Abstract
G protein–coupled receptors (GPCRs) transduce many important physiological signals and are targets for a large fraction of therapeutic drugs. Members of the largest family of GPCRs (family A) are thought to self-associate as dimers and higher-order oligomers, although the significance of such quaternary structures for signaling or receptor trafficking is known for only a few examples. One outstanding question is the physical stability of family A oligomers in cell membranes. Stable oligomers would be expected to move through cellular compartments and membrane domains as intact groups of protomers. Here, we test this prediction by recruiting subsets of affinity-tagged family A protomers into artificial microdomains on the surface of living cells and asking if untagged protomers move into these domains (are corecruited) at the same time. We find that tagged β2 adrenergic and μ-opioid protomers are unable to corecruit untagged protomers into microdomains. In contrast, tagged metabotropic glutamate receptor protomers do corecruit untagged protomers into such microdomains, which is consistent with the known covalent mechanism whereby these family C receptors dimerize. These observations suggest that interactions between these family A protomers are too weak to directly influence subcellular location, and that mechanisms that move these receptors between subcellular compartments and domains must operate on individual protomers.
Introduction
The largest family of transmembrane receptors is the G protein–coupled receptors (GPCRs), also known as seven-transmembrane receptors, which includes several hundred individual gene products (Pierce et al., 2002). Recent studies have determined the tertiary structures of many of these receptors and have uncovered conserved structural mechanisms of ligand binding, receptor activation, and G protein–coupling (Rosenbaum et al., 2009; Kobilka, 2011; Rasmussen et al., 2011b). The quaternary structure of these receptors has also been studied extensively, as many GPCRs have been shown to associate as homodimers, heterodimers, or higher-order oligomers (Angers et al., 2002; Milligan, 2008; Palczewski, 2010). However, these studies have not revealed conserved structural mechanisms or functional consequences of oligomerization for the largest subset of GPCRs, the family A (rhodopsin-like) receptors. Individual receptor protomers are capable of binding ligands and coupling to downstream signaling molecules (Hanson et al., 2007; Whorton et al., 2007, 2008; Bayburt et al., 2011). Therefore, the impact of family A receptor oligomerization may be restricted to allosteric regulation of receptor function (Han et al., 2009), or regulation of receptor trafficking and localization mediated by lateral interactions between protomers (Milligan, 2010). Methodological limitations have hindered the physical characterization of GPCR oligomers, and the stability and specificity of interactions between most family A protomers remain unknown or are controversial.
It is notoriously difficult to study interactions between integral membrane proteins in vitro, largely because it is difficult to isolate and solubilize intact proteins. Moreover, solubilization removes two-dimensional constraints on membrane proteins, removes lipid components that may contribute to their structure, and alters the availability of other membrane proteins that may compete for or modify interactions. Therefore, interactions between isolated membrane proteins may differ substantially from those that occur in intact cellular membranes (Hong and Bowie, 2011). Resonance energy transfer (fluorescence resonance energy transfer and bioluminescence resonance energy transfer) can be used to infer direct or indirect interactions between labeled membrane proteins in intact cells (Milligan and Bouvier, 2005; Loura and Prieto, 2011). However, distinguishing energy transfer due to a specific interaction from that due to random proximity is especially challenging when both labeled proteins are associated with a lipid bilayer (Vogel et al., 2006). Energy transfer between the same family A GPCRs has been attributed to specific association by some (Mercier et al., 2002; Bouvier et al., 2007) and random proximity by others (James et al., 2006), highlighting the need for additional methods to study interactions between transmembrane proteins in cells.
Here we use micron-sized beads to recruit affinity-tagged class A protomers into microscopic domains on the surface of living cells while monitoring corecruitment of untagged protomers. This assay is conceptually similar to in vitro assays such as coimmunoprecipitation, but does not involve removal of receptors from the plasma membrane. We focus on β2 adrenergic receptors (β2ARs), because these are prototypical family A receptors whose oligomerization has been studied in detail (Hebert et al., 1996). We also study μ-opioid receptors (μORs), as these receptors crystallized with two large interprotomer interfaces that could mediate oligomerization in membranes (Manglik et al., 2012). We find that lateral interactions between protomers of these family A receptors are too weak to mediate corecruitment to bead-induced domains. This implies that oligomerization will also be unable to mediate significant corecruitment of these protomers into clathrin-coated pits or other cell surface domains.
Materials and Methods
Plasmid DNA Constructs.
SNAP-β2AR was purchased from New England Biolabs (Ipswich, MA); metabotropic glutamate (mGlu)2 and β2AR plasmids were purchased from the Missouri S & T cDNA Resource Center (Rolla, MO). Hexahistidine (His) or the SNAP tag (preceded by the signal sequence from the 5HT3 receptor) was fused to the N terminus, and cerulean (C) or venus (V) were fused to the C terminus of mGlu2 and β2AR. Hexahistidine-cerulean or venus (preceded by the signal sequence from the human growth hormone) were fused to the N terminus of the rat μOR. The membrane marker red fluorescent protein (RFP)-membrane was TagRFP-fused to the final 25 amino acids of H-Ras. All constructs were made using a two-step polymerase chain reaction protocol and were verified by automated sequencing.
Cell Culture, Transfection, and Labeling.
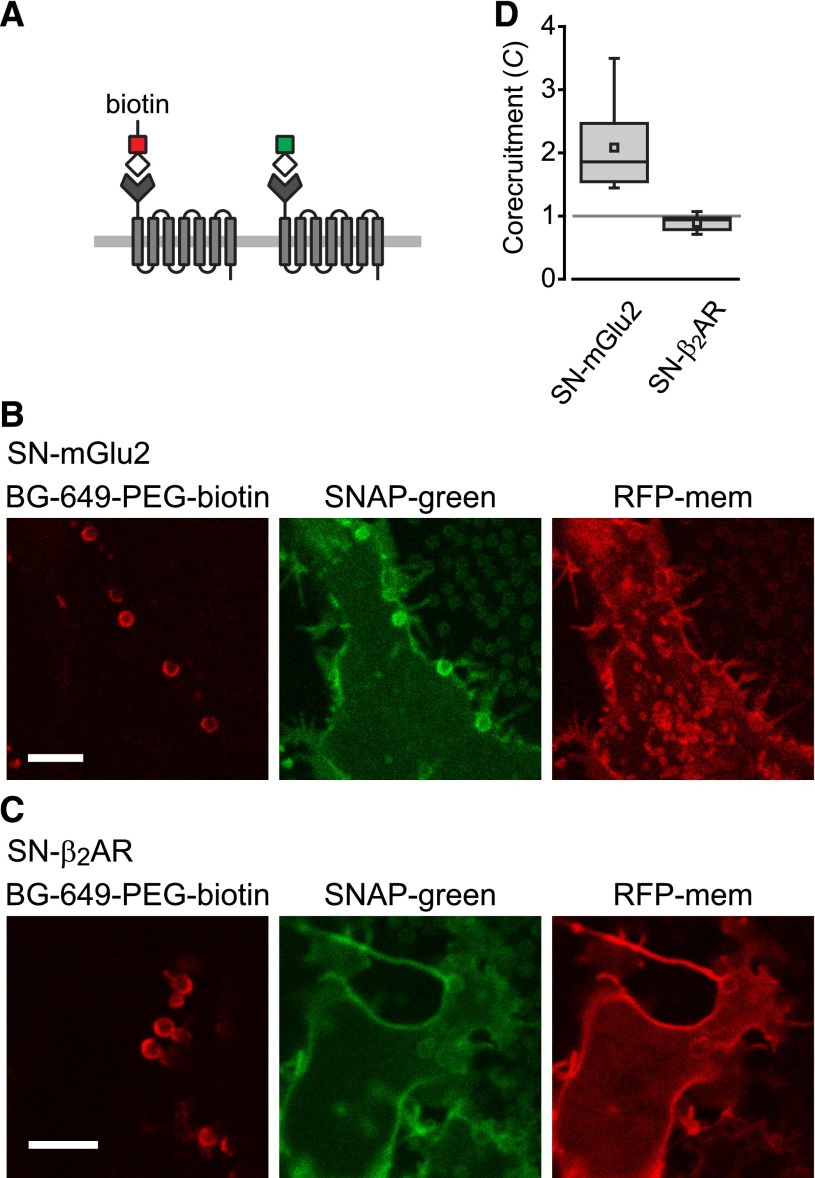
Human embryonic kidney 293, Chinese hamster ovary-K1, and COS-7 (American Type Culture Collection, Manassas, VA) cells were propagated in plastic flasks according to the supplier’s protocol. Cells were split onto polylysine-coated glass coverslips 24–72 hours prior to transfection. Cells were transfected using 25 kDa linear polyethyleneimine (Polysciences, Inc., Warrington, PA) at an nitrogen/phosphate ratio of 20, and were used for experiments 12–48 hours later. In most experiments the ratio of plasmid DNA encoding tagged receptor protomers to that encoding untagged protomers was 1:1. To estimate levels of receptor expression we measured β2AR-V fluorescence intensity in populations of transiently transfected cells using a fluorometer, and calculated receptor expression from a standard curve constructed using radioligand binding and a stable cell line that expressed β2AR-V under the control of an inducible promoter. Correction for 30% transfection efficiency (measured using flow cytometry) yielded an average value of ∼195,000 β2AR-V receptor protomers per cell, or ∼290 μm−1. Judging from fluorescence intensity individual cells expressed a similar number of His-β2AR-C protomers. Cells expressing SNAP-tagged protomers were labeled with benzylguanine (BG) SNAP substrates in complete growth medium for 2 hours prior to imaging. For the experiment shown in Fig. 5, cells were labeled with a mixture of 100 nM BG-649-PEG-biotin (New England Biolabs) and 5 nM SNAP-green (Cisbio, Codolet, France). The percentage of protomers labeled with each of these dyes was calculated by comparing green fluorescence emitted by cells labeled with this combination to that emitted by cells labeled with 100 nM SNAP-green alone using a plate-reading fluorometer. This concentration of SNAP-green saturates labeling of competent SNAP tags.
Fig. 5.
Corecruitment of SNAP-mGlu2 but not SNAP-β2AR protomers labeled with dye (SNAP-green) by protomers labeled with dye-biotin (BG-649-PEG-biotin). (A) SNAP-tagged protomers are labeled with BG-649-PEG-biotin or SNAP-green after incubation with a mixture of both dyes. (B and D) SNAP-mGlu2 (SN-mGlu2) protomers labeled with BG-649-PEG-biotin corecruit protomers labeled with SNAP-green to sAV beads. (C and D) SN-β2AR protomers labeled with BG-649-PEG-biotin do not corecruit protomers labeled with SNAP-green. Scale bars in B and C, 5 μm.
Bead Application and Confocal Imaging.
Immobilized metal affinity chromatography (IMAC) and streptavidin (sAV) beads (Dynabeads His-Tag and sAV C1; Life Technologies, Carlsbad, CA) were washed in phosphate-buffered saline, resuspended without dilution, and manually pipetted onto cells (10 μl/25-mm coverslip). According to the information supplied by the manufacturer, streptavidin beads can bind ∼400 × 10−12 moles of biotin/mg, which equates to approximately 80,000 molecules/μm2 of bead surface. Assuming that IMAC beads and streptavidin beads are similar (∼109 beads mg−1), the corresponding value for IMAC beads would be ∼275,000 molecules μm−2. Both of these values exceed the maximum possible packing density of seven transmembrane receptors. Given the levels of receptor expression likely to be achieved in our experiments, the degree of recruitment observed (2- to 3-fold; e.g., Supplemental Fig. 5), and the absence of bead saturation, the density of recruited protomers in our experiments is likely to be far less than these figures.
For imaging cells were bathed in phosphate-buffered saline and imaged at room temperature (20–22°C) using an SP2 laser scanning confocal microscope (Leica, Wetzlar, Germany) and a 63×, 1.4 numerical aperture objective. Excitation/emission wavelengths for the various fluorophores were (nm): cerulean, 458/465–500; venus, 514/520–650; TagRFP, 543/550–625; BG-649–PEG-biotin, 633/640–800; and SNAP-green, 488/495–550. Image analysis was performed with ImageJ (NIH, Bethesda, MD). Recruitment and corecruitment indices were calculated from background-subtracted regions of interest as indicated in Fig. 1. Numeric values throughout the text refer to the mean ± S.E.M.
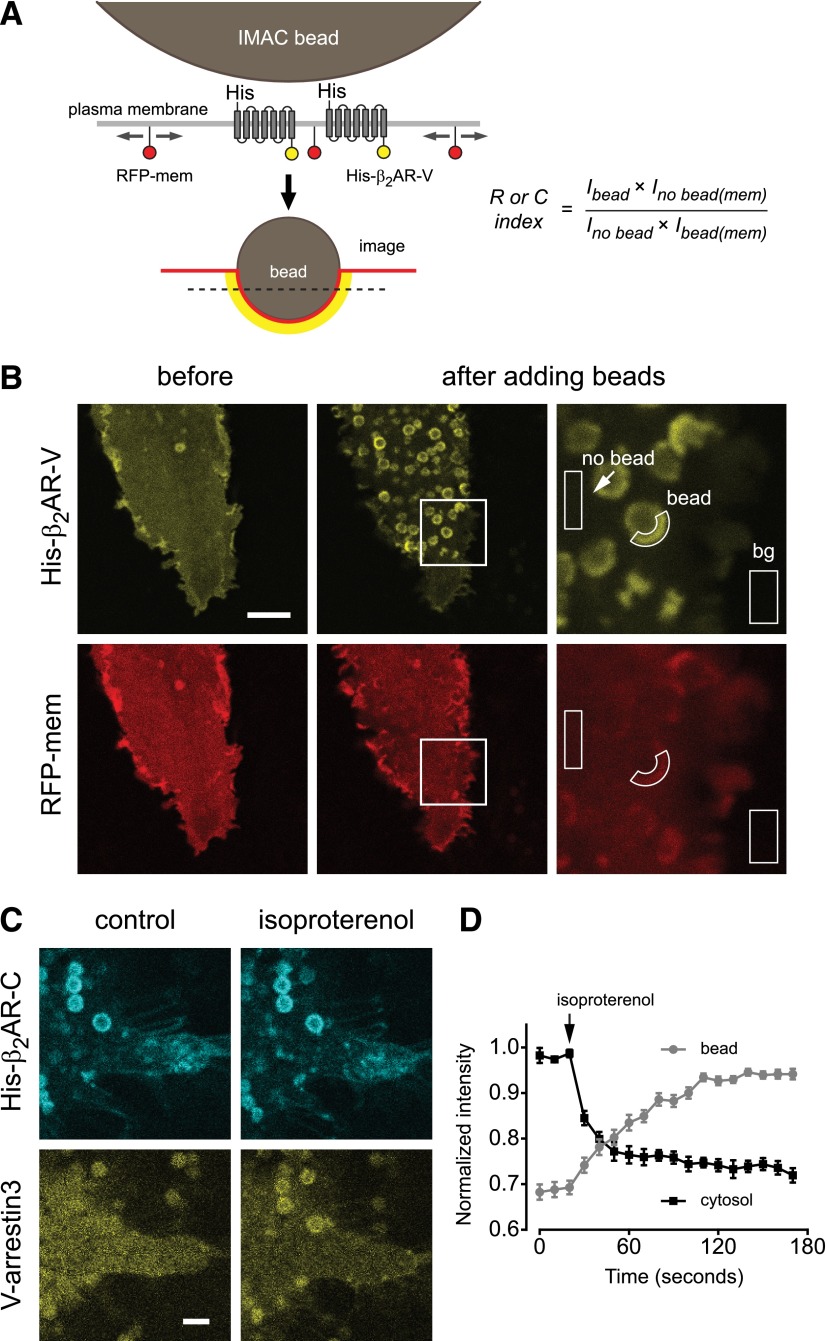
Fig. 1.
IMAC beads recruit functional β2 adrenergic receptors. (A) Schematic illustrating recruitment of His-β2AR-V protomers to the surface of IMAC beads. The dashed line indicates the typical optical plane of section. Intensity of RFP-membrane (RFP-mem) in bead-associated [Ibead(mem)] and surrounding [Ino bead(mem)] ROIs is used to correct for distortion of the plasma membrane by the bead and calculated recruitment (R) and corecruitment (C) indices. (B) Confocal images showing His-β2AR-V and RFP-mem before and after recruitment to IMAC beads, and illustration of regions of interest used for analysis and background (bg) subtraction; scale bar, 5 μm. (C and D) Bead-associated His-β2AR-C recruits V-arrestin3 from the cytosol to the plasma membrane in response to 10 μM isoproterenol; scale bar, 2 μm. Data points in D represent the mean ± S.E.M. of 5 cytosol and 27 bead ROIs from five cells.
Results
To establish an on-cell corecruitment assay we fused a hexahistidine tag to the N terminus and a fluorescent protein (cerulean or venus; C or V) to the C terminus of the human β2AR. The N- and C-terminal extensions of this receptor are relatively long (∼30 and 80 amino acids, respectively) and unstructured, and have been shown previously to tolerate such additions with minimal disruption of receptor function (Barak et al., 1997b). Confocal imaging showed that His-tagged β2ARs (e.g., His-β2AR-V) were expressed uniformly at the plasma membrane of transiently transfected human embryonic kidney 293 cells (Fig. 1B). We then applied a suspension of cobalt-based IMAC beads, which were allowed to settle onto the surface of transfected cells (Fig. 1A). After contacting the cell surface individual beads accumulated fluorescence over the course of ∼1–2 minutes, which is consistent with diffusion to and capture of His-tagged protomers at the bead-cell interface (Supplemental Movie 1). In most cases the optical plane of section was parallel to the cell surface (Fig. 1A), thus hemispheric domains of His-β2AR-V appeared as fluorescent circles or crescents depending on whether the bead came to rest on the cell center (circles) or margin (crescents). Bead image profiles decreased in diameter as the image plane was moved toward the cell center, thus protomers were captured at the entire bead-membrane interface (Supplemental Fig. 1). Bead-induced domains were easily identifiable by their size (1 μM in diameter), nonfluorescent interiors, and immobility relative to structures such as intracellular vesicles. In many cases fluorescence originating from tagged proteins in regions of the plasma membrane that were not associated with a bead was barely detectable above background, suggesting that in these cases almost all of the His-tagged proteins were recruited to a bead-induced domain. Because beads deformed the plasma membrane it was necessary to normalize background-subtracted fluorescence intensity in bead-associated (Ibead) and surrounding (Ino bead) regions of interest to the amount of membrane present in the same regions using an inert marker of the plasma membrane (Fig. 1B). For this purpose we used tagRFP bearing the C-terminal 25 amino acids of H-Ras (RFP-mem), which is both prenylated and palmitoylated. Corrected recruitment (R, for tagged protomers) and corecruitment (C, for untagged protomers) indices were defined as the fold fluorescence enhancement at the bead compared with the surrounding plasma membrane. Values of R or C greater than 1 indicate recruitment or corecruitment, values less than 1 indicate exclusion, and values of 1 indicate neither.
We then asked if receptors retained their ability to bind ligands and signal to intracellular proteins while tethered to beads. We first transfected cells with His-tagged β2AR-cerulean and venus-arrestin3, recruited His-β2AR-C to beads, and then stimulated these receptors with the agonist isoproterenol (10 μM; Fig. 1C). Agonist stimulation rapidly redistributed V-arrestin3 from the cytosol to the plasma membrane (Barak et al., 1997a), and in particular to regions of the plasma membrane associated with beads (Fig. 1D; Supplemental Movie 2). We then transfected cells with His-β2AR-C and nanobody 80–green fluorescent protein (GFP), an engineered biosensor that binds to the active conformation of the β2AR (Rasmussen et al., 2011a; Irannejad et al., 2013). Agonist stimulation rapidly redistributed nanobody 80–GFP from the cytosol to regions of the plasma membrane associated with beads (Supplemental Fig. 2; Supplemental Movie 3). These results suggest that β2ARs concentrated at cell surface domains by IMAC beads remain functional, and are consistent with a previous study that showed that isolated β2ARs remained functional after tethering to a solid substrate via an N-terminal tag (Neumann et al., 2002).
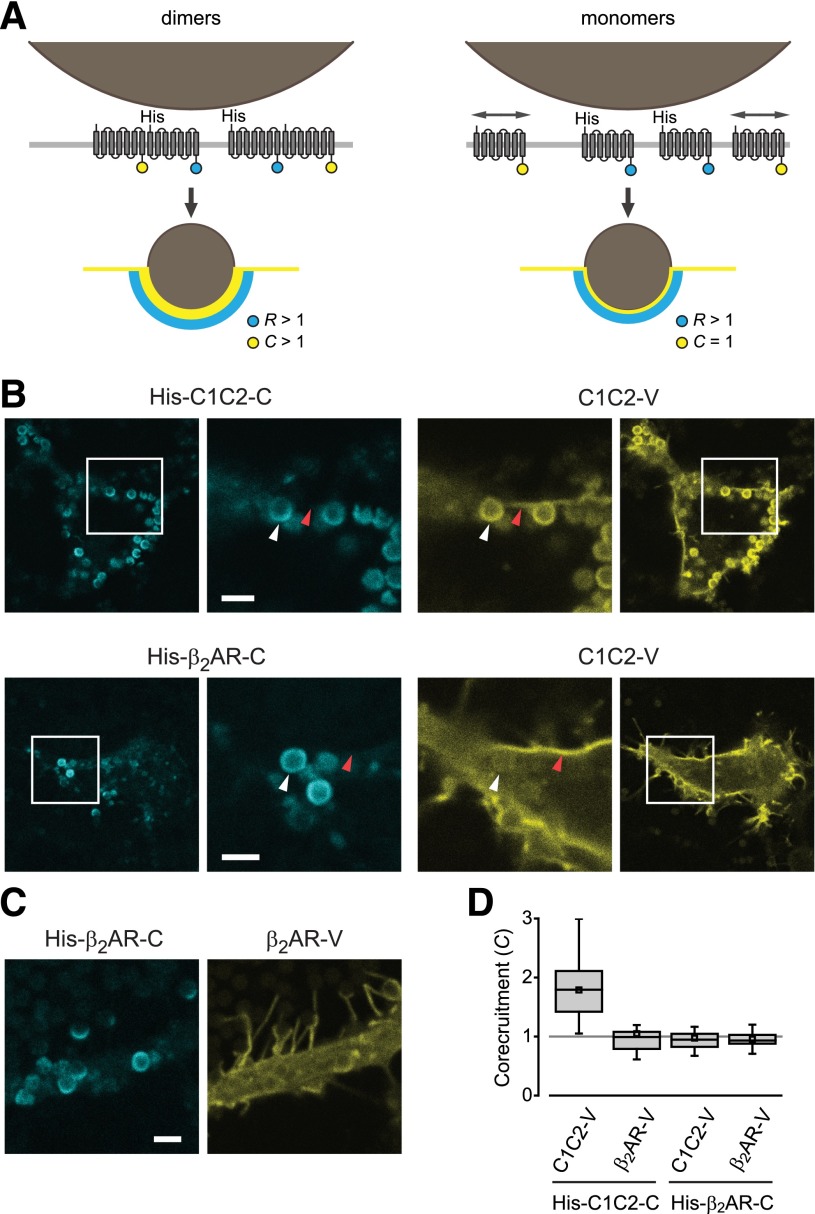
We reasoned that if GPCRs formed sufficiently stable dimers (or higher-order oligomers) at the cell surface, then recruitment of His-tagged protomers to bead-induced domains would corecruit untagged protomers to the same domains (Fig. 2A). As a positive homodimeric control we used the channelrhodopsin chimera C1C2. This seven-transmembrane protein was chosen because it closely resembles family A GPCRs in overall structure and topology, but has a conserved and structurally well-defined mechanism of homodimerization mediated by 2 or 3 disulfide bonds in a short N-terminal extension (Kato et al., 2012). His-tagged C1C2-cerulean (His-C1C2-C) corecruited untagged C1C2-venus to IMAC beads (C = 1.79 ± 0.09, mean ± S.E.M.; n = 28; Fig. 2B). In contrast, His-β2AR-C did not corecruit C1C2-V (C = 0.97 ± 0.05; n = 24; Fig. 2B), suggesting that association of C1C2-V fluorescence with IMAC beads depended on dimerization with His-C1C2-C protomers. In a similar manner, bead-associated His-C1C2-C did not corecruit untagged β2AR-V (C = 1.05 ± 0.09; n = 25; Fig. 2D). Finally, we found that recruitment of His-β2AR-C did not corecruit β2AR-V (C = 0.96 ± 0.02; n = 40; Fig. 2, C and D). Similar results for His-β2AR-C and β2AR-V were obtained in the presence of isoproterenol (10 μM; C = 0.97 ± 0.04; n = 10) or the inverse agonist 3-(isopropylamino)-1-[(7-methyl-4-indanyl)oxy]butan-2-ol (ICI 118,551) (10 μM; C = 1.12 ± 0.07; n = 10). Recruitment of His-β2AR-C also failed to corecruit β2AR-V in Chinese hamster ovary-K1 and COS-7 cell lines (Supplemental Fig. 3). These results suggest that C1C2, but not β2AR protomers, assemble as stable dimers on the cell surface.
Fig. 2.
Corecruitment of C1C2 but not β2AR protomers to IMAC beads. (A) Stable dimers corecruit (C > 1) untagged protomers to IMAC beads (left), whereas monomers leave untagged protomers distributed throughout the surrounding plasma membrane (C = 1; right). (B and D) His-C1C2-C corecruits C1C2-V, but not β2AR-V to bead-associated regions (white arrowheads). Plasma membrane regions not apposed to IMAC beads (red arrowheads) are severely depleted of His-C1C2-C protomers. (C and D) His-β2AR-C does not corecruit β2AR-V to IMAC beads. Scale bars in B and C, 2 μm. In D, bars represent median, 25th, and 75th percentiles; small squares represent mean values; and whiskers represent minimum and maximum values.
Because the recent crystal structure of the μOR included two parallel dimer interfaces (Manglik et al., 2012) we performed similar experiments with His-cerulean-μOR and venus-μOR, both of which carried N-terminal fluorescent proteins. We have previously shown that similar fluorescent protein-μOR fusion proteins are functional when immobilized on the cell surface (Lober et al., 2006). Accordingly, agonist stimulation (10 μM DAMGO) rapidly redistributed V-arrestin3 from the cytosol to the bead-associated plasma membrane in cells expressing His-C-μOR (Supplemental Fig. 4). However, recruitment of His-C-μOR to IMAC beads failed to corecruit V-μOR (C = 0.94 ± 0.04; n = 23; Supplemental Fig. 4), suggesting that these family A receptors also do not assemble as highly stable dimers or higher-order oligomers in the plasma membrane.
Because His-tagged protomers were highly concentrated at the bead-membrane interface in these experiments, we considered the possibility that untagged protomers were sterically excluded from the membrane apposed to the bead surface. However, in cases where corecruitment was not observed C remained close to 1, indicating that untagged protomers were as abundant in regions of the plasma membrane associated with beads as in the surrounding regions. Exclusion of untagged protomers from bead-associated membrane regions would be expected to produce values of C that are less than 1. In addition, the nearly complete depletion of His-tagged protomers from nonbead membrane regions (e.g., Fig. 2B) suggested that bead-binding capacity was not saturated, and that additional protomers could therefore have been accommodated in the membrane opposite the bead surface. To directly test this idea we cotransfected His-β2AR-C with either β2AR-V (as before) or His-β2AR-V and imaged cells with standardized illumination and detection parameters. The absolute intensity of bead-associated His-β2AR-C was the same with β2AR-V (125 ± 9 arbitrary units; n = 10) and His-β2AR-V (131 ± 7 arbitrary units; n = 13; P = 0.62, unpaired t test), suggesting that His-tagged protomers did not compete for limited binding sites or space in the bead-apposed membrane. Untagged β2AR-V was not corecruited in these experiments (C = 0.97 ± 0.04), whereas His-β2AR-V was heavily recruited (R = 2.32 ± 0.20; Supplemental Fig. 5). Finally, we observed that β2AR-V fluorescence remained the same in bead-apposed and surrounding regions during the process of recruitment, that is when His-β2AR-C was still in the process of being captured by the beads (Supplemental Fig. 6; Supplemental Movie 4). Disruption of dimers by steric exclusion would be expected to occur only as beads reached their full binding capacity, thus the absence of corecruitment at early time points is unlikely to be due to such a mechanism.
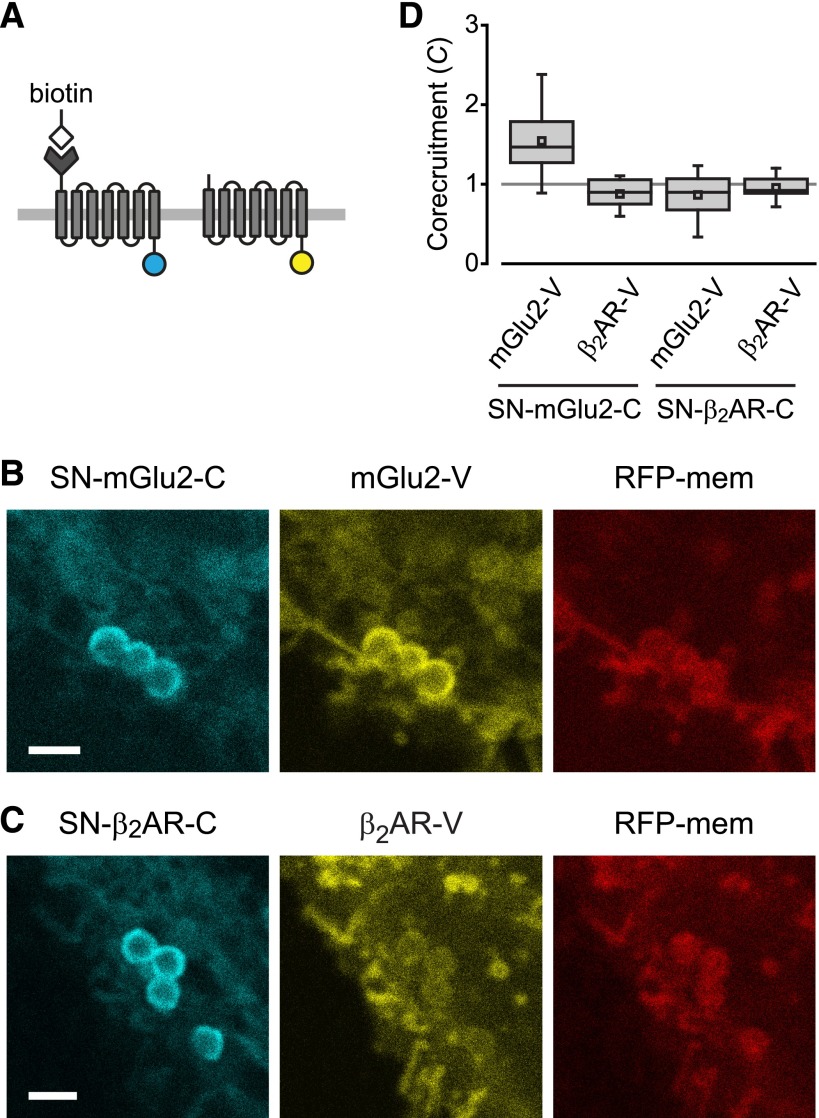
Unlike family A receptors, family C GPCRs dimerize via well-understood mechanisms, and dimerization has obvious functional consequences for these receptors (Pin et al., 2003). We were unable to demonstrate dimerization of the class C mGlu2 or GABAB receptors with our original corecruitment strategy because we found that both receptors, unlike the family A receptors we tested, were nonspecifically recruited to IMAC beads even when they were not tagged with hexahistidine (Supplemental Fig. 7). This prompted us to explore other bead matrices and affinity tags, including biotin-avidin. To specifically biotinylate cell surface receptors we made use of the alkyltransferase SNAP-tag (Keppler et al., 2003), which has been used in several previous studies of GPCR oligomerization. In these experiments protomers were fused to either the SNAP-tag at their N terminus and to cerulean at their C terminus, or solely to venus at their C terminus (Fig. 3A). SNAP-tagged protomers were covalently biotinylated with a biotin substrate (SNAP-biotin) and recruited to streptavidin-conjugated beads. For example, biotinylated SNAP-mGlu2-cerulean (SN-mGlu2-C) corecruited mGlu2-venus to sAV beads (C = 1.54 ± 0.07, n = 27; Fig. 3, B and D). In contrast, biotinylated SN-mGlu2-C did not corecruit β2AR-V (C = 0.88 ± 0.04; n = 17), indicating the specificity of the mGlu2-V corecruitment. Conversely, when biotinylated SNAP-β2AR-cerulean was recruited to sAV beads, neither mGlu2-V (C = 0.87 ± 0.06; n = 19) nor β2AR-V (C = 0.96 ± 0.03; n = 17; Fig. 3, C and D) was corecruited. These results demonstrate the utility of this general strategy for GPCRs, and further suggest that β2ARs are not held tightly together on the cell surface.
Fig. 3.
Corecruitment of mGlu2 but not β2AR protomers to sAV beads. (A) SNAP-tagged (SN-) protomers are covalently labeled with BG-biotin and fused to cerulean, whereas protomers without SNAP tags are fused to venus. (B and D) Biotinylated SN-mGlu2-C corecruits mGlu2-V to sAV beads. (C and D) Biotinylated SN-β2AR-C does not corecruit β2AR-V. Scale bars in B and C, 2 μm.
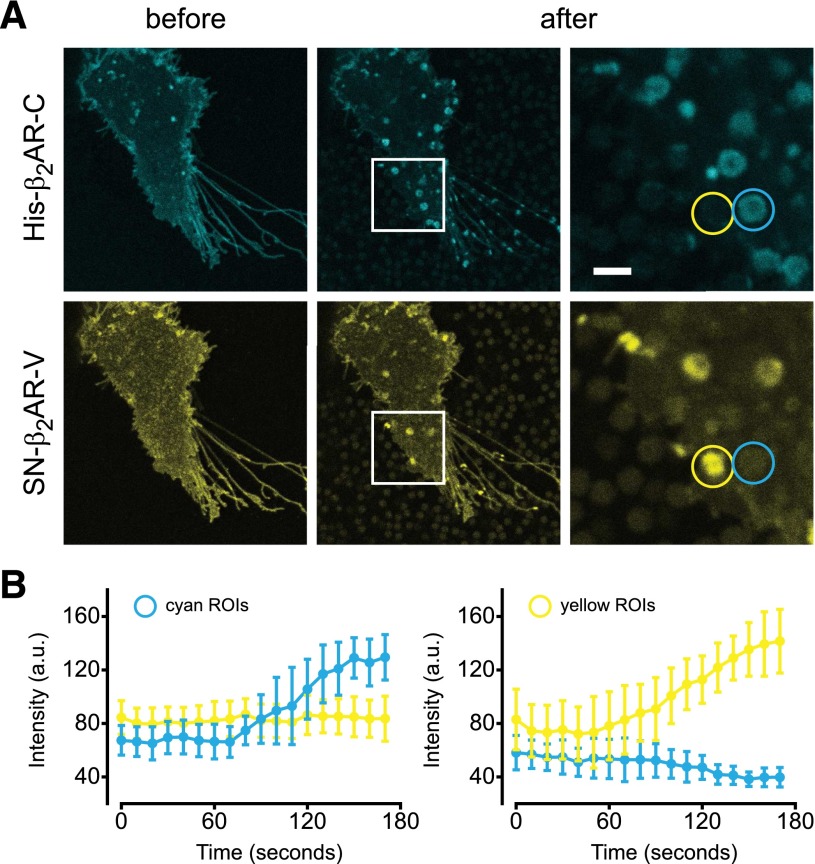
The availability of two different recruitment methods allowed us to simultaneously recruit different protomers to different cell surface domains. We transfected cells with His-β2AR-C and SN-β2AR-V, biotinylated the latter, and applied a mixture of IMAC and sAV beads. This resulted in the accumulation of cerulean and venus fluorescence at different regions of interest (ROIs) corresponding to IMAC beads and sAV beads (Fig. 4A; Supplemental Movie 5). Analysis of these regions over time indicated that IMAC beads (cyan ROIs) did not accumulate venus fluorescence, and sAV beads (yellow ROIs) did not accumulate cerulean fluorescence at any point during the recruitment process (Fig. 4B).
Fig. 4.
Segregation of His-β2AR-cerulean and SN-β2AR-venus by mixed IMAC and sAV beads. (A) Mixed IMAC and sAV beads are applied to cells expressing His-β2AR-C and SN-β2AR-V. Cyan and yellow ROIs are indicated; scale bar, 2 μm. (B) Development of cyan and yellow fluorescence over time in ROIs designated cyan or yellow. Beads are pipetted over cells at time = 30 seconds, and settle onto the cell surface over the next minute. Individual data points represent the mean ± S.D. of 15 cyan and 15 yellow ROIs from two cells.
It is conceivable that only identical protomers assemble into stable homodimers, particularly if, as has been suggested, dimers form shortly after protein translation in the endoplasmic reticulum (Salahpour et al., 2004). In this case we would not expect corecruitment of protomers bearing different genetically encoded tags and fluorescent proteins. To address this possibility we exploited the versatility of the SNAP-tag to perform corecruitment experiments using a protocol that required expression of only a single type of protomer. We transfected cells with either SN-mGlu2 or SN-β2AR (without C-terminal fluorescent proteins), and simultaneously labeled these receptors with a combination of spectrally distinct SNAP substrates. One substrate (BG-649-PEG-biotin; Supplemental Fig. 8) included a biotin moiety and an infrared dye, whereas the other substrate (SNAP-green) was simply a green dye (Fig. 5A). The labeling ratio was adjusted so that ∼60% of the protomers were labeled with BG-649-PEG-biotin, ensuring that a sufficient fraction of any dimers formed would bear both labels. When SN-mGlu2 protomers labeled with BG-649-PEG-biotin were recruited to sAV beads, SN-mGlu2 protomers labeled with SNAP-green were corecruited (C = 2.08 ± 0.25; n = 8; Fig. 5, B and D). In contrast, when SN-β2AR protomers labeled with BG-649-PEG-biotin were recruited to sAV beads, SN-β2AR protomers labeled with SNAP-green were not corecruited (C = 0.89 ± 0.04; n = 9; Fig. 5, B and D). These results suggest that the absence of β2AR corecruitment cannot be explained by obligatory homodimerization of identical protomers.
Discussion
The idea that family A GPCR protomers interact with each other is now firmly established, but many questions concerning the functional significance and physical properties of these interactions remain unanswered. Here we address the physical stability of interactions between β2AR and μOR protomers using a quantitative imaging approach in living cells. We find that recruiting a subset of protomers into artificial cell surface domains does not lead to detectable indirect corecruitment of other protomers, suggesting that the interactions between protomers are not sufficiently stable to dictate residence in such domains. Measuring steady-state corecruitment of untethered protomers to domains of tethered protomers does not yield an association lifetime, but rather indicates the state of the association-dissociation equilibrium. Our results suggest that, for the receptors we studied, this equilibrium greatly favors dissociation. This appears to be the case even though the receptors we studied were overexpressed, and were likely to be more concentrated than receptors expressed in most native tissues. We cannot rule out the possibility that noncovalent dimers are in some way disrupted when one protomer of a pair binds to a bead. This type of disruption would have to apply to three types of N-terminal affinity tags (His-, His-cerulean-, and SNAP-) and two types of beads (IMAC and streptavidin) to completely account for our failure to observe corecruitment of untagged β2AR and μOR. Moreover, we have shown that bead-attached protomers are functionally intact to the extent that they still bind agonists and undergo the conformational changes associated with signaling. This suggests that tethering to a solid substrate via an N-terminal tag does not substantially alter receptor structure or dynamics, and therefore it seems unlikely that it would disrupt dimerization.
The absence of corecruitment into bead-induced domains is consistent with our previous observation that internalization of agonist-bound β2AR protomers does not lead to significant cointernalization of unbound protomers (Lan et al., 2011) and is also consistent with our previous fluorescence recovery after photobleaching study of D2 dopamine receptors, which indicated that protomers of these monoamine receptors do not detectably influence one another’s mobility in the membrane (Fonseca and Lambert, 2009). On the other hand, other studies using fluorescence recovery after photobleaching and single-molecule imaging methods have concluded that β2ARs form relatively stable (∼5-second lifetime) higher-order oligomers, and that the equilibrium favors association even with low (subphysiological) levels of receptor expression (Dorsch et al., 2009; Calebiro et al., 2013). The reason(s) for these discrepant results are not immediately apparent. Similar unresolved discrepancies exist between different fluorescence resonance energy transfer and bioluminescence resonance energy transfer studies of β2AR oligomerization (Mercier et al., 2002; James et al., 2006; Salahpour and Masri, 2007; Felce and Davis, 2012; Kawano et al., 2013).
The physiologic function of GPCRs involves the regulated movement of receptors between subcellular compartments and retention of receptors in discrete domains. Our findings suggest that the mechanisms that direct the family A receptors we studied into subcellular compartments must operate on individual protomers, as opposed to dimers or oligomers. Like many GPCRs, these receptors are recruited into clathrin-coated pits by binding to arrestin, which in turn interacts with clathrin and the clathrin adapter AP2 (Goodman et al., 1996; Kang et al., 2013). Our results suggest that each protomer will need to bind directly to an arrestin molecule to be recruited into a coated pit. In a similar manner, β2ARs are not distributed randomly on the surface of cardiomyocytes, but are confined in plasma membrane microdomains via an interaction between the receptor C terminus and an A-kinase anchoring protein (Valentine and Haggie, 2011). Our results suggest that each protomer will be retained separately by this mechanism. Likewise, postendocytic sorting of β2ARs and other GPCRs relies on interactions with adapter proteins and elements of the cytoskeleton (Cao et al., 1999; Hanyaloglu and von Zastrow, 2008), and our results suggest that individual protomers, rather than groups of protomers, will be sorted by these mechanisms. It is quite possible that interactions between other family A protomers will be found to be much more stable than those that we studied here. However, at least for the receptors that we studied, our results suggest that the functional significance of oligomerization will not include localization in cellular compartments and domains.
Supplementary Material
Acknowledgments
The authors thank Jan Steyaert and Mark von Zastrow for providing a plasmid encoding nanobody 80–GFP.
Abbreviations
- β2AR
β2 adrenergic receptor
- BG
benzylguanine
- C1C2
channelrhodopsin 1 – channelrhodopsin 2 chimera
- C
corrected corecruitment index
- C
cerulean fluorescent protein
- GFP
green fluorescent protein
- GPCR
G protein–coupled receptor
- His
hexahistidine
- ICI 118,551
3-(isopropylamino)-1-[(7-methyl-4-indanyl)oxy]butan-2-ol
- IMAC
immobilized metal affinity chromatography
- mGlu
metabotropic glutamate
- μOR
μ-opioid receptor
- RFP
red fluorescent protein
- ROI
region of interest
- sAV
streptavidin
- V
venus fluorescent protein
Authorship Contributions
Participated in research design: Javitch, Lambert.
Conducted experiments: Gavalas, Lan, Lambert.
Contributed new reagents or analytic tools: Liu, Corrêa, Jr.
Performed data analysis: Gavalas, Lan, Lambert.
Wrote or contributed to the writing of the manuscript: Javitch, Lambert.
Footnotes
This work was supported by the National Institutes of Health National Institute of General Medical Sciences [Grants GM078319 and GM096762]; the National Institutes of Health National Institute on Drug Abuse [Grant DA022413]; and the National Institutes of Health National Institute of Mental Health [Grant MH54137].
 This article has supplemental material available at molpharm.aspetjournals.org.
This article has supplemental material available at molpharm.aspetjournals.org.
References
- Angers S, Salahpour A, Bouvier M. (2002) Dimerization: an emerging concept for G protein-coupled receptor ontogeny and function. Annu Rev Pharmacol Toxicol 42:409–435 [DOI] [PubMed] [Google Scholar]
- Barak LS, Ferguson SS, Zhang J, Caron MG. (1997a) A beta-arrestin/green fluorescent protein biosensor for detecting G protein-coupled receptor activation. J Biol Chem 272:27497–27500 [DOI] [PubMed] [Google Scholar]
- Barak LS, Ferguson SS, Zhang J, Martenson C, Meyer T, Caron MG. (1997b) Internal trafficking and surface mobility of a functionally intact beta2-adrenergic receptor-green fluorescent protein conjugate. Mol Pharmacol 51:177–184 [DOI] [PubMed] [Google Scholar]
- Bayburt TH, Vishnivetskiy SA, McLean MA, Morizumi T, Huang CC, Tesmer JJ, Ernst OP, Sligar SG, Gurevich VV. (2011) Monomeric rhodopsin is sufficient for normal rhodopsin kinase (GRK1) phosphorylation and arrestin-1 binding. J Biol Chem 286:1420–1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvier M, Heveker N, Jockers R, Marullo S, Milligan G. (2007) BRET analysis of GPCR oligomerization: newer does not mean better. Nat Methods 4:3–4, author reply 4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calebiro D, Rieken F, Wagner J, Sungkaworn T, Zabel U, Borzi A, Cocucci E, Zürn A, Lohse MJ. (2013) Single-molecule analysis of fluorescently labeled G-protein-coupled receptors reveals complexes with distinct dynamics and organization. Proc Natl Acad Sci USA 110:743–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao TT, Deacon HW, Reczek D, Bretscher A, von Zastrow M. (1999) A kinase-regulated PDZ-domain interaction controls endocytic sorting of the beta2-adrenergic receptor. Nature 401:286–290 [DOI] [PubMed] [Google Scholar]
- Dorsch S, Klotz KN, Engelhardt S, Lohse MJ, Bünemann M. (2009) Analysis of receptor oligomerization by FRAP microscopy. Nat Methods 6:225–230 [DOI] [PubMed] [Google Scholar]
- Felce JH, Davis SJ. (2012) Unraveling receptor stoichiometry using bret. Front Endocrinol (Lausanne) 3:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca JM, Lambert NA. (2009) Instability of a class a G protein-coupled receptor oligomer interface. Mol Pharmacol 75:1296–1299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman OB, Jr, Krupnick JG, Santini F, Gurevich VV, Penn RB, Gagnon AW, Keen JH, Benovic JL. (1996) Beta-arrestin acts as a clathrin adaptor in endocytosis of the beta2-adrenergic receptor. Nature 383:447–450 [DOI] [PubMed] [Google Scholar]
- Han Y, Moreira IS, Urizar E, Weinstein H, Javitch JA. (2009) Allosteric communication between protomers of dopamine class A GPCR dimers modulates activation. Nat Chem Biol 5:688–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson SM, Gurevich EV, Vishnivetskiy SA, Ahmed MR, Song X, Gurevich VV. (2007) Each rhodopsin molecule binds its own arrestin. Proc Natl Acad Sci USA 104:3125–3128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanyaloglu AC, von Zastrow M. (2008) Regulation of GPCRs by endocytic membrane trafficking and its potential implications. Annu Rev Pharmacol Toxicol 48:537–568 [DOI] [PubMed] [Google Scholar]
- Hebert TE, Moffett S, Morello JP, Loisel TP, Bichet DG, Barret C, Bouvier M. (1996) A peptide derived from a beta2-adrenergic receptor transmembrane domain inhibits both receptor dimerization and activation. J Biol Chem 271:16384–16392 [DOI] [PubMed] [Google Scholar]
- Hong H, Bowie JU. (2011) Dramatic destabilization of transmembrane helix interactions by features of natural membrane environments. J Am Chem Soc 133:11389–11398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irannejad R, Tomshine JC, Tomshine JR, Chevalier M, Mahoney JP, Steyaert J, Rasmussen SG, Sunahara RK, El-Samad H, Huang B, et al. (2013) Conformational biosensors reveal GPCR signalling from endosomes. Nature 495:534–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- James JR, Oliveira MI, Carmo AM, Iaboni A, Davis SJ. (2006) A rigorous experimental framework for detecting protein oligomerization using bioluminescence resonance energy transfer. Nat Methods 3:1001–1006 [DOI] [PubMed] [Google Scholar]
- Kang DS, Tian X, Benovic JL. (2013) β-Arrestins and G protein-coupled receptor trafficking. Methods Enzymol 521:91–108 [DOI] [PubMed] [Google Scholar]
- Kato HE, Zhang F, Yizhar O, Ramakrishnan C, Nishizawa T, Hirata K, Ito J, Aita Y, Tsukazaki T, Hayashi S, et al. (2012) Crystal structure of the channelrhodopsin light-gated cation channel. Nature 482:369–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano K, Yano Y, Omae K, Matsuzaki S, Matsuzaki K. (2013) Stoichiometric analysis of oligomerization of membrane proteins on living cells using coiled-coil labeling and spectral imaging. Anal Chem 85:3454–3461 [DOI] [PubMed] [Google Scholar]
- Keppler A, Gendreizig S, Gronemeyer T, Pick H, Vogel H, Johnsson K. (2003) A general method for the covalent labeling of fusion proteins with small molecules in vivo. Nat Biotechnol 21:86–89 [DOI] [PubMed] [Google Scholar]
- Kobilka BK. (2011) Structural insights into adrenergic receptor function and pharmacology. Trends Pharmacol Sci 32:213–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan TH, Kuravi S, Lambert NA. (2011) Internalization dissociates β2-adrenergic receptors. PLoS ONE 6:e17361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lober RM, Pereira MA, Lambert NA. (2006) Rapid activation of inwardly rectifying potassium channels by immobile G-protein-coupled receptors. J Neurosci 26:12602–12608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loura LM, Prieto M. (2011) FRET in Membrane Biophysics: An Overview. Front Physiol 2:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manglik A, Kruse AC, Kobilka TS, Thian FS, Mathiesen JM, Sunahara RK, Pardo L, Weis WI, Kobilka BK, Granier S. (2012) Crystal structure of the µ-opioid receptor bound to a morphinan antagonist. Nature 485:321–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercier JF, Salahpour A, Angers S, Breit A, Bouvier M. (2002) Quantitative assessment of beta 1- and beta 2-adrenergic receptor homo- and heterodimerization by bioluminescence resonance energy transfer. J Biol Chem 277:44925–44931 [DOI] [PubMed] [Google Scholar]
- Milligan G (2008) A day in the life of a G protein-coupled receptor: the contribution to function of G protein-coupled receptor dimerization. Br J Pharmacol 153 Suppl 1: S216–229. [DOI] [PMC free article] [PubMed]
- Milligan G. (2010) The role of dimerisation in the cellular trafficking of G-protein-coupled receptors. Curr Opin Pharmacol 10:23–29 [DOI] [PubMed] [Google Scholar]
- Milligan G, Bouvier M. (2005) Methods to monitor the quaternary structure of G protein-coupled receptors. FEBS J 272:2914–2925 [DOI] [PubMed] [Google Scholar]
- Neumann L, Wohland T, Whelan RJ, Zare RN, Kobilka BK. (2002) Functional immobilization of a ligand-activated G-protein-coupled receptor. ChemBioChem 3:993–998 [DOI] [PubMed] [Google Scholar]
- Palczewski K. (2010) Oligomeric forms of G protein-coupled receptors (GPCRs). Trends Biochem Sci 35:595–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce KL, Premont RT, Lefkowitz RJ. (2002) Seven-transmembrane receptors. Nat Rev Mol Cell Biol 3:639–650 [DOI] [PubMed] [Google Scholar]
- Pin JP, Galvez T, Prézeau L. (2003) Evolution, structure, and activation mechanism of family 3/C G-protein-coupled receptors. Pharmacol Ther 98:325–354 [DOI] [PubMed] [Google Scholar]
- Rasmussen SG, Choi HJ, Fung JJ, Pardon E, Casarosa P, Chae PS, Devree BT, Rosenbaum DM, Thian FS, Kobilka TS, et al. (2011a) Structure of a nanobody-stabilized active state of the β(2) adrenoceptor. Nature 469:175–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen SG, DeVree BT, Zou Y, Kruse AC, Chung KY, Kobilka TS, Thian FS, Chae PS, Pardon E, Calinski D, et al. (2011b) Crystal structure of the β2 adrenergic receptor-Gs protein complex. Nature 477:549–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum DM, Rasmussen SG, Kobilka BK. (2009) The structure and function of G-protein-coupled receptors. Nature 459:356–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salahpour A, Angers S, Mercier JF, Lagacé M, Marullo S, Bouvier M. (2004) Homodimerization of the beta2-adrenergic receptor as a prerequisite for cell surface targeting. J Biol Chem 279:33390–33397 [DOI] [PubMed] [Google Scholar]
- Salahpour A, Masri B. (2007) Experimental challenge to a ‘rigorous’ BRET analysis of GPCR oligomerization. Nat Methods 4:599–600, author reply 601 [DOI] [PubMed] [Google Scholar]
- Valentine CD, Haggie PM. (2011) Confinement of β(1)- and β(2)-adrenergic receptors in the plasma membrane of cardiomyocyte-like H9c2 cells is mediated by selective interactions with PDZ domain and A-kinase anchoring proteins but not caveolae. Mol Biol Cell 22:2970–2982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel SS, Thaler C, Koushik SV. (2006) Fanciful FRET. Sci STKE 2006:re2. [DOI] [PubMed] [Google Scholar]
- Whorton MR, Bokoch MP, Rasmussen SG, Huang B, Zare RN, Kobilka B, Sunahara RK. (2007) A monomeric G protein-coupled receptor isolated in a high-density lipoprotein particle efficiently activates its G protein. Proc Natl Acad Sci USA 104:7682–7687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whorton MR, Jastrzebska B, Park PS, Fotiadis D, Engel A, Palczewski K, Sunahara RK. (2008) Efficient coupling of transducin to monomeric rhodopsin in a phospholipid bilayer. J Biol Chem 283:4387–4394 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.