Abstract
Repeated cocaine exposure induces long-lasting neuroadaptations that alter subsequent responsiveness to the drug. However, systems-level investigation of these neuroplastic consequences is limited. We employed a rodent model of drug addiction to investigate neuroadaptations associated with prolonged forced abstinence after long-term cocaine self-administration (SA). Since natural rewards also activate the mesolimbic reward system in a partially over-lapping fashion as cocaine, our design also included a sucrose SA group. Rats were trained to self-administer cocaine or sucrose using a fixed-ratio one, long-access schedule (6 h/day for 20 days). A third group of naïve, sedentary rats served as a negative control. After 30 days of abstinence, the reactivity of the reward system was assessed with functional magnetic resonance imaging (fMRI) following an intravenous cocaine injection challenge. A strong positive fMRI response, as measured by fractional cerebral blood volume changes relative to baseline (CBV%), was seen in the sedentary control group in such cortico-limbic regions as medial prefrontal cortex and anterior cingulate cortex. In contrast, both the cocaine and sucrose SA groups demonstrated a very similar initial negative fMRI response followed by an attenuated positive response. The magnitude of the mPFC response was significantly correlated with the total amount of reinforcer intake during the training sessions for the cocaine SA but not for the sucrose SA group. Given that the two SA groups had identical histories of operant training and handling, this region-specific group difference revealed by regression analysis may reflect the development of neuroadaptive mechanisms specifically related to the emergence of addiction-like behavior that occurs only in cocaine SA animals.
Keywords: Neuroadaptation, Self-administration, Abstinence, Pharmacological MRI
Introduction
Relapse to drug use, even after prolonged withdrawal, remains a major challenge in addiction treatment and has been attributed, at least in part, to long-lasting neuroadaptations within the mesocorticolimbic (MCL) system (Kalivas, 2004; O'Brien, 1997). Neurochemically, acute cocaine exerts its action by blocking monoamine transporters, potentiating the actions of synaptically released neurotransmitters (Ritz et al., 1990). However, following chronic cocaine exposure, neuroadaptations occur such that glutamate levels in a number of mesocorticolimbic structures, including ventral tegmental area (VTA), nucleus accumbens (NAc) and prefrontal cortex, are also elevated by subsequent cocaine challenge (Pierce et al., 1996; You et al., 2007). This enhancement in glutamatergic transmission has been hypothesized to be an important mechanism in mediating the transition from recreational cocaine use to a compulsive use pattern (Baker et al., 2003). Electrophysiological studies have demonstrated that chronic cocaine administration modulates the firing properties and firing patterns of neurons in a number of MCL regions, including medial prefrontal cortex (mPFC), VTA and NAc (Hollander and Carelli, 2007; Peoples et al., 2007b; Trantham et al., 2002; Ungless et al., 2001).
Given the broad spectrum of neurochemical, electrophysiological and behavioral alterations, it is likely that the overall neuroadaptive response to chronic cocaine is the product of multiple processes at several anatomical foci. In order to determine their neural substrates, a technique capable of identifying neural events in circuits and pathways at a systems level is required. As such, we take advantage of the high spatial and temporal resolution of functional magnetic resonance imaging (fMRI), along with its ability to measure changes in neuronal activity throughout the neuraxis, to examine the neuroplastic consequences of chronic cocaine self-administration (SA). Unlike most pharmacological MRI studies employing an acute drug challenge in naïve animals (Febo et al., 2004; Lu et al., 2007b; Luo et al., 2003; Marota et al., 2000), we used a model of extended SA plus prolonged abstinence prior to acute drug injection, analogous to that used in preclinical reinstatement models (Epstein et al., 2006). Neuroadaptations that occur in this model are believed to capture at least part of the neurobiological aspects of human relapse, including persistent drug seeking, increased motivation to obtain drug and relapse to drug use when exposed to stress, drug or drug related cues (Ahmed and Cador, 2006; Vanderschuren and Everitt, 2004).
In addition to cocaine's unconditioned physiological effects, the environmental context paired with repeated cocaine SA, such as the operant chamber and the housing light, could have profound learned conditioned effects (Stewart et al., 1984). Both the drug injection context and the drug itself reinstate drug-seeking behavior after an extended period of abstinence (Bossert et al., 2005). Importantly, only contextual cue-induced cocaine-seeking behavior incubates overtime, while re-exposure to cocaine itself does not (Lu et al., 2004), suggesting that neuroadaptation processes develop differentially during the abstinence period. To control for non-specific contextual and learning effects and motor behavior from those specifically caused by cocaine SA and abstinence, a separate group of animals underwent similar SA behavioral training procedures using oral sucrose as a reinforcer. Given that the brain reward circuitry activated by natural reward, such as sucrose, partially overlaps with that activated by cocaine (Kelley and Berridge, 2002), we hypothesized that differences in the reactivity of the reward system between these two groups as measured by fMRI likely reflect effects specific to cocaine SA and abstinence. Additionally, we also included a training- and drug-naïve group to study the behaviorally unbiased central effects of acute cocaine administration, an evaluation not likely to be determined in naïve human subjects due to ethical concerns. Results demonstrate that only in mPFC did the neuronal response to an acute cocaine challenge significantly correlate with total cocaine, but not sucrose intake during the SA training sessions, despite virtually identical response numbers and patterns between the two SA groups. Such cocaine SA-specific adaptations may be candidate mechanisms that reflect pathological cocaine-induced drug-seeking behavior.
Materials and methods
Subjects
Long Evans rats were obtained from Charles River Laboratories (Wilmington, MA). Animals started the study at ~12 weeks of age at which time they were food restricted (400–450 g body weight) to ensure they would fit into the MRI animal holder. All animal procedures were reviewed and approved by the Animal Care and Use Committee of both the University of Pennsylvania and the National Institute on Drug Abuse Intramural Research Program (NIDA-IRP).
Experimental design summary
The experiments involved a SA phase and an imaging phase, with the former conducted at the University of Pennsylvania and the latter at NIDA-IRP. Rats destined for cocaine SA underwent jugular catheter implantation. On the sixth day post-surgery, animals were trained to self-administer cocaine intravenously. A second group of animals, absent of jugular catheters, was trained to self-administer sucrose orally. A third group was a housing control group, and underwent neither surgery nor any training, but were otherwise treated the same as the two SA groups. After 14 days of training, the treatment groups were subject to discrimination test sessions, followed by 20 days of long-access SA sessions and finally 30–34 days of abstinence. Animals were transported to the NIDA-IRP after the initial 11–17 days of abstinence. The caging, bedding, food, and colony environmental conditions (e.g., light cycle) were identical at both the University of Pennsylvania and NIDA-IRP. Following the remaining abstinence period, imaging procedures were conducted.
Behavioral procedures
Cocaine SA: Rats were trained to self-administer cocaine in daily 1-h sessions under a fixed-ratio 1 (FR1) schedule of reinforcement for 4 days. Prior to placement of an animal into the chamber, a contextual odor cue was applied underneath the chamber floor. The odor was provided by a cotton swab soaked in either a lemon or a vanilla scent (Pure Lemon Extract and Pure Vanilla Extract; McCormick). Scents were counterbalanced between animals. A lever press was followed by delivery of a cocaine infusion (0.75 mg/kg), which was paired with a 7-s illumination of a light above the lever. An 8-s time-out period followed each reinforced lever press, during which responding was recorded but not reinforced.
Long-access SA sessions
Following odor discrimination test sessions, the duration of daily cocaine SA sessions was extended to 6-h per day (referred to as long access (LgA) sessions). Animals were trained under LgA sessions with the S+ odor for an additional 20 days (5 days per week). To maintain representation of the S− odor, rats were also trained 2 days per week with the S− odor for 15min.
Sucrose SA group
Animals in the sucrose SA group were exposed to the same pre-training, odor discrimination and LgA training except that: 1) each reinforced lever press was followed by delivery of a sucrose solution into a drinking well (0.2 ml of 32% sucrose over 10 s) and 2) the number of sucrose infusions was matched to the daily number of cocaine infusions earned by a paired animal in the cocaine group.
Imaging procedures
Animal preparation procedures for MRI were similar to those previously reported (Lu et al., 2007a). Briefly, on the scan day, rats were anesthetized with 2% isoflurane in 1:1 mixture of oxygen and air. A femoral artery was catheterized for blood sampling and blood pressure monitoring. A tracheotomy was performed for artificial ventilation. A customized T-shaped trachea tube was used to bypass the exhaled air to a gas analyzer for continuous monitoring of end-tidal CO2 and O2. Core body temperature was maintained at 37.5±0.5 °C with a temperature-controlled water-circulating pad. After surgery, anesthesia was switched to continuous intravenous (IV) infusion of propofol (35 mg/kg/h). A neuromuscular blocking agent (pancuronium bromide, 1.5 g/kg/h, IV) was administered to further minimize motion artifacts. Two infusion pumps were employed to continuously infuse propofol and pancuronium bromide into bilateral femoral veins. For each animal, arterial blood gases were sampled right before and after the scan. An iron-oxide contrast agent was administered (IV) at an iron dose of 25 mg/kg to achieve cerebral blood volume (CBV) weighted contrast. This iron dose enhances the sensitivity and offsets the positive BOLD effect in CBV-weighted fMRI signal at high field (Lu et al., 2007a).
Scan methods
fMRI experiments were performed using a Bruker Biospin 9.4T scanner (Bruker Medizintechnik, Karlsruhe, Germany). A volume coil was used for RF excitation and a circular surface coil was used for signal reception. The anterior commissure (−0.36mm from bregma; Paxinos and Watson, 2005) was used as the landmark to localize slices. Following initial localization scans, a Rapid Acquisition with Relaxation Enhancement (RARE) sequence was used to acquire high-resolution anatomical images in the coronal plane, which was used for subsequent image registration. Scan parameters were: TR/effective TE=2650/40 ms, field of view (FOV)=30mm, matrix size=192×192, data were zero-padded to 256×256 for image reconstruction, the total number of slices was 23 with a slice thickness of 1 mm. CBV-weighted fMRI data were acquired using a traditional gradient echo sequence. This sequence provides excellent spatial coverage with minimal distortion or signal dropout even in the amygdala, where magnetic field inhomogeneity is severe, and allowed for accurate image registration across animals. Scan parameters were: TR/TE=314/6 ms, FOV=30 mm, matrix size=96×96, data were zero-padded to 128×128 for image reconstruction (voxel size: 0.234×0.234×1 mm3), the total number of slices was 13 with a slice thickness of 1 mm. It took 30 s to acquire one volume of data.
The scan paradigm was as follows: after 5 min of baseline acquisition, rats received a saline injection, followed 5 min thereafter by a cocaine injection (0.75 mg/kg, IV). The entire fMRI session lasted for 36 min. fMRI data were continuously acquired, reconstructed and displayed using a real-time sequence developed in our lab (Lu et al., 2008). Of the 29 SA rats (14 for cocaine and 15 for sucrose), one was used for pilot development, three had experimental errors (surgical preparation, MION dose, scan parameters) and three had abnormal arterial blood gases and blood pressure during the scans. These animals were excluded, resulting in a total of 22 successful scans (n=10 for cocaine and n=12 for sucrose). We also scanned 12 sedentary control rats.
Data analysis
Images from individual animals were registered to a common space using a method previously described (Lu et al., 2007b, 2010), spatially smoothed using a Gaussian kernel with a full width at half maximum (FWHM) of 0.8 mm, and linear detrended. Raw fMRI signal from individual voxels was converted to fractional CBV changes using the following equation (Mandeville et al., 1998):
| (1) |
Here Spre and Spost are baseline signal intensity pre- and post-contrast agent injections, and S(t) is the time course after acute cocaine or saline injection. fMRI response in this paper refers to percent CBV change unless otherwise specified.
IV saline injection
Recent evidence suggests that an IV saline injection is capable of producing a peripheral sensory stimulus, which is transmitted to the central nervous system, producing a rapid, transient EEG desynchronization, and in cocaine SA experienced animals, such an IV injection could serve as a conditioned interoceptive cue (Kiyatkin and Lenoir, 2011; Wise et al., 2008). To explore the effect of vehicle (saline) injection, the 5-min CBV time course (10 data points) following saline injection was averaged on a voxel-wise basis; data across all three groups (cocaine, sucrose and naïve) were collapsed and then a t-tested against zero was performed, yielding a main effect of IV saline injection. We then performed one-factor repeated measures ANOVA to examine group effects.
IV cocaine injection
Since we found a significant main effect of IV saline injection in a number of regions but no difference across the three experimental groups (see Supplemental Fig. 1), to control for the effect of saline administration, CBV-fMRI time courses following cocaine injections were normalized to the mean of the post-saline injection time course (10 data points). This was done by subtracting voxel-wise mean fractional saline CBV signal from the cocaine CBV time courses on a voxel-wise basis. Theoretically this normalization operation cancels out any potential differences in fMRI responses to vehicle injections across the three experimental groups.
Since different drug exposure history can lead to differential temporal response patterns that may not conform to a single, simple fMRI response model, post-cocaine injection data were further analyzed with a model-free data analysis approach as described below.
As an exploratory first step, the 24-min post-cocaine injection CBV time courses were temporally smoothed and binned into 2-min windows (see Fig. 4). Mean fMRI responses within each time bin were calculated by averaging the four 30-sec data points. A one-way ANOVA was performed across the three treatment groups for each of the 12 bins, resulting in a total of 12 ANOVA statistical maps.
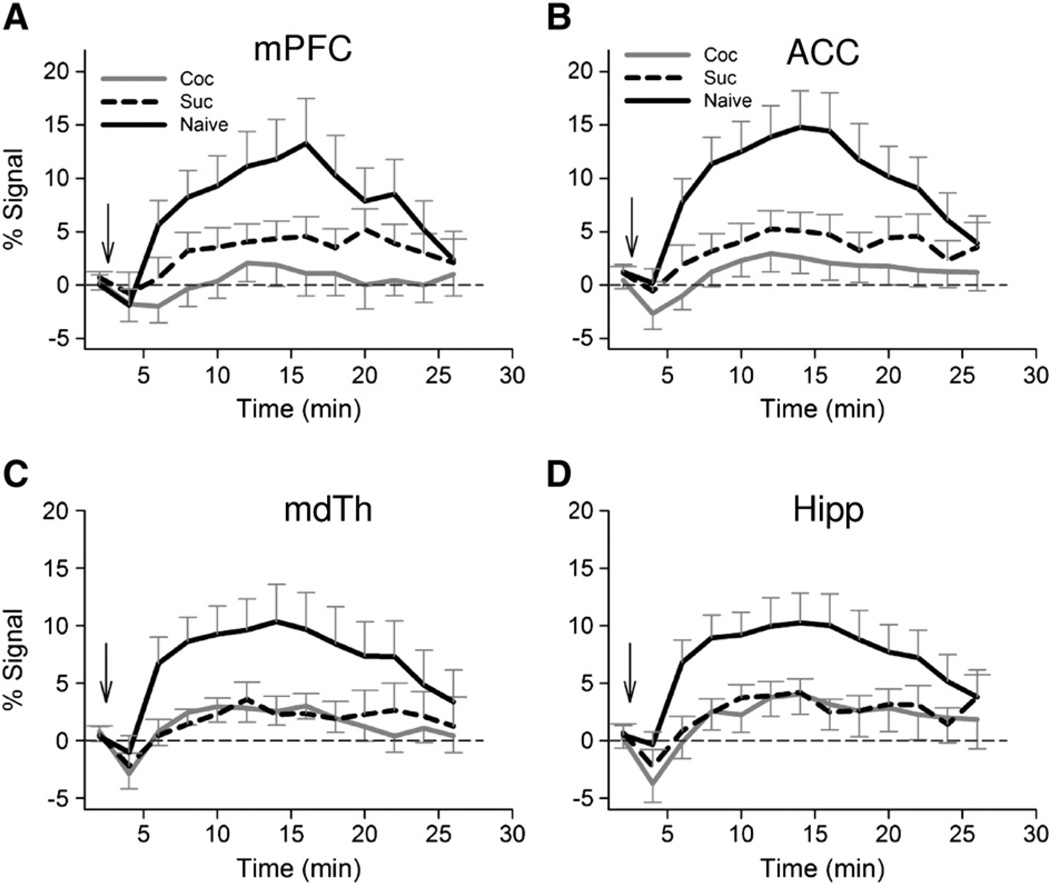
Fig. 4.
Averaged fMRI time courses from three experimental groups (cocaine SA: n=10; sucrose SA: n=12; cocaine naïve group: n=12). Note the early negative CBV response and a greatly reduced positive response in cocaine SA rats relative to naïve rats. Arrows indicate cocaine injection. Error bars represent SEM. Abbreviations: mPFC, medial prefrontal cortex (PrL and IL); ACC, anterior cingulate cortex; Hipp, hippocampus; mdTH, medial dorsal thalamic nuclei; Coc, cocaine; Suc, sucrose.
Visual inspection of the maps and the fMRI time courses across regions clearly suggested the existence of a 3-epoch temporal response pattern, namely a rising, plateau and a trailing phase. As such, in step two we re-binned the response curves into three time windows (2–8, 8–16, and 16–24 min post-injection), corresponding to the three response phases and averaged the fMRI responses within each phase. Mean epoch responses across the three treatment groups were then subject to a 3×3 ANOVA (GROUP×TIME) with time window as a repeated measure. Since we are interested in differences in fMRI responses across the three treatment groups within individual time windows, we also performed an ANOVA across each time window, resulting in a total of three ANOVA maps, one for each phase. To visualize the map patterns across the three phases, we color-coded the significant main effects of treatment based on temporal phases. This resulted in an overlapped activation map with a total of seven possible overlapping schemes, color-coded as explained in Fig. 2. Post-hoc t-tests were performed to further compare responses across the three experimental groups.
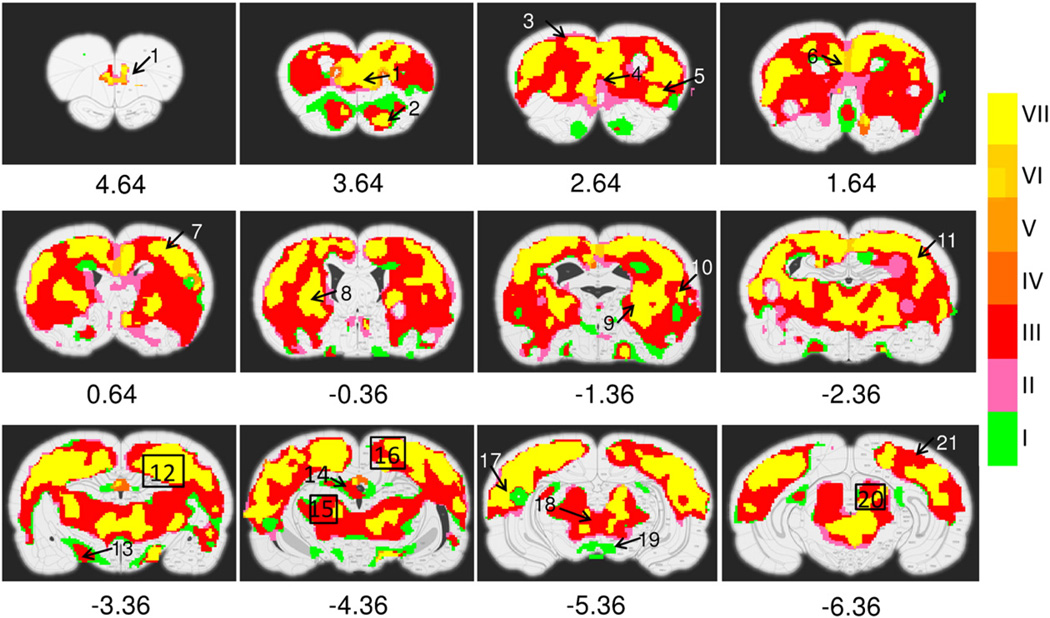
Fig. 2.
Activation maps showing the ANOVA main effect of cocaine INJECTION. fMRI time courses are binned into rising, plateau and trailing phases. Activation maps across the three phases are combined. Color bar indicates 7 possible temporal patterns: I-> rising, II-> plateau, III-> rising and plateau, IV-> trailing, V-> rising and trailing, VI-> plateau and trailing, VII-> rising, plateau and trailing phases. Numbers below figures are coordinates (in mm) relative to bregma. Activated structures include: 1, prelimbic cortex; 2, olfactory cortex; 3, motor cortex; 4, infralimbic cortex; 5, insular cortex; 6, cingulate cortex; 7, primary somatosensory forelimb region (S1FL); 8, caudate putamen; 9, globus pallidus; 10, secondary somatosensory cortex (S2); 11, primary somatosensory barrel field (S1BF); 12, hippocampus; 13, lateral hypothalamic nuclei; 14, habenular nuclei, 15, ventral posterior lateral and medial thalamic nuclei (VPL, VPM); 16, retrosplenial cortex; 17, auditory cortex; 18, periaqueductal gray (PAG); 19, fasciculus retroflexus and paranigral nucleus of the ventral tegmental area; 20, superior colliculus; 21, visual cortex.
In step three, we derived statistical maps of the main effect of cocaine challenge by collapsing all fMRI responses across the three treatment groups and performed t-tests against zero. Similar to the treatment effect ANOVA described above, cocaine main effect analyses were also performed for each of the three fMRI response phases, leading to three t-test maps. The same color-coding strategy was used to visualize the temporal patterns of cocaine main effects across the three phases.
Significance threshold and minimum cluster size were based on statistical simulation using AlphaSim in AFNI (Cox, 1996). A corrected p<0.05 (uncorrected p<0.01 with a cluster size of 38) was considered significant. Activation maps from the above analyses were overlaid onto a digital rat atlas (Paxinos and Watson, 2005) and co-registered with high-resolution anatomical images to accurately identify activated structures (Lu et al., 2010).
We also performed region-of-interest (ROI) analysis. This was done in two steps: first, a priori ROIs were anatomically defined based on the digital rat brain atlas registered to MRI images (Lu et al., 2010). Second, voxels within individual anatomical ROIs that demonstrated a significant group main effect were averaged to generate fMRI response curves. To explore behavioral correlates of these fMRI responses, we performed linear regression analyses of fMRI responses within each of the three temporal phases (rising, plateau and trailing phases) versus previous cocaine or sucrose intake history, including total number of reinforcers, escalation ratio, and difference in the number of reinforcers between the last and first SA day. Regression was performed using the R software package (http://www.r-project.org) and included group as a factor, intake history as a covariate and their interaction. A significant interaction would indicate a group difference in fMRI versus intake slopes.
Results
Behavior
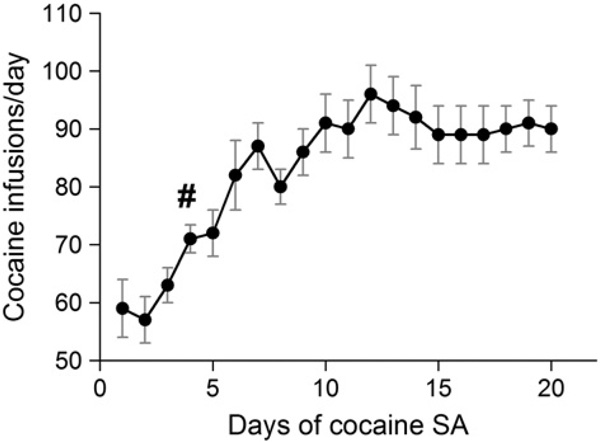
Daily cocaine infusions during the 20-day training period are shown in Fig. 1 (data from one rat unavailable). The number of infusions on the first day was 59±5 during the 6-h SA sessions. One-way repeated measures ANOVA with a post-hoc Dunnett's test revealed that starting on day 4, there was a significant increase in cocaine infusions (p<0.05), which stabilized at about 90 infusions for days 15–20. fMRI experiments were successfully performed on 10 rats; reinforcer intake data from one rat were not available, resulting in 9 cocaine SA rats in this analysis.
Fig. 1.
Daily cocaine infusions escalate over time using an FR-1 long-access (6 h/day) self-administration (SA) schedule (n=9; SA dose: 0.75 mg/kg per infusion,). #, The number of infusions on the fourth day is significantly higher than on the first day (p<0.05, one-way repeated measures ANOVA with post-hoc Dunnett's test).
fMRI
Fig. 2 illustrates the significant cocaine main effects. Of the three temporal phases (rising, plateau and trailing), several discrete regions, notably part of olfactory tubercle (bregma 3.64 mm), paranigral nuclei (PN) of the ventral tegmental area (VTA) and fasciculus retroflexus (fr) (bregma −5.36 mm) exhibit significant cocaine effects, but only in the rising phase. A large part of the caudate putamen (CPu), hippocampus, and thalamus as well as the habenular nuclei (bregma −4.36) also exhibited significant drug effects, but in both the rising and plateau phases. Finally, a number of regions, including prelimbic (PrL) and infralimbic (IL) cortex, olfactory tubercle, anterior cingulate cortex (ACC: CG1/CG2), retrospenial cortex (RSD/RSG), dorsal lateral CPu, thalamus and periaqueductal gray (PAG) as well as the primary and secondary somatosensory, visual and auditory cortex all exhibited significant main effects across all three phases.
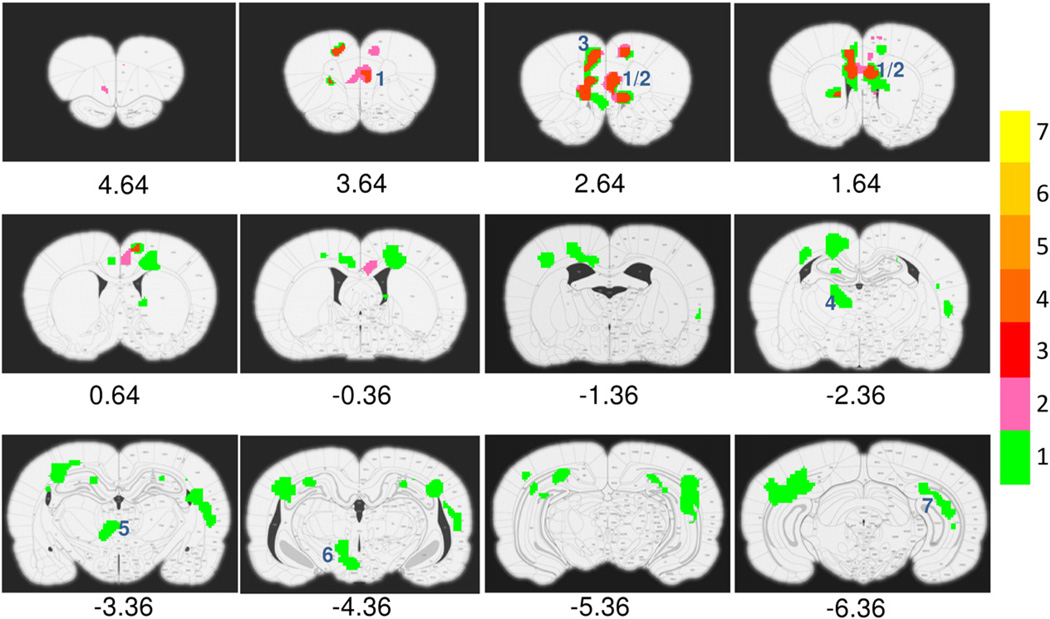
The fMRI responses differed during the rising phase as a function of GROUP (cocaine, sucrose, naïve) in such structures as medial prefrontal cortex (mPFC, PrL+IL), ACC, medial dorsal thalamus, hypothalamus and hippocampus. Significant differences during the plateau phase were seen only in mPFC and a small extension into ACC. There was no difference during the trailing phase between the 3 groups. Fig. 3 summarizes these results, which are very similar to the GROUP main effect derived from the GROUP×TIME ANOVA (data not shown). To further quantify the CBV responses, time courses of activated voxels within individual ROIs were averaged. Fig. 4 shows averaged responses across groups in mPFC, ACC, medial dorsal thalamus and hippocampus.
Fig. 3.
Statistical activation maps showing ANOVA main effect of GROUP (cocaine, sucrose and naïve). Binning and color bar are identical to Fig. 2. Note only in prelimbic, infralimbic and anterior cingulate cortices did the effect last through the rising and plateau phases. Numbers below figures are coordinates (in mm) relative to bregma. Numbers (blue color) inside each figure indicates activated structures: 1 and 2, prelimbic and infralimbic cortex; 3, anterior cingulate cortex; 4, medial dorsal thalamus; 5, central medial thalamic nuclei; 6, hypothalamic nuclei; 7, hippocampus.
Given the distinct physiological effects of cocaine and sucrose, we had hypothesized that rats with different SA histories would exhibit distinct neuronal response patterns to an acute cocaine injection. To our surprise, and in contrast to the large magnitude response seen in drug-naïve rats, we found similarly attenuated responses in both the cocaine- and sucrose SA groups (Fig. 4) to the acute cocaine challenge, which was further confirmed by a voxel-wise post-hoc t-test between these two groups. Post-hoc tests between naïve and either cocaine- or sucrose SA groups showed essentially the same results, which were very similar to Fig. 3.
Correlation of the fMRI signal with SA behavior
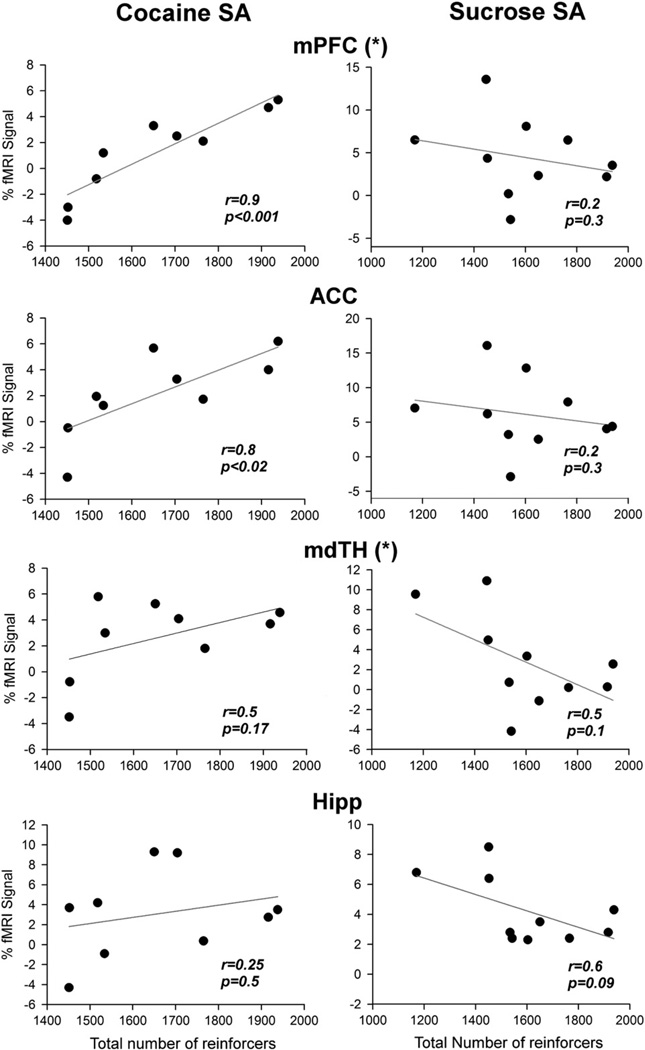
To further explore for neuroplastic changes specific to cocaine or sucrose SA, we performed linear regression analysis between the fMRI response amplitude and cocaine or sucrose SA history. Of the 10 cocaine SA rats and 12 sucrose SA rats successfully scanned, reinforcer intake data from one cocaine rat and 2 sucrose rats were not available, resulting in 9 data points in the cocaine SA group and 10 data points in the sucrose SA group for the linear regression analysis. As shown in Fig. 5, the fMRI response during the plateau phase in both mPFC and ACC was positively correlated with the total amount of cocaine intake during the cocaine SA sessions. In contrast, the responses in hippocampus and medial dorsal thalamus did not correlate with total cocaine intake. Furthermore, in bothmPFC and medial dorsal thalamus, the slopes resulting from linear regression analysis were significantly different between the cocaine and the sucrose SA groups (F(1,15)=5.95; p<0.03 and F(1,15)=5.17; p<0.04 respectively). There was no significant correlation between fMRI response and cocaine intake amount for either the first or the last day. These data suggest that prior cocaine SA history uniquely modified the fMRI responses in these areas, and that the plasticity was induced by extended cocaine exposure rather than by preexisting inter-subject difference.
Fig. 5.
Linear regression analysis of fMRI response versus the total amount of reinforcers self-administered during the entire training period. Abbreviations: mPFC, medial prefrontal cortex (PrL+IL); ACC, anterior cingulate cortex; mdTH, medial dorsal thalamic nuclei; Hipp, hippocampus. (*) Indicates the regression slope in the cocaine SA group is significantly different from that in the sucrose group (p<0.05).
Discussion
In this study we demonstrate that, using fMRI, rats experiencing 20 days of long-access cocaine SA followed by 30 days of forced abstinence exhibited attenuated neuronal responses to an acute IV cocaine injection in the mPFC and ACC. Surprisingly, a group of rats trained to SA sucrose and behaviorally matched to the cocaine SA group, also demonstrated a similarly attenuated response to cocaine in the same areas, even in the absence of any prior lifetime cocaine exposure. However, two major findings distinguish the two SA groups. First, linear regression analyses of the fMRI responses in mPFC and medial dorsal thalamus revealed significant differences in response slope between cocaine and sucrose SA groups as a function of lifetime reinforcer received, suggesting that cocaine SA history uniquely modified the neuronal response to drug challenge. Second, in both mPFC and ACC, the fMRI responses in rats trained to self-administer cocaine were correlated with individual differences in the total amount of cocaine intake during the training sessions. Given that the two SA groups had, by design, the same history of operant training, number of reinforcers and handling, the region-specific group differences cannot be attributed to general operant conditioning learning mechanisms or other common housing, handling or procedural matters. Rather, they may reflect the development of neuroadaptive mechanisms specifically related to the emergence of addiction-like behaviors that occur in the LgA cocaine, but not in the LgA sucrose rats.
Several lines of evidence suggest that chronic cocaine exposure leads to neuroadaptations in the striato-thalamo-cortical circuits. Previous studies in human cocaine addicts documented reduction in basal cerebral blood perfusion and glucose metabolism in the frontal cortex (Volkow et al., 1988, 1992) and decreases in dopamine release induced by systemic methylphenidate or amphetamine administration (Martinez et al., 2007; Volkow et al., 1997). Nonhuman primates trained to self-administer cocaine also demonstrate a decrease in local cerebral glucose utilization in the striatum (Beveridge et al., 2006; Porrino et al., 2004). Furthermore, rats experiencing repeated cocaine SA exhibit decreased basal neuronal activity in mPFC (Sun and Rebec, 2006) and NAc (Peoples et al., 2007a). Recently, resting state fMRI, a technique that detects functional connectivity based on synchronized low frequency fluctuations in the fMRI time course (Biswal et al., 1995), also revealed decreases in functional connectivity between mPFC and amygdala, and between mPFC and hippocampus in cocaine dependent individuals compared to healthy control subjects (Gu et al., 2010), suggesting neuroadaptations in a number of neural circuits within the MCL system. The attenuated fMRI response to cocaine challenge seen in our abstinent cocaine SA rats reinforces the critical role of striato-thalamo-cortical circuits in addiction.
It is somewhat surprising to see that sucrose SA rats, like cocaine SA rats, had generally similar averaged temporal response profiles following acute cocaine challenge (Fig. 4). The similar profiles could reflect either a general effect of the operant conditioning procedures to which both groups were exposed or common plastic neural adaptations to repeated sucrose and cocaine exposure and sustained abstinence (Avena et al., 2008). However, the magnitude of an individual's fMRI signal in both mPFC and ACC was positively correlated with cocaine, but not sucrose, SA intake history. Furthermore, in both mPFC and medial dorsal thalamus, cocaine SA history uniquely modified the fMRI signals, as demonstrated by different regression slopes between the two SA groups (Fig. 5). Notably, mPFC is the major recipient of medial dorsal thalamus output (Price, 2007), and in turn projects via glutamatergic outputs to key striatal components within cortico-striato-thalamo-cortical circuits. These circuits are thought to help translate motivational stimuli into adaptive motor responses and have been implicated in compulsive drug-seeking behavior (Goldstein and Volkow, 2002; Mogenson et al., 1980; Vanderschuren and Everitt, 2005). Excitotoxic lesion of the mediodorsal thalamic nucleus attenuates cocaine SA in rats (Weissenborn et al., 1998), while mediodorsal thalamic ablation impairs visual stimulus-reward associative learning in monkeys (Gaffan and Murray, 1990). Activation of GABAergic transmission in mediodorsal thalamus decreases dopamine transmission in mPFC (Churchill et al., 1996). Enhanced thalamic response to the indirect dopamine agonist methylphenidate is seen in human cocaine addicts (Volkowet al., 1997), although the imaging resolution did not allow differentiation of mediodorsal thalamus from other thalamic nuclei. Our data along with previous studies collectively suggest that the mediodorsal thalamic nucleus is a key locus where cocaine-specific neuroadaptations occur, and warrants further investigation.
Although natural rewards, such as sucrose, are thought to activate the MCL system in a similar fashion as addictive drugs (Volkow and Wise, 2005), there is a growing literature suggesting that cocaine SA and matched sucrose SA induce distinct neuroadaptive responses within brain regions implicated in drug addiction (Chen et al., 2008; Jones et al., 2008; Levy et al., 2007). In this regard, the report by Levy et al. (2007) is particularly relevant. In that study, repeated high frequency pulses to the frontal (prelimbic) cortex reduced both cocaine-seeking behavior and the motivation for its consumption as measured by both fix-ratio and progressive ratio cocaine SA schedules, but the stimulation did not affect sucrose reward-related behaviors, suggesting differential neural processes within the PFC that mediate cocaine versus sucrose seeking and taking behavior. Our current study demonstrates unique correlations in both mPFC and mediodorsal thalamus that differentiate cocaine SA from sucrose SA, suggesting a persistent pharmacologically mediated cocaine-induced neuroadaptation. Plasticity specific to cocaine SA, particularly when uniquely correlational with the emergence of addiction-like behavior (e.g. escalation of intake), may be candidate mechanisms that contribute specifically to pathological cocaine directed behavior.
Several preclinical studies suggest that acute cocaine increases metabolic activity in PFC, with previous cocaine history blunting this effect (Clow and Hammer, 1991; Febo et al., 2005; Hammer et al., 1993). While these studies employed experimenter-delivered injections and short periods of abstinence, the enforced abstinence model employed herein, one shown to engender addiction-like behaviors (Ahmed and Cador, 2006), demonstrates the neuroadaptive persistence, manifest here as an attenuated cocaine challenge response. Similar findings across both experimenter-delivered and self-administered drug delivery also support a pharmacological rather than a behavioral interpretation to our change drug response. The parallel findings of attenuatedmPFC activity in both human and preclinical models suggest that these neuroplastic changes may serve as a translational biomarker target in treatment.
Functional implications
As shown in Figs. 3 and 5, compared with drug-naïve animals, animals previously trained to self-administer cocaine exhibited a generally attenuated fMRI response to an acute cocaine injection. However, in mPFC and ACC, the amplitude of an individual animal's neuronal response was positively correlated with the total amount of cocaine intake during the SA sessions, suggesting that the cocaine training procedures might have induced two opposing processes, one that decreased the fMRI signal, and another that increased it. These two opposing processes may be related to differential adaptations in D1 and D2 receptor functions (Bowers et al., 2004; Nestler, 2001), since activation of D1 and D2 receptors differentially alters the CBV-fMRI signal: D1 antagonism and D2 agonism both decrease CBV-fMRI signal whereas D1 agonism and D2 antagonism increase CBV (Chen et al., 2005; Choi et al., 2006). In this regard, our data are in line with the growing literature implicating maladaptations in mPFC dopamine transmission and mPFC-NAc glutamate transmission as core mechanisms regulating cocaine-seeking behavior (Capriles et al., 2003; McFarland et al., 2003; Park et al., 2002), supporting the hypothesis that frontostriatal dysfunction may account for a core feature of addiction (Jentsch and Taylor, 1999; Kalivas et al., 2005). Indeed, a recent human imaging study demonstrated a reduction in dorsal ACC-striatal functional connectivity as a function of nicotine addiction severity (Hong et al., 2009).
An interesting question is whether the attenuated responses to cocaine challenge seen in the two SA groups were a result of a reward negative state following the enforced abstinence. In humans, acute cocaine administration induces euphoria whereas cocaine withdrawal leads to dysphoria and anhedonia (Gawin and Kleber, 1986). A recent study (Leventhal et al., 2010)with 43,093 American adults indicated that anhedonia appears to be uniquely associated with lifetime use of cocaine and amphetamines and lifetime progression from use to dependence, suggesting the generalizability and specificity of the anhedonia–stimulant relationship. In rodent studies, anhedonia associated with cocaine withdrawal has utilized intracranial self-stimulation (ICSS) protocols (Markou and Koob, 1991), a measure of the state of the animal's reward system. Although the magnitude and duration of the anhedonia state has been shown to be proportional to the amount of cocaine consumed during the binge, the ICSS threshold typically returns to pre-drug baseline following a relatively short duration of abstinence (<10 days), and does so for a variety of drugs tested, including cocaine, amphetamine and nicotine (Barr andMarkou, 2005; Markou and Koob, 1991). In the present study, animals experienced 30 days of forced abstinence, thus it seems unlikely that the reduced fMRI response to drug challenge were a result of a reward negative state. Instead, the attenuated responses may have been a manifestation of neuroadaptations revealed by forced abstinence. Further study with different abstinence durations, both longer and shorter, combined with ICSS or other paradigms, may help clarify this question.
Temporal patterns of fMRI responses to IV cocaine
Acute cocaine administration induced a brief negative response (reduced CBV), followed by a positive response. The early negative response was modest in the naïve group, but particularly prominent in the cocaine SA group. A brief negative response has been reported in drug-naïve rats receiving acute cocaine challenge (Marota et al., 2000), and is likely caused by cocaine's vasoconstrictive effects. In contrast, the positive CBV response was likely caused by enhanced neuronal activity as a result of cocaine. These two opposite effects on the vascular compete, leading to the observed biphasic response patterns. This conjecture is supported by a recentmultimodal optical imaging study showing a similar biphasic CBF response profile and enhanced calcium activity (an indicator of neuronal activity) immediately following cocaine injection (Yuan et al., 2011). Since we observed no differences in the arterial blood pressure profile across the three groups (Supplemental Fig. 2), cocaine likely induced similar vasoconstrictive effects independent of behavioral training. The more prominent negative response seen in the cocaine SA group might have been caused by reduced vasodilation rather than enhanced vasoconstriction as a result of neuronal firing adaptations. As the temporal resolution of this study was 30 s, higher temporal resolution imaging techniques (such as echo planar imaging)may provide further insight into this phenomenon.
Limitations and technical considerations
Both the cocaine and sucrose SA groups exhibited similar fMRI responses, while the sedentary control group demonstrated higher activation levels across the regions depicted. Given that addictive drugs such as cocaine and natural rewards such as sucrose activate similar reward system components, the similar fMRI responses between the two SA groups could reflect overlapping neuradaptation processes to these seemingly distinct reinforcers. Alternatively, this similar response pattern could be due to general operant conditioning procedures as well as the daily handling and enrichment to which both SA groups were exposed. To disambiguate this possibility would require a group of rats self-administering saline with operant procedures similar to the cocaine and sucrose SA rats.
Substantial clinical and preclinical data have demonstrated that stress is an important factor in drug addiction (Sinha et al., 2011). In this study, only cocaine SA rats were catheterized for training. As such, stress differentially associated with catheterization of this group may have partially confounded the results, although anesthesia during the fMRI experiments and the time between catheterization and fMRI data collection (more than 2 months) likely mitigated such effects. Additionally, since the goal of this study was to assess the reactivity of the brain reward system after LgA cocaine SA and one month of abstinence, the sucrose SA rats were intended to control for non-specific contextual and learning effects and motor behavior. In so far as both the cocaine and sucrose SA groups received only IV cocaine injection, although unlikely, we cannot discount the possibility that the correlation found in the cocaine SA group might reflect patterns of activation following exposure to a previously encountered reward by one of the two SA groups. Further experimentation is needed to address this possibility. Furthermore, due to a number of technical considerations during data acquisition, the saline and cocaine injections were not counterbalanced, which may confound a clear interpretation of the challenge data. Finally, this study was conducted under general anesthesia, and both basal neuronal activity and that induced by cocaine administration may have been modulated by anesthetics. However, any such modulatory effect should be similar across treatment groups, and thus it is unlikely that the group differences as seen in Fig. 3 were the results of general anesthesia.
In summary, we employed a rodent SA-abstinence model to investigate neural consequences of long-term cocaine exposure. In comparison to a treatment naïve group, an acute cocaine challenge produced an attenuated group averaged fMRI response in rats previously trained to self-administer either cocaine or sucrose in such MCL regions as mPFC and ACC. What did separate the two SA groups, however, was that the total amount of cocaine intake during SA training was positively correlated with the amplitude of fMRI response for individual animals, while the sucrose SA history did not bias subsequent cocaine-induced activation. Given that animals in the two SA groups experienced the same operant training and handling, this group difference may reflect specific neuroplasticity underlying the “addiction-like” behavior that occurs in LgA cocaine SA. Further study is needed to elucidate the underlying mechanisms mediating the attenuated cocaine challenge response.
Acknowledgments
We thank William Rea for his excellent technical assistance. This work was supported by the Intramural Research Program of the National Institute on Drug Abuse (NIDA) and by an NIH Director's Bench-to- Bedside grant to L. L. Peoples and E. A. Stein. It was also partially supported by P50DA012756, and R01 552981 (L.L.P.), Institute for Translational Medicine and Therapeutics of the University of Pennsylvania, and grant UL1RR024134 from the National Center for Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Footnotes
Appendix A. Supplementary data
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.neuroimage.2012.05.076.
References
- Ahmed SH, Cador M. Dissociation of psychomotor sensitization from compulsive cocaine consumption. Neuropsychopharmacology. 2006;31:563–571. doi: 10.1038/sj.npp.1300834. [DOI] [PubMed] [Google Scholar]
- Avena N, Rada P, Hoebel B. Evidence for sugar addiction: behavioral and neurochemical effects of intermittent, excessive sugar intake. Neurosci. Biobehav. Rev. 2008;32:20–39. doi: 10.1016/j.neubiorev.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DA, McFarland K, Lake RW, Shen H, Tang XC, Toda S, Kalivas PW. Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. Nat. Neurosci. 2003;6:743–749. doi: 10.1038/nn1069. [DOI] [PubMed] [Google Scholar]
- Barr AM, Markou A. Psychostimulant withdrawal as an inducing condition in animal models of depression. Neurosci. Biobehav. Rev. 2005;29:675–706. doi: 10.1016/j.neubiorev.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Beveridge TJ, Smith HR, Daunais JB, Nader MA, Porrino LJ. Chronic cocaine self-administration is associated with altered functional activity in the temporal lobes of non human primates. Eur. J. Neurosci. 2006;23:3109–3118. doi: 10.1111/j.1460-9568.2006.04788.x. [DOI] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn. Reson. Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Ghitza UE, Lu L, Epstein DH, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: an update and clinical implications. Eur. J. Pharmacol. 2005;526:36–50. doi: 10.1016/j.ejphar.2005.09.030. [DOI] [PubMed] [Google Scholar]
- Bowers MS, McFarland K, Lake RW, Peterson YK, Lapish CC, Gregory ML, Lanier SM, Kalivas PW. Activator of G protein signaling 3: a gatekeeper of cocaine sensitization and drug seeking. Neuron. 2004;42:269–281. doi: 10.1016/s0896-6273(04)00159-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capriles N, Rodaros D, Sorge RE, Stewart J. A role for the prefrontal cortex in stress- and cocaine-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl.) 2003;168:66–74. doi: 10.1007/s00213-002-1283-z. [DOI] [PubMed] [Google Scholar]
- Chen YC, Choi JK, Andersen SL, Rosen BR, Jenkins BG. Mapping dopamine D2/D3 receptor function using pharmacological magnetic resonance imaging. Psychopharmacology (Berl.) 2005;180:705–715. doi: 10.1007/s00213-004-2034-0. [DOI] [PubMed] [Google Scholar]
- Chen B, Bowers M, Martin M, Hopf F, Guillory A, Carelli R, Chou J, Bonci A. Cocaine but not natural reward self-administration nor passive cocaine infusion produces persistent LTP in the VTA. Neuron. 2008;59:288–297. doi: 10.1016/j.neuron.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JK, Chen YI, Hamel E, Jenkins BG. Brain hemodynamic changes mediated by dopamine receptors: role of the cerebral microvasculature in dopamine-mediated neurovascular coupling. Neuroimage. 2006;30:700–712. doi: 10.1016/j.neuroimage.2005.10.029. [DOI] [PubMed] [Google Scholar]
- Churchill L, Zahm DS, Duffy P, Kalivas PW. The mediodorsal nucleus of the thalamus in rats .2. Behavioral and neurochemical effects of GABA agonists. Neuroscience. 1996;70:103–112. doi: 10.1016/0306-4522(95)00352-j. [DOI] [PubMed] [Google Scholar]
- Clow DW, Hammer RP., Jr Cocaine abstinence following chronic treatment al-ters cerebral metabolism in dopaminergic reward regions. Bromocriptine en-hances recovery. Neuropsychopharmacology. 1991;4:71–75. [PubMed] [Google Scholar]
- Cox R. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Epstein DH, Preston KL, Stewart J, Shaham Y. Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacology (Berl.) 2006;189:1–16. doi: 10.1007/s00213-006-0529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Febo M, Segarra AC, Tenney JR, Brevard ME, Duong TQ, Ferris CF. Imaging cocaine-induced changes in the mesocorticolimbic dopaminergic system of conscious rats. J. Neurosci. Methods. 2004;139:167–176. doi: 10.1016/j.jneumeth.2004.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Febo M, Segarra AC, Nair G, Schmidt K, Duong TQ, Ferris CF. The neural consequences of repeated cocaine exposure revealed by functional MRI in awake rats. Neuropsychopharmacology. 2005;30:936–943. doi: 10.1038/sj.npp.1300653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffan D, Murray EA. Amygdalar interaction with the mediodorsal nucleus of the thalamus and the ventromedial prefrontal cortex in stimulus reward associative learning in the monkey. J. Neurosci. 1990;10:3479–3493. doi: 10.1523/JNEUROSCI.10-11-03479.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawin FH, Kleber HD. Abstinence symptomatology and psychiatric-diagnosis in cocaine abusers—clinical observations. Arch. Gen. Psychiatry. 1986;43:107–113. doi: 10.1001/archpsyc.1986.01800020013003. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am. J. Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu H, Salmeron BJ, Ross TJ, Geng X, Zhan W, Stein EA, Yang Y. Mesocorticolimbic circuits are impaired in chronic cocaine users as demonstrated by resting-state functional connectivity. Neuroimage. 2010;53:593–601. doi: 10.1016/j.neuroimage.2010.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer RP, Pires WS, Markou A, Koob GF. Withdrawal following cocaine self-administration decreases regional cerebral metabolic rate in critical brain reward regions. Synapse. 1993;14:73–80. doi: 10.1002/syn.890140110. [DOI] [PubMed] [Google Scholar]
- Hollander JA, Carelli RM. Cocaine-associated stimuli increase cocaine seeking and activate accumbens core neurons after abstinence. J. Neurosci. 2007;27:3535–3539. doi: 10.1523/JNEUROSCI.3667-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong LE, Gu H, Yang Y, Ross TJ, Salmeron BJ, Buchholz B, Thaker GK, Stein EA. Association of nicotine addiction and nicotine's actions with separate cingulate cortex functional circuits. Arch. Gen. Psychiatry. 2009;66:431–441. doi: 10.1001/archgenpsychiatry.2009.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology (Berl.) 1999;146:373–390. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- Jones JL, Wheeler RA, Carelli RM. Behavioral responding and nucleus accumbens cell firing are unaltered following periods of abstinence from sucrose. Synapse. 2008;62:219–228. doi: 10.1002/syn.20486. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. Glutamatesystems incocaineaddiction. Curr. Opin. Pharmacol. 2004;4:23–29. doi: 10.1016/j.coph.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Kalivas P, Volkow N, Seamans J. Unmanageable motivation in addiction: a pathology in prefrontal-accumbens glutamate transmission. Neuron. 2005:647–650. doi: 10.1016/j.neuron.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Berridge KC. The neuroscience of natural rewards: relevance to addictive drugs. J. Neurosci. 2002;22:3306–3311. doi: 10.1523/JNEUROSCI.22-09-03306.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyatkin EA, Lenoir M. Intravenous saline injection as an interoceptive signal in rats. Psychopharmacology (Berl.) 2011;217:387–396. doi: 10.1007/s00213-011-2294-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, Brightman M, Ameringer KJ, Greenberg J, Mickens L, Ray LA, Sun P, Sussman S. Anhedonia associated with stimulant use and dependence in a population-based sample of American adults. Exp. Clin. Psychopharmacol. 2010;18:562–569. doi: 10.1037/a0021964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy D, Shabat-Simon M, Shalev U, Barnea-Ygael N, Cooper A, Zangen A. Repeated electrical stimulation of reward-related brain regions affects cocaine but not"Natural" reinforcement. J. Neurosci. 2007;27:14179–14189. doi: 10.1523/JNEUROSCI.4477-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Grimm JW, Dempsey J, Shaham Y. Cocaine seeking over extended withdrawal periods in rats: different time courses of responding induced by cocaine cues versus cocaine priming over the first 6 months. Psychopharmacology (Berl.) 2004;176:101–108. doi: 10.1007/s00213-004-1860-4. [DOI] [PubMed] [Google Scholar]
- Lu H, Scholl CA, Zuo Y, Stein EA, Yang Y. Quantifying the blood oxygenation level dependent effect in cerebral blood volume-weighted functional MRI at 9.4 T. Magn. Reson. Med. 2007a;58:616–621. doi: 10.1002/mrm.21354. [DOI] [PubMed] [Google Scholar]
- Lu H, Xi ZX, Gitajn L, Rea W, Yang Y, Stein EA. Cocaine-induced brain activation detected by dynamic manganese-enhanced magnetic resonance imaging (MEMRI) Proc. Natl. Acad. Sci. U. S. A. 2007b;104:2489–2494. doi: 10.1073/pnas.0606983104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Yang S, Zuo Y, Demny S, Stein EA, Yang Y. Real-time animal functional magnetic resonance imaging and its application to neuropharmacological studies. Magn. Reson. Imaging. 2008;26:1266–1272. doi: 10.1016/j.mri.2008.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Scholl CA, Zuo Y, Demny S, Rea W, Stein EA, Yang Y. Registering and analyzing rat fMRI data in the stereotaxic framework by exploiting intrinsic anatomical features. Magn. Reson. Imaging. 2010;28:146–152. doi: 10.1016/j.mri.2009.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo F, Wu G, Li Z, Li SJ. Characterization of effects of mean arterial blood pressure induced by cocaine and cocaine methiodide on BOLD signals in rat brain. Magn. Reson. Med. 2003;49:264–270. doi: 10.1002/mrm.10366. [DOI] [PubMed] [Google Scholar]
- Mandeville JB, Marota JJ, Kosofsky BE, Keltner JR, Weissleder R, Rosen BR, Weisskoff RM. Dynamic functional imaging of relative cerebral blood volume during rat forepaw stimulation. Magn. Reson. Med. 1998;39:615–624. doi: 10.1002/mrm.1910390415. [DOI] [PubMed] [Google Scholar]
- Markou A, Koob GF. Postcocaine anhedonia—an animal-model of cocaine withdrawal. Neuropsychopharmacology. 1991;4:17–26. [PubMed] [Google Scholar]
- Marota JJ, Mandeville JB, Weisskoff RM, Moskowitz MA, Rosen BR, Kosofsky BE. Cocaine activation discriminates dopaminergic projections by temporal response: an fMRI study in rat. Neuroimage. 2000;11:13–23. doi: 10.1006/nimg.1999.0520. [DOI] [PubMed] [Google Scholar]
- Martinez D, Narendran R, Foltin RW, Slifstein M, Hwang DR, Broft A, Huang Y, Cooper TB, Fischman MW, Kleber HD, Laruelle M. Amphetamine-induced dopamine release: markedly blunted in cocaine dependence and predictive of the choice to self-administer cocaine. Am. J. Psychiatry. 2007;164:622–629. doi: 10.1176/ajp.2007.164.4.622. [DOI] [PubMed] [Google Scholar]
- McFarland K, Lapish CC, Kalivas PW. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J. Neurosci. 2003;23:3531–3537. doi: 10.1523/JNEUROSCI.23-08-03531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogenson GJ, Jones DL, Yim CY. From motivation to action: functional interface between the limbic system and the motor system. Prog. Neurobiol. 1980;14:69–97. doi: 10.1016/0301-0082(80)90018-0. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Molecular basis of long-term plasticity underlying addiction. Nat. Rev. Neurosci. 2001;2:119–128. doi: 10.1038/35053570. [DOI] [PubMed] [Google Scholar]
- O'Brien CP. Progress in the science of addiction. Am. J. Psychiatry. 1997;154:1195–1197. doi: 10.1176/ajp.154.9.1195. [DOI] [PubMed] [Google Scholar]
- Park WK, Bari AA, Jey AR, Anderson SM, Spealman RD, Rowlett JK, Pierce RC. Cocaine administered into the medial prefrontal cortex reinstates cocaine-seeking behavior by increasing AMPA receptor-mediated glutamate transmission in the nucleus accumbens. J. Neurosci. 2002;22:2916–2925. doi: 10.1523/JNEUROSCI.22-07-02916.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 6th ed. Elsevier Inc.; 2005. [DOI] [PubMed] [Google Scholar]
- Peoples LL, Kravitz AV, Guillem K. The role of accumbal hypoactivity in cocaine addiction. ScientificWorldJournal. 2007a;7:22–45. doi: 10.1100/tsw.2007.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peoples LL, Kravitz AV, Lynch KG, Cavanaugh DJ. Accumbal neurons that are activated during cocaine self-administration are spared from inhibitory effects of repeated cocaine self-administration. Neuropsychopharmacology. 2007b;32:1141–1158. doi: 10.1038/sj.npp.1301203. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Bell K, Duffy P, Kalivas PW. Repeated cocaine augments excitatory amino acid transmission in the nucleus accumbens only in rats having developed behavioral sensitization. J. Neurosci. 1996;16:1550–1560. doi: 10.1523/JNEUROSCI.16-04-01550.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porrino L, Lyons D, Smith H, Daunais J, Nader M. Cocaine self-administration produces a progressive involvement of limbic, association, and sensorimotor striatal domains. J. Neurosci. 2004;24:3554–3562. doi: 10.1523/JNEUROSCI.5578-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JL. Definition of the orbital cortex in relation to specific connections with limbic and visceral structures and other cortical regions. Ann. N. Y. Acad. Sci. 2007;1121:54–71. doi: 10.1196/annals.1401.008. [DOI] [PubMed] [Google Scholar]
- Ritz M, Cone E, Kuhar M. Cocaine inhibition of ligand binding at dopamine, norepinephrine and serotonin transporters: a structure–activity study. Life Sci. 1990;46:635–645. doi: 10.1016/0024-3205(90)90132-b. [DOI] [PubMed] [Google Scholar]
- Sinha R, Shaham Y, Heilig M. Translational and reverse translational research on the role of stress in drug craving and relapse. Psychopharmacology (Berl.) 2011;218:69–82. doi: 10.1007/s00213-011-2263-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart J, Dewit H, Eikelboom R. Role of unconditioned and conditioned drug effects in the self-administration of opiates and stimulants. Psychol. Rev. 1984;91:251–268. [PubMed] [Google Scholar]
- Sun W, Rebec GV. Repeated cocaine self-administration alters processing of cocaine-related information in rat prefrontal cortex. J. Neurosci. 2006;26:8004–8008. doi: 10.1523/JNEUROSCI.1413-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trantham H, Szumlinski KK, McFarland K, Kalivas PW, Lavin A. Repeated cocaine administration alters the electrophysiological properties of prefrontal cortical neurons. Neuroscience. 2002;113:749–753. doi: 10.1016/s0306-4522(02)00246-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungless M, Whistler J, Malenka R, Bonci A. Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature. 2001;411:583–587. doi: 10.1038/35079077. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Everitt BJ. Drug seeking becomes compulsive after prolonged cocaine self-administration. Science. 2004;305:1017–1019. doi: 10.1126/science.1098975. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Everitt BJ. Behavioral and neural mechanisms of compulsive drug seeking. Eur. J. Pharmacol. 2005;526:77–88. doi: 10.1016/j.ejphar.2005.09.037. [DOI] [PubMed] [Google Scholar]
- Volkow N, Wise R. How can drug addiction help us understand obesity? Nat. Neurosci. 2005;8:555–560. doi: 10.1038/nn1452. [DOI] [PubMed] [Google Scholar]
- Volkow N, Mullani N, Gould K, Adler S, Krajewski K. Cerebral blood-flow in chronic cocaine users—a study with positron emission tomography. BrJ. Psychiatry. 1988:641–648. doi: 10.1192/bjp.152.5.641. [DOI] [PubMed] [Google Scholar]
- Volkow N, Hitzemann R, Wang G, Fowler J, Wolf A, Dewey S, Handlesman L. Long-term frontal brain metabolic changes in cocaine abusers. Synapse. 1992:184–190. doi: 10.1002/syn.890110303. [DOI] [PubMed] [Google Scholar]
- Volkow N, Wang G, Fowler J, Logan J, Gatley S, Hitzemann R, Chen A, Dewey S, Pappas N. Decreased striatal dopaminergic responsiveness in detoxified cocaine-dependent subjects. Nature. 1997:830–833. doi: 10.1038/386830a0. [DOI] [PubMed] [Google Scholar]
- Weissenborn R, Whitelaw RB, Robbins TW, Everitt BJ. Excitotoxic lesions of the mediodorsal thalamic nucleus attenuate intravenous cocaine self administration. Psychopharmacology. 1998;140:225–232. doi: 10.1007/s002130050761. [DOI] [PubMed] [Google Scholar]
- Wise RA, Wang B, You ZB. Cocaine serves as a peripheral interoceptive conditioned stimulus for central glutamate and dopamine release. PLoS One. 2008;3 doi: 10.1371/journal.pone.0002846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You ZB, Wang B, Zitzman D, Azari S, Wise RA. A role for conditioned ventral tegmental glutamate release in cocaine seeking. J. Neurosci. 2007;27:10546–10555. doi: 10.1523/JNEUROSCI.2967-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Z, Luo Z, Volkow ND, Pan Y, Du C. Imaging separation of neuronal from vascular effects of cocaine on rat cortical brain in vivo. Neuroimage. 2011;54:1130–1139. doi: 10.1016/j.neuroimage.2010.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]