Abstract
Leukemia inhibitory factor (LIF) is a pleiotropic growth factor that regulates several biological functions. This review focuses on the LIF-dependent STAT activation and its impact on modulation of trophoblast functions during embryo implantation. LIF is mainly produced by the maternal endometrium at the time of implantation while its receptors are present both on the endometrium and trophoblasts. It might influence blastocyst attachment through STAT3 activation and expression of integrins. After attachment of the blastocyst, trophoblasts undergo proliferation and differentiation into invasive EVTs and non-invasive STBs. Under in vitro conditions, LIF regulates all these processes through activation of STAT- and MAPK-dependent signaling pathways. The observations that LIF and STAT3 knockout mice are infertile further strengthen the notion about the critical involvement of LIF-mediated signaling during embryo implantation. Hence, a better understanding of LIF-STAT signaling would help in improving fertility as use of LIF in in vitro blastocyst culture improves the implanting ability of blastocyst after IVF.
Keywords: leukemia inhibitory factor, embryo implantation, JAK-STAT, trophoblast, pregnancy, trophoblast invasion, syncytialization
Introduction
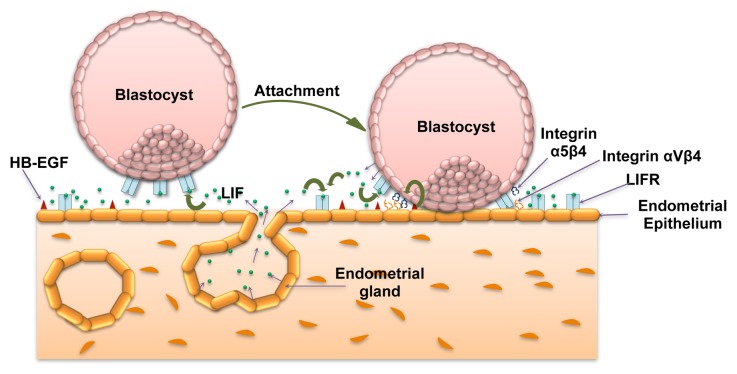
After fertilization, the zygote undergoes several rounds of cell division to form the blastocyst during its journey from the fallopian tube to the uterine cavity. Attainment of successful implantation of blastocyst depends upon the synchronized changes in the endometrium before and after arrival of the blastocyst into the uterine cavity. The cues obtained from the receptive endometrium initially helps in the attachment of the blastocyst to the endometrial epithelium and later proliferation and differentiation of trophoblasts to form functional placenta.1 After blastocyst hatching, the trophectodermal cells become accessible for paracrine signaling through growth factors and other soluble bioactive molecules present in the uterine fluid.2 The blastocyst, freed from the zona pellucida, can now interact with uterine luminal epithelium where there could be possibility for the juxtracrine signaling through the growth factors like HB-EGF present on the cell surface that induce the expression of integrin α5β1 on the trophoblasts (Fig. 1).3,4 This imparts competence to the blastocyst to make an attachment with the fibronectin present in the extracellular matrix (ECM), marking the beginning of the cross-signaling across the trophoblast and endometrial cells that lead to firm attachment of the blastocyst.2 Adhesion of the blastocyst to the maternal endometrium acts as anchor and trigger for differentiation of trophoblasts into the outer syncytiotrophoblast (STB) and the inner cytotrophoblast (CTB).2 STBs have the inherent ability to produce several lytic enzymes, which degrade the ECM and secrete factors that trigger apoptosis of the endometrial epithelial cells. This way, they enter through the endometrial epithelium and breach the barrier of basal lamina to embed the blastocyst into the stroma of the endometrium. In addition, STBs perform many different functions, including exchange of substrates, gases, and other factors between the maternal and fetal circulation and synthesis and secretion of protein and steroid hormones, growth factors, and other substances vital for regulation of maternal and fetal metabolism. With this, the process of early implantation events finishes by the end of the 2nd week of fertilization.1 After this, CTBs undergo extensive proliferation to form a compact cell column and later differentiate into highly invasive form of extravillous trophoblasts (EVTs). These are produced either to anchor the chorionic villi into the Nitabuch layer or to profoundly infiltrate the endometrial decidua. Invasive trophoblast cells ultimately reach to the maternal spiral arteries and replace the existing endothelial layer by forming the endovascular trophoblasts.5
Figure 1. Significance of LIF-mediated signaling in blastocyst attachment. LIF is expressed by the receptive endometrial luminal and glandular epithelium. At the same time LIFR is expressed by the endometrial luminal epithelium as well as by the blastocyst. At the time of implantation, endometrial epithelial cells express integrin αVβ3 as well as osteopontin (not shown in figure) that forms the part of pinopodes essential for the initiation of implantation. A juxtacrine signaling through HB-EGF expressed on the endometrial epithelium leads to the expression of integrin α5β3 by trophoblast cells. These changes collectively bring out attachment of the blastocyst to the endometrium. Once the blastocyst gets attached, it also starts expressing LIF that can act in autocrine or paracrine way on trophoblast and endometrial cells, respectively.
LIF, interleukin (IL)-6, IL-11, HGF, IGF, IL-1, IL-15, IL-8, EGF, cytokines of TGF-β super family, IFN-α, IFN-γ, etc. are the major cytokines and growth factors present during the peri-implantation period and play vital role in the accomplishment of successful pregnancy through influencing the trophoblast function.6-9 Apart from these, studies have also been performed which suggest that for attainment of successful implantation, activation of signaling pathways like JAK-STAT, MAPK, Notch, Smad, PI3K, etc. plays a crucial role.1,5-7,9-12 In this review, we will focus on the LIF-mediated activation of the STAT signaling pathway in the regulation of blastocyst attachment followed by trophoblast proliferation, invasion, and differentiation during the course of implantation.
Leukemia Inhibitory Factor
LIF is a pleiotropic cytokine of IL-6 family that is considered as one of the cytokines essential for the successful completion of human pregnancy.13,14 It was initially identified as a cytokine having the ability to inhibit proliferation of mouse myeloid leukemic cells and induce their differentiation into macrophages.15 However, in humans, it is also produced by several other cell types like endometrial cells, fibroblasts, hepatocytes, osteoblasts, monocytes, macrophages, T cells, etc. to regulate varying degree of functions.16-18 It is also expressed by granulosa-lutein and ovarian stroma cells.19 A higher concentration of LIF in follicular fluid correlates with the embryo quality suggesting an important role of LIF in the physiology of ovulation and early embryo development. In humans, LIF controls the uterine receptivity for blastocyst implantation, trophoblast behavior by promoting proliferation, invasion, and differentiation.11,20 In the endometrium, both glandular and luminal epithelial cells express LIF with a higher expression by glandular epithelium, which peaks during the secretory/postovulatory phase of the menstrual cycle (Fig. 1).20-22 In contrast to LIF, expression of LIFR is higher in the endometrial epithelial cells as compared with glandular epithelial cells. After blastocyst attachment to the endometrium, trophoblasts also start expressing LIF that might influence their physiological functions in an autocrine way.20,23-25 Both villous and extravillous trophoblasts express LIF and its receptor throughout pregnancy.26
LIF and Its Influence on Pregnancy
First observation about the critical involvement of LIF in embryo implantation came through experimentation in LIF knockout mouse. LIF-deficient female mice showed inability to attach the implanting blastocyst and it was fascinating to note that infusion of LIF into the uterus allowed the blastocyst to attach and grow until the full term.27 However, mice knocked out for LIF receptor (LIFR) had normal implantation but newborns died within 24 h of birth due to impaired placental function.28 Disruption of gp130, the STAT3-activating subunit shared by all members of the IL-6 receptor family, leads to an identical phenotype as knocking out of LIF.29 This suggests that for the initial phase of implantation, LIF might be influencing the trophoblast function. Clinically, it has been observed that the endometrial cells obtained from several cases of infertility have a diminished expression of LIF and these are represented with repeated abortions or unexplained infertility.30,31 Not only the expression of LIF but, mutations in the LIF gene expression have been associated with unexplained infertility in woman. In a case study, analysis of the mutations in the coding region and critical regulatory regions of the LIF gene has revealed that there were increasing number of heterozygous point mutations in close proximity to the start codon of exon 1 and in exon 3.32 These mutations could also be the reason for the poor outcome of fertility following IVF than the control group of woman.33 These regions are functionally important for the biological activity of LIF. Thus, heterozygosity for a LIF gene mutation could contribute to rising level of functionally inactive LIF in the uterus leading to implantation failure. But, mutation in the LIF gene is not the only reason for the unexplained infertility or recurrent implantation failure.34
LIF-STAT Signaling
LIF, like other members of IL-6 family of cytokines transduces its signal through formation of heterodimer with specific LIFR and the common co-receptor for IL-6 family (gp130).13 Binding of the LIF to its receptor leads to activation of both STAT and RAS/MAPK signaling cascade in trophoblasts.35,36 In this section, of all the signaling pathways getting activated in trophoblast cells, STAT-dependent downstream signaling pathways will be discussed in detail.
The JAK-STAT pathway was first defined as the signal transduction pathway downstream of cytokine receptors. Later, it was demonstrated that this pathway is responsible for the control of several biological responses, including cell growth, differentiation, longevity, and migration.37 STATs were discovered as molecules associated with interferon-γ-mediated signaling and gene expression with DNA-binding ability.38 STAT proteins are made up of about 750 to 848 amino acids (90–155 kDa). Out of the six STAT families of proteins, STAT1, STAT3, and STAT5 also generate their splice variants.39 All six STAT proteins are encoded by separate genes. All STAT proteins have a typical six domain structure, namely an oligomerization domain, a coiled-coil domain, a DNA-binding domain that determines the DNA-binding specificity, a linker domain, a SH2 domain that allows receptor binding and dimerization, and a transcription activation domain that contains a conserved serine residue (except in STAT2 and STAT6). In addition, all STAT proteins possess a critical tyrosine near the SH2 domain, approximately at amino acid position 700 (at position 705 in STAT3). Phosphorylation of this tyrosine is essential for dimerization, nuclear translocation, and DNA binding of STAT proteins. Apart from tyr phosphorylation, STAT1, STAT3, and STAT5 proteins also get phosphorylated at a C-terminal serine (for example, ser727 in STAT3) that is required for maximal transcriptional activity. Truncated isoforms of STAT proteins that lack the C-terminal transcription activation domain may act as dominant-negative isoforms and regulate the STATs biological activity.
STATs are present in latent form in the cytoplasm until the time they get activated by extracellular ligands like cytokines, growth factors, and hormones. Each of them is differentially activated by specific extracellular ligands, allowing differential intracellular processing of signals transduced across the plasma membrane. They regulate distinct biological functions in normal human physiology and development but also regulate oncogenic signaling in many different tumors.40 STAT3 and STAT5 are the STAT proteins that have been mostly implicated in the progression of cancer. In cancer cells, activation of STAT3 and STAT5 leads to increased expression of downstream target genes, which increases proliferation, survival, angiogenesis, and immune evasion.41 STAT target genes that regulate cell survival and proliferation include the B-cell lymphoma 2 (Bcl-2) family members, survivin, cyclin D1, and myc. STAT3 activation also promotes the cellular invasion by activating the transcription of MMP1, MMP2, MMP9, and MMP10.42,43 In certain circumstances, STATs can be activated independent of JAKs by other non-receptor tyrosine kinases.44 This kind of activation is mostly linked with the downstream signaling activation through growth factor receptors. For example, STAT1, STAT3, and STAT5 are activated directly through EGFR while PDGFR can directly activate STAT5.45-48
Most of the information about the functional role of STAT proteins in the regulation of biological function comes from the studies conducted on knockout mouse for a specific STAT protein.49 Among these, only STAT3 knockout mouse showed remarkable loss of fertility due to embryonic lethality in early gestation. STAT3 knockout embryos degenerate and die in the early post-implantation period on E7.5 but, can be rescued through substitution with an alternative splice form of STAT3, STAT3β, in which the C-terminal transactivation domain is replaced with a seven amino acid extension.49-51 Furthermore, the inhibition of STAT3 activation in the mouse endometrium also prevents the embryo implantation.52 This invites the hypothesis that LIF-STAT signaling might have a critical involvement in the process of implantation, possibly acting as a critical modulator of trophoblast invasion.27,28,49,52
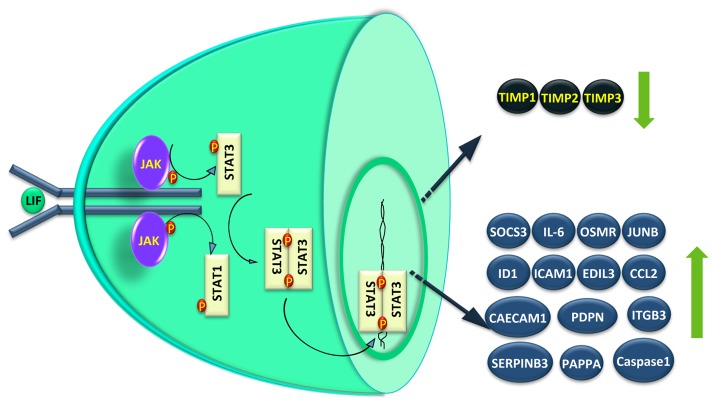
STAT3 gets activated through phosphorylation at tyrosine residue 705 as well at serine residue 727 in response to external ligands.53 Tyrosine(705) phosphorylation facilitates STAT3 dimerization and translocation to the nucleus, where they bind to the specific DNA response elements and enable gene transcription.53,54 A phosphorylation event at serine residue 727 also modulates the transcriptional activity of STAT3, and is required for maximal transcriptional activity.53,55 In J774.2 macrophages, leptin-induced ERK activation corresponded with an increase in both phosphorylation of ser727 and STAT3 DNA binding activity.56 However, there is also a notion that STAT3(ser727) phosphorylation has no bearing on their DNA binding or transcriptional activity.57 Phosphorylation of STAT3(ser727) has been linked with the activation of MAPK family members, whose activation are mainly dependent in the cellular context and the stimulus used.58-62 Although, it is undetermined whether serine phosphorylation is dependent on tyrosine phosphorylation, phosphorylation at ser727 of STAT3 may be essential for STAT3 activity.53,55,63 For example, serine phosphorylation of STAT3 is essential for post-natal survival and growth, since knock-in of STAT3SA cDNA, which replaces serine residue 727 with an alanine, into STAT3 knockout mice fails to compensate the phenotype.64 In addition, STAT3β, a truncated form of STAT3 without the serine residue 727-containing C-terminus, works as a negative regulator of STAT3-mediated activity in breast cancer cells.39,65,66 Phosphorylation of STAT3 at ser727 is associated with the malignant phenotype of several cancers including breast cancer.67 In human first trimester placentae, pSTAT3(ser727) protein is detectable in both cytoplasm and nuclei of CTB, STB, and vCTB while this expression profile disappears in the term placenta.68 Placental trophoblastic cancers also show higher nuclear pSTAT3(ser727) localization than their normal trophoblast counterpart. In trophoblastic cells, activation of STAT3 by serine phosphorylation is mainly mediated via mammalian target of rapamycin (mTOR).62 STAT3 expression or activation profile is constitutively higher in several malignancies, including those pertaining to the reproductive system.37,69,70 Choriocarcinoma cells also have higher STAT3 DNA binding activity which correlated with their malignant phenotype.68 In trophoblasts, upon LIF treatment, cytoplasmic STATs get phosphorylated by activated JAKs (tyrosine kinase) through phosphorylation at STAT3(tyr705) or STAT1(tyr727) (Fig. 2). Extent of phosphorylation of STAT1 has always been lower as compared with STAT3 upon LIF stimulation. In JEG-3 choriocarcinoma cells, STAT3 also gets phosphorylated at ser727 by activated ERK1/2 as inhibition of ERK1/2 activation following LIF treatment abrogated the LIF-mediated STAT3(ser727) phosphorylation (unpublished observation). Activated STATs form homo-/hetero- dimers through binding of the phosphotyrosine of one STAT molecule to the SH2 domain of its partner (Fig. 2). Upon dimerization, the STATs are translocated to the nucleus, where they act as transcription factors. One of the transcribed proteins is the SOCS3 that can negatively modulate the duration of the cytokine signaling response by binding to phosphotyrosine residues on JAKs (Fig. 2).71-74 LIF suppresses its own effects by means of negative feedback regulation of the JAK-STAT pathway through SOCS3.73,75 In trophoblast cells, STAT3-dependent expression of SOCS3 is essential for the negative regulation of trophoblast giant cell differentiation.76 We have observed a significant increase in the expression of SOCS3 in JEG-3 choriocarcinoma as well as in HTR-8/SVneo trophoblastic cells treated with LIF [unpublished observations].
Figure 2. STAT-dependent signaling and gene expression in LIF treated trophoblastic cells. LIF upon binding to the gp130-LIF receptor complex present on the plasma membrane of trophoblastic cells activate JAKs that ultimately phosphorylate the STAT3 and or STAT1 in the cytoplasm. These activated STATs form the homo- or hetero-dimers and move inside the nucleus to influence the expression of various genes that could regulate different functions like cytokine and signaling (IL-6, OSMR, SOCS3, and JUNB), adhesion (CECAM1, PDPN, and ITGB3), invasion (PAPPA, Caspase1, SERPINB3, TIMP1, TIMP2, and TIMP3), angiogenesis (ID1, ICAM1, EDIL3, and CCL2), etc. The genes whose expression is downregulated following LIF treatment are shown with down-arrow, while those showing upregulation are depicted with an up arrow.
Role of LIF in Blastocyst Attachment and Implantation
In humans, appearance of pinopodes (ectoplasmic protrusions from the endometrial epithelial cells) is considered as morphologic marker for the uterine receptivity.77 It is present for a very brief period of time and coincides with the window of implantation. Role of LIF in the initial phase of blastocyst attachment and implantation becomes more speculative with the fact that the peak of LIF and LIFR expression by uterine epithelium coincides with the appearance of pinopodes.22 In addition to LIFR, pinopodes also have higher expression of molecules like osteopontin and integrin αVβ3 that help in embryo implantation (Fig. 1).77-79 Establishment of a possible regulatory role of LIFR mediated signaling in the expression of osteopontin and integrin αVβ3 would help in understanding the LIF-STAT signaling in embryo implantation. Another way to confirm the significance of LIF in embryo implantation is to ablate the LIF-mediated signaling that can be achieved by neutralization of LIF present in the uterine lumen by using antibodies. Infusion of LIF antibodies in the uterine horn of pregnant mice led to reduced number of embryos implanted on day 8 of pregnancy.80 Under in vitro conditions, treatment of blastocyst with LIF enhances the blastocyst outgrowth which also gets compromised upon addition of LIF antibodies. In rhesus monkeys, infusion of anti-LIF monoclonal antibodies also reduced the implantation rate and the pregnancy in experimental group as compared with controls.81
In mouse, LIF-dependent activation of downstream signaling in endometrial cells is essential for the blastocyst to establish attachment with the endometrium. Co-immunoprecipitation experiments for LIFR and gp130 at the time of blastocyst attachment showed formation of heterodimers that is required for the LIF-mediated downstream signaling.82 In mouse luminal epithelial cells, LIF increases the activation of STAT3 through binding to LIFR and gp130 heterodimer.13 The activation of STAT3 takes place by phosphorylation at tyr705 residue. Phosphorylated STAT3 undergo nuclear localization and binds specifically to the STAT3 consensus recognition sequence. It was interesting to note that LIF treatment to the purified luminal epithelial cells only activated STAT3 and did not increase the phosphorylation of ERK1/2 (which was activated to a higher extent even before LIF treatment). The authors reasoned that a higher level of activation of ERK1/2 even before treatment of LIF could be due to the presence of EGF that mainly act by activation of ERK1/2 and less through activation of STAT3. This suggests that activation of STAT3 and not the ERK1/2 is critical for the embryo to implant. In the luminal epithelium, STAT3 activation showed a peculiar temporal activation pattern as in spite of the presence of LIF after day 4 (day on which implantation occurs in mouse) there was no activation of STAT3. This suggests that for uterine receptivity and blastocyst attachment not only the presence of LIF but activation of STAT3 is also important.13 However, the molecular basis behind the refractoriness of LIFR-mediated signaling in the luminal epithelial cells beyond the “window of implantation” are still speculative.
The human blastocyst expresses the LIFR at the time of implantation, the time when endometrial concentration of LIF reaches to the peak and this way trophoblasts might respond to the incoming stimuli from the endometrium (Fig. 1).24 In addition to this, LIF-mediated autocrine or paracrine signaling in the endometrial cells might aid in preparing the endometrial cells to attach with the incoming blastocyst (Fig. 1).20 Considering its significance in the regulation of early implantation, its usefulness has been shown in the IVF application. In IVF, it can be used as an in vitro supplement to the culture medium to enhance the quality of embryo at the stage of transfer into the uterus (United States Patent 5962321; Inventors: Gough, Nicholas Martin; Willson, Tracey Ann, Seamark, Robert Frederick [Beulah Park, AU], http://www.freepatentsonline.com/5962321.html).
Trophoblast Proliferation and Invasion: Regulation by LIF-STAT Signaling
After implantation, the trophoblast cells proliferate and breach the epithelial barrier to invade through the decidua thereby establishes close physical contact with the various cellular components of the maternal endometrium. A controlled proliferation, invasion, and self-renewal of trophoblast cells during this phase of development are important for successful establishment of pregnancy. There is extensive cross-talk between the trophoblast and the decidual cells to direct the process of proliferation and invasion. Several endometrium-derived molecules including LIF alter the proliferative and invasive potential of the trophoblasts in vitro.83,84 LIF increases the proliferation and invasion of JEG-3 choriocarcinoma as well as trophoblastic HTR-8/SVneo cells under in vitro conditions.7,35,36 Proliferation of different cell types is regulated by the activation of both STAT and ERK1/2 dependent signaling pathways. However, in trophoblasts, activation of ERK1/2 and not the STAT3 has been shown to regulate their proliferation upon treatment with LIF.35 Silencing of STAT3 expression in trophoblastic cells did not alter the LIF-mediated increase in proliferation.
LIF increases the invasiveness of trophoblastic cells through activation of STAT3 as silencing of STAT3 expression in JEG-3 choriocarcinoma cells resulted in a dramatic reduction in their invasive potential regardless of LIF supplementation.85 Recently, we have reported that in trophoblastic HTR-8/SVneo cells, LIF activates not only STAT3 but also STAT1 to a significant extent to increase their invasiveness across the Matrigel matrix.36 This led to a dose dependent increase in their invasiveness through increase in the expression of several invasion-associated molecules. LIF increased the expression of novel regulatory molecules like pappalysin 1 or PAPPA, podoplanin, SERPINB3, ICAM1, ID1, and integrin β3 (which are also expressed by human placenta) and decreased the expression of TIMP1, TIMP2, and TIMP3 (Fig. 2). Silencing of pappalysin 1 expression by siRNA led to abrogation of LIF-mediated invasion of HTR-8/SVneo cells. Earlier, in JEG-3 choriocarcinoma cells, LIF-mediated increase in the invasiveness was shown to be associated with increase in the expression of caspase 1 and decrease in the expression of TIMP1(Fig. 2).7
Syncytialization of Trophoblasts: Relevance of LIF-STAT Signaling
Syncytialization of trophoblastic cells is one of the essential attributes that holds the key for successful implantation of the embryo. Syncytial fusion enables the transfer of CTB-derived nuclei and other organelles, proteins, and RNA as well as cytoplasm and membranes into the STB. Permanent acquisition of fresh cellular components, however, requires continuous disposal of aged cytosolic content to maintain the homeostasis of the STB. Thus, apoptotic material is packed into the syncytial knots at the apical plasma membrane of the STB, where these corpuscular structures are released as sealed membrane vesicles into the maternal circulation.86 Restricted fusion, in contrast, may result in depletion of fresh cellular components within the STB, leading to exhaustion of the syncytial layer. Hence, trophoblast turnover has to be regulated within a tight range, avoiding excessive as well as restricted cytotrophoblast-syncytiotrophoblast fusion. Deregulated CTB to STB fusion may lead to preeclampsia, intrauterine growth retardation (IUGR) and implantation failure.87,88
LIF has been shown to regulate differentiation of trophoblast like BeWo (choriocarcinoma) cells through activation of JAK-STAT and MAPK3/1 signaling pathways. It shows a synergistic effect on forskolin-induced BeWo cell fusion.89 It activates other signaling pathways, such as MAPK and phosphatidylinositol-3-kinase (PIK3)/AKT.90 MAPK3/1 modulates both fusion and hCG secretion in primary STBs and BeWo cells.91,92 Based on the fact that LIF enhances MAPK3/1 pathway activation, it is likely that the effect of LIF on cell fusion relies on a cooperative cross-talk between LIF-induced activation of MAPK3/1 and forskolin-induced activation of PKA signaling pathways. A similar converging mechanism could be proposed for the JAK-STAT pathway, where it can be speculated that homodimers and/or heterodimers of STAT1 and STAT3 directly or indirectly act as co-activators of fusion-related gene promoters, as the fusogenic capacity of BeWo cells is greatly affected by inhibiting the activation of both MAPK3/1 and JAK-STAT signaling pathways.89
SOCS is also an important regulator of the embryo implantation as genetic deletion of SOCS3 resulted in embryonic lethality due to placental insufficiency at around embryonic day (E)13 in mice.93-95 In the SOCS3-null placenta, chorio-allantoic fusion occurred normally, but the labyrinthine and spongiotrophoblast layers of the mouse placenta were poorly formed, while trophoblast giant cells were increased in number and in size. To emphasize the fact that embryonic lethality associated with absence of SOCS3 is due to compromised placental differentiation, use of wild-type extraembryonic tissues, either in complementation via aggregation with tetraploid embryos (which contribute to extraembryonic tissues but not to embryo proper) or generation of chimeras composed of SOCS3-null embryos and wild-type trophoblast stem cells rescued the lethal phenotype.95,96 Genetic crosses between mice heterozygous for deletion of SOCS3 and LIFRα (null mutants for each is lethal) revealed that the phenotype is due to dysregulation of signaling downstream of the LIFR and that the ligand responsible for this, LIF, is produced by embryonic tissues and acts in a paracrine fashion.28,95 In human placenta SOCS1, SOCS2, and SOCS3 mRNA and protein are detectable in pre-term and term villous placenta and immunohistochemical analysis localized all three SOCS proteins to all decidual cells.97,98 Interestingly, decreased SOCS3 expression has also been observed in the villous tissue of placentae obtained from women with preeclampsia.99 These studies suggest that SOCS3 expression might have implication for the trophoblast invasion and their deficiency might lead to shallow invasion of the deciduas.100 Presence of an excess of proliferative immature trophoblast in preeclampsia is indicative of lack of invasive differentiation of trophoblast cells and SOCS3 could be one of the regulator for this kind of differentiation.
Conclusion
Present understanding suggests that LIF is one of the factors behind most of the physiological changes in trophoblasts during the course of embryo implantation. Its influence ranges from embryo adhesion to the regulation of trophoblast proliferation, invasion, and syncytialization. Presence of LIF in the in vitro culture medium improves the quality of the implanting blastocyst. LIF through activation of JAK-STAT signaling pathway bring out the above mentioned physiological changes. To better understand the STAT mediated invasion and differentiation of trophoblasts, substantial research efforts should be directed toward understanding the regulation of STAT responsive gene expression and their physiological relevance during these processes.
Acknowledgments
The authors are thankful to the Indian Council of Medical Research (ICMR), New Delhi, India and National Institute of Immunology, New Delhi for financial support. SS Malhotra is recipient of the Junior Research Fellowship of the Council of Scientific and Industrial Research (CSIR), Government of India.
Glossary
Abbreviations:
- CAECAM1
carcinoembryonic antigen-related cell adhesion molecule 1
- CCL2
chemokine (C-C motif) ligand 2
- CTB
cytotrophoblast
- EDIL3
EGF-like repeats and discoidin I-like domains 3
- EGF
epidermal growth factor
- EVT
extravillous trophoblast
- HB-EGF
heparin binding-epidermal growth factor
- HGF
hepatocyte growth factor
- ICAM1
intercellular adhesion molecule 1
- ID1
DNA-binding inhibitor protein
- IFN
interferon
- IGF
insulin-like growth factor
- ITGB3
integrin β3
- IVF
in vitro fertilization
- JAK
Janus kinase
- LIF
leukemia inhibitory factor
- MAPK
mitogen activated protein kinase
- MMP
matrix metalloproteinase
- OMR
oncostatin M receptor
- PAPPA
pregnancy associated plasma protein A
- PDGFR
platelet-derived growth factor receptor
- PDPN
podoplanin
- PI3K
phosphatidylinositol-3-kinase
- RAS
rat sarcoma
- SERPINB3
squamous cell carcinoma antigen-1
- Smad
mothers against decapentaplegic homolog
- SOCS
suppressor of cytokine signaling
- STAT
signal transducer and activator of transcription
- STB
syncytiotrophoblast
- TGF-β
transforming growth factor-beta
- TIMP
tissue inhibitor of metalloproteinase
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/jak-stat/article/25155
References
- 1.Modi DN, Godbole G, Suman P, Gupta SK. Endometrial biology during trophoblast invasion. Front Biosci (Schol Ed) 2012;4:1151–71. doi: 10.2741/S323. [DOI] [PubMed] [Google Scholar]
- 2.Armant DR. Blastocysts don’t go it alone. Extrinsic signals fine-tune the intrinsic developmental program of trophoblast cells. Dev Biol. 2005;280:260–80. doi: 10.1016/j.ydbio.2005.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raab G, Kover K, Paria BC, Dey SK, Ezzell RM, Klagsbrun M. Mouse preimplantation blastocysts adhere to cells expressing the transmembrane form of heparin-binding EGF-like growth factor. Development. 1996;122:637–45. doi: 10.1242/dev.122.2.637. [DOI] [PubMed] [Google Scholar]
- 4.Wang J, Mayernik L, Schultz JF, Armant DR. Acceleration of trophoblast differentiation by heparin-binding EGF-like growth factor is dependent on the stage-specific activation of calcium influx by ErbB receptors in developing mouse blastocysts. Development. 2000;127:33–44. doi: 10.1242/dev.127.1.33. [DOI] [PubMed] [Google Scholar]
- 5.Soares MJ, Chakraborty D, Renaud SJ, Kubota K, Bu P, Konno T, et al. Regulatory pathways controlling the endovascular invasive trophoblast cell lineage. J Reprod Dev. 2012;58:283–7. doi: 10.1262/jrd.2011-039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dimitriadis E, White CA, Jones RL, Salamonsen LA. Cytokines, chemokines and growth factors in endometrium related to implantation. Hum Reprod Update. 2005;11:613–30. doi: 10.1093/humupd/dmi023. [DOI] [PubMed] [Google Scholar]
- 7.Fitzgerald JS, Tsareva SA, Poehlmann TG, Berod L, Meissner A, Corvinus FM, et al. Leukemia inhibitory factor triggers activation of signal transducer and activator of transcription 3, proliferation, invasiveness, and altered protease expression in choriocarcinoma cells. Int J Biochem Cell Biol. 2005;37:2284–96. doi: 10.1016/j.biocel.2005.02.025. [DOI] [PubMed] [Google Scholar]
- 8.Guzeloglu-Kayisli O, Kayisli UA, Taylor HS. The role of growth factors and cytokines during implantation: endocrine and paracrine interactions. Semin Reprod Med. 2009;27:62–79. doi: 10.1055/s-0028-1108011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knöfler M. Critical growth factors and signalling pathways controlling human trophoblast invasion. Int J Dev Biol. 2010;54:269–80. doi: 10.1387/ijdb.082769mk. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pollheimer J, Knöfler M. Signalling pathways regulating the invasive differentiation of human trophoblasts: a review. Placenta. 2005;26(Suppl A):S21–30. doi: 10.1016/j.placenta.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 11.Fitzgerald JS, Poehlmann TG, Schleussner E, Markert UR. Trophoblast invasion: the role of intracellular cytokine signalling via signal transducer and activator of transcription 3 (STAT3) Hum Reprod Update. 2008;14:335–44. doi: 10.1093/humupd/dmn010. [DOI] [PubMed] [Google Scholar]
- 12.Zhao WX, Lin JH. Notch signaling pathway and human placenta. Int J Med Sci. 2012;9:447–52. doi: 10.7150/ijms.4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng JG, Chen JR, Hernandez L, Alvord WG, Stewart CL. Dual control of LIF expression and LIF receptor function regulate Stat3 activation at the onset of uterine receptivity and embryo implantation. Proc Natl Acad Sci U S A. 2001;98:8680–5. doi: 10.1073/pnas.151180898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Güney M, Oral B, Karahan N, Mungan T. Expression of leukaemia inhibitory factor (LIF) during the window of implantation in copper T380A intrauterine device users. Eur J Contracept Reprod Health Care. 2007;12:212–9. doi: 10.1080/13625180701441261. [DOI] [PubMed] [Google Scholar]
- 15.Gearing DP, Gough NM, King JA, Hilton DJ, Nicola NA, Simpson RJ, et al. Molecular cloning and expression of cDNA encoding a murine myeloid leukaemia inhibitory factor (LIF) EMBO J. 1987;6:3995–4002. doi: 10.1002/j.1460-2075.1987.tb02742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taupin JL, Pitard V, Dechanet J, Miossec V, Gualde N, Moreau JF. Leukemia inhibitory factor: part of a large ingathering family. Int Rev Immunol. 1998;16:397–426. doi: 10.3109/08830189809043003. [DOI] [PubMed] [Google Scholar]
- 17.Metcalf D. The unsolved enigmas of leukemia inhibitory factor. Stem Cells. 2003;21:5–14. doi: 10.1634/stemcells.21-1-5. [DOI] [PubMed] [Google Scholar]
- 18.Mathieu ME, Saucourt C, Mournetas V, Gauthereau X, Thézé N, Praloran V, et al. LIF-dependent signaling: new pieces in the Lego. Stem Cell Rev. 2012;8:1–15. doi: 10.1007/s12015-011-9261-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arici A, Oral E, Bahtiyar O, Engin O, Seli E, Jones EE. Leukaemia inhibitory factor expression in human follicular fluid and ovarian cells. Hum Reprod. 1997;12:1233–9. doi: 10.1093/humrep/12.6.1233. [DOI] [PubMed] [Google Scholar]
- 20.Cullinan EB, Abbondanzo SJ, Anderson PS, Pollard JW, Lessey BA, Stewart CL. Leukemia inhibitory factor (LIF) and LIF receptor expression in human endometrium suggests a potential autocrine/paracrine function in regulating embryo implantation. Proc Natl Acad Sci U S A. 1996;93:3115–20. doi: 10.1073/pnas.93.7.3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laird SM, Tuckerman EM, Dalton CF, Dunphy BC, Li TC, Zhang X. The production of leukaemia inhibitory factor by human endometrium: presence in uterine flushings and production by cells in culture. Hum Reprod. 1997;12:569–74. doi: 10.1093/humrep/12.3.569. [DOI] [PubMed] [Google Scholar]
- 22.Aghajanova L, Stavreus-Evers A, Nikas Y, Hovatta O, Landgren BM. Coexpression of pinopodes and leukemia inhibitory factor, as well as its receptor, in human endometrium. Fertil Steril. 2003;79(Suppl 1):808–14. doi: 10.1016/S0015-0282(02)04830-6. [DOI] [PubMed] [Google Scholar]
- 23.Conquet F, Brûlet P. Developmental expression of myeloid leukemia inhibitory factor gene in preimplantation blastocysts and in extraembryonic tissue of mouse embryos. Mol Cell Biol. 1990;10:3801–5. doi: 10.1128/mcb.10.7.3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Charnock-Jones DS, Sharkey AM, Fenwick P, Smith SK. Leukaemia inhibitory factor mRNA concentration peaks in human endometrium at the time of implantation and the blastocyst contains mRNA for the receptor at this time. J Reprod Fertil. 1994;101:421–6. doi: 10.1530/jrf.0.1010421. [DOI] [PubMed] [Google Scholar]
- 25.Kojima K, Kanzaki H, Iwai M, Hatayama H, Fujimoto M, Narukawa S, et al. Expression of leukaemia inhibitory factor (LIF) receptor in human placenta: a possible role for LIF in the growth and differentiation of trophoblasts. Hum Reprod. 1995;10:1907–11. doi: 10.1093/oxfordjournals.humrep.a136205. [DOI] [PubMed] [Google Scholar]
- 26.Sharkey AM, King A, Clark DE, Burrows TD, Jokhi PP, Charnock-Jones DS, et al. Localization of leukemia inhibitory factor and its receptor in human placenta throughout pregnancy. Biol Reprod. 1999;60:355–64. doi: 10.1095/biolreprod60.2.355. [DOI] [PubMed] [Google Scholar]
- 27.Stewart CL, Kaspar P, Brunet LJ, Bhatt H, Gadi I, Köntgen F, et al. Blastocyst implantation depends on maternal expression of leukaemia inhibitory factor. Nature. 1992;359:76–9. doi: 10.1038/359076a0. [DOI] [PubMed] [Google Scholar]
- 28.Ware CB, Horowitz MC, Renshaw BR, Hunt JS, Liggitt D, Koblar SA, et al. Targeted disruption of the low-affinity leukemia inhibitory factor receptor gene causes placental, skeletal, neural and metabolic defects and results in perinatal death. Development. 1995;121:1283–99. doi: 10.1242/dev.121.5.1283. [DOI] [PubMed] [Google Scholar]
- 29.Akira S, Yoshida K, Tanaka T, Taga T, Kishimoto T. Targeted disruption of the IL-6 related genes: gp130 and NF-IL-6. Immunol Rev. 1995;148:221–53. doi: 10.1111/j.1600-065X.1995.tb00100.x. [DOI] [PubMed] [Google Scholar]
- 30.Delage G, Moreau JF, Taupin JL, Freitas S, Hambartsoumian E, Olivennes F, et al. In-vitro endometrial secretion of human interleukin for DA cells/leukaemia inhibitory factor by explant cultures from fertile and infertile women. Hum Reprod. 1995;10:2483–8. doi: 10.1093/oxfordjournals.humrep.a136328. [DOI] [PubMed] [Google Scholar]
- 31.Hambartsoumian E. Endometrial leukemia inhibitory factor (LIF) as a possible cause of unexplained infertility and multiple failures of implantation. Am J Reprod Immunol. 1998;39:137–43. doi: 10.1111/j.1600-0897.1998.tb00345.x. [DOI] [PubMed] [Google Scholar]
- 32.Giess R, Tanasescu I, Steck T, Sendtner M. Leukaemia inhibitory factor gene mutations in infertile women. Mol Hum Reprod. 1999;5:581–6. doi: 10.1093/molehr/5.6.581. [DOI] [PubMed] [Google Scholar]
- 33.Novotný Z, Krízan J, Síma R, Síma P, Uher P, Zech N, et al. Leukaemia inhibitory factor (LIF) gene mutations in women diagnosed with unexplained infertility and endometriosis have a negative impact on the IVF outcome. A pilot study. Folia Biol (Praha) 2009;55:92–7. [PubMed] [Google Scholar]
- 34.Steck T, Giess R, Suetterlin MW, Bolland M, Wiest S, Poehls UG, et al. Leukaemia inhibitory factor (LIF) gene mutations in women with unexplained infertility and recurrent failure of implantation after IVF and embryo transfer. Eur J Obstet Gynecol Reprod Biol. 2004;112:69–73. doi: 10.1016/S0301-2115(03)00315-4. [DOI] [PubMed] [Google Scholar]
- 35.Golla JP, Suman P, Morales Prieto DM, Markert UR, Gupta SK. Leukaemia inhibitory factor mediated proliferation of HTR-8/SVneo trophoblastic cells is dependent on activation of extracellular signal-regulated kinase 1/2. Reprod Fertil Dev. 2011;23:714–24. doi: 10.1071/RD10315. [DOI] [PubMed] [Google Scholar]
- 36.Suman P, Shembekar N, Gupta SK. Leukemia inhibitory factor increases the invasiveness of trophoblastic cells through integrated increase in the expression of adhesion molecules and pappalysin 1 with a concomitant decrease in the expression of tissue inhibitor of matrix metalloproteinases. Fertil Steril. 2012;99:533–42. doi: 10.1016/j.fertnstert.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 37.Bromberg JF. Activation of STAT proteins and growth control. Bioessays. 2001;23:161–9. doi: 10.1002/1521-1878(200102)23:2<161::AID-BIES1023>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 38.Schindler C, Shuai K, Prezioso VR, Darnell JE., Jr. Interferon-dependent tyrosine phosphorylation of a latent cytoplasmic transcription factor. Science. 1992;257:809–13. doi: 10.1126/science.1496401. [DOI] [PubMed] [Google Scholar]
- 39.Schaefer TS, Sanders LK, Park OK, Nathans D. Functional differences between Stat3α and Stat3β. Mol Cell Biol. 1997;17:5307–16. doi: 10.1128/mcb.17.9.5307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Turkson J, Kim JS, Zhang S, Yuan J, Huang M, Glenn M, et al. Novel peptidomimetic inhibitors of signal transducer and activator of transcription 3 dimerization and biological activity. Mol Cancer Ther. 2004;3:261–9. [PubMed] [Google Scholar]
- 41.Yu H, Jove R. The STATs of cancer--new molecular targets come of age. Nat Rev Cancer. 2004;4:97–105. doi: 10.1038/nrc1275. [DOI] [PubMed] [Google Scholar]
- 42.Xie TX, Wei D, Liu M, Gao AC, Ali-Osman F, Sawaya R, et al. Stat3 activation regulates the expression of matrix metalloproteinase-2 and tumor invasion and metastasis. Oncogene. 2004;23:3550–60. doi: 10.1038/sj.onc.1207383. [DOI] [PubMed] [Google Scholar]
- 43.Itoh T, Ikeda T, Gomi H, Nakao S, Suzuki T, Itohara S. Unaltered secretion of beta-amyloid precursor protein in gelatinase A (matrix metalloproteinase 2)-deficient mice. J Biol Chem. 1997;272:22389–92. doi: 10.1074/jbc.272.36.22389. [DOI] [PubMed] [Google Scholar]
- 44.Olayioye MA, Beuvink I, Horsch K, Daly JM, Hynes NE. ErbB receptor-induced activation of stat transcription factors is mediated by Src tyrosine kinases. J Biol Chem. 1999;274:17209–18. doi: 10.1074/jbc.274.24.17209. [DOI] [PubMed] [Google Scholar]
- 45.Fu XY, Zhang JJ. Transcription factor p91 interacts with the epidermal growth factor receptor and mediates activation of the c-fos gene promoter. Cell. 1993;74:1135–45. doi: 10.1016/0092-8674(93)90734-8. [DOI] [PubMed] [Google Scholar]
- 46.Ruff-Jamison S, Zhong Z, Wen Z, Chen K, Darnell JE, Jr., Cohen S. Epidermal growth factor and lipopolysaccharide activate Stat3 transcription factor in mouse liver. J Biol Chem. 1994;269:21933–5. [PubMed] [Google Scholar]
- 47.Ruff-Jamison S, Chen K, Cohen S. Epidermal growth factor induces the tyrosine phosphorylation and nuclear translocation of Stat 5 in mouse liver. Proc Natl Acad Sci U S A. 1995;92:4215–8. doi: 10.1073/pnas.92.10.4215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Paukku K, Valgeirsdóttir S, Saharinen P, Bergman M, Heldin CH, Silvennoinen O. Platelet-derived growth factor (PDGF)-induced activation of signal transducer and activator of transcription (Stat) 5 is mediated by PDGF beta-receptor and is not dependent on c-src, fyn, jak1 or jak2 kinases. Biochem J. 2000;345:759–66. doi: 10.1042/0264-6021:3450759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Akira S. Functional roles of STAT family proteins: lessons from knockout mice. Stem Cells. 1999;17:138–46. doi: 10.1002/stem.170138. [DOI] [PubMed] [Google Scholar]
- 50.Duncan SA, Zhong Z, Wen Z, Darnell JE., Jr. STAT signaling is active during early mammalian development. Dev Dyn. 1997;208:190–8. doi: 10.1002/(SICI)1097-0177(199702)208:2<190::AID-AJA6>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 51.Takeda K, Noguchi K, Shi W, Tanaka T, Matsumoto M, Yoshida N, et al. Targeted disruption of the mouse Stat3 gene leads to early embryonic lethality. Proc Natl Acad Sci U S A. 1997;94:3801–4. doi: 10.1073/pnas.94.8.3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Catalano RD, Johnson MH, Campbell EA, Charnock-Jones DS, Smith SK, Sharkey AM. Inhibition of Stat3 activation in the endometrium prevents implantation: a nonsteroidal approach to contraception. Proc Natl Acad Sci U S A. 2005;102:8585–90. doi: 10.1073/pnas.0502343102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wen Z, Zhong Z, Darnell JE., Jr. Maximal activation of transcription by Stat1 and Stat3 requires both tyrosine and serine phosphorylation. Cell. 1995;82:241–50. doi: 10.1016/0092-8674(95)90311-9. [DOI] [PubMed] [Google Scholar]
- 54.Darnell JE., Jr. STATs and gene regulation. Science. 1997;277:1630–5. doi: 10.1126/science.277.5332.1630. [DOI] [PubMed] [Google Scholar]
- 55.Ceresa BP, Pessin JE. Insulin stimulates the serine phosphorylation of the signal transducer and activator of transcription (STAT3) isoform. J Biol Chem. 1996;271:12121–4. doi: 10.1074/jbc.271.21.12121. [DOI] [PubMed] [Google Scholar]
- 56.O’Rourke L, Shepherd PR. Biphasic regulation of extracellular-signal-regulated protein kinase by leptin in macrophages: role in regulating STAT3 Ser727 phosphorylation and DNA binding. Biochem J. 2002;364:875–9. doi: 10.1042/BJ20020295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wen Z, Darnell JE., Jr. Mapping of Stat3 serine phosphorylation to a single residue (727) and evidence that serine phosphorylation has no influence on DNA binding of Stat1 and Stat3. Nucleic Acids Res. 1997;25:2062–7. doi: 10.1093/nar/25.11.2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sengupta TK, Talbot ES, Scherle PA, Ivashkiv LB. Rapid inhibition of interleukin-6 signaling and Stat3 activation mediated by mitogen-activated protein kinases. Proc Natl Acad Sci U S A. 1998;95:11107–12. doi: 10.1073/pnas.95.19.11107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kuroki M, O’Flaherty JT. Extracellular signal-regulated protein kinase (ERK)-dependent and ERK-independent pathways target STAT3 on serine-727 in human neutrophils stimulated by chemotactic factors and cytokines. Biochem J. 1999;341:691–6. doi: 10.1042/0264-6021:3410691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schuringa JJ, Jonk LJ, Dokter WH, Vellenga E, Kruijer W. Interleukin-6-induced STAT3 transactivation and Ser727 phosphorylation involves Vav, Rac-1 and the kinase SEK-1/MKK-4 as signal transduction components. Biochem J. 2000;347:89–96. doi: 10.1042/0264-6021:3470089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Aggarwal BB, Kunnumakkara AB, Harikumar KB, Gupta SR, Tharakan ST, Koca C, et al. Signal transducer and activator of transcription-3, inflammation, and cancer: how intimate is the relationship? Ann N Y Acad Sci. 2009;1171:59–76. doi: 10.1111/j.1749-6632.2009.04911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Busch S, Renaud SJ, Schleussner E, Graham CH, Markert UR. mTOR mediates human trophoblast invasion through regulation of matrix-remodeling enzymes and is associated with serine phosphorylation of STAT3. Exp Cell Res. 2009;315:1724–33. doi: 10.1016/j.yexcr.2009.01.026. [DOI] [PubMed] [Google Scholar]
- 63.Ng J, Cantrell D. STAT3 is a serine kinase target in T lymphocytes. Interleukin 2 and T cell antigen receptor signals converge upon serine 727. J Biol Chem. 1997;272:24542–9. doi: 10.1074/jbc.272.39.24542. [DOI] [PubMed] [Google Scholar]
- 64.Shen Y, Schlessinger K, Zhu X, Meffre E, Quimby F, Levy DE, et al. Essential role of STAT3 in postnatal survival and growth revealed by mice lacking STAT3 serine 727 phosphorylation. Mol Cell Biol. 2004;24:407–19. doi: 10.1128/MCB.24.1.407-419.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Caldenhoven E, van Dijk TB, Solari R, Armstrong J, Raaijmakers JA, Lammers JW, et al. STAT3β, a splice variant of transcription factor STAT3, is a dominant negative regulator of transcription. J Biol Chem. 1996;271:13221–7. doi: 10.1074/jbc.271.22.13221. [DOI] [PubMed] [Google Scholar]
- 66.Burke WM, Jin X, Lin HJ, Huang M, Liu R, Reynolds RK, et al. Inhibition of constitutively active Stat3 suppresses growth of human ovarian and breast cancer cells. Oncogene. 2001;20:7925–34. doi: 10.1038/sj.onc.1204990. [DOI] [PubMed] [Google Scholar]
- 67.Yeh YT, Ou-Yang F, Chen IF, Yang SF, Wang YY, Chuang HY, et al. STAT3 ser727 phosphorylation and its association with negative estrogen receptor status in breast infiltrating ductal carcinoma. Int J Cancer. 2006;118:2943–7. doi: 10.1002/ijc.21771. [DOI] [PubMed] [Google Scholar]
- 68.Chan HY, Siu MK, Zhang HJ, Wong ES, Ngan HY, Chan KY, et al. Activated Stat3 expression in gestational trophoblastic disease: correlation with clinicopathological parameters and apoptotic indices. Histopathology. 2008;53:139–46. doi: 10.1111/j.1365-2559.2008.03089.x. [DOI] [PubMed] [Google Scholar]
- 69.Corvinus FM, Fitzgerald JS, Friedrich K, Markert UR. Evidence for a correlation between trophoblast invasiveness and STAT3 activity. Am J Reprod Immunol. 2003;50:316–21. doi: 10.1034/j.1600-0897.2003.00099.x. [DOI] [PubMed] [Google Scholar]
- 70.Ranger JJ, Levy DE, Shahalizadeh S, Hallett M, Muller WJ. Identification of a Stat3-dependent transcription regulatory network involved in metastatic progression. Cancer Res. 2009;69:6823–30. doi: 10.1158/0008-5472.CAN-09-1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Magrangeas F, Boisteau O, Denis S, Jacques Y, Minvielle S. Negative regulation of onconstatin M signaling by suppressor of cytokine signaling (SOCS-3) Eur Cytokine Netw. 2001;12:309–15. [PubMed] [Google Scholar]
- 72.Kamura T, Sato S, Haque D, Liu L, Kaelin WG, Jr., Conaway RC, et al. The Elongin BC complex interacts with the conserved SOCS-box motif present in members of the SOCS, ras, WD-40 repeat, and ankyrin repeat families. Genes Dev. 1998;12:3872–81. doi: 10.1101/gad.12.24.3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hilton DJ. Negative regulators of cytokine signal transduction. Cell Mol Life Sci. 1999;55:1568–77. doi: 10.1007/s000180050396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fitzgerald JS, Toth B, Jeschke U, Schleussner E, Markert UR. Knocking off the suppressors of cytokine signaling (SOCS): their roles in mammalian pregnancy. J Reprod Immunol. 2009;83:117–23. doi: 10.1016/j.jri.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 75.Naka T, Narazaki M, Hirata M, Matsumoto T, Minamoto S, Aono A, et al. Structure and function of a new STAT-induced STAT inhibitor. Nature. 1997;387:924–9. doi: 10.1038/43219. [DOI] [PubMed] [Google Scholar]
- 76.Isobe A, Takeda T, Sakata M, Yamamoto T, Minekawa R, Hayashi M, et al. STAT3-mediated constitutive expression of SOCS3 in an undifferentiated rat trophoblast-like cell line. Placenta. 2006;27:912–8. doi: 10.1016/j.placenta.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 77.Nardo LG, Sabatini L, Rai R, Nardo F. Pinopode expression during human implantation. Eur J Obstet Gynecol Reprod Biol. 2002;101:104–8. doi: 10.1016/S0301-2115(01)00523-1. [DOI] [PubMed] [Google Scholar]
- 78.Ceydeli N, Kaleli S, Calay Z, Erel CT, Akbas F, Ertungealp E. Difference in alpha(v)beta3 integrin expression in endometrial stromal cell in subgroups of women with unexplained infertility. Eur J Obstet Gynecol Reprod Biol. 2006;126:206–11. doi: 10.1016/j.ejogrb.2005.11.034. [DOI] [PubMed] [Google Scholar]
- 79.Tei C, Maruyama T, Kuji N, Miyazaki T, Mikami M, Yoshimura Y. Reduced expression of alphavbeta3 integrin in the endometrium of unexplained infertility patients with recurrent IVF-ET failures: improvement by danazol treatment. J Assist Reprod Genet. 2003;20:13–20. doi: 10.1023/A:1021254620888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cai LQ, Cao YJ, Duan EK. Effects of leukaemia inhibitory factor on embryo implantation in the mouse. Cytokine. 2000;12:1676–82. doi: 10.1006/cyto.2000.0758. [DOI] [PubMed] [Google Scholar]
- 81.Sengupta J, Lalitkumar PG, Najwa AR, Ghosh D. Monoclonal anti-leukemia inhibitory factor antibody inhibits blastocyst implantation in the rhesus monkey. Contraception. 2006;74:419–25. doi: 10.1016/j.contraception.2006.05.070. [DOI] [PubMed] [Google Scholar]
- 82.Song H, Lim H. Evidence for heterodimeric association of leukemia inhibitory factor (LIF) receptor and gp130 in the mouse uterus for LIF signaling during blastocyst implantation. Reproduction. 2006;131:341–9. doi: 10.1530/rep.1.00956. [DOI] [PubMed] [Google Scholar]
- 83.Johnstone ED, Mackova M, Das S, Payne SG, Lowen B, Sibley CP, et al. Multiple anti-apoptotic pathways stimulated by EGF in cytotrophoblasts. Placenta. 2005;26:548–55. doi: 10.1016/j.placenta.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 84.Magariños MP, Sánchez-Margalet V, Kotler M, Calvo JC, Varone CL. Leptin promotes cell proliferation and survival of trophoblastic cells. Biol Reprod. 2007;76:203–10. doi: 10.1095/biolreprod.106.051391. [DOI] [PubMed] [Google Scholar]
- 85.Poehlmann TG, Fitzgerald JS, Meissner A, Wengenmayer T, Schleussner E, Friedrich K, et al. Trophoblast invasion: tuning through LIF, signalling via Stat3. Placenta. 2005;26(Suppl A):S37–41. doi: 10.1016/j.placenta.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 86.Gauster M, Huppertz B. Fusion of cytotrophoblast with syncytiotrophoblast in the human placenta: factors involved in syncytialization. J Reprod Med Endocrinol. 2008;5:76–82. [Google Scholar]
- 87.Langbein M, Strick R, Strissel PL, Vogt N, Parsch H, Beckmann MW, et al. Impaired cytotrophoblast cell-cell fusion is associated with reduced Syncytin and increased apoptosis in patients with placental dysfunction. Mol Reprod Dev. 2008;75:175–83. doi: 10.1002/mrd.20729. [DOI] [PubMed] [Google Scholar]
- 88.Chen CP, Wang KG, Chen CY, Yu C, Chuang HC, Chen H. Altered placental syncytin and its receptor ASCT2 expression in placental development and pre-eclampsia. BJOG. 2006;113:152–8. doi: 10.1111/j.1471-0528.2005.00843.x. [DOI] [PubMed] [Google Scholar]
- 89.Leduc K, Bourassa V, Asselin E, Leclerc P, Lafond J, Reyes-Moreno C. Leukemia inhibitory factor regulates differentiation of trophoblastlike BeWo cells through the activation of JAK/STAT and MAPK3/1 MAP kinase-signaling pathways. Biol Reprod. 2012;86:54. doi: 10.1095/biolreprod.111.094334. [DOI] [PubMed] [Google Scholar]
- 90.Heinrich PC, Behrmann I, Haan S, Hermanns HM, Müller-Newen G, Schaper F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J. 2003;374:1–20. doi: 10.1042/BJ20030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Daoud G, Amyot M, Rassart E, Masse A, Simoneau L, Lafond J. ERK1/2 and p38 regulate trophoblasts differentiation in human term placenta. J Physiol. 2005;566:409–23. doi: 10.1113/jphysiol.2005.089326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Delidaki M, Gu M, Hein A, Vatish M, Grammatopoulos DK. Interplay of cAMP and MAPK pathways in hCG secretion and fusogenic gene expression in a trophoblast cell line. Mol Cell Endocrinol. 2011;332:213–20. doi: 10.1016/j.mce.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 93.Marine JC, McKay C, Wang D, Topham DJ, Parganas E, Nakajima H, et al. SOCS3 is essential in the regulation of fetal liver erythropoiesis. Cell. 1999;98:617–27. doi: 10.1016/S0092-8674(00)80049-5. [DOI] [PubMed] [Google Scholar]
- 94.Roberts AW, Robb L, Rakar S, Hartley L, Cluse L, Nicola NA, et al. Placental defects and embryonic lethality in mice lacking suppressor of cytokine signaling 3. Proc Natl Acad Sci U S A. 2001;98:9324–9. doi: 10.1073/pnas.161271798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Takahashi Y, Carpino N, Cross JC, Torres M, Parganas E, Ihle JN. SOCS3: an essential regulator of LIF receptor signaling in trophoblast giant cell differentiation. EMBO J. 2003;22:372–84. doi: 10.1093/emboj/cdg057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Takahashi Y, Dominici M, Swift J, Nagy C, Ihle JN. Trophoblast stem cells rescue placental defect in SOCS3-deficient mice. J Biol Chem. 2006;281:11444–5. doi: 10.1074/jbc.C600015200. [DOI] [PubMed] [Google Scholar]
- 97.Blumenstein M, Bowen-Shauver JM, Keelan JA, Mitchell MD. Identification of suppressors of cytokine signaling (SOCS) proteins in human gestational tissues: differential regulation is associated with the onset of labor. J Clin Endocrinol Metab. 2002;87:1094–7. doi: 10.1210/jc.87.3.1094. [DOI] [PubMed] [Google Scholar]
- 98.Blumenstein M, Keelan JA, Bowen-Shauver JM, Mitchell MD. Suppressors of cytokine signaling proteins in human preterm placental tissues. J Mol Endocrinol. 2005;35:165–75. doi: 10.1677/jme.1.01767. [DOI] [PubMed] [Google Scholar]
- 99.Zhao S, Gu Y, Dong Q, Fan R, Wang Y. Altered interleukin-6 receptor, IL-6R and gp130, production and expression and decreased SOCS-3 expression in placentas from women with pre-eclampsia. Placenta. 2008;29:1024–8. doi: 10.1016/j.placenta.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Redline RW, Patterson P. Pre-eclampsia is associated with an excess of proliferative immature intermediate trophoblast. Hum Pathol. 1995;26:594–600. doi: 10.1016/0046-8177(95)90162-0. [DOI] [PubMed] [Google Scholar]