Abstract
A quantitative understanding of the complex interactions between cells, soluble factors, and the biological and mechanical properties of biomaterials is required to guide cell remodeling towards regeneration of healthy tissue rather than fibrocontractive tissue. In the present study, we characterized the combined effects of boundary stiffness and transforming growth factor-β1 (TGF-β1) on cell-generated forces and collagen accumulation. We first generated a quantitative map of cell-generated tension in response to these factors by culturing valvular interstitial cells (VICs) within micro-scale fibrin gels between compliant posts (0.15–1.05 nN/nm) in chemically-defined media with TGF-β1 (0–5 ng/mL). The VICs generated 100 to 3000 nN/cell after one week of culture, and multiple regression modeling demonstrated, for the first time, quantitative interaction (synergy) between these factors in a three-dimensional culture system. We then isolated passive and active components of tension within the micro-tissues and found that cells cultured with high levels of stiffness and TGF-β1 expressed myofibroblast markers and generated substantial residual tension in the matrix yet, surprisingly, were not able to generate additional tension in response to membrane depolarization signifying a state of continual maximal contraction. In contrast, negligible residual tension was stored in the low stiffness and TGF-β1 groups indicating a lower potential for shrinkage upon release. We then studied if ECM could be generated under the low tension environment and found that TGF-β1, but not EGF, increased de novo collagen accumulation in both low and high tension environments roughly equally. Combined, these findings suggest that isometric cell force, passive retraction, and collagen production can be tuned by independently altering boundary stiffness and TGF-β1 concentration. The ability to stimulate matrix production without inducing high active tension will aid in the development of robust tissue engineered heart valves and other connective tissue replacements where minimizing tissue shrinkage upon implantation is critical.
Keywords: Valvular interstitial cells, tissue engineering, heart valves, stiffness, isometric force, contraction, fibrin, 3D, TGF-β1, EGF, collagen
1. INTRODUCTION
Advanced scaffolds for tissue engineering and regenerative medicine have the potential to be remodeled by cells and thus be adapted to the patient’s local chemomechanical environment. However, complex interactions between soluble factors, the biological properties of the biomaterial (such as polymer/protein type and ligand density), and the mechanical properties of biomaterial [1, 2] govern the cells’ ability to degrade the biomaterial, synthesize extracellular matrix (ECM), and generate forces. Approaches aimed at increasing the rate of growth in tissue engineered structures often result in unstable and uncontrolled fibrocontractive remodeling involving excessive contraction of the resident cells [3], build-up of residual stress [4], and ECM synthesis (i.e., scarring) [5] rather than regeneration of normal tissue. To achieve the desired tissue regeneration, there is a need to understand how biochemical and mechanical signals interact in the regulation of cell-generated forces and ECM remodeling.
Neo-tissue bulking [6–9] and retraction [10, 11] resulting from fibrocontractive remodeling are especially detrimental in the development of tissue engineered heart valves (TEHV), since even a slight shortening or thickening of the leaflets can lead to regurgitation (back flow) [3, 10, 11]. Leaflet thickening, excessive active cell contraction, and passive residual tension in the tissue [4, 10, 11] have been attributed to large numbers of myofibroblasts in TEHV leaflets since they are a highly contractile and synthetic phenotype. Excessive activation of myofibroblasts and an increase in ECM stiffness are also associated with fibrosis and calcification of native heart valves [12]. Myofibroblast activation is triggered when TEHVs are mechanically conditioned in bioreactors and/or treated with growth factors to accelerate the development of de novo tissue [13]. Activation is also observed in native heart valves as a result of abrupt changes in pressure loading [14].
Mechanical tension and transforming growth factor-β1 (TGF-β1) are the two main regulators of myofibroblast activation [5, 15, 16]. Culture conditions involving externally applied stress or high substrate elastic modulus lead to formation of stress fibers in the cytoplasm which in turn generate intracellular tension [15–17]. Under high intracellular tension, TGF-β1 stimulates recruitment of alpha-smooth muscle actin (α-SMA) in the stress fibers [18], the defining hallmark for the myofibroblast phenotype, which contributes to further increased intracellular tension [19]. Few studies explicitly quantify the forces involved in myofibroblast activation, however it has been shown that cell-generated tension and expression of α-SMA in stress fibers are positively correlated to substrate modulus [20, 21] over certain modulus thresholds [17, 22, 23] and below saturation limits at high modulus levels [17]. TGF-β1 also increases fibroblast traction forces in a dose-dependent manner if the substrate is sufficiently stiff [21]. Analogous to two-dimensional (2D) substrate modulus, the ability of three-dimensional (3D) scaffolds to resist deformation due to cell-generated tension also strongly regulates myofibroblast activation. Most strikingly, TGF-β1 induces α-SMA expression in cells in anchored collagen gels but not in floating gels [24, 25]. TGF-β1 acts as an agonist which increases the rate of compaction of free-floating gels (to smaller diameter) [26–28] and anchored gels (to lower thickness) [29] in a dose-dependent manner. Further, when cells are pre-treated with TGF-β1 prior to seeding into collagen gels, they compact the floating gels to a higher extent, which indicates an increased ability to generate traction [28]. Similarly, TGF-β1 treatment of VICs [30] and fibroblasts [28] for several days results in higher rate and extent of gel retraction upon release of anchored gels.
While high tension resulting from myofibroblast activation is undesired in tissue engineering, TGF-β1 and mechanical stimulation are potent stimulants of ECM production and are widely used in tissue engineering to augment growth [31–33]. For example, collagen production by nenonatal smooth muscle cells increases 4 fold with 1 ng/mL TGF-β1 treatment [34]. ECM protein expression increases when cells are cyclically stretched [35–37] and decreases when contraction is inhibited in fibroblasts [38]. These findings demonstrate that both growth factors and tension modulate ECM production, but how growth factor stimulation of ECM production is regulated by tension in 3D remains understudied. It is possible that optimal combinations of these two factors - tension and growth factors - may be utilized to induce the formation of robust tissue without excessive active contraction or residual matrix stress
The most direct and functional measure of a cell’s contractile state is the force it generates against the substrate or scaffold. However, in the majority of collagen gel assays the cell forces have not been directly measured, thus quantitative relationship between tension and myofibroblast differentiation have not been determined in 3D gels. Whereas measurement of the traction exerted by single cells against compliant 2D substrates utilizing traction force microscopy (TFM) is now commonplace, measuring single-cell traction force in 3D scaffolds remains challenging [39]. Alternatively, culture force monitors and compliant-anchored systems have been developed to directly measure cell-generated forces of populations of cells within biopolymer gels [40, 41]. Compliant-anchored systems have the additional benefit of allowing one to modulate the cell-generated tension by altering the resistance to cell-generated forces. Using these systems, we and others [42–44] have shown that increasing boundary stiffness leads to an increase in the cell contractile force and an increased sensitivity of fibroblasts to TGF-β1 [42]. In these investigations, a very limited number of stiffness levels and soluble factor concentrations have been studied (generally “low” and “high”). Establishment of quantitative relationships between cell behavior and specific biochemical and mechanical stimuli requires each factor to be tested at multiple levels in combination.
The goal of the present study is to quantitatively characterize the combined effects of boundary stiffness and TGF-β1 on VIC-generated forces within a 3D matrix. To this end, we cultured VICs in fibrin gels under graded levels of boundary stiffness with multiple concentrations of TGF-β1 in a micro-scale compliant-anchored system and measured cell-generated isometric force, stimulated contraction and residual matrix tension. We also investigated the combined effects of boundary stiffness and growth factors on the cells’ ability to accumulate collagen.
2. MATERIALS AND METHODS
2.1. Isolation of valvular interstitial cells
Pig hearts were obtained from a local slaughter house. Aortic VICs were isolated according to a reported protocol [45] within two hours of death. Briefly, aortic valve leaflets were removed from the aortic root, and washed with cold sterile Dulbecco’s phosphate buffered saline (DPBS, Cellgro, Manassas, VA). Leaflets were submerged in cold collagenase solution made up of 600 U/mL solution of Type II collagenase (Worthington Biochemical, Lakewood, NJ) in Dulbecco’s Modified Eagle’s Medium (DMEM, Life Technologies, Grand Island, New York) with 1% penicillin/ streptomycin/ ampothericin B (PSA, Life Technologies, Grand Island, New York) and 10% fetal bovine serum (FBS, Life Technologies, Grand Island, New York). Valvular endothelial cells were removed by rubbing the leaflet surfaces using sterile cotton swabs, and the valve leaflets were washed with cold collagenase solution once more and incubated in collagenase solution at 37°C overnight. After enzymatic digestion of the leaflets, cells were plated on tissue culture flasks in DMEM with 1% PSA and 10% FBS. VICs at passage 5–8 were used for all experiments.
2.2. Micro-tissue gauges
To modulate and measure the forces applied by cells in a 3D environment, sub-millimeter-sized VIC-populated fibrin gels were cultured suspended between flexible posts. To create the posts and micro-culture wells, the micro-tissue gauge (μTUG) system was used as described by Legant et al. [46] (molds generously provided by Prof. Chris Chen, University of Pennsylvania). This system is composed of an array of micro-wells (dimensions of each well: 800 μm × 400 μm × 250 μm) containing two flexible cantilevered posts made of poly(dimethylsiloxane) (PDMS, Dow Corning, Midland, MI) (Figure 1). Cells were suspended in a biopolymer gel solution within the micro-wells. As cells compacted the gel, 3D constructs were formed between the flexible posts (Figure 1C), and the total force applied by the cells was calculated using post deflections and beam bending equations (Supplementary Materials Eqn. 1). Force-per-cell values were calculated by dividing the force exerted on one post by the average number of representative volume elements (RVEs) spanning a cross-sectional area in the middle of micro-tissues [2]. An RVE contains one cell and surrounding volume of ECM (see Supplementary Materials for detailed description of RVEs). The cell number was determined by counting the nuclei in the central region which are stained with Hoechst (Figure 1D).
Figure 1.
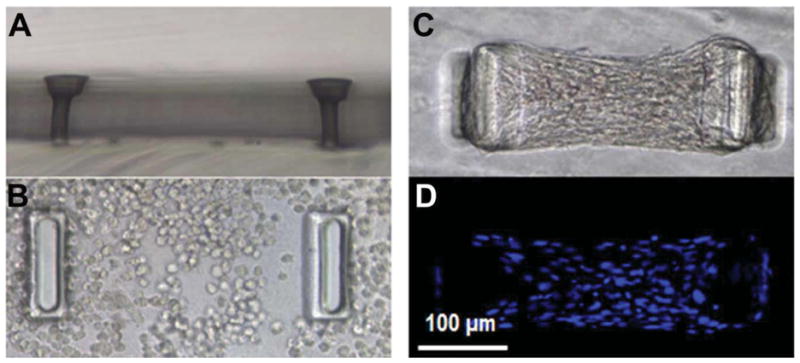
A) micro-tissue gauge (μTUG) well shown A) from the side without cells and gel, B) from the top filled with a cell-populated gel at t=1 hours and C & D) at t=72 hours. In (D) the nuclei are stained by Hoechst to facilitate cell counting utilizing fluorescent microscopy. All panels imaged at 20X.
To obtain different boundary stiffness values, the PDMS post elastic modulus was modulated by the monomer-to-curing agent ratios and heat treatments (Supplementary Materials, Table 1). PDMS moduli were measured by uniaxial tensile testing (EP1000, Instron, Norwood, MA).
2.3. Fabrication of VIC-seeded fibrin gel micro-tissues
Empty μTUG wells were treated with 1% Pluronic F-127 (Invitrogen, Eugene, Oregon) for 8 minutes to create a hydrophobic surface and prevent cell attachment to the PDMS. VICs (300,000 cells/mL final concentration) were re-suspended in a fibrin gel solution consisting of fibrinogen from bovine plasma (Sigma, St. Louis, MO, 3.25 mg/mL final concentration) and 1 U/mL thrombin (Sigma, St. Louis, MO), and this suspension was centrifuged into the μTUGs. After centrifugation, excess gel solution was aspirated and the micro-tissues were incubated at 37°C for 1 hour to allow for polymerization of the fibrinogen. Following the polymerization period, chemically defined media with different growth factor concentrations were added into μTUG dishes. A defined medium previously reported by our group [47] was selected over serum-containing media to avoid masking the effects of exogenous growth factors while allowing robust cell contraction and matrix production. The defined medium consists of a 3:1 ratio of DMEM (high glucose; 4.5 g/L) and Ham’s F12 with the addition of 5 μg/ml insulin, 5 ng/ml selenious acid, 10−4 M ethanolamine, 250 μg/ml L-ascorbic acid phosphate magnesium salt n-hydrate, 2×10−10 M L-3,3′,5-triiodothyronine, 4×10−3 M of Glutamax™ (Life Technologies, Grand Island, New York), and 1% penicillin/ streptomycin/ amphotericin B. To prevent premature fibrin degradation, 20 μg/mL Aprotinin (Sigma, St. Louis, MO) was added to the media. The lowest PDMS stiffness utilized was 600 kPa since softer posts collapse, and the highest stiffness value was kept under 5000 kPa to allow visually detectable deflection of the posts. TGF-β1 concentrations were chosen between zero and 5 ng/mL, a concentration range reported in tissue engineering applications [31, 33, 48]. Epidermal growth factor (EGF, Sigma, St. Louis, MO) supplementation was utilized as an alternative supplement to stimulate matrix production without myofibroblast activation [26, 49, 50]. We previously demonstrated that the concentration of EGF chosen for this study (5 ng/mL) stimulates maximal ECM production from dermal fibroblasts [49, 50].
2.4. VIC-generated force measurements
VIC-populated gels were cultured under four different post stiffness levels (0.15, 0.33, 0.56 and 1.05 nN/nm) and with four different TGF-β1 concentrations (zero, 0.05, 0.5 and 5 ng/mL). Homeostatic tissue tension was measured every 24 hours for seven days by monitoring the post deflections as previously described [46]. In a separate set of experiments, micro-tissues were cultured with two levels of post stiffness (0.15 and 1.05 nN/nm) and two levels of TGF-β1 (zero and 5 ng/mL) for four days. On day 4, micro-tissues were stimulated with 90 mM potassium chloride (KCl) for 10 minutes to measure the maximal cell contraction. KCl depolarizes the cell membrane and activates contraction in muscle or muscle-like cells [51, 52]. Following the stimulated contraction measurements, the micro-tissues were treated with 6 mM Cytochalasin-D (Cyto-D) for two hours to inhibit F-actin polymerization and eliminate active cell tension to determine the residual matrix tension. The residual tension is a functional metric for cell-mediated matrix remodeling [53, 54]. In the present study we defined stimulated contraction as the increase in force-per-cell above the homeostatic tension in response to KCl (FSTIMULATED). The passive and active components of homeostatic tissue tension were defined as active-isometric cell force (FCELL) and residual tension. Active-isometric cell force is equal to the difference between the homeostatic tissue tension at a certain remodeling time (FSTATIC) and residual tension (FRESIDUAL); i.e., FCELL = FSTATIC − FRESIDUAL.
In an additional set of experiments aimed at comparing the effects of TGF-β1 and EGF on cell-generated forces and collagen synthesis, micro-tissues were cultured with either soft (k = 0.15 nN/nm) or stiff (k = 1.05 nN/nm) posts. TGF-β1+ groups were supplemented with 5 ng/mL TGF-β1, and EGF+ groups were supplemented with 5 ng/mL EGF. Homeostatic tissue tension was measured on day 4.
2.5. Inhibition of contraction by Blebbistatin
In one set of experiments, cell-generated forces were inhibited by adding 5 μM Blebbistatin (Myosin II inhibitor, Sigma, St Louis, MO) to the culture media on the second day of culture. Micro-tissues were then cultured with either 0 or 5 ng/mL TGF-β1 and either 0 or 5 μM Blebbistatin for five days. De novo collagen accumulation was assessed as described below.
2.7. Immunofluorescent staining
Micro-tissues were fixed in 4% paraformaldehyde for 15 minutes and permeabilized with 0.25% Triton X-100 (Sigma) for 20 minutes. To visualize α-SMA or collagen, tissues were blocked with 1.5% normal goat serum in PBS for 30 min, and incubated with primary anti-α-SMA (Sigma, St. Louis, MO) or anti-collagen (Abcam, Cambridge, MA) antibody, respectively, for one hour. Fluorescently labeled secondary antibody (Alexa-546, Invitrogen, Carlsbad, CA) was then applied and imaged with Leica SP5 point scanning confocal/DMI6000 inverted microscope. In a subset of experiments, the de novo collagen accumulation was semi-quantitatively measured by calculating the mean intensity in region of interests (ROI) in the central region of micro-tissue with equal area using the Leica Application Suit software. The mean intensity was divided by the total number of cells in the ROI to calculate collagen intensity per cell. F-actin was labeled by Phalloidin (Alexa-488, Life Technologies, Grand Island, New York).
2.8. Statistical analysis
All values are reported as mean ± standard deviation. Statistical comparisons were made using two-way analysis of variance (ANOVA) with p < 0.05 considered significant. When a significant difference was found, experimental groups were compared with Tukey’s HSD (Honestly Significant Difference) post-hoc test (Sigmaplot 11.0, Systat Software).
The entire force data set from the 16-group stiffness/TGF-β1 experiment was fit with a 2-factor interaction model to quantitatively describe the effects of stiffness and TGF-β1 concentration (and their interactions) on the force generated per cell (Eqn. 1). A log transformation was applied to the TGF-β1 concentration levels normalized to the maximum concentration level (5 ng/mL). Design of experiment (DOE) software was utilized to design the study and to model and plot the data (Design-Expert 8.0.7.1, Stat-Ease, Inc., MN). The model with the minimum number of parameters which fit the data with lowest p-value and highest R2 value was determined by the analysis to be:
| (1) |
where F is force per cell (nN), [TGFβ] is TGF-β1 concentration (ng/mL), k is post stiffness (nN/nm), and c1..c4 are model parameters (fitting constants).
3. RESULTS
3.1 Micro-tissue generation
VICs compact the fibrin gels and form dense dog bone-shaped micro-tissues between the cantilevered posts within 20–30 hours (Figure 1C). Due to compositional differences in our micro-tissues from those in previous studies [43, 46, 55] (pure fibrin, VICs, and chemically-defined media (without serum)), the μTUG system methodology was substantially modified. For direct comparisons between the groups, the seeding conditions were kept constant despite wide ranges of stiffness and growth factors; this constraint lead to large differences in force and ECM generation between the treatment groups and, consequently, the rate of success in forming tissues was lower than previously reported. The success rate of micro-tissues lasting more than four days was as low as 10% in groups with high TGF-β1 concentration and 20–30% in other groups.
3.2 Tension generation by VICs within micro-tissues
Posts are visibly deflected by the tension within the tissue during gel compaction as a function of both TGF-β1 concentration and post stiffness. Under soft boundaries, force-per-cell values reach a maximum after 24–36 hours then start to decrease slightly on the 2nd day without TGF-β1 (Figure 2). Under stiff boundaries with less than 0.5 ng/mL TGF-β1, tension decreases after 24 hours, but with TGF-β1 concentrations of 0.5 and 5 ng/mL, tension increases dramatically and keeps increasing until the end of the experiment at seven days. Under the stiffest boundary (k=1.05 nN/nm), 5 ng/mL TGF-β1 increases force per cell 8.6 fold compared to the no-TGF-β1 group under the same boundary stiffness. Boundary stiffness has no significant effect on cell forces in the absence of TGF-β1 (Figure 3, please see Supplementary Material for ANOVA results). Similarly, TGF-β1 does not alter cell-generated forces significantly under soft boundaries (k < 0.33 mN/mm, Figure 3).
Figure 2.
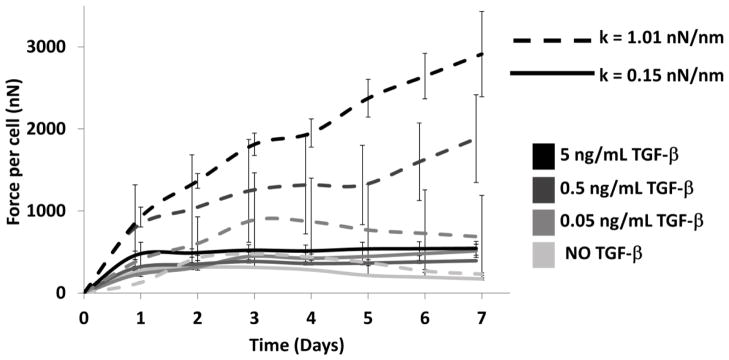
Average force-per-cell values over seven days of culture (n=8–12 per group). Under soft boundary homeostatic tissue tension reaches a plateau after second day at any TGF-β1 concentration. Under stiff boundary, tension increases day by day in a dose-dependent manner. See Supplementary Material for the results of the ANOVA.
Figure 3.
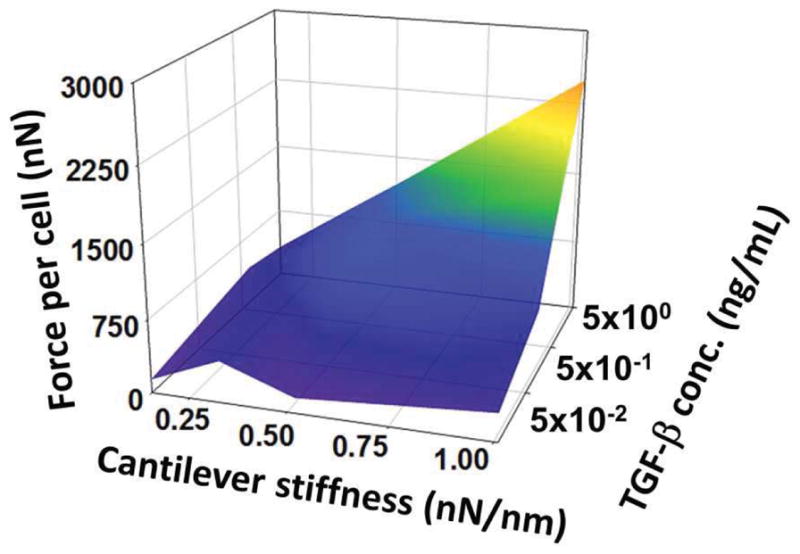
Response surface of force-per-cell values on 7th day for all groups (n=8–12 per group). Under the softest boundary (k = 0.15 nN/nm), increasing TGF-β1 concentration does not significantly affect homeostatic tension. Similarly, in the absence of TGF-β1, increasing boundary stiffness does not alter force-per-cell values. At higher levels, these factors interacted synergistically.
3.3. TGF-β1 and stiffness interaction model
The optimal form of the regression model determined utilizing DOE software fit the complete stiffness-TGF-β1-force data set with high correlation with only four parameters. The statistical analysis indicates that each parameter has significant impact with little interaction between parameters (Table 1). Strong interaction between TGF-β1 and boundary stiffness is quantitatively demonstrated by the model (p < 0.0001), and the interaction term provided substantially higher correlation with the data (r2= 0.92 with the interaction term compared to r2= 0.69 without the interaction term). A surface plot of the data predicted by the model is provided in the Supplementary Materials.
TABLE 1.
Model parameters for the regression model (Eqn 1); ANOVA results indicate that each parameter has significant impact on the fit to the dataset.
| c1 | 2140 (nN) |
| c2 | 780 (nN) |
| c3 | 1660 (nm) |
| c4 | 710 (nm) |
| F value | P value | |
|---|---|---|
| Model | 48 | <0.0001 |
| k | 73 | 0.0001 |
| TGFβ1 | 64 | <0.0001 |
| Interaction | 36 | <0.0001 |
3.4. Active isometric cell force, stimulated contraction and residual tension
Active isometric cell force (FCELL), stimulated contraction by activation of VICs with 90 mM KCl (FSTIMULATED), and residual tension per cell in the matrix after Cyto-D treatment (FRESIDUAL) are shown in Figure 4. The active isometric cell force is significantly higher under stiff boundaries with TGF-β1 than any other group. Stimulated contraction as a response to KCl, on the other hand, is significantly lower in TGF-β1+ groups, either under soft or stiff boundaries, and it is the highest under stiff boundary without TGF-β1. Increasing boundary stiffness increases FSTIMULATED significantly without TGF-β1 treatment. Videos showing response to stimulation and inhibition with and without TGF-β1 are provided in the Supplementary Materials, Movie S1 and Movie S2, respectively).
Figure 4.
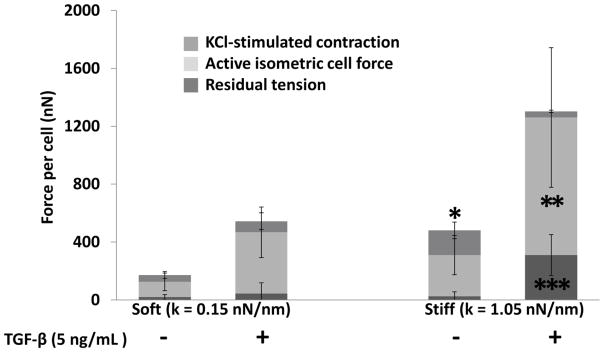
Active isometric cell force, KCl-stimulated contraction, and residual tension in micro-tissues cultured against soft (0.15 nN/nm) or stiff (1.05 nN/m) posts with or without TGF-β1 (5 ng/mL) after four days of culture (n ≥ 3). Against the stiff boundary with TGF-β1, cells are in a state of high static contraction and produce negligible additional force in response to KCl stimulation but generate large residual tension in the matrix compared to other groups. Cells cultured against stiff boundaries without TGF-β1 provide a stronger response to KCl than the other groups. Two-way ANOVA p<0.05: *: significantly higher KCl-stimulated contraction from all other groups, **: significantly higher active isometric cell force from all other groups. ***: Significantly higher residual tension from all other groups.
3.5. α-SMA expression
TGF-β1 and boundary stiffness do not alter the total amount of staining or brightness of α-SMA staining. However, the distribution is dramatically different between TGF-β1- and TGF-β1+ groups. In TGF-β1+ groups, α-SMA is incorporated in stress fibers, while in TGF-β1+ groups α-SMA staining is punctate and in a short rod-like shape (Figure 5).
Figure 5.
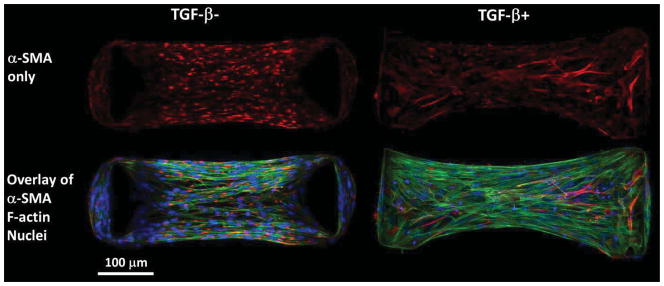
In fibrin gels cultured between stiff posts in chemically defined media for four days, α-SMA staining is punctate and not localized on stress fibers in the absence of TGF-β1 (left), whereas, α-SMA staining is bright and co-localized with stress fibers in the presence of 5 ng/ml TGF-β1 (right) indicating the myofibroblast phenotype.
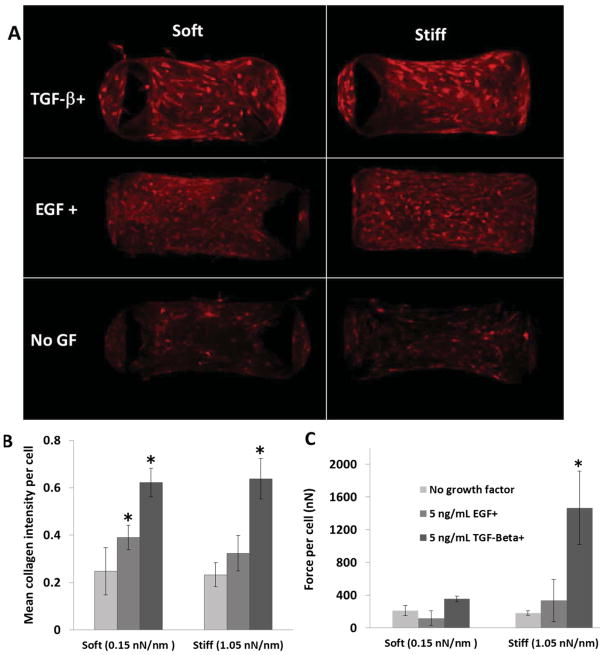
3.6. Effect of tension on collagen accumulation stimulated by TGF-β1 and EGF
Figure 6A shows representative images of collagen staining in micro-tissues. TGF-β1 increases the mean collagen intensity per cell significantly for both soft and stiff boundaries. EGF causes a significant increase in mean collagen intensity only for the soft boundary (Figure 6B). TGF-β1 increases force-per-cell in the case of stiff boundaries, but not soft boundaries; whereas EGF does not significantly affect force-per-cell values at any concentration tested regardless of boundary stiffness (Figure 6C). When cell-generated forces are inhibited with 5 μM Blebbistatin, TGF-β1 still increases total de novo collagen intensity in micro-tissues; however, enhancement of collagen production with TGF-β1 is attenuated (Figure 7A).
Figure 6.
A) De novo collagen stained in micro-tissues after five days in culture against soft (0.15 nN/nm) and stiff (1.05 nN/nm) boundaries. B) TGF-β1 increases collagen production per cell equally under soft and stiff boundaries. EGF increases collagen intensity significantly only under soft boundary. Two-way ANOVA, p<0.05: *: Significantly higher than control groups (p<0.05) (n=3). C) Force-per-cell values after four days of culture. EGF does not alter isometric cell force significantly. *: Significantly higher force per cell than all other groups (n=4).
Figure 7.
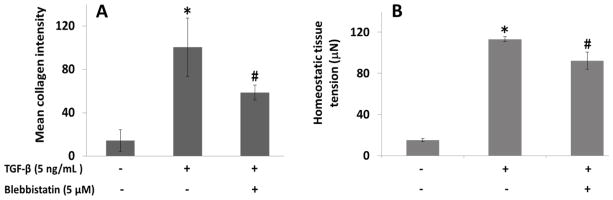
A) Mean collagen intensity in equal ROIs on day 6 and B) mean homeostatic tissue tension in micro-tissues cultured with either 0 or 5 ng/mL TGF-β1 and either 0 or 5 μM Blebbistatin against stiff boundaries (1.05 nN/nm). Enhancement of tension and collagen production with TGF-β1 are attenuated by Blebbistatin. One-way ANOVA, p<0.005: *: Significantly higher from all other groups, #: Significantly higher from control (no-TGF-β1/no-Blebbistatin) group.
4. DISCUSSION
This study represents the first quantitative characterization of the combined effects of stiffness and TGF-β1 on myofibroblast activation in a 3D tissue model. Our regression analysis indicates that TGF-β1 and boundary stiffness increase the homeostatic level of tension in VIC-populated fibrin gels both independently and synergistically. However, increasing only one of these factors with minimal levels of the other does not increase the cell-generated forces significantly. We also found that when cultured with a high concentration of TGF-β1 against stiff boundaries, VICs are in a state of nearly maximal contraction and impart significant residual tension on the matrix during remodeling. Finally, we observed that collagen production is stimulated by TGF-β1 treatment in a tension-dependent manner, yet collagen accumulation is significant even under low tension with TGF-β1 but not EGF supplementation. Taken together, the results indicate the possibility of generating collagen-rich engineered tissues under a low tension environment to produce TEHV leaflets with minimal retraction.
4.1. Interaction between TGF-β1 and stiffness
Although it is clear from the literature that both TGF-β1 and resistance to cell-generated tension (i.e., stiffness) of the 2D substrate or 3D matrix are necessary factors for myofibroblast activation [5, 15, 16], quantitative relationships between these parameters have not been developed. It is generally accepted that with increasing TGF-β1 concentration (4 levels), α-SMA protein levels increase in VICs cultured on rigid tissue culture plastic or glass [30, 56]. In our previous study utilizing compliant 2D substrates (15 different moduli spanning a large range), we observed a sigmoidal relationship between substrate modulus and α-SMA-positive stress fibers, and we also observed synergy with TGF-β1 (0 or 5 ng/mL) [17]. It appears that α-SMA-positive stress fibers are formed only over a threshold substrate modulus (5–9 kPa for VICs [17] and 20 kPa for dermal fibroblasts [22]). Further, Anseth et al. found that dynamically decreasing substrate modulus decreases the number of α-SMA positive VICs, which implies that myofibroblast differentiation is reversible under a threshold of stiffness [23]. In anchored collagen gels that are released from their rigid boundaries, TGF-β1 significantly increases both gel compaction rate and α-SMA expression in a dose dependent manner [24, 28, 30]. In contrast, in free floating gels (not cultured against rigid anchors then released) where high tension cannot be generated by the cells, α-SMA is not expressed. Combined these findings clearly demonstrate an interaction between stiffness and TGF-β1 in terms of myofibroblast activation, yet it is not possible to identify the functional relationship between these factors since only one stimulus was tested at multiple levels in each study and cell forces have not been directly measured.
High force generation is a powerful functional measure of the myofibroblast phenotype [18]. Traction force microscopy studies demonstrate that increasing stiffness increases traction forces applied to the substrate by fibroblasts [20–22]. Hinz and colleagues report that a minimum force per focal adhesion (125 nN) is required for recruitment of α-SMA into stress fibers [22], implying a minimum stiffness for myofibroblast differentiation. Tschumperlin and colleagues [21] found that TGF-β1 does not affect lung fibroblast-generated forces when substrate modulus is less than 13 kPa, while it causes a dramatic stiffness-dependent increase over this stiffness. Here we observed a similar trend in cell-generated forces in our 3D culture system in that TGF-β1 has no significant effect under soft boundaries (k < 0.33 N/m) but has a strong dose-dependent effect for higher stiffness posts. For the first time, by using graded levels of both stiffness and TGF-β1 simultaneously, we obtained complete force response surface as a function of these factors in 3D which can be used to determine scaffold and culture parameters to obtain a desired level of tension.
4.2 Effect of stiffness in the absence of exogenous TGF-β1
Previous studies utilizing the micro-tissue system report increases in the forces generated by fibroblasts [42, 46] and cardiomyocytes [43] with increasing post stiffness in the absence of exogenous TGF-β1. In contrast, our findings indicate that stiffness only has a strong dose-dependent effect at concentrations of TGF-β1 higher than 0.05 ng/mL. Further, in the absence of TGF-β1, the α-SMA distribution is diffuse in the cytoplasm of ours cells, even under the stiffest boundary where the force per cell is approximately 300 nN. The sensitivity to stiffness observed in previous investigations without explicit growth factor supplementation may be due to the presence of growth factors in serum [57]. In this study, we utilize chemically defined medium to avoid growth factors contained in serum without “starving” the cells of other essential components in serum.
4.3 VIC-generated forces and micro-tissue failure
The force-per-cell values obtained in the present study (130–2500 nN) are significantly higher than values reported in previous studies using μTUG system (15–25 nN for per 3T3 fibroblast) [46, 55] and culture force monitors (50–200 nN per fibroblast) [20]. This discrepancy is mainly due to differences in how the force-per-cell is calculated. In previous studies, the force per cell is generally determined by dividing the force by the total number of cells in the gel (i.e., assuming all cells act in parallel), which is an oversimplification. Alternatively, as we have previously described [2, 42] we divide the total tension applied to one post by the average number of representative volume elements (RVEs) parallel to each other in the central portion of the tissue which assumes that the representative number of cells in a given cross-section of the tissue act in parallel [2] (Figure S2, see Supplementary Materials for details). When calculated using the standard method of dividing the force by the total number of cells in the gel, the force-per-cell values in the present study range from 19–422 nN. These values similar to previously reported, but are slightly higher possibly due to the contractility of the VIC cell type, a soluble factor in the chemically defined medium (especially the high concentration of TGF-β1), and/or our use of higher boundary stiffness than previous μTUG systems (but lower than the isometric CFM systems).
The high force per cell relative to the amount of ECM production by the cells was one of the main reasons for tissue failures in the device, especially with high post stiffness and TGF-β1 concentration. Many tissues slipped of the soft posts under high tension conditions (Supplementary Materials Figure S3A), and tissue thinning due to fibrinolysis and/or low ECM production in the absence of growth factors lead to ripping of the tissues (Figure S3B). To prevent micro-tissues from slipping off of the posts, the cell density was decreased to reduce tension, and in turn, post deflection; however, lower cell numbers limit the maximum stiffness of posts that can be utilized. To minimize tissue thinning and breaking, aminocaproic acid was added to culture media, but it did not prevent tissue breaking. Adding aprotinin decreased tissue breaking significantly. Tissues also stuck to the PDMS walls due to the adhesive nature of fibrin and lead to failure (Figure S3C). Increasing Pluronic concentration was utilized to prevent fibrin from sticking to the PDMS, but too high concentration led to tissues popping out from the wells. Due to this limitation, Pluronic concentration was kept under 2% for all groups.
4.4. Effect of TGF-β1 on active isometric and KCl-stimulated cell force
To separate the cells’ ability to generate and hold isometric force against the posts from their ability to actively contract in response to an agonist (i.e., contractility), we stimulated the cells with KCl following four days of culture. One of the most striking findings of this analysis is that the cells cultured against stiff posts with TGF-β1, identified as myofibroblasts by their α-SMA-positive stress fibers (Figure 5), are the least contractile of all groups (3.3 % increase in force over homeostatic tension with KCl stimulation, Figure 4). In the absence of TGF-β1, the cells generated lower total forces against stiff posts than with the growth factor but were significantly more contractile than all other groups (54 % increase in force over homeostatic tension with KCl stimulation), even greater than TGF-β1-treated cells cultured against soft posts. Recently, it was reported that cyclic distension of fibroblast-populated fibrin constructs does not alter the effect of TGF-β1 on KCl-stimulated contraction or number of α-SMA positive cells [39]. We speculate that cells are already able generate substantial tension against stiff boundaries and differentiate into myofibroblasts, thus additional mechanical conditioning has little additional effect on their contractile ability. Grinnell and colleagues [28] have also found that, in anchored fibroblast-populated collagen gels, cells pretreated with TGF-β1 for five days appear to be unable to contract in response to agonists (lysophosphatidic acid (LPA) or additional TGF-β1) when released from the rigid boundary. Taken together, myofibroblasts appear to exist in a nearly maximally contracted state - they apply high isometric force to the matrix yet have little additional ability to contract in response to external stimulation.
4.5 Combined effect of TGF-β1 and boundary stiffness on residual tension
The other striking result from our analysis of the components of the total force applied to the posts is that the residual tension in the matrix is much higher under stiff boundaries with TGF-β1 than other groups (7–15 fold over other groups). This result indicates that myofibroblasts remodel the tissue much more extensively than non-activated fibroblasts. The effect of residual tension on tissue retraction has been investigated in previous studies utilizing fibroblast-populated protein gels [50] and polymer scaffolds [4] by eliminating active cell contraction with Cyto-D or Rho-associated protein kinase (ROCK) inhibitors prior to release of anchored matrices. When active cell force is inhibited, total retraction upon release is decreased by 75% in fibrin gels [50], 80% in collagen gels [58] and 40% in PGA/P4HB scaffolds [4]. To decrease retraction of fibrin-based TE heart valve leaflets, Tranquillo and colleagues treated the engineered tissues with Blebbistatin (Myosin II inhibitor) 2–4 days prior to implantation at the end of a 3-week static culture period [3]. The treatment provided a transient retraction and the valves were functional initially; however, after four weeks in vivo regurgitation due to tissue shrinkage caused valve failure. More recently, the cells have been removed from TEHVs altogether in an attempt to eliminate retraction, and decellularized TEHVs have been repopulated with non-contractile cells [59, 60], but these valves also retracted after in vivo remodeling [60].
Taken together, these results imply that cell-generated forces must be reduced throughout the remodeling period to eliminate residual tension (and thus passive retraction) rather than simply blocking cell contractile activity immediately prior to implant. Yet it remains unclear whether robust TE constructs rich in organized collagen can be created under low tension since tension is needed to stabilize collagen against enzymatic degradation [61, 62] and the effect of tension on collagen production of VICs is not known. To explore the feasibility of generating collagenous tissues under low tension, we investigated the effects of boundary stiffness and growth factors on collagen accumulation in our fibrin gel micro-tissues.
4.6. Combined effect of TGF-β1 and tension on collagen accumulation
Our data indicate that boundary stiffness alone, in the absence of growth factors (and serum), does not significantly affect collagen accumulation (Figure 6A, B). This result parallels the lack of difference in homeostatic tension between stiffness groups in the absence of TGF-β1 (Figure 6 C). These data are consistent with previous studies which show that cyclic stretching alone, in the absence of serum or growth factors, does not affect procollagen synthesis in fibroblasts [36]. With the addition of 5 ng/mL TGF-β1, however, we observed a similar (approximately 2.5-fold) increase in collagen staining under both stiff and soft boundaries even though the growth factor does not stimulate a significant increase in tension against soft posts. This lack of mechanical effect is in contrast to studies which show that dynamic stretch enhances the stimulation of procollagen synthesis by serum or TGF-β1 [36, 63]. When we decreased the cell-generated tension with a low concentration of Blebbistatin, as an alternative to utilizing low boundary stiffness, the augmentation of collagen production with TGF-β1 was significantly attenuated. Taken together, these findings indicate that both mechanical forces and growth factors are necessary for stimulation of collagen production, but there may be particular combinations (e.g., TGF-β1 stimulation under low mechanical loading) which stimulate sufficient collagen accumulation without inducing unwanted tension in the tissue. Although our results are promising, our collagen accumulation measurements were based on optical evaluations after only five days in culture, an early stage for tissue remodeling. The effects of cell generated forces on ECM synthesis and de novo tissue integrity must be elucidated in long-term studies.
EGF also increases collagen accumulation under our experimental conditions, but the increase is significantly less than for TGF-β1 supplementation. Surprisingly, EGF has less of an effect on collagen accumulation for cells cultured against stiff posts (1.4-fold) than soft posts (1.6-fold). Previously, we showed that EGF increases collagen accumulation in fibroblast-populated fibrin gels cultured against rigid boundaries to the same extent as an equivalent concentration of TGF-β1 (5 ng/mL) [49, 50]. In the aforementioned study, the EGF-treated samples retracted significantly less than both TGF-treated and serum-fed control samples suggesting lower cell-generated forces in the EGF group. However, in the retraction assay, the isometric force is not measured prior to release and the total retraction is affected by residual matrix stress, stiffness of matrix, and cell contractility. Data from the current study indicate that EGF likely stimulated lower cell-generated force than TGF-β1 in the rigidly anchored fibrin gels. Assuming that the tension was lower throughout the culture duration, it is unclear why collagen accumulation was equivalent with EGF and TGF-β1 supplementation in the previous study whereas it is much lower in this study. It is possible that the balance between collagen production and degradation changes in its tension dependency over long-term culture (21 days in the previous study compared to five days in this study).
Overall, when comparing the force and collagen per cell, it is clear that the level of tension in the gel modulates collagen accumulation, but that these parameters can be uncoupled with the use of different growth factors. For example, the average force per cell in the EGF/stiff group is approximately the same as for the TGF-β1/soft group (Figures 6B, C), yet the collagen accumulation is dramatically lower for the EFG/stiff group.
5. Conclusions
In the present study, we quantified the interaction between the boundary stiffness and TGF-β1 on VIC-generated forces in a 3D tissue model. We showed that higher active isometric-cell force leads to larger residual tension in the tissues, which implies that to prevent built-up of large residual tension, cell contractility must be regulated through entire culture period. We also observed that collagen accumulation per cell can be stimulated in similar rates under significantly different tension levels. The ability to increase collagen production under low tension is promising for TE applications; since increasing ECM production without inducing myofibroblast activation will likely decrease retraction. Obtaining robust tissues without excessive tissue tension might be possible by augmenting ECM synthesis by growth factors together with regulation of cell-generated forces by drugs. More research is needed to determine optimum tension levels in the tissue, suitable drugs to regulate cell-generated forces and collagen degradation through entire culture period, and optimum growth factor concentrations. High throughput experiments by using micro-tissue systems can be used to determine these optimum conditions, and, these conditions can be tried for organ-size constructs.
Supplementary Material
Acknowledgments
The authors gratefully thank the Chen Lab (University of Pennsylvania) for the use of the micro-TUG system. The authors also thank Thomas Boudou, Michael Borochin, Adrian West and Mahmut Selman Sakar for sharing their experience with micro-TUG system and acknowledge partial financial support from National Institutes of Health (1R15HL087257-01A2) and U.S. Army Medical Research and Materiel Command (USAMRC, Grant W81XWH-11-1-0631).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pedersen JA, Swartz MA. Mechanobiology in the third dimension. Ann Biomed Eng. 2005;33:1469–90. doi: 10.1007/s10439-005-8159-4. [DOI] [PubMed] [Google Scholar]
- 2.Kural MH, Billiar KL. Regulating tension in three-dimensional culture environments. Exp Cell Res. doi: 10.1016/j.yexcr.2013.06.019. http://dx.doi.org/10.1016/j.yexcr.2013.06.019. [DOI] [PMC free article] [PubMed]
- 3.Syedain ZH, Lahti MT, Johnson SL, Robinson PS, Ruth GR, Bianco RW, et al. Implantation of a tissue-engineered heart valve from human fibroblasts exhibiting short term function in the sheep pulmonary artery. Cardiovasc Eng Technol. 2011;2:101–12. [Google Scholar]
- 4.van Vlimmeren MA, Driessen-Mol A, Oomens CW, Baaijens FP. Passive and active contributions to generated force and retraction in heart valve tissue engineering. Biomech Model Mechanobiol. 2012;11:1015–27. doi: 10.1007/s10237-011-0370-7. [DOI] [PubMed] [Google Scholar]
- 5.Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3:349–63. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 6.Lundberg MS. Cardiovascular tissue engineering research support at the National Heart, Lung, and Blood Institute. Circ Res. 2013;112:1097–103. doi: 10.1161/CIRCRESAHA.112.300638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoenig MR, Campbell GR, Rolfe BE, Campbell JH. Tissue-engineered blood vessels: alternative to autologous grafts? Arteriocler Thromb Vacs Biol. 2005;25:1128–34. doi: 10.1161/01.ATV.0000158996.03867.72. [DOI] [PubMed] [Google Scholar]
- 8.Gauvin R, Larouche D, Marcoux H, Guignard R, Auger FA, Germain L. Minimal contraction for tissue-engineered skin substitutes when matured at the air-liquid interface. J Tissue Eng Regen Med. 2013;7:452–60. doi: 10.1002/term.543. [DOI] [PubMed] [Google Scholar]
- 9.Roy-Chaudhury P, Kelly BS, Miller MA, Reaves A, Armstrong J, Nanayakkara N, et al. Venous neointimal hyperplasia in polytetrafluoroethylene dialysis grafts. Kidney Int. 2001;59:2325–34. doi: 10.1046/j.1523-1755.2001.00750.x. [DOI] [PubMed] [Google Scholar]
- 10.van Vlimmeren MA, Driessen-Mol A, Oomens CW, Baaijens FP. An in vitro model system to quantify stress generation, compaction, and retraction in engineered heart valve tissue. Tissue Eng Part C Methods. 2011;17:983–91. doi: 10.1089/ten.TEC.2011.0070. [DOI] [PubMed] [Google Scholar]
- 11.Schmidt D, Dijkman PE, Driessen-Mol A, Stenger R, Mariani C, Puolakka A, et al. Minimally-invasive implantation of living tissue engineered heart valves: a comprehensive approach from autologous vascular cells to stem cells. J Am Coll Cardiol. 2010;56:510–20. doi: 10.1016/j.jacc.2010.04.024. [DOI] [PubMed] [Google Scholar]
- 12.Rabkin E, Aikawa M, Stone JR, Fukumoto Y, Libby P, Schoen FJ. Activated interstitial myofibroblasts express catabolic enzymes and mediate matrix remodeling in myxomatous heart valves. Circulation. 2001;104:2525–32. doi: 10.1161/hc4601.099489. [DOI] [PubMed] [Google Scholar]
- 13.Hoerstrup SP, Kadner A, Melnitchouk S, Trojan A, Eid K, Tracy J, et al. Tissue engineering of functional trileaflet heart valves from human marrow stromal cells. Circulation. 2002;106:I143–50. [PubMed] [Google Scholar]
- 14.Schoen FJ. Evolving concepts of cardiac valve dynamics: the continuum of development, functional structure, pathobiology, and tissue engineering. Circulation. 2008;118:1864–80. doi: 10.1161/CIRCULATIONAHA.108.805911. [DOI] [PubMed] [Google Scholar]
- 15.Hinz B. Formation and function of the myofibroblast during tissue repair. J Invest Dermatol. 2007;127:526–37. doi: 10.1038/sj.jid.5700613. [DOI] [PubMed] [Google Scholar]
- 16.Hinz B, Mastrangelo D, Iselin CE, Chaponnier C, Gabbiani G. Mechanical tension controls granulation tissue contractile activity and myofibroblast differentiation. Am J Pathol. 2001;159:1009–20. doi: 10.1016/S0002-9440(10)61776-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quinlan AM, Billiar KL. Investigating the role of substrate stiffness in the persistence of valvular interstitial cell activation. J Biomed Mater Res A. 2012;100:2474–82. doi: 10.1002/jbm.a.34162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hinz B, Celetta G, Tomasek JJ, Gabbiani G, Chaponnier C. Alpha-smooth muscle actin expression upregulates fibroblast contractile activity. Mol Biol Cell. 2001;12:2730–41. doi: 10.1091/mbc.12.9.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen J, Li H, SundarRaj N, Wang JH. Alpha-smooth muscle actin expression enhances cell traction force. Cell Motil Cytoskeleton. 2007;64:248–57. doi: 10.1002/cm.20178. [DOI] [PubMed] [Google Scholar]
- 20.Han SJ, Bielawski KS, Ting LH, Rodriguez ML, Sniadecki NJ. Decoupling substrate stiffness, spread area, and micropost density: a close spatial relationship between traction forces and focal adhesions. Biophys J. 2012;103:640–8. doi: 10.1016/j.bpj.2012.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marinkovic A, Mih JD, Park JA, Liu F, Tschumperlin DJ. Improved throughput traction microscopy reveals pivotal role for matrix stiffness in fibroblast contractility and TGF-beta responsiveness. Am J Physiol Lung Cell Mol Physiol. 2012;303:L169–80. doi: 10.1152/ajplung.00108.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goffin JM, Pittet P, Csucs G, Lussi JW, Meister JJ, Hinz B. Focal adhesion size controls tension-dependent recruitment of alpha-smooth muscle actin to stress fibers. J Cell Biol. 2006;172:259–68. doi: 10.1083/jcb.200506179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kloxin AM, Benton JA, Anseth KS. In situ elasticity modulation with dynamic substrates to direct cell phenotype. Biomaterials. 2010;31:1–8. doi: 10.1016/j.biomaterials.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arora PD, Narani N, McCulloch CA. The compliance of collagen gels regulates transforming growth factor-beta induction of alpha-smooth muscle actin in fibroblasts. The American journal of pathology. 1999;154:871–82. doi: 10.1016/s0002-9440(10)65334-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vaughan MB, Howard EW, Tomasek JJ. Transforming growth factor-beta1 promotes the morphological and functional differentiation of the myofibroblast. Exp Cell Res. 2000;257:180–9. doi: 10.1006/excr.2000.4869. [DOI] [PubMed] [Google Scholar]
- 26.Inoue M, Ono I, Tateshita T, Kuroyanagi Y, Shioya N. Effect of a collagen matrix containing epidermal growth factor on wound contraction. Wound Repair Regen. 1998;6:213–22. doi: 10.1046/j.1524-475x.1998.60307.x. [DOI] [PubMed] [Google Scholar]
- 27.Murray MM, Rice K, Wright RJ, Spector M. The effect of selected growth factors on human anterior cruciate ligament cell interactions with a three-dimensional collagen-GAG scaffold. J Orthop Res. 2003;21:238–44. doi: 10.1016/S0736-0266(02)00142-0. [DOI] [PubMed] [Google Scholar]
- 28.Grinnell F, Ho CH. Transforming growth factor beta stimulates fibroblast-collagen matrix contraction by different mechanisms in mechanically loaded and unloaded matrices. Exp Cell Res. 2002;273:248–55. doi: 10.1006/excr.2001.5445. [DOI] [PubMed] [Google Scholar]
- 29.Tuan T, Song A, Chang A, Younai S, Nimni ME. In vitro fibroplasia: Matrix contraction, cell growth, and collagen production of fibroblasts cultured in fibrin gels. Exp Cell Res. 1996;223:127–34. doi: 10.1006/excr.1996.0065. [DOI] [PubMed] [Google Scholar]
- 30.Walker GA, Masters KS, Shah DN, Anseth KS, Leinwand LA. Valvular myofibroblast activation by transforming growth factor-beta: implications for pathological extracellular matrix remodeling in heart valve disease. Circ Res. 2004;95:253–60. doi: 10.1161/01.RES.0000136520.07995.aa. [DOI] [PubMed] [Google Scholar]
- 31.Yao L, Swartz DD, Gugino SF, Russell JA, Andreadis ST. Fibrin-based tissue-engineered blood vessels: differential effects of biomaterial and culture parameters on mechanical strength and vascular reactivity. Tissue Eng. 2005;11:991–1003. doi: 10.1089/ten.2005.11.991. [DOI] [PubMed] [Google Scholar]
- 32.Roberts AB, Sporn MB, Assoian RK, Smith JM, Roche NS, Wakefield LM, et al. Transforming growth factor type beta: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. PNAS. 1986;83:4167–71. doi: 10.1073/pnas.83.12.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Neidert MR, Lee ES, Oegema TR, Tranquillo RT. Enhanced fibrin remodeling in vitro with TGF-beta1, insulin and plasmin for improved tissue-equivalents. Biomaterials. 2002;23:3717–31. doi: 10.1016/s0142-9612(02)00106-0. [DOI] [PubMed] [Google Scholar]
- 34.Grassl ED, Oegema TR, Tranquillo RT. A fibrin-based arterial media equivalent. J Biomed Mater Res A. 2003;66:550–61. doi: 10.1002/jbm.a.10589. [DOI] [PubMed] [Google Scholar]
- 35.Breen EC. Mechanical strain increases type I collagen expression in pulmonary fibroblasts in vitro. J Appl Physiol. 2000;88:203–9. doi: 10.1152/jappl.2000.88.1.203. [DOI] [PubMed] [Google Scholar]
- 36.Parsons M, Kessler E, Laurent GJ, Brown RA, Bishop JE. Mechanical load enhances procollagen processing in dermal fibroblasts by regulating levels of procollagen C-proteinase. Exp Cell Res. 1999;252:319–31. doi: 10.1006/excr.1999.4618. [DOI] [PubMed] [Google Scholar]
- 37.Yang G, Crawford RC, Wang JH. Proliferation and collagen production of human patellar tendon fibroblasts in response to cyclic uniaxial stretching in serum-free conditions. J Biomech. 2004;37:1543–50. doi: 10.1016/j.jbiomech.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 38.Hinz B, Gabbiani G, Chaponnier C. The NH2-terminal peptide of alpha-smooth muscle actin inhibits force generation by the myofibroblast in vitro and in vivo. J Cell Biol. 2002;157:657–63. doi: 10.1083/jcb.200201049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hall MS, Long R, Feng X, Huang Y, Hui C-Y, Wu M. Toward single cell traction microscopy within 3D collagen matrices. Exp Cell Res. doi: 10.1016/j.yexcr.2013.06.009. http://dx.doi.org/10.1016/j.yexcr.2013.06.009. [DOI] [PMC free article] [PubMed]
- 40.Delvoye P, Wiliquet P, Leveque JL, Nusgens BV, Lapiere CM. Measurement of mechanical forces generated by skin fibroblasts embedded in a three-dimensional collagen gel. J Invest Dermatol. 1991;97:898–902. doi: 10.1111/1523-1747.ep12491651. [DOI] [PubMed] [Google Scholar]
- 41.Kolodney MS, Wysolmerski RB. Isometric contraction by fibroblasts and endothelial cells in tissue culture: a quantitative study. J Cell Biol. 1992;117:73–82. doi: 10.1083/jcb.117.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.John J, Quinlan AT, Silvestri C, Billiar K. Boundary stiffness regulates fibroblast behavior in collagen gels. Ann Biomed Eng. 2010;38:658–73. doi: 10.1007/s10439-009-9856-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boudou T, Legant WR, Mu A, Borochin MA, Thavandiran N, Radisic M, et al. A microfabricated platform to measure and manipulate the mechanics of engineered cardiac microtissues. Tissue Eng Part A. 2012;18:910–9. doi: 10.1089/ten.tea.2011.0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Freyman TM, Yannas IV, Yokoo R, Gibson LJ. Fibroblast contraction of a collagen–GAG matrix. Biomaterials. 2001;22:2883–91. doi: 10.1016/s0142-9612(01)00034-5. [DOI] [PubMed] [Google Scholar]
- 45.Throm Quinlan AM, Sierad LN, Capulli AK, Firstenberg LE, Billiar KL. Combining dynamic stretch and tunable stiffness to probe cell mechanobiology in vitro. PLoS One. 2011;6:e23272. doi: 10.1371/journal.pone.0023272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Legant WR, Pathak A, Yang MT, Deshpande VS, McMeeking RM, Chen CS. Microfabricated tissue gauges to measure and manipulate forces from 3D microtissues. PNAS. 2009;106:10097–102. doi: 10.1073/pnas.0900174106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ahlfors JE, Billiar KL. Biomechanical and biochemical characteristics of a human fibroblast-produced and remodeled matrix. Biomaterials. 2007;28:2183–91. doi: 10.1016/j.biomaterials.2006.12.030. [DOI] [PubMed] [Google Scholar]
- 48.Tranquillo R, Isenberg B. Artificial Soft Tissue Fabrication from Cell-Contracted Biopolymers. In: Guilak F, Butler D, Goldstein S, Mooney D, editors. Functional Tissue Engineering. Springer; New York: 2003. pp. 305–17. [Google Scholar]
- 49.Throm AM, Liu WC, Lock CH, Billiar KL. Development of a cell-derived matrix: effects of epidermal growth factor in chemically defined culture. J Biomed Mater Res A. 2010;92:533–41. doi: 10.1002/jbm.a.32369. [DOI] [PubMed] [Google Scholar]
- 50.Grouf JL, Throm AM, Balestrini JL, Bush KA, Billiar KL. Differential effects of EGF and TGF-beta1 on fibroblast activity in fibrin-based tissue equivalents. Tissue Eng. 2007;13:799–807. doi: 10.1089/ten.2006.0206. [DOI] [PubMed] [Google Scholar]
- 51.Kershaw JD, Misfeld M, Sievers HH, Yacoub MH, Chester AH. Specific regional and directional contractile responses of aortic cusp tissue. J Heart Valve Dis. 2004;13:798–803. [PubMed] [Google Scholar]
- 52.Merryman DW, Shadow HS, Schoen FJ, Sacks MS. The effects of cellular contraction on aortic valve leaflet flexural stiffness. J Biomech. 2006;39:88–96. doi: 10.1016/j.jbiomech.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 53.Ingber DE, Prusty D, Sun Z, Betensky H, Wang N. Cell shape, cytoskeletal mechanics, and cell cycle control in angiogenesis. J Biomech. 1995;28:1471–84. doi: 10.1016/0021-9290(95)00095-x. [DOI] [PubMed] [Google Scholar]
- 54.Tamariz E, Grinnell F. Modulation of fibroblast morphology and adhesion during collagen matrix remodeling. Mol Biol Cell. 2002;13:3915–29. doi: 10.1091/mbc.E02-05-0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.West AR, Zaman N, Cole DJ, Walker MJ, Legant WR, Boudou T, et al. Development and characterization of a 3D multicell microtissue culture model of airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2013;304:L4–16. doi: 10.1152/ajplung.00168.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cushing MC, Liao J-T, Anseth KS. Activation of valvular interstitial cells is mediated by transforming growth factor-β1 interactions with matrix molecules. Matrix Biol. 2005;24:428–37. doi: 10.1016/j.matbio.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 57.Thermoscientific. Growth Factors in Cell Culture Serum. Retrieved 08.13.13, 2013, from http://www.thermoscientific.com/ecomm/servlet/newsdetail?storeId=11152&contentId=56974.
- 58.Tomasek JJ, Haaksma CJ, Eddy RJ, Vaughan MB. Fibroblast contraction occurs on release of tension in attached collagen lattices: Dependency on an organized actin cytoskeleton and serum. Anat Rec. 1992;232:359–68. doi: 10.1002/ar.1092320305. [DOI] [PubMed] [Google Scholar]
- 59.Dijkman PE, Driessen-Mol A, Frese L, Hoerstrup SP, Baaijens FPT. Decellularized homologous tissue-engineered heart valves as off-the-shelf alternatives to xeno- and homografts. Biomaterials. 2012;33:4545–54. doi: 10.1016/j.biomaterials.2012.03.015. [DOI] [PubMed] [Google Scholar]
- 60.Weber B, Dijkman PE, Scherman J, Sanders B, Emmert MY, Grunenfelder J, et al. Off-the-shelf human decellularized tissue-engineered heart valves in a non-human primate model. Biomaterials. 2013;34:7269–80. doi: 10.1016/j.biomaterials.2013.04.059. [DOI] [PubMed] [Google Scholar]
- 61.Flynn BP, Bhole AP, Saeidi N, Liles M, DiMarzio CA, Ruberti JW. Mechanical strain stabilizes reconstituted collagen fibrils against enzymatic degradation by mammalian collagenase matrix metalloproteinase 8 (MMP-8) PLoS ONE. 2010;5:e12337. doi: 10.1371/journal.pone.0012337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chiquet M. Regulation of extracellular matrix gene expression by mechanical stress. Matrix Biol. 1999;18:417–26. doi: 10.1016/s0945-053x(99)00039-6. [DOI] [PubMed] [Google Scholar]
- 63.Butt RP, Bishop JE. Mechanical load enhances the stimulatory effect of serum growth factors on cardiac fibroblast procollagen synthesis. J Mol Cell Cardiol. 1997;29:1141–51. doi: 10.1006/jmcc.1996.0347. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.