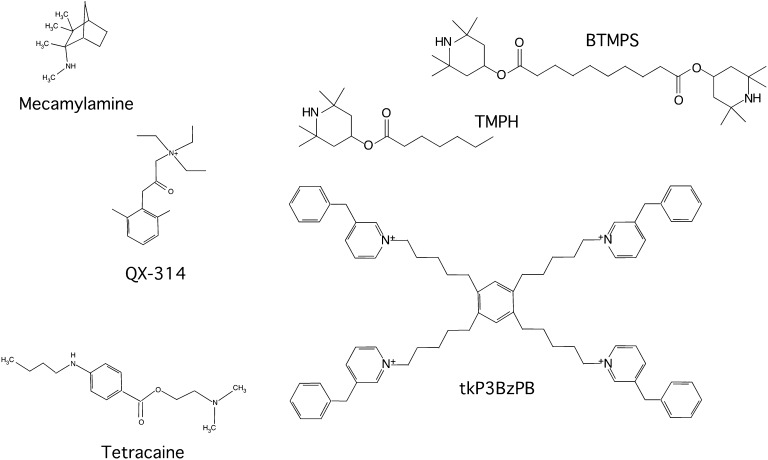
Abstract
Positive allosteric modulators (PAMs) of α7 nicotinic acetylcholine receptors can enhance ion channel currents and downstream effects of α7 stimulation. We investigated the approach of using noncompetitive antagonists to regulate α7 receptor function, potentially distinguishing effects requiring ion channel currents from signaling induced by nonconducting states. Three small readily reversible antagonists, (1S,2R,4R)-N,2,3,3-tetramethylbicyclo[2.2.1]heptan-2-amine (mecamylamine), N-(2.6-dimethylphenylcarbamoylmethyl)triethylammonium bromide (QX-314), and 2-(dimethylamino)ethyl 4-(butylamino)benzoate (tetracaine), as well as three large slowly reversible antagonists, bis-(2,2,6,6-tetramethyl-4-piperidinyl) sebacate (BTMPS), 2,2,6,6-tetramethylpiperidin-4-yl heptanoate (TMPH), and 1,2,4,5-tetra-{5-[1-(3-benzyl)pyridinium]pent-1-yl}benzene tetrabromide (tkP3BzPB), were investigated for their effectiveness and voltage dependence in the inhibition of responses evoked by acetylcholine alone or augmented by the α7-selective PAM N-(5-chloro-2,4-dimethoxyphenyl)-N′-(5-methyl-3-isoxazolyl)-urea (PNU-120596). Analyses of the small antagonists on PNU-120596–potentiated single-channel bursts indicated that each agent had a distinct mechanism of inhibition and only that of QX-314 was consistent with simple open channel block. In addition to decreasing channel open times and burst durations, mecamylamine and tetracaine induced unique subconductance states. To determine whether channel-blocking activity alone would be sufficient to prevent cell death, the antagonists were tested for their ability to protect α7-expressing cells from cytotoxic effects of the α7 agonist choline in combination with PNU-120596. Only tetracaine and tkP3BzPB, the two agents that had effects least consistent with simple ion channel block, were fully cytoprotective at concentrations that gave submaximal inhibition of macroscopic currents in oocytes. Further analyses indicated that toxicity produced by PNU-120596 and choline was calcium independent and likely an apoptotic event. Our results are consistent with the hypothesis that PAMs may modulate conformational states important for both channel activity and ion channel-independent signaling.
Introduction
Homomeric α7 nicotinic acetylcholine receptors (nAChRs) have many properties that make them unique. Although they constitute one of the two major types of nAChRs in the brain, they are also widely expressed in non-neuronal tissues, suggesting that they have evolved to serve multiple functions in different tissues and represent a sort of primordial ligand-gated ion channel. Consistent with this hypothesis, α7 nAChRs are not strictly receptors for acetylcholine (ACh), since they can also be activated by choline, the ubiquitous ACh precursor. The α7 receptors lack many of the specializations normally associated with ligand-gated ion channels that mediate fast synaptic transmission. Even under optimized conditions, α7 receptors have a probability of synchronized openings that is two orders of magnitude lower than that of the nAChRs at neuromuscular junctions or autonomic ganglia (Williams et al., 2012). They have five rather than two agonist binding sites, and although they desensitize rapidly at high levels of agonist occupancy, their desensitization is rapidly reversible and is not associated with the induction of a high-affinity nonconducting state.
The unique properties and broad distribution of α7 nAChRs in neuronal and non-neuronal cell types challenge the conventional assumption that all signaling mediated by these receptors must be due to the activation of ion channel currents. The activated ion channel of α7 nAChRs has a remarkably high permeability to calcium under normal conditions (Séguelá et al., 1993), and in some contexts α7 signaling is likely to be associated with ion channel activation. However, α7-activation in some non-neuronal cell types appears to occur independently of or even in the absence of ion channel activation (de Jonge and Ulloa, 2007), supporting the hypothesis that ligand-bound conducting states may mediate signal transduction via cascades such as those associated with the JAK/STAT pathway (Marrero and Bencherif, 2009).
The discovery of α7-selective positive allosteric modulators (PAMs), such as N-(5-chloro-2,4-dimethoxyphenyl)-N′-(5-methyl-3-isoxazolyl)-urea (PNU-120596), has enhanced our ability to probe the conformational dynamics and signaling properties of α7 receptors. PNU-120596 has been classified as a “type II” PAM (Grønlien et al., 2007): it both increases the probability of transient activation by agonists, and also destabilizes one or more of the conformational states that would be associated with desensitization in the absence of the PAM. We have identified two classes of ligand-bound nonconducting “desensitized” states: Ds states, which can be converted to conducting states by PNU-120596 (sensitive to the effects of the PAM), and Di state(s), which are insensitive to the effects of the PAM. The differential sensitivity of these desensitized states to PNU-120596 has allowed us to probe the conformational trajectory of α7 receptors after agonist binding and has led to the hypothesis that in the absence of a PAM, ion channel-independent signal transduction may be mediated by receptors in either or both of these ligand-bound nonconducting states (Williams et al., 2011b).
If there is a dichotomy between channel-dependent and channel-independent signaling, it might be assumed that a PAM such as PNU-120596 would amplify only the channel-dependent signaling. However, a comparison of the microscopic (i.e., single-channel) and macroscopic effects of PNU-120596 indicated that there is a very large effect on the open probability of a very small percentage of the channels modified by the PAM (Williams et al., 2011b), suggesting that the distribution and properties of the nonconducting states may also be strongly affected by the PAM.
Excitotoxicity is a phenomenon that has been associated with neuronal cell death as a consequence of overstimulation of calcium-permeable N-methyl-d-aspartic acid (NMDA)–type glutamate receptors (Rothman and Olney, 1987). We recently demonstrated cytotoxic effects of PNU-120596 combined with choline on α7-expressing human embryonic kidney 293 (HEK 293) cells (Williams et al., 2012). This effect was blocked by the α7-selective competitive antagonist methyllycaconitine and was hypothesized to be due to ion channel-mediated calcium overload, similar to the NMDA receptor-mediated excitotoxicity. To investigate this hypothesis, we characterized the sensitivity of α7-mediated currents to six noncompetitive antagonists (Fig. 1) under control conditions and with coapplications of PNU-120596. We distinguished not only differences in the potency and mechanism of inhibition for these agents but also differences in their effectiveness at inhibiting currents evoked by ACh alone or those potentiated by PNU-120596. Our data suggest that the ion conduction pathway differs in the PNU-120596–potentiated currents compared with controls, both in regard to the binding of antagonists and intrinsic factors associated with the inward rectification of neuronal nAChRs. We then extended our studies of these antagonists to determine their potential ability to act as cytoprotective agents against α7-mediated cytotoxic effects of PNU-120596 and choline, and further experiments demonstrate that this toxicity is calcium independent.
Fig. 1.
Structures of small noncompetitive antagonists mecamylamine, QX-314, and tetracaine (left) and slowly reversible inhibitors BTMPS, TMPH, and tkP3BzPB (right).
Materials and Methods
Chemicals.
Solvents and reagents were purchased from Sigma-Aldrich (St. Louis, MO). Cell culture supplies were purchased from Life Technologies (Grand Island, NY). The Hanks’ balanced salt solution (HBSS) (Life Technologies) contained the following (in mM): 1.26 CaCl2, 0.493 MgCl2, 0.407 MgSO4, 5.33 KCl, 0.441 KH2PO4, 4.17 NaHCO3, 137.93 NaCl, 0.338 Na2HPO4, and 5.56 d-glucose. PNU-120596 was synthesized by Dr. Jingyi Wang and Kinga Chojnacka as previously described (Williams et al., 2011b). Mecamylamine [(1S,2R,4R)-N,2,3,3-tetramethylbicyclo[2.2.1]heptan-2-amine], QX-314 [N-(2,6-dimethylphenylcarbamoylmethyl)triethylammonium bromide], and tetracaine [2-(dimethylamino)ethyl 4-(butylamino)benzoate] were purchased from Sigma-Aldrich. BTMPS (bis-(2,2,6,6-tetramethyl-4-piperidinyl) sebacate) was provided by Ciba-Geigy (Summit, NJ). TMPH (2,2,6,6-tetramethylpiperidin-4-yl heptanoate) was purchased from Tocris (c/o R&D Systems, Minneapolis, MN). tkP3BzPB (1,2,4,5-tetra-{5-[1-(3-benzyl)pyridinium]pent1-yl}benzene tetrabromide) was synthesized as previously published (Papke et al., 2005). Fresh ACh and choline stock solutions were made each day of experimentation. PNU-120596 stock solutions were prepared in dimethylsulfoxide (DMSO), stored at −20°C, and used for up to 1 month. PNU-120596 solutions were prepared freshly each day at the desired concentration from the stored stock.
Heterologous Expression of α7 nAChRs in Xenopus Oocytes.
The cDNA clones of human α7 nAChR and human resistance-to-cholinesterase 3 (RIC-3) were provided by Dr. Jon Lindstrom (University of Pennsylvania, Philadelphia, PA) and Dr. Millet Treinin (Hebrew University, Jerusalem, Israel), respectively. Subsequent to linearization and purification of the plasmid cDNAs, cRNAs were prepared using the mMessage mMachine in vitro RNA transfection kit (Ambion, Austin, TX).
Oocytes were surgically removed from mature female Xenopus laevis frogs (Nasco, Ft. Atkinson, WI) and injected with cRNAs of α7 nAChR and RIC-3 as described previously (Papke and Stokes, 2010). The RIC-3 chaperone protein can improve and accelerate α7 expression with no effects on the pharmacological properties of the receptors (Halevi et al., 2003). Frogs were maintained in the Animal Care Service facility of the University of Florida, and all procedures were approved by the University of Florida Institutional Animal Care and Use Committee. In brief, the frog was first anesthetized for 15–20 min in 1.5 l frog-tank water containing 1 g ethyl 3-aminobenzoate methanesulfonate (MS-222) buffered with sodium bicarbonate. The harvested oocytes were treated with 1.25 mg/ml collagenase (Worthington Biochemicals, Freehold, NJ) for 2 hours at room temperature in a calcium-free Barth’s solution (88 mM NaCl, 1 mM KCl, 2.38 mM NaHCO3, 0.82 mM MgSO4, 15 mM HEPES, and 12 mg/l tetracycline, pH 7.6) to remove the follicular layer. Stage V oocytes were subsequently isolated and injected with 50 nl of 6 ng α7 nAChR subunit cRNA and 3 ng RIC-3 cRNA. Recordings were carried out 1–7 days after injection.
Two-Electrode Voltage Clamp Electrophysiology.
Experiments were conducted using OpusXpress 6000A (Molecular Devices, Union City, CA) (Papke and Stokes, 2010). Both the voltage and current electrodes were filled with 3 M KCl. Oocytes were voltage-clamped at −60 mV except when determining the effect of voltage on inhibition by different antagonists. To determine voltage dependence in the absence of PNU-120596, cells were held at −80 or −40 mV, whereas in the presence of PNU-120596, cells were held at −60 or +50 mV. The oocytes were bath-perfused with Ringer’s solution (115 mM NaCl, 2.5 mM KCl, 1.8 mM CaCl2, 10 mM HEPES, and 1 μM atropine, pH 7.2) with a flow rate of 2 ml/min. To evaluate the effects of noncompetitive antagonists on ACh-evoked responses of α7 nAChRs expressed in oocytes in the absence and presence of PNU-120596, the initial control conditions were set up by two applications of ACh alone or ACh plus PNU-120596 before coapplications of experimental drugs with the control ACh or ACh plus PNU-120596. The agonist solutions were applied from a 96-well plate via disposable tips, and the antagonists and PNU-120596 were either coapplied with ACh by the OpusXpress pipette delivery system or bath-applied using the OpusXpress system to switch the running buffer. For the concentration-response study, drug applications were 12 seconds followed by a 181-second washout period and usually alternated between controls and test solutions containing experimental antagonists of various concentrations. A typical recording for each oocyte contained two initial control applications of ACh or ACh plus PNU-120596, an experimental drug application, and then a follow-up control application of ACh with or without PNU-120596 to determine the desensitization or rundown of the receptors.
Data were collected at 50 Hz, filtered at 20 Hz, analyzed by Clampfit 9.2 (Molecular Devices) and Excel (Microsoft, Redmond WA), and normalized to the averaged current of the two initial control responses (Papke and Porter Papke, 2002). Data were expressed as the mean ± S.E.M. from at least four oocytes for each experiment. For the concentration-response relationships, responses were normalized to the net charge of the most adjacent prior control. Data were plotted by Kaleidagraph 3.0.2 (Abelbeck Software, Reading, PA) and curves were generated as the best fit of the average values from the Hill equation, using negative Hill slopes.
Cell Culture of A7R3HC10 Cells.
The A7R3HC10 cells stably expressing human α7 and human RIC-3 were generated from low passage number HEK 293 cells obtained from American Type Culture Collection (Manassas, VA) (Williams et al., 2012). The A7R3HC10 cells were routinely cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum in the presence of 0.45 mg/ml O-2-amino-2,7-dideoxy-D-glycero-α-D-gluco-heptopyranosyl-(1→4)-O-[3-deoxy-4-C-methyl-3-(methylamino)-β-L-arabinopyranosyl-(1→6)]-2-deoxy-D-streptamine disulfate (G418) and 0.015 mg/ml hygromycin at 37°C with 5% CO2. For normal passaging, cells were dissociated with 1 mM EDTA in calcium-free and magnesium-free HBSS to avoid nonselective damage to the α7 nAChRs expressed on the cell surface. Cells with fewer than 27 passages after stable transfection were used for patch clamp recordings, and cells with fewer than 30 passages were used in cytotoxicity experiments.
Outside-Out Patch Clamp Electrophysiology.
Single-channel currents were recorded in the outside-out configuration at room temperature using an Axopatch 200A amplifier (Molecular Devices) as described previously (Williams et al., 2011a,b). One day before recording, A7R3HC10 cells were plated onto 12-mm glass coverslips (Thermo Fisher Scientific) coated with 0.1 mg/ml poly-d-lysine (Sigma-Aldrich). Cells were recorded 1–5 days after plating. Briefly, cells were bathed in an external solution containing (in mM) 165 NaCl, 5 KCl, 2 CaCl2, 10 glucose, and 5 HEPES, and 1 µM atropine, pH 7.35. Patch pipettes were pulled using the P-97 micropipette puller (Sutter Instruments, Novato, CA), fire-polished to 5–10 MΩ, coated with SigmaCote (Sigma-Aldrich), and filled with an internal solution containing (in mM) 147 CsCl, 2 MgCl2, 1 CaCl2, 10 EGTA, 10 HEPES, and 2 Mg-ATP, pH 7.35. Recordings were low-pass filtered to 10 kHz with the built-in amplifier filter (4-pole Bessel) and digitized at 100 kHz with a DigiData 1440 digitizer (Molecular Devices) using Clampex 10.3 data acquisition software (Molecular Devices).
Fast solution exchange to outside-out patches was performed via a Burleigh piezoelectric stepper (EXFO, Concord, ON, Canada) conditioned by an RC circuit (τ = 2 milliseconds) to reduce oscillations and avoid damage to the crystal (Kabakov and Papke, 1998). A double-barrel drug pipette was made from borosilicate theta glass (Sutter Instruments) and mounted on the piezoelectric stepper. Two 60-ml syringes (Monoject; Sherwood Medical Company, St. Louis, MO) were connected to each channel of the drug pipette via polyethylene tubing. Channel 1 of the drug pipette was connected to syringes containing either 300 μM ACh and 10 μM PNU-120596 or external solution, whereas channel 2 was connected to syringes containing either 300 μM ACh and 10 μM PNU-120596 plus the antagonist or 50% external solution. The time required to switch solutions in a channel was approximately 15 seconds. To maintain undisturbed laminar flow from the drug pipette and minimize solution mixing, external solution was continuously perfused through the recording chamber (Warner Instruments, Hamden, CT) at 4 ml/min, and the drug pipette should be correctly aligned such that drug streams would directly perfuse through the outside-out patch in the recording pipette. All solutions in the four syringes were degassed under vacuum to eliminate air bubbles that could damage the patch seal. The solution exchange time (10–90% rise time) was determined to be typically <1 milliseconds by measuring changes in holding current upon moving 50% external solution over the open recording pipette tip with the piezoelectric stepper (Williams et al., 2011b).
Once a stable outside-out patch was obtained and the drug and recording pipettes were positioned, the streams of 300 μM ACh and 10 μM PNU-120596 from channel 1 and 300 μM ACh, 10 μM PNU-120596 plus the antagonist from channel 2 were turned on. The piezoelectric stepper was used to move the 300 μM ACh and 10 μM PNU-120596 stream over the patch to obtain the PNU-120596–potentiated control response. After initial periods of multichannel openings, the stepper was then used to move the 300 μM ACh, 10 μM PNU-120596, and the antagonist stream over the patch to investigate the effect of each antagonist on single-channel bursts potentiated by PNU-120596, after which the 300 μM ACh and 10 μM PNU-120596 stream was moved back over the patch for the postantagonist control response. The drug application pipette usually switched back and forth to allow the solution from either barrel to flow over the patch and record under the control and test conditions. After data collection, each patch was blown off the tip of the recording pipette, and the solution exchange profile was determined. Data were analyzed only if the solution exchange was clean and occurred rapidly.
Data from at least seven individual patches for each condition were pooled to obtain sufficient numbers of events for analysis. Sections of data traces containing single-channel activity and flanked by ≥100 milliseconds of closed time were selected for analysis (Colquhoun and Sakmann, 1995; Williams et al., 2011b). Apparent subconductances that occurred occasionally were ignored. All traces were filtered to 5 kHz, corrected for baseline drift, idealized, and analyzed with Clampfit 10.3 (Molecular Devices). Burst analysis was conducted to define groups of one or more apparent channel openings and closures that arose from an individual channel.
Whole-Cell Patch Clamp Electrophysiology.
The 12-mm glass coverslips were coated with 0.1 mg/ml poly-d-lysine at 37°C for 5 minutes. A7R3HC10 cells were plated onto the coverslips 1–4 days before recording. Whole-cell voltage clamp recordings were performed at room temperature using an Axopatch 200B amplifier (Molecular Devices). Briefly, cells were bathed in the same external solution as used in the outside-out single-channel recordings. Patch pipettes (3–5 MΩ) were filled with an internal solution containing (in mM) 120 CsCl, 2 MgCl2, 10 EGTA, 10 HEPES, and 5 Mg-ATP, pH 7.35. The current-voltage (I-V) relationships of currents evoked by ACh alone or potentiated by PNU-120596 were investigated. Drug solutions were applied by Picospritzer III (General Valve, Fairfield, NJ) via pressure (10–20 psi). The application pipette was positioned approximately 10–15 μm from the cell. Drug applications were 3 seconds in duration and were made every 60 seconds. Three baseline responses induced by 1 mM ACh or 100 μM ACh plus 10 μM PNU-120596 were initially recorded at −70 mV, after which responses were recorded as the membrane potential (Vm) increased from −70 to +60 mV with 10-mV increments. Recordings were filtered to 5 kHz and digitized at 20 kHz with a DigiData 1322A (Molecular Devices) using Clampex 9.2. Both the input resistance and access resistance were monitored by a 10-ms/10-mV pulse before each response. The whole-cell recordings were analyzed with Clampfit 10.3. Cells with access resistance >40 MΩ were excluded from analysis. In experiments evaluating the voltage dependence of currents evoked by ACh alone, input resistances were usually 1–2 GΩ. However, the whole-cell seals decreased upon the increase in holding potential when determining the current-voltage relationship of the PNU-120596–potentiated responses. Thus, a more relaxed criterion was adopted for this condition to include whole-cell recordings with input resistance >100 MΩ in the analysis. Responses were measured as peak currents. The steady-state I-V curves were obtained by normalizing the current at each Vm to the averaged response at −70 mV, which was defined as −1. I-V curves were plotted by Kalidagraph 3.0.2, and data were represented as the mean ± S.E.M. of five cells.
Cytotoxicity Assay.
A7R3HC10 cells were grown in normal culture medium, and experiments were conducted in HBSS because DMEM contained approximately 30 μM choline, which would act as an α7 agonist and perturb our manipulation of agonist concentration in the assay (Williams et al., 2012). Briefly, A7R3HC10 cells and untransfected HEK 293 cells with the same passage number were plated into 96-well plates at a density of 1.5 × 104 cells per well in DMEM with 10% fetal bovine serum and incubated overnight at 37°C. After removing the medium, cells were washed twice with HBSS. Experimental compounds prepared in HBSS were then applied to the cells. For PNU-120596 and BTMPS, the 10 mM stock solutions were respectively dissolved in DMSO and methanol, and their working solutions were diluted with HBSS. Both the A7R3HC10 cells and untransfected HEK 293 cells were incubated at 28°C for 2 hours, because the onset of PNU-120596 cytotoxicity was revealed to be very fast and the maximal toxicity occurred within 2 hours (Williams et al., 2012). After the 2-hour treatment, the experimental solutions were replaced with 100 μl HBSS and 20 μl CellTiter96 solution (Promega, Madison, WI) per well and incubated at 37°C overnight. The absorbance at 490 nm was read by a microplate spectrophotometer (BioTek, Winooski, VT). The cytotoxicity is defined as the reduction in cell viability, which is directly associated with the reduction of a tetrazolium salt by dehydrogenase enzymes in metabolically active cells to form a soluble formazan dye that has specific absorbance at 490 nm. Each condition was tested in three wells to obtain an averaged absorbance value. Background absorbance was measured from cell-free wells and subtracted from all control and test conditions. The absorbance values from test conditions were normalized to the absorbance of the 0.5% DMSO vehicle control, which was defined as 100% cell viability. Statistical significance was analyzed using the two-tailed Student's t test.
We expanded our analysis of cytotoxicity to determine whether the 100 µM choline and 10 µM PNU-120596 treatment of 2 hours at 28°C can cause death of A7R3HC10 cells in the absence of calcium. We performed the cytotoxicity assay in a modified HBSS solution containing (in mM) 1.26 BaCl2, 0.9 MgCl2, 5.33 KCl, 0.441 KH2PO4, 4.17 NaHCO3, 137.93 NaCl, 0.338 Na2HPO4, and 5.56 d-glucose. In this formula, we replaced the CaCl2 with BaCl2, increased the concentration of MgCl2, and removed the MgSO4 to avoid forming precipitates with barium. The modified HBSS containing 1.26 mM CaCl2, 0.9 mM MgCl2, and other components without MgSO4, as well as the normal HBSS containing 1.26 mM CaCl2, 0.493 mM MgCl2, 0.407 mM MgSO4, and other components, were also used as controls. The absorbance at 490 nm was read after 4 hours and overnight incubation at 37°C.
We also measured the induction of apoptosis by the 100 µM choline and 10 µM PNU-120596 treatment via the Apo-ONE Homogeneous Caspase-3/7 Assay (Promega). In brief, 100 µM choline and 10 µM PNU-120596 prepared in the normal HBSS solution was applied to the A7R3HC10 cells and incubated at 28°C for 2 hours. After the 2-hour treatment, the experimental solution was replaced with 50 µl HBSS and 50 µl Apo-ONE Caspase-3/7 Reagent (Promega) per well, gently mixed using a plate shaker for 15 minutes, and incubated at room temperature for 4 hours. The fluorescence was read by a microplate spectrophotometer (BioTek) at an excitation wavelength range of 485 ± 20 nm and an emission wavelength range of 528 ± 20 nm. Each condition was tested in three wells. The averaged background fluorescence was subtracted from all control and test conditions. The fluorescence values from test conditions were normalized to the averaged fluorescence of the 0.5% DMSO vehicle control, which was defined as 100% caspase-3/7 activity. Statistical significance was analyzed using the Student's t test.
Results
Macroscopic Studies of Small Antagonists in Oocytes.
Homomeric α7 nAChRs have recently been recognized as a potential target for therapeutics leading to the development of α7-selective ligands (agonists, partial agonists, competitive antagonists). Because the α7 receptors are allosteric membrane proteins with dynamic interconversions between multiple functional states, these ligands are valuable experimental tools to investigate different models for the activation and desensitization of α7 nAChRs. Unlike the competitive antagonists that bind to the conventional agonist binding site, the inhibition by noncompetitive antagonists showed a distinctly different mechanism in that the antagonists bind to other modulatory sites and thus will not prevent agonist binding. An understanding of the unique mechanisms of different forms of noncompetitive antagonism will increase our knowledge of receptor-mediated signaling, including whether ligand binding may have effects that are independent of ion conduction. However, due to the intrinsic low Popen and rapid desensitization of α7 receptors, the direct characterization of potential α7 noncompetitive antagonists is challenging.
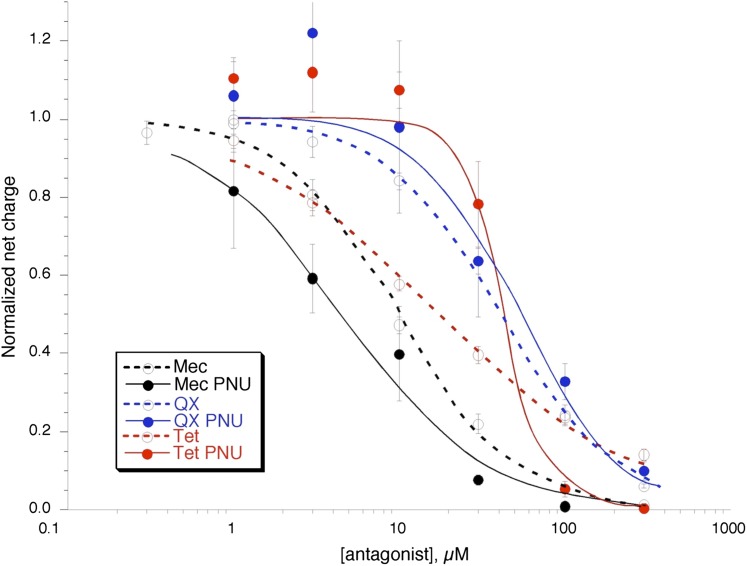
The capability of type II α7 PAMs to prolong the kinetics of α7 activation by destabilization of desensitized states (Grønlien et al., 2007) provides a new opportunity to study these receptors and raises a question of whether the potentiated currents have similar sensitivity to noncompetitive antagonists as the currents evoked by agonist alone. Here we examined the effects of three small antagonists to inhibit α7-mediated currents expressed in X. laevis oocytes evoked by either ACh alone or ACh coapplied with PNU-120596, one of the most effective and well-characterized α7 selective PAMs (Hurst et al., 2005; Grønlien et al., 2007; Young et al., 2008; Gopalakrishnan et al., 2011; Williams et al., 2011b). Normalized net charge data were obtained from 4–8 cells when each antagonist was coapplied with 60 or 10 μM ACh and 10 μM PNU-120596 at various concentrations (Fig. 2). The IC50 values calculated from the curves fit for the inhibition of ACh-evoked responses in the absence and presence of PNU-120596 by three small noncompetitive antagonists are shown in Table 1. Mecamylamine inhibited the currents evoked by ACh alone and by ACh plus PNU-120596 with IC50 values of 10 ± 1 and 4.8 ± 0.7 μM, respectively, indicating that the inhibitory effect of mecamylamine for PNU-120596–potentiated currents was approximately 2-fold greater than for currents evoked by ACh. In contrast, when applied at concentrations ranging from 1 to 300 μM, tetracaine’s inhibitory effect was much greater for responses evoked by ACh alone than those potentiated by PNU-120596 (IC50 values of which were 18 ± 2 and 43 ± 8 μM, respectively). Coapplication of QX-314 through the same concentration range as tetracaine produced inhibition of the currents evoked by ACh alone or ACh plus PNU-120596, with comparable IC50 values of 42 ± 2 and 55 ± 16 μM, respectively.
Fig. 2.
Inhibition of ACh-evoked responses in the absence and presence of PNU-120596 (PNU) of human α7 nAChRs expressed in Xenopus oocytes by coapplication of mecamylamine (Mec), QX-314 (QX), and tetracaine (Tet). Data are normalized to the averaged net charge of control 60 or 10 µM ACh plus 10 µM PNU-120596 responses. Each point represents the mean (± S.E.M.) of at least four oocytes for each condition.
TABLE 1.
IC50 values for the inhibition of α7-mediated currents in Xenopus oocytes
| Agent | Control | PNU-120596 Potentiated |
|---|---|---|
| μM | ||
| Mecamylamine | 10 ± 1 | 4.8 ± 0.7 |
| QX-314 | 42 ± 2 | 55 ± 16 |
| Tetracaine | 18 ± 2 | 43 ± 8 |
Inhibition of PNU-120596–Potentiated Single-Channel Bursts with Small Reversible Antagonists.
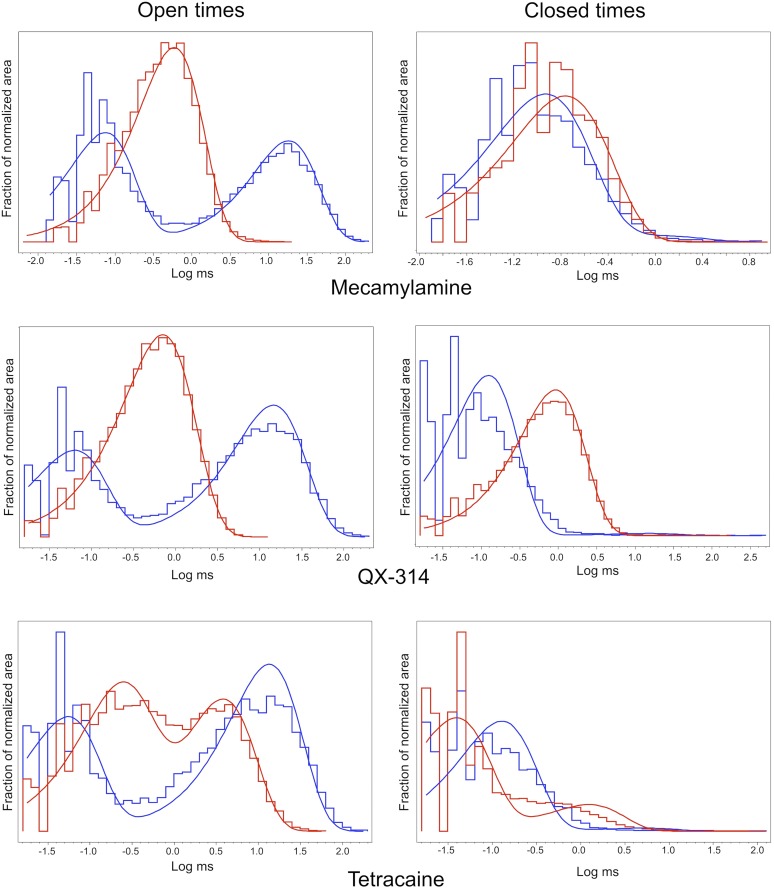
We recently investigated the ACh-evoked responses of α7 nAChRs expressed in BOSC23 cells in the absence and presence of PNU-120596 from outside-out patches (Williams et al., 2011b). In this study, we aimed at measuring the effects of the three small reversible antagonists on the properties of single-channel bursts enhanced by PNU-120596 because ACh alone only evoked short-lived single-channel openings that quickly diminished after exposure to the agonist (Williams et al., 2011b).
Consistent with our previous studies with transiently transfected cells, in the presence of 300 μM ACh and 10 μM PNU-120596, the α7-mediated currents in outside-out patches pulled from A7R3HC10 cells decayed over the course of several seconds to intermittent prolonged single-channel bursts. Control data were obtained both from patches that were not exposed to any antagonist and from patches that were alternately exposed to antagonist-free (control) and antagonist-containing (experimental) solution. There were no significant differences in the control burst data under these varying conditions (not shown).
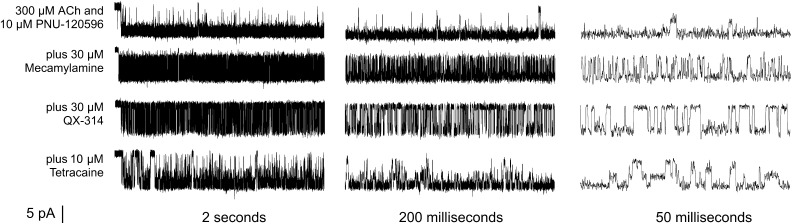
Consistent with our previous observations, channels were activated with the highest probability immediately after application of ACh and PNU-120596. Subsequently, channels approached a steady-state condition with the intermittent appearance of prolonged bursts. The prolonged application of ACh and PNU-120596 along with mecamylamine induced the majority of channels to enter the Di state or blocked state, revealing single-channel bursts potentiated by PNU-120596 but modified by the antagonist. Each of the small antagonists produced distinct alterations in the pattern of intraburst openings and closures, as illustrated in Fig. 3.
Fig. 3.
Representative single-channel traces of outside-out patches from α7 receptors before and after antagonist cotreatment as indicated in the different time resolutions. Currents were sampled at 100 kHz and low-pass filtered at 5 kHz.
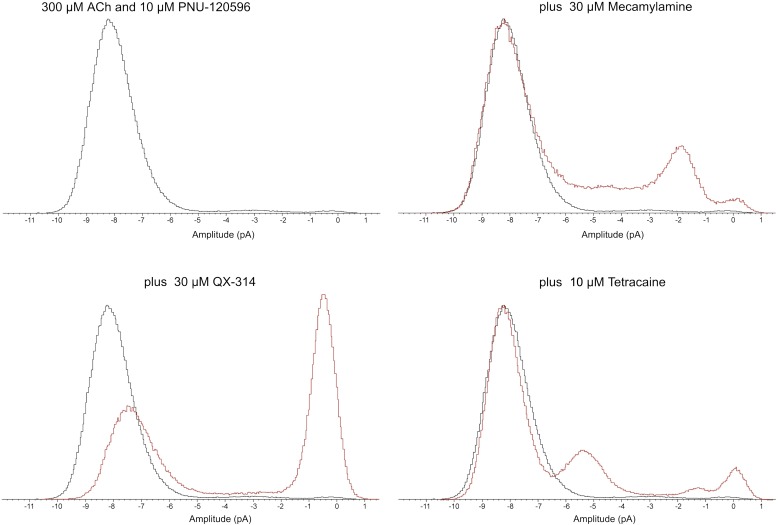
As shown in the all-point histograms in Fig. 4, under control conditions, the majority of openings were fit to a full conductance state of approximately 8 pA. There were rare subconductance events of about 3 pA, and relatively little time was spent in the closed state within a burst. The introduction of 30 µM mecamylamine introduced at least one new subconductance state at 1.7 pA, representing 16% as many events as in the full conductance state. In addition, a similar amount of time was spent with intermediate conductance that did not resolve into discrete elements.
Fig. 4.
All-point histograms of the representative recordings shown in Fig. 3. Burst durations were 5.78, 2.70, 2.63, and 3.92 seconds for the control, mecamylamine, QX-314, and tetracaine bursts, respectively. For clarity of comparisons, the histograms were autoscaled so that the largest components were of the same amplitude. For the QX-314 data, the largest component was the blocked closed state, whereas the largest component was the primary open state in the other histograms.
When 30 µM QX-314 was applied to a patch, 61% of the time within a burst showed a nearly fully closed state, and the amplitude of the fully open state appeared to be reduced. The apparent shifts in the full open and closed amplitudes in Fig. 4 were likely due to the fact that the filter settings prevented complete resolution of either class of events in these rapidly changing recordings.
The presence of 10 µM tetracaine introduced a distinct subconductance that was 70% of the amplitude of the full conductance state and accounted for 22% as much area of the all-point histogram as the full conductance state.
We examined the effects of these antagonists on the intraburst open and closed times as well as the burst duration and frequency. For these analyses, control data were obtained from the same patches as exposed to a specific antagonist to make allowance for any effects that might be due to patch-to-patch variability. In the composite of all control data (not shown), there were two main open times [τ1 = 65 µs (44%), τ2 = 18 milliseconds (46%)] and two closed times [τ1 = 80 µs (74%), τ2 = 280 µs (25%)].
As shown in Fig. 5 and Table 2, the control bursts in all three data sets were similar to the pooled data, although in some cases the open time histograms were best fit by including a minor third component. In the presence of 30 µM mecamylamine, open times shifted toward a single component with an average open time of 700 µs, possibly representing an obscuring of the normal brief opening and a shortening of the longer events. The intraburst closed times in mecamylamine shifted toward a single longer closed time of 160 µs. It should be noted that our event detection threshold would accept the mecamylamine-associated subconductance state indicated in Fig. 4 as channel closed time.
Fig. 5.
Effects of small reversible antagonists on the apparent open and closed durations in the PNU-120596–potentiated α7 single-channel bursts. Control bursts were evoked by 300 μM ACh and 10 μM PNU-120596 either before the antagonist coapplication or after the removal of external antagonist from the same patch. Note that although low-amplitude infrequent subconductance events were observed, for these analyses the event detection thresholds were set to capture only the main conductance state of the receptor, and times in the subconductance states were taken as closures. (Top) Fit histograms displaying intraburst open and closed durations of 32 control bursts (blue line) from 7 patches (19,850 events), and 35 bursts from 8 patches (32,104 events) treated by 300 μM ACh, 10 μM PNU-120596, and 30 μM mecamylamine (red line). (Middle) Fit histograms displaying intraburst open and closed durations of 26 control bursts (blue line) from 16 patches (9104 events), and 57 bursts from 18 patches (85,090 events) treated by 300 μM ACh, 10 μM PNU-120596, and 30 μM QX-314 (red line). (Bottom) Fit histograms displaying intraburst open and closed durations of 30 control bursts (blue line) from 10 patches (9232 events), and 44 bursts from 9 patches (28,835 events) treated by 300 μM ACh, 10 μM PNU-120596, and 10 μM tetracaine (red line). Only the bursts lasting longer than 1 second and with no obvious subconductance were selected for analysis to ensure enough intraburst events. Fit parameters are listed in Table 2. To compare data sets with varying numbers of events, each of the histograms was normalized to have a total area equal to one so that the y scaling was adjusted automatically for each histogram.
TABLE 2.
Time constants and areas of the event duration distributions
Data are presented as τ (% of total area).
| Agent | Bursts | Open Times |
Closed Times |
||||
|---|---|---|---|---|---|---|---|
| τ1 | τ2 | τ3 | τ1 | τ2 | |||
| n | μs | ms | ms | μs | μs | ||
| Control | 32 | 60 (49) | 1 (5) | 18 (45) | 30 (21) | 100 (79) | |
| Mecamylamine | 35 | 700 (100) | 160 (100) | ||||
| Control | 26 | 50 (38) | 3 (12) | 17 (49) | 38 (53) | 210 (47) | |
| QX-314 | 57 | 700 (100) | 900 (100) | ||||
| Control | 30 | 50 (40) | 14 (60) | 40 (51) | 200 (49) | ||
| Tetracaine | 44 | 200 (46) | 2.5 (37) | 5.9 (17) | 30 (77) | 540 (22) | |
The presence of 30 µM QX-314 also had the effect of moving the open time distribution toward a single component of approximately 700 µs. The intraburst closed time distribution in QX-314 was shifted to a single component with a time constant of 900 µs. In contrast to mecamylamine and QX-314, tetracaine had the effect of introducing components into the open time distribution as an intermediate duration, rather than simply shortening the duration of the longer openings. It should also be noted that our event detection threshold would accept the tetracaine-associated subconductance state indicated in Fig. 4 as channel open times. Events within tetracaine bursts were sorted by amplitude, and those in the range of 4–6 pA were extracted as the subconductance state. These events had an average open time of 570 μs, making them most closely correspond to the briefest open time of the full distribution. Tetracaine also introduced a novel longer component into the closed time distribution.
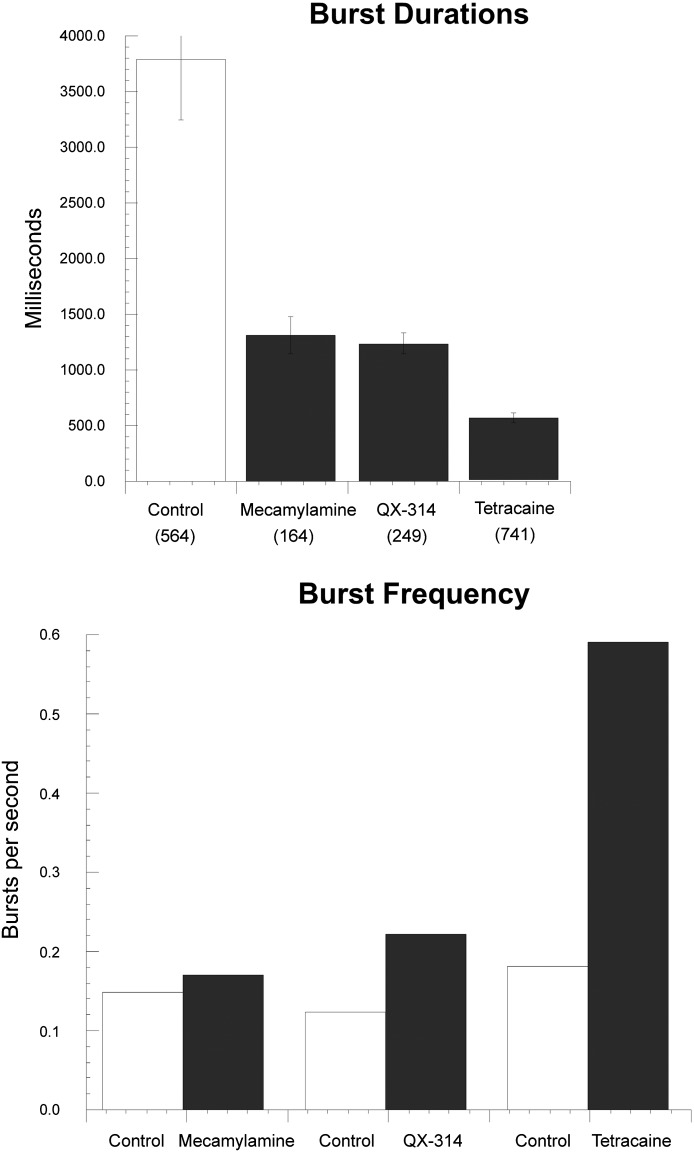
To evaluate the roles of the small reversible antagonists on influencing the burst duration and burst frequency, we analyzed all apparent bursts (clusters of events isolated by intervals of 100 milliseconds or more) recorded under each condition, including the ones that appeared as relatively brief events that primarily occurred in isolation and those that contained simultaneous openings and subconductance events. The average durations of the PNU-120596–potentiated α7 control bursts were 3116.72 ± 363.12 milliseconds (n = 162), 4656.53 ± 464.11 milliseconds (n = 222), and 3606.50 ± 313.46 milliseconds (n = 180) for the mecamylamine, QX-314, and tetracaine data sets, respectively. Overall the average duration of control bursts of α7 receptors potentiated by 10 µM PNU-120596 persisted for 3879.13 ± 234.19 milliseconds (n = 564), with no significant differences among the data sets.
All of the antagonist cotreatments significantly shortened the length of the bursts (Fig. 6). The mecamylamine-, QX-314-, and tetracaine-treated bursts only lasted for 1309.21 ± 164.43 milliseconds (n = 164), 1236.81 ± 92.80 milliseconds (n = 249), and 554.38 ± 41.67 (n = 741), respectively. Only tetracaine produced a marked increase in the burst frequency (0.59 versus 0.18 bursts per second), suggesting that tetracaine produced blocked times longer than the 100-millisecond criterion used to define our interburst interval. These data are therefore consistent with the hypothesis that ACh can dissociate or the channels can desensitize or otherwise close before the dissociation of tetracaine, indicating that tetracaine is a parallel blocker, as previously reported for muscle nAChR (Papke and Oswald, 1989) and neuronal nAChR with channel mutations (Papke et al., 2001a).
Fig. 6.
Summary of the effects of antagonist treatment on α7 burst duration and burst frequency of responses produced by 300 μM ACh and 10 μM PNU-120596. The number of bursts under each condition is indicated in parentheses.
Effects of Slowly Reversible Antagonists on α7 Responses with or without PNU-120596.
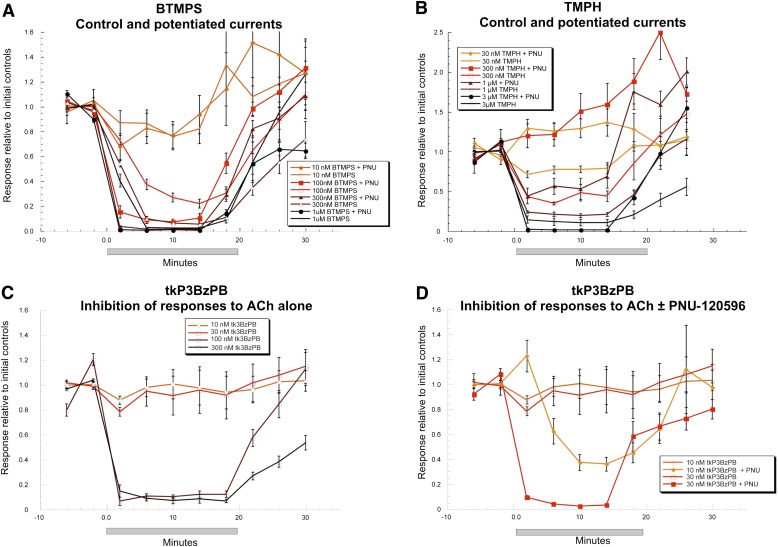
Although many noncompetitive antagonists work rapidly enough for their effects to be apparent on the same time scale of the α7 single-channel events in the presence of PNU-120596, other antagonists such as BTMPS, TMPH, and tkP3BzPB have such slow reversibility that once they affected a channel, it would be unlikely that the same channel would be observed to reopen in the same recording, and if it did reopen, it would be impossible to discriminate it from the opening of any other channels in the patch. Moreover, the potency of such agents can only be appreciated under equilibrium conditions [i.e., when concentration-driven on-rates (M−1s−1) are on the same time scale as the off-rates], so they have the opportunity to work at low concentrations over prolonged periods of time. Therefore, we tested the effects of extended bath applications of these three slowly reversible antagonists on the ACh-evoked responses of human α7 receptors expressed in oocytes with or without PNU-120596 potentiation. The experimental data shown in Fig. 7 are averaged peak current responses obtained from at least four cells when each antagonist was bath-applied at different concentrations (and coapplied with ACh). Each point was normalized to the averaged peak current of two initial 60 µM ACh control responses from the same oocytes.
Fig. 7.
Effects of bath-applied slowly reversible antagonists BTMPS (A), TMPH (B), and tkP3BzPB (C and D) on responses of α7 nAChRs expressed in oocytes to 60 or 10 µM ACh plus 10 μM PNU-120596 (PNU). After measuring two baseline responses evoked by ACh alone or ACh plus PNU-120596, the antagonist solution was added to the bath for 15 minutes, followed by a 10- to 15-minute washout. The oocytes were repeatedly probed for their ACh or ACh plus PNU-120596 responses. All points represent averaged normalized data (± S.E.M., n ≥ 4) of peak current responses for each condition. Note that since low concentrations of TMPH were ineffective and inhibited a run up of potentiated responses, the scale of (B) is expanded relative to the other plots.
At a concentration of 100 nM, the use-dependent inhibitor BTMPS (Papke et al., 1994) was partially effective at inhibiting the responses evoked by 60 μM ACh alone. The peak current response measured 14 minutes after BTMPS bath application was approximately 25% that of the baseline control, and the response recovered slowly with a time constant of approximately 15 minutes. In contrast, 100 nM BTMPS was significantly more effective at inhibiting the responses evoked by coapplication of 10 μM ACh and 10 μM PNU-120596, with faster onset of inhibition and, interestingly, a quicker recovery. The peak current response to ACh plus PNU-120596 determined 2 minutes after BTMPS bath application was 15% that of the control, and the response fully recovered to baseline after an approximately 7-minute washout. When bath-applied at concentrations of 300 nM and 1 μM, BTMPS showed persistent inhibition of peak currents evoked by ACh alone and ACh plus PNU-120596 in a dose-dependent manner, and recovery of these responses was also relatively slow (Fig. 7A). In general, BTMPS demonstrated greater inhibitory effect and faster onset of inhibition on α7 responses to ACh plus PNU-120596 than on responses to ACh alone, which may be due to the prolonged openings of PNU-120596–potentiated α7 ion channels for BTMPS to block (Williams et al., 2011b). BTMPS also revealed a quicker recovery profile on PNU-120596–potentiated α7 currents, except that when bath-applied at 1 μM, responses evoked by ACh alone recovered to baseline after a 9-minute washout, whereas responses evoked by ACh and PNU-120596 only recovered to approximately 60% of controls after a 15-minute washout. These data suggest that both the blocking and unblocking rates of BTMPS for α7 receptors are faster for the open state, including the one promoted by PNU-120596.
When the use-independent inhibitor TMPH (Papke et al., 2005) was bath-applied at a relatively low concentration (300 nM), TMPH produced partial inhibition of the ACh-evoked responses of α7 nAChRs, reducing them to approximately 40% of the pretreatment currents. Somewhat unexpectedly, the PNU-120596–potentiated responses of α7 receptors were not inhibited in the presence of bath-applied 300 nM TMPH (Fig. 7B), and, in fact, responses increased rather than decreased, due to the accumulating effects of the potentiator, as previously reported (Williams et al., 2011b). The 1- and 3-μM TMPH treatments significantly inhibited 75 and 90% of the responses evoked by ACh, respectively. The PNU-120596–potentiated α7 receptors showed an approximately 50% blockade at bath concentration of 1 μM TMPH, whereas full inhibition of α7-mediated responses potentiated by PNU-120596 was achieved with 3 μM TMPH, the highest concentration tested. Taken together, these data suggest that TMPH may be a more potent antagonist for inhibiting α7 nAChRs evoked by ACh alone than for PNU-120596–potentiated responses. After bath applications of TMPH, progressive concentration-dependent recovery of responses evoked by ACh as well as ACh plus PNU-120596 was observed within 10 minutes.
The α7-selective antagonist tkP3BzPB (López-Hernández et al., 2009) bath-applied at concentrations of 10 and 30 nM showed little effect at blocking the responses evoked by ACh alone. However, nearly complete inhibition was seen when tkP3BzPB was bath-applied at 100 and 300 nM (Fig. 7C). In contrast to the effects of TMPH, tkP3BzPB was more effective at blocking the potentiated α7 receptors. At 10 and 30 nM, concentrations that were largely ineffective at inhibiting the responses elicited by ACh alone, responses evoked by ACh and PNU-120596 were blocked approximately 60 and 98%, respectively, compared with the controls (Fig. 7D). The inhibition was slowly reversible; the higher the concentration of tkP3BzPB applied to the bath, the longer the currents took to recover to full control responses.
Voltage Dependence of α7 Responses and Antagonism.
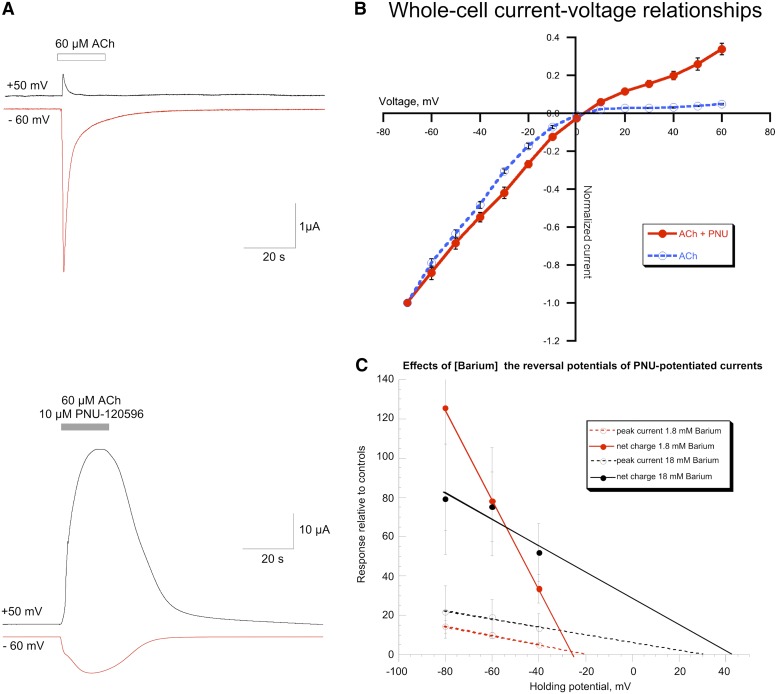
A feature of channel-blocking antagonists that are charged at physiologic pH is a voltage-dependent enhancement of inhibition if the binding site of the antagonist is within the membrane's electric field (Leonard et al., 1988). Since under typical physiologic conditions α7 and other neuronal nAChRs have inward rectifying current-voltage relationships attributed to blockade of outward currents by intracellular magnesium ions (Ifune and Steinbach, 1990; Mathie et al., 1990; Sands and Barish, 1992) or intracellular polyamines (Haghighi and Cooper, 1998), voltage dependence of antagonist activity can usually only be assessed over a limited range of voltage (Papke et al., 2001a). The typical inward rectification of α7 responses recorded under control conditions is illustrated in the upper traces of Fig. 8A. Peak current responses to 60 µM ACh recorded at +50 mV were only 7.9% ± 1.7% of control responses recorded at −60 mV, and net charge responses were reduced to 3.4% ± 1.1% of the controls (n = 6).
Fig. 8.
Effects of PNU-120596 on the current-voltage relationships of α7-mediated currents. (A) The upper traces illustrate the strong inward rectification of control α7 currents for receptors expressed in Xenopus oocytes. The net charge of responses measured at the depolarizing voltage (+50 mV) was a small fraction (3.4% ± 1.0%, n = 6) of that of responses recorded at the standard holding potential of −60 mV. In contrast, as shown in the lower traces, when measured relative to initial ACh controls, the absolute net charge of the responses evoked by 60 μM ACh plus 10 µM PNU-120596 at +50 mV was not smaller than those recorded at −60 mV. The potentiation factors determined from multiple cells were 286-fold ± 85-fold and 177-fold ± 36-fold larger than −60 mV controls for +50 and −60 mV responses, respectively (n = 4). These sample traces were obtained from different cells but were scaled to the ACh control response of the respective cells. (B) The current-voltage relationships of whole-cell responses of α7-expressing A7R3HC10 cells to the pressure application of 1 mM ACh alone (n = 5) or 100 µM ACh plus 10 µM PNU-120596 (n = 5). (C) Barium permeability of PNU-120596–potentiated α7 currents. Calcium-free Ringer’s solutions were made as previously described (Francis and Papke, 1996) with either 1.8 mM BaCl2 (and 48.6 mM sucrose) or 18 mM BaCl2. Cells were first tested for responses to control applications of 60 µM ACh alone in the low barium solution and then either kept in the low barium Ringer’s, or switched to the high barium solution for a series of stimulations with 30 µM ACh plus 6 µM PNU-120596 at different holding potentials. Current-voltage relationships were calculated by linear regression.
PNU-120596–potentiated currents of α7 receptors expressed in oocytes show reduced inward rectification compared with control responses (Sitzia et al., 2011). As shown in the lower traces in Fig. 8A, responses produced by 60 µM ACh and 10 µM PNU-120596 were not reduced at the depolarized voltage compared with those at the control voltage. Normalized to initial ACh controls, the PNU-120596–potentiated responses at +50 mV, in fact, tended to be larger (61-fold ± 19-fold and 286-fold ± 65-fold larger for peak current and net charge, respectively, n = 4) than those at the standard voltage (which were increased 29 ± 8 and 177 ± 35 times for peak current and net charge, respectively, n = 6). However, the amplitudes of the potentiated currents recorded on the same day from oocytes of the same injection set were not statistically different at the two voltages. The relief of inward rectification in the PNU-120596–potentiated currents is consistent with the hypothesis that the ion permeation pathway of α7 receptors potentiated by PNU-120596 is qualitatively different from that of the normal activated state. However, strong inward rectification has also been reported for PNU-120596–potentiated single-channel bursts in outside-out patches from transiently transfected BOSC23 cells (Williams et al., 2011b). We therefore evaluated the voltage dependence of control and PNU-120596–potentiated whole-cell responses in the stably transfected A7R3HC10 cells. As shown in Fig. 8B, there was significantly less inward rectification of PNU-120596–potentiated whole-cell responses than in the responses evoked by ACh alone.
It has previously been reported that for heteromeric nAChRs (Francis and Papke, 1996) and AMPA-type glutamate receptors (Hollmann et al., 1991) that there are correlations between inward rectification and calcium permeability, such that manipulations which reduce rectification also reduce calcium permeability. Since PNU-120596 removes the inward rectification of the α7-mediated currents, we used barium-substituted Ringer’s solution as previously described (Francis and Papke, 1996) to determine the divalent ion permeability of the PNU-120596–potentiated receptors. Specifically, barium was substituted for calcium at either 1.8 mM or increased to 18 mM, with the low barium solution osmotically compensated with sucrose. Barium was used as a reporter of divalent permeability to avoid the contribution of calcium-dependent chloride currents to the measurements of reversal potentials. As shown in Fig. 8C, the reversal potentials of both the net charge and peak current measurements were strongly influenced by the external barium concentration, indicating that the channels of the potentiated receptors retained high divalent ion permeability.
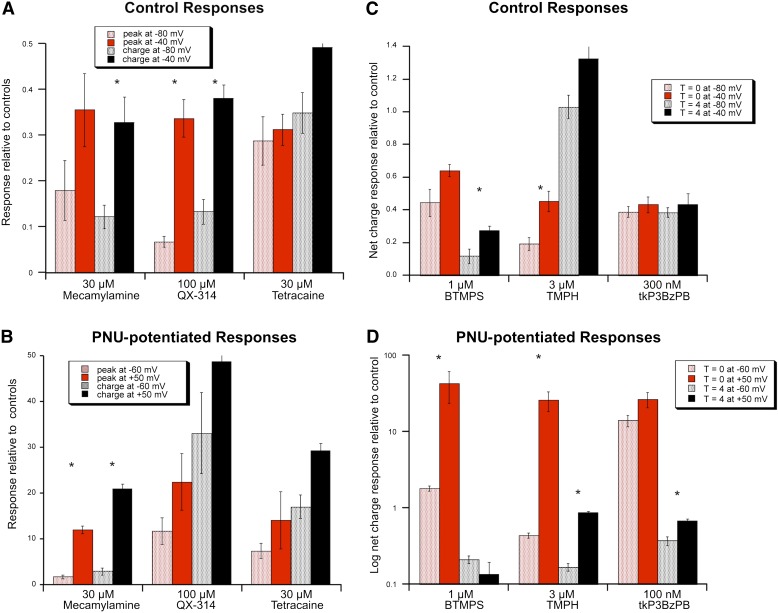
We evaluated the voltage dependence of the antagonism of control and potentiated responses by both the small and large antagonists. Control responses were studied at either −80 or −40 mV, and the effects of antagonist coapplication were compared with responses evoked by ACh alone at the same potentials. PNU-120596–potentiated currents were evoked at either −60 or +50 mV, and responses were measured relative to initial control responses to ACh alone obtained at −60 mV. For clarity of presentation and analysis, outward currents recorded at +50 mV and inward currents at −60 mV are expressed as absolute magnitude.
There was no significant effect of holding potential on the inhibition of control responses by tetracaine. There was a significant effect of voltage in the inhibition of net charge by mecamylamine and a similar trend in the inhibition of peak current (Fig. 9A). Inhibition of peak current and net charge by 100 µM QX-314 was greater at −80 than at −40 mV for the control responses. The results for the potentiated currents were similar for tetracaine and mecamylamine (Fig. 9B), but, interestingly, there was no significant effect of voltage for the inhibition of potentiated currents by QX-314.
Fig. 9.
The voltage dependence of antagonist activity. (A) The inhibition of 60 µM ACh-evoked α7-mediated absolute peak current and net charge responses in Xenopus oocytes recorded at either −80 or −40 mV by the small reversible antagonists. (B) Effects of the small reversible antagonists on α7-mediated responses evoked by the application of 60 µM ACh plus 10 µM PNU-120596 (PNU) recorded at either −60 or +50 mV. (C) The inhibition of 60 µM ACh-evoked α7-mediated net charge responses by large slowly reversible antagonists measured at either −80 or −40 mV. Effects were measured both on the responses evoked during the ACh-antagonist coapplication and on the responses to control applications of ACh 4 minutes after the coapplication responses. (D) Effects of the large slowly reversible antagonists on α7-mediated absolute net charge responses evoked by the application of 60 µM ACh plus 10 µM PNU-120596 recorded at either −60 or +50 mV. Effects were measured both on the responses evoked during the ACh/PNU-120596-antagonist coapplication and on the responses to control applications of ACh 4 minutes after the coapplication responses. Data on the inhibition of control ACh responses (A and C) were normalized to ACh-evoked responses recorded at the same holding potentials as the experimental responses. Data on the inhibition of responses to ACh plus PNU-120596 (B and D) were normalized to the average of two ACh-evoked control responses recorded at −60 mV prior to the coapplication of ACh, PNU-120596, and antagonist at the indicated voltage. Note that in D, due to the large magnitude of both the PNU-120596 and the antagonist effects, response data are displayed on a log scale. All bars represent the mean (± S.E.M.) of at least four oocytes. Asterisks indicate conditions in which there was significantly less (P < 0.05) inhibition of responses recorded at the more positive of the two potentials compared.
Effects of the large antagonists were measured at the time of coapplication and on the ACh-evoked control responses recorded after the standard 4-minute washout (at the standard −60 mV holding potential). Consistent with previous studies of BTMPS (Papke, 1993), the inhibition measured for responses evoked by ACh alone was greater on post-treatment controls than during coapplication, and the residual inhibition was voltage dependent (Fig. 9C). Consistent with previous studies of TMPH (Papke et al., 2005), inhibition of α7 control responses was reduced after washout, and the inhibition during coapplication was voltage dependent. Inhibition of control responses by a single application of 300 nM tkP3BzPB showed no voltage or time dependence.
To compare control responses after the application of the large antagonists to the PNU-120596–potentiated responses, the y-axis in Fig. 9D is a log scale. The coapplication of 1 µM BTMPS with 60 µM ACh and 10 µM PNU-120596 at −60 mV reduced the net charge to a level comparable to the ACh control responses (close to 1 on the log scale), but when the coapplication was made at +50 mV, there was relatively little inhibition. However, control responses after washout were reduced under both conditions and were not significantly different. The voltage dependence of TMPH during the coapplication step was similar to that of BTMPS (Fig. 9D), but there was less recovery when the drug was first applied at −60 mV, unlike the results with BTMPS. Inhibition by tkP3BzPB showed no voltage dependence during the coapplication, but there was a significant, albeit small, voltage dependence in the recovery after washout.
Effects of Antagonists on α7-Mediated Cytotoxicity in the Presence of PNU-120596.
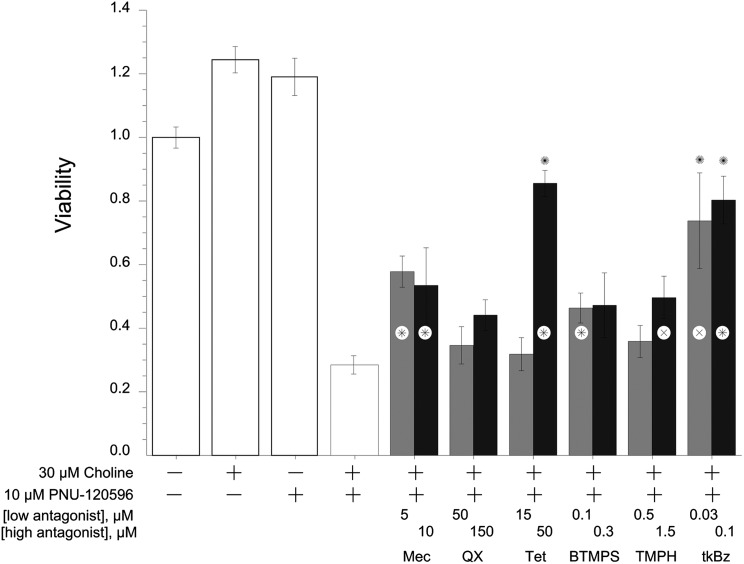
Our previous study revealed that α7-expressing A7R3HC10 cells were sensitive to excitotoxicity when stimulated with the α7-selective agonist choline and PNU-120596 (Williams et al., 2012). In those experiments, we confirmed that the PNU-120596 toxic effects were dependent on α7 receptor activation since they were sensitive to the α7-selective competitive antagonist methyllycaconitine. To further explore whether the PNU-120596 cytotoxicity mediated by α7 receptors strictly requires α7 ion channel currents and to further discriminate the activity profiles of antagonists characterized, the effects of the six noncompetitive antagonists on the PNU-120596 toxicity were examined by coapplication of each antagonist with 30 μM choline and 10 μM PNU-120596. As shown in Fig. 10, treatments with either 30 μM choline alone or 10 μM PNU-120596 alone did not show any cytotoxicity compared with cells treated with the 0.5% DMSO vehicle, whereas the 30 μM choline and 10 μM PNU-120596 cotreatment resulted in significant toxicity, approximately 30% cell viability relative to that of the control cells.
Fig. 10.
Protective effects of different α7 nAChR noncompetitive antagonists on the in vitro cytotoxicity profile of 10 μM PNU-120596 with 30 μM choline in A7R3HC10 cells incubated in HBSS at 28°C. ×P < 0.05; *P < 0.01 compared with viability of choline and PNU-120596–treated cells; ✺P > 0.05 compared with viability of treatment-free cells. Each bar represents the mean ± S.E.M. (n = 3). Untransfected HEK 293 cells were treated in parallel and were unaffected by all treatments (data not shown). Mec, mecamylamine; QX, QX-314; Tet, tetracaine; tkBZ, tkP3BzPB.
We observed that the six antagonists showed distinct protective effects on the in vitro cytotoxicity profile of PNU-120596. The agents were all tested at two concentrations, and all agents except for QX-314 provided some level of protection at one or both of the concentrations tested. Only tetracaine showed a significant concentration-dependent increase in the protection. Only the higher concentrations of tetracaine and tkP3BzPB produced a level of protection that restored viability of the treatment-free controls.
Mechanisms of PNU-120596–Mediated Cytotoxicity.
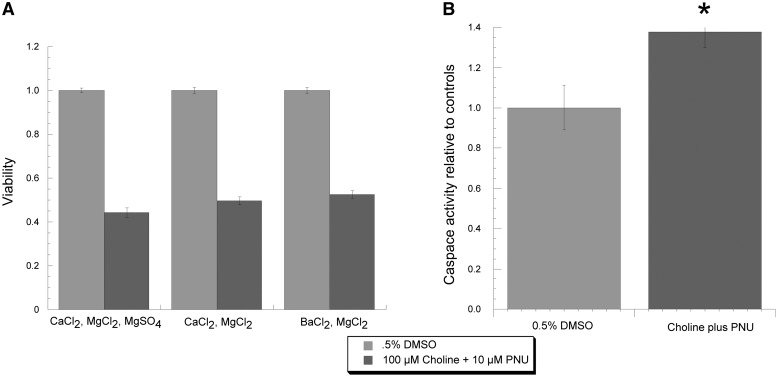
Due to the high calcium permeability of α7 ion channels under control conditions, it has been considered a reasonable hypothesis that the toxic effect on A7R3HC10 cells produced by the choline and PNU-120596 treatment would be due to a calcium-mediated sort of excitoxicity. However, our results with the antagonists call this into question. Therefore, we tested whether the cytotoxic effect observed on A7R3HC10 cells required the presence of extracellular calcium. It was not possible to prepare calcium-free HBSS by simply substituting the CaCl2 with BaCl2 since the presence of MgSO4 in the HBSS led to the precipitation of barium sulfate. Therefore, as a further control, we made and tested a modified HBSS with additional MgCl2 to replace the MgSO4, in addition to the normal HBSS control that contained CaCl2, MgCl2, and MgSO4. As shown in Fig. 11A, 4-hour treatments with choline and PNU-120596 were equally toxic with all three salt solutions. Similar results were obtained with overnight treatments (data not shown).
Fig. 11.
Mechanistic studies of cytotoxicity produced by choline and PNU-120596 (PNU) treatments to A7R3HC10 cells. (A) Treatments previously shown to be toxic to A7R3HC10 cells were compared with the 0.5% DMSO vehicle controls with solutions of different salt composition. In addition to HBSS with standard composition, we tested an HBSS with additional MgCl2 to replace MgSO4, and a calcium- and SO42−-free HBSS containing barium and additional MgCl2. (B) Caspase-3/7 activity was measured in A7R3HC10 cells that were treated with either 0.5% DMSO or 100 µM choline plus 10 µM PNU-120596 for 4 hours. *P < 0.05.
To determine whether the cytotoxic effects of the choline and PNU-120596 treatments reflected activation of pathways for apoptosis, we measured the level of caspase-3/7 activity, indicative of apoptotic cell death (Mazumder et al., 2008), in A7R3HC10 cells treated with 0.5% DMSO or choline and PNU-120596. As shown in Fig. 11B, there was a significant (P < 0.05) increase in caspase-3/7 activity in the choline and PNU-120596–treated cells.
Discussion
One approach for probing ion channel structure and function is to use noncompetitive antagonists that may vary in their interaction with the ion channel in ways that may be dependent on conformational states or specific amino acid residues exposed in the ion conduction pathway. For example, the lidocaine derivative QX-314 inhibits muscle type nAChRs through channel block that is conditional on channels being in the open state (Neher and Steinbach, 1978), whereas the alternative local anesthetic tetracaine can block both open and closed conformations of the channel through a parallel blocking mechanism (Papke and Oswald, 1989). Studies of macroscopic currents mediated by the ganglionic analog α3β4 expressed in Xenopus oocytes suggested that both of these agents could block wild-type receptors in a voltage-dependent manner consistent with open channel block, but that conversion of the α3β4 transmembrane domain to more closely resemble that of the muscle receptor had qualitatively different effects on the blocking properties of these agents, changing the potency of QX-314 and the mechanism of inhibition by tetracaine (Papke et al., 2001a).
Mecamylamine is widely used as a nonselective nAChR antagonist. Mecamylamine's inhibition of a broad range of nAChR subtypes has been demonstrated to be noncompetitive and in some cases also voltage dependent (Papke et al., 2001b), consistent with open channel block. However, single-channel data on mecamylamine are limited. Although one study of α3-containing receptors was consistent with channel block (Nelson and Lindstrom, 1999), in that study the data were sufficient to calculate forward blocking rates but unique blocked times were not apparent.
Single-channel studies of any of the small noncompetitive, putatively channel-blocking antagonists require open times of sufficient duration to measure the effects of the agents on open times and bursting properties. Due to the infrequent and extremely brief nature of α7 single-channel events, it has not been possible to characterize these agents on the level of single channels. However, the open times of α7 nAChRs are greatly prolonged in the presence of type II PAMs such as PNU-120596 (Williams et al., 2011b), making it feasible to characterize these agents and distinguish their molecular mechanisms on allosterically modulated receptors. We summarized our findings for the six agents studied on control and PNU-120596–potentiated α7 currents in Table 3.
TABLE 3.
Summary of hypotheses related to inhibitory mechanisms
| Agent | Voltage Dependence |
Comments | |
|---|---|---|---|
| Control | PNU-120596 | ||
| Mecamylamine | Net charge only | Strong | In the presence of the PAM, decreases open times and burst durations. Produces small-amplitude subconductance events. Most likely open channel block but produces some longer block times as well. |
| QX-314 | Strong | None | Fast channel blocker, no subconductance events. However, since burst durations are decreased, there must be some long block times as well. |
| Tetracaine | None | None | Most likely blocks both open and closed channels. Multiple forms of blockade suggested by closed times, the increase in burst frequency, and the appearance of subconductance states. |
| BTMPS | Net charge only | Peak only | Inhibition is use-dependent but not strongly voltage dependent. The longer channels are open, as in the presence of the PAM, the more likely they are to be blocked. |
| TMPH | Peak only | Strong | Likely to preferentially block open channels; however, the off-rate for α7 receptors is relatively fast compared with what has been reported for heteromeric channels. |
| tkP3BzPB | None | Weak | Probably not an open channel blocker. Shows preferential inhibition of the PNU-120596–potentiated receptors. Probably affects channels in both closed and open states. |
Our data indicate that the three small antagonists tested have unique channel-blocking mechanisms. Although all three shortened intraburst open time durations, only tetracaine maintained the bimodal distribution of open times. Likewise, all three shortened burst durations but only tetracaine increased the apparent burst frequency, suggesting that it produced blocked times longer than our threshold value of 100 millieconds for interburst closures. Tetracaine also introduced an additional element into the intraburst closed time distribution, consistent with multiple mechanisms of inhibition. In addition, one mechanism for inhibition by tetracaine appeared to be associated with incomplete channel openings, leading to one or more levels of subconductance. The observation that inhibition by tetracaine did not show significant voltage dependence would also suggest that this agent may bind outside the membrane's electric field and perhaps have some effects at the agonist binding sites.
The second class of nAChR noncompetitive antagonists studied (BTMPS, TMPH, and tkP3BzPB) has mechanisms that can be distinguished based on conformational state dependence and ion channel sequence (Papke, 1993; Papke et al., 2005; López-Hernández et al., 2009). They produced blocked times far too long to be resolved on the level of single-channel currents, even those that have been protracted by the application of a PAM. Of these three agents, only BTMPS has been shown to require channel activation to produce long-lasting use-dependent inhibition. Whereas TMPH and tkP3BzPB can inhibit nAChRs in both closed and open states (use-independent inhibition), studies of α7 receptors under control conditions (i.e., in the absence of PAMs) suggest that among the neuronal nAChRs studied, α7 receptors are relatively insensitive to TMPH and most sensitive to tkP3BzPB.
Interestingly, the inhibitory effects of tkP3BzPB at low concentrations were enhanced by potentiation of the currents with PNU-120596, while those of TMPH at low concentrations were reduced with PNU-120596, and the effects of BTMPS were relatively similar under both conditions. Although inhibition of heteromeric nAChRs by TMPH was not strictly use dependent, the inhibition of α7 receptors by this agent did appear to be voltage dependent. Inhibition by BTMPS, which is voltage independent for heteromeric neuronal nAChRs (Francis et al., 1998), showed an interesting pattern of voltage dependence in the inhibition of α7 currents. BTMPS is strictly use dependent, and under control conditions when single-channel currents would have been extremely short, there was no apparent effect of voltage on the onset of inhibition, but there was a significant effect of voltage on the recovery of currents, consistent with faster dissociation of the blocker from the channels with depolarization. There was relatively little effect of voltage on inhibition by tkP3BzPB under any conditions.
As the six antagonists studied differed in their detailed mechanisms of α7 ion channel inhibition, they also differed in their effects on the cytotoxicity produced by PNU-120596 and choline, an effect we initially hypothesized to be directly related to α7-mediated current influx. It is interesting to note that although both NMDA-type glutamate receptors and α7 nAChRs have high permeability to calcium that might produce excitotoxicity, these two receptors differ in several key properties under physiologic conditions. For example, NMDA receptors have a relatively high open probability, but they normally conduct little current in the absence of other depolarizing stimuli, due to the outward rectification of their current-voltage relationship associated with voltage-dependent block by magnesium ions. In contrast, under control conditions, α7 receptors have very low probability of being open and an inward rectifying current-voltage relationship. Both of these features would put a check on the possibility of excessive α7-mediated calcium influx under normal conditions, since if there was sufficient channel activation to depolarize a cell, the inward rectification of the channel would provide a negative feedback. This is the opposite of what occurs with NMDA-type glutamate receptor-mediated excitotoxicity, in which depolarization relieves channel block by magnesium and provides positive feedback, amplifying channel-mediated calcium currents. Our data indicate that PNU-120596 alters both of the features of α7 currents that would potentially limit cytotoxic calcium currents under normal conditions, open probability and inward rectification.
Although all of the antagonists tested were effective at reducing α7 ion channel currents, they were not all effective at eliminating the cytotoxic effect of PNU-120596 and choline. Several of the agents provided partial protection, but only the agents that had mechanisms that were least consistent with simple ion channel blockade, tetracaine and tkP3BzPB, fully protected the cells. QX-314 and the related lidocaine derivative 2-[(2,6-dimethylphenyl)amino]-N,N,N-trimethyl-2-oxoethaniminium chloride (QX-222) are agents normally associated with the form of open channel blockade that shortens open times and increases burst durations (Neher and Steinbach, 1978). However, even though we confirmed that QX-314 shortened the duration of PNU-120596–potentiated α7 bursts, it provided no significant protection from the toxic effect of the PNU-120596 and choline treatment. Interestingly, in addition to shortening burst and single-channel openings, mecamylamine and tetracaine induced novel subconductance states, and were cytoprotective to varying degrees. Likewise, BTMPS, a very effective antagonist of α7 ion channel currents, was relatively ineffective as a cytoprotective agent. Therefore, our results failed to support the hypothesis that the cytotoxic effect of PNU-120596 and choline is mediated by ion channel currents alone; in fact, the toxicity does not appear to require extracellular calcium.
The apparent dissociation between ion channel inhibition and cytoprotection may reflect back on the data that indicated that for each channel actively exhibiting PNU-120596–enhanced currents there are likely to be at least 100-fold more receptors that have been modified by the antagonist but are in nonconducting states. Our data indicate that at least some cytotoxicity is likely to come from alterations in the downstream signaling associated with nonconducting states regulating apoptotic pathways.
Our data indicate that the ion channel of the PNU-120596–potentiated conducting state must be significantly different from the pore formed in receptors that have not been modified by PNU-120596. The most solid proof of this is the reduction or elimination of the inward rectification in the channel modified by PNU-120596. The concept that PNU-120596 produces global effects on the structure of all conformational states, and not just changes the equilibrium between conducting and nonconducting states, is also consistent with the different IC50 values of mecamylamine and tetracaine for control and PNU-120596–modified receptors and the fact that the IC50 values for these antagonists changed in opposite directions.
PAMs of α7 are being tested in numerous preclinical models related to a range of therapeutic indications from cognitive function (McLean et al., 2012) to neuropathic pain and inflammation (Bencherif et al., 2011). The conventional interpretation of the data in all of these disparate areas is that the effects of PAMs are due to the increased ion channel activity alone. Our data are consistent with the hypothesis that the PAMs may have more global effects on α7 function that could extend to signaling associated with nonconducting states of the receptor.
Acknowledgments
The authors thank Dr. Jingyi Wang and Kinga Chojnacka for the synthesis of PNU-120596. They also thank Dr. Nicole A. Horenstein for many helpful discussions, as well as Clare Stokes for editorial comments and Dustin K. Williams and Jeffrey S. Thinschmidt for technical advice. OpusXpress experiments were conducted by Shehd Abdullah Abbas Al Rubaiy, Matthew Isaacson, Lu Wenchi Corrie, and Sarah Pinheiro.
Abbreviations
- ACh
acetylcholine
- BTMPS
bis-(2,2,6,6-tetramethyl-4-piperidinyl) sebacate
- Di
PNU-120596–insensitive desensitization
- DMEM
Dulbecco’s modified Eagle’s medium
- DMSO
dimethylsulfoxide
- Ds
PNU-120596–sensitive desensitization
- G418
O-2-amino-2,7-dideoxy-D-glycero-α-D-gluco-heptopyranosyl-(1→4)-O-[3-deoxy-4-C-methyl-3-(methylamino)-β-L-arabinopyranosyl-(1→6)]-2-deoxy-D-streptamine disulfate
- HBSS
Hanks’ balanced salt solution
- HEK 293
human embryonic kidney 293 cells
- I-V
current-voltage relationship
- mecamylamine
(1S,2R,4R)-N,2,3,3-tetramethylbicyclo[2.2.1]heptan-2-amine
- MS-222
ethyl 3-aminobenzoate methanesulfonate
- nAChR
nicotinic acetylcholine receptor
- NMDA
N-methyl-d-aspartic acid
- PAM
positive allosteric modulator
- PNU-120596
N-(5-chloro-2,4-dimethoxyphenyl)-N′-(5-methyl-3-isoxazolyl)-urea
- QX-222
2-[(2,6-dimethylphenyl)amino]-N,N,N-trimethyl-2-oxoethaniminium chloride
- QX-314
N-(2,6-dimethylphenylcarbamoylmethyl)triethylammonium bromide
- RIC-3
resistance-to-cholinesterase 3
- tetracaine
2-(dimethylamino)ethyl 4-(butylamino)benzoate
- tkP3BzPB
1,2,4,5-tetra-{5-[1-(3-benzyl)pyridinium]pent-1-yl}benzene tetrabromide
- TMPH
2,2,6,6-tetramethylpiperidin-4-yl heptanoate
Authorship Contributions
Participated in research design: Peng, Papke.
Conducted experiments: Peng, Kimbrell, Tian.
Contributed new reagents or analytic tools: Crooks, Fifer.
Performed data analysis: Peng, Pack, Papke.
Wrote or contributed to the writing of the manuscript: Peng, Papke.
Footnotes
This work was supported by the National Institutes of Health National Institute of General Medical Sciences [Grant R01-GM57481].
References
- Bencherif M, Lippiello PM, Lucas R, Marrero MB. (2011) Alpha7 nicotinic receptors as novel therapeutic targets for inflammation-based diseases. Cell Mol Life Sci 68:931–949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D, Sakmann B. (1995) Fitting and statistical analysis of single-channel records, in Single-Channel Recording (Sakmann B, Neher E, eds) pp 483–585, Plenum Press, New York, NY [Google Scholar]
- de Jonge WJ, Ulloa L. (2007) The alpha7 nicotinic acetylcholine receptor as a pharmacological target for inflammation. Br J Pharmacol 151:915–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis MM, Choi KI, Horenstein BA, Papke RL. (1998) Sensitivity to voltage-independent inhibition determined by pore-lining region of the acetylcholine receptor. Biophys J 74:2306–2317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis MM, Papke RL. (1996) Muscle-type nicotinic acetylcholine receptor delta subunit determines sensitivity to noncompetitive inhibitors, while gamma subunit regulates divalent permeability. Neuropharmacology 35:1547–1556 [DOI] [PubMed] [Google Scholar]
- Gopalakrishnan SM, Philip BM, Gronlien JH, Malysz J, Anderson DJ, Gopalakrishnan M, Warrior U, and Burns DJ (2011) Functional characterization and high-throughput screening of positive allosteric modulators of α7 nicotinic acetylcholine receptors in IMR-32 neuroblastoma cells. Assay Drug Dev Technol 9:635–645 [DOI] [PubMed]
- Grønlien JH, Håkerud M, Ween H, Thorin-Hagene K, Briggs CA, Gopalakrishnan M, Malysz J. (2007) Distinct profiles of alpha7 nAChR positive allosteric modulation revealed by structurally diverse chemotypes. Mol Pharmacol 72:715–724 [DOI] [PubMed] [Google Scholar]
- Haghighi AP, Cooper E. (1998) Neuronal nicotinic acetylcholine receptors are blocked by intracellular spermine in a voltage-dependent manner. J Neurosci 18:4050–4062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halevi S, Yassin L, Eshel M, Sala F, Sala S, Criado M, Treinin M. (2003) Conservation within the RIC-3 gene family. Effectors of mammalian nicotinic acetylcholine receptor expression. J Biol Chem 278:34411–34417 [DOI] [PubMed] [Google Scholar]
- Hollmann M, Hartley M, Heinemann S. (1991) Ca2+ permeability of KA-AMPA—gated glutamate receptor channels depends on subunit composition. Science 252:851–853 [DOI] [PubMed] [Google Scholar]
- Hurst RS, Hajós M, Raggenbass M, Wall TM, Higdon NR, Lawson JA, Rutherford-Root KL, Berkenpas MB, Hoffmann WE, Piotrowski DW, et al. (2005) A novel positive allosteric modulator of the alpha7 neuronal nicotinic acetylcholine receptor: in vitro and in vivo characterization. J Neurosci 25:4396–4405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ifune CK, Steinbach JH. (1990) Rectification of acetylcholine-elicited currents in PC12 pheochromocytoma cells. Proc Natl Acad Sci USA 87:4794–4798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabakov AY and Papke RL (1998) Ultra fast solution applications for prolonged gap-free recordings: Controlling a Burleigh piezo-electric positioner with Clampex7. AxoBits January:6–9.
- Leonard RJ, Labarca CG, Charnet P, Davidson N, Lester HA. (1988) Evidence that the M2 membrane-spanning region lines the ion channel pore of the nicotinic receptor. Science 242:1578–1581 [DOI] [PubMed] [Google Scholar]
- López-Hernández GY, Thinschmidt JS, Zheng G, Zhang Z, Crooks PA, Dwoskin LP, Papke RL. (2009) Selective inhibition of acetylcholine-evoked responses of alpha7 neuronal nicotinic acetylcholine receptors by novel tris- and tetrakis-azaaromatic quaternary ammonium antagonists. Mol Pharmacol 76:652–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrero MB, Bencherif M. (2009) Convergence of alpha 7 nicotinic acetylcholine receptor-activated pathways for anti-apoptosis and anti-inflammation: central role for JAK2 activation of STAT3 and NF-kappaB. Brain Res 1256:1–7 [DOI] [PubMed] [Google Scholar]
- Mathie A, Colquhoun D, Cull-Candy SG. (1990) Rectification of currents activated by nicotinic acetylcholine receptors in rat sympathetic ganglion neurones. J Physiol 427:625–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazumder S, Plesca D, Almasan A. (2008) Caspase-3 activation is a critical determinant of genotoxic stress-induced apoptosis. Methods Mol Biol 414:13–21 [DOI] [PubMed] [Google Scholar]
- McLean SL, Idris NF, Grayson B, Gendle DF, Mackie C, Lesage AS, Pemberton DJ, Neill JC. (2012) PNU-120596, a positive allosteric modulator of α7 nicotinic acetylcholine receptors, reverses a sub-chronic phencyclidine-induced cognitive deficit in the attentional set-shifting task in female rats. J Psychopharmacol 26:1265–1270 [DOI] [PubMed] [Google Scholar]
- Neher E, Steinbach JH. (1978) Local anaesthetics transiently block currents through single acetylcholine-receptor channels. J Physiol 277:153–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson ME, Lindstrom J. (1999) Single channel properties of human alpha3 AChRs: impact of beta2, beta4 and alpha5 subunits. J Physiol 516:657–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke RL (1993) Use-dependent inhibition of neuronal nicotinic ACHR by Tinuvin 770 (BIS (2, 2, 6, 6, - Tetramethyl-4-Piperidinyl) Sebacacate), a possible additive to laboratory plastics. Biophys J 64:W-pos421. [Google Scholar]
- Papke RL, Buhr JD, Francis MM, Choi KI, Thinschmidt JS, Horenstein NA. (2005) The effects of subunit composition on the inhibition of nicotinic receptors by the amphipathic blocker 2,2,6,6-tetramethylpiperidin-4-yl heptanoate. Mol Pharmacol 67:1977–1990 [DOI] [PubMed] [Google Scholar]
- Papke RL, Craig AG, Heinemann SF. (1994) Inhibition of nicotinic acetylcholine receptors by bis (2,2,6,6-tetramethyl- 4-piperidinyl) sebacate (Tinuvin 770), an additive to medical plastics. J Pharmacol Exp Ther 268:718–726 [PubMed] [Google Scholar]
- Papke RL, Horenstein BA, Placzek AN. (2001a) Inhibition of wild-type and mutant neuronal nicotinic acetylcholine receptors by local anesthetics. Mol Pharmacol 60:1365–1374 [DOI] [PubMed] [Google Scholar]
- Papke RL, Oswald RE. (1989) Mechanisms of noncompetitive inhibition of acetylcholine-induced single-channel currents. J Gen Physiol 93:785–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke RL, Porter Papke JK. (2002) Comparative pharmacology of rat and human alpha7 nAChR conducted with net charge analysis. Br J Pharmacol 137:49–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke RL, Sanberg PR, Shytle RD. (2001b) Analysis of mecamylamine stereoisomers on human nicotinic receptor subtypes. J Pharmacol Exp Ther 297:646–656 [PubMed] [Google Scholar]
- Papke RL, Stokes C. (2010) Working with OpusXpress: methods for high volume oocyte experiments. Methods 51:121–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman SM, Olney JW. (1987) Excitotoxicity and the NMDA receptor. Trends Neurosci 10:299–302 [DOI] [PubMed] [Google Scholar]
- Sands SB, Barish ME. (1992) Neuronal nicotinic acetylcholine receptor currents in phaeochromocytoma (PC12) cells: dual mechanisms of rectification. J Physiol 447:467–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Séguéla P, Wadiche J, Dineley-Miller K, Dani JA, Patrick JW. (1993) Molecular cloning, functional properties, and distribution of rat brain alpha 7: a nicotinic cation channel highly permeable to calcium. J Neurosci 13:596–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitzia F, Brown JT, Randall AD, Dunlop J. (2011) Voltage- and temperature-dependent allosteric modulation of α7 nicotinic receptors by PNU120596. Front Pharmacol 2:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DK, Peng C, Kimbrell MR, Papke RL. (2012) Intrinsically low open probability of α7 nicotinic acetylcholine receptors can be overcome by positive allosteric modulation and serum factors leading to the generation of excitotoxic currents at physiological temperatures. Mol Pharmacol 82:746–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DK, Stokes C, Horenstein NA, Papke RL. (2011a) The effective opening of nicotinic acetylcholine receptors with single agonist binding sites. J Gen Physiol 137:369–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DK, Wang J, Papke RL. (2011b) Investigation of the molecular mechanism of the α7 nicotinic acetylcholine receptor positive allosteric modulator PNU-120596 provides evidence for two distinct desensitized states. Mol Pharmacol 80:1013–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young GT, Zwart R, Walker AS, Sher E, Millar NS. (2008) Potentiation of alpha7 nicotinic acetylcholine receptors via an allosteric transmembrane site. Proc Natl Acad Sci USA 105:14686–14691 [DOI] [PMC free article] [PubMed] [Google Scholar]