Abstract
Background
CXCL10 (IP-10) is a potent chemoattractant for T cells that has been postulated to play arole in infection and acute cellular rejection (ACR) in animal models. We measured CXCL10 (IP-10) (and other cytokines previously implicated in the pathogenesis of ACR) in the bronchoalveolar lavage (BAL) of lung transplant recipients (LTRs) to determine the association between CXCL10 (IP-10) and ACR in LTRs.
Methods
In a prospective study of 85 LTRs, expression of cytokines (TNF, IFNγ, IL-6, IL-8, IL-15, IL-16, IL-17, CXCL10 (IP-10) and MCP-1 (CCL2)) in BAL samples (n=233) from patients with episodes of ACR (n=44), infection (Infect) (n=25), concomitant ‘Infect +ACR’ (n=10), and ‘No Infect & No ACR’ (n=154) were analyzed.
Results
The levels of both CXCL10 (IP-10) and IL-16 were significantly increased in histologically proven ACR, as compared to the ‘No Infect & No ACR’ group (CXCL10 [IP-10]: 107.0 vs. 31.9 pg/mL [p=0.001]; IL-16: 472.1 vs. 283.01 [p=0.01]).However, in a linear mixed effects model, significant association was found only between CXCL10 (IP-10)] and ACR. A 1-log increase of CXCL10 (IP-10) was associated with a 40% higher risk of ACR (OR 1.4; 95% CI 1.12-1.84).
Conclusion
Higher values of CXCL10 (IP-10) in BAL fluid are associated with ACR in LTRs suggesting a potential mechanistic role in the pathogenesis of ACR in LTRs. These results suggest that therapeutic strategies to inhibit CXCL10 (IP-10) and or its cognate receptor, CXCR3, warrant investigation to prevent and/or treat ACR in clinical lung transplantation.
Keywords: CXCL10 (IP-10), lung transplantation, acute cellular rejection (ACR), cytokines
Introduction
Lung transplant recipients (LTRs) are at a higher risk of both infection and rejection, as compared to other categories of solid organ transplant recipients (SOTRs) (1). It is important to elucidate the underlying mechanisms of clinical syndromes causing lung allograft dysfunction and failure in this patient population. CXCL10 (IP-10) is a member of the CXC chemokine subgroup of the chemokine superfamily and exerts its biological effects by binding to its cognate receptor, CXCR3. CXCL10 (IP-10) plays a critical role in the regulation of T cell chemotaxis during inflammatory immune responses and has been implicated as an important mediator of acute rejection in organ transplant recipients (2). The role of CXCL10 (IP-10) role in the pathogenesis of organ rejection was initially highlighted in a MHC-mismatched mouse allograft model in which CXCR3-/- mice survived longer than wild type mice (3). Similarly, another study in a rat model of orthotropic lung transplantation and ACR noted increased expression of CXCL10 (IP-10) and CXCR3 associated with recruitment of mononuclear cells in the allograft during ACR (4).
Relatively few studies have specifically evaluated the role played by CXCL10 (IP-10) in human LTRs. One recently published study noted higher serum levels of CXCL10 (IP-10) in LTRs who developed grade 3 primary graft dysfunction (5). Previous studies examining whether CXCL10 (IP-10) levels in the BAL are associated with ACR in LTRs have yielded conflicting results (5, 6).
Results
Clinical Characteristics
Of the 85 patients enrolled in the study, 35 received alemtuzumab induction immunosuppression (Table 1). The median number of samples analyzed per patient was 3. Median number of samples/patient in the alemtuzumab and non-alemtuzumab groups were not significantly different (4 vs. 2; p=0.4). Median time to first sample was 51 days (IQR 22-97). Patients with subclinical rejection (minimal) were followed clinically with augmentation in calcineurin inhibitor therapy.
Table 1.
Basic demographics of LTRs in the study.
| Characteristic | Alemtuzumab (N=35) | Non- Alemtuzumab (N=50) | P value |
|---|---|---|---|
| Age (Years): Interquartile range | 56.5 (45-62) | 61 (52-64) | 0.6 |
| Sex | 0.36 | ||
| Male | 69% (24/35) | 58% (29/50) | |
| Female | 31% (11/35) | 42% (21/50) | |
| Underlying Disease % | |||
| Emphysema | 25% (8/35) | 35% (16/50) | 0.46 |
| Idiopathic Pulmonary Fibrosis | 20% (7/35) | 29% (14/50) | 0.45 |
| Cystic Fibrosis | 20% (7/35) | 8% (4/50) | .018 |
| Interstitial Lung diseases | 11% (4/35) | 6% (3/50) | 0.44 |
| Antitrypsin deficiency | 0% | 4% (2/50) | |
| Obliterative bronchiolitis | 3% (1/35) | 4% (2/50) | |
| Others | 23% (8/35) | 18% (9/50) | |
| Primary mismatch (Donor +/Recipient -) for Cytomegalovirus | 14% (4/35) | 20% (10/50) | 0.38 |
NS: not statistically significant; p≥.05
In this cohort, 25 episodes were classified as infection-only episodes (Infect) and 44 episodes were classified as ACR-only episodes (ACR). Ten episodes of concomitant infection and ACR (Infect+ACR) were noted, while 154 episodes were categorized as normal (No Infect & No ACR). The details of various infectious syndromes are provided in Table 2. Individuals who received alemtuzumab induction were more likely to develop CMV pneumonitis while patients in the non-alemtuzumab induction group almost exclusively had CMV infection. Episodes of ACR were more common in the alemtuzumab group. The values of CXCXL10 (IP10) and TNF were not significantly different between patients who did and did not receive alemtuzumab induction. The median values of cytokines among various clinical syndromes are reported in Figure 1.
Table 2.
Important clinical events (number of episodes in patients) in the study cohort.
| Alemtuzumab Group N=35 | Non-Alemtuzumab Group N=50 | P value | |
|---|---|---|---|
| Infectious syndromes % (n/N) | |||
| Probable invasive aspergillosis | 3% (1/35) | 4% (2/50) | 1.0 |
| CMV Infection/Disease | 9% (3/35) | 28% (14/50) | 0.07 |
| Gram negative Pneumonia | 14% (5/35) | 2% (1/50) | 0.07 |
| Gram positive Pneumonia | 11% (4/35) | 2% (1/50) | 0.15 |
| Culture negative Tracheobronchitis | 11% (4/35) | 0% (0/50) | - |
| Culture negative Pneumonia | 2% (1/50) | 10% (5/50) | 0.3 |
| Histologically proven (ACR) | |||
| Minimal | 48% (18/35) | 24% (12/50) | 0.012 |
| Mild | 31% (11/35) | 6% (3/50) | 0.03 |
| Moderate | 22% (8/35) | 0% (0/50) | - |
| Severe | 6% (2/35) | 0% (0/50) | - |
Figure 1.
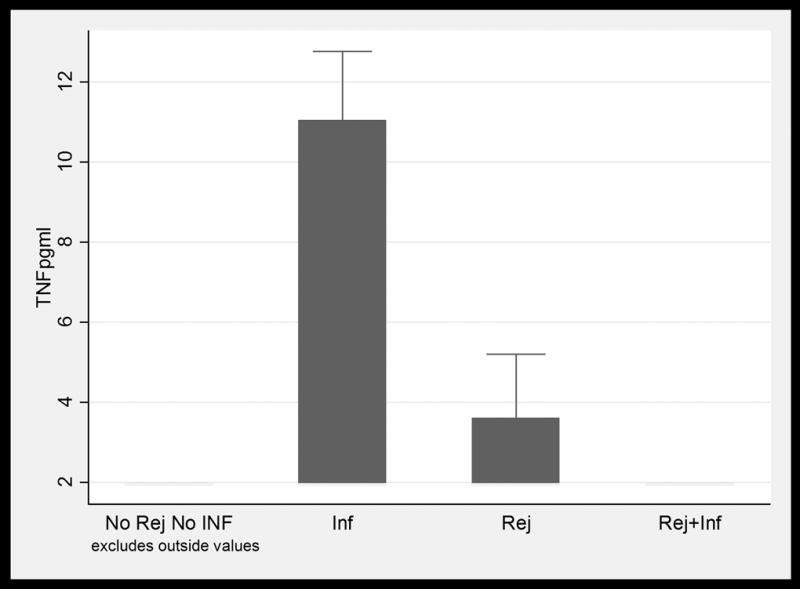
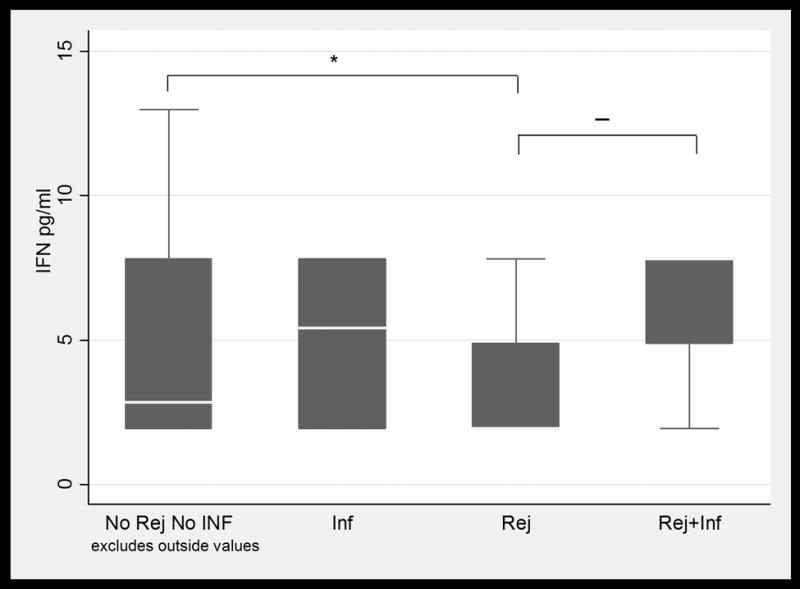
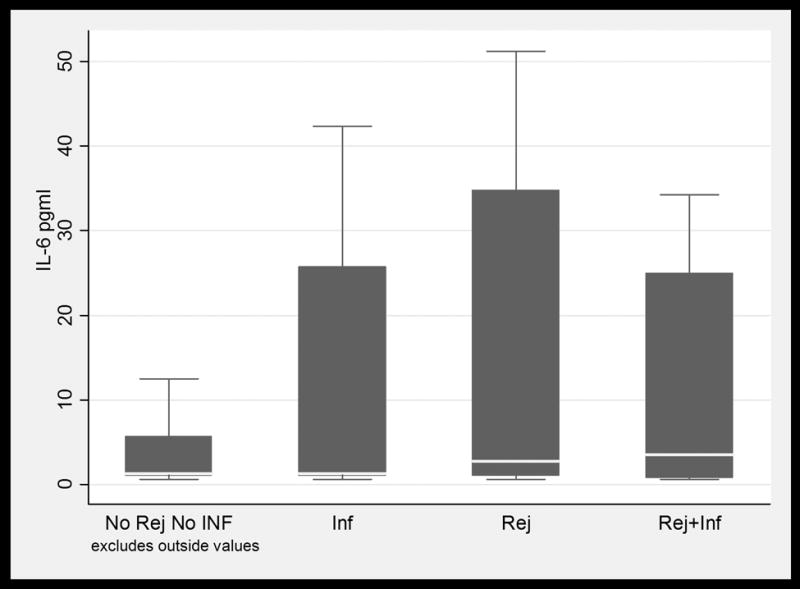

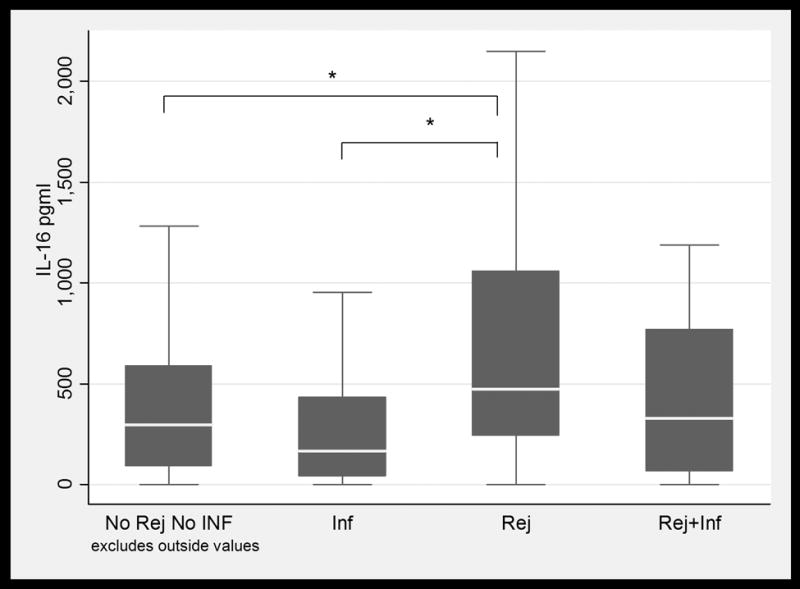
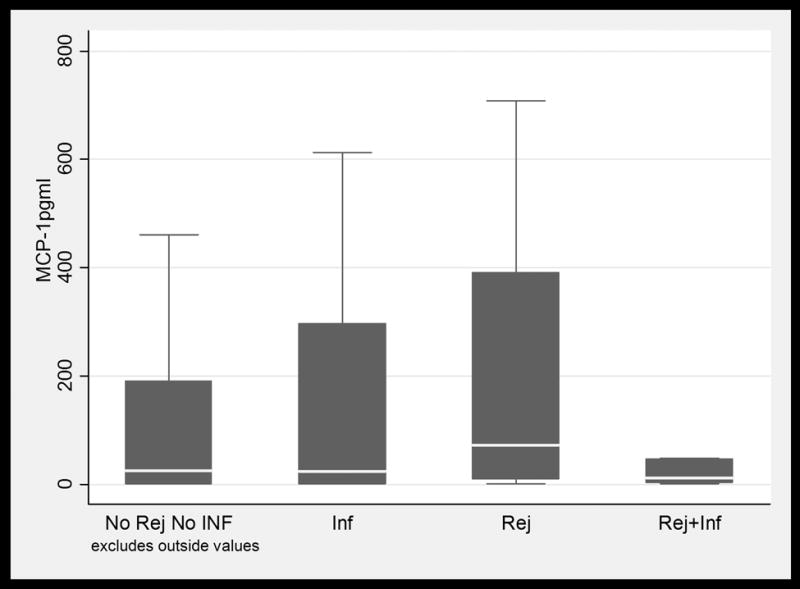
BAL fluid concentrations values in LTRs with and without infection and /or ACR. Only IL-16 values were significantly higher in patients with ACR when compared to patients with infection. (1a) TNFα; (1b) IFNγ; (1c) IL-6; (1d) IL-15; (1e) IL-16; and (1f) MCP-1. Horizontal line represents the median, the box encompasses the 25-75 percentile and the error bars encompass the 10th -90th percentile. * Represents Pairwise Wilcoxon Rank Sum Tests (p ≤ 0.01):
BAL Fluid Cytokine Levels in ACR
Among patients with biopsy proven ACR (all grades), CXCL10 (IP-10) and IL-16 were significantly elevated when compared to patients with No Infect & No ACR. The values for CXCL10 (IP-10) were 107.0 pg/mL in the ACR group as compared to 31.98 pg/mL in the No Infect & No ACR group (p=0.0001) (Figure 2), while IL-16 values were 472.1 pg/mL in patients with ACR as compared to 283.09 pg/mL (p=0.01) in patients with No Infect & No ACR Figure 1(e).
Figure 2.
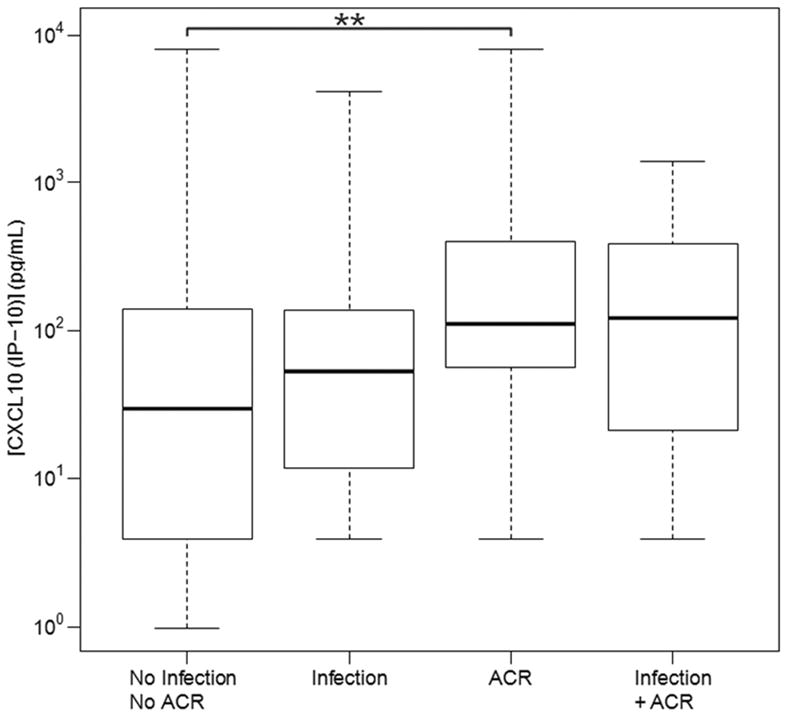
BAL fluid CXCL10 (IP-10) concentration values in LTRs with and without infection and/or ACR. CXCL10 (IP -10) values were significantly higher in BAL fluid from patients with ACR when compared to BAL fluid from patients with infection, or no infection or ACR. Horizontal line represents the median, the box encompasses the 25th to 75th percentile and the error bars encompass the 1090 percentile for CXCL10 (IP-10). ** Represents Pairwise Wilcoxon Rank Sum Tests (p ≤ 0.001):
However, of the 8 cytokines tested, the only significant association using the linear mixed effects model was between CXCL10 (IP-10) and presence of ACR (Table 3). CXCL10 (IP-10) did not exhibit a significant association with infection, nor was there any significant effect due to differences in immunosuppressive protocols between institutes or any additional significant interactions. The association between CXCL10 (IP-10) and ACR was corroborated by the Mann-Whitney test (Table 4). The latter also suggested an association between IL-16 and ACR. However, this effect was confounded by a significant association between IL-16 and institute (Table 3). Therefore, we view CXCL10 (IP-10) as the most promising biomarker of ACR of the cytokines/chemokines examined in this study. Overall, there was a trend towards higher CXCL10 (IP10) values among lung transplant with increasing grades of rejection (minimal to moderate). The CXCL10 (IP10) median values in patients with minimal, mild, moderate or severe rejection were 86 pg/mL (IQR 26-165), 218 pg/mL (IQR 65-636), 409 pg/mL (IQR 86-1302), and 217 pg/mL (IQR 7.81-426), respectively. Among the patients who received alemtuzumab induction, a similar trend towards a higher concentration of CXCL10 (IP10) with increasing severity or grade of rejection was noted. The CXCL10 (IP10) median values in patients with minimal, mild, moderate were 81 pg/mL (IQR 25-234), 295 pg/mL (IQR 104-956), and 409 pg/mL (IQR 86-1302), respectively. The median CXCL10 (IP10) value for severe rejection was 217 pg/mL (IQR 7.81-426) based on only two observations. No episodes of severe rejection were noted in patients without alemtuzumab induction. The CXCL10 (IP-10) ranges are summarized in Table 4 and Figure 2. The CXCLl0 (IP10) values declined after the resolution of ACR episodes. The median CXCL10 (IP10) values during and after ACR episodes were 293 pg/mL (IQR 76-517) and 39 pg/mL (IQR 13-141), respectively. The CXCL10 (IP10) values were statistically significant in linear mixed effect model with a p value = 0.001. A CXCL10 (IP-10) value of ≥306 pg/mL yielded a 90% specificity and 34% sensitivity for the prediction of ACR (Figure 4).
Table 3.
Mixed linear effects model showing effects of various cytokine concentrations in ACR and/or infection.
| Fixed Effects | TNF | IFN-γ | IL-6 | IL-15 | IL-16 | IL-17 | CXCL10 (IP-10) | MCP-1 (CCL2) |
|---|---|---|---|---|---|---|---|---|
| ACR (yes; no) | 0.32 | 0.30 | 0.32 | 0.53 | 0.53 | 0.53 | 0.0023 | 0.30 |
| Infection (yes; no) | 0.070 | 0.96 | 0.36 | 0.74 | 0.10 | 0.93 | 0.86 | 0.96 |
| Institute (TGH; UPMC) | 0.60 | 2.3E-18 | 0.89 | 0.74 | 7.5E-08 | 0.068 | 0.60 | 0.89 |
| Interactions | ||||||||
| ACR:Infection | 0.061 | 0.33 | 0.83 | 1.00 | 1.00 | 0.52 | 0.52 | 1.00 |
| ACR:Institute | 0.84 | 0.68 | 0.68 | 0.84 | 0.96 | 0.68 | 0.84 | 0.68 |
| Infection:Institute | 0.53 | 0.92 | 0.64 | 0.64 | 0.53 | 0.64 | 0.79 | 1.00 |
| ACR:Infection:institute | 0.61 | 0.75 | 0.81 | 0.95 | 0.95 | 0.61 | 0.95 | 0.95 |
Table 4.
BAL fluid cytokine median values (interquartile range) in histologically proven ACR.
| Cytokine median pg/mL |
No ACR (N=173) |
ACR (N=62) |
min detectable threshold |
FDR from linear model |
FDR from Mann-Whitney |
|---|---|---|---|---|---|
| TNF | 0.98 (0.98 - 1.72) | 0.98 (0.98 - 2.21) | 1.95 | 0.32 | 0.47 |
| IFN-γ | 3.51 (0.98 - 3.91) | 0.98 (0.98 - 3.91) | 1.95; 7.81 | 0.30 | 0.24 |
| IL-6 | 1.28 (0.59 - 6.52) | 3.05 (0.59 - 28.53) | 0.58; 1.17 | 0.32 | 0.10 |
| IL-15 | 2.28 (0.98 - 5.60) | 2.20 (0.98 - 3.69) | 0.97; 1.95 | 0.53 | 0.18 |
| IL-16 | 251.13 (85.70 - 570.48) | 456.99 (194.63 - 995.54) | 1.95; 3.9 | 0.53 | 0.038 |
| IL-17 | 0.49 (0.49 - 0.49) | 0.49 (0.49 - 0.49) | 0.97 | 0.53 | 0.038 |
| CXCL10 (IP-10) | 33.00 (3.91 - 138.40) | 111.23 (46.89 - 384.80) | 1.95; 7.81 | 0.0023 | 0.000081 |
| MCP-1 (CCL2) | 24.34 (1.49 - 198.26) | 41.44 (9.05 - 330.33) | 0.97; 1.95 | 0.30 | 0.28 |
Figure 4.
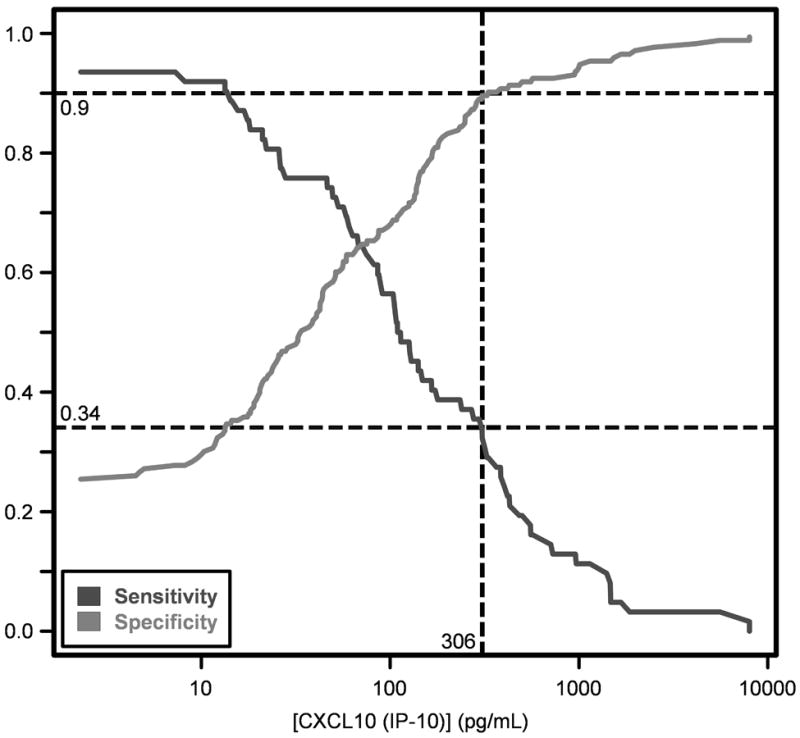
Sensitivity and specificity of CXCL10 (IP-10) values for the diagnosis of ACR. A CXCL10 (IP-10) of 306 pg/mL or higher corresponds to 90% specificity and 34% sensitivity.
Discussion
Our data suggest that elevated CXCL10 (IP-10) in BAL fluid is associated with ACR. Clinical studies evaluating BAL fluid levels CXCL10 (IP-10) in ACR in LTRs have been contradictory (6, 7). Although highly specific, CXCL10 (IP-10) has relatively poor sensitivity for ACR (Figure 4). In a multivariate analysis of the cytokines studied, a one log increase in CXCL10 (IP-10) was associated with a 40% increase in the probability of ACR.
In a study involving 54 biopsies of 24 human LTRs, immunohistochemical examination showed that areas characterized by ACR were infiltrated by T-cells expressing CXCR3. T-cells in BAL of LTRs with ACR were CXCR3+ and demonstrated a strong in vitro migratory capacity in response to CXCL10 (IP-10). Furthermore, alveolar macrophages obtained from individuals with ACR expressed and secreted levels of CXCL10 (IP-10) which were capable of inducing chemotaxis of CXCR3+ T-cells (8). This study, however, did not measure levels of CXCL10 (IP-10) in the BAL fluid. Our findings extend those from previous studies by directly demonstrating that CXCL10 (IP-10) is elevated in the BAL supernatant of patients with ACR, suggesting a critical role for CXCL10 (IP-10) in the pathogenesis of ACR in LTRs.
To date, two other studies have measured CXCL10 (IP-10) levels in the BAL fluid from LTRs. Belperio et al. reported higher CXCL10 (IP-10) levels among LTRs with ACR as compared to healthy individuals (6). However, this study failed to include evaluation of LTRs experiencing infections or to provide a multivariate analysis to account for confounding variables. The other previously published study reported that elevated CXCL10 (IP-10) in BAL fluid was associated with poor clinical outcome and development of bronchiolitis obliterans syndrome (BOS), but failed to show any association with ACR in generalized estimating equation (GEE) regression analysis (7). Our study adds to the existing data by addressing the limitations of the previously reported studies as stated earlier. We showed that CXCL10 (IP-10) was associated with ACR in LTRs and not with infection in a multicenter prospective study of 85 well-characterized patients.
In experimental animal models, elevated levels of TNF, IFN-γ, IL-2 and IL-6 have been reported in the BAL fluid of transplanted lungs during ACR episodes (9-11). Human studies, however, have yielded inconsistent results (12-16). Other studies reported IFN-γ mRNA expression by BAL fluid cells from LTRs within the first three months of graft dysfunction (17, 18). It should be noted that previous studies reporting an association of IFN-γ, IL-6 or TNF expression with ACR in LTRs did not measure CXCL10 (IP-10) (14, 19, 20). Failure to detect an association of TNF with ACR in our study may be attributed to the relatively low levels of TNF in BAL fluid which may in turn be related to fewer numbers of macrophages in our BAL fluids, especially in the cohorts of patients who received alemtuzumab induction (21, 22). In addition, the ability to detect mRNA expression in cells may be much more sensitive than assays of cytokine protein in BAL supernatant.
Association between higher values of IL-16 and ACR observed by Mann-Whitney analysis was not substantiated in the mixed linear effects model due to the significant interaction with the institution where lung transplantation was performed. The exact reason for this observation remains unclear to us. However, our study is consistent with a matched controlled study of 28 LTRs, in which the authors failed to find significantly higher values of IL-16 in the BALs preceding or during ACR (23).
There are several limitations of our current study. The absolute cytokine values were dependent on the BAL procedures performed. At both institutions, the practice was generally standardized. However, the volume of BAL fluid returned by the procedure may vary with technique, thereby affecting the absolute cytokine values observed and possibly contributing to some degree of variation in the observed cytokine values. We were able to analyze only 233 available samples that were much lower than the potential 500 samples. The lower numbers of analyzed samples do not reflect selection bias as the investigators were blinded to CXCL10 (IP10) results. However, inter-institutional differences in clinical variables (e.g., CMV pneumonia and acute rejection rates) may have resulted from individual institutional practice styles. We also did not measure the cell count in BAL as a correlate for rejection. Previous reports have found that cell count measurements might not be specific for rejection and may vary with immunosuppressive regimens and time from transplant (24). We also chose to analyze fewer cytokines in our cohort; we would however argue that these cytokines were reported to be associated with acute or chronic rejection in LTRs in previous studies. Indeed, this comment could not be made regarding other cytokines that were not studied. However, CXCL10 (IP-10) is an important cytokine in TH1 responses, which have been implicated in the pathogenesis of ACR. The significant association of CXCL10 (IP-10) in the presence of other established TH1 cytokines (IFN-γ, TNF) involved in the process of ACR supports a crucial role of CXCL10 (IP-10) in the TH1 pathway.
In conclusion, our data suggests that higher values of CXCL10 (IP-10) in BAL are independently associated with ACR in LTRs. Indeed, future studies in a larger cohort are warranted to further validate our findings. Additional studies assessing the chemokine pathway of CXCL10 (IP-10) may delineate the critical mechanistic role played by CXCL10 (IP-10) in the pathogenesis of ACR in lung allografts. Furthermore, our studies suggest CXCL10 (IP-10) and CXCR3 as potential targets for novel drug development to prevent and/or treat ACR in LTRs (and possibly ACR in other SOTRs).
Material and Methods
Study Design and Setting
Patients
LTRs who were ≥ 18 years of age at the University of Pittsburgh Medical Center (UPMC, Pittsburgh, PA, USA) and the Toronto General Hospital/University of Toronto (TGH, Toronto, ON, CA) from July 2007 to January 2010 were enrolled in the study. Patients were followed prospectively for one year after lung transplantation. Patients underwent scheduled bronchoscopy with BAL or at the discretion of their primary physician for investigation of clinical symptoms suggestive or graft dysfunction. Subjects underwent surveillance bronchoscopy with BAL 2 weeks, and 2, 4, 6, 8, 10 and 12 months during the first year following transplantation at the UPMC. Surveillance bronchoscopy was performed at 2 and 6 weeks, then 3, 6, 9, and 12 months following transplantation at the TGH. Eighty-five patients were enrolled in the study, and a total of 233 BAL samples were obtained for analysis, of which 132 were from UPMC and 101 samples were from TGH. The respective Institutional Review Boards of University of Pittsburgh (0212095) and TGH/University Health Network (08-0723-TE) approved the study in advance, and all patients provided written informed consent for their participation in the study.
Immunosuppression Protocol
All LTRs at UPMC (n=35) received induction immunosuppression with alemtuzumab and maintenance triple immunosuppression with tacrolimus, mycophenolate mofetil and prednisone as described previously (25). Patients enrolled at TGH (n=50) did not receive induction immunosuppression and were maintained on a regimen of cyclosporine, prednisone and azathioprine as reported previously (26).
Prophylaxis Regimens
Cytomegalovirus (CMV)
At UPMC, all patients received a minimum of 6 months of CMV prophylaxis with valganciclovir adjusted for renal function. At TGH, CMV-seronegative recipients with CMV-seropositive donors received 6 months of prophylaxis with valganciclovir adjusted for renal function while CMV-seropositive recipients received 3 months of valganciclovir prophylaxis.
Antifungal Prophylaxis
All patients at UPMC received voriconazole 200 mg twice a day for a minimum of 120 days (27), while at TGH, prophylaxis with voriconazole was administered for 3 months only to individuals with a of history of pre-transplant colonization with Aspergillus spp. or in the presence of post-transplant Aspergillus spp. colonization.
All patients received Pneumocystis jirovecii prophylaxis with trimethoprim-sulfamethoxazole (TMP-SMX) single strength daily. Inhaled pentamidine or oral dapsone was used for patients allergic to TMP-SMX. All patients were prospectively followed for one year after transplantation.
Definitions
Infections
Infectious syndromes (CMV, aspergillosis and pneumonia) were defined as per the standardized recommendations of the American Society of Transplantation (AST) and International Society for Heart and Lung Transplantation (ISHLT) (28, 29). The classification of each infection episode was based on bronchoscopy and BAL analysis.
Acute Cellular Rejection (ACR)
All ACR episodes reported were biopsy proven and graded as minimal, mild, moderate or severe based on the histology findings according to the ISHLT guidelines (30). Clinically suspected ACR and antibody-mediated rejection episodes were excluded from the analysis.
Bronchoscopy with BAL
The patients were prepared with 20% benzocaine spray and 1% lidocaine solution applied to the oropharynx. A total of two 50 mL and one 25 mL aliquots of normal saline solution were instilled separately and immediately aspirated into separate traps. Patients with marginal oxygenation may not have received the entire 125 mL BAL. The aspirates were pooled (BAL fluid), and the total volume instilled and recovered was recorded. Of the possible 500 BAL samples in this cohort, 233 samples with adequate volume were available for analysis.
Sample Processing
Approximately 6 mL of the pooled BAL fluid sample was sent to the clinical virology and microbiology laboratories. The remaining pooled BAL fluid sample was separated into two aliquots; one was sent to the clinical microbiology laboratory for cell count and differential and the other was centrifuged at 400 × g for 20 minutes at 4°C. The supernatant was separated from the cell pellet and a 4 mL aliquot of the supernatant was stored at -80°C for future analysis.
BAL Fluid Cytokine Assays
BAL fluid concentrations of tumor necrosis factor (TNF), interferon-γ (IFN-γ), interleukin-6 (IL-6), IL-15, IL-16, IL-17, CXCL10 (IP-10) and CCL2 (MCP-1) were measured in BAL fluid by specific enzyme-linked imunosorbent assay (ELISA) using commercially available, validated matched monoclonal antibody pairs (R&D Systems; Minneapolis, MN, USA) in a blinded fashion. IFN-γ and IL-15 were measured without dilution, IL-6 and CXCL10 (IP-10) were measured at 1:2 dilution, while MCP-1 (CCL2) CXC and IL-16 were measured at 1:4 and 1:8 dilution, respectively. The absorbency was measured in an ELISA reader (Model 450, Bio-Rad) at 450 nm. The respective cytokine concentration was determined by interpolation from standard curves and expressed as pg/mL.
In summary, Maxisorp 96 well plates (VWR, cat# CA-62409-002) were coated with 50 μl/well of capture antibody at the suggested dilution by the manufacturer and incubated at room temperature overnight. Plates were washed 7X with PBS pH 7.2-7.4/0.05% tween-20 and then blotted against paper towels. Plates were blocked with 200 μl/well PBS pH 7.2-7.4/0.1% BSA (Reagent Diluent) for 2 hours at room temperature. Block solution was removed and 50 μl/well of standards diluted in reagent diluents were added to plates along with samples diluted with reagent diluents to appropriate dilutions. Plates were incubated at 4°C overnight. Plates were washed 7x with PBS pH 7.2-7.4/0.05% Tween-20, blotted against paper towel 50 μl/well of detection antibody (at the suggested dilution by the manufacturer), added and then incubated at room temperature for 2 hours. Plates were washed 7X with PBS pH 7.2-7.4/0.05% tween-20 and then blotted against paper towels. Extravidin Alkaline Phosphatase (Sigma, cat# E2636) was diluted to 1/2000 in Reagent Diluents and 50 μl/well was added to plates and incubated for 1 hour at room temperature. Plates were washed 7X with PBS pH 7.2-7.4/0.05% tween-20, 2X with distilled water and then blotted against paper towels. Plates were then incubated with 100 μl/well of PNPP Sigma Fast (Sigma, cat# N2770) prepared as per manufacturer’s instructions away from light. Plates were read at OD 405 at various time points.
Statistics
All statistical analyses were performed in R version 2.14.0. Preliminary analysis suggested insufficient statistical power to resolve multiple grades of ACR or infection type. Therefore, we used a simple binary classification (positive or negative) for both ACR and infection. Data were log transformed prior to analysis. Concentrations too low to detect by ELISA assay (Table 4) were assigned a value of 0 or, for log transformation, one half of the minimum detection threshold.
We used a linear mixed effects model to determine if there were any significant associations between each of the cytokine concentrations and ACR or infection. The model defined infection, ACR, and institution as fixed effects and patient ID as a random effect. Because of skew in the data, association with ACR was corroborated using the non-parametric Mann-Whitney test. To correct for testing of multiple cytokines, we used a Benjamin & Hochberg false discovery rate (FDR) adjustment for all p values and defined FDR ≤ 0.05 as significant.
Figure 3.
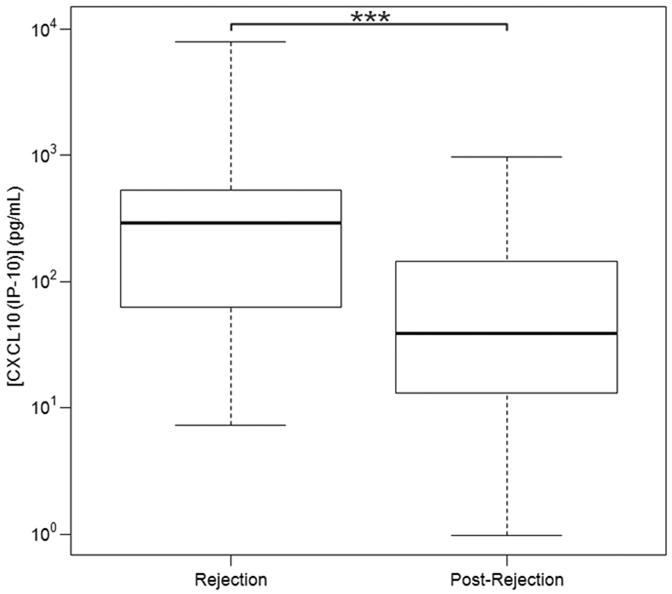
BAL fluid CXCL10 (IP-10) concentration values in LTRs during and post-ACR based on linear mixed effect model. CXCL10 (IP -10) values were significantly higher in BAL fluid from patients with ACR when compared to BAL fluid after ACR. Horizontal line represents the median, the box encompasses the 25-75 percentile and the error bars encompass the 10 -90 percentile for CXCL10 (IP-10). *** Represents Pairwise Wilcoxon Rank Sum Tests (p ≤ 0.0001):
Acknowledgments
Funding: Funded by NIH grant R03 AI067412-01A2. (SH) WCL is the recipient of the Canada Research Chair in Infectious Diseases and Inflammation from the Canadian Institutes for Health Research (CIHR)
Principal investigator of NIH grant R03 AI067412-01A2.
Footnotes
Participated in research design, patient recruitment, data analysis, and writing of the manuscript
Participated in patient recruitment, data analysis, contributed analytic tools
Participated in data analysis and contributed analytic tools
Participated in data analysis and writing of the manuscript
Participated in patient recruitment, data analysis, and writing of the manuscript
Participated in patient recruitment
Participated in patient recruitment and data analysis
Participated in research design, patient recruitment.
Participated in research design, data analysis, and writing of the manuscript
Participated in patient recruitment and data analysis
Participated in research design, data analysis, contributed analytic tools and writing of the manuscript
No conflict of interest
Reference List
- 1.Pappas PG, Alexander BD, Andes DR, Hadley S, Kauffman CA, Freifeld A, Anaissie EJ, Brumble LM, Herwaldt L, Ito J, Kontoyiannis DP, Lyon GM, Marr KA, Morrison VA, Park BJ, Patterson TF, Perl TM, Oster RA, Schuster MG, Walker R, Walsh TJ, Wannemuehler KA, Chiller TM. Invasive fungal infections among organ transplant recipients: results of the Transplant-Associated Infection Surveillance Network (TRANSNET) Clin Infect Dis. 2010;50(8):1101–1111. doi: 10.1086/651262. [DOI] [PubMed] [Google Scholar]
- 2.Romagnani P, Crescioli C. CXCL10: A candidate biomarker in transplantation. Clin Chim Acta. 2012 doi: 10.1016/j.cca.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 3.Hancock WW, Gao W, Csizmadia V, Faia KL, Shemmeri N, Luster AD. Donor-derived IP-10 initiates development of acute allograft rejection. J Exp Med. 2001;193(8):975–980. doi: 10.1084/jem.193.8.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belperio JA, Keane MP, Burdick MD, Lynch JP, III, Zisman DA, Xue YY, Li K, Ardehali A, Ross DJ, Strieter RM. Role of CXCL9/CXCR3 chemokine biology during pathogenesis of acute lung allograft rejection. J Immunol. 2003;171(9):4844–4852. doi: 10.4049/jimmunol.171.9.4844. [DOI] [PubMed] [Google Scholar]
- 5.Hoffman SA, Wang L, Shah CV, Ahya VN, Pochettino A, Olthoff K, Shaked A, Wille K, Lama VN, Milstone A, Ware LB, Orens J, Weinacker A, Demissie E, Bellamy S, Kawut SM, Hancock WW, Christie JD. Plasma cytokines and chemokines in primary graft dysfunction post-lung transplantation. Am J Transplant. 2009;9(2):389–396. doi: 10.1111/j.1600-6143.2008.02497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belperio JA, Keane MP, Burdick MD, Lynch JP, III, Xue YY, Li K, Ross DJ, Strieter RM. Critical role for CXCR3 chemokine biology in the pathogenesis of bronchiolitis obliterans syndrome. J Immunol. 2002;169(2):1037–1049. doi: 10.4049/jimmunol.169.2.1037. [DOI] [PubMed] [Google Scholar]
- 7.Neujahr DC, Perez SD, Mohammed A, Ulukpo O, Lawrence EC, Fernandez F, Pickens A, Force SD, Song M, Larsen CP, Kirk AD. Cumulative exposure to gamma interferon-dependent chemokines CXCL9 and CXCL10 correlates with worse outcome after lung transplant. Am J Transplant. 2012;12(2):438–446. doi: 10.1111/j.1600-6143.2011.03857.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agostini C, Calabrese F, Rea F, Facco M, Tosoni A, Loy M, Binotto G, Valente M, Trentin L, Semenzato G. Cxcr3 and its ligand CXCL10 are expressed by inflammatory cells infiltrating lung allografts and mediate chemotaxis of T cells at sites of rejection. Am J Pathol. 2001;158(5):1703–1711. doi: 10.1016/S0002-9440(10)64126-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shalaby MR, Waage A, Espevik T. Cytokine regulation of interleukin 6 production by human endothelial cells. Cell Immunol. 1989;121(2):372–382. doi: 10.1016/0008-8749(89)90036-1. [DOI] [PubMed] [Google Scholar]
- 10.Chang SC, Hsu HK, Perng RP, Shiao GM, Lin CY. Significance of biochemical markers in early detection of canine lung allograft rejection. Transplantation. 1991;51(3):579–584. doi: 10.1097/00007890-199103000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Rolfe MW, Kunkel S, Lincoln P, Deeb M, Lupinetti F, Strieter R. Lung allograft rejection: role of tumor necrosis factor-alpha and interleukin-6. Chest. 1993;103(2 Suppl):133S. doi: 10.1378/chest.103.2_supplement.133s. [DOI] [PubMed] [Google Scholar]
- 12.Rondeau E, Cerrina J, Delarue F, Ladurie FL, Herve P, Chapelier A, Dartevelle P, Sraer JD. Tumor necrosis factor alpha (TNF-alpha) production by cells of bronchioloalveolar lavage (BAL) and peripheral blood mononuclear cells (PBMC) in cardiopulmonary transplant recipients. Transplant Proc. 1990;22(4):1855–1856. [PubMed] [Google Scholar]
- 13.Magnan A, Mege JL, Escallier JC, Brisse J, Capo C, Reynaud M, Thomas P, Meric B, Garbe L, Badier M, Viard L, Bongrand P, Giudicelli R, Metras D, Fuentes P, Vervloet D, Noirclerc M. Balance between alveolar macrophage IL-6 and TGF-beta in lung-transplant recipients. Marseille and Montreal Lung Transplantation Group. Am J Respir Crit Care Med. 1996;153(4 Pt 1):1431–1436. doi: 10.1164/ajrccm.153.4.8616577. [DOI] [PubMed] [Google Scholar]
- 14.Iacono A, Dauber J, Keenan R, Spichty K, Cai J, Grgurich W, Burckart G, Smaldone G, Pham S, Ohori NP, Yousem S, Williams P, Griffith B, Zeevi A. Interleukin 6 and interferon-gamma gene expression in lung transplant recipients with refractory acute cellular rejection: implications for monitoring and inhibition by treatment with aerosolized cyclosporine. Transplantation. 1997;64(2):263–269. doi: 10.1097/00007890-199707270-00015. [DOI] [PubMed] [Google Scholar]
- 15.Whitehead BF, Stoehr C, Wu CJ, Patterson G, Burchard EG, Theodore J, Clayberger C, Starnes VA. Cytokine gene expression in human lung transplant recipients. Transplantation. 1993;56(4):956–961. doi: 10.1097/00007890-199310000-00034. [DOI] [PubMed] [Google Scholar]
- 16.Sundaresan S, Alevy YG, Steward N, Tucker J, Trulock EP, Cooper JD, Patterson GA, Mohanakumar T. Cytokine gene transcripts for tumor necrosis factor-alpha, interleukin-2, and interferon-gamma in human pulmonary allografts. J Heart Lung Transplant. 1995;14(3):512–518. [PubMed] [Google Scholar]
- 17.Ross DJ, Moudgil A, Bagga A, Toyoda M, Marchevsky AM, Kass RM, Jordan SC. Lung allograft dysfunction correlates with gamma-interferon gene expression in bronchoalveolar lavage. J Heart Lung Transplant. 1999;18(7):627–636. doi: 10.1016/s1053-2498(99)00007-8. [DOI] [PubMed] [Google Scholar]
- 18.Moudgil A, Bagga A, Toyoda M, Nicolaidou E, Jordan SC, Ross D. Expression of gamma-IFN mRNA in bronchoalveolar lavage fluid correlates with early acute allograft rejection in lung transplant recipients. Clin Transplant. 1999;13(2):201–207. doi: 10.1034/j.1399-0012.1999.130208.x. [DOI] [PubMed] [Google Scholar]
- 19.Hodge G, Hodge S, Chambers D, Reynolds PN, Holmes M. Acute lung transplant rejection is associated with localized increase in T-cell IFNgamma and TNFalpha proinflammatory cytokines in the airways. Transplantation. 2007;84(11):1452–1458. doi: 10.1097/01.tp.0000290679.94163.e1. [DOI] [PubMed] [Google Scholar]
- 20.Magnan A, Mege JL, Reynaud M, Thomas P, Capo C, Garbe L, Meric B, Badier M, Bongrand P, Viard L. Monitoring of alveolar macrophage production of tumor necrosis factor-alpha and interleukin-6 in lung transplant recipients. Marseille and Montreal Lung Transplantation Group. Am J Respir Crit Care Med. 1994;150(3):684–689. doi: 10.1164/ajrccm.150.3.8087338. [DOI] [PubMed] [Google Scholar]
- 21.Rizzo M, SivaSai KS, Smith MA, Trulock EP, Lynch JP, Patterson GA, Mohanakumar T. Increased expression of inflammatory cytokines and adhesion molecules by alveolar macrophages of human lung allograft recipients with acute rejection: decline with resolution of rejection. J Heart Lung Transplant. 2000;19(9):858–865. doi: 10.1016/s1053-2498(00)00165-0. [DOI] [PubMed] [Google Scholar]
- 22.Fattal-German M, Le Roy LF, Lecerf F, Berrih-Aknin S. Expression of ICAM-1 and TNF alpha in human alveolar macrophages from lung-transplant recipients. Ann N Y Acad Sci. 1996;796(138-48):138–148. doi: 10.1111/j.1749-6632.1996.tb32575.x. [DOI] [PubMed] [Google Scholar]
- 23.Laan M, Linden A, Riise GC. IL-16 in the airways of lung allograft recipients with acute rejection or obliterative bronchiolitis. Clin Exp Immunol. 2003;133(2):290–296. doi: 10.1046/j.1365-2249.2003.02196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tiroke AH, Bewig B, Haverich A. Bronchoalveolar lavage in lung transplantation. State of the art. Clin Transplant. 1999;13(2):131–157. doi: 10.1034/j.1399-0012.1999.130201.x. [DOI] [PubMed] [Google Scholar]
- 25.Shyu S, Dew MA, Pilewski JM, DeVito Dabbs AJ, Zaldonis DB, Studer SM, Crespo MM, Toyoda Y, Bermudez CA, McCurry KR. Five-year outcomes with alemtuzumab induction after lung transplantation. J Heart Lung Transplant. 2011;30(7):743–754. doi: 10.1016/j.healun.2011.01.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hadjiliadis D, Steele MP, Govert JA, Davis RD, Palmer SM. Outcome of lung transplant patients admitted to the medical ICU. Chest. 2004;125(3):1040–1045. doi: 10.1378/chest.125.3.1040. [DOI] [PubMed] [Google Scholar]
- 27.Husain S, Paterson DL, Studer S, Pilewski J, Crespo M, Zaldonis D, Shutt K, Pakstis DL, Zeevi A, Johnson B, Kwak EJ, McCurry KR. Voriconazole prophylaxis in lung transplant recipients. Am J Transplant. 2006;6(12):3008–3016. doi: 10.1111/j.1600-6143.2006.01548.x. [DOI] [PubMed] [Google Scholar]
- 28.Humar A, Michaels M. American Society of Transplantation recommendations for screening, monitoring and reporting of infectious complications in immunosuppression trials in recipients of organ transplantation. Am J Transplant. 2006;6(2):262–274. doi: 10.1111/j.1600-6143.2005.01207.x. [DOI] [PubMed] [Google Scholar]
- 29.Husain S, Mooney ML, Danziger-Isakov L, Mattner F, Singh N, Avery R, Ison M, Humar A, Padera RF, Lawler LP, Fisher A, Drew RJ, Gould KF, Sole A, Studer S, Munoz P, Singer LG, Hannan M. A 2010 working formulation for the standardization of definitions of infections in cardiothoracic transplant recipients. J Heart Lung Transplant. 2011;30(4):361–374. doi: 10.1016/j.healun.2011.01.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yousem SA, Berry GJ, Cagle PT, Chamberlain D, Husain AN, Hruban RH, Marchevsky A, Ohori NP, Ritter J, Stewart S, Tazelaar HD. Revision of the 1990 working formulation for the classification of pulmonary allograft rejection: Lung Rejection Study Group. J Heart Lung Transplant. 1996;15(1 Pt 1):1–15. [PubMed] [Google Scholar]


