Abstract
After over a century of progress, phototropism research still presents some fascinating challenges.
Though few and far between, phototropism studies through 1937 established a number of important principles. (1) Blue light is the active spectral region. (2) The phototropic stimulus is perceived by the coleoptile tip, and the consequences of the stimulation progress down into the growing region. (3) Lateral transport of auxin mediates the curvature response. (4) The reciprocity law holds for first positive curvature, whereas second positive curvature is time dependent. (5) Red light treatment had a major effect on phototropic sensitivity. Later studies established the following. (6) Seven blue light receptors (cryptochromes, phototropins, and three F-box proteins) were identified and characterized. (7) A flavin was established as the photoreceptor chromophore for all seven. (8) The chromophore domain, designated the LOV domain (for light, oxygen, or voltage), carries out a unique photochemistry. (9) LOV domains must be truly ancient chromophore domains. There remain some puzzles. The fluence-response threshold level for first positive curvature is far below that for phototropin photochemistry. Likewise, the fluence-response threshold level for the red light effect on coleoptile phototropism is far below those for phytochrome phototransformation. Cytological effects of red light are also very insensitive compared with the physiological effects of red light. What is the mechanism allowing for this extraordinary photosensitivity? How is phototropin specificity controlled? What are the functions of the phytochrome kinase substrate proteins in both phytochrome and phototropin responses? What mechanism leads to lateral auxin transport? Finally, are LOV domain proteins true photoreceptors in all of the bacteria in which they occur? If so, what is their biological function?
Even in the ancient world, astute observers noted that plants could turn to face the sunlight. What was originally designated heliotropism for plants that followed the sun eventually became divided into two distinct response categories: solar tracking (the real heliotropism), a repetitive and completely reversible turgor-driven process; and phototropism, an irreversible directional growth response determined by light direction. Over the past 200 years, a large number of brilliant biologists, including Julius Sachs (1864), Charles Darwin (1881), Frits Went (1928), and Kenneth Thimann (Went and Thimann, 1937) have applied their talents to examining and elucidating the mechanisms accounting for both of these responses. The entire history of phototropism and solar tracking parallels and is intertwined with that for a number of other blue light responses, found not just in higher plants but in bryophytes, ferns, algae, fungi, and, most recently bacteria. For a detailed account of this history, see Briggs (2006).
Progress in research on blue light-activated processes over the last half century was severely hampered by a lack of knowledge of the relevant blue light receptors. By contrast, the discovery and initial characterization of a red/far-red-reversible phytochrome (Butler et al., 1959) nurtured an enormous and sophisticated body of knowledge about these photoreceptors: their structure, their chromophores, their photophysics and photochemistry, and the extraordinary signal transduction networks that they rule. Meanwhile, although a huge and scattered body of knowledge on blue light responses in plants and fungi accumulated, there was scarcely a clue to what the photoreceptor(s) might be, and competing hypotheses abounded. Attention focused on the physical and physiological characterization of responses to blue light and, eventually, biochemical investigations of intriguing in vitro photochemistry. It was only with the advent of modern molecular genetics and its extraordinary capabilities that research on blue light photobiology finally began to catch up with phytochrome photobiology 20 years ago and the first blue light receptor, cryptochrome1 (cry1), became identified (Ahmad and Cashmore, 1993).
This review will go back to some of the puzzles of earlier years, a few over 100 years old, and provide a glimpse of the status of these puzzles today. They arose from studies of response kinetics, action spectroscopy, interactions between blue and red regions of the visible spectrum, and discrepancies between in vivo and in vitro results. Although the focus will be on higher plant phototropism, several other blue light responses will contribute to the discussion. No effort will be made to be comprehensive. Any effort here would be redundant with that of three recent excellent reviews on phototropism (Sakai and Haga, 2012; Christie and Murphy, 2013; Hohm et al., 2013) and on LOV (for light, oxygen, or voltage) domain photochemistry (Losi and Gärtner, 2011, 2012). The article concludes with a look at the unexpected role of LOV domains in prokaryotes.
WHAT ARE THE KINETIC PROPERTIES OF HIGHER PLANT PHOTOTROPISM?
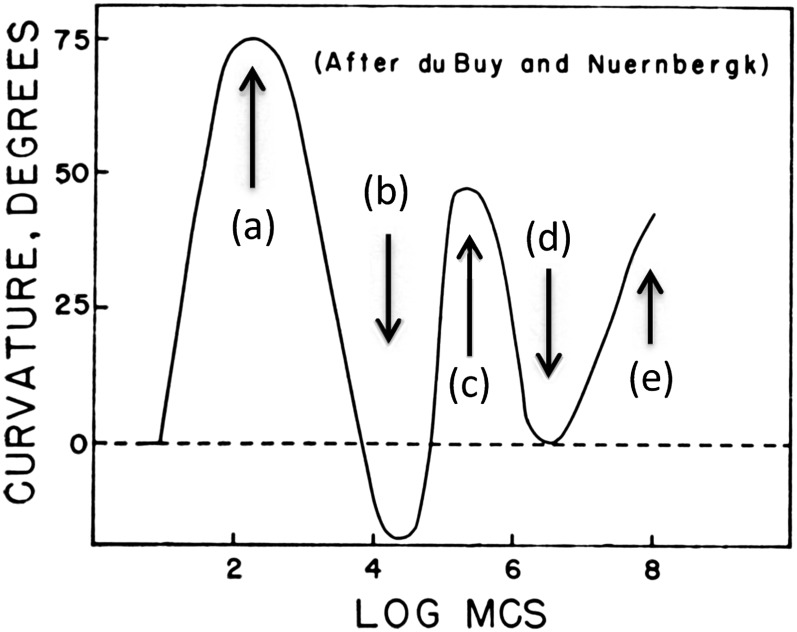
Over a century ago, Fröschel (1908) and Blaauw (1909) first reported that the phototropic responses of Lepidium spp. seedlings and Avena spp. coleoptiles, respectively, obeyed the reciprocity law of Bunsen and Roscoe (1862). The law states that as long as the light dose (fluence: intensity × time) is held constant, a given light response, in these two cases phototropism, will remain the same over a broad range of intensity × time combinations. In both cases, the authors were investigating threshold responses, something that later investigators tended to forget. Meanwhile, du Buy and Nuernbergk (1934) collected data from a number of authors and published the fluence-response curve shown in Figure 1 for the phototropic responses of Avena spp. coleoptiles over an immense range of fluences (8 orders of magnitude). The first maximum was designated first positive curvature, the immediately subsequent minimum first negative curvature, the second maximum second positive curvature, the next minimum a “zone of indifference,” and the final ascent third positive curvature. First positive curvature was usually located only a short distance below the coleoptile tip, whereas second positive curvature extended somewhat farther down the coleoptiles. For some reason, reciprocity tests were never carried out at any fluences above the threshold assays used by Fröschel (1908) and Blaauw (1909), although their conclusions, unfortunately, became accepted and applied to higher plant phototropism in general. All of this early work was with white light from a variety of sources, and the unit of fluence at the time was the meter-candle second (MCS).
Figure 1.
Fluence-response curve for the phototropic responses of Avena spp. coleoptiles assembled from the literature by du Buy and Nuernbergk (1934). From Briggs (1960).
Over 50 years ago, Briggs (1960) finally reinvestigated the reciprocity relationships of phototropism for maize (Zea mays) and oat (Avena sativa) coleoptiles over a wide range of fluences and demonstrated that the reciprocity law was valid only for first positive curvature but not for second. In all cases, for fluences above 10,000 MCS, maximum phototropic curvature for both species was attained after about 20 min of unilateral light stimulus irrespective of fluence. Indeed, a flashing-light experiment with a total fluence of just over 30,000 MCS showed that with increasing dark periods but constant flash duration, maximum curvature for maize coleoptiles was only achieved when the total exposure time, dark plus light, was 20 min or more.
Zimmerman and Briggs (1963a, 1963b) then developed a kinetic model for the phototropic responses of oat coleoptiles, based on a detailed series of fluence-response curves, developing a set of differential equations to describe the various curves. The model for first positive phototropism involved an initial photoreceptor activation and its subsequent photoinactivation, a two-step photoresponse. The model they developed for second positive curvature involved the production of an activated photoreceptor followed by a dark reaction that led to the curvature response. The model, therefore, incorporated a thermal reaction and hence time dependence for the response, accounting for the lack of reciprocity. It also required a second photoreaction that reversed the first photoactivation.
By the early 1960s, it was well established that first positive curvature is responding strictly according to first-order photochemistry: the response is linear with respect to the log of the number of photoreceptor molecules activated. Like first positive curvature, blue light activation of phototropin1 (phot1) phosphorylation also obeys the Bunsen-Roscoe reciprocity law both in vivo and in vitro (Briggs et al., 2001), consistent with this model. The descent of the response curve following the first positive maximum is then thought to involve a decrease in the difference between photoactivation of the photoreceptor on irradiated versus shaded sides of the coleoptiles. As fluences are increased, molecules of the photoreceptor on both sides of the coleoptiles all eventually become photoactivated and the differential disappears. This current hypothesis does not exactly match the model from Zimmerman and Briggs (1963b) for first positive phototropism. However, activation of photoreceptor molecules on the shaded side could replace the photoreceptor inactivation their model requires.
Let us examine the situation for second positive curvature. As exposure time increases, the activated phototropin molecules are postulated to relax gradually to their dark state and can now be reactivated. As there is a light gradient across the organ, reactivation is greater on the illuminated side than on the shaded side of the coleoptiles and a persistent differential becomes established, leading to second positive curvature, time dependent because it depends on the thermal decay rate for the activated photoreceptors. Again, the precise model of Zimmerman and Briggs (1963b) is not directly supported, but the differential reactivation of photoreceptors on the irradiated versus the shaded side of the responding organ could replace the reverse photoreaction they postulated.
Evidence for light-induced differential phosphorylation of phot1 across unilaterally illuminated oat coleoptiles (Salomon et al., 1997) supports the hypothesis that a light gradient leads to a biochemical gradient in an activated photoreceptor, both for first and second positive curvature. Differential activation of auxin-induced gene expression in Brassica oleracea hypocotyls between irradiated and shaded sides by blue light, activity downstream from light-activated phototropin (Esmon et al., 2006), likewise supports this hypothesis. Phototropin phosphorylation has been shown to be required for functional phototropism (Inoue et al., 2008, 2011). Thus, the time dependence for recovery of phosphorylated phototropin to its unphosphorylated (and physiologically inactive) dark state could account for the time dependence of second positive curvature. Indeed, the measured capacity of phot1 to undergo light-activated phosphorylation in vivo recovers over a period of minutes following a brief saturating light pulse (Briggs et al., 2001). (A saturating pulse is one that is thought to activate all of the photoreceptor molecules at once on both irradiated and shaded sides of the responding organ, a pulse that fails to induce curvature, the first minimum in the fluence-response curve for phototropism [for details, see Briggs et al., 2001].) The recovery of phototropic sensitivity after such a pulse follows roughly the same time course (Briggs, 1960).
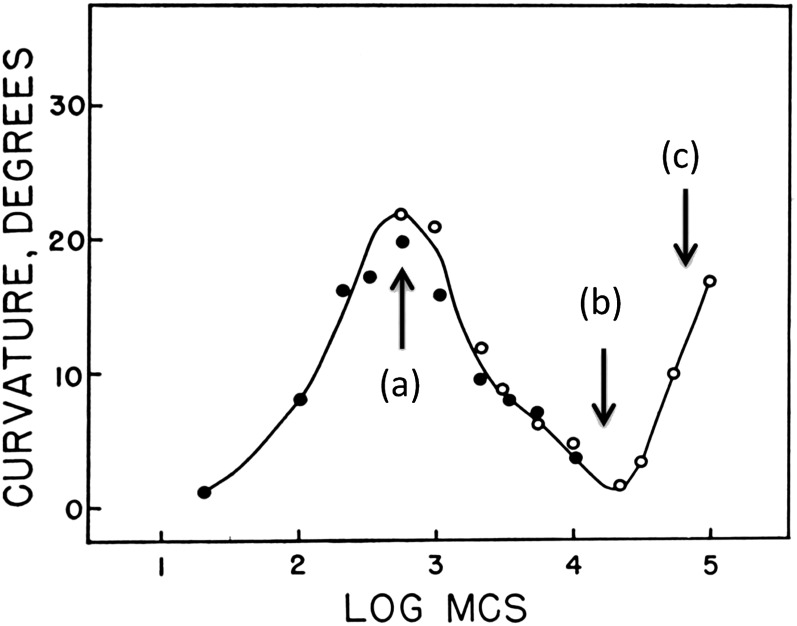
Two features of the du Buy and Nuernbergk (1934) fluence-response curve remain unexplained: first negative curvature and third positive curvature. First negative curvature has only been demonstrated for oat coleoptiles, is lacking for maize coleoptiles, and has never been reported for dicot seedlings. It remains a puzzle, although Zimmerman and Briggs (1963b) developed a model, similar to that for first positive curvature, that provided a good fit to the data. Third positive curvature might be explained by the fact that the data sets leading to this response differed widely in fluence rate and exposure time used. These differing light conditions could easily have led to curious anomalies in response to yield an apparent “third positive curvature.” The longer exposure times required to achieve these fluences varied, as did the light sources. Indeed, since 1937, there has been no further evidence for a third positive curvature in any species investigated. However, first and second positive curvatures appear to be universal among monocot coleoptiles and dicot seedlings (Fig. 2; Briggs, 1960; Zimmerman and Briggs 1963a; Chon and Briggs, 1966; Baskin and Iino, 1987; Iino, 2001; Esmon et al., 2006), including Arabidopsis (Arabidopsis thaliana; Konjević et al., 1989).
Figure 2.
Fluence-response curve for the phototropic responses of maize coleoptiles. From Briggs (1960).
There is a distressing feature in attempts to correlate light-activated phototropin phosphorylation in vivo with phototropism: the threshold and saturation fluences for blue light activation of first positive curvature lie between 1 and 2 orders of magnitude below those for phosphorylation (Briggs et al., 2001). The physiological response curve is simply not congruent with the photochemical response curve. This apparent paradox is currently unresolved.
HOW DOES RED LIGHT AFFECT PHOTOTROPISM?
Given that blue light activates phototropic curvature and red light normally does not (but see below), a majority of the early phototropism studies depended on red light as a safelight. However, Curry (1957) noted for the first time that small fluences of red light actually reduced the phototropic sensitivity of oat coleoptiles in the first positive curvature range by 1 order of magnitude. Zimmerman and Briggs (1963a, 1963b) confirmed this dramatic effect both for first positive curvature (and for first negative curvature) of oat coleoptiles and demonstrated in addition that red light had the opposite effect on second positive curvature: it increased its sensitivity by a factor of 3. Chon and Briggs (1966) reported similar results with maize coleoptiles. Chon and Briggs (1966) also demonstrated that the maize response to red light was fully far-red reversible and appeared to be a classic phytochrome response.
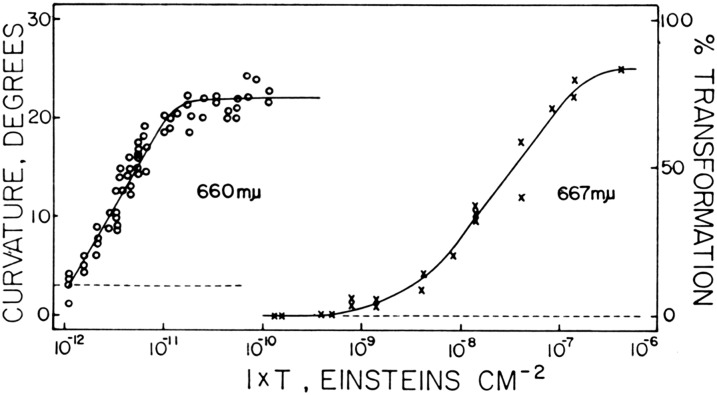
There is a problem with a simple interpretation of the results based on phytochrome. Quantitative experiments with maize coleoptiles led to a surprising finding: Briggs and Chon (1966) measured fluence-response relationships for the red light effect on first positive phototropism and compared it with those relationships for phytochrome transformation in vivo for maize coleoptiles. Surprisingly, phototropism was altered by fluences of red light 3 orders of magnitude too low to cause measureable phototransformation of phytochrome (Fig. 3). This discrepancy parallels the paradox in phototropism: blue light induces phototropic curvature at fluences far below those that induce detectable phototropin phosphorylation either in vitro or in vivo (see above). This result is all the more paradoxical in that the red light effect on phototropism is fully far-red reversible if the far-red light exposures are sufficiently short. (Longer far-red exposures themselves caused the same shift in phototropic sensitivity that red exposures did.) These highly sensitive responses were later designated “very low fluence” responses (see Mandoli and Briggs, 1981, who used slightly different terminology). This apparent paradox is also currently unresolved.
Figure 3.
Fluence-response curve for the phototropic curvature of maize coleoptiles (left) and for phytochrome phototransformation in vivo (right). From Briggs and Chon (1966).
The situation in Arabidopsis is somewhat different. Red light actually sensitizes both first and second positive curvature. Janoudi and Poff (1991) first showed that red light treatment enhanced phototropic curvature in the first positive range, subsequently confirmed and shown to be mediated by phyA (Hennig, 1996; Parks et al., 1996). Janoudi et al. (1992) showed that red light pretreatment actually shortened the lag period between the start of blue light irradiation and the onset of curvature, again an enhancement. Whippo and Hangarter (2004) demonstrated that the role of phytochromes was more complex and that phyB and phyD could also mediate the sensitization of Arabidopsis phototropism to higher blue light fluences (for further details, see Han et al., 2008). To date, there is no evidence for opposite effects of red light on first and second positive curvature in Arabidopsis.
Another effect of red light on etiolated coleoptiles might be related to red light-induced changes in phototropic sensitivity. Briggs (1963a) found that continuous red light treatment of etiolated maize coleoptiles reduced the amount of diffusible auxin that could be collected in agar blocks to roughly one-half of that from dark controls. The decrease was gradual, reaching a stable lower level after about 2 h. During a dark period after a 2-h red light pretreatment, the diffusible auxin yield gradually returned to the dark level, again over a recovery period of about 2 h. The time courses for these auxin changes were essentially parallel to the red light-induced decrease in phototropic sensitivity (2 h) and its subsequent recovery in the dark (2 h).
More recently, studies with phot1 tagged with GFP provided one possible mechanism for the red light-induced increase in phototropic sensitivity found in etiolated Arabidopsis hypocotyls. Sakamoto and Briggs (2002) first noted that blue light induced the movement of phot1-GFP from the plasma membrane into the cytoplasm, a response explored in detail by Wan et al. (2008). The response required 10 to 15 min to go to completion. Subsequently, Kong et al. (2006) reported a similar movement of phot2-GFP from the plasma membrane in Arabidopsis hypocotyl cells. Han et al. (2008) then found that red light given prior to phototropic induction almost completely eliminated the blue light-induced movement of phot1. The photoreceptor remained closely associated with the plasma membrane. Given that lateral auxin transport is certain to be associated with plasma membrane proteins, retention of a blue light receptor following red light treatment could well account for the observed sensitization of phototropism in Arabidopsis. Interestingly, this red light effect could only be observed in the actively growing region of the hypocotyls.
While the above mechanism may be valid for the red light-induced sensitization observed for Arabidopsis, it can hardly be the whole story. First, red light dramatically desensitizes coleoptiles in the first positive curvature range, rather than sensitizing them. Second, while first positive curvature loses sensitivity, second positive curvature gains it. Third, the threshold for this red light effect in coleoptiles is far below that reported by Han et al. (2008) for inducing the changes in phot1 subcellular relocalization in Arabidopsis (Briggs and Chon, 1966). Thus, the proposed mechanism, retention of phototropin at the plasma membrane, is clearly not the whole story. For the third time, an apparent discrepancy between physiology and photochemistry remains unresolved.
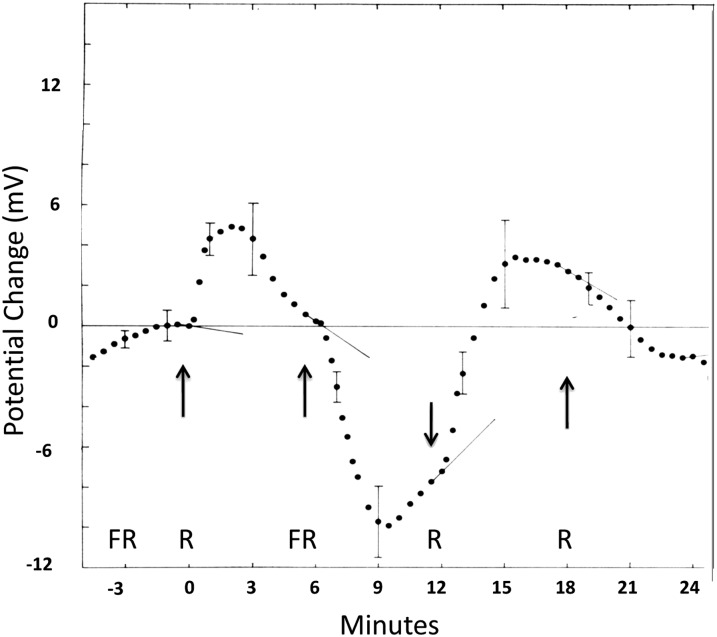
Newman and Briggs (1972) reported that red light treatment increased the electrical potential between the top 1 cm of etiolated oat coleoptiles by about 6 mV (with respect to a reference electrode in a 10 mm KCl solution containing the roots). The increase began about 10 s after the onset of red light and recovered to the dark level after about 6 min (Fig. 4). At that time, a pulse of far-red light led after about 15 s to a decrease in potential of about 10 mV followed by dark recovery over several minutes. A second red light pulse had no effect if there had not been an intervening far-red light pulse. These electrophysiological changes are clearly phytochrome mediated and indicate some sort of rapid action at the plasma membrane. Haupt (1959, 1960) some years earlier had done his classic experiments with the alga genus Mougeotia, demonstrating red/far-red-reversible effects on chloroplast movement that could only be explained by parallel-oriented phytochrome molecules in close proximity to the cell surface. Most recently, Jaedicke et al. (2012) have demonstrated an association of phytochrome with phototropin at the plasma membrane in protonemata of the moss Physcomitrella patens. They also used a split yellow fluorescent protein assay to demonstrate an association of Arabidopsis phytochrome with Arabidopsis phototropin when they were both injected into onion (Allium cepa) epidermal cells, although they failed to obtain evidence for such an association in a yeast two-hybrid test. Hughes (2013) has recently written a comprehensive review on cytoplasmic signaling mediated by phytochrome, covering the early history as well as recent developments. Work on the phytochromes and their signal transduction pathways has in recent years focused overwhelmingly on their effects on transcription (Franklin and Quail, 2010; Quail, 2010; Leiver and Quail, 2011; Zhong et al., 2012). It is timely that attention is being returned to possible roles of phytochromes at the plasma membrane.
Figure 4.
Red (R)/far-red (FR)-reversible changes in potential (mV) between the top 1 cm of an oat coleoptile and a reference electrode in a 10 mm solution around the roots. After Newman and Briggs (1972).
In 2006, Lariguet et al. (2006) reported an important finding: the Arabidopsis PHYTOCHROME KINASE SUBSTRATE1 (PKS1) protein, strongly induced by blue light but phyA dependent, interacts both in vivo and in vitro with phot1 and NONPHOTOTROPIC HYPOCOTYL3 (NPH3). Like phot1 and NPH3, PKS1 is plasma membrane localized. PKS1 also was shown to regulate root phototropism and root gravitropism (Boccalandro et al., 2008). A second family member, PKS2, was observed to regulate both leaf flattening and leaf positioning, two phototropin-dependent responses (de Carbonnel et al., 2010). Finally, Kami et al. (2012) showed that some nuclear signaling involving phyA is needed for a maximal phototropic response. Thus, the two PKS proteins may well provide the missing link between phytochrome and phot1, a link that strongly impacts phototropism.
We are still left with the large discrepancy between physiology and photochemistry, regardless of mechanism. However, it is possible that the discrepancies observed between the photosensitivity of phytochrome phototransformation and both phototropic sensitivity changes and phototropin phosphorylation could be explained by some trigger mechanism at the plasma membrane, an amplification mechanism somehow already charged. This mechanism could be fired when only a very few photoreceptor molecules are activated. Such a mechanism might be analogous to the visual system where a very few photons are sufficient to activate signaling in a dark-adapted mammalian eye (Pelli, 1990).
AUXIN DISTRIBUTION AND PHOTOTROPISM
Frits Went (1928) originally proposed that the phototropism of coleoptiles was mediated by light-induced lateral transport of auxin, a conclusion based on measurements of diffusible auxin from irradiated and shaded sides of coleoptile tips. Because the total auxin recovered was less than that from dark controls, however, differential inactivation of auxin, higher on the irradiated side than on the shaded side, was not eliminated as a potential mechanism leading to curvature. The auxin mechanism in coleoptiles remained controversial until Briggs et al. (1957) demonstrated that an impermeable barrier (a coverslip) placed between shaded and irradiated halves of split maize coleoptile tips eliminated any auxin differential without affecting overall auxin yield, confirming Went’s result and conclusion. Briggs (1963b) and Pickard and Thimann (1964), who used radiolabeled auxin, confirmed and extended these observations.
Several somewhat more recent studies have demonstrated that the growth differential between irradiated and shaded sides of phototropically curving organs is compensatory: a decrease in growth rate on the irradiated side is matched by an increase in growth rate on the shaded side. Iino and Briggs (1984) documented this response in maize coleoptiles, and Baskin et al. (1985) further resolved it with high-resolution time-course measurements of coleoptiles as curvature developed. Baskin (1986) then demonstrated a similar compensatory growth differential in phototropically stimulated epicotyls of pea (Pisum sativum). All three of these studies are consistent with a model that involves lateral transport of auxin without any net loss or gain of the hormone itself during the response.
As is well known, Darwin (1881) first demonstrated the importance of the grass coleoptile tip in perceiving the phototropic light stimulus. The coleoptile tip itself had to be irradiated in order for curvature of the lower regions of the coleoptile to develop curvature. Sierp and Seybold (1926) and Lange (1927) narrowed the photosensitive region to a fraction of 1 mm of the extreme coleoptile tip. Boysen-Jensen (1928) then demonstrated that physical contact between the illuminated and shaded sides of the oat coleoptile was also essential in order to obtain phototropic curvature. When he slit the coleoptile tip and inserted a piece of foil (opaque) or a chip of glass (transparent) into the slit prior to unilateral illumination at right angles to the slit, curvature development was blocked. If the slit was parallel to the direction of light, curvature developed even with the insertion of the barrier. Finally, if he split the tip and pushed the two halves back together without any barrier, he obtained curvature. Briggs (1963b) repeated the slit-tip experiment with maize coleoptiles using a glass barrier and obtained a similar result. When the barrier was oriented at right angles to the incident light, second positive curvature was significantly reduced and first positive curvature was completely eliminated. Briggs (1963b) then showed that with only 0.5 mm of 5-mm coleoptile tips intact, unilateral light still induced as great a differential in diffusible auxin between illuminated and shaded sides of 5-mm coleoptiles tips as when the apical 4 mm of the 5-mm coleoptile tips was intact. More recently, Palmer et al. (1993) showed that the apical 1 mm of maize coleoptiles showed the highest level of light-activated phosphorylation of what was subsequently identified as phot1.
All of these results indicate for phototropism (1) that photoperception in the coleoptile apex is essential, (2) that cell-cell lateral communication between lighted and shaded sides is essential, and (3) that a phototropin is likely the photoreceptor. These three conclusions appear to be unambiguous. Unfortunately, the large discrepancy between the fluence for threshold phototropism and the fluence for threshold blue light-driven phototropin phosphorylation casts something of a pall over the “unambiguous” conclusion implicating a phototropin in coleoptiles.
The growth measurements (Baskin, 1986) demonstrating compensatory growth on irradiated and shaded sides of pea epicotyls clearly established that lateral transport of auxin could be functioning in dicots as well as monocots. In addition, as mentioned above, Esmon et al. (2006) demonstrated a difference in auxin-activated transcription between irradiated and shaded sides of B. oleracea hypocotyls. In the past decade, a great deal of information has accumulated about the several proteins involved in auxin transport (Sakai and Haga, 2012; Christie and Murphy, 2013). However, an unanswered question remained: is the site of perception of the light signal in the responding tissue itself or, as in coleoptiles, is it perceived in the upper nonelongating tissues and transported basipitally, as in the case of coleoptiles? Christie et al. (2011) used dark-adapted deetiolated Arabidopsis carrying the DR5rev:GFP reporter protein, a system that responds visually to auxin, to address this question. They found that unilateral blue light induces, first, an inhibition of the flow of auxin from the cotyledons into the vascular tissue and epidermis below, accompanied by an inhibition of growth. Lateral displacement of auxin into the shaded epidermis above the elongation zone followed, and the auxin differential generated between epidermis on irradiated and shaded sides moved down into the elongation zone, accompanied by the development of curvature. Although blue light-induced inhibition of the auxin efflux transporter ABCB19 accounted for the initial growth inhibition, which auxin transporter(s) are involved in the actual lateral transport is not currently known. However, the model developed over a century ago by Darwin for coleoptiles is evidently also valid for Arabidopsis and likely for other dicots: light perception above the growing tissue and generation by lateral transport of an auxin gradient that moves down into the growing tissue.
WHAT CHROMOPHORE DETECTS THE BLUE LIGHT?
At the beginning of the 19th century, Sebastiani Poggioli (1817) first asked what color of light was responsible for inducing plant movements. He noted that the leaves of Mimosa pudica favored violet light for inducing a change in leaf orientation to place the lamina at right angles to the light source (heliotropism or solar tracking), likely the first hint that there might be a specialized photoreceptor that required “violet” light for its activation and downstream signaling. Some years later, Payer (1842) demonstrated that true phototropism of watercress (Nasturtium officinale) seedlings was also maximally sensitive in the blue region of the spectrum. Other workers followed with more detailed studies (Briggs, 2006), but it took until the middle of the 20th century for Shropshire and Withrow (1958) and Thimann and Curry (1960) to publish the first definitive action spectra for first positive curvature of oat coleoptile, covering the wavelength range 330 to 550 nm. Everett and Thimann (1968) then produced an action spectrum for second positive curvature that covered activity over the same wavelength range.
In subsequent years, action spectra for phenomena activated by blue light proliferated, with spectra for responses as diverse as phototropism in the two fungi Phycomyces blakesleeanus and Pilobulus kleinii, phototaxis in Euglena gracilis, stimulation of carotenoid synthesis by the fungi Neurospora crassa and Fusarium aqueductuum, chloroplast rearrangement in the moss Funaria hygrometrica, suppression of a circadian rhythm of conidiation in N. crassa (Sargent and Briggs, 1967), and several other phenomena (Presti and Delbrück, 1978). The general features of all of these action spectra were similar: a single broad band of activity in the UV-A near 360 nm, a more complex band in the blue with a maximum near 450 nm, and fine structure indicative of different vibrational modes of the photoreceptor.
Baskin and Iino (1987), using the Okazaki Large Spectrograph, produced with alfalfa (Medicago sativa) seedlings what remains technically the best action spectrum by far for first positive phototropism. It extends from 530 nm well into the UV-B region of the spectrum (down to 260 nm) and documented for the first time a peak of activity around 280 to 290 nm, about one-third as high as the 450-nm maximum. Their action spectrum coincided almost exactly with that obtained by Thimann and Curry (1960) for oat coleoptiles over the entire wavelength range covered by both studies.
Conventional wisdom for many decades was that there was a single blue light receptor common to all of these responses. The overriding controversy was whether the chromophore was a carotene, a flavin, or something entirely different. It was only in 1993 that the first blue light receptor was identified, cry1 in Arabidopsis (Ahmad and Cashmore, 1993). Lin et al. (1995) soon identified a second cryptochrome (cry2). The cryptochromes are structurally related to DNA photolyases, two-chromophore proteins involved in DNA repair and binding both a flavin and either a pterin (methenyltetrahydrofolate) or a deazaflavin as chromophores (Sancar, 2003). Cryptochromes were found to bind a flavin and most likely methenyltetrahydrofolate as the second chromophore (Lin et al., 1995; Malhotra et al., 1995), an initial win for flavins.
After the discovery of the cryptochromes, identification of the photoreceptor chromophore for phototropism remained controversial. Quiñlones and Zeiger (1994) proposed that the carotinoid zeaxanthin, an important component of the photoprotective xanthophyll cycle for photosynthesis, might be the functioning chromophore for phototropism in maize coleoptiles. However, Palmer et al. (1996) found that the coleoptiles of maize seedlings grown on the inhibitor of carotenoid biosynthesis norflurazon showed normal phototropism in the absence of any detectable carotenoids. Ahmad et al. (1998) then presented evidence that cryptochromes themselves might be carrying out the photoreceptor function for phototropism. Lascève et al. (1999) then investigated the stomatal opening responses to both blue light and phototropism in Arabidopsis mutants in cryptochromes (cry1, cry2, and a cry1 cry2 double mutant), a blocked xanthophyll cycle mutant (npq1), and the recently discovered phot1 mutants nph1, nph3, and nph4 (Liscum and Briggs, 1995, 1996). As only the nph mutants suppressed first positive phototropism in response to blue light (Liscum and Briggs, 1996; Lascève et al., 1999), the authors ruled out members of the xanthophyll cycle and both cryptochromes for phototropism.
Lascève et al. (1999) also eliminated phot1 as mediating stomatal opening because the phot1 (nph1) mutants showed significant stomatal opening in the absence of a phototropic response. Thus, they concluded that there must be a minimum of four different blue light receptors in this model species: two cryptochromes, phot1, and an unknown photoreceptor that functioned for stomatal opening but not phototropism. The source of this error is now obvious. The less sensitive phot2 (Jarillo et al., 1998; Kagawa et al., 2001) had not been identified at the time. Lascève et al. (1999) had used a fluence rate in the stomatal studies that was high enough to activate phot2, whereas the fluence they used for the phototropic studies was much too low to activate phot2 (Sakai et al., 2001). Hence, they missed phot2’s contribution to phototropism but detected it for stomatal opening. Thus, the fourth blue light receptor has turned out to be another phototropin, phot2, and not a completely different molecule.
Currently unanswered is what the photoreceptor is for the UV-B peak described by Baskin and Iino (1987). It could be the consequence of photoexcitation of the UV-B-absorbing peak of the flavin chromophore of one or both phototropins or of one or more aromatic residues in a currently unknown photoreceptor protein. It could also be the consequence of the photoexcitation of UVR8, a recently described UV-B photoreceptor in Arabidopsis (Rizzini et al., 2011). Time will tell.
Finally, there have been two reports of phytochrome-mediated phototropism. Iino et al. (1984) reported very weak phototropic curvatures of maize mesocotyls. It could be significantly enhanced by placing the seedlings horizontally on a clinostat to eliminate curvature elicited by gravitropism. Red light from above prior to unilateral red light completely eliminated the phototropic response, leading the authors to conclude that the photoreceptor was a phytochrome. Likewise, Parker et al. (1989) observed a weak phototropic response to unilateral red light from etiolated pea epicotyls that had developed in complete darkness. They reported a sharp decrease in overall growth rate about 15 min after the onset of red light and hypothesized that the curvature was the consequence of differential growth inhibition across the mesocotyl. Red light from above also eliminated the response, leading the authors to the same conclusion. It appears that a phytochromobilin chromophore functions for a photoreceptor for phototropism, although phototropism is hardly its major assignment.
FINALLY, A PHOTORECEPTOR PROTEIN FOR PHOTOTROPISM: A SHORT SUMMARY
Identification of the phototropins finally became feasible with the use of Arabidopsis mutants that were deficient in phototropism (Khurana and Poff, 1989). Reymond et al. (1992) found that one of the mutants of Khurana and Poff (1989), JK224, was severely impaired in its light-inducible phosphorylation. Liscum and Briggs (1995) then isolated a phototropism mutant designated nph1 that failed to respond to unilateral light in the first positive curvature range and lacked the light-activated phosphorylation reaction. Huala et al. (1997) then used this mutant to identify and sequence the gene encoding the protein that was the substrate for light-induced phosphorylation of a membrane protein. It contained two domains about 100 amino acids long with very similar amino acid sequences, which they designated LOV domains (as they were similar to domains in other proteins that were sensitive to light, oxygen, or voltage). Downstream was a protein kinase domain, a member of the AGC-VIIIb protein kinase subfamily (Bögre et al., 2003). A year later, Christie et al. (1998) expressed the gene in insect cells and demonstrated (1) that it bound a flavin and (2) that light activated its phosphorylation in the absence of any other plant proteins. These authors thus concluded that it was a photoreceptor for phototropism. Christie et al. (1999) then demonstrated that the LOV domains were both binding sites for FMN. It was not long before a second phototropin, phot2, was identified (Jarillo et al., 1998; Kagawa et al., 2001).
As we now know (see below), there is a third family of blue light receptors in Arabidopsis, the Zeitlupe family (ZTL [Somers et al., 2000], LKP2 [Schultz et al., 2001], and FKF1 [Nelson et al., 2000]). Rather than playing a role in phototropism, they are involved in modulating circadian rhythms and flowering through posttranslational regulation (Fujiwara, 2008). These proteins are all F-box proteins that use the same unique LOV domain photochemistry now elucidated for the phototropins and many bacterial blue light receptors (Losi and Gärtner, 2012). As both cryptochromes, both phototropins, and all three F-box proteins use a flavin as chromophore, the long, drawn-out carotenoid versus flavin controversy has been settled unanimously in favor of flavins. Furthermore, a putative single blue light receptor has now disintegrated into seven demonstrable blue light receptors: two cryptochromes (possibly a third), two phototropins, and three F-box proteins.
PHOTOTROPIN-MEDIATED SIGNAL TRANSDUCTION PATHWAYS: FIERCELY INDEPENDENT?
There is a surprising disconnect between the elements identified for one phototropin-activated signal transduction pathway and another. For example, the protein phosphatase PP2A A1 is required to dephosphorylate (and hence deactivate) phot2 but evidently plays no role in the dephosphorylation of phot1 (Tseng and Briggs, 2010). The 14-3-3 λ protein isoform is required for optimal stomatal opening mediated by phot2 but has no effect on stomatal opening mediated by phot1. Furthermore, a mutation at the 14-3-3 λ site fails to affect phot2-mediated phototropism, leaf flattening, or chloroplast movement (Tseng et al., 2012). Only phot1 mediates the rapid inhibition of stem growth of etiolated seedlings (Folta and Spalding, 2001). Both the chloroplast-avoidance response and nuclear positioning are regulated only by phot2 (Demarsy and Fankhauser, 2009). Thus, although many responses can be activated by either phototropin, perhaps with different sensitivities, many others depend exclusively on only one of the two photoreceptors. Furthermore, even for a single phototropin, signal transduction pathways for different responses must actually differ at the level of the photoreceptor. A phototropin-interacting protein required for one response is completely dispensable for another response. For discussion of these and several other examples, see Demarsy and Fankhauser (2009).
LOV DOMAINS: CHROMOPHORE DOMAINS WITH A UNIQUE PHOTOCHEMISTRY
Purified LOV domains have provided a rich new source of material for investigating chromophore structure and photophysics. Salomon et al. (2000) demonstrated that they carried out a unique photoreaction: the formation of a covalent bond between a highly conserved Cys and the C-4a carbon of the FMN to form a cysteinyl adduct. Swartz et al. (2001) first reported that the LOV domain photocycle involved the formation of a flavin triplet intermediate prior to cysteinyl adduct formation. Swartz et al. (2002) also reported protein and chromophore structural changes induced by light using Fourier-transform infrared spectroscopy. Corchnoy et al. (2003) showed next that LOV domain photoactivation led to about a 30% decrease in α-helicity, based on circular dichroism measurements. Harper et al. (2003) then showed that adduct formation led to an unfolding of an amphipathic α-helix just C terminal from the downstream LOV domain, likely the step necessary to activate the C-terminal kinase domain (likely accounting for the α-helicity loss reported by Corchnoy et al. [2003]). A number of LOV domain structures have now been solved by x-ray crystallography. Readers should consult the reviews by Möglich et al. (2010) and Losi and Gärtner (2011, 2012) for a summary of progress in this active area.
SOME FINAL THOUGHTS
Although incredible progress has been made in the past two decades in understanding plant blue light receptors and the processes they regulate, there remain a few puzzles that have stubbornly resisted solving to date or have been almost totally ignored. The huge discrepancy between the amount of light that activates a particular physiological response and the amount that it takes to activate the responsible photoreceptor is still unexplained. Indeed, even when a great deal is known about the activation and relaxation of a photoreceptor, be it a phototropin or a phytochrome, the discrepancy persists unexplained. Is there a specialized trigger mechanism? (The phytochrome community is actually worse off than the phototropin community. Their discrepancy has the physiology and photochemistry separated by about 3 orders of magnitude, whereas the phototropin community must only cope with a discrepancy of somewhere between 1 and 2 orders of magnitude.) How is phototropin specificity regulated? What determines why a given response is mediated by phot1 alone, phot2 alone, or both phototropins? What determines whether a protein plays a role in one signaling pathway but not another? Do different sites or patterns of phototropin phosphorylation play a role? Are there other posttranslational modifications that lend specificity? How does unilateral light really activate the lateral transport of auxin? What auxin transporters are involved? These fascinating questions should keep inquisitive plant biologists busy for a long time.
POSTSCRIPT: LOV BEYOND THE PLANT KINGDOM
Since the original discovery of flavin-binding LOV domains in phototropins (Christie et al., 1999) and of their unique photochemistry, light-activated formation of a flavin-cysteinyl adduct (Salomon et al., 2000), putative LOV domains have been identified in proteins not only from fungi (Idnurm et al., 2010) but in proteins from over 10% of all bacteria thus far sequenced (Losi and Gärtner, 2012; Pathak et al., 2012). The first functional fungal photoreceptor to be characterized was White collar1 (Wc1) from N. crassa (Froehlich et al., 2002), and homologs of Wc1 are now known from all major groups of fungi (Idnurm et al., 2010), including P. blakesleeanus (Idnurm et al., 2006), the sporangiophores of which were the subject of decades of phototropism research. Wc1 binds the promoters and regulates the transcription of a number of light-regulated genes in a blue light-dependent fashion.
The first bacterial protein reported to have a LOV domain was a LOV-STAS protein, YtvA, from Bacillus subtilis (Losi et al., 2002). Putative LOV domains are found upstream from a large number of His kinases (LOV-HK), the commonest LOV proteins, cyclic-di-GMP domains (GGDEF; EAL domains), STAS domains, DNA-binding domains, and phosphatase domains. There are also many short LOV proteins with no known function (Krauss et al., 2009). Recent metagenome-based screening, largely from the Sargasso Sea but also from the open ocean, resulted in the assignment of 578 putative LOV domains on the basis of a highly conserved core region, a minimum of 14 amino acids thought to be essential for photoactivity, and a minimal length of 80 amino acids (Pathak et al., 2012).
There is a vast reservoir of proteins harboring LOV domains in nonphotosynthetic as well as photosynthetic bacteria. (In addition to proteins containing LOV domains, there are many that contain putative phytochromes or BLUF [for blue light sensing using FAD] domain proteins. Indeed, some bacteria carry two different types of putative photoreceptors and some even carry three [Gomelsky and Hoff, 2011].) Whenever it has been possible to express either the LOV domain itself or the entire LOV protein, they all show normal LOV domain photochemistry: cysteinyl adduct formation on photoexcitation. However, a major question remains: what are the functions of these many putative LOV domain photoreceptors? Finding a phenotype has been a challenging task. However, in a number of recent cases, the efforts have been successful. Light activation of a LOV domain HK in the animal pathogen Brucella abortus increases its virulence 10-fold in a macrophage assay (Swartz et al., 2007). Light induces the activity of a cyclic di-GMP phosphodiesterase activity in the cyanobacterium Synechococcus elongatus (Cao et al., 2010). Light activates a general stress response through the LOV-STAS protein YtvA in B. subtilis (Avila-Pérez et al., 2006). A LOV-HK mediates host colonization by the plant pathogen Xanthomonas axonopodis pv citri (Kraiselburd et al., 2012). Likewise, a LOV-HK enzyme mediates light-activated cell attachment for Caulobacter crescentum (Purcell et al., 2007). A LOV-HK mediates exopolysaccharade production, attachment, and nodulation in Rhizobium leguminosarum (Bonomi et al., 2012). Light plays an important role in regulating swarming motility in Pseudomonas syringae through a LOV-HK, a response that also involves a bacterial phytochrome (Wu et al., 2013). Finally a LOV-STAS protein confers light-dependent transcription, swimming motility, and invasiveness to the animal pathogen Listeria monocytogenes (Ondrusch and Kreft, 2011). This list will no doubt increase.
While information on the signal transduction pathways that these LOV proteins activate is still scant, it is clear that there is a whole rich world of bacterial photophysiology wide open for investigation. Thus, basic research on plant photoreceptors not only still poses tantalizing questions about plant photoreceptors themselves but has uncovered an exciting new field for microbiologists.
Acknowledgments
I thank Drs. Tong-Seung Tseng and Rajnish Khanna for their careful review of the manuscript and Dr. Tseng for help in scanning and improving the quality of published figures. I also thank Michael R. Blatt for providing the motive farce for this article.
Glossary
- LOV
light, oxygen, or voltage
- MCS
meter-candle second
References
- Ahmad M, Cashmore AR. (1993) HY4 gene of A. thaliana encodes a protein with characteristics of a blue-light photoreceptor. Nature 366: 162–166 [DOI] [PubMed] [Google Scholar]
- Ahmad M, Jarillo JA, Smirnova O, Cashmore AR. (1998) Cryptochrome blue-light photoreceptors of Arabidopsis implicated in phototropism. Nature 392: 720–723 [DOI] [PubMed] [Google Scholar]
- Avila-Pérez M, Hellingwerf KJ, Kort R. (2006) Blue light activates the sigmaB-dependent stress response of Bacillus subtilis via YtvA. J Bacteriol 188: 6411–6414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskin TI. (1986) Redistribution of growth during phototropism and nutation in the pea epicotyl. Planta 169: 406–414 [DOI] [PubMed] [Google Scholar]
- Baskin TI, Iino M. (1987) An action spectrum in the blue and ultraviolet for phototropism in alfalfa. Photochem Photobiol 46: 127–136 [Google Scholar]
- Baskin TI, Iino M, Green PB, Briggs WR. (1985) High-resolution measurement of growth during first positive phototropism in maize. Plant Cell Environ 8: 595–603 [Google Scholar]
- Blaauw AH. (1909) Die Perzeption des Lichtes. Rec Trav Bot Néerl 5: 209–372 [Google Scholar]
- Boccalandro HE, De Simone SN, Bergmann-Honsberger A, Schepens I, Fankhauser C, Casal JJ. (2008) PHYTOCHROME KINASE SUBSTRATE1 regulates root phototropism and gravitropism. Plant Physiol 146: 108–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bögre L, Okrész L, Henriques R, Anthony RG. (2003) Growth signalling pathways in Arabidopsis and the AGC protein kinases. Trends Plant Sci 8: 424–431 [DOI] [PubMed] [Google Scholar]
- Bonomi HR, Posadas DM, Paris G, Carrica MdC, Frederickson M, Pietrasanta LI, Bogomolni RA, Zorreguieta A, Goldbaum FA. (2012) Light regulates attachment, exopolysaccharide production, and nodulation in Rhizobium leguminosarum through a LOV-histidine kinase photoreceptor. Proc Natl Acad Sci USA 109: 12135–12140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boysen-Jensen P. (1928) Die phototropische Induktion in der Spitze der Avenakoleoptile. Planta 5: 464–477 [Google Scholar]
- Briggs WR. (1960) Light dosage and the phototropic responses of corn and oat coleoptiles. Plant Physiol 35: 951–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs WR. (1963a) Red light, auxin relationships, and the phototropic responses of corn and oat coleoptiles. Am J Bot 50: 196–207 [Google Scholar]
- Briggs WR. (1963b) Mediation of phototropic responses of corn coleoptiles by lateral transport of auxin. Plant Physiol 38: 237–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs WR (2006) Blue-UV-A receptors: historical overview. In E Schäfer, F Nagy, eds, Photomorphogenesis in Plants and Bacteria, Ed 3. Springer, Dordrecht, The Netherlands, pp 171–197 [Google Scholar]
- Briggs WR, Chon H-P. (1966) The physiological versus the spectrophotometric status of phytochrome in corn coleoptiles. Plant Physiol 41: 1159–1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs WR, Christie JM, Salomon M. (2001) Phototropins: a new family of flavin-binding blue light receptors in plants. Antioxid Redox Signal 3: 775–788 [DOI] [PubMed] [Google Scholar]
- Briggs WR, Tocher RD, Wilson JF. (1957) Phototropic auxin redistribution in corn coleoptiles. Science 126: 210–212 [DOI] [PubMed] [Google Scholar]
- Bunsen R, Roscoe H. (1862) Photochemische Unterzuchungen. Ann Phys Chem 17: 529–562 [Google Scholar]
- Butler WL, Norris KH, Siegelman HW, Hendricks SB. (1959) Detection, assay and preliminary purification of the pigment controlling photoresponsive development of plants. Proc Natl Acad Sci USA 45: 1703–1708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Z, Livoti E, Losi A, Gärtner W. (2010) A blue light-inducible phosphodiesterase activity in the cyanobacterium Synechococcus elongatus. Photochem Photobiol 86: 606–611 [DOI] [PubMed] [Google Scholar]
- Chon HP, Briggs WR. (1966) Effect of red light on the phototropic sensitivity of corn coleoptiles. Plant Physiol 41: 1715–1724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie JM, Murphy AS. (2013) Shoot phototropism in higher plants: new light through old concepts. Am J Bot 100: 35–46 [DOI] [PubMed] [Google Scholar]
- Christie JM, Reymond P, Powell GK, Bernasconi P, Raibekas AA, Liscum E, Briggs WR. (1998) Arabidopsis NPH1: a flavoprotein with the properties of a photoreceptor for phototropism. Science 282: 1698–1701 [DOI] [PubMed] [Google Scholar]
- Christie JM, Salomon M, Nozue K, Wada M, Briggs WR. (1999) LOV (light, oxygen, or voltage) domains of the blue-light photoreceptor phototropin (nph1): binding sites for the chromophore flavin mononucleotide. Proc Natl Acad Sci USA 96: 8779–8783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie JM, Yang H, Richter GL, Sullivan S, Thomson CE, Lin J, Titapiwatanakun B, Ennis M, Kaiserli E, Lee OR, et al. (2011) phot1 inhibition of ABCB19 primes lateral auxin fluxes in the shoot apex required for phototropism. PLoS Biol 9: e1001076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corchnoy SB, Swartz TE, Lewis JW, Szundi I, Briggs WR, Bogomolni RA. (2003) Intramolecular proton transfers and structural changes during the photocycle of the LOV2 domain of phototropin 1. J Biol Chem 278: 724–731 [DOI] [PubMed] [Google Scholar]
- Curry GM (1957) Studies on the spectral sensitivity of phototropism. PhD thesis. Harvard University, Cambridge, MA [Google Scholar]
- Darwin C (1881) The Power of Movement in Plants. Da Capo Press, New York (Reprint Edition, 1966) [Google Scholar]
- de Carbonnel M, Davis P, Roelfsema MR, Inoue S-I, Schepens I, Lariguet P, Geisler M, Shimazaki K-I, Hangarter R, Fankhauser C. (2010) The Arabidopsis PHYTOCHROME KINASE SUBSTRATE2 protein is a phototropin signaling element that regulates leaf flattening and leaf positioning. Plant Physiol 152: 1391–1405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demarsy E, Fankhauser C. (2009) Higher plants use LOV to perceive blue light. Curr Opin Plant Biol 12: 69–74 [DOI] [PubMed] [Google Scholar]
- du Buy HG, Nuernbergk E. (1934) Phototropismus und Wachstum der Pflanzen II. Ergeb Biol 10: 207–322 [Google Scholar]
- Esmon CA, Tinsley AG, Ljung K, Sandberg G, Hearne LB, Liscum E. (2006) A gradient of auxin and auxin-dependent transcription precedes tropic growth responses. Proc Natl Acad Sci USA 103: 236–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett M, Thimann KV. (1968) Second positive phototropism in the Avena coleoptile. Plant Physiol 43: 1786–1792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folta KM, Spalding EP. (2001) Unexpected roles for cryptochrome 2 and phototropin revealed by high-resolution analysis of blue light-mediated hypocotyl growth inhibition. Plant J 26: 471–478 [DOI] [PubMed] [Google Scholar]
- Franklin KA, Quail PH. (2010) Phytochrome functions in Arabidopsis development. J Exp Bot 61: 11–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froehlich AC, Liu Y, Loros JJ, Dunlap JC. (2002) White Collar-1, a circadian blue light photoreceptor, binding to the frequency promoter. Science 297: 815–819 [DOI] [PubMed] [Google Scholar]
- Fröschel P. (1908) Unterzuchungen über die heliotropische Präzentationszeit. I Sitzber Mathnaturwiss Kl Kais Akad Wiss 107: 235–256 [Google Scholar]
- Fujiwara S. (2008) Novel blue light receptors with an F-box: their direct control of the circadian clock and flower timing in Arabidopsis. Plant Biotechnol 25: 123–129 [Google Scholar]
- Gomelsky M, Hoff WD. (2011) Light helps bacteria make important lifestyle decisions. Trends Microbiol 19: 441–448 [DOI] [PubMed] [Google Scholar]
- Han I-S, Tseng T-S, Eisinger W, Briggs WR. (2008) Phytochrome A regulates the intracellular distribution of phototropin 1-green fluorescent protein in Arabidopsis thaliana. Plant Cell 20: 2835–2847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper SM, Neil LC, Gardner KH. (2003) Structural basis of a phototropin light switch. Science 301: 1541–1544 [DOI] [PubMed] [Google Scholar]
- Haupt W. (1959) Die Chloroplastendrehung bei Mougeotia. I. Über den quantitiven und qualitiven Lichtbedarf der Swachlightbewegung. Planta 53: 484–501 [Google Scholar]
- Haupt W. (1960) Die Chloroplastendrehung bei Mougeotia. II. Die Unduktion der Schwachlichtbewegung durch linear polarizierten Licht. Planta 55: 465–479 [Google Scholar]
- Hennig L (1996) Phytochrome degradation and dark reversion. In E Schäfer, F Nagy, eds, Photomorphogenesis in Plants and Bacteria, Ed 3. Springer, Dordrecht, The Netherlands, pp 131–153 [Google Scholar]
- Hohm T, Preuten T, Fankhauser C. (2013) Phototropism: translating light into directional growth. Am J Bot 100: 47–59 [DOI] [PubMed] [Google Scholar]
- Huala E, Oeller PW, Liscum E, Han I-S, Larsen E, Briggs WR. (1997) Arabidopsis NPH1: a protein kinase with a putative redox-sensing domain. Science 278: 2120–2123 [DOI] [PubMed] [Google Scholar]
- Hughes J. (2013) Phytochrome cytoplasmic signaling. Annu Rev Plant Biol 64: 377–402 [DOI] [PubMed] [Google Scholar]
- Idnurm A, Rodríguez-Romero J, Corrochano LM, Sanz C, Iturriaga EA, Eslava AP, Heitman J. (2006) The Phycomyces madA gene encodes a blue-light photoreceptor for phototropism and other light responses. Proc Natl Acad Sci USA 103: 4546–4551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idnurm A, Verma S, Corrochano LM. (2010) A glimpse into the basis of vision in the kingdom Mycota. Fungal Genet Biol 47: 881–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iino M (2001) Phototropism in higher plants. In DP Häder, M Lebert, eds, Photomovement. Elsevier Science, Amsterdam, pp 659–811 [Google Scholar]
- Iino M, Briggs WR. (1984) Growth distribution during first positive phototropic curvature of maize coleoptiles. Plant Cell Environ 7: 97–104 [Google Scholar]
- Iino M, Schäfer E, Briggs WR. (1984) Photo-perception sites for phytochrome-mediated phototropism of maize coleoptiles. Planta 162: 477–479 [DOI] [PubMed] [Google Scholar]
- Inoue S, Kinoshita T, Matsumoto M, Nakayama KI, Doi M, Shimazaki K-I. (2008) Blue light-induced autophosphorylation of phototropin is a primary step for signaling. Proc Natl Acad Sci USA 105: 5626–5631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue S-I, Matsushita T, Tomokiyo Y, Matsumoto M, Nakayama KI, Kinoshita T, Shimazaki K-I. (2011) Functional analyses of the activation loop of phototropin2 in Arabidopsis. Plant Physiol 156: 117–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaedicke K, Lichtenthäler AL, Meyberg R, Zeidler M, Hughes J. (2012) A phytochrome-phototropin light signaling complex at the plasma membrane. Proc Natl Acad Sci USA 109: 12231–12236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janoudi A-K, Konjević R, Apel P, Poff KL. (1992) Time threshold for second positive phototropism is decreased by preirradiation with red light. Plant Physiol 99: 1422–1425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janoudi A-K, Poff KL. (1991) Characterization of adaptation in phototropism of Arabidopsis thaliana. Plant Physiol 95: 517–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarillo J, Ahmad M, Cashmore AR. (1998) NPL1 (accession no. AF053401): a second member of the NPH serine/threonine kinase family of Arabidopsis. Plant Physiol 117: 719 [Google Scholar]
- Kagawa T, Sakai T, Suetsugu N, Oikawa K, Ishiguro S, Kato T, Tabata S, Okada K, Wada M. (2001) Arabidopsis NPL1: a phototropin homolog controlling the chloroplast high-light avoidance response. Science 291: 2138–2141 [DOI] [PubMed] [Google Scholar]
- Kami C, Hersch M, Trevisan M, Genoud T, Hiltbrunner A, Bergmann S, Fankhauser C. (2012) Nuclear phytochrome A signaling promotes phototropism in Arabidopsis. Plant Cell 24: 566–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khurana JP, Poff KL. (1989) Mutants of Arabidopsis thaliana with altered phototropism. Planta 178: 400–406 [PubMed] [Google Scholar]
- Kong S-G, Suzuki T, Tamura K, Mochizuki N, Hara-Nishimura I, Nagatani A. (2006) Blue light-induced association of phototropin 2 with the Golgi apparatus. Plant J 45: 994–1005 [DOI] [PubMed] [Google Scholar]
- Konjević R, Steinitz B, Poff KL. (1989) Dependence of the phototropic response of Arabidopsis thaliana on fluence rate and wavelength. Proc Natl Acad Sci USA 86: 9876–9880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraiselburd I, Alet AI, Tondo ML, Petrocelli S, Daurelio LD, Monzón J, Ruiz OA, Losi A, Orellano EG. (2012) A LOV protein modulates the physiological attributes of Xanthomonas axonopodis pv. citri relevant for host plant colonization. PLoS ONE 7: e38226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauss U, Minh BQ, Losi A, Gärtner W, Eggert T, von Haeseler A, Jaeger K-E. (2009) Distribution and phylogeny of light-oxygen-voltage-blue-light-signaling proteins in the three kingdoms of life. J Bacteriol 191: 7234–7242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange S. (1927) Die Verteilung der Lightempfindlichkeit in der Spitze der Haferkoleoptile. Jarhb Wiss Botan 67: 1–51 [Google Scholar]
- Lariguet P, Schepens I, Hodgson D, Pedmale UV, Trevisan M, Kami C, de Carbonnel M, Alonso JM, Ecker JR, Liscum E, et al. (2006) PHYTOCHROME KINASE SUBSTRATE 1 is a phototropin 1 binding protein required for phototropism. Proc Natl Acad Sci USA 103: 10134–10139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lascève GL, Leymarie J, Olney MA, Liscum E, Christie JM, Vavasseur A, Briggs WR. (1999) Arabidopsis contains at least four independent blue-light-activated signal transduction pathways. Plant Physiol 120: 605–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leivar P, Quail PH. (2011) PIFs: pivotal components in a cellular signaling hub. Trends Plant Sci 16: 19–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CT, Robertson DE, Ahmad M, Raibekas AA, Jorns MS, Dutton PL, Cashmore AR. (1995) Association of flavin adenine dinucleotide with the Arabidopsis blue light receptor CRY1. Science 269: 968–970 [DOI] [PubMed] [Google Scholar]
- Liscum E, Briggs WR. (1995) Mutations in the NPH1 locus of Arabidopsis disrupt the perception of phototropic stimuli. Plant Cell 7: 473–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liscum E, Briggs WR. (1996) Mutations of Arabidopsis in potential transduction and response components of the phototropic signaling pathway. Plant Physiol 112: 291–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losi A, Gärtner W. (2011) Old chromophores, new photoactivation paradigms, trendy applications: flavins in blue light-sensing photoreceptors. Photochem Photobiol 87: 491–510 [DOI] [PubMed] [Google Scholar]
- Losi A, Gärtner W. (2012) The evolution of flavin-binding photoreceptors: an ancient chromophore serving trendy blue-light sensors. Annu Rev Plant Biol 63: 49–72 [DOI] [PubMed] [Google Scholar]
- Losi A, Polverini E, Quest B, Gärtner W. (2002) First evidence for phototropin-related blue-light receptors in prokaryotes. Biophys J 82: 2627–2634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra K, Kim ST, Batschauer A, Dawut L, Sancar A. (1995) Putative blue-light photoreceptors from Arabidopsis thaliana and Sinapis alba with a high degree of sequence homology to DNA photolyase contain the two photolyase cofactors but lack DNA repair activity. Biochemistry 34: 6892–6899 [DOI] [PubMed] [Google Scholar]
- Mandoli DF, Briggs WR. (1981) Phytochrome control of two low-irradiance responses in etiolated oat seedlings. Plant Physiol 67: 733–739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möglich A, Yang X, Ayers RA, Moffat K. (2010) Structure and function of plant photoreceptors. Annu Rev Plant Biol 61: 21–47 [DOI] [PubMed] [Google Scholar]
- Nelson DC, Lasswell J, Rogg LE, Cohen MA, Bartel B. (2000) FKF1, a clock-controlled gene that regulates the transition to flowering in Arabidopsis. Cell 101: 331–340 [DOI] [PubMed] [Google Scholar]
- Newman IA, Briggs WR. (1972) Phytochrome-mediated electric potential changes in oat seedlings. Plant Physiol 50: 687–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ondrusch N, Kreft J. (2011) Blue and red light modulates SigB-dependent gene transcription, swimming motility and invasiveness in Listeria monocytogenes. PLoS ONE 6: e16151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer JM, Short TW, Briggs WR. (1993) Correlation of blue light-induced phosphorylation to phototropism in Zea mays L. Plant Physiol 102: 1219–1225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer JM, Warpeha KMF, Briggs WR. (1996) Evidence that zeaxanthin is not the phototreceptor for phototropism in maize coleoptiles. Plant Physiol 110: 1323–1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker K, Baskin TI, Briggs WR. (1989) Evidence for a phytochrome-mediated phototropism in etiolated pea seedlings. Plant Physiol 89: 493–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks BM, Quail PH, Hangarter RP. (1996) Phytochrome A regulates red-light induction of phototropic enhancement in Arabidopsis. Plant Physiol 110: 155–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak GP, Losi A, Gärtner W. (2012) Metagenome-based screening reveals worldwide distribution of LOV-domain proteins. Photochem Photobiol 88: 107–118 [DOI] [PubMed] [Google Scholar]
- Payer J (1842) Mémoire sur la tendance des tiges vers la lumiere. Compte Rendu des Seances de l’Académie des Seances July 4: 1194–1196 [Google Scholar]
- Pelli DG (1990) The quantum efficiency of vision. In C Blackmore, ed, Vision: Coding and Efficiency. Cambridge University Press, Cambridge, UK, pp 3–24 [Google Scholar]
- Pickard BG, Thimann KV. (1964) Transport and distribution of auxin during tropistic responses. II. The lateral migration of auxin in phototropism of coleoptiles. Plant Physiol 39: 341–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poggioli S. (1817) Della influenze che ha il raggio magnetic sulla vegetation della piante. Coi Tipi di Annesio nobili Opusc Scientif Fasc 1: 9–23 [Google Scholar]
- Presti D, Delbrück M. (1978) Photoreceptors for biosynthesis, energy storage and vision. Plant Cell Environ 1: 81–100 [Google Scholar]
- Purcell EB, Siegal-Gaskins D, Rawling DC, Fiebig A, Crosson S. (2007) A photosensory two-component system regulates bacterial cell attachment. Proc Natl Acad Sci USA 104: 18241–18246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quail PH. (2010) Phytochromes. Curr Biol 20: R504–R507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiñlones MA, Zeiger E. (1994) A putative role of the xanthophyll, zeaxanthin, in blue light photoreception of corn coleoptiles. Science 264: 558–561 [DOI] [PubMed] [Google Scholar]
- Reymond P, Short TW, Briggs WR, Poff KL. (1992) Light-induced phosphorylation of a membrane protein plays an early role in signal transduction for phototropism in Arabidopsis thaliana. Proc Natl Acad Sci USA 89: 4718–4721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzini L, Favory J-J, Cloix C, Faggionato D, O’Hara A, Kaiserli E, Baumeister R, Schäfer E, Nagy F, Jenkins GI, et al. (2011) Perception of UV-B by the Arabidopsis UVR8 protein. Science 332: 103–106 [DOI] [PubMed] [Google Scholar]
- Sachs J. (1864) Wirkungen farbigen Lichts auf Pflanzen. Botanische Zeitung 47: 353–358 [Google Scholar]
- Sakai T, Haga K. (2012) Molecular genetic analysis of phototropism in Arabidopsis. Plant Cell Physiol 53: 1517–1534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai T, Kagawa T, Kasahara M, Swartz TE, Christie JM, Briggs WR, Wada M, Okada K. (2001) Arabidopsis nph1 and npl1: blue light receptors that mediate both phototropism and chloroplast relocation. Proc Natl Acad Sci USA 98: 6969–6974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto K, Briggs WR. (2002) Cellular and subcellular localization of phototropin 1. Plant Cell 14: 1723–1735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon M, Christie JM, Knieb E, Lempert U, Briggs WR. (2000) Photochemical and mutational analysis of the FMN-binding domains of the plant blue light receptor, phototropin. Biochemistry 39: 9401–9410 [DOI] [PubMed] [Google Scholar]
- Salomon M, Zacherl M, Rüdiger W. (1997) Assymetric, blue light-dependent phoshorylation of a 116-kilodalton plasma membrane protein can be correlated with first- and second-positive curvature of oat coleoptiles. Plant Physiol 15: 485–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancar A. (2003) Structure and function of DNA photolyase and cryptochrome blue-light photoreceptors. Chem Rev 103: 2203–2237 [DOI] [PubMed] [Google Scholar]
- Sargent ML, Briggs WR. (1967) The effects of light on a circadian rhythm of conidiation in Neurospora. Plant Physiol 42: 1504–1510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz TF, Kiyosue T, Yanovsky M, Wada M, Kay SA. (2001) A role for LKP2 in the circadian clock of Arabidopsis. Plant Cell 13: 2659–2670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shropshire W, Jr, Withrow RB. (1958) Action spectrum for phototropic tip-curvature of Avena. Plant Physiol 33: 360–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierp H, Seybold A. (1926) Unterzuchungen über die Lichtempfindlichkeit der Spitze und des Stumpfes in der Koleoptile von Avena sativa. Jarhb Wiss Botan 65: 592–610 [Google Scholar]
- Somers DE, Schultz TF, Milnamow M, Kay SA. (2000) ZEITLUPE encodes a novel clock-associated PAS protein from Arabidopsis. Cell 101: 319–329 [DOI] [PubMed] [Google Scholar]
- Swartz TE, Corchnoy SB, Christie JM, Lewis JW, Szundi I, Briggs WR, Bogomolni RA. (2001) The photocycle of a flavin-binding domain of the blue light photoreceptor phototropin. J Biol Chem 276: 36493–36500 [DOI] [PubMed] [Google Scholar]
- Swartz TE, Tseng T-S, Frederickson MA, Paris G, Comerci DJ, Rajashekara G, Kim J-G, Mudgett MB, Splitter GA, Ugalde RA, et al. (2007) Blue-light-activated histidine kinases: two-component sensors in bacteria. Science 317: 1090–1093 [DOI] [PubMed] [Google Scholar]
- Swartz TE, Wenzel PJ, Corchnoy SB, Briggs WR, Bogomolni RA. (2002) Vibration spectroscopy reveals light-induced chromophore and protein structural changes in the LOV2 domain of the plant blue-light receptor phototropin 1. Biochemistry 41: 7183–7189 [DOI] [PubMed] [Google Scholar]
- Thimann KV, Curry GM. (1960) Phototropism and phototaxis. Comparative Biochemistry 1: 243–306 [Google Scholar]
- Tseng T-S, Briggs WR. (2010) The Arabidopsis rcn1-1 mutation impairs dephosphorylation of Phot2, resulting in enhanced blue light responses. Plant Cell 22: 392–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng T-S, Whippo C, Hangarter RP, Briggs WR. (2012) The role of a 14-3-3 protein in stomatal opening mediated by PHOT2 in Arabidopsis. Plant Cell 24: 1114–1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Y-L, Eisinger W, Ehrhardt D, Kubitscheck U, Baluska F, Briggs WR. (2008) The subcellular localization and blue-light-induced movement of phototropin 1-GFP in etiolated seedlings of Arabidopsis thaliana. Mol Plant 1: 103–117 [DOI] [PubMed] [Google Scholar]
- Went FW. (1928) Wuchsstoff und Wachstum. Rec Trav Bot Neerl 25: 1–119 [Google Scholar]
- Went FW, Thimann KV (1937) Phytohormones. Macmillan, New York [Google Scholar]
- Whippo CW, Hangarter RP. (2004) Phytochrome modulation of blue-light-induced phototropism. Plant Cell Environ 27: 1223–1228 [Google Scholar]
- Wu L, McGrane RS, Beattie GA. (2013) Light regulation of swarming motility in Pseudomonas syringae integrates signaling pathways mediated by a bacteriophytochrome and a LOV protein. MBio 4: e00334–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong S, Shi H, Xue C, Wang L, Xi Y, Li J, Quail PH, Deng XW, Guo H. (2012) A molecular framework of light-controlled phytohormone action in Arabidopsis. Curr Biol 22: 1530–1535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman BK, Briggs WR. (1963a) Phototropic dosage-response curves for oat coleoptiles. Plant Physiol 38: 248–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman BK, Briggs WR. (1963b) A kinetic model for phototropic responses of oat coleoptiles. Plant Physiol 38: 253–261 [DOI] [PMC free article] [PubMed] [Google Scholar]