Abstract
The yeast transcriptional coactivator GCN5 (yGCN5), a histone acetyltransferase (HAT), is part of large multimeric complexes that are required for chromatin remodeling and transcriptional activation. Like other eukaryotes, the malaria parasite DNA is organized into nucleosomes and the genome encodes components of chromatin-remodeling complexes. Here we show that GCN5 is conserved in Plasmodium species and that the most homologous regions are within the HAT domain and the bromodomain. The Plasmodium falciparum GCN5 homologue (PfGCN5) is spliced with three introns, encoding a protein of 1,464 residues. Mapping of the ends of the PfGCN5 transcript suggests that the mRNA is 5.2 to 5.4 kb, consistent with the result from Northern analysis. Using free core histones, we determined that recombinant PfGCN5 proteins have conserved HAT activity with a substrate preference for histone H3. Using substrate-specific antibodies, we determined that both Lys-8 and -14 of H3 were acetylated by the recombinant PfGCN5. In eukaryotes, GCN5 homologues interact with yeast ADA2 homologues and form large multiprotein HAT complexes. We have identified an ADA2 homologue in P. falciparum, PfADA2. Yeast two-hybrid and in vitro binding assays verified the interactions between PfGCN5 and PfADA2, suggesting that they may be associated with each other in vivo. The conserved function of the HAT domain in PfGCN5 was further illustrated with yeast complementation experiments, which showed that the PfGCN5 region corresponding to the full-length yGCN5 could partially complement the yGCN5 deletion mutation. Furthermore, a chimera comprising the PfGCN5 HAT domain fused to the remainder of yeast GCN5 (yGCN5) fully rescued the yGCN5 deletion mutant. These data demonstrate that PfGCN5 is an authentic GCN5 family member and may exist in chromatin-remodeling complexes to regulate gene expression in P. falciparum.
In eukaryotes, nuclear DNA is packaged in a hierarchical structure of 30- to 400-nm chromatin fibers composed of core histones and nonhistone proteins. Nucleosome, the primary building block of chromatin, can inhibit several processes of eukaryotic transcription, from activator binding to the promoter, to transcriptional initiation, elongation, and termination (69). The relief of nucleosome-mediated repression of transcription requires the modification of the chromatin structures, which is often accomplished by the action of two major classes of multiprotein complexes. One class, represented by the SWI/SNF complex, perturbs nucleosome structure in an ATP-dependent manner (65). The other class remodels the chromatin structure through covalent modifications (acetylation, phosphorylation, methylation, and ubiquitination) of the amino termini of the core histones in the nucleosomes (5). Within the second class, histone acetyltransferase (HAT) complexes are the best characterized (reviewed in references 8, 24, and 56). HATs acetylate lysine residues at the N-terminal tails of core histones, thereby neutralizing their positive charges. This presumably reduces their affinity for the negatively charged DNA or other chromatin proteins, which may cause nucleosomes to unfold and increase access to transcriptional factors (reviewed in references 26, 56, and 60). While numerous studies correlate transcriptional activation with nucleosomal histone acetylation, the identification of a HAT enzyme in Tetrahymena as a homologue of the yeast transcriptional coactivator protein yGCN5 has directly linked histone acetylation to transcriptional activation (9). Since this discovery, many eukaryotic transcriptional factors including the human TATA-binding protein-associated factor TAFII250, p300/CBP (CREB-binding protein), and PCAF (p300/CBP-associated factor), SRC1 (steroid receptor coactivator 1), ACTR (activator of thyroid and retinoic acid receptor) (reviewed in reference 60), and the transcriptional factor ATF-2 (37) have been identified as HATs, further emphasizing the importance of histone acetylation in transcriptional activation.
Transcriptional coactivators or adaptors have been hypothesized to provide a physical bridge between the upstream activators and the transcriptional machinery at the promoter (27). This hypothesis is supported by the ability of adaptors to associate with activation domains (3, 14, 64) and TATA-binding protein (3, 57). The yeast transcriptional adaptor GCN5 (general control nonrepressed protein 5) and ADA (alteration/deficiency in activation) proteins (ADA1, ADA2, ADA3, and ADA5/Spt20) were originally identified genetically because mutations in these proteins confer resistance to toxicity caused by overexpression of the acidic activator chimera GAL4-VP16 fusion protein (6, 44). As a HAT, GCN5 alone acetylates only free histones; but as the catalytic subunit of two yeast native multiprotein HAT complexes, GCN5 acetylates histones in nucleosomes (25, 52). One complex has a molecular mass of 0.8 MDa and was named the ADA complex; the other has a molecular mass of 1.8 MDa, possesses adaptor components as well as Spt (suppressor of Ty) proteins, and was hence termed Spt-Ada-Gcn5-acetyltransferase (SAGA) complex (25). Both complexes contain ADA2, ADA3, and GCN5, which have been shown to interact physically and functionally to form a trimeric catalytic core (10, 12, 22, 29, 44, 58). Homologues of GCN5 have been identified in a wide range of eukaryotes, including humans (11, 67), mice (70), Drosophila (55), Tetrahymena (9), Toxoplasma (28, 61), and Arabidopsis (59). Interestingly, both humans and mice harbor two GCN5 homologues, GCN5 and PCAF (11, 50, 70), which appear to function in distinct HAT complexes. Even more complicated is the presence of two isoforms of GCN5 in mammalians and Drosophila as the result of alternative splicing (55, 70). Taken together, the evolutionary conservation of GCN5 suggests that similar transcriptional activation pathways may exist in different eukaryotes.
The malaria parasite Plasmodium falciparum is responsible for over one million deaths each year. Its life cycle involves many morphologically distinct stages alternating between a vertebrate and an invertebrate host (21). In both hosts, parasite gene expression is strictly regulated, which is responsible for the distinct RNA profiles observed at different developmental stages (7, 42). Despite this, transcriptional regulation in this parasite remains largely unknown. Although a GCN5 family member has been documented in a closely related parasite, Toxoplasma gondii (28, 61), the Plasmodium homologue and the effect of histone acetylation on transcriptional regulation have not been characterized. Yet, the presence of a histone deacetylase (HDAC) in P. falciparum (36) and antiparasitic activities of HDAC inhibitors such as the fungal metabolite apicidin underscore the importance of balanced histone acetylation and deacetylation in parasite development (1, 17).
To understand the role of histone acetylation in regulating global gene expression in Plasmodium, we began to characterize the homologues of the yeast transcriptional coactivator or adaptor complexes. Here we report the cloning of the yeast GCN5 homologue from P. falciparum, referred to as PfGCN5. We have shown that PfGCN5 and orthologues in other Plasmodium species share significant homology to other GCN5 family members with conserved HAT activity. In addition, we have demonstrated interactions between PfGCN5 and PfADA2 by using in vitro pull-down assays and the yeast two-hybrid system, which suggests that PfGCN5 may exist as the catalytic subunit of HAT complexes in Plasmodium. Finally we have determined that the HAT domains in PfGCN5 and yGCN5 are interchangeable.
MATERIALS AND METHODS
Parasite culture.
The malaria parasite P. falciparum 3D7 clone was cultured in human red blood cells in RPMI 1640 medium supplemented with 25 mM HEPES, 50 mg of hypoxanthine/liter, 25 mM NaHCO3, and 10% (vol/vol) heat-inactivated type A human serum. For most purposes, the culture was not synchronized. To isolate the parasite, the culture was treated with 0.05% saponin to lyse the red blood cell membrane and the released parasites were pelleted by centrifugation and washed twice with cold phosphate-buffered saline (15).
RNA extraction, RT-PCR, and Northern analysis.
Total RNA was extracted from the parasite pellet with Trizol Reagent (Invitrogen, Calsbad, Calif.). To isolate the PfGCN5, cDNA was synthesized with oligo(dT) primer from 2 μg of total RNA by using SuperScript II reverse transcriptase (RT) (Invitrogen) in 20 μl of reaction mixture (16). One microliter of the cDNA reaction was used to PCR amplify the 5′ and 3′ fragments of the PfGCN5 open reading frame (ORF) with KlenTaq (Clontech, Palo Alto, Calif.) with primers 5′-GATTATTTGATTAGGAATAATG-3′ and 5′-GTTTATCCACATGCCCGTCATC-3′ and primers PGCN5 and PGCN3 (Table 1). The PCR products were cloned in pCR2.1-TOPO vector (Invitrogen) and sequenced by using BigDye termination mix (Applied Biosystems, Foster City, Calif.).
TABLE 1.
Primers used for expression constructs, yeast two-hybrid analysis, and yeast complementationa
| Primer | Sequence |
|---|---|
| Protein expression in E. coli | |
| PGCN5 | 5′ GCGTCCATGGGATCCATGCTGTTTATAAATGCCACG 3′ |
| PGCN3 | 5′ GCGCTCGAGTGCTGTATCAGTTATAGCTTC 3′ |
| PfGCN-F2 | 5′ CGCGGATCCATGGAAGAGAATATGGGTATTAT 3′ |
| PfGCN-R2 | 5′ ACGCGTCGACTATAAAATGTATAGCTTTCTTTAC 3′ |
| PfADEF | 5′ CTCGAGGATAGTAATTACCATTGTGA 3′ |
| PADAR1 | 5′ CCCAAGCTTATTTTACCATCTCTTTAGCTCTTC 3′ |
| PADAR2 | 5′ CCCAAGCTTATATTTCATATGGATTTCCATCCG 3′ |
| PADAF1 | 5′ CCGGAATTCCTCGAGAGGACACAAATTATTGGATACTG 3′ |
| PfH3-F | 5′ CATATGGCACGAACTAAACAAAC 3′ |
| PfH3-R | 5′ CTCGAGAGATCTTTCTCCACGAATAC 3′ |
| Yeast two-hybrid constructs | |
| PgcnTh1 | 5′ CGCGGATCCGAATTCATGGAATGTTACATTTTCCCA 3′ |
| PgenTh2 | 5′ GCGCTCGAGTTATTTCAATTGAACTTCTTTATGT 3′ |
| Yeast complementation constructs | |
| yGCN-F1 | 5′ CGCGGATCCATGGTCACAAAACATCAGA 3′ |
| yGCN-R1 | 5′ GGAAGATCTTTAATCAATAAGGTGAGAA 3′ |
| PfGCN-F1 | 5′ CGCGGATCCGATAGGGAACCAGATCATT 3′ |
| PfGCN-R1 | 5′ GGAAGATCTTTATTTTGCTGTATCAGTTATAGCTTC 3′ |
| yGCN-F2 | 5′ CGCGGATCCATGTTGGACGCAGGTAAGATTCT 3′ |
| yGCN-R2 | 5′ ACGCGTCGACTTAATATCGTATTCTTGGTAAC 3′ |
| Chimera 1 | |
| PGCN5 | 5′ GCGTCCATGGGATCCATGCTGTTTATAAATGCCACG 3′ |
| Pf-YgF | 5′ CCCAAACATCAATTATTTGGACGCAGGTAAGATTCT 3′ |
| Pf-YgR | 3′ TAAAAGGGTTTGTAGTTAATAAACCTGCGTCCATTC 5′ |
| yGCN-R1 | 5′ GGAAGATCTTTAATCAATAAGGTGAGAA 3′ |
| Chimera 2 | |
| PfGCN-F1 | 5′ CGCGGATCCGATAGGGAACCAGATCATT 3′ |
| Pf-YgF | 5′ CCCAAACATCAATTATTTGGACGCAGGTAAGATTCT 3′ |
| Pf-YgR | 3′ TAAAAGGGTTTGTAGTTAATAAACCTGCGTCCATTC 5′ |
| yGCN-R1 | 5′ GGAAGATCTTTAATCAATAAGGTGAGAA 3′ |
| Chimera 3 | |
| yGCN-F1 | 5′ CGCGGATCCATGGTCACAAAACATCAGA 3′ |
| Y-PfgR | 3′ CAATGGTTCTTATGCTATAAACTCTGAAAGCCTTTAC 5′ |
| Y-PfgF | 5′ CCAAGAATACGATATTTGAGACTTTCGGAAATGTT 3′ |
| PfGCN-R1 | 5′ GGAAGATCTTTATTTTGCTGTATCAGTTATAGCTTC 3′ |
Underlined sequences indicate the cloning sites. For the three chimeric constructs, the primers at the fusion junctions are aligned to show the overlapping sequences and the yGCN5 sequences are shown in boldface type.
Northern hybridization was done with 15 μg of total RNA from mixed blood stage parasites by standard procedures (53). For probe labeling, a 710-bp cDNA fragment of PfGCN5 was amplified by PCR with primers PfGCN5-F2 and PgcnTh2 (Table 1). The PCR product was purified from a 1% agarose gel and labeled with [32P]dATP with the Prime-A-Gene system (Promega, Madison, Wis.). Hybridization and washes were performed under high stringency (16, 53).
Sequence analysis.
Sequence analysis was performed with the Genetics Computer Group program, version 10.1 (Madison, Wis.) and the Lasergene software (DNASTAR, Madison, Wis.). The PfGCN5 cDNA sequence was compared with the genomic DNA sequence in PlasmoDB (http://plasmodb.org) to identify the introns. It was also used to identify GCN5 homologues in other Plasmodium species by searching the PlasmoDB with various BLAST algorithms. These sequences were retrieved for further analysis to predict protein coding sequences based on the intron positions in PfGCN5. Multiple-sequence alignments of the Plasmodium GCN5 homologues were performed with yeast and T. gondii GCN5 as reference sequences (28, 61).
RLM-RACE.
To determine the 5′ and 3′ ends of the PfGCN5 transcript, RNA ligase-mediated rapid amplification of cDNA ends (RLM-RACE) was performed with 10 μg of total RNA isolated from a mixed blood stage culture by using the FirstChoice RLM-RACE kit (Ambion, Austin, Tex.) according to the manufacturer's instructions. For 5′ RLM-RACE, cDNA was synthesized with random decamers and Moloney murine leukemia virus RT. A nested PCR was performed with two PfGCN5-specific primers (outer primer, 5′-GTCCTCTTTTCCAAGAAGAT-3′, and inner primer, 5′-GGACAAATCTCATTGTCAAA-3′) and two primers from the 45-base RNA adaptor. To determine the 3′ end of the mRNA, cDNA was synthesized by using an oligo(dT) primer with a 3′ RACE adaptor. Two PfGCN5-specific primers near the 3′ end (outer primer, 5′-CAACAGTCTGCATGGCCATT-3′, and inner primer, 5′-GGTATTGAGCTTAAGAGAAT-3′) and the oligo(dT) primer were used to perform a nested PCR to amplify the 3′ end of the cDNA. The PCR products from the 5′ and 3′ RACEs were cloned in the pCR2.1-TOPO vector and sequenced.
Protein expression in bacteria.
To determine whether PfGCN5 has the conserved GCN5 family HAT activity, two truncated PfGCN5 versions were expressed in bacteria. Two cDNA fragments corresponding to amino acids (aa) 1090 to 1463 and aa 1124 to 1310 (the HAT domain) were amplified with primer pairs PGCN5-PGCN3 and PfGCN5-F2-PfGCN5-R2 (Table 1) and cloned separately into the pGEX-6P-1 vector at the BamHI and XhoI sites to produce glutathione S-transferase (GST) fusion proteins. Recombinant proteins expressed in bacterial strain BL21 were purified from 1 liter of culture with glutathione-Sepharose 4B (Amersham, Piscataway, N.J.). All proteins for HAT assays were dialyzed in 1× HAT assay buffer (50 mM Tris-HCl [pH 8.0], 5% glycerol, 0.1 mM EDTA, 50 mM KCl, 1 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride, 10 mM sodium butyrate) and stored at −80°C.
For in vitro protein interaction assays, DNA fragments of PfADA2 encoding a putative full-length ADA2 domain (aa 1524 to 1842), the GCN5 binding domain (aa 1524 to 1664), and the ADA3 binding domain (aa 1688 to 1842) were amplified from P. falciparum genomic DNA with primer pairs PADEF-PADAR1, PADEF-PADAR2, and PADAF1-PADAR1, respectively (Table 1), and cloned into pGEX-6P-1 at the XhoI and HindIII sites. GST fusion proteins were purified similarly with glutathione-Sepharose 4B. The C-terminal PfGCN5 (aa 1090 to 1463) fragment was amplified from P. falciparum cDNA with primers PGCN5-PGCN3 and cloned into the pET28a vector at BamHI and XhoI sites to produce a recombinant protein with double six-His tags. Recombinant PfGCN5 protein was purified by using Ni-nitrilotriacetic acid resin (Qiagen, Valencia, Calif.) as previously described (16) and dialyzed in the binding buffer (20 mM HEPES [pH 7.8], 10 mM MgCl2, 100 mM NaCl, 15% glycerol, and 1 mM phenylmethylsulfonyl fluoride) for in vitro protein interaction assays. The purity of the purified recombinant proteins was checked by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE). Protein concentrations were determined by using the Bio-Rad (Hercules, Calif.) protein assay reagents, with bovine serum albumin as the standard.
HAT assays.
Liquid HAT assays were performed essentially as described by Brownell et al. (9). Briefly, a 30-μl reaction mixture containing 1× HAT assay buffer, 5 μg of calf thymus histones (Sigma, St. Louis, Mo.), 0.25 μCi of [3H]acetyl coenzyme A ([3H]acetyl-CoA; Amersham), and either 2 μg of purified GST-PfGCN5 (aa 1124 to 1310), 1.8 μg of purified GST-PfGCN5 (aa 1090 to 1464), or 5 μg of purified GST (as the negative control) was incubated at 30°C for 30 min. Subsequently, the reactions were stopped by adding 6 μl of 6× SDS-PAGE loading buffer, and 15 μl of each sample was separated by SDS-18% PAGE. Gels were stained with Coomassie brilliant blue R-250. For fluorography, the stained gel was soaked in autoradiographic enhancer (Perkin Elmer Life Sciences, Boston, Mass.), vacuum dried, and exposed to X-ray film for 48 h. The autoradiograph was compared with the Coomassie blue-stained gel to identify the acetylated histone bands.
To determine the residue substrate specificity of the recombinant PfGCN5 HAT domain, liquid HAT assays were performed as described above with nonradioactive acetyl-CoA, and acetylated histones were separated by SDS-15% PAGE for immunoblotting with antibodies specific for either acetylated Lys 9 or Lys 14 of histone H3 (Upstate Biotechnology, Lake Placid, N.Y.).
Yeast two-hybrid analysis.
To test whether PfADA2 interacts with PfGCN5, we employed the yeast two-hybrid system. A region in PfGCN5 (aa 1278 to 1361, containing the putative ADA2 binding domain) was amplified with primer pair PgcnTh1-PgcnTh2 (Table 1) and cloned into the yeast two-hybrid vector pHybLex/Zeo (Invitrogen) to produce a bait fusion protein LexA-GCN5. Three fragments of PfADA2 (aa 1524 to 1842, full-length ADA2 domain; aa 1524 to 1664, putative GCN5-binding domain; and aa 1688 to 1842, putative ADA3 binding domain) were amplified with primer pairs PADEF-PADAR1, PADEF-PADAR2, and PADAF1-PADAR1, respectively (Table 1), and cloned into the vector pYESTrp2 to produce the prey protein hybrid B42-ADA2. Two plasmids, pHybLex/Zeo-Fos2 and pYESTrp-Jun, were included as the positive control. Competent yeast SKY48/pLacGUS was transformed with a bait and prey plasmid by the lithium acetate protocol (23). The transformants were plated on YC-UW medium (Obiogene, Carlsbad, Calif.) with Zeocin to select yeast colonies carrying both plasmids. The interaction between the bait and prey will result in the expression of the reporter genes, Leu2 and β-galactosidase. To determine whether the PfADA2 and PfGCN5 domains interact, single yeast colonies were picked and grown in liquid YC-UW (plus Zeocin) medium, and 3 μl of 10-fold serial dilutions of the cultures beginning with 6 × 106 cells/ml was spotted on YC-UWL plates (without Leu) with Zeocin and galactose. The growth of the yeast colonies was recorded. The strength of the interactions was further determined by measuring the β-galactosidase activity by a standard method (62). Three yeast colonies for each two-hybrid combination were assayed, and the results were plotted by using KaleidaGraph 3.5 (Synergy Software, Reading, Pa.).
In vitro protein interaction assays.
To further confirm the direct interaction between PfGCN5 and PfADA2, recombinant proteins were produced and used in in vitro pull-down assays. Three recombinant GST-PfADA2 proteins and GST alone coupled to glutathione-Sepharose 4B beads were washed extensively with the binding buffer. The amount of protein on each column was estimated to be 15 μg by SDS-PAGE separation of aliquots of beads. For each column, 15 μg of recombinant His-tagged PfGCN5 was added to Sepharose 4B beads. The mix was incubated for 1.5 h at 4°C with rotation. After extensive washes with the binding buffer, beads were washed in the same buffer containing 0.25 M NaCl. Material remaining on beads was eluted with 20 μl of 1× SDS sample buffer and boiled for 5 min. For each sample, 6 μl was loaded onto an SDS-10% PAGE gel and analyzed by Western blotting with a monoclonal anti-His antibody.
Construction of yeast expression plasmids.
Primers used for plasmid construction are listed in Table 1. To test whether the PfGCN5 could complement the yeast gcn5− mutant, eight plasmids were constructed in the yeast expression vector pG1(HA)2. The yeast expression vector pG1(HA)2 is a derivative of pG1 (54) which contains double hemagglutinin (HA) epitopes, the constitutive glyceraldehyde-3-phosphate dehydrogenase promoter, and the phosphoglycerate kinase termination sequence. The constructs have been schematically illustrated (see Fig. 5). The full-length yGCN5 was amplified with primer pair YGCN-F1-YGCN-R1 and cloned at the BamHI site of pG1(HA)2 to create the expression plasmid yGCN5, which was used as a positive control for full complementation of the gcn5− mutant. Similarly, PfGCN5-1 (aa 1090 to 1464) and PfGCN5-2 (aa 1138 to 1464) were constructed with primers PGCN5-PfGCN-R1 and PfGCN-F1-PfGCN-R1, respectively. To create three chimeras, we used a strategy employing PCR-mediated recombination so that no restriction sites at the junction of the chimera are needed (33). At the junction, the two primers were designed in a way that both PCR products shared the same sequence at one end (Table 1). Chimeras 1, 2, and 3 correspond to PfGCN5 aa 1090 to 1288 plus yGCN5 aa 261 to 439, PfGCN5 aa 1138 to 1288 plus yGCN5 aa 261 to 439, and yGCN5 aa 1 to 260 plus PfGCN5 aa 1289 to 1464, respectively. To verify that the complementary effects of the chimeras were not due to the mere presence of partial yGCN5 domains, two yGCN5 constructs, yGCN5-N (N-terminal region containing the HAT domain, aa 1 to 260) and yGCN5-C (aa 261 to 439) were constructed by using yGCN-F1-yGCN-R2 and yGCN-F2-yGCN-R2, respectively.
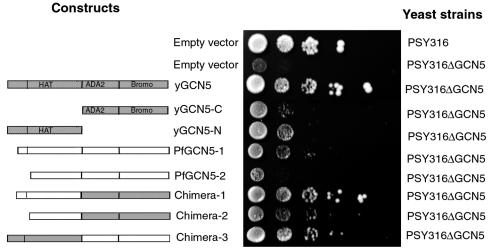
FIG. 5.
Growth complementation assays of yeast gcn5− mutant by PfGCN5 fragments and PfGCN5-yGCN5 chimeras. The wild-type strain PSY316 and the strain with the GCN5 deletion (PSY316 ΔGCN5) transformed with the empty vector were used as controls. The yeast strain PSY316 ΔGCN5 was transformed with one of the following constructs: three versions of yGCN5 (yGCN5 [aa 1 to 439], yGCN5-N [aa 1 to 260], and yGCN5-C [aa 261 to 439]), two versions of PfGCN5 (PfGCN5-1 [aa 1090 to 1464] and PfGCN5-2 [aa 1138 to 1464]), and three chimeric constructs (chimera 1 [PfGCN5 aa 1090 to 1288 plus yGCN5 aa 261 to 439], chimera 2 [PfGCN5 aa 1138 to 1288 plus yGCN5 aa 261 to 439], and chimera 3 [yGCN5 aa 1 to 260 plus PfGCN5 aa 1289 to 1464]). The constructs are schematically illustrated on the left, with the yGCN5 fragments shown as shaded boxes and the PfGCN5 fragments shown as open boxes. Tenfold dilutions of the transformants were spotted on minimum synthetic medium. Plates were photographed after incubation at 30°C for 4 days.
Complementation of yeast mutants.
The yeast wild-type strain PSY316 (MATα ade-2-101 his3-Δ200 leu2-3,2-112 lys2 ura3-53) and the gcn5− mutant strain PSY316ΔGCN5 (MATα ade-2-101 his3-Δ200 leu2-3,2-112 lys2 ura3-53 trp1 Δgcn5) (11) were kindly provided by Jerry Workman. Yeast transformation was carried out by the lithium acetate protocol (23). Protein expression was verified by immunoblotting with monoclonal anti-HA antibody. To test whether the transformation could complement the growth of the yeast, transformed cells were plated on fully supplemented synthetic dropout (SD) medium. Single colonies were picked and grown in fully supplemented liquid SD medium overnight at 30°C. Tenfold serial dilutions starting with 6 × 106 cells/ml were spotted on plates with SD minimal medium. Plates were photographed after incubation at 30°C for 4 days.
Nucleotide sequence accession number.
The complete PfGCN5 cDNA sequence was deposited in GenBank under accession no. AY498855.
RESULTS
Plasmodium parasites have a conserved GCN5 homologue.
Many eukaryotes have the conserved HAT proteins homologous to yCGN5. A BLAST search of PlasmoDB with yGCN5 revealed a homologue in the P. falciparum genome on chromosome 8, herein referred to as PfGCN5 (Fig. 1A). The PfGCN5 (PF08_0034) was noted to have three introns with a coding region of 4,455 bp, encoding a protein of 1,484 aa. To verify the computer prediction, we have amplified and sequenced PfGCN5 cDNA fragments by using RNA from mixed asexual stages. Comparison of the cDNA and genomic sequences confirmed the correct prediction of the first and third introns, but the second intron was 60 bp longer than predicted. The positions and lengths of the introns are shown in Fig. 1B. In accordance, the correct PfGCN5 ORF is 4,395 nucleotides and encodes a protein of 1,464 residues with a molecular mass of 170.8 kDa and a pI of 6.5.
FIG. 1.
Comparison of Plasmodium GCN5 homologues. (A) Schematic illustration of PfGCN5 and yGCN5 (not to scale). The positions of the HAT domain, ADA2-binding domain, and bromodomain are indicated by filled, open, and checkered boxes, respectively. The hatched box in PfGCN5 indicates the conserved region in the N-terminal extension among all Plasmodium species (Fig. 2B). The numbers shown above the scheme indicate the boundaries of each domain. Note that PfGCN5 has an ∼1,100-residue N-terminal extension. (B) Alignments of four predicted Plasmodium GCN5 proteins (Pf, P. falciparum; Pk, P. knowlesi; Pv, P. vivax; Py, P. yoelii) and yGCN5 (Sc, S. cerevisiae). The alignment starts at aa 676 of PfGCN5 to show a conserved central region among all Plasmodium species (corresponding to the hatched box in panel A). Boundaries of the HAT domain, ADA2-binding domain, and bromodomain are marked with block arrows with reference to the corresponding domains in yGCN5. The four motifs (I to IV) defining the GCN5 family members are double underlined, and the four motifs (C, D, A, and B) defining the GNAT superfamily are marked above the sequences. The glutamic acid proposed to be the catalytic residue is marked with an asterisk. Residues identical among three species are shaded, and dashes have been inserted to optimize the alignments. The positions and lengths of the three introns in PfGCN5 are indicated by triangles.
BLAST searches of other Plasmodium genomic sequences available in PlasmoDB identified a GCN5 homologue from each species. Based on the genomic organization of PfGCN5, we predicted the ORFs for Plasmodium knowlesi, Plasmodium vivax, and Plasmodium yoelii GCN5 to encode proteins of 1,554, 1,488, and 1,402 aa, respectively (Fig. 1). Among all Plasmodium GCN5 proteins, the 485-residue C-terminal sequences share more than 90% amino acid identity. In addition, a central region corresponding to aa 695 to 755 of PfGCN5 is also homologous among all Plasmodium GCN5s. Comparison of Plasmodium GCN5s with the GCN5 from a closely related parasite, T. gondii (28, 61), revealed significant homology near the C terminus (data not shown). Compared with GCN5 from lower eukaryotes such as yGCN5 (439 aa), the predicted Apicomplexa GCN5 proteins have a very long N-terminal extension that shows little homology to known sequences. Even among Plasmodium species, the N-terminal extensions share limited sequence homology; it is only between very closely related species such as P. vivax and P. knowlesi that extensive homology in the N-terminal extension exists.
In the Plasmodium GCN5s, the ∼360-aa carboxyl-terminal sequence is homologous to 70 to 436 aa of the best-characterized yGCN5. This region includes two domains that are highly conserved among all GCN5 family proteins: the HAT domain (∼220 residues) and the bromodomain (∼100 residues) (Fig. 1). Three-dimensional (3-D) structures have been resolved for several HATs and bromodomains (reviewed in references 45 and 46). The HAT domain is the catalytic center of the enzyme, and the bromodomain of HAT proteins probably promotes interaction with acetylated histones (46). The HAT domains of all Plasmodium GCN5s have four highly conserved motifs (I to IV) that define the GCN5 family (8). Besides, they also share various degrees of homology in four sequence motifs (C, D, A, and B in N- to C-terminal order) that define the GCN5-related N-acetyltransferase (GNAT) superfamily (Fig. 1B) (19, 49). The most conserved motif among GNAT superfamily members is motif A, whereas motif C is the least homologous and was initially considered absent in GCN5 family proteins (49). In addition, all Plasmodium GCN5 proteins share a conserved Glu residue corresponding to Glu-173 of yGCN5 (Fig. 1B), which is essential for the HAT activity in vitro and GCN5 function in vivo, and implicated from the 3-D structure to function as a general base for catalysis (63, 66). Between the HAT domain and the bromodomain is the domain that interacts with another coactivator protein, ADA2. This domain is highly homologous among the Plasmodium species, but it shows limited homology (∼22% amino acid identity) to that of yGCN5. All of these conserved features in the HAT domain and the bromodomain suggest that these Plasmodium homologues are members of the GCN5 family.
PfGCN5 gene expression and transcriptional initiation sites.
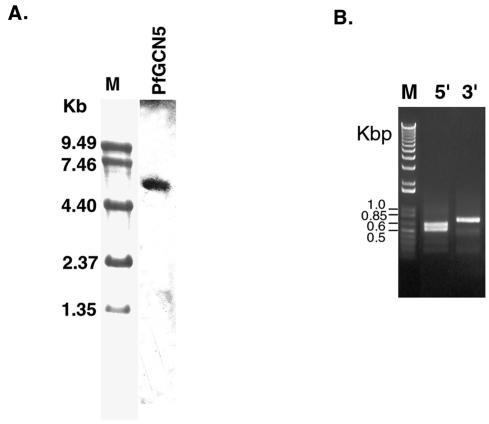
DNA microarray analysis indicates that PfGCN5 is expressed in all developmental stages of P. falciparum (42). Semiquantitative RT-PCR analysis with RNA from synchronized blood stages also confirmed the constitutive expression of PfGCN5 (data not shown). Northern analysis with total RNA from mixed asexual stages identified an ∼5.4-kb mRNA (Fig. 2A).
FIG. 2.
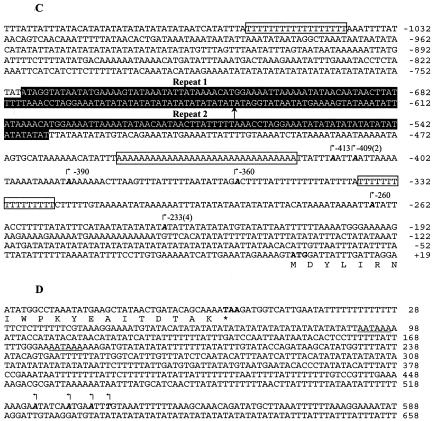
Expression of PfGCN5. (A) Northern blot of PfGCN5. Total RNA (15 μg) from mixed asexual stages was separated in a 1% formaldehyde-agarose gel and hybridized to the [32P]dATP-labeled PfGCN5 probe. The molecular markers (M) were stained with methylene blue. (B) Mapping of 5′ and 3′ ends of PfGCN5 transcript. PCR products from RLM-RACE and 3′ RACE were separated in a 1% agarose gel. M, 1-kb ladder in kilobase pairs (Invitrogen); 5′, RLM-RACE for mapping TISs of PfGCN5; 3′, 3′ RACE for mapping 3′ end of PfGCN5. (C) Nucleotide sequence at the 5′ end of PfGCN5. TISs are indicated by boldface italic letters and marked with small arrows. Numbering is with respect to the translational initiation codon ATG (in boldface type). Numbers in parentheses indicate the numbers of clones present in the 10 random clones sequenced. Three dA-dT tracts are boxed. Two 108-bp tandem repeats are shaded. The end of repeat 1 and the start of repeat 2 are marked with an upright arrow. (D) Nucleotide sequence at the 3′ end of PfGCN5. Arrows indicate the termination sites that cluster in a 14-bp region. The stop codon TAA is in boldface type and marked with an asterisk. Two polyadenylation signals are underlined.
To map the 5′ and 3′ ends of the PfGCN5 mRNA, RACEs were performed. RLM-RACE amplified two fragments of ∼500 and 600 bp, and 3′ RACE produced one cDNA fragment of ∼650 bp (Fig. 2B). Sequencing of 10 independent 5′ RACE products indicated that the cDNA sequences were colinear with the genomic sequence but heterogeneous in their 5′ ends (Fig. 2C). While four transcriptional initiation sites (TISs) were clustered from −390 to −413 bp upstream of the ATG translation initiation codon, an additional four TISs were found at a single site, −233. These two clusters are in agreement with the two fragments amplified by RLM-RACE, indicating that PfGCN5 transcription in asexual stage parasites was initiated at two major sites approximately 410 and 233 bp upstream of the ATG codon. Furthermore, two minor TISs were located between the two major initiation sites. Since RLM-RACE is designed to amplify only the 5′ end of mRNA with an intact cap structure (47), this result suggested that multiple TISs were used for PfGCN5. This finding is in agreement with the conclusion from a comprehensive transcriptome analysis with a similar oligo-capping method, which indicates that all transcripts of P. falciparum genes use diverse TISs (68).
We have analyzed ∼1,100 bp upstream of the ATG codon for identification of promoter elements. Although the extremely AT-rich promoter region defied attempts to find transcription factor-binding motifs, we have identified two types of elements that may play roles in transcriptional regulation. One is the 32-nucleotide run of dAs immediately upstream of the distal major TISs (Fig. 2C). Two dT tracts of >15 nucleotides are also located in the promoter region. Such dA-dT tracts are found highly enriched in noncoding regions of the P. falciparum genome (18). While their exact roles are not defined, the rigid DNA curvature and changes in nucleosomal structure at the dA-dT tracts may be important for modulating gene expression in malaria parasites (51). The other element is the two 108-bp tandem repeats located between −534 and −748. In some Plasmodium promoters, long repeats have been found to contain nuclear factor-binding sequences that are essential for promoter activity (31). The repeats in the PfGCN5 promoter do not resemble those in known promoters and may contain novel regulatory elements.
We have sequenced four clones of the 3′ RACE products, all of which were terminated in a 14-bp region 524 bp downstream from the PfGCN5 stop codon (Fig. 2D). Two putative polyadenylation signals are located 92 and 177 bp downstream of the stop codon. According to the lengths of the 5′ and 3′ untranslated regions, the PfGCN5 mRNA is estimated to be 5.2 to 5.4 kb, consistent with the result from Northern analysis. The complete PfGCN5 cDNA sequence was deposited in GenBank (accession no. AY498855).
PfGCN5 has HAT activity.
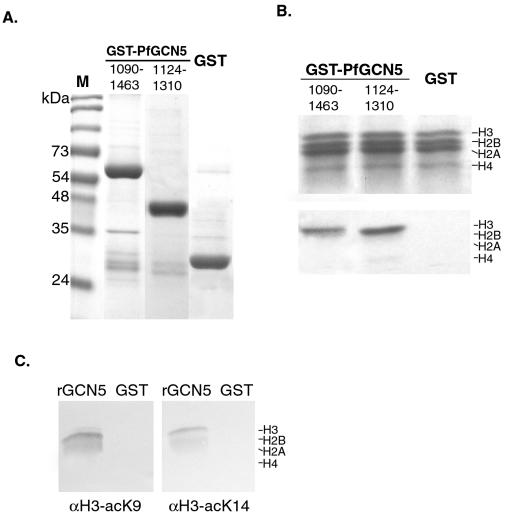
GCN5 family proteins are able to acetylate core histones in vitro. To determine whether PfGCN5 has the conserved HAT activity, histone acetylation assays were conducted with recombinant PfGCN5. Two truncated versions of PfGCN5, aa 1090 to 1464 and aa 1124 to 1310, both containing the HAT domain, were fused to N-terminal GST, and the fusion proteins were expressed and purified (Fig. 3A). The recombinant proteins and the GST control were incubated with calf thymus core histones and [3H]acetyl-CoA. The results showed that both truncated PfGCN5 polypeptides preferentially and effectively acetylated histone H3 and, to a lesser extent, H4 (Fig. 3B), a characteristic of GCN5 family members (38). As expected, the control GST protein purified in the same way showed no HAT activity. Using two antibodies specific for either K8 or K14 of histone H3, we determined that the recombinant PfGCN5 HAT domain could acetylate these Lys residues (Fig. 3C). These two residues are highly preferred acetylation sites for Gcn5 proteins (38). Together, these data demonstrate that PfGCN5 is a bona fide GCN5 homologue with similar substrate specificity to other GCN5 proteins.
FIG. 3.
HAT activity of PfGCN5. (A) Expression and purification of recombinant GST-PfGCN5 proteins. A Coomassie blue-stained gel shows the purified GST fusion proteins and GST control. Two PfGCN5 truncations (residues 1090 to 1463 and 1124 to 1310) correspond to the full-length yGCN5 and the HAT domain, respectively. The left lane (M) shows a protein size standard. (B) Liquid HAT assay. The recombinant GST-PfGCN5 proteins and the GST control were incubated with [3H]acetyl-CoA and 5 μg of calf thymus histones. The histones were analyzed by SDS-18% PAGE and fluorography. The upper panel shows Coomassie blue staining of the core histones, and the lower panel is the fluorogram showing preferential acetylation of H3. The four core histones, H3, H2B, H2A, and H4, are labeled. (C) Residue specificity of the recombinant PfGCN5 HAT domain for histone H3. Core histones incubated with recombinant GST-PfGCN5 HAT domain (rGCN5) or GST protein were separated by SDS-15% PAGE and blotted with antibodies specific for either acetylated K8 (αH3-acK8) or K14 (αH3-acK14) of histone H3.
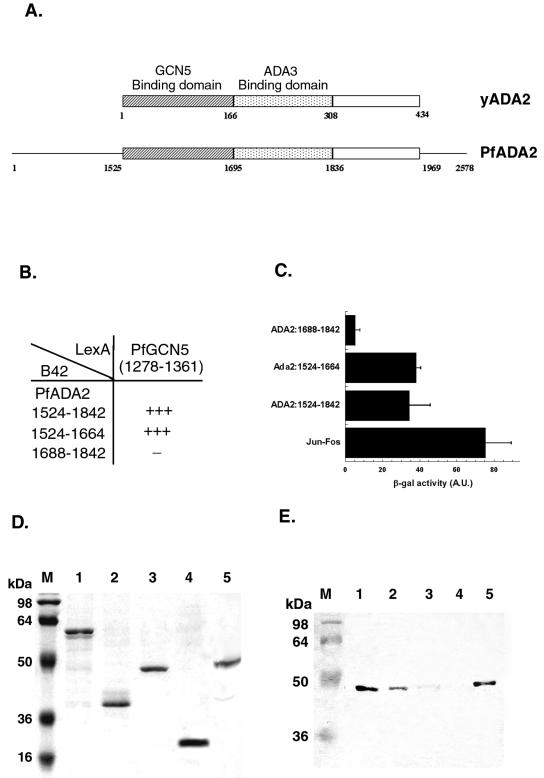
PfGCN5 interacts with PfADA2.
Although yGCN5 alone is a potent acetylase for free histones, it fails to acetylate histones contained in nucleosomes. In vivo, yGCN5 and the coactivators ADA2 and ADA3 form a catalytic core of two different HAT complexes, the SAGA and ADA complexes (25), to acetylate the nucleosomal histones. The region interacting with yGCN5 is located in the SANT domain of yADA2 (within the first 118 residues) (58), and the ADA2-binding domain is located within aa 250 to 280 of yGCN5 (12). Using a combination of RT-PCR and library-screening methods, we have isolated the P. falciparum ADA2 homologue, referred to as PfADA2 (Q. Fan and L. Cui, unpublished data). PfADA2 encodes a large protein of 2,578 residues, and the region homologous to yADA2 is located at aa 1525 to 1969. Sequence comparison assisted the delineation of putative GCN5- and ADA3-binding domains in PfADA2 (Fig. 4A). To determine whether PfADA2 and PfGCN5 interact with each other, we employed the yeast two-hybrid system. The putative ADA2-binding domain in PfGCN5 (aa 1278 to 1360) was fused to the bacterial LexA DNA-binding domain in the vector pHybLex/Zeo. Three regions of PfADA2 (the full-length ADA2 domain, the putative GCN5-binding domain, and the putative ADA3-binding domain) were cloned into the pYESTrp2 to produce B42-ADA2 fusion proteins. Both plasmids were transformed into the yeast strain SKY48/pLacGus. Two plasmids, pHybLex/Zeo-Fos2 and pYESTrp-Jun, were included as positive controls. In the yeast growth assays, clones transformed with the LexA-GCN5 bait plasmid and either B42-ADA2 (aa 1524 to 1842) or B42-ADA2 (aa 1524 to 1664) grew as vigorously as the positive control on selective medium lacking leucine, whereas clones with the LexA-GCN5 bait plasmid and B42-ADA2 (aa 1688 to 1842) grew very poorly (Fig. 4B). To further determine the strength of the interactions, the same transformants were grown in liquid medium to test for β-galactosidase activity (Fig. 4C). The results indicated that either the full-length ADA2 domain or the putative GCN5-binding domain, but not the putative ADA3-binding domain, interacted with the putative ADA2-binding domain of PfGCN5. In all cases, the Leu2 and β-galactosidase induction correlated well.
FIG. 4.
PfGCN5 and PfADA2 interactions. (A) Schematic comparison between yADA2 and PfADA2 (not to scale). The ADA2-like domain is located near the C terminus, between aa 1525 and 1969 in PfADA2. The conserved GCN5-binding and ADA3-binding domains are shown as hatched and stippled boxes, respectively. (B) Yeast two-hybrid analysis growth assay on selective medium. A yeast strain transformed with both LexA-PfGCN5 bait plasmid and the B42-PfADA2 prey plasmid was seeded on the leucine-deficient medium YC-UWL. The growth of the yeast strain was compared to that of the positive control with LexA-Fos and B42-Jun. Vigorous growth is indicated by +++, and very poor growth is indicated by −. (C) β-Galactosidase assay with the same yeast transformants as in panel B. At least three colonies were assayed for β-galactosidase production to show the mean + standard deviation. A.U., arbitrary units. (D) Coomassie blue staining of the purified recombinant proteins used to analyze binding of purified PfGCN5 to PfADA2. Each lane represents a portion of the glutathione beads used for binding assays in panel E. Protein standard markers in kilodaltons are shown on the left (lane M). Lanes: 1, GST-PfADA2 (aa 1524 to 1842); 2, GST-PfADA2 (aa 1524 to 1664); 3, GST-PfADA2 (aa 1688 to 1842); 4, GST alone; 5, His-PfGCN5 (aa 1090 to 1463). (E) Western blot analysis of binding of purified His-PfGCN5 to GST-PfADA2 (various deletion derivatives). Purified His-PfGCN5 was incubated with GST fusions and GST alone as shown in panel D. Materials remaining on the glutathione beads after washing with 0.25 M NaCl were separated by SDS-PAGE and probed with monoclonal anti-His antibody to detect His-PfGCN5. The left lane (M) shows protein mass markers in kilodaltons. Lanes 1 to 4 are as described for panel D. Lane 5 shows the purified His-PfGCN5 as the positive control.
In vitro GST pull-down assays were performed to biochemically confirm the interaction between PfGCN5 and PfADA2. GST-fused PfADA2 fragments were expressed in bacteria, purified with glutathione-Sepharose (Fig. 4D), and incubated with recombinant His-tagged PfGCN5 protein (aa 1090 to 1464). Figure 4E shows that the full-length ADA2 domain (aa 1524 to 1842) interacted most efficiently with PfGCN5 (lane 1). The interacting region was further refined to the putative GCN5-binding domain in PfADA2 (aa 1524 to 1664) (lane 2). In contrast, the putative ADA3-binding domain only retained trace amounts of recombinant PfGCN5 (lane 3). GST protein alone did not retain detectable amounts of recombinant PfGCN5 (lane 4). Altogether, both the yeast two-hybrid and the GST pull-down experiments indicated that PfADA2 and PfGCN5 interact and that the interacting domains correspond to those mapped in the yeast proteins.
Growth complementation of yeast gcn5− mutant by PfGCN5 domains.
GCN5 homologues in eukaryotes are functionally similar to yGCN5, as shown from the yeast complementation assay (28, 67). To determine whether PfGCN5 can functionally replace its yeast homologues in vivo, we transformed the gcn5− mutant of S. cerevisiae with yGCN5, PfGCN5, and yeast-Plasmodium chimeric GCN5 constructs. The ygcn5− mutant exhibits retarded growth in minimal medium. Wild-type PSY316 and the gcn5− deletion mutant transformed with the empty vector and grown on minimum medium served as the wild-type and retarded-growth phenotypes, respectively. The results showed that the wild type and gcn5− deletion mutant transformed with the plasmid bearing the yGCN5 gene displayed normal growth, whereas the growth of the gcn5− mutant transformed with the empty vector was severely retarded (Fig. 5). The PfGCN5-1 construct (aa 1090 to 1464), having an N-terminal extension beyond the HAT domain, could partially complement the yeast gcn5− mutant, whereas PfGCN5-2 (aa 1138 to 1464), with a 14-residue N-terminal deletion in the HAT domain, failed to complement the gcn5− phenotype (Fig. 5). To further test the ability of different domains of PfGCN5 to rescue the ygcn5− deletion, three chimeric GCN5 constructs were examined in the gcn5− mutant. Chimera 1 had an intact HAT domain from PfGCN5 fused to the remainder of yGCN5 (aa 261 to 439), and chimera 2 had a 14-aa deletion in the PfGCN5 HAT domain. Again, the intact PfGCN5 HAT domain with a 34-aa N-terminal extension (chimera 1) was fully functional in replacing the HAT domain of yGCN5; transformation with chimera 1 almost restored the gcn5− mutant to the wild-type phenotype. In contrast, the deletion in the PfGCN5 HAT domain in chimera 2 significantly compromised its ability to rescue the ygcn5− mutant. Although chimera 3 contains the intact HAT domain of yGCN5 (aa 1 to 260) fused to the remainder of PfGCN5, it was able to provide partial complementation to ygcn5−. To demonstrate that the effect of these chimeras in complementing the ygcn5− mutant was not due to the mere existence of the N or C terminus of yGCN, constructs only bearing these yGCN5 domains (yGCN5-N, aa 1 to 260, and yGCN5-C, aa 261 to 439) were transformed into the ygcn5− mutant. However, these constructs had minimum effects on rescuing the ygcn5− mutation. These results demonstrate that HAT function is conserved in PfGCN5 and that the most conserved HAT domains are expected to be interchangeable between members of the GCN5 family.
DISCUSSION
Homologues of yGCN5 have been identified in various eukaryotes and now in the protozoan malaria parasites. Here we show that all malaria parasite genomes encode a GCN5 family HAT protein, and together with the T. godii GCN5, they probably constitute a subgroup of this protein family with a long N-terminal extension. We further show that PfGCN5 is expressed constitutively in asexual stages, and transcription is initiated at two major sites. Both in vitro HAT activity assays and yeast complementation experiments have confirmed that PfGCN5 possesses conserved HAT activity. Moreover, we have identified another homologue of the GCN5 catalytic core, PfADA2, in the P. falciparum genome and demonstrated the interaction between PfGCN5 and PfADA2, further implying that PfGCN5 may exist as complexes in P. falciparum.
Apicomplexan GCN5 homologues.
The gene PF08_0034 in the P. falciparum genome has been annotated as the putative GCN5 homologue by virtue of its resemblance to other GCN5 family members. Regions of the highest homology are the C-terminal HAT domain and bromodomain, two domains typical of all GCN5 members. The GCN5 homologue is present in all Plasmodium species examined, and these proteins are perfectly conserved at the C terminus, suggesting that they are functionally similar. This finding prompted us to examine whether the region in PfGCN5 that is homologous to the HAT domain also possesses HAT activity. As expected, two recombinant PfGCN5 proteins showed similar enzymatic activity to other members of the GCN5 protein family, with a preference for histone H3 as the substrate (55, 59, 67, 70). Furthermore, the two preferred sites for yGCN5, K8 and K14 of histone H3, were also acetylated by the recombinant PfGCN5 protein (38). This provides strong evidence that Plasmodium GCN5s are novel members of the GCN5 family with other distinctive features that may be shared among Apicomplexa GCN5 members. Like the T. gondii GCN5, all Plasmodium GCN5s have a long N-terminal extension. Remarkably, it bears no homology to the N-terminal extension present in the human GCN5 and PCAF and the Drosophila GCN5 (55, 61, 70). Even among all Plasmodium species, the homology among the N-terminal extensions is low except for a short conserved central region (aa 695 to 755 in PfGCN5) or between very closely related species (e.g., between P. vivax and P. knowlesi). This finding indicates that the N-terminal extension is not unique to GCN5 family members from higher eukaryotes (55). Although the functions of the N-terminal extensions in GCN5 members are not completely understood, these regions in the mammalian GCN5 members mediate protein-protein interactions. For example, both the mammalian GCN5 and PCAF contain domains in the N-terminal extension that interact with the transcription factor CBP (70, 71) and the Notch receptor (34). In addition, the N-terminal extension may be important for recognition of nucleosomal substrates because the full-length mouse GCN5 readily acetylates both free and nucleosomal histones (70). Unfortunately, the extremely AT-rich P. falciparum genome does not allow us to express the full-length PfGCN5 protein to assess this possibility. The divergence in the N-terminal sequences of GCN5 members among different eukaryotes suggests they might interact with different partners and perform different functions.
The recombinant PfGCN5 proteins have displayed histone H3 acetylase activity characteristic of GCN5 family HATs. Yet, despite the overall sequence conservation between PfGCN5 and yGCN5, it only partially complemented the growth defect of the yeast mutant with yGCN5 deletion. This result is nonetheless similar to those obtained with the human and T. gondii GCN5 genes, which also failed to complement the yeast gcn5− mutant (28, 67). Though it is not clear why heterologous GCN5s corresponding to the full-length yGCN5 could not functionally replace the yGCN5, we could envisage that sequence divergence in the ADA2-binding domain of PfGCN5 from that of yGCN5 may have markedly reduced its ability to interact with yADA2. In fact, only 22% amino acid identity is observed between the ADA2-binding domains of Plasmodium GCN5s and yGCN5. In sharp contrast, the most conserved HAT domain in PfGCN5 is interchangeable with that of the yGCN5. Chimera 1, comprising the PfGCN5 HAT domain and the remainder of yGCN5, fully rescued the yeast gcn5− mutant. Because constructs lacking the N-terminal 14 aa of the HAT domain had significantly decreased their complementing activity, the integrity of the N terminus of the HAT domain (motif C of the GNAT superfamily) seems essential for the in vivo function of the PfGCN5 HAT domain in yeast. In the 3-D structure, this region is upstream of the first α-helix (19). Taken together, our data demonstrate the functional conservation of the PfGCN5 HAT domain.
HAT complexes.
Although the GCN5 protein or the HAT domain alone has been shown to efficiently acetylate free histones, it requires interactions with other proteins to acetylate histones in nucleosomes. Genetic and biochemical studies demonstrate that the three adaptor proteins ADA2, ADA3, and GCN5 interact to form a catalytic core (11, 12, 22, 29, 44), which is sufficient to acetylate nucleosomal histones in vitro (2). This catalytic trimer has been identified in at least two yeast native HAT complexes, the ADA and SAGA complexes (25). Although GCN5-containing complexes may vary from one organism to another, many components of the HAT complexes are evolutionarily conserved. For instance, almost all GNAT complexes so far characterized contained ADA3 (reviewed in reference 8). Besides, ADA2 homologues have been documented in humans (4, 11), Drosophila (37, 46), Arabidopsis (57), and Plasmodium (Fan and Cui, unpublished). Interestingly, two ADA2 homologues are present in higher eukaryotes such as Arabidopsis (57), Drosophila (39, 48), and humans (4, 11). Moreover, these ADA2 paralogues appear to exist in different multiprotein complexes (4, 39), which perform distinct functions in vivo. In P. falciparum, we have identified a single GCN5 and ADA2 homologue and established direct interactions between them, suggesting that PfADA2 and PfGCN5 might be associated with each other in vivo.
Nucleosome organization and chromatin-remodeling complexes in Plasmodium.
Like other eukaryotes, P. falciparum has four core histones, H2A, H2B, H3, and H4, and its nucleosomal organization is typical of eukaryotes with a nucleosome phasing of 155 ± 5 bp (13, 40, 41, 43). Chromosomes generally maintain such a typical nucleosomal organization (40), and only in the extreme telomeric regions does a short nonnucleosomal chromatin structure exist, which is similar to those reported in a number of lower eukaryotes (20). Although it is not clear how nucleosome structure influences gene expression in malaria parasites, indirect evidence indicates that it may be involved in global gene regulation. An earlier study suggested that P. falciparum chromosomes are functionally compartmentalized into an actively transcribed central domain and transcriptionally silent subtelomeric domains (40). Recent microarray analysis showed that genes involved in growth and maintenance of the parasite are restricted to the central regions of the chromosomes, whereas clusters that function in erythrocyte-remodeling processes are located near the ends of the chromosomes (42). These observations of nonrandom distribution of genes highlight the possibility that gene regulation in malaria is partially chromatin dependent. More direct evidence for the role of chromatin structure in gene regulation came from promoter analysis. Promoter analysis by transient transfection assays shows that plasmid DNA, shortly after being electroporated into the parasite, is not organized into phased nucleosomal arrays, resulting in improper promoter function (30). It is not until the episomal plasmid DNA assembles into nucleosomes or is stably integrated into the chromosome that normal promoter activity is achieved (32). These data strongly suggest that nucleosome organization in Plasmodium is important for regulating gene expression during its life cycle.
In model systems, chromatin remodeling at the promoter region causes dynamic conformational changes of the chromatin during the activation or deactivation of the gene. Consequently, changes in the chromatin structures at the promoter region have a profound effect on the activity of the promoter. Although such remodeling promoters have not been identified in P. falciparum, the parasite genome does encode essential components of two major chromatin-remodeling complexes, the ATP-dependent SWI/SNF and HAT complexes. First, a homologue of the Snf-2 family (SNF2L) belonging to the SWI/SNF complex is located on chromosome 11 and transcribed throughout the asexual stages (35). Subsequently, an HDAC has been identified from the parasite which may be responsible for histone hypoacetylation, repressed nucleosome structure, and gene inactivation (36). In this report, we have characterized the HAT protein PfGCN5, an antagonist to HDAC, and provided compelling evidence about the functional conservation of PfGCN5 and PfADA2. The finding that perturbation of histone acetylation by HDAC inhibitors has various levels of antiparasitic effect against Apicomplexan parasites (1, 17) further stresses the importance of histone acetylation and deacetylation in transcriptional regulation. Collectively, these data indicate that chromatin-remodeling complexes are present in P. falciparum and may play an essential role in regulating gene expression during parasite development. Research in this area may yield significant leads in the discovery of novel chemotherapeutic drugs for battling parasitic diseases.
Acknowledgments
We thank Jerry Workman at the Stowers Institute for providing the yeast strains and Joe Reese of the Department of Biochemistry and Molecular Biology, The Pennsylvania State University, for providing the yeast expression vectors and very helpful suggestions for the yeast complementation experiments. We also thank Jinfang Li for assisting with the RLM-RACE experiment and Anthony Pomicter for maintaining the malaria culture.
This research is partially supported by the National Institutes of Health grant AI46472 to L.C.
REFERENCES
- 1.Andrews, K. T., A. Walduck, M. J. Kelso, D. P. Fairlie, A. Saul, and P. G. Parsons. 2000. Anti-malarial effect of histone deacetylation inhibitors and mammalian tumour cytodifferentiating agents. Int. J. Parasitol. 30:761-768. [DOI] [PubMed] [Google Scholar]
- 2.Balasubramanian, R., M. G. Pray-Grant, W. Selleck, P. A. Grant, and S. Tan. 2002. Role of the Ada2 and Ada3 transcriptional coactivators in histone acetylation. J. Biol. Chem. 277:7989-7995. [DOI] [PubMed] [Google Scholar]
- 3.Barlev, N. A., R. Candau, L. Wang, P. Darpino, N. Silverman, and S. L. Berger. 1995. Characterization and physical interactions of the putative transcriptional adaptor, ADA2, with acidic activation domains and TATA-binding protein. J. Biol. Chem. 270:19337-19344. [DOI] [PubMed] [Google Scholar]
- 4.Barlev, N. A., A. V. Emelyanov, P. Castagnino, P. Zegerman, A. J. Bannister, M. A. Sepulveda, F. Robert, L. Tora, T. Kouzarides, B. K. Birshtein, and S. L. Berger. 2003. A novel human Ada2 homologue functions with Gcn5 or Brg1 to coactivate transcription. Mol. Cell. Biol. 23:6944-6957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berger, S. L. 2002. Histone modifications in transcriptional regulation. Curr. Opin. Genet. Dev. 12:142-148. [DOI] [PubMed] [Google Scholar]
- 6.Berger, S. L., B. Pina, N. Silverman, G. A. Marcus, J. Agapite, J. L. Regier, S. J. Triezenberg, and L. Guarente. 1992. Genetic isolation of Ada2: a potential transcriptional adaptor required for function of certain acidic activation domains. Cell 70:251-265. [DOI] [PubMed] [Google Scholar]
- 7.Bozdech, Z., M. Llinas, B. L. Pulliam, E. D. Wong, J. Zhu, and J. L. DeRisi. 18 August 2003. The transcriptome of the intraerythrocytic developmental cycle of Plasmodium falciparum. PloS Biol. 10.1371/journal.pbio.0000005. [DOI] [PMC free article] [PubMed]
- 8.Brown, C. E., T. Lechner, L. Howe, and J. L. Workman. 2000. The many HATs of transcription coactivators. Trends Biochem. Sci. 25:15-19. [DOI] [PubMed] [Google Scholar]
- 9.Brownell, J. E., J. Zhou, T. Ranalli, R. Kobayashi, D. G. Edmondson, S. Y. Roth, and C. D. Allis. 1996. Tetrahymena histone acetyltransferase A: a homolog to yeast Gcn5p linking histone acetylation to gene activation. Cell 84:843-851. [DOI] [PubMed] [Google Scholar]
- 10.Candau, R., and S. L. Berger. 1996. Structural and functional analysis of yeast putative adaptors. Evidence for an adaptor complex in vivo. J. Biol. Chem. 271:5237-5245. [DOI] [PubMed] [Google Scholar]
- 11.Candau, R., P. A. Moore, L. Wang, N. Barlev, C. Y. Ying, C. A. Rosen, and S. L. Berger. 1996. Identification of human proteins functionally conserved with the yeast putative adaptors ADA2 and GCN5. Mol. Cell. Biol. 16:593-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Candau, R., J. Zhou, C. D. Allis, and S. L. Berger. 1997. Histone acetyltransferase activity and interaction with ADA2 are critical for GCN5 function in vivo. EMBO J. 16:555-565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cary, C., D. Lamont, J. P. Dalton, and C. Doerig. 1994. Plasmodium falciparum chromatin: nucleosomal organisation and histone-like proteins. Parasitol. Res. 80:255-258. [DOI] [PubMed] [Google Scholar]
- 14.Chiang, Y. C., P. Komarnitsky, D. Chase, and C. L. Denis. 1996. ADR1 activation domains contact the histone acetyltransferase GCN5 and the core transcriptional factor TFIIB. J. Biol. Chem. 271:32359-32365. [DOI] [PubMed] [Google Scholar]
- 15.Cui, L., K. A. Rzomp, Q. Fan, S. K. Martin, and J. Williams. 2001. Plasmodium falciparum: Differential display analysis of gene expression during gametocytogenesis. Exp. Parasitol. 99:244-254. [DOI] [PubMed] [Google Scholar]
- 16.Cui, L., Q. Fan, and J. Li. 2002. The malaria parasite Plasmodium falciparum encodes members of the Puf RNA-binding protein family with conserved RNA binding activity. Nucleic Acids Res. 30:4607-4617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Darkin-Rattray, S. J., A. M. Gurnett, R. W. Myers, P. M. Dulski, T. M. Crumley, J. J. Allocco, C. Cannova, P. T. Meinke, S. L. Colletti, M. A. Bednarek, S. B. Singh, M. A. Goetz, A. W. Dombrowski, J. D. Polishook, and D. M. Schmatz. 1996. Apicidin: a novel antiprotozoal agent that inhibits parasite histone deacetylase. Proc. Natl. Acad. Sci. USA 93:13143-13147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dechering, K. J., K. Cuelenaere, R. N. H. Honinkgs, and J. A. M. Leunissen. 1998. Distinct frequency-distributions of homopolymeric DNA tracts in different genomes. Nucleic Acids Res. 26:4056-4062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dyda, F., D. C. Klein, and A. B. Hickman. 2000. GCN5-related N-acetyltransferases: a structural overview. Annu. Rev. Biophys. Biomol. Struct. 29:81-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Figueiredo, L. M., L. A. Pirritt, and A. Scherf. 2000. Genomic organisation and chromatin structure of Plasmodium falciparum chromosome ends. Mol. Biochem. Parasitol. 106:169-174. [DOI] [PubMed] [Google Scholar]
- 21.Garnham, P. C. C. 1988. Malaria parasites of man: life cycles and morphology, p. 61-96. In W. H. Wernsdorfer and I. McGrego (ed.), Malaria: principles and practice of malariology. Churchill Livingstone, Oxford, United Kingdom.
- 22.Georgakopoulos, T., N. Gounalaki, and G. Thireos. 1995. Genetic evidence for the interaction of yeast transcriptional co-activator proteins GCN5 and ADA2. Mol. Gen. Genet. 246:723-728. [DOI] [PubMed] [Google Scholar]
- 23.Gietz, D., A. St. Jean, R. A. Woods, and R. H. Schiestl. 1992. Improved method for high efficiency transformation of intact yeast cells. Nucleic Acids Res. 20:1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grant, P. A., and S. L. Berger. 1999. Histone acetyltransferase complexes. Semin. Cell Dev. Biol. 10:169-177. [DOI] [PubMed] [Google Scholar]
- 25.Grant, P. A., L. Duggan, J. Cote, S. M. Roberts, J. E. Brownell, R. Candau, R. Ohba, T. Owen-Hughes, C. D. Allis, F. Winston, S. L. Berger, and J. L. Workman. 1997. Yeast Gcn5 functions in two multisubunit complexes to acetylate nucelosomal histones: characterization of an Ada complex and the SAGA (Spt/Ada) complex. Genes Dev. 11:1640-1650. [DOI] [PubMed] [Google Scholar]
- 26.Grunstein, M. 1997. Histone acetylation in chromatin structure and transcription. Nature 389:349-352. [DOI] [PubMed] [Google Scholar]
- 27.Guarente, L. 1995. Transcriptional coactivators in yeast and beyond. Trends Biochem. Sci. 20:517-521. [DOI] [PubMed] [Google Scholar]
- 28.Hettmann, C., and D. Soldati. 1999. Cloning and analysis of a Toxoplasma gondii histone acetyltransferase: a novel chromatin remodeling factor in Apicomplexan parasites. Nucleic Acids Res. 27:4344-4352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Horiuki, J., N. Silverman, G. A. Marcus, and L. Guarente. 1995. ADA2, a putative transcriptional adaptor, consists of two separable domains and interacts with ADA3 and GCN5 in a trimeric complex. Mol. Cell. Biol. 15:1203-1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Horrocks, P., and M. Lanzer. 1999. Differences in nucleosome organization over episomally located plasmids coincides with aberrant promoter activity in P. falciparum. Parasitol. Int. 48:55-61. [DOI] [PubMed] [Google Scholar]
- 31.Horrocks, P., and M. Lanzer. 1999. Mutational analysis identifies a five base pair cis-acting sequence essential for GBP130 promoter activity in Plasmodium falciparum. Mol. Biochem. Parasitol. 99:77-87. [DOI] [PubMed] [Google Scholar]
- 32.Horrocks, P., R. Pinches, N. Kriek, and C. Newbold. 2002. Stage-specific promoter activity from stably maintained episomes in Plasmodium falciparum. Int. J. Parasitol. 32:1203-1206. [DOI] [PubMed] [Google Scholar]
- 33.Horton, R. M., Z. Cai, S. N. Ho, and L. R. Pease. 1990. Gene splicing by overlap extension: tailor-made genes using the polymerase chain reaction. BioTechniques 8:528-535. [PubMed] [Google Scholar]
- 34.Hurooka, H., and T. Honji. 2000. Functional interaction between the mouse Notch1 intracellular region and histone acetyltransferases PCAF and GCN5. J. Biol. Chem. 275:17211-17220. [DOI] [PubMed] [Google Scholar]
- 35.Ji, D.-D., and D. E. Arnot. 1997. A Plasmodium falciparum homologue of the ATPase subunit of a multi-protein complex involved in chromatin remodeling for transcription. Mol. Biochem. Parasitol. 88:151-162. [DOI] [PubMed] [Google Scholar]
- 36.Joshi, M. B., D. T. Lin, P. H. Chiang, N. D. Goldman, H. Fujioka, M. Aikawa, and C. Syin. 1999. Molecular cloning and nuclear localization of a histone deacetylase homologue in Plasmodium falciparum. Mol. Biochem. Parasitol. 99:11-19. [DOI] [PubMed] [Google Scholar]
- 37.Kawasaki, H., L. Schlitz, R. Chiu, K. Itakura, K. Taira, Y. Nakatani, and K. K. Yokoyama. 2000. ATF-2 has intrinsic histone acetyltransferase activity which is modulated by phosphorylation. Nature 405:195-200. [DOI] [PubMed] [Google Scholar]
- 38.Kuo, M.-H., J. Brownell, R. E. Sobel, T. A. Ranalli, D. G. Edmonson, S. Y. Roth, and C. D. Allis. 1996. Transcription-linked acetylation by Gcn5p of histone H3 and H4 at specific lysines. Nature 383:269-272. [DOI] [PubMed] [Google Scholar]
- 39.Kusch, T., S. Guelman, S. M. Abmayr, and J. L. Workman. 2003. Two Drosophila Ada2 homologues function in different multiprotein complexes. Mol. Cell. Biol. 23:3305-3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lanzer, M., D. de Bruin, and J. V. Ravetch. 1993. Transcriptional differences in polymorphic and conserved domains of a complete cloned P. falciparum chromosome. Nature 361:654-657. [DOI] [PubMed] [Google Scholar]
- 41.Lanzer, M., D. de Bruin, S. P. Wertheimer, and J. V. Ravetch. 1994. Transcriptional and nucleosomal characterization of a subtelomeric gene cluster flanking a site of chromosomal rearrangements in Plasmodium falciparum. Nucleic Acids Res. 22:4176-4182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.La Roch, K. G., Y. Zhou, P. L. Blair, M. Grainger, J. K. Moch, J. D. Haynes, P. De la Vega, A. A. Holder, S. Batalov, D. J. Carucci, and E. A. Winzeler. 2003. Discovery of gene function by expression profiling of the malaria parasite life cycle. Science 301:1503-1508. [DOI] [PubMed] [Google Scholar]
- 43.Longhurst, H. J., and A. A. Holder. 1997. The histones of Plasmodium falciparum: identification, purification and a possible role in the pathology of malaria. Parasitology 114:413-419. [DOI] [PubMed] [Google Scholar]
- 44.Marcus, G. A., N. Silverman, S. L. Berger, J. Horiuki, and L. Guarente. 1994. Functional similarity and physical association between GCN5 and ADA2: putative transcriptional adaptors. EMBO J. 13:4807-4815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marmorstein, R. 2001. Structure of histone acetyltransferases. J. Mol. Biol. 311:433-444. [DOI] [PubMed] [Google Scholar]
- 46.Marmorstein, R., and S. L. Berger. 2001. Structure and function of bromodomains in chromatin-regulation complexes. Gene 272:1-9. [DOI] [PubMed] [Google Scholar]
- 47.Maruyama, K., and S. Sugano. 1994. Oligo-capping: a simple method to replace the cap structure of eukaryotic mRNAs with oligoribonucleotides. Gene 138:171-174. [DOI] [PubMed] [Google Scholar]
- 48.Muratoglu, S., S. Georgieva, G. Pápai, E. Scheer, I. Enünlü, O. Komonyi, I. Cserpán, L. Lebedeva, E. Nabirochkina, A. Udvardy, L. Tora, and I. Boros. 2003. Two different Drosophila ADA2 homologues are present in distinct GCN5 histone acetyltransferase-containing complexes. Mol. Cell. Biol. 23:306-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Neuwald, A. F., and D. Landsman. 1997. GCN5-related histone N-acetyltransferases belong to a diverse superfamily that includes the yeast SPT10 protein. Trends Biochem. Sci. 22:154-155. [DOI] [PubMed] [Google Scholar]
- 50.Ogryzko, V. V., R. L. Schlitz, V. Russanova, B. H. Howard, and Y. Nakatani. 1996. The transcriptional coactivators p300 and CBP are histone acetyltransferases. Cell 87:953-959. [DOI] [PubMed] [Google Scholar]
- 51.Poter, M. 2001. The DNA polymerase δ promoter from Plasmodium falciparum contains an unusually long 5′ untranslated region and intrinsic DNA curvature. Mol. Biochem. Parasitol. 114:249-255. [DOI] [PubMed] [Google Scholar]
- 52.Ruiz-Garcia, A. B., R. Sendra, M. Pamblanco, and V. Tordera. 1997. Gcn5p is involved in the acetylation of histone H3 in nucleosomes. FEBS Lett. 403:186-190. [DOI] [PubMed] [Google Scholar]
- 53.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 54.Schena, M., D. Picard, and K. R. Yamamoto. 1991. Vectors for constitutive and inducible gene expression in yeast. Methods Enzymol. 194:389-398. [DOI] [PubMed] [Google Scholar]
- 55.Smith, E. R., J. M. Belote, R. L. Schiltz, X.-J. Yang, P. A. Moore, S. L. Berger, Y. Nakatani, and C. D. Allis. 1998. Cloning of Drosophila GCN5: conserved features among metazoan GCN5 family members. Nucleic Acids Res. 26:2948-2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sterner, D. E., and S. L. Berger. 2000. Acetylation of histones and transcription-related factors. Microbiol. Mol. Biol. Rev. 64:435-459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sterner, D. E., P. A. Grant, S. M. Roberts, L. J. Duggan, R. Belotserkovskaya, L. A. Pacella, F. Winston, J. L. Workman, and S. L. Berger. 1999. Functional organization of the yeast SAGA complex: distinct components involved in structural integrity, nucleosome acetylation, and TATA-binding protein interaction. Mol. Cell. Biol. 19:86-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sterner, D. E., X. Wang, M. H. Bloom, G. M. Simon, and S. L. Berger. 2002. The SANT domain of Ada2 is required for normal acetylation of histones by the yeast SAGA complex. J. Biol. Chem. 277:8178-8186. [DOI] [PubMed] [Google Scholar]
- 59.Stockinger, E. J., Y. Mao, M. K. Regier, S. J. Triezenberg, and M. F. Thomashow. 2001. Transcriptional adaptor and histone acetyltransferase proteins in Arabidopsis and their interactions with CBF1, a transcriptional activator involved in cold-regulated gene expression. Nucleic Acids Res. 29:1524-1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Struhl, K. 1998. Histone acetylation and transcriptional regulatory mechanisms. Genes Dev. 12:599-606. [DOI] [PubMed] [Google Scholar]
- 61.Sullivan, W. J., Jr., and C. K. Smith II. 2000. Cloning and characterization of a novel histone acetyltransferase homologue from the protozoan parasite Toxoplasma gondii reveals a distinct GCN5 family member. Gene 242:193-200. [DOI] [PubMed] [Google Scholar]
- 62.Te Heesen, S., and I. Stagljar. 2000. Two-hybrid system applicable to membrane protein, p. 117-127. In L. Zhu and G. J. Hannon (ed.), Yeast hybrid technologies. Eaton Publishing, Natick, Mass.
- 63.Trievel, R. C., J. R. Rojas, D. E. Sterner, R. Venkataramani, L. Wang, J. Zhou, C. D. Allis, S. L. Berger, and R. Marmorstein. 1999. Crystal structure and mechanism of histone acetylation of the yeast GCN5 transcriptional coactivator. Proc. Natl. Acad. Sci. USA 96:8931-8936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Utley, R. T., K. Ikeda, P. A. Grant, J. Cote, D. J. Steger, A. Eberharter, S. John, and J. L. Workman. 1998. Transcriptional activators direct histone acetyltransferase complexes to nucleosomes. Nature 394:498-502. [DOI] [PubMed] [Google Scholar]
- 65.Vagnali, M., A. H. Hassan, K. E. Neely, and J. L. Workman. 2000. ATP-dependent chromatin-remodeling complexes. Mol. Cell. Biol. 20:1899-1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang, L., L. Liu, and S. L. Berger. 1998. Critical residues for histone acetylation by Gcn5, functioning in Ada and SAGA complexes, are also required for transcriptional function in vivo. Genes Dev. 12:640-653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang, L., C. Mizzen, C. Ying, R. Candau, N. Barlev, J. Brownell, C. D. Allis, and S. L. Berger. 1997. Histone acetyltransferase activity is conserved between yeast and human GCN5 and is required for complementation of growth and transcriptional activation. Mol. Cell. Biol. 17:519-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Watanabe, J., M. Sasaki, Y. Suzuki, and S. Sugano. 2002. Analysis of transcriptomes of human malaria parasite Plasmodium falciparum using full-length enriched library: identification of novel genes and diverse transcription start sites of messenger RNAs. Gene 291:105-113. [DOI] [PubMed] [Google Scholar]
- 69.Workman, J. L., and R. E. Kingston. 1998. Alteration of nucleosome structure as a mechanism of transcriptional regulation. Annu. Rev. Biochem. 67:545-579. [DOI] [PubMed] [Google Scholar]
- 70.Xu, W., D. G. Edmondson, and S. Y. Roth. 1998. Mammalian GCN5 and P/CAF acetyltransferases have homologous amino-terminal domains important for recognition of nucleosomal substrates. Mol. Cell. Biol. 18:5659-5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yang, X.-J., V. V. Ogryzko, J. Nishikawa, B. H. Howard, and Y. Nakatani. 1996. A p300/CBP-associated factor that competes with the adenoviral E1A oncoprotein. Nature 382:319-324. [DOI] [PubMed] [Google Scholar]