Abstract
Mitogen-activated protein (MAP) kinase signaling pathways are ubiquitous and evolutionarily conserved in eukaryotic organisms. MAP kinase pathways are composed of a MAP kinase, a MAP kinase kinase, and a MAP kinase kinase kinase; activation is regulated by sequential phosphorylation. Components of three MAP kinase pathways have been identified by genome sequence analysis in the filamentous fungus Neurospora crassa. One of the predicted MAP kinases in N. crassa, MAK-2, shows similarity to Fus3p and Kss1p of Saccharomyces cerevisiae, which are involved in sexual reproduction and filamentation, respectively. In this study, we show that an N. crassa mutant disrupted in mak-2 exhibits a pleiotropic phenotype: derepressed conidiation, shortened aerial hyphae, lack of vegetative hyphal fusion, female sterility, and autonomous ascospore lethality. We assessed the phosphorylation of MAK-2 during conidial germination and early colony development. Peak levels of MAK-2 phosphorylation were most closely associated with germ tube elongation, branching, and hyphal fusion events between conidial germlings. A MAP kinase kinase kinase (NRC-1) is the predicted product of N. crassa nrc-1 locus and is a homologue of STE11 in S. cerevisiae. An nrc-1 mutant shares many of the same phenotypic traits as the mak-2 mutant and, in particular, is a hyphal fusion mutant. We show that MAK-2 phosphorylation during early colony development is dependent upon the presence of NRC-1 and postulate that phosphorylation of MAK-2 is required for hyphal fusion events that occur during conidial germination.
Mitogen-activated protein (MAP) kinases are ubiquitous and evolutionarily conserved enzymes in eukaryotic organisms connecting cell surface receptors to critical regulatory targets within cells that result in various morphogenetic processes (11, 36, 64). MAP kinase activity is regulated through a tiered cascade composed of a MAP kinase, MAP kinase kinase (MEK), and a MEK kinase (MEKK). These enzymes are regulated by a characteristic phosphorelay system in which a series of three protein kinases phosphorylate and activate one another. Intracellular targets are subsequently regulated by phosphorylation and include transcription factors and cytoskeletal proteins (52). In Saccharomyces cerevisiae, 5 MAP kinase pathways have been identified (22, 24). The SMK1 MAP kinase pathway is involved in sporulation. The HOG1 pathway is involved in adaptation to high osmotic conditions, and the SLT2 MAP kinase pathway is involved in cell wall integrity. The remaining two MAP kinase pathways share MEKK and MEK components (Ste11p and Ste7p) but have different MAP kinase proteins. The MAP kinase Kss1p mediates a switch from budding growth to filamentation in response to nitrogen limitation and other environmental signals. The FUS3 MAP kinase pathway is activated in response to the binding of a peptide-mating pheromone to a cell type-specific pheromone receptor. In addition to activating transcription, transduction of the mating response results in reorientation of the cytoskeleton and secretory apparatus to polarize growth towards a mating partner.
In filamentous ascomycete fungi, such as Neurospora crassa, three MAP kinase modules have been identified by genome sequence analysis (17); the various components of MAP kinase signal transduction pathways can be assembled into different modules to perform different biological functions (2). An N. crassa mutant containing lesions in the putative ortholog of HOG1 shows defects in adaptation to conditions of high osmolarity (67). Candida albicans, Aspergillus nidulans, and Magnaporthe grisea mutants with lesions in the cell wall integrity-associated pathway (SLT2 pathway) show pleiotropic effects, including increased sensitivity to cell wall degrading enzymes (9, 39, 66). Mutants constructed in a number of pathogenic filamentous fungi that contain lesions in MAP kinase genes that are orthologous to FUS3/KSS1 are nonpathogenic. In C. albicans, cek1 mutants show defects in switching from unicellular budding growth to invasive hyphal growth under some conditions, and virulence in a mouse model is attenuated (12). Mutations in other predicted components in this MAP kinase pathway (STE7, STE20, and STE12 homologs) in C. albicans result in mutants that show a similar phenotype to cek1 mutants (30, 34). In plant pathogenic fungi, such as M. grisea, Colletotrichum lagenarium, and Cochliobolus heterostrophus, strains that contain disruptions of the FUS3/KSS1 orthologs fail to make infection structures called appressoria, which are required for host penetration (37, 51, 65). These mutants also fail to grow extensively in the host plant, even when inoculated into wound sites, which bypasses the requirement for appressoria. The C. lagenarium and C. heterostrophus MAP kinase mutants are compromised in conidiation, and the C. heterostrophus mutant has female sterility. Mutations in FUS3/KSS1 homologs in fungal plant pathogens that do not form appressoria, such as the necrotrophic pathogen Botrytis cinerea and the vascular wilt pathogen Fusarium oxysporum, also result in mutants that are unable to effectively colonize plants (15, 68).
The aim of this study was to analyze the phenotype of a mutant in a FUS3/KSS1 ortholog, mak-2, in the saprotrophic filamentous fungus N. crassa. We observed that phosphorylation of MAK-2 was associated with morphological events occurring during early colony development. In addition, we examined the phosphorylation of MAK-2 in an N. crassa nrc-1 mutant (31); nrc-1 encodes a STE11 ortholog and is predicted to be in the same MAP kinase pathway as mak-2. We show that mak-2 and nrc-1 are hyphal fusion mutants and that phosphorylation of MAK-2 is correlated with germ tube elongation, branching, and fusion events between conidial germlings.
MATERIALS AND METHODS
Strains and growth conditions.
N. crassa strains used in this study are listed in Table 1. The Δmak-2 strain PB-1 was constructed by gene replacement of the entire mak-2 open reading frame (ORF) with the gene for hygromycin phosphotransferase (P. Bobrowicz and D. J. Ebbole, unpublished data) (see Fig. 2), including 25 bp upstream of the mak-2 ATG and 278 bp downstream of the stop codon for the mak-2 ORF (accession number AF348490). All strains were grown on Vogel's medium (57) with or without supplements depending on the strains used. The nrc-1 mutant (31) was grown on Vogel's dextrose medium (the nrc-1 strain contains an invertase mutation, inv).
TABLE 1.
N. crassa strains
| Strain | Genotype | Reference or source |
|---|---|---|
| Xa-2 | het-cPA arg-5; pan-2 a | 63 |
| 1-1-83 | ad3A his-3 A | A. J. F. Griffiths |
| 9-1-5 | pyr-4 A | 63 |
| FGSC 4347 | fl a | FGSCa |
| FGSC 4317 | fl A | FGSC |
| R1-08 | a | R. L. Metzenberg |
| RLM 40-27 | 74 ORS A | R. L. Metzenberg |
| FGSC 4564 | ad-3B cyh-1 am1 | FGSC |
| FGSC 1814 | al-1 aro-8 A | FGSC |
| DJ912-99 | arg-1; trp-4 A | This study |
| RLM 32-27 | cyh-1 nic-2 lys-4 A | R. L. Metzenberg |
| PB-1 | mak-2 a | P. Bobrowicz and D. Ebbole |
| 1822 | ad3A nic-2 a | A. J. F. Griffiths |
| 12-21-388 | ad3B al-2; cot-1; pan-2 A | A. J. F. Griffiths |
| APJ-2 | al-1; mak-2 a | This study |
| APJ-4 | lys-4 nic-2; mak-2 A | This study |
| APJ-3 | arg-1; trp-4; mak-2 a | This study |
| APJ-5 | lys-4 nic-2; mak-2 A | This study |
| APJ-1 | al-2; mak-2 pan-2 a | This study |
| nrc-1 | al-2; aro-9; nrc-1; inv; qa-2 a (qa-2) | 31 |
FGSC, Fungal Genetic Stock Center.
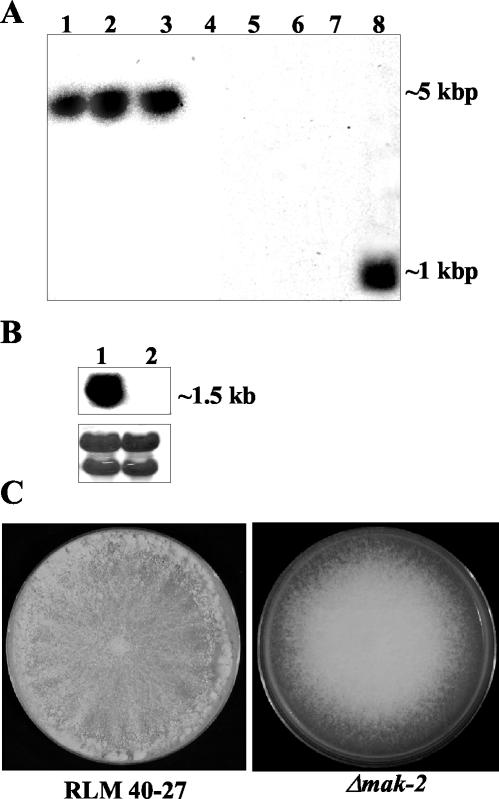
FIG. 2.
Analysis of wild-type and Δmak-2 strains. (A) Southern analysis of genomic DNA from Δmak-2 strains and RLM 40-27 (wild-type strain). Genomic DNA was digested with EcoRV and probed with a hygromycin phosphotransferase gene fragment (lanes 1 to 4) or a mak-2 PCR fragment (lanes 5 to 8). Δmak-2 progeny were APJ-2 (lanes 1 and 5), APJ-1 (lanes 2 and 6), PB-1 (lanes 3 and 7), and RLM 40-27 (lanes 4 and 8). (B) Northern analysis of mak-2 transcription in RLM 40-27 versus Δmak-2 strain PB-1. Two-day-old mycelia were used for RNA extraction. A mak-2 PCR fragment was used as a probe (see Materials and Methods). Lane 1, RLM 40-27; lane 2, PB-1. The same blot was stained with methylene blue as an RNA loading control (lower panel). The rRNA bands are shown. (C) Growth characteristics and morphology of a Δmak-2 mutant compared to wild-type (RLM 40-27). Conidia from RLM 40-27 and PB-1 were inoculated onto plates containing Vogel's (57) minimal medium and assessed for growth and conidiation. Δmak-2 mutants grow slower than the wild type (2.2 versus 7 cm/day) and have derepressed conidiation.
All crosses were performed on Westergaard's synthetic cross medium (60), in some cases with limiting amounts of nutritional supplements. The Δmak-2 strain PB-1 is female sterile but male fertile and was therefore used as a male in crosses. The helper strain, am1 (FGSC 4564) was used in heterokaryons for crosses where complementation of auxotrophic markers was required in the female parent (43). To isolate the APJ-1 Δmak-2 progeny, a heterokaryon between (FGSC 4564 + 12-21-388) (Table 1) was used as a female and fertilized with conidia from PB-1. One-tenth of the normally added supplement of adenine was added to mating medium, since both strains used as a female were auxotrophic for adenine. The Δmak-2 APJ-2 progeny was obtained by crossing (FGSC 4564 + FGSC 1814) with PB-1 conidia. APJ-3 was obtained by crossing (FGSC 4564 + DJ912-99) as a female, which was fertilized with PB-1 conidia. APJ-4 and APJ-5 Δmak-2 progeny were obtained from a (FGSC 4564 + RLM 32-27) heterokaryon fertilized with PB-1 conidia. Ascospores were heat shocked at 60°C for 40 min to induce germination followed by plating on Bdes plates (6) with or without 200 μg of hygromycin/ml plus various supplements, as required.
Escherichia coli strain DH5α (F endAI hsdR17 supE44 lacZM13) (Bethesda Research Laboratory, Gaithersburg, Md.) was used for all DNA manipulations (46).
PCR and Southern blot analysis.
The genotype of the Δmak-2 progeny was confirmed by PCR amplification of genomic DNA from putative Δmak-2 progeny by using mak-2 and hygromycin phosphotransferase gene-specific primers. Genomic DNA was isolated as described previously (35). The primer for the hygromycin phosphotransferase gene was 5′-GTAGAAGTACTCGCCGATAGTGGAA-3′, and the primer for the 5′ flank of mak-2 was 5′-TCCTTTACCTTCACTCTTTCTAC-3′. mak-2-specific primers were 5′-GCCTATGGTGTCTGGTAAGTTG-3′ and 5′-ACTGCTCCTTGCTCAGGTTATCC-3′. Ten micrograms of genomic DNA from RLM 40-27 (wild type), APJ-1, APJ-2, APJ-3, APJ-4, and the original Δmak-2 strain was digested overnight with EcoRV and BglII and subsequently used for Southern blot analysis (46). A 1.4-kbp hygromycin phosphotransferase fragment (isolated from pCB1004 by restriction digestion with HpaII) (10) and a mak-2 PCR fragment (see above for primer sequences) were used as probes for Southern blot analyses. RNA isolation and Northern analyses were done according to the method described in reference 40.
Heterokaryon tests.
Heterokaryons were forced by spotting approximately 106 conidia from two auxotrophic strains onto a petri plate containing Vogel's minimal medium. In hyphal fusion-competent strains, vigorous heterokaryotic growth is observed within 24 h. A modified heterokaryon test was performed for hyphal fusion mutants (63). Briefly, conidia of two strains with different nutritional requirements were spotted 1 to 2 cm apart on plates containing Vogel's minimal medium. One-one hundredth of the normal concentration of the required supplement was cospotted with the conidia. This amount of nutrient was sufficient for conidial germination and sparse growth. Successful heterokaryon formation was observed by the formation of a dense patch of mycelia at the intersection of the two sparse colonies.
Preparation of protein extracts and immunoblot analysis.
Four-day-old conidia (107) were suspended in 300 μl of sterile H2O and were spotted onto sterile cellophane that had been placed onto duplicate plates containing Vogel's medium plus any required supplements. All plates were incubated at 24°C. One set of plates was examined microscopically for development during conidial germination, and the second set of plates was used for protein extraction. Fungal tissue, along with the cellophane membrane, was harvested at various time points, depending on the experimental design. For protein extraction, cellophane containing conidia, germlings, or hyphae (depending upon the stage of growth) was peeled off the plate and plunged into liquid nitrogen. The samples were subsequently ground in liquid nitrogen, and extraction buffer (50 mM HEPES [pH 7.5], 2 mM EGTA, 2 mM EDTA, 1% Triton X-100, 10% glycerol, 100 mM NaCl, 1 mM phenylmethylsulfonyl fluoride), protease inhibitor cocktail (Roche), and phosphatase inhibitor (1 mM sodium orthovanadate and 1 mM sodium fluoride) were added. After centrifugation at 2,390 × g for 30 min, supernatants were transferred to clean tubes. All extraction and centrifugation steps were carried out at 4°C. The concentration of protein extract was determined by using a Bio-Rad protein assay kit with bovine serum albumin (Sigma) as the standard.
For immunoblot analysis, 30 μg of total protein (per lane) was separated on a sodium dodecyl sulfate (SDS)-10% polyacrylamide gel by electrophoresis. Protein was transferred onto nitrocellulose membranes by wet electroblotting (Bio-Rad). Transferred protein was assessed with reversible Ponceau-S dye (Fluka Biochemica). Blocking of membranes was performed for 1 h in Tris-buffered saline (50 mM Tris [pH 7.5], 150 mM NaCl), 0.2% Tween 20, and 5% nonfat dried milk (Nestle Carnation) at room temperature. The membranes were subsequently incubated with either anti-p44/42 MAP kinase (1:3,000 dilution) or anti-phospho p44/42 MAP kinase antibodies (1:3,000 dilution) (PhosphoPlus antibody kit; Cell Signaling Technology) in Tris-buffered saline (50 mM Tris [pH 7.5], 150 mM NaCl), 0.1% Tween 20, and 1% nonfat dried milk overnight at 4°C. The anti-phospho p44/42 antibodies recognize phosphorylated threonine (amino acid 202, Erk1) and tyrosine (amino acid 204, Erk1) residues in Erk1/2. The TEY sites are highly conserved in MAK-2 (Fig. 1). The anti-p44/42 antibodies were produced by immunizing rabbits with a synthetic peptide derived from the carboxy terminus sequence of Erk2; the exact epitope used for production of antibodies is proprietary (Cell Signaling Technology). Following hybridization, blots were washed three times for 5 min each in Tris-buffered saline and 0.2% Tween 20 and subsequently incubated with horseradish peroxidase-conjugated secondary antibody (1:2,000 dilution) (PhosphoPlus antibody kit; Cell Signaling Technology) for 1 h at room temperature. The antibody complex was visualized by using an enhanced chemiluminescence PhosphoPlus antibody kit (Cell Signaling Technology) and an ECL kit (Amersham) according to the manufacturer's instructions. For equal protein loading controls, blots were stripped and probed with monoclonal antibody (clone TU27) against β-tubulin (dilution 1:2,000; BabCo).
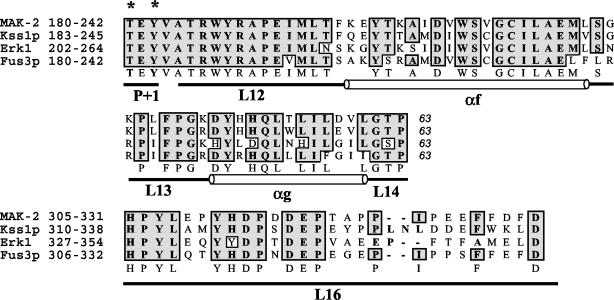
FIG. 1.
Alignment of MAK-2, Kss1p, Erk1, and Fus3p. All four of the MAP kinases are aligned by Clustal W with identity-based alignment. The regions presented are the catalytic core (63 amino acids, P+1 to L14) and the substrate interaction and docking domain (L16). All four of the MAP kinases are activated by phosphorylation at T*EY* in the P+1 loop in the catalytic domain. The phosphorylation lip starts from amino acids Asp-Phe-Gly (DFG) in subdomain VII and ends at TEY in subdomain VIII. Structural domains (L, loop; α, α-helix) are designated according established nomenclature (1). National Center for Biotechnology Information accession numbers are as follows: mak-2, AF348490; FUS3, Z35777; KSS1, Z72825; Erk1, P27361. Asterisks indicate phosphorylated residues.
Transformation assays and protoplast fusion experiments.
Protoplasts were prepared as described previously (47). Strain APJ-1 (Table 1) was used as a recipient for transformation assays with pOKE103 constructs (62). Neurospora strains were transformed as described previously (62). Transformation plates were incubated at 30°C for 2 days. Pantothenate prototrophic transformants were identified and transferred to Vogel's plates (57) and incubated at 24°C, and the phenotypic and growth characteristics of transformants were monitored.
For protoplast fusion experiments, an equal amount of protoplasts from two strains with different auxotrophic markers were incubated with 30% (wt/vol) polyethylene glycol 6000, 50 mM CaCl2, and 10 mM morpholinepropanesulfonic acid (MOPS) at 30°C for 40 min. The protoplasts were then mixed with prewarmed top agar and poured onto plates containing FIGS minimal medium (47). The plates were incubated at 30°C for 2 to 3 days. Heterokaryotic colonies were subsequently transferred to minimal medium and incubated at 24°C. Growth characteristics of the colonies were monitored for a week.
For complementation experiments, a 2,385-bp mak-2 fragment was PCR amplified with mak-2-specific primers (5′-CAATGACAGCCCCTGGACACTGACAT-3′ and 5′-ACGCCTAGCCATAAGTGCCACAGCCAA-3′) from genomic DNA of RLM 40-27 (wild type). The PCR product was than ligated into the pGEM-T vector. An EcoRI mak-2 fragment was ligated into an EcoRI linearized plasmid vector pOKE103 (62) (containing the panthothenate gene, pan-2+, gift from R. L. Metzenberg). This plasmid, pOKEmak-2, was used for complementation experiments of Δmak-2 strains via transformation.
Microscopy.
For light microscopy, conidia, germinating conidia, and hyphae were observed on a Zeiss Axioskop 2 fluorescence microscope with bright field or differential interference contrast (DIC) optics and under identical growth conditions used for protein extraction (see above). Conidial germination, branching, and hyphal fusion events were quantified at hourly intervals over a 12-h period with a Nikon TE2000 microscope with an oil immersion 40× (N.A. 1.0) plan fluor objective. At each time point, >100 conidia or germlings were analyzed on each of 8 replicate slides taken from two petri plates sampled throughout the time course. The percentage of germinated conidia and germlings involved in hyphal fusion events was calculated.
Confocal laser scanning microscopy was performed with a Nikon PCM2000 confocal microscope equipped with an argon ion laser. Cells were stained with FM4-64 as described previously (26) and excited at 514 nm, and fluorescence was detected at >550 nm. Oil immersion 60× (N.A. 1.4) or dry 20× (N.A. 0.75) plan apo objectives were used for imaging. Captured images were finally processed with Photoshop software (version 6.0; Adobe).
RESULTS
Alignment of MAK-2 with Fus3p, Kss1p, and Erk1 shows conservation of catalytic residues.
A putative ortholog of KSS1/FUS3, called mak-2 (Bobrowicz and Ebbole, unpublished) encodes a 352-amino-acid protein in N. crassa. This protein is predicted to encode a MAP kinase on the basis of sequence alignment and the presence of a highly conserved catalytic core, i.e., threonine and tyrosine residues T*EY* (Fig. 1). A MEK is predicted to phosphorylate the T and Y resides in the catalytic core of MAK-2, resulting in activation. The predicted MAK-2 amino acid sequence shows high sequence identity to predicted MAP kinases from other filamentous fungi, such as PMK1 from M. grisea (65), CMK1 from C. lagenarium (51), BMP1 from B. cinerea (68), fmk1 in F. oxysporum (15), and CHK1 in C. heterostrophus (37) (>90% identity). The MAP kinases in these plant pathogens are required for appressorium formation and/or colonization of host tissues. MAK-2 was also highly similar to Erk1 (extracellular signal-regulated kinase 1) (73% identity), Erk2 (55% identity), and S. cerevisiae Fus3p (59% identity) and Kss1p (59% identity). Erk1 and Erk2 are MAP kinases involved in the regulation of meiosis, mitosis, and postmitotic functions in differentiated mammalian cells (11, 42). All known kinases have a regulatory and a catalytic domain; the former is absent in MAP kinases. The catalytic domain is composed of 11 major subdomains, and the catalytic core lies in subdomain VIII. As evident from the alignment data (Fig. 1), all four kinases (i.e., MAK-2, Erk1, Kss1p, and Fus3p) have identical amino acid residues in the catalytic core Thr-Glu-Tyr (TEY).
A mak-2 deletion mutant exhibits a pleiotropic phenotype.
The Δmak-2 mutant strain PB-1 was isolated by the replacement of the predicted mak-2 ORF with the gene for hygromycin phosphotransferase (hph) (Bobrowicz and Ebbole, unpublished). We confirmed the disruption of mak-2 and the linkage of the hph gene to sequences flanking the mak-2 locus in PB-1 by PCR with one primer specific for hph and the other for sequences flanking mak-2; amplification of an ∼500-bp band was apparent in PB-1 but was absent in wild-type strains (data not shown). Southern blot analysis of genomic DNA from the wild type (RLM 40-27) and PB-1 showed the presence of a single copy of the hph gene in PB-1 (Fig. 2A). A band corresponding to mak-2 was detected only in the wild type; PB-1 lacks mak-2 hybridizing sequences (Fig. 2A). By Northern analysis, a transcript from mak-2 was identified in RLM 40-27 but not in the Δmak-2 strain PB-1 (Fig. 2B). We were successful in obtaining a number of hygromycin-resistant Δmak-2 progeny from a variety of crosses (APJ-1, APJ-2, APJ-3, APJ-4, and APJ-5) (see Materials and Methods and below). The molecular analysis of the mak-2 gene disruption in these progeny gave a pattern identical to that of the original Δmak-2 strain PB-1 (Fig. 2A).
The Δmak-2 deletion strain PB-1 and its Δmak-2 progeny displayed a pleiotropic phenotype. A wild-type strain (RLM 40-27) had a growth rate of 7 ± 0.5 cm/day compared to 2.3 ± 0.5 cm/day (24°C) for strains containing the Δmak-2 mutation. N. crassa normally sporulates upon carbon depletion (55) and conidiates at the edges of a petri dish approximately 2 days postinoculation (Fig. 2C). By contrast, the Δmak-2 mutant has stunted aerial hyphae and conidiates profusely as it grows across the petri plate (Fig. 2C). In addition to vegetative growth and conidiation phenotypes, the Δmak-2 mutant and its progeny failed to make female reproductive structures, protoperithecia, and are therefore female sterile.
To assess whether female sterility, growth rate, and conidiation defects of Δmak-2 can be complemented by heterokaryon formation with a mak-2+ strain, we attempted to force heterokaryons between Δmak-2 progeny and mak-2+ strains containing different auxotrophic markers by using mixed conidial suspensions. In all cases, recovery of heterokaryons failed. This observation suggested that the Δmak-2 mutants could be defective in hyphal fusion. We therefore used a modified heterokaryon test (63) to assess the ability of the Δmak-2 strains to form a heterokaryon with a wild-type strain by using complementing auxotrophic markers. Conidial suspensions from auxotrophic strains were inoculated 1 cm apart on a plate with limiting amounts of supplements. Successful heterokaryon formation between Δmak-2 auxotrophic strains (Table 1) and mak-2+ auxotrophic strains was visualized by vigorous growth occurring in the region of contact between Δmak-2 and mak-2+ strains. Such heterokaryons showed a wild-type growth and conidiation phenotype, indicating that the mutation in mak-2 is recessive. By contrast, we were unable to recover heterokaryons between Δmak-2 strains with different nutritional requirements by using either conidial suspensions or modified heterokaryon tests.
Δmak-2 mutants are hyphal fusion defective.
Filamentous fungi normally form an interconnected mycelium network during growth, a process that is mediated by hyphal fusion events (8, 26). Our inability to recover (Δmak-2 and Δmak-2) heterokaryons suggested that mak-2 might be required for hyphal fusion. We therefore assessed the ability of Δmak-2 mutants to undergo self-fusion by bright field, DIC, and confocal microscopy. The hyphae at the periphery of the Δmak-2 colony (PB-1) showed some differences in apical extension and branching frequency compared to the hyphae in the periphery of a wild-type colony (Fig. 3A). The hyphal density in the Δmak-2 mutant was reduced, and individual hyphae were typically thinner and exhibited a more meandering growth pattern. Although hyphae at the periphery of a wild-type colony of N. crassa undergo apical extension and branching, these hyphae are refractory for hyphal fusion (8, 26). The interior hyphae of a wild-type colony also undergo apical extension and branching, but they additionally undergo numerous hyphal fusion events (26).
FIG. 3.
The Δmak-2 mutant fails to undergo hyphal fusion. Hyphae of wild-type RLM 40-27 and Δmak-2 strains were stained with FM4-64 and imaged by confocal microscopy. Bars, 20 μm. (A) Morphologies of wild-type (RLM 40-27) and Δmak-2 PB-1 strains in the colony periphery. Hyphae do not undergo fusion in this region of the colony in either strain. The Δmak-2 mutant PB-1 shows a branching frequency defect and more meandering hyphae. (B) Morphologies of wild-type (RLM 40-27) and Δmak-2 PB-1 strains in the colony interior. Hyphae of the wild type have undergone many fusion events (each indicated by a white star). In the Δmak-2 mutant, hyphal fusions are absent, although hyphae frequently make physical contact with each other (indicated by a dagger). This figure was provided by David Jacobson.
The interior of the Δmak-2 colony was significantly different from that of a wild-type colony (Fig. 3). In the Δmak-2 mutant, hyphal fusion in the colony interior did not take place, although hyphae frequently made contact with each other. These data are consistent with our previous observation that heterokaryons between two Δmak-2 strains with forcing auxotrophic markers were unrecoverable and indicate that the mak-2 MAP kinase pathway is required for hyphal fusion in N. crassa. To confirm that mak-2 is required for hyphal fusion and is not involved in postfusion events, such as maintenance of a heterokaryon, we performed protoplast fusion experiments with wild-type and Δmak-2 strains carrying different auxotrophic markers. Protoplast fusion between two wild-type strains 1-1-83 (ad3A his-3 A) and 9-1-5 (pyr-4 A) resulted in the formation of vigorous heterokaryotic colonies on minimal medium plates. Protoplast fusion between APJ-1 (al-2 mak-2 pan-2 a) and APJ-3 (arg-1 trp-4 mak-2 a) protoplasts resulted in heterokaryotic colonies on minimal medium that grew at a rate similar to that of the Δmak-2 mutant itself. These data indicate that mutations in mak-2 disrupt the ability to form a heterokaryon, not heterokaryon maintenance. These data are consistent with the hypothesis that the mak-2 MAP kinase pathway is required for hyphal fusion in N. crassa.
Δmak-2 mutants show an ascospore autonomous lethal phenotype.
We assessed sexual phenotypes of Δmak-2 strains as a female by forcing a heterokaryon between a Δmak-2 strain containing auxotrophic markers and a strain containing a mutation at the mating type locus am1 (21, 43) by using a modified heterokaryon procedure (see Materials and Methods). The (am1 + Δmak-2) heterokaryon formed female reproductive structures and protoperithecia and was fertilized with conidia from the opposite mating type. Normal numbers of perithecia and ascospores were produced. However, very few of the heat-shocked ascospores germinated on plates containing 200 μg of hygromycin/ml. Similarly, normal numbers of perithecia and ascospores were produced when conidia Δmak-2 mutants were used as the fertilizing parent; very few hygromycin-resistant progeny were recovered. In all, we performed 5 replicate crosses and screened many thousands of ascospore progeny, but we successfully isolated only 5 mak-2 progeny containing auxotrophic markers (Table 1). When these five Δmak-2 hygromycin-resistant progeny (APJ-1 to -5) were crossed as either males or females to a wild-type strain, they all displayed the ascospore autonomous lethal phenotype of the original Δmak-2 mutant. These data suggest that a functional mak-2 MAP kinase pathway is essential for ascospore germination and/or maturation.
The Δmak-2 vegetative phenotypes were complemented by the introduction of a wild-type copy of mak-2 into APJ-1 by selection for Pan+ transformants (pOKEmak-2) (see Material and Methods and reference 62). The growth rate of the mak-2-complemented transformants was almost equal to that of RLM 40-27 (wild-type strain, approximately 7 cm/day). Aerial hyphae in the mak-2-complemented transformants were still short but not as short as Δmak-2 mutants, and the conidiation pattern was mostly like that of the wild type, although some sporulation still occurred at the plate center. Hyphal fusion was restored in all analyzed transformants (Fig. 4D). Complemented transformants also formed abundant protoperithecia when grown on synthetic crossing medium. Complementation for ascospore maturation and germination was not performed due to the occurrence of genome surveillance mechanisms in N. crassa that effectively silence or mutate ectopic copies of genes (48, 49).
FIG. 4.
The Δmak-2 mutant lacks a protein that is recognized by anti-p44/42 and anti-phospho p44/42 antibodies. (A) Total protein (30 μg) was extracted from wild-type strains (RLM 40-27 and Xa-2), Δmak-2 (PB-1), Δmak-2 progeny (APJ-1, APJ-3, and APJ-4), and nrc-1 and separated on an SDS-10% polyacrylamide gel electrophoresis gel. The blot was probed with anti-p44/42 (α-p44/42) antibodies (PhosphoPlus antibody kit; Cell Signaling Technology). Lane 1, nrc-1; lane 2, APJ-4; lane 3, APJ-3; lane 4, APJ-1; lane 5, RLM 40-27; lane 6, Xa-2; lane 7, PB-1. The lower panel shows blots probed with anti-β-tubulin antibodies for protein loading controls. (B) The introduction of mak-2 into Δmak-2 progeny APJ-1 restores the production of a protein that is recognized by anti-p44/42 antibodies. Total protein (30 μg) was extracted from APJ-1 transformants containing pOKEmak-2 (4C, 6C, and 7C), the wild type (RLM 47-20), and PB-1 Δmak-2 mycelia and separated by SDS-10% polyacrylamide gel electrophoresis followed by transfer onto a nitrocellulose membrane. Anti-p44/42 antibody (Cell Signaling Technology) was used for probing the blot. Lane 1, RLM 40-27; lane 2, Δmak-2 (PB-1); lane 3, APJ-1 transformant (4C) containing pOKEmak-2; lane 4, APJ-1 transformant (6C) containing pOKEmak-2; lane 5, APJ-1 transformant (7C) containing pOKEmak-2. (C) The Δmak-2 mutants lack a protein that is recognized by anti-phospho p44/42 antibodies. Total protein (30 μg) was extracted from the wild-type strain (RLM 40-27), Δmak-2 progeny (APJ-1, APJ-2, and APJ-4) and nrc-1 grown for 20 h as described in Materials and Methods and separated on an SDS-10% polyacrylamide gel electrophoresis gel. The blot was probed with anti-phospho p44/42 antibodies (PhosphoPlus antibody kit; Cell Signaling Technology). Lane 1, nrc-1; lane 2, APJ-1; lane 3, APJ-4; lane 4, APJ-2; lane 5, RLM 40-27. (D) The APJ-1 transformant (6C) containing mak-2 shows restoration of hyphal fusion. Conidia from the APJ-1 transformant containing pOKEmak-2 (6C) were inoculated onto cellophane layered onto plates containing Vogel's minimal medium. Colonies were assessed for hyphal fusion events after 24 h of growth. Arrows indicate hyphal fusion events. Bar, 10 μm.
Δmak-2 mutants lack a protein that cross-reacts with anti-p44/42 (Erk1/Erk2) antibodies.
Because of the similarity of MAK-2 to mammalian Erk1 and Erk2, we used commercially available antibodies against mammalian Erk1/Erk2 (anti-p44/42 antibodies, PhosphoPlus antibody kit; Cell Signaling Technology) to assess cross-reactivity with MAK-2. When anti-p44/42 antibodies were used as a probe in Western blots of protein extracts from a wild-type N. crassa strains, the anti-p44/42 antibodies hybridized to a protein of approximately 43 kDa (Fig. 4A, lanes 5 and 6). The predicted molecular mass of MAK-2 is approximately 41 kDa. In the Δmak-2 mutant PB-1 and in Δmak-2 progeny (APJ-1 to -4), this ∼43 kDa band was not detectable (Fig. 4A, lanes 2 to 4 and 7). To determine whether the introduction of mak-2 into PB-1 restored the presence of a cross-reacting ∼43-kDa protein, we subjected protein extracts from Δmak-2-complemented transformants carrying pOKEmak-2 to Western analysis with anti-p44/42 antibodies (Fig. 4B, lanes 3 to 5). Varying levels of an ∼43-kDa protein was observed in the Δmak-2-complemented transformants.
Activation of MAK-2 is correlated with germ tube elongation, branching, and fusion between conidial germlings.
The phenotype of the Δmak-2 mutant suggested that activation of MAK-2 via phosphorylation could be both temporally and spatially regulated in a growing colony. Phospho-specific anti-p44/42 (phospho-p44/42) antibody is available that recognizes phosphorylated threonine (T) and tyrosine (Y) residues in Erk1/Erk2. In MAK-2, these residues are highly conserved; the predicted phosphorylation sites occur at amino acid positions 180 (T) and 182 (Y) (Fig. 1). As with anti-p44/42 antibody, the Δmak-2 mutants lack a protein that is recognized by the phospho-p44/42 antibody (Fig. 4C, lanes 2 to 4), although a protein was identified in extracts from RLM 40-27 (Fig. 4C, lane 5). We therefore assessed whether variations in phosphorylation of MAK-2 in a wild-type strain (RLM 40-27) occurred during conidial germination and early colony development. As shown in Fig. 5A, MAK-2 phosphorylation was evident at 4 h postinoculation. Phosphorylation of MAK-2 increased until 8 h, followed by a decrease in phosphorylation at 12, 16, 20, and 24 h (Fig. 5A, I). The blot was stripped and reprobed with anti-tubulin and anti-p44/42 antibodies (Fig. 5A, II and III); an approximately equal level of protein is present at each time point. A similar pattern of MAK-2 phosphorylation during germination was observed in replicate trials with conidia from RLM 40-27 and also when equal amounts of conidia from 1-1-83 (ad3A his-3 A) and 9-1-5 (pyr-4 A) (Table 1) were coinoculated onto minimal medium to force heterokaryon formation (8 separate trials) (data not shown). These observations suggest a role for the mak-2 MAP kinase pathway in events associated with conidial germination and/or early colony development in N. crassa.
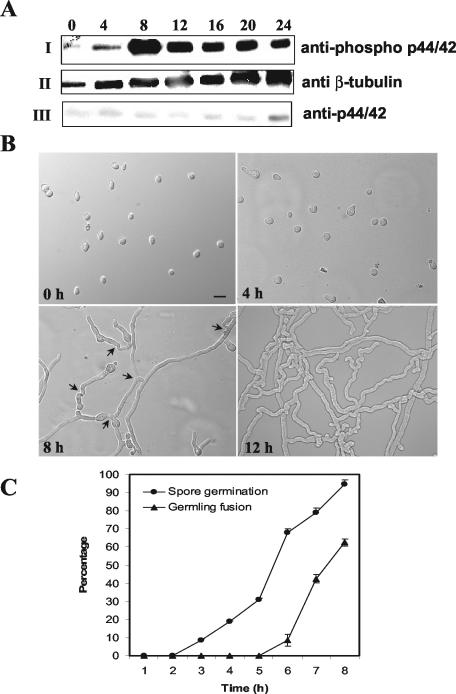
FIG. 5.
MAK-2 phosphorylation is associated with germ tube elongation and hyphal fusion between germlings. (A) Conidia from a wild-type strain (RLM 40-27) were inoculated onto a cellophane membrane layered on Vogel's minimal medium plates and incubated at 24°C. Total protein (30 μg) isolated at 0, 4, 8, 12, 16, 20, and 24 h postinoculation of conidia was used for Western blot analysis. (I) Protein extracts from the different time points probed with anti-phospho p44/42 antibodies (PhosphoPlus antibody kit; Cell Signaling Technology). (II) The blot in panel I was stripped and reprobed with anti-β-tubulin monoclonal antibodies (clone TU27; BabCo). (III) The blot in panel II was restripped and probed with anti-p44/42 antibodies. The anti-phospho p44/p42 antibodies (Cell Signaling Technology) recognize highly conserved phosphorylated T and Y residues in MAP kinases (residues 202 to 204 in Erk1 and residues 180 to 182 in MAK-2) (Fig. 1). The anti-p44/42 antibodies were raised to a peptide synthesized based on the C-terminal amino acid sequence of Erk1. The phospho-p44/42 antibody gave a stronger signal on Western blots than the anti-p44/42 antibody, presumably because of the highly conserved TEY site in MAK-2. The same experiment was repeated with unstripped blots for anti-p44/42 antibodies, and identical results were obtained. (B) DIC images of conidia and conidial germlings from the wild type (RLM 40-27) at 0, 4, 8, and 12 h postinoculation. Bar, 30 μm. (C) Quantitation of germination and hyphal fusion in conidial germlings in the wild type (RLM 40-27) over the first 8 h postinoculation. Error bars represent standard errors.
We microscopically examined wild-type conidia during germination and early colony development, focusing on events that correlated with the increase in phosphorylation of MAK-2, approximately 4 to 12 h postinoculation. Conidia began to form germ tubes between 2 and 3 h postinoculation; the majority of conidia had germinated by the 8-h time point (Fig. 5B and C). From 2 to 8 h postinoculation, germ tubes elongated and branched. After 5 h postinoculation, many of the germ tubes and germling branches had fused with other branches or germ tubes. After 8 h, 62% ± 1.9% of the conidial germlings were involved in hyphal fusion events with other germlings (Fig. 5B and C). At the 12-h time point, the interconnected hyphal network was too dense to accurately measure the percentage of germlings involved in fusion. The increase in MAK-2 phosphorylation observed between the 4- and 8-h time points was associated with a period in which germ tubes were elongating and branching. During this same time period, the number of hyphal fusion events rapidly increased.
We evaluated the germination of Δmak-2 conidia compared with wild-type at time points similar to those for RLM 40-27. Germination of Δmak-2 conidia was initiated at a similar time point (between 2 and 3 h) to that of wild-type conidia (but occurred over a longer period [2 to 12 h] than the 2 to 8 h in the case of the wild type) (Fig. 5C). Following germination, Δmak-2 germ tubes grew at a much slower rate than the wild type and branching of the germ tube was delayed (Fig. 6A). At the 4-h time point, the average germ tube length of Δmak-2 conidia was only 3.5 ± 0.1 μm; for the wild-type, the average germ tube length at this time point was 6.45 ± 0.5 μm. Most significantly, hyphal fusion events between conidial germlings were completely absent in the Δmak mutant.
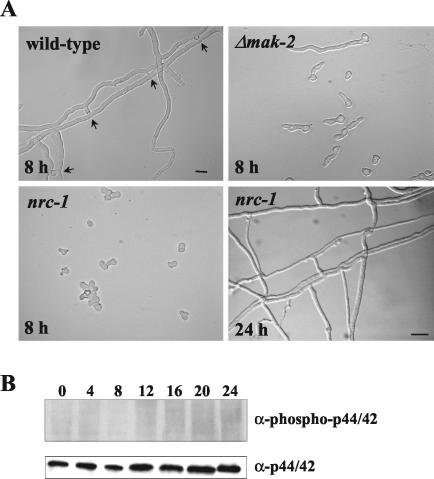
FIG. 6.
nrc-1 is required for phosphorylation of MAK-2 during conidial germination. (A) DIC image of germinating conidia from wild-type (RLM 40-27), Δmak-2, and nrc-1 strains 8 h postinoculation and from the nrc-1 strain 24 h postinoculation. Arrows indicate fusion events observed in RLM 40-27. Hyphal fusion was not evident in nrc-1, although hyphae frequently made contact or grew over each other (24 h). Bar, 30 μm. (B) Conidia from nrc-1 were inoculated on a cellophane membrane layered onto plates containing Vogel's minimal medium with dextrose at 24°C (nrc-1 is an invertase mutant). Total protein (30 μg) from nrc-1 mycelia was harvested at the 0-, 4-, 8-, 12-, 16-, 20-, and 24-h time points. Protein extracts were separated by SDS-10% polyacrylamide gel electrophoresis and blotted onto nitrocellulose membranes. (Top panel) Blot probed with anti-phospho p44/42 antibodies (PhosphoPlus antibody kit; Cell Signaling Technology). (Bottom panel) Blot from the panel above stripped and probed with anti-p44/42 antibodies. The experiment was repeated three times with identical results.
nrc-1 is an upstream component of the mak-2 pathway.
N. crassa strains that have mutations in a MEKK gene, nrc-1, display a phenotype similar to that of Δmak-2 mutants, i.e., slow growth, nonrepressed conidiation, lack of aerial hyphae and protoperithecia, and an autonomous ascospore lethal phenotype (31). nrc-1 encodes a protein that is an ortholog of STE11 of S. cerevisiae; Ste11p phosphorylates the MEK Ste7p, which subsequently phosphorylates Fus3p or Kss1p (2, 25). Therefore, NRC-1 is predicted to be in the same phosphorylation cascade as MAK-2. We assessed hyphal fusion capability in the nrc-1 mutant and observed that it failed to form heterokaryons with a wild-type strain under all conditions tested (data not shown). Conidial germination in nrc-1 was delayed (cf. nrc-1 in Fig. 6A, which was imaged 8 h after hydration, to a wild-type strain), as was germ tube elongation and branching. As with the Δmak-2 mutant, hyphal fusion during conidial germination and colony establishment was absent in the nrc-1 mutant (Fig. 6A).
MAK-2 was detected in protein extracts from a 20-h culture of nrc-1 with anti-p44/42 antibody (Fig. 4A, lane 1); phosphorylated MAK-2 was not detectable in protein extracts from the nrc-1 mutant at the same time point (Fig. 4C, lane 1). These data also supported the hypothesis that NRC-1 is in the same signaling pathway and upstream of MAK-2. We therefore assessed phosphorylation of MAK-2 during conidial germination to colony establishment in an nrc-1 mutant. As shown in Fig. 6B, MAK-2 was not phosphorylated at any time point over a 24-h time period in the nrc-1 mutant. However, when the same blot was probed with anti-p44/42 antibodies, MAK-2 protein was detected at all time points. Thus, mutations in nrc-1 affect phosphorylation levels of MAK-2 during conidial germination and colony establishment. These results suggest that mak-2 and nrc-1 belong to a common signal transduction pathway in N. crassa which is involved in early germination events, including germ tube elongation, branching, and hyphal fusion.
DISCUSSION
In Neurospora, hyphal fusion occurs at two stages during colony development: (i) between germlings during the establishment of fungal colonies (29, 33) and (ii) between hyphae in subapical parts of mature colonies (8, 26). In this paper, we provide evidence that a MAP kinase pathway in N. crassa, involving MAK-2 and NRC-1, regulates hyphal fusion between germlings and hyphae in mature fungal colonies. These results are based on an analysis of mak-2 and nrc-1 mutants, time course studies of phosphorylation of MAK-2, and quantitation of the cytological events occurring during early colony development. Fusions between conidia and germlings is not unique to Neurospora but has also been described for other fungi (23, 29, 33, 45). We observed numerous hyphal fusion events between germlings during early stages of colony development, which correlated with a peak of MAK-2 phosphorylation. Our hypothesis is that, during conidial germination, the MAK-2 MAP kinase pathway is induced by a diffusible substance elaborated by germinating conidia, which triggers hyphal fusion between conidial germlings. We suggest that the decreased, yet significant, level of MAK-2 phosphorylation after 12+ h postinoculation may reflect the nonsynchronous and lower rate of hyphal fusion events in the colony as it grows older. In this study, it was not possible to quantify the rate of hyphal fusion in a mature colony because the hyphal density became too great to obtain reliable measurements.
Live cell imaging of hyphal fusion events in N. crassa (26) and microscopic analyses in a number of filamentous fungi (8, 18, 29, 33) show that attraction between hyphae involved in fusion events is mediated by diffusible substances. At present, there are no clues as to what controls the frequency or the spatial and temporal distribution of hyphal fusion within a fungal colony. It is generally assumed that hyphal fusion, by networking hyphae, is important for intrahyphal communication, translocation of water and nutrients, and general homeostasis (20, 44). It is also possible that hyphal fusion is required to appropriately regulate developmental processes, such as conidiation and protoperithecial development. Hyphal fusion events between germlings could be a way to optimize resource exploitation by increasing the biomass of genetically identical individuals.
Because conidial germlings and hyphae within a single colony are genetically identical, hyphal fusion is a self-signaling phenomenon. The nature of diffusible substances in filamentous fungi that mediate self-signaling during either hyphal attraction or hyphal avoidance (53) is unknown. During sexual reproduction, pheromones are involved in the attraction of hyphae to each other or between specialized structures of different genotypes and are well characterized for many fungi (3, 28, 32, 50). The mating type locus of N. crassa, which regulates the expression of mating type-specific pheromone genes (4), apparently does not play a role in hyphal fusion; mat mutants are hyphal fusion competent (13, 43). However, many of the processes required for hyphal fusion in filamentous fungi are also required by S. cerevisiae cells during mating, a process that has been well characterized, i.e., signaling by diffusible substances, redirected growth of fusing cells, attachment, production and targeting of wall-degrading enzymes to the attachment site, fusion of the plasma membrane, and cytoplasmic mixing (2, 19, 32). Significantly, these components include MAP kinase pathways.
In this paper, we show that during conidial germination, NRC-1 is required for phosphorylation of MAK-2. The nrc-1 mutant has many of the same phenotypic traits as the mak-2 mutant (31), including an inability to undergo hyphal fusion. Our data support the hypothesis that NRC-1 and MAK-2 are components of the same signal transduction pathway. Mutations in an A. nidulans nrc-1 homolog, steC, resulted in the isolation of a pleiotropic mutant that also failed to form heterokaryons (58). By using phosphospecific antibodies, the authors showed that phosphorylation of two MAP kinases were associated with conidiation in A. nidulans and require functional SteCp. However, neither of these two kinases were in the 40-kDa range, the size range predicted for the KSS1/FUS3 ortholog in A. nidulans. A downstream component of the pheromone response pathway in S. cerevisiae is STE12, which encodes a transcription factor (16). Mutations in STE12 homologs in filamentous fungi results in mutants that are affected in sexual or pathogenic development on host plants (5, 41, 56). An N. crassa strain containing a mutation in the STE12 ortholog has recently been identified (Bobrowicz and Ebbole, unpublished). The N. crassa ste12 mutant is similar in phenotype to nrc-1 and mak-2 mutants and also fails to undergo hyphal fusion (D. J. Jacobson and N. L. Glass, unpublished data). These data suggest that other components of the pheromone response pathway that are present in the genomes of filamentous fungi may also be involved in hyphal fusion and colony establishment.
It has recently been shown that the SLT2 cell wall integrity MAP kinase pathway may be important for heterokaryon formation in filamentous fungi. In Fusarium graminearum, disruption of the SLT2 (MAP kinase) ortholog resulted in mutants affected in cell wall integrity. These mutants also failed to form heterokaryons (27). During mating in S. cerevisiae, the SLT2 MAP kinase pathway is downstream of the FUS3 MAP kinase pathway and is required for remodeling of the cell wall during shmoo formation (7). These observations suggest that a similar relationship between the orthologous FUS3 and SLT2 MAP kinase pathways may also exist in filamentous fungi and may be important for hyphal fusion.
Conidial germination in filamentous fungi depends on various factors such as nutrient deprivation, hydration, physical stimuli, contact stimulation, and proximity to other conidia (14). Mutational analysis of genes in filamentous fungi that are orthologous to those required in S. cerevisiae for budding indicate that many of the functions involved in polarization and bud site selection affect germ tube emergence in filamentous fungi (for reviews, see references 38 and 59). Mutations in mak-2 did not significantly affect the timing of the initiation of germination or germ tube formation, and thus, MAK-2 does not play an essential role in breaking conidial dormancy or formation of the germ tube. However, mak-2 mutants showed a much slower rate of germ tube elongation, indicating that a functional MAK-2 MAP kinase pathway is required for optimal apical extension of a hypha, a process apparently unrelated to hyphal fusion. In addition, the nrc-1 and mak-2 mutants are female sterile and show nonrepressible conidiation. It is unclear whether these phenotypes are due to an inability to form an interconnected fungal colony or if the MAK-2 MAP kinase pathway has a role in these developmental processes that is separable from its role in hyphal fusion. All hyphal fusion mutants so far described for N. crassa have pleiotropic phenotypes that share some, but not all, of the developmental abnormalities of mak-2 and nrc-1 mutants (31, 61, 63).
Plant pathogens containing mutations in mak-2 homologs, such as M. grisea pmk1 (65), C. lagenarium cmk1 (51), and C. heterostrophus chk1 (37) affect appressorium formation and host colonization. We predict that all of these MAP kinase mutants are also hyphal fusion mutants, which may affect the ability of these organisms to colonize host tissue. Hyphal fusion and appressorium formation typically involve cell adhesion, swelling, and cell wall digestion (26, 54). It may be that these two processes between pathogenic and saprotrophic fungi use common signal transduction mechanisms and may be evolutionarily related. Further comparisons between pathogenic and saprotrophic fungi with regard to requirements for signal transduction, hyphal fusion, and pathogenicity may reveal the answer.
Acknowledgments
We gratefully acknowledge Piotr Bobrowicz and Dan Ebbole for providing the mak-2 mutant. We thank the members of the Glass laboratory for critical reading of the manuscript, especially Natalie Catlett and David Jacobson. We thank Girdhar Pandey for help with RNA isolation.
Funding for this project was provided by a National Science Foundation Grant to N.L.G. (MCB-01311355). Thanks are also due to the Biotechnology and Biological Sciences Research Council for funding (grant number 15/P18594) to N.D.R.
Part of the research involved the use microscopy equipment in the Collaborative, Optical, Spectroscopy, Micromanipulation and Imaging Centre (COSMIC) facility, which is a Nikon-Partners-in-Research laboratory at the University of Edinburgh.
REFERENCES
- 1.Atienza, J. M., M. Suh, I. Xenarios, R. Landgraf, and J. Colicelli. 2000. Human ERK1 induces filamentous growth and cell wall remodeling pathways in Saccharomyces cerevisiae. J. Biol. Chem. 275:20638-20646. [DOI] [PubMed] [Google Scholar]
- 2.Banuett, F. 1998. Signalling in the yeasts: an informational cascade with links to the filamentous fungi. Microbiol. Mol. Biol. Rev. 62:249-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bistis, G. N. 1981. Chemotropic interactions between trichogynes and conidia of opposite mating-type in Neurospora crassa. Mycologia 73:959-975. [Google Scholar]
- 4.Bobrowicz, P., R. Pawlak, A. Correa, D. Bell-Pedersen, and D. J. Ebbole. 2002. The Neurospora crassa pheromone precursor genes are regulated by the mating type locus and the circadian clock. Mol. Microbiol. 45:795-804. [DOI] [PubMed] [Google Scholar]
- 5.Borneman, A. R., M. J. Hynes, and A. Andrianopoulos. 2001. An STE12 homolog from the asexual, dimorphic fungus Penicillium marneffei complements the defect in sexual development of an Aspergillus nidulans steA mutant. Genetics 157:1003-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brockman, H. E., F. J. DeSerres, T. M. Ong, and C. Y. Hung. 1987. Two N-hydroxylaminopurines are highly mutagenic in the ad-3 forward-mutation test in growing cultures of heterokaryon 12 of Neurospora crassa. Mutat. Res. 177:61-75. [DOI] [PubMed] [Google Scholar]
- 7.Buehrer, B. M., and B. Errede. 1997. Coordination of the mating and cell integrity mitogen-activated protein kinase pathways in Saccharomyces cerevisiae. Mol. Cell. Biol. 17:6517-6525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buller, A. H. R. 1933. Researches on fungi, vol. 5. Longman, London, England.
- 9.Bussink, H., and S. A. Osmani. 1999. A mitogen-activated protein kinase (MPKA) is involved in polarized growth in the filamentous fungus, Aspergillus nidulans. FEMS Microbiol. Lett. 173:117-125. [DOI] [PubMed] [Google Scholar]
- 10.Carroll, A. M., J. A. Sweigard, and B. Valent. 1994. Improved vectors for selecting resistance to hygromycin. Fungal Genet. Newsl. 41:22. [Google Scholar]
- 11.Chang, L., and M. Karin. 2001. Mammalian MAP kinase signalling cascades. Nature 410:37-40. [DOI] [PubMed] [Google Scholar]
- 12.Csank, C., K. Schroppel, E. Leberer, D. Harcus, O. Mohamed, S. Meloche, D. Y. Thomas, and M. Whiteway. 1998. Roles of the Candida albicans mitogen-activated protein kinase homologue, Cek1p, in hyphal development and systemic candidiasis. Infect. Immun. 66:2713-2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DeLange, A. M., and A. J. Griffiths. 1975. Escape from mating-type incompatibility in bisexual (A + a) Neurospora heterokaryons. Can. J. Genet. Cytol. 17:441-449. [DOI] [PubMed] [Google Scholar]
- 14.Denfert, C. 1997. Fungal spore germination-insights from the molecular genetics of Aspergillus nidulans and Neurospora crassa. Fungal Genet. Biol. 21:163-172. [Google Scholar]
- 15.Di Pietro, A., F. I. Garcia-MacEira, E. Meglecz, and M. I. G. Roncero. 2001. A MAP kinase of the vascular wilt fungus Fusarium oxysporum is essential for root penetration and pathogenesis. Mol. Microbiol. 39:1140-1152. [PubMed] [Google Scholar]
- 16.Errede, B., and G. Ammerer. 1989. STE12, a protein involved in cell-type-specific transcription and signal transduction in yeast, is part of protein-DNA complexes. Genes Dev. 3:1349-1361. [DOI] [PubMed] [Google Scholar]
- 17.Galagan, J. E., S. E. Calvo, K. A. Borkovich, E. U. Selker, N. D. Read, D. Jaffe, W. FitzHugh, L. J. Ma, S. Smirnov, S. Purcell, B. Rehman, T. Elkins, R. Engels, S. G. Wang, C. B. Nielsen, J. Butler, M. Endrizzi, D. Y. Qui, P. Ianakiev, D. B. Pedersen, M. A. Nelson, M. Werner-Washburne, C. P. Selitrennikoff, J. A. Kinsey, E. L. Braun, et al. 2003. The genome sequence of the filamentous fungus Neurospora crassa. Nature 422:859-868. [DOI] [PubMed] [Google Scholar]
- 18.Giovannetti, M., D. Azzolini, and A. S. Citernesi. 1999. Anastomosis formation and nuclear and protoplasmic exchange in arbuscular mycorrhizal fungi. Appl. Environ. Microbiol. 65:5571-5575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glass, N. L., D. J. Jacobson, and K. T. Shiu. 2000. The genetics of hyphal fusion and vegetative incompatibility in filamentous ascomycetes. Annu. Rev. Genet. 34:165-186. [DOI] [PubMed] [Google Scholar]
- 20.Gregory, P. H. 1984. The fungal mycelium: a historical perspective. Trans. Br. Mycol. Soc. 82:1-11. [Google Scholar]
- 21.Griffiths, A. J. F., and A. M. Delange. 1978. Mutations of the a mating-type gene in Neurospora crassa. Genetics 88:239-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gustin, M. C., J. Albertyn, M. Alexander, and K. Davenport. 1998. MAP kinase pathways in the yeast Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 62:1264-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hay, T. S. 1995. Unusual germination of spores of Arthrobotrys conoides and A. cladodes. Mycol. Res. 99:981-982. [Google Scholar]
- 24.Herskowitz, I. 1995. MAP kinase pathways in yeast: for mating and more. Cell 80:187-197. [DOI] [PubMed] [Google Scholar]
- 25.Herskowitz, I. 1989. A regulatory hierarchy for cell specialization in yeast. Nature 342:749-757. [DOI] [PubMed] [Google Scholar]
- 26.Hickey, P. C., D. J. Jacobson, N. D. Read, and N. L. Glass. 2002. Live-cell imaging of vegetative hyphal fusion in Neurospora crassa. Fungal Genet. Biol. 37:109-119. [DOI] [PubMed] [Google Scholar]
- 27.Hou, Z. M., C. Y. Xue, Y. L. Peng, T. Katan, H. C. Kistler, and J. R. Xu. 2002. A mitogen-activated protein kinase gene (MGV1) in Fusarium graminearum is required for female fertility, heterokaryon formation, and plant infection. Mol. Plant-Microbe Interact. 15:1119-1127. [DOI] [PubMed] [Google Scholar]
- 28.Kim, H., R. L. Metzenberg, and M. A. Nelson. 2002. Multiple functions of mfa-1, a putative pheromone precursor gene of Neurospora crassa. Eukaryot. Cell 1:987-999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Köhler, E. 1930. Zur kenntnis der vegetativen anastomosen der pilze, II. Planta 10:495-522. [Google Scholar]
- 30.Kohler, J. R., and J. R. Fink. 1996. Candida albicans strains heterozygous and homozygous for mutations in mitogen-activated protein kinase signaling components have defects in hyphal development. Proc. Nat. Acad. Sci. USA 93:13223-13228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kothe, G. O., and S. J. Free. 1998. The isolation and characterization of nrc-1 and nrc-2, two genes encoding protein kinases that control growth and development in Neurospora crassa. Genetics 149:117-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kurjan, J. 1993. The pheromone response pathway in Saccharomyces cerevisiae. Annu. Rev. Genet. 27:147-179. [DOI] [PubMed] [Google Scholar]
- 33.Laibach, F. 1928. Ueber Zellfusionen bei Pilzen. Planta 5:340-359. [Google Scholar]
- 34.Leberer, E., D. Harcus, I. D. Broadbent, K. L. Clark, D. Dignard, K. Ziegelbauer, A. Schmidt, and N. A. R. Gow. 1997. Signal transduction through homologues of the Ste20p and Ste7p protein kinases can trigger hyphal formation in the pathogenic fungus Candida albicans. Proc. Nat. Acad. Sci. USA 93:13217-13222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee, S. B., and J. T. Taylor. 1990. Isolation of DNA from fungal mycelia and single spores, p. 282-287. In M. A. Innis, D. H. Gelfand, J. J. Sninsky, and T. J. White (ed.), PCR protocols a guide to methods and applications. Academic Press, New York, N.Y.
- 36.Lengeler, K. B., R. C. Davidson, C. D'Souza, T. Harashima, W. C. Shen, P. Wang, X. Pan, M. Waugh, and J. Heitman. 2000. Signal transduction cascades regulating fungal development and virulence. Microbiol. Mol. Biol. Rev. 64:746-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lev, S., A. Sharon, R. Hadar, H. Ma, and B. A. Horwitz. 1999. A mitogen-activated protein kinase of the corn leaf pathogen Cochliobolus heterostrophus is involved in conidiation, appressorium formation, and pathogenicity: diverse roles for mitogen-activated protein kinase homologs in foliar pathogens. Proc. Natl. Acad. Sci. USA 96:13542-13547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Momany, M. 2002. Polarity in filamentous fungi: establishment, maintenance and new axes. Curr. Opin. Microbiol. 5:580-585. [DOI] [PubMed] [Google Scholar]
- 39.Navarro-Garcia, F., M. Sanches, J. Pla, and C. Nombela. 1995. Functional characterization of the MKC1 gene of Candida albicans, which encodes a mitogen-activated protein kinase homolog related to cell integrity. Mol. Cell. Biol. 15:2197-2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pandey, G. K., V. S. Reddy, M. K. Reddy, R. Deswal, A. Bhattacharya, and S. K. Sopory. 2002. Transgenic tobacco expressing Entamoeba histolytica calcium binding protein exhibits enhanced growth and tolerance to salt stress. Plant Sci. 162:41-47. [Google Scholar]
- 41.Park, G., C. Xue, L. Zheng, S. Lam, and J.-R. Xu. 2002. MST12 regulates infectious growth but not appressorium formation in the rice blast fungus Magnaporthe grisea. Mol. Plant-Microbe Interact. 15:183-192. [DOI] [PubMed] [Google Scholar]
- 42.Pearson, G., F. Robinson, T. B. Gibson, B. E. Xu, M. Karandikar, K. Berman, and M. H. Cobb. 2001. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr. Rev. 22:153-183. [DOI] [PubMed] [Google Scholar]
- 43.Perkins, D. D. 1984. Advantages of using the inactive-mating-type am1 strain as a helper component in heterokaryons. Fungal Genet. Newsl. 31:41-42. [Google Scholar]
- 44.Rayner, A. D. M. 1996. Interconnectedness and individualism in fungal mycelia, p. 193-232. In B. C. Sutton (ed.), A century of mycology. University of Cambridge Press, Cambridge, United Kingdom.
- 45.Roca, M. G., L. C. Davide, M. C. Mendes-Costa, and A. Wheals. 2003. Conidial anastomosis tubes in Colletotrichum. Fungal Genet. Biol. 40:138-145. [DOI] [PubMed] [Google Scholar]
- 46.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 47.Schweizer, M., M. E. Case, C. C. Dykstra, N. H. Giles, and S. R. Kushner. 1981. Identification and characterization of recombinant plasmids carrying the complete qa-2+ cluster from Neurospora crassa including the qa-1+ regulatory gene. Proc. Natl. Acad. Sci. USA 78:5086-5090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Selker, E. U. 1997. Epigenetic phenomena in filamentous fungi: useful paradigms or repeat-induced confusion? Trends Genet. 13:296-301. [DOI] [PubMed] [Google Scholar]
- 49.Shiu, P., N. B. Raju, D. Zickler, and R. L. Metzenberg. 2001. Meiotic silencing of unpaired DNA. Cell 107:905-916. [DOI] [PubMed] [Google Scholar]
- 50.Snetselaar, K. M., M. Bolker, and R. Kahmann. 1996. Ustilago maydis mating hyphae orient their growth toward pheromone sources. Fungal Genet. Biol. 20:299-312. [DOI] [PubMed] [Google Scholar]
- 51.Takano, Y., T. Kikuchi, Y. Kubo, J. E. Hamer, K. Mise, and I. Furusawa. 2000. The Colletotrichum lagenarium MAP kinase gene CMK1 regulates diverse aspects of fungal pathogenesis. Mol. Plant-Microbe Interact. 13:374-383. [DOI] [PubMed] [Google Scholar]
- 52.Treisman, R. 1996. Regulation of transcription by MAP kinase cascades. Curr. Opin. Cell Biol. 8:205-215. [DOI] [PubMed] [Google Scholar]
- 53.Trinci, A. P. J. 1984. Regulation of hyphal branching and hyphal orientation, p. 23-52. In D. H. Jennings and A. D. M. Rayner (ed.), The ecology and physiology of the fungal mycelium. Cambridge University Press, Cambridge, United Kingdom.
- 54.Tucker, S. L., and N. J. Talbot. 2001. Surface attachment and pre-penetration stage development by plant pathogenic fungi. Annu. Rev. Phytopathol. 39:385-417. [DOI] [PubMed] [Google Scholar]
- 55.Turian, G., and D. E. Bianchi. 1972. Conidiation in Neurospora. Bot. Rev. 38:119-154. [Google Scholar]
- 56.Vallim, M. A., K. Y. Miller, and B. L. Miller. 2000. Aspergillus SteA (sterile12-like) is a homeodomain-C2/H2-Zn+2 finger transcription factor required for sexual reproduction. Mol. Microbiol. 36:290-301. [DOI] [PubMed] [Google Scholar]
- 57.Vogel, H. J. 1964. Distribution of lysine pathways among fungi: evolutionary implications. Am. Nat. 98:435-446. [Google Scholar]
- 58.Wei, H. J., N. Requena, and R. Fischer. 2003. The MAPKK kinase SteC regulates conidiophore morphology and is essential for heterokaryon formation and sexual development in the homothallic fungus Aspergillus nidulans. Mol. Microbiol. 47:1577-1588. [DOI] [PubMed] [Google Scholar]
- 59.Wendlend, J. 2001. Comparison of morphogenetic networks of filamentous fungi and yeast. Fungal Genet. Biol. 34:63-82. [DOI] [PubMed] [Google Scholar]
- 60.Westergaard, M., and H. K. Mitchell. 1947. Neurospora V. A synthetic medium favoring sexual reproduction. Am. J. Bot. 34:573-577. [Google Scholar]
- 61.Wilson, J. F., and J. A. Dempsey. 1999. A hyphal fusion mutant in Neurospora crassa. Fungal Genet. Newsl. 46:31. [Google Scholar]
- 62.Wu, J., and N. L. Glass. 2001. Identification of specificity determinants and generation of alleles with novel specificity at the het-c heterokaryon incompatibility locus of Neurospora crassa. Mol. Cell. Biol. 21:1045-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xiang, Q., C. Rasmussen, and N. L. Glass. 2002. The ham-2 locus, encoding a putative transmembrane protein, is required for hyphal fusion in Neurospora crassa. Genetics 160:169-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xu, J.-R. 2000. MAP kinases in fungal pathogens. Fungal Genet. Biol. 31:137-152. [DOI] [PubMed] [Google Scholar]
- 65.Xu, J. R., and J. E. Hamer. 1996. MAP kinase and cAMP signaling regulate infection structure formation and pathogenic growth in the rice blast fungus Magnaporthe grisea. Genes Dev. 10:2696-2706. [DOI] [PubMed] [Google Scholar]
- 66.Xu, J. R., C. J. Staiger, and J. E. Hamer. 1998. Inactivation of the mitogen-activated protein kinase Mps1 from the rice blast fungus prevents penetration of host cells but allows activation of plant defense responses. Proc. Nat. Acad. Sci. USA 95:12713-12718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang, Y., R. Lamm, C. Pillonel, S. Lam, and J. R. Xu. 2002. Osmoregulation and fungicide resistance: the Neurospora crassa os-2 gene encodes a HOG1 mitogen-activated protein kinase homologue. Appl. Environ. Microbiol. 68:532-538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zheng, L., M. Campbell, J. Murphy, S. Lam, and J. R. Xu. 2000. The BMP1 gene is essential for pathogenicity in the gray mold fungus Botrytis cinerea. Mol. Plant-Microbe Interact. 13:724-732. [DOI] [PubMed] [Google Scholar]