Abstract
Background
The mTOR is an important regulator of HSCs self-renewal and its overactivation contributes to HSCs premature exhaustion in part via induction of HSCs senescence. Inhibition of mTOR with rapamycin has the potential to promote long term hematopoiesis of ex vivo expanded HSCs to facilitate the clinical application of HSCs transplantation for various hematological diseases.
Methods
A well-established ex vivo expansion system for mouse bone marrow HSCs was utilized to investigate whether inhibition of overactivated mTOR with rapamycin can promote long term hematopoiesis of ex vivo expanded HSCs and to elucidate the mechanisms of action of rapamycin.
Results
HSCs-enriched mouse bone marrow LSK cells exhibited a time-dependent activation of mTOR after ex vivo expansion in a serum-free medium supplemented with SCF, TPO and FL. The overactivation of mTOR was associated with induction of senescence but not apoptosis in LSK cells and a significant reduction in the ability of HSCs to produce long-term hematopoietic reconstitution. Inhibition of overactivated mTOR with rapamycin promoted ex vivo expansion and long term hematopoietic reconstitution of HSCs. The increase in long term hematopoiesis of expanded HSCs is likely attributable in part to rapamycin-mediated upregulation of Bmi1 and downregulation of p16, which prevent HSCs from undergoing senescence during ex vivo expansion.
Conclusions
These findings suggest that mTOR plays an important role in the regulation of HSCs self-renewal in vitro and inhibition of mTOR hyperactivation with rapamycin may represent a novel approach to promote ex vivo expansion and their long term hematopoietic reconstitution of HSCs.
Keywords: hematopoietic stem cells, ex vivo expansion, long term hematopoietic reconstitution, mTOR, rapamycin, senescence
INTRODUCTION
Hematopoietic stem cell (HSC) transplantation is an effective treatment and sometime the only cure for many hematological disorders. Unfortunately, its therapeutic potential has not been fulfilled because of lacking of a suitable donor or insufficient numbers of HSCs for transplantation (1, 2). Ex vivo expansion of HSCs could potentially generate ample HSCs to overcome these obstacles. So far, moderate ex vivo expansion of HSCs has been achieved by incubation of HSCs with various hematopoietic growth factors, cytokines, Notch ligands, Wnt3a, or angiopoietin-like protein (3–6). Coculture of HSCs with bone marrow stromal cells and endothelial cells also increases expansion of HSCs (7, 8). In addition, ectopic expression of various transcription factors such as HoxB4 by gene transfection can induce robust expansions of HSCs in vitro (9). However, these methods have limited utility in clinical practice because of the concerns about the 1) high costs of hematopoietic growth factors, 2) difficulty in standardizing stromal elements to meet FDA regulations, and 3) risks of HSC transformation by gene transfection. In addition, ex vivo expansion of HSCs usually occurs at the expense of HSC self-renewal, which leads to a significant reduction in the ability of the expanded HSCs to produce long-term hematopoietic reconstitution after transplantation (10). Therefore, increasing efforts have been devoted to identify small molecules that can help to overcome the shortcomings of these existing methods. Our recent studies showed that ex vivo expansion of both mouse bone marrow and human cord blood HSCs activated p38 (10, 11). Activation of p38 was associated with a significant increase in apoptosis and cellular senescence in HSCs and their progeny. Inhibition of p38 with a specific inhibitor can promote HSCs ex vivo expansion while preserving HSCs long-term hematopoietic activity. These findings encouraged us to expand our study to uncover other molecular pathways that could be activated to inhibit HSCs self-renewal during ex vivo expansion and thus, potentially be targeted by a small molecule inhibitor to promote ex vivo expansion and long term hematopoietic reconstitution of HSCs.
The mTOR, a member of the family of PI3K-related kinases, is a central regulator of cellular response to stress and changes in environmental cues, such as changes in nutrients, oxygen tension, and growth factor stimulation (12). It has also emerged as an important regulator for HSCs self-renewal. Activation of mTOR has been found in HSCs during aging or under various pathological conditions such as deletion of the genes encoding PTEN, TSC1 and glycogen synthase kinase 3 (GSK3) (13–15). This activation contributes to premature exhaustion of HSCs in part via induction of apoptosis and senescence, while inhibition of mTOR with rapamycin has been shown to prevent premature exhaustion of HSCs caused by the genetic deletion of Pten, Tsc1 or Gsk3 in mice and to rejuvenate aging HSCs to extend the lifespan of old mice (13–15).
During ex vivo expansion, HSCs are subjected to a variety of stressors, including increases in oxygen tension, fluctuations in various nutrients and growth factor concentrations, and accumulation of toxic metabolites (16). Any of these stressors may cause hyperactivation of mTOR to inhibit HSCs self-renewal and expansion ex vivo. Therefore, we hypothesized that rapamycin has the potential to be used as a small molecule enhancer to promote HSCs ex vivo expansion. The present study was designed to test this hypothesis using our well-established ex vivo expansion system for mouse bone marrow HSCs.
The results from our study showed that mouse bone marrow LSK cells enriched with HSCs exhibited a time-dependent activation of mTOR after culture in a serum-free medium supplemented with SCF, TPO and FL. The activation of mTOR was associated with a significant reduction in HSCs activity and induction of senescence in LSK cells. Addition of rapamycin to the culture inhibited the activation of mTOR in LSK cells, which led to promotion in ex vivo expansion and long term hematopoietic reconstitution of HSCs as shown by the CAFC and competitive repopulation assays. The promotion in HSCs expansion is likely attributable in part to rapamycin-mediated upregulation of Bmi1 and downregulation of p16, which prevent HSCs from undergoing senescence during ex vivo expansion. These findings suggest that mTOR plays an important role in the regulation of HSCs self-renewal in vitro and inhibition of overactivated mTOR with rapamycin may represent a novel approach to promote hematopoiesis of ex vivo expanded HSCs.
RESULTS
Ex vivo expansion of HSCs activates mTOR in a time-dependent manner
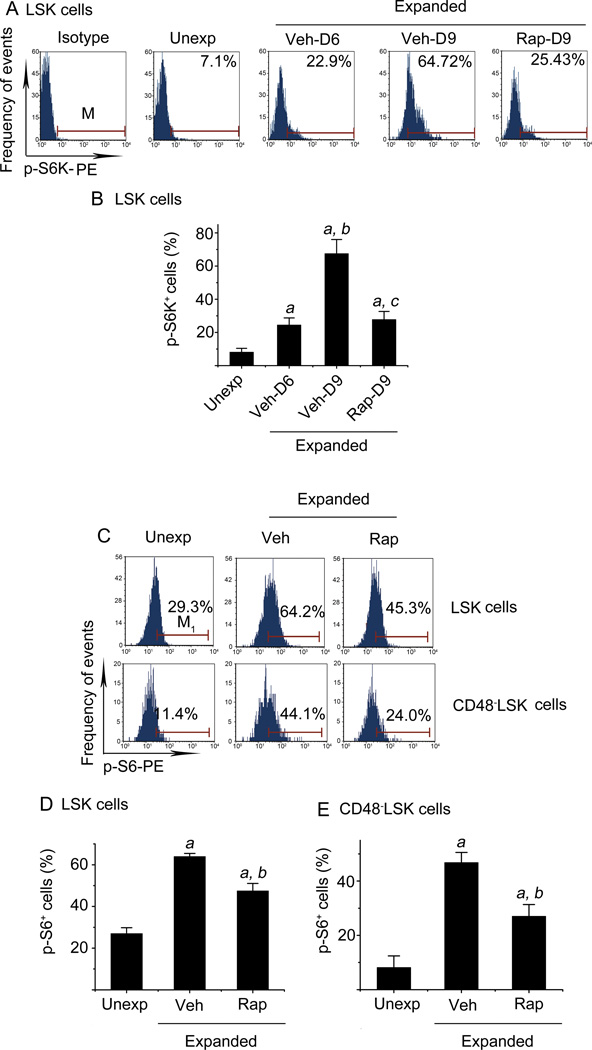
S6K1 is a direct substrate of mTOR complex 1 (mTORC1) that consists of mTOR and four other components (12). Its phosphorylation has been widely used as an indicator of mTOR activation. To determine whether ex vivo expansion of HSCs activates the mTOR pathway, we analyzed S6K1 phosphorylation in freshly isolated LSK cells and those harvested from HSCs ex vivo expansion cultures at various times by the phosphor-flow cytometric method. As shown in Figure 1 A and B, a small percentage (8.19% ± 2.2%) of unexpanded LSK cells showed low levels of S6K1 phosphorylation, indicating limited activation of the mTOR pathway. The percentage of LSK cells stained positive for phosphorylated S6K1 (p-S6K1) increased to 24.55% ± 4.2% and 67.51% ± 8.5% after the cells were cultured in vitro for 6 and 9 days with vehicle, respectively. Rapamycin treatment (20 ng/ml) between day 6 and day 9 of the culture resulted in a significant reduction in positive p-S6K1 staining in LSK cells compared to vehicle-treated cells (vehicle: 67.51% ± 8.5% vs. rapamycin: 27.85% ± 4.8%) (Figure 1 A & B). This finding suggests that ex vivo expansion of HSCs activates mTOR in LSK cells in a time-dependent manner and the activation can be inhibited by rapamycin. This suggestion is further supported by the observations that the phosphorylation of S6, a substrate of S6K, was also increased in LSK cells after 9 days of culture and the increase was attenuated by rapamycin treatment (Figure 1 C & D). Similar findings were observed in CD48−LSK cells (Figure 1 C & E), a primitive hematopoietic cell population that more closely represents HSCs after ex vivo expansion by phenotyping (17).
Figure 1. Ex vivo expansion of HSCs activates mTOR in a time-dependent manner.
(A) Representative flow cytometric analyses of phosphorylated S6K (p-S6K) in freshly isolated unexpanded Lin−Sca1+c-kit+ cells (LSK cells) (Unexp) and ex vivo expanded LSK cells after 6-day (Veh-D6) and 9-day (Veh-D9) culture with vehicle (0.1% DMSO) or 9-day (Rap-D9) culture with rapamycin (20 ng/ml) started on day 6 are shown. Cells stained with an isotype control antibody (Isotype) were included as a control for the staining. (B) Percentages of p-S6K+ LSK cells from three independent assays are presented as mean ± SE. a, p<0.05 vs. unexpanded cells; b, p<0.05 vs. cells expanded with vehicle for 6 days; c, p<0.05 vs. cells expanded with vehicle for 9 days. (C) Representative flow cytometric analyses of phosphorylated S6 (p-S6) in freshly isolated LSK and CD48−LSK cells (Unexp) and ex vivo expanded LSK and CD48−LSK cells after 9-day culture with vehicle (Veh, 0.1% DMSO) or rapamycin (Rap, 20 ng/ml) started on day 6 are shown. (D) Percentages of p-S6+ LSK cells from three independent assays are presented as mean ± SE. (E) Percentages of p-S6+ CD48−LSK cells from three independent assays are presented as mean ± SE. a, p<0.05 vs. unexpanded cells; and b, p<0.05 vs. cells expanded with vehicle.
Inhibition of mTOR with rapamycin promotes ex vivo expansion of HSCs
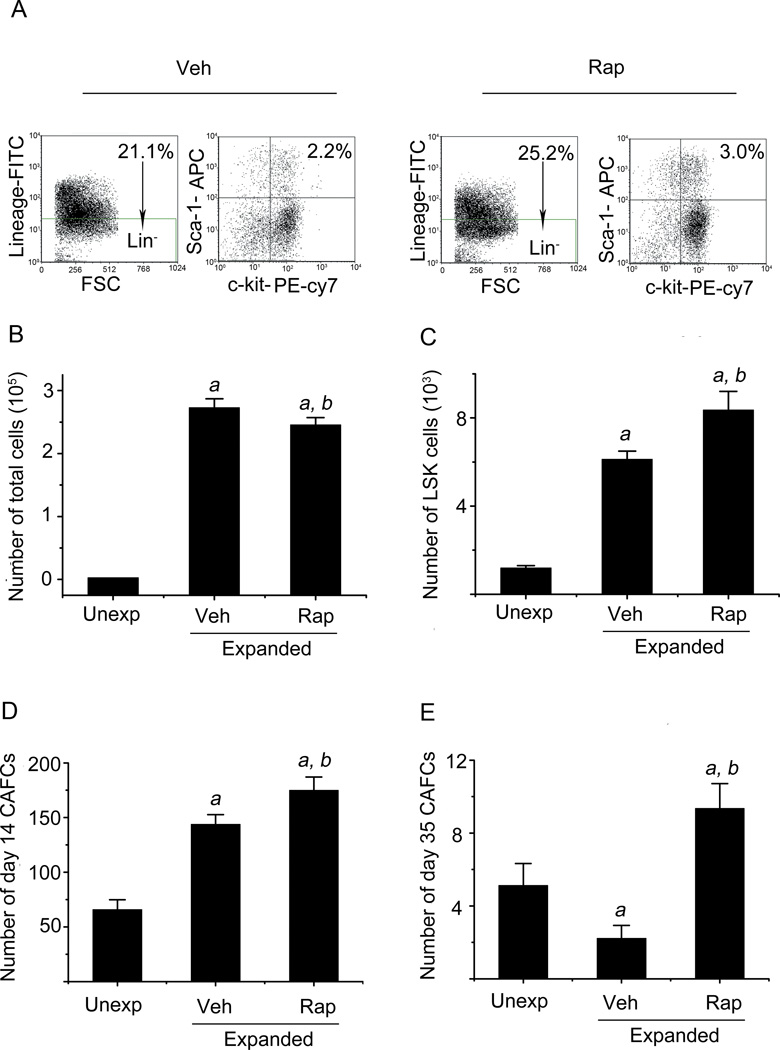
Since activation of mTOR has been shown to impair HSCs self-renewal and inhibition of hyperactivated mTOR with rapamycin can prevent HSCs premature exhaustion caused by the genetic deletion of Pten, Tsc1 and Gsk3 in mice and rejuvenate aging HSCs (13–15), we investigated whether rapamycin can also promote ex vivo expansion of HSCs by inhibiting mTOR overactivation. In a preliminary study, we found that 20 ng/ml rapamycin was the optimal concentration to inhibit mTOR overactivation and promote ex vivo expansion of HSCs (data not shown). In addition, we found that rapamycin inhibited cell proliferation if it was added to the ex vivo HSC expansion cultures earlier than 6 days after the initiation of the culture (data not shown). Therefore, we added rapamycin (20 ng/ml) or vehicle into HSCs expansion cultures on day 6 when LSK cells exhibited a significant activation of mTOR (Figure 1). On day 10, all cells were harvested from the cultures and counted to calculate the number of total nucleated cells produced from the expansion. The percentage of LSK cells in the harvested cells was analyzed by flow cytometry after immunostaining in order to calculate LSK cell number for the estimation of HSCs expansion because LSK cells are enriched for HSCs (Figure 2A) . As shown in Figure 2B, compared to input cells, the numbers of total nucleated cells increased about 80- and 90-fold after 9 days of culture in the presence of and absence of rapamycin, respectively, indicating that rapamycin treatment slightly reduced the production of nucleated cells in comparison with the cells without rapamycin treatment. However, the cells cultured with rapamycin contained significantly more LSK cells than those without rapamycin (Figure 2C). Compared to the number of input LSK cells, the number of LSK cells increased 9- and 6-fold after 9 days of ex vivo expansion with and without rapamycin, respectively. This finding suggests that overactivated mTOR inhibition by rapamycin may promote HSCs ex vivo expansion.
Figure 2. Inhibition of mTOR with rapamycin promotes ex vivo expansion of HSCs.
Lin−Sca1+ cells were cultured in vitro for 5 days without any treatment and then cultured with either vehicle (Veh, 0.1% DMSO) or rapamycin (Rap, 20 ng/ml) from day 6 to day 9. The cells harvested from the cultures on day 10 were enumerated and analyzed by flow cytometry and CAFC assay. (A) Representative flow cytometric analyses of LSK cells in the cultures with Veh or Rap. The numbers presented in the flow charts are frequencies for each cell population indicated. (B) Number of total nucleated cells. (C) Number of LSK cells. (D) Number of day-14 CAFCs. (E) Number of day-35 CAFCs. The data in B-E are presented as mean ± SE (n=3 independent cultures) recovered from the cultures with 3000 input (Unexp) Lin−Sca1+ cells. a, p<0.05 vs. unexpanded cells; and b, p<0.05 vs. cells expanded with vehicle.
LSK cells contain both HSCs and multiple potent progenitors (MPPs). To determine whether rapamycin can increase HSCs expansion ex vivo, we quantified the frequencies of CAFCs by CAFC assay on day 35, because the day-35 CAFC assay is considered one of the best in vitro assays for measuring HSCs. In addition, day-14 CAFCs were analyzed to quantify HPCs (10). As shown in Figure 2 D & E, cell cultures with rapamycin contained 4.2- and 1.2-fold more day-35 and day-14 CAFCs, respectively, than cultures without rapamycin. More importantly, when the numbers of day-35 and day-14 CAFCs in the cultured cells were expressed as a ratio to those in input, it was found that the majority of HSCs cultured without rapamycin could not self-renew, resulting in a reduction in day-35 CAFCs by about 55% compared to input. This finding agrees with the previous observation showing that HSCs from mouse bone marrow lose radioprotective and long-term engraftment potential after ex vivo expansion in the presence of multiple cytokines (10). In contrast, HSCs cultured with rapamycin underwent self-renewing proliferation, leading to a 1.8-fold increase in day-35 CAFCs compared to input, indicating that the inhibition of overactivated mTOR with rapamycin promotes HSCs ex vivo expansion.
Inhibition of mTOR with rapamycin increases HSCs long-term engraftment after transplantation
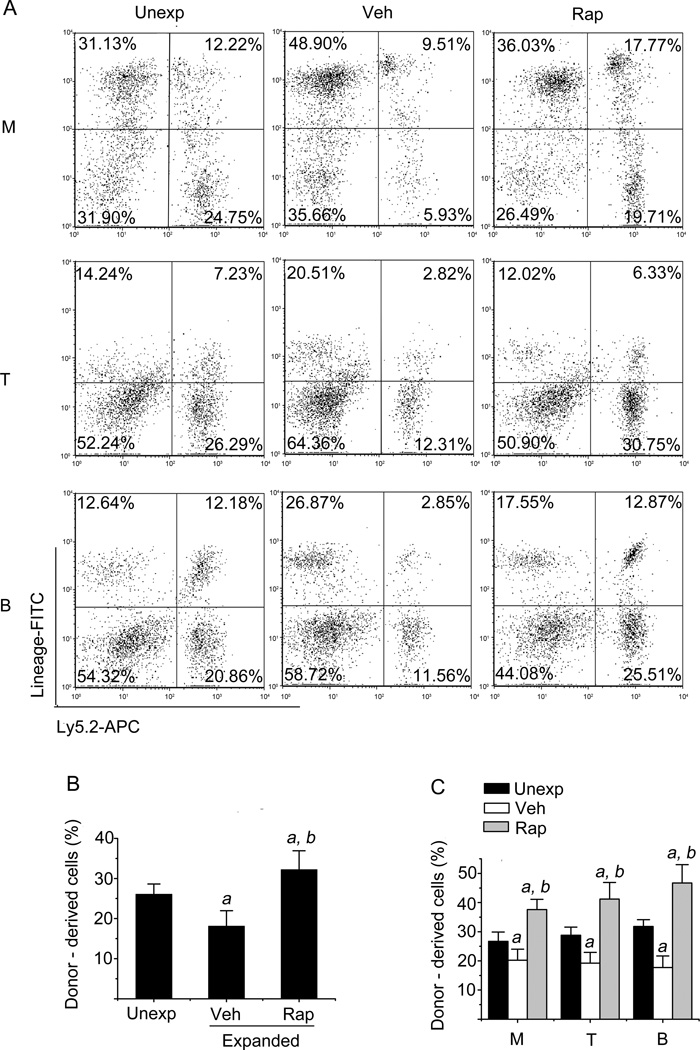
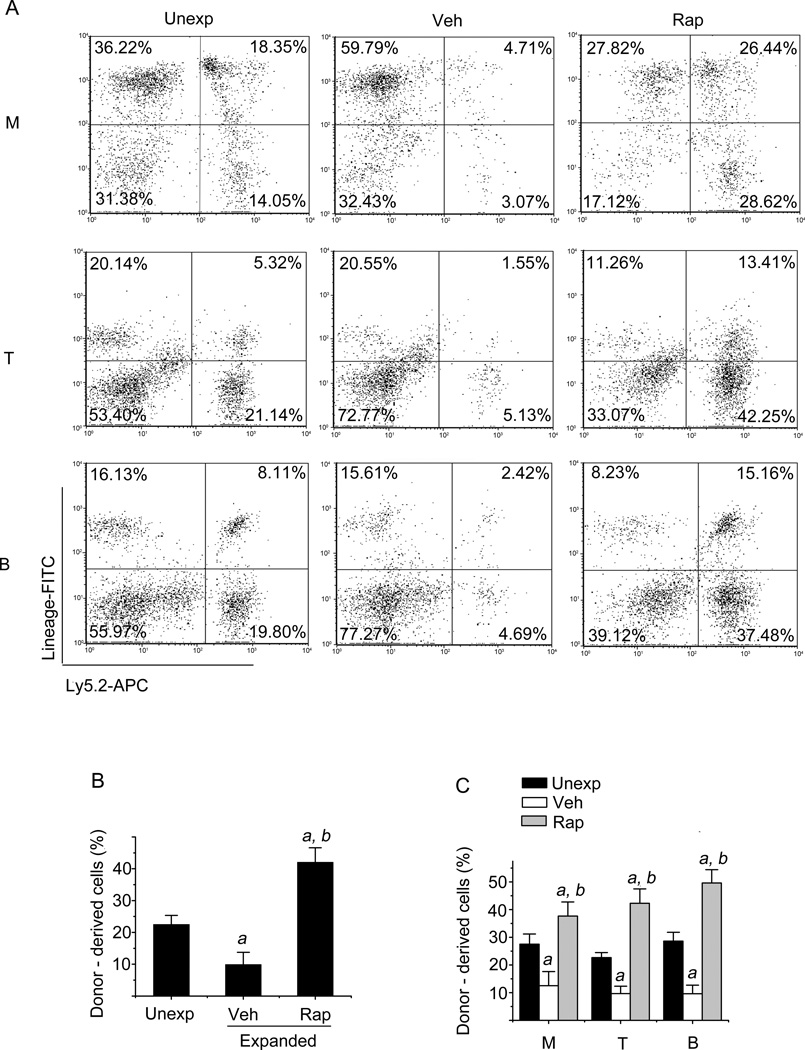
HSCs transplantation is considered the gold standard assay for long-term repopulating HSCs (10). To validate if inhibition of overactivated mTOR with rapamycin can really promote HSCs ex vivo expansion, we performed a competitive repopulation assay by transplanting 2000 freshly isolated Lin−Sca1+ cells or the progeny harvested from a 9-day culture with the same number of input Lin−Sca1+ cells in the presence or absence of rapamycin into lethally irradiated recipients along with competitors. Peripheral blood from these recipients was analyzed for donor-cell engraftment at 8 and 32 weeks after transplantation. As shown in Figures 3 and 4, a 9-day ex vivo expansion of Lin−Sca1+ cells in the absence of rapamycin resulted in about 31% and 56% decreases in donor cell engraftment at 8 weeks and 32 weeks after transplantation compared to the engraftment produced by the unexpanded input cells, respectively. Rapamycin treatment from day 6 to day 9 of ex vivo expansion of Lin−Sca1+ cells led to 2-fold and 4-fold increases in donor cell engraftment 8 weeks and 32 weeks after transplantation compared to vehicle treatment, respectively. More importantly, we observed a 2-fold increase in donor cell engraftment from rapamycin-expanded cells compared to unexpanded input cells (Figures 3 & 4). In addition, rapamycin treatment also improved the long-term reconstitution of myeloid, B- and T-lymphocyte lineage blood cells in the recipients 8 and 32 weeks after transplantation (Figures 3 & 4). Based on the 32-week long-term engraftment data, we calculated the repopulating unit (RU) for the input and expanded cells with vehicle or rapamycin according to the formulation reported previously (18, 19). It was found that the input cells (e.g. 2000 freshly isolated Lin−Sca1+ cells) contained 0.56 RU. Ex vivo expansion of the cells with vehicle resulted in about 60% reduction of RU compared to that of the unexpanded input cells (reduced from 0.56 to 0.22 RU). In contrast, the cells expanded with rapamycin exhibited about 250% increases in RU compared to that of the input cells (increased from 0.56 to 1.38 RU). Taken together, these results demonstrate that inhibition of hyper-activated mTOR with rapamycin promotes long term hematopoietic reconstitution of ex vivo expanded HSCs.
Figure 3. Inhibition of mTOR with rapamycin increases HSC engraftment after transplantation.
Lin−Sca1+ cells were expanded as described above with vehicle (Veh) or rapamycin (Rap). The progeny of 2×103 Lin−Sca1+ input cells from Ly5.2 mice after 9 days ex vivo expansion along with 2×105 competitive bone marrow cells from Ly5.1 mice were transplanted into lethally irradiated Ly5.1 mice. Unexpanded 2×103 Lin−Sca1+ cells mixed with the same number of bone marrow cells from Ly5.1 mice were used as controls. Donor cell engraftment in peripheral blood was determined 8 weeks after transplantation by flow cytometry. (A) Representative flow cytometric analyses of donor-derived cells in the peripheral blood of recipients 8 weeks after transplantation. Donor-derived (Ly5.2) myeloid cells (M), T cells (T), and B cells (B) were analyzed by flow cytometry after immunostaining with APC-conjugated anti-Ly5.2 and FITC-conjugated anti-Gr-1/Mac-1, anti-Thy-1.2, and anti-B220 antibodies, respectively. The numbers presented in the flow charts are percentages of each of these cell populations derived from the donor cells (Ly5.2 positive cells) and competitors (Ly5.1 positive cells). (B) The percentage of total donor-derived cells in the peripheral blood at 8 weeks after transplantation, respectively. (C) The percentage of donor-derived myeloid cells (M), T cells (T), and B cells (B) in the peripheral blood at 8 weeks after transplantation, respectively. The data are presented as mean ± SE of three independent transplantation experiments with cells from separate cultures and each transplantation consisting of 7 mice per group. a, p<0.05 vs. unexpdnded cells; and b, p<0.05 vs. cells expanded with vehicle.
Figure 4. Inhibition of mTOR with rapamycin increases HSC long-term engraftment after transplantation.
Lin−Sca1+ cells were expanded with vehicle (Veh) or rapamycin (Rap) and transplanted as described above. Donor cell long-term engraftment in peripheral blood was determined 32 weeks after transplantation by flow cytometry. (A) Representative flow cytometric analyses of donor-derived cells in the peripheral blood of recipients 32 weeks after transplantation. Donor-derived (Ly5.2) myeloid cells (M), T cells (T), and B cells (B) were analyzed by flow cytometry after immunostaining with APC-conjugated anti-Ly5.2 and FITC-conjugated anti-Gr-1/Mac-1, anti-Thy-1.2, and anti-B220 antibodies, respectively. The numbers presented in the flow charts are percentages of each of these cell populations derived from the donor cells (Ly5.2 positive cells) and competitors (Ly5.1 positive cells). (B) The percentage of total donor-derived cells in the peripheral blood at 32 weeks after transplantation, respectively. (C) The percentage of donor-derived myeloid cells (M), T cells (T), and B cells (B) in the peripheral blood at 32 weeks after transplantation, respectively. The data are presented as mean ± SE of three independent transplantation experiments with cells from separate cultures and each transplantation consisting of 7 mice per group. a, p<0.05 vs. unexpdnded cells; and b, p<0.05 vs. cells expanded with vehicle.
Inhibition of mTOR with rapamycin promotes HSCs ex vivo expansion in part by inhibiting HSCs senescence
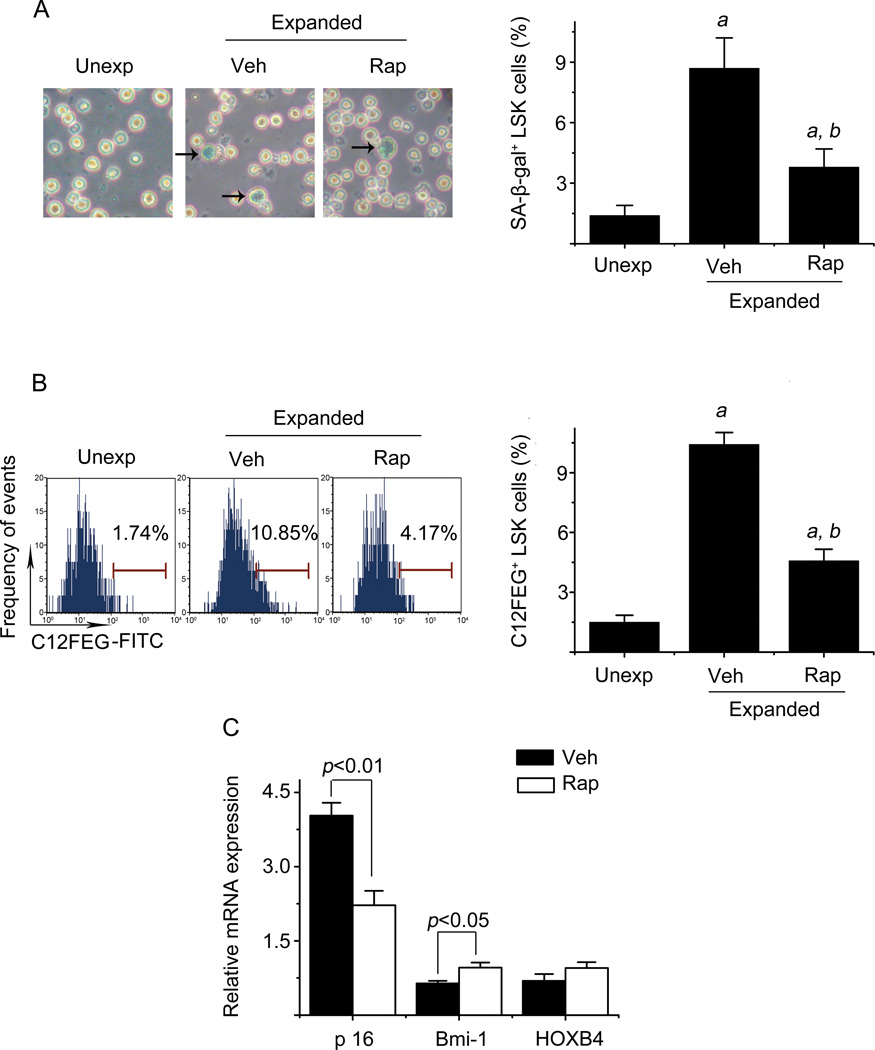
Ex vivo expansion of hematopoietic stem cells (HSCs) depends on HSCs self-renewing proliferation, which can be negatively affected by HSCs differentiation, apoptosis, and senescence (3, 10, 11). To elucidate the mechanisms by which mTOR inhibition promotes long term hematopoiesis of expanded HSCs, we analyzed the effects of rapamycin treatment on HSCs differentiation, apoptosis, and senescence by CFC assay, annexin-V staining, and SA-β-gal staining, respectively. As shown in SDC, Supplemental figure 1, rapamycin treatment had no significant effect on the formation of CFU-GM, BFU-E, and CFU-GEMM from the progeny of HSCs, nor did it reduce the percentage of annexin-V positive cells in LSK cell population. However, rapamycin reduced the number of LSK cells that underwent senescence during ex vivo expansion, as significantly less LSK cells from rapamycin culture stained positive for SA-β-gal (Figure 5A), a widely used biomarker of senescent cells, than those from vehicle culture. Similar findings were also observed in Figure 5B when we used C12FDG to measure SA-β-gal activity in LSK cells, which can be more sensitively and better quantitatively measured by flow cytometry (20). These findings suggest that inhibition of mTOR with rapamycin promotes ex vivo HSCs expansion not by inhibition of HSCs differentiation and apoptosis but probably via suppression of HSCs senescence. This suggestion is in agreement with the finding that rapamycin treatment increased the expression of Bmi1 mRNA while decreasing the expression of p16Ink4a (p16) mRNA in LSK cells (Figure 5C), since both Bmi1 and p16 play an important role in regulation of HSCs senescence but in an opposite direction, e.g. Bmi1 inhibits senescence by repressing the expression of p16 and p19 while p16 is an effector of cellular senescence (21).
Figure 5. Inhibition of mTOR with rapamycin reduces HSCs senescence after ex vivo expansion.
(A) Analysis of HSCs senescence by SA-β-gal staining. Left panel: Representative photomicrographs of SA-β-gal staining in freshly isolated unexpanded LSK cells (Unexp) and sorted LSK cells after ex vivo expansion with vehicle (Veh) or rapamycin (Rap) are shown. The cells pointed to by arrows are SA-β-gal positive cells. Right panel: percentages of SA-β-gal positive LSK cells are presented as mean ± SE (n = 3). (B) Analysis of HSCs senescence by flow cytometry after C12FEG staining. Left panel: Representative flow cytometric analyses of C12FEG staining in freshly isolated unexpanded LSK cells (Unexp) and ex vivo expanded LSK cells with vehicle (Veh, 0.1% DMSO) or rapamycin (Rap, 20 ng/ml) started on day 6 are shown. Right panel: percentages of C12FEG positive LSK cells are presented as mean ± SE (n = 3). (C) Analysis of p16, Bmi1, and HoxB4 mRNA expression in sorted LSK cells after ex vivo expansion with vehicle (Veh) or rapamycin (Rap) by real-time RT-PCR. The data are expressed as a ratio of these to freshly isolated LSK cells and presented as mean ± SE (n = 3). a, p < 0.05 vs. unexpanded cells; and b, p<0.05 vs. cells expanded with vehicle.
DISCUSSION
It has been well-established that overactivation of mTOR impairs HSCs function and causes HSCs depletion in vivo under various pathological conditions and during aging (13, 22). To our knowledge, our study is the first to show that ex vivo expansion of HSCs also causes overactivation of mTOR in a time-dependent manner in mouse bone marrow LSK cells that are enriched with HSCs. We showed that the activation of mTOR was associated with a significant reduction in the ability of HSCs to produce long-term hematopoietic reconstitution after transplantation of the ex vivo expanded cells. Addition of rapamycin after 6 days of ex vivo culture when mTOR became significantly activated in LSK cells promoted HSCs expansion, which led to an approximate 2- to 4-fold increase in long-term hematopoietic engraftment by the expanded cells treated with rapamycin compared to unexpanded input cells and the cells expanded without rapamycin treatment, respectively. These findings demonstrate that mTOR plays an important role in HSCs self-renewing proliferation during ex vivo expansion and that inhibition of overactivated mTOR with rapamycin has the potential to be employed as a novel strategy to promote hematopoiesis of ex vivo expanded HSCs, which can facilitate the clinical application of HSCs transplantation for various hematological diseases.
However, the timing of rapamycin treatment may be important in determining whether inhibition of mTOR can promote hematopoiesis of ex vivo expanded HSCs. Rohrabaugh SL et al. recently showed that the addition of rapamycin at the beginning of ex vivo expansion of human cord blood CD34+ cells decreased the expansion of CD34+ cells but improved their engraftment in non-obese diabetic, severe combined immunedeficient, and IL2 receptor γ chain null mice (23). In addition, Huang J et al. reported that suppression of mTOR with rapamycin in combination with the activation of the canonical Wnt–β-catenin pathway by a glycogen synthase kinase 3 inhibitor when Lin−Sca1+ or LSK cells were cultured ex vivo in a cytokine-free condition only permitted the maintenance but not expansion of mouse long-term HSCs (24). Therefore, we suspect that a moderate level of mTOR activation at the beginning of HSCs ex vivo expansion may be required for HSCs proliferation and HSCs expansion, whereas a high level of mTOR activation at a later stage of HSCs ex vivo expansion can impair long term hematopoiesis of expanded HSCs by induction of HSCs senescence. Therefore, to effectively promote ex vivo expansion of HSCs, rapamycin should be added to the culture of ex vivo expansion of HSCs when mTOR becomes significantly activated in HSCs.
In addition, our studies revealed some of the fundamental mechanisms by which rapamycin promotes HSCs ex vivo expansion. Our results showed that inhibition of mTOR with rapamycin reduced HSCs senescence while having no significant effects on HSCs apoptosis and differentiation during ex vivo expansion. The inhibition of HSCs senescence may be attributable to rapamycin-mediated upregulation of Bmi1 and downregulation of p16, since Bmi1 can inhibit senescence by repressing the expression of p16 and p19 while p16 is an effector of cellular senescence (21).
Although we showed that inhibition of mTOR by rapamycin can promote ex vivo expansion of HSCs, it is possible that the strategies that can inhibit the upstream signaling pathways leading to mTOR activation can also be utilized to increase HSCs expansion either alone or in combination with rapamycin. It has been well-established that mTOR is a central regulator of cellular response to stress and to changes in environmental cues. During ex vivo expansion, HSCs are constantly subjected to a variety of stressors, including increases in oxygen tension, fluctuations in various nutrients and growth factor concentrations, and accumulation of toxic metabolites. All of these may cause activation of mTOR via oxidative stress (15), activation of p38 (25) and other unknown mechanisms. Recently, we showed that p38 inhibition can promote ex vivo expansion of both mouse bone marrow and human cord blood HSCs (10, 11). It remains to be determined whether the effect of p38 inhibition is mediated in part by inhibition of mTOR and whether simultaneous inhibition of p38 and mTOR can synergistically promote HSCs ex vivo expansion. Therefore, further study to elucidate the mechanisms by which ex vivo expansion of HSCs causes activation of mTOR in HSCs may lead to the identification of additional molecular targets for intervention to promote HSCs self-renewal and expansion in an ex vivo cell culture environment.
DESIGN AND METHODS
Reagents
All the antibodies used to stain various types of hematopoietic cells are presented in Supplemental Table S1. Recombinant mouse SCF, TPO and FL were purchased from Peprotech (Rocky Hill, NJ, USA). StemSpan® SFEM medium was obtained from StemCell Technologies (Vancouver, BC, Canada). Rapamycin was purchased from Cell Signaling Technology (Beverly, MA, USA).
Mice
C57BL/6-Ly-5.1 (Ly5.1) mice were originally obtained from the Jackson Laboratories (Bar Harbor, ME, USA) and were bred in the Laboratory Animal Research Centre (LARC) of Huazhong University of Science and Technology (HUST) in China. C57BL/6-Ly-5.2 (Ly5.2) mice were obtained from LARC and the Jackson Laboratories, respectively, to be used at HUST and at the University of Arkansas for Medical Sciences (UAMS) in the US. Mice received food and water ad libitum. All mice were used at 8–12 weeks of age in compliance with the standards for humane care and use of laboratory animals and with the approval of the Institutional Animal Care and Use Committees at HUST and UAMS.
Ex vivo expansion of mouse bone marrow HSCs
Mouse bone marrow Lin−Sca1+ cells were obtained by a combination of negative selection for Lin− cells and positive selection for Sca1+ cells using MicroBeads (Miltenyi Biotec Inc, Auburn, CA, USA) following the protocols provided by the manufacturer. They were seeded into wells of 12-well plates at 3×103 cells per well and incubated with 1.5 ml StemSpan® SFEM supplemented with 100 ng/ml SCF, TPO and FL (HSC expansion medium) at 37°C, 5% CO2 and 100% humidity. The cells were fed with 1 ml fresh HSCs expansion medium every 3 days during the culture. Rapamycin (20 ng/ml in 0.1% dimethyl sulfoxide [DMSO]) was added to the culture on day 6, while DMSO (0.1%) was used as a vehicle control. All progeny harvested from each culture at various times were analyzed as described below.
Flow cytometric analysis of intracellular phosphorylated proteins
Flow cytometric analysis of intracellular phosphorylated proteins were performed as described in supplemental methods.
Colony-forming cell (CFC) and cobblestone-area-forming cell (CAFC) assays
The CFC and CAFC assays were performed as described in supplemental methods.
Competitive repopulation assay
Cells harvested from a 9-day culture of 2000 input Lin−Sca1+cells or 2000 freshly isolated Lin−Sca1+cells from Ly5.2 mice were mixed with 2×105 competitive bone marrow mononuclear cells (BMMNCs) from Ly5.1 mice. The cell mixture was transplanted into lethally irradiated (9.5 Gy TBI) Ly5.1 mice by tail-vein injection. Peripheral blood samples were obtained from the retro-orbital plexus using heparin-coated micropipets (Drummond Scientific, Broomall, PA, USA) at 8 and 32 weeks post transplantation. Following lysis of red blood cells with 0.15 M NH4Cl, the blood samples were stained with APC-conjugated anti-Ly5.2 antibodies and FITC-conjugated anti-Thy-1.2, anti-CD45R/B220, or anti-Gr-1/Mac-1 antibodies and then analyzed for donor-derived nuclear cells, T-cells, B-cells, and myeloid cells (including granulocytes and monocytes/macrophages), respectively, on a FACS Caliber. We repeated the competitive repopulation assay three times with cells harvested from three separate cultures. Each assay consisted of 7 mice per transplantation group. The donor cell engraftment is presented as mean ± SE of three independent transplantation experiments. In addition, we calculated the repopulating unit (RU) of donor cells according to the original formula proposed by Dr. Harrison’s group (19). Specifically, each RU is the relative repopulating ability of 105 fresh marrow cells from the same standard competitor pool. Numbers of RU from each testing donor cell population are calculated from the percentage of testing donor cells where the number of fresh competitor marrow cells used per 105 equalled C. RU = % (C) /(100 -%) or RU = Testing cells % × C / Competitors %.
Apoptosis assay
Apoptosis was performed as described in supplemental methods.
Senescence-associated β galactosidase (SA-β-gal) staining
SA-β-gal staining was performed as described in supplemental methods.
Analysis of senescence by C12FDG staining
The analysis of senescence by C12FDG staining was performed as described in supplemental methods.
Quantitative reverse transcriptase PCR (qRT-PCR)
qRT-PCR was performed as described in supplemental methods.
Statistical analysis
The statistical software package SPSS 13.0 was used to analyze data by analysis of variance (ANOVA). In the events that ANOVA justified post hoc comparisons between group means, these were performed using the LSD-t test for multiple comparisons. For experiments in which only single experimental and control group were used, group differences were examined by unpaired or paired Student’s t test. A P value of < 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
Funding
This work was supported by grants from the National Natural Science Foundation of China (No.30871097 and No.81170524) to Dr. Lingbo Liu and a grant from the National Institutes of Health of the US (AI080421) and a grant from the Edward P. Evan's Foundation to Dr. Daohong Zhou. Dr. Daohong Zhou is also supported by the Winthrop Rockefeller Endowment for Leukemia Research and the Arkansas Research Alliance Scholarship from the Arkansas Science & Technology Authority.
The authors would like to thank Mr. Zhihui Liang and Mrs. Huifen Zhu for the flow cytometric analysis and the Laboratory Animal Research Centre of Huazhong University of Science and Technology for their support in animal care. The Office of Grants and Scientific Publications of the University of Arkansas for Medical Sciences provided editing services.
Abbreviations
- BFU-E
burst-forming unit-erythroid
- CFU-GEMM
CFU-granulocyte, -erythrocyte, -monocyte, and -megakaryocyte
- CAFC
cobblestone area forming cell
- CFU-GM
colony-forming unit-granulocyte macrophage
- FL
Flt3 ligand
- GSK3
glycogen synthase kinase 3
- HSCs
hematopoietic stem cells
- LSK cells
Lin-Sca1+c-kit+ cells
- mTOR
mammalian target of rapamycin
- p38
p38 mitogen-activated protein kinase
- PTEN
phosphatase and tensin homolog
- PI3K
phosphoinositide-3-kinase
- SCF
stem cell factor
- TPO
thrombopoietin
- TSC1
tuberous sclerosis complex 1
- Wnt3a
Member 3A of Wingless-type MMTV integration site family
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contribution: LBL and DZ designed the research; YL and LL performed most of the experiments and JW and LS performed some of the experiments. YL and LL collected data. YL, LL, PZ, DZ, and LBL analyzed the data. YL, DZ and LBL wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Reference
- 1.Srour EF, Abonour R, Cornetta K, Traycoff CM. Ex vivo expansion of hematopoietic stem and progenitor cells: are we there yet? Journal of hematotherapy. 1999;8:93. doi: 10.1089/106161299320370. [DOI] [PubMed] [Google Scholar]
- 2.Gluck S. Ex vivo expansion of hematopoietic stem cells. Journal of hematotherapy & stem cell research. 1999;8:575. doi: 10.1089/152581699319722. [DOI] [PubMed] [Google Scholar]
- 3.Dahlberg A, Delaney C, Bernstein ID. Ex vivo expansion of human hematopoietic stem and progenitor cells. Blood. 2011;117:6083. doi: 10.1182/blood-2011-01-283606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim JA, Kang YJ, Park G, et al. Identification of a stroma-mediated Wnt/beta-catenin signal promoting self-renewal of hematopoietic stem cells in the stem cell niche. Stem cells. 2009;27:1318. doi: 10.1002/stem.52. [DOI] [PubMed] [Google Scholar]
- 5.Huynh H, Iizuka S, Kaba M, et al. Insulin-like growth factor-binding protein 2 secreted by a tumorigenic cell line supports ex vivo expansion of mouse hematopoietic stem cells. Stem cells. 2008;26:1628. doi: 10.1634/stemcells.2008-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller CL, Eaves CJ. Expansion in vitro of adult murine hematopoietic stem cells with transplantable lympho-myeloid reconstituting ability. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:13648. doi: 10.1073/pnas.94.25.13648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khoury M, Drake A, Chen Q, et al. Mesenchymal stem cells secreting angiopoietin-like-5 support efficient expansion of human hematopoietic stem cells without compromising their repopulating potential. Stem Cells Dev. 2011;20:1371. doi: 10.1089/scd.2010.0456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kobayashi H, Butler JM, O'Donnell R, et al. Angiocrine factors from Akt-activated endothelial cells balance self-renewal and differentiation of haematopoietic stem cells. Nat Cell Biol. 2010;12:1046. doi: 10.1038/ncb2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Antonchuk J, Sauvageau G, Humphries RK. HOXB4-induced expansion of adult hematopoietic stem cells ex vivo. Cell. 2002;109:39. doi: 10.1016/s0092-8674(02)00697-9. [DOI] [PubMed] [Google Scholar]
- 10.Wang Y, Kellner J, Liu L, Zhou D. Inhibition of p38 mitogen-activated protein kinase promotes ex vivo hematopoietic stem cell expansion. Stem Cells Dev. 2011;20:1143. doi: 10.1089/scd.2010.0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zou J, Zou P, Wang J, et al. Inhibition of p38 MAPK activity promotes ex vivo expansion of human cord blood hematopoietic stem cells. Annals of hematology. 2012;91:813. doi: 10.1007/s00277-011-1397-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nature reviews Molecular cell biology. 2011;12:21. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee JY, Nakada D, Yilmaz OH, et al. mTOR activation induces tumor suppressors that inhibit leukemogenesis and deplete hematopoietic stem cells after Pten deletion. Cell Stem Cell. 2010;7:593. doi: 10.1016/j.stem.2010.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang J, Zhang Y, Bersenev A, et al. Pivotal role for glycogen synthase kinase-3 in hematopoietic stem cell homeostasis in mice. J Clin Invest. 2009;119:3519. doi: 10.1172/JCI40572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen C, Liu Y, Liu Y, Zheng P. The axis of mTOR-mitochondria-ROS and stemness of the hematopoietic stem cells. Cell Cycle. 2009;8:1158. doi: 10.4161/cc.8.8.8139. [DOI] [PubMed] [Google Scholar]
- 16.Csaszar E, Kirouac DC, Yu M, et al. Rapid expansion of human hematopoietic stem cells by automated control of inhibitory feedback signaling. Cell Stem Cell. 2012;10:218. doi: 10.1016/j.stem.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 17.Noda S, Horiguchi K, Ichikawa H, Miyoshi H. Repopulating activity of ex vivo-expanded murine hematopoietic stem cells resides in the CD48-c-Kit+Sca-1+lineage marker-cell population. Stem cells. 2008;26:646. doi: 10.1634/stemcells.2007-0623. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y, Taylor BR, Shannon K, Clapp DW. Quantitative effects of Nf1 inactivation on in vivo hematopoiesis. J Clin Invest. 2001;108:709. doi: 10.1172/JCI12758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen J, Astle CM, Muller-Sieburg CE, Harrison DE. Primitive hematopoietic stem cell function in vivo is uniquely high in the CXB-12 mouse strain. Blood. 2000;96:4124. [PubMed] [Google Scholar]
- 20.Debacq-Chainiaux F, Erusalimsky JD, Campisi J, Toussaint O. Protocols to detect senescence-associated beta-galactosidase (SA-betagal) activity, a biomarker of senescent cells in culture and in vivo. Nature protocols. 2009;4:1798. doi: 10.1038/nprot.2009.191. [DOI] [PubMed] [Google Scholar]
- 21.Park IK, Qian D, Kiel M, et al. Bmi-1 is required for maintenance of adult self-renewing haematopoietic stem cells. Nature. 2003;423:302. doi: 10.1038/nature01587. [DOI] [PubMed] [Google Scholar]
- 22.Chen C, Liu Y, Liu R, et al. TSC-mTOR maintains quiescence and function of hematopoietic stem cells by repressing mitochondrial biogenesis and reactive oxygen species. The Journal of experimental medicine. 2008;205:2397. doi: 10.1084/jem.20081297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rohrabaugh SL, Campbell TB, Hangoc G, Broxmeyer HE. Ex vivo rapamycin treatment of human cord blood CD34+ cells enhances their engraftment of NSG mice. Blood cells, molecules & diseases. 2011;46:318. doi: 10.1016/j.bcmd.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang J, Nguyen-McCarty M, Hexner EO, Danet-Desnoyers G, Klein PS. Maintenance of hematopoietic stem cells through regulation of Wnt and mTOR pathways. Nat Med. 2012;18:1778. doi: 10.1038/nm.2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu XN, Wang XK, Wu SQ, et al. Phosphorylation of Raptor by p38beta participates in arsenite-induced mammalian target of rapamycin complex 1 (mTORC1) activation. J Biol Chem. 2011;286:31501. doi: 10.1074/jbc.M111.233122. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.