Abstract
A rapid and simple PCR-based assay for detection of the group 2 capsule synthesis gene kpsM of Escherichia coli was designed and validated. When combined with the published group 2 primers (kpsIIf, 5′-GCGCATTTGCTGATACTGTTG-3′; kpsIIr, 5′-CATCCAGACGATAAGCATGAGCA-3′), the new primers (the kpsIIf primer and a new reverse primer K2r, 5′-AGGTAGTTCAGACTCACACCT-3′) allowed specific identification by exclusion of the heretofore elusive K2 kpsM variant. The primers yielded the predicted amplicon when multiplexed with other primers and used under varied assay conditions, including a range of concentrations of individual reaction mixture ingredients and of annealing temperatures (from 54 to 64°C).
The polysaccharide capsules of extraintestinal pathogenic Escherichia coli (ExPEC) are important both pathogenetically and taxonomically (9, 12). The low-molecular-weight, high-charge density group 2 and 3 capsules that are associated with ExPEC, in contrast to the high-molecular-weight, low-charge density group 1 capsules that are associated with commensal E. coli, protect ExPEC against phagocytosis and complement-mediated killing, thereby contributing to extraintestinal virulence (4, 7, 9, 10, 24, 32). In addition, the approximately 80 defined K (capsular) antigens of E. coli, particularly those that are epidemiologically associated with virulence, serve as useful markers for identifying virulent ExPEC clonal groups such as E. coli O18:K1:H7, O6:K2:H1, O4:K12:H5, and O15:K52:H1 (10, 18, 26, 27).
However, K-antigen typing is a highly specialized technique performed only in a few reference laboratories. In contrast, molecular detection of kps variants can be done in any molecular epidemiology laboratory. Group 2 and group 3 kps operons share a somewhat similar organization, including moderately to highly conserved regions (e.g., kpsDMTE) that encode transport and assembly functions, combined with highly variable type-specific regions that encode the synthesis and/or polymerization of the specific component sugars of the particular polysaccharide (5, 28-31, 33). Probe hybridization discriminates readily between group 2 and group 3 kpsM variants but, because of extensive within-group conservation, does not resolve individual capsular types within each group (22).
In contrast, PCR-based detection, because of its greater sequence specificity, is more discriminating than probe hybridization and can detect single nucleotide variants of certain genes (22, 23). PCR assays also are easier to perform and interpret than are blot-based assays and can be multiplexed to increase efficiency (22, 23). However, until recently K1 and K5 were the only sequenced group 2 kps variants, which hindered the development of group-specific and type-specific primers. Primers designed to detect only the K1 kpsM variant did exhibit K1 specificity (22). In contrast, kpsM primers designed to detect only the K5 variant instead detected all group 2 variants except K1 and K2; other primers designed to detect all group 2 variants failed to detect the K2 variant, which thus still required probe hybridization for detection (22).
The recent report of the complete genome sequence for archetypal ExPEC strain CFT073 (38), a member of the (sepsis- and pyelonephritis-associated) O6:K2:H1 clonal group (22, 23, 27), suggested the possibility of devising K2-specific primers. Here we describe our efforts toward this end and our validation of the resulting PCR assay.
Strains.
A total of 140 E. coli isolates were selected from diverse collections to provide a range of K antigens and kpsM genotypes. From five published collections for which O:K:H serotypes were determined and communicated to the authors by the International Escherichia and Klebsiella Centre (IEKC) of the World Health Organization (Statens Seruminstitut, Copenhagen, Denmark) 30 isolates were selected, including multiple representatives of the two available O:K:H serotypes containing the K2 antigen (for O6:K2:H1, n = 4; for O25:K2:H2, n = 2), one or more representatives of diverse non-K2 group 2 capsular types (K1, K5, K7, K12, K16, K52, K53, and K100; n = 16), seven group 3 capsule isolates (for K3, n = 3; for K10,54/96, n = 3; for K54, n = 1), and one capsule-minus isolate known to contain group 3 kpsM sequences (strain J96) (18-20, 22, 23). These isolates were defined as the IEKC reference set. Twenty-eight other K-type isolates were studied, for which K antigens had been determined by various (or unspecified) laboratories and/or methods, and were as published (34, 35) or communicated to the authors. Finally, to provide ample representatives from each category, we selected from various collections 82 additional isolates for which the K-antigen status was unknown but the kpsM genotype (group 2 non-K2, group 2 K2, group 3, or negative) was defined by a combination of PCR and probe hybridization (22).
kps genotyping.
Isolates were amplified by using published kpsM II (i.e., group 2) and kpsM III (i.e., group 3) primers (22) and were subjected to dot blot hybridization by using digoxigenin-labeled probes derived from these same primers, as previously described (22). The PCR mixture (total, 25 μl) included 2 μl of boiled lysate template DNA, 4 mM MgCl2, 0.8 mM concentrations of four deoxynucleoside triphosphates, 0.6 μM concentrations of the primers, and 0.75 U of AmpliTaq Gold (or 1.25 U for multiplex PCR) in 1× PCR buffer (Perkin Elmer) (22). Cycling conditions were as follows: 95°C for 12 min, followed by 25 cycles of 94°C for 30 s, 58°C for 30 s, and 72°C for 3 min, with extension at 72°C for 10 min (22). Of the isolates that hybridized with the group 2 probe, those that also reacted with the group 2 primers in PCR were defined as kpsM group 2, non-K2, whereas those that were nonreactive with these primers were defined as kpsM K2. Isolates that hybridized with the group 3 probe and reacted with the group 3 primers were defined as kpsM group 3. Isolates that failed to react with either the group 2 or group 3 probe or primers were defined as kpsM negative (i.e., for group 2 and group 3 kpsM variants). Selected strains underwent PCR analysis for the K1 kpsM variant and/or neuS, which participates in the synthesis of the K1 antigen, by using established primers and PCR conditions (22, 37). Duplicate boiled lysates of each isolate, as prepared independently from separate colonies, were used as target DNA. Appropriate positive and negative controls were included in each blot or PCR run. For selected isolates, the kpsM sequences were directly determined along both strands. These sequences were aligned with reference kpsM sequences (as described below) to identify polymorphisms.
New primer design.
Sequence alignments of the published K1, K2 (CFT073), and K5 kpsM variants (group 2; GenBank accession no. M57382, AE016766, and X53819, respectively) and the K10,K54/96 kpsM variant (group 3; GenBank accession no. AF007777) were made by using CLUSTAL-X (36). Alignments were inspected for regions common to all three group 2 variants, but not the group 3 variant, or specific to the K2 variant only. Candidate primers were selected according to standard criteria (11).
Inclusive group 2 kpsM primers.
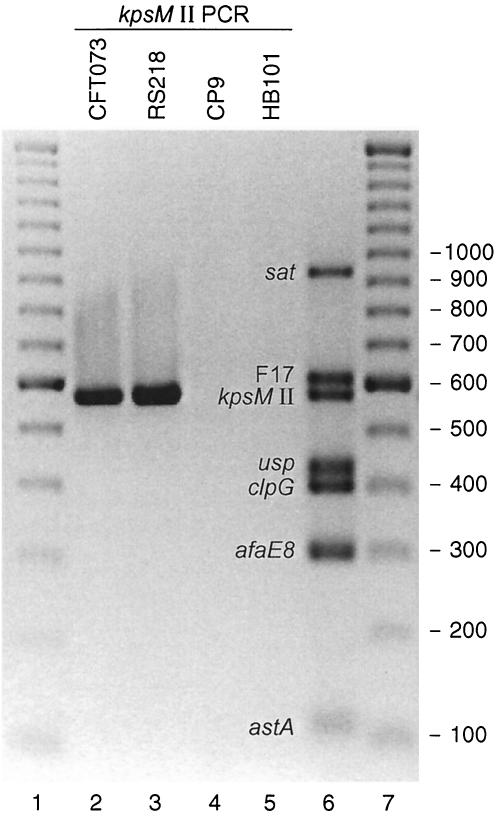
Efforts to devise K2-specific primers proved unsuccessful. Therefore, we instead sought to devise inclusive group 2 primers. Primers targeting conserved regions in the aligned K1, K2, and K5 kpsM sequences, i.e., our existing forward primer kpsIIf (5′-GCGCATTTGCTGATACTGTTG-3′) (kpsM bases 121 to 141) and a new reverse primer K2r (5′-AGGTAGTTCAGACTCACACCT-3′) (bases 698 to 678), were tested against the 30-member IEKC reference set and then against the remaining 110 test isolates (Table 1). The new group 2 primers yielded PCR results that were 100% concordant with dot blot hybridization results obtained by using the (old) group 2 probe. That is, they detected all group 2 blot-positive isolates regardless of whether these did (non-K2; n = 45) or did not (K2; n = 44) react with the old group 2 primers, but they did not detect any group 2 blot-negative isolates (kpsM group 3 [n = 29] and kpsM negative [n = 22]) (Table 1). Thus, a combined result of “negative with the old, positive with the new group 2 primers” identified the same (putative K2) subset within group 2 as did a combined result of “blot positive with the group 2 probe, PCR negative with the old group 2 primers.” This result demonstrated the accurate detection by PCR alone of the K2 kpsM variant as genetically defined. The specificity of the new group 2 kpsM primers for group 2 capsule variants, including the K2 variant, was illustrated by their detection of group 2 strains RS218 (O18:K1:H7) and CFT073 (O6:K2:H1) but not group 3 strain CP9 (O4:K10,54/96:H5) or laboratory strain HB101 (kpsM negative) (Fig. 1).
TABLE 1.
Comparison of dot blot and PCR results for detection of kpsM variants and putative K-antigen phenotypes
| No. of isolates | Category |
kpsM genotypea
|
No. of isolates identified by putative K-antigen phenotypeb
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Dot blot result by indicated probe
|
PCR result by indicated primers
|
||||||||||
| Group 2
|
Group 3 | Non-K2 group 2 (n = 27) | K2 (n = 11) | Group 3 (n = 14) | K− or otherc (n = 6) | Unknown | |||||
| Group 2 | Group 3 | Old | New | ||||||||
| 47 | Group 2, non-K2 | + | − | + | + | − | 25b | 3d | 1e | 1 | 17 |
| 42 | Group 2, K2 | + | − | − | + | − | 0 | 8b | 0 | 1 | 33 |
| 29 | Group 3 | − | + | − | − | + | 0 | 0 | 13b | 1f | 15 |
| 22 | Negative | − | − | − | − | − | 2g | 0 | 0 | 3b | 17 |
Plus and minus signs indicate the presence or absence, respectively, of a trait. Old, previously published selective primers; new, inclusive primers developed in the present study.
Results in bold are for cells containing members of the IEKC reference set.
Includes four capsule-minus (K−) isolates, a putative K36 (group 1) isolate, and a K80 (obsolete; lipopolysaccharide-associated capsular antigen) isolate.
Includes two putative O4:K2:H5 isolates and an O6:K2:H31 isolate.
Putative K3.
Strain J96, variously reported as capsular minus (K−) and K6.
Putative K1.
FIG. 1.
PCR detection of the group 2 kpsM variant in various reference strains of E. coli and as part of a multiplex virulence factor assay. Lanes 1 and 7, 100-bp ladder; lanes 2 to 5, new group 2 kpsM primers only, with reference strains CFT073 (lane 2), RS218 (lane 3), CP9 (lane 4), and HB101 (lane 5); lane 6, primer pool 6 (including the new group 2 kpsM primers) and pooled template DNA from reference strains positive for sat (6), F17 (3), usp (1), clpG (2), afaE8 (25), and astA (39). F17, F17 fimbriae.
Genotype-phenotype comparison.
We next assessed the correspondence of the kpsM genotype, as defined by a combination of PCR and blot, with the K-antigen phenotype among the 58 isolates for which a putative K-antigen status was available. Correspondence was observed for 49 of the 58 (84%) isolates, as shown in Table 1(boldface values). Notably, 29 of the 30 IEKC reference set isolates fell within these four cells, i.e., they exhibited precise genotype-phenotype concordance. The sole exception was strain J96, which although previously reported to express the (group 2) K6 antigen (8), contains group 3 kps sequences and is capsule minus according to the IEKC (19).
The other eight putative genotype-phenotype discrepancies, which were limited to non-IEKC reference set isolates (P < 0.001), included three putative phenotype K2 isolates that exhibited a non-K2 genotype, one capsule-minus isolate that exhibited the K2 kpsM genotype, and two putative K1 phenotype isolates that were kpsM negative (Table 1). Five of these isolates were investigated further. The two putative K1 phenotype isolates that were kpsM negative also were PCR negative with primers specific for the K1 kpsM variant and for neuS (K1 antigen synthesis). The three putative K2 phenotype isolates that exhibited a non-K2 group 2 genotype exhibited kpsM sequences that imprecisely matched all of the reference group 2 kpsM sequences (K2, 96 to 97% identity; K5, 94% identity, K1, 92% identity). Interestingly, the two isolates that putatively represented the same serotype (O4:K2:H5) exhibited slightly different (four single-nucleotide polymorphisms) kpsM sequences, one of which was identical to kpsM from the third isolate, which putatively represented a different serotype (O6:K2:H31).
Multiplex PCR and sensitivity analysis.
The addition of the new (inclusive) group 2 kpsM primers to pool 6 of our multiplex virulence factor PCR assay yielded, with a control template DNA pool, a robust PCR product of the expected size (577 bp) in addition to the six other virulence factor-specific products for sat (secreted autotransporter toxin) (6), F17 (F17 fimbriae) (3), usp (uropathogenic specific protein) (1), clpG (CS31A adhesin) (2), afaE8 (afimbrial adhesin) (25), and astA (enteroaggregative E. coli stable toxin) (39) (Fig. 1). The kpsM band was readily detected when present together with various combinations of the other six bands (data not shown). Assay performance was not appreciably affected by halving or doubling the concentrations of individual reaction mixture ingredients (MgCl2, deoxynucleoside triphosphates, polymerase, primers, and template DNA) or by varying the annealing temperatures from 54 to 64°C (data not shown).
Comment.
We designed and validated PCR primers that detect all group 2 kpsM (capsule synthesis) variants, as defined by probe hybridization, including the heretofore elusive K2 variant. Comparison of the results obtained with the new versus our old group 2 primers provided accurate discrimination of the K2 kpsM variant, which is detected by the new but not the old primers. Identification of the K2 kpsM variant is relevant for the molecular epidemiological identification of ExPEC clonal groups such as O6:K2:H1, a prominent cause of pyelonephritis and sepsis and the source of archetypal ExPEC strains CFT073 and AD110 (17, 18, 22, 23, 27). Assay performance was stable over a range of reagent concentrations and annealing temperatures.
The difficulty we encountered in this and a previous study (22) in devising type-specific and group-specific kpsM primers contrasts with the comparatively straightforward development of primers specific for the 13 known alleles of papA (P fimbrial structural subunit) (13, 21, 23). The difference lies largely in the availability of DNA sequences for all of the papA alleles (21) but, until recently, for only the K1 and K5 kpsM variants among the various group 2 capsular types. Consequently, we used instead the newly available K2 sequence to devise new consensus group 2 primers (38). Presumably, the regions we selected are highly conserved among group 2 kpsM variants in general, thereby allowing PCR detection to accomplish what previously was possible only with probe hybridization. The resulting primers can be multiplexed, permitting the simultaneous detection of multiple virulence factors, for expedited molecular epidemiological screening (13, 15-17, 22).
Some of the observed genotype-phenotype discrepancies may reflect true biological differences (as in the case of strain J96, which fails to express its known group 3 capsule genes) (23). However, serotyping errors and/or strain substitutions have been amply documented in other contexts (13, 14, 23) and must be considered here as well. This concern is supported by the observations that none of the discrepancies (other than for strain J96) involved the IEKC reference set isolates and that most involved specific K antigens that, when encountered within the IEKC reference set, were accurately aligned with the appropriate kpsM genotype. Additionally, for several isolates (putative K1 phenotype and kpsM negative [n = 2] and putative K2 phenotype and non-K2 group 2 kps genotype [n = 3]) genetic analysis supported the kpsM PCR results over the phenotypic data.
In summary, we have devised and extensively validated a rapid and simple PCR-based assay for the broad detection of group 2 kpsM variants in E. coli. When combined with our previously published group 2 primers, the new group 2 primers allow identification by exclusion of the K2 kpsM variant, which is detected only by the new primers. True genotype-phenotype discrepancies, although observed, were rare. The new primers function well when multiplexed with other primers and used over a broad range of assay conditions. Because of the considerable clinical significance of K2-expressing ExPEC clonal groups such as O6:K2:H1, these primers should provide a useful new tool for E. coli molecular epidemiology.
Acknowledgments
This material is based upon work supported by an Office of Research and Development, Medical Research Service, Department of Veterans Affairs, National Research Initiative (NRI) Competitive Grants Program/U. S. Department of Agriculture grant 00-35212-9408, the Minnesota Department of Health, and National Institutes of Health grant DK-47504.
Dave Prentiss (Minneapolis VA Medical Center) helped prepare the figure.
REFERENCES
- 1.Bauer, R. J., L. Zhang, B. Foxman, A. Siitonen, M. E. Jantunen, H. Saxen, and C. F. Marrs. 2002. Molecular epidemiology of 3 putative virulence genes for Escherichia coli urinary tract infection-usp, iha, and iroNE. coli. J. Infect. Dis. 185:1521-1524. [DOI] [PubMed] [Google Scholar]
- 2.Bertin, Y., C. Martin, J.-P. Girardieu, P. Pohl, and M. Conterpois. 1998. Association of genes encoding P fimbriae, CS31A antigen and EAST 1 toxin among CNF1-producing Escherichia coli strains from cattle with septicemia and diarrhea. FEMS Microbiol. Lett. 162:235-239. [DOI] [PubMed] [Google Scholar]
- 3.Bertin, Y., C. Martin, E. Oswald, and J.-P. Girardieu. 1996. Rapid and specific detection of F17-related pilin and adhesin genes in diarrheic and septicemic Escherichia coli strains by multiplex PCR. J. Clin. Microbiol. 34:2921-2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burns, S. M., and S. I. Hull. 1999. Loss of resistance to ingestion and phagocytic killing by O- and K-mutants of a uropathogenic Escherichia coli O75:K5 strain. Infect. Immun. 67:3757-3762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drake, C. R., G. J. Boulnois, and I. S. Roberts. 1993. The Escherichia coli serA-linked capsule locus and its flanking sequences are polymorphic, genetic evidence for the existence of more than two groups of capsule gene clusters. J. Gen. Microbiol. 139:1707-1714. [DOI] [PubMed] [Google Scholar]
- 6.Guyer, D. M., I. R. Henderson, J. P. Nataro, and H. L. T. Mobley. 2000. Identification of Sat, an autotransporter toxin produced by uropathogenic Escherichia coli. Mol. Microbiol. 38:53-56. [DOI] [PubMed] [Google Scholar]
- 7.Hoffman, J. A., C. Wass, M. F. Stins, and K. S. Kim. 1999. The capsule supports survival but not traversal of Escherichia coli K1 across the blood-brain barrier. Infect. Immun. 67:3566-3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hull, R. A., R. E. Gill, P. Hsu, B. H. Minshew, and S. Falkow. 1981. Construction and expression of recombinant plasmids encoding type 1 or d-mannose-resistant pili from a urinary tract infection Escherichia coli isolate. Infect. Immun. 33:933-938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jann, K., and B. Jann. 1992. Capsules of Escherichia coli, expression and biological significance. Can. J. Microbiol. 38:705-710. [DOI] [PubMed] [Google Scholar]
- 10.Jann, K., and B. Jann. 1983. The K antigens of Escherichia coli. Prog. Allergy 33:53-79. [DOI] [PubMed] [Google Scholar]
- 11.Johnson, J. R. 2000. Development of polymerase chain reaction-based assays for bacterial gene detection. J. Microbiol. Methods 41:201-209. [DOI] [PubMed] [Google Scholar]
- 12.Johnson, J. R. 1991. Virulence factors in Escherichia coli urinary tract infection. Clin. Microbiol. Rev. 4:80-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson, J. R., P. Delavari, M. Kuskowski, and A. L. Stell. 2001. Phylogenetic distribution of extraintestinal virulence-associated traits in Escherichia coli. J. Infect. Dis. 183:78-88. [DOI] [PubMed] [Google Scholar]
- 14.Johnson, J. R., P. Delavari, A. L. Stell, G. Prats, U. Carlino, and T. A. Russo. 2001. On the integrity of archival strain collections, including the ECOR collection. ASM News 67:288-289. [Google Scholar]
- 15.Johnson, J. R., H. A. Lockman, K. Owens, S. Jelacic, and P. I. Tarr. 2003. High-frequency secondary mutations after suicide-driven allelic exchange mutagenesis in extraintestinal pathogenic Escherichia coli. J. Bacteriol. 185:5301-5305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson, J. R., A. C. Murray, A. Gajewski, M. Sulliman, P. Snippes, M. A. Kuskowski, and K. E. Smith. 2003. Isolation and molecular characterization of nalidixic acid-resistant extraintestinal pathogenic Escherichia coli from retail chicken products. Antimicrob. Agents Chemother. 47:2161-2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson, J. R., T. T. O'Bryan, M. A. Kuskowski, and J. N. Maslow. 2001. Ongoing horizontal and vertical transmission of virulence genes and papA alleles among Escherichia coli blood isolates from patients with diverse-source bacteremia. Infect. Immun. 69:5363-5374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson, J. R., I. Orskov, F. Orskov, P. Goullet, B. Picard, S. L. Moseley, P. L. Roberts, and W. E. Stamm. 1994. O, K, and H antigens predict virulence factors, carboxylesterase B pattern, antimicrobial resistance, and host compromise among Escherichia coli strains causing urosepsis. J. Infect. Dis. 169:119-126. [DOI] [PubMed] [Google Scholar]
- 19.Johnson, J. R., T. A. Russo, F. Scheutz, J. J. Brown, L. Zhang, K. Palin, C. Rode, C. Bloch, C. F. Marrs, and B. Foxman. 1997. Discovery of disseminated J96-like strains of uropathogenic Escherichia coli O4:H5 containing genes for both PapGJ96 (“Class I”) and PrsGJ96 (“Class III”) Gal(α1-4)Gal-binding adhesins. J. Infect. Dis. 175:983-988. [DOI] [PubMed] [Google Scholar]
- 20.Johnson, J. R., A. E. Stapleton, T. A. Russo, F. S. Scheutz, J. J. Brown, and J. N. Maslow. 1997. Characteristics and prevalence within serogroup O4 of a J96-like clonal group of uropathogenic Escherichia coli O4:H5 containing the Class I and Class III alleles of papG. Infect. Immun. 65:2153-2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson, J. R., A. Stell, and P. Delavari. 2001. Canine feces as a reservoir of extraintestinal pathogenic Escherichia coli. Infect. Immun. 69:1306-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson, J. R., and A. L. Stell. 2000. Extended virulence genotypes of Escherichia coli strains from patients with urosepsis in relation to phylogeny and host compromise. J. Infect. Dis. 181:261-272. [DOI] [PubMed] [Google Scholar]
- 23.Johnson, J. R., A. L. Stell, F. Scheutz, T. T. O'Bryan, T. A. Russo, U. B. Carlino, C. C. Fasching, J. Kavle, L. van Dijk, and W. Gaastra. 2000. Analysis of F antigen-specific papA alleles of extraintestinal pathogenic Escherichia coli using a novel multiplex PCR-based assay. Infect Immun. 68:1587-1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim, K. S., H. Itabashi, P. Gemski, J. Sadoff, R. L. Warren, and A. S. Cross. 1992. The K1 capsule is the critical determinant in the development of Escherichia coli meningitis in the rat. J. Clin. Investig. 90:897-905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Le Bougenec, C., L. Lalioui, L. du Merle, M. Jouve, P. Courcoux, S. Bouzari, R. Selvarangan, B. J. Nowicki, G. Y., A. Andremont, P. Gounon, and M.-I. Garcia. 2001. Characterization of AfaE adhesins produced by extraintestinal and intestinal human Escherichia coli isolates: PCR assays for detection of Afa adhesins that do or do not recognize Dr blood group antigens. J. Clin. Microbiol. 39:1738-1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Orskov, I., and F. Orskov. 1985. Escherichia coli in extra-intestinal infections. J. Hyg. 95:551-575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Orskov, I., F. Orskov, A. Birch-Andersen, M. Kanamori, and C. Svanborg Eden. 1982. O, K, H and fimbrial antigens in Escherichia coli serotypes associated with pyelonephritis and cystitis. Scand. J. Infect. Dis. 33:18-25. [PubMed] [Google Scholar]
- 28.Pearce, R., and I. S. Roberts. 1995. Cloning and analysis of gene clusters for production of the Escherichia coli K10 and K54 antigens: identification of a new group of serA-linked capsule gene clusters. J. Bacteriol. 177:3992-3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roberts, I., R. Mountford, N. High, D. Bitter-Suermann, K. Jann, K. Timmis, and G. Boulnois. 1986. Molecular cloning and analysis of genes for production of K5, K7, K12, and K92 capsular polysaccharides in Escherichia coli. J. Bacteriol. 168:1228-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roberts, I. A., R. Mountford, R. Hodge, K. B. Jann, and G. J. Boulnois. 1988. Common organization of gene clusters for production of different capsular polysaccharides (K antigens) in Escherichia coli. J. Bacteriol. 170:1305-1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roberts, M., I. Roberts, T. K. Korhonen, K. Jann, D. Bitter-Suermann, G. J. Boulnois, and P. H. Williams. 1988. DNA probes for K-antigen (capsule) typing of Escherichia coli. J. Clin. Microbiol. 26:385-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Russo, T. A., Y. Liang, and A. S. Cross. 1994. The presence of K54 capsular polysaccharide increases the pathogenicity of Escherichia coli in vivo. J. Infect. Dis. 169:112-118. [DOI] [PubMed] [Google Scholar]
- 33.Russo, T. A., S. Wenderoth, U. B. Carlino, J. M. Merrick, and A. J. Lesse. 1998. Identification, genomic organization, and analysis of the group III capsular polysaccharide genes kpsD, kpsM, kpsT, and kpsE from an extraintestinal isolate of Escherichia coli (CP9, O4/K54/H5). J. Bacteriol. 180:338-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Senior, D. F., P. deMan, and C. Svanborg. 1992. Serotype, hemolysin production, and adherence characteristics of strains of Escherichia coli causing urinary tract infection in dogs. Am. J. Vet. Res. 53:494-498. [PubMed] [Google Scholar]
- 35.Starcic, M., J. R. Johnson, A. L. Stell, J. van der Goot, H. G. Hendriks, C. van Vorstenbosch, L. van Dijk, and W. Gaastra. 2002. Haemolytic Escherichia coli isolated from dogs with diarrhea have characteristics of both uropathogenic and necrotoxigenic strains. Vet. Microbiol. 85:361-377. [DOI] [PubMed] [Google Scholar]
- 36.Thompson, J. D., T. J. Gibson, F. Plewniak, F. Jeanmougin, and D. G. Higgins. 1998. The CLUSTAL-X windows interface: flexible strategies for multiple sequence alignment aided quality analysis tools. Nucleic Acids Res. 25:4876-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsukamoto, T. 1997. PCR method for detection of K1 antigen and serotype of Escherichia coli isolated from extraintestinal infection. Kansenshogaku Zasshi 71:125-129. (In Japanese.) [DOI] [PubMed]
- 38.Welch, R., V. Burland, G. Plunkett III, P. Redford, P. Roesch, D. Rasko, E. L. Buckles, S.-R. Liou, A. Boutin, J. Hackett, D. Stroud, G. F. Mayhew, D. J. Rose, S. Zhou, D. C. Schwartz, N. T. Perna, H. L. T. Mobley, M. S. Donnenberg, and F. R. Blattner. 2002. Extensive mosaic structure revealed by the complete genome sequence of uropathogenic Escherichia coli. Proc. Natl. Acad. Sci. USA. 99:17020-17024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamamoto, T., and P. Echeverria. 1996. Detection of the enteroaggregative Escherichia coli heat-stable enterotoxin 1 gene sequences in enterotoxigenic E. coli strains pathogenic for humans. Infect. Immun. 64:1441-1445. [DOI] [PMC free article] [PubMed] [Google Scholar]