Abstract
Cytokines are key mediators of the development and homeostasis of hematopoietic cells, critical for host defense, but also for the development of autoimmune and inflammatory diseases like psoriasis or rheumatoid arthritis (RA). Blocking cytokines activity by interfering with the ligand-receptor association has been successfully employed to treat several immune disorders. A subgroup of cytokines signals through receptors requiring the association with a family of cytoplasmic protein tyrosine kinases known as Janus kinases (Jaks). Jaks have recently gained significant attention as therapeutic targets in inflammation and autoimmunity and several Jak inhibitory small molecules have been developed. The first two Jak inhibitors, tofacitinib and ruxolitinib, have been approved for the treatment of RA and primary myelofibrosis, respectively. Efficacy and safety data suggest that some of these oral Jak inhibitors as well as their topical formulations may soon enter the daily clinical practice for treating patients with psoriasis, lupus erythematosus or other inflammatory skin diseases. While biologics typically target one single cytokine, these new immunomodulators can inhibit signals from multiple cytokines intracellularly and therefore could be useful when other therapies are ineffective. Thus, Jak inhibitors may replace some traditional immunosuppressive agents and help patients not responding to previous therapies.
Introduction
Given the importance that cytokines have in development and homeostasis of the immune system it is not surprising that these soluble factors are critical players in immune mediated disorders including inflammatory autoimmune diseases. For example, the pathogenesis of psoriasis is characterized by the activation of numerous immune cells, which interact with resident tissue cells. In the skin, these cells are primarily composed by keratinocytes and endothelia, in the joints by synoviocytes, fibroblasts and osteoblasts (1, 2). Cellular contact and, more importantly, secreted factors like cytokines, can cause persistent inflammation of skin and joints. The cytokines network in psoriasis’ pathogenesis is well studied (1, 3) and some similarities are found in other pathologies like RA or ulcerative colitis (4, 5). Innate cytokines determining lineage-specification of CD4+ T helper (Th) cells, such as interleukin (IL)-1 and IL-6, and cytokines released by T cells and resident tissue cells like tumor necrosis factor (TNF), interferons (IFNs), IL-17 or IL-23 are indispensable for disease manifestation and perpetuation.
Given the role these molecules play in inflammatory pathologies limiting their interaction with their specific receptors has been successfully exploited for therapeutic purposes through the use of biologics. For immunological purposes, biologics such as monoclonal antibodies, recombinant soluble receptors and fusion proteins of receptor moieties with antibodies fragments, block the interaction of a specific cytokine with its receptor. In the past 15 years, biologics have completely revolutionized the clinical approach to the treatment of autoimmunity and inflammatory pathologies.
The basis for this success was our better understanding of cellular and molecular players. In psoriasis, clearance was observed after bone marrow transplantation, in those receiving the immunosuppressant cyclosporine or antibodies targeting CD4 suggesting a prominent role for T cells (6, 7) and, in particular, IFN-γ-producing Th1 cells (8, 9). Based on such analysis of disease-associated or tissue-infiltrating Th cells in patients and in animal models, Th1 cells were thought to drive the inflammatory responses in organ-specific autoimmune diseases and inflammatory pathologies like psoriasis.
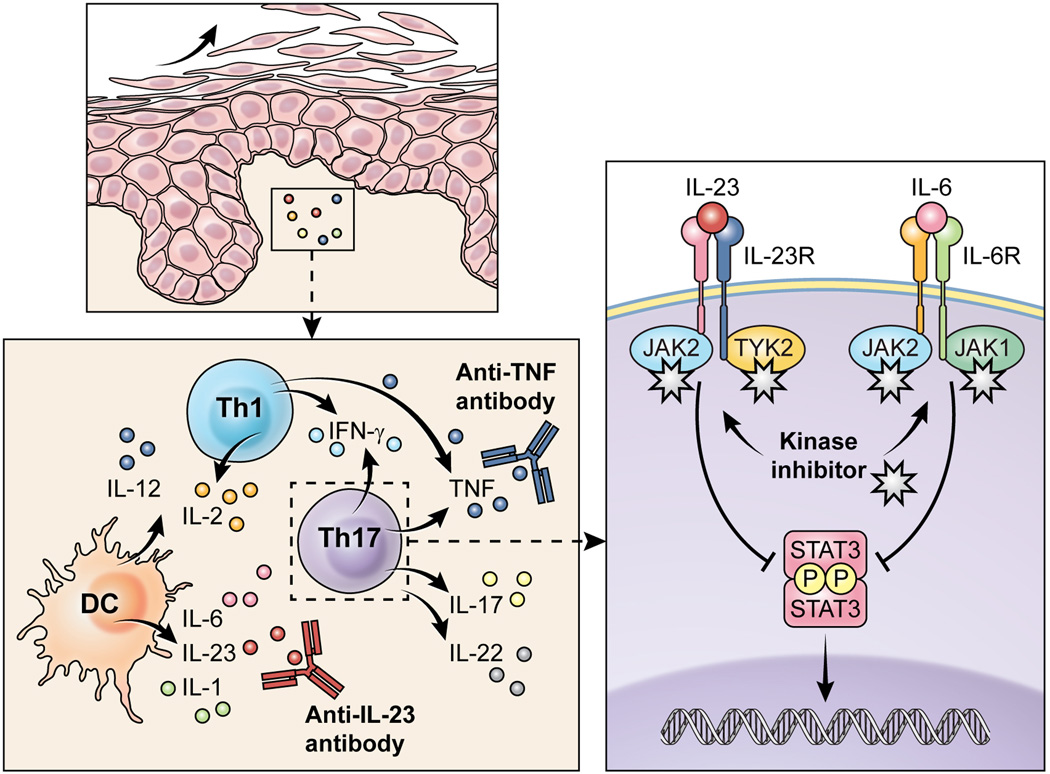
Surprisingly, mice lacking IFN-γ, the IFN-γ receptor or its downstream signaling element signal transducer and activator of transcription (STAT)1 exhibit exaggerated autoimmune inflammation (10). Furthermore, the Th1-promoting cytokines IFN-α and IFN-γ both exacerbate psoriasis whereas therapies with recombinant IL-4, IL-10 or IL-11 showed some clinical improvements (9, 11, 12). At the beginning of this millennium, a second CD4+ T cell population known as Th17, a T cell subset producing IL-17, IL-22 and TNF was recognized to play a major role. Th17 cells with potential to induce inflammatory pathology typically require signals from IL-23 (13, 14). High numbers of IL-17-expressing Th17 cells and IL-23-expressing dendritic cells (DC) are present in psoriatic skin (Figure 1) (15, 16). The knowledge on the underlying cytokine network in psoriasis allowed the establishment of therapies with biologics targeting TNF (17–19) or the IL-12/IL-23 p40 subunit (20). Targeting IL-17 or its receptor is being evaluated in phase III studies for psoriasis (21–23). In RA, biologics targeting TNF, IL-1 or the IL-6 receptor have been approved (2).
Figure 1. The critical role of cytokines in the pathogenesis of psoriasis: Targeted therapies inhibiting extracellular cytokine actions and the new approach of blocking intracellular cytokine signaling.
Immune sensors like dendritic cells release innate cytokines including IL-12, IL-1, IL-6 or IL-23 that drive the differentiation of T cells towards pathogenic Th1 and Th17 cells. Th1 cells produce IFN-γ, IL-2 and TNF, while Th17 cells express IL-17, IL-22, TNF and, in humans, IFN-γ. The resident tissue cells respond to the inflammatory cells and their secreted cytokines by uncontrolled proliferation. Biologics can neutralize single cytokines to dampen the inflammation. In contrast, Jak inhibitors interfere with cytokine signaling by blocking signal transmission in innate and adaptive immune cells, keratinocytes as well as endothelial cells.
The knowledge on the underlying cytokine network in inflammatory diseases allowed the establishment of therapies with biologics. Conversely, therapeutic targeting of single cytokines has also led to great advancements in the understanding of the distinctive roles cytokines play in autoimmune diseases. Nonetheless, these drugs have limitations. The endovenous administration, which most of these drugs require, is less than ideal. Not all patients show sufficient response and fulfill the expected treatment goals. Furthermore, these proteins harbor immunogenic potential which sometimes results in loss of response during continuous therapy (24). Notably, biologics with long half-life bear some risks during infections and may increase the risk of cancer and major cardiovascular events. Finally, their costs is beyond the means for some patients and health care systems.
Thus, development of alternative therapeutic strategies is desirable particularly for patients not responding to classical immunosuppressive drugs or targeted antibody therapies. Upon receptor binding, cytokines trigger signaling events involving several cytosolic substrates. These substrates, and their enzymatic activities, are ideal targets for the development of small molecules aiming at modulating cytokine-driven cellular responses.
In this viewpoint we discuss the arrival, in the clinical arena, of a new class of intracellular immunomodulators, which block kinases of the Janus family, better known as Jaks, which may re-revolutionize the treatment of autoimmune diseases and inflammatory disorders beyond biologics.
Cytokine signaling - moving from the cell surface to the inside
The diverse functions of cytokines in tissues are initiated by the binding of the cytokines to their binding to specific receptors and subsequent signal transmission. The subgroup of cytokines that uses type I and type II cytokine receptors comprises more than 60 members including hormone-like mediators, interleukins and IFNs. Among these, IL-6, IL-12, IL-19, IL-20, IL-21, IL-22 and IL-23 as well as IFNs are relevant in the pathogenesis of psoriasis, RA and many other inflammatory and autoimmune pathologies (25). Cytokine signaling is also important for the biology of non-immune cells like keratinocytes, fibroblasts, osteoblasts, synoviocytes or endothelial cells.
Upon binding of these cytokines to their specific type I or type II receptor, the receptor undergoes oligomerization. Since the receptor itself lacks intrinsic kinase activity, this oligomerization recruits Jaks which autophosphorlyate and then phosphorylate tyrosine residues within the receptor’s chains. This provides binding sites for STATs which bind and also become phosphorylated by Jaks. Activated STATs then dimerize and translocate into the nucleus where they regulate gene expression (Figure 1) Complexity in signaling in part is due to the fact that one or more members of the Jak family (Jak1, Jak2, Jak3, and Tyk2) and one or more members of STATs may be recruited by any specific receptor. For example, while receptors for hormone-like cytokines (erythropoietin (EPO), granulocyte macrophage colony-stimulating factor (GMCSF) use exclusively Jak2 (26), the type I IFNs use a combination of Jak1 and Tyk2, the IFN-γ receptor uses a combination of Jak1 and Jak2. The p40-sharing cytokines IL-12 and IL-23 activate receptors associating with a combination of Jak2 and Tyk2 (27). The group of γc-chain containing receptors, utilized by IL-2, IL-4, IL-7, IL-9, IL-15, and IL-21, use Jak1 and Jak3 (Table 1) (28–30).
Table 1.
Cytokines, their associated JAK/STAT proteins and their role in Th cell differentiation and function.
| Ligand (Cytokine) |
Associated Jak kinases |
Activated Stat proteins |
Important for differentiation of |
Effector cytokine secreted by |
|---|---|---|---|---|
| IL-2 | Jak1, Jak3 | Stat5, Stat3 | Treg, Th2 | Th1 |
| IL-4 | Jak1, Jak3 | Stat6 | Th2, Th9 | Th2 |
| IL-6 | Jak1, Jak2, Tyk2 | Stat3, Stat1 | Th17 | |
| IL-7 | Jak1, Jak3 | Stat5, Stat3 | ||
| IL-9 | Jak1, Jak3 | Stat5, Stat3 | Th9 | |
| IL-10 | Jak1, Tyk2 | Stat3, Stat1 | Treg, Th2, Th17 | |
| IL-12 | Jak2, Tyk2 | Stat4 | Th1 | |
| IL-13 | Jak1, Jak2, Tyk2 | Stat6 | Th2 | |
| IL-15 | Jak1, Jak3 | Stat5, Stat3 | ||
| IL-21 | Jak1, Jak3 | Stat3, Stat1 | Th17 | Th17 |
| IL-22 | Jak1, Tyk2 | Stat3, Stat1 | Th17, Th22 | |
| IL-23 | Jak2, Tyk2 | Stat3, Stat4 | Th17 | |
| IL-27 | Jak1, Jak2, Tyk2 | Stat1, Stat3 | Th1 | |
| IFNα/β | Jak1, Tyk2 | Stat1, Stat2 | Th1 | |
| IFN-γ | Jak1, Jak2 | Stat1 | Th1 | Th1 |
| EPO | Jak2 | Stat5 | ||
| GM-CSF | Jak2 | Stat3, Stat5 |
Inhibiting protein tyrosine kinases - a new old concept
Protein kinases like Jaks are enzymes, which transfer phosphate groups from adenosine triphosphate (ATP) or guanosine triphosphate to hydroxyl groups of amino acids of their substrates. Tyrosine kinases phosphorylate tyrosine residues and blocking their enzymatic activity has proved to be a successful strategy. Imatinib, a small, orally available, competitive molecule which blocks the ATP binding activity of the BCR-Abl tyrosine kinase, was developed for the treatment of patients with chronic myeloid leukemia (CML) (31). Imatinib has become a successful therapy in CML, demonstrating that the development of specific kinase inhibitors, which inhibit the activity of the cytokines involved in Th1/Th17-mediated diseases was clearly feasible.
Individuals with mutations within the JAK3 gene suffer from severe combined immunodeficiency, lacking T and NK and having reduced numbers of B cells (32, 33). These patients present with recurrent infections but do not have any other organ abnormalities. Thus, Jak3 was viewed as an excellent target for immunosuppressive therapies and resulted in the development of tofacitinib (CP-690,550). Preclinical trials with tofacitinib showed significant improvement in experimental models of autoimmunity and organ transplantation (34–36)
Currently, tofacitinib is approved in the USA, Japan, Switzerland, Russia, Argentina, Kuwait and the United Arab Emirates for the treatment of patients with RA. The European Medicines Agency (EMA) has yet to approve the marketing of tofacitinib in the European Union for RA. Clinical phase 2 and phase 3 trials showed efficacy in RA with American College of Rheumatology responses in patients failing disease-modifying antirheumatic drugs (DMARDs) or even TNF antagonists. Importantly, clinical responses were similar to those achieved with TNF antagonists (37–42). In psoriasis, oral tofacitinib showed a dose-dependent efficacy in phase 2 trials (43). Furthermore, tofacitinib demonstrated clinical effectiveness in a phase 2 study for ulcerative colitis (44). Adverse events such as leukopenia or lymphopenia were only observed in a subset of patients, arguing against a profound block of T or B cell homoeostasis. Moreover, neutropenia occurred rarely in tofacitinib-treated patients. Notably, anemia was documented in some patients (37–40, 45, 46). This adverse event could not be explained by selective Jak3 inhibition, since the erythropoietin receptor associates with Jak2 (26). In fact, when tofacitinib’s activity was carefully analyzed in vitro and in vivo, the results indicated that, although Jak3 was inhibited at nanomolar potency, other Jak family members especially Jak1, but also Jak2 and to a much lesser degree also Tyk2 were affected (35, 47). Thus, tofacitinib is a potent inhibitor of Jak3 and Jak1 with some activity against Jak2.
Targeting Jak1 or Jak2 was thought to be helpful for interfering with the signaling pathways implicated in the generation of pathogenic Th1 and Th17 cells in autoimmunity and inhibition of Jak2 was the initial intent, which resulted in the development of ruxolitinib (INCB018424). In 2005, independent groups identified the importance of a mutated Jak2 variant in myeloproliferative disorders (MPD) such as primary polycythemia vera (PV), essential thrombocythemia (ET) and primary myelofibrosis (48–51). These hematologic malignancies are characterized by excess proliferation of one or more myeloid lineages. The most common cause is a point mutation (JAK2V617F) present in 95% of patients with PV and over 50% of patients with ET and PMF.
Jak2 inhibitors like ruxolitinib SB1518, SAR302503 and lestaurtinib can interfere with the autophosphorylation of Jak2 in JAK2V617F-positive cells. In patients with primary myelofibrosis, ruxolitinib demonstrated significant clinical benefits reducing spleen size, myelofibroisis-related symptoms and prolonging overall survival (52–54). As described for tofacitinib, the selectivity of these inhibitors for Jak2 was analyzed in detail. Ruxolitinib has been reported to inhibit both Jak2 and Jak1; nonetheless, the toxicity of ruxolitinib in humans seems to be limited. As expected, Jak2 inhibition frequently resulted in anemia and thrombocytopenia. As a Jak1/Jak2 inhibitor, ruxolitinib became the first Jak inhibitor approved by the Food and Drug Administration and EMA with therapeutic indication for the treatment of intermediate or high-risk myelofibrosis (55). In several preclinical studies, ruxolitinib showed efficacy in rodent models of arthritis and in animal models of skin inflammation (56, 57), and, therefore is also under clinical evaluation for RA and psoriasis.
To be or not to be… selective?
Imatinib was designed and thought to be a selective BCR-Abl inhibitor. In reality it blocks multiple kinases (47) and this off-target activity allowed the use of imatinib in other diseases (58). Similarly, most of the Jak inhibitors developed so far are selective for these kinases, but, as mentioned above, do not discriminate well among the Jak family members (47, 58). The Jak inhibitors approved so far target at least two Jaks with nanomolar potency. Such non-specificity may indeed explain their efficacy in Th1/Th17-mediated autoimmunity and pathologies. Such promiscuity in inhibition leads often to concerns of toxicity, however, although not yet assessed in long-term studies, the toxicity of the Jak inhibitors seems to be limited. The limited toxicity observed so far could be due to the rapid kinetics of action of such compounds. It is likely that Jak inhibitors lower the degree of STAT activation in vivo without causing permanent or total blockade of this pathway. Furthermore, cytokines have in immune cells their major targets and therefore Jak inhibitors could preferentially act on activated immune cells.
The generation of highly selective inhibitors, with no off-target activity against other Jaks, may result in increased efficacy and safety, as some of the anti-inflammatory cytokines could be spared. For instance, a selective Tyk2 inhibitor would target the signals of IL-12 and IL-23 and probably mimic, at least to some extent, the therapeutic effects of an anti-p40 antibody.
Immunological mode of action of Jak inhibitors
Tofacitinib and ruxolitinib both inhibit STAT3 phosphorylation (Table 1) (36, 56). In the case of tofacitinib, it was predicted that this Jak3/Jak1 inhibitor would block IL-17 production. Yet, surprisingly, when naïve Th cells were treated with the Th17-promoting cytokines, a combination of IL-6 and TGF-β, in the presence of tofacitinib, IL-17 production was dramatically enhanced (36). This observation can be explained by the opposing roles that STAT3 and STAT5 have on Th17 differentiation (59). STAT5 inhibition by tofacitinib enhances IL-17 expression. In contrast, in the absence of TGF-β, tofacitinib is a potent inhibitor of Th17 cells (36). Tofacitinib limits the production of IL-17A, IL-17F and IL-22, expression of the IL-23R as well as differentiation of Th1 cells. Other Jak1 inhibitors have similar effects on pathogenic Th1 and Th17 responses. Ruxolitinib can reduce the expression of the Th1 cytokine IFN-γ and the Th17 cytokines IL-17, IL-21 and IL-22 in a rodent model of arthritis (56). In patients with myelofibrosis, ruxolitinib decreased circulating inflammatory cytokines like IL-6 and TNF (52).
However, additional actions on other cell types of the immune system like macrophages, NK and B cells may contribute to the antirheumatic and antipsoriatic activity of Jak inhibitors. Decreased numbers of NK cells were observed in tofacitinib-treated individuals (45). Surprisingly, tofacitinib can even modulate innate immune responses as demonstrated in a model of LPS-induced endotoxemic shock (36).
Lowering both innate and adaptive immune responses seems to be advantageous for the efficacy of oral immunomodulating compounds in psoriasis and arthritis (60, 61). Some of the immunological effects observed when using Jak inhibitors resemble the effects when inhibiting the IL-6 receptor or p40. Yet, only highly selective inhibitors for single Jaks or STAT3-targeting compounds may allow decipher the relevance of each signaling molecule.
Jak inhibitors in dermatology
As mentioned above, oral tofacitinib is already in phase 3 for the treatment of patients with psoriasis. Baricitinib (also known as LY3009104) and the Jak1 inhibitor GSK2586184 are both in phase 2 (Table 2). Interestingly, both orally approved Jak inhibitors tofacitinib and ruxolitinib are also studied as topical formulations in psoriasis (62, 63). The data on efficacy and safety of topical Jak inhibitors formulations are important since these therapeutics could revolutionize the treatment modalities in dermatology. Topical treatment of skin inflammation is mainly done with corticosteroids and their continuous use can result in skin atrophy and teleangiectasia. So far, only a few immunomodulating ointments are available like topical vitamin D derivatives for psoriasis (64) and topical calcineurin inhibitors for atopic dermatitis (65).
Table 2.
Clinical stage of some Jak inhibitors in inflammatory autoimmune diseases and myeloproliferative disorders
| Inhibitor | Primary targets |
Indications and developmental stage |
|---|---|---|
| Tofacitinib (Xeljanz®) | Jak1, Jak3 | PSO (phase 3), RA*, UC (phase 2) |
| Ruxolitinib (Jakafi®) | Jak1, Jak2 | PSO (phase 2), RA (phase 2), PMF#, PV and ET (phase 3) |
| Baricitinib | Jak1, Jak2 | PSO (phase 2), RA (phase 3), |
| R333 and R348 | Jak3, Syk | LUPUS (phase 2), DED (phase 1) |
| SAR302503 | Jak2 | PMF (phase 2), PV and ET (phase 2) |
| VX-509 | Jak3 | RA (phase 2) |
| GLPG0634 | Jak1 | RA (phase 2) |
| GSK2586184 | Jak1 | PSO (phase 2), LUPUS (phase 2) |
| Lestaurtinib | Jak2, Flt3 | PSO (Phase 2), PMF (phase 2), PV and ET (phase 2) |
| CYT387 | Jak1, Jak2 | PMF (phase 2), PV and ET (phase 2) |
| AZD 1480 | Jak1, Jak2 | PMF, PV and ET (phase 2) |
| Pacritinib | Jak2 | PMF (phase 2) |
| AC430 | Jak2 | RA (phase 1) |
RA, rheumatoid arthritis; PSO, psoriasis; PMF, primary myelofibrosis; ET, essential thrombocythemia; PV, polycythemia vera; LUPUS, lupus erythematosus; DED, drye eye disease;
approved in US and Japan;
approved in US and EU
Interestingly, both compounds also affect keratinocyte biology. In psoriatic skin, IL-22-mediated STAT3 activation in keratinocytes results in acanthosis (66). Topical Jak inhibitors could directly dampen STAT3 activation in keratinocytes and affect the expression of numerous genes implicated in epidermal differentiation (67, 68). The anti-inflammatory and anti-proliferative potential of topical Jak inhibitors, if accompanied with limited side effects, could be helpful in a number of skin diseases like psoriasis, contact dermatitis, atopic dermatitis, lupus erythematosus, lichen planus and alopecia.
Conclusions
Given the success of tofacitinib and ruxolitinib, several other compounds are in various phases of development (Table 2). Inhibitors currently in trials for RA include baricitinib (Jak1/Jak2), VX-509 (Jak3), GLPG0634 (Jak1) and AC430 (Jak2). Safety and efficacy in the setting of myelofibrosis was also recently demonstrated for the presumably more selective Jak2 inhibitor SAR302503 (69). Other Jak inhibitors tested for myeloproliferative disorders include AZD1480 and CYT387.
It has been 20 years since we started to understand signaling downstream of cytokine receptors. Importantly we can now manipulate cytokine activity and limit their actions by inhibiting proximal cytoplasmic molecules like the Jaks. So far, the clinical results are promising and we believe that with newer and more specific inhibitors safety and efficacy will improve. The biologics were a huge clinical success and we may be on the brink of another therapeutic revolution. We may, once again… hit the Jakpot!
Acknowledgements
This work was supported by the Deutsche Forschungsgemeinschaft (DFG) Sonderforschungsbereich 685 (KG) and by the NIAMS Intramural Research Program of (MG).
Footnotes
Author contributions
Kamran Ghoreschi and Massimo Gadina wrote the manuscript, provided intellectual input, critically reviewed and approved the final version of the manuscript.
Conflict of interests
Kamran Ghoreschi has been a consultant or investigator for Pfizer, Eli Lilly and Company, Abbott, Biogen Idec, Celgene, Janssen-Cilag, MSD Sharp & Dohme and Novartis Pharmaceuticals. The U.S. National Institutes of Health holds a patent related to Janus family kinases and identification of immune modulators, and has a Collaborative Research Agreement and Development Award with Pfizer.
References
- 1.Nestle FO, Kaplan DH, Barker J. Psoriasis. The New England journal of medicine. 2009;361:496–509. doi: 10.1056/NEJMra0804595. [DOI] [PubMed] [Google Scholar]
- 2.McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. The New England journal of medicine. 2011;365:2205–2219. doi: 10.1056/NEJMra1004965. [DOI] [PubMed] [Google Scholar]
- 3.Ghoreschi K, Weigert C, Rocken M. Immunopathogenesis and role of T cells in psoriasis. Clin Dermatol. 2007;25:574–580. doi: 10.1016/j.clindermatol.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 4.Feldmann M, Maini SR. Role of cytokines in rheumatoid arthritis: an education in pathophysiology and therapeutics. Immunological reviews. 2008;223:7–19. doi: 10.1111/j.1600-065X.2008.00626.x. [DOI] [PubMed] [Google Scholar]
- 5.Strober W, Fuss IJ. Proinflammatory cytokines in the pathogenesis of inflammatory bowel diseases. Gastroenterology. 2011;140:1756–1767. doi: 10.1053/j.gastro.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nicolas JF, Chamchick N, Thivolet J, Wijdenes J, Morel P, Revillard JP. CD4 antibody treatment of severe psoriasis. Lancet. 1991;338:321. doi: 10.1016/0140-6736(91)90465-2. [DOI] [PubMed] [Google Scholar]
- 7.Prinz J, Braun-Falco O, Meurer M, et al. Chimaeric CD4 monoclonal antibody in treatment of generalised pustular psoriasis. Lancet. 1991;338:320–321. doi: 10.1016/0140-6736(91)90464-z. [DOI] [PubMed] [Google Scholar]
- 8.Schlaak JF, Buslau M, Jochum W, et al. T cells involved in psoriasis vulgaris belong to the Th1 subset. J Invest Dermatol. 1994;102:145–149. doi: 10.1111/1523-1747.ep12371752. [DOI] [PubMed] [Google Scholar]
- 9.Ghoreschi K, Thomas P, Breit S, et al. Interleukin-4 therapy of psoriasis induces Th2 responses and improves human autoimmune disease. Nature medicine. 2003;9:40–46. doi: 10.1038/nm804. [DOI] [PubMed] [Google Scholar]
- 10.Bettelli E, Sullivan B, Szabo SJ, Sobel RA, Glimcher LH, Kuchroo VK. Loss of T-bet, but not STAT1, prevents the development of experimental autoimmune encephalomyelitis. The Journal of experimental medicine. 2004;200:79–87. doi: 10.1084/jem.20031819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Asadullah K, Sterry W, Stephanek K, et al. IL-10 is a key cytokine in psoriasis. Proof of principle by IL-10 therapy: a new therapeutic approach. J Clin Invest. 1998;101:783–794. doi: 10.1172/JCI1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trepicchio WL, Ozawa M, Walters IB, et al. Interleukin-11 therapy selectively downregulates type I cytokine proinflammatory pathways in psoriasis lesions. J Clin Invest. 1999;104:1527–1537. doi: 10.1172/JCI6910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McGeachy MJ, Bak-Jensen KS, Chen Y, et al. TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat Immunol. 2007;8:1390–1397. doi: 10.1038/ni1539. [DOI] [PubMed] [Google Scholar]
- 14.Ghoreschi K, Laurence A, Yang XP, et al. Generation of pathogenic T(H)17 cells in the absence of TGF-beta signalling. Nature. 2010;467:967–971. doi: 10.1038/nature09447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee E, Trepicchio WL, Oestreicher JL, et al. Increased expression of interleukin 23 p19 and p40 in lesional skin of patients with psoriasis vulgaris. The Journal of experimental medicine. 2004;199:125–130. doi: 10.1084/jem.20030451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilson NJ, Boniface K, Chan JR, et al. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol. 2007;8:950–957. doi: 10.1038/ni1497. [DOI] [PubMed] [Google Scholar]
- 17.Mease PJ, Goffe BS, Metz J, VanderStoep A, Finck B, Burge DJ. Etanercept in the treatment of psoriatic arthritis and psoriasis: a randomised trial. Lancet. 2000;356:385–390. doi: 10.1016/S0140-6736(00)02530-7. [DOI] [PubMed] [Google Scholar]
- 18.Chaudhari U, Romano P, Mulcahy LD, Dooley LT, Baker DG, Gottlieb AB. Efficacy and safety of infliximab monotherapy for plaque-type psoriasis: a randomised trial. Lancet. 2001;357:1842–1847. doi: 10.1016/s0140-6736(00)04954-0. [DOI] [PubMed] [Google Scholar]
- 19.Gordon KB, Langley RG, Leonardi C, et al. Clinical response to adalimumab treatment in patients with moderate to severe psoriasis: double-blind, randomized controlled trial and open-label extension study. J Am Acad Dermatol. 2006;55:598–606. doi: 10.1016/j.jaad.2006.05.027. [DOI] [PubMed] [Google Scholar]
- 20.Leonardi CL, Kimball AB, Papp KA, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1) Lancet. 2008;371:1665–1674. doi: 10.1016/S0140-6736(08)60725-4. [DOI] [PubMed] [Google Scholar]
- 21.Hueber W, Patel DD, Dryja T, et al. Effects of AIN457, a fully human antibody to interleukin-17A, on psoriasis, rheumatoid arthritis, and uveitis. Sci Transl Med. 2010;2:52ra72. doi: 10.1126/scitranslmed.3001107. [DOI] [PubMed] [Google Scholar]
- 22.Leonardi C, Matheson R, Zachariae C, et al. Anti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. The New England journal of medicine. 2012;366:1190–1199. doi: 10.1056/NEJMoa1109997. [DOI] [PubMed] [Google Scholar]
- 23.Papp KA, Leonardi C, Menter A, et al. Brodalumab, an anti-interleukin-17-receptor antibody for psoriasis. The New England journal of medicine. 2012;366:1181–1189. doi: 10.1056/NEJMoa1109017. [DOI] [PubMed] [Google Scholar]
- 24.Garces S, Demengeot J, Benito-Garcia E. The immunogenicity of anti-TNF therapy in immune-mediated inflammatory diseases: a systematic review of the literature with a meta-analysis. Ann Rheum Dis. 2012 doi: 10.1136/annrheumdis-2012-202220. [DOI] [PubMed] [Google Scholar]
- 25.Ujiie H, Shimizu H. Evidence for pathogenicity of autoreactive T cells in autoimmune bullous diseases shown by animal disease models. Exp Dermatol. 2012;21:901–905. doi: 10.1111/exd.12011. [DOI] [PubMed] [Google Scholar]
- 26.Parganas E, Wang D, Stravopodis D, et al. Jak2 is essential for signaling through a variety of cytokine receptors. Cell. 1998;93:385–395. doi: 10.1016/s0092-8674(00)81167-8. [DOI] [PubMed] [Google Scholar]
- 27.Muller M, Briscoe J, Laxton C, et al. The protein tyrosine kinase JAK1 complements defects in interferon-alpha/beta and -gamma signal transduction. Nature. 1993;366:129–135. doi: 10.1038/366129a0. [DOI] [PubMed] [Google Scholar]
- 28.Johnston JA, Kawamura M, Kirken RA, et al. Phosphorylation and activation of the Jak-3 Janus kinase in response to interleukin-2. Nature. 1994;370:151–153. doi: 10.1038/370151a0. [DOI] [PubMed] [Google Scholar]
- 29.Ghoreschi K, Laurence A, O'Shea JJ. Janus kinases in immune cell signaling. Immunological reviews. 2009;228:273–287. doi: 10.1111/j.1600-065X.2008.00754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O'Shea JJ, Gadina M, Schreiber RD. Cytokine signaling in 2002: new surprises in the Jak/Stat pathway. Cell. 2002;109(Suppl):S121–S131. doi: 10.1016/s0092-8674(02)00701-8. [DOI] [PubMed] [Google Scholar]
- 31.Druker BJ, Tamura S, Buchdunger E, et al. Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr-Abl positive cells. Nature medicine. 1996;2:561–566. doi: 10.1038/nm0596-561. [DOI] [PubMed] [Google Scholar]
- 32.Macchi P, Villa A, Giliani S, et al. Mutations of Jak-3 gene in patients with autosomal severe combined immune deficiency (SCID) Nature. 1995;377:65–68. doi: 10.1038/377065a0. [DOI] [PubMed] [Google Scholar]
- 33.Pesu M, Candotti F, Husa M, Hofmann SR, Notarangelo LD, O'Shea JJ. Jak3, severe combined immunodeficiency, and a new class of immunosuppressive drugs. Immunological reviews. 2005;203:127–142. doi: 10.1111/j.0105-2896.2005.00220.x. [DOI] [PubMed] [Google Scholar]
- 34.Changelian PS, Flanagan ME, Ball DJ, et al. Prevention of organ allograft rejection by a specific Janus kinase 3 inhibitor. Science. 2003;302:875–878. doi: 10.1126/science.1087061. [DOI] [PubMed] [Google Scholar]
- 35.Meyer DM, Jesson MI, Li X, et al. Anti-inflammatory activity and neutrophil reductions mediated by the JAK1/JAK3 inhibitor, CP-690,550, in rat adjuvant-induced arthritis. J Inflamm (Lond) 7:41. doi: 10.1186/1476-9255-7-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ghoreschi K, Jesson MI, Li X, et al. Modulation of Innate and Adaptive Immune Responses by Tofacitinib (CP-690,550) Journal of immunology. 186:4234–4243. doi: 10.4049/jimmunol.1003668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kremer JM, Bloom BJ, Breedveld FC, et al. The safety and efficacy of a JAK inhibitor in patients with active rheumatoid arthritis: Results of a double-blind, placebo-controlled phase IIa trial of three dosage levels of CP-690,550 versus placebo. Arthritis and rheumatism. 2009;60:1895–1905. doi: 10.1002/art.24567. [DOI] [PubMed] [Google Scholar]
- 38.Fleischmann R, Cutolo M, Genovese MC, et al. Phase IIb dose-ranging study of the oral JAK inhibitor tofacitinib (CP-690,550) or adalimumab monotherapy versus placebo in patients with active rheumatoid arthritis with an inadequate response to disease-modifying antirheumatic drugs. Arthritis and rheumatism. 2012;64:617–629. doi: 10.1002/art.33383. [DOI] [PubMed] [Google Scholar]
- 39.Fleischmann R, Kremer J, Cush J, et al. Placebo-controlled trial of tofacitinib monotherapy in rheumatoid arthritis. The New England journal of medicine. 2012;367:495–507. doi: 10.1056/NEJMoa1109071. [DOI] [PubMed] [Google Scholar]
- 40.van Vollenhoven RF, Fleischmann R, Cohen S, et al. Tofacitinib or adalimumab versus placebo in rheumatoid arthritis. The New England journal of medicine. 2012;367:508–519. doi: 10.1056/NEJMoa1112072. [DOI] [PubMed] [Google Scholar]
- 41.Burmester GR, Blanco R, Charles-Schoeman C, et al. Tofacitinib (CP-690,550) in combination with methotrexate in patients with active rheumatoid arthritis with an inadequate response to tumour necrosis factor inhibitors: a randomised phase 3 trial. Lancet. 2013;381:451–460. doi: 10.1016/S0140-6736(12)61424-X. [DOI] [PubMed] [Google Scholar]
- 42.van der Heijde D, Tanaka Y, Fleischmann R, et al. Tofacitinib (CP-690,550) in patients with rheumatoid arthritis receiving methotrexate: twelve-month data from a twenty-four-month phase III randomized radiographic study. Arthritis and rheumatism. 2013;65:559–570. doi: 10.1002/art.37816. [DOI] [PubMed] [Google Scholar]
- 43.Papp KA, Menter A, Strober B, et al. Efficacy and safety of tofacitinib, an oral Janus kinase inhibitor, in the treatment of psoriasis: a Phase 2b randomized placebo-controlled dose-ranging study. Br J Dermatol. 2012;167:668–677. doi: 10.1111/j.1365-2133.2012.11168.x. [DOI] [PubMed] [Google Scholar]
- 44.Sandborn WJ, Ghosh S, Panes J, et al. Tofacitinib, an oral Janus kinase inhibitor, in active ulcerative colitis. The New England journal of medicine. 2012;367:616–624. doi: 10.1056/NEJMoa1112168. [DOI] [PubMed] [Google Scholar]
- 45.van Gurp E, Weimar W, Gaston R, et al. Phase 1 dose-escalation study of CP-690 550 in stable renal allograft recipients: preliminary findings of safety, tolerability, effects on lymphocyte subsets and pharmacokinetics. Am J Transplant. 2008;8:1711–1718. doi: 10.1111/j.1600-6143.2008.02307.x. [DOI] [PubMed] [Google Scholar]
- 46.Strober B, Buonanno M, Clark JD, et al. Effect of tofacitinib, a Janus kinase inhibitor, on haematological parameters during 12 weeks of psoriasis treatment. Br J Dermatol. 2013 doi: 10.1111/bjd.12517. [DOI] [PubMed] [Google Scholar]
- 47.Karaman MW, Herrgard S, Treiber DK, et al. A quantitative analysis of kinase inhibitor selectivity. Nat Biotechnol. 2008;26:127–132. doi: 10.1038/nbt1358. [DOI] [PubMed] [Google Scholar]
- 48.Baxter EJ, Scott LM, Campbell PJ, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365:1054–1061. doi: 10.1016/S0140-6736(05)71142-9. [DOI] [PubMed] [Google Scholar]
- 49.Levine RL, Wadleigh M, Cools J, et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell. 2005;7:387–397. doi: 10.1016/j.ccr.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 50.James C, Ugo V, Le Couedic JP, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005;434:1144–1148. doi: 10.1038/nature03546. [DOI] [PubMed] [Google Scholar]
- 51.Kralovics R, Passamonti F, Buser AS, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. The New England journal of medicine. 2005;352:1779–1790. doi: 10.1056/NEJMoa051113. [DOI] [PubMed] [Google Scholar]
- 52.Verstovsek S, Kantarjian H, Mesa RA, et al. Safety and efficacy of INCB018424, a JAK1 and JAK2 inhibitor, in myelofibrosis. The New England journal of medicine. 363:1117–1127. doi: 10.1056/NEJMoa1002028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Harrison C, Kiladjian JJ, Al-Ali HK, et al. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. The New England journal of medicine. 2012;366:787–798. doi: 10.1056/NEJMoa1110556. [DOI] [PubMed] [Google Scholar]
- 54.Verstovsek S, Mesa RA, Gotlib J, et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. The New England journal of medicine. 2012;366:799–807. doi: 10.1056/NEJMoa1110557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Verstovsek S, Mesa RA, Gotlib J, et al. The clinical benefit of ruxolitinib across patient subgroups: analysis of a placebo-controlled, Phase III study in patients with myelofibrosis. Br J Haematol. 2013;161:508–516. doi: 10.1111/bjh.12274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fridman JS, Scherle PA, Collins R, et al. Selective inhibition of JAK1 and JAK2 is efficacious in rodent models of arthritis: preclinical characterization of INCB028050. Journal of immunology. 184:5298–5307. doi: 10.4049/jimmunol.0902819. [DOI] [PubMed] [Google Scholar]
- 57.Fridman JS, Scherle PA, Collins R, et al. Preclinical evaluation of local JAK1 and JAK2 inhibition in cutaneous inflammation. J Invest Dermatol. 2011;131:1838–1844. doi: 10.1038/jid.2011.140. [DOI] [PubMed] [Google Scholar]
- 58.Ghoreschi K, Laurence A, O'Shea JJ. Selectivity and therapeutic inhibition of kinases: to be or not to be? Nat Immunol. 2009;10:356–360. doi: 10.1038/ni.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang XP, Ghoreschi K, Steward-Tharp SM, et al. Opposing regulation of the locus encoding IL-17 through direct, reciprocal actions of STAT3 and STAT5. Nat Immunol. 2011;12:247–254. doi: 10.1038/ni.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ghoreschi K, Bruck J, Kellerer C, et al. Fumarates improve psoriasis and multiple sclerosis by inducing type II dendritic cells. The Journal of experimental medicine. 2011;208:2291–2303. doi: 10.1084/jem.20100977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McCann FE, Palfreeman AC, Andrews M, et al. Apremilast, a novel PDE4 inhibitor, inhibits spontaneous production of tumour necrosis factor-alpha from human rheumatoid synovial cells and ameliorates experimental arthritis. Arthritis research & therapy. 2010;12:R107. doi: 10.1186/ar3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ports WC, Khan S, Lan S, et al. A randomised Phase 2a efficacy and safety trial of the topical Janus kinase inhibitor tofacitinib in the treatment of chronic plaque psoriasis. Br J Dermatol. 2013 doi: 10.1111/bjd.12266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Punwani N, Scherle P, Flores R, et al. Preliminary clinical activity of a topical JAK1/2 inhibitor in the treatment of psoriasis. J Am Acad Dermatol. 2012;67:658–664. doi: 10.1016/j.jaad.2011.12.018. [DOI] [PubMed] [Google Scholar]
- 64.Kragballe K, Gjertsen BT, De Hoop D, et al. Double-blind, right/left comparison of calcipotriol and betamethasone valerate in treatment of psoriasis vulgaris. Lancet. 1991;337:193–196. doi: 10.1016/0140-6736(91)92157-w. [DOI] [PubMed] [Google Scholar]
- 65.Ruzicka T, Bieber T, Schopf E, et al. A short-term trial of tacrolimus ointment for atopic dermatitis. European Tacrolimus Multicenter Atopic Dermatitis Study Group. The New England journal of medicine. 1997;337:816–821. doi: 10.1056/NEJM199709183371203. [DOI] [PubMed] [Google Scholar]
- 66.Zheng Y, Danilenko DM, Valdez P, et al. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445:648–651. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
- 67.Mattiuzzo NR, Toulza E, Jonca N, Serre G, Guerrin M. A large-scale multi-technique approach identifies forty-nine new players of keratinocyte terminal differentiation in human epidermis. Exp Dermatol. 2011;20:113–118. doi: 10.1111/j.1600-0625.2010.01188.x. [DOI] [PubMed] [Google Scholar]
- 68.Terazawa S, Nakajima H, Shingo M, Niwano T, Imokawa G. Astaxanthin attenuates the UVB-induced secretion of prostaglandin E2 and interleukin-8 in human keratinocytes by interrupting MSK1 phosphorylation in a ROS depletion-independent manner. Exp Dermatol. 2012;21(Suppl 1):11–17. doi: 10.1111/j.1600-0625.2012.01496.x. [DOI] [PubMed] [Google Scholar]
- 69.Pardanani A, Gotlib JR, Jamieson C, et al. Safety and efficacy of TG101348, a selective JAK2 inhibitor, in myelofibrosis. J Clin Oncol. 29:789–796. doi: 10.1200/JCO.2010.32.8021. [DOI] [PMC free article] [PubMed] [Google Scholar]