Abstract
BACKGROUND:
Nonalcoholic fatty liver disease (NAFLD) exhibits tight links with insulin resistance (IR) and the metabolic syndrome (MetS), a cluster of cardiovascular risk factors. Compared with non-Hispanic whites, non-Hispanic black adolescents have more IR but a lower prevalence of NAFLD and MetS. Our hypothesis was that IR would be a better predictor of alanine aminotransferase (ALT) elevations than is MetS among non-Hispanic blacks.
METHODS:
We analyzed data from 4124 adolescents aged 12 to 19 years in the 1999 to 2010 NHANES, using unexplained elevations in ALT (>30 U/L) to characterize presumed NAFLD and using a pediatric adaptation of the Adult Treatment Panel III definition of MetS.
RESULTS:
Prevalence of elevated ALT varied by race/ethnicity (Hispanics 13.7%, non-Hispanic white 8.6%, non-Hispanic blacks 5.4%, P < .0001). Among non-Hispanic whites and Hispanics, a classification of MetS performed well in identifying adolescents with elevated ALT (odds ratios [ORs] 9.53 and 5.56, respectively), as did MetS-related indices. However, among non-Hispanic blacks, the association between MetS and ALT elevations was smaller in magnitude and technically nonsignificant (OR = 3.24, P = .051). Furthermore, among non-Hispanic blacks, the presence of IR and elevated waist circumference performed more poorly at identifying ALT elevations (ORs 3.93 and 2.28, respectively: significantly smaller than ORs for non-Hispanic whites, P < .05), with triglyceride elevations being a better predictor (OR = 4.44).
CONCLUSIONS:
Non-Hispanic black adolescents exhibit a lower relationship between IR and elevated ALT, supporting racial/ethnic differences in the link between MetS and NAFLD. These data may have implications regarding triggers for screening for NAFLD among non-Hispanic black adolescents, focusing particularly on those with triglyceride elevations.
Keywords: metabolic syndrome, visceral obesity, inflammation, racial/ethnic difference
What’s Known on This Subject:
Evaluating for elevations in alanine aminotransferase (ALT) is a common screening test for the presence of nonalcoholic fatty liver disease (NAFLD). NAFLD is less common among non-Hispanic blacks. Better predictors of NAFLD are needed to identify individuals in most need of screening.
What This Study Adds:
Relative to other ethnicities, metabolic syndrome and insulin resistance performed poorly at identifying non-Hispanic black adolescents with ALT elevations. The presence of metabolic syndrome may therefore not be an adequate trigger for NAFLD screening. Triglyceride elevations performed similarly between groups in identifying ALT elevations.
Pediatric nonalcoholic fatty liver disease (NAFLD) is a spectrum of liver tissue abnormalities involving hepatic fatty infiltration, ranging from simple steatosis to hepatitis to end-stage liver disease.1,2 Inflammation and cellular injury are often indicated by elevations in liver enzymes, particularly alanine aminotransferase (ALT).3–5 Pediatric NAFLD has evolved into a serious public health issue, affecting between 7% to 17% of adolescents.6,7 Although treatments are limited, lifestyle intervention and weight loss appear beneficial.8–10 Long-term prognosis without intervention is variable, but many children may ultimately progress to nonalcoholic steatohepatitis or cirrhosis in childhood or young adulthood.11,12 Because the diagnosis of NAFLD involves an invasive procedure (liver biopsy) for its diagnosis, alternative markers have been identified for the presence of NAFLD, of which ALT is the best marker.5,7,13 Although elevations in ALT are in no way diagnostic of the presence of NAFLD, elevated ALT in 1 study had a sensitivity of 0.92 for identifying fatty-fibrotic findings on ultrasound.14
NAFLD is tightly linked to obesity. Obese children have a sevenfold higher prevalence of NAFLD compared with normal-weight children.6 NAFLD is also independently linked to metabolic syndrome (MetS), a cluster of clinical signs and symptoms associated with insulin resistance (IR), including dyslipidemia (elevated triglycerides, low high-density lipoprotein cholesterol [HDL-C]), elevated blood pressure (BP), central/abdominal obesity, and elevated fasting glucose.15–17 Obese adolescents with MetS have a fivefold increase in NAFLD compared with obese adolescents without MetS.17
The link between NAFLD and IR has raised speculation regarding interrelationships between NAFLD and IR, with shared features of visceral obesity (frequently assessed as elevations in waist circumference [WC]),18 elevated triglycerides, and underlying inflammation, indicated by increases in high-sensitivity C-reactive protein (hsCRP)19–21 and γ-glutamyl transferase (GGT).22,23 The tight link between MetS and NAFLD has prompted some to suggest screening for NAFLD (via assessment for unexplained ALT elevations >30 U/L) among adolescents with MetS.17
However, racial/ethnic discrepancies in MetS may affect its relationship to NAFLD. Non-Hispanic black adolescents have a lower prevalence of MetS24–27 and NAFLD5,18,28–30 despite having more IR.26,31–35 Conversely, Hispanics have a higher prevalence of NAFLD despite similar rates of MetS.5,7,13,18,24,25
Given these intergroup differences, our goal was to assess relationships among MetS, IR, and NAFLD between racial/ethnic groups. We evaluated adolescents from the NHANES for unexplained elevations in ALT as a potential marker for NAFLD. Our hypothesis was that given the poor accuracy of MetS for identifying IR in non-Hispanic blacks, MetS would be an overall poor predictor of unexplained ALT elevations among non-Hispanic blacks. We also hypothesized that IR itself would be a better predictor of elevated ALT among non-Hispanic black adolescents. Such data regarding interethnic differences may lead to insights into the underlying processes linking NAFLD and IR.
Methods
Data were obtained from NHANES (1999–2010), a multistage probability sample of the US population. These cross-sectional surveys are conducted by the National Center for Health Statistics of the Centers for Disease Control, with randomly selected subjects undergoing anthropometric and BP measurements, answering questionnaires, and undergoing phlebotomy (http://www.cdc.gov/nchs/nhanes.htm). The National Center for Health Statistics ethics review board reviewed and approved the survey, and participants gave informed consent before participation. Measurement of weight, height, BMI, WC, BP, and laboratory measures of ALT, aspartate aminotransferase, GGT, hsCRP, triglycerides, HDL-C, and glucose were obtained by using standardized protocols and calibrated equipment.7,36 Only blood samples obtained after an ≥8-hour fast were analyzed. Participants were classified as overweight if their BMI z score was in the 85th to 95th percentile for age and obese if their BMI z score was ≥95th percentile.
We excluded individuals with positive serologic studies associated with viral hepatitis, including those with a positive hepatitis B core antibody, hepatitis B surface antigen, hepatitis C confirmed antibody, and hepatitis D antibody. Subjects were also excluded if they were pregnant, had known diabetes, or were taking antidiabetic or antihyperlipidemic medications because these situations are all likely to alter lipid and insulin levels in a manner that may not reflect baseline insulin-NAFLD correlations. Individuals taking antihypertensive medication were classified as having hypertension. Data were not readily available regarding alcohol intake in this age range.
Outcome Variable: ALT
We used an upper limit value for ALT of 30 U/L, derived as the 97th percentile from NHANES III37 and used previously.7 Data from non-Hispanic white, non-Hispanic black, or Hispanic (Mexican-American/other Hispanic) adolescents, aged 12 to 19 years were analyzed. Children <12 years were excluded because fasting laboratory values were only obtained in participants ≥12 years.
MetS Classification
MetS was defined by a commonly used pediatric/adolescent adaptation of the Adult Treatment Panel III criteria.36 Participants had to meet ≥3 of the following 5 criteria: (1) concentration of triglycerides ≥110 mg/dL; (2) HDL-C ≤40 mg/dL; (3) WC ≥90th percentile for age/gender (or Adult Treatment Panel III limit of 102 cm for males and 88 cm for females, whichever was lower)16,38; (4) glucose concentration ≥100 mg/dL; and (5) systolic or diastolic BP ≥90th percentile (age, height, and gender-specific).39 Hypertension was defined as systolic or diastolic BP ≥90th percentile for age, height, and gender. The homeostasis model of IR (HOMA-IR) was calculated as [(fasting insulin in mU/mL) × fasting glucose in mg/dL/405]. IR was defined as HOMA-IR ≥4.0 as done previously in adolescents.40
Statistical Analysis
Statistical significance was defined as a P < .05. Statistical analysis was performed using SAS (version 9.3, Cary, NC), using survey procedures (eg, SURVEYREG) that account for the survey design when estimating SEs to obtain population-based estimates. We combined data sets from the six 2-year cycles (1999–2010) for statistical analyses to increase sample size. Prevalence rates of MetS, NAFLD (classified as ALT ≥30), and elevated HOMA (≥4.0) were calculated by gender and race/ethnicity and compared via χ2 tests. Mean levels of relevant continuous measures were compared among groups by using either t tests or analysis of variance. Various regression models were fit to the data, all including gender, education (highest level obtained for any household member), and household income-to-needs ratio as covariates. Logistic regression was used to assess effects of gender, race/ethnicity, and MetS status on the odds of NAFLD. All interactions of the 3 covariates (gender, race/ethnicity, and MetS status) were initially included in the model but removed in a stepwise fashion if the associated interaction P value was >.10. Separate logistic models of NAFLD were also fit to the data, examining the impact of MetS, MetS components, and other elevations (HOMA-IR, hsCRP) on the odds of NAFLD by race/ethnicity. Odds ratios (ORs) adjusting for the aforementioned covariates were estimated to compare odds of NAFLD for increasing numbers of MetS elevations. Linear regression was used to compare mean levels of triglycerides, hsCRP, and GGT between those with ALT ≥30 versus <30. Comparisons between the 2 ALT groups were allowed to vary by race/ethnicity. We stratified subjects by age (boys ≥16 years and girls ≥14 years) to assess for differences in these findings among children likely to be in later pubertal stages.41,42 Natural log transformations were used to achieve normality; geometric means from these models were reported. Finally, OR of ALT elevations were estimated comparing quartiles of various MetS or MetS-related measures.
Results
Prevalence of MetS and Unexplained ALT Elevations
We evaluated 4124 adolescents, including 1207 non-Hispanic whites, 1233 non-Hispanic blacks, and 1684 Hispanics. The prevalence of abnormal ALT levels in adolescents differed by race/ethnicity: Hispanics, 13.7%; non-Hispanic whites, 8.6%; and non-Hispanic blacks, 5.4% (P < .0001; Table 1). Similarly, the prevalence of MetS also differed by race/ethnicity: Hispanics, 10.6%; non-Hispanic whites, 8.4%; and non-Hispanic blacks, 4.2%. Both elevated ALT levels and MetS were more common among overweight and obese adolescents than normal-weight adolescents for each racial/ethnic group (Supplemental Table 5). IR defined as HOMA-IR ≥4.040 was higher in non-Hispanic blacks and Hispanics compared with non-Hispanic whites (Table 1). These findings were similar when fasting insulin was evaluated (data not shown). Mean values for MetS components, insulin, ALT and aspartate aminotransferase, and inflammatory markers are shown by gender and race/ethnicity in Supplemental Table 6. Values differed significantly by race/ethnicity for multiple variables. Non-Hispanic blacks had the highest values of high-density lipoprotein, systolic BP, and GGT, and the lowest values of glucose, triglycerides, and ALT.
TABLE 1.
NHANES 1999–2010 Characteristics: Children Aged 12 to 19 Years With Data on All MetS Components (n = 4124)
| n | % With Elevated ALTa (95% CI) | % With MetSb (95% CI) | % With IRc (95% CI) | |
|---|---|---|---|---|
| Overall | 4124 | 9.0 (7.5–10.5) | 8.1 (6.8–9.5) | 16.0 (14.4–17.5) |
| By gender | ||||
| Female | 1946 | 3.3 (2.4–4.2) | 5.3 (4.1–6.5) | 15.8 (13.5–18.1) |
| Male | 2178 | 14.4 (11.8–17.1) | 10.9 (8.7–13.0) | 16.1 (13.7–18.5) |
| Pd | <.0001 | <.0001 | .8879 | |
| By race/ethnicity | ||||
| Non-Hispanic white | 1207 | 8.6 (6.6–10.5) | 8.4 (6.5–10.2) | 12.9 (10.8–15.0) |
| Non-Hispanic black | 1233 | 5.4 (3.5–7.3) | 4.2 (2.9–5.6) | 19.4 (17.1–21.6) |
| Hispanic | 1684 | 13.7 (11.5–15.8) | 10.6 (8.4–12.7) | 24.3 (21.2–27.4) |
| Pe | <.0001 | .0003 | <.0001 |
CI, confidence interval.
ALT ≥30.
Pediatric Adaptation of Adult Treatment Panel III of Metabolic Syndrome.36
HOMA-IR ≥4.0.
χ2 test comparing rates between males and females.
χ2 test comparing rates among the race/ethnicity groups.
OR of Elevated ALT
In a model adjusting for household education and income, the factors associated with risk of ALT elevation included male gender (OR = 4.59, 95% CI = 3.24–6.49, P < .0001), Hispanic ethnicity (OR = 1.8, 95% CI = 1.24–2.61, P < .01), and MetS (OR = 7.81, 95% CI = 5.24–11.65, P < .0001; Table 2). Notably, there was no significant interaction between gender, race/ethnicity, and MetS in their relationship to elevated ALT, suggesting that MetS correlated similarly between males and females (and among race/ethnic groups) in its relationship to NAFLD.
TABLE 2.
Logistic Model of Odds of ALT ≥30
| Model Covariate | OR Estimate | 95% CI | P |
|---|---|---|---|
| Household education | |||
| Less than high school | 0.93 | (0.55–1.58) | .8002 |
| High school | 0.95 | (0.53–1.71) | .8712 |
| More than high school | 1 (ref) | ||
| Household income | 0.99 | (0.87–1.11) | .8285 |
| Gender | |||
| Male | 4.59 | (3.24–6.49) | <.0001 |
| Female | 1 (ref) | ||
| Race/ethnicity | |||
| Non-Hispanic black | 0.74 | (0.48–1.13) | .1648 |
| Hispanic | 1.80 | (1.24–2.61) | .0022 |
| Non-Hispanic white | 1 (ref) | ||
| MetS | 7.81 | (5.24–11.65) | <.0001 |
All interactions among race/ethnicity, gender, and MetS were not significant (P > .20) and were removed from the model. CI, confidence interval.
Even though the race/ethnicity × MetS interaction was not significant (P = .22), we kept this interaction in the next set of models to allow for the impact of MetS (and its components in separate models each) on the odds of ALT elevation to vary by race/ethnicity (Table 3). Here, MetS was a significant predictor of ALT elevations among non-Hispanic white (OR = 9.53, 95% CI = 5.58–16.27, P < .0001) and Hispanic (OR = 5.56, 95% CI = 3.10–9.95, P < .0001) adolescents. Among non-Hispanic blacks, the relationship between MetS and elevated ALT was lower in magnitude and not technically significant (OR 3.24, 0.99–10.54, P = .0514; Table 3). Abnormalities in individual MetS components, IR, and elevated hsCRP also performed well in general as predictors of ALT elevations. However, elevations in WC and HOMA-IR had significantly lower OR for elevated ALT among non-Hispanic blacks compared with non-Hispanic whites (P = .0044 and P = .0189 for interethnicity comparison, respectively), whereas elevations in triglycerides were the best predictor of ALT elevations among non-Hispanic blacks (Table 3).
TABLE 3.
Adjusted ORs of ALT ≥ 30 by Race/Ethnicity
| OR (95% CI) | |||
|---|---|---|---|
| Non-Hispanic White | Non-Hispanic Black | Hispanic | |
| MetS | 9.53 (5.58–16.27) | 3.24 (0.99–10.54) | 5.56 (3.10–9.95) |
| MetS components | |||
| Elevated WC | 7.24 (4.23–12.37) | 2.28** (1.27–4.10) | 5.72 (3.56–9.19) |
| Elevated BP | 3.83 (2.18–6.72) | 1.98 (0.98–4.01) | 1.91 (0.90–4.05) |
| Low HDL-C | 3.34 (1.94–5.73) | 3.01 (1.37–6.65) | 2.68 (1.57–4.59) |
| Elevated triglycerides | 2.48 (1.50–4.11) | 4.44 (2.32–8.47) | 3.52 (2.16–5.76) |
| Elevated glucose | 2.20 (1.28–3.80) | 2.92 (1.43–5.95) | 1.91 (1.19–3.06) |
| Other outcomes | |||
| Elevated HOMA-IR (≥4.0) | 8.23 (4.94–13.72) | 3.93* (2.31–6.70) | 5.59 (3.28–9.50) |
| Elevated hsCRP (>4.5) | 2.36 (0.90–6.21) | 0.95 (0.43–2.09) | 4.24 (2.09–8.90) |
Adjusted for gender, household income, and highest household education. CI, confidence interval.
P < .05 (Comparing OR to Non-Hispanic whites).
P < .01 (Comparing OR to Non-Hispanic whites).
Inflammation and Elevated ALT
We next assessed for differences in systemic inflammation among adolescents with and without unexplained ALT elevations by racial/ethnic group, using hsCRP and GGT as inflammatory measures and adjusting for household education and income (Table 4). Geometric means of these measures estimated from the linear models of the log-transformed outcomes were compared between those with and without elevated ALT. Among each racial/ethnic group, there was a higher degree of systemic inflammation among adolescents with ALT elevations. Non-Hispanic black adolescents without ALT elevations had higher mean levels of GGT than non-Hispanic whites, whereas Hispanics had lower hsCRP levels than non-Hispanic whites. Levels of inflammatory markers were otherwise similar by race/ethnicity.
TABLE 4.
Comparisons Between Normal Versus Elevated ALT, by Race/Ethnicity
| Non-Hispanic White | Non-Hispanic Black | Hispanic | |
|---|---|---|---|
| Triglycerides, GM (95% CI) | |||
| ALT ≥30 | 111.1 (99.5–125.2) | 76.7a (64.7–90.0) | 104.6 (89.1–122.7) |
| ALT <30 | 82.3 (79.0–85.6) | 60.3a (58.6–62.2) | 77.5a (74.4–79.8) |
| Ratio of GMs | 1.3 (1.2–1.5)b | 1.3 (1.1–1.5)b | 1.4 (1.2–1.6)b |
| HsCRP, GM (95% CI) | |||
| ALT ≥30 | 1.1 (0.9–1.5) | 0.8 (0.7–1.0) | 1.4 (1.0–1.8) |
| ALT <30 | 0.5 (0.5–0.6) | 0.5 (0.5–0.6) | 0.6a (0.5–0.7) |
| Ratio of GMs | 2.2 (1.6–3.0)b | 1.6 (1.2–2.0)b | 2.3 (1.7–3.1)b |
| GGT, GM (95% CI) | |||
| ALT ≥30 | 23.3 (19.1–28.8) | 24.3 (20.3–29.1) | 24.8 (21.8–28.2) |
| ALT <30 | 12.6 (12.1–13.1) | 15.0a (14.4–15.8) | 12.7 (12.2–13.2) |
| Ratio of GMs | 1.9 (1.5–2.3)b | 1.9 (1.3–1.9)b | 2.0 (1.7–2.2)b |
Model of log(outcome), including race/ethnicity, ALT status, and their interaction, adjusting for household education and income. CI, confidence interval; GM, geometric mean.
Geometric mean significantly different than non-Hispanic white (P < .05).
Ratio significantly (P < .05) >1 (ie, significant difference between the ALT groups, within race/ethnicity group).
ALT Elevation by HOMA-IR, WC, and Triglyceride Quartiles
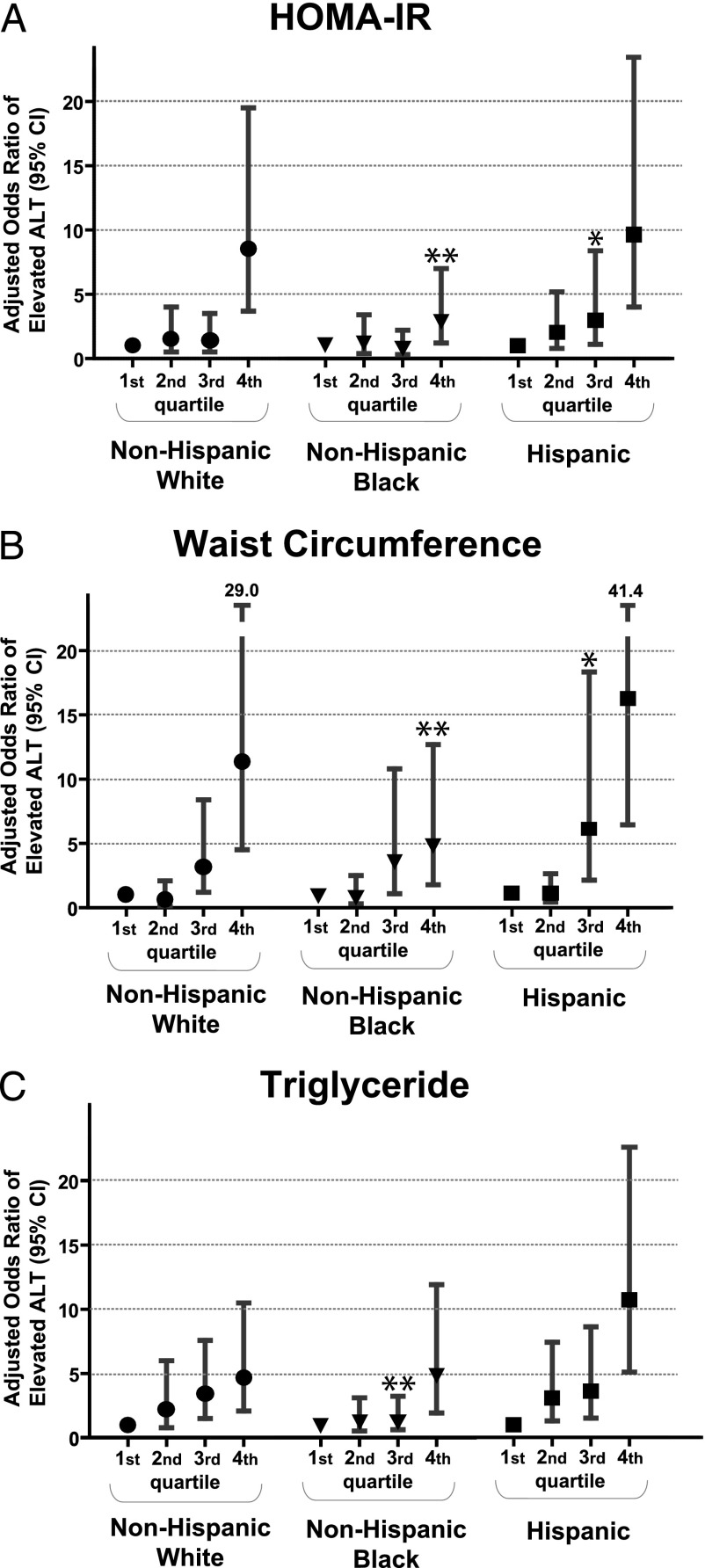
Finally, we divided HOMA-IR, triglycerides, and WC into quartiles among the overall sample to assess for increases in the odds of unexplained ALT elevations with increasing degree of these measures while adjusting for gender, household education, and income. Although all racial/ethnic groups exhibited higher adjusted ORs of ALT elevation among individuals in the highest quartile for HOMA-IR compared with the lowest, this OR was significantly lower among non-Hispanic blacks (2.9) compared with non-Hispanic whites (8.5) but not compared with Hispanics (9.6; Fig 1A). The same was true for OR of ALT elevation by quartiles of WC, with a lower adjusted OR for elevated ALT among non-Hispanic blacks (4.8) compared with non-Hispanic whites (11.4, P < .01) and Hispanics (16.1, P > .05; Fig 1B). Although lower levels of triglycerides carried low OR of ALT elevation in non-Hispanic black adolescents, non-Hispanic blacks with triglycerides in the top quartile exhibited a similar OR (4.8) to that seen in non-Hispanic whites (4.7). Hispanics with triglycerides in the top quartile had the highest OR of ALT elevations of any of the racial/ethnic groups (10.7, P < .01 versus non-Hispanic whites; Fig 1C). When fasting insulin was substituted for HOMA-IR, this yielded nearly identical results (data not shown). We did not have data regarding pubertal status in this database; however, when we stratified by age group to assess these findings among children likely to be in late puberty (boys aged ≥16 years; girls aged ≥14 years41,42), we found similar findings regarding these interethnicity differences (data not shown).
FIGURE 1.
Odds of elevated ALT by racial/ethnic group and by quartiles of insulin, WC, and triglycerides. Odds of ALT elevation are shown for each racial/ethnic group evaluated by quartile of (A) HOMA-IR, (B) WC, and (C) triglycerides, with the first quartile being the referent group. Significance relative to non-Hispanic whites: * P < .05; ** P < .01.
Discussion
Racial/ethnic variation in NAFLD and/or ALT elevations5,7,18,28–30,43,44 and MetS24–27,32–34 have been noted previously, suggesting clear differences in obesity-related changes in fatty liver and IR. Non-Hispanic blacks in particular have a low prevalence of NAFLD5,18 and MetS24–27 despite a high degree of IR. Given known inaccuracies of current MetS criteria in identifying IR and inflammation among non-Hispanic black adolescents, our original hypothesis was that the degree of elevation in HOMA-IR would prove to be better than MetS at predicting of elevations in ALT. This was technically true, with a nonsignificant OR for MetS predicting ALT elevations (OR = 3.24, P = .05), compared with a significant OR for elevated insulin (OR = 3.45, P = .02), although with a larger sample size statistical correlation may have been noted for both. Nevertheless, what was much more striking was that HOMA-IR did not have as close of an association with ALT elevations among non-Hispanic blacks as among the other ethnicities. Although other reports have shown less NAFLD in non-Hispanic blacks, this report represents the first demonstration of a weaker link between HOMA-IR and ALT elevations as a marker for suspected NAFLD among non-Hispanic blacks. That these findings are present (1) at a relatively early age, (2) in a population largely free from medical comorbidities, and (3) after adjustment for socioeconomic factors supports the presence of clinically significant racial/ethnic differences in hepatic response to obesity and IR.
Notably, non-Hispanic blacks are less likely to have visceral adiposity as assessed by computed tomography31,45 and elevated WC,25 potentially producing a lower propensity toward hepatic fat accumulation.18,46 Non-Hispanic blacks also have a low prevalence of MetS diagnosis, predominantly related to having lower triglyceride levels than other racial/ethnic groups,47 making MetS a poorer marker of IR in non-Hispanic blacks.26,32,35,48 Our present findings build on these assertions by revealing that even in the presence of elevated WC and HOMA-IR, non-Hispanic black adolescents have a lower OR than non-Hispanic whites for suspected NAFLD.
In considering these associations, it is notable that the cause-effect relationship between NAFLD and MetS likely involves some degree of overlap. Clearly the presence of NAFLD is associated with elevated levels of inflammation19,20 and IR17,49,50 and, when present, is likely to contribute to the findings associated with MetS.17 In this sense, previous studies would suggest that NAFLD may be a cause of worsening MetS. Conversely, the underlying processes of obesity, inflammation, and adipokines that drive abnormalities associated with MetS are also associated with NAFLD in basic science models51,52 and may contribute to liver fat accumulation through related pathways.15 In this sense, individuals with a high degree of IR from these processes would be expected to have a higher risk of NAFLD, highlighting how NAFLD may be an effect of processes behind MetS.
Nevertheless, both of these cause-effect situations appear diminished among non-Hispanic black adolescents compared with other groups in that the lower prevalence of NAFLD was not associated with lower levels of inflammation or IR, and a similar or higher degree of IR in non-Hispanic blacks compared with other groups did not correlate with higher rates of NAFLD. Although the cross-sectional nature of these data limits the conclusions we can make regarding these relationships, these findings overall suggest a lower degree of association among MetS, IR, and NAFLD in non-Hispanic black adolescents compared with non-Hispanic whites and Hispanics, as illustrated by Table 3 and Fig 1A.
These findings may pertain to differences in body fat distribution among non-Hispanic blacks, who notably have less visceral adiposity.31,46 Although visceral obesity is tightly linked to IR, this link does not appear necessary in non-Hispanic blacks.53 However, non-Hispanic blacks have more subcutaneous fat.46 Basic science studies have suggested that the ability to deposit large quantities of fat in subcutaneous tissue protects against deposition in visceral and hepatic compartments.18,54 Nevertheless, we noted that even when WC is elevated in non-Hispanic black adolescents, suggesting increase in visceral adipose tissue, there were lower ORs for suspected NAFLD than seen for non-Hispanic whites (Fig 1B). This would appear to additionally dissociate visceral adiposity from NAFLD in non-Hispanic black adolescents.
The most notable difference in MetS between non-Hispanic blacks and other ethnicities is a lower prevalence of hypertriglyceridemia, which may be due in part to lower activity of lipoprotein lipase.48 Interestingly, one of the pathophysiologic processes associated with NAFLD is an increase in free fatty acids from the liver, also noted in elevations in triglycerides,17 which are elevated on average >40% among adolescents with suspected NAFLD compared with control subjects.7 We noted that despite lower triglyceride levels overall among non-Hispanic blacks, when non-Hispanic black adolescents do have high triglyceride levels, they had a similar OR for elevated ALT as non-Hispanic whites (Fig 1C). Thus, although ALT increases are associated overall with central obesity, low HDL-C, and high triglycerides, it appears that among non-Hispanic black adolescents, high triglycerides constitute a particularly good marker of elevated ALT risk. It is unclear if non-Hispanic blacks with such elevations in triglycerides represent a subgroup with a particularly progressed condition of MetS or if their triglyceride elevations represent differences in genetic propensity to both manifestation of MetS and development of NAFLD. Regardless, suspicion of potential NAFLD among non-Hispanic blacks should rise when elevated triglycerides are present, more so than other features of MetS, potentially representing a new indicator for screening for NAFLD among non-Hispanic blacks.
In contrast to these findings regarding non-Hispanic blacks, our findings support a relationship between IR and NAFLD that is at least as strong, if not stronger, among Hispanics as among non-Hispanic whites. Hispanics have been noted to have more NAFLD with worsened severity and higher rates of nonalcoholic steatohepatitis.13,55 Although a lower relationship between IR and NAFLD has been suggested among Hispanics versus non-Hispanic whites in past studies,43 other researchers have found that, when matched for the degree of adiposity, Hispanics and non-Hispanic whites exhibited similar relationships between IR and fatty liver disease severity.44 Our data support overall similarities in the relationship between IR and NAFLD among Hispanics and non-Hispanic white adolescents, with, if anything, a tendency toward higher OR for NAFLD in Hispanics with fasting insulin and WC in the highest quartile.
Our study had multiple limitations. We used cross-sectional data from NHANES. This permitted analysis of a large US population-based sample of adolescents, but the cross-sectional nature does not permit conclusions on the temporal nature of MetS and NAFLD. Although powerful, the NHANES data we used also do not contain information regarding pubertal status, which is of clear importance in the consideration of IR; however, when we stratified by age to evaluate children more likely to be in late puberty (boys aged ≥16 years; girls aged ≥14 years41,42) we found similar results, suggesting against a puberty-related cause of these findings. We used unexplained ALT elevations to assess for suspected NAFLD. Although we excluded individuals with serological findings of hepatitis, there still may have been non-NAFLD causes of these ALT elevations such as hepatitis types not evaluated in NHANES, Wilson’s disease, and chronic alcohol use. Nevertheless, use of ALT elevations has been validated in previous studies as a strong predictor of NAFLD in children4 and adults3 and has been widely used in other epidemiologic studies.5,7,13 Finally, we were not able to directly measure IR, instead using HOMA-IR and fasting insulin as measures of IR. Although still an imperfect means of estimating insulin sensitivity, HOMA-IR and fasting insulin overall correlate with the hyperinsulinemic-euglycemic clamp method, the gold standard for assessing IR.56
NAFLD has become the most common liver disease in children. Because of the epidemic increase in childhood obesity, screening mechanisms should be in place for surveying for the potential for NAFLD in children. These data underscore the importance of screening among children with central obesity and MetS, with implications that these children could be targeted for weight loss and other treatments to potentially prevent future complications. Among non-Hispanic black children, elevations in triglycerides may be another striking sign.
Conclusions
We found racial/ethnic differences in the relationship between elevated ALT and IR. The presence of IR and elevated WC were poorer predictors of elevated ALT overall among non-Hispanic blacks compared with non-Hispanic whites and may not be as effective of triggers for screening for NAFLD in non-Hispanic blacks, whereas elevated triglyceride levels may be a better indication for screening among non-Hispanic black adolescents. These data add to accumulating evidence of differences in how MetS and related diseases are manifest in non-Hispanic blacks and may reflect a need for ethnicity-based screening criteria for adolescents at risk for NAFLD. Clearly more research is necessary.
Supplementary Material
Glossary
- ALT
alanine aminotransferase
- BP
blood pressure
- GGT
γ-glutamyl transferase
- HDL-C
high-density lipoprotein cholesterol
- HOMA-IR
homeostasis model of insulin resistance
- hsCRP
high-sensitivity C-reactive protein
- IR
insulin resistance
- MetS
metabolic syndrome
- NAFLD
nonalcoholic fatty liver disease
- OR
odds ratio
- WC
waist circumference
Footnotes
Drs DeBoer, Wiener, and Barnes were involved in the design, interpretation, and write-up of the study; and Dr Gurka was involved in the design, analysis, interpretation, and write-up of the study. All authors approved the final manuscript as submitted.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Supported by National Institutes of Health grants 5K08HD060739-04 (MDD), U54GM104942 (MJG), and 1R21DK085363 (MJG and MDD). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Angulo P, Lindor KD. Non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2002;17(suppl):S186–S190 [DOI] [PubMed] [Google Scholar]
- 2.Mencin AA, Lavine JE. Nonalcoholic fatty liver disease in children. Curr Opin Clin Nutr Metab Care. 2011;14(2):151–157 [DOI] [PubMed] [Google Scholar]
- 3.Dixon JB, Bhathal PS, O’Brien PE. Nonalcoholic fatty liver disease: predictors of nonalcoholic steatohepatitis and liver fibrosis in the severely obese. Gastroenterology. 2001;121(1):91–100 [DOI] [PubMed] [Google Scholar]
- 4.Fishbein MH, Miner M, Mogren C, Chalekson J. The spectrum of fatty liver in obese children and the relationship of serum aminotransferases to severity of steatosis. J Pediatr Gastroenterol Nutr. 2003;36(1):54–61 [DOI] [PubMed] [Google Scholar]
- 5.Schwimmer JB, McGreal N, Deutsch R, Finegold MJ, Lavine JE. Influence of gender, race, and ethnicity on suspected fatty liver in obese adolescents. Pediatrics. 2005;115(5). Available at: www.pediatrics.org/cgi/content/full/115/5/e561 [DOI] [PubMed] [Google Scholar]
- 6.Schwimmer JB, Deutsch R, Kahen T, Lavine JE, Stanley C, Behling C. Prevalence of fatty liver in children and adolescents. Pediatrics. 2006;118(4):1388–1393 [DOI] [PubMed] [Google Scholar]
- 7.Fraser A, Longnecker MP, Lawlor DA. Prevalence of elevated alanine aminotransferase among US adolescents and associated factors: NHANES 1999–2004. Gastroenterology. 2007;133(6):1814–1820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koot BG, van der Baan-Slootweg OH, Tamminga-Smeulders CL, et al. Lifestyle intervention for non-alcoholic fatty liver disease: prospective cohort study of its efficacy and factors related to improvement. Arch Dis Child. 2011;96(7):669–674 [DOI] [PubMed] [Google Scholar]
- 9.Nobili V, Manco M, Devito R, et al. Lifestyle intervention and antioxidant therapy in children with nonalcoholic fatty liver disease: a randomized, controlled trial. Hepatology. 2008;48(1):119–128 [DOI] [PubMed] [Google Scholar]
- 10.Suzuki A, Lindor K, St Saver J, et al. Effect of changes on body weight and lifestyle in nonalcoholic fatty liver disease. J Hepatol. 2005;43(6):1060–1066 [DOI] [PubMed] [Google Scholar]
- 11.Adams LA, Lymp JF, St Sauver J, et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129(1):113–121 [DOI] [PubMed] [Google Scholar]
- 12.Molleston JP, White F, Teckman J, Fitzgerald JF. Obese children with steatohepatitis can develop cirrhosis in childhood. Am J Gastroenterol. 2002;97(9):2460–2462 [DOI] [PubMed] [Google Scholar]
- 13.Graham RC, Burke A, Stettler N. Ethnic and sex differences in the association between metabolic syndrome and suspected nonalcoholic fatty liver disease in a nationally representative sample of US adolescents. J Pediatr Gastroenterol Nutr. 2009;49(4):442–449 [DOI] [PubMed] [Google Scholar]
- 14.Tazawa Y, Noguchi H, Nishinomiya F, Takada G. Serum alanine aminotransferase activity in obese children. Acta Paediatr. 1997;86(3):238–241 [DOI] [PubMed] [Google Scholar]
- 15.Bremer AA, Mietus-Snyder M, Lustig RH. Toward a unifying hypothesis of metabolic syndrome. Pediatrics. 2012;129(3):557–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grundy SM, Cleeman JI, Daniels SR, et al. American Heart Association. National Heart, Lung, and Blood Institute . Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112(17):2735–2752 [DOI] [PubMed] [Google Scholar]
- 17.Schwimmer JB, Pardee PE, Lavine JE, Blumkin AK, Cook S. Cardiovascular risk factors and the metabolic syndrome in pediatric nonalcoholic fatty liver disease. Circulation. 2008;118(3):277–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guerrero R, Vega GL, Grundy SM, Browning JD. Ethnic differences in hepatic steatosis: an insulin resistance paradox? Hepatology. 2009;49(3):791–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oliveira AC, Oliveira AM, Almeida MS, Silva AM, Adan L, Ladeia AM. Alanine aminotransferase and high sensitivity C-reactive protein: correlates of cardiovascular risk factors in youth. J Pediatr. 2008;152(3):337–342 [DOI] [PubMed] [Google Scholar]
- 20.Mandato C, Lucariello S, Licenziati MR, et al. Metabolic, hormonal, oxidative, and inflammatory factors in pediatric obesity-related liver disease. J Pediatr. 2005;147(1):62–66 [DOI] [PubMed] [Google Scholar]
- 21.DeBoer MD. Obesity, systemic inflammation, and increased risk for cardiovascular disease and diabetes among adolescents: a need for screening tools to target interventions. Nutrition. 2013;29(2):379–386 [DOI] [PMC free article] [PubMed]
- 22.Turgut O, Tandogan I, Gurlek A. Association of gamma-glutamyltransferase with cardiovascular risk: a prognostic outlook. Arch Med Res. 2009;40(4):318–320 [DOI] [PubMed] [Google Scholar]
- 23.Verrijken A, Francque S, Mertens I, Talloen M, Peiffer F, Van Gaal L. Visceral adipose tissue and inflammation correlate with elevated liver tests in a cohort of overweight and obese patients. Int J Obes (Lond). 2010;34(5):899–907 [DOI] [PubMed] [Google Scholar]
- 24.Park YW, Zhu S, Palaniappan L, Heshka S, Carnethon MR, Heymsfield SB. The metabolic syndrome: prevalence and associated risk factor findings in the US population from the Third National Health and Nutrition Examination Survey, 1988–1994. Arch Intern Med. 2003;163(4):427–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walker SE, Gurka MJ, Oliver MN, Johns DW, DeBoer MD. Racial/ethnic discrepancies in the metabolic syndrome begin in childhood and persist after adjustment for environmental factors. Nutr Metab Cardiovasc Dis. 2012;22(2):141–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DeBoer MD, Dong L, Gurka MJ. Racial/ethnic and sex differences in the ability of metabolic syndrome criteria to predict elevations in fasting insulin levels in adolescents. J Pediatr. 2011;159(6):975–981.e3 [DOI] [PMC free article] [PubMed]
- 27.Gurka MJ, Ice CL, Sun SS, Deboer MD. A confirmatory factor analysis of the metabolic syndrome in adolescents: an examination of sex and racial/ethnic differences. Cardiovasc Diabetol. 2012;11:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Browning JD, Szczepaniak LS, Dobbins R, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40(6):1387–1395 [DOI] [PubMed] [Google Scholar]
- 29.Caldwell SH, Harris DM, Patrie JT, Hespenheide EE. Is NASH underdiagnosed among African Americans? Am J Gastroenterol. 2002;97(6):1496–1500 [DOI] [PubMed] [Google Scholar]
- 30.Mohanty SR, Troy TN, Huo D, O’Brien BL, Jensen DM, Hart J. Influence of ethnicity on histological differences in non-alcoholic fatty liver disease. J Hepatol. 2009;50(4):797–804 [DOI] [PubMed] [Google Scholar]
- 31.Bacha F, Saad R, Gungor N, Janosky J, Arslanian SA. Obesity, regional fat distribution, and syndrome X in obese black versus white adolescents: race differential in diabetogenic and atherogenic risk factors. J Clin Endocrinol Metab. 2003;88(6):2534–2540 [DOI] [PubMed] [Google Scholar]
- 32.Deboer MD. Ethnicity, obesity and the metabolic syndrome: implications on assessing risk and targeting intervention. Expert Rev Endocrinol Metab. 2011;6(2):279–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.DeBoer MD, Gurka MJ. Low sensitivity for the metabolic syndrome to detect uric acid elevations in females and non-Hispanic-black male adolescents: an analysis of NHANES 1999–2006. Atherosclerosis. 2012;220(2):575–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DeBoer MD, Gurka MJ, Sumner AE. Diagnosis of the metabolic syndrome is associated with disproportionately high levels of high-sensitivity C-reactive protein in non-Hispanic black adolescents: an analysis of NHANES 1999–2008. Diabetes Care. 2011;34(3):734–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee S, Bacha F, Gungor N, Arslanian SA. Racial differences in adiponectin in youth: relationship to visceral fat and insulin sensitivity. Diabetes Care. 2006;29(1):51–56 [DOI] [PubMed] [Google Scholar]
- 36.Ford ES, Li C, Cook S, Choi HK. Serum concentrations of uric acid and the metabolic syndrome among US children and adolescents. Circulation. 2007;115(19):2526–2532 [DOI] [PubMed] [Google Scholar]
- 37.Strauss RS, Barlow SE, Dietz WH. Prevalence of abnormal serum aminotransferase values in overweight and obese adolescents. J Pediatr. 2000;136(6):727–733 [PubMed] [Google Scholar]
- 38.Fernández JR, Redden DT, Pietrobelli A, Allison DB. Waist circumference percentiles in nationally representative samples of African-American, European-American, and Mexican-American children and adolescents. J Pediatr. 2004;145(4):439–444 [DOI] [PubMed] [Google Scholar]
- 39.The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114(suppl 2):555–576 [PubMed]
- 40.Reinehr T, Andler W. Changes in the atherogenic risk factor profile according to degree of weight loss. Arch Dis Child. 2004;89(5):419–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anderson SE, Must A. Interpreting the continued decline in the average age at menarche: results from two nationally representative surveys of U.S. girls studied 10 years apart. J Pediatr. 2005;147(6):753–760 [DOI] [PubMed] [Google Scholar]
- 42.Herman-Giddens ME, Steffes J, Harris D, et al. Secondary sexual characteristics in boys: data from the Pediatric Research in Office Settings Network. Pediatrics. 2012;130(5). Available at: www.pediatrics.org/cgi/content/full/130/5/e1058 [DOI] [PubMed] [Google Scholar]
- 43.Bambha K, Belt P, Abraham M, et al. Nonalcoholic Steatohepatitis Clinical Research Network Research Group . Ethnicity and nonalcoholic fatty liver disease. Hepatology. 2012;55(3):769–780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lomonaco R, Ortiz-Lopez C, Orsak B, et al. Role of ethnicity in overweight and obese patients with nonalcoholic steatohepatitis. Hepatology. 2011;54(3):837–845 [DOI] [PubMed] [Google Scholar]
- 45.Hill JO, Sidney S, Lewis CE, Tolan K, Scherzinger AL, Stamm ER. Racial differences in amounts of visceral adipose tissue in young adults: the CARDIA (Coronary Artery Risk Development in Young Adults) study. Am J Clin Nutr. 1999;69(3):381–387 [DOI] [PubMed] [Google Scholar]
- 46.Liska D, Dufour S, Zern TL, et al. Interethnic differences in muscle, liver and abdominal fat partitioning in obese adolescents. PLoS ONE. 2007;2(6):e569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sumner AE, Cowie CC. Ethnic differences in the ability of triglyceride levels to identify insulin resistance. Atherosclerosis. 2008;196(2):696–703 [DOI] [PubMed] [Google Scholar]
- 48.Sumner AE. Ethnic differences in triglyceride levels and high-density lipoprotein lead to underdiagnosis of the metabolic syndrome in black children and adults. J Pediatr. 2009;155(3):S7.e7–e11 [DOI] [PMC free article] [PubMed]
- 49.Cusi K. Role of obesity and lipotoxicity in the development of nonalcoholic steatohepatitis: pathophysiology and clinical implications. Gastroenterology. 2012;142(4):711–725.e6 [DOI] [PubMed]
- 50.D’Adamo E, Cali AM, Weiss R, et al. Central role of fatty liver in the pathogenesis of insulin resistance in obese adolescents. Diabetes Care. 2010;33(8):1817–1822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gaemers IC, Stallen JM, Kunne C, et al. Lipotoxicity and steatohepatitis in an overfed mouse model for non-alcoholic fatty liver disease. Biochim Biophys Acta. 2011;1812(4):447–458 [DOI] [PubMed] [Google Scholar]
- 52.Nagarajan P, Mahesh Kumar MJ, Venkatesan R, Majundar SS, Juyal RC. Genetically modified mouse models for the study of nonalcoholic fatty liver disease. World J Gastroenterol. 2012;18(11):1141–1153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Goran MI, Bergman RN, Gower BA. Influence of total vs. visceral fat on insulin action and secretion in African American and white children. Obes Res. 2001;9(8):423–431 [DOI] [PubMed] [Google Scholar]
- 54.Kim JY, van de Wall E, Laplante M, et al. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J Clin Invest. 2007;117(9):2621–2637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Williams CD, Stengel J, Asike MI, et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140(1):124–131 [DOI] [PubMed] [Google Scholar]
- 56.Schwartz B, Jacobs DR, Jr, Moran A, Steinberger J, Hong CP, Sinaiko AR. Measurement of insulin sensitivity in children: comparison between the euglycemic-hyperinsulinemic clamp and surrogate measures. Diabetes Care. 2008;31(4):783–788 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.