Abstract
The late stages of human breast cancer development are poorly understood complex processes associated with the expression of genes by cancers that promote specific tumorigenic activities, such as angiogenesis. Here, we describe the identification of periostin as a mesenchyme-specific gene whose acquired expression by human breast cancers leads to a significant enhancement in tumor progression and angiogenesis. Undetectable in normal human breast tissues, periostin was found to be overexpressed by the vast majority of human primary breast cancers examined. Tumor cell lines engineered to overexpress periostin showed a phenotype of accelerated growth and angiogenesis as xenografts in immunocompromised animals. The underlying mechanism of periostin-mediated induction of angiogenesis was found to derive in part from the up-regulation of the vascular endothelial growth factor receptor Flk-1/KDR by endothelial cells through an integrin αvβ3-focal adhesion kinase-mediated signaling pathway. These findings demonstrate the presence of a novel mechanism by which tumor angiogenesis is acquired with the expression of a mesenchyme-specific gene as a crucial step in late stages of tumorigenesis.
The development of human cancers is a multistep complex process by which cancer cells acquire the ability to overcome the restraints imposed by the surrounding normal tissue microenvironment (7). This process is believed to be driven by the intrinsic genomic instability of cancer cells to express genes that confer selective advantages under the adverse growth conditions associated with a rapidly expanding tumor mass, such as hypoxia and a poor supply of nutrients. After reaching a critical mass, cancer cells have to find ways to promote angiogenesis in order to progress and expand during late stages of tumorigenesis (4, 5). To this end, a number of genes, such as the vascular endothelial growth factor (VEGF), have been demonstrated to play critical roles in the development of tumor vasculature (4, 5, 10). However, much more remains to be learned about the molecular nature of still unidentified players and their modes of action in promoting tumor angiogenesis.
Recently, large-scale efforts have been made to determine gene expression pattern differences between various types of human cancers and their corresponding normal tissues by using the serial analysis of gene expression (SAGE) and gene array analyses (14, 33-35, 37). Indeed, significant differences in gene expression patterns have been revealed by these studies. In breast cancer, for example, such investigations have led to the application of gene array analysis in the diagnosis, prognosis, and design of rational treatment of patients according to the molecular signatures of the individual tumors (21, 22, 32, 35). In the meantime, although the alterations of oncogenes and tumor suppressor genes have shown a close association with the progression of human cancers based on their defined functions, less is known about the specific contributions of a large number of genes whose expression patterns are also significantly changed during the tumorigenic process. Particularly interesting is the observation that mesenchyme-specific genes, normally associated with osteoblasts, are highly expressed by various types of human cancers (17, 31). However, the expression of mesenchyme-specific genes has not been functionally linked to the development of specific tumor phenotypes.
To address this question, we sought to determine the potential contributions of such candidate genes to specific phenotypic changes associated with the progression of late-stage tumorigenesis and identified a mesenchyme-specific gene product, periostin, as a novel angiogenic factor whose overexpression by human breast cancers leads to the significant enhancement of angiogenesis. The angiogenic activity of periostin correlated with the increased expression of the VEGF receptor Flk-1/KDR by endothelial cells through an integrin αvβ3-focal adhesion kinase (FAK)-mediated signaling pathway. These findings indicate that epithelial cell-derived tumors may gain the capabilities to generate more blood vessels, invade, and metastasize during late stages of tumorigenesis by the acquired expression of genes whose functions are normally associated only with mesenchymal cells.
MATERIALS AND METHODS
Gene array analysis.
Total RNA from 50 primary breast cancers and three normal primary mammary epithelial cultures was labeled and hybridized to Affymetrix Gene Chips (FL arrays and 6,800 genes). Data were expressed as the average differences between the perfect match and mismatch probes for the periostin gene (see http://data.cgt.duke.edu for the raw gene array data).
Generation of periostin-producing cells.
Full-length human periostin cDNA (MluI-XhoI) was subcloned into a retroviral pCMV-neo-vector. 293T retroviral packaging cells were transfected with the periostin construct or vector control in the presence of pCL 10A1 vector by using Fugene 6 as the delivery vehicle. Forty-eight hours after transfection, the supernatant was harvested and filtered through a 0.4-μm-pore-size filter, and the virus-containing medium was used to infect cells. Selection with 800 μg of G418/ml was started 48 h after infection. For 293T, B16F1, and MDA-MB-231 cell lines, the drug-resistant cell populations were used for subsequent studies of tumor formation. For MCF-7 cells, a single stable clone that expresses periostin was isolated.
Tumor xenograft analysis in mice.
Four-week-old female SCID-Beige mice (Charles River, Wilmington, Mass.) were subcutaneously injected with control or periostin-producing 293T (2.5 × 107), MDA-MB-231 (1 × 107), or B16F1 (0.4 × 107) cells in 0.2 ml of Hank's balanced buffer without calcium and magnesium. The growth of solid tumors from the injected cells was monitored daily for up to 2 or 4 weeks before the animals were sacrificed to remove tumors for analysis. For testing the effect of Flk-1/KDR inhibitor SU5416 on tumor growth, mice were injected subcutaneously to the opposite flank of the tumor cell injection with SU5416 (20 mg/kg) in suspension of a diluent (0.5% carboxymethylcellulose sodium, 0.9% sodium chloride, 0.4% polysorbate 80, 0.9% benzyl alcohol) every other day for 5 weeks. Control mice were treated with the diluent alone. The removed tumors were measured and calculated as follows: volume = length × width2 × 0.52.
Hemoglobin content measurement.
Tumor tissue (0.25 g) was removed immediately after animal sacrifice, and blood was mechanically extracted in phosphate-buffered saline containing heparin. Hemoglobin concentration was determined as previously described (2).
Immunohistochemical analysis.
For frozen tissue samples, tumor slides (thickness, 6 to 10 μm) were fixed in 2% paraformaldehyde. Sections were stained for the presence of CD31 (Becton Dickinson Labware, Bedford, Mass.), Flk-1/KDR (Research Santa Cruz, Santa Cruz, Calif.), or periostin according to the manufacturer's instructions (Vector Laboratories, Burlingame, Calif.).
Generation of recombinant periostin.
Full-length human periostin cDNA with a His tag was subcloned into pFastBac1 vector (Life Technologies, Rockville, Md.). Following transformation and amplification in Escherichia coli DH10Bac, bacmid DNA containing periostin was transfected into Sf9 insect cells (Invitrogen, Carlsbad, Calif.) by using Cellfectin reagent (Life Technologies), and baculoviral medium was produced. Recombinant periostin was generated 48 h after infection of High-5 cells with viral medium. A Ni-nitrilotriacetic acid column was used to purify recombinant periostin according to the manufacturer's instructions (Life Technologies), and pure periostin was finally produced through a PD-10 column (Amersham Pharmacia Biotech, Piscataway, N.J.).
Cell migration assay.
Human microvascular endothelial cells (HMVEC) (2 × 105) were preincubated with serum-free medium for 12 h and transferred onto transwells (24-well plates) for migration assays as previously described (30).
[3H]thymidine incorporation assay.
HMVEC were grown in 12-well plates to subconfluence, and the culture medium was changed to conditioned medium from the parental, control, and periostin-producing MCF-7 cells for 12 h. After washing, VEGF (10 ng/ml) was added for 12 h. A total of 2 μCi of [3H]thymidine was then added to each well for 6 h. After being thoroughly washed with phosphate-buffered saline, the cells were scraped and precipitated with 200 μl of 10% trichloroacetic acid. 3H radioactivity was solubilized in 0.3 ml of 0.3 M NaOH and quantitated by liquid scintillation count.
Western blot analysis.
Serum-free media from cultured confluent cells were collected, and the presence of secreted periostin was determined by immunoblotting with a polyclonal antiperiostin antibody. The antibody was generated by immunizing the rabbits with recombinant periostin protein and was purified through an affinity column. For the measurement of Flk-1/KDR and FAK activation, an anti-phospho-Tyr 951 antibody (Research Santa Cruz) and an anti-phospho-Tyr 681 FAK antibody (Biosource, Camarillo, Calif.) were used to detect phosphorylated Flk-1/KDR or FAK in cell lysates.
RESULTS
Identification of periostin as a mesenchymal gene overexpressed in human cancers.
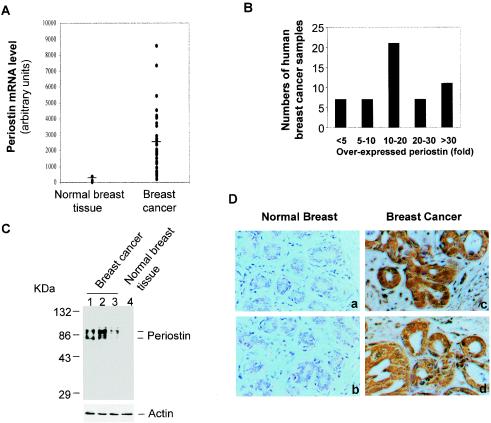
To understand the nature of genes that are responsible for the pathological progression associated with late stages of human cancer development, we searched the database derived from recently published results in the SAGE library in order to identify candidate genes that are highly expressed in various types of human cancers in comparison to normal tissue (14, 33, 34, 37). Among several candidates we found one gene, periostin (also termed OSF-2 for osteoblast-specific factor-2), that was originally defined as a secreted factor associated with osteoblastic cell function during bone development (8, 14, 29) and that was overexpressed in a broad range of human cancer types, including breast cancer (6, 9, 25, 26). To establish further a more comprehensive profile for periostin expression in human breast cancer, we took advantage of the available data from gene expression array analyses generated from both normal breast tissues and primary breast tumor samples from patients (35). Consistent with previously reported findings that periostin is specifically expressed by osteoblasts, the level of periostin expression was undetectable in normal breast tissues or in an immortalized cell line derived from normal mammary epithelial cells (Fig. 1A). However, the expression of periostin was readily detected in the vast majority of breast tumor samples, with an average level of periostin expression 20-fold higher than the baseline as defined by the value of gene array data obtained from normal breast tissues (Fig. 1A). As shown in Fig. 1B, 86% of the tumor samples overexpressed periostin at levels fivefold higher than the baseline. Among the samples, 11 (20%) had extremely high levels of periostin mRNA expression (>30-fold over baseline).
FIG. 1.
High levels of periostin expression are associated with human breast cancers. (A) Periostin expression pattern based on gene array data. The raw data from gene array analysis of the expression of periostin in normal (3 samples) and breast cancer (50 samples) tissues are plotted (the mean value for normal tissues at 106 as a baseline versus that for breast cancer samples at 2,100). (B) The same gene array data on periostin expression by 50 breast cancers were categorized into different groups based on levels of periostin overexpression. (C) Periostin protein expression in normal and tumor tissue samples. Tissue extracts from normal or breast cancer tissue samples were subjected to immunoblot analysis with a polyclonal antiperiostin antibody. The results shown in lanes 1 and 2 are from tumor samples from the group with the highest levels of periostin mRNA expression (>30-fold). The result shown in lane 3 was from tumor tissue with less than a fivefold increase in periostin mRNA expression. The higher band of the doublet is likely the unprocessed form of periostin, and the lower band is likely the secreted form of periostin with its signal sequence cleaved from the N terminus. (D) Tissue sections from human normal breast and breast cancer samples were analyzed by immunohistochemical staining and representative sections of each type of sample are shown. Red immunostaining represents positive staining for periostin protein in tumor tissues but not in normal tissues (magnification, ×200).
To determine if the higher levels of periostin mRNA expression revealed by gene array analysis were directly linked to increased levels of periostin protein expression, we performed Western blot analysis with protein extracts from several tumor samples. As shown in Fig. 1C, both an unprocessed and a processed shorter form of periostin were found to be highly expressed in tumor tissues from three different patients, whereas periostin was absent from normal breast tissue. Breast tumor tissues are known to contain both epithelial cancer cells and stromal cells. To determine the distribution of periostin within breast cancer tissues, we performed an immunohistochemical analysis by using a specific antiperiostin antibody. As shown in Fig. 1D, extensive staining of periostin was found mainly in areas containing carcinoma cells within the tumor tissue sections. The source of periostin production appeared to be the epithelial cancer cells, based on the results of RNA in situ hybridization analysis (data not shown). In contrast, periostin was undetectable in normal breast tissues (Fig. 1D). Taken together, these data demonstrate that periostin is highly expressed in a vast majority of the breast cancer samples examined and in a number of other types of human cancers (9, 14, 25, 26).
Overexpression of periostin is associated with enhanced tumor growth and angiogenesis.
The acquired expression of periostin by various types of cancers including breast cancer suggests that periostin may be intimately associated with the progression of tumor development. To test this hypothesis, tumor cells were engineered to produce periostin and injected into immunocompromised animals, and the growth characteristics of the resulting solid tumors were examined. Specifically, we used three cell lines that do not express endogenous periostin at a detectable level: the 293T cell line derived from human kidney epithelial cells, the highly invasive mouse melanoma cell line B16F1, and the metastatic human breast cancer line MDA-MB-231. After introducing the periostin gene into these cells by means of a retroviral vector infection system, we examined the expression of periostin by performing Western blot analysis on the conditioned media harvested from the stably infected cell populations.
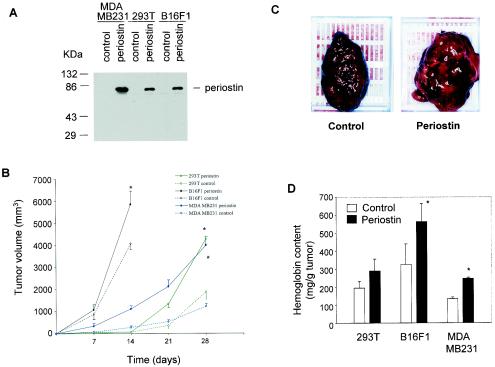
As shown in Fig. 2A, all three cell populations, compared to control vector-infected cells, secreted significant amounts of periostin to the media. Interestingly, the proliferation rate of the periostin-producing cells was found to be noticeably slower than that of the control cells in culture (data not shown), suggesting that periostin does not confer a proliferation-promoting effect on tumor cells in vitro. These cell populations were then injected subcutaneously into SCID-Beige mice, and the growth characteristics of resulting tumors were analyzed over a period of 14 or 28 days. For 293T cells, the volume of tumors derived from periostin-producing cells was two- to fourfold higher than that of tumors from control cells on day 28 (Fig. 2B). Strikingly, expression of periostin also enhanced the growth of tumors derived from the B16F1 and MDA-MB-231 cells (Fig. 2B), despite the fact that those cell lines are known to be among the most aggressive types in tumor formation when they are grown as xenografts. Interestingly, we found that the growth of tumors derived from the vector-transfected control cells was not affected by the presence of periostin-producing tumors in the same animal when the two cell populations were injected into opposite flanks (data not shown), suggesting that periostin exerts its effect locally rather than systematically.
FIG. 2.
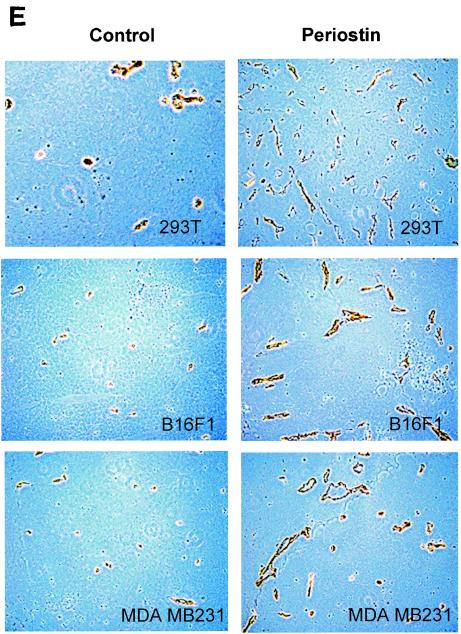
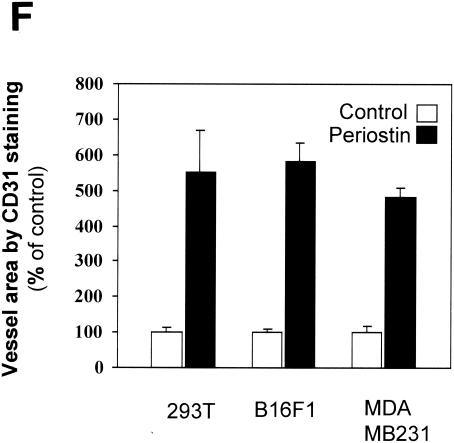
Periostin enhances tumor growth and angiogenesis of xenografts in immunocompromised mice. (A) Generation of periostin-producing cells. The production of periostin by cell populations from 293T, B16F1, and MDA-MB-231 cell lines was examined by immunoblotting with conditioned culture media. (B) Time course of tumor growth. The growth rate of tumors derived from periostin-producing or control 293T, B16F1, or MDA-MB-231 cells was monitored weekly by the measurement of tumor volume. (C) Higher levels of hemorrhage were associated with tumors derived from periostin-producing cells than from control cells. As an example, a pair of tumors derived from B16F1 cells was photographed. (D) Higher levels of hemoglobin content were associated with tumors derived from periostin-producing cells. *, P was <0.05 in a comparison with control groups. (E) Higher levels of CD31 expression were detected in tumors derived from periostin-producing cells. Tumor sections were stained with an anti-CD31 antibody, and representative sections for each type of sample are shown. Brown staining distributed along the vasculature network represents CD31-positive endothelial cells (magnification, ×200). (F) Vessel area was determined by quantitation of CD31-positive staining in fixed-size areas of tumor sections by using an image-scanning program developed by the National Institutes of Health.
In the process of dissecting the tumors, we noticed that the tumor mass derived from periostin-producing cells had an appearance of higher levels of hemorrhage compared to levels in tumors from control cells (Fig. 2C). Based on this observation, we measured the content of hemoglobin as a reflection of the amount of blood contained within the tumors. As shown in Fig. 2D, the hemoglobin content in tumors derived from periostin-producing cells was on average 30% higher than that in tumors derived from control cells, suggesting a possible difference in the density of blood vessels between the two forms of tumors.
To probe this possibility further, we performed an immunohistochemical analysis on tumor sections by utilizing an antibody against vascular endothelial cell marker CD31, an assay commonly used to detect the presence of vascular endothelial cells. Consistent with the results of the hemoglobin content measurement, we found that tumors derived from periostin-producing cells were much more intensively vascularized than control tumors as determined by the more intense staining of the marker CD31 (Fig. 2E and F). This result firmly established that the presence of periostin is intimately associated with the presence of a higher density of vasculature and endothelial cells in tumors grown as xenografts. Taken together, our data strongly suggest that periostin may act to promote tumor growth by inducing tumor angiogenesis.
Periostin promotes angiogenesis via up-regulation of VEGF receptor 2 expression in endothelial cells through an integrin αvβ3-FAK-mediated signaling pathway.
To explore the underlying mechanism by which periostin promotes tumor angiogenesis, we examined the effect of periostin on the activity of HMVEC that are known to play an essential role in the formation of new blood vessels. Since primary HMVEC can grow in culture for a very limited number of passages before adapting a senescence phenotype, hampering our analysis of periostin on HMVEC biology, we introduced the human telomerase catalytic subunit (hTERT) into the primary HMVEC to extend their life span in culture. As with other cell types (11, 20), hTERT expression extended the life span but had no effect on the proliferative and migration profiles of HMVEC (data not shown), and we have consequently used them in all of our subsequent experiments.
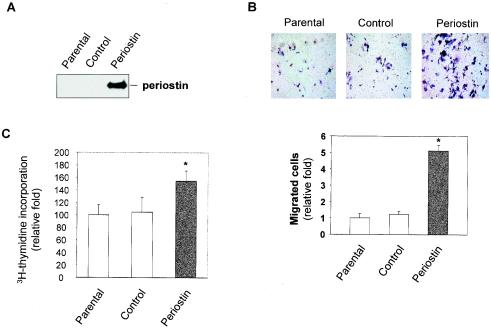
To test the possibility that periostin derived from the tumor tissue could exert angiogenic effects on vascular endothelial cells in vivo via a paracrine loop, we developed an in vitro coculture system to evaluate the angiogenic activity of periostin secreted by tumor cells. Cells from a stable clone of the MCF-7 human breast cancer cell line expressing high levels of periostin (Fig. 3A) were grown to reach confluence in the bottom chamber of transwells, and then HMVEC were plated onto the upper chamber. After incubation for 4 h, we found that the number of migrated HMVEC in the well containing periostin-producing MCF-7 cells was fivefold higher than that in the wells containing either parental or vector control MCF-7 cells (Fig. 3B). Consistent with the positive activity of periostin on endothelial cells observed with enhancement in cell migration, the proliferation of HMVEC was also stimulated by culturing the cells in periostin-containing conditioned media (Fig. 3C).
FIG. 3.
Periostin secreted by tumor cells promotes angiogenic activities of HMVEC. (A) The conditioned media from parental, vector-transfected, or periostin-producing MCF-7 cells were collected, and periostin expression was determined by immunoblotting. (B) Tumor cell-derived periostin enhances HMVEC migration. The three types of cells indicated in panel A were grown in serum-free media for 48 h in the lower chamber of transwells. HMVEC were transferred onto the upper chamber, and trapped cells were counted 4 h later. (C) Tumor cell-derived periostin enhances proliferation of HMVEC. HMVEC were incubated with conditioned media derived from the three types of cells described in panel A for 12 h, and [3H]thymidine incorporation was measured following a labeling period of 6 h. *, P was <0.05 compared with parental or control cells.
To explore further the molecular mechanism of periostin-induced angiogenesis, we examined whether periostin could have an effect on the regulation of expression or activity of certain angiogenic factors and their receptors that are known to play important roles in tumor angiogenesis. VEGF and its receptor Flk-1/KDR have been extensively documented to be involved in the induction of angiogenesis during the development of solid tumors (13, 19, 28, 36). VEGF secreted from tumor cells, as well as stromal cells, exerts its angiogenic effects on endothelial cells by the activation of Flk-1/KDR. We found by Western blot analysis that the level of VEGF in conditioned medium from periostin-producing MCF-7 cells was no different from the amount in medium derived from control cells (data not shown). In addition, treatment of parental MCF-7 cells that lack the expression of endogenous periostin with recombinant periostin produced in a baculovirus system did not enhance VEGF production (data not shown).
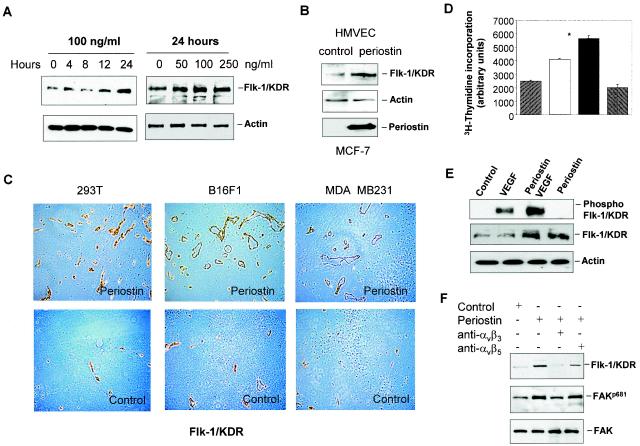
In contrast, incubation of HMVEC with recombinant periostin or the conditioned medium from periostin-producing MCF-7 cells resulted in up-regulation of Flk-1/KDR expression in a dose- and time-dependent manner (Fig. 4A and B). Consistent with the results shown in Fig. 2E, immunostaining of tumor sections with the anti-Flk-1/KDR antibody also confirmed the presence of a higher level of presence of this VEGF receptor associated with the higher density of blood vessels in tumors derived from periostin-producing cells (Fig. 4C). To determine if up-regulation of Flk-1/KDR expression leads to an increase in the sensitivity of endothelial cells to VEGF, we pretreated HMVEC with recombinant periostin and subsequently measured the cellular proliferative response to VEGF. As shown in Fig. 4D, the proliferation of HMVEC was increased in response to VEGF in comparison to untreated control cells, and this stimulatory response was further potentiated by the periostin pretreatment. To confirm that the significant enhancement in response to VEGF by the periostin-pretreated HMVEC was the result of the increased activity of Flk-1/KDR due to its up-regulated expression, we examined the potential changes in the kinase autophosphorylation activity of Flk-1/KDR by using a specific anti-phospho-Tyr 951 antibody (3). As shown in Fig. 4E, periostin pretreatment of HMVEC led to a significant increase in the amount of phosphorylated Flk-1/KDR as a direct reflection of the increase in Flk-1/KDR expression and, consequently, enhancement in its activation in response to VEGF.
FIG. 4.
Up-regulation of Flk-1/KDR is at least in part responsible for periostin-induced angiogenesis. (A) Recombinant periostin induces the expression of Flk-1/KDR in a time- and dose-dependent manner. HMVEC were treated with periostin (100 ng/ml) for up to 24 h or treated with different concentrations of periostin for 24 h. Protein samples from the treated cells were analyzed for Flk-1/KDR expression by immunoblotting. Actin was used as a loading control. (B) Periostin derived from conditioned medium of MCF-7 cells induces Flk-1/KDR expression. Control or periostin-producing MCF-7 cells shown at the bottom panel were incubated with serum-free medium for 48 h. HMVEC were incubated with the conditioned media for 24 h prior to analysis for Flk-1/KDR expression. (C) Increased presence of Flk-1/KDR associated with blood vessels is detected in tumors derived from periostin-producing cells. Tumor sections derived from 293T, B16F1, or MDA-MB-231 cells were subjected to immunohistochemical staining analysis with an anti-Flk-1/KDR antibody. (D) Periostin pretreatment potentiates HMVEC proliferation in response to VEGF. HMVEC were pretreated with periostin (100 ng/ml) for 12 h. After the cells were washed, VEGF (10 ng/ml) was added for 12 h to determine the effect on cell proliferation. *, P was <0.05 compared with VEGF treatment alone. (E) Periostin pretreatment potentiates Flk-1/KDR phosphorylation in response to VEGF. The same assay condition was used as described above except that the incubation time for VEGF was 5 min, followed by the quantitative determination of phosphorylated Flk-1/KDR by immunoblotting with a specific anti-phospho-Tyr 951 antibody.
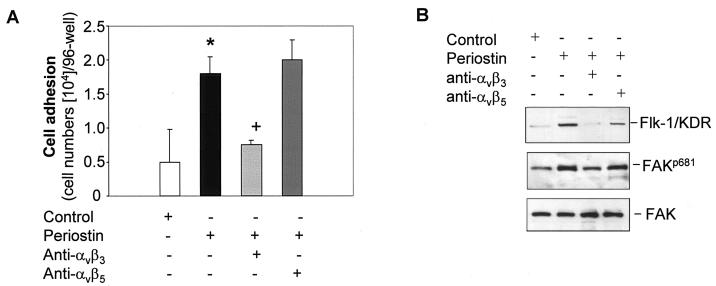
A recent report suggested that periostin could functionally interact with integrins to mediate the adhesion and migration of human ovarian carcinoma cells (6). To test the possibility that periostin may induce Flk-1/KDR expression through interaction with integrins in endothelial cells, we examined the profile of integrin expression in HMVEC and found those cells to express predominantly αvβ3 integrins (data not shown). Treatment of HMVEC with recombinant periostin did not alter the expression profile of the integrins (data not shown). We next probed if interference with the function of integrins by specific anti-integrin antibodies has an effect on the ability of periostin to mediate cell adhesion and induction of Flk-1/KDR in HMVEC. As shown in Fig. 5A, treatment of HMVEC with a specific anti-αvβ3 integrin antibody inhibited adhesion of these cells to the culture wells precoated with periostin. Consistent with this result, treatment of HMVEC with periostin in the presence of the anti-αvβ3 integrin antibody prevented the induction of Flk-1/KDR (Fig. 5B). The specificity of the blockage of periostin activity achieved by interfering with the function of αvβ3 integrin was demonstrated by the lack of an effect on the periostin-mediated cell adhesion and induction of Flk-1/KDR expression when a specific anti-αvβ5 integrin antibody was used in the same assays (Fig. 5). The initial step of integrin signaling involves the activation of FAK. Consistent with this notion, we found that transient stimulation of HMVEC with periostin augmented the phosphorylation of FAK on tyrosine 681 (Fig. 5B), an event indicative of the activation of FAK (1). The increase in FAK phosphorylation on Tyr 681 was reversed to the basal level by the presence of the anti-αvβ3 integrin antibody but not the anti-αvβ5 integrin antibody. Taken together, these results strongly suggest that the αvβ3 integrin-FAK signaling pathway plays an essential role in mediating the effect of periostin on the up-regulation of Flk-1/KDR expression in HMVEC.
FIG. 5.
The αvβ3 integrin-FAK signaling pathway mediates the induction of Flk-1/KDR expression. (A) HMVEC (5 × 104 cells/well) were cultured in a 96-well plate precoated with periostin (10 μg/ml) in the presence of anti-αvβ3 or anti-αvβ5 integrin antibody (10 μg/ml) for 1 h (noncoated wells were used as the control). Adhesive cells were counted following washing. *, P was <0.05 compared with the control cells; +, P was <0.05 compared with the cells plated on periostin-coated wells alone. (B) in the presence or absence of the indicated specific anti-integrin antibodies, HMVEC were treated with periostin (100 ng/ml) for 12 h to examine the effect on the up-regulation of Flk-1/KDR as determined by Western blotting. Alternatively, the same assay conditions were used except that the time of incubation was reduced to 15 min to observe a change in FAK autophosphorylation on Tyr 681 (FAKp681). An antibody against FAK was used as a loading control.
Inhibition of VEGF receptor abolished periostin-induced enhancement in tumor growth and angiogenesis.
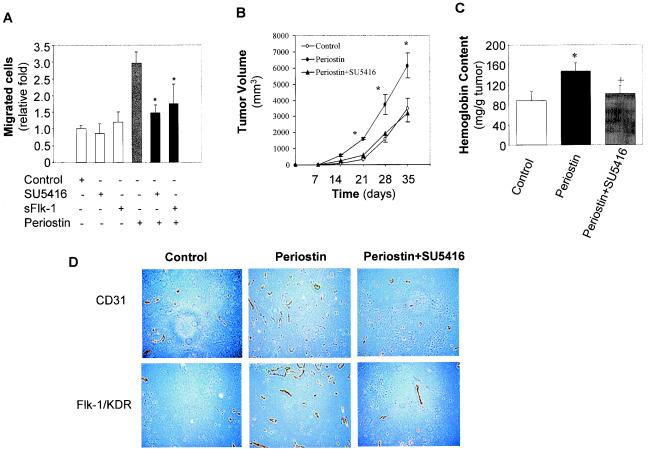
Finally, to establish a firm role for Flk-1/KDR up-regulation in the mediation of the proangiogenic activity of periostin, we employed two specific inhibitors of Flk-1/KDR in our functional assays. As shown in Fig. 6A, we found that the periostin-induced increases in cellular migration by the HMVEC were significantly inhibited by the presence of two inhibitors, SU5416, a compound that has been previously reported to specifically block the kinase activity of Flk-1/KDR (12), and sFlk, the soluble form of a VEGF receptor that has been demonstrated to sequester VEGF (16). Importantly, the increased tumor growth that resulted from the production of periostin was completely reversed by the presence of the inhibitor SU5416 in our xenograft model system (Fig. 6B). This decrease in tumor growth correlated directly with a reduction in angiogenic activity within the tumor mass derived from animals treated with the inhibitor SU5416, since the extent of vasculature density in those tumors was reduced to a level similar to that detected in control tumor sections (Fig. 6C and D).
FIG. 6.
Inhibition of Flk-1/KDR abolished periostin-induced enhancement in tumor growth and angiogenesis. (A) Inhibition of Flk-1/KDR blocks periostin-induced cell migration. HMVEC were employed for a migration assay in the presence of SU5416 (20 μM), sFlk-1 (100 ng/ml), periostin (100 ng/ml), or different combinations as indicated. *, P was <0.05 compared with the group treated with periostin alone. (B) The enhanced tumor growth by periostin-producing 293T cells was completely reversed by the Flk-1/KDR inhibitor SU5416. The time course of 293T tumor growth as measured by tumor volume was plotted with control, periostin-producing cells, or periostin plus SU5416 as described in Materials and Methods. *, P was <0.05 compared with control or periostin plus SU5416. (C and D) Reduced tumor growth in the presence of SU5416 was correlated with a reduction in angiogenesis. Hemoglobin content in tumors was measured as previously described. *, P was <0.05 compared with control; +, P was <0.05 compared with periostin. Tumor sections were immunohistochemically stained with anti-CD31 and Flk-1/KDR antibodies, and representative sections of each type of sample are shown (magnification, ×200).
Hence, periostin acts to increase the expression of the VEGF receptor (Flk-1/KDR), which in turn renders the cells more sensitive to the action of VEGF as demonstrated by a significant increase in both biological and biochemical responses by the HMVEC. Most importantly, the increased activity of the VEGF receptor is directly linked to the increased growth of tumor xenografts as a consequence of periostin production. Taken together, these data strongly support the notion that the up-regulation of Flk-1/KDR expression and, consequently, the sensitization of endothelial cells to the potent angiogenic factor VEGF are at least partially responsible for periostin-mediated tumor angiogenesis.
DISCUSSION
The present results reveal a novel mechanism by which angiogenesis is promoted in tumor progression in vivo. In this case, the acquired expression of periostin, an osteoblast-specific secreted protein known to be associated with cell adhesion activities for bone formation and development, by the epithelial cell-derived tumors leads to a significant enhancement in angiogenesis and tumor progression. Periostin produced by the breast carcinoma cells within the tumor mass acts in a paracrine manner to enhance the responsiveness of microvessel endothelial cells that have been recruited into the tumor and coopted to generate the neovascularization associated with the rapid expansion of the tumor mass and possibly subsequent metastasis. In fact, another study has found that overexpression of periostin is associated with the increased metastasis of colon cancers (S. Bao, G. Ouyang, X. Bai, Z. Huang, C. Ma, M. Liu, R. Shao, R. M. Anderson, J. N. Rich, and X.-F. Wang, submitted for publication). Mechanistically, the up-regulation of expression of a critical receptor for the potent angiogenic factor VEGF represents a novel signaling pathway through which tumors can act to promote the proliferation, migration, and vessel formation activities of endothelial cells.
Importantly, the acquired expression of periostin by tumors of epithelial origin described here is certainly not an isolated and rare event during tumorigenesis, since a number of other examples of acquired expression of mesenchymal genes by epithelial-cell-derived tumors can be found in the literature (17, 18, 23). For example, osteonectin, also termed SPARC, whose physiological activity is believed to be associated mainly with osteoblast function, has been found to be overexpressed by a wide range of human cancer types (15, 24, 31). As a secreted polypeptide without sequence homology with periostin, osteonectin has been implicated to promote tumor progression and angiogenesis (10). Thus, the common feature for this group of structurally unrelated and functionally diverse molecules, either as secreted or extracellular matrix-associated proteins, is that their physiological functions are normally associated strictly with cells that derive from mesenchymal origins such as osteoblasts. By acquiring the expression of such mesenchymal genes, the epithelial carcinoma cells gain the abilities that are normally associated with mesenchymal cells, an event which appears to correlate with the progression into a more aggressive cancer phenotype.
Our immunohistochemical staining data suggest that periostin is present predominantly in areas containing cancer cells within the tumor mass. Based on the result of RNA in situ hybridization, the source of periostin production was determined to be the carcinoma cells, a notion that is consistent with the conclusion of a recent study on the production of periostin by human ovarian cancer cells (6). However, another recent report suggested that the localization of periostin mRNA was mainly associated with the stromal portion of the tumor tissue sample isolated from a breast cancer patient (27). In any event, periostin produced by either cell type within the tumor tissue could exert a similar paracrine effect on the endothelial cells recruited to the tumor mass to promote angiogenesis. As a related matter, we have also observed that the tumor cells engineered to produce periostin in culture containing a normal level of fetal bovine serum had a growth disadvantage in comparison to control cells (data not shown). In fact, we had to resort to the use of tumor cell populations in which ectopic periostin expression was engineered by retroviral infection and transient drug selection since we could not obtain multiple lines of stable clones that overexpress periostin; the exception was a single stable clone from MCF7 cells which was used as a source of periostin production in a number of the experiments described above. Thus, although periostin may confer an advantage for the growth of breast tumors in vivo by altering the microenvironment through the induction of angiogenesis, its overexpression may impose a growth disadvantage when the tumor cells are grown in culture.
These observations reveal the vital importance of relying on evidence derived from primary human cancer samples rather than established cell lines to draw major conclusions on the mechanism and involvement of specific genes in tumorigenesis. Furthermore, the tumor-promoting effect of molecules such as periostin can only be revealed by in vivo analysis in animal models, rather than solely by in vitro studies in cell culture. The normal functions of this type of gene are often not associated with the promotion of cell proliferation, in contrast to the roles of many defined oncogenes. Instead, this group of proteins may exert their influence on tumorigenesis by changing the microenvironment of tumor growth through the regulation or alteration of cell adhesion, composition of the extracellular matrix, and the activities of stromal cells within and surrounding the tumor mass.
Based on the results of this study with periostin as an example, we suspect that different types of human cancers derived from epithelial origins, and even cancers from the same tissue type but derived from different individual patients, may acquire the expression of different sets of mesenchymal-specific genes to gain different mesenchymal-associated capabilities during late stages of tumorigenesis. In other words, the heterogeneity in the functions of this group of genes may confer different tumorigenic capabilities on the cancer cells that acquire the expression of such genes, creating another layer of heterogeneity and complexity for each type and even each case of cancer development. Thus, identification and characterization of such genes will become crucial for a full understanding of the molecular events associated with late stages of tumorigenesis, particularly angiogenesis and metastasis, and for the future development of specific and effective regimens for a cancer treatment tailored to each individual patient. To this end, the availability of a vast amount of data on gene expression profiles derived from the SAGE library and gene array analyses of a broad range of human cancer types in comparison to their counterparts of normal tissue has provided us with a golden opportunity through functional genomics to search for and identify candidate genes that fit this profile. Since the activities of these molecules may not be associated with the promotion of cell proliferation, the foremost criterion for evaluating the potential contribution of any candidate genes to the progression of tumorigenesis will have to be based on an assessment of the ability of those genes to promote tumorigenesis in in vivo studies of xenografts or transgenic animal model systems.
Acknowledgments
We thank J. Nevins, C. Counter and T.-P. Yao for critical comments, S. Cheng for assistant in immunostaining assays, C. Counter for providing hTERT viral medium, and J. Rich and Q. Shi for assistance in animal treatment.
This study was supported in part by NIH grant CA 83770 and DOD grant BC-980188 to X.-F.W. and by DOD postdoctoral fellowship grant DAMD17-00-100228 to R.S.
REFERENCES
- 1.Abu-Ghazaleh, R., J. Kabir, H. Jia, M. Lobo, and I. Zachary. 2001. Src mediates stimulation by vascular endothelial growth factor of the phosphorylation of focal adhesion kinase at tyrosine 861, and migration and anti-apoptosis in endothelial cells. Biochem. J. 360:255-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Biroccio, A., A. Candiloro, M. Mottolese, O. Sapora, A. Albini, G. Zupi, and D. Del Bufalo. 2000. Bcl-2 overexpression and hypoxia synergistically act to modulate vascular endothelial growth factor expression and in vivo angiogenesis in a breast carcinoma line. FASEB J. 14:652-660. [DOI] [PubMed] [Google Scholar]
- 3.Dougher, M., and B. I. Terman. 1999. Autophosphorylation of KDR in the kinase domain is required for maximal VEGF-stimulated kinase activity and receptor internalization. Oncogene 18:1619-1627. [DOI] [PubMed] [Google Scholar]
- 4.Folkman, J. 1996. Tumor angiogenesis and tissue factor. Nat. Med. 2:167-168. [DOI] [PubMed] [Google Scholar]
- 5.Folkman, J., K. Watson, D. Ingber, and D. Hanahan. 1989. Induction of angiogenesis during the transition from hyperplasia to neoplasia. Nature 339:58-61. [DOI] [PubMed] [Google Scholar]
- 6.Gillan, L., D. Matei, D. A. Fishman, C. S. Gerbin, B. Y. Karlan, and D. D. Chang. 2002. Periostin secreted by epithelial ovarian carcinoma is a ligand for αVβ3 and αVβ5 integrins and promotes cell motility. Cancer Res. 62:5358-5364. [PubMed] [Google Scholar]
- 7.Hanahan, D., and R. A. Weinberg. 2000. The hallmarks of cancer. Cell 100:57-70. [DOI] [PubMed] [Google Scholar]
- 8.Horiuchi, K., N. Amizuka, S. Takeshita, H. Takamatsu, M. Katsuura, H. Ozawa, Y. Toyama, L. F. Bonewald, and A. Kudo. 1999. Identification and characterization of a novel protein, periostin, with restricted expression to periosteum and periodontal ligament and increased expression by transforming growth factor beta. J. Bone Miner. Res. 14:1239-1249. [DOI] [PubMed] [Google Scholar]
- 9.Ismail, R. S., R. L. Baldwin, J. Fang, D. Browning, B. Y. Karlan, J. C. Gasson, and D. D. Chang. 2000. Differential gene expression between normal and tumor-derived ovarian epithelial cells. Cancer Res. 60:6744-6749. [PubMed] [Google Scholar]
- 10.Jendraschak, E., and E. H. Sage. 1996. Regulation of angiogenesis by SPARC and angiostatin: implications for tumor cell biology. Semin. Cancer Biol. 7:139-146. [DOI] [PubMed] [Google Scholar]
- 11.Jiang, X. R., G. Jimenez, E. Chang, M. Frolkis, B. Kusler, M. Sage, M. Beeche, A. G. Bodnar, G. M. Wahl, T. D. Tlsty, and C. P. Chiu. 1999. Telomerase expression in human somatic cells does not induce changes associated with a transformed phenotype. Nat. Genet. 21:111-114. [DOI] [PubMed] [Google Scholar]
- 12.Kasahara, Y., R. M. Tuder, L. Taraseviciene-Stewart, T. D. Le Cras, S. Abman, P. K. Hirth, J. Waltenberger, and N. F. Voelkel. 2000. Inhibition of VEGF receptors causes lung cell apoptosis and emphysema. J. Clin. Investig. 106:1311-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim, K. J., B. Li, J. Winer, M. Armanini, N. Gillett, H. S. Phillips, and N. Ferrara. 1993. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature 362:841-844. [DOI] [PubMed] [Google Scholar]
- 14.Lal, A., A. E. Lash, S. F. Altschul, V. Velculescu, L. Zhang, R. E. McLendon, M. A. Marra, C. Prange, P. J. Morin, K. Polyak, N. Papadopoulos, B. Vogelstein, K. W. Kinzler, R. L. Strausberg, and G. J. Riggins. 1999. A public database for gene expression in human cancers. Cancer Res. 59:5403-5407. [PubMed] [Google Scholar]
- 15.Ledda, M. F., S. Adris, A. I. Bravo, C. Kairiyama, L. Bover, Y. Chernajovsky, J. Mordoh, and O. L. Podhajcer. 1997. Suppression of SPARC expression by antisense RNA abrogates the tumorigenicity of human melanoma cells. Nat. Med. 3:171-176. [DOI] [PubMed] [Google Scholar]
- 16.Lin, P., S. Sankar, S. Shan, M. W. Dewhirst, P. J. Polverini, T. Q. Quinn, and K. G. Peters. 1998. Inhibition of tumor growth by targeting tumor endothelium using a soluble vascular endothelial growth factor receptor. Cell Growth Differ. 9:49-58. [PubMed] [Google Scholar]
- 17.Lochter, A., Z. Werb, and M. J. Bissell. 1999. Transcriptional regulation of stromelysin-1 gene expression is altered during progression of mouse mammary epithelial cells from functionally normal to malignant. Matrix Biol. 18:455-467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lyden, D., K. Hattori, S. Dias, C. Costa, P. Blaikie, L. Butros, A. Chadburn, B. Heissig, W. Marks, L. Witte, Y. Wu, D. Hicklin, Z. Zhu, N. R. Hackett, R. G. Crystal, M. A. Moore, K. A. Hajjar, K. Manova, R. Benezra, and S. Rafii. 2001. Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat. Med. 7:1194-1201. [DOI] [PubMed] [Google Scholar]
- 19.Millauer, B., M. P. Longhi, K. H. Plate, L. K. Shawver, W. Risau, A. Ullrich, and L. M. Strawn. 1996. Dominant-negative inhibition of Flk-1 suppresses the growth of many tumor types in vivo. Cancer Res. 56:1615-1620. [PubMed] [Google Scholar]
- 20.Morales, C. P., S. E. Holt, M. Ouellette, K. J. Kaur, Y. Yan, K. S. Wilson, M. A. White, W. E. Wright, and J. W. Shay. 1999. Absence of cancer-associated changes in human fibroblasts immortalized with telomerase. Nat. Genet. 21:115-118. [DOI] [PubMed] [Google Scholar]
- 21.Perou, C. M., S. S. Jeffrey, M. van de Rijn, C. A. Rees, M. B. Eisen, D. T. Ross, A. Pergamenschikov, C. F. Williams, S. X. Zhu, J. C. Lee, D. Lashkari, D. Shalon, P. O. Brown, and D. Botstein. 1999. Distinctive gene expression patterns in human mammary epithelial cells and breast cancers. Proc. Natl. Acad. Sci. USA 96:9212-9217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perou, C. M., T. Sorlie, M. B. Eisen, M. van de Rijn, S. S. Jeffrey, C. A. Rees, J. R. Pollack, D. T. Ross, H. Johnsen, L. A. Akslen, O. Fluge, A. Pergamenschikov, C. Williams, S. X. Zhu, P. E. Lonning, A. L. Borresen-Dale, P. O. Brown, and D. Botstein. 2000. Molecular portraits of human breast tumours. Nature 406:747-752. [DOI] [PubMed] [Google Scholar]
- 23.Putz, E., K. Witter, S. Offner, P. Stosiek, A. Zippelius, J. Johnson, R. Zahn, G. Riethmuller, and K. Pantel. 1999. Phenotypic characteristics of cell lines derived from disseminated cancer cells in bone marrow of patients with solid epithelial tumors: establishment of working models for human micrometastases. Cancer Res. 59:241-248. [PubMed] [Google Scholar]
- 24.Rich, J. N., Q. Shi, M. Hjelmeland, T. J. Cummings, C. T. Kuan, D. D. Bigner, C. M. Counter, and X. F. Wang. 2003. Bone-related genes expressed in advanced malignancies induce invasion and metastasis in a genetically defined human cancer model. J. Biol. Chem. 278:15951-15957. [DOI] [PubMed] [Google Scholar]
- 25.Sasaki, H., D. Auclair, M. Kaji, I. Fukai, M. Kiriyama, Y. Yamakawa, Y. Fujii, and L. B. Chen. 2001. Serum level of the periostin, a homologue of an insect cell adhesion molecule, in thymoma patients. Cancer Lett. 172:37-42. [DOI] [PubMed] [Google Scholar]
- 26.Sasaki, H., M. Dai, D. Auclair, I. Fukai, M. Kiriyama, Y. Yamakawa, Y. Fujii, and L. B. Chen. 2001. Serum level of the periostin, a homologue of an insect cell adhesion molecule, as a prognostic marker in nonsmall cell lung carcinomas. Cancer 92:843-848. [DOI] [PubMed] [Google Scholar]
- 27.Sasaki, H., C. Y. Yu, M. Dai, C. Tam, M. Loda, D. Auclair, L. B. Chen, and A. Elias. 2003. Elevated serum periostin levels in patients with bone metastases from breast but not lung cancer. Breast Cancer Res. Treat. 77:245-252. [DOI] [PubMed] [Google Scholar]
- 28.Stacker, S. A., C. Caesar, M. E. Baldwin, G. E. Thornton, R. A. Williams, R. Prevo, D. G. Jackson, S. Nishikawa, H. Kubo, and M. G. Achen. 2001. VEGF-D promotes the metastatic spread of tumor cells via the lymphatics. Nat. Med. 7:186-191. [DOI] [PubMed] [Google Scholar]
- 29.Takeshita, S., R. Kikuno, K. Tezuka, and E. Amann. 1993. Osteoblast-specific factor 2: cloning of a putative bone adhesion protein with homology with the insect protein fasciclin I. Biochem. J. 294:271-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tang, S., Y. Gao, and J. A. Ware. 1999. Enhancement of endothelial cell migration and in vitro tube formation by TAP20, a novel beta 5 integrin-modulating, PKC theta-dependent protein. J. Cell Biol. 147:1073-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vajkoczy, P., M. D. Menger, R. Goldbrunner, S. Ge, T. A. Fong, B. Vollmar, L. Schilling, A. Ullrich, K. P. Hirth, J. C. Tonn, P. Schmiedek, and S. A. Rempel. 2000. Targeting angiogenesis inhibits tumor infiltration and expression of the pro-invasive protein SPARC. Int. J. Cancer 87:261-268. [DOI] [PubMed] [Google Scholar]
- 32.van 't Veer, L. J., H. Dai, M. J. van de Vijver, Y. D. He, A. A. Hart, M. Mao, H. L. Peterse, K. van der Kooy, M. J. Marton, A. T. Witteveen, G. J. Schreiber, R. M. Kerkhoven, C. Roberts, P. S. Linsley, R. Bernards, and S. H. Friend. 2002. Gene expression profiling predicts clinical outcome of breast cancer. Nature 415:530-536. [DOI] [PubMed] [Google Scholar]
- 33.Velculescu, V. E., B. Vogelstein, and K. W. Kinzler. 2000. Analysing uncharted transcriptomes with SAGE. Trends Genet. 16:423-425. [DOI] [PubMed] [Google Scholar]
- 34.Velculescu, V. E., L. Zhang, B. Vogelstein, and K. W. Kinzler. 1995. Serial analysis of gene expression. Science 270:484-487. [DOI] [PubMed] [Google Scholar]
- 35.West, M., C. Blanchette, H. Dressman, E. Huang, S. Ishida, R. Spang, H. Zuzan, J. A. Olson, Jr., J. R. Marks, and J. R. Nevins. 2001. Predicting the clinical status of human breast cancer by using gene expression profiles. Proc. Natl. Acad. Sci. USA 98:11462-11467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yancopoulos, G. D., S. Davis, N. W. Gale, J. S. Rudge, S. J. Wiegand, and J. Holash. 2000. Vascular-specific growth factors and blood vessel formation. Nature 407:242-248. [DOI] [PubMed] [Google Scholar]
- 37.Zhang, L., W. Zhou, V. E. Velculescu, S. E. Kern, R. H. Hruban, S. R. Hamilton, B. Vogelstein, and K. W. Kinzler. 1997. Gene expression profiles in normal and cancer cells. Science 276:1268-1272. [DOI] [PubMed] [Google Scholar]