Abstract
Previous studies identified a family of organic anion transport proteins (OATPs), many of which have C-terminal PDZ binding consensus sequences. In particular, the C-terminal four amino acids of Oatp1a1, a transporter on rat and mouse hepatocytes, comprise a consensus binding site for PDZK1. In PDZK1 knockout mice and in transfected cells where PDZK1 expression was knocked down, Oatp1a1 accumulates in intracellular vesicles. The present study tests the hypothesis that Oatp1a1 traffics to and from the cell surface in vesicles along microtubules, and that PDZK1 guides recruitment of specific motors to these vesicles. Oatp1a1-containing vesicles were prepared from wild-type and PDZK1 knockout mice. As seen by immunofluorescence, kinesin-1, a microtubule plus-end directed motor, was largely associated with vesicles from wild-type mouse liver, whereas dynein, a minus-end directed motor, was largely associated with vesicles from PDZK1 knockout mouse liver. Quantification of motility on directionally marked microtubules following addition of 50 µM ATP showed that wild-type vesicles moved equally toward the plus and minus ends whereas PDZK1 knockout vesicles moved predominantly toward the minus end, consistent with net movement toward the cell interior. These studies provide a novel mechanism by which PDZK1 regulates intracellular trafficking of Oatp1a1 by recruiting specific motors to Oatp1a1-containing vesicles. In the absence of PDZK1, Oatp1a1-containing vesicles cannot recruit kinesin-1 and associate with dynein as a predominant minus-end directed motor. Whether this is a result of direct interaction of the Oatp1a1 cytoplasmic domain with dynein or with a dynein-containing protein complex remains to be established.
Introduction
The hepatocyte serves as a major site of organic anion clearance from the circulation (Scharschmidt et al., 1975; Wolkoff, 2012). Many of these compounds are poorly soluble in aqueous solution, and circulate bound to proteins such as albumin (Wolkoff et al., 1987; Choi et al., 2009). Uptake involves extraction from the protein carrier and is mediated by a specific transporter(s) on the basolateral (sinusoidal) surface of the cell (Meier et al., 1997; Wolkoff, 2012). Past studies have identified a family of organic anion transport proteins (OATPs) that can mediate this uptake process (Jacquemin et al., 1994; Wolkoff, 2012). These proteins have been shown to play a role in clearance of anionic drugs from the circulation, and mutations have been associated with several reports of toxicity in patients due to reduced clearance of drug by the liver (Link et al., 2008; Clarke and Cherrington, 2012; Nakanishi and Tamai, 2012). Many of the members of the OATP family have been found to have PDZ binding consensus sequences at their C termini (Choi et al., 2009). Previous studies of the rat oatp1a1 transporter showed that its C-terminal four amino acids (KTKL) comprise a consensus site that binds PDZK1 (Wang et al., 2005; Choi et al., 2011). The mouse homolog has the same C-terminal sequence, and traffics poorly to the hepatocyte surface in PDZK1 knockout mice, accumulating in vesicular structures within cells (Wang et al., 2005). These mice have no change in total expression of the transporter, but the altered subcellular distribution correlated with reduced uptake of the organic anion, sulfobromophthalein, as compared with wild-type mice (Wang et al., 2005).
Further studies were performed in 293T cells expressing oatp1a1 in the presence or absence of PDZK1 (Choi et al., 2011). Similar to results in mice, in the absence of PDZK1, distribution of oatp1a1 was largely intracellular. This contrasted with its abundant plasma membrane expression in the presence of PDZK1 as seen by immunofluorescence as well as cell surface biotinylation and immunoanalysis (Choi et al., 2011). Based on these findings, we hypothesized that oatp1a1 in vesicles could traffic to and from the cell surface along microtubules (Sarkar et al., 2006), and that motor activity was regulated by its interaction with PDZK1. This was tested in the present study in which intracellular vesicles were prepared from wild-type and PDZK1 knockout mouse livers (Nath et al., 2007). Vesicles that were associated with Oatp1a1 were identified using oatp1a1 antibody and fluorescent secondary antibody. Motility chambers with a volume of approximately 5 µl were coated with polymerized taxol-stabilized fluorescent microtubules. Vesicles were flowed into the chambers and attached to the microtubules. Motors were activated by addition of ATP, and motile Oatp1a1-associated vesicles were quantified (Murray et al., 2000; Murray and Wolkoff, 2005). The direction of movement toward the plus or minus ends of microtubules was quantified using directionally labeled microtubules (Nath et al., 2007). Immunofluorescence colocalization permitted identification of vesicle-associated motors.
Materials and Methods
Animals
Wild-type C57BL/6J male mice were purchased from The Jackson Laboratory (Bar Harbor, ME). PDZK1 knockout mice with a C57BL background were provided by Dr. David L. Silver (formerly of the Albert Einstein College of Medicine, now at the Duke-National University of Singapore Graduate Medical School) and bred in the animal facility at the Albert Einstein College of Medicine. Animals were studied at approximately 14 weeks of age, and all procedures were approved by the Animal Use Committee of the Albert Einstein College of Medicine.
Reagents and Antibodies
Rabbit antibody raised against the N-terminal 14 amino acids of rat oatp1a1 and previously shown to react with mouse Oatp1a1 was affinity purified as reported previously (Wang et al., 2005). Mouse monoclonal antibodies against dynein (SC-13524), nonimmune mouse IgG (SC-2025), and nonimmune rabbit IgG (SC-2027) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Mouse monoclonal antibody to KifC1 (MCA4060Z) was purchased from AbD Serotec (Oxford, UK). Mouse monoclonal antibody to kinesin-1 heavy chain (Kif5B; MAB1613) was obtained from Chemicon International (Temecula, CA), now a subsidiary of EMD Millipore (Boston, MA). Mouse monoclonal antibody against KifC3 (SAB1406060) was purchased from Sigma (St. Louis, MO). Mouse monoclonal antibody against PDZK1 (AB89452) was purchased from Abcam (Cambridge, MA). Fluorescent secondary antibodies were purchased from Jackson ImmunoResearch (West Grove, PA). Tubulin (TL238) and rhodamine tubulin (TL331M) were purchased from Cytoskeleton Inc. (Denver, CO).
Isolation of Endocytic Vesicles
Endocytic vesicles were prepared from groups of three to five male mouse livers as previously described (Nath et al., 2007). In brief, mice were anesthetized with 100 mg/kg ketamine-HCl. The livers were removed and Dounce homogenized in magnesium, EGTA, PIPES, sucrose (MEPS) buffer (pH7.4) containing 5 mM MgCl2, 5 mM EGTA, 35 mM 1,4-piperazinediethanesulfonic acid (PIPES), and 5 mM sucrose. Protease inhibitor cocktail from Sigma was added at a dilution of 1:50 along with cOmplete mini EDTA-free tablets from Roche (Basel, Switzerland) at 1 tablet per 10 ml of sucrose MEPS buffer. After a 10-minute centrifugation at 1800g, the postnuclear supernatant was subjected to Sephacryl S200 (Pharmacia, Uppsala, Sweden) column chromatography. Vesicle-enriched fractions were adjusted to 1.4 M sucrose using 2.5 M sucrose MEPS buffer with 2 mM phenylmethylsulfonyl fluoride and protease inhibitors. Samples were layered at the bottom of a discontinuous sucrose gradient composed of 1.2 and 0.25 M sucrose in MEPS buffer. Following centrifugation at 100,000g for 2 hours, vesicles were collected from the 1.2–0.25 M sucrose interface. Aliquots of vesicles were stored at −80°C until use.
Immunofluorescence Analysis of Vesicles
Fluorescent vesicles were flowed into an optical chamber. In previous studies, we found that vesicles bound avidly to the unprocessed glass surface (Murray and Wolkoff, 2005). Unattached vesicles were removed by washing with PMEE buffer (35 mM PIPES-K2, 2 mg/ml bovine serum albumin, 5 mM MgCl2, 1 mM EGTA, 0.5 mM EDTA, 4 mM DTT, 5 mg/ml casein, pH 7.4). Vesicles were incubated on ice for 6 minutes with (Oatp1a1) antibody diluted 1:100 in PMEE buffer and washed in PMEE buffer. This was repeated with the second primary antibody (e.g., dynein antibody). Following the final wash, appropriate fluorescent secondary antibodies were flowed in, incubated for 5 minutes, and washed in PMEE buffer. Final washes were conducted in PMEE buffer with 2 mg/ml ascorbic acid in the absence of casein.
Analysis of Microtubule-Based Motility of Oatp1a1-Associated Vesicles
Preparation of Microtubules.
Fluorescent microtubules were polymerized from tubulin in buffer containing 80 mM PIPES-K2, 1 mM MgCl2, 1 mM EGTA, 1 mM GTP, 3% glycerol, pH 7.0 in a ratio of 7:1 mixture of unlabeled tubulin to rhodamine-tubulin at 37°C. To prepare polarity-marked fluorescent microtubules, dim seeds with a 75:1 ratio of tubulin to rhodamine-tubulin were polymerized for 5 minutes at 37°C. These seeds were then sheared by rapidly pipetting 2 µl of the mixture up and down five times in a 10-µl Eppendorf pipette (Eppendorf, Hamburg, Germany). Microtubules were then polymerized at 37°C for 6 minutes with a 6:1 mixture of unlabeled to rhodamine-labeled tubulin, then stabilized with 20 µM taxol. Polymerized microtubules were centrifuged at 15 psi for 3 minutes in a Beckman (Brea, CA) Airfuge to remove unpolymerized tubulin.
Vesicle Motility Assay.
Fluorescent microtubules were flowed into a DEAE-dextran–coated optical chamber (Bananis et al., 2000). Unattached microtubules were removed by washing with PMEE buffer containing 20 µM taxol. Vesicles were then flowed into the chamber and allowed to bind to microtubules at room temperature for 10 minutes. Unattached vesicles were removed by washing. Vesicles were labeled with Oatp1a1 antibody as described earlier, except that 20 µM taxol was added to the PMEE buffer. The motility chamber was then placed on the fluorescence microscope stage maintained at 37°C. Addition of 50 µM ATP was used to initiate vesicle motility. In studies of inhibition of motility, antibodies to motor proteins were flowed into the chamber prior to the addition of ATP while drug inhibitors were added with ATP.
Image and Statistical Analysis
Optical chambers were imaged using a 60× 1.4 numerical aperture Olympus objective on an inverted Olympus IX71 microscope (Olympus, Tokyo, Japan). Images were captured by a CoolSNAP HQ cooled charge-coupled device camera (Photometrics, Roper Scientific, Tuscon, AZ) controlled by MetaMorph software (Molecular Devices, Sunnyvale, CA). Data were analyzed using ImageJ 1.39u (National Institutes of Health public domain; http://rsb.info.nih.gov/ij/) and Adobe Photoshop CS2 version 9.0.2 (Adobe Systems, San Jose, CA). Colocalization of fluorescent proteins on vesicles was quantified using the autoscore colocalization macro for ImageJ as previously described (Murray et al., 2002; Mukhopadhyay et al., 2011). Time-lapsed movies of motility experiments were taken at 1 frame per second for 90 seconds starting before addition of ATP. Movies were scored manually using ImageJ 1.39u. Statistical analysis was performed using χ2 or Student’s t test as appropriate.
Results
Motility of Oatp1a1-Associated Vesicles on Microtubules
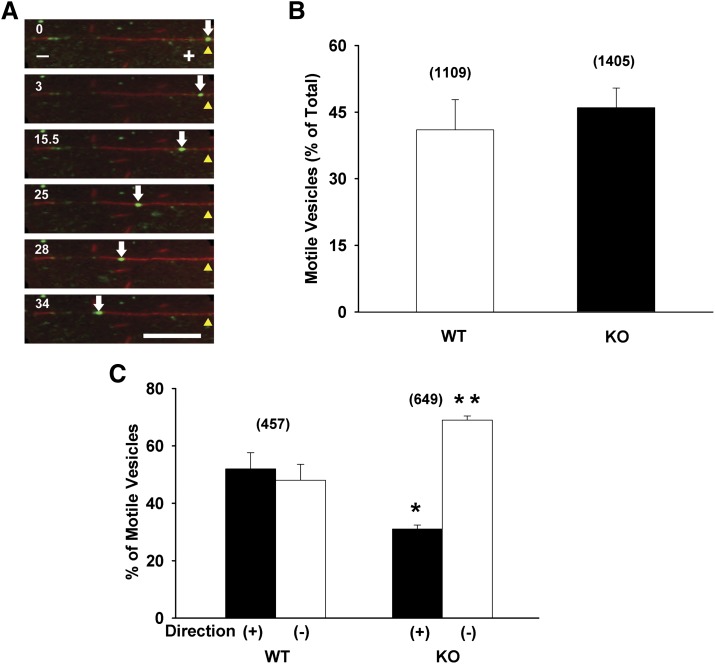
Initial experiments were performed to examine whether Oatp1a1-containing vesicles bind to and move along microtubules. Figure 1A (also see Supplemental Movie 1) shows a representative time series of images of an Oatp1a1-associated vesicle, prepared from a PDZK1 knockout mouse, moving on a polarity-marked microtubule. The plus and minus microtubule ends are indicated by long and short regions of bright fluorescence, respectively. In this example, the vesicle moved toward the minus end. In the 34 seconds shown in this figure, it moved approximately 20 µm. Similar analysis of multiple vesicles prepared from wild-type and PDZK1 knockout mice was performed. As seen in Fig. 1B, approximately 45% of microtubule-bound Oatp1a1-associated vesicles obtained from wild-type or PDZK1 knockout mice moved upon addition of 50 µM ATP. However, quantification of direction of movement shows substantially different behavior between these two populations of vesicles. As seen in Fig. 1C, Oatp1a1-associated vesicles prepared from wild-type mouse liver moved equally toward the plus and minus ends of polarity-marked microtubules. In contrast, vesicles prepared from PDZK1 knockout mouse liver had a significant bias toward the microtubule minus end, with approximately 70% of motile vesicles moving in the minus-end direction.
Fig. 1.
Microtubule-based motility of Oatp1a1-associated endocytic vesicles. Oatp1a1-containing endocytic vesicles were prepared from livers of wild-type (WT) and PDZK1 knockout (KO) mice and flowed into microchambers that had been coated with polarity-marked fluorescent microtubules. After the binding of vesicles to microtubules, motility was initiated with the addition of 50 µM ATP. (A) Representative images demonstrating minus-end directed movement of an Oatp1a1-containing vesicle prepared from PDZK1 knockout mouse liver. A red microtubule with attached green Oatp1a1-labeled vesicles runs horizontally and contains markings for microtubule polarity. The polarity marks were generated by polymerizing brightly fluorescent tubulin from short, dimly fluorescent microtubule seeds, allowing the growth of long microtubule plus ends. Visible from left to right is the microtubule minus end (−), a dimly fluorescent seed, and the microtubule plus end (+) to which a green, motile vesicle is bound. The white arrow follows this vesicle as it moves toward the minus end of the microtubule. The yellow arrowhead indicates the starting point for the vesicle. Time in seconds after addition of ATP is indicated at the top left of each panel. In the 34 seconds of this study, the vesicle moved approximately 18 µm (approximately 0.5 µm/s). Scale bar = 10 µm. (B) The percentage of microtubule-bound vesicles that moved following ATP addition is indicated by the bars for wild-type–derived (open bars) and PDZK1 knockout–derived (solid bars) vesicles. (C) The percentage of motile vesicles from the studies in (B) moving toward the plus (closed bars) or minus (open bars) ends of microtubules is indicated. Numbers in parentheses represent the number of motile vesicles that were examined. Error bars represent the mean ± S.E.M. *P < 0.0001 as compared with plus-end motility of wild-type vesicles; **P < 0.0001 as compared with minus-end motility of wild-type vesicles.
Immunolocalization of Microtubule-Based Motors on Oatp1a1-Associated Vesicles
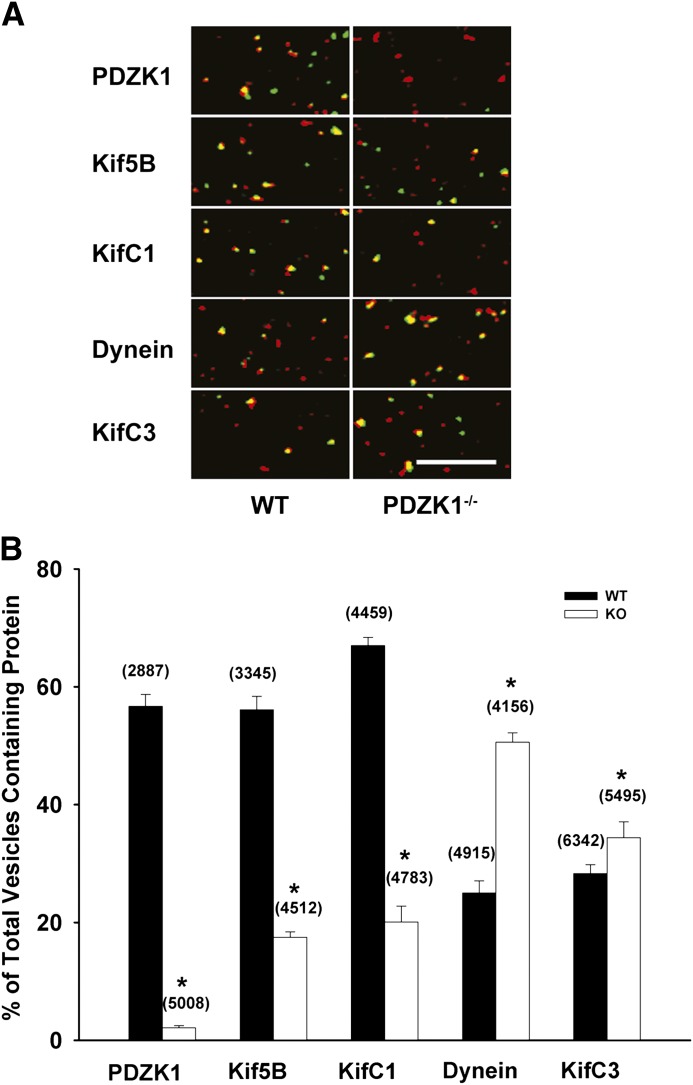
The preceding studies indicate that Oatp1a1-containing vesicles are associated with microtubule-based motors that can mediate both plus- and minus-end directed motility. Identification of candidate motors that are associated with these vesicles was determined by immunofluorescence microscopy. Representative studies are shown in Fig. 2A, and quantitation of multiple studies is shown in Fig. 2B. When association of Oatp1a1-containing vesicles with PDZK1 was examined, close to 60% of Oatp1a1-containing vesicles prepared from wild-type mouse liver were also associated with PDZK1. As expected, there was no colocalization of PDZK1 with vesicles prepared from PDZK1 knockout mice. As seen in Fig. 2B, there were several substantial differences in motor protein distribution between vesicles from wild-type and PDZK1 knockout mice. Kinesin-1 (Kif5B), a plus-end directed kinesin motor, and KifC1, a minus-end directed kinesin, were present in approximately 60% of vesicles from wild-type mice, but fewer than 20% of vesicles from PDZK1 knockout mice. In contrast, dynein, a minus-end directed motor, was present in approximately 50% of the vesicles from PDZK1 knockout mice, but only 25% of vesicles from wild-type mice. Further studies were performed to determine whether these motors mediate motility of these vesicles.
Fig. 2.
Colocalization of motor proteins and PDZK1 with Oatp1a1-associated vesicles. Endocytic vesicles isolated from wild-type (WT) and PDZK1 knockout (KO) mouse livers were attached to the glass surface of microchambers and immunostained for Oatp1a1 and motor proteins or PDZK1 as described in Materials and Methods. (A) Representative images are shown in which Oatp1a1 is in red and PDZK1 or motor proteins are in green. Vesicles in yellow represent colocalization of the two. Scale bar = 10 µm. (B) Quantification of protein colocalization with Oatp1a1-containing vesicles. The percentage of Oatp1a1-containing vesicles that colocalized with each of the proteins indicated in the figure is represented by filled (wild-type) or open (PDZK1 knockout) bars. The number of Oatp1a1-associated vesicles examined is in parentheses. Error bars represent the mean ± S.E.M. *P < 0.0001 as compared with colocalization in wild-type vesicles.
Activity of Vesicle-Associated Motors
Analysis of Total Motility.
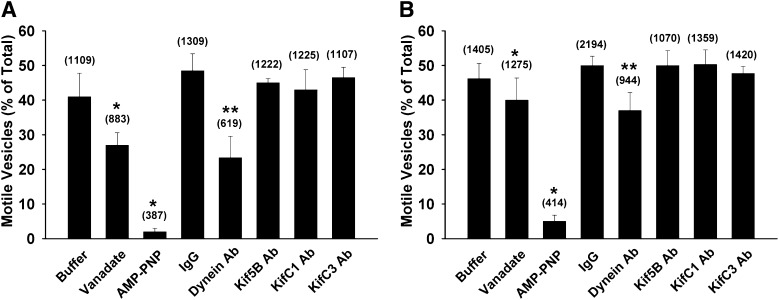
Studies were performed to examine the effect of inclusion of 5 µM vanadate or 1 mM adenosine 5′-(β,γ-imido) triphosphate lithium salt hydrate (AMP-PNP) on motility. Based upon previous observations, at the concentrations used, these compounds inhibit dynein and kinesins, respectively (Murray et al., 2000). As seen in Fig. 3, inclusion of vanadate reduced the number of motile vesicles, more so in vesicles prepared from wild-type as compared with PDZK1 knockout mice. Addition of AMP-PNP resulted in almost complete inhibition of motility of vesicles from both wild-type and PDZK1 knockdown mice. Of the motor antibodies tested, only antibody to dynein reduced the number of motile vesicles prepared from wild-type and PDZK1 knockout mice. Studies quantifying changes in total motility do not take into account potential complex interactions of motors and colocalization of motors of opposite direction on a single vesicle as we and others have observed previously (Nath et al., 2007; Soppina et al., 2009; Hendricks et al., 2010; Schuster et al., 2011). Subsequent motility studies were performed on polarity-marked microtubules to quantify directional motility.
Fig. 3.
Effects of chemical and motor protein antibody inhibitors on the fraction of Oatp1a1-containing vesicles that move. The proportion of Oatp1a1-associated vesicles derived from wild-type (A) and PDZK1 knockout (B) mouse liver that move on microtubules in the presence of chemical or motor protein antibody inhibitors are represented by the bars. Buffer addition serves as the control for vanadate and AMP-PNP studies, and nonimmune IgG addition serves as the control for antibody studies. The total number of Oatp1a1-associated vesicles counted is in parentheses. *P < 0.0001 for total motility versus buffer control; **P < 0.0001 for total motility versus IgG control. Ab, antibody.
Analysis of Directional Motility on Polarity-Marked Microtubules
Effects of Chemical Inhibitors.
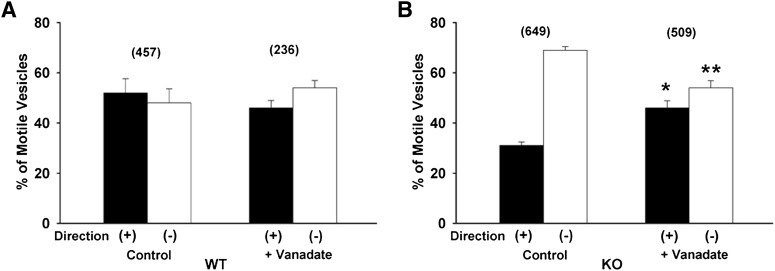
As seen in Fig. 4A, there was no effect of addition of vanadate on the proportion of Oatp1a1-containing vesicles prepared from wild-type mice moving in either direction, although the total number of motile vesicles was reduced (Fig. 3A). In contrast, as seen in Fig. 4B, there was a significant reduction in minus-end directed motility of vesicles prepared from PDZK1 knockout mice, and a corresponding increase in plus-end directed motility. As noted earlier, addition of AMP-PNP resulted in almost complete inhibition of motility of vesicles from both wild-type and PDZK1 knockdown mice (Fig. 3), and there were not enough motile vesicles to accurately assess directional movement.
Fig. 4.
Effect of vanadate on directional motility of Oatp1a1-associated vesicles. One micromolar vanadate, an inhibitor of dynein, was included in the motility assay performed on polarity-marked microtubules. The proportion of motile vesicles moving toward the plus or minus ends of microtubules was quantified for wild-type (WT) vesicles (A) and PDZK1 knockout (KO) vesicles (B). The total number of motile vesicles observed is shown in parentheses. Error bars represent the mean ± S.E.M. *P < 0.0001 as compared with plus-end motility; **P < 0.0001 as compared with minus-end motility.
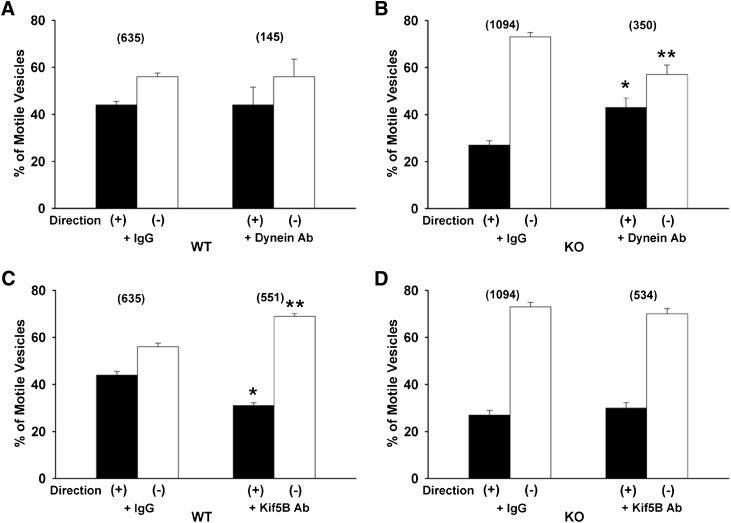
Roles of Kinesin-1 and Dynein.
To better assess the role of specific motors in mediating motility of these vesicles, effects of motor antibodies on directional movement of Oatp1a1-containing vesicles were quantified. As seen in Fig. 5A, there was no effect of dynein antibody on directional motility of Oatp1a1-containing vesicles prepared from wild-type mice. Similar to results with vanadate, dynein antibody preincubation significantly reduced minus-end motility of vesicles prepared from PDZK1 knockout mice, with proportionately more plus-end motility remaining (Fig. 5B). In contrast to results for dynein inhibition, preincubation with antibody to the plus-end kinesin Kif5B (kinesin-1) resulted in reduced plus-end directed motility of wild-type vesicles and a corresponding proportional increase in movement toward the minus end (Fig. 5C). There was no effect of Kif5B antibody on directional motility of Oatp1a1-containing vesicles prepared from PDZK1 knockout mice (Fig. 5D).
Fig. 5.
Effect of antibodies to dynein or kinesin-1 (Kif5B) on directional motility of Oatp1a1-associated vesicles. Vesicles prepared from wild-type (WT) (A and C) or PDZK1 knockout (KO) (B and D) mouse livers were bound to polarity-marked microtubules in a microscopy chamber and preincubated for 5 minutes with nonimmune IgG or antibodies to dynein (A and B) or kinesin-1 (C and D). The proportion of moving vesicles going toward the plus or minus ends of the microtubules was quantified following addition of 50 µM ATP. The total number of motile vesicles observed is shown in parentheses. Error bars represent the mean ± S.E.M. *P < 0.0001 as compared with plus-end motility; **P < 0.0001 as compared with minus-end motility. Ab, antibody.
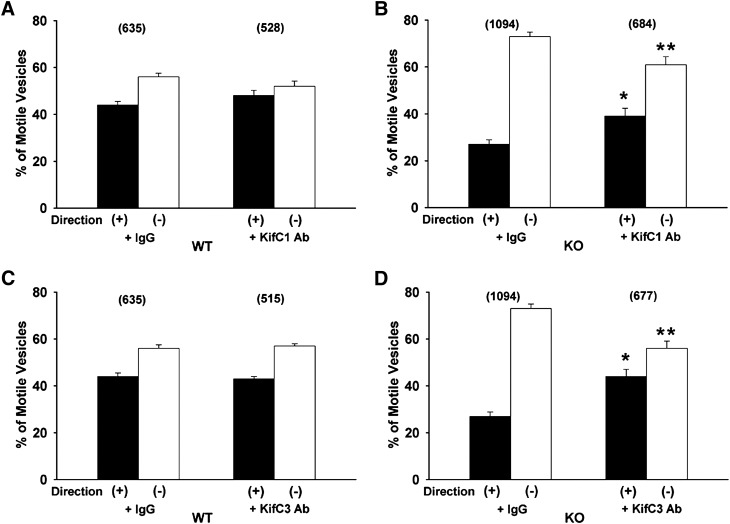
Roles of the Minus-End Kinesins KifC1 and KifC3.
Preincubation of wild-type vesicles with antibody to the minus-end kinesin KifC1 had no significant effect on their total or directional motility (Figs. 3A and 6A). Incubation with this antibody resulted in a significant reduction in the proportion of minus-end motility in vesicles from PDZK1 knockout mice (Fig. 6B). This suggests that KifC1 may mediate some of the minus-end directed motility in these vesicles, and that when its function is inhibited by antibody, compensatory plus-end directed motor activity becomes apparent. Similarly, there was no effect of antibody to the minus-end kinesin KifC3 on total or directional motility of wild-type vesicles (Figs. 3A and 5C), but there was a significant reduction in minus-end motility of vesicles from knockout mice (Fig. 5D), although their total motility was unchanged (Fig. 3B).
Fig. 6.
Effect of antibodies to the minus-end kinesins KifC1 and KifC3 on directional motility of Oatp1a1-associated vesicles. Vesicles prepared from wild-type (WT) (A and C) or PDZK1 knockout (KO) (B and D) mouse livers were bound to polarity-marked microtubules in a microscopy chamber and preincubated for 5 minutes with nonimmune IgG or antibodies to KifC1 (A and B) or KifC3 (C and D). The proportion of vesicles moving toward the plus or minus ends of the microtubules was quantified following addition of 50 µM ATP. The total number of motile vesicles observed is shown in parentheses. Error bars represent the mean ± S.E.M. *P < 0.0001 as compared with plus-end motility; **P < 0.0001 as compared with minus-end motility. Ab, antibody.
Discussion
OATPs are a superfamily of transport proteins responsible for sodium-independent uptake of compounds that include hormones, bile salts, xenobiotics, and drugs (Hagenbuch and Meier, 2003, 2004; Iusuf et al., 2012; Hagenbuch and Stieger, 2013). The superfamily is composed of six families based on 40% amino acid sequence identity divided into subfamilies that have 60% amino acid homology (Iusuf et al., 2012; Hagenbuch and Stieger, 2013). OATP family members are expressed in multiple organs, including the brain, heart, kidney, intestine, and liver, and they share structural similarities that include predicted 12 transmembrane domains (Wang et al., 2008; Hagenbuch and Stieger, 2013) and 3–4 N-linked glycosylation sites (Wang et al., 2008; Yao et al., 2012). Reduced transport activity of OATPs, accompanied by adverse drug reactions, have been described with a number of genetic polymorphisms (Link et al., 2008; Clarke and Cherrington, 2012; Nakanishi and Tamai, 2012). It is probable that any condition in which plasma membrane localization of OATPs is reduced could have a similar effect.
Oatp1a1, first identified in rats (Jacquemin et al., 1994), is a prototypical member of the OATP family. Our previous studies indicated that oatp1a1 can traffic from the cell surface following phosphorylation at serines 634 and 635 (Xiao et al., 2006), resulting in downregulation of transport function (Glavy et al., 2000; Choi et al., 2011). In addition, several members of the OATP family, including Oatp1a1 contain a PDZ consensus binding motif (KTKL) at the C terminus (Choi et al., 2009). We also showed that this consensus motif mediated binding of Oatp1a1 to PDZK1 (Wang et al., 2005). Mice in which the gene for PDZK1 was knocked out had reduced plasma disappearance of the Oatp1a1 ligand sulfobromophthalein (Wang et al., 2005). Oatp1a1 in these mice accumulated in intracellular vesicles rather than at the cell surface (Wang et al., 2005). Further studies were performed in cell lines transfected with oatp1a1 with or without cotransfection of PDZK1. In the presence of PDZK1, oatp1a1 was predominantly on the cell surface as determined morphologically and by cell surface biotinylation (Choi et al., 2011). In the absence of PDZK1 expression, oatp1a1 was predominantly intracellular (Wang et al., 2005; Choi et al., 2011). The present study examined whether changes in microtubule-based motility of Oatp1a1-containing vesicles could represent the mechanism behind these observations.
Trafficking of a number of proteins between the plasma membrane and an intracellular pool has been shown to require intact microtubules (Oda et al., 1995; Sarkar et al., 2006 Soldati and Schliwa, 2006). Based on this, we hypothesized that Oatp1a1-containing vesicles traffic through the cell along microtubules, and that their direction of movement is determined by specific vesicle-associated motors. In the present study, we tested this hypothesis and examined the role of PDZK1 on motor recruitment and directional motility of Oatp1a1-containing vesicles. These studies used a system that we developed to reconstitute microtubule-based vesicle motility in vitro (Bananis et al., 2000; Murray et al., 2000; Murray and Wolkoff, 2005). The present studies were performed using Oatp1a1-containing vesicles that were prepared from livers of wild-type or PDZK1 knockout mice. These studies quantified the directional motility of vesicles on microtubules in the absence of cytosol or other subcellular components, and provide a platform on which to examine vesicle-associated proteins, including specific microtubule-based motors and regulatory factors (Murray et al., 2002). It is notable that, as we have described previously, native vesicles can be studied by antibody labeling of cell surface transporter without inhibiting their ability to move on microtubules (Bananis et al., 2000; Sarkar et al., 2006). This obviates concerns that could arise with overexpression of a fluorescently labeled protein, such as Oatp1a1- that could alter stoichiometry of association with other proteins. This could be important for studies of PDZK1 function. PDZK1 is a 70-kDa scaffolding protein that can assemble protein complexes, and plays an important role in regulating expression of the high-density lipoprotein scavenger receptor class B type 1 (Silver, 2002; Kocher et al., 2003) as well as cell surface expression of Oatp1a1- in the liver (Wang et al., 2005).
Our studies indicate that PDZK1 is not required for binding of Oatp1a1-containing vesicles to microtubules (Fig. 1B), but that it regulates the direction in which these vesicles move (Fig. 1C). The plus end of microtubules in most cells, including hepatocytes (Novikoff et al., 1996), is near the cell surface, whereas the minus end is within the cell at the microtubule organizing center. In the absence of PDZK1, Oatp1a1-containing vesicles move predominantly toward the minus end of microtubules, which is consistent with their localization within the cell interior. Several microtubule-based motors that are associated with these vesicles were identified. As seen in Fig. 2, their distribution on vesicles varies in the presence or absence of PDZK1. Interestingly, Oatp1a1-containing vesicles from wild-type mice are highly associated with the plus-end motor kinesin-1 (Kif5B), whereas Oatp1a1-containing vesicles from PDZK1 knockout mice have little kinesin-1. Oatp1a1-containing vesicles from both wild-type and PDZK1 knockout mice were associated with dynein, a minus-end directed motor, but dynein was significantly more abundant on vesicles from knockout mice (Fig. 2B). In both groups, the number of motile vesicles was reduced by inhibition of dynein activity either with vanadate or with antibody (Fig. 2B). However, inhibition of the knockout vesicles was less effective. Both vanadate and dynein antibody–treated motile vesicles from wild-type mice continued to move approximately equally toward the plus and minus directions, whereas those from knockout mice lost their minus-end bias and had motility similar to that of wild-type vesicles (Figs. 4 and 5). Previous studies showed colocalization and coordination of motor activities of dynein and kinesin-1 (Gross et al., 2002; Ligon et al., 2004; Uchida et al., 2009; Deng et al., 2010). We hypothesize that a population of vesicles from wild-type mice is associated with both motors, and that inhibition of dynein on these vesicles results in dysfunction of this regulatory mechanism, producing loss of both plus- and minus-end motility. In contrast, there is little kinesin-1 associated with knockout vesicles. Consequently, activity of dynein is not coregulated with that of kinesin-1 so that inhibition of dynein results in reduced minus-end motility (Fig. 5). In further studies, we found that KifC1 and KifC3, two minus-end directed kinesins, are also associated with Oatp1a1-containing vesicles from wild-type and PDZK1 knockout mice (Fig. 2B). Inclusion of antibodies to these motors during motility assays resulted in a decrease in minus-end directed motility of vesicles from PDZK1 knockout, but not wild-type mice (Figs. 3 and 6, A and C). This suggests that these minus-end kinesins are active in the absence of PDZK1, consistent with the relatively large amount of residual minus-end directed motility in these vesicles when dynein is inhibited with vanadate or antibody. The reason for their lack of activity in vesicles from wild-type mice is not known at this time, but could be related to interaction with kinesin-1, as we described in a previous study (Nath et al., 2007). Vesicles from PDZK1 knockout mice exhibit a different profile of motor proteins, with increased KifC3 and dynein but decreased KifC1 and Kif5B. These results again suggest that PDZK1 is responsible for motor protein recruitment, and in the absence of PDZK1, the profile of motor proteins and their regulation are altered.
That members of the kinesin family are associated with all motile Oatp1a1-containing vesicles is suggested by experiments in which AMP-PNP was included in the motility assay. AMP-PNP is known to associate with kinesins resulting in strong binding to microtubules and complete inhibition of motility at high ratios of AMP-PNP to ATP (Uemura et al., 2002; Subramanian and Gelles, 2007). As seen in Fig. 3, this compound inhibited virtually all motility for both wild-type and PDZK1 knockout vesicles. This suggests that AMP-PNP functionally anchors these vesicles to microtubules via their kinesins, making them unable to move, even if they are also associated with dynein.
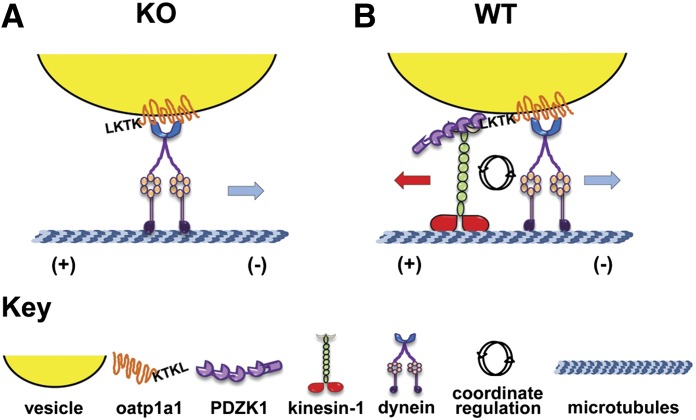
In summary, the present study presents a novel mechanism by which PDZK1 regulates intracellular trafficking of Oatp1a1- by recruiting specific motors to intracellular Oatp1a1-containing vesicles. Our previous studies showed that Oatp1a1- binds to only two of the four independent PDZ binding domains on PDZK1 (Wang et al., 2005). As shown in the illustration in Fig. 7, we suggest that this enables PDZK1 to form complexes with proteins that recruit kinesin-1 to the vesicle. In the absence of PDZK1, Oatp1a1-containing vesicles have reduced ability to recruit kinesin-1 and instead associate with dynein as a predominant minus-end directed motor. We know that the terminal four amino acids on the cytoplasmic domain of Oatp1a1 are required to recruit PDZK1. Whether this cytoplasmic domain contains sequences that facilitate recruitment of motors and regulatory factors in the absence of PDZK1 is the subject of ongoing investigation.
Fig. 7.
Proposed role of PDZK1 in selective recruitment of motors to Oatp1a1-associated vesicles. As seen in (A), we propose that one or more of the cytosolic domains of vesicle-associated Oatp1a1 bind a protein complex that contains dynein, independent of the presence of PDZK1. As seen in (B), PDZK1 bound to the C-terminal PDZ binding consensus sequence (KTKL) of vesicle-associated Oatp1a1 recruits a complex of proteins that includes kinesin-1 (Kif5B). In this situation, activity of these motors is coordinately regulated, such that inhibition of one type of motor will affect activity of the other. KO, knockout; WT, wild-type.
Supplementary Material
Abbreviations
- AMP-PNP
adenosine 5′-(β,γ-imido) triphosphate lithium salt hydrate
- MEPS buffer
5 mM MgCl2, 5 mM EGTA, 35 mM PIPES, 0.25 M sucrose pH 7.4
- OATP
organic anion transport protein
- PDZ
postsynaptic density protein, drosophila disc large tumor suppressor, zonula occludens-1 protein
- PIPES
1,4-piperazinediethanesulfonic acid
- PMEE (PIPES, Magnesium, EGTA, EDTA) buffer
35 mM PIPES-K2, 2 mg/ml bovine serum albumin, 5 mM MgCl2, 1 mM EGTA, 0.5 mM EDTA, 4 mM (diothiothreitol) DTT, 5 mg/ml casein, pH7.4
Authorship Contributions
Participated in research design: Wang, Murray, Wolkoff.
Conducted experiments: Wang.
Contributed new reagents or analytic tools: Murray.
Performed data analysis: Wang, Wolkoff.
Wrote or contributed to the writing of the manuscript: Wang, Murray, Wolkoff.
Footnotes
This work was supported by the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases [Grants DK23026, DK41296, and DK41918].
 This article has supplemental material available at dmd.aspetjournals.org.
This article has supplemental material available at dmd.aspetjournals.org.
References
- Bananis E, Murray JW, Stockert RJ, Satir P, Wolkoff AW. (2000) Microtubule and motor-dependent endocytic vesicle sorting in vitro. J Cell Biol 151:179–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JH, Murray JW, Wolkoff AW.(2009) Hepatocyte basolateral membrane organic anion transporters, in The Liver: Biology and Pathobiology (Arias IM, Alter HJ, Boyer JL, Cohen DE, Fausto N, Shafritz DA, Wolkoff AW. eds) pp 305–321, Wiley-Blackwell, Chichester [Google Scholar]
- Choi JH, Murray JW, Wolkoff AW. (2011) PDZK1 binding and serine phosphorylation regulate subcellular trafficking of organic anion transport protein 1a1. Am J Physiol Gastrointest Liver Physiol 300:G384–G393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke JD, Cherrington NJ. (2012) Genetics or environment in drug transport: the case of organic anion transporting polypeptides and adverse drug reactions. Expert Opin Drug Metab Toxicol 8:349–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W, Garrett C, Dombert B, Soura V, Banks G, Fisher EM, van der Brug MP, Hafezparast M. (2010) Neurodegenerative mutation in cytoplasmic dynein alters its organization and dynein-dynactin and dynein-kinesin interactions. J Biol Chem 285:39922–39934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glavy JS, Wu SM, Wang PJ, Orr GA, Wolkoff AW. (2000) Down-regulation by extracellular ATP of rat hepatocyte organic anion transport is mediated by serine phosphorylation of oatp1. J Biol Chem 275:1479–1484 [DOI] [PubMed] [Google Scholar]
- Gross SP, Welte MA, Block SM, Wieschaus EF. (2002) Coordination of opposite-polarity microtubule motors. J Cell Biol 156:715–724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagenbuch B, Meier PJ. (2003) The superfamily of organic anion transporting polypeptides. Biochim Biophys Acta 1609:1–18 [DOI] [PubMed] [Google Scholar]
- Hagenbuch B, Meier PJ. (2004) Organic anion transporting polypeptides of the OATP/ SLC21 family: phylogenetic classification as OATP/ SLCO superfamily, new nomenclature and molecular/functional properties. Pflugers Arch 447:653–665 [DOI] [PubMed] [Google Scholar]
- Hagenbuch B, Stieger B. (2013) The SLCO (former SLC21) superfamily of transporters. Mol Aspects Med 34:396–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks AG, Perlson E, Ross JL, Schroeder HW, 3rd, Tokito M, Holzbaur EL. (2010) Motor coordination via a tug-of-war mechanism drives bidirectional vesicle transport. Curr Biol 20:697–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iusuf D, van de Steeg E, Schinkel AH. (2012) Functions of OATP1A and 1B transporters in vivo: insights from mouse models. Trends Pharmacol Sci 33:100–108 [DOI] [PubMed] [Google Scholar]
- Jacquemin E, Hagenbuch B, Stieger B, Wolkoff AW, Meier PJ. (1994) Expression cloning of a rat liver Na(+)-independent organic anion transporter. Proc Natl Acad Sci USA 91:133–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocher O, Yesilaltay A, Cirovic C, Pal R, Rigotti A, Krieger M. (2003) Targeted disruption of the PDZK1 gene in mice causes tissue-specific depletion of the high density lipoprotein receptor scavenger receptor class B type I and altered lipoprotein metabolism. J Biol Chem 278:52820–52825 [DOI] [PubMed] [Google Scholar]
- Ligon LA, Tokito M, Finklestein JM, Grossman FE, Holzbaur EL. (2004) A direct interaction between cytoplasmic dynein and kinesin I may coordinate motor activity. J Biol Chem 279:19201–19208 [DOI] [PubMed] [Google Scholar]
- Link E, Parish S, Armitage J, Bowman L, Heath S, Matsuda F, Gut I, Lathrop M, Collins R, SEARCH Collaborative Group (2008) SLCO1B1 variants and statin-induced myopathy—a genomewide study. N Engl J Med 359:789–799 [DOI] [PubMed] [Google Scholar]
- Meier PJ, Eckhardt U, Schroeder A, Hagenbuch B, Stieger B. (1997) Substrate specificity of sinusoidal bile acid and organic anion uptake systems in rat and human liver. Hepatology 26:1667–1677 [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay A, Nieves E, Che FY, Wang J, Jin L, Murray JW, Gordon K, Angeletti RH, Wolkoff AW. (2011) Proteomic analysis of endocytic vesicles: Rab1a regulates motility of early endocytic vesicles. J Cell Sci 124:765–775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray JW, Bananis E, Wolkoff AW. (2000) Reconstitution of ATP-dependent movement of endocytic vesicles along microtubules in vitro: an oscillatory bidirectional process. Mol Biol Cell 11:419–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray JW, Bananis E, Wolkoff AW. (2002) Immunofluorescence microchamber technique for characterizing isolated organelles. Anal Biochem 305:55–67 [DOI] [PubMed] [Google Scholar]
- Murray JW, Wolkoff AW. (2005) Assay of Rab4-dependent trafficking on microtubules. Methods Enzymol 403:92–107 [DOI] [PubMed] [Google Scholar]
- Nakanishi T, Tamai I. (2012) Genetic polymorphisms of OATP transporters and their impact on intestinal absorption and hepatic disposition of drugs. Drug Metab Pharmacokinet 27:106–121 [DOI] [PubMed] [Google Scholar]
- Nath S, Bananis E, Sarkar S, Stockert RJ, Sperry AO, Murray JW, Wolkoff AW. (2007) Kif5B and Kifc1 interact and are required for motility and fission of early endocytic vesicles in mouse liver. Mol Biol Cell 18:1839–1849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novikoff PM, Cammer M, Tao L, Oda H, Stockert RJ, Wolkoff AW, Satir P. (1996) Three-dimensional organization of rat hepatocyte cytoskeleton: relation to the asialoglycoprotein endocytosis pathway. J Cell Sci 109:21–32 [DOI] [PubMed] [Google Scholar]
- Oda H, Stockert RJ, Collins C, Wang H, Novikoff PM, Satir P, Wolkoff AW. (1995) Interaction of the microtubule cytoskeleton with endocytic vesicles and cytoplasmic dynein in cultured rat hepatocytes. J Biol Chem 270:15242–15249 [DOI] [PubMed] [Google Scholar]
- Sarkar S, Bananis E, Nath S, Anwer MS, Wolkoff AW, Murray JW. (2006) PKCzeta is required for microtubule-based motility of vesicles containing the ntcp transporter. Traffic 7:1078–1091 [DOI] [PubMed] [Google Scholar]
- Scharschmidt BF, Waggoner JG, Berk PD. (1975) Hepatic organic anion uptake in the rat. J Clin Invest 56:1280–1292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster M, Lipowsky R, Assmann MA, Lenz P, Steinberg G. (2011) Transient binding of dynein controls bidirectional long-range motility of early endosomes. Proc Natl Acad Sci USA 108:3618–3623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver DL. (2002) A carboxyl-terminal PDZ-interacting domain of scavenger receptor B, type I is essential for cell surface expression in liver. J Biol Chem 277:34042–34047 [DOI] [PubMed] [Google Scholar]
- Soldati T, Schliwa M. (2006) Powering membrane traffic in endocytosis and recycling. Nat Rev Mol Cell Biol 7:897–908 [DOI] [PubMed] [Google Scholar]
- Soppina V, Rai AK, Ramaiya AJ, Barak P, Mallik R. (2009) Tug-of-war between dissimilar teams of microtubule motors regulates transport and fission of endosomes. Proc Natl Acad Sci USA 106:19381–19386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian R, Gelles J. (2007) Two distinct modes of processive kinesin movement in mixtures of ATP and AMP-PNP. J Gen Physiol 130:445–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida A, Alami NH, Brown A. (2009) Tight functional coupling of kinesin-1A and dynein motors in the bidirectional transport of neurofilaments. Mol Biol Cell 20:4997–5006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura S, Kawaguchi K, Yajima J, Edamatsu M, Toyoshima YY, Ishiwata S. (2002) Kinesin-microtubule binding depends on both nucleotide state and loading direction. Proc Natl Acad Sci USA 99:5977–5981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Hata S, Xiao Y, Murray JW, Wolkoff AW. (2008) Topological assessment of oatp1a1: a 12-transmembrane domain integral membrane protein with three N-linked carbohydrate chains. Am J Physiol Gastrointest Liver Physiol 294:G1052–G1059 [DOI] [PubMed] [Google Scholar]
- Wang P, Wang JJ, Xiao Y, Murray JW, Novikoff PM, Angeletti RH, Orr GA, Lan D, Silver DL, Wolkoff AW. (2005) Interaction with PDZK1 is required for expression of organic anion transporting protein 1A1 on the hepatocyte surface. J Biol Chem 280:30143–30149 [DOI] [PubMed] [Google Scholar]
- Wolkoff AW. (2012) Mechanisms of hepatocyte organic anion transport, in Physiology of the Gastrointestinal Tract (Johnson LR, Ghishan FK, Kaunitz JD, Merchant JL, Said HM, Wood JD. eds) pp 1485–1506, Academic Press, San Diego [Google Scholar]
- Wolkoff AW, Samuelson AC, Johansen KL, Nakata R, Withers DM, Sosiak A. (1987) Influence of Cl- on organic anion transport in short-term cultured rat hepatocytes and isolated perfused rat liver. J Clin Invest 79:1259–1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y, Nieves E, Angeletti RH, Orr GA, Wolkoff AW. (2006) Rat organic anion transporting protein 1A1 (Oatp1a1): purification and phosphopeptide assignment. Biochemistry 45:3357–3369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J, Hong W, Huang J, Zhan K, Huang H, Hong M. (2012) N-Glycosylation dictates proper processing of organic anion transporting polypeptide 1B1. PLoS ONE 7:e52563. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.