Abstract
Fibroblast growth factor (FGF) receptor (FGFR) signaling controls the migration of glial, mesodermal, and tracheal cells in Drosophila melanogaster. Little is known about the molecular events linking receptor activation to cytoskeletal rearrangements during cell migration. We have performed a functional characterization of Downstream-of-FGFR (Dof), a putative adapter protein that acts specifically in FGFR signal transduction in Drosophila. By combining reverse genetic, cell culture, and biochemical approaches, we demonstrate that Dof is a specific substrate for the two Drosophila FGFRs. After defining a minimal Dof rescue protein, we identify two regions important for Dof function in mesodermal and tracheal cell migration. The N-terminal 484 amino acids are strictly required for the interaction of Dof with the FGFRs. Upon receptor activation, tyrosine residue 515 becomes phosphorylated and recruits the phosphatase Corkscrew (Csw). Csw recruitment represents an essential step in FGF-induced cell migration and in the activation of the Ras/MAPK pathway. However, our results also indicate that the activation of Ras is not sufficient to activate the migration machinery in tracheal and mesodermal cells. Additional proteins binding either to the FGFRs, to Dof, or to Csw appear to be crucial for a chemotactic response.
Fibroblast growth factor (FGF) receptors (FGFRs) control diverse cellular processes, including cell proliferation, differentiation, migration, and survival during metazoan development (for reviews, see references 29 and 31). FGFRs belong to a large family of transmembrane receptor tyrosine kinases (RTK) and activate a variety of downstream signaling molecules. The transcriptional responses to FGFR activation are elicited, at least in part, via the Ras/mitogen-activated protein kinase (MAPK) pathway and the phosphorylation of Ets-domain-containing transcriptional regulators (25). Specific responses in the nucleus are then most likely determined by interactions with preexisting nuclear components on genomic enhancers or repressor elements (1, 20, 40). Much less is known about how RTK signaling or FGFR signaling in particular regulates cytoplasmic events in the control of cell movement.
In Drosophila melanogaster, FGFR signaling has been implicated in the migration of several tissues, notably, in the spreading of mesoderm, the migration of tracheal cells and glial cells in the embryo, and the recruitment of mesodermal cells into the genital disk and in the formation of air sacs during larval stages (2, 4, 11, 12, 18, 36). In vivo imaging in live Drosophila embryos has shown that FGFR signaling indeed leads to dynamic cytoskeletal reorganizations during migration, resulting in the formation of filopodial extensions in tip cells (33, 36). Despite the importance of FGFR signaling in cell migration in Drosophila, the molecular events linking signal reception to these cytoskeletal events remain elusive.
Similar to other RTKs, FGFRs are activated by receptor dimerization upon ligand binding, which then leads to activation of the intracellular kinase domain and autophosphorylation of the RTK on specific tyrosine residues (for a review, see reference 38). Some of these phosphorylated tyrosines provide docking sites for Src-homology 2 (SH2) domain-containing proteins that propagate the signal inside the cell.
In contrast to most other RTKs, FGFRs lack a Grb2 binding site and thus need to recruit an intermediate docking molecule to activate the MAPK pathway. In vertebrates, many of the cellular responses of FGFs, including the activation of the MAPK pathway, are mediated through the recruitment of the membrane-linked docking protein SNT/FRS2 (19, 26, 43). SNT/FRS2 constitutively interacts with the juxtamembrane domain of the FGFR through its phosphotyrosine binding domain (27, 44) and recruits other signaling molecules upon tyrosine phosphorylation. Recruitment of the Grb2-Sos complex or the phosphatase SHP2 on SNT/FRS2 leads to activation of the MAPK pathway, whereas recruitment of Grb2-Gab1 leads to activation of the cell survival pathway via phosphatidylinositol 3-kinase (PI3K) interaction (14, 19, 28).
In Drosophila, genetic studies have identified a potential, FGFR-specific signaling mediator, Downstream-of-FGFR (Dof) (also known as Heartbroken or Stumps [16, 21, 42]). Dof is expressed exclusively in the tissues expressing the two FGFRs Heartless (Htl) and Breathless (Btl), i.e., in mesodermal, tracheal, and a subset of glial cells (42). Dof encodes an ankyrin-repeat- and coiled-coil-containing protein and represents the founding member of a small family of proteins including BCAP and BANK, two vertebrate proteins that have recently been identified and shown to regulate B-cell-receptor-specific PI3K activation and calcium mobilization, respectively (24, 45). Genetic epistasis experiments in Drosophila have shown that Dof functions downstream of the activated FGFR and upstream of Ras (16, 21, 42), but neither the role of Dof in the interpretation of the chemotactic response to FGFR signaling has been addressed so far, nor has Dof been analyzed at the molecular level. Since the FGFR signaling system plays such a dominant role in cell migration in several tissues in Drosophila, a first step towards understanding the complex events linking receptor activation to cytoskeletal rearrangements is the elucidation of the role of Dof.
In this work, we first show that Dof is a cellular substrate of the Drosophila FGFRs, becomes phosphorylated upon receptor activation, and recruits the tyrosine phosphatase Corkscrew (Csw), an event essential for Ras/MAPK activation. Using Dof variant constructs and in vivo rescue assays, we find that although Ras activation is required for chemoattraction, Ras activation is not sufficient to induce tracheal cell migration. Our studies incorporate the Dof protein into the chemotactic response downstream of FGF and demonstrate that both Ras-dependent and Ras-independent events contribute to efficient cytoskeletal reorganizations, leading to cell migration.
MATERIALS AND METHODS
Drosophila strains and genetics.
The generation of the transgenic UASdof flies was described previously (42). The dof mutant dof032, identified in a mutant collection generated in the laboratory of C. Klämbt (15), contains a stop codon at position 225 of the Dof amino acid sequence. The UAStorso4021-btl (UASbtlact) flies were provided by S. Leevers and E. Hafen (derived from the hs-torso4021-FGFR1 construct described in reference 32). The btl-Gal4 line (39) was used to drive upstream activated sequence (UAS) transgenes in tracheal cells, the twist-Gal4 line (obtained from M. Akam) was used for studies of mesodermal cells, and the pannier-Gal4 line was used for studies of ectodermal cells. The mutated UASdof transgenes were introduced into the Drosophila genome by P-element-mediated germ line transformation (35). At least three independent transformant lines were analyzed for each construct. The expression of each deletion mutant protein was assayed with anti-Dof antibodies, except for transgenes 1 to 368, 1 to 484, and 485 to 600, in which expression was checked at the level of RNA.
The rescue assay was described previously (42). Briefly, UASdof-transgenes/+;dof032/+ flies were crossed to either btl-Gal4/+; dof032/+ or twi-Gal4/+; dof032/+ flies and embryos were collected overnight at 25°C. Homozygous dof mutant embryos were identified either by the lack of Eve-expressing cells at the dorsal midline, which indicate defects in mesoderm spreading (btl-Gal4 crosses), or by the lack of tracheal cell migration (twi-Gal4 crosses). Rescue of mesodermal cell migration was scored with the Evenskipped antibody (see Results).
Embryo stainings.
Embryo fixation, staining, and light and confocal fluorescence microscopy were performed as previously described (6). We used the following primary antibodies: monoclonal antibody 2A12, which stains the lumen of the tracheal tree (kindly provided by N. Patel), anti-Evenskipped (kindly provided by M. Frasch), mouse monoclonal anti-DSRF (13), and anti-double-phosphorylated extracellular signal-regulated kinase (anti-dpERK) (Sigma). Weak fluorescent signals were amplified by the use of biotinylated tyramide (NEN Life Science Product) followed by streptavidin-Texas Red or streptavidin-fluorescein secondary antibodies. Fluorescent images were captured by confocal microscopy (Leica TCS) and processed by use of Photoshop software (Adobe) and represent projections of sections on one focal plane. For the other stainings, we used biotinylated anti-mouse immunoglobulin G and biotinylated anti-mouse immunoglobulin M secondary antibodies, which were revealed by using the biotinylated horseradish peroxidase ABC kit (Vectastain).
Plasmid construction.
The constructs prepared for the rescue assay were generated by PCR using the appropriate oligonucleotides (sequences of oligonucleotides on request) and subcloned into the pUASt vector. For expression in Schneider cells, cDNA were cloned in the pMT/V5-HisA vector (Invitrogen) containing the heavy metal methalothionein promoter. All constructs were verified by sequencing before embryo injection or before transfection of S2 cells.
Cell transfection, cell lysates, and immunoprecipitation.
Drosophila Schneider (S2) cells were cultured at 25°C in Schneider's Drosophila medium (Gibco) supplemented with 10% heat-inactivated fetal calf serum, 2 mM glutamine, penicillin (50 U/ml), and streptomycin (50 μg/ml) (complete medium). For transfection experiments, 3 × 106 S2 cells were plated per 35-mm-diameter dish in complete medium. Cells were transiently transfected with different combinations of plasmids (0.6 μg total) using the Effectene reagent following the manufacturer's instructions (Qiagen). Protein expression was induced 24 h after transfection by addition of 0.6 mM CuSO4 for the indicated times. Cells were lysed in modified RIPA buffer (50 mM Tris [pH 8], 150 mM NaCl, 1% Nonidet P-40, 0.5% deoxycholate, 0.1% sodium dodecyl sulfate [SDS], 0.4 mM EDTA, 10% glycerol, 0.2 mM sodium orthovanadate, 1 mM phenylmethylsulfonyl fluoride, Protease Inhibitors Cocktail [Roche]). For immunoprecipitation assays, cell lysates were precleared with protein G-Sepharose beads (Amersham Pharmacia) for 3 h at 4°C on a rocking platform and then incubated with the primary antibody overnight at 4°C on a rocking platform. Protein-antibody complexes were recovered by incubation with protein G-Sepharose beads for 1 h at 4°C. Bead-bound complexes were washed four times with cold lysis buffer and boiled with 2× SDS sample buffer. For Western blot analysis of total cell lysates, equal amounts of protein were separated by SDS-polyacrylamide gel electrophoresis (PAGE) under reducing conditions, transferred on nitrocellulose or polyvinylidene difluoride Immobilon (Millipore) membranes, and probed using appropriate antibodies in 5% dry milk in TBS-T (20 mM Tris-HCl [pH 7.6]; 150 mM NaCl, 0.1% Tween 20). Proteins were visualized using enhanced chemiluminescence (ECL kit; Amersham Corp.) following the manufacturer's instructions. The following primary antibodies were used for immunoprecipitation and Western blot experiments: anti-Myc 9B11 (Cell Signaling), anti-V5 (Invitrogen), anti-dpERK (Sigma), anti-PY20 (Transduction Laboratories), and anti-Csw (kindly provided by L. A. Perkins).
RESULTS
Functional dissection of the Dof protein in vivo.
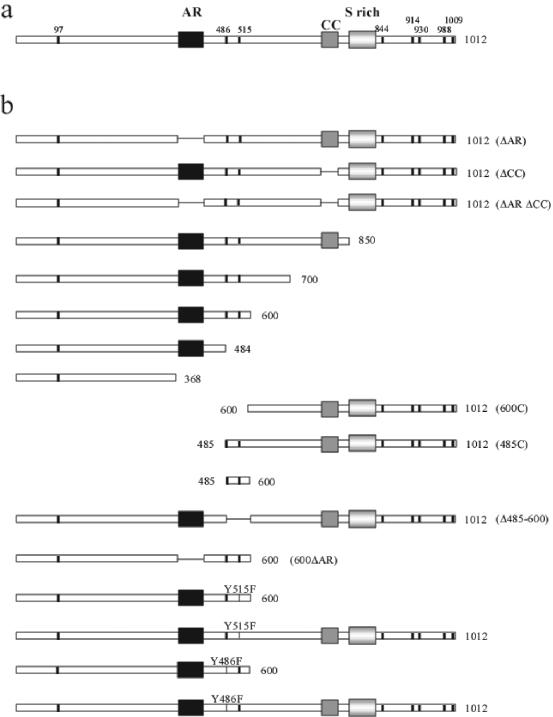
Expression of a dof cDNA under the indirect control of tissue-specific enhancers is sufficient to rescue migration defects of tracheal and mesodermal cells in a dof mutant embryo (42). We used this rescue assay for a functional analysis of the Dof protein. Modified UAS-dof transgenes were expressed with either the btl-Gal4 or the twi-Gal4 drivers in dof mutant embryos in order to identify regions of Dof that are important for its function in tracheal and mesodermal cell migration, respectively (Fig. 1; Table 1). For each construct, several transgenic lines were established and tested (see Materials and Methods). The capacity of each transgene to rescue tracheal cell migration was scored by counting the formation of different tracheal branches in rescued embryos (Table 1).
FIG. 1.
(a) Schematic representation of the Dof protein. Black box, ankyrin repeats (amino acids 373 to 431); dark grey box, coiled-coil domain (amino acids 703 to 741); light grey box, serine-rich region (amino acids 768 to 828). Tyrosine residues that could serve as SH2 domain binding sites are shown as thick vertical black lines. (b) Schematic representation of the different constructs used in the rescue assays. Each construct was placed downstream of UAS sequences.
TABLE 1.
Rescue capacities of the transgenes analyzed in this studya
| Construct | Rescue in trachea | Efficiency (arbitrary)b | Analysis of rescued embryos
|
DSRF expression | Rescue in mesodermd | ||
|---|---|---|---|---|---|---|---|
| DT breaksc | DB (n = 10) | GB (n = 10) | |||||
| Full dof | + | +++ | 0-1 (n = 24) | 10 | 9-10 | + | + |
| 1-850 | + | ++ | 2-3 (n = 25) | 8 | 6-7 | + | + |
| 1-700 | + | + | 3 (n = 29) | 8 | 7 | + | + |
| 1-600 | + | + | 4 (n = 32) | 6 | 5-6 | + | + |
| 1-484 | − | − | − | ||||
| 1-368 | − | − | − | ||||
| 600-1012 | − | − | − | ||||
| 485-1012 | − | − | − | ||||
| 485-600 | − | ND | − | ||||
| dof Δ485-600 | + | ++ | 1-2 (n = 21) | 9 | 8-9 | + | + |
| ΔAR | + | ++ | 1-2 (n = 41) | 9-10 | 9 | + | + |
| ΔCC | + | ++ | 1 (n = 15) | 9-10 | 9 | + | + |
| ΔARΔCC | + | ++ | 1-2 (n = 36) | 9-10 | 9 | + | + |
| 600ΔAR | − | + | − | ||||
| 600Y515F | − | − | − | ||||
| Y515F | + | ++ | 1-2 (n = 27) | 9 | 8 | + | + |
| 600Y486F | + | + | 5 (n = 40) | 4 | 3 | + | +/− |
| Y486F | + | ++ | 1-2 (n = 21) | 9-10 | 9-10 | + | + |
The capacity of each transgene to rescue tracheal cell migration was scored by counting the formation of different tracheal branches per side of rescued embryos. Mesodermal rescue was scored by counting Evenskipped-positive cells on the dorsal side of the embryo. Abbreviations: DT, dorsal trunk; DB, dorsal branch; GB, ganglionic branch; ND, not done.
Symbols: +, positive; ++, high; +++, very high.
n, number of rescued embryos.
Symbols: +, more than 75% of cells positive for Evenskipped compared to wild-type embryos; +/−, between 20 and 75% of cells positive for Evenskipped; −, less than 10% of cells positive for Evenskipped.
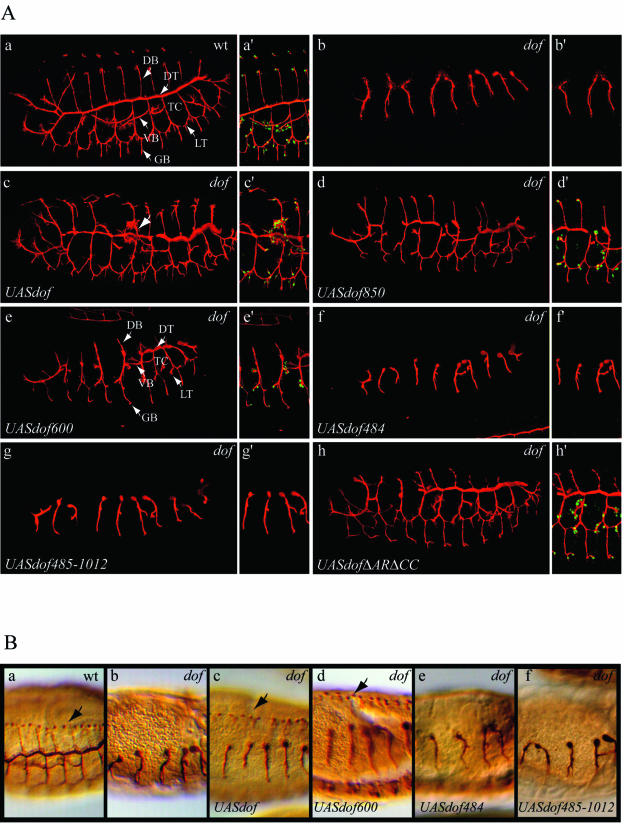
Expression of a full-length dof transgene in the tracheal system of dof mutant embryos rescues most aspects of tracheal patterning, except for the visceral branches, which do not extend properly (42) (Fig. 2A, subpanel c). This is most likely due to the fact that the underlying visceral mesoderm is not correctly specified in dof mutant embryos and tracheal cells lack the appropriate support along their migratory route (5, 42). In addition, since the mesoderm is used by most tracheal branches as a migratory substrate (8), the tracheal network of rescued mutant embryos has a less well organized appearance than that of a wild-type embryo (42) (Fig. 2A, subpanels a and c).
FIG. 2.
(A) Rescue of tracheal cell migration by altered versions of the Dof protein. The lumen of the tracheal system of embryos was visualized with the 2A12 antibody (red) (subpanels a to h and a′ to h′), and terminal cells were visualized with the anti-DSRF antibody (green) (subpanels a′ to h′). Confocal projections of a representative embryo are shown for a wt embryo (subpanels a and a′), for a dof−/− embryo (subpanels b and b′), and for rescued embryos expressing the following transgenes in a dof−/− background under the control of the btlGal4 driver: UASdof (subpanels c and c′), UASdof850 (subpanels d and d′), UASdof600 (subpanels e and e′), UASdof484 (subpanels f and f′), UASdof485-1012 (subpanels g and g′), and UASdofΔARΔCC (subpanels h and h′). Abbreviations: DB, dorsal branch; TC, transverse connective; VB, visceral branch; LT, lateral trunk; GB, ganglionic branch. The arrow in subpanel c points to the misrouted VB. (B) Rescue of mesodermal cell migration by altered versions of the Dof protein. The lumen of the tracheal system of embryos was visualized with the 2A12 antibody and the pericardial cells with anti-Evenskipped antibodies (subpanels a to f). Sections of representative embryos are shown for a wt embryo (subpanel a), for a dof−/− embryo (subpanel b), and for rescued embryos expressing the following transgenes in a dof−/− background under the control of the twiGal4 driver: UASdof (subpanel c), UASdof600 (subpanel d), UASdof484 (subpanel e), and UASdof485-1012 (subpanel f). Arrows point to the presence of Eve-positive cells (subpanels a, c, and d).
Deletion from the C terminus showed that transgenes coding for Dof proteins containing at least the N-terminal 600 amino acids were able to substitute for the lack of endogenous Dof function in vivo and allowed for a significant rescue of tracheal cell migration, albeit with somewhat different quantitative efficiencies (Fig. 2A; Table 1). One of the major defects observed in these rescued tracheal trees was a discontinuity of the dorsal trunk (DT), with an average of two to three DT breaks for the Dof850 transgene and of four breaks for the Dof600 transgene (Fig. 2A; Table 1). In addition, the transgenes displayed various efficiencies in rescuing the formation of dorsal branches (DB) and ganglionic branches (GB) (Table 1). Expression of the Drosophila Serum Response Factor/blistered (DSRF/bs) gene, a transcriptional target of Bnl/FGF signaling in terminal tracheal cells (13, 22, 23), was also recovered in the tracheal trees of rescued embryos (Fig. 2A; Table 1). Although expression of the first 600 amino acids of the protein (Dof600) allowed only for a partial rescue of tracheal morphogenesis, all six types of primary branches were formed, and branch fusion occurred in many segments (Fig. 2A, subpanel e), suggesting that this part of the protein retains the capacity to read out the activation state of the FGFR and to relay the signal to the migration machinery. Thus, Dof600 will be considered as a minimal Dof protein. Deletion of an additional 116 amino acids from the C terminus of Dof600 abolished the function of this minimal Dof600 protein (construct dof1-484 [Fig. 2A; Table 1]).
The loss of rescue activity upon the deletion of amino acids 485 to 600 in Dof600 suggests that this region is crucial for the function of Dof in tracheal cell migration. However, this region was found to be sufficient neither to rescue tracheal cell migration of dof mutant embryos by itself (construct dof485-600) nor when associated with the C-terminal part of the protein (construct dof485-1012) (Fig. 2; Table 1). These results demonstrate that the first 484 amino acids of the Dof protein are essential for its function. A full-length Dof protein, in which amino acids 485 to 600 were deleted (construct dofΔ485-600), also rescued tracheal cell migration (Table 1). This demonstrates that although the region of amino acids 485 to 600 plays a crucial role in the minimal Dof600 protein, its function might be redundantly contained in the C terminus of the Dof protein.
Somewhat surprisingly, expression of transgenes lacking the two recognizable domains of Dof, the coiled-coil domain and the ankyrin repeats, allowed for an efficient rescue of tracheal cell migration, suggesting either that these domains are not necessary for Dof function or, alternatively, that they might carry out functions that are redundant with other parts of the molecule. We favor the second hypothesis since deletion of the ankyrin repeats in the Dof600 protein completely abolished the function of the minimal Dof600 protein in the migration rescue assay (construct dof600ΔAR [Table 1] [also see below and Fig. 6A, subpanel e]).
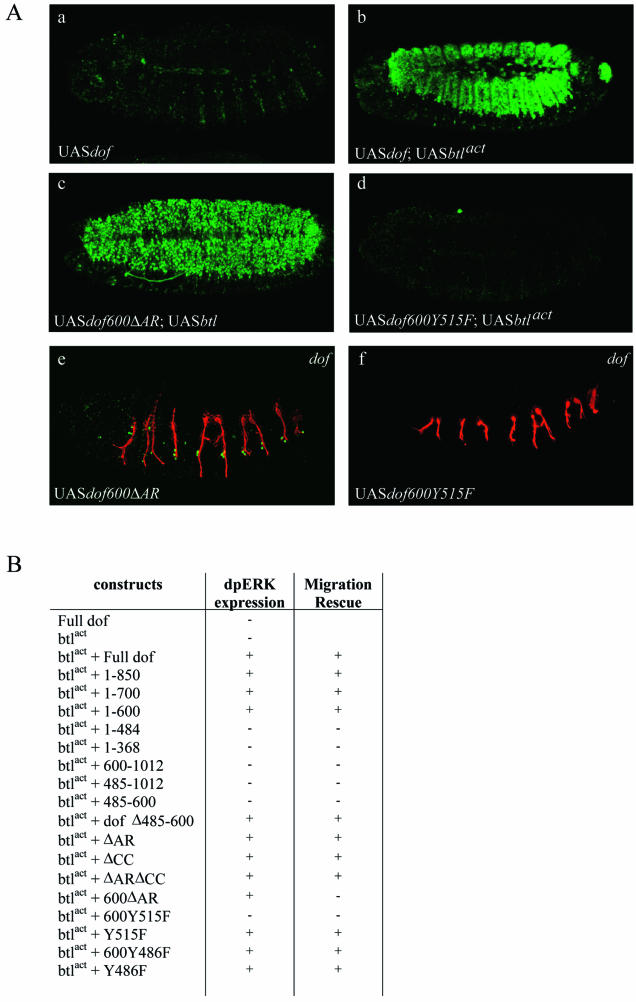
FIG.6.
(A) Activation of the MAPK pathway is important but not sufficient to direct cell migration. Activation of the MAPK pathway wasvisualized with anti-dpERK antibodies (subpanels a to d), the lumen of the tracheal system with the 2A12 antibody in red and terminal cells with the anti-DSRF antibody in green (subpanels e and f). Confocal projections are shown for embryos expressing the following transgenes under the control of the pannierGal4 driver: UASdof (subpanel a), UASdof and UASbtlact (subpanel b), UASdof600ΔAR and UASbtlact (subpanel c) and UASdof600Y515F and UASbtlact (subpanel d). (Subpanels e and f) Embryos expressing UASdof600ΔAR or UASdof600Y515F in a dof−/− background under the control of the btlGal4 driver are shown. (B) Summary table of the capacity of each transgene to activate dpERK in combination with an activated form of btl. The capacity of each dof transgene to rescue cell migration in dof−/− embryos is also shown in the second column.
As already mentioned, Dof is also essential for mesodermal cell spreading and migration, and this requirement can also be rescued by expression of a full-length dof transgene in the mesoderm of dof mutant embryos with the twi-Gal4 driver (42) (Fig. 2B, subpanel c). Using the same strategy as for the tracheal system, we tested the capacity of the different dof transgenes to rescue mesodermal cell migration of dof mutant embryos, monitoring the development of pericardial cells that express Evenskipped in the dorsal region of the embryo (Fig. 2B; Table 1). Similar to the results observed for tracheal cell migration, the first 600 amino acids of Dof are sufficient to rescue mesoderm migration and pericardial development of dof mutant embryos; deletion of either the region containing amino acids 485 to 600 or the first 484 amino acids from the minimal Dof600 protein completely abolished mesodermal rescue activity (Fig. 2B; Table 1). The coiled-coil region and the ankyrin repeats were also dispensable when deleted in the context of full-length Dof (Table 1); however, deletion of the ankyrin repeat in the context of the minimal Dof600 protein abolished activity in vivo.
Based on these observations, we conclude that the first 484 amino acids of Dof are absolutely essential for its function in tracheal and mesodermal cell migration. When fused to these N-terminal sequences, the region between residues 485 and 600 of Dof (or alternatively the much longer region between 600 and the C terminus) can mediate the migratory response in tracheal and mesodermal cells upon FGFR activation. Based on these results, we concentrated our efforts on the identification of the role of the N-terminal sequences (amino acids 1 to 484) and the C-terminal sequences (amino acids 485 to 600) of the minimal Dof600 protein in the chemotactic response.
Dof is specifically phosphorylated upon FGF signaling in Schneider cells.
In order to address molecular aspects of the function of Dof in FGF signaling, we activated the FGF signaling pathway in Drosophila Schneider cells (S2) by expressing constitutive active versions of either of the two FGFRs (Btl or Htl) together with Dof under the control of a heavy metal-inducible promoter (see Materials and Methods).
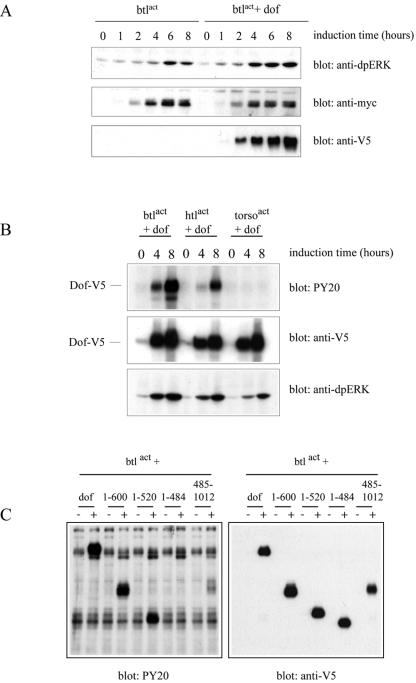
It has previously been argued that Dof is an essential signaling component for the FGF pathway since in its absence, FGF-dependent ERK phosphorylation and hence activation of the Ras/MAPK pathway are not observed (16, 21, 42). Furthermore, Dof was found to be exclusively needed for FGF-mediated signal transduction since other RTK signaling systems, such as Torso or EGF, can activate the MAPK cascade in dof mutant embryos and do not rely on Dof for efficient signaling (6, 21, 42). To characterize our reconstituted cell culture system, we first investigated the requirement for Dof to activate the MAPK pathway in S2 cells upon FGF induction. FGF signaling was induced in S2 cells by expressing constitutive active versions of Btl or Htl, which consisted of fusions between the transmembrane and the extracellular domain of the Torso4021 protein and the intracellular domain of each FGFR (32). Compared with an activated form of Btl (Torso4021-Btl; Btlact) expressed alone in S2 cells, coexpression of Btlact with Dof significantly increased MAPK activity as revealed by anti-dpERK antibody detection on Western blot (Fig. 3A) (9, 10). These results suggest that similar to the situation observed in vivo, Dof is important to fully activate ERK upon FGFR activation in S2 cells.
FIG. 3.
(A) Dof is important to activate ERK upon FGF signaling. S2 cells transfected with myc-btlact alone or in combination with dof-V5 were lysed after induction of expression for the indicated times with 0.6 mM CuSO4. Whole-cell lysates were subjected to SDS-PAGE, and ERK phosphorylation levels were examined by immunoblot analysis with anti-dpERK antibodies. Protein levels were evaluated for myc-Btlact and Dof-V5. (B) Dof is specifically phosphorylated upon FGF signaling. S2 cells were transfected with the indicated combination of constructs and lysed after induction of expression for the indicated times. Tyrosine phosphorylation levels were investigated on whole cell extracts by antiphosphotyrosine immunoblotting (upper panel), Dof-V5 expression levels by anti-V5 immunodetection (middle panel) and activation of ERK by anti-dpERK immunoblotting (bottom panel). (C) The different Dof proteins are not phosphorylated to the same extent. S2 cells were transfected with the indicated combination of expression plasmids and lysed after 8 h of induction (+). Transfected cells left untreated for the same time were used as controls (−). Tyrosine phosphorylation of the different constructs was assayed with antiphosphotyrosine antibodies (left panel) and protein expression levels by anti-V5 immunoblotting (right panel).
We then asked whether Dof was a specific substrate of FGF signaling and investigated its phosphorylation level upon FGFR or Torso signaling in S2 cells. As shown in Fig. 3B, expression of constitutive active Btl or Htl led to strong tyrosine phosphorylation of Dof, demonstrating that Dof is indeed a phosphotarget, either a direct or an indirect one, of both Drosophila FGFRs (see below). Interestingly, Dof remained unphosphorylated upon signaling from the Torso RTK (Fig. 3B), demonstrating that Dof is not a target of all RTKs.
The first 600 amino acids of the protein, defined through the in vivo rescue experiments as the minimal Dof600 protein, were phosphorylated on tyrosine residues in response to Btl/FGFR activation to a similar extent as the full-length protein (Fig. 3C). In contrast, no tyrosine phosphorylation was detected on the shorter Dof484 protein under the same conditions of induction (Fig. 3C). These observations link our results obtained with the in vivo rescue assays to the state of phosphorylation of Dof in S2 cells, demonstrating that the region between amino acids 485 and 600 is necessary for Dof to be recognized or used as a substrate for FGFR-dependent phosphorylation. Interestingly, Dof derivatives (Dof485C and Dof600C) comprising only the C-terminal part of Dof (thus missing the first 484 amino acids) were found to be phosphorylated to a much lesser extent (Fig. 3C and data not shown; see also below).
Dof interacts with both FGF receptors.
Since Dof becomes phosphorylated upon FGF signaling (above) and genetically acts downstream of both FGFRs (21, 42), we investigated whether Dof interacted with the FGFRs Btl and Htl.
S2 cells were cotransfected with myc-tagged versions of btlact or htlact together with V5-tagged wild-type dof or deletion mutant forms of dof. To investigate the ability of Dof to form a complex with the receptor, we analyzed the capacity of these two proteins to coimmunoprecipitate from S2 cell lysates. As shown in Fig. 4, full-length Dof or the minimal Dof600 protein formed a complex with the activated version of both Drosophila FGFRs upon induction. With the aim to determine whether this interaction was dependent on the constitutive kinase activity of Btlact, we generated a wild-type btl construct. However, expression of this construct in S2 cells led to ligand-independent kinase activation, possibly due to dimer formation caused by overexpression of the receptor (data not shown). Therefore, we could not assay the interaction of Dof with a nonactivated form of the receptor. However, Dof has recently been shown to directly interact with the kinase domain of both Htl and Btl in yeast two-hybrid assays (3), suggesting that this interaction might be constitutive and not dependent on the activation state of the FGFRs. More recently, we have now shown that a kinase-dead version of Btl still interacts with Dof, despite its inability to phosphorylate itself or the associated Dof protein (data not shown).
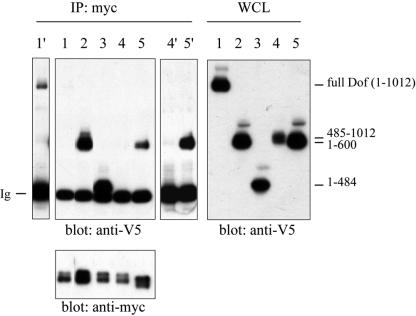
FIG. 4.
Dof interacts with both Drosophila FGFRs. S2 cells transfected with various plasmids (lane 1, myc-btlact + dof-V5; lane 2, myc-btlact + dof600-V5; lane 3, myc-btlact + dof485-V5; lane 4, myc-btlact + dof485-1012-V5; lane 5, myc-htlact + dof600-V5) were lysed after 8 h of induction with 0.6 mM CuSO4. Whole-cell lysates (WCL) were immunoprecipitated (IP) with anti-myc antibodies, subjected to SDS-PAGE and immunoblotted with anti-V5 antibodies (left top panel) or anti-myc antibodies (left bottom panel). Protein expression levels were assayed by anti-V5 immunoblotting (right panel). Overexposure of the anti-V5 immunoblot is shown for lane 1 (lane 1′) and lanes 4 and 5 (lanes 4′ and 5′). The weaker interaction of Dof compared to Dof600 might be due to an intramolecular interaction of the N and C terminus of Dof (3). Ig, immunoglobulins.
The shorter Dof484 protein was also coimmunoprecipitated with Btl (Fig. 4), although it is not phosphorylated upon FGF signaling (Fig. 3). No interaction with Btl was detected when the Dof600C or the Dof485C proteins, consisting of the C-terminal parts of the Dof protein, were coexpressed with the receptor in S2 cells (Fig. 4 and data not shown). These results indicate that the region of Dof required to form an efficient complex with the receptor resides in the most N-terminal part of the protein, in the first 484 amino acids. These observations also suggest that the inability of the C-terminal part of Dof (lacking the first 484 amino acids) to rescue tracheal or mesodermal migration might be due to the incapacity of these proteins to form a complex with the FGFRs and to become efficiently phosphorylated.
In order to fine-map the interaction domain between Dof and the FGFRs, a series of deletion constructs were generated in the context of the minimal Dof600 protein, coexpressed with Btlact in S2 cells and tested for their ability to coimmunoprecipitate with the FGFR. The following deletion constructs were generated: dof120-600, dof600Δ120-240, dof600Δ217-288, dof600Δ240-373, dof600Δ373-436 (corresponding to dof600ΔAR), and dof600Δ436-486. All these deletion mutant versions of Dof600 retained the ability to form a complex with Btl (data not shown), suggesting that Dof600 might contain several redundant receptor interaction domains.
Tyrosine 515 is essential for the function of the Dof600 protein in vivo and serves as a Csw binding site.
We have shown above that the region between amino acids 485 and 600 of the minimal Dof protein is important to mediate mesodermal and tracheal cell migration in vivo and that this region is essential for Dof to become a phosphoprotein upon FGF signaling. These observations suggest that this region either becomes phosphorylated on tyrosine residues upon FGF signaling or is important for Dof to be recognized by the activated receptor as a substrate.
Dof contains five tyrosine residues in the region 485 to 600 (Y486, Y515, Y563, Y564, and Y592 [42]). One of these tyrosines is embedded in an environment mimicking a putative consensus binding site for the SH2 domain of PI3K (Y486, pYMEM) and one represents a potential binding site for the SH2 domain of the nonreceptor tyrosine phosphatase Csw (Y515, LNpYISV). To find out whether these putative SH2 binding sites are critical for Dof function when phosphorylated, we replaced each of the two tyrosines with the nonphosphorylable residue phenylalanine and tested the capacity of the altered proteins to mediate tracheal and mesodermal cell migration in our in vivo rescue assay.
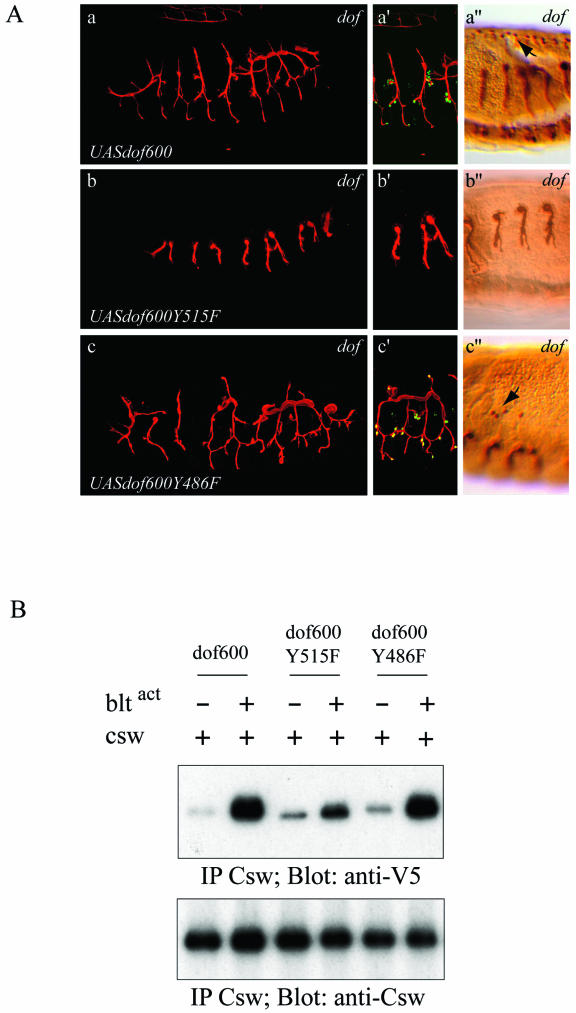
Expression of a transgene coding for the minimal Dof600 protein, in which tyrosine 515 is replaced by phenylalanine, did not allow rescue of tracheal cell migration in dof mutant embryos, and no activation of DSRF was observed (Fig. 5A, subpanels b and b′). This demonstrates that residue 515 plays an important role for the function of the minimal Dof protein in vivo. In contrast, mutation of tyrosine 486 only slightly affected the function of the minimal Dof protein, and rescue of tracheal cell migration and DSRF expression was still readily observed (Fig. 5A, subpanels c and c′; Table 1). The same transgenes were also tested for their ability to restore mesodermal cell migration, and consistent with our finding in the trachea, we observed that dof600Y515F did not rescue mesodermal spreading in dof mutant embryos (Fig. 5A, subpanels b", and c"; Table 1). In contrast, the Dof600Y486F protein, although somewhat less efficient than the Dof600 protein, retained the ability to rescue mesodermal cell migration. These observations demonstrate that tyrosine 515 of Dof600 plays a crucial role in both tracheal and mesodermal cell migration.
FIG.5.
(A) Rescue of tracheal and mesodermal cell migration by altered versions of the Dof600 protein. The lumen of the tracheal system of embryos was visualized with the 2A12 antibody in red (subpanels a to c and a′ to c′) or in brown (subpanels a" to c"), terminal cells were visualizedwith the anti-DSRF antibody in green (subpanels a′ to c′) and the pericardial cells with anti-Evenskipped antibodies in brown (subpanels a" to c"). Confocal projections are shown for a representative rescued embryo expressing the following transgenes in a dof−/− background under the control of the btlGal4 driver: UASdof600 (subpanels a and a′), UASdof600Y515F (b and b′) and UASdof600Y486F (subpanels c and c′). Sections are shown for representative rescued embryos expressing the following transgenes in a dof−/− background under the control of the twiGal4 driver: UASdof600 (subpanel a"), UASdof600Y515F (subpanel b"), and UASdof600Y486F (subpanel c"). (B) Tyrosine 515 serves as a Csw binding site. S2 cells cotransfected with csw and the different forms of V5-tagged dof600 as indicated on the figure, with or without myc-btlact, were lysed after 8 h of induction with 0.6 mM CuSO4. Whole-cell lysates were immunoprecipitated with anti-Csw antibodies, subjected to SDS-PAGE and immunoblotted with anti-V5 antibodies (upper panel) and anti-Csw antibodies (bottom panel).
Since residue 515 is part of a putative consensus binding site for SH2 domains of the phosphatase Csw, we investigated the capacity of Dof and Csw to form a protein complex in S2 cells. No constitutive interaction was detected between Csw and Dof600 in S2 cell extracts in the absence of FGF signaling (Fig. 5B, lane 1). However, Csw and Dof600 formed a complex upon FGF stimulation (Fig. 5B, lane 2). Substitution of the tyrosine 515 with phenylalanine significantly weakened the formation of a Csw/Dof complex upon FGF stimulation, demonstrating that tyrosine 515 of Dof plays an important role in the recruitment of Csw. Mutation of tyrosine 486 had no effect on the recruitment of Csw (Fig. 5B, lane 6).
Based on these observations and the in vivo rescue experiments, we conclude that the recruitment of Csw by Dof upon FGF signaling is important for tracheal and mesodermal cell migration. These results are in line with genetic experiments showing that mutations in csw produce a phenotype identical to bnl, btl, and dof; i.e., tracheal and mesodermal cells fail to migrate (17, 30) (see Discussion).
Recruitment of Csw via Dof is essential for the activation of the MAPK pathway.
The cellular responses to FGF signaling in tracheal and mesodermal cells include the formation of dynamic filopodial extensions (33, 36), the activation of the Ras/MAPK pathway (9, 10), and the induction of nuclear gene expression—for example, the activation of DSRF/bs in terminal tracheal cells (13, 41). In order to find out whether each of the deletion constructs we generated affected these responses in a similar manner, we assayed their capacity to activate the Ras/MAPK pathway and the DSRF/bs gene (Fig. 6; Table 1).
To measure the in vivo activation of the Ras/MAPK pathway, we made use of the observation (Alain Jung, unpublished data) that simultaneous expression of the activated form of the btl receptor and a dof transgene in dorsal ectodermal cells under the indirect control of the pannier-Gal4 driver is sufficient to activate the MAPK pathway, as revealed with anti-dpERK immunostaining (Fig. 6A, subpanel b). Expression of either construct alone was not sufficient to lead to an accumulation of dpERK (Fig. 6A, subpanel a). Using this assay, we tested all the transgenes described above (Table 1) for dpERK staining in the dorsal ectoderm. As shown in Fig. 6, we observed a rather strict correlation between the transgenes rescuing tracheal cell migration and the ones that activated the MAPK cascade upon FGF signaling, with the exception of dof600ΔAR (see below). The failure of the dof600Y515F transgene to activate the Ras/MAPK pathway, combined with the previous observations that tyrosine 515 is essential for rescue function in vivo and interaction with Csw in S2 cells, suggest that the recruitment of Csw to the activated receptor complex is a crucial step in the FGFR-dependent activation of Ras and the MAPK pathway, and that Ras signaling might play a crucial role in FGF-guided cell migration.
Activation of the MAPK cascade is not sufficient to direct cell migration.
Surprisingly, coexpression of the dof600ΔAR transgene with btlact resulted in the phosphorylation of ERK, despite the failure of this transgene to rescue tracheal cell migration (Fig. 6A, subpanels c and e). When we assayed the tracheal system in dof mutant embryos expressing Dof600ΔAR, we indeed found that despite the lack of migration, DSRF/Bs was expressed in a subset of terminal tracheal cells (Fig. 6A, subpanels c and e). These results suggest that local activation of Ras/MAPK pathway is sufficient to activate transcriptional target genes of FGFR signaling, but not tracheal cell migration. A number of other observations support this interpretation; these will be outlined in detail in the Discussion.
DISCUSSION
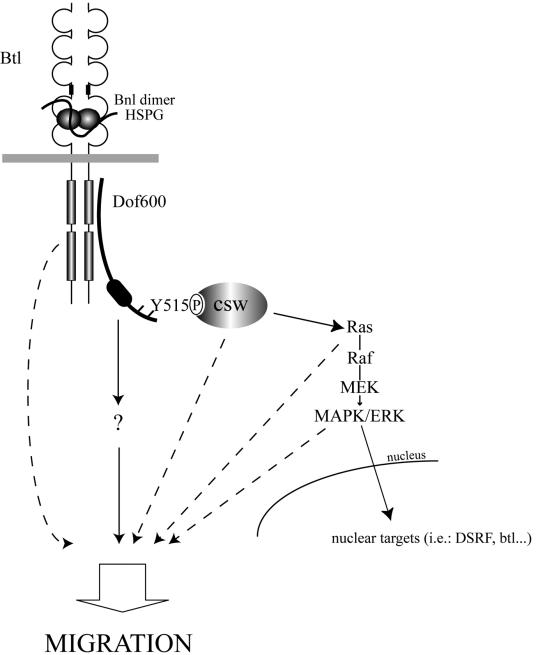
We have performed a functional characterization of Dof, a putative adapter protein that functions specifically in FGF signal transduction in Drosophila. By combining reverse genetics, cell culture assays, and biochemical approaches we have demonstrated that Dof is indeed a substrate for the Drosophila FGFRs. After defining a minimal Dof rescue protein, we identified two regions of Dof important for its function in mesodermal and tracheal cell migration. The N-terminal 484 amino acids are required for the recruitment of Dof to the FGFRs, while tyrosine 515 becomes phosphorylated upon signaling and recruits the phosphatase Csw. Although Csw recruitment represents an essential step towards the activation of the Ras/MAPK pathway, the activation of the latter does not appear to be sufficient to signal to the migration machinery in tracheal and mesodermal cells. Additional proteins binding to the activated receptor, to Dof, or unidentified substrates for Csw appear to be crucial for a chemotactic response (schematically represented in Fig. 7).
FIG. 7.
Schematic model of the FGF signaling pathway in the control of cell migration in Drosophila. HSPG, heparan sulfate proteoglycan. See Results and Discussion for details.
Dof is an FGFR-specific scaffolding protein.
Genetic epistasis experiments have shown that Dof functions downstream of the activated FGFRs and upstream or in parallel to Ras (16, 21, 42). However, the biochemical function of Dof in the interpretation of the chemotactic response to FGFR signaling has not been addressed so far. Using in vivo rescue assays, we first identified a minimal Dof protein containing the first 600 amino acids of Dof that allows rescue of both mesodermal and tracheal cell migration. Although the rescue in the tracheal system is not as efficient as the rescue observed with the wild-type construct, all six branches can migrate out, demonstrating that the first 600 amino acids of Dof retains the capacity to read out the local activation state of the FGFRs and to relay the signal to the migration machinery, albeit with somewhat reduced efficiency. Deletion from the C terminus of this dof minigene, as well as internal deletions, resulted in loss of rescue capacity, thus identifying regions of functional importance.
We have built on the results obtained in this in vivo assay and analyzed all of the constructs in a Drosophila S2 cell culture assay, in which we activated either the FGFR or the Torso signaling system. We found that both full-length Dof and Dof600 are phosphorylated on tyrosine residues upon FGF signaling, but that Torso cannot use Dof as a substrate. These results are consistent with in vivo data showing that Dof is exclusively needed for FGF-mediated signal transduction and that Torso is able to activate the MAPK cascade in the absence of Dof in dof mutant embryos (6, 21, 42). Using coimmunoprecipitation experiments, we show that Dof forms a complex with both FGFRs and that the first 484 amino acids, although not phosphorylated upon FGF signaling, are required and sufficient for the association with the FGFRs, demonstrating that phosphorylation of Dof is not necessary for complex formation. Our cell culture analysis is in line with a recent report showing that the N-terminal part of Dof directly interacts with the kinase domains of Btl and Htl in yeast two hybrid assays (3). In addition, we have observed that both the juxtamembrane and the C terminus of Btl can be deleted without affecting considerably the quality of the rescue capacity of the receptor (data not shown). Thus, it appears that Dof directly docks onto the kinase domain of the FGF receptor, in contrast to the vertebrate FGFR adapter SNT/FRS2, which interacts with a sequence motif in the juxtamembrane region of the receptor (27, 44).
Csw is recruited to the FGFR signaling complex via Dof and is required for FGF guided cell migration.
Since Dof becomes phosphorylated upon FGFR signaling in S2 cells, we asked whether we could identify functionally important phosphorylation sites, the proteins recognizing these sites in the phosphorylated state, and confirm our results in vivo by making use of the rescue assay and genetic analysis. Two potential phosphorylation target sites were identified by sequence analysis in the essential region comprising amino acids 485 to 600. While mutation of each individual site resulted in reduced phosphorylation of Dof600 in S2 cells upon FGFR signaling (data not shown), mutation of only one of these sites, tyrosine 515, abolished the migration rescue capacity in vivo. Since the functionally required tyrosine residue was part of a putative consensus binding site for the SH2 domain of the nonreceptor tyrosine phosphatase Csw/SHP-2, we tested the interaction of Csw with Dof using coimmunoprecipitation experiments and confirmed that Csw was indeed recruited to the activated signaling complex via Dof. We found in our rescue assays that both the region 485 to 600 as well as the region from 600 to the C terminus (construct dofΔ485-600) were able to confer function to the signaling-deficient N terminus (residues 1 to 484). We know that the C-terminal sequences also recruit the Csw phosphatase in the absence of tyrosine 515 (data not shown), but we do not know whether they do so directly or indirectly. Further deletion analyses and biochemical studies will be required to address this question.
Genetic evidence supporting an interaction between Dof and Csw was provided some time ago by the finding that mutations in csw produce a phenotype identical to bnl, btl, and dof; i.e., tracheal and mesodermal cells fail to migrate (17, 30). The sum of these results clearly assign a crucial role for both Dof and Csw downstream of the FGFRs in the migratory response, indicating that the ligand-dependent phosphorylation of Dof leads to the recruitment of Csw to the signaling complex, ultimately triggering cell locomotion. SHP-2, the vertebrate homologue of Csw, has been shown to be required at the initial steps of gastrulation, as mesodermal cells migrate away from the primitive streak in response to chemotactic signals initiated by fibroblast growth factors (37). In addition, SHP-2 has also been found to be crucial for tubulogenesis and for the sustained stimulation of the ERK/MAPK pathway upon induction of another chemotactic factor, the hepatocyte growth factor/scatter factor (for a review, see reference 34), thus placing SHP-2/Csw as a key player in branching morphogenesis induced by diverse chemotactic factors. Therefore, it appears that both in invertebrates and vertebrates, SHP-2/Csw plays a major role in RTK signaling in the control of cell migration. The similarity of the Drosophila FGF signal transduction pathway to the vertebrate FGF pathway make the fly system accessible to address future issues not resolved in vertebrates, such as the targets of SHP-2/Csw involved in Ras activation and/or cell migration.
Ras/MAPK activation is required but not sufficient for FGF-guided cell migration.
Using the dpERK antibody as a readout for the activation of the Ras/MAPK pathway in vivo, we find that abolishing the interaction between the Dof600 minimal protein and Csw abolishes the activation of the MAPK cascade upon FGFR signaling. The strong correlation we found between migration and MAPK activation when analyzing all mutant dof constructs in this assay (Fig. 6B) might indicate that local activation of the Ras/MAPK pathway in tracheal tip cells is sufficient to trigger the migratory response upon Btl signaling (see also (33). However, two lines of evidence make us believe that this might not be the case.
On the one hand, we previously observed that under conditions in which all tracheal cells sustain high levels of Ras/MAPK activity (upon RasV12 overexpression), tracheal cells migrate normally in wild-type embryos (data not shown; see also (16). In sharp contrast, ectopic expression of the Bnl ligand leads to a complete disruption of directed migration (data not shown; see reference 41). Therefore, high levels of Ras/MAPK activity do not appear to produce the same migratory response as ligand-activated FGFR signaling. Indeed, and again in contrast to ectopic Bnl (see reference 33), overexpression of RasV12 in wild-type embryos does not produce significant filopodial activity in DT tracheal cells (Marc Neumann, unpublished results), confirming that the activation of Ras is not sufficient to produce cytoskeletal rearrangements by itself.
On the other hand, we also observed that while the Dof600 protein lacking the ankyrin repeats did allow FGFR-dependent activation of the Ras/MAPK pathway and downstream nuclear response genes, this protein failed to induce migration. Thus, even local Ras activation under the control of the endogenous ligand Bnl, Btl, and Dof600ΔAR is unable to activate the migratory machinery. Interestingly, it has also been reported that Ras activation is insufficient to guide RTK-mediated border cell migration during Drosophila oogenesis (7).
Is Ras activation then required at all for cells to produce a cytoskeletal response and migrate directionally? Unfortunately, genetic analysis cannot be used to directly address this question in the embryo since maternal and zygotic loss of Ras activity results in embryos that do not develop far enough to analyze the tracheal system. However, when activated Ras (RasV12) is expressed in the tracheal system or in the mesoderm of dof mutant embryos, a certain rescue of migration can be obtained (data not shown; see also references 16, 21, and 42). This suggests that Ras signaling is essential but not sufficient for efficient FGFR-dependent cell migration; additional proteins binding to the receptor, to Dof or to Csw appear to be crucial for a chemotactic response. To analyze the role of Ras experimentally and in detail, mitotic clones lacking Ras activity should be analyzed with regard to their migration properties. Recent reports concerning the role of FGF signaling in the migration of mesodermal and tracheal cells during late larval development might provide the basis for such analyses (2, 36).
Acknowledgments
We thank Christian Klämbt for allowing us to screen the mutant collection from which the dof032 allele was isolated. We thank Stéphane Vincent and Alain Jung for their contributions in an early phase of the project as well as Kristi Wharton and Carlos Ribeiro for comments on the manuscript. Many thanks also go to Marc Neumann and Carlos Ribeiro for discussion. Special thanks go to Shigeo Hayashi, Ernst Hafen, Sally Leevers, Michael Akam, Liz Perkins, Nipam Patel, and Manfred Frasch for providing stocks, plasmids, and antibodies used in this study.
This work was supported by grants form the Swiss National Science Foundation and by the Kantons Basel-Stadt and Basel-Land.
REFERENCES
- 1.Affolter, M., and R. Mann. 2001. Development. Legs, eyes, or wings-selectors and signals make the difference. Science 292:1080-1081. [DOI] [PubMed] [Google Scholar]
- 2.Ahmad, S. M., and B. S. Baker. 2002. Sex-specific deployment of FGF signaling in Drosophila recruits mesodermal cells into the male genital imaginal disc. Cell 109:651-661. [DOI] [PubMed] [Google Scholar]
- 3.Battersby, A., A. Csiszar, M. Leptin, and R. Wilson. 2003. Isolation of proteins that interact with the signal transduction molecule Dof and identification of a functional domain conserved between Dof and vertebrate BCAP. J. Mol. Biol. 329:479-493. [DOI] [PubMed] [Google Scholar]
- 4.Beiman, M., B. Z. Shilo, and T. Volk. 1996. Heartless, a Drosophila FGF receptor homolog, is essential for cell migration and establishment of several mesodermal lineages. Genes Dev. 10:2993-3002. [DOI] [PubMed] [Google Scholar]
- 5.Boube, M., M. D. Martin-Bermudo, N. H. Brown, and J. Casanova. 2001. Specific tracheal migration is mediated by complementary expression of cell surface proteins. Genes Dev. 15:1554-1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dossenbach, C., S. Rock, and M. Affolter. 2001. Specificity of FGF signaling in cell migration in Drosophila. Development 128:4563-4572. [DOI] [PubMed] [Google Scholar]
- 7.Duchek, P., and P. Rorth. 2001. Guidance of cell migration by EGF receptor signaling during Drosophila oogenesis. Science 291:131-133. [DOI] [PubMed] [Google Scholar]
- 8.Franch-Marro, X., and J. Casanova. 2000. The alternative migratory pathways of the Drosophila tracheal cells are associated with distinct subsets of mesodermal cells. Dev. Biol. 227:80-90. [DOI] [PubMed] [Google Scholar]
- 9.Gabay, L., R. Seger, and B. Z. Shilo. 1997. In situ activation pattern of Drosophila EGF receptor pathway during development. Science 277:1103-1106. [DOI] [PubMed] [Google Scholar]
- 10.Gabay, L., R. Seger, and B. Z. Shilo. 1997. MAP kinase in situ activation atlas during Drosophila embryogenesis. Development 124:3535-3541. [DOI] [PubMed] [Google Scholar]
- 11.Gisselbrecht, S., J. B. Skeath, C. Q. Doe, and A. M. Michelson. 1996. heartless encodes a fibroblast growth factor receptor (DFR1/DFGF-R2) involved in the directional migration of early mesodermal cells in the Drosophila embryo. Genes Dev. 10:3003-3017. [DOI] [PubMed] [Google Scholar]
- 12.Glazer, L., and B. Z. Shilo. 1991. The Drosophila FGF-R homolog is expressed in the embryonic tracheal system and appears to be required for directed tracheal cell extension. Genes Dev. 5:697-705. [DOI] [PubMed] [Google Scholar]
- 13.Guillemin, K., J. Groppe, K. Ducker, R. Treisman, E. Hafen, M. Affolter, and M. A. Krasnow. 1996. The pruned gene encodes the Drosophila serum response factor and regulates cytoplasmic outgrowth during terminal branching of the tracheal system. Development 122:1353-1362. [DOI] [PubMed] [Google Scholar]
- 14.Hadari, Y. R., N. Gotoh, H. Kouhara, I. Lax, and J. Schlessinger. 2001. Critical role for the docking-protein FRS2 alpha in FGF receptor-mediated signal transduction pathways. Proc. Natl. Acad. Sci. USA 98:8578-8583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hummel, T., K. Schimmelpfeng, and C. Klambt. 1999. Commissure formation in the embryonic CNS of Drosophila. Dev. Biol. 209:381-398. [DOI] [PubMed] [Google Scholar]
- 16.Imam, F., D. Sutherland, W. Huang, and M. A. Krasnow. 1999. stumps, a Drosophila gene required for fibroblast growth factor (FGF)-directed migrations of tracheal and mesodermal cells. Genetics 152:307-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson Hamlet, M. R., and L. A. Perkins. 2001. Analysis of corkscrew signaling in the Drosophila epidermal growth factor receptor pathway during myogenesis. Genetics 159:1073-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klambt, C., L. Glazer, and B. Z. Shilo. 1992. breathless, a Drosophila FGF receptor homolog, is essential for migration of tracheal and specific midline glial cells. Genes Dev. 6:1668-1678. [DOI] [PubMed] [Google Scholar]
- 19.Kouhara, H., Y. R. Hadari, T. Spivak-Kroizman, J. Schilling, D. Bar-Sagi, I. Lax, and J. Schlessinger. 1997. A lipid-anchored Grb2-binding protein that links FGF-receptor activation to the Ras/MAPK signaling pathway. Cell 89:693-702. [DOI] [PubMed] [Google Scholar]
- 20.Mann, R. S., and S. B. Carroll. 2002. Molecular mechanisms of selector gene function and evolution. Curr. Opin. Genet. Dev. 12:592-600. [DOI] [PubMed] [Google Scholar]
- 21.Michelson, A. M., S. Gisselbrecht, E. Buff, and J. B. Skeath. 1998. Heartbroken is a specific downstream mediator of FGF receptor signalling in Drosophila. Development 125:4379-4389. [DOI] [PubMed] [Google Scholar]
- 22.Montagne, J., J. Groppe, K. Guillemin, M. A. Krasnow, W. J. Gehring, and M. Affolter. 1996. The Drosophila Serum Response Factor gene is required for the formation of intervein tissue of the wing and is allelic to blistered. Development 122:2589-2597. [DOI] [PubMed] [Google Scholar]
- 23.Nussbaumer, U., G. Halder, J. Groppe, M. Affolter, and J. Montagne. 2000. Expression of the blisteref/DSRF gene is controlled by different morphogens during Drosophila trachea and wing development. Mech. Dev. 96:27-36. [DOI] [PubMed] [Google Scholar]
- 24.Okada, T., A. Maeda, A. Iwamatsu, K. Gotoh, and T. Kurosaki. 2000. BCAP: the tyrosine kinase substrate that connects B cell receptor to phosphoinositide 3-kinase activation. Immunity 13:817-827. [DOI] [PubMed] [Google Scholar]
- 25.O'Neill, E. M., I. Rebay, R. Tjian, and G. M. Rubin. 1994. The activities of two Ets-related transcription factors required for Drosophila eye development are modulated by the Ras/MAPK pathway. Cell 78:137-147. [DOI] [PubMed] [Google Scholar]
- 26.Ong, S. H., K. C. Goh, Y. P. Lim, B. C. Low, P. Klint, L. Claesson-Welsh, X. Cao, Y. H. Tan, and G. R. Guy. 1996. Suc1-associated neurotrophic factor target (SNT) protein is a major FGF-stimulated tyrosine phosphorylated 90-kDa protein which binds to the SH2 domain of GRB2. Biochem. Biophys. Res. Commun. 225:1021-1026. [DOI] [PubMed] [Google Scholar]
- 27.Ong, S. H., G. R. Guy, Y. R. Hadari, S. Laks, N. Gotoh, J. Schlessinger, and I. Lax. 2000. FRS2 proteins recruit intracellular signaling pathways by binding to diverse targets on fibroblast growth factor and nerve growth factor receptors. Mol. Cell. Biol. 20:979-989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ong, S. H., Y. R. Hadari, N. Gotoh, G. R. Guy, J. Schlessinger, and I. Lax. 2001. Stimulation of phosphatidylinositol 3-kinase by fibroblast growth factor receptors is mediated by coordinated recruitment of multiple docking proteins. Proc. Natl. Acad. Sci. USA 98:6074-6079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ornitz, D. M., and N. Itoh. 2001. Fibroblast growth factors. Genome Biol. 2:3005.1-3005.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perkins, L. A., M. R. Johnson, M. B. Melnick, and N. Perrimon. 1996. The nonreceptor protein tyrosine phosphatase corkscrew functions in multiple receptor tyrosine kinase pathways in Drosophila. Dev. Biol. 180:63-81. [DOI] [PubMed] [Google Scholar]
- 31.Powers, C. J., S. W. McLeskey, and A. Wellstein. 2000. Fibroblast growth factors, their receptors and signaling. Endocrine-Related Cancer 7:165-197. [DOI] [PubMed] [Google Scholar]
- 32.Reichman-Fried, M., B. Dickson, E. Hafen, and B. Z. Shilo. 1994. Elucidation of the role of breathless, a Drosophila FGF receptor homolog, in tracheal cell migration. Genes Dev. 8:428-439. [DOI] [PubMed] [Google Scholar]
- 33.Ribeiro, C., A. Ebner, and M. Affolter. 2002. In vivo imaging reveals different cellular functions for FGF and Dpp signaling in tracheal branching morphogenesis. Dev. Cell 2:677-683. [DOI] [PubMed] [Google Scholar]
- 34.Rosario, M., and W. Bircmeier. 2003. How to make tubes: signaling by the Met receptor tyrosine kinas. Trends Cell Biol. 13:328-335. [DOI] [PubMed] [Google Scholar]
- 35.Rubin, G. M., and A. C. Spradling. 1982. Genetic transformation of Drosophila with transposable element vectors. Science 218:348-353. [DOI] [PubMed] [Google Scholar]
- 36.Sato, M., and T. B. Kornberg. 2002. FGF is an essential mitogen and chemoattractant for the air sacs of the drosophila tracheal system. Dev. Cell 3:195-207. [DOI] [PubMed] [Google Scholar]
- 37.Saxton, T. M., and T. Pawson. 1999. Morphogenetic movements at gastrulation require the SH2 tyrosine phosphatase Shp2. Proc. Natl. Acad. Sci. USA 96:3790-3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schlessinger, J. 2000. Cell signaling by receptor tyrosine kinases. Cell 103:211-225. [DOI] [PubMed] [Google Scholar]
- 39.Shiga, Y., Tanaka-Matakatsu, and S. Hayashi. 1996. A nuclear GFP/beta-galactosidase fusion protein as a marker for morphogenesis in living Drosophila. Dev. Growth Differ. 38:99-106. [Google Scholar]
- 40.Simon, M. A. 2000. Receptor tyrosine kinases: specific outcomes from general signals. Cell 103:13-15. [DOI] [PubMed] [Google Scholar]
- 41.Sutherland, D., C. Samakovlis, and M. A. Krasnow. 1996. branchless encodes a Drosophila FGF homolog that controls tracheal cell migration and the pattern of branching. Cell 87:1091-1101. [DOI] [PubMed] [Google Scholar]
- 42.Vincent, S., R. Wilson, C. Coelho, M. Affolter, and M. Leptin. 1998. The Drosophila protein Dof is specifically required for FGF signaling. Mol. Cell 2:515-525. [DOI] [PubMed] [Google Scholar]
- 43.Wang, J. K., H. Xu, H. C. Li, and M. Goldfarb. 1996. Broadly expressed SNT-like proteins link FGF receptor stimulation to activators of Ras. Oncogene 13:721-729. [PubMed] [Google Scholar]
- 44.Xu, H., K. W. Lee, and M. Goldfarb. 1998. Novel recognition motif on fibroblast growth factor receptor mediates direct association and activation of SNT adapter proteins. J. Biol. Chem. 273:17987-17990. [DOI] [PubMed] [Google Scholar]
- 45.Yokoyama, K., I.-H. Su, T. Tezuka, T. Yasuda, K. Mikoshiba, A. Tarakhovsky, and T. Yamamoto. 2002. BANK regulates BCR-induced calcium mobilization by promoting tyrosine phosphorylation of IP(3) receptor. EMBO J. 21:83-92. [DOI] [PMC free article] [PubMed] [Google Scholar]