Abstract
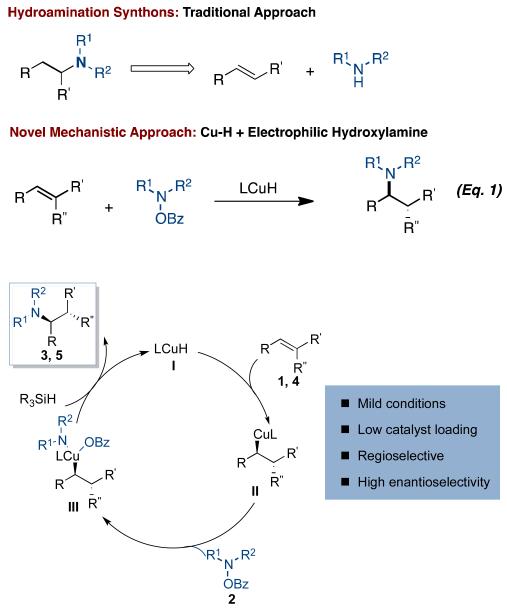
A highly enantio- and regioselective copper-catalyzed hydroamination reaction of alkenes has been developed using diethoxy(methyl)silane (DEMS) and esters of hydroxylamines. The process tolerates a wide variety of substituted styrenes, including trans-, cis-, and β,β-disubstituted styrenes to yield α–branched amines. In addition, aliphatic alkenes coupled to generate exclusively the anti-Markovnikov hydroamination products.
Hydroamination, the direct formation of a C-N bond by the formal addition of an amine to an alkene, is a powerful synthetic procedure with the potential to gain access to amine products which are widely featured in pharmaceutically active compounds.1 Although great progress has been made in the field of late transition metal-catalyzed hydroamination,2 several challenges still exist. For example, the intermolecular process requires activated alkenes such as vinyl arenes.2a,i,h or acrylic acid derivatives,2c while asymmetric variants are limited to the addition of aryl amines to simple β-unsubstituted styrene derivatives and achieve only moderate levels of enantiomeric excess.2a,3 In addition, there are limited methods available to obtain the anti-Markovnikov product in hydroamination reactions of aliphatic amines.4 Thus, there remains a need for the development of asymmetric hydroamination reactions that tolerate a wide variety of substitution patterns on the alkene component and proceed with high regio- and enantioselectivity.
Over the last decade, our laboratory has reported several examples of asymmetric reactions involving copper-hydride (CuH) intermediates.5a.e We postulated that this CuH strategy could serve as a platform for the hydroamination of alkenes (Eq. 1). In our approach for asymmetric intermolecular hydroamination, we propose that insertion of an alkene (1, 4) into a chiral ligand-bound LCu(I)H species (I) would form an alkyl-copper complex (II) (Figure 1).6 Sub-sequent oxidative addition of an electrophilic amine source, such as a hydroxylamine 2,7 followed by reductive elimination, would form the C-N bond enantioselectively. The copper (I) species generated would then undergo transmetalation with an external hydride-transfer reagent to reform I.10 This mechanism (Figure 1) comes in a straightforward manner from a combination of our previous work in two are-as.5a,11 Herein, we report a mild copper-catalyzed hydroamination strategy using a chiral copper catalyst with a broad substrate scope. We note that toward the end of our work, a paper describing a method similar to the first portion (asymmetric) of this chemistry by Hirano and Miura was reported. 2a
Figure 1.
Proposed catalytic cycle for CuH catalyzed hydroamination of alkenes
We began our investigation by attempting the hydroamination of styrene (1a) using readily available Cu(OAc)2 and easily accessible O-benzoylhydroxylamine 2a (Table 1). Various ligands and hydride-transfer reagents were tested. We were able to achieve the desired cross-coupled products in up to 74% ee using polymethylhydrosiloxane (PMHS) or diethoxymethylsilane (DEMS) in conjunction with the commercially available ligand BINAP (L1) (entries 2-3). DEMS generated the desired product in the highest yield (entry 3), and thus was chosen as the hydride transfer reagent of choice in the examination of other chiral ligands (entries 4-8). We were able to realize up to 97% ee when using (R)-DTBM-SEGPHOS (L5) as the ligand (entry 7). Further optimization revealed that the reaction proceeds with low catalyst loading (2 mol%) at 40 °C (entry 8), without diminishing the yield or enantioselectivity. The reaction exclusively generated an α-branched amine, which is consistent with the proposed catalytic cycle (Figure 1) because the hydride migration from the copper catalyst to the alkene would generate the more stable α-bond Cu species.12
Table 1. Reaction Optimization.
| Entry | Cu(OAc)2 | Hydride Reagent | L | Yield 4aa |
ee |
|---|---|---|---|---|---|
| 1 | 10 mol% | HBpin | L1 | 2% | ndb |
| 2 | 10 mol% | PMHS | L1 | 40% | 741% |
| 3 | 10 mol% | DEMS | L1 | 64% | 73% |
| 4 | 10 mol% | DEMS | L2 | 83% | 65>% |
| 5 | 10 mol% | DEMS | L3 | 99% | 95>% |
| 6 | 10 mol% | DEMS | L4 | 99% | 79% |
| 7 | 10 mol% | DEMS | L5 | 99% | 97% |
| 8c | 2 mol% | DEMS | L5 | 97% | 97% |
GC yields with dodecane as the internal standard.
Not determined.
Reaction was carried out at 40 °C.
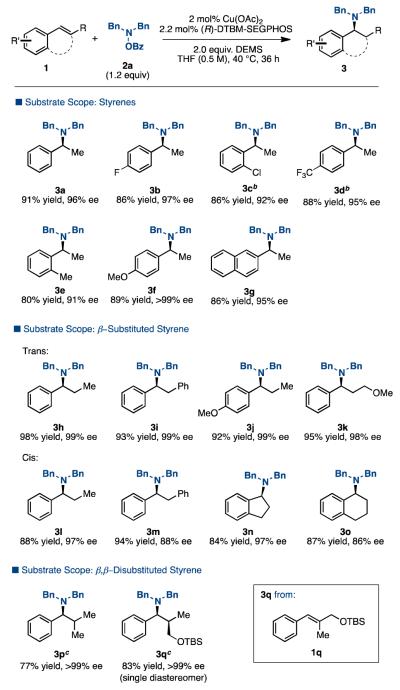
With an optimized protocol in hand, we then explored the substrate scope with respect to the styrene component (Table 2). This hydroamination tolerates a variety of substituents on the aryl ring of styrene (3b-g). The reaction also works efficiently with both trans- and cis-β-substituted styrenes (3h-o). Even hindered β,β-disubstituted styrenes undergo hydroamination in high yield and ee in this reaction (3p-q). Notably, the hydroamination of β,β-disubstituted styrene 1q gave the product 3q as a single diastereomer.
Table 2. Scope of Different Styrene Derivatives a.
|
lsolated yields (average of two runs). 2 (1 mmol), O-benzoyl-N,N-dibenzylhydroxylamine (1.2 mmol), Cu(OAc)2 (2 mol %), (R-DTBM-SEGPHOS (2.2 mol %), DEMS (2 mmol), THF (0.5 M), 40 °C, up to 36 h.
Cu(OAc)2 (4 mol %), (R-DTBM-SEGPHOS (4.4 mol %).
THF (1 M).
We next explored the use of other amine electrophiles in this reaction. We found that this reaction is applicable to several alkyl- and dialkyl-N-OBz amines (Table 3). N-(OBz)azepane and other heterocyclic-N-OBz amines also furnished the respective hydroamination products in high yields and enantioselectivities.
Table 3. Scope of Amine Electrophiles with Styrene a.
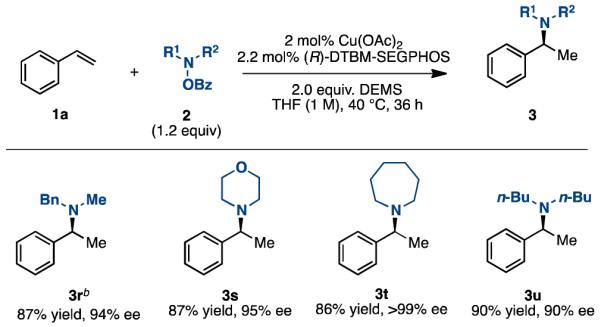
|
lsolated yields (average of two runs). 2 (1 mmol), hydroxyla-mine (1.2 mmol), Cu(OAc)2 (2 mol %), (R-DTBM-SEGPHOS (2.2 mol %), DEMS (2 mmol), THF (1 M), 40 °C, up to 36 h.
THF (0.5 M).
Since hydroamination of unactivated alkenes remains a challenge, we examined whether the developed protocol would be applicable with aliphatic alkenes.4b We found that terminal aliphatic alkenes could be effectively hydroaminated under the same conditions (Tables 4 and 5). In every case, the reaction exclusively produces the anti-Markovnikov products. Like the reaction with styrene, this protocol tolerated alkenes containing a primary alkyl bromide (5c), an epoxide (5g), and was compatible with alkenes containing a tosylamine (5d), an amide (5e), a pyridine (5f), a tert-butyldimethylsilyl ether (5i), and ones with geminal substituents (5h-i). Additionally, a number of amine electrophiles, including the sterically hindered tetramethylpiperidine N-OBz (5m), cross-coupled efficiently. Our hypothesis for the observed selectivity for the anti-Markovnikov products is that the hydride migration from the copper catalyst proceeds to form the less sterically crowded terminal copper intermediate (Scheme 1); here there is no electronic advantage as for styrenes to form the 2°-alkyl-Cu intermediate. Oxidative addition of the hydroxylamine and subsequent reductive elimination would generate the un-branched tertiary amines.
Table 4. Hydroamination of Terminal Aliphatic Al-kenesa.
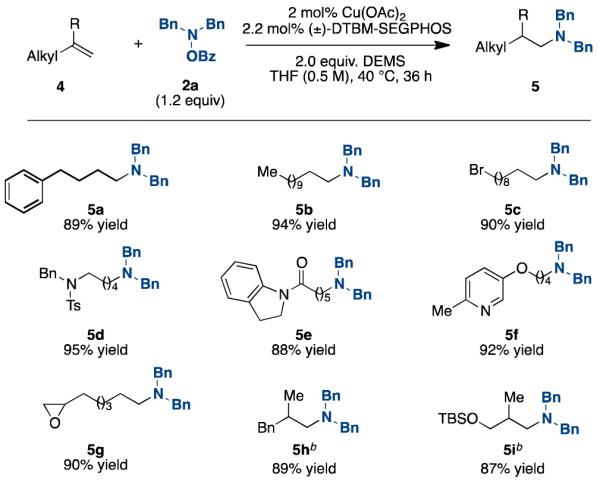
|
Isolated yields (average of two runs). 2 (1 mmol), O-benzoyl-N,N-dibenzylhydroxylamine (1.2 mmol), Cu(OAc)2 (2 mol %), (±)-DTBM-SEGPHOS (2.2 mol %), DEMS (2 mmol), THF (0.5 M), 40 °C, up to 36 h.
THF (1 M).
Table 5. Scope of Amine Electrophiles with 4-Phenyl-1-butenea.
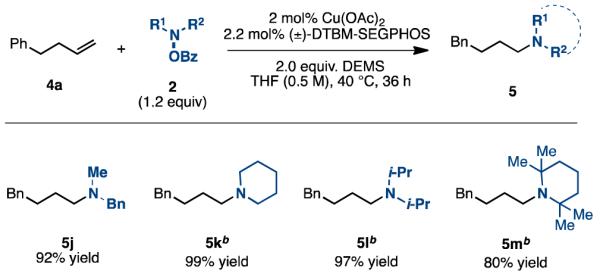
|
Isolated yields (average of two runs). 2 (1 mmol), hydroxyla-mine (1.2 mmol), Cu(OAc)2 (2 mol %), (±)-DTBM-SEGPHOS (2.2 mol %), DEMS (2 mmol), THF (0.5 M), 40 °C, up to 36 h.
Cu(OAc)2 (4 mol %), (±)-DTBM-SEGPHOS (4.4 mol %) were used.
As a demonstration of the robustness and practicality of this method, it was carried out at 10 mmol scale (Scheme 2) using the β-substituted styrene ((E)-(3-methoxyprop-1-en-1-yl)benzene) as β-substituted styrenes are known to be difficult substrates in asymmetric hydroamination reactions.3 We were able to lower the catalyst loading to 1 mol% with no decrease in the yield or enantioselectivity.
Scheme 1.
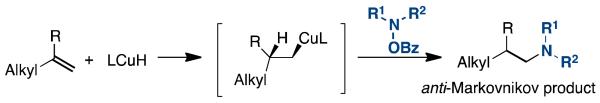
Anti-Markovnikov Hydroamination of Aliphatic Alkene
In summary, we have reported a mild method for synthesizing chiral tertiary amines by employing an asymmetric copper-catalyzed hydroamination. Substitution occurs in a regioselective manner to generate a C-N bond at the α-position of styrene derivatives. This method has been shown to be compatible with various substituted styrene derivatives, and styrenes with β–substitution. Additionally, this method allows the development of copper-catalyzed anti-Markovnikov hydroaminations of terminal aliphatic alkenes. We are currently investigating the asymmetric version of internal aliphatic alkene hydroamination, which will be reported in due course.
Supplementary Material
Scheme 2.
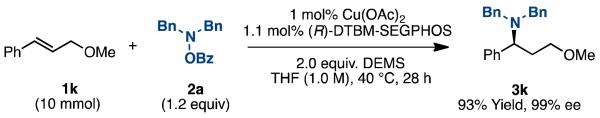
Large-scale Hydroamination Reaction of β-substituted Styrene
ACKNOWLEDGMENT
Research reported in this publication was supported by the National Institutes of Health under award number GM58160. We would like to thank Phillip J. Milner and Dr. Daniel T. Cohen for their advice in the preparation of this manuscript.
Footnotes
ASSOCIATED CONTENT
Experimental procedures and characterization data for all compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.Severin R, Doye S. Chem. Soc. Rev. 2007;36:1407. doi: 10.1039/b600981f. [DOI] [PubMed] [Google Scholar]
- 2.(a) Miki Y, Hirano K, Satoh T, Miura M. Angew. Chem. Int. Ed. 2013;52 doi: 10.1002/anie.201304365. doi: 10.1002/anie.201304365. [DOI] [PubMed] [Google Scholar]; (b) Pawlas J, Nakao Y, Kawatsura M, Hartwig JF. J. Am. Chem. Soc. 2002;124:3669. doi: 10.1021/ja017575w. [DOI] [PubMed] [Google Scholar]; (c) Kawatsura M, Hartwig JF. Organometallics. 2001;20:1960. [Google Scholar]; (d) Senn HM, Blochl PE, Togni A. J. Am. Chem. Soc. 2000;122:4098. [Google Scholar]; (e) Muller TE, Beller M. Chem. Rev. 1998;98:675. doi: 10.1021/cr960433d. [DOI] [PubMed] [Google Scholar]; (f) Muller TE, Hultzch KC, Yus M, Foubelo F, Tada M. Chem. Rev. 2006;108:3795. doi: 10.1021/cr0306788. [DOI] [PubMed] [Google Scholar]; (g) Dorta R;, Egli P, Zurcher F, Togni A. J. Am. Chem. Soc. 1997;119:10857. [Google Scholar]; (h) Casalnuovo AL, Calabrese JC, Milstein D. J. Am. Chem. Soc. 1988;110:6738. [Google Scholar]; (i) Zhou J, Hartwig JF. J. Am. Chem. Soc. 2008;130:12220. doi: 10.1021/ja803523z. [DOI] [PubMed] [Google Scholar]; (j) Lober O, Kawatsura M, Hartwig JF. J. Am. Chem. Soc. 2001;123:4366. doi: 10.1021/ja005881o. [DOI] [PubMed] [Google Scholar]; (k) Li K, Horton PN, Hursthouse MB, Hii KK. J. Organomet. Chem. 2003;665:250. [Google Scholar]; (l) Hu A, Ogasawara M, Sakamoto T, Okada A;, Nakajima K, Takahashi T, Lin W. Adv. Synth. Catal. 2006;348:2051. [Google Scholar]; (m) Nettekoven U, Hartwig JF. J. Am. Chem. Soc. 2002;124:1166. doi: 10.1021/ja017521m. [DOI] [PubMed] [Google Scholar]
- 3.Utsunomiya M, Kuwano R, Kawatsura M, Hartwig JF. J. Am. Chem. Soc. 2003;125:5608. doi: 10.1021/ja0293608. Kawatsura M, Hartwig JF. J. Am. Chem. Soc. 2000;122:9546. (c) The work of Miura (see ref. 2a), which was reported concurrent to the preparation to this manuscript, was successful in the asymmetric hydroamination of β–substituted styrenes.
- 4.Nobis M, Drieβen.Hölscher B. Angew. Chem. Int. Ed. 2001;40:3983. doi: 10.1002/1521-3773(20011105)40:21<3983::aid-anie3983>3.0.co;2-8. (b) Lalic has described an elegant Cu-catalyzed hydroboration/transmetallation sequence to primary amines: Rucker RP, Whittaker AM, Dang H, Lalic G. J. Am. Chem. Soc. 2012;134:6571. doi: 10.1021/ja3023829.
- 5.Appella DH, Moritani Y, Shintani R, Ferreira EM, Buchwald SL. J. Am. Chem. Soc. 1999;121:9473. Moritani Y, Appella DH, Jurkauskas V, Buchwald SL. J. Am. Chem. Soc. 2000;122:6797. Yun J, Buchwald SL. Org. Lett. 2001;3:1129. doi: 10.1021/ol015577f. Jurkauskas V, Buchwald SL. J. Am. Chem. Soc. 2002;124:2892. doi: 10.1021/ja025603k. Rainka MP, Aye Y, Buchwald SL. Proc. Natl. Ac. Sci. U.S.A. 2004;101:5821. doi: 10.1073/pnas.0307764101. For other examples of Cu.catalyzed asymmetric hydrogenation reactions of vinyl heteroarenes (See ref. 5f-g). Rupnicki L, Saxena A, Lam HW. J. Am. Chem. Soc. 2009;131:10386. doi: 10.1021/ja904365h. Saxena A, Choi B, Lam HW. J. Am. Chem. Soc. 2012;134:8428. doi: 10.1021/ja3036916.
- 6.Similar Cu.catalyzed reactions invoking CuH intermediacy has been reported for asymmetric hydroboration of styrenes: Who D, Chea H, Ju J, Yun J. Angew. Chem. Int. Ed. 2009;48:6062. doi: 10.1002/anie.200902015. Feng X, Jeon H, Yun J. Angew. Chem. Int. Ed. 2013;52:3989. doi: 10.1002/anie.201208610. Grigg RD, Hoveln, Schomaker JM. J. Am. Chem. Soc. 2012;134:16131. doi: 10.1021/ja306446m. Sakae R, Hirano K, Satoh T, Miura M. Chem. Lett. doi:10.1246/cl.130485.
- 7.(a) We initially selected O-benzoyl protected secondary hydroxylamine electrophiles due to their previous demonstration as effective electrophiles in copper-catalysis (ref. 6b-d). Berman AM, Johnson JS. J. Am. Chem. Soc. 2004;126:5680. doi: 10.1021/ja049474e. Berman AM, Johnson JS. J. Org. Chem. 2006;71:219. doi: 10.1021/jo051999h. Campbell M, Johnson JS. Org. Lett. 2007;9:1521. doi: 10.1021/ol0702829. (e) Other amine electrophiles previously demonstrated in transition-metal catalyzed cross-coupling reactions include O-sulfonyl protected secondary hydroxylamines (ref. 8) and secondary haloamines (ref. 9). See ref. 7f for a review on various electrophilic amine sources, and ref. 7g a review specific to transition-metal catalyzed system. Erdik E, Ay M. Chem. Rev. 1989;1947;89 Barker TJ, Jarvo ER. Synthesis. 2011;24:3958. Use of copper intermediates to form amine products: Matsuda N, Hirano K, Satoh T, Miura M. Angew. Chem. Int. Ed. 2012;51:3642. doi: 10.1002/anie.201108773. Rucker RP, Whittaker AM, Dang H, Lalic G. Angew. Chem. Int. Ed. 2012;51:3953. doi: 10.1002/anie.201200480. Matsuda N, Hirano K, Satoh T, Miura M. J. Am. Chem. Soc. 2013;135:4934. doi: 10.1021/ja4007645.
- 8.Boche G, Mayer N, Bernheim M, Wagner K. Angew. Chem. Int. Ed. 1978;17:687. [Google Scholar]
- 9.(a) Kovacic P, Lowry MK, Field KW. Chem. Rev. 1970;70:639. [Google Scholar]; (b) He C, Chen C, Cheng J, Liu C, Liu W, Li Q, Lei A. Angew. Chem. Int. Ed. 2008;47:6414. doi: 10.1002/anie.200801427. [DOI] [PubMed] [Google Scholar]; (c) Barker TJ, Jarvo ER. J. Am. Chem. Soc. 2009;131:15598. doi: 10.1021/ja907038b. [DOI] [PubMed] [Google Scholar]
- 10.The catalytic pathway we described here is different from the one reported in the hydroamination of aliphatic alkene using 9.BBN.H (see ref. 4b). We proposed the intermediacy of Cu.H as a species that transfers the hydride onto the alkene.
- 11.Strieter ER, Bhayana B, Buchwald SL. J. Am. Chem. Soc. 2009;131:78–88. doi: 10.1021/ja0781893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.For calculations on the regiochemistry of insertion reaction of alkenes with LCu(boryl): Dang L, Zhao H, Lin Z, Marder TB. Organometallics. 2007;26:2824.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.