Summary
Present medications for epilepsy have substantial limitations, such as medical intractability in many patients and lack of antiepileptogenic properties to prevent epilepsy. Drugs with novel mechanisms of action are needed to overcome these limitations. The mammalian target of rapamycin (mTOR) signaling pathway has emerged as a possible therapeutic target for epilepsy. Preliminary clinical trials suggest that mTOR inhibitors reduce seizures in tuberous sclerosis complex (TSC) patients with intractable epilepsy. Furthermore, mTOR inhibitors have antiepileptogenic properties in preventing epilepsy in animal models of TSC. Besides TSC, accumulating preclinical data suggest that mTOR inhibitors may have antiseizure or antiepileptogenic actions in other types of epilepsy, including infantile spasms, neonatal hypoxic seizures, absence epilepsy, and acquired temporal lobe epilepsy following brain injury, but these effects depend on a number of conditions. Future clinical and basic research is needed to establish whether mTOR inhibitors are an effective treatment for epilepsy.
Keywords: seizures, epilepsy, epileptogenesis, mTOR, rapamycin, everolimus, tuberous sclerosis, traumatic brain injury
Introduction
Epilepsy represents a chronic neurological disorder involving recurrent seizures and caused by a large variety of genetic and acquired etiologies. While a variety of medical and non-medical treatments exist, the first line therapy for epilepsy typically is medication. Over twenty drugs are presently approved for treating epilepsy. Despite a large number of options, there are a couple significant limitations to available medications. Although many epilepsy patients are seizure-free with medication, about a third of patients still experience seizures despite treatment and have medically-intractable epilepsy [1]. In addition, even when seizures are controlled with medication, currently available drugs are generally believed to be only symptomatic therapy in suppressing seizures (antiseizure or anticonvulsant). There is minimal evidence that these medications have disease-modifying properties for preventing or slowing the development of epilepsy (antiepileptogenic) [2]. Thus, novel treatments need to be developed to address the problems of medical intractability and the lack of disease-modifying therapies in epilepsy.
The successful development of better medications for epilepsy will likely involve targeting mechanisms of action that are novel and different compared with existing therapies [3,4]. Current medications directly decrease neuronal excitability primarily by modulating ion channels and neurotransmitter receptors. For example, many established seizure medications antagonize sodium channels, such as phenytoin and carbamazepine, or potentiate GABAa receptors, such as phenobarbital and benzodiazepines. The mechanisms that cause medical intractability are not completely understood, but clearly result in resistance to the conventional actions of seizure medications on neuronal excitability [1]. Furthermore, it is not surprising that mechanisms that are involved in the initial development of the epileptic state, or epileptogenesis, are distinct from mechanisms of increased neuronal excitability that directly stimulate ongoing seizures.
Rather than targeting the end-stage mechanisms that directly control neuronal excitability, a rational strategy for addressing the issues of medical intractability and disease modification is to modulate initial signaling pathways that activate downstream mechanisms involved in epileptogenesis and seizure generation. The mammalian target of rapamycin (mTOR) pathway has emerged as a signaling mechanism involved in epileptogenic processes and as a potential novel target for epilepsy treatments. While there have been other summaries of this topic [5–9], this Expert Review will critically analyze the most recent evidence from animal models and initial clinical trials for both potential antiseizure and antiepileptogenic effects of mTOR inhibitors.
The mTOR Pathway under Physiological Conditions
The mTOR protein is a protein kinase with an interesting history leading up to its initial discovery. In the 1960’s, a Canadian scientific expedition explored the famous Easter Island, best known for its hillside Moa statues. Analysis of soil samples taken from Easter island were subsequently found to contain a bacteria that produced a novel antifungal agent, which was named rapamycin after the Polynesian name for the island, Rapa Nui. This drug was also found to have potent immunosuppressive properties and was eventually developed and approved as an immunosuppressant drug for kidney transplant patients in 1999. While initially the mechanism of action of rapamycin was not known, rapamycin was found to inhibit a specific protein kinase, which was aptly named target of rapamycin (TOR). TOR is strongly conserved across species, including a mammalian form, mTOR.
mTOR represents a common protein kinase implicated in a large variety of key physiological functions, including regulation of cellular growth, proliferation, apoptosis, autophagy, metabolism, and cytoskeleton [10,11]. mTOR is part of two main signaling complexes, mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2). mTORC1 is made up of the catalytic subunit mTOR itself, as well as the regulatory protein, raptor, and additional binding proteins, and is inhibited by rapamycin. mTORC2 consists of mTOR, the regulatory protein rictor, and accessory binding proteins, and is relatively insensitive to rapamycin, although it can be inhibited with prolonged exposure to rapamycin [12]. mTORC1 regulates cell growth and metabolism, as well as other physiological functions, primarily by modulating protein synthesis via activation of a couple of downstream pathways: S6 kinase and ribosomal S6, involved in ribosomal biosynthesis, and 4EBP1/eIF4E, translation initiating factors. mTORC2 regulates cell survival, metabolism and structure through modulation of other downstream protein kinases and cytoskeletal elements.
Many functions of the mTOR pathway involve responding to environmental stimuli or signals via regulation of mTOR by upstream signaling pathways (Fig. 1). For example, in anabolic states with growth factor or nutrient surplus, the phosphoinositide-3 kinase (PI3K)/Akt pathway activates the mTOR pathway, which stimulates metabolism and cell growth. By comparison, in catabolic states of nutrient or energy shortage, other pathways, such as the LKB1/AMPK pathway, may inhibit mTOR activity resulting in reduced metabolism and cell growth.
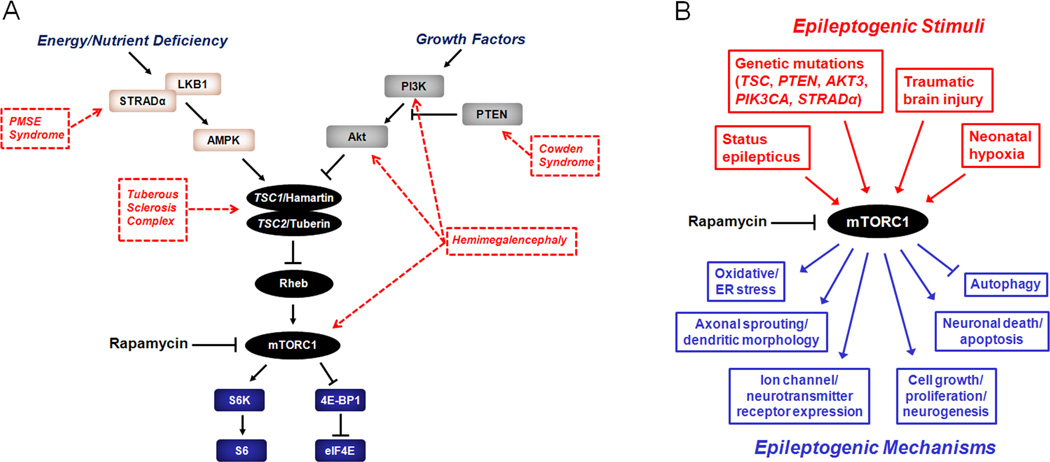
Figure 1.
The mTOR pathway and epilepsy. A. The mTOR pathway involves two complexes, the rapamycin-sensitive, mTORC1, and the relatively rapamycin-insensitive, mTORC2 (not shown), is regulated by a number of upstream signaling pathways, and activates a series of downstream effectors. Upstream regulators (PI3K/Akt, LKB1/AMPK) modulate the mTOR pathway in response to various physiological stimuli involved in energy, nutrient, and growth regulation. In turn, mTORC1 stimulates downstream signaling mechanisms primarily involved in ribosomal biogenesis (S6K, S6) and protein translation (4E-BP1, eIF4E). Genetic mutations in different components of the mTOR pathway have been identified that cause hyperactivation of mTORC1 and may lead to various disease phenotypes, including epilepsy. B. The mTOR pathway may coordinate multiple mechanisms of epileptogenesis due to diverse causes of epilepsy. mTORC1 may be abnormally activated by a variety of genetic defects or acquired injuries. In turn, mTORC1 hyperactivation may trigger multiple downstream mechanisms of epileptogenesis via regulation of protein synthesis and other cellular processes, such as expression of ion channels, axonal sprouting, oxidative stress, autophagy, neuronal death, and neurogenesis. Inhibition of mTORC1 may represent a rational therapy for multiple types of epilepsy involving abnormal mTOR pathway activation. AMPK - 5' adenosine monophosphate-activated protein kinase; eIF4E, elongation initiation factor 4E; LKB1 - liver kinase B1; mTORC1 - mammalian target of rapamycin complex 1; PI3K - phosphoinositide-3 kinase; PTEN - phosphatase and tensin homolog on chromosome 10; Rheb - Ras homolog enriched in brain; STRADα - STE20-related kinase adapter alpha; S6 - ribosomal protein S6; S6K - ribosomal S6 kinase; TSC1 - tuberous sclerosis complex 1 protein; TSC2 - tuberous sclerosis complex 2 protein; 4E-BP1 - elongation factor 4E binding protein 1.
Although mTOR is involved in a range of functions throughout the body, several important brain-specific roles of mTOR have been identified. Given mTOR’s general role in cell growth and proliferation, mTOR is centrally involved in normal brain development. Similarly, mTOR is critical for mechanisms of synaptic plasticity and learning [13,14], as well as dendritic and axonal morphology of neurons [15–17]. mTOR may also regulate neuronal excitability and signaling via modulation of the expression of ion channels and neurotransmitter receptors [18, 19]. Overall, the large variety of physiological functions of mTOR in the brain make the mTOR pathway a logical candidate for mediating pathophysiological mechanisms of specific neurological diseases, including epilepsy.
The mTOR Pathway under Pathological Conditions
Dysfunction of the mTOR pathway is implicated in the pathophysiology of a number of neurological and non-neurological disorders, ranging from cancer and diabetes to neurodegenerative diseases [10,20–22]. The prototypic disorder featuring dysregulated mTOR signaling is the genetic disease, tuberous sclerosis complex (TSC). TSC is an autosomal dominant disorder involving the development of tumors or hamartomas in various organs throughout the body, including cortical malformations, called tubers, and tumors, in particular subependymal giant cell astrocytomas (SEGAs), in the brain [23,24]. The clinical manifestations of TSC may vary widely, depending on the organs affected. However, neurological involvement typically constitutes the most disabling symptoms in TSC, including refractory epilepsy, autism, and mental retardation. Mutations in two distinct genes, TSC1 and TSC2, cause TSC. TSC1 and TSC2 produce the proteins hamartin and tuberin, respectively. Hamartin and tuberin bind together to form a complex, which inhibits mTOR. Thus, mutation of either TSC1 or TSC2 results in disinhibition of the mTOR pathway. Abnormal activation of the mTOR pathway can stimulate excessive cell proliferation and growth, which promotes tumorigenesis in TSC patients. The discovery of the mechanistic link between mTOR and the TSC genes immediately suggested the potential of rapamycin as a treatment for TSC. Within the last several years since this discovery, clinical trials have demonstrated that mTOR inhibitors reduce tumor growth in TSC, and the mTOR inhibitor, everolimus, has now been approved by the United States Food and Drug Administration for treating SEGAs and kidney tumors in TSC patients [25–28].
mTOR pathway dysregulation represents a rational mechanistic basis for brain tumors and possibly cortical tubers in TSC. Other malformations of cortical development share similar histopathological and molecular features as TSC, including disordered cortical lamination and cytomegalic immature cells, leading to the hypothesis that abnormal mTOR signaling could represent a shared pathophysiological mechanism [29–31]. In fact, recent clinical studies have provided evidence that a group of related developmental structural lesions of the brain have defects in various upstream or downstream aspects of mTOR signaling (Fig. 1A). Hemimegalencephaly, a severe cortical malformation characterized by overgrowth, disorganized lamination, and enlarged cells involving much of one cerebral hemisphere, has been associated with somatic mutations in different elements of the PI3K/AKT/mTOR pathway [32,33]. Polyhydramnios, megalencephaly, and symptomatic epilepsy (PMSE) syndrome, is caused by mutations in the STRADα gene, which results in dysregulated mTOR signaling due a decrease in the inhibitory upstream LKB1/AMPK pathway [34]. Finally, although definitive pathogenic mutations have yet not been established, isolated focal cortical dysplasias or related neoplastic brain lesions, such as gangliogliomas and dysembryoplastic neuroepithelial tumors, also exhibit abnormalities in mTOR signaling elements [35–39]. Thus, this group of related developmental brain malformations and tumors appear to share an underlying molecular pathogenesis involving the mTOR pathway and have collectively been referred to as “TORopathies” [29–31]
A common clinical feature of these developmental brain disorders is the frequent occurrence of intractable epilepsy, suggesting that mTOR could be a central mechanism involved in epileptogenesis. Many physiological functions of the mTOR pathway, such as regulation of synaptic plasticity, cellular growth, apoptosis, and expression of ion channels and other proteins related to neuronal excitability, could promote seizures under pathological conditions (Fig. 1B). In addition to cortical malformations, the widespread functions of mTOR in the brain also make it a rationale candidate for influencing mechanisms of acquired epilepsies, such as due to head trauma, stroke, or other injuries to the brain. The availability of rapamycin and other mTOR inhibitors represents a powerful tool for testing the role of the mTOR pathway in models of epilepsy and ultimately may represent novel antiseizure or antiepileptogenic treatments for different types of epilepsy. In the following two sections, evidence will be reviewed that mTOR signaling contributes to various mechanisms of epilepsy and that mTOR inhibitors have either antiseizure (effective in reducing or eliminating seizures in patients with established epilepsy) (Table 1) or antiepileptogenic effects (effective in preventing the development of epilepsy in patients at risk but who have never had a seizure) (Table 2).
Table 1.
Potential Antiseizure Effects of mTOR Inhibitors in Animal Models and Clinical Studies
| Epilepsy Type/Model | Effect of mTOR Inhibitor on Seizures | Proposed Mechanism(s) of Action | References |
|---|---|---|---|
| Animal Models | |||
| Acute Anticonvulsant Effects | |||
| Maximal electroshock threshold | Increase in seizure threshold 3–6 hr after a single dose, but not 3 daily doses, of rapamycin in 4 wk old mice | Unknown | 43 |
| 6 Hz stimulation test | No effect in 4 wk old mice | n/a | 43 |
| Pentylenetetrazole (PTZ) seizures | No effect in 4 wk old mice or adult rats | n/a | 43–45 |
| Increase in seizure latency 4 hr after a single dose, but not 3 daily doses, of rapamycin in 15 d old rats | Decreased neuropeptide Y expression | 44 | |
| Paradoxical decrease in seizure threshold after 3 daily doses of rapamycin in 3–4 wk old, but not adult, rats | Reduction in KCC2 expression | 45 | |
| Fluorothyl-induced seizures | Increase in seizure threshold 4 hr after a single dose of rapamycin in 15 d old and adult rats | Decreased neuropeptide Y expression | 44 |
| Kainate-induced acute seizures | Reduction in seizure severity after 3 daily doses of rapamycin and 90 min after kainate in 4 wk old mice | Unknown | 43 |
| No effect in 15 d old, 3–4 wk old, and adult rats | n/a | 44,45 | |
| Pilocarpine-induced acute seizures | Paradoxical increase in seizure severity after 3 daily doses of rapamycin in 3–4 wk old, but not adult, rats | Reduction in KCC2 expression | 45 |
| Multiple-hit model of infantile spasms | Reduction in acutely-induced spasms in rats. | Unknown | 46 |
| NMDA-induced infantile spasms | No effect on acutely-induced spasms in rats. | n/a | 44,47 |
| TSC-related Epilepsy | |||
| Tuberous sclerosis complex knock-out mice | Reduction in chronic seizure frequency in Tsc1 KO mice after the onset of epilepsy | Inhibition of cell growth/proliferation, restored astrocyte glutamate transport. | 48 |
| Pten knock-out mice | Reduction in chronic seizure frequency and duration in Pten KO mice after the onset of epilepsy | Decreased megalencephaly, cell size | 49–52 |
| Non-TSC-related Epilepsy | |||
| WAG/Rij rat model of genetic absence epilepsy | Reduction in frequency of spike-wave discharges within 30 minutes of single dose of rapamycin in adult WAG/Rij rats. | Unknown | 56 |
| Temporal lobe epilepsy following pilocarpine status epilepticus | Reduction in chronic spontaneous seizure frequency in rats following status epilepticus | Inhibition of mossy fiber sprouting | 57 |
| Clinical Studies | |||
| Tuberous sclerosis complex | Reduction in seizure frequency in TSC patients with intractable epilepsy. | Unknown | 26,53–55 |
Table 2.
Potential Antiepileptogenic Effects of mTOR Inhibitors in Animal Models
| Epilepsy Type/Model | Effect on Epilepsy | Proposed Mechanism(s) of Action | References |
|---|---|---|---|
| TSC-related Epilepsy | |||
| Tuberous sclerosis complex knock-out mice | Prevention of epilepsy in Tsc KO mice when initiated prior to onset of seizures | Inhibition of cell growth/proliferation, restored astrocyte glutamate transport, decreased inflammation/ER stress, restored myelination. | 48,59–63 |
| Temporal Lobe Epilepsy | |||
| Kainate status epilepticus model of temporal lobe epilepsy | Reduction in frequency of spontaneous seizures in rats following status epilepticus | Inhibition of mossy fiber sprouting | 69 |
| Pilocarpine status epilepticus model of temporal lobe epilepsy | Reduction in mossy fiber sprouting, but no effect on spontaneous seizures in mice | Inhibition of mossy fiber sprouting | 66,71 |
| Angular bundle electrical stimulation model of temporal lobe epilepsy | Reduction in frequency of spontaneous seizures in rats following status epilepticus | Inhibition of mossy fiber sprouting, reduction in neuronal death, decreased blood-brain barrier leakage | 70 |
| Amydala electrical stimulation model of temporal lobe epilepsy | No effect on spontaneous seizures in rats following status epilepticus | No effect on mossy fiber sprouting | 72 |
| Other Types of Epilepsy | |||
| Neonatal hypoxia | Reduction in chronic seizures in rats following hypoxic neonatal seizures. | Inhibition of enhanced glutamate EPSCs | 74 |
| WAG/Rij rat model of genetic absence epilepsy | Reduction in frequency of spike-wave discharges at 6 and 10 months of age after treatment with rapamycin from 45 d to 5 mo in WAG/Rij rats. | Unknown | 56 |
| Controlled cortical impact injury model of posttraumatic epilepsy | Reduction in frequency of spontaneous seizures in mice following controlled cortical impact injury | Inhibition of mossy fiber sprouting, neuronal death | 78 |
| Organotypic slice culture injury model of posttraumatic epilepsy | Reduction in electrographic seizures in organotypic slice cultures | Inhibition of axonal sprouting, neuronal death | 79 |
Potential Antiseizure Effects of mTOR Inhibitors
Existing seizure medications act primarily by directly inhibiting neuronal excitability through modulation of neurotransmitter receptors or ion channels, such as GABAA receptors or voltage-gated sodium channels. These medications predominantly suppress seizures, but they don’t seem to reverse the underlying mechanisms of epileptogenesis that originally cause epilepsy. By comparison, mTOR inhibitors seem not to work like conventional seizure medications. For example, with direct administration to neurons, rapamycin has very limited or no acute effect on the electrical properties of neurons in vitro [40,41]. There have been no established reports that mTOR inhibitors directly bind ion channels or neurotransmitter receptors like many existing seizure medications. On the other hand, modulation of the mTOR pathway could potentially influence neuronal excitability indirectly by regulating protein synthesis of specific ion channels or other proteins controlling neuronal signaling. For instance, the mTOR pathway can modulate the expression of potassium channels and glutamate receptors [18,19]. Furthermore, mTOR is also involved in mechanisms of synaptic structure and plasticity [14–16], which could lead to abnormal neuronal signaling under pathological conditions. Thus, mTOR inhibitors do have the potential to affect neuronal excitability and exert therapeutic effects against seizures (Table 1).
Acute Anticonvulsant Effects of mTOR Inhibitors
Many current seizure medications were initially discovered via screening for anticonvulsant properties in standard preclinical rodent models of seizures acutely provoked by electrical stimulation or convulsant drugs [42]. A few recent studies have similarly assessed the anticonvulsant efficacy of rapamycin in a battery of these acute seizure models. In electrical stimulation models in 4 week old mice, rapamycin was found to increase seizure threshold in the maximal electroshock threshold (MES-T) test within 3–6 hours after a single rapamycin injection, but not after three consecutive days of rapamycin; also, single or repetitive rapamycin treatment had no effect in the 6 Hz stimulation test [43]. In chemical convulsant models, rapamycin had no effect on pentylenetetrazole (PTZ)-induced seizures and had limited mixed effects on acute kainate-induced seizures in 4 week old mice, causing a decrease in seizure latency after one dose of rapamycin but decreasing seizure severity after 3 consecutive daily doses of rapamycin [43]. A second study compared effects of rapamycin on immature (postnatal day 15) and adult (~8 week old) rats in several acute pharmacological seizure models. Rapamycin had limited anticonvulsant effects against flurothyl-induced seizures in both immature and adult rats, which were dependent on the timing and number of doses of rapamycin administration [44]. While rapamycin had no effect in adult rats in the PTZ model, it had an acute anticonvulsant effect against PTZ-induced tonic-clonic seizures in immature rats, which again was dependent on the duration of pretreatment [44]. In contrast, another study also found no acute effects of rapamycin on PTZ, pilocarpine, and kainate-induced seizures in adult rats, but reported a paradoxical exacerbation of seizure susceptibility or severity in the PTZ and pilocarpine models in younger (3–4 weeks) rats [45]. Finally, rapamycin has also been tested in a couple pharmacological models of infantile spasms. In the severe multiple-hit model of symptomatic infantile spasms, rapamycin inhibited spasms and also decreased cognitive deficits [46], but in another model, rapamycin had no effect on NMDA-induced flexor spasms [47].
Thus, overall, rapamycin may have limited, variable effects on acutely-induced seizures, which are dependent on a number of factors, including the age of the animal, the timing of the treatment paradigm, and the seizure model. When acute effects of rapamycin have been observed, they have usually occurred in immature animals. The mechanisms of the anticonvulsant effects of rapamycin are largely unknown. Based on the differences in effects with single versus repetitive doses of rapamycin, there does not appear to be a direct correlation with anticonvulsant properties and the suppression of mTOR activity [43]. One study has correlated the anticonvulsant effects of rapamycin in immature rats with decreased neuropeptide Y expression [44]. Again, there is minimal evidence that rapamycin binds to or directly modulates ion channels or neurotransmitter receptors like many traditional anticonvulsant drugs, but mTOR inhibitors could indirectly affect neuronal excitability by regulating mTOR-dependent protein synthesis and expression of molecules that mediate neuronal signaling.
Antiseizure Effects of mTOR Inhibitors in TSC-Related Epilepsy
While the use of acute seizure models has been successful in identifying many effective anticonvulsant agents, these models have a number of limitations and do not closely mimic the epileptic brain, as they acutely induce seizures in non-epileptic animals. Testing the efficacy of drugs on chronic epilepsy models in animals with spontaneous seizures secondary to a preexisting genetic defect or acquired brain injury likely represents a more clinically-relevant approach and may promote discovery of drugs with more diverse mechanisms of action. In this regard, the potential antiseizure effects of mTOR inhibitors have been tested in a variety of animal models of genetic and acquired epilepsy.
Among the wide variety of types and causes of epilepsy and a corresponding range of animal models of epilepsy, the most rational choice for testing effects of mTOR inhibitors would be in epilepsies with genetic defects causing abnormally increased mTOR activity. Multiple animal models of TSC have been generated, involving inactivation of the Tsc1 or Tsc2 gene in different subtypes of brain cells, including neurons, glia or progenitor cells. While much focus has been placed on potential antiepileptogenic actions of early treatment with mTOR inhibitors for preventing epilepsy in these models (see next section), late treatment with rapamycin has been found to decrease seizure frequency in mice with established established epilepsy in at least one of these TSC models [48]. Furthermore, other animal models involving increased mTOR activity due to deletion of the Pten gene, an upstream modulator of the mTOR pathway, also have severe epilepsy. mTOR inhibitors reduce the frequency of epileptiform abnormalities and seizures of Pten knock-out mice [49–52]. While the downstream mechanisms responsible for these effects have not been proven, these preclinical studies demonstrate that mTOR inhibitors can effectively treat seizures in mice with established epilepsy related to mTOR pathway hyperactivation from a genetic defect.
The possible clinical applications of mTOR inhibitors for treating seizures in TSC patients have started to be investigated. Case reports of TSC patients with epilepsy have demonstrated a reduction in seizures after initiation of an mTOR inhibitor for treating SEGAs [53,54]. Seizure frequency was also assessed as a secondary outcome measure in clinical trials of the mTOR inhibitor everolimus for the treatment of SEGAs. Everolimus significantly reduced seizure frequency in a subset of TSC patients in this study [26]. Since the effects on seizures in these clinical cases could be indirectly related to an inhibition of SEGA growth and a corresponding reduction in hydrocephalus, a separate clinical trial was recently performed examining the effect of everolimus on seizures as the primary outcome measure in TSC patients without SEGAs. In a preliminary report of this uncontrolled trial, treatment with everolimus was associated with at least a 50% reduction in seizures in 14 of 20 (70%) TSC patients with intractable epilepsy, including 4 (20%) becoming seizure-free [55]. A multicenter placebo-controlled trial is now in planning.
Antiseizure Effects of mTOR Inhibitors in Non-TSC-Related Epilepsies
In addition to genetic epilepsies involving intrinsic hyperactivation of mTOR, abnormalities in the mTOR pathway could also be involved in other types and causes of epilepsy. In a genetic model of absence epilepsy, WAG/Rij rats, rapamycin treatment causes a rapid decrease in the number and duration of spike-wave discharges, the EEG correlate of absence seizures, within 30 minutes [56]. In the pilocarpine model of acquired limbic epilepsy, an episode of pilocarpine-induced status epilepticus results in brain injury and emergence of spontaneous seizures. Rapamycin treatment reduced seizure frequency in rats with chronic epilepsy following pilocarpine [57]. Thus, there is preclinical evidence that mTOR inhibitors may have efficacy as antiseizure treatment in diverse forms of epilepsy, including acquired epilepsy due to brain injury (Table 1). Presently, however, there are no clinical studies specifically testing efficacy of mTOR inhibitors in patients with other types of epilepsy, besides TSC.
Potential Antiepileptogenic Effects of mTOR Inhibitors
Current antiseizure medications have proven efficacy for treating seizures in patients with established epilepsy. However, available medications appear to act only as symptomatic therapies in suppressing seizures, but none have been definitively demonstrated to exert antiepileptogenic or disease-modifying effects for preventing the development of epilepsy in high risk patients or for slowing the underlying progression of epilepsy. For instance, while phenytoin and valproate have been shown to decrease acute symptomatic seizures in the immediate period following head trauma, treatment with these medications do not improve the subsequent risk of developing posttraumatic epilepsy [2]. The development of true antiepileptogenic and disease-modifying drugs has become a top priority for basic epilepsy research in recent years, but no such treatments have emerged in clinical practice at this time [3,4]. mTOR inhibitors represent a rational candidate for a potential antiepileptogenic therapy for a number of reasons, at least in some forms of epilepsy [5–9]. While ictogenesis or seizure generation most closely entails mechanisms immediately controlling brain excitability, epileptogenesis is a more complex, diverse process in response to an initial injury or defect of the brain and may involve more chronic, progressive mechanisms, such as regulation of gene transcription and protein synthesis, structural changes to neurons, neuronal death and apoptosis, neurogenesis, synaptic and circuit reorganization, and non-neuronal processes (e.g., inflammation, gliosis, breakdown of blood-brain barrier). The mTOR pathway is implicated in regulating a number of these mechanisms and therefore mTOR inhibitors could possibly possess antiepileptogenic or disease-modifying properties for epilepsy (Table 2).
Antiepileptogenic Effects of mTOR Inhibitors in TSC-Related Epilepsy
Rational disorders to test for antiepileptogenic properties of mTOR inhibitors are again genetic diseases with intrinsic abnormal signaling, such as TSC. Since mTOR inhibitors have already been demonstrated to treat tumors in TSC, the mTOR-mediated mechanisms that promote tumorigenesis may overlap or have similarities with mechanisms of epileptogenesis in TSC [5–8]. A large number of molecular, cellular, and histological abnormalities have been discovered in animal models and pathological brain samples from TSC patients, which have been implicated in epileptogenesis, including the presence of dysmorphic neurons and giant cells, gliosis, inflammatory processes, altered astrocyte glutamate transporter expression, and tuber formation [58]. Again, given the known role of the mTOR pathway in regulating a variety of cellular and molecular processes, it is plausible that mTOR inhibitors may be able to reverse or prevent many of these abnormalities. In fact, in several animal models of TSC, treatment with rapamycin at an early stage before the onset of seizures inhibits many pathological and cellular abnormalities in these models and, in most cases, decreases or completely prevents the development of epilepsy [48,59–63]. However, it is important to note that cessation of treatment usually resulted in the delayed development of seizures and pathological abnormalities, suggesting that long-term mTOR inhibitor therapy may be necessary to sustain efficacy. In addition to epilepsy, another potential therapeutic application of mTOR inhibitors in TSC is for the cognitive neurological comorbidities of this disease. Remarkably, mTOR inhibitors can prevent or reverse cognitive deficits in TSC mouse models [63,64]. Overall, these preclinical studies in animal models of TSC provide convincing evidence of “proof-of-principle” that mTOR inhibitors may have antiepileptogenic and disease-modifying effects in TSC and support the initiation of clinical trials in TSC patients for such indications. However, as the design of antiepileptogenic drug trials face a number of practical, technical, and ethical issues (see 5-Year View), there are currently no significant clinical data on potential antiepileptogenic or disease-modifying effects of mTOR inhibitors in TSC patients or other patients at high risk for epilepsy.
Antiepileptogenic Effects of mTOR Inhibitors in Non-TSC-Related Epilepsy
Beyond TSC, accumulating preclinical data suggest that the mTOR pathway can influence epileptogenic mechanisms in other types of epilepsy and that mTOR inhibitors might have more widespread applications as an antiepileptogenic therapy [5–9]. Human temporal lobe epilepsy related to hippocampal sclerosis represents one of the most clinically-relevant and medically-intractable types of epilepsy. From a basic science standpoint, animal models of temporal lobe epilepsy and hippocampal injury due to drug-induced status epilepticus or electrical stimulation have historically been among the most popular models of epilepsy to study. Histological analysis of both animal models or human specimens of hippocampal sclerosis consistently exhibit stereotypical pathological changes, including selective neuronal death, neurogenesis, and synaptic reorganization, particularly in the form of mossy fiber sprouting in the dentate gyrus. Although the mechanisms of epileptogenesis in these cases of temporal lobe epilepsy are still controversy, these pathological abnormalities, as well as specific molecular changes in neurons and glia that affect excitability, have been implicated in promoting hyperexcitable, aberrant hippocampal circuitry that leads to seizures.
A number of recent studies have investigated the potential involvement of the mTOR pathway in epileptogenesis in rodent models of temporal lobe epilepsy. First of all, selective hyperactivation of the mTOR pathway by postnatal deletion of the Pten gene specifically in dentate gyrus granule neurons is sufficient to cause temporal lobe epilepsy in mice [65]. In addition, other recent studies of rodent models of temporal lobe epilepsy induced by status epilepticus indicate that the mTOR pathway is abnormally activated following status epilepticus and that mTOR inhibition retards mossy fiber sprouting and the associated increased excitability of hippocampal circuits [57,66–70]. A couple of these studies have reported that early rapamycin treatment decreases development of spontaneous seizures in the rat kainate and angular bundle electrical stimulation status epilepticus models [69,70], but other studies in the mouse pilocarpine and amygdala stimulation models found no effect of rapamycin on epilepsy development [71,72]. The differing results in these studies may relate to differences in epilepsy model, animal species/strain, and timing and dose of rapamycin. Furthermore, similar to TSC, it appears that continued treatment with rapamycin may be necessary to maintain effectiveness, at least for pathological abnormalities associated with temporal lobe epilepsy, particularly mossy fiber sprouting [57,73]. Despite some controversy, there is substantial preclinical evidence from animal models that, at least under some conditions, mTOR inhibitors may have antiepileptogenic properties in preventing pathological mechanisms and the development of acquired temporal lobe epilepsy
The potential role of the mTOR pathway in animal models of other, diverse types of epilepsy has also been investigated. Brain insults occurring around the neonatal period often lead to long-term epilepsy and cognitive deficits. In a rodent model of neonatal hypoxia-induced seizures, rapamycin treatment inhibited early increases in mTOR activation, glutamatergic synaptic transmission and seizure susceptibility and attenuated the subsequent development of epilepsy, as well as autistic-like behavior [74]. In the WAG/Rij rat model of absence epilepsy, early rapamycin treatment starting at 45 days of life and continuing for about 4 months significantly reduced the subsequent development of absence seizures in adult rats, including up to 5 months after the rapamycin was stopped, consistent with a persistent antiepileptogenic effect [56]. Another very clinically relevant disorder is posttraumatic epilepsy following traumatic brain injury (TBI), which often has a long latent period allowing the opportunity for antiepileptogenic intervention. In animal models, mTOR pathway activity is clearly increased following different types of experimental TBI and mTOR inhibitors have neuroprotective actions against neuronal death and behavioral deficits following TBI [75–77]. While final studies on the effect of mTOR inhibitors on posttraumatic epilepsy have not yet been published, preliminary reports suggest that rapamycin has antiepileptogenic properties in attenuating pathological abnormalities and the development of posttraumatic seizures in both in vivo and in vitro models of TBI [78,79]. If these preliminary studies are confirmed, this could have tremendous clinical impact for patients with TBI at risk for posttraumatic epilepsy and other neurological deficits. However, while markers of abnormal mTOR activation have been identified in brain specimens of patients with non-TSC-related epilepsy [80], presently no clinical studies have been published on the use of mTOR inhibitors for these other types of epilepsy.
Expert Commentary
Currently-available seizure medications have substantial limitations, including medical intractability in a significant proportion of epilepsy patients and the lack of antiepileptogenic or disease-modifying properties to prevent epilepsy or slow its progression. To overcome these limitations, novel drugs are needed that have completely different mechanisms of action than current medications. In theory, the mTOR pathway constitutes a rational therapeutic target for epilepsy, based on its significant role in regulating a range of physiological and pathological processes that may influence epileptogenesis and seizure generation. In practice, there is substantial preclinical evidence that mTOR inhibitors exert both antiseizure and antiepileptogenic actions, especially in mouse models of TSC, but also in animal models of a number of other diverse types of epilepsy (Tables 1 & 2). Clinical studies of mTOR inhibitors tested in epilepsy patients are more limited, primarily involving uncontrolled data that suggest an antiseizure effect of mTOR inhibitors in TSC patients with intractable epilepsy. Randomized, placebo-controlled trials are required to establish more clearly if mTOR inhibitors reduce seizure frequency of TSC patients. Moreover, there are no existing clinical studies specifically testing if mTOR inhibitors have efficacy for non-TSC-related epilepsy or possess antiepileptogenic properties in any type of epilepsy.
Before the potential therapeutic uses of mTOR inhibitors can be realized, several issues need to be investigated further on both the basic science and clinical levels. The overriding problem is clearly defining the clinical scenarios in which mTOR inhibitors would be expected to be most effective for epilepsy. For example, are mTOR inhibitors clinically useful as standard antiseizure/anticonvulsant drugs in patients that already have epilepsy? Or are they much better suited as novel antiepileptogenic therapies for preventing epilepsy? Are mTOR inhibitors effective against many types of epilepsy, irrespective of underlying etiology, or are they primarily indicated for specific types or etiologies of epilepsy that intrinsically feature increased mTOR activity? Is there a critical timing or duration of treatment with mTOR inhibitors necessary for efficacy? Even if mTOR inhibitors are efficacious, do the benefits outweigh the side effects? Based on the available preclinical data, the efficacy of mTOR inhibitors in different contexts does appear to vary depending on the underlying etiology or pathophysiological mechanisms of the epilepsy and the timing of drug administration. These complex questions and issues will be discussed further below.
Are mTOR inhibitors useful as standard anticonvulsant drugs?
The standard clinical approach to treating epilepsy is, of course, to initiate therapy after a patient presents with seizures, with the goal of inhibiting future seizures. In most cases, this traditional approach basically amounts to a relatively non-specific, symptomatic treatment of the end-stage symptoms of epilepsy. When effective, antiseizure drugs directly decrease neuronal excitability and simply suppress seizures in a relatively non-specific fashion, often irrespective of the underlying etiology and pathogenesis of the patient’s epilepsy. With regard to mTOR inhibitors, the preclinical data are mixed as to whether mTOR inhibitors have general anticonvulsant properties, independent of underlying etiology. The three recent studies that assessed rapamycin for anticonvulsant effects in standard batteries of acute seizure models in normal animals found variable, sometimes contradictory, results [43–45] (Table 1). When rapamycin was seen to have an anticonvulsant effect in these studies, the effects were usually mild and dependent on particular parameters, such as the age of the animal, the seizure model tested, and the timing of drug administration. Ironically, in at least the MES-T model, there seemed to be a lack of correlation between anticonvulsant efficacy and the suppression of mTOR activity, as longer rapamycin treatment (which should have greater mTOR inhibition) was not as effective as a single dose [43]. This suggests that these mild anticonvulsant effects of rapamycin may be mechanistically non-specific. Although rapamycin may be able to influence neuronal excitability by regulating protein synthesis of ion channels or other relevant proteins, there is no convincing evidence that mTOR inhibitors directly modulate ion channels or neurotransmitter receptors like many conventional antiseizure drugs. Overall, rapamycin appears to be a relatively weak anticonvulsant, at best, and only under particular circumstances. Therefore, mTOR inhibitors may have limited clinical utility as a standard, non-specific antiseizure treatment for epilepsy in general. However, it is still feasible that mTOR inhibitors could have stronger antiseizure efficacy in specific disorders, like TSC, that involve intrinsic mTOR hyperactivation (see below).
Are mTOR inhibitors useful as antiepileptogenic drugs?
No currently available seizure medications have been definitively demonstrated to possess antiepileptogenic or disease-modifying properties for epilepsy in people. Thus, identifying an effective antiepileptogenic drug is a tall order and would be a major advance. Despite these caveats, there is significant reason to believe that mTOR inhibitors may have true antiepileptogenic properties, at least under some conditions. Many mechanisms of epileptogenesis likely are completely different than mechanisms of ictogenesis/seizure generation, and drugs that are effective anticonvulsants may not have any antiepileptogenic potential. From a mechanistic standpoint, inhibition of the mTOR pathway is a very attractive, rational candidate for possessing antiepileptogenic properties. Rather than direct, acute modulation of ion channels and neurotransmitter receptors, mTOR inhibitors have the potential to impact more chronic, progressive brain processes that may mediate epileptogenesis, such as axonal sprouting, neuronal death, inflammation, autophagy, and expression of a variety of proteins. Evidence for antiepileptogenic effects of mTOR inhibitors is most convincing in multiple mouse models of TSC, where early treatment prior to seizure onset can completely prevent the development of epilepsy and, importantly, the underlying pathological, cellular, and molecular defects that mediate epileptogenesis [48,59–63]. The caveat is that long-term treatment appears to be necessary to maintain effectiveness, as discontinuation of rapamycin therapy results in subsequent emergence of epilepsy and associated pathological changes in TSC mouse models. This is not surprising, as mTOR inhibitors are not correcting the underlying genetic defect of TSC, and so once the drug is stopped, the mTOR pathway is free to activate abnormally. Despite the need for continued treatment in TSC, the prevention of underlying pathological abnormalities causing epilepsy should qualify as a true antiepileptogenic or disease-modifying effect, completely different from the symptomatic antiseizure effects of standard anticonvulsant drugs.
In some animal models of non-TSC epilepsy, there is also substantial evidence for antiepileptogenic properties, but the effects are more variable and dependent on specific circumstances. Rapamycin treatment reduced the development of epilepsy and mossy fiber sprouting in the kainate status epilepticus model of temporal lobe epilepsy [69], but only inhibited mossy fiber sprouting, not epilepsy, in the closely-related pilocarpine model [71]. Similarly, rapamycin appeared to attenuate epileptogenesis following status epilepticus triggered electrically by angular bundle stimulation [70], but not stimulation of the amydala [72]. These differences could be related to a number of experimental variables, such as the epilepsy model, species, or dose and timing of rapamycin. However, this emphasizes the point that, in contrast to genetic epilepsies with intrinsically abnormal mTOR activation, the relationship between mTOR and acquired epilepsies following brain injury is not as direct and consistent, and the utility of mTOR inhibitors is less certain.
Is the efficacy of mTOR inhibitors dependent on the underlying etiology?
About a decade ago, the identification of the mechanistic link between the TSC genes and the mTOR pathway immediately raised the possibility of utilizing rapamycin as a disease-specific treatment for TSC [23,24]. More recently, other related malformations of cortical development have also been found to involve genetic mutations in different components of the mTOR pathway [32–34]. This group of disorders all feature intractable epilepsy as a major phenotype and collectively have been referred to as “TORopathies” [29–31]. As abnormal mTOR signaling appears to be central to the pathophysiology of these genetic disorders, it makes sense that mTOR inhibitors may have better efficacy for epilepsy from these etiologies compared with other types of epilepsy. However, it is also possible that other types of epilepsy may also involve abnormal mTOR signaling as an “acquired TORopathy”. Evidence from animal models indicates that multiple, diverse pathologic stimuli that trigger epileptogenesis activate the mTOR pathway [68–70,74,75]. In fact, seizures themselves in the absence of other associated pathology increase mTOR activity [81], raising the possibility that mTOR could participate in a circular process of progressive epileptogenesis. Thus, while mTOR inhibitors may have only limited efficacy as general anticonvulsant drugs (see above), mTOR inhibitors may be particularly effective for epilepsy in disorders involving genetic or acquired hyperactivation of mTOR.
Is there a critical timing or duration of treatment with mTOR inhibitors necessary for efficacy?
The timing and duration of treatment with mTOR inhibitors may be critical in affecting the efficacy for epilepsy, especially for potential antiepileptogenic actions. In general, the earlier the treatment can be initiated in the process of epileptogenesis, the more likely it will have an effect. For TSC, pathological abnormalities, such as cortical tubers, arise prenatally during early brain development. Assuming these pathological lesions are necessary for epileptogenesis, in theory, prenatal treatment would maximize efficacy (but also increase adverse effects – see next section). In terms of duration of treatment, as mentioned above, long-term therapy may be necessary to maintain effectiveness in genetic disorders, like TSC, as mTOR reactivation may trigger epileptogenesis after mTOR inhibitors are stopped. In contrast, mTOR activation following brain injury appears to be transient [69] and therefore a self-limited treatment with mTOR inhibitors during a critical part of the latent period following injury may be sufficient to block epileptogenesis. Interestingly, however, mossy fiber sprouting following status epilepticus, which was inhibited by rapamycin treatment, recurred after rapamycin was stopped [73], suggesting that long-term treatment may also be necessary for acquired epilepsies. On the other hand, the antiepileptogenic effect of rapamycin in decreasing absence seizures in WAG/Rij rats persisted for at least 5 months after rapamycin was discontinued [56].
Even if mTOR inhibitors are efficacious, do the benefits outweigh the side effects?
As rapamycin (sirolimus) was initially approved by the FDA in 1999 as an immunosuppressant agent for organ transplant patients, the side effects of mTOR inhibitors are well-documented. While overall mTOR inhibitors are generally well-tolerated, significant adverse effects may occur, including opportunistic infections, hyperlipidemia, thrombocytopenia, and apthous ulcers. In addition, mTOR inhibitors may decrease somatic growth and interrupt critical mechanisms of brain development and learning, such as long-term potentiation and synaptic plasticity [82]. Thus, the potential benefit of early antiepileptogenic treatment should be weighed against the increased risk of adverse side effects, especially at early developmental stages. One option to lessen this concern might involve using “drug holidays” or treatment protocols in which mTOR inhibitors are administered intermittently. Preclinical studies in animals indicate that treatment paradigms involving intermittent administration of mTOR inhibitors remain efficacious, while decreasing adverse effects, such as immunosuppression [51,83,84].
Five-year View
With the above limitations and unresolved issues in mind, a logical plan can be made to continue to move the field forward over the next five years. The scientific rationale, preclinical data, and initial clinical studies of mTOR inhibitors, which support a role of the mTOR pathway in epilepsy, are strongest for TSC. To follow the encouraging preliminary results from uncontrolled studies on the effects of everolimus in TSC patients with intractable epilepsy [26,53–55], a multicenter placebo-controlled trial of everolimus in TSC patients with refractory partial-onset seizures is currently in planning (EXIST-3, ClinicalTrials.gov NCT01713946). Since everolimus is already approved for the treatment of SEGAs and kidney tumors in TSC, encouraging results from a placebo controlled epilepsy trial could lead quickly to an additional approval of this mTOR inhibitor for intractable seizures in TSC patients. If this occurs, the emergence of mTOR inhibitors as a proven therapy for epilepsy in TSC would represent a novel advance from a mechanistic standpoint, compared to existing antiseizure medications.
On the other hand, even if mTOR inhibitors are demonstrated to be an effective therapy for seizures in TSC patients, the existing preliminary studies suggest that efficacy of mTOR inhibitors in TSC may not be much different than other standard seizure medications used in intractable epilepsy patients; i.e., they may help reduce seizure frequency, but most patients do not become seizure-free, which is the ultimate goal. So, despite the novel mechanism of action, the ultimate clinical impact of mTOR inhibitors as antiseizure agents in TSC may be relatively modest. However, mTOR inhibitors might have a more significant impact as a potential preventative, antiepileptogenic therapy. No preventative, disease-modifying treatment has been demonstrated to work for any type of epilepsy in people. Furthermore, compared with standard antiseizure drug trials in intractable epilepsy patients, clinical studies addressing antiepileptogenesis are very difficult to design and conduct. One major issue is selecting an appropriate high-risk, but presymptomatic/asymptomatic, patient population to target with antiepileptogenic therapy. TSC patients may actually represent an ideal population for antiepileptogenic drug trials, because some patients are diagnosed with TSC at an early age before the onset of epilepsy [85]. At the same time, since the majority (up to 90%) of TSC patients will develop epilepsy in the future [86], it may be more justifiable to expose asymptomatic patients to an experimental therapy that may have adverse effects.
Despite the positive theoretical rationale, several practical issues must be addressed prior to initiating an antiepileptogenic clinical trial of mTOR inhibitors in TSC patients. As mentioned above, the treatment duration will need to be carefully considered and weighed against potential long-term side effects, as prolonged therapy may be necessary to maintain effectiveness. Again the use of drug holidays may decrease the risks of chronic use of mTOR inhibitors. Further preclinical animal studies could determine the optimal treatment regimens that minimize side effects but still maintain efficacy. Finally, most TSC patients do ultimately develop epilepsy, but a subgroup will not, resulting in the unnecessary treatment of some patients. To minimize unnecessary exposure, biomarkers could be developed that identify the patients at highest risk for epilepsy and thus the most appropriate candidates for initiating antiepileptogenic treatment. While this is still an area of active research, EEG, neuroimaging, and biochemical assays are being investigated as potential biomarkers for predicting epilepsy in TSC.
Outside of TSC, there are other patient populations that might be appropriate candidates for treatment with mTOR inhibitors, either as antiseizure or antiepileptogenic therapy. For example, there is some rationale for hypothesizing that mTOR inhibitors could have antiepileptogenic properties for preventing epilepsy in patients with traumatic or other types of acquired brain injury. To be prudent, an extension to non-TSC populations should await more definitive results from clinical trials in TSC, as well as further preclinical animal studies that better delineate the particular circumstances and types of non-TSC epilepsy most responsive to mTOR inhibitors. However, given the growing number of other neurological and non-neurological diseases in which mTOR has been implicated [87,88], it seems likely that future research may identify a variety of neurological applications for mTOR inhibitors.
Key Issues.
Current seizure medications have significant limitations, such as intractability and lack of antiepileptogenic properties, indicating that new drugs for epilepsy are needed with novel mechanisms of action.
The mammalian target of rapamycin (mTOR) pathway regulates a number of important physiological process, such as synaptic plasticity and ion channel expression, which could influence epileptogenesis under pathological conditions.
mTOR inhibitors have limited anticonvulsant effects in animal models of acutely-provoked seizures.
mTOR inhibitors have antiseizure effects in decreasing seizure frequency in animal models of tuberous sclerosis complex (TSC), temporal lobe epilepsy, and absence epilepsy.
mTOR inhibitors have antiepileptogenic effects in preventing the development of epilepsy in animal models of TSC, as well as other types of epilepsy, but the effects depend on specific conditions, such as model type and timing of treatment.
Preliminary clinical studies indicate that mTOR inhibitors reduce seizure frequency in TSC patients with intractable epilepsy.
Future research should identify the specific clinical applications of mTOR inhibitors for epilepsy, most likely in TSC, but potentially for other types of epilepsy as well.
Acknowledgments
Financial Disclosures/Acknowledgements
The author currently receives grant support from the National Institutes of Health/NINDS (R01NS056872, R01NS079321, P20NS080199) and the Department of Defense (Tuberous Sclerosis Complex Research Program).
References
- 1.Kwan P, Brodie MJ. Refractory epilepsy: mechanisms and solutions. Expert Rev. Neurother. 2012;6:397–406. doi: 10.1586/14737175.6.3.397. [DOI] [PubMed] [Google Scholar]
- 2.Temkin NR. Antiepileptogenesis and seizure prevention trials with antiepileptic drugs: meta-analysis of controlled trials. Epilepsia. 2001;42:515–524. doi: 10.1046/j.1528-1157.2001.28900.x. [DOI] [PubMed] [Google Scholar]
- 3.Pitkanen A, Lukasiuk K. Mechanisms of epileptogenesis and potential treatment targets. Lancet Neurol. 2011;10:173–186. doi: 10.1016/S1474-4422(10)70310-0. [DOI] [PubMed] [Google Scholar]
- 4.Loscher W, Schmidt D. Modern antiepileptic drug development has failed to deliver: ways out of the current dilemma. Epilepsia. 2011;52:657–678. doi: 10.1111/j.1528-1167.2011.03024.x. [DOI] [PubMed] [Google Scholar]
- 5.Wong M. Mammalian target of rapamycin (mTOR) inhibition as a potential antiepileptogenic therapy: From tuberous sclerosis to common acquired epilepsies. Epilepsia. 2010;51:27–36. doi: 10.1111/j.1528-1167.2009.02341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McDaniel SS, Wong M. Therapeutic role of mammalian target of rapamycin (mTOR) inhibition in preventing epileptogenesis. Neurosci. Lett. 2011;497:231–239. doi: 10.1016/j.neulet.2011.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cho CH. Frontier of epilepsy research – mTOR signaling pathway. Exp. Mol. Med. 2011;31:231–274. doi: 10.3858/emm.2011.43.5.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galanopoulou AS, Gorter JA, Cepeda C. Finding a better drug for epilepsy: the mTOR pathway as an antiepileptogenic target. Epilepsia. 2012;53:1119–1130. doi: 10.1111/j.1528-1167.2012.03506.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ryther RC, Wong M. Mammalian target of rapamycin (mTOR) inhibition: potential for antiseizure, antiepileptogenic, and epileptostatic therapy. Curr. Neurol. Neurosci. Rep. 2012;12:410–418. doi: 10.1007/s11910-012-0276-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weber JD, Gutmann DH. Deconvoluting mTOR biology. Cell Cycle. 2012;11:236–248. doi: 10.4161/cc.11.2.19022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sarbassov DD, Ali SM, Sengupta S, et al. Prolonged rapamycin treatment inhibitors mTORC2 assembly and Akt/PBK . Mol. Cell. 2006;22:159–169. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 13.Bekinschtein P, Katche C, Slipczuk LN, et al. mTOR signaling in the hippocampus is necessary for memory formation. Neurobiol. Learn. Mem. 2007;87:303–307. doi: 10.1016/j.nlm.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 14.Tang SJ, Reis G, Kang H, Gingras AC, Sonenberg N, Schuman EM. A rapamycin-sensitive signaling pathway contributes to long-term synaptic plasticity in the hippocampus. Proc. Natl. Acad. Sci. USA. 2002;99:467–472. doi: 10.1073/pnas.012605299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jaworski J, Spangler S, Seeburg DP, Hoogenraad CC, Sheng MM. Control of dendritic arborization by the phosphoinositide-3’-kinase-Akt-mammlian target of rapamycin pathway. J. Neurosci. 2005;25:11300–11312. doi: 10.1523/JNEUROSCI.2270-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumar V, Zhang MX, Swank MW, Kunz J, Wu GY. Regulation of dendritic morphogenesis by Ras-PI3K-Akt-mTOR and Ras-MAPK signaling pathways. J. Neurosci. 2005;25:11288–11299. doi: 10.1523/JNEUROSCI.2284-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sherman DL, Krois M, Wu LM, et al. Arrest of myelination and reduced axon growth when Schwann cells lack mTOR. J. Neurosci. 2012;32:1817–1825. doi: 10.1523/JNEUROSCI.4814-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Graham KF, Haddick PC, Jan YN, Jan LY. Activity- and mTOR-dependent suppression of Kv1.1 channel mRNA translation in dendrites. Science. 2006;314:144–148. doi: 10.1126/science.1131693. [DOI] [PubMed] [Google Scholar]
- 19.Wang Y, Barbaro MF, Baraban SC. A role for the mTOR pathway in surface expression of AMPA receptors. Neurosci. Lett. 2006;401:35–39. doi: 10.1016/j.neulet.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 20.Zoncu R, Efevan A, Sabitini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat. Rev. Mol. Cell Biol. 2011;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dazert E, Hall MN. mTOR signaling in disease. Current Opin. Cell Biol. 2011;23:744–755. doi: 10.1016/j.ceb.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 22.Bove J, Martinez-Vicente M, Vila M. Fighting neurodegeneration with rapamycin: mechanistic insights. Nat. Rev. Neurosci. 2011;12:437–452. doi: 10.1038/nrn3068. [DOI] [PubMed] [Google Scholar]
- 23.Orlova KA, Crino PB. The tuberous sclerosis complex. Ann. NY Acad. Sci. 2010;1184:87–105. doi: 10.1111/j.1749-6632.2009.05117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crino PB, Nathanson KL, Henske EP. The tuberous sclerosis complex. N. Engl. J. Med. 2006;355:1345–1356. doi: 10.1056/NEJMra055323. [DOI] [PubMed] [Google Scholar]
- 25.Franz DN, Leonard J, Tudor C, et al. Rapamycin causes regression of astrocytomas in tuberous sclerosis complex. Ann. Neurol. 2006;59:490–498. doi: 10.1002/ana.20784. [DOI] [PubMed] [Google Scholar]
- 26. Krueger DA, Care MM, Holland K, et al. Everolimus for subependymal giant-cell astrocytomas in Tuberous Sclerosis. N. Engl. J. Med. 2010;363:1801–1811. doi: 10.1056/NEJMoa1001671. *Clinical trial demonstrating efficacy of a mTOR inhibitor for inhibiting brain tumor growth in TSC patients, as well as decreasing seizure frequency as a secondary measure.
- 27.Bissler JJ, McCormack FX, Young LR, et al. Sirolimus for angiomyolipoma in tuberous sclerosis complex or lymphangioleiomyomatosis. N. Engl. J. Med. 2008;358:140–151. doi: 10.1056/NEJMoa063564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Franz DN, Belousova E, Sparagana S, et al. Efficacy and safety of everolimus for subependymal giant cell astrocytomas associated with tuberous sclerosis complex (EXIST-1): a multicentre, randomized, placebo-controlled phase 3 trial. Lancet. 2013;381:125–132. doi: 10.1016/S0140-6736(12)61134-9. [DOI] [PubMed] [Google Scholar]
- 29.Crino PB. Focal brain malformations: seizures, signaling, sequencing. Epilepsia. 2009;50(Suppl 9):3–8. doi: 10.1111/j.1528-1167.2009.02289.x. [DOI] [PubMed] [Google Scholar]
- 30.Crino PB. mTOR: a pathogenic signaling pathway in developmental brain malformations. Trends Mol. Med. 2011;17:734–742. doi: 10.1016/j.molmed.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 31.Wong M, Crino PB. In: mTOR and epileptogenesis in developmental brain malformations. In: Jasper’s Basic Mechanisms of the Epilepsies. Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV, editors. 2012. [PubMed] [Google Scholar]
- 32.Lee JH, Huynh M, Silhaw JL, et al. De novo somatic mutations in components of the PI3K-AKT3-mTOR pathway cause hemimegalencephaly. Nat. Genet. 2012;44:941–945. doi: 10.1038/ng.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Poduri A, Evrony D, Cai X, et al. Somatic activation of AKT3 causes hemispheric developmental brain malformations. Neuron. 2012;74:41–48. doi: 10.1016/j.neuron.2012.03.010. *One of two recent studies demonstrating somatic mutations in the PI3K–AKT3-mTOR pathway as a cause of hemimegalencephaly
- 34.Orlova KA, Parker WE, Heuer GG, et al. STRADα deficiency results in aberrant mTORC1 signaling during corticogenesis in humans and mice. J. Clin. Invest. 2010;120:1591–1602. doi: 10.1172/JCI41592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baybis M, Yu J, Lee A, et al. mTOR cascade activation distinguishes tubers from focal cortical dysplasia. Ann. Neurol. 2004;56:478–487. doi: 10.1002/ana.20211. [DOI] [PubMed] [Google Scholar]
- 36.Ljungberg MC, Bhattacharjee MB, Lu Y, et al. Activation of mammalian target of rapamycin in cytomegalic neurons of human cortical dysplasia. Ann. Neurol. 2006;60:420–429. doi: 10.1002/ana.20949. [DOI] [PubMed] [Google Scholar]
- 37.Schick V, Majores M, Engels G, et al. Differential Pi3K-pathway activation in cortical tubers and focal cortical dysplasias with balloon cells. Brain Pathol. 2007;17:165–173. doi: 10.1111/j.1750-3639.2007.00059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Samadani U, Judkins A, Alkpalu A, Aronica E, Crino PB. Differential gene expression in ganglioglioma. Epilepsia. 2007;48:646–653. doi: 10.1111/j.1528-1167.2007.00925.x. [DOI] [PubMed] [Google Scholar]
- 39.Boer K, Troost D, Timmermans W, van Rijen PC, Spliet WG, Aronica E. Pi3K-mTOR signaling and AMOG expression in epilepsy-associated glioneuronal tumors. Brain Pathol. 2010;20:234–244. doi: 10.1111/j.1750-3639.2009.00268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Daoud D, Scheld HH, Speckmann EJ, Gorji A. Rapamycin: brain excitability studied in vitro. Epilepsia. 2007;48:834–836. doi: 10.1111/j.1528-1167.2006.00976.x. [DOI] [PubMed] [Google Scholar]
- 41.Ruegg S, Baybis M, Juul H, Dichter M, Crino PB. Effects of rapamycin on gene expression, morphology, and electrophysiological properties of rat hippocampal neurons. Epilepsy Res. 2007;77:85–92. doi: 10.1016/j.eplepsyres.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.White HS. Preclinical development of antiepileptic drugs: past, present, and future directions. Epilepsia. 2003;44(Suppl 7):2–8. doi: 10.1046/j.1528-1157.44.s7.10.x. [DOI] [PubMed] [Google Scholar]
- 43. Hartman AL, Santos P, Dolce A, Hardwick JM. The mTOR inhibitor rapamycin has limited acute anticonvulsant effects in mice. PLoS One. 2012;7:e45156. doi: 10.1371/journal.pone.0045156. *Recent study screening for anticonvulsant properties of rapamycin in a battery of animal models of acutely-induced seizures.
- 44.Chachua T, Poon KL, Yum MS, et al. Rapamycin has age-, treatment paradigm-, and modelspecific anticonvulsant effects and modulates neuropeptide Y expression in rats. Epilepsia. 2012;53:2015–2025. doi: 10.1111/j.1528-1167.2012.03674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang X, McMahon J, Yang J, Shin D, Huang Y. Rapamycin down-regulates KCC2 expression and increases seizure susceptibility to convulsants in immature rats. Neuroscience. 2012;219:33–47. doi: 10.1016/j.neuroscience.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Raffo E, Coppola A, Ono T, Briggs Sw, Galanopoulo AS. A pulse rapamycin therapy for infantile spasms and associated cognitive decline. Neurobiol. Dis. 2011;43:322–328. doi: 10.1016/j.nbd.2011.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chachua T, Yum MS, Veliskova J, Velisek L. Validation of the rat model of cryptogenic infantile spasms. Epilepsia. 2011;52:1666–1677. doi: 10.1111/j.1528-1167.2011.03220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zeng L, Xu L, Gutmann DH, Wong M. Rapamycin prevents epilepsy in a mouse model of Tuberous Sclerosis Complex. Ann. Neurol. 2008;63:444–453. doi: 10.1002/ana.21331. * First study demonstrating both antiepileptogenic and antiseizure effects of rapamycin in a mouse model of TSC.
- 49.Kwon C, Zhu X, Zhang J, Baker SJ. mTor is required for hypertrophy of Pten-deficient neuronal soma in vivo . Proc. Natl. Acad. Sci. USA. 2003;100:12923–12928. doi: 10.1073/pnas.2132711100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ljungberg MC, Sunnen CN, Lugo JN, Anderson AE, D'Arcangelo G. Rapamycin suppresses seizures and neuronal hypertrophy in a mouse model of cortical dysplasia. Dis. Model Mech. 2009;2:389–398. doi: 10.1242/dmm.002386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sunnen CN, Brewster AL, Lugo JN, et al. Inhibition of the mammalian target of rapamycin blocks epilepsy progression in NS-Pten conditional knockout mice. Epilepsia. 2011;52:2065–2075. doi: 10.1111/j.1528-1167.2011.03280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhou J, Blundell J, Ogawa S, et al. Pharmacological inhibition of mTORC1 suppresses anatomical, cellular, and behavioral abnormalities in neural-specific Pten knock-out mice. J. Neurosci. 2009;29:1773–1783. doi: 10.1523/JNEUROSCI.5685-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Muncy J, Butler IJ, Koenig M. Rapamycin reduces seizure frequency in Tuberous Sclerosis Complex. J. Child Neurol. 2009;24:477. doi: 10.1177/0883073808324535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Perek-Poinik M, Jozwiak S, Jurkiewicz E, Perek D, Kotulska K. Effective everolimus treatment of inoperable, life-threatening subependymal giant cell astrocytoma and intractable epilepsy in a patient with tuberous sclerosis complex. Eur. J. Paediatr. Neurol. 2012;16:83–85. doi: 10.1016/j.ejpn.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 55.Krueger DA, Wilfong AA, Holland-Bouley K, et al. Everolimus improves seizure control in tuberous sclerosis complex. American Epilepsy Society Annual Meeting Abstracts. 2012 #1.237. [Google Scholar]
- 56.Russo E, Citaro R, Donato G, et al. mTOR inhibition modulates epileptogenesis, seizures and depressive behavior in a genetic rat model of absence epilepsy. Neuropharm. 2012 Oct 22; doi: 10.1016/j.neuropharm.2012.09.019. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 57.Huang X, Zhang H, Yang J, et al. Pharmacological inhibition of the mammalian target of rapamycin pathway suppresses acquired epilepsy. Neurobiol. Dis. 2010;40:193–199. doi: 10.1016/j.nbd.2010.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Holmes GL, Stafstrom CE. Tuberous Sclerosis Study Group. Tuberous sclerosis complex and epilepsy: recent developments and future challenges. Epilepsia. 2007;48:617–630. doi: 10.1111/j.1528-1167.2007.01035.x. [DOI] [PubMed] [Google Scholar]
- 59.Meikle L, Pollizzi K, Egnor A, et al. Response of a neuronal model of Tuberous Sclerosis to mammalian target of Rapamycin (mTOR) inhibitors: effects on mTORC1 and Akt signaling lead to improved survival and function. J. Neurosci. 2008;28:5422–5432. doi: 10.1523/JNEUROSCI.0955-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Goto J, Talos DM, Klein P, et al. Regulable neural progenitor-specific TSC1 loss yields giant cells with organellar dysfunction in a model of tuberous sclerosis complex. Proc. Natl. Acad. Sci. USA. 2011;108:1070–1079. doi: 10.1073/pnas.1106454108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zeng L, Rensing NR, Zhang B, Gutmann DH, Gambello MJ, Wong M. TSC2 gene inactivation causes a more severe epilepsy phenotype than TSC1 inactivation in a mouse model of Tuberous Sclerosis Complex. Hum. Mol. Genet. 2011;20:445–454. doi: 10.1093/hmg/ddq491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Carson RP, Van Nielen DL, Winzenburger PA, Ess KC. Neuronal and glia abnormalities in TSC1-deficient forebrain and partial rescue by rapamycin. Neurobiol. Dis. 2012;45:369–380. doi: 10.1016/j.nbd.2011.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Way SW, Rozas NS, Wu HC, et al. The differential effects of prenatal and/or postnatal rapamycin on neurodevelopmental defects and cognition in a neuroglial mouse model of tuberous sclerosis complex. Hum. Mol. Genet. 2012;21:3226–3236. doi: 10.1093/hmg/dds156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ehninger D, Han S, Shilyansky C, et al. Reversal of learning deficits in a TSC2 +/− mouse model of tuberous sclerosis. Nat. Med. 2008;14:843–848. doi: 10.1038/nm1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Pun RY, Rolle IJ, Lasarge CL, et al. Excessive activation of mTOR in postnatally generated granule cells is sufficient to cause epilepsy. Neuron. 2012;75:1022–1034. doi: 10.1016/j.neuron.2012.08.002. *Study demonstrating that selective hyperactivation of the mTOR pathway in postnatal dentate gyrus granule neurons is sufficient to cause temporal lobe epilepsy in mice.
- 66.Buckmaster PS, Ingram EA, Wen X. Inhibition of the mammalian target of rapamycin signaling pathway suppresses dentate granule cell axon sprouting in a rodent model of temporal lobe epilepsy. J. Neurosci. 2009;29:8259–8269. doi: 10.1523/JNEUROSCI.4179-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tang H, Long H, Zeng C, et al. Rapamycin suppresses the recurrent excitatory circuits of dentate gyrus in a mouse model of temporal lobe epilepsy. Biochem. Biophys. Res. Commun. 2012;420:199–204. doi: 10.1016/j.bbrc.2012.02.143. [DOI] [PubMed] [Google Scholar]
- 68.Sha LZ, Xing XL, Zhang D, et al. Mapping the spatio-temporal pattern of the mammalian target of rapamycin (mTOR) activation in temporal lobe epilepsy. PLoS One. 2012;7:e39152. doi: 10.1371/journal.pone.0039152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zeng LH, Rensing NR, Wong M. The mammalian target of rapamycin signaling pathway mediates epileptogenesis in a model of temporal lobe epilepsy. J. Neurosci. 2009;29:6964–72. doi: 10.1523/JNEUROSCI.0066-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.van Vliet EA, Forte G, Holtman L, et al. Inhibition of mammalian target of rapamycin reduces epileptogenesis and blood-brain barrier leakage but not microglia activation. Epilepsia. 2012;53:1254–1263. doi: 10.1111/j.1528-1167.2012.03513.x. [DOI] [PubMed] [Google Scholar]
- 71.Buckmaster PS, Lew FH. Rapamycin suppresses mossy fiber sprouting but not seizure frequency in a mouse model of temporal lobe epilepsy. J. Neurosci. 2011;31:2337–47. doi: 10.1523/JNEUROSCI.4852-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sliwa A, Plucinska G, Bednarczyk J, Lukasiuk K. Post-treatment with rapamycin does not prevent epileptogenesis in the amygdala stimulation model of temporal lobe epilepsy. Neurosci. Lett. 2012;509:105–105. doi: 10.1016/j.neulet.2011.12.051. [DOI] [PubMed] [Google Scholar]
- 73.Lew FH, Buckmaster PS. Is there a critical period for mossy fiber sprouting in a mouse model of temporal lobe epilepsy? Epilepsia. 2011;52:2326–2332. doi: 10.1111/j.1528-1167.2011.03315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Talos DM, Sun H, Zhou X, et al. The interaction between early life epilepsy and autistic-like behavioral consequences: a role for the mammalian target of rapamycin (mTORC1) pathway. PLoS One. 2012;7:e35885. doi: 10.1371/journal.pone.0035885. *Study demonstrating that mTOR inhibition attenuates epilepsy and autistic behavior following neonatal hypoxic seizures.
- 75.Chen S, Atkins CM, Liu CL, et al. Alterations in mammalian target of rapamycin signaling pathways after traumatic brain injury. J. Cereb. Blood Flow Metab. 2007;27:939–949. doi: 10.1038/sj.jcbfm.9600393. [DOI] [PubMed] [Google Scholar]
- 76.Erlich S, Alexandrovich A, Shohami E, Pinkas-Kramarski R. Rapamycin is a neuroprotective treatment for traumatic brain injury. Neurobiol. Dis. 2007;26:86–93. doi: 10.1016/j.nbd.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 77.Park J, Zhang J, Qiu J, et al. Combination therapy targeting Akt and mammalian target of rapamycin improves functional outcome after controlled cortical impact in mice. J. Cereb. Blood Flow Metab. 2012;32:330–340. doi: 10.1038/jcbfm.2011.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Guo D, Zeng LH, Brody DL, Wong M. mTOR inhibition has potential antiepileptogenic effects in a controlled cortical impact model of traumatic brain injury. Epilepsy Currents. 2011;11(Suppl 1) Abstract #1.014. [Google Scholar]
- 79.Berdichevsky Y, Saponjian Y, Mail M, Staley KJ. Organotypic culture model of post-traumatic epileptogenesis used as a medium-throughput screen of antiepileptic drugs. Epilepsy Currents. 2012;12(Suppl 1) Abstract #3.026. [Google Scholar]
- 80.Sosunov AA, Wu X, McGovern RA, et al. The mTOR pathway is activated in glial cells in mesial temporal sclerosis. Epilepsia. 2012;53(Suppl 1):78–165. doi: 10.1111/j.1528-1167.2012.03478.x. [DOI] [PubMed] [Google Scholar]
- 81.Zhang B, Wong M. Pentylenetetrazole-induced seizures cause acute, but not chronic, mTOR pathway activation in rat. Epilepsia. 2012;53:506–511. doi: 10.1111/j.1528-1167.2011.03384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jaworski J, Sheng M. The growing role of mTOR in neuronal development and plasticity. Mol. Neurobiol. 2012;34:205–219. doi: 10.1385/MN:34:3:205. [DOI] [PubMed] [Google Scholar]
- 83.Boulay A, Zumstein-Mecker S, Stephan C, et al. Antitumor efficacy of intermittent treatment schedules with the rapamycin derivative RAD001 correlates with prolonged inactivation of ribosomal protein S6 kinase 1 in peripheral blood mononuclear cells. Cancer Res. 2004;64:252–261. doi: 10.1158/0008-5472.can-3554-2. [DOI] [PubMed] [Google Scholar]
- 84.Rivera VM, Squillace RM, Miller D, et al. Ridaforolimus (AP23573; MK-8669), a potent mTOR inhibitor, has broad antitumor activity and can be optimally administered using intermittent dosing regimens. Mol. Cancer Ther. 2011;10:1059–1071. doi: 10.1158/1535-7163.MCT-10-0792. [DOI] [PubMed] [Google Scholar]
- 85.Datta AN, Hahn CD, Sahin M. Clinical presentation and diagnosis of tuberous sclerosis complex in infancy. J. Child Neurol. 2008;23:268–273. doi: 10.1177/0883073807309250. [DOI] [PubMed] [Google Scholar]
- 86.Chu-Shore CJ, Major P, Camposano S, Muzykewicz D, Thiele EA. The natural history of epilepsy in tuberous sclerosis complex. Epilepsia. 2010;51:1236–1241. doi: 10.1111/j.1528-1167.2009.02474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dazert E, Hall MN. mTOR signaling in disease. Current Opin. Cell Biol. 2011;23:744–755. doi: 10.1016/j.ceb.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 88.Bove J, Martinez-Vicente M, Vila M. Fighting neurodegeneration with rapamycin: mechanistic insights. Nat. Rev. Neurosci. 2011;12:437–452. doi: 10.1038/nrn3068. [DOI] [PubMed] [Google Scholar]