontario. Vitamin B12 and cognitive function: an evidence-based analysis. Ont Health Tech Assess Ser [Internet]. 2013 November;13(23):1–45. Available from http://www.hqontario.ca/evidence/publications-and-ohtac-recommendations/ontario-health-technology-assessment-series/B12-cognitive-function
Permission Requests
All inquiries regarding permission to reproduce any content in the Ontario Health Technology Assessment Series should be directed to EvidenceInfo@hqontario.ca.
How to Obtain Issues in the Ontario Health Technology Assessment Series
All reports in the Ontario Health Technology Assessment Series are freely available in PDF format at the following URL: http://www.hqontario.ca/evidence/publications-and-ohtac-recommendations/ontario-health-technology-assessment-series.
Indexing
The Ontario Health Technology Assessment Series is currently indexed in MEDLINE/PubMed, Excerpta Medica/Embase, and the Centre for Reviews and Dissemination database.
Conflict of Interest Statement
All reports in the Ontario Health Technology Assessment Series are impartial. There are no competing interests or conflicts of interest to declare.
Peer Review
All reports in the Ontario Health Technology Assessment Series are subject to external expert peer review. Additionally, Health Quality Ontario posts draft reports and recommendations on its website for public comment prior to publication. For more information, please visit: http://www.hqontario.ca/evidence/evidence-process/evidence-review-process/professional-and-public-engagement-and-consultation.
About Health Quality Ontario
Health Quality Ontario is an arms-length agency of the Ontario government. It is a partner and leader in transforming Ontario’s health care system so that it can deliver a better experience of care, better outcomes for Ontarians, and better value for money.
Health Quality Ontario strives to promote health care that is supported by the best available scientific evidence. The Evidence Development and Standards branch works with expert advisory panels, clinical experts, scientific collaborators, and field evaluation partners to conduct evidence-based reviews that evaluate the effectiveness and cost-effectiveness of health interventions in Ontario.
Based on the evidence provided by Evidence Development and Standards and its partners, the Ontario Health Technology Advisory Committee—a standing advisory subcommittee of the Health Quality Ontario Board—makes recommendations about the uptake, diffusion, distribution, or removal of health interventions to Ontario’s Ministry of Health and Long-Term Care, clinicians, health system leaders, and policy-makers.
Health Quality Ontario’s research is published as part of the Ontario Health Technology Assessment Series, which is indexed in MEDLINE/PubMed, Excerpta Medica/Embase, and the Centre for Reviews and Dissemination database. Corresponding Ontario Health Technology Advisory Committee recommendations and other associated reports are also published on the Health Quality Ontario website. Visit http://www.hqontario.ca for more information.
About the Ontario Health Technology Assessment Series
To conduct its comprehensive analyses, Evidence Development and Standards and its research partners review the available scientific literature, making every effort to consider all relevant national and international research; collaborate with partners across relevant government branches; consult with expert advisory panels, clinical and other external experts, and developers of health technologies; and solicit any necessary supplemental information.
In addition, Evidence Development and Standards collects and analyzes information about how a health intervention fits within current practice and existing treatment alternatives. Details about the diffusion of the intervention into current health care practices in Ontario add an important dimension to the review.
The Ontario Health Technology Advisory Committee uses a unique decision determinants framework when making recommendations to the Health Quality Ontario Board. The framework takes into account clinical benefits, value for money, societal and ethical considerations, and the economic feasibility of the health care intervention in Ontario. Draft Ontario Health Technology Advisory Committee recommendations and evidence-based reviews are posted for 21 days on the Health Quality Ontario website, giving individuals and organizations an opportunity to provide comments prior to publication. For more information, please visit: http://www.hqontario.ca/evidence/evidence-process/evidence-review-process/professional-and-public-engagement-and-consultation.
Disclaimer
This report was prepared by the Evidence Development and Standards branch at Health Quality Ontario or one of its research partners for the Ontario Health Technology Advisory Committee and was developed from analysis, interpretation, and comparison of scientific research. It also incorporates, when available, Ontario data and information provided by experts and applicants to Health Quality Ontario. This report is current as of the date of the literature search specified in the Research Methods section. Health Quality Ontario makes no representation that the literature search captured every publication that was or could be applicable to the subject matter of the report. It is possible that relevant scientific findings may have been reported since the completion of the review. This analysis may be superseded by an updated publication on the same topic. Please check the Health Quality Ontario website for a list of all publications: http://www.hqontario.ca/evidence/publications-and-ohtac-recommendations.
Health Quality Ontario
130 Bloor Street West, 10th Floor
Toronto, Ontario
M5S 1N5
Tel: 416-323-6868
Toll Free: 1-866-623-6868
Fax: 416-323-9261
Email: EvidenceInfo@hqontario.ca
ISSN 1915-7398 (online)
ISBN 978-1-4606-2026-7 (PDF)
© Queen’s Printer for Ontario, 2013
List of Tables
| Table 1. Volume of Vitamin B12 Laboratory Tests in Ontario for Fiscal Years 2005/2006 to 2010/2011 |
| Table 2. Summary of Laboratory Tests to Assess Vitamin B12 Deficiency |
| Table 3. Guidelines for Assessment of Vitamin B12 Levels |
| Table 4. Body of Evidence Examined According to Study Design |
| Table 5. Summary of Systematic Reviews Examining the Association Between Vitamin B12 (or Hyperhomocysteinemia) and the Onset of Dementia |
| Table 6. Studies Included in Highest-Scoring Systematic Reviews, Sorted by Length of Observation Period |
| Table 7. Studies Included in Systematic Reviews of the Effectiveness of Vitamin B12 on Cognitive Function |
| Table 8. Randomized Controlled Trials Comparing Oral Vitamin B12 to Intramuscular Vitamin B12 in Patients with Vitamin B12 Deficiency |
| Table A1. Systematic Reviews of Treatment with B12 for Cognitive Function |
| Table A2. GRADE Evidence Profile for the Association Between Homocysteine and the Onset of Dementia |
| Table A3. Risk of Bias Among Observational Trials for the Association Between Homocysteine and the Onset of Dementia |
| Table A4. GRADE Evidence Profile for Comparison of B Vitamins Versus Placebo |
| Table A5. Risk of Bias Among Randomized Controlled Trials for Comparison of B Vitamins Versus Placebo |
| Table A6. GRADE Evidence Profile for Comparison of Oral Vitamin B12 and Parenteral Vitamin B12 |
| Table A7. Risk of Bias Among Randomized Controlled Trials for Comparison of Oral Vitamin B12 and Parenteral Vitamin B12 |
List of Figures
List of Abbreviations
- AD
Alzheimer disease
- CADTH
Canadian Agency for Drugs and Technologies in Health
- CCCDTD
Canadian Consensus Conference on the Diagnosis and Treatment of Dementia
- CI
Confidence interval
- CINAHL
EBSCO Cumulative Index to Nursing & Allied Health Literature
- CRD
Centre for Reviews and Dissemination
- EBA
Evidence-based analysis
- Hcy
Homocysteine
- HQO
Health Quality Ontario
- MCV
Mean corpuscular volume
- MMA
Methylmalonic acid
- NHANES
National Health and Nutritional Examination Survey
- RCT
Randomized controlled trial
- SIGN
Scottish Intercollegiate Guidelines Network
References
- 1.Snow CF. Laboratory diagnosis of vitamin B12 and folate deficiency. Arch Intern Med. 1999;159(6):1289–98. doi: 10.1001/archinte.159.12.1289. [DOI] [PubMed] [Google Scholar]
- 2.Rumsey SE, Hokin B, Magin PJ, Pond D. Macrocytosis: an Australian general practice perspective. Aust Fam Physician. 2007;36(7):571–2. [PubMed] [Google Scholar]
- 3.Aslinia F, Mazza JJ, Yale SH. Megaloblastic anemia and other causes of macrocytosis. Clin Med Res. 2006;4(3):236–41. doi: 10.3121/cmr.4.3.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaferle J, Strzoda CE. Evaluation of macrocytosis. Am Fam Physician. 2009;79(3):203–8. [PubMed] [Google Scholar]
- 5.Carmel R. Pernicious anemia: the expected findings of very low serum cobalamin levels, anemia, and macrocytosis are often lacking. Arch Intern Med. 1988;148:1712–4. doi: 10.1001/archinte.148.8.1712. [DOI] [PubMed] [Google Scholar]
- 6.Lindenbaum J, Healton EB, Savage DG, Brust JCM, Garrett TJ, Podell ER, et al. Neuropsychiatric disorders caused by cobalamin deficiency in the absence of anemia or macrocytosis. N Engl J Med. 1988;318:1720–8. doi: 10.1056/NEJM198806303182604. [DOI] [PubMed] [Google Scholar]
- 7.Carmel R. Prevalence of undiagnosed pernicious anemia in the elderly. Arch Intern Med. 1996;156:1097–100. [PubMed] [Google Scholar]
- 8.Guralnik JM, Eisenstaedt RS, Ferrucci L, Klein HG, Woodman RC. Prevalence of anemia in persons 65 years and older in the United States: evidence for a high rate of unexplained anemia. Blood. 2004;104:2263–8. doi: 10.1182/blood-2004-05-1812. [DOI] [PubMed] [Google Scholar]
- 9.3rd Canadian Consensus Conference on Diagnosis and Treatment of Dementia. Canadian Consensus Conference on Diagnosis and Treatment of Dementia. 2007. [[cited: 2012 Oct 10]]. Available from: http://www.cccdtd.ca/pdfs/Final_Recommendations_CCCDTD_2007.pdf .
- 10.Chertkow H. Diagnosis and treatment of dementia: introduction. Can Med Assoc J. 2008;178(3):316–21. doi: 10.1503/cmaj.070795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feldman HH, Jacova C, Robillard A, Garcia A, Chow T, Borrie M, et al. Diagnosis and treatment of dementia: 2. Diagnosis. Can Med Assoc J. 2008;178(7):825–36. doi: 10.1503/cmaj.070798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garcia A. Cobalamin and homocysteine in older adults: do we need to test for serum levels in the work-up of dementia? Alzheimers Dement. 2007;3(4):318–24. doi: 10.1016/j.jalz.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 13.Clarfield AM. The decreasing prevalence of reversible dementias: an updated meta-analysis. Arch Intern Med. 2003;163:2219–29. doi: 10.1001/archinte.163.18.2219. [DOI] [PubMed] [Google Scholar]
- 14.BC Guidelines and Protocols Advisory Committee. Cognitive impairment in the elderly--recognition, diagnosis and management. Vancouver, BC: Guidelines and Protocols Advisory Committee. 2007. [[cited: 2012 Oct 10]]. Available from: http://www.bcguidelines.ca/pdf/cognitive.pdf .
- 15.Willis CD, Elshaug AG, Milverton JL, Watt AM, Metz MP, Hiller JE. Diagnostic performance of serum cobalamin tests: a systematic review and meta-analysis. Pathology. 2011;43(5):472–81. doi: 10.1097/PAT.0b013e3283486435. [DOI] [PubMed] [Google Scholar]
- 16.Hvas A-M, Nexo E. Diagnosis and treatment of vitamin B12 deficiency. An update. Haematologica. 2006;91(11):1506–12. [PubMed] [Google Scholar]
- 17.Bailey RL, Carmel R, Green R, Pfeiffer CM, Cogswell ME, Osterloh JD, et al. Monitoring of vitamin B-12 nutritional status in the United States by using plasma methylmalonic acid and serum vitamin B-12. Am J Clin Nutr. 2011;94(2):552–61. doi: 10.3945/ajcn.111.015222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yetley EA, Pfeiffer CM, Phinney KW, Bailey RL, Blackmore S, Bock JL, et al. Biomarkers of vitamin B-12 status in NHANES: a roundtable summary. Am J Clin Nutr. 2011;94(1):313S–21S. doi: 10.3945/ajcn.111.013243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.BC Guidelines and Protocols Advisory Committee. Cobalamin (vitamin B12) deficiency: investigation and management. British Columbia: BC Ministry of Health. 2012. [[cited: 2012 Aug 28]]. p. 4 p. Available from: http://www.bcguidelines.ca/guideline_cobalamin.html .
- 20.Smellie WSA, Wilson D, McNulty CAM, Galloway MJ, Spickett GA, Finnigan DI, et al. Best practice in primary care pathology: review 1. J Clin Pathol. 2005;58(10):1016–24. doi: 10.1136/jcp.2004.025049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andres E, Loukili NH, Noel E, Kaltenbach G, Abdelgheni MB, Perrin AE, et al. Vitamin B12 (cobalamin) deficiency in elderly patients. Can Med Assoc J. 2004;171(3):251–9. doi: 10.1503/cmaj.1031155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.AGREE Working Group. Appraisal of guidelines for research and evaluation II (AGREE). Hamilton, ON: AGREE Working Group. 2009. [[cited: 2012 Aug 28]]. p. 56 p. Available from: http://www.agreetrust.org/
- 23.Scottish Intercollegiate Guidelines Network. Management of patients with dementia. Edinburgh: NHS Scotland. [[cited: 2012 Dec 12]];2006 57:p. 86. Available from: http://www.sign.ac.uk/pdf/sign86.pdf . [Google Scholar]
- 24.Guyatt GH, Oxman AD, Schuenemann HJ, Tugwell P, Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol. 2011;64(4):380–2. doi: 10.1016/j.jclinepi.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 25.Goodman C. Literature searching and evidence interpretation for assessing health care practices. Stockholm, Sweden: Swedish Council on Technology Assessment in Health Care. [1996 SBU. Report No. 119E]. [DOI] [PubMed]
- 26.Moore E, Mander A, Ames D, Carne R, Sanders K, Watters D. Cognitive impairment and vitamin B12: a review. Int Psychogeriatr. 2012;24(4):541–56. doi: 10.1017/S1041610211002511. [DOI] [PubMed] [Google Scholar]
- 27.Ho RC, Cheung MW, Fu E, Win HH, Zaw MH, Ng A, et al. Is high homocysteine level a risk factor for cognitive decline in elderly? A systematic review, meta-analysis, and meta-regression. Am J Geriatr Psychiatry. 2011;19(7):607–17. doi: 10.1097/JGP.0b013e3181f17eed. [DOI] [PubMed] [Google Scholar]
- 28.Wald DS, Kasturiratne A, Simmonds M. Serum homocysteine and dementia: meta-analysis of eight cohort studies including 8669 participants. Alzheimers Dement. 2011;7(4):412–7. doi: 10.1016/j.jalz.2010.08.234. [DOI] [PubMed] [Google Scholar]
- 29.Dangour AD, Whitehouse PJ, Rafferty K, Mitchell SA, Smith L, Hawkesworth S, et al. Bvitamins and fatty acids in the prevention and treatment of Alzheimer’s disease and dementia: a systematic review. J Alzheimers Dis. 2010;22(1):205–24. doi: 10.3233/JAD-2010-090940. [DOI] [PubMed] [Google Scholar]
- 30.Van Dam F, Van Gool WA. Hyperhomocysteinemia and Alzheimer’s disease: a systematic review. Arch Gerontol Geriatr. 2009;48(3):425–30. doi: 10.1016/j.archger.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 31.Raman G, Tatsioni A, Chung M, Rosenberg IH, Lau J, Lichtenstein AH, et al. Heterogeneity and lack of good quality studies limit association between folate, vitamins B-6 and B-12, and cognitive function. J Nutr. 2007;137(7):1789–94. doi: 10.1093/jn/137.7.1789. [DOI] [PubMed] [Google Scholar]
- 32.Ellinson M, Thomas J, Patterson A. A critical evaluation of the relationship between serum vitamin B, folate and total homocysteine with cognitive impairment in the elderly. J Hum Nutr Diet. 2004;17(4):371–83. doi: 10.1111/j.1365-277X.2004.00532.x. [DOI] [PubMed] [Google Scholar]
- 33.Zylberstein DE, Lissner L, Bjorkelund C, Mehlig K, Thelle DS, Gustafson D, et al. Midlife homocysteine and late-life dementia in women. A prospective population study. Neurobiol Aging. 2011;32:380–6. doi: 10.1016/j.neurobiolaging.2009.02.024. [DOI] [PubMed] [Google Scholar]
- 34.Seshadri S, Beiser A, Selhub J, Jacques PF, Rosenberg IH, D’Agostino RB, et al. Plasma homocysteine as a risk factor for dementia and Alzheimer’s disease. N Engl J Med. 2002;346(7):476–83. doi: 10.1056/NEJMoa011613. [DOI] [PubMed] [Google Scholar]
- 35.Kivipelto M, Annerbo S, Hultdin J, Backman L, Viitanen M, Fratiglioni L, et al. Homocysteine and holo-transcobalamin and the risk of dementia and Alzheimers disease: a prospective study. Eur J Neurol. 2009;16(7):808–13. doi: 10.1111/j.1468-1331.2009.02590.x. [DOI] [PubMed] [Google Scholar]
- 36.Nurk E, Refsum H, Tell GS, Engedal K, Vollset SE, Ueland PM, et al. Plasma total homocysteine and memory in the elderly: The Hordaland homocysteine study. Ann Neurol. 2005;58(6):847–57. doi: 10.1002/ana.20645. [DOI] [PubMed] [Google Scholar]
- 37.Luchsinger JA, Tang MX, Shea S, Miller J, Green R, Mayeux R. Plasma homocysteine levels and risk of Alzheimer disease. Neurology. 2004;62(11):1972–6. doi: 10.1212/01.wnl.0000129504.60409.88. [DOI] [PubMed] [Google Scholar]
- 38.Haan MN, Miller JW, Aiello AE, Whitmer RA, Jagust WJ, Mungas DM, et al. Homocysteine, B vitamins, and the incidence of dementia and cognitive impairment: results from the Sacramento Area Latino Study on Aging. Am J Clin Nutr. 2007;85(2):511–7. doi: 10.1093/ajcn/85.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ravaglia G, Forti P, Maioli F, Martelli M, Servadei L, Brunetti N, et al. Homocysteine and folate as risk factors for dementia and Alzheimer disease. Am J Clin Nutr. 2005;82(3):636–43. doi: 10.1093/ajcn.82.3.636. [DOI] [PubMed] [Google Scholar]
- 40.Wang H-X, Wahlin A, Basun H, Fastbom J, Windlad B, Fratiglioni L. Vitamin B12 and folate in relation to the development of Alzheimer’s disease. Neurology. 2001;56:1188–94. doi: 10.1212/wnl.56.9.1188. [DOI] [PubMed] [Google Scholar]
- 41.Kalmijn S, Launer LJ, Lindemans J, Bots ML, Hofman A, Breteler MMB. Total homocysteine and cognitive decline in a community-based sample of elderly subjects. Am J Epidemiol. 1999;150(3):283–9. doi: 10.1093/oxfordjournals.aje.a010000. [DOI] [PubMed] [Google Scholar]
- 42.Kim JM, Stewart R, Kim SW, Shin IS, Yang SJ, Shin HY, et al. Changes in folate, vitamin B12 and homocysteine associated with incident dementia. J Neurol Neurosurg Psychiatry. 2008;79(8):864–8. doi: 10.1136/jnnp.2007.131482. [DOI] [PubMed] [Google Scholar]
- 43.Rowan EN, Dickinson HO, Stephens S, Ballard C, Kalaria R, Kenny RA. Homocysteine and post-stroke cognitive decline. Age Ageing. 2007;36(3):339–43. doi: 10.1093/ageing/afm006. [DOI] [PubMed] [Google Scholar]
- 44.Ford AH, Almeida OP. Effect of homocysteine lowering treatment on cognitive function: a systematic review and meta-analysis of randomized controlled trials. J Alzheimers Dis. 2012;29(1):133–49. doi: 10.3233/JAD-2012-111739. [DOI] [PubMed] [Google Scholar]
- 45.Jia X, McNeill G, Avenell A. Does taking vitamin, mineral and fatty acid supplements prevent cognitive decline? A systematic review of randomized controlled trials. J Human Nutr Diet. 2008;21(4):317–36. doi: 10.1111/j.1365-277X.2008.00887.x. [DOI] [PubMed] [Google Scholar]
- 46.Balk EM, Raman G, Tatsioni A, Chung M, Lau J, Rosenberg IH. Vitamin B6, B12, and folic acid supplementation and cognitive function: a systematic review of randomized trials. Arch Intern Med. 2007;167(1):21–30. doi: 10.1001/archinte.167.1.21. [DOI] [PubMed] [Google Scholar]
- 47.Malouf R, Areosa SA. Vitamin B12 for cognition. Cochrane Database Syst Rev. 2003. p. CD004326. [DOI] [PubMed]
- 48.de Jager CA, Oulhaj A, Jacoby R, Refsum H, Smith AD. Cognitive and clinical outcomes of homocysteine-lowering B-vitamin treatment in mild cognitive impairment: a randomized controlled trial. Int J Geriatr Psychiatry. 2012;27(6):592–600. doi: 10.1002/gps.2758. [DOI] [PubMed] [Google Scholar]
- 49.Kwok T, Lee J, Law CB, Pan PC, Yung CY, Choi KC, et al. A randomized placebo controlled trial of homocysteine lowering to reduce cognitive decline in older demented people. Clin Nutr. 2011;30(3):297–302. doi: 10.1016/j.clnu.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 50.Ford AH, Flicker L, Alfonso H, Thomas J, Clarnette R, Martins R, et al. Vitamins B(12), B(6), and folic acid for cognition in older men. Neurology. 2010;75(17):1540–7. doi: 10.1212/WNL.0b013e3181f962c4. [DOI] [PubMed] [Google Scholar]
- 51.Aisen PS, Schneider LS, Sano M, Diaz-Arrastia R, van Dyck CH, Weiner MF, et al. High-dose B vitamin supplementation and cognitive decline in Alzheimer disease: a randomized controlled trial. JAMA: J Am Med Assoc. 2008;300(15):1774–83. doi: 10.1001/jama.300.15.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kang JH, Cook N, Manson J, Buring JE, Albert CM, Grodstein F. A trial of B vitamins and cognitive function among women at high risk of cardiovascular disease. Am J Clin Nutr. 2008;88(6):1602–10. doi: 10.3945/ajcn.2008.26404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Eussen SJ, de Groot LC, Joosten LW, Bloo RJ, Clarke R, Ueland PM, et al. Effect of oral vitamin B-12 with or without folic acid on cognitive function in older people with mild vitamin B-12 deficiency: a randomized, placebo-controlled trial. Am J Clin Nutr. 2006;84(2):361–70. doi: 10.1093/ajcn/84.1.361. [DOI] [PubMed] [Google Scholar]
- 54.McMahon JA, Green TJ, Skeaff CM, Knight RG, Mann JI, Williams SM. A controlled trial of homocysteine lowering and cognitive performance. N Engl J Med. 2006;354(26):2764–72. doi: 10.1056/NEJMoa054025. [DOI] [PubMed] [Google Scholar]
- 55.Lewerin C, Matousek M, Steen G, Johansson B, Steen B, Nilsson-Ehle H. Significant correlations of plasma homocysteine and serum methymalonic acid with movement and cognitive performance in elderly subjects but no improvement from short-term vitamin therapy: a placebo-controlled randomized study. Am J Clin Nutr. 2005;81:1155–62. doi: 10.1093/ajcn/81.5.1155. [DOI] [PubMed] [Google Scholar]
- 56.Stott DJ, MacIntosh G, Lowe GD, Rumley A, McMahon AD, Langhorne P, et al. Randomized controlled trial of homocysteine-lowering vitamin treatment in elderly patients with vascular disease. Am J Clin Nutr. 2005;82(6):1320–6. doi: 10.1093/ajcn/82.6.1320. [DOI] [PubMed] [Google Scholar]
- 57.Garcia A, Pulman K, Zanibbi K, Day A. Cobalamin reduces homocysteine in older adults on folic acid-fortified diet: pilot, double-blind, randomized, placebo-controlled trial. J Am Geriatr Soc. 2004;52(8):1410–2. doi: 10.1111/j.1532-5415.2004.52379_10.x. [DOI] [PubMed] [Google Scholar]
- 58.Hvas AM, Juul S, Lauritzen L, Nexo E, Ellegaard J. No effect of vitamin B-12 treatment on cognitive function and depression: a randomized placebo controlled study. J Affect Disord. 2004;81(3):269–73. doi: 10.1016/S0165-0327(03)00169-1. [DOI] [PubMed] [Google Scholar]
- 59.Clarke R, Harrison G, Richards S. Vital Trial Collaborative Group. Effect of vitamins and aspirin on markers of platelet activation, oxidative stress and homocysteine in people at high risk of dementia. J Intern Med. 2003;254(1):67–75. doi: 10.1046/j.1365-2796.2003.01154.x. [DOI] [PubMed] [Google Scholar]
- 60.Bryan J, Calvaresi E, Hughes D. Short-term folate, vitamin B12 or B6 supplementation slightly affects memory performance but not mood in women of various ages. J Nutr. 2002;132:1345–56. doi: 10.1093/jn/132.6.1345. [DOI] [PubMed] [Google Scholar]
- 61.Seal EC, Metz J, Flicker L, Melny J. A randomized, double-blind, placebo-controlled study of oral vitamin B12 supplementation in older patients with subnormal or borderline serum vitamin B12 concentrations. J Am Geriatr Soc. 2002;50:146–51. doi: 10.1046/j.1532-5415.2002.50020.x. [DOI] [PubMed] [Google Scholar]
- 62.Kwok T, Tang C, Woo J, Lai WK, Law LK, Pang CP. Randomized trial of the effect of supplementation on the cognitive function of older people with subnormal cobalamin levels. Int J Geriat Psychiatry. 1998;13:611–6. doi: 10.1002/(sici)1099-1166(199809)13:9<611::aid-gps832>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 63.de la Fourniere F, Ferry M, Cnockaert X. Vitamin B12 deficiency and dementia status: multicentre epidemiological study therapy. Sem Hop. 1997;73(5-6):133–40. [Google Scholar]
- 64.Kral VA, Solyom L, Enesco H, Ledwidge B. Relationship of vitamin B12 and folic acid to memory function. Bio Psychiatry. 1970;2(19-26) [PubMed] [Google Scholar]
- 65.Smith AD, Smith SM, de Jager CA, Whitbread P, Johnston C, Agacinski G, et al. Homocysteine-lowering by B vitamins slows the rate of accelerated brain atrophy in mild cognitive impairment: a randomized controlled trial. PLoS ONE [Electronic Resource] 2010;5(9):e12244. doi: 10.1371/journal.pone.0012244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Heneghan C. TrustTheEvidence.net: [[updated 2012; cited 2012 Dec 12]]. Vitamin B and slowing the rate of brain atrophy: the numbers don’t add up. [Internet] Available from: http://blogs.trusttheevidence.net/carl-heneghan/vitamin-b-and-slowing-the-rate-of-brain-atrophy-the-numbers-dont-add-up/100909109 . [Google Scholar]
- 67.Vidal-Alaball J, Butler CC, Cannings-John R, Goringe A, Hood K, McCaddon A, et al. Oral vitamin B12 versus intramuscular vitamin B12 for vitamin B12 deficiency. Cochrane Database Syst Rev. 2005. p. CD004655. [DOI] [PMC free article] [PubMed]
- 68.Kuzminski AM, Del Giacco EJ, Allen RH, Stabler SP, Lindenbaum J. Effective treatment of cobalamin deficiency with oral cobalamin. Blood. 1998;92(4):1191–8. [PubMed] [Google Scholar]
- 69.Bolaman Z, Kadikoylu G, Yukselen V, Yavasoglu I, Barutca S, Senturk T. Oral versus intramuscular cobalamin treatment in megaloblastic anemia: a single-center, prospective, randomized, open-label study. Clin Ther. 2003;25(12):3124–34. doi: 10.1016/s0149-2918(03)90096-8. [DOI] [PubMed] [Google Scholar]
- 70.Canadian Agency for Drugs and Technologies in Health. Oral versus injectable vitamin B12 supplementation: clinical and cost-effectiveness. Ottawa, ON: Canadian Agency for Drugs and Technologies in Health. 2007. p. 7 p. Canadian Agency for Drugs and Technologies in Health. Available from: http://www.cadth.ca/media/pdf/htis/Oral%20vs%20Injectable%20Vitamin%20B12%20Suppleme ntation%20Clinical%20and%20Cost%20Effectiveness.pdf .
- 71.Castelli MC, Friedman K, Sherry J, Brazzillo K, Genoble L, Bhargava P, et al. Comparing the efficacy and tolerability of a new daily oral vitamin B12 formulation and intermittent intramuscular vitamin B12 in normalizing low cobalamin levels: a randomized, open-label, parallel-group study. Clin Ther. 2011;33(3):358–71. doi: 10.1016/j.clinthera.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 72.Smith AD, Refsum H. Do we need to reconsider the desirable blood level of vitamin B12? J Intern Med. 2011;271:179–82. doi: 10.1111/j.1365-2796.2011.02485.x. [DOI] [PubMed] [Google Scholar]
- 73.McLean E, de Benoist B, Allen LH. Review of the magnitude of folate and vitamin B12 deficiencies worldwide. Food Nutr Bull. 2008;29(2):S38–S51. doi: 10.1177/15648265080292S107. [DOI] [PubMed] [Google Scholar]
- 74.Allen LH. How common is vitamin B12 deficiency? Am J Clin Nutr. 2009;89(2):693S–6S. doi: 10.3945/ajcn.2008.26947A. [DOI] [PubMed] [Google Scholar]
- 75.Tucker KL, Rich S, Rosenberg I, Jacques P, Dallal G, Wilson PWF, et al. Plasma vitamin B12 concentrations relate to intake source in the Framingham Offspring Study. Am J Clin Nutr. 2000;71(2):514–22. doi: 10.1093/ajcn/71.2.514. [DOI] [PubMed] [Google Scholar]
- 76.Metz J, McNeil AR, Levin M. The relationship between serum cobalamin concentration and mean red cell volume at varying concentrations of serum folate. Clin Lab Haem. 2004;26:323–5. doi: 10.1111/j.1365-2257.2004.00631.x. [DOI] [PubMed] [Google Scholar]
- 77.Canadian Agency for Drugs and Technologies in Health. Methylcobalamin ultra (vitamin B12) and vitamin C supplementation for the general population: clinical evidence. Ottawa, ON: Canadian Agency for Drugs and Technologies in Health. 2012. [[cited: 2012 Dec 12]]. Available from: http://www.cadth.ca/media/pdf/htis/sept-2012/RB0530%20Vitamins%20B12%20and%20C%20Final.pdf .
- 78.Tierney MS, Lermer MA. Computerized cognitive assessment in primary care to identify patients with suspected cognitive impairment. J Alzheimers Dis. 2010;20(3):823–32. doi: 10.3233/JAD-2010-091672. [DOI] [PubMed] [Google Scholar]
- 79.Martin-Khan M, Wootton R, Gray L. A systematic review of the reliability of screening for cognitive impairment in older adults by use of standardised assessment tools administered via the telephone. J Telemed Telecare. 2010;16(8):422–8. doi: 10.1258/jtt.2010.100209. [DOI] [PubMed] [Google Scholar]
- 80.Kaszas B, Kovacs N, Balas I, Kallai J, Aschemann Z, Kerekes Z, et al. Sensitivity and specificity of Addenbrooke’s Cognitive Examination, Mattis Dementia Rating Scale, Frontal Assessment Battery and Mini Mental State Examination for diagnosing dementia in Parkinson’s disease. Parkinsonism Relat Disord. 2012;18(5):553–6. doi: 10.1016/j.parkreldis.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 81.Stabler SP. Vitamin B12 deficiency. N Engl J Med. 2013;368(2):149–60. doi: 10.1056/NEJMcp1113996. [DOI] [PubMed] [Google Scholar]
- 82.Food and Nutrition Board. DRI Dietary reference intakes for thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, pantothenic acid, biotin, and choline. [[updated 1998; cited 2013 Jan 15]]. [Internet] Available from: http://www.nap.edu/openbook.php?record_id=6015&page=339 . [PubMed]


