Abstract
The ubiquitin-proteolytic system (UPS) regulates a variety of cellular and biological processes by controlling the stability of regulatory proteins, in space and time. Not surprisingly, defects in this system have been associated with various syndromes and pathologies, including cancer, illustrating the importance of understanding the regulation and the multiple functions of this system. C. elegans is a powerful model system to identify components of the UPS and to study their function during development in multicellular organisms. In C. elegans, the evolutionarily conserved CRL2LRR-1 E3-ligase is critical for the development of the germline. Inactivation of the CUL-2 scaffold or the LRR-1 substrate-recognition subunit leads to a cell cycle arrest in germline stem cells resulting in sterility. Through a genetic screen, we have identified a cul-2 temperature-sensitive allele and we have used this allele to show that CUL-2 plays multiple roles in the development of the germline. CUL-2 (1) promotes germ cell proliferation, (2) influences the balance between mitotic proliferation and meiotic differentiation, and (3) inhibits the first step of meiotic prophase. Here, we discuss how CUL-2 regulates and coordinates these different processes. We suggest that ubiquitin-mediated protein degradation constitutes an important additional layer of regulation that contributes to the spatial organization of the germline.
Keywords: CUL-2, LRR-1, E3 ubiquitin-ligase, C. elegans, DNA replication checkpoint, germline, proliferation, differentiation
CRL2LRR-1 E3-ligase is Essential for Germline Development and Embryogenesis in C. elegans
The ubiquitin-proteolytic system (UPS) comprises a series of enzymatic reactions leading to the covalent addition of ubiquitin chains onto lysine residues of protein substrates, targeting their subsequent degradation by the 26S proteasome, a large macromolecular complex with protease activities.1 Polyubiquitination of the substrate requires the coordinated action of three enzymes: E1 ubiquitin-activating enzymes, E2 ubiquitin conjugating-enzymes, and E3 ubiquitin-ligases.2,3 Together, these enzymes activate and transfer ubiquitin to target proteins by promoting the formation of an isopeptide bond between a lysine residue of the substrate and the C terminus of ubiquitin. Reiteration of this catalytic cycle assembles ubiquitin chains on the substrate, targeting its recognition and degradation by the 26S proteasome.
The paramount regulatory step in the system is the selective recognition of the substrate, which is achieved by E3-enzymes. The most prominent family of E3-enzymes is composed of multisubunit Cullin-RING E3-Ligases (CRLs) comprising exchangeable substrate-recognition modules nucleated by a specific Cullin RING-based catalytic core.4-8 Eukaryotic genomes encode several cullin subunits, which function as platform of specific E3-enzymes. Whereas most cullins are evolutionarily conserved from yeast to human, Cul2 is present only in multicellular organisms.9,10 However, the role of Cul2 and the majority of its targets remain to be found.
In C. elegans, CUL-2 is highly expressed in the germline where it is essential for cell cycle progression.11 cul-2 loss-of-function animals are defective in germline stem cell (GSC) proliferation resulting in animal sterility. To further dissect cul-2 function in the germline, we searched for the substrate-recognition subunit (SRS) acting together with CUL-2 to regulate GSC proliferation. Substrate-recognition modules of CRL2 complexes comprise the core subunits ELC-1 and ELB-1 that link the N-terminal part of CUL-2 to specific SRS termed BC-Cul2 box because these proteins share small regions, termed BC and Cul2 box, which are required for binding ELC-1 and CUL-2, respectively.12,13 We and others identified the evolutionarily conserved Leucine Rich Repeat protein LRR-1 as the specific SRS acting together with CUL-2 to regulate germ cell proliferation.14,15 LRR-1 is abundant in the germline and lrr-1-null [lrr-1(0)] mutant animals, similarly to cul-2 mutants, are defective in germ cell proliferation resulting in animal sterility.
Hyperactivation of the DNA Replication Checkpoint Causes Germ Cell Cycle Arrest and Sterility in lrr-1 and cul-2 Mutants
In order to understand the cause of the cell cycle arrest in lrr-1(0) mutant germ cells, we performed a visual RNAi-based suppressor screen. We constructed an lrr-1(0) strain expressing the Histone H2B fused to GFP under the control of the germline-specific promoter pie-1 and screened for genes whose inactivation by RNAi suppressed the germ cell cycle arrest of lrr-1(0) mutant, and restored their fertility (Fig. 1A). This visual screen led to the identification of CHK-1 (Chk1 in humans for checkpoint kinase 1) and ATL-1 (ATR, Ataxia telangiectasia and Rad3 related) kinases, which are core components of the DNA replication checkpoint pathway.16-18 This checkpoint pathway is typically activated in response to defects in DNA replication, such as stalled replication forks, and blocks cell cycle progression in G2 phase.19,20 These observations indicated that the DNA replication checkpoint is hyperactivated in lrr-1(0) mutants and prevents mitotic proliferation of GSC resulting in animal sterility.14
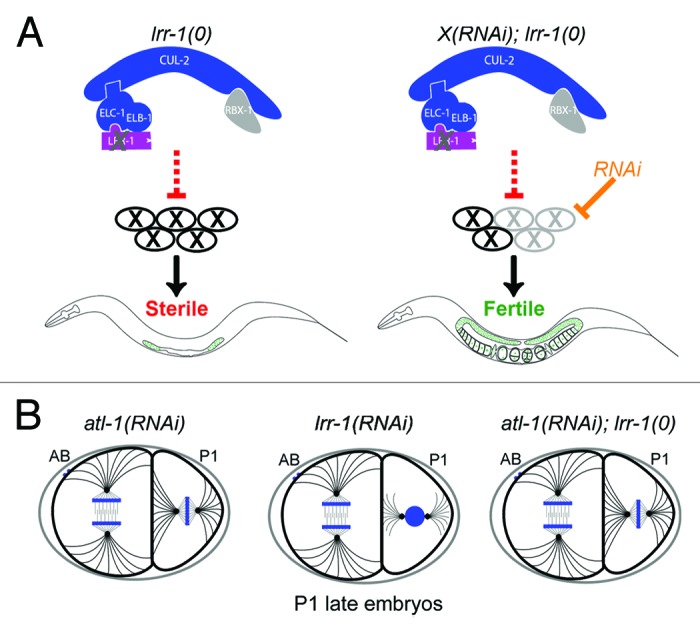
Figure 1. Hyperactivation of the DNA replication checkpoint pathway in lrr-1(0) mutant animals. (A) Flow-chart of the RNAi-based visual suppressor screen employed to search for lrr-1(0) suppressors. The CRL2LRR-1 complex is presented, the scaffold CUL-2, the adaptor ELC-1 and ELB-1 are in blue, RBX-1 in gray, and the LRR-1 substrate-recognition subunit in purple. X, substrates or pathways activated upon loss of lrr-1 function. The screen was designed to search for genes whose inactivation by RNAi suppress lrr-1(0) mutant sterility. (B) Schematic of dividing embryos of the indicated genotypes. Note that inactivation of lrr-1 delays division of the P1 blastomere and this delay is suppressed by inactivation of the DNA replication checkpoint.
Hyperactivation of the DNA replication pathway in absence of lrr-1 function is not only occurring in the germline but also in early embryos where RNAi-mediated knockdown of lrr-1 causes activation of the DNA replication checkpoint resulting in a severe delay in the division of the P1 blastomere at the two-cell stage (aka P1 late phenotype, Fig. 1B).14
To further dissect the role of the CRL2LRR-1 E3-enzyme, we searched for temperature-sensitive (ts) mutants affecting the function of this enzyme. We took advantage of the unique phenotype of the lrr-1(0) mutant to screen for ts mutants presenting similar phenotypes. More specifically, we screened a collection of temperature-sensitive mutants presenting a P1 late phenotype and searched for mutants that are sterile at restrictive temperature (25 °C) but fertile in absence of DNA replication checkpoint, like lrr-1(0) mutants. By this approach, we identified or209, the first cul-2 temperature-sensitive allele. cul-2(or209ts) mutant animals are largely sterile at restrictive temperature but recover fertility in absence of DNA replication checkpoint pathway components. The finding of a cul-2 allele in this simple genetic screen confirmed our previous observations, indicating that the DNA replication checkpoint blocks cell cycle progression in the germline upon inactivation of the CRL2LRR-1 E3-enzyme.14
Why is the ATL-1 checkpoint pathway hyperactivated when CRL2LRR-1 function is compromised? We believe that one function of this E3-ligase is to regulate DNA replication integrity in germ cells and in early embryos. The ATL-1 pathway is most likely primarily activated in response to DNA replication defects in the lrr-1(0)14 and cul-2(or209ts) mutants.21 Consistent with this hypothesis, ssDNA accumulates in lrr-1 mutant germ cells, as revealed by the appearance of RPA-1 foci.14 RPA-1 binds single-stranded (ss) DNA and ssDNA-RPA-1 complexes contribute to the recruitment and activation of the DNA replication checkpoint pathway leading to cell cycle arrest in germline stem cells.
What causes the accumulation of ssDNA? We have shown that a fraction of germ cells accumulate with a DNA content greater than 4N in lrr-1(0) mutants suggesting that some regions of the genome might undergo re-replication;14 however, what causes DNA re-replication in these animals is currently unknown. LRR-1 may regulate the stability of factor(s) required for DNA replication. For instance, it has been shown in budding yeast that several key factors are limiting for DNA replication initiation22 and LRR-1 might control the stability of one of these factors. Alternatively, LRR-1 might regulate the activity of cyclin-dependent kinase 1, which regulates DNA replication initiation. The Cdk inhibitor CKI-1 has been proposed to be one of the targets of the CRL2LRR-1 complex in the germline.15 However, in our hands, inactivation of cki-1 by RNAi using different methods failed to suppress the germ cell proliferation defect observed in lrr-1(0) or cul-2ts mutant animals indicating that CKI-1 might not be the critical LRR-1 target in the germline. Consistent with this hypothesis, CKI-1 is apparently not expressed in the germline.23 Therefore, the CRL2LRR-1 substrate involved in DNA replication remains to be found. The role of the CRL2LRR-1 enzyme in DNA replication integrity is likely identical in germ cells and in early embryos. In summary, the CRL2LRR-1 enzyme promotes germ cell proliferation, most likely by regulating DNA replication integrity and, thereby, prevents activation of the DNA replication checkpoint.
Beyond Germ Cell Proliferation: Role of the CRL2LRR-1 E3-ligase in the Spatial Organization of the Germline
lrr-1- and cul-2-null mutants are defective in germ cell proliferation, therefore analyzing a role of the CRL2LRR-1 complex in later steps of germ cell development was not possible until the identification of the cul-2ts allele. Using this allele, we discovered additional phenotypes associated with the loss of cul-2 function. In particular, we obtained evidences indicating that CRL2LRR-1 influences the balance between germline stem cell proliferation and meiotic differentiation and we showed that this enzyme inhibits the assembly of the synaptonemal complex (SC).
In C. elegans, the balance between germ cell proliferation and meiotic differentiation is controlled by the GLP-1/Notch signaling pathway.24 The distal tip cell (DTC), which caps the distal end of the germline, provides the notch ligand. Downstream of Notch signaling, a regulatory network of post-transcriptional nature coordinates the decision to either proliferate or differentiate by entry into meiosis. Central in this network are the almost identical FBF-1/2 (pumilio) RNA-binding proteins, which repress in GSCs the translation of meiotic-promoting factors, including GLD-1, GLD-2/3, and CKI-2.23,25,26
We started to investigate a potential role of cul-2 in regulating the balance between germ cell proliferation and meiotic differentiation when we found that cul-2ts animals are hypersensitive to RNAi-mediated inactivation of FBF-1/2, an observation that we confirmed using genetic alleles. These results suggest that FBF-1/2 and CUL-2 may share common targets to inhibit meiotic differentiation by acting at the mRNA and protein level, respectively. Such complementary mechanisms would provide robustness to the meiotic entry decision. However, the CUL-2 target(s) involved in this process remains to be identified.
The decision to enter into meiosis must be coordinated with the timely production of meiotic chromosomal proteins, such as SC components, and their recruitment on meiotic chromosomes. FBF-1/2 plays a role in this coordination by repressing in GSC the expression of the SC components, including HIM-3, HTP-1/2, and SYP-1.27 However, the recruitment of these proteins on chromosomes depends on the activity of the HORMA-domain protein HTP-3.28,29 HTP-3 is expressed at low levels in GCSs but in contrast to the other structural components of meiotic chromosomes, HTP-3 expression is not regulated by FBF-1/2.27 We obtained substantial evidences indicating that HTP-3 is a substrate of CRL2LRR-1 E3-ligase. HTP-3 physically interacts with LRR-1 and accumulates upon inactivation of cul-2 or proteasome subunits. Whereas, inappropriate HTP-3 accumulation in GSC promotes the recruitment of HIM-3 on chromosomes, it is however not sufficient to trigger meiotic entry. These observations suggest that several independent pathways act together to regulate and coordinate meiotic entry. Notably, the cyclinE/Cdk2 kinase (CYE-1/CDK-2 in C. elegans) recently emerged as a critical regulator of the mitosis to meiosis entry decision.30,31 Indeed, cye-1 inactivation force mitotic-proliferating germ cells to enter into meiosis,21,30 suggesting that CYE-1/CDK-2 might inhibit the different pathways promoting meiotic entry.
Concluding Remarks
The identification of a cul-2 temperature-sensitive revealed exciting new insights into the role of the CRL2LRR-1 E3-ligase in the development of germline. This E3-enzyme promotes germ cell proliferation, most likely by controlling DNA replication integrity, but also influences the balance between mitotic proliferation and meiotic differentiation and prevents the premature assembly of the synaptonemal complex by regulating HTP-3 stability. It will be critical to identify the molecular mechanisms providing spatial regulation of HTP-3 degradation. Given that LRR-1 is expressed throughout the germline, we suspect that HTP-3 post-translational modifications in germline stem cells might promote its interaction with LRR-1. Equally important will be the identification of the other targets of the CRL2LRR-1 enzyme. Finally, given that LRR-1 is evolutionarily conserved, it will be interesting to determine whether LRR-1 plays similar functions in other organisms.
Acknowledgments
This work was supported grants from the CNRS (ATIP), the French National Research Agency (ANR) under grant No ANR-2012-BSV2-0001-01 and the City of Paris.
Submitted
07/05/13
Accepted
07/10/13
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/worm/article/25716
References
- 1.Hershko A, Heller H, Elias S, Ciechanover A. Components of ubiquitin-protein ligase system. Resolution, affinity purification, and role in protein breakdown. J Biol Chem. 1983;258:8206–14. [PubMed] [Google Scholar]
- 2.Ye Y, Rape M. Building ubiquitin chains: E2 enzymes at work. Nat Rev Mol Cell Biol. 2009;10:755–64. doi: 10.1038/nrm2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schulman BA, Harper JW. Ubiquitin-like protein activation by E1 enzymes: the apex for downstream signalling pathways. Nat Rev Mol Cell Biol. 2009;10:319–31. doi: 10.1038/nrm2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Willems AR, Schwab M, Tyers M. A hitchhiker’s guide to the cullin ubiquitin ligases: SCF and its kin. Biochim Biophys Acta. 2004;1695:133–70. doi: 10.1016/j.bbamcr.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 5.Petroski MD, Deshaies RJ. Function and regulation of cullin-RING ubiquitin ligases. Nat Rev Mol Cell Biol. 2005;6:9–20. doi: 10.1038/nrm1547. [DOI] [PubMed] [Google Scholar]
- 6.Bosu DR, Kipreos ET. Cullin-RING ubiquitin ligases: global regulation and activation cycles. Cell Div. 2008;3:7. doi: 10.1186/1747-1028-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Merlet J, Burger J, Gomes J-E, Pintard L. Regulation of cullin-RING E3 ubiquitin-ligases by neddylation and dimerization. Cell Mol Life Sci. 2009;66:1924–38. doi: 10.1007/s00018-009-8712-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deshaies RJ, Joazeiro CAP. RING domain E3 ubiquitin ligases. Annu Rev Biochem. 2009;78:399–434. doi: 10.1146/annurev.biochem.78.101807.093809. [DOI] [PubMed] [Google Scholar]
- 9.Marín I, Ferrús A. Comparative genomics of the RBR family, including the Parkinson’s disease-related gene parkin and the genes of the ariadne subfamily. Mol Biol Evol. 2002;19:2039–50. doi: 10.1093/oxfordjournals.molbev.a004029. [DOI] [PubMed] [Google Scholar]
- 10.Sarikas A, Hartmann T, Pan Z-Q. The cullin protein family. Genome Biol. 2011;12:220. doi: 10.1186/gb-2011-12-4-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feng H, Zhong W, Punkosdy G, Gu S, Zhou L, Seabolt EK, et al. CUL-2 is required for the G1-to-S-phase transition and mitotic chromosome condensation in Caenorhabditis elegans. Nat Cell Biol. 1999;1:486–92. doi: 10.1038/70272. [DOI] [PubMed] [Google Scholar]
- 12.Mahrour N, Redwine WB, Florens L, Swanson SK, Martin-Brown S, Bradford WD, et al. Characterization of Cullin-box sequences that direct recruitment of Cul2-Rbx1 and Cul5-Rbx2 modules to Elongin BC-based ubiquitin ligases. J Biol Chem. 2008;283:8005–13. doi: 10.1074/jbc.M706987200. [DOI] [PubMed] [Google Scholar]
- 13.Okumura F, Matsuzaki M, Nakatsukasa K, Kamura T. The Role of Elongin BC-Containing Ubiquitin Ligases. Front Oncol. 2012;2:10. doi: 10.3389/fonc.2012.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Merlet J, Burger J, Tavernier N, Richaudeau B, Gomes J-E, Pintard L. The CRL2LRR-1 ubiquitin ligase regulates cell cycle progression during C. elegans development. Development. 2010;137:3857–66. doi: 10.1242/dev.054866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Starostina NG, Simpliciano JM, McGuirk MA, Kipreos ET. CRL2(LRR-1) targets a CDK inhibitor for cell cycle control in C. elegans and actin-based motility regulation in human cells. Dev Cell. 2010;19:753–64. doi: 10.1016/j.devcel.2010.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aoki H, Sato S, Takanami T, Ishihara T, Katsura I, Takahashi H, et al. Characterization of Ce-atl-1, an ATM-like gene from Caenorhabditis elegans. Mol Gen Genet. 2000;264:119–26. doi: 10.1007/s004380000291. [DOI] [PubMed] [Google Scholar]
- 17.Brauchle M, Baumer K, Gönczy P. Differential activation of the DNA replication checkpoint contributes to asynchrony of cell division in C. elegans embryos. Curr Biol. 2003;13:819–27. doi: 10.1016/S0960-9822(03)00295-1. [DOI] [PubMed] [Google Scholar]
- 18.Kalogeropoulos N, Christoforou C, Green AJ, Gill S, Ashcroft NR. chk-1 is an essential gene and is required for an S-M checkpoint during early embryogenesis. Cell Cycle. 2004;3:1196–200. doi: 10.4161/cc.3.9.1116. [DOI] [PubMed] [Google Scholar]
- 19.Nyberg KA, Michelson RJ, Putnam CW, Weinert TA. Toward maintaining the genome: DNA damage and replication checkpoints. Annu Rev Genet. 2002;36:617–56. doi: 10.1146/annurev.genet.36.060402.113540. [DOI] [PubMed] [Google Scholar]
- 20.Lin JJ, Dutta A. ATR pathway is the primary pathway for activating G2/M checkpoint induction after re-replication. J Biol Chem. 2007;282:30357–62. doi: 10.1074/jbc.M705178200. [DOI] [PubMed] [Google Scholar]
- 21.Burger J, Merlet J, Tavernier N, Richaudeau B, Arnold A, Ciosk R, et al. CRL(2LRR-1) E3-ligase regulates proliferation and progression through meiosis in the Caenorhabditis elegans germline. PLoS Genet. 2013;9:e1003375. doi: 10.1371/journal.pgen.1003375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mantiero D, Mackenzie A, Donaldson A, Zegerman P. Limiting replication initiation factors execute the temporal programme of origin firing in budding yeast. EMBO J. 2011;30:4805–14. doi: 10.1038/emboj.2011.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kalchhauser I, Farley BM, Pauli S, Ryder SP, Ciosk R. FBF represses the Cip/Kip cell-cycle inhibitor CKI-2 to promote self-renewal of germline stem cells in C. elegans. EMBO J. 2011;30:3823–9. doi: 10.1038/emboj.2011.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kimble J, Crittenden SL. Controls of germline stem cells, entry into meiosis, and the sperm/oocyte decision in Caenorhabditis elegans. Annu Rev Cell Dev Biol. 2007;23:405–33. doi: 10.1146/annurev.cellbio.23.090506.123326. [DOI] [PubMed] [Google Scholar]
- 25.Crittenden SL, Bernstein DS, Bachorik JL, Thompson BE, Gallegos M, Petcherski AG, et al. A conserved RNA-binding protein controls germline stem cells in Caenorhabditis elegans. Nature. 2002;417:660–3. doi: 10.1038/nature754. [DOI] [PubMed] [Google Scholar]
- 26.Kershner A, Crittenden SL, Friend K, Sorensen EB, Porter DF, Kimble J. Germline Stem Cells and Their Regulation in the Nematode Caenorhabditis elegans. Adv Exp Med Biol. 2013;786:29–46. doi: 10.1007/978-94-007-6621-1_3. [DOI] [PubMed] [Google Scholar]
- 27.Merritt C, Seydoux G. The Puf RNA-binding proteins FBF-1 and FBF-2 inhibit the expression of synaptonemal complex proteins in germline stem cells. Development. 2010;137:1787–98. doi: 10.1242/dev.050799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goodyer W, Kaitna S, Couteau F, Ward JD, Boulton SJ, Zetka M. HTP-3 links DSB formation with homolog pairing and crossing over during C. elegans meiosis. Dev Cell. 2008;14:263–74. doi: 10.1016/j.devcel.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 29.Severson AF, Ling L, van Zuylen V, Meyer BJ. The axial element protein HTP-3 promotes cohesin loading and meiotic axis assembly in C. elegans to implement the meiotic program of chromosome segregation. Genes Dev. 2009;23:1763–78. doi: 10.1101/gad.1808809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fox PM, Vought VE, Hanazawa M, Lee M-H, Maine EM, Schedl T. Cyclin E and CDK-2 regulate proliferative cell fate and cell cycle progression in the C. elegans germline. Development. 2011;138:2223–34. doi: 10.1242/dev.059535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jeong J, Verheyden JM, Kimble J. Cyclin E and Cdk2 control GLD-1, the mitosis/meiosis decision, and germline stem cells in Caenorhabditis elegans. PLoS Genet. 2011;7:e1001348. doi: 10.1371/journal.pgen.1001348. [DOI] [PMC free article] [PubMed] [Google Scholar]


