Summary
The SLC10A transporter gene family consists of seven members and substrates transported by three members (SLC10A1, SLC10A2 and SLC10A6) are Na+-dependent. SLC10A1 (sodium taurocholate cotransporting polypeptide or NTCP) and SLC10A2 (apical sodium-dependent bile salt transporter or ASBT) transport bile salts and play an important role in maintaining enterohepatic circulation of bile salts. Solutes other than bile salts are also transported by NTCP. However, ASBT has not been shown to be a transporter for non-bile salt substrates. While the transport function of NTCP can potentially be used as liver function test, interpretation of such a test may be complicated by altered expression of NTCP in diseases and presence of drugs that may inhibit NTCP function. Transport of bile salts by NTCP and ASBT is inhibited by a number of drugs and it appears that ASBT is more permissive to drug inhibition than NTCP. The clinical significance of this inhibition in drug disposition and drug-drug interaction remains to be determined. Both NCTP and ASBT undergo post-translational regulations that involve phosphorylation/dephosphorylation, translocation to and retrieval from the plasma membrane and degradation by the ubiquitin-proteasome system. These posttranslational regulations are mediated via signaling pathways involving cAMP, calcium, nitric oxide, phosphoinositide-3-kinase (PI3K), protein kinase C (PKC) and protein phosphatases. There appears to be species difference in the substrate specificity and the regulation of plasma membrane localization of human and rodent NTCP. These differences should be taken into account when extrapolating rodent data for human clinical relevance and developing novel therapies. NTCP has recently been shown to play an important role in HBV and HDV infection by serving as a receptor for entry of these viruses into hepatocytes.
Introduction
Currently, the SLC10A gene family consists of seven members (30,41). Sodium-dependent bile salt transport in rat hepatocytes was first reported in 1978 (6). The transporter was first cloned from rat using a functional expression cloning approach (61,64) and is known as the sodium taurocholate cotransporting polypeptide (NTCP). It is the founding member of this family and is named Slc10a1(59). Other members of the family include apical bile salt transporter (ASBT; SLC10A2), SLC10A3, SLC10A4, SLC10A5, Na+-dependent organic anion transporter (SOAT; SLC10A6) and SLC10A7(30,41,50). NTCP is localized at the basolateral membrane of hepatocytes, while ASBT is localized at the apical membrane of cholangiocytes, ileum and renal proximal tubules (32,41). SOAT transports sulfated taurolithocholate and sulfated steroid metabolites (34). Neither transport activity nor specific substrates have been identified for the gene products of SLC10A3, SLC10A4, SLC10A5 and SLC10a7 and these are currently considered orphan transporters. Of the seven members of the SLC10A family, NTCP and ASBT have been extensively characterized with established role in the enterohepatic circulation of bile salts.
Bile salts are the major constituents of bile and play an important role in the intestinal digestion of fat and absorption of fat soluble vitamins (75). They may also play an important role in systemic energy homeostasis (123). Bile acids are synthesized in hepatocytes from cholesterol in a complex series of biochemical reactions (129). The resulting primary bile acids, cholic acid and chenodeoxycholic acid, are conjugated to glycine or to taurine (75). These conjugated bile salts have lower pKa values and are consequently more hydrophilic and less cytotoxic than their unconjugated forms (75). Inborn errors of bile acid biosynthesis can cause severe liver diseases (31,71,143). In hepatocytes, the newly synthesized bile salts mix with bile salts entering the cells via the basolateral membrane and are exported against a steep concentration gradient into the canaliculus. The canaliculi are the starting point of the biliary tree from where bile salts reach the duodenum to assist fat digestion. Bile salts are reabsorbed along the small intestine and transported via the portal circulation back to the liver for uptake and resecretion (76). This circling of bile salts is called enterohepatic circulation and bile salts can be used as a model for understanding the journey of drugs undergoing enterohepatic circulation (36,77,148).
NTCP and ASBT are the two members of the SCLC10 transporter family involved in maintaining uninterrupted enterohepatic circulation of bile salts. Uptake of bile salts into the hepatocytes occurs predominantly by NTCP (SLC10A1) and to a lesser extent by OATPs (SLCOs)(105,146). So far, no inherited severe diseases associated with these transporters have been reported. Export of bile salts across the canalicular membrane is mediated by the ATP-dependent canalicular bile salt export pump BSEP (ABCB11)(105,146). Mutations in this transporter lead to progressive familial intrahepatic cholestasis type 2 suggesting a lack of backup transporter(s) for canalicular efflux of bile salts (80,92). Uptake across the apical membrane of enterocytes is mediated by the apical bile salt transporter ASBT (SLC10A2)(34,36), while the efflux from enterocyte at the basolateral membrane is facilitated by the heterodimeric transporter OSTα/OSTβ (35,36). Mutations in the gene coding for ASBT may lead to primary bile acid malabsorption (7). During the passage through the bile duct, a small fraction of bile salts undergoes reabsorption through cholangiocytes into the portal blood by transporters located at the luminal side (ASBT) and the basolateral side (MRP3/4 and OSTα/β) and then into the hepatocytes by NTCP. This cycling of bile salts between cholangiocytes and hepatocytes is known as cholehepatic shunting (30,75).
This review will focus on NTCP and ASBT highlighting latest developments and post-translational regulations. Aspects of NTCP and ASBT not included in this review can be found in a number of excellent recent reviews on the family of SLC10A transporters (30,34,41,50,65,88).
2. Transport function of NTCP
NTCP is the only hepatocellular sodium-dependent uptake system for bile salts, at least in rats if not in all mammals and this is based on results that a rat NTCP-specific antisense oligonucleotide co-injected with total rat liver mRNA into Xenopus laevis oocytes blocked the expression of Na+-dependent taurocholate (TC) uptake by approx. 95% (62). To date, no information on mice with a disrupted Slc10a1 gene has been published, which would allow to further support this previous finding. Also, in humans so far no patients with mutations rendering the transport activity of human NTCP (hNTCP) non-functional have been published. Hence, a definitive answer on the number of sodium dependent transport system(s) for uptake of bile salts into hepatocytes is still lacking.
From a thermodynamic point of view, NTCP is a typical secondary active transporter and has been shown to be strictly sodium-dependent. NTCP necessitates the binding of two sodium-ions together with a bile salt molecule for the transport step. In the case of the monoanionic TC, as well as the monoanionic fluorescent bile salt cholyl-Nε-NBD-lysine, rat NTCP (rNTCP) transport activity has been shown to be electrogenic, while it is electroneutral for the dianionic fluorescent bile salt cholylglycylamidofluorescein (164). Hence, depending on the charge of the substrate, the driving force of the uptake may vary and may consist of the energy available from the chemical and/or the electrochemical gradient of sodium.
The list of substrates transported by NTCP has been extensively reviewed (30,41,146). Solutes other than bile salts including steroid hormones, thyroid hormones, drugs and drugs conjugated with bile acids can be transported by hNTCP/rNTCP (SLC10A1/Slc10a1)(41,44). Comparison of published Km values for bile salts obtained from intact livers and heterologously expressed hNTCP/rNTCP (41,146) suggests that the properties of the cloned NTCP/Ntcp reflect reasonably well the affinity of NTCP/Ntcp in in-vivo situations. A study reported that human hepatocellular carcinomas express NTCP, which mediates high-affinity uptake of chlorambucil-taurocholate (94). Thus, cytotoxic drugs conjugated with TC could provide a potential therapeutic strategy to deliver these drugs to hepatocellular carcinomas.
In addition to endogenous substrates, drugs including statins and recently micafungin (170), an antifungal agent, have been reported to be transported by NTCP (41). Interestingly, rosuvastatin is transported by hNTCP, but not by rNtcp (73). This is one example, which demonstrates that despite a large substrate overlap among rat, mouse and human NTCP (146) transport data should be extrapolated with caution across different species.
The physiological relevance of a transporter is raised when a solute is transported by multiple transporters. For example, statins are also transported by organic anion transporting polypeptides (OATPs) (117) and this may also be true for other solutes not yet described. While the question of the physiologically relevant transporter(s) for drugs cannot be answered now, limited information is available for bile acids and bile salts as endogenous substrates. Upon heterologous expression of hNTCP/rNTCP, this transporter is capable of transporting conjugated bile salts as well as unconjugated bile acids (146). The same is observed for OATPs expressed in liver with a preference for unconjugated bile acids (63). Thus, one could conclude that NTCP might, with respect to bile salt transport, replace hepatocellular OATPs. However and interestingly, mice with an inactivated locus for the Slco1a/1b subfamily display normal serum levels of conjugated bile salts, but have 13-fold elevated levels of unconjugated bile acids (157). Knocking out only Slco1b2 in mice also leads to some elevation of unconjugated bile acids with normal bile salt levels in serum (33). These findings suggest that, at least in mice, NTCP is a very poor, if at all a transporter, for unconjugated bile acids.
In order to address the issue on transporter overlap for conjugated bile salts, data from Slc10a1 knock-out mice would be needed.
The structure of NTCP has not yet been resolved. The current knowledge derived from modeling and mutation experiments is still very limited and summarized elsewhere (30,41). In cases where exact structural information is lacking, chemical modifications of substrates with subsequent transport/inhibition studies may yield further information on transport properties. To this end, the side chain of bile salts was identified as very important for substrate interaction with hNTCP (9). Later, it was found by using a series of bile acids analogs with modifications at the C-3 and C-7 position that modifications at the C-7 but not at the C-3 position abolished hNTCP mediated transport with comparable results for hASBT (87). Two recent studies reported on a pharmacophore model and a structure-activity relationship for hNTCP, respectively (40,55). The first study (55) identified two hydrogen bond acceptors and three hydrophobic elements as important structural features. Thereby the distance between the hydrogen bond acceptor features as well as the presences of one or two negative charges (absent in their features) are essential for successful interaction of compounds with hNTCP. The second study (40) used inhibition studies with drugs and found that two hydrophobic elements and one hydrogen bond acceptor are of importance for the interaction with hNTCP. Hence, the two models show considerable differences. One reason could be the use of IC50 values for model development. IC50 values give no information on the type of inhibition (23) and hence may include substance binding distant to the substrate binding site of hNTCP.
3. Transport function of ASBT
Different from NTCP, the substrate specificity of ASBT is considerably narrower. Traditionally, bile acids and bile salts were identified as sole ASBT substrates (34,41). Lately, evidence has been presented supporting the concept that bile salt transport capacity of ASBT is phylogenetically conserved (101). Interestingly, a bacterial homologue of ASBT is also capable of mediating bile salt transport (78). New studies have expanded the list of substrates to two uncharged non-bile salt substrates containing an amide bond and an alcohol expanding the substrate spectrum of hASBT to solutes other than bile salts (87). ASBT has been proposed to be used as target for mediating intestinal absorption of drugs conjugated to bile salts to increase the bioavailability of such drugs. This “Trojan horse” approach has, however, met with limited success (8). This observation further supports the concept of ASBT having a rather narrow substrate specificity. Mechanistically, ASBT is electrogenic requiring cotransport of two sodium ions together with a bile salt molecule (34).
The structure of a mammalian ASBT has not yet been determined. However, recently a crystal structure of ASBT from Neisseria meningitis was elucidated at 2.2 Å. ASBTNM from N. meningitis is 26 % identical and 54 % similar to human ASBT and transports TC in a sodium-dependent manner with a Km-value of 52 μM (78). This structure, obtained in the presence of TC and sodium, revealed 10 transmembrane domains and a hydrophobic inward-facing binding cavity. This bacterial ASBT structure differs considerably from the currently favored model for mammalian ASBT thought to cross the plasma membrane 7 to 9 times (30,41). Hence, clearly much more work is needed to understand the structure of mammalian ASBT.
As ASBT is a candidate for enhancing intestinal absorption of (pro)drugs, extensive structure function studies aimed at determining a pharmacophore structure for ASBT substrates have been conducted. Based on functional studies using intestinal perfusion and everted intestine sac models, several requirements for ASBT substrates were found to be important (34) and these include a negatively charged side chain, at least one hydroxyl group at the steroid nucleus position 3, 7 or 12 and a cis-configuration of the steroid backbone. Extensive further studies led to the development of a first pharmacophore model, which differed considerably from the early model. This model consists of a hydrogen bond donor function, a hydrogen bond acceptor function and three hydrophobic features. Importantly, neither the 3-hydroxygroup of the steroid ring nor a cis-orientation of the A-ring was found to be important (9). The current state of knowledge on the chemical properties of ASBT substrates shows the following main features (87,90): 1) binding of a bile salt to ASBT involves one hydrogen bond donor, one hydrogen bond acceptor and three hydrophobic interactions, 2) lack of a negatively charged C-24 side chain maintains some affinity for ASBT, but the negatively charged side chain is necessary for high affinity and 3) the 3-position is the preferred position for drug targeting to ASBT.
4. Role of NTCP in Liver function tests
Anionic dyes have been known to be extracted from blood by the liver and that some of them may undergo enterohepatic circulation has been known for more than 100 years (1). Bromosulfophthalein (BSP) and indocyanine green (ICG) have been used clinically, whereby the latter is still in use today (131). BSP was indeed the substrate used when the first Oatp (rat Oatp1a1) was identified by expression cloning (81). Today, the role of transport systems in liver function tests is increasingly appreciated (74,147). Rat Ntcp was found to transport BSP if expressed in Xenopus laevis oocytes (60) and displayed a Km for BSP of 3.7 μM when stably expressed in HeLa cells (68). Also human NTCP transports BSP (43) and in addition mediates uptake of ICG into stably transfected cells (37). A comparative study (97) using the three heptaocellular OATPs and NTCP found that NTCP can mediate uptake, albeit weakly, of the magnetic resonance imaging contrast agent gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid (Gd-EOB-DTPA). Hence several exogenous compounds used to assess dynamic liver functions in humans are (among other transporters) taken up by NTCP into human hepatocytes. Naturally, endogenous substrates are a viable option for dynamically assessing liver function, as there will be no issues related to potential toxicity, as for example for BSP (131). Indeed, radioactively labeled cholylglycine shows a decreased clearance in patients with liver disease in comparison to healthy controls (96). In addition, there is a good correlation between clearances of cholyltaurine and ICG (119). Instead of using radioactively labeled bile salts, fluorescent bile salts could potentially be an alternative, given that NTCP does not exclusively transport bile salts. A pilot study in a small number of patients with liver cirrhosis proved this concept for cholyl-lysyl-fluorescein (CLF) (108). An investigation of CLF as potential substrate for organic anion transporters present in hepatocytes did however demonstrate that CLF is not a substrate for NTCP, but rather for OATP1B3 and to a lesser extent for OATP1B1 (38). The same study however found that CLF is a very good inhibitor of NTCP. This exemplifies that the modifications of NTCP substrates have to be carefully considered and support the concept that the side chain of bile salts is a critical determinant for their recognition by NTCP as a transported substrate (9). The following fluorescent bile salts have been shown to be substrates of rat or human NTCP: cholylglycylfluorescein and chenodeoxycholyllysyl-NBD (22), NBD-cholyltaurine (122), cholylglycylamidofluorescein and chenodeoxycholylglycylamidofluorescein (109,114).
The fact that several substances used to perform dynamic liver function tests are transported by NTCP has several implications: 1. The sodium-gradient across the plasma membrane constitutes a driving force for NTCP. This sodium gradient is maintained by (Na+, K+)-ATPase. In some forms of liver diseases, the activity of (Na+, K+)-ATPase may be impaired as shown in an animal model of endotoxin-induced cholestasis (156). Also, treatment of rats with pharmacologic doses of ethinylestradiol has been found by some to reduce the activity of (Na+, K+)-ATPase (11,107) but not by others (17,18,95) 2. NTCP expression can be up or down regulated in certain forms of human liver diseases. Thus, NTCP is down-regulated in progressive familial intrahepatic cholestasis (84), inflammatory cholestasis, primary biliary cirrhosis and chronic hepatitis C (67,115,173,174), while NTCP is up-regulated in nonalcoholic steatohepatitis (10), end-stage primary biliary cirrhosis (153) and in patients with late-stage obstructive cholestasis (25). 3. Polymorphic variants of the SLC10A1 gene lead to the expression of NTCPs with different transport properties (72). 4. mRNA expression of NTCP is subject to considerable inter-individual variability (85) and may translate into variable protein expression as demonstrated for hepatocellular ABC-transporters (106). 5. Certain drugs may interfere with the transport activity of NTCP. Hence, while some liver function tests may be affected by a disease and patient dependent altered expression and/or function of NTCP, clearly more information is needed to take advantage of NTCP as a molecular determinant in dynamic liver function tests.
5. Clinical implication of inhibition of NTCP/ASBT by drugs
Drugs may inhibit solute transport by NTCP/ASBT in two major ways: 1) drugs may be transported by the transporters resulting in competitive inhibition and 2) drugs may inhibit solute uptake by the transporters without being transported by the transporter. Indeed, there are drugs that inhibit hNTCP/rNTCP mediated bile salt uptake, but are not transported by hNTCP/rNTCP (41,89,146). Drugs that inhibit solute transport by transporters with or without being transported can have significant effects on the disposition of endogenous substrates and drugs. These effects are important considerations in drug development, as they may result in adverse effects or provide therapeutic strategies. Thus, inhibition of NTCP would be expected to impair disposition of bile salts resulting in higher systemic concentration of bile salts and consequent adverse effects (103,109). Similarly, inhibition of ASBT will result in disruption of enterohepatic circulation of bile salts and increased fecal loss of bile salts and their metabolites resulting in undesirable effects of bile acids in the colon, such as diarrhea (124). Inhibition of rodent ASBT also results in effects secondary to decreased intestinal bile acid absorption, such as decreased plasma cholesterol (100). Thus, an experimental drug (SC-435) inhibits TC uptake by ASBT and reduces atherosclerosis in apolipoprotein E null mice (14). In addition, inhibitors of ASBT (A3309 & 264W94) have been shown to produce beneficial effects in patients with chronic idiopathic constipation (27,144) and alleviate diabetes in Zucker Diabetic Fatty (ZDF) rats (26). A number of drugs have been shown to inhibit ABST (40,54,172) and these include calcium channel blockers (nifedipine, isradipine, diltiazem, verapamil), HMG-CoA reductase inhibitors (simvastatin, mevastatin, lovastatin), diuretics (spironolactone, bumetadine, althazide, but not furosemide) and others (dibucaine, indomethacin, mesoridazine, quinine). However, the clinical implications of inhibition of ASBT by these drugs are yet to be determined.
The effect on drug disposition can also lead to drug-drug interaction (DDI). DDI is an important determinant of drug safety and efficacy, and is influenced by factors that affect drug disposition. It is becoming evident that transporters often work together with drug metabolizing enzymes in drug disposition. While interactions with drug metabolizing enzymes have long been considered to be important in drug disposition, a role for transporters in drug disposition, therapeutic efficacy and adverse drug reactions has increasingly been recognized (90). Some transporters are considered to be clinically important in drug absorption and disposition and therefore could mediate DDIs (51). Although a number of SLC transporters are considered to have clinical importance in the absorption and disposition of drugs (51), NTCP and ASBT are not included. This is mainly because the clinical importance of these transporters is not well established. Since drugs can be transported by NTCP and can also inhibit solute transport by NTCP, a potential role of NTCP in drug-drug interaction is very likely and is reported (69,170). Similarly, ASBT may also be involved in drug-drug interactions that may be revealed in future studies.
There appears to be a difference in the ability of drugs to inhibit NTCP and ASBT. A previous study in rabbit suggested that more drugs inhibited NTCP than ASBT (91). A recent study compared the inhibitory potentials of a number of FDA approved drugs for NTCP and ASBT (40). This study identified 27 drugs as novel NTCP inhibitors, including a variety of therapeutic classes including antifungal, antihyperlipidemic, antihypertensive, anti-inflammatory, and glucocorticoid drugs. Of the 72 drugs tested, a total of 31 drugs inhibited NTCP, while 51 drugs inhibited ASBT with overlapping inhibition. These results suggest that ASBT is more permissive to drug inhibition than NTCP and this may be related to NTCP possessing fewer pharmacophore features. Whether the differences in the permissiveness to drug inhibition between the two transporters are due to species difference remains to be established. The possibility of species differences is consistent with the findings that rosuvastatin is a human NTCP substrate but not a substrate for rat NTCP (73) and bosentan is a more potent inhibitor for rat NTCP than human NTCP (98).
6. Post-Translational Regulation of NTCP and ASBT
Both NTCP and ASBT undergo transcriptional and post-transcriptional regulations. Transcriptional regulation involving various nuclear receptors has recently been reviewed by others (30,88) and hence the focus of this review will be post translational regulation. Plasma membrane transporters, after translation, need to be translocated to the plasma membrane to carry out their intended functions. The level of a transporter in the plasma membrane may be increased or decreased based on the need to regulate solute transport by the transporter. This is a highly regulated post-translational event requiring involvement of various cellular signaling pathways and is best exemplified by the regulation of glucose transporter (GLUT4) by insulin in adipocytes and skeletal muscle (45,99). The post-translation event also involves protein quality control to select and target dysfunctional proteins for degradation by the ubiquitin-proteasome system (29). Studies to date suggest that the post-translational regulation of NTCP as well as ASBT involves plasma membrane transport/retrieval and degradation by the ubiquitin-proteasome system. Several signaling pathways have been implicated in the post-translational regulation of these transporters and these pathways include cAMP, intracellular Ca2+, nitric oxide, and activation of phosphoinositide-3-kinase (PI3K), proteins kinase Cs (PKCs) and protein phosphatases. The role of these pathways is better understood for NTCP (Fig. 1) than ASBT because of limited studies with ASBT. Some of these pathways have previously been reviewed (4,20,88).
Figure 1.
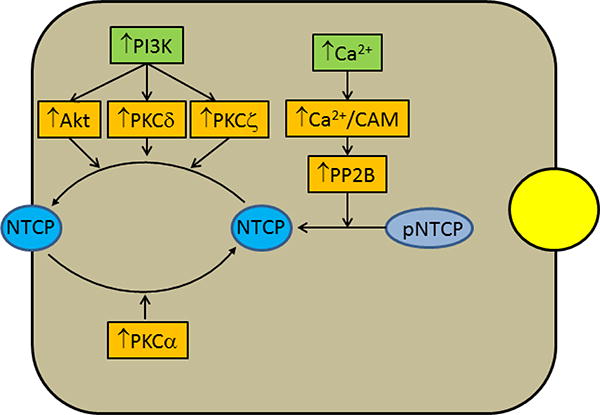
Postulated signaling pathways regulating NTCP insertion into and retrieval from the plasma membrane. Cyclic AMP increases hepatic uptake of bile acid by increasing plasma membrane level of NTCP. The effect of cAMP is mediated via two major pathways; activation of PI3K and increase in intracellular Ca2+. Activation of PI3K leads to activations of Akt, PKCδ and PKCζ, which in turn induce translocation of NTCP from intracellular compartment to the plasma membrane most likely by stimulating vesicular trafficking of NTCP containing vesicles. Rat NTCP is a phosphoprotein and the dephosphorylated NTCP undergoes translocation to the plasma membrane. Increases in intracellular Ca2+ by cAMP leads to activation of Ca2+-dependent protein phosphatase 2B (PP2B) via Ca2+-calmodulin kinase (Ca2+-CAM). PP2B dephosphorylates rat NTCP allowing translocation to the plasma membrane. On the other hand, activation of PKCα by a cholestatic bile acid, taurochenodeoxycholate (TCDC), stimulates retrieval of rat NTCP from the plasma membrane by an as yet unknown mechanism except that inhibition of PI3K and PP2B enhances the effect of TCDC.
Plasma membrane level of NTCP and thereby hepatic TC uptake is regulated by cAMP and bile acids. Cyclic AMP stimulates TC uptake (19,57,118,139) and increases plasma membrane level of NTCP in rat hepatocytes and in hepatic cells stably transfected with human NTCP (42,111,139). In contrast, cholestatic bile salts (taurochenodeoxycholate (TCDC) and taurolithocholate (TLC)) inhibit TC uptake (110,142) and decrease plasma membrane rNTCP (110,141). Thus, bile salts uptake by NTCP undergoes dynamic regulation. The increase in uptake by cAMP, increased by glucagon, may allow the hepatocytes to efficiently absorb bile salts returning from the intestine under physiological conditions, while the decrease in uptake may protect hepatocytes from further increases in intracellular bile salts observed in cholestasis (125). Functionally important polymorphisms in hNTCP may result in decreased plasma expression. Multiple single nucleotide polymorphisms in NTCP have been shown to be present in populations of European, African, Chinese, and Hispanic Americans, and the altered transport activity of Ile223 to Thr variant seen only in African Americans was due at least in part to decreased plasma membrane expression (72).
A role of PI3K in bile formation is evident from a study (46) showing that wortmannin, a specific inhibitor of PI3K, inhibits bile formation, bile acid secretion and vesicle trafficking in isolated perfused rat liver. Since then various studies have provided evidence supporting a role for PI3K/Akt pathway in cell survival and translocation of hepatocellular transporters to the plasma membrane, indicating a beneficial role of PI3K in hepatic cells (4,48,130,160). PI3Ks are a family of lipid kinases (classes I, II, and III) that phosphorylate the inositol ring of phosphatidylinositides (PIs) at 3 position known as D3 phosphorylation (24). The resulting phosphorylated PIs (PIPs), acting in concert with phosphoinositide-dependent kinases (PDKs), are involved in the activation of downstream kinases, such as PKCζ/λ, Akt/PKB, and p70S6K (154) The PI3K/Akt pathway is involved in cell swelling and cAMP-induced increases in TC uptake and rNTCP translocation to the plasma membrane (159,163) and cAMP inhibits TLC-induced inhibition of TC uptake by activating the PI3K pathway (141). The effect of cAMP on hNTCP/rNTCP translocation is also mediated via PI3K/PKCδ (118,136) and PI3K/PKCζ (102) pathways (see below).
NTCP translocation to the plasma membrane is regulated by phosphorylation and dephosphorylation, although kinase(s) involved in phosphorylation of NTCP has not yet been identified. Rat NTCP is a serine/threonine phosphorylated protein (112) and cAMP-induced increases in TC uptake and plasma membrane rNTCP is associated with NTCP dephosphorylation (113) at Ser226(5). Cyclic AMP increases intracellular Ca2+(57) and the ability of cAMP to dephosphorylate rNTCP is inhibited by a calcium chelator (112). Cyclic AMP activates protein phosphatase 2B (PP2B), a Ca2+/calmodulin-dependent serine and threonine protein phosphatase (86) by increasing intracellular Ca2+(161). PP2B directly dephosphorylates rNTCP and an inhibitor of PP2B inhibits cAMP-induced dephosphorylation and translocation of rNTCP (161). Taken together, these results suggest that cAMP activates PP2B by increasing intracellular Ca2+ and PP2B in turn dephosphorylates rNTCP facilitating its translocation to the plasma membrane (Fig. 1). Interestingly, inhibition of PP2B has been shown to reverse TCDC-induced retrieval of rNTCP (110). Whether TCDC-induced retrieval of rNTCP involves PP2B mediated dephosphorylation of NTCP remains to be established. It is however possible that inhibition of PP2B results in increased phosphorylation of a mediator that is involved in the retrieval of rNTCP.
Cyclic AMP-dependent increases in apical ASBT in rat cholangiocytes (3) and rat ileum (128) have been reported. Secretin, acting via cAMP, increases apical ASBT in rat cholangiocytes, which is reversible and requires intact microtubules (3). This result suggests acute stimulation of vesicular translocation of ASBT to the apical membrane and this may explain secretin induced increased cholehepatic shunting and associated increases in the choleretic effect of bile acids (165). It does not appear that ASBT translocation is affected by phosphorylation. ASBT has not been shown to be phosphorylated. However, mutation of potential phosphorylation sites (S335 and T339) in the cytoplasmic tail of rASBT by non-phosphorylatable alanine results in decreased initial bile acid transport activity and increases the basolateral distribution of the mutants without significantly decreasing apical distribution (152). Thus, phosphorylation, if it occurs, may inhibit basolateral targeting of ASBT in rat cholangiocytes.
Like NTCP (93), ASBT in human cholangiocyte cell lines undergoes degradation via the ubiquitin-proteasome system (166). NTCP is shown to be degraded by the ubiquitin-proteasome system at the level of ER-associated degradation. It is speculated that dysfunction of this pathway may result in intracellular NTCP deposits in cholestatic livers (93). Since mutation of S335 and T339 to alanine in human ASBT reduces the rate of ASBT disposal under basal conditions as well as IL-1β -dependent ubiquitination and disposal of ASBT, it is suggested that human ABST degradation by proteasome involves phosphorylation followed by ubiquitination (166). However, role of phosphorylation has not been directly assessed.
Protein kinase C isoforms have been shown to mediate NTCP/Ntcp translocation to and retrieval from the plasma membrane (Fig. 1). Protein kinase C belongs to a family of serine/threonine protein kinases that are involved in the regulation of diverse cellular functions and consists of at least 12 isoforms (116,127). Activation of most PKCs, if not all, is PI3K dependent (116). PKCs shown to be present in rat hepatocytes include PKCα, PKCδ, PKCε, and PKCζ; with the presence of PKCβII being controversial. (13,83,149). Initial studies with PMA, activator of conventional and novel PKCs, suggested that activation of PKCs inhibits TC uptake in hepatocytes (4,21,57). More recent studies suggest that TC uptake and NTCP translocation/retrieval are differentially affected by PKC isoforms. Thus, PKCδ (118,136) and PKCζ (102) mediate cAMP-induced hNTCP/rNTCP translocation, while conventional PKCs (most likely PKCα) mediate rNTCPp retrieval (110,150). The activation of PKCδ and PKCζ by cAMP is PI3K-dependent (102,118,136), while TCDC-induced rNTCP retrieval is enhanced when PI3K is inhibited (110). Thus, it does not appear that TCDC-induced activation of PKCα is dependent on PI3K. On the other hand, TLC induces retrieval of NTCP in rat hepatocytes (141), but inhibits PKCα (12). Thus, the retrieval of rNTCP by cholestatic bile acids may involve mediators in addition to PKCα. TLC, which activates PKCε in rat hepatocytes (13,141), also inhibits TC uptake in rat hepatocytes (142) and in HuH-NTCP cell (141). However, TLC-induced inhibition of TC uptake is not mediated via PKCε (141). In contrast, TLC-induced retrieval of MRP2 is mediated via PKCε (140). Thus, the canalicular membrane may be the target of PKCε and this is consistent with the finding that TLC translocates PKCε to the canalicular membrane (13). PMA, an activator of PKCs, has been shown to inhibit TC uptake and decrease plasma membrane ASBT in Caco-2 cells transfected with ileal ASBT (133). This effect of PMA appears to be mediated via PKCζ. It is speculated that this pathway may underlie the pathophysiology of diseases associated with disruptions in bile acid homeostasis.
There are limited studies exploring the mechanism by which PKCs affect NTCP translocation. These studies suggest a role for Rab4, a small GTPase, which cycles between GTP bound (Rab4-GTP) active form and GDP bound (Rab4-GDP) inactive form and is involved in endocytosis (2). Thus, PKCδ facilitates hNTCP translocation by activating Rab4 (118) and PKCζ increases motility of rNTCP containing vesicles along the microtubules (132). Other studies showed that Rab4 facilitates cAMP-induced rNTCP translocation (139), rNTCP containing vesicles co-localize with Rab4 (132), cAMP induced rNTCP translocation is dependent on PI3K dependent (162) and actin cytoskeleton and microtubules (42,162), and activation of PKCδ and PKCζ is PI3K dependent (102,118,136). Taken together, these results may suggest that activation of PI3K/PKCδ by cAMP leads to activation of Rab4 in NTCP containing Rab4 vesicles and this is followed by PI3K/PKCζ mediated increased motility of NTCP/Rab4 containing vesicles along the microtubules to the plasma membrane (Fig. 2).
Figure 2.
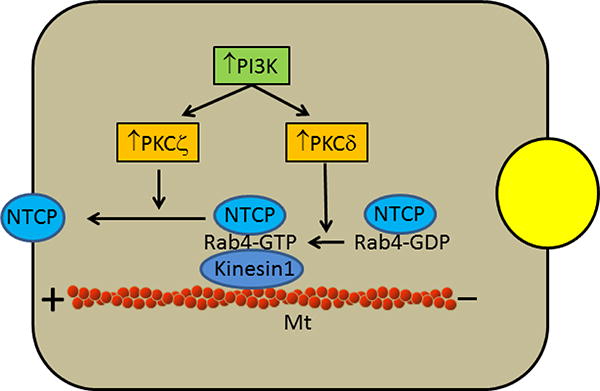
Postulated role of Rab4, PI3K/PKCδ and PI3K/PKCζ in the translocation of NTCP. NTCP containing vesicles co-localize with Rab4, which cycles between GTP bound (Rab4-GTP) active form and GDP bound (Rab4-GDP) inactive form. Activation of PKCδ leads to the conversion of inactive Rab4-GDP to active Rab4-GTP, which moves NTCP containing vesicles towards the plus end of microtubules (Mt) by interacting with kinesin-1. This movement is further facilitated by PKCζ.
Post translation modification affecting plasma membrane localization of NTCP also involves S-nitrosylation. Treatment with thiol-binding agents inhibits TC uptake in isolated rat hepatocytes (15,16) and by NTCP and ASBT (66) indicating a role for cysteine residues in transport function. Nitric oxide has been shown to inhibit TC uptake in rat hepatocytes (145), HuH7 cells stably transfected with human NTCP (138) and human hepatocytes (53). One of the ways that NO exerts its cellular effects is through a posttranslational modification termed S-nitrosylation, which involves binding of NO to reactive thiol groups on cysteine residues (70,135). A number of proteins (transcription factors, kinases, phosphatases, membrane receptors and transporters) are regulated by S-nitrosylation (39,70) and abnormal S-nitrosylation of proteins has been implicated in a number of human diseases including sepsis, diabetes and cystic fibrosis (28,47,58,120,126,137). Human NTCP has four cysteine residues (Cys44, Cys98, Cys125, and Cys266) that are conserved in rat, mouse, and rabbit (171). Thus, inhibition of TC uptake by NO may involve S-nitrosylation of NTCP. Indeed, NO has been shown to S-nitrosylate NTCP (53,138) and this is associated with a decrease in plasma membrane NTCP (138). Moreover, dithiothreitol reverses NO-mediated inhibition of TC uptake and S-nitrosylation of NTCP (138). A follow up study showed that the inhibition of TC uptake by NO may involve S-nitrosylation of rNTCP-Cys96(155). Such a mechanism may be involved in LPS-induced cholestasis, which is associated with a burst of NO production by iNOS in liver cells (49).
NO also produces its cellular effects by nitration of tyrosine residues (79) and has been shown to increase tyrosine nitration of hNTCP (53). However, the effect of tyrosine nitration on TC uptake and plasma membrane NTCP has not been directly studied. It may be noted that two tyrosine residues on the cytoplasmic tail of rat NTCP appear to be involved in targeting to the basolateral membrane (151). Thus, it is possible that tyrosine nitration of NTCP by NO may also result in decreased translocation to the basolateral membrane resulting in decreased TC uptake.
There appears to be species differences in the regulation of plasma membrane localization of hNTCP and rNTCP. TLC inhibits TC uptake by hNTCP and rNTCP. However, TLC decreases plasma membrane rat NTCP but not human NTCP (141). Like Bosentan (98), TLC inhibits TC uptake by rNTCP and hNTCP non-competitively (142) and competitively (141), respectively. NO inhibits TC uptake by hNTCP/rNTCP. However, NO decreases plasma membrane level of human NTCP (138) and does not affect plasma membrane level of rat NTCP (155). In contrast, cAMP-induced increases in TC uptake are associated with translocation of hNTCP as well as rNTCP. These differences should be taken into account when extrapolating mechanisms regulating rat NTCP to human NTCP for the purpose of human drug development and also for understanding the mechanistic relevance in human diseases.
7. A novel function of NTCP
A novel function for NTCP as a receptor and transporter for hepatitis B virus (HBV) and hepatitis D virus (HDV) has recently been described (169). Both HBV and HDV contain three envelope proteins known as small (S), middle (M), and large (L). These proteins share the same C-terminal domain corresponding to the S protein, but differ at the N-terminal domains by the presence of pre-S2 domain in M protein and pre-S1 and pre-S2 domains in L protein. The entry of both HBV and HDV is determined by the pre-S1 domain of L protein after interacting with cellular receptor(s) on hepatocytes (52), as evidenced by inhibition of viral entry and infection by myristoylated N-terminal 47 amino acids of the pre-S1 domain of L protein (56,104). Myrcludex B, a drug based on myristoylated pre-S1 domain, has entered clinical trials after demonstration of efficacy against both HBV and HDV infection in an uPA-SCID mouse model (121), indicating potential clinical significance of viral entry inhibition in the treatment of HBV/HDV. Despite these advances, the identity of the receptor(s) remained elusive until the studies by Yan et al (169).
In an elegant series of experiments Yan et al showed (169) that the receptor-binding region of pre-S1 domain of L protein specifically interacts with NTCP and amino acids 157 to 165 of NTCP (KGIVISLVL) are critical for viral entry. A follow up study showed that mouse NTCP can bind pre-S1 domain of HBV L protein, but does not support HBV or HDV infection (168). However, substitution of mouse Ntcp residues 84 to 87 by human counterparts effectively supports viral infections. Thus, there may be multiple domains of NTCP involved in supporting viral infections. In addition, insensitive hepatic cell lines (HuH-7 and HepG-2) transfected with human NTCP supported HBV/HDV infection (168), although the extent of infection was much lower than in hepatocytes. Thus, human NTCP plays an important role in viral entry and consequent infection. However, viral entry is only one step in efficient viral infection and replication in human hepatocytes and other factors, such as host components, are likely to be involved (134,158,167).
These results raise interesting questions about the transport function of NTCP. One possibility may be that these viruses are transported by NTCP the same way as it transports bile salts. In that case, it would be important to know whether the transport of HBV and HDV into hepatocytes is Na+ dependent and is inhibited by known substrates/inhibitors of NTCP. It is however possible that this is a novel function of NTCP with molecular mechanism of the transport function yet to be determined. A likely possibility is that NTCP is internalized by endocytosis following binding to the virus allowing the virus to enter the cell, as also suggested by others (158,167). Internalization of HBV/HDV may be initiated by protein-protein interactions between the envelop protein of the virus and NTCP leading to endocytosis. In that case, what would be the signal for internalization and how is that signal generated? Calcium dependent conventional PKCs and most likely PKCα have been shown to induce rNTCP retrieval (110,150). Whether PKCα or other signaling molecules may be involved remains to be investigated. Irrespective of the transport mechanism involved, the finding that NTCP is involved in HBV and HDV infection is expected to inspire further studies leading to a better understanding of the role of NTCP in HBV/HDV infection and development of agents to inhibit viral entry and infection.
8. Future perspectives
NTCP and ASBT were originally described to transport bile salts and play an important role in enterohepatic circulation of bile salts. It is now apparent that these transporters are capable of transporting other solutes including drugs and viruses. The physiological and clinical relevance of NTCP for drugs transported by additional transporters requires further examination. The ability of drugs to be transported or to inhibit solute transport by these transporters is likely to affect drug disposition resulting drug-drug interactions, provide strategies for novel drug development and alter disposition of endogenous substrates. Further studies are needed to fully explore the clinical relevance of such interactions. The ability of NTCP to transport HBV/HDV and thereby facilitate hepatic viral infection should lead to further studies providing better understanding of the transport mechanism and regulation of NTCP as well as mechanisms of and therapy against HBV/HVD infection. Further studies are needed to define the conditions and substrates for NTCP to be used for liver function tests. Our understanding of cellular mechanisms involved in the post-translational regulation of NTCP/ASBT has been steadily increasing. It is apparent that solute transport by these transporters is acutely regulated by translocation to and retrieval from the plasma membrane. However, signaling pathways involved are incompletely understood, especially for ileal ASBT. A better understanding of the signaling pathways regulating plasma membrane localization should allow us to target specific pathways to limit or enhance solute transport and thereby limit hepatic toxicity or enhance hepatic functions as needed. It may be noted that most of these studies are conducted in rodents. While there are some similarities (based on limited studies) in substrate specificity and regulatory mechanisms between rodents and humans, there are also differences. Thus, caution should be exercised in extrapolating rodent data to humans until findings in rodents are confirmed for the human isoforms. Most of the SLC10A transporters are considered to be orphan transporters and hence very little is known about the regulation of these orphan transporters. It is anticipated that substantial progress in these areas will be made in the near future.
Acknowledgments
This work was supported in part by grants from NIH to MSA (DK033436 and DK090010) and from the Swiss National Science Foundation to BS (310030_144195).
References
- 1.Abel JJ, Rowntree LG. On the pharmacological action of some phthaleins and their derivatives, with especial reference to their behavior as purgatives. J Pharmacol Exp Ther. 1909;1:231–264. [Google Scholar]
- 2.Agola JO, Jim PA, Ward HH, BasuRay S, Wandinger-Ness A. Rab GTPases as regulators of endocytosis, targets of disease and therapeutic opportunities. Clin Genet. 2011;80:305–318. doi: 10.1111/j.1399-0004.2011.01724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alpini G, Glaser S, Baiocchi L, Francis H, Xia X, Lesage G. Secretin activation of the apical Na+-dependent bile acid transporter is associated with cholehepatic shunting in rats. Hepatology. 2005;41:1037–1045. doi: 10.1002/hep.20653. [DOI] [PubMed] [Google Scholar]
- 4.Anwer MS. Cellular Regulation of hepatic bile acid transport in health and cholestasis. Hepatology. 2004;39:581–589. doi: 10.1002/hep.20090. [DOI] [PubMed] [Google Scholar]
- 5.Anwer MS, Gillin H, Mukhopadhyay S, Balasubramaniyan N, Suchy FJ, Ananthanarayanan M. Dephosphorylation of Ser-226 facilitates plasma membrane retention of Ntcp. J Biol Chem. 2005;280:33687–33692. doi: 10.1074/jbc.M502151200. [DOI] [PubMed] [Google Scholar]
- 6.Anwer MS, Hegner D. Effect of Na+ on bile acid uptake by isolated rat hepatocytes. Hoppe-Zeyler's Z Physiol Chem. 1978;359:181–192. [PubMed] [Google Scholar]
- 7.Bajor A, Gillberg PG, Abrahamsson H. Bile acids: short and long term effects in the intestine. Scand J Gastroenterol. 2010;45:645–664. doi: 10.3109/00365521003702734. [DOI] [PubMed] [Google Scholar]
- 8.Balakrishnan A, Polli JE. Apical sodium dependent bile acid transporter (ASBT, SLC10A2): a potential prodrug target. Mol Pharm. 2006;3:223–230. doi: 10.1021/mp060022d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baringhaus KH, Matter H, Stengelin S, Kramer W. Substrate specificity of the ileal and the hepatic Na(+)/bile acid cotransporters of the rabbit. II. A reliable 3D QSAR pharmacophore model for the ileal Na(+)/bile acid cotransporter. J Lipid Res. 1999;40:2158–2168. [PubMed] [Google Scholar]
- 10.Bechmann LP, Kocabayoglu P, Sowa JP, Sydor S, Best J, Schlattjan M, Beilfuss A, Schmitt J, Hannivoort RA, Kilicarslan A, Rust C, Berr F, Tschopp O, Gerken G, Friedman SL, Geier A, Canbay A. Free fatty acids repress small heterodimer partner (SHP) activation and adiponectin counteracts bile acid-induced liver injury in superobese patients with nonalcoholic steatohepatitis. Hepatology. 2013;57:1394–1406. doi: 10.1002/hep.26225. [DOI] [PubMed] [Google Scholar]
- 11.Berr F, Simon FR, Reichen J. Ethynylestradiol impairs bile salt uptake and Na-K pump function of rat hepatocytes. Am J Physiol. 1984;247:G437–G443. doi: 10.1152/ajpgi.1984.247.4.G437. [DOI] [PubMed] [Google Scholar]
- 12.Beuers U, Bilzer M, Chittattu A, Kullak-Ublick GA, Keppler D, Paumgartner G, Dombrowski F. Tauroursodeoxycholic acid inserts the apical conjugate export pump, Mrp2, into canalicular membranes and stimulates organic anion secretion by protein kinase C-dependent mechanisms in cholestatic rat liver. Hepatology. 2001;33:1206–1216. doi: 10.1053/jhep.2001.24034. [DOI] [PubMed] [Google Scholar]
- 13.Beuers U, Probst I, Soroka C, Boyer JL, Kullak-Ublick GA, Paumgartner G. Modulation of protein kinase C by taurolithocholic acid in isolated rat hepatocytes. Hepatology. 1999;29:477–482. doi: 10.1002/hep.510290227. [DOI] [PubMed] [Google Scholar]
- 14.Bhat BG, Rapp SR, Beaudry JA, Napawan N, Butteiger DN, Hall KA, Null CL, Luo Y, Keller BT. Inhibition of ileal bile acid transport and reduced atherosclerosis in apoE-/- mice by SC-435. J Lipid Res. 2003;44:1614–1621. doi: 10.1194/jlr.M200469-JLR200. [DOI] [PubMed] [Google Scholar]
- 15.Blumrich M, Petzinger E. Membrane transport of conjugated and unconjugated bile acids into hepatocytes is susceptible to SH-blocking reagents. Biochim Biophys Acta. 1990;1029:1–12. doi: 10.1016/0005-2736(90)90430-v. [DOI] [PubMed] [Google Scholar]
- 16.Blumrich M, Petzinger E. Two distinct types of SH-groups are necessary for bumetanide and bile acid uptake into isolated rat hepatocytes. Biochim Biophys Acta. 1993;1149:278–284. doi: 10.1016/0005-2736(93)90211-h. [DOI] [PubMed] [Google Scholar]
- 17.Boelsterli UA, Rakhit G, Balazs T. Modulation by S-adenosyl-L-methionine of hepatic Na+,K+-ATPase, membrane fluidity, and bile flow in rats with ethinyl estradiol-induced cholestasis. Hepatology. 1983;3:12–17. doi: 10.1002/hep.1840030102. [DOI] [PubMed] [Google Scholar]
- 18.Bossard R, Stieger B, O'Neill B, Fricker G, Meier PJ. Ethinylestradiol treatment induces multiple canalicular membrane transport alterations in rat liver. J Clin Invest. 1993;91:2714–2720. doi: 10.1172/JCI116511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Botham KM, Suckling KE. The effect of dibutyryl cyclic AMP on the uptake of taurocholic acid by isolated rat liver cells. Biochim Biophys Acta. 1986;883:26–32. doi: 10.1016/0304-4165(86)90130-3. [DOI] [PubMed] [Google Scholar]
- 20.Bouscarel B, Kroll SD, Fromm H. Signal transduction and hepatocellular bile acid transport: cross talk between bile acids and second messengers. Gastroenterology. 1999;117:433–452. doi: 10.1053/gast.1999.0029900433. [DOI] [PubMed] [Google Scholar]
- 21.Bouscarel B, Reza S, Dougherty LA, Fromm H, Nussbaum R. Regulation of taurocholate and ursodeoxycholate uptake in hamster hepatocytes by Ca2+- mobilizing agents. Am J Physiol. 1996;271:G1084–G1095. doi: 10.1152/ajpgi.1996.271.6.G1084. [DOI] [PubMed] [Google Scholar]
- 22.Boyer JL, Ng OC, Ananthanarayanan M, Hofmann AF, Schteingart CD, Hagenbuch B, Stieger B, Meier PJ. Expression and characterization of a functional rat liver Na+ bile acid cotransport system in COS-7 cells. Am J Physiol. 1994;266:G382–G387. doi: 10.1152/ajpgi.1994.266.3.G382. [DOI] [PubMed] [Google Scholar]
- 23.Brouwer KL, Keppler D, Hoffmaster KA, Bow DA, Cheng Y, Lai Y, Palm JE, Stieger B, Evers R. In vitro methods to support transporter evaluation in drug discovery and development. Clin Pharmacol Ther. 2013;94:95–112. doi: 10.1038/clpt.2013.81. [DOI] [PubMed] [Google Scholar]
- 24.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 25.Chen HL, Liu YJ, Chen HL, Wu SH, Ni YH, Ho MC, Lai HS, Hsu WM, Hsu HY, Tseng HC, Jeng YM, Chang MH. Expression of hepatocyte transporters and nuclear receptors in children with early and late-stage biliary atresia. Pediatr Res. 2008;63:667–673. doi: 10.1203/PDR.0b013e318170a6b5. [DOI] [PubMed] [Google Scholar]
- 26.Chen L, Yao X, Young A, McNulty J, Anderson D, Liu Y, Nystrom C, Croom D, Ross S, Collins J, Rajpal D, Hamlet K, Smith C, Gedulin B. Inhibition of apical sodium-dependent bile acid transporter as a novel treatment for diabetes. Am J Physiol Endocrinol Metab. 2012;302:E68–E76. doi: 10.1152/ajpendo.00323.2011. [DOI] [PubMed] [Google Scholar]
- 27.Chey WD, Camilleri M, Chang L, Rikner L, Graffner H. A randomized placebo-controlled phase IIb trial of a3309, a bile acid transporter inhibitor, for chronic idiopathic constipation. Am J Gastroenterol. 2011;106:1803–1812. doi: 10.1038/ajg.2011.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chung KK, Dawson VL, Dawson TM. S-nitrosylation in Parkinson's disease and related neurodegenerative disorders. Methods Enzymol. 2005;396:139–150. doi: 10.1016/S0076-6879(05)96014-X. [DOI] [PubMed] [Google Scholar]
- 29.Claessen JH, Kundrat L, Ploegh HL. Protein quality control in the ER: balancing the ubiquitin checkbook. Trends Cell Biol. 2012;22:22–32. doi: 10.1016/j.tcb.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Claro da ST, Polli JE, Swaan PW. The solute carrier family 10 (SLC10): beyond bile acid transport. Mol Aspects Med. 2013;34:252–269. doi: 10.1016/j.mam.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clayton PT. Disorders of bile acid synthesis. J Inherit Metab Dis. 2011;34:593–604. doi: 10.1007/s10545-010-9259-3. [DOI] [PubMed] [Google Scholar]
- 32.Craddock AL, Love MW, Daniel RW, Kirby LC, Walters HC, Wong MH, Dawson PA. Expression and transport properties of the human ileal and renal sodium-dependent bile acid transporter. Am J Physiol. 1998;274:G157–G169. doi: 10.1152/ajpgi.1998.274.1.G157. [DOI] [PubMed] [Google Scholar]
- 33.Csanaky IL, Lu H, Zhang Y, Ogura K, Choudhuri S, Klaassen CD. Organic anion-transporting polypeptide 1b2 (Oatp1b2) is important for the hepatic uptake of unconjugated bile acids: Studies in Oatp1b2-null mice. Hepatology. 2011;53:272–281. doi: 10.1002/hep.23984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dawson PA. Role of the intestinal bile acid transporters in bile acid and drug disposition. Handb Exp Pharmacol. 2011:169–203. doi: 10.1007/978-3-642-14541-4_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dawson PA, Hubbert ML, Rao A. Getting the mOST from OST: Role of organic solute transporter, OSTalpha-OSTbeta, in bile acid and steroid metabolism. Biochim Biophys Acta. 2010;1801:994–1004. doi: 10.1016/j.bbalip.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dawson PA, Lan T, Rao A. Bile acid transporters. J Lipid Res. 2009;50:2340–2357. doi: 10.1194/jlr.R900012-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Graaf W, Hausler S, Heger M, van Ginhoven TM, van CG, Bennink RJ, Kullak-Ublick GA, Hesselmann R, van Gulik TM, Stieger B. Transporters involved in the hepatic uptake of (99m)Tc-mebrofenin and indocyanine green. J Hepatol. 2011;54:738–745. doi: 10.1016/j.jhep.2010.07.047. [DOI] [PubMed] [Google Scholar]
- 38.de Waart DR, Hausler S, Vlaming ML, Kunne C, Hanggi E, Gruss HJ, Oude Elferink RP, Stieger B. Hepatic transport mechanisms of cholyl-L-lysyl-fluorescein. J Pharmacol Exp Ther. 2010;334:78–86. doi: 10.1124/jpet.110.166991. [DOI] [PubMed] [Google Scholar]
- 39.Derakhshan B, Hao G, Gross SS. Balancing reactivity against selectivity: the evolution of protein S-nitrosylation as an effector of cell signaling by nitric oxide. Cardiovasc Res. 2007;75:210–219. doi: 10.1016/j.cardiores.2007.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dong Z, Ekins S, Polli JE. Structure-activity relationship for FDA approved drugs as inhibitors of the human sodium taurocholate cotransporting polypeptide (NTCP) Mol Pharm. 2013;10:1008–1019. doi: 10.1021/mp300453k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Doring B, Lutteke T, Geyer J, Petzinger E. The SLC10 carrier family: transport functions and molecular structure. Curr Top Membr. 2012;70:105–168. doi: 10.1016/B978-0-12-394316-3.00004-1. [DOI] [PubMed] [Google Scholar]
- 42.Dranoff JA, McClure M, Burgstahler AD, Denson LA, Crawford AR, Crawford JM, Karpen SJ, Nathanson MH. Short-term regulation of bile acid uptake by microfilament-dependent translocation of ntcp to the plasma membrane. Hepatology. 1999;30:223–229. doi: 10.1002/hep.510300136. [DOI] [PubMed] [Google Scholar]
- 43.Engel A, Oswald S, Siegmund W, Keiser M. Pharmaceutical excipients influence the function of human uptake transporting proteins. Mol Pharm. 2012;9:2577–2581. doi: 10.1021/mp3001815. [DOI] [PubMed] [Google Scholar]
- 44.Faber KN, Muller M, Jansen PL. Drug transport proteins in the liver. Adv Drug Deliv Rev. 2003;55:107–124. doi: 10.1016/s0169-409x(02)00173-4. [DOI] [PubMed] [Google Scholar]
- 45.Foley K, Boguslavsky S, Klip A. Endocytosis, recycling, and regulated exocytosis of glucose transporter 4. Biochemistry. 2011;50:3048–3061. doi: 10.1021/bi2000356. [DOI] [PubMed] [Google Scholar]
- 46.Folli F, Alvaro D, Gigliozzi A, Bassotti C, Kahn CR, Pontiroli AE, Capocaccia L, Jezequel AM, Benedetti A. Regulation of endocytic-transcytotic pathways and bile secretion by phosphatidylinositiol 3-kinase in rats. Gastroenterology. 1997;113:954–965. doi: 10.1016/s0016-5085(97)70192-6. [DOI] [PubMed] [Google Scholar]
- 47.Foster MW, Hess DT, Stamler JS. Protein S-nitrosylation in health and disease: a current perspective. Trends Mol Med. 2009;15:391–404. doi: 10.1016/j.molmed.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gates A, Hohenester S, Anwer MS, Webster CR. cAMP-GEF cytoprotection by Src tyrosine kinase activation of phosphoinositide-3-kinase p110 {beta}/{alpha} in rat hepatocytes. Am J Physiol Gastrointest Liver Physiol. 2009;296:G764–G774. doi: 10.1152/ajpgi.90622.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Geier A, Fickert P, Trauner M. Mechanisms of disease: mechanisms and clinical implications of cholestasis in sepsis. Nat Clin Pract Gastroenterol Hepatol. 2006;3:574–585. doi: 10.1038/ncpgasthep0602. [DOI] [PubMed] [Google Scholar]
- 50.Geyer J, Wilke T, Petzinger E. The solute carrier family SLC10: more than a family of bile acid transporters regarding function and phylogenetic relationships. Naunyn Schmiedebergs Arch Pharmacol. 2006;372:413–431. doi: 10.1007/s00210-006-0043-8. [DOI] [PubMed] [Google Scholar]
- 51.Giacomini KM, Huang SM, Tweedie DJ, Benet LZ, Brouwer KL, Chu X, Dahlin A, Evers R, Fischer V, Hillgren KM, Hoffmaster KA, Ishikawa T, Keppler D, Kim RB, Lee CA, Niemi M, Polli JW, Sugiyama Y, Swaan PW, Ware JA, Wright SH, Yee SW, Zamek-Gliszczynski MJ, Zhang L. Membrane transporters in drug development. Nat Rev Drug Discov. 2010;9:215–236. doi: 10.1038/nrd3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Glebe D, Urban S. Viral and cellular determinants involved in hepadnaviral entry. World J Gastroenterol. 2007;13:22–38. doi: 10.3748/wjg.v13.i1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gonzalez R, Cruz A, Ferrin G, Lopez-Cillero P, Fernandez-Rodriguez R, Briceno J, Gomez MA, Rufian S, Mata ML, Martinez-Ruiz A, Marin JJ, Muntane J. Nitric oxide mimics transcriptional and post-translational regulation during alpha-tocopherol cytoprotection against glycochenodeoxycholate-induced cell death in hepatocytes. J Hepatol. 2011;55:133–144. doi: 10.1016/j.jhep.2010.10.022. [DOI] [PubMed] [Google Scholar]
- 54.Grandvuinet AS, Vestergaard HT, Rapin N, Steffansen B. Intestinal transporters for endogenic and pharmaceutical organic anions: the challenges of deriving in-vitro kinetic parameters for the prediction of clinically relevant drug-drug interactions. J Pharm Pharmacol. 2012;64:1523–1548. doi: 10.1111/j.2042-7158.2012.01505.x. [DOI] [PubMed] [Google Scholar]
- 55.Greupink R, Nabuurs SB, Zarzycka B, Verweij V, Monshouwer M, Huisman MT, Russel FG. In silico identification of potential cholestasis-inducing agents via modeling of Na(+)-dependent taurocholate cotransporting polypeptide substrate specificity. Toxicol Sci. 2012;129:35–48. doi: 10.1093/toxsci/kfs188. [DOI] [PubMed] [Google Scholar]
- 56.Gripon P, Cannie I, Urban S. Efficient inhibition of hepatitis B virus infection by acylated peptides derived from the large viral surface protein. J Virol. 2005;79:1613–1622. doi: 10.1128/JVI.79.3.1613-1622.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Grüne S, Engelking LR, Anwer MS. Role of intracellular calcium and protein kinases in the activation of hepatic Na+/taurocholate cotransport by cyclic AMP. J Biol Chem. 1993;268:17734–17741. [PubMed] [Google Scholar]
- 58.Gu Z, Kaul M, Yan B, Kridel SJ, Cui J, Strongin A, Smith JW, Liddington RC, Lipton SA. S-nitrosylation of matrix metalloproteinases: signaling pathway to neuronal cell death. Science. 2002;297:1186–1190. doi: 10.1126/science.1073634. [DOI] [PubMed] [Google Scholar]
- 59.Hagenbuch B, Dawson P. The sodium bile salt cotransport family SLC10. Pflugers Arch. 2004;447:566–570. doi: 10.1007/s00424-003-1130-z. [DOI] [PubMed] [Google Scholar]
- 60.Hagenbuch B, Jacquemin E, Meier PJ. Na+-dependent and Na+-independent bile acid uptake systems in the liver. Cell Physiol Biochem. 1994;4:198–205. [Google Scholar]
- 61.Hagenbuch B, Lubbert H, Stieger B, Meier PJ. Expression of the hepatocyte Na+/bile acid cotransporter in Xenopus laevis oocytes. J Biol Chem. 1990;265:5357–5360. [PubMed] [Google Scholar]
- 62.Hagenbuch B, Scharschmidt BF, Meier PJ. Effect of antisense oligonucleotides on the expression of hepatocellular bile acid and organic anion uptake systems in Xenopus laevis oocytes. Biochem J. 1996;316(Pt 3):901–904. doi: 10.1042/bj3160901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hagenbuch B, Stieger B. The SLCO (former SLC21) superfamily of transporters. Mol Aspects Med. 2013;34:396–412. doi: 10.1016/j.mam.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hagenbuch B, Stieger B, Foguet M, Lubbert H, Meier PJ. Functional expression cloning and characterization of the hepatocyte Na+/bile acid cotransport system. Proc Natl Acad Sci (USA) 1991;88:10629–10633. doi: 10.1073/pnas.88.23.10629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Halilbasic E, Claudel T, Trauner M. Bile acid transporters and regulatory nuclear receptors in the liver and beyond. J Hepatol. 2013;58:155–168. doi: 10.1016/j.jhep.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hallen S, Fryklund J, Sachs G. Inhibition of the human sodium/bile acid cotransporters by side-specific methanethiosulfonate sulfhydryl reagents: substrate-controlled accessibility of site of inactivation. Biochemistry. 2000;39:6743–6750. doi: 10.1021/bi000577t. [DOI] [PubMed] [Google Scholar]
- 67.Hanada K, Nakai K, Tanaka H, Suzuki F, Kumada H, Ohno Y, Ozawa S, Ogata H. Effect of nuclear receptor downregulation on hepatic expression of cytochrome P450 and transporters in chronic hepatitis C in association with fibrosis development. Drug Metab Pharmacokinet. 2012;27:301–306. doi: 10.2133/dmpk.dmpk-11-rg-077. [DOI] [PubMed] [Google Scholar]
- 68.Hata S, Wang P, Eftychiou N, Ananthanarayanan M, Batta A, Salen G, Pang KS, Wolkoff AW. Substrate specificities of rat oatp1 and ntcp: implications for hepatic organic anion uptake. Am J Physiol Gastrointest Liver Physiol. 2003;285:G829–G839. doi: 10.1152/ajpgi.00352.2002. [DOI] [PubMed] [Google Scholar]
- 69.Hebert MF, Townsend RW, Austin S, Balan G, Blough DK, Buell D, Keirns J, Bekersky I. Concomitant cyclosporine and micafungin pharmacokinetics in healthy volunteers. J Clin Pharmacol. 2005;45:954–960. doi: 10.1177/0091270005278601. [DOI] [PubMed] [Google Scholar]
- 70.Hess DT, Stamler JS. Regulation by S-nitrosylation of Protein Posttranslational Modification. J Biol Chem. 2011 doi: 10.1074/jbc.R111.285742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Heubi JE, Setchell KD, Bove KE. Inborn errors of bile acid metabolism. Semin Liver Dis. 2007;27:282–294. doi: 10.1055/s-2007-985073. [DOI] [PubMed] [Google Scholar]
- 72.Ho RH, Leake BF, Roberts RL, Lee W, Kim RB. Ethnicity-dependent polymorphism in Na+-taurocholate cotransporting polypeptide (SLC10A1) reveals a domain critical for bile acid substrate recognition. J Biol Chem. 2004;279:7213–7222. doi: 10.1074/jbc.M305782200. [DOI] [PubMed] [Google Scholar]
- 73.Ho RH, Tirona RG, Leake BF, Glaeser H, Lee W, Lemke CJ, Wang Y, Kim RB. Drug and bile acid transporters in rosuvastatin hepatic uptake: function, expression, and pharmacogenetics. Gastroenterology. 2006;130:1793–1806. doi: 10.1053/j.gastro.2006.02.034. [DOI] [PubMed] [Google Scholar]
- 74.Hoekstra LT, de Graaf W, Nibourg GA, Heger M, Bennink RJ, Stieger B, van Gulik TM. Physiological and biochemical basis of clinical liver function tests: a review. Ann Surg. 2013;257:27–36. doi: 10.1097/SLA.0b013e31825d5d47. [DOI] [PubMed] [Google Scholar]
- 75.Hofmann AF. The enterohepatic circulation of bile acids in mammals: form and functions. Front Biosci. 2009;14:2584–2598. doi: 10.2741/3399. [DOI] [PubMed] [Google Scholar]
- 76.Hofmann AF, Hagey LR. Bile acids: chemistry, pathochemistry, biology, pathobiology, and therapeutics. Cell Mol Life Sci. 2008;65:2461–2483. doi: 10.1007/s00018-008-7568-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hofmann AF, Molino G, Milanese M, Belforte G. Description and simulation of a physiological pharmacokinetic model for the metabolism and enterohepatic circulation of bile acids in man. Cholic acid in healthy man. J Clin Invest. 1983;71:1003–1022. doi: 10.1172/JCI110828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hu NJ, Iwata S, Cameron AD, Drew D. Crystal structure of a bacterial homologue of the bile acid sodium symporter ASBT. Nature. 2011;478:408–411. doi: 10.1038/nature10450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ischiropoulos H. Protein tyrosine nitration--an update. Arch Biochem Biophys. 2009;484:117–121. doi: 10.1016/j.abb.2008.10.034. [DOI] [PubMed] [Google Scholar]
- 80.Jacquemin E. Progressive familial intrahepatic cholestasis. Clin Res Hepatol Gastroenterol. 2012;36(Suppl 1):S26–S35. doi: 10.1016/S2210-7401(12)70018-9. [DOI] [PubMed] [Google Scholar]
- 81.Jacquemin E, Hagenbuch B, Stieger B, Wolkoff AW, Meier PJ. Expression cloning of a rat liver Na(+)-independent organic anion transporter. Proc Natl Acad Sci U S A. 1994;91:133–137. doi: 10.1073/pnas.91.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jiang Y, Chen C, Li Z, Guo W, Gegner JA, Lin S, Han J. Characterization of the structure and function of a new mitogen-activated protein kinase (p38beta) J Biol Chem. 1996;271:17920–17926. doi: 10.1074/jbc.271.30.17920. [DOI] [PubMed] [Google Scholar]
- 83.Jones BA, Rao YP, Stravitz RT, Gores GJ. Bile salt-induced apoptosis of hepatocytes involves activation of protein kinase C. Am J Physiol. 1997;272:G1109–G1115. doi: 10.1152/ajpgi.1997.272.5.G1109. [DOI] [PubMed] [Google Scholar]
- 84.Keitel V, Burdelski M, Warskulat U, Kuhlkamp T, Keppler D, Haussinger D, Kubitz R. Expression and localization of hepatobiliary transport proteins in progressive familial intrahepatic cholestasis. Hepatology. 2005;41:1160–1172. doi: 10.1002/hep.20682. [DOI] [PubMed] [Google Scholar]
- 85.Kim RB, Leake B, Cvetkovic M, Roden MM, Nadeau J, Walubo A, Wilkinson GR. Modulation by drugs of human hepatic sodium-dependent bile acid transporter (sodium taurocholate cotransporting polypeptide) activity. J Pharmacol Exp Ther. 1999;291:1204–1209. [PubMed] [Google Scholar]
- 86.Klee CB, Ren H, Wang X. Regulation of the calmodulin-stimulated protein phosphatase, calcineurin. J Biol Chem. 1998;273:13367–13370. doi: 10.1074/jbc.273.22.13367. [DOI] [PubMed] [Google Scholar]
- 87.Kolhatkar V, Polli JE. Structural requirements of bile acid transporters: C-3 and C-7 modifications of steroidal hydroxyl groups. Eur J Pharm Sci. 2012;46:86–99. doi: 10.1016/j.ejps.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kosters A, Karpen SJ. Bile acid transporters in health and disease. Xenobiotica. 2008;38:1043–1071. doi: 10.1080/00498250802040584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kouzuki H, Suzuki H, Stieger B, Meier PJ, Sugiyama Y. Characterization of the transport properties of organic anion transporting polypeptide 1 (oatp1) and Na(+)/taurocholate cotransporting polypeptide (Ntcp): comparative studies on the inhibitory effect of their possible substrates in hepatocytes and cDNA-transfected COS-7 cells. J Pharmacol Exp Ther. 2000;292:505–511. [PubMed] [Google Scholar]
- 90.Kramer W. Transporters, Trojan horses and therapeutics: suitability of bile acid and peptide transporters for drug delivery. Biol Chem. 2011;392:77–94. doi: 10.1515/BC.2011.017. [DOI] [PubMed] [Google Scholar]
- 91.Kramer W, Stengelin S, Baringhaus KH, Enhsen A, Heuer H, Becker W, Corsiero D, Girbig F, Noll R, Weyland C. Substrate specificity of the ileal and the hepatic Na(+)/bile acid cotransporters of the rabbit. I. Transport studies with membrane vesicles and cell lines expressing the cloned transporters. J Lipid Res. 1999;40:1604–1617. [PubMed] [Google Scholar]
- 92.Kubitz R, Droge C, Stindt J, Weissenberger K, Haussinger D. The bile salt export pump (BSEP) in health and disease. Clin Res Hepatol Gastroenterol. 2012;36:536–553. doi: 10.1016/j.clinre.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 93.Kuhlkamp T, Keitel V, Helmer A, Haussinger D, Kubitz R. Degradation of the sodium taurocholate cotransporting polypeptide (NTCP) by the ubiquitin-proteasome system. Biol Chem. 2005;386:1065–1074. doi: 10.1515/BC.2005.122. [DOI] [PubMed] [Google Scholar]
- 94.Kullak-Ublick GA, Glasa J, Boker C, Oswald M, Grutzner U, Hagenbuch B, Stieger B, Meier PJ, Beuers U, Kramer W, Wess G, Paumgartner G. Chlorambucil-taurocholate is transported by bile acid carriers expressed in human hepatocellular carcinomas. Gastroenterology. 1997;113:1295–1305. doi: 10.1053/gast.1997.v113.pm9322525. [DOI] [PubMed] [Google Scholar]
- 95.Landmann L, Angermuller S, Rahner C, Stieger B. Expression, distribution, and activity of Na+,K+-ATPase in normal and cholestatic rat liver. J Histochem Cytochem. 1998;46:405–410. doi: 10.1177/002215549804600315. [DOI] [PubMed] [Google Scholar]
- 96.LaRusso NF, Hoffman NE, Hofmann AF, Korman MG. Validity and sensitivity of an intravenous bile acid tolerance test in patients with liver disease. N Engl J Med. 1975;292:1209–1214. doi: 10.1056/NEJM197506052922303. [DOI] [PubMed] [Google Scholar]
- 97.Leonhardt M, Keiser M, Oswald S, Kuhn J, Jia J, Grube M, Kroemer HK, Siegmund W, Weitschies W. Hepatic uptake of the magnetic resonance imaging contrast agent Gd-EOB-DTPA: role of human organic anion transporters. Drug Metab Dispos. 2010;38:1024–1028. doi: 10.1124/dmd.110.032862. [DOI] [PubMed] [Google Scholar]
- 98.Leslie EM, Watkins PB, Kim RB, Brouwer KL. Differential inhibition of rat and human Na+-dependent taurocholate cotransporting polypeptide (NTCP/SLC10A1)by bosentan: a mechanism for species differences in hepatotoxicity. J Pharmacol Exp Ther. 2007;321:1170–1178. doi: 10.1124/jpet.106.119073. [DOI] [PubMed] [Google Scholar]
- 99.Leto D, Saltiel AR. Regulation of glucose transport by insulin: traffic control of GLUT4. Nat Rev Mol Cell Biol. 2012;13:383–396. doi: 10.1038/nrm3351. [DOI] [PubMed] [Google Scholar]
- 100.Lewis MC, Brieaddy LE, Root C. Effects of 2164U90 on ileal bile acid absorption and serum cholesterol in rats and mice. J Lipid Res. 1995;36:1098–1105. [PubMed] [Google Scholar]
- 101.Lionarons DA, Boyer JL, Cai SY. Evolution of substrate specificity for the bile salt transporter ASBT (SLC10A2) J Lipid Res. 2012;53:1535–1542. doi: 10.1194/jlr.M025726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.McConkey M, Gillin H, Webster CR, Anwer MS. Cross-talk between protein kinases Czeta and B in cyclic AMP-mediated sodium taurocholate co-transporting polypeptide translocation in hepatocytes. J Biol Chem. 2004;279:20882–20888. doi: 10.1074/jbc.M309988200. [DOI] [PubMed] [Google Scholar]
- 103.McRae MP, Lowe CM, Tian X, Bourdet DL, Ho RH, Leake BF, Kim RB, Brouwer KL, Kashuba AD. Ritonavir, saquinavir, and efavirenz, but not nevirapine, inhibit bile acid transport in human and rat hepatocytes. J Pharmacol Exp Ther. 2006;318:1068–1075. doi: 10.1124/jpet.106.102657. [DOI] [PubMed] [Google Scholar]
- 104.Meier A, Mehrle S, Weiss TS, Mier W, Urban S. Myristoylated PreS1-domain of the hepatitis B virus L-protein mediates specific binding to differentiated hepatocytes. Hepatology. 2013;58:31–42. doi: 10.1002/hep.26181. [DOI] [PubMed] [Google Scholar]
- 105.Meier PJ, Stieger B. Bile salt transporters. Annu Rev Physiol. 2002;64:635–661. doi: 10.1146/annurev.physiol.64.082201.100300. [DOI] [PubMed] [Google Scholar]
- 106.Meier Y, Pauli-Magnus C, Zanger UM, Klein K, Schaeffeler E, Nussler AK, Nussler N, Eichelbaum M, Meier PJ, Stieger B. Interindividual variability of canalicular ATP-binding-cassette (ABC)-transporter expression in human liver. Hepatology. 2006;44:62–74. doi: 10.1002/hep.21214. [DOI] [PubMed] [Google Scholar]
- 107.Miccio M, Orzes N, Lunazzi GC, Gazzin B, Corsi R, Tiribelli C. Reversal of ethinylestradiol-induced cholestasis by epomediol in rat. The role of liver plasma-membrane fluidity. Biochem Pharmacol. 1989;38:3559–3563. doi: 10.1016/0006-2952(89)90128-7. [DOI] [PubMed] [Google Scholar]
- 108.Milkiewicz P, Saksena S, Cardenas T, Mills CO, Elias E. Plasma elimination of cholyl-lysyl-fluorescein (CLF): a pilot study in patients with liver cirrhosis. Liver. 2000;20:330–334. doi: 10.1034/j.1600-0676.2000.020004330.x. [DOI] [PubMed] [Google Scholar]
- 109.Mita S, Suzuki H, Akita H, Hayashi H, Onuki R, Hofmann AF, Sugiyama Y. Inhibition of bile acid transport across Na+/taurocholate cotransporting polypeptide (SLC10A1) and bile salt export pump (ABCB 11)-coexpressing LLC-PK1 cells by cholestasis-inducing drugs. Drug Metab Dispos. 2006;34:1575–1581. doi: 10.1124/dmd.105.008748. [DOI] [PubMed] [Google Scholar]
- 110.Muhlfeld S, Domanova O, Berlage T, Stross C, Helmer A, Keitel V, Haussinger D, Kubitz R. Short-term feedback regulation of bile salt uptake by bile salts in rodent liver. Hepatology. 2012;56:2387–2397. doi: 10.1002/hep.25955. [DOI] [PubMed] [Google Scholar]
- 111.Mukhopadhayay S, Ananthanarayanan M, Stieger B, Meier PJ, Suchy FJ, Anwer MS. cAMP increases liver Na+-taurocholate cotransport by translocating transporter to plasma membranes. Am J Physiol. 1997;273:G842–G848. doi: 10.1152/ajpgi.1997.273.4.G842. [DOI] [PubMed] [Google Scholar]
- 112.Mukhopadhayay S, Ananthanarayanan M, Stieger B, Meier PJ, Suchy FJ, Anwer MS. Sodium taurocholate cotransporting polypeptide is a serine, threonine phosphoprotein and is dephosphorylated by cyclic AMP. Hepatology. 1998;28:1629–1636. doi: 10.1002/hep.510280624. [DOI] [PubMed] [Google Scholar]
- 113.Mukhopadhayay S, Webster CRL, Anwer MS. Role of protein phosphatase in cyclic AMP-mediated stimulation of hepatic Na+/taurocholate cotransport. J Biol Chem. 1998;273:30039–30045. doi: 10.1074/jbc.273.45.30039. [DOI] [PubMed] [Google Scholar]
- 114.Murray JW, Thosani AJ, Wang P, Wolkoff AW. Heterogeneous accumulation of fluorescent bile acids in primary rat hepatocytes does not correlate with their homogenous expression of ntcp. Am J Physiol Gastrointest Liver Physiol. 2011;301:G60–G68. doi: 10.1152/ajpgi.00035.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nakai K, Tanaka H, Hanada K, Ogata H, Suzuki F, Kumada H, Miyajima A, Ishida S, Sunouchi M, Habano W, Kamikawa Y, Kubota K, Kita J, Ozawa S, Ohno Y. Decreased expression of cytochromes P450 1A2, 2E1, and 3A4 and drug transporters Na+-taurocholate-cotransporting polypeptide, organic cation transporter 1, and organic anion-transporting peptide-C correlates with the progression of liver fibrosis in chronic hepatitis C patients. Drug Metab Dispos. 2008;36:1786–1793. doi: 10.1124/dmd.107.020073. [DOI] [PubMed] [Google Scholar]
- 116.Newton AC. Regulation of the ABC kinases by phosphorylation: protein kinase C as a paradigm. Biochem J. 2003;370:361–371. doi: 10.1042/BJ20021626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Niemi M, Pasanen MK, Neuvonen PJ. Organic anion transporting polypeptide 1B1: a genetically polymorphic transporter of major importance for hepatic drug uptake. Pharmacol Rev. 2011;63:157–181. doi: 10.1124/pr.110.002857. [DOI] [PubMed] [Google Scholar]
- 118.Park SW, Schonhoff CM, Webster CR, Anwer MS. Protein kinase Cdelta differentially regulates cAMP-dependent translocation of NTCP and MRP2 to the plasma membrane. Am J Physiol Gastrointest Liver Physiol. 2012;303:G657–G665. doi: 10.1152/ajpgi.00529.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Paumgartner G, Vasella DL, Herz R, Reichen J, Preisig R. [Hepatic extraction of taurocholate and indocyanine green in patients with liver disease (author's transl)] Z Gastroenterol. 1979;17:753–761. [PubMed] [Google Scholar]
- 120.Pawloski JR, Hess DT, Stamler JS. Impaired vasodilation by red blood cells in sickle cell disease. Proc Natl Acad Sci U S A. 2005;102:2531–2536. doi: 10.1073/pnas.0409876102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Petersen J, Dandri M, Mier W, Lutgehetmann M, Volz T, von WF, Haberkorn U, Fischer L, Pollok JM, Erbes B, Seitz S, Urban S. Prevention of hepatitis B virus infection in vivo by entry inhibitors derived from the large envelope protein. Nat Biotechnol. 2008;26:335–341. doi: 10.1038/nbt1389. [DOI] [PubMed] [Google Scholar]
- 122.Platte HD, Honscha W, Schuh K, Petzinger E. Functional characterization of the hepatic sodium-dependent taurocholate transporter stably transfected into an immortalized liver-derived cell line and V79 fibroblasts. Eur J Cell Biol. 1996;70:54–60. [PubMed] [Google Scholar]
- 123.Pols TW, Noriega LG, Nomura M, Auwerx J, Schoonjans K. The bile acid membrane receptor TGR5 as an emerging target in metabolism and inflammation. J Hepatol. 2011;54:1263–1272. doi: 10.1016/j.jhep.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Potter GD. Bile acid diarrhea. Dig Dis. 1998;16:118–124. doi: 10.1159/000016855. [DOI] [PubMed] [Google Scholar]
- 125.Prekeris R, Klumperman J, Scheller RH. A Rab11/Rip11 protein complex regulates apical membrane trafficking via recycling endosomes. Mol Cell. 2000;6:1437–1448. doi: 10.1016/s1097-2765(00)00140-4. [DOI] [PubMed] [Google Scholar]
- 126.Que LG, Liu L, Yan Y, Whitehead GS, Gavett SH, Schwartz DA, Stamler JS. Protection from experimental asthma by an endogenous bronchodilator. Science. 2005;308:1618–1621. doi: 10.1126/science.1108228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Reyland ME. Protein kinase C isoforms: Multi-functional regulators of cell life and death. Front Biosci. 2009;14:2386–2399. doi: 10.2741/3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Reymann A, Braun W, Drobik C, Woermann C. Stimulation of bile acid active transport related to increased mucosal cyclic AMP content in rat ileum in vitro. Biochim Biophys Acta. 1989;1011:158–164. doi: 10.1016/0167-4889(89)90203-6. [DOI] [PubMed] [Google Scholar]
- 129.Russell DW. Fifty years of advances in bile acid synthesis and metabolism. J Lipid Res. 2009;50(Suppl):S120–S125. doi: 10.1194/jlr.R800026-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Rust C, Bauchmuller K, Fickert P, Fuchsbichler A, Beuers U. Phosphatidylinositol 3-kinase-dependent signaling modulates taurochenodeoxycholic acid-induced liver injury and cholestasis in perfused rat livers. Am J Physiol Gastrointest Liver Physiol. 2005;289:G88–G94. doi: 10.1152/ajpgi.00450.2004. [DOI] [PubMed] [Google Scholar]
- 131.Sakka SG. Assessing liver function. Curr Opin Crit Care. 2007;13:207–214. doi: 10.1097/MCC.0b013e328012b268. [DOI] [PubMed] [Google Scholar]
- 132.Sarkar S, Bananis E, Nath S, Anwer MS, Wolkoff AW, Murray JW. PKCzeta is required for microtubule-based motility of vesicles containing the ntcp transporter. Traffic. 2006;7:1078–1091. doi: 10.1111/j.1600-0854.2006.00447.x. [DOI] [PubMed] [Google Scholar]
- 133.Sarwar Z, Annaba F, Dwivedi A, Saksena S, Gill RK, Alrefai WA. Modulation of ileal apical Na+-dependent bile acid transporter ASBT by protein kinase C. Am J Physiol Gastrointest Liver Physiol. 2009;297:G532–G538. doi: 10.1152/ajpgi.00052.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Schieck A, Schulze A, Gahler C, Muller T, Haberkorn U, Alexandrov A, Urban S, Mier W. Hepatitis B virus hepatotropism is mediated by specific receptor recognition in the liver and not restricted to susceptible hosts. Hepatology. 2013;58:43–53. doi: 10.1002/hep.26211. [DOI] [PubMed] [Google Scholar]
- 135.Schonhoff CM, Gaston B, Mannick JB. Nitrosylation of cytochrome c during apoptosis. J Biol Chem. 2003;278:18265–18270. doi: 10.1074/jbc.M212459200. [DOI] [PubMed] [Google Scholar]
- 136.Schonhoff CM, Gillin H, Webster CR, Anwer MS. Protein kinase Cdelta mediates cyclic adenosine monophosphate-stimulated translocation of sodium taurocholate cotransporting polypeptide and multidrug resistant associated protein 2 in rat hepatocytes. Hepatology. 2008;47:1309–1316. doi: 10.1002/hep.22162. [DOI] [PubMed] [Google Scholar]
- 137.Schonhoff CM, Matsuoka M, Tummala H, Johnson MA, Estevez AG, Wu R, Kamaid A, Ricart KC, Hashimoto Y, Gaston B, Macdonald TL, Xu Z, Mannick JB. S-nitrosothiol depletion in amyotrophic lateral sclerosis. Proc Natl Acad Sci U S A. 2006;103:2404–2409. doi: 10.1073/pnas.0507243103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Schonhoff CM, Ramasamy U, Anwer MS. Nitric oxide-mediated inhibition of taurocholate uptake involves S-nitrosylation of NTCP. Am J Physiol Gastrointest Liver Physiol. 2011;300:G364–G370. doi: 10.1152/ajpgi.00170.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Schonhoff CM, Thankey K, Webster CR, Wakabayashi Y, Wolkoff AW, Anwer MS. Rab4 facilitates cyclic adenosine monophosphate-stimulated bile acid uptake and Na(+)-taurocholate cotransporting polypeptide translocation. Hepatology. 2008;48:1665–1670. doi: 10.1002/hep.22495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Schonhoff CM, Webster CR, Anwer MS. Taurolithocholate-induced MRP2 retrieval involves MARCKS phosphorylation by protein kinase C in HUH-NTCP Cells. Hepatology. 2013;58:284–292. doi: 10.1002/hep.26333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Schonhoff CM, Yamazaki A, Hohenester S, Webster CR, Bouscarel B, Anwer MS. PKC{epsilon}-dependent and -independent effects of taurolithocholate on PI3K/PKB pathway and taurocholate uptake in HuH-NTCP cell line. Am J Physiol Gastrointest Liver Physiol. 2009;297:G1259–G1267. doi: 10.1152/ajpgi.00177.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Schwenk M, Schwarz LR, Greim H. Taurolithocholate inhibits taurocholate uptake by isolated hepatocytes at low concentrations. Naunyn Schmiedebergs Arch Pharmacol. 1977;298:175–179. doi: 10.1007/BF00508626. [DOI] [PubMed] [Google Scholar]
- 143.Setchell KD, Heubi JE, Shah S, Lavine JE, Suskind D, Al-Edreesi M, Potter C, Russell DW, O'Connell NC, Wolfe B, Jha P, Zhang W, Bove KE, Knisely AS, Hofmann AF, Rosenthal P, Bull LN. Genetic defects in bile acid conjugation cause fat-soluble vitamin deficiency. Gastroenterology. 2013;144:945–955. doi: 10.1053/j.gastro.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Simren M, Bajor A, Gillberg PG, Rudling M, Abrahamsson H. Randomised clinical trial: The ileal bile acid transporter inhibitor A3309 vs. placebo in patients with chronic idiopathic constipation--a double-blind study. Aliment Pharmacol Ther. 2011;34:41–50. doi: 10.1111/j.1365-2036.2011.04675.x. [DOI] [PubMed] [Google Scholar]
- 145.Song IS, Lee IK, Chung SJ, Kim SG, Lee MG, Shim CK. Effect of nitric oxide on the sinusoidal uptake of organic cations and anions by isolated hepatocytes. Arch Pharm Res. 2002;25:984–988. doi: 10.1007/BF02977024. [DOI] [PubMed] [Google Scholar]
- 146.Stieger B. The role of the sodium-taurocholate cotransporting polypeptide (NTCP) and of the bile salt export pump (BSEP) in physiology and pathophysiology of bile formation. Handb Exp Pharmacol. 2011;201:205–259. doi: 10.1007/978-3-642-14541-4_5. [DOI] [PubMed] [Google Scholar]
- 147.Stieger B, Heger M, de Graaf W, Paumgartner G, van GT. The emerging role of transport systems in liver function tests. Eur J Pharmacol. 2012;675:1–5. doi: 10.1016/j.ejphar.2011.11.048. [DOI] [PubMed] [Google Scholar]
- 148.Stieger B, Meier PJ. Pharmacogenetics of drug transporters in the enterohepatic circulation. Pharmacogenomics. 2011;12:611–631. doi: 10.2217/pgs.11.53. [DOI] [PubMed] [Google Scholar]
- 149.Stravitz RT, Rao YP, Vlahcevic ZR, Gurley EC, Jarvis WD, Hylemon PB. Hepatocellular protein kinase C activation by bile acids: implications for regulation of cholesterol 7 alpha-hydroxylase. Am J Physiol. 1996;271:G293–G303. doi: 10.1152/ajpgi.1996.271.2.G293. [DOI] [PubMed] [Google Scholar]
- 150.Stross C, Helmer A, Weissenberger K, Gorg B, Keitel V, Haussinger D, Kubitz R. Protein kinase C induces endocytosis of the sodium taurocholate cotransporting polypeptide. Am J Physiol Gastrointest Liver Physiol. 2010;299:G320–G328. doi: 10.1152/ajpgi.00180.2010. [DOI] [PubMed] [Google Scholar]
- 151.Sun AQ, Arrese MA, Zeng L, Swaby I, Zhou MM, Suchy FJ. The rat liver Na(+)/bile acid cotransporter. Importance of the cytoplasmic tail to function and plasma membrane targeting. J Biol Chem. 2001;276:6825–6833. doi: 10.1074/jbc.M008797200. [DOI] [PubMed] [Google Scholar]
- 152.Sun AQ, Salkar R, Sachchidanand, Xu S, Zeng L, Zhou MM, Suchy FJ. A 14-amino acid sequence with a beta-turn structure is required for apical membrane sorting of the rat ileal bile acid transporter. J Biol Chem. 2003;278:4000–4009. doi: 10.1074/jbc.M207163200. [DOI] [PubMed] [Google Scholar]
- 153.Takeyama Y, Kanegae K, Inomata S, Takata K, Tanaka T, Ueda S, Yokoyama K, Morihara D, Nishizawa S, Anan A, Irie M, Iwata K, Shakado S, Sohda T, Sakisaka S. Sustained upregulation of sodium taurocholate cotransporting polypeptide and bile salt export pump and downregulation of cholesterol 7alpha-hydroxylase in the liver of patients with end-stage primary biliary cirrhosis. Med Mol Morphol. 2010;43:134–138. doi: 10.1007/s00795-009-0480-9. [DOI] [PubMed] [Google Scholar]
- 154.Toker A. Protein kinases as mediators of phosphoinositide 3-kinase signaling. Mol Pharmacol. 2000;57:652–658. [PubMed] [Google Scholar]
- 155.Umadevi R, Anwer MS, Schonhoff CM. Cysteine 96 of Ntcp is Responsible for NO-Mediated Inhibition of Taurocholate Uptake. Am J Physiol Gastrointest Liver Physiol. 2013 doi: 10.1152/ajpgi.00089.2013. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Utili R, Abernathy CO, Zimmerman HJ. Inhibition of Na+, K+-adenosinetriphosphatase by endotoxin: a possible mechanism for endotoxin-induced cholestasis. J Infect Dis. 1977;136:583–587. doi: 10.1093/infdis/136.4.583. [DOI] [PubMed] [Google Scholar]
- 157.van de Steeg E, Wagenaar E, van der Kruijssen CM, Burggraaff JE, de Waart DR, Elferink RP, Kenworthy KE, Schinkel AH. Organic anion transporting polypeptide 1a/1b-knockout mice provide insights into hepatic handling of bilirubin, bile acids, and drugs. J Clin Invest. 2010;120:2942–2952. doi: 10.1172/JCI42168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Warner N, Locarnini S. The new front-line in hepatitis B/D research: Identification and blocking of a functional receptor. Hepatology. 2013;58:9–12. doi: 10.1002/hep.26292. [DOI] [PubMed] [Google Scholar]
- 159.Webster CR, Srinivasulu U, Ananthanarayanan M, Suchy FJ, Anwer MS. Protein kinase B/Akt mediates cAMP- and cell swelling-stimulated Na+/taurocholate cotransport and Ntcp translocation. J Biol Chem. 2002;277:28578–28583. doi: 10.1074/jbc.M201937200. [DOI] [PubMed] [Google Scholar]
- 160.Webster CR, Usechak P, Anwer MS. cAMP inhibits bile acid-induced apoptosis by blocking caspase activation and cytochrome c release. Am J Physiol Gastrointest Liver Physiol. 2002;283:G727–G738. doi: 10.1152/ajpgi.00410.2001. [DOI] [PubMed] [Google Scholar]
- 161.Webster CRL, Blanch C, Anwer MS. Role of PP2B in cAMP-induced dephosphorylation and translocation of NTCP. Am J Physiol Gastrointest Liver Physiol. 2002;283:G44–G50. doi: 10.1152/ajpgi.00530.2001. [DOI] [PubMed] [Google Scholar]
- 162.Webster CRL, Anwer MS. Role of the PI3K/PKB signaling pathway in cAMP-mediated translocation of rat liver Ntcp. Am J Physiol. 1999;277:G1165–G1172. doi: 10.1152/ajpgi.1999.277.6.G1165. [DOI] [PubMed] [Google Scholar]
- 163.Webster CRL, Blanch CJ, Philips J, Anwer MS. Cell swelling-induced translocation of rat liver Na+/taurocholate cotransport polypeptide is mediated via the phosphoinositide 3-kinase signaling pathway. J Biol Chem. 2000;275:29754–29760. doi: 10.1074/jbc.M002831200. [DOI] [PubMed] [Google Scholar]
- 164.Weinman SA. Electrogenicity of Na(+)-coupled bile acid transporters. Yale J Biol Med. 1997;70:331–340. [PMC free article] [PubMed] [Google Scholar]
- 165.Xia X, Francis H, Glaser S, Alpini G, Lesage G. Bile acid interactions with cholangiocytes. World J Gastroenterol. 2006;12:3553–3563. doi: 10.3748/wjg.v12.i22.3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Xia X, Roundtree M, Merikhi A, Lu X, Shentu S, Lesage G. Degradation of the apical sodium-dependent bile acid transporter by the ubiquitin-proteasome pathway in cholangiocytes. J Biol Chem. 2004;279:44931–44937. doi: 10.1074/jbc.M400969200. [DOI] [PubMed] [Google Scholar]
- 167.Xiao F, McKeating JA, Baumert TF. A bile acid transporter as a candidate receptor for hepatitis B and D virus entry. J Hepatol. 2013;58:1246–1248. doi: 10.1016/j.jhep.2013.01.036. [DOI] [PubMed] [Google Scholar]
- 168.Yan H, Peng B, He W, Zhong G, Qi Y, Ren B, Gao Z, Jing Z, Song M, Xu G, Sui J, Li W. Molecular determinants of hepatitis B and d virus entry restriction in mouse sodium taurocholate cotransporting polypeptide. J Virol. 2013;87:7977–7991. doi: 10.1128/JVI.03540-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Yan H, Zhong G, Xu G, He W, Jing Z, Gao Z, Huang Y, Qi Y, Peng B, Wang H, Fu L, Song M, Chen P, Gao W, Ren B, Sun Y, Cai T, Feng X, Sui J, Li W. Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. Elife. 2012;1:1–28. doi: 10.7554/eLife.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Yanni SB, Augustijns PF, Benjamin DK, Jr, Brouwer KL, Thakker DR, Annaert PP. In vitro investigation of the hepatobiliary disposition mechanisms of the antifungal agent micafungin in humans and rats. Drug Metab Dispos. 2010;38:1848–1856. doi: 10.1124/dmd.110.033811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Zahner D, Eckhardt U, Petzinger E. Transport of taurocholate by mutants of negatively charged amino acids, cysteines, and threonines of the rat liver sodium-dependent taurocholate cotransporting polypeptide Ntcp. Eur J Biochem. 2003;270:1117–1127. doi: 10.1046/j.1432-1033.2003.03463.x. [DOI] [PubMed] [Google Scholar]
- 172.Zheng X, Ekins S, Raufman JP, Polli JE. Computational models for drug inhibition of the human apical sodium-dependent bile acid transporter. Mol Pharm. 2009;6:1591–1603. doi: 10.1021/mp900163d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Zollner G, Fickert P, Silbert D, Fuchsbichler A, Marschall HU, Zatloukal K, Denk H, Trauner M. Adaptive changes in hepatobiliary transporter expression in primary biliary cirrhosis. J Hepatol. 2003;38:717–727. doi: 10.1016/s0168-8278(03)00096-5. [DOI] [PubMed] [Google Scholar]
- 174.Zollner G, Fickert P, Zenz R, Fuchsbichler A, Stumptner C, Kenner L, Ferenci P, Stauber RE, Krejs GJ, Denk H, Zatloukal K, Trauner M. Hepatobiliary transporter expression in percutaneous liver biopsies of patients with cholestatic liver diseases. Hepatology. 2001;33:633–646. doi: 10.1053/jhep.2001.22646. [DOI] [PubMed] [Google Scholar]


