This work identifies a Sclerotinia sclerotiorum elicitor that is sensed by RECEPTOR-LIKE PROTEIN30 and evokes MAMP-triggered immunity via the BRASSINOSTEROID INSENSITIVE1-ASSOCIATED RECEPTOR KINASE1 and SUPPRESSOR OF BIR1-1/EVERSHED signaling pathway. Thus, this work demonstrates the relevance of pattern recognition receptor–triggered immunity in resistance to necrotrophic fungi.
Abstract
Effective plant defense strategies rely in part on the perception of non-self determinants, so-called microbe-associated molecular patterns (MAMPs), by transmembrane pattern recognition receptors leading to MAMP-triggered immunity. Plant resistance against necrotrophic pathogens with a broad host range is complex and yet not well understood. Particularly, it is unclear if resistance to necrotrophs involves pattern recognition receptors. Here, we partially purified a novel proteinaceous elicitor called SCLEROTINIA CULTURE FILTRATE ELICITOR1 (SCFE1) from the necrotrophic fungal pathogen Sclerotinia sclerotiorum that induces typical MAMP-triggered immune responses in Arabidopsis thaliana. Analysis of natural genetic variation revealed five Arabidopsis accessions (Mt-0, Lov-1, Lov-5, Br-0, and Sq-1) that are fully insensitive to the SCFE1-containing fraction. We used a forward genetics approach and mapped the locus determining SCFE1 sensitivity to RECEPTOR-LIKE PROTEIN30 (RLP30). We also show that SCFE1-triggered immune responses engage a signaling pathway dependent on the regulatory receptor-like kinases BRASSINOSTEROID INSENSITIVE1-ASSOCIATED RECEPTOR KINASE1 (BAK1) and SUPPRESSOR OF BIR1-1/EVERSHED (SOBIR1/EVR). Mutants of RLP30, BAK1, and SOBIR1 are more susceptible to S. sclerotiorum and the related fungus Botrytis cinerea. The presence of an elicitor in S. sclerotiorum evoking MAMP-triggered immune responses and sensed by RLP30/SOBIR1/BAK1 demonstrates the relevance of MAMP-triggered immunity in resistance to necrotrophic fungi.
INTRODUCTION
Plants face constant threats from pathogenic microorganisms, but they defeat the majority of these threats upon detection of microbe-associated molecular patterns (MAMPs). Plants detect MAMPs through pattern recognition receptors (PRRs), which provide an important basis for broad-spectrum resistance against both nonadapted and adapted pathogens (Akira et al., 2006). Arabidopsis thaliana, like all higher plants, has evolved a large number of cell surface receptors that play major roles in inherent and adaptive processes by regulating growth, development, organogenesis, and responses to external stimuli (Kaul et al., 2000). The largest family (>600 genes) of surface receptors in Arabidopsis is formed by receptor-like kinase proteins (RLKs), transmembrane proteins with a cytoplasmic kinase domain participating in intracellular signal transduction and an extracellular domain potentially responsible for ligand perception. A few RLKs have been demonstrated to sense MAMPs and to participate in plant defense (Boller and Felix, 2009). The best studied examples are FLAGELLIN SENSING2 (FLS2) and EF-TU RECEPTOR (EFR), two receptors with an extracellular domain harboring tandem leucine-rich repeats (eLRRs) that bind an ∼22–amino acid fragment of bacterial flagellin, flg22, and an ∼18–amino acid fragment of bacterial elongation factor thermo-unstable, elf18, respectively (Gomez-Gomez and Boller, 2000; Zipfel et al., 2006). In addition to RLKs, receptor-like proteins (RLPs) function as crucial players in plant immunity (Yang et al., 2012). RLPs and RLKs have similar structures, except that the RLPs lack the cytoplasmic kinase domain (Wang et al., 2008). RLP-type PRRs include the tomato (Solanum lycopersicum) Eix1 and Eix2, which mediate perception of the cell wall–derived ethylene-inducing xylanase (Eix) from Trichoderma species (Ron and Avni, 2004), and Ve1, which is involved in the recognition of the protein Ave1 from various fungal species (de Jonge et al., 2012). RLPs carrying lysin motif ectodomains function in the perception of N-acetylglucosamine–containing glycan structures, such as peptidoglycan or chitin, two major structural components of bacterial or fungal cell walls, respectively (reviewed in Gust et al., 2012).
In Arabidopsis, a reverse genetic study of the role of the whole repertoire of eLRR-RLPs in development and stress responses identified only a few RLPs with an apparent biological function (Wang et al., 2008). Loss-of-function experiments have shown that RLP30, RLP51, and RLP52 are involved in basal resistance against a bacterium (Pseudomonas syringae pv phaseolicola), an oomycete (Hyaloperonospora arabidopsidis), and a fungus (Erysiphe cichoracearum), respectively (Ramonell et al., 2005; Wang et al., 2008; Zhang et al., 2010). Additionally, eLRR-RLPs function in development. For example, TOO MANY MOUTHS (RLP17) regulates stomata distribution and initiation of stomatal precursor cells (Yang and Sack, 1995; Nadeau and Sack, 2002), and CLAVATA2 (RLP10) is required for proper meristem and organ development (Kayes and Clark, 1998; Jeong et al., 1999).
The absence of the intracellular signaling domain suggests that, to activate immune and developmental responses, RLPs interact with additional components, most likely RLKs. For example, BRI1-ASSOCIATED RECEPTOR KINASE1 (BAK1), an eLRR-RLK structurally similar to FLS2 and EFR, is involved in Ve1-mediated resistance in tomato to Verticillium dahlia and V. albo-atrum (Fradin et al., 2009). BAK1 was identified as an interactor of BRASSINOSTEROID INSENSITIVE1, another eLRR-RLK that recognizes the plant hormone brassinolide and controls plant growth and development (Li et al., 2002; Nam and Li, 2002). In addition, BAK1 forms a complex with FLS2 and EFR in a ligand-dependent manner (Chinchilla et al., 2007; Heese et al., 2007; Roux et al., 2011), underlining its capacity to interact with different types of eLRR-containing receptors and its role as key regulator of immune and developmental responses. The eLRR-RLK SUPPRESSOR OF BIR1-1/EVERSHED (SOBIR1/EVR) associates with RLPs, such as Ve1 and Cf-4, and plays an essential role in RLP-mediated resistance to fungal pathogens in tomato (Liebrand et al., 2013). SOBIR1 functions as an activator of a cell death pathway that is suppressed by the eLRR-RLK BAK1-INTERACTING RECEPTOR-LIKE KINASE1 (BIR1), a negative regulator of multiple plant resistance signaling pathways (Gao et al., 2009). SOBIR1 also inhibits abscission and was called EVR (Leslie et al., 2010).
Arabidopsis provides an experimental system for examining the fundamental aspects of microbe perception, ligand–receptor interaction, and intracellular reprogramming. The genomes of hundreds of different Arabidopsis accessions have been sequenced (http://1001genomes.org/). Polymorphisms reflect the natural genetic variation among these accessions, driven in part by adaptation to environmental factors, including biotic stresses. Hence, Arabidopsis accessions offer an important reservoir of receptors with specific and diverse recognition specificities awaiting their functional characterization. Only the chitin receptor CHITIN ELICITOR RECEPTOR KINASE1 (CERK1) (Miya et al., 2007; Wan et al., 2008) has been identified as a surface PRR involved in the recognition of fungal-derived MAMPs or effectors in Arabidopsis. The white mold fungus Sclerotinia sclerotiorum is one of the most devastating necrotrophic pathogens of many economically important plants, including soybean (Glycine max), sunflower (Helianthus annuus), and agricultural Brassicaceae (Bolton et al., 2006). S. sclerotiorum secretes oxalic acid and produces an arsenal of hydrolytic enzymes and toxins that enable this fungus to infect a broad host range (>400 species) of mainly dicotyledonous plant species (Boland and Hall, 1994). Necrotrophs typically trigger rapid host cell death; thus, these pathogens are regarded as using unsophisticated colonization strategies. However, recent results clearly indicate that plant interactions with necrotrophic pathogens are more complex than initially hypothesized (Laluk and Mengiste, 2010). The development of a pathosystem between Arabidopsis and S. sclerotiorum (Dickman and Mitra, 1992) or the taxonomically closely related fungus Botrytis cinerea (Koch and Slusarenko, 1990) helped the investigation of plant resistance against necrotrophic fungi (reviewed in Laluk and Mengiste, 2010), but the exact molecular mechanisms underlying this type of interaction remain to be elucidated.
In this study, we describe a biochemical and genetic approach that allowed the rapid identification of a novel elicitor-containing preparation from S. sclerotiorum and of its corresponding potential Arabidopsis PRR. We used the culture medium of axenically grown S. sclerotiorum and a conventional chromatography approach to purify partially a proteinaceous elicitor activity that we named SCLEROTINIA CULTURE FILTRATE ELICITOR1 (SCFE1). The SCFE1-containing fraction activates typical MAMP-induced immune responses in Arabidopsis. Next, we took advantage of the natural variation in SCFE1 responsiveness among different Arabidopsis accessions to identify the eLRR-RLP RLP30 as being required for SCFE1 perception/sensitivity. We also provide genetic evidence that SCFE1-triggered immune responses require the eLRR-RLKs BAK1 and SOBIR1. Most importantly, rlp30, bak1, and sobir1 mutants are hypersusceptible to fungal infections with S. sclerotiorum and B. cinerea. The isolation of the SCFE1-containing fraction and RLP30 provides the basis for the genetic and molecular dissection of BAK1- and SOBIR1-dependent RLP-mediated signal perception and transduction in Arabidopsis immunity. RLP30, as a central component of a pattern recognition system, confers partial resistance to necrotrophic fungi in Arabidopsis and offers the possibility to engineer disease resistance in crop plants through molecular breeding.
RESULTS
The SCFE1-Containing Fraction Induces Typical MAMP-Dependent Immune Responses
To identify Arabidopsis immune receptors involved in the perception of S. sclerotiorum, we first had to isolate fungal-derived elicitor activities evoking MAMP-induced defense responses. We used induction of the plant stress hormone ethylene as a bioassay to monitor elicitor activities throughout the purification. The ethylene assay has been successfully used as a robust, rapid, and highly sensitive method for the identification of MAMPs like flg22, elf18, and Eix (Avni et al., 1994; Felix et al., 1999; Kunze et al., 2004). In a multistep approach combining two separations on cation-exchange chromatography columns, we partially purified a single elicitor-containing fraction from the culture medium of axenically grown S. sclerotiorum; we called this the SCFE1-containing fraction (Figure 1A; see Supplemental Figures 1A and 1B online). Further characterization showed that the elicitor activity is selectively sensitive to protease treatment, but resistant to deglycosylation, heat, and SDS treatment (see Supplemental Figures 1C and 1D online), suggesting that the elicitor activity is most likely associated with a linear peptide motif acting as an immunogenic epitope and that protein folding or enzymatic activity is not important. Dose–response analysis of the SCFE1-containing fraction revealed a concentration of ∼0.15 µg/mL to be sufficient to trigger half-maximal ethylene production (see Supplemental Figure 1E online).
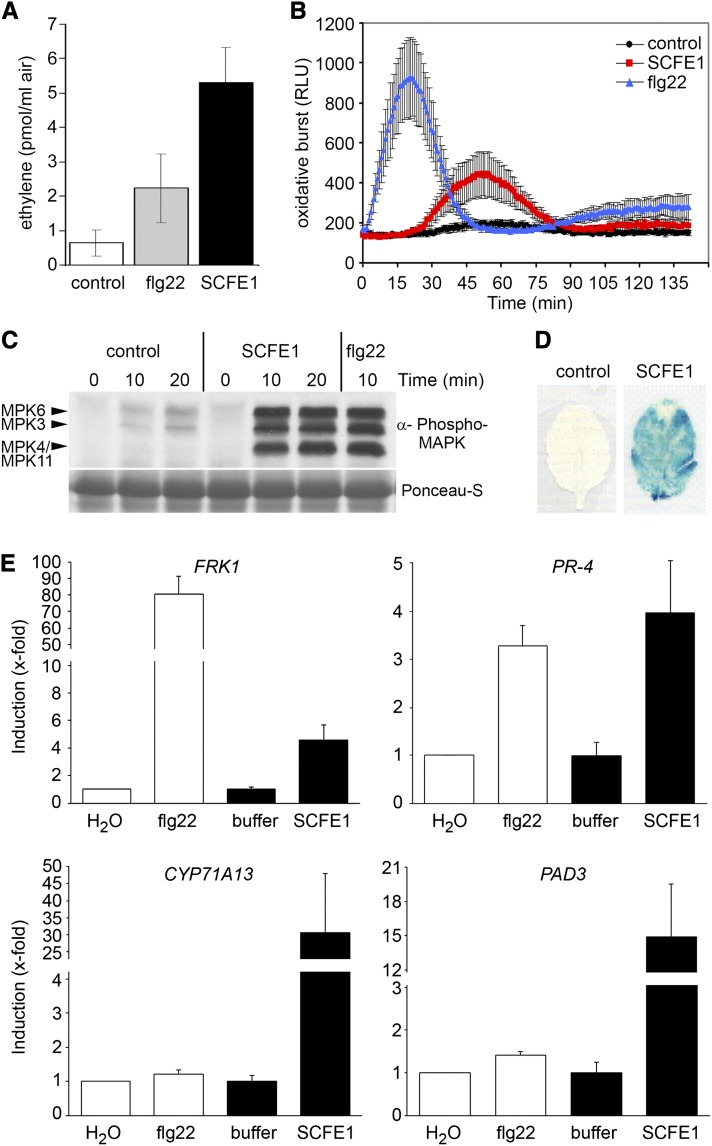
Figure 1.
The SCFE1-Containing Fraction Generates Typical Immune Responses in Arabidopsis.
(A) Arabidopsis Col-0 leaf pieces were treated with 0.5 µg/mL of the SCFE1-containing fraction or 100 nM flg22, and ethylene emission was measured by gas chromatography after 3 h. Bars represent average values ± sd (n = 2). The experiment was repeated at least three times with similar results.
(B) Oxidative burst triggered by 0.12 µg/mL of the SCFE1-containing fraction (red) or 100 nM flg22 (blue) in Arabidopsis Col-0 leaf discs, measured in relative light units (RLU). Results are means ± sd (n = 12).
(C) Immunoblotting of activated MAPK with anti-phospho p44/p42 antibody in Arabidopsis Col-0 leaf extracts. Leaf samples were collected 0, 10, or 20 min after infiltration with buffer (control), 0.5 µg/mL of the SCFE1-containing fraction, or 100 nM flg22. Ponceau S Red staining served as a loading control. Arrowheads indicate the position of MAPKs 3, 4/11, and 6.
(D) GUS activity in pPR-1:GUS transgenic Arabidopsis Col-0 plants. Leaves were infiltrated with 0.5 µg/mL of the SCFE1-containing fraction or buffer and collected 24 h later for histochemical GUS staining.
(E) Transcriptional profiling of defense-related genes by qRT-PCR. Arabidopsis Col-0 leaves were infiltrated with water, 100 nM flg22, or 0.5 µg/mL of the SCFE1-containing fraction and the corresponding buffer and collected 6 h after treatment. Expression of the indicated genes was normalized to the levels of EF-1α transcript and is presented as fold induction compared with the respective control set to 1. Error bars indicate sd (n = 3).
The stability of SCFE1 activity in the presence of SDS prompted us to perform one-dimensional SDS-PAGE with the SCFE1-containing fraction eluted from the last cation exchange chromatography step followed by the elution of the SCFE1 activity from gel slices. We recovered the ethylene-inducing activity from gel slices corresponding to proteins with a molecular mass of 16 to ∼22 kD (see Supplemental Figure 2 online). To date, all subsequent attempts to purify to homogeneity and to characterize SCFE1 have failed, mainly because of the low abundance of the protein. However, we can exclude contamination of the SCFE1 activity with flg22, elf18, or, more likely, fungal chitin, as the fls2 efr and cerk1 mutant plants responded normally to the SCFE1-containing fraction (see Supplemental Figure 3A online). In addition, the activity of the SCFE1-containing fraction did not depend on generation of the danger-associated molecular pattern Pep1 (Krol et al., 2010) (see Supplemental Figure 3B online). We could also rule out a toxic function of the SCFE1-containing fraction as it did not trigger any cell death, in contrast with infiltration with 3 µM of a necrosis- and ethylene-inducing like protein (Liberti et al., 2008) (see Supplemental Figure 3C online).
Early cellular events following the perception of MAMPs such as flg22 include the induced production of reactive oxygen species (ROS) and the posttranslational activation of mitogen-activated protein kinase (MAPK) cascades (Boller and Felix, 2009). To find out the extent of overlap of cellular changes triggered by the SCFE1-containing fraction and the changes triggered by well-characterized early MAMP signal transduction, we used flg22 for comparative studies. After treatment of Arabidopsis Columbia-0 (Col-0) leaves with the SCFE1-containing fraction, we obtained a significant increase in ROS production within 45 min, although the kinetics and intensity of the oxidative burst differed from the oxidative burst induced by treatment with flg22 (Figure 1B). Also, the SCFE1-containing fraction triggered similar levels of ROS in the wild type and the fls2 efr mutant, showing further that FLS2 and EFR are not involved in SCFE1 perception (see Supplemental Figure 4 online). MAPK activity was analyzed by immunoblotting assays using a p44/42 antibody raised against phosphorylated MAPKs. As shown in Figure 1C, the SCFE1-containing fraction strongly activated the defense-associated MAPKs MPK3, MPK4/MPK11, and MPK6, which was indistinguishable from the induction pattern obtained with flg22.
A typical late plant response to pathogen infection or treatment with MAMPs is the induction of the synthesis of pathogenesis-related (PR) proteins, such as PR-1 (Ferreira et al., 2007). To visualize PR-1 expression, we used a transgenic pPR-1:GUS reporter line, in which the PR-1 promoter is fused to the uidA gene from Escherichia coli coding for β-glucuronidase (GUS) (Shapiro and Zhang, 2001). As shown in Figure 1D, treatment with the SCFE1-containing fraction resulted in a strong GUS activity, indicating that SCFE1 induces the expression of PR-1.
Plant adaptation to biotic stresses promotes transcriptional upregulation of large sets of genes that are involved in disease resistance (Navarro et al., 2004; Zipfel et al., 2006). The expression profile of typical early MAMP-regulated genes after application of the SCFE1-containing fraction revealed that the expression of PR-4 (jasmonate/ethylene [JA/ET] dependent) is similarly induced 6 h after treatment with flg22 or with the SCFE1-containing fraction (Figure 1E). However, quantitative differences were observed with the upregulation of FLG22-INDUCED RECEPTOR-LIKE KINASE1 (FRK1) expression (salicylic acid [SA] dependent), which is stronger in response to flg22. The opposite was observed for the expression of CYTOCHROME P450, FAMILY71, SUBFAMILY A, POLYPEPTIDE13 (CYP71A13) and PHYTOALEXIN DEFICIENT3 (PAD3) (camalexin biosynthetic pathway), which is higher with the SCFE1-containing fraction than with flg22 at the investigated time point (Figure 1E). The reason for this is unclear, but it may reflect kinetic differences in gene expression and dose-dependent effects between SCFE1 and flg22 rather than being the consequence of activation of different signaling branches. Together, our initial results suggest that despite the quantitative differences in the ability of the SCFE1-containing fraction and flg22 to trigger defense responses, the two elicitors induce qualitatively similar changes.
Fast Forward Genetics Identifies RLP30 as Being Required for SCFE1 Perception/Sensitivity
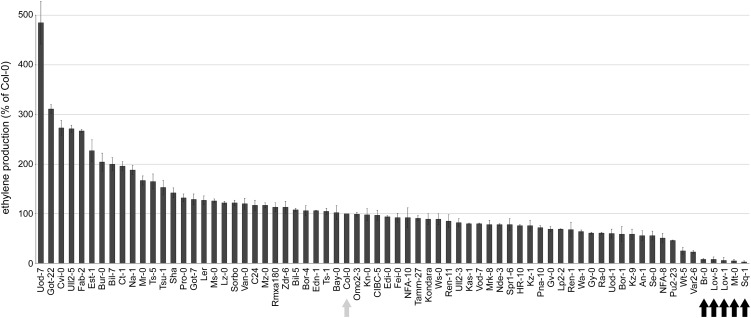
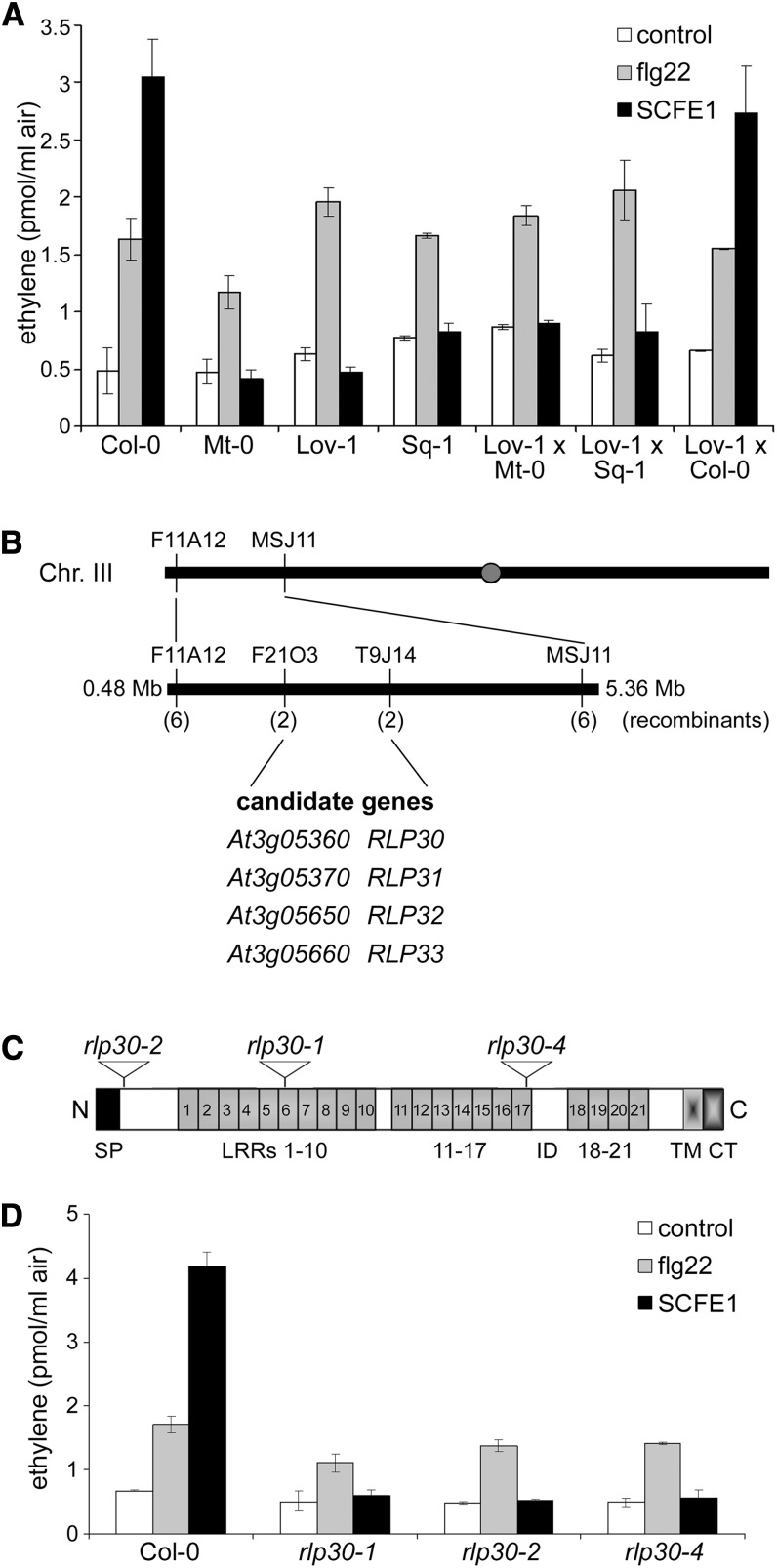
Although we have not yet identified the protein/peptide corresponding to SCFE1, we have correlative evidence that the elicitor activity is associated with one molecular pattern rather than a mixture of different elicitors. Based on this hypothesis, we used a forward genetic approach to identify Arabidopsis genes required for SCFE1 perception or signal transduction. Natural genetic variation between different Arabidopsis accessions may produce accessions that are partially or fully insensitive to the SCFE1-containing fraction and thus enable the identification of, for instance, the SCFE1 receptor. For example, this approach identified the flg22-insensitive accession Ws-0, which lacks a functional FLS2 receptor (Gomez-Gomez and Boller, 2000). We tested the ethylene response to treatment with the SCFE1-containing fraction in 70 different Arabidopsis accessions (Nordborg collection, Nottingham Arabidopsis Stock Centre) and found the accessions Br-0, Lov-1, Lov-5, Mt-0, and Sq-1 to be fully insensitive to the SCFE1-containing fraction (Figure 2). These accessions were not impaired in their ability to produce ethylene, since they retained full responsiveness to flg22 (Figure 3A). The F1 progeny of Col-0 crossed with the three insensitive accessions (Lov-1, Mt-0, and Sq-1) displayed a normal response to the SCFE1-containing fraction (Figure 3A; see Supplemental Table 1 online), indicating the recessive nature of this trait. Crossing the insensitive accessions Lov-1, Mt-0, and Sq-1 to each other and analysis of the resulting F1 progeny indicated that the nonresponsiveness to the SCFE1-containing fraction is allelic in all three accessions (Figure 3A; see Supplemental Table 1 online). F2 populations from the Lov-1 × Col-0 cross showed a segregation ratio of 3:1 (206 sensitive versus 64 insensitive plants; see Supplemental Table 1 online), suggesting that the insensitivity phenotype to the SCFE1-containing fraction in the Lov-1 accession is controlled by a single recessive nuclear gene.
Figure 2.
SCFE1-Induced Ethylene Response in Different Arabidopsis Accessions.
The SCFE1-containing fraction (0.25 µg/mL) was tested for ethylene-inducing activity on leaf pieces of indicated Arabidopsis accessions. Bars represent relative ethylene production of the respective accession compared with Col-0, which was set to 100% (gray arrow). Black arrows indicate SCFE1-insensitive accessions. Shown are means of two replicates ± sd. The experiment was performed two times with similar results.
Figure 3.
SCFE1 Perception Depends on RLP30.
(A) The SCFE1-containing fraction (0.25 µg/mL) was tested for ethylene-inducing activity on leaf pieces from Arabidopsis Col-0, the accessions Mt-0, Lov-1, and Sq-1, and the F1 population of the crosses Lov-1 × Mt-0, Lov-1 × Sq-1, and Lov-1 × Col-0. Leaf pieces left untreated (control) or treated with 500 nM flg22 served as controls. Bars represent average values ± sd (n = 2). The experiment was repeated three times with similar results.
(B) Map-based cloning strategy for identifying the gene required for SCFE1 perception from F2 mapping populations of the Lov-1 × Col-0 cross. Available simple sequence length polymorphism and restriction fragment length polymorphism markers on chromosome 3 (F11A12 and MSJ11) and restriction fragment length polymorphism markers developed in this study (F21O3 and T9J14) are indicated. The number of recombinants from the mapping population of 270 F2 plants is shown in parentheses. The SCFE1 insensitivity locus was mapped to a 1-Mb region between markers F21O3 and T9J14. Candidate genes within this region are shown at the bottom.
(C) Schematic representation of the RLP30 protein organization. Indicated are the positions of the predicted signal peptide (SP), LRRs 1 to 21, which are interrupted by an island domain (ID), the C-terminal transmembrane domain (TM), and the short cytoplasmic tail (CT). The positions of the three rlp30 T-DNA insertions are indicated.
(D) Ethylene assay in Arabidopsis Col-0 plants compared with rlp30 mutants. Leaf pieces were treated with 0.25 µg/mL of the SCFE1-containing fraction or as a control with 500 nM flg22 prior to measurement of ethylene production. Bars represent average values ± sd (n = 2). The experiment was repeated three times with similar results.
We further used ethylene production as a trait to map the locus involved in sensitivity to SCFE1 in the Lov-1 × Col-0 cross. Crude mapping located this locus on the upper arm of chromosome 3 in a region between markers F11A12 and MSJ11 (Figure 3B). Further fine-mapping narrowed the SCFE1 sensitivity locus to a region of ∼1 Mb between markers F21O3 and T9J14 containing a total of 249 genes. Among these genes, we found only four genes encoding eLRR-RLPs (RLP30 to RLP33) but no members of the eLRR-RLK protein family (Figure 3B). The selection of these candidate genes was driven by our knowledge about the well-established function of eLRR ectodomains in perception of proteinaceous MAMPs, as known for FLS2/flg22 and EFR/elf18.
Independent T-DNA insertion lines for RLP30 (At3g05360), RLP31 (At3g05370), and RLP33 (At3g05660) were obtained from the collection described by Wang et al. (2008) and tested for ethylene production in the presence of SCFE1. The T-DNA line with an insertion in RLP32 (At3g05650, FLAG_588C11) did not grow and could not be included in the assay. Only the rlp30 mutants were affected in SCFE1-dependent ethylene production; the knockout lines for RLP31 and RLP33 displayed a normal response to the SCFE1-containing fraction (Figure 3D; see Supplemental Figure 5 online). We did not find the predicted T-DNA insertion in the rlp30-3 line, and the plants were sensitive to treatment with the SCFE1-containing fraction (see Supplemental Figures 5 and 6 online). Importantly, all the rlp30 mutants remained fully responsive to flg22 (Figure 3D; see Supplemental Figure 5 online). To confirm that RLP30 is responsible for the sensitivity to the SCFE1-containing fraction, we tested different rlp30-1 mutant lines complemented with genomic DNA or cDNA constructs encoding the Col-0 RLP30 protein with/without a C-terminally fused yellow fluorescent protein tag (Wang et al., 2008). All the completed lines displayed a wild-type level of ethylene production after treatment with the SCFE1-containing fraction (see Supplemental Figure 7 online).
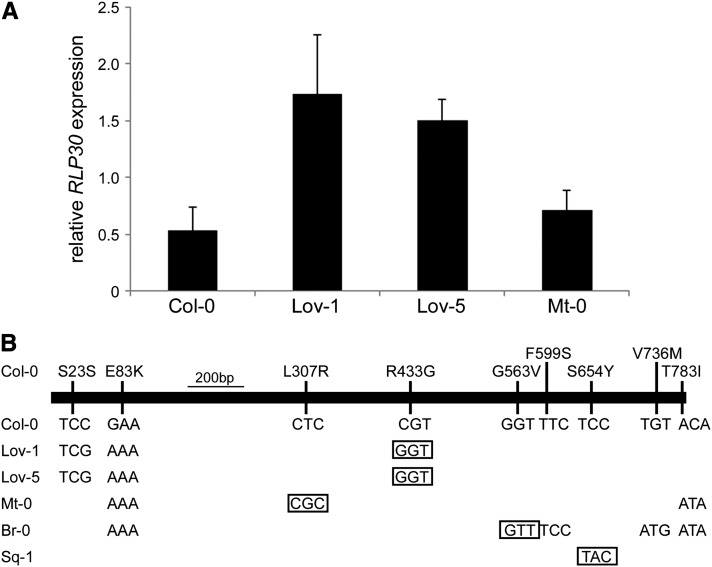
We next examined the RLP30 locus in the insensitive Arabidopsis accessions. Transcriptional profiling of RLP30 in Lov-1, Lov-5, and Mt-0 showed that deletion or pseudogenization of RLP30 is not responsible for the SCFE1 insensitivity in these accessions (Figure 4A). The RLP30 alleles of the five SCFE1-insensitive accessions were sequenced, and we identified different patterns of missense mutations but no nonsense mutation that would yield a truncated RLP30 protein (Figure 4B). We used the polymorph tools (http://signal.salk.edu/atg1001/3.0/gebrowser.php) to compare the RLP30 DNA and protein sequences from SCFE1-insensitive and -sensitive accessions (shown in Figure 2). All the loss-of-function mutations in RLP30 result from different single amino acid replacements within the eLRR domain. Mutations that are specific to SCFE1-insensitive accessions are L307R (Mt-0), R433G (Lov-1 and Lov-5), G563V (Br-0), and S654Y (Sq-1) (Figure 4B). Together, these data demonstrate the specific involvement of RLP30 in SCFE1-mediated perception or signaling.
Figure 4.
Analysis of RLP30 in SCFE1-Insensitive Arabidopsis Accessions.
(A) Transcriptional profiling of RLP30 in different Arabidopsis accessions by qRT-PCR. Total RNA from leaves of 6- to 8-week-old plants was subjected to qRT-PCR using RLP30-specific primers RLP30-fd and RLP30-rv, and gene expression was normalized to the levels of Actin transcript. Error bars indicate sd (n = 3).
(B) The intronless RLP30 gene was amplified from genomic DNA of the Arabidopsis accessions Col-0, Lov-1, Lov-5, Mt-0, Br-0, and Sq-1 and sequenced after subcloning into the vector pCR8. The genomic region is indicated by a bold black line showing all amino acid replacements on top in the Col-0 accession resulting from single nucleotide polymorphisms. Single nucleotide polymorphisms occurring in individual accessions are indicated below, and boxes indicate single nucleotide polymorphisms specific to SCFE1-insensitive accessions upon extension of the comparative analysis of DNA and protein sequences to all the SCFE1-sensitive accessions shown in Figure 2 with the polymorph tool (http://signal.salk.edu/atg1001/3.0/gebrowser.php).
SCFE1 Sensitivity Requires BAK1
RLP30 presents all the hallmarks of a cell surface–located RLP with an N-terminal signal peptide, an extracellular domain containing 21 LRRs (which possibly act as the SCFE1 binding site), a single transmembrane domain, and a short cytoplasmic tail of 25 amino acid residues (Figure 3C). It has been previously published that RLP30 localizes to the plasma membrane and that RLP30 expression is strongly induced upon MAMP treatment or pathogen infection (Wang et al., 2008).
However, the absence of a cytosolic signaling domain indicates that RLP30 is most likely part of a heteromeric receptor complex that requires additional components for intracellular signaling. Assaying mutants for ENHANCED DISEASE SUSCEPTIBILITY1 (EDS1), PHYTOALEXIN DEFICIENT4 (PAD4), and NON-RACE SPECIFIC DISEASE RESISTANCE1 (NDR1), which are crucial for Effector-triggered immunity by Toll/interleukin1 receptor-nucleotide binding-leucine rich repeat (TIR-NB-LRR) and coiled/coil-nucleotide binding-LRR type of resistance proteins in Arabidopsis (Hammond-Kosack and Parker, 2003), indicated that these components are not involved in ethylene production triggered by the SCFE1-containing fraction (see Supplemental Figure 8 online).
FLS2 and EFR belong to the subfamily XII of LRR-RLKs containing eight additional members of unknown function, some of them being induced during infection or MAMP treatment (Zipfel et al., 2006; Postel et al., 2010). We could also exclude a role for these proteins in SCFE1 signaling, since none of the homozygous mutant lines in the fls2 efr1 cerk1 background displayed an altered response to the SCFE1-containing fraction (see Supplemental Figure 9 online).
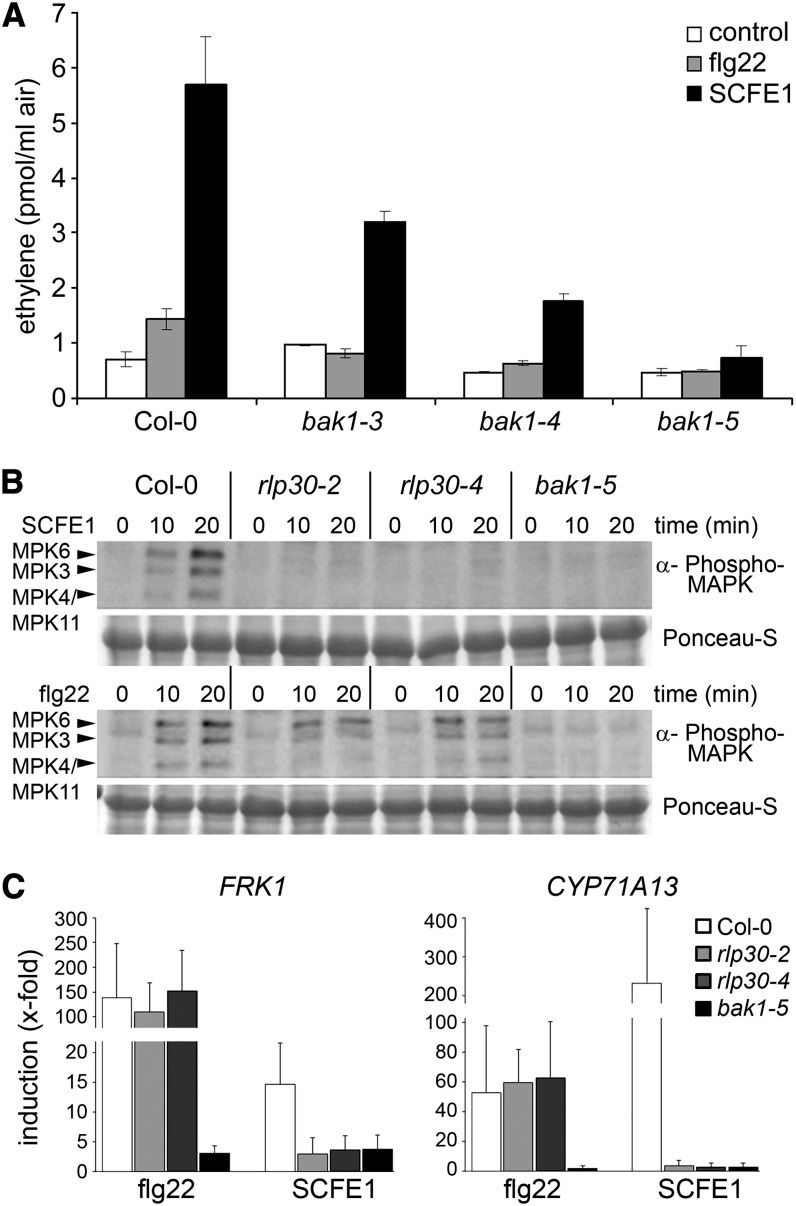
The eLRR-RLK BAK1 is a key regulator of immune and developmental signaling pathways. BAK1 physically interacts with various other eLRR-RLKs both in a ligand-dependent and -independent manner, suggesting a role as adapter or coreceptor in plant heteromeric receptor complexes (Nam and Li, 2002; Chinchilla et al., 2007; Heese et al., 2007; Roux et al., 2011). Several mutant alleles of BAK1 exist: bak1-3 and bak1-4 insertional mutants are partially impaired in brassinosteroid signaling, MAMP-triggered responses, and cell death control (Nam and Li, 2002; Chinchilla et al., 2007; Heese et al., 2007; Kemmerling et al., 2007), whereas the bak1-5 allele, encoding a BAK1 protein with an altered kinase activity, has a dominant-negative effect that affects only MAMP signaling but not brassinosteroid responses and cell death (Schwessinger et al., 2011). The ethylene response in bak1-3 and bak1-4 plants was only partially abolished, but bak1-5 plants were fully insensitive to treatment with the SCFE1-containing fraction (Figure 5A). The partial insensitivity to SCFE1 in bak1-3 and bak1-4 mutants might be due to functionally redundant genes, such as BAK1-LIKE1 (BKK1). Therefore, we also measured SCFE1-induced (and flg22) ethylene response in the bkk1-1 mutant. However, in contrast with the bak1 mutants, we did not find any significant alteration in ethylene production (see Supplemental Figure 10 online), suggesting that BAK1 is more important than BKK1 for SCFE1-mediated immune responses, as previously observed for flg22/FLS2 (Roux et al., 2011).
Figure 5.
SCFE1-Mediated Immune Responses Require BAK1.
(A) SCFE1-induced ethylene accumulation in bak1-3, bak1-4, and bak1-5 mutants. Leaves were treated with 0.25 µg/mL of the SCFE1-containing fraction or 500 nM flg22. Bars represent average values ± sd (n = 2). The experiment was repeated three times with similar results.
(B) Immunoblotting of activated MAPK with anti-phospho p44/p42 antibody in Arabidopsis seedling extracts. Seedlings were collected 0, 10, or 20 min after treatment with 1 µg/mL of the SCFE1-containing fraction (top panels) or 100 nM flg22 (bottom panels). Ponceau S staining served as a loading control. The identity of individual MAPKs as determined by size is indicated by arrowheads.
(C) Transcriptional profiling of the defense-related genes FRK1 and CYP71A13 by qRT-PCR. Arabidopsis seedlings were treated with 100 nM flg22 or 1 µg/mL of the SCFE1-containing fraction and collected 6 h after treatment. Gene expression was normalized to the levels of EF-1α transcript and is presented as fold induction compared with the respective control as described in Figure 1E. Error bars indicate sd (n = 3).
In the next step, we wanted to obtain a more detailed view on the role of BAK1 in SCFE1-mediated early signaling. We found that, similar to treatment with flg22, posttranslational MAPK activation by the SCFE1-containing fraction was absent in bak1-5 plants (Figure 5B). Parallel assays performed with two independent rlp30 mutant alleles (rlp30-2 and rlp30-4) confirmed that MAPK 3, 4, 6, and 11 were not activated in the mutants by the SCFE1-containing fraction but responded normally to flg22 (Figure 5B). Furthermore, the expression of MAMP-induced marker genes was equally dramatically reduced in bak1-5 plants upon treatment with flg22 or the SCFE1-containing fraction, whereas rlp30 mutants were specifically affected in their response to the SCFE1-containing fraction (Figure 5C). Together, these data clearly demonstrate that both FLS2- and RLP30-mediated immune responses share BAK1 and that SCFE1 is specifically sensed by RLP30.
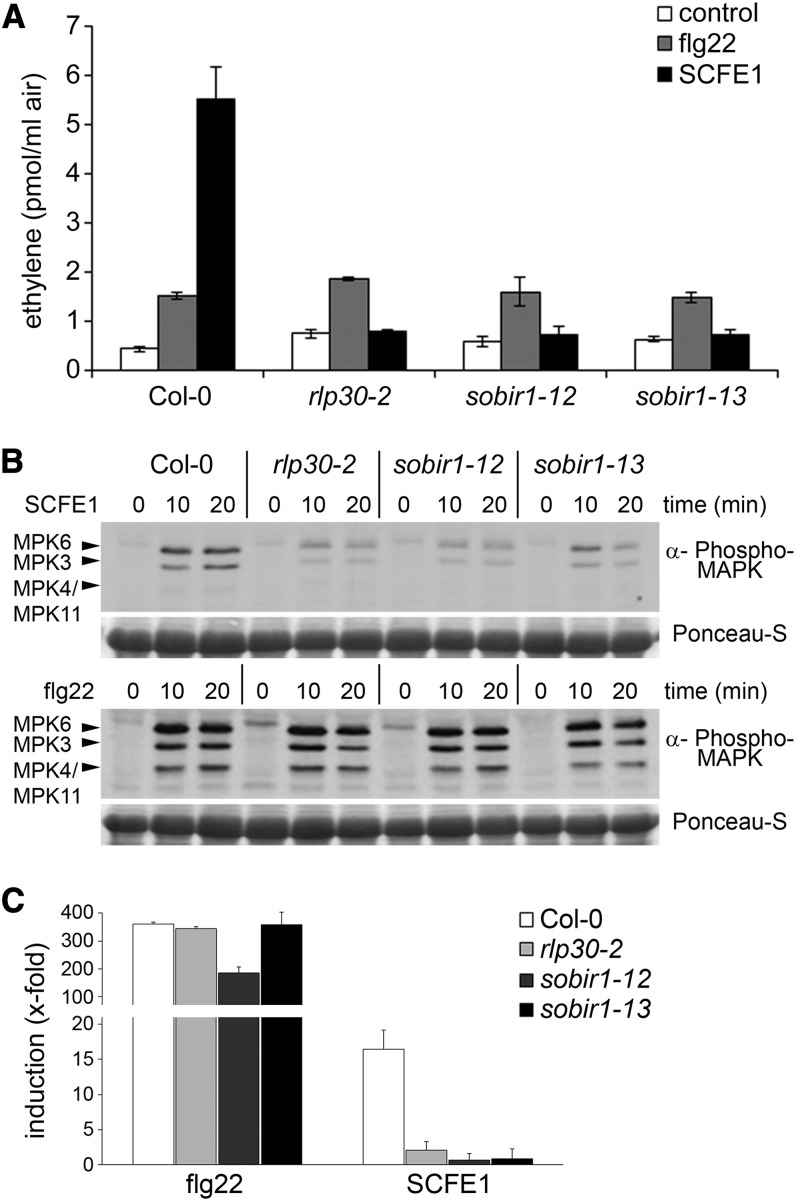
SOBIR1/EVR: A Third Component Involved in SCFE1 Perception/Sensitivity
To search for additional RLP30 interacting or signaling partners, we mined the public database of membrane protein interactors (http://www.associomics.org/Associomics/Home.html). Out of 66 putative interactors, this yeast two-hybrid–based data set showed that five RLKs interact with RLP30 (see Supplemental Table 2 online). From the Nottingham Arabidopsis Stock Centre, we obtained T-DNA insertion lines for three RLP30-interacting RLKs (SOBIR1/EVR, At2g31880; LRR PROTEIN KINASE FAMILY, At2g37050 and At5g59650). Only the sobir1 mutants were affected in SCFE1-dependent ethylene production; the knockout lines corresponding to the other RLKs displayed a wild-type response upon treatment with the SCFE1-containing fraction (see Supplemental Figure 11 online). Unlike bak1 but similar to rlp30 mutants, sobir1 mutants produced a wild-type level of ethylene in response to elicitation with flg22 (Figure 6A). Furthermore, we found that SCFE1-dependent posttranslational MAPK activation and the expression of the MAMP-induced marker gene FRK1 were impaired in the sobir1 mutant lines but not in the presence of flg22 (Figures 6B and 6C). These data corroborate the results from recent studies demonstrating that the tomato SOBIR1 homolog acts as a novel coreceptor and regulator for PRRs of the eLRR-RLP type (Liebrand et al., 2013). It also suggests that SOBIR1 is not involved in eLRR-RLK–mediated signaling. In conclusion, our results prove the existence of an SCFE1-mediated immune pathway in Arabidopsis recruiting the eLRR-RLP RLP30 and both eLRR-RLKs BAK1 and SOBIR1/EVR.
Figure 6.
SCFE1-Mediated Immune Responses Require SOBIR1/EVR.
(A) SCFE1-induced ethylene accumulation in rlp30-2, sobir1-12, and sobir1-13 mutants. Leaves were treated with 0.25 µg/mL of the SCFE1-containing fraction or 500 nM flg22. Bars represent average values ± sd (n = 2). The experiment was repeated three times with similar results.
(B) Immunoblotting of activated MAPK with anti-phospho p44/p42 antibody in Arabidopsis seedling extracts. Seedlings were collected 0, 10, or 20 min after treatment with 1 µg/mL of the SCFE1-containing fraction (top panels) or 100 nM flg22 (bottom panels). Ponceau S staining served as a loading control. The identity of individual MAPKs as determined by size is indicated by arrowheads.
(C) Transcriptional profiling of the defense-related gene FRK1 by qRT-PCR. Arabidopsis seedlings were treated with 100 nM flg22 or 1 µg/mL of the SCFE1-containing fraction and collected 6 h after treatment. Gene expression was normalized to the levels of EF-1α transcript and is presented as fold induction compared with the respective control as described in Figure 1E. Error bars indicate sd (n = 3).
RLP30, BAK1, and SOBIR1/EVR Contribute to Resistance against Necrotrophic Fungi
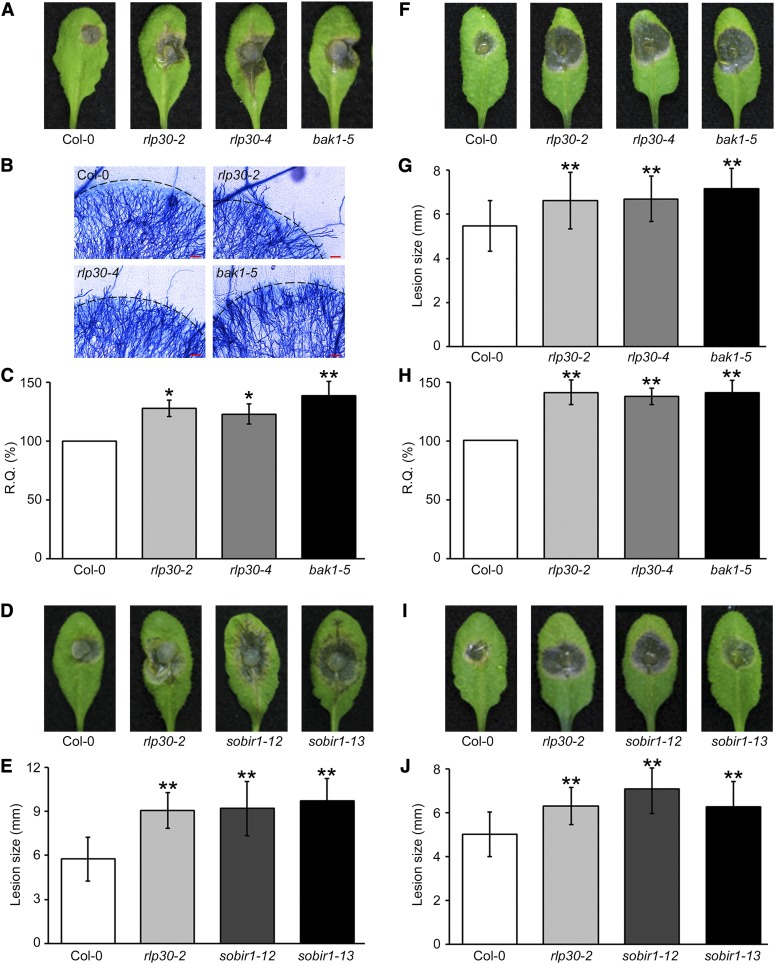
To test whether RLP30 is required for basal resistance against S. sclerotiorum, the knockout mutants rlp30-2 and rlp30-4 were challenged with the wild-type S. sclerotiorum strain 1980. As shown in Figure 7A, inoculation of both rlp30 mutant lines with S. sclerotiorum resulted in increased disease symptoms and cell death compared with wild-type Col-0. Microscopy examination of infected leaves after trypan blue staining revealed that mycelium growth was restricted to the necrotizing zone in Col-0 plants, but it spreads beyond this zone in the rlp30 mutants (Figure 7B). Fungal growth, assessed by measuring S. sclerotiorum genomic DNA levels by quantitative real-time PCR (qRT-PCR), was higher in rlp30 plants than in Col-0 (Figure 7C).
Figure 7.
Resistance to Necrotrophic Fungal Pathogens Requires RLP30, BAK1, and SOBIR1.
Infections with S. sclerotiorum ([A] to [E]) or B. cinerea ([F] to [J]).
(A) Symptom development on Col-0 plants or rlp30-2, rlp30-4, and bak1-5 mutant plants 2 d after inoculation with S. sclerotiorum strain 1980.
(B) Trypan blue staining of fungal hyphae 2 d after inoculation. A ring of blue-stained dead plant cells is indicated by a black line. Red bars = 100 µm.
(C) Fungal biomass determined by qRT-PCR (relative quantity [R.Q.]) 2 d after S. sclerotiorum infection in wild-type Col-0 plants or the indicated mutant line. S. sclerotiorum ITS genomic DNA levels are shown relative to the level of the Arabidopsis chloroplast-encoded reference gene ribulose-1,5-bis-phosphate carboxylase/oxygenase large subunit. Col-0 wild type is set to 100% relative quantity. Shown are means ± sd (n = 4). One-way analysis of variance (ANOVA) [F(3,12) = 7.902, P = 0.0036] followed by Dunnett’s multiple comparison test indicate a significant difference (*P < 0.05 and **P < 0.01) between the data sets from mutant plants compared with Col-0.
(D) and (E) For (D), symptom development on Col-0 plants or rlp30-2, sobir1-12, and sobir1-13 mutant plants was measured as described in (A), and lesion sizes determined 2 d after inoculation (E). Results represent means ± sd (n ≥ 16). One-way ANOVA [F(3,71) = 28.34, P < 0.0001] and Dunnett’s test (**P < 0.01) were performed.
(F) and (G) Col-0 plants or rlp30-2, rlp30-4, and bak1-5 mutants were drop-inoculated with 5 μL of a 2 × 106 spores mL−1 solution of B. cinerea and symptom development (F) or lesion sizes (G) were determined 3 d after inoculation. Results represent means ± sd (n = 20). One-way ANOVA [F(3,79) = 8.243, P < 0.0001] and Dunnett’s test (**P < 0.01) were performed.
(H) Fungal biomass at 3 d after inoculation was determined using B. cinerea Actin genomic DNA levels relative to Arabidopsis ribulose-1,5-bis-phosphate carboxylase/oxygenase levels as described in (C). One-way ANOVA [F(3,12) = 8.042, P = 0.0033] and Dunnett’s test (**P < 0.01) were performed.
(I) and (J) Symptom development (I) and lesion sizes (J) on Col-0 plants or rlp30-2, sobir1-12, and sobir1-13 mutants were measured as described in (F) and (G). Results represent means ± sd (n = 20). One-way ANOVA [F(3,80) = 14.22, P < 0.0001] and Dunnett’s test (**P < 0.01) were performed.
Likewise, B. cinerea spore inoculation of rlp30 plants resulted in increased cell death and lesion size when compared with Col-0 plants (Figures 7F and 7G). The enhanced growth of B. cinerea observed visually correlated with an elevated fungal DNA content in infected rlp30 leaves (Figure 7H).
BAK1 controls plant programmed cell death and immunity to necrotrophic fungi, and the insertional mutants bak1-3 and bak1-4 are more susceptible to B. cinerea and Alternaria brassicicola (Kemmerling et al., 2007). A clear conclusion on the role of BAK1 in mediating immunity to these necrotrophic fungi was impeded by the enhanced cell death phenotype of these alleles. However, the SCFE1-insensitive bak1-5 line, which does not display increased cell death (Schwessinger et al., 2011), was also more susceptible to S. sclerotiorum and B. cinerea (Figures 7A to 7C and 7F to 7H), revealing that BAK1 indeed plays a critical role mediating basal immunity to these pathogens.
SOBIR1 was proven to be an important factor in the control of tomato resistance against pathogenic fungi (Liebrand et al., 2013). Virus-induced gene silencing assays in tomato revealed its contribution to Cf-4– and Ve1-mediated resistance to the fungal pathogens Cladosporium fulvum and Verticillium dahliae (Liebrand et al., 2013). As shown in Figures 7D, 7E, 7I, and 7J, in Arabidopsis, the sobir1 mutation causes hypersusceptibility to S. sclerotiorum and B. cinerea, suggesting that SOBIR1 fills a major function in immunity against a broader range of fungal pathogens.
Together, our results indicate that RLP30, BAK1, and SOBIR1 play roles in defense likely recognizing a non-race-specific elicitor(s) against at least two different species among the most common necrotrophic fungal pathogens.
DISCUSSION
In this study, we demonstrated that in Arabidopsis, RLP30, BAK1, and SOBIR1 are required for the perception of a novel S. sclerotiorum elicitor that triggers genuine MAMP-induced immune responses. Lack of RLP30 and the two RLKs in Arabidopsis mutants enhanced susceptibility to S. sclerotiorum and to the closely related necrotrophic fungus B. cinerea. We generated evidence that SCFE1 is very likely a peptide with immunogenic properties and that SCFE1 does not act as a necrosis-inducing toxin, a common weapon of necrotrophic pathogens to colonize host plants. The molecular identification of SCFE1 remains an important aim for future work since it would be necessary to uncover biochemical aspects of ligand-receptor association and establish its role in the fitness or virulence of S. sclerotiorum. However, pure SCFE1 protein is not mandatory for studying molecular mechanisms of the signaling involving RLP30, BAK1, and SOBIR1/EVR or for application-oriented plant immunity research. An alternative to the isolation of SCFE1 by column chromatography is offered by the development of bioinformatic tools that allow high-throughput prediction of proteinaceous elicitors when sufficient genome information is available (McCann et al., 2012). With the sequencing of the S. sclerotiorum genome (Amselem et al., 2011), it is conceivable that an in silico approach would provide a list of putative SCFE1 candidates.
We observed that the expression of JA/ET-regulated genes and camalexin biosynthetic genes is more strongly induced by the SCFE1-containing fraction than by flg22. In addition, EDS1, PAD4, and NDR1, which are essential for SA-mediated resistance (Hammond-Kosack and Parker, 2003), are not involved in SCFE1-induced ethylene production. This is in accordance with the general view that the JA/ET and SA pathway act antagonistically with a prominent role of JA/ET in resistance against necrotrophs, while SA appears to be more important for resistance against biotrophs (Glazebrook, 2005). Therefore, it is tempting to hypothesize that SCFE1-mediated signaling preferentially activates the JA/ET branch of the immune system, and a comprehensive analysis of global metabolomic, transcriptomic, and proteomic changes will help to answer how the SCFE1 signal is integrated and to which extend it overlaps or differs from flg22 signaling.
With the identification of an RLP participating in resistance against S. sclerotiorum and B. cinerea in Arabidopsis, we genetically defined a PRR that recognizes a proteinaceous elicitor potentially conserved in necrotrophic fungal pathogens. Our study also demonstrates the value of Arabidopsis as a genomic resource for isolating and exploiting new genes that could be transferred into economically important crop plants lacking an obvious ortholog of the Arabidopsis locus to confer disease resistance. The completion of the genome sequencing of more than 400 Arabidopsis accessions (http://1001genomes.org/) and the existence of large collections of F2 segregating mapping populations and knockout lines for nearly all the putative Arabidopsis receptor-encoding genes permit high-throughput identification of receptors for microbial elicitors. Gene presence/absence, deletion/insertion, and single nucleotide polymorphisms increase the degree of genetic variability among the different accessions (Cao et al., 2011; Gan et al., 2011) facilitating the association of receptors with their biological function and their exploitation for biotechnological applications.
RLPs represent a unique class of cell surface receptors, as they lack a functional cytoplasmic domain. We have shown that both BAK1 and SOBIR1 are genetically required for RLP30-induced signal transduction. The contribution of BAK1 and SOBIR1 in RLP-mediated signaling seems to be complex. BAK1 interacts with tomato Eix1 (but not Eix2), which acts as a decoy receptor and attenuates EIX induced internalization and signaling of the Eix2 receptor (Bar et al., 2010). Therefore, BAK1 seems to act as a negative regulator in EIX signaling, in contrast with its positive effect on SCFE1- or Ve1-mediated signaling (Fradin et al., 2011). Similarly, SOBIR1 acts as a positive regulator of cell death in Arabidopsis (Gao et al., 2009) and tomato, in which silencing SOBIR1 compromised the Cf-4/Avr4– and Ve1/Ave1-induced hypersensitive response (Liebrand et al., 2013). This would be in contrast with its implication in RLP30-mediated resistance against necrotrophic pathogens that gain advantage by promoting cell death/necrosis, suggesting a negative role of SOBIR1 in cell death control. Future experiments focusing on the molecular mechanism underlying the immune signaling pathway mediated by RLP30, BAK1, and SOBIR1/EVR should uncover the precise role of each component in resistance against S. sclerotiorum and B. cinerea.
Our screen identified five accessions with responsiveness to the SCFE1-containing fraction below 10% of the wild type, and for three of them (Mt-0, Lov-1, and Sq-1), the loss of response maps to the RLP30 locus. We did not verify whether the two other accessions (Br-0 and Lov-5) are allelic, but sequencing of RLP30 revealed mutation patterns that are unique to the SCFE1-insensitive accessions. The mutations in the extracellular domain probably affect either the stability of RLP30, SCFE1 binding, and/or the association with BAK1 and/or SOBIR1. In addition to SOBIR1 relaying the SCFE1 signal, other components might be identified through mining of the yeast two-hybrid interactors database. The possible identification of components like the thioredoxin CITRX coupling the tomato Cf9 with the cytoplasmic RLK ACIK (Rivas et al., 2004; Rowland et al., 2005; Nekrasov et al., 2006) should provide insight into RLP-mediated immune signaling in Arabidopsis and allow us to investigate possible interactions with RLK-mediated immunity programs recruiting BAK1.
In general, plant resistance against necrotrophic pathogens is quantitative and multilayered, rendering its genetic dissection extremely challenging. Work by Perchepied et al. (2010) highlighted the large variation in natural resistance to S. sclerotiorum infection among more than 50 Arabidopsis accessions. Accessions responding normally to the SCFE1-containing fraction were found in both hypersusceptible and resistant categories. Among the five SCFE1-insensitive accessions identified in this study, only Mt-0 was listed in Perchepied et al., and the accession displayed intermediate resistance to S. sclerotiorum (Perchepied et al., 2010). Thus, redundant perception systems involving other PRRs than RLP30 might have evolved to render quantitative resistance against S. sclerotiorum and B. cinerea.
A previous report (Wang et al., 2008) did not notice any altered resistance to necrotrophic fungi, including S. sclerotiorum and B. cinerea, in the rlp30 mutants. The use of S. sclerotiorum isolates with a different level of aggressiveness and different experimental settings of the patho-assay might account for these discrepancies. Interestingly, in the same report, rlp30 mutants were shown to be more susceptible to infection with the nonadapted bacterial pathogen P. syringae pv phaseolicola strain 1448A (Wang et al., 2008). One possible hypothesis that would explain the bacterial phenotype is that the elusive SCFE1 protein is conserved in both fungi and bacteria. It is indeed possible that bacteria and fungi may very well share orthologous proteins that were acquired by horizontal gene transfer and that are detected by the plant immune system. This has been for example recently shown for the elicitor Ave1 (for Avirulence on Ve1 tomato), which triggers resistance in tomato plants carrying the LRR-RLP Ve1. Ave1 is present in various fungal species (Verticillium spp, Colletotrichum higginsianum, and Cercospora beticola) and in the bacterium Xanthomonas axonopodis where it is known as the virulence factor XacPNP (Nembaware et al., 2004; de Jonge et al., 2012) Therefore, RLP30 might be a promising candidate to enhance pathogen perception and to improve resistance in crop plants that lack a perception system for SCFE1 against diseases not only caused by fungi such as Sclerotinia and Botrytis species but also by bacteria.
In conclusion, the identification of the SCFE1-containing fraction and RLP30 as potential elicitor-receptor partners is an important step toward understanding the molecular mechanisms underlying plant resistance to necrotrophic pathogens, a field that remains largely uncovered. We predict that partial purification of microbial extracts, as performed in the case of the SCFE1-containing fraction and eMAX, a novel bacterial MAMP from Xanthomonas (Jehle et al., 2013), will permit the successful identification of many more receptors directed against various types of pathogens in a relatively short period of time. The wealth of genetic resources available in Arabidopsis offers a major advantage over other plant systems, allowing us to increase our fundamental understanding of receptor-dependent immunity and an unparalleled opportunity to turn surface receptors, used individually, stacked or generated by hybrid technology, into tools to engineer durable broad-spectrum resistance in agricultural crops (Gust et al., 2010; Lacombe et al., 2010).
METHODS
Fungal Culture and Purification of the SCFE1-Containing Fraction
The Sclerotinia sclerotiorum strain 1980 was kindly provided by Henrik Stotz (University of Würzburg, Germany). S. sclerotiorum strain 1946 and Botrytis cinerea isolate BO5-10 were purchased from the Deutsche Sammlung von Mikroorganismen und Zellkulturen. The fungi were maintained on malt-peptone agar plates (10 g malt, 2.5 g peptone, and 15 g agar/L) or on Moser B agar in 20-mL McCartney bottles at 15°C in the dark. To prepare axenic liquid cultures, 8 to 10 liters of malt-peptone medium was divided into 1-liter flasks (400 mL/flask), inoculated with two to three mycelium plugs, and incubated without shaking at room temperature in the dark for 2 to 3 weeks until the mycelium fully covered the liquid surface. Culture medium was harvested by filtration through a 75-µm nylon mesh and freeze-dried for 3 to 4 d. Dried culture filtrate material was stored in air-tight bottles until processing.
To obtain the SCFE1-containing fraction, dried material was resuspended in 100 mM MES buffer at pH 5.4 (6 mL/g dry weight) and centrifuged twice for 20 min at 10,000g and 4°C to remove insoluble particles. In a first step, the resulting supernatant was subjected to cation-exchange chromatography and loaded onto a XK16 column (GE Healthcare) packed with Sepharose SP FastFlow matrix (GE Healthcare) to a bed volume of 15 mL and equilibrated with buffer A (100 mM MES buffer at pH 5.4). After washing with buffer A, elution was performed with buffer B (100 mM MES buffer, pH 5.4, and 500 mM KCl). A single fraction corresponding to the elution peak monitored with OD280nm was collected manually. In a second step, the eluate of the first column was diluted 10× in buffer A and rechromatographed onto a Source 15S 4.6/100 PE column (GE Healthcare) equilibrated with buffer A. After washing with buffer A, SCFE1 was eluted with a linear gradient of buffer B (0 to 60% in 40 column volumes). The 500-μL fractions corresponding to the whole elution peak monitored with OD280nm were collected using automated fractionation. Active fractions containing SCFE1 were identified by measurement of their ability to elicit ethylene production in Arabidopsis thaliana leaves.
Plant Material
Arabidopsis plants were grown on soil or half-strength Murashige and Skoog medium as described (Brock et al., 2010). Arabidopsis accession Col-0 was the background for all mutants and transgenic lines used in this study as listed in Supplemental Table 3 online.
Seeds of the Nordborg accession collection (Nordborg et al., 2005) were obtained from the Nottingham Arabidopsis Stock Centre. Accession crosses were obtained by crossing the individual accessions and collecting F1 and F2 populations. F2 populations of the Lov-1 × Col-0 cross were kindly provided by Marco Todesco and Detlef Weigel (MPI Tuebingen, Germany). Map-based cloning was performed with the primers listed in Supplemental Table 4 online.
Elicitation of Immune Responses
The detection of ethylene and ROS in leaf pieces of 5-week-old Arabidopsis plants was performed as described (Felix et al., 1999; Albert et al., 2010). For the ROS assay, SCFE1-containing fractions were first dialyzed against water to remove buffer. Immunoblot analyses using the anti-phospho p44/42 MAP kinase antibody (Cell Signaling Technology) were performed as described (Brock et al., 2010).
RNA isolation and qRT-PCR analysis of plant material treated with the SCFE1-containing fraction or its buffer (100 mM MES, pH 5.4, and 500 mM KCl) were performed as described previously (Gust et al., 2007; Brock et al., 2010). Briefly, total RNA was isolated using the Tri Reagent method (Sigma-Aldrich). To perform first-strand cDNA synthesis using Moloney Murine Leukemia Virus RT RevertAid (Fermentas), 2 to 5 µg of total RNA was used. Quantitative PCR reactions and measurements were performed with the iQ5 Multi-color real-time PCR detection system (Bio-Rad) using the SYBR green Fluorescein Mix (Thermo Scientific). Relative quantification of gene expression data was performed using the 2−ΔΔCt (cycle threshold) method. EF-1α transcripts served for normalization, and controls were set to 1. All quantifications were made in duplicate on RNA samples obtained from three independent experiments, each performed with a pool of two leaves. The sequences of the primers used for PCR amplifications are listed in Supplemental Table 5 online.
The histochemical detection of GUS enzyme activity in PR-1:GUS transgenic plants (Shapiro and Zhang, 2001) was performed as described (Gust et al., 2007). In all assays, 100 to 500 nM synthetic flg22 was used as a control (Felix et al., 1999).
Arabidopsis Infection Assays
S. sclerotiorum strain 1980 was used for infection assays, and cultures were freshly prepared on potato dextrose agar (Sigma-Aldrich) from a −80°C stock (Guo and Stotz, 2007). Prior to inoculation, S. sclerotiorum was grown on minimal medium to reduce S. sclerotiorum aggressiveness as previously described (Guo and Stotz, 2007). An agar plug (5 mm in diameter) containing actively growing S. sclerotiorum mycelium was placed on the adaxial surface of rosette leaves of 4- to 5-week-old Arabidopsis plants. Plants were afterwards maintained at high humidity. Plant cell death and fungal growth at the cellular level were monitored by the trypan blue staining method (Torres et al., 2005). B. cinerea isolate BO5-10 was grown on synthetic media and used for infection assays on Arabidopsis leaves of 4- to 5-week-old plants as described previously (Kemmerling et al., 2007).
For fungal DNA quantification, four S. sclerotiorum– or B. cinerea–infected leaves per genotype were harvested and pooled after 2 or 3 d of inoculation, respectively. The samples were frozen in liquid nitrogen and ground to powder. Total DNA was isolated using cetyltrimethylammonium bromide buffer (1.4 M NaCl, 20 mM EDTA, pH 8, 100 mM Tris-HCl, pH 8, and 3% CTAB). Fungal biomass was determined by qRT-PCR using the SYBR Green qPCR Master Mix (Fermentas). The relative concentration of S. sclerotiorum internal transcribed spacer (ITS) or B. cinerea Actin genomic DNA levels to Arabidopsis ribulose-1,5-bisphosphate carboxylase/oxygenase (large subunit) levels was used to quantify fungal biomass (Fradin et al., 2011). Specific primers are listed in Supplemental Table 5 online.
Accession Numbers
Sequence data from this article can be found in the Arabidopsis Genome Initiative or GenBank/EMBL databases under the following accession numbers: RLP30, At3g05360; RLP31, At3g05370; RLP33, At3g05660; BAK1, At4g33430; BKK1, At2g13790; SOBIR1, At2g31880; LRR protein kinase family, At2g37050; LRR protein kinase family, At5g59650; FLS2, At5g46330; EFR, At5g20480; XII1, At1g35710; XII2, At2g24130; XII3, At3g47090; XII4, At3g47110; XII5, At3g47570; XII6, At3g47580; XII7, At4g08850; PEPR1, At1g73080; PEPR2, At1g17750; CERK1, At3g21630; PAD4, At3g52430; EDS1, At3g48090; NDR1, At3g20600; FRK1, At2g19190; PAD3, At3g26830; PR-1, At2g14610; PR-4, At3g04720; CYP71A13, At2g30770; EF-1α, At1g07920/30/40; ACTIN7, At5g09810; RUBISCO, Atcg00490; S. sclerotiorum ITS, KC748491; and B. cinerea ACTIN, BC1G_08198.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. Isolation and Physico-Chemical Properties of the SCFE1-Containing Fraction.
Supplemental Figure 2. Elicitor Activity of SCFE1 Can Be Purified from SDS-PAGE.
Supplemental Figure 3. The SCFE1-Containing Fraction Does Not Induce Necrosis and Triggers Ethylene Production Independently of Known MAMP or DAMP Receptors.
Supplemental Figure 4. The SCFE1-Containing Fraction Triggers ROS Production Independently of Known MAMP Receptors.
Supplemental Figure 5. SCFE1-Triggered Ethylene Production in Candidate rlp Mutants.
Supplemental Figure 6. Analysis of Arabidopsis rlp30 Mutants.
Supplemental Figure 7. Ethylene Response in Complemented rlp30-1 Mutant Plants.
Supplemental Figure 8. The Activity of the SCFE1-Containing Fraction Does Not Depend on Known ETI Components.
Supplemental Figure 9. SCFE1 Perception Does Not Require LRR-RLK XII Family Members.
Supplemental Figure 10. The Activity of the SCFE1-Containing Fraction Does Not Depend on BKK1.
Supplemental Figure 11. The Activity of the SCFE1-Containing Fraction Depends on SOBIR1/EVR.
Supplemental Table 1. SCFE1 Sensitivity Is Controlled by a Single Recessive Gene Locus in Different Arabidopsis Accessions.
Supplemental Table 2. Putative Interactors of RLP30.
Supplemental Table 3. Arabidopsis Mutant and Transgenic Lines Used in This Study.
Supplemental Table 4. Primers Used for Map-Based Cloning.
Supplemental Table 5. Primers Used in qPCR Analyses.
Supplementary Material
Acknowledgments
We thank Henrik Stotz (University of Würzburg, Germany) and Marco Todesco and Detlef Weigel (Max Planck Institute for Developmental Biology, Tuebingen, Germany) for providing the S. sclerotiorum strain 1980 and Lov-1 × Col-0 crosses, respectively. We thank Bart Thomma (University of Wageningen, The Netherlands) and Birgit Kemmerling (Center for Plant Molecular Biology-University of Tübingen, Germany) for sharing the RLP and SOBIR1/EVR T-DNA lines, respectively. We thank Georg Felix and Thorsten Nürnberger (Center for Plant Molecular Biology-University of Tübingen, Germany) for providing flg22 and NLP from Phytophthora parasitica, respectively. We thank Christiane Karasch-Wittmann (University of Tübingen) for assistance with fungal growth and maintenance conditions and Andreas Kulik (University of Tübingen) for helping to process the culture filtrate. We thank Georg Felix (Center for Plant Molecular Biology-University of Tübingen) and Erika Isono (Technical University of Munich, Germany) for critical reading of the article. This work was supported by the Deutsche Forschungsgemeinschaft (BR 3875 1-1 to F.B. and A.A.G., as part of the ERA-NET Plant Genomics Pattern Recognition Receptors: Discovery, Function and Application in Crops for Durable Disease Control (PRR CROP) consortium, and SFB766 to A.A.G.) and by grants from the UK Biotechnology and Biological Sciences Research Council (BB/G024936/1, as part of the ERA-PG PRR CROP consortium) and the Gatsby Charitable Foundation to C.Z.
AUTHOR CONTRIBUTIONS
F.B. and A.A.G. conceived and designed the study and analyzed the data. W.Z., M.F., D.K., B.L., Y.D., and F.F.G.B. performed the experiments and analyzed the data. M.T. provided materials. C.Z. provided materials and analyzed the data. F.B., A.A.G., M.F., and W.Z. wrote the article. All the authors critically read and commented on the article and approved of its final version for submission.
Glossary
- MAMP
microbe-associated molecular pattern
- PRR
pattern recognition receptor
- RLK
receptor-like kinase
- eLRR
extracellular domain harboring tandem leucine-rich repeat
- RLP
receptor-like protein
- ROS
reactive oxygen species
- MAPK
mitogen-activated protein kinase
- Col-0
Columbia-0
- GUS
β-glucuronidase
- LRR
leucine-rich repeat
- qRT-PCR
quantitative real-time PCR
- JA/ET
jasmonate/ethylene
- SA
salicylic acid
- ANOVA
analysis of variance
References
- Akira S., Uematsu S., Takeuchi O. (2006). Pathogen recognition and innate immunity. Cell 124: 783–801 [DOI] [PubMed] [Google Scholar]
- Albert M., Jehle A.K., Mueller K., Eisele C., Lipschis M., Felix G. (2010). Arabidopsis thaliana pattern recognition receptors for bacterial elongation factor Tu and flagellin can be combined to form functional chimeric receptors. J. Biol. Chem. 285: 19035–19042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amselem J., et al. (2011). Genomic analysis of the necrotrophic fungal pathogens Sclerotinia sclerotiorum and Botrytis cinerea. PLoS Genet. 7: e1002230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avni A., Bailey B.A., Mattoo A.K., Anderson J.D. (1994). Induction of ethylene biosynthesis in Nicotiana tabacum by a Trichoderma viride xylanase is correlated to the accumulation of 1-aminocyclopropane-1-carboxylic acid (ACC) synthase and ACC oxidase transcripts. Plant Physiol. 106: 1049–1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar M., Sharfman M., Ron M., Avni A. (2010). BAK1 is required for the attenuation of ethylene-inducing xylanase (Eix)-induced defense responses by the decoy receptor LeEix1. Plant J. 63: 791–800 [DOI] [PubMed] [Google Scholar]
- Boland G.J., Hall R. (1994). Index of plant hosts of Sclerotinia sclerotiorum. Can. J. Plant Pathol. 16: 93–108 [Google Scholar]
- Boller T., Felix G. (2009). A renaissance of elicitors: Perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annu. Rev. Plant Biol. 60: 379–406 [DOI] [PubMed] [Google Scholar]
- Bolton M.D., Thomma B.P., Nelson B.D. (2006). Sclerotinia sclerotiorum (Lib.) de Bary: Biology and molecular traits of a cosmopolitan pathogen. Mol. Plant Pathol. 7: 1–16 [DOI] [PubMed] [Google Scholar]
- Brock A.K., Willmann R., Kolb D., Grefen L., Lajunen H.M., Bethke G., Lee J., Nürnberger T., Gust A.A. (2010). The Arabidopsis mitogen-activated protein kinase phosphatase PP2C5 affects seed germination, stomatal aperture, and abscisic acid-inducible gene expression. Plant Physiol. 153: 1098–1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J., et al. (2011). Whole-genome sequencing of multiple Arabidopsis thaliana populations. Nat. Genet. 43: 956–963 [DOI] [PubMed] [Google Scholar]
- Chinchilla D., Zipfel C., Robatzek S., Kemmerling B., Nürnberger T., Jones J.D., Felix G., Boller T. (2007). A flagellin-induced complex of the receptor FLS2 and BAK1 initiates plant defence. Nature 448: 497–500 [DOI] [PubMed] [Google Scholar]
- de Jonge R., van Esse H.P., Maruthachalam K., Bolton M.D., Santhanam P., Saber M.K., Zhang Z., Usami T., Lievens B., Subbarao K.V., Thomma B.P. (2012). Tomato immune receptor Ve1 recognizes effector of multiple fungal pathogens uncovered by genome and RNA sequencing. Proc. Natl. Acad. Sci. USA 109: 5110–5115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickman M.B., Mitra A. (1992). Arabidopsis thaliana as a model for studying Sclerotinia sclerotiorum pathogenesis. Physiol. Mol. Plant Pathol. 41: 255–263 [Google Scholar]
- Felix G., Duran J.D., Volko S., Boller T. (1999). Plants have a sensitive perception system for the most conserved domain of bacterial flagellin. Plant J. 18: 265–276 [DOI] [PubMed] [Google Scholar]
- Ferreira R.B., Monteiro S., Freitas R., Santos C.N., Chen Z., Batista L.M., Duarte J., Borges A., Teixeira A.R. (2007). The role of plant defence proteins in fungal pathogenesis. Mol. Plant Pathol. 8: 677–700 [DOI] [PubMed] [Google Scholar]
- Fradin E.F., Abd-El-Haliem A., Masini L., van den Berg G.C., Joosten M.H., Thomma B.P. (2011). Interfamily transfer of tomato Ve1 mediates Verticillium resistance in Arabidopsis. Plant Physiol. 156: 2255–2265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fradin E.F., Zhang Z., Juarez Ayala J.C., Castroverde C.D., Nazar R.N., Robb J., Liu C.M., Thomma B.P. (2009). Genetic dissection of Verticillium wilt resistance mediated by tomato Ve1. Plant Physiol. 150: 320–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan X.C., et al. (2011). Multiple reference genomes and transcriptomes for Arabidopsis thaliana. Nature 477: 419–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao M.H., Wang X., Wang D.M., Xu F., Ding X.J., Zhang Z.B., Bi D.L., Cheng Y.T., Chen S., Li X., Zhang Y.L. (2009). Regulation of cell death and innate immunity by two receptor-like kinases in Arabidopsis. Cell Host Microbe 6: 34–44 [DOI] [PubMed] [Google Scholar]
- Glazebrook J. (2005). Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu. Rev. Phytopathol. 43: 205–227 [DOI] [PubMed] [Google Scholar]
- Gómez-Gómez L., Boller T. (2000). FLS2: An LRR receptor-like kinase involved in the perception of the bacterial elicitor flagellin in Arabidopsis. Mol. Cell 5: 1003–1011 [DOI] [PubMed] [Google Scholar]
- Guo X., Stotz H.U. (2007). Defense against Sclerotinia sclerotiorum in Arabidopsis is dependent on jasmonic acid, salicylic acid, and ethylene signaling. Mol. Plant Microbe Interact. 20: 1384–1395 [DOI] [PubMed] [Google Scholar]
- Gust A.A., Biswas R., Lenz H.D., Rauhut T., Ranf S., Kemmerling B., Götz F., Glawischnig E., Lee J., Felix G., Nürnberger T. (2007). Bacteria-derived peptidoglycans constitute pathogen-associated molecular patterns triggering innate immunity in Arabidopsis. J. Biol. Chem. 282: 32338–32348 [DOI] [PubMed] [Google Scholar]
- Gust A.A., Brunner F., Nürnberger T. (2010). Biotechnological concepts for improving plant innate immunity. Curr. Opin. Biotechnol. 21: 204–210 [DOI] [PubMed] [Google Scholar]
- Gust A.A., Willmann R., Desaki Y., Grabherr H.M., Nürnberger T. (2012). Plant LysM proteins: Modules mediating symbiosis and immunity. Trends Plant Sci. 17: 495–502 [DOI] [PubMed] [Google Scholar]
- Hammond-Kosack K.E., Parker J.E. (2003). Deciphering plant-pathogen communication: Fresh perspectives for molecular resistance breeding. Curr. Opin. Biotechnol. 14: 177–193 [DOI] [PubMed] [Google Scholar]
- Heese A., Hann D.R., Gimenez-Ibanez S., Jones A.M., He K., Li J., Schroeder J.I., Peck S.C., Rathjen J.P. (2007). The receptor-like kinase SERK3/BAK1 is a central regulator of innate immunity in plants. Proc. Natl. Acad. Sci. USA 104: 12217–12222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jehle A.K., Lipschis M., Albert M., Fallahzadeh-Mamaghani V., Fürst U., Mueller K., Felix G. (2013). The receptor-like protein ReMAX of Arabidopsis detects the microbe-associated molecular pattern eMax from Xanthomonas. Plant Cell 25: 2330–2340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong S., Trotochaud A.E., Clark S.E. (1999). The Arabidopsis CLAVATA2 gene encodes a receptor-like protein required for the stability of the CLAVATA1 receptor-like kinase. Plant Cell 11: 1925–1934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul S., et al. Arabidopsis Genome Initiative (2000). Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature 408: 796–815 [DOI] [PubMed] [Google Scholar]
- Kayes J.M., Clark S.E. (1998). CLAVATA2, a regulator of meristem and organ development in Arabidopsis. Development 125: 3843–3851 [DOI] [PubMed] [Google Scholar]
- Kemmerling B., et al. (2007). The BRI1-associated kinase 1, BAK1, has a brassinolide-independent role in plant cell-death control. Curr. Biol. 17: 1116–1122 [DOI] [PubMed] [Google Scholar]
- Koch E., Slusarenko A.J. (1990). Fungal pathogens of Arabidopsis thaliana (L) Heyhn. Bot. Helv. 100: 257–268 [Google Scholar]
- Krol E., Mentzel T., Chinchilla D., Boller T., Felix G., Kemmerling B., Postel S., Arents M., Jeworutzki E., Al-Rasheid K.A., Becker D., Hedrich R. (2010). Perception of the Arabidopsis danger signal peptide 1 involves the pattern recognition receptor AtPEPR1 and its close homologue AtPEPR2. J. Biol. Chem. 285: 13471–13479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunze G., Zipfel C., Robatzek S., Niehaus K., Boller T., Felix G. (2004). The N terminus of bacterial elongation factor Tu elicits innate immunity in Arabidopsis plants. Plant Cell 16: 3496–3507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacombe S., Rougon-Cardoso A., Sherwood E., Peeters N., Dahlbeck D., van Esse H.P., Smoker M., Rallapalli G., Thomma B.P., Staskawicz B., Jones J.D., Zipfel C. (2010). Interfamily transfer of a plant pattern-recognition receptor confers broad-spectrum bacterial resistance. Nat. Biotechnol. 28: 365–369 [DOI] [PubMed] [Google Scholar]
- Laluk K., Mengiste T. (2010). Necrotroph attacks on plants: Wanton destruction or covert extortion? Arabidopsis Book 8: e0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie M.E., Lewis M.W., Youn J.Y., Daniels M.J., Liljegren S.J. (2010). The EVERSHED receptor-like kinase modulates floral organ shedding in Arabidopsis. Development 137: 467–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Wen J., Lease K.A., Doke J.T., Tax F.E., Walker J.C. (2002). BAK1, an Arabidopsis LRR receptor-like protein kinase, interacts with BRI1 and modulates brassinosteroid signaling. Cell 110: 213–222 [DOI] [PubMed] [Google Scholar]
- Liberti D., Qutob D., Gijzen M., Dobinson K.F. (2008). Functional characterization of necrosis and ethylene-inducing like proteins (NLPs) from a necrotrophic fungus Sclerotinia sclerotiorum. Phytopathology 98: S90 [Google Scholar]
- Liebrand T.W., van den Berg G.C., Zhang Z., Smit P., Cordewener J.H., America A.H., Sklenar J., Jones A.M., Tameling W.I., Robatzek S., Thomma B.P., Joosten M.H. (2013). Receptor-like kinase SOBIR1/EVR interacts with receptor-like proteins in plant immunity against fungal infection. Proc. Natl. Acad. Sci. USA 110: 10010–10015 Erratum. Proc. Natl. Acad. Sci. USA 110: 13228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann H.C., Nahal H., Thakur S., Guttman D.S. (2012). Identification of innate immunity elicitors using molecular signatures of natural selection. Proc. Natl. Acad. Sci. USA 109: 4215–4220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miya A., Albert P., Shinya T., Desaki Y., Ichimura K., Shirasu K., Narusaka Y., Kawakami N., Kaku H., Shibuya N. (2007). CERK1, a LysM receptor kinase, is essential for chitin elicitor signaling in Arabidopsis. Proc. Natl. Acad. Sci. USA 104: 19613–19618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadeau J.A., Sack F.D. (2002). Control of stomatal distribution on the Arabidopsis leaf surface. Science 296: 1697–1700 [DOI] [PubMed] [Google Scholar]
- Nam K.H., Li J. (2002). BRI1/BAK1, a receptor kinase pair mediating brassinosteroid signaling. Cell 110: 203–212 [DOI] [PubMed] [Google Scholar]
- Navarro L., Zipfel C., Rowland O., Keller I., Robatzek S., Boller T., Jones J.D. (2004). The transcriptional innate immune response to flg22. Interplay and overlap with Avr gene-dependent defense responses and bacterial pathogenesis. Plant Physiol. 135: 1113–1128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nekrasov V., Ludwig A.A., Jones J.D. (2006). CITRX thioredoxin is a putative adaptor protein connecting Cf-9 and the ACIK1 protein kinase during the Cf-9/Avr9- induced defence response. FEBS Lett. 580: 4236–4241 [DOI] [PubMed] [Google Scholar]
- Nembaware V., Seoighe C., Sayed M., Gehring C. (2004). A plant natriuretic peptide-like gene in the bacterial pathogen Xanthomonas axonopodis may induce hyper-hydration in the plant host: a hypothesis of molecular mimicry. BMC Evol. Biol. 4: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordborg M., et al. (2005). The pattern of polymorphism in Arabidopsis thaliana. PLoS Biol. 3: e196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perchepied L., Balagué C., Riou C., Claudel-Renard C., Rivière N., Grezes-Besset B., Roby D. (2010). Nitric oxide participates in the complex interplay of defense-related signaling pathways controlling disease resistance to Sclerotinia sclerotiorum in Arabidopsis thaliana. Mol. Plant Microbe Interact. 23: 846–860 [DOI] [PubMed] [Google Scholar]
- Postel S., Küfner I., Beuter C., Mazzotta S., Schwedt A., Borlotti A., Halter T., Kemmerling B., Nürnberger T. (2010). The multifunctional leucine-rich repeat receptor kinase BAK1 is implicated in Arabidopsis development and immunity. Eur. J. Cell Biol. 89: 169–174 [DOI] [PubMed] [Google Scholar]
- Ramonell K., Berrocal-Lobo M., Koh S., Wan J., Edwards H., Stacey G., Somerville S. (2005). Loss-of-function mutations in chitin responsive genes show increased susceptibility to the powdery mildew pathogen Erysiphe cichoracearum. Plant Physiol. 138: 1027–1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivas S., Rougon-Cardoso A., Smoker M., Schauser L., Yoshioka H., Jones J.D. (2004). CITRX thioredoxin interacts with the tomato Cf-9 resistance protein and negatively regulates defence. EMBO J. 23: 2156–2165 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Ron M., Avni A. (2004). The receptor for the fungal elicitor ethylene-inducing xylanase is a member of a resistance-like gene family in tomato. Plant Cell 16: 1604–1615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux M., Schwessinger B., Albrecht C., Chinchilla D., Jones A., Holton N., Malinovsky F.G., Tör M., de Vries S., Zipfel C. (2011). The Arabidopsis leucine-rich repeat receptor-like kinases BAK1/SERK3 and BKK1/SERK4 are required for innate immunity to hemibiotrophic and biotrophic pathogens. Plant Cell 23: 2440–2455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland O., Ludwig A.A., Merrick C.J., Baillieul F., Tracy F.E., Durrant W.E., Fritz-Laylin L., Nekrasov V., Sjölander K., Yoshioka H., Jones J.D.G. (2005). Functional analysis of Avr9/Cf-9 rapidly elicited genes identifies a protein kinase, ACIK1, that is essential for full Cf-9-dependent disease resistance in tomato. Plant Cell 17: 295–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwessinger B., Roux M., Kadota Y., Ntoukakis V., Sklenar J., Jones A., Zipfel C. (2011). Phosphorylation-dependent differential regulation of plant growth, cell death, and innate immunity by the regulatory receptor-like kinase BAK1. PLoS Genet. 7: e1002046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro A.D., Zhang C. (2001). The role of NDR1 in avirulence gene-directed signaling and control of programmed cell death in Arabidopsis. Plant Physiol. 127: 1089–1101 [PMC free article] [PubMed] [Google Scholar]
- Torres M.A., Jones J.D., Dangl J.L. (2005). Pathogen-induced, NADPH oxidase-derived reactive oxygen intermediates suppress spread of cell death in Arabidopsis thaliana. Nat. Genet. 37: 1130–1134 [DOI] [PubMed] [Google Scholar]
- Wan J., Zhang X.C., Neece D., Ramonell K.M., Clough S., Kim S.Y., Stacey M.G., Stacey G. (2008). A LysM receptor-like kinase plays a critical role in chitin signaling and fungal resistance in Arabidopsis. Plant Cell 20: 471–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G., et al. (2008). A genome-wide functional investigation into the roles of receptor-like proteins in Arabidopsis. Plant Physiol. 147: 503–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M., Sack F.D. (1995). The too many mouths and four lips mutations affect stomatal production in Arabidopsis. Plant Cell 7: 2227–2239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Deng F., Ramonell K.M. (2012). Receptor-like kinases and receptor-like proteins: Keys to pathogen recognition and defense signaling in plant innate immunity. Front. Biol. 7: 155–166 [Google Scholar]
- Zhang Y., Yang Y., Fang B., Gannon P., Ding P., Li X., Zhang Y. (2010). Arabidopsis snc2-1D activates receptor-like protein-mediated immunity transduced through WRKY70. Plant Cell 22: 3153–3163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipfel C., Kunze G., Chinchilla D., Caniard A., Jones J.D., Boller T., Felix G. (2006). Perception of the bacterial PAMP EF-Tu by the receptor EFR restricts Agrobacterium-mediated transformation. Cell 125: 749–760 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.