Significance
Release of endogenous danger signals has been found to be a key mechanism of many conventional, broadly used adjuvants. Yet extracellular ATP has so far not been linked to vaccination success. Here we show that ATP release from muscle is crucial for the mechanism of action of the vaccine adjuvant MF59, leading to efficient CD4 T-cell priming and high antibody titers.
Keywords: vaccine adjuvants, danger associated molecular pattern, DAMP, inflammation
Abstract
Vaccines are the most effective agents to control infections. In addition to the pathogen antigens, vaccines contain adjuvants that are used to enhance protective immune responses. However, the molecular mechanism of action of most adjuvants is ill-known, and a better understanding of adjuvanticity is needed to develop improved adjuvants based on molecular targets that further enhance vaccine efficacy. This is particularly important for tuberculosis, malaria, AIDS, and other diseases for which protective vaccines do not exist. Release of endogenous danger signals has been linked to adjuvanticity; however, the role of extracellular ATP during vaccination has never been explored. Here, we tested whether ATP release is involved in the immune boosting effect of four common adjuvants: aluminum hydroxide, calcium phosphate, incomplete Freund’s adjuvant, and the oil-in-water emulsion MF59. We found that intramuscular injection is always associated with a weak transient release of ATP, which was greatly enhanced by the presence of MF59 but not by all other adjuvants tested. Local injection of apyrase, an ATP-hydrolyzing enzyme, inhibited cell recruitment in the muscle induced by MF59 but not by alum or incomplete Freund’s adjuvant. In addition, apyrase strongly inhibited influenza-specific T-cell responses and hemagglutination inhibition titers in response to an MF59-adjuvanted trivalent influenza vaccine. These data demonstrate that a transient ATP release is required for innate and adaptive immune responses induced by MF59 and link extracellular ATP with an enhanced response to vaccination.
Vaccine adjuvants are used to enhance immune responses toward coadministered antigens, thereby improving vaccine potency, immunological memory, or cross-protection (1, 2). Experimental adjuvants range from simple molecules such as calcium phosphate (CaPi) to very complex mixtures such as incomplete Freund’s adjuvant (IFA), made of a water-in-oil emulsion, or complete Freund’s adjuvant, which also includes killed Mycobacteria (3). However, for human vaccines, adjuvants of highly defined properties that combine efficacy with complete safety are needed; to date, very few compounds have been licensed. Some of the safest and most efficient adjuvants licensed for human use, such as aluminum hydroxide (alum) and the oil-in-water squalene-based emulsion MF59, have been empirically identified, and their mechanism of action is still not fully understood (4, 5). A better understanding of their mechanism of action is needed to develop improved adjuvants that further enhance vaccine efficacy. This is particularly important for diseases for which protective vaccines do not exist (6).
An examination of the chemical nature of four major vaccine adjuvants (alum, CaPi, IFA, and MF59) suggested they could interact with the phospholipid bilayer of cell membranes via hydrogen bonding or ionic interactions with the head groups of phospholipids/glycolipids and/or via hydrophobic interactions with the hydrocarbon chains of lipids. As vaccines are frequently administered by intramuscular (i.m.) injection, we posited that a high local concentration of adjuvant is generated in a confined portion of the muscle and that the first cell membrane they come in contact with is the sarcolemma. Because we found that the muscle injection of membrane-interacting snake phospholipase A2 myotoxin induces the release of ATP, which is contained in large amounts inside muscle fibers (7, 8), we decided to evaluate the possibility that other putative membrane-interacting agents such as the major adjuvants mentioned earlier might similarly induce ATP release. This possibility would be particularly relevant in the context of adjuvanticity, as ATP is a “danger signal” acting on a variety of purinergic P2 receptors and, as such, is a strong modulator of immune responses (9–11).
Results
Adjuvant-Induced ATP Release from Injected Mouse Muscles.
To test our hypothesis, we monitored the adjuvant-stimulated ATP release in mice using the reporter system luciferase-luciferin. In the presence of ATP, luciferase catalyzes oxidation of luciferin with an emission of photons that can be recorded by an appropriate imaging apparatus.
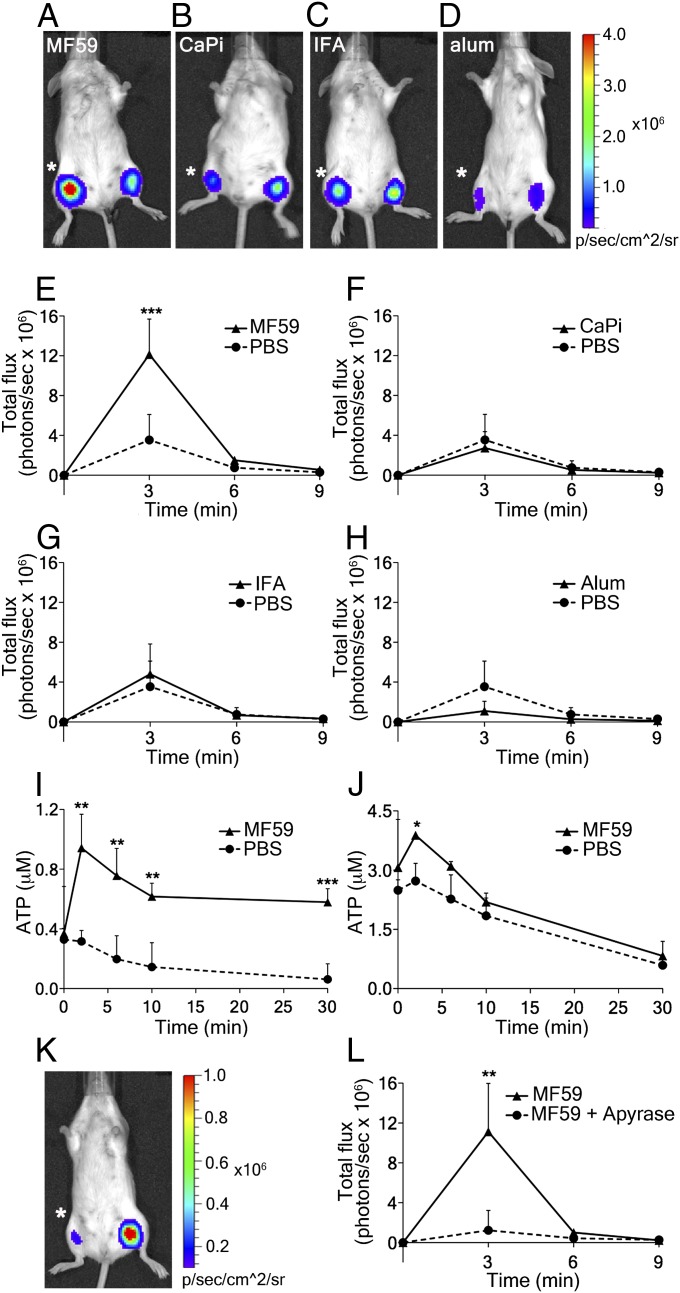
Recent work has shown that cells engineered to stably express luciferase on their plasma membrane (PmeLUC cells) are suitable for detecting changes in extracellular ATP concentration in vivo (12). However, in preliminary experiments, we found that i.m. injected luciferase adsorbs onto muscle fibers in vivo and efficiently reports ATP changes within the muscle. Furthermore, administration of soluble luciferase causes a smaller perturbation of tissue homeostasis than injection of the PmeLUC cell suspension. This read-out is so sensitive that even the low ATP release resulting from needle injury can be detected at the injection site. Testing the different adjuvants, we found that only MF59 injection induces a fast and prominent ATP signal that is significantly higher than ATP release caused by PBS injection in the contralateral muscle (Fig. 1 A and E). In contrast, CaPi or IFA inoculation do not increase ATP release over buffer control (Fig. 1 B, C, F, and G), and alum even appears to decrease the signal (Fig. 1 D and H). However, alum readily binds many proteins. Because we observed that ATP binds to alum in vitro, the luminescence reduction could be a consequence of ATP and/or luciferin-luciferase adsorption onto the adjuvant surface.
Fig. 1.
Adjuvant-induced ATP release in mouse muscles and the effect of apyrase. (A–D) Representative images taken 3 min after intramuscular injection of adjuvants (right hind limb; asterisk) or PBS (left hind limb) in BALB/c mice together with the mixture luciferase-luciferin that reports on ATP changes. (E–H) Corresponding quantitative analyses of chemiluminescence emission over time (number of photons per second in the region of interest). (A and E) MF59 (40% vol/vol), (B and F) CaPi (50 μg), (C and G) IFA (40% vol/vol), (D and H) alum (100 μg), and (I and J) ATP release from ex vivo mouse muscles injected with MF59. Mouse tibialis anterior (I) and quadriceps (J) muscles were exposed and injected with MF59 (40% vol/vol), continuous lines; the dotted line refers to the injection of the same volume of PBS. Muscles were rapidly removed and suspended in oxygenated buffer at 37 °C. ATP released into buffer was quantified at the given times, using the luciferin-luciferase assay and a known ATP standard. (K) Representative image taken 3 min after intramuscular injection of MF59 + apyrase (10 U; asterisk) or MF59 alone (contralateral muscle). (L) Corresponding quantitative analysis over time. Data show mean values + SD from at least four independent experiments. Unpaired, two-tailed Student’s t test (T): *P < 0.05, **P < 0.01, ***P < 0.001.
Quantitative photoemission evaluation revealed that MF59 injection increased extracellular ATP levels about threefold compared with those triggered by PBS- (CaPi-, IFA-, or alum-) containing mixtures (Fig. 1 E to H). Extracellular ATP increases within 2–3 min after MF59 injection and then declines over the next 5–6 min. Signal decline is most likely a result of ATP dilution in tissue fluids and the ATP-hydrolyzing activity of ecto-ATPases, which are present on the surface of sarcolemma and stromal cells (13). To test this latter possibility and to have an independent evaluation of ATP release, we injected ex vivo tibialis anterior and quadriceps muscles with MF59 or buffer control and measured ATP release into the medium (Fig. 1 I and J). The assay confirms prior findings, yet ATP release lasts longer, possibly because of the different diffusion kinetics of the fluids within the perimuscle milieu in the two different set-ups. Furthermore, in the ex vivo assay, ATP freely diffuses into the bathing solution, and therefore partially escapes hydrolysis, indirectly supporting the explanation that in the in vivo experiments, ATP is immediately exposed to the degrading activity of ecto-ATPases. Next, we tested which of the individual ingredients of MF59 would be responsible for its activity. Not surprisingly, ATP release is caused by Tween 80 and Span 85, but not by squalene oil alone (Fig. S1).
Taken together, our results clearly document that MF59 displays a unique capacity to greatly increase ATP release from injected muscles. To assess whether this ATP release would be essential for the adjuvant effect of MF59, we quenched extracellular ATP by coinjection of MF59 with apyrase, an enzyme that rapidly hydrolyzes ATP to AMP (14). Fig. 1 K and L shows that apyrase completely abolishes the MF59-induced ATP signal. On the basis of this result, we could proceed to determining whether coinjection of apyrase would alter induction of innate and adaptive immune responses by MF59.
Immune Cell Recruitment Induced by MF59 Injection Is Inhibited by Apyrase.
Over the years, ATP has emerged as an important activator and modulator of immune responses, among several other danger molecules that are released from cells by a variety of pathogens of differing physical, chemical, and biological nature (10, 11, 15). In particular, it was shown that ATP released by stressed or dying cells promotes recruitment and activation of phagocytes. Therefore, we investigated whether and how ATP release could contribute to the activity of MF59.
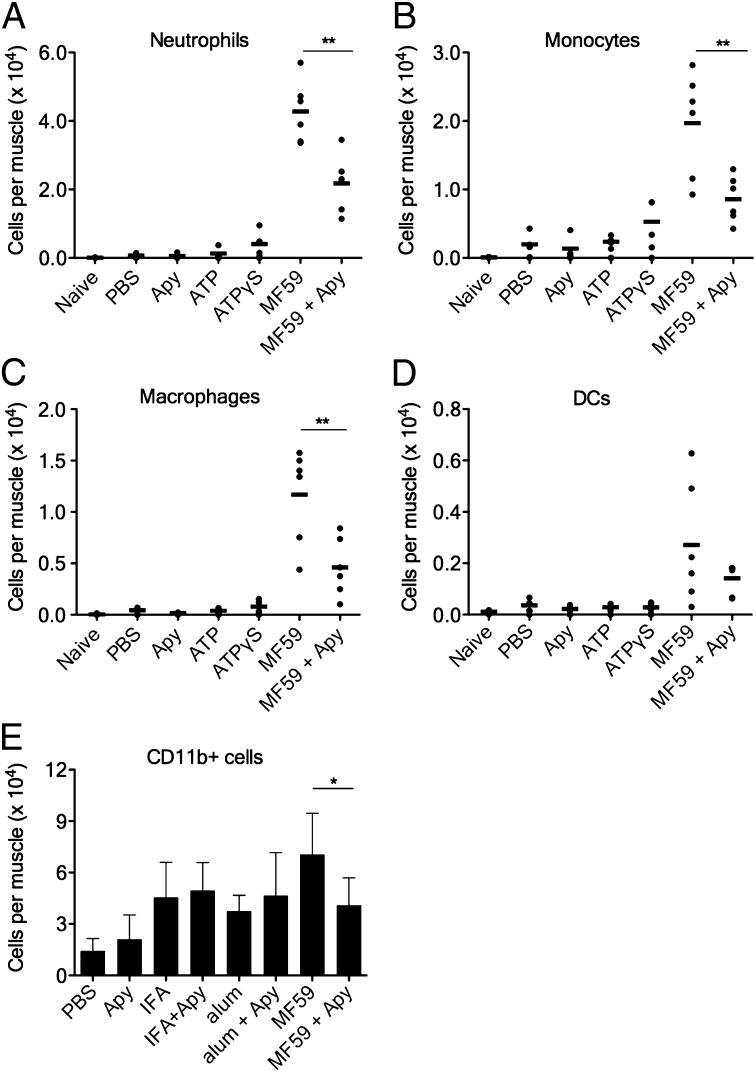
The strong adjuvant effect of MF59 (16, 17) has been ascribed to its capability to induce an immunocompetent environment in the muscle, characterized by a rapid and transient influx of a large number of CD11b+ immune cells participating in antigen uptake and transport to draining lymph nodes (18–21). To clarify the role of ATP in MF59-induced cell recruitment, mice were injected i.m. with MF59 in the presence or absence of apyrase. After 24 h, muscles were harvested and their content of neutrophils, monocytes, dendritic cells, and macrophages was determined by flow cytometry (the gating strategy is shown in Fig. S2). Coinjection of apyrase clearly lowered MF59-induced cell recruitment (Fig. 2), indicating that ATP release is in part responsible for cell influx. However, ATP by itself does not have an appreciable effect, which is true for both degradation-sensitive ATP and degradation-resistant ATP-γS. However, this is not surprising, as the injection of a single ATP bolus does not reproduce the graded concentration of extracellular ATP that appears to be necessary to support chemotaxis (11, 22). In addition, MF59 might induce the release of additional danger- or damage-associated signals and chemotactic factors that may synergize with ATP, as shown, for example, for mesenchymal stem cell responses to CXC chemokine ligand (CXCL)12 and ATP (23). In favor of this hypothesis is the finding that the injection of alum and IFA also resulted in a significant recruitment of CD11b+ cells in the injected muscle. However, for all adjuvants, cell influx was significantly lower compared with MF59 (Fig. 2E) and reached levels similar to those of mice treated with MF59 and apyrase. At the same time, coinjection of apyrase did not have any effect on alum- and IFA-mediated cell recruitment, which is consistent with the previous finding that they are not good ATP inducers in the muscle (Fig. 1 G and H).
Fig. 2.
Coinjection of apyrase reduces immune cell recruitment induced by MF59, but not by IFA or alum. (A–D) Groups of mice were injected i.m. with the indicated compounds at the following doses: MF59 (40% vol/vol), ATP or ATP-γS (5mM), apyrase (Apy) (10 U per leg), or PBS. Single-cell suspensions of treated muscles were analyzed by FACS 24 h postinjection. Dots show numbers of the respective cell type per individual muscle (N ≥ 4 per group), whereas black bars indicate arithmetic means. (A) Neutrophils, (B) monocytes, (C) macrophages, and (D) dendritic cells (DCs). (E) Groups of mice were injected i.m. with the indicated compounds at the following doses: MF59 (20% vol/vol), IFA (40% vol/vol), alum (100 μg), apyrase (10 U per leg), or PBS, all in presence of OVA (10 μg per mouse). Numbers of CD11b+ cells are reported, data show mean values + SD from eight to 12 muscles per group. Unpaired, two-tailed Student’s t test (T): *P < 0.05, **P < 0.01.
ATP Release Contributes to Adjuvanticity of MF59.
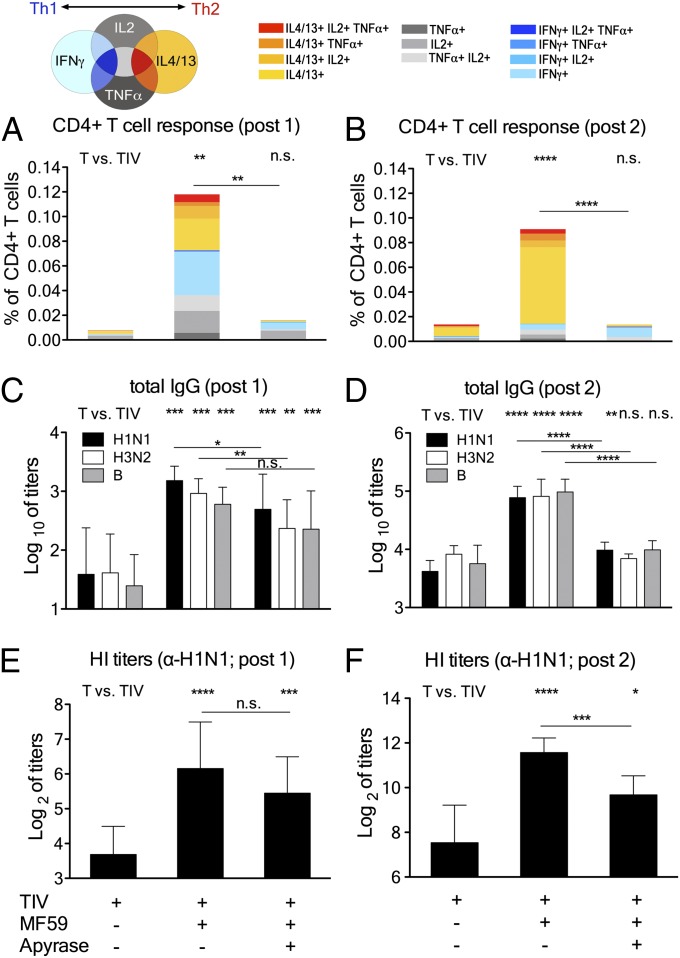
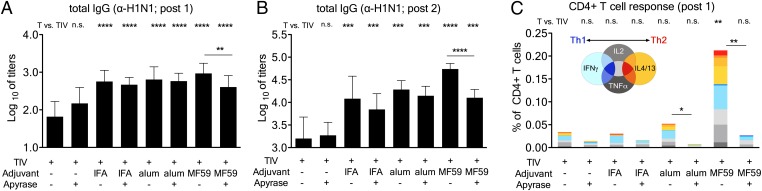
A strong recruitment of immune cells at the injection site leads to enhanced antigen uptake and transport to draining lymph nodes, which translates into overall strongly enhanced adaptive immune responses (18, 19). Accordingly, we assessed the effect of MF59-induced ATP release on CD4+ T helper (Th) responses and antibody titers. Groups of mice were immunized with an experimental trivalent influenza vaccine (TIV), either as plain antigens or together with MF59 in the presence or absence of apyrase. Control experiments showed that the injection of TIV antigens alone does not induce ATP release (Fig. S3). We found that MF59-induced ATP release strongly contributes to adjuvanticity both for Th cell responses and vaccine-specific antibody titers. Vaccine-specific Th cells were reactivated by in vitro stimulation of splenocytes from immunized mice and assessed by FACS for intracellular cytokine expression (gating strategy and representative FACS blots are shown in Fig. S4). MF59-adjuvanted vaccine induced high T-cell responses after the first immunization, but this effect was completely abolished by coinjection of apyrase (Fig. 3A). Similar results were observed after the booster dose (Fig. 3B). MF59 induced a mixed Th1/Th2 profile, but apyrase-mediated abrogation of ATP signaling had more effect on Th2 (IL4/13+) responses compared with Th1 (IFNγ+). This is even more evident after booster vaccination.
Fig. 3.
Coinjection of apyrase inhibits adjuvanticity of MF59 to a trivalent influenza vaccine (TIV). (A–F) twelve mice per group were immunized twice (4 wk apart) with TIV and adjuvants, as indicated: MF59 (40% vol/vol), apyrase (10 U per leg), and TIV (0.1 μg each antigen). (A and B) Spleens from 4 mice per group were taken 2 wk after each immunization, and vaccine-specific CD4+ T helper cells were reactivated by in vitro stimulation. Their individual cytokine profile was assessed by intracellular cytokine staining and FACS analysis. The bars show cumulative numbers of vaccine-specific cytokine expressing cells after the first (A) and second (B) immunization, and the individual color code indicates the type of cytokines expressed by the respective cells, as indicated. (C–F) Serum samples were drawn 2 wk after each immunization, and vaccine-specific antibody titers were measured. Total IgG antibody titers toward H1N1/California, H3N2/Perth, and B/Brisbane after the first (C) and second (D) immunization. Values represent mean logarithmic titers (log 10) of eight to 12 mice per group + SD. Hemagglutination inhibition titers toward H1N1/California after the first (E) and second (F) immunization; values represent means of Log2 titers of eight to 12 mice per group + SD. Unpaired, two-tailed Student’s t test (T): *P < 0.05,**P < 0.01, ***P < 0.001,****P < 0.0001.
MF59 significantly enhanced antibody titers against all three vaccine antigens compared with TIV alone after the first vaccination (Fig. 3C), with a mixed IgG1/IgG2 profile (Fig. S5). The prominent adjuvant effect of MF59 not only led to a large increase in total antibody titers but also increased functional hemagglutination inhibition (HI) titers, which are considered a correlate of protection for influenza vaccinations (24) (Fig. 3E). Apyrase coinjection modestly reduced the antibody responses induced by the first injection of MF59-TIV. However, the adjuvant effect of MF59 was still significant (Fig. 3 C and E). Only after the booster immunization did the apyrase-mediated reduction of total IgG, IgG1, and HI titers become highly significant (Fig. 3 D and F and Fig. S5). Similar results were observed using other antigens such as ovalbumin (OVA; Fig. S6).
We wondered whether the partial effect of apyrase after the first immunization with TIV-MF59 was a result of the use of a high dose of adjuvant that cannot be blocked entirely. Therefore, we titrated down MF59 from 40% (standard dose used here) to as little as 2.5%. At all conditions tested, a significant reduction of antibody titers by apyrase was detected after the booster vaccination, but not after the first dose (Fig. S7). In summary, our data demonstrate that ATP is required for CD4+ T-cell responses induced by MF59 and for secondary antibody responses, but not for primary antibody responses.
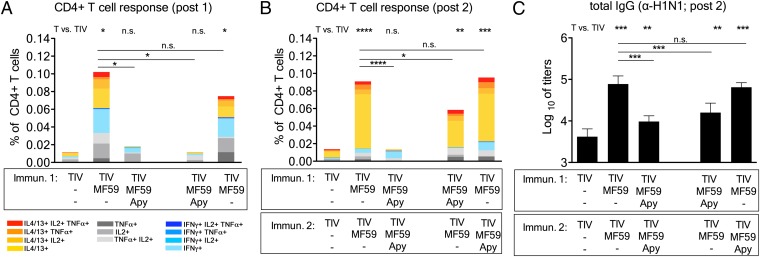
To better dissect the effect of apyrase on MF59 adjuvanticity, we performed an additional experiment using TIV. One group of mice received two doses of MF59+apyrase, as before. In the other groups, apyrase was added to MF59 only during prime or only during boost (Fig. 4). The group receiving MF59+apyrase twice had severely reduced Th cell responses after the first and the second vaccination, as previously shown. Apyrase coinjection only during prime had a very significant effect on CD4 responses after the first dose, but it only modestly reduced CD4 T-cell frequencies after the second dose. Apyrase added only during boost did not have any significant effect on CD4 T-cell frequencies (Fig. 4B). Antibody titers were significantly reduced in mice that received MF59+apyrase either during both immunizations or during the prime (Fig. 4C), whereas apyrase addition during the booster dose had no significant effect. Interestingly, we did not detect any difference in antibody titers in the group of mice that received apyrase twice compared with the mice that received apyrase only during the prime.
Fig. 4.
ATP release induced by MF59 is essential during the first vaccination. Mice were immunized as before with TIV and adjuvants, as indicated. One group of mice received two doses of MF59+apyrase, whereas in the other groups, apyrase (Apy) was added to MF59 only during prime or only during boost. (A and B) Spleens from 4 mice per group were taken 2 wk after each immunization, and vaccine-specific CD4+ T helper cells were reactivated by in vitro stimulation, as before. The bars show cumulative numbers of vaccine-specific cytokine expressing cells after the first (A) and second (B) immunization, whereas the individual color code indicates the type of cytokines expressed by the respective cells. (C) Serum samples were drawn 2 wk after the second immunization, and total IgG antibody titers toward H1N1/California were measured by ELISA. Values represent mean logarithmic titers (log 10) of eight mice per group + SD. Unpaired, two-tailed Student’s t test (T): *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
From this experiment, we concluded that the effect of apyrase on antibody responses induced by MF59 results from an inhibition of T-cell priming during the first vaccination.
Next, we asked whether the effect of apyrase was specific for MF59 or whether it could be extended to other adjuvants (Fig. 5). We found that apyrase did not significantly reduce antibody responses generated by TIV antigens alone or in combination with IFA and alum, which do not stimulate ATP release in the muscle (Fig. 1 C and D). This is true both for primary antibody responses after the first dose (Fig. 5A) and for secondary responses (Fig. 5B and Fig. S8A), as well as for functional HI titers (Fig. S8B).
Fig. 5.
Apyrase inhibits antibody responses induced by TIV adjuvanted with MF59, but not with alum and IFA. (A–C) Twelve mice per group were immunized as before with TIV and adjuvants, as indicated. The following doses were used: MF59 (40% vol/vol), IFA (40% vol/vol), alum (100 μg), apyrase (10 U per leg), and TIV (0.1 μg each antigen). (A and B) Serum samples were drawn 2 wk after the first (A) or second (B) immunization, and total IgG antibody titers toward H1N1/California were measured by ELISA. Values represent mean logarithmic titers (log 10) of eight to 12 mice per group + SD. (C) Spleens from 4 mice per group were taken 2 wk after the first immunization, and vaccine-specific CD4+ T helper cells were reactivated by in vitro stimulation. Their individual cytokine profile was assessed by intracellular cytokine staining and FACS analysis. The bars show cumulative numbers of vaccine-specific cytokine expressing cells, as indicated. Unpaired, two-tailed Student’s t test (T): *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
The analysis of T-cell responses revealed that inhibition of antigen-specific Th cells by apyrase is predominantly observed in the presence of MF59. Addition of apyrase to TIV antigens alone or formulated with IFA resulted in a nonsignificant reduction of vaccine-specific CD4 T cells. However, apyrase significantly reduced CD4 T cells induced by TIV formulated in alum (Fig. 5C). Apyrase-mediated hydrolysis of the baseline level of extracellular ATP induced by injury might be responsible for the observed reduction of specific CD4 T cells in the groups that did not receive MF59 as adjuvant.
Our results clearly show that ATP release contributes to adjuvanticity of MF59. We questioned whether ATP by itself could induce a measurable adjuvant effect. Therefore, we immunized mice with TIV and different concentrations of ATP or the hydrolysis-resistant ATP-γS (Fig. S9 A–C). As expected, MF59 increased total IgG titers in response to all three influenza antigens, whereas ATP coadministration did not boost antibody responses at any concentration tested. The efficient immune modulation by ATP might depend on timely graded and local concentrations of ATP and/or on synergies with other alarmins released by MF59 injection. It is probable that the injection of ATP does not mimic the localized tissue release of ATP and of other factors induced by MF59 in the muscle.
Discussion
Taken together, the present results provide strong evidence that ATP released from injected muscle is a crucial contributor to the adjuvant activity of MF59. The immunological step in which ATP is required is naive T-cell priming after the administration of the first dose of vaccine. Efficient T-cell priming is required for an optimal antibody response after the boost. The effect of apyrase was more evident for CD4+ T-cell responses in the Th2 compartment. Accordingly, we observed a strong reduction of IgG1 antibodies after the booster dose of MF59-TIV vaccine. The data available at this stage do not address directly the question concerning which cells release ATP around the injection site. However, previous studies have indicated that MF59 injection induces activation of muscle fibers, as revealed by JunB translocation and Pentraxin 3 expression (21), suggesting that skeletal muscle fibers, with their high content of ATP, are a major target of MF59. At variance from MF59, neither alum nor CaPi and IFA were found to increase ATP release from muscles over injection-induced background level. However, this negative result leaves open the possibility that other “danger signaling” molecules could be involved in their adjuvant activities. Indeed, recent reports have implicated endogenous uric acid and DNA release in the adjuvanticity of alum (25–27). Similar to the role of ATP for MF59, alum-driven DNA release has been implicated in the priming of naive T cells after the first dose of vaccine and has only a partial effect on antibody responses, suggesting that additional mechanisms are involved in alum adjuvanticity (27). Even in the case of MF59, the results presented here indicate that ATP is a crucial contributor, but not the sole factor involved in MF59 adjuvanticity.
Exogenous ATP is active on an array of purinergic receptors that can modulate intracellular signaling. In particular, ATP is known to activate the NLRP3 inflammasome complex via the P2X7 receptor, inducing the maturation and release of proinflammatory cytokines such as IL-1β (28, 29). Interestingly, NLRP3 has also been linked to alum mechanism of action (30, 31), and therefore P2X7 may represent an obvious link between ATP and adjuvanticity. However, two independent studies have shown that the mechanism of MF59 is independent from NLRP3 inflammasome activation (32, 33), and therefore we propose that other events triggered by purinergic receptors are required for adjuvanticity. We have reported that MF59 adjuvanticity depends on signaling pathways that involve the Myeloid differentiation primary response protein MyD88 (32). MyD88 is a common adaptor of most toll-like receptors and of IL-1 family cytokine receptors such as IL-1R, IL-18R, or IL-33R ST2. As MF59 does not trigger toll-like receptor signaling in vitro (33), it is more likely that MyD88 signaling downstream of IL-1 family receptors could be crucial for MF59 activity. Interestingly, it was shown that ATP-induced signaling via purinergic receptors not only leads to inflammasome-dependent IL-1 release but also can induce the inflammasome-independent release of IL-33 (34). It would be interesting to ascertain whether apyrase treatment in MyD88 KO mice might lead to total abrogation of all hallmarks of MF59 action, including generation of an immunocompetent environment, cell recruitment, antigen uptake and translocation, and activation of adaptive immunity.
This work supports the model that adjuvanticity involves induction of host molecules acting as danger signals. The latter activate the immune system to the advantage of a more sustained and protective immune reaction to vaccines. These findings lead to a large area of investigation to identify compounds that induce the most appropriate and advantageous danger signals boosting immune response to vaccines. We could show that ATP can contribute to a broad panel of immune events ranging from innate immunity to adaptive humoral and cellular responses, representing an attractive target to improve vaccine responses.
Materials and Methods
Mice.
Pathogen-free BALB/c mice (purchased from Charles River) aged 6–8 wk were used in this study in agreement with institutional and European guidelines. All experimental procedures involving animals were carried out in accordance with the Italian Animal Welfare Act and were approved by the local authority veterinary service at the University of Padova, Ferrara and at the Animal Ethical Committee (AEC) of Novartis in Siena.
Adjuvants.
MF59, a Novartis proprietary oil-in-water emulsion consisting of 4.3% (vol/vol) squalene, 0.5% Tween 80, and 0.5% Span 85 in citrate buffer (10 mM), was prepared as described earlier (19). The mean particle size of the emulsion droplets determined with a Mastersizer X (Malvern Instruments) was 194 ± 76 nm. Aluminum hydroxide was from Novartis, calcium phosphate from Brenntag Biosector, and IFA from Difco Laboratories.
In vivo Bioluminescence Imaging.
In vivo bioluminescent imaging was performed with an ultra-low-noise, high-sensitivity cooled CCD camera mounted on a light-tight imaging chamber (IVIS Lumina System, Caliper, Perkin-Elmer). Tracking, monitoring, and quantification of signals were controlled by the acquisition and analysis software Living Image. Mice were anesthetized with a continuous flux of isoflurane, positioned in the instrument chamber, and injected with a 50-µL syringe fitted with a 29-gauge needle (Hamilton). For each mouse, one leg was injected intramuscularly with a mixture composed of the reporter (luciferase-luciferin mix, Promega) and of the adjuvant to be tested, MF59 (40% vol/vol), alum (100 µg), CaPi (50 µg), and IFA (40% vol/vol), in a total volume of 20 µL. The individual components of MF59 (squalene, Span 85, and Tween 80) were formulated in PBS at the same dose as within the MF59 emulsion. The contralateral leg was injected with the reporter solution plus the PBS used for adjuvant dilution. One mouse per each experimental run was monitored immediately after injections; luminescent images were obtained with constant exposure times of 3 min, for a total of 9 min; regions of interest were defined manually around the injection site to determine the total photon flux as number of photons per second. Experiments with apyrase were performed in the same conditions: For each mouse, one leg was injected with a mixture composed of the luciferase-luciferin mix plus MF59 (40% vol/vol) and apyrase (10U, Sigma), whereas the contralateral leg was injected with the reporter solution plus MF59 (40% vol/vol) alone. For experiments with TIV antigens, mice were i.m. injected with the reporter plus TIV antigens (0.1 μg each antigen) alone, TIV antigens adjuvated with MF59 (20% vol/vol), or PBS. For all injections, the final volume was 20 µL.
Muscle Isolation and ex Vivo ATP Measurement.
Mouse hind limb muscles (tibialis anterior, or quadriceps) were injected with 25 µL MF59 (40% vol/vol) diluted in PBS in one leg or with the same volume of PBS in the contralateral one, and then rapidly isolated from mice and immediately transferred to vials containing 1 mL oxygenated (95% O2, 5% CO2) physiological buffer (139 mM NaCl, 12 mM NaHCO3, 4 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 1 mM KH2PO4, and 11 mM glucose at pH 7.4) at 37 °C. The ATP released in the buffer was determined with the ATPlite luciferase assay (Perkin-Elmer) as previously described (8).
Cell Recruitment into Muscle.
Groups of mice were injected with 25 μL per muscle of MF59 (40% or 20% vol/vol), alum (100 µg), and IFA (40% vol/vol), all with or without apyrase (10 U per muscle), with ATP-γS or ATP (5 mM, Sigma), or PBS buffer control. Experiments were performed injecting either adjuvants alone or in presence of the model antigen ovalbumin (OVA-AF647; Molecular Probes, Invitrogen). Twenty-four hours postinjection, mice were killed and quadriceps muscles were processed, as previously described (19). Cells were stained with Live/Dead Fixable Yellow (Invitrogen) and combinations of the following antibodies: α-Ly6C-FITC, α-CD11b-PE-Cy7, α-Ly6G-PE, α-CD3-PerCpCy5.5 (all from BD Pharmingen) and α-I-A/I-E-AlexaFluor700, α-F4/80-PacificBlue, α-CD11c-APC-eFluor780 (all from eBioscience). The stained cells were analyzed using a FACS LSR II Special Order System (BD Biosciences), using BD DIVA software (BD Biosciences).
Vaccine Formulation and Immunization.
Experimental trivalent influenza vaccine composed of equal amounts hemagglutinin (HA) from influenza strains H1N1 A/California/7/2009, H3N2 A/Perth/16/2009, and B/Brisbane/60/2008 was used in immunogenicity experiments. The vaccine contains purified subunit antigens and is standardized for HA content by single radial immunodiffusion. For adjuvanticity experiments, groups of eight to 12 animals were immunized two times on days 0 and 28 in the quadriceps muscles of both hind legs with 25 μL vaccine per leg (50 μL total per mouse). Doses were 0.3 μg (0.1 μg each antigen) of either influenza-soluble trivalent egg-derived antigen alone; antigens mixed with research grade MF59 (40% vol/vol), alum (100 µg), or IFA (40% vol/vol), all with or without apyrase (10 U per muscle), apyrase alone, ATP-γS, or ATP (1 or 5 mM). Serum samples of individual mice were collected 2 wk after each immunization and evaluated for total IgG antibody titers by ELISA and HI titers by the HI assay. All formulations were optimized for pH and osmolality to physiological conditions.
For some experiments, mice were immunized as described before with 10 µg per mouse Endograde OVA (Hyglos).
ELISA.
Titration of HA-specific or OVA-specific total IgG (IgG) was performed on individual serum samples as previously described (19). Antibody titers are dilutions that give an optical density higher than the mean plus five times the SD of the average optical density obtained in the preimmune sera. The titers were normalized with respect to the reference serum assayed in parallel.
Determination of Antibodies by HI Assay.
The HI assay was carried out on individual sera taken 2 wk after the second immunization, as described elsewhere (17).
In Vitro Restimulation of Ag-Specific CD4+ T Cells.
Four mice per group were killed 2 wk after immunization, and spleens were collected to assess the frequency and phenotype of Ag-specific CD4+/CD44+ T cells induced by vaccination. The assay was performed as described elsewhere (17).
Statistical Analysis.
All statistics were performed using GraphPad Prism software. The unpaired two-sample Student’s t test was used. P values less than 0.05 were considered significant.
Supplementary Material
Acknowledgments
We thank S. Calabrò, Y. Teplova, D. Casini, I. Vicenti, A. Bonci, S. Aprea, and the Novartis FACS and Animal facilities for technical assistance; S. Bertholet for discussions; and M. Scarletti for help with IVIS technique and animal care. M.V. is supported by a Novartis Ph.D. Fellowship. These studies were supported by grants from the University of Padova (to C.M.), from IARC (IG 5354), Telethon-Italy (GGP06070), MIUR (FIRB, RBAP11FXBC; PRIN, 2009LMEEEH, and PON01_00117), a grant from the European Commission of the Seventh Framework Program (Advanced Immunization Technologies, 280873), the European Community (ERA-NET Nanostroke), and institutional funds from the University of Ferrara (to F.D.V.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1319784110/-/DCSupplemental.
References
- 1.McKee AS, MacLeod MKL, Kappler JW, Marrack P. Immune mechanisms of protection: Can adjuvants rise to the challenge? BMC Biol. 2010;8:37. doi: 10.1186/1741-7007-8-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McKee AS, Munks MW, Marrack P. How do adjuvants work? Important considerations for new generation adjuvants. Immunity. 2007;27(5):687–690. doi: 10.1016/j.immuni.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 3.Coffman RL, Sher A, Seder RA. Vaccine adjuvants: Putting innate immunity to work. Immunity. 2010;33(4):492–503. doi: 10.1016/j.immuni.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lambrecht BN, Kool M, Willart MA, Hammad H. Mechanism of action of clinically approved adjuvants. Curr Opin Immunol. 2009;21(1):23–29. doi: 10.1016/j.coi.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 5.Tritto E, Mosca F, De Gregorio E. Mechanism of action of licensed vaccine adjuvants. Vaccine. 2009;27(25-26):3331–3334. doi: 10.1016/j.vaccine.2009.01.084. [DOI] [PubMed] [Google Scholar]
- 6.Rappuoli R, Aderem A. A 2020 vision for vaccines against HIV, tuberculosis and malaria. Nature. 2011;473(7348):463–469. doi: 10.1038/nature10124. [DOI] [PubMed] [Google Scholar]
- 7.Cintra-Francischinelli M, et al. Calcium imaging of muscle cells treated with snake myotoxins reveals toxin synergism and presence of acceptors. Cell Mol Life Sci. 2009;66(10):1718–1728. doi: 10.1007/s00018-009-9053-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cintra-Francischinelli M, et al. Bothrops snake myotoxins induce a large efflux of ATP and potassium with spreading of cell damage and pain. Proc Natl Acad Sci USA. 2010;107(32):14140–14145. doi: 10.1073/pnas.1009128107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burnstock GAB. Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev. 2007;87(2):659–797. doi: 10.1152/physrev.00043.2006. [DOI] [PubMed] [Google Scholar]
- 10.Di Virgilio F. Purinergic mechanism in the immune system: A signal of danger for dendritic cells. Purinergic Signal. 2005;1(3):205–209. doi: 10.1007/s11302-005-6312-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Junger WG. Immune cell regulation by autocrine purinergic signalling. Nat Rev Immunol. 2011;11(3):201–212. doi: 10.1038/nri2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pellegatti P, et al. Increased level of extracellular ATP at tumor sites: In vivo imaging with plasma membrane luciferase. PLoS ONE. 2008;3(7):e2599. doi: 10.1371/journal.pone.0002599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kannan S, Elimban V, Fandrich RR, Kardami E, Dhalla NS. Immunolocalization of the sarcolemmal Ca2+/Mg2+ ecto-ATPase (myoglein) in rat myocardium. Mol Cell Biochem. 1999;197(1-2):187–194. doi: 10.1023/a:1006982708128. [DOI] [PubMed] [Google Scholar]
- 14.Komoszyński M, Wojtczak A. Apyrases (ATP diphosphohydrolases, EC 3.6.1.5): Function and relationship to ATPases. Biochim Biophys Acta. 1996;1310(2):233–241. doi: 10.1016/0167-4889(95)00135-2. [DOI] [PubMed] [Google Scholar]
- 15.Pugin J. How tissue injury alarms the immune system and causes a systemic inflammatory response syndrome. Ann Intensive Care. 2012;2(1):27. doi: 10.1186/2110-5820-2-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wack A, et al. Combination adjuvants for the induction of potent, long-lasting antibody and T-cell responses to influenza vaccine in mice. Vaccine. 2008;26(4):552–561. doi: 10.1016/j.vaccine.2007.11.054. [DOI] [PubMed] [Google Scholar]
- 17.Caproni E, et al. MF59 and Pam3CSK4 boost adaptive responses to influenza subunit vaccine through an IFN type I-independent mechanism of action. J Immunol. 2012;188(7):3088–3098. doi: 10.4049/jimmunol.1101764. [DOI] [PubMed] [Google Scholar]
- 18.Seubert A, Monaci E, Pizza M, O’Hagan DT, Wack A. The adjuvants aluminum hydroxide and MF59 induce monocyte and granulocyte chemoattractants and enhance monocyte differentiation toward dendritic cells. J Immunol. 2008;180(8):5402–5412. doi: 10.4049/jimmunol.180.8.5402. [DOI] [PubMed] [Google Scholar]
- 19.Calabro S, et al. Vaccine adjuvants alum and MF59 induce rapid recruitment of neutrophils and monocytes that participate in antigen transport to draining lymph nodes. Vaccine. 2011;29(9):1812–1823. doi: 10.1016/j.vaccine.2010.12.090. [DOI] [PubMed] [Google Scholar]
- 20.Pimorady-Esfahani A, Grounds MD, McMenamin PG. Macrophages and dendritic cells in normal and regenerating murine skeletal muscle. Muscle Nerve. 1997;20(2):158–166. doi: 10.1002/(sici)1097-4598(199702)20:2<158::aid-mus4>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 21.Mosca F, et al. Molecular and cellular signatures of human vaccine adjuvants. Proc Natl Acad Sci USA. 2008;105(30):10501–10506. doi: 10.1073/pnas.0804699105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rayah A, Kanellopoulos JM, Di Virgilio F. P2 receptors and immunity. Microbes Infect. 2012;14(14):1254–1262. doi: 10.1016/j.micinf.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferrari D, et al. Purinergic stimulation of human mesenchymal stem cells potentiates their chemotactic response to CXCL12 and increases the homing capacity and production of proinflammatory cytokines. Exp Hematol. 2011;39(3):360–374, e1–e5. doi: 10.1016/j.exphem.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 24.Ott G, Barchfeld GL, Van Nest G. Enhancement of humoral response against human influenza vaccine with the simple submicron oil/water emulsion adjuvant MF59. Vaccine. 1995;13(16):1557–1562. doi: 10.1016/0264-410x(95)00089-j. [DOI] [PubMed] [Google Scholar]
- 25.Kool M, et al. Alum adjuvant boosts adaptive immunity by inducing uric acid and activating inflammatory dendritic cells. J Exp Med. 2008;205(4):869–882. doi: 10.1084/jem.20071087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marichal T, et al. DNA released from dying host cells mediates aluminum adjuvant activity. Nat Med. 2011;17(8):996–1002. doi: 10.1038/nm.2403. [DOI] [PubMed] [Google Scholar]
- 27.McKee AS, et al. Host DNA released in response to aluminum adjuvant enhances MHC class II-mediated antigen presentation and prolongs CD4 T-cell interactions with dendritic cells. Proc Natl Acad Sci USA. 2013;110(12):E1122–E1131. doi: 10.1073/pnas.1300392110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bours MJ, Dagnelie PC, Giuliani AL, Wesselius A, Di Virgilio F (2011) P2 receptors and extracellular ATP: A novel homeostatic pathway in inflammation. Front Biosci (Schol Ed) 3:1443-1456. [DOI] [PubMed]
- 29.Di Virgilio F. The therapeutic potential of modifying inflammasomes and NOD-like receptors. Pharmacol Rev. 2013;65(3):872–905. doi: 10.1124/pr.112.006171. [DOI] [PubMed] [Google Scholar]
- 30.Eisenbarth SC, Colegio OR, O’Connor W, Sutterwala FS, Flavell RA. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature. 2008;453(7198):1122–1126. doi: 10.1038/nature06939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li H, Willingham SB, Ting JP, Re F. Cutting edge: Inflammasome activation by alum and alum’s adjuvant effect are mediated by NLRP3. J Immunol. 2008;181(1):17–21. doi: 10.4049/jimmunol.181.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seubert A, et al. Adjuvanticity of the oil-in-water emulsion MF59 is independent of Nlrp3 inflammasome but requires the adaptor protein MyD88. Proc Natl Acad Sci USA. 2011;108(27):11169–11174. doi: 10.1073/pnas.1107941108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ellebedy AH, et al. Inflammasome-independent role of the apoptosis-associated speck-like protein containing CARD (ASC) in the adjuvant effect of MF59. Proc Natl Acad Sci USA. 2011;108(7):2927–2932. doi: 10.1073/pnas.1012455108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kouzaki H, Iijima K, Kobayashi T, O’Grady SM, Kita H. The danger signal, extracellular ATP, is a sensor for an airborne allergen and triggers IL-33 release and innate Th2-type responses. J Immunol. 2011;186(7):4375–4387. doi: 10.4049/jimmunol.1003020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.