This study reveals that the moss Physcomitrella patens has a cuticle that is compositionally and structurally similar to those of later diverging land plants. A genetic knockout of a moss putative ABCG transporter, ABCG7, is deficient in cuticular wax accumulation and susceptible to desiccation stress. The cuticle appears to be a highly conserved evolutionary adaptation to life on land.
Abstract
The plant cuticle is thought to be a critical evolutionary adaptation that allowed the first plants to colonize land, because of its key roles in regulating plant water status and providing protection from biotic and abiotic stresses. Much has been learned about cuticle composition and structure through genetic and biochemical studies of angiosperms, as well as underlying genetic pathways, but little is known about the cuticles of early diverging plant lineages. Here, we demonstrate that the moss Physcomitrella patens, an extant relative of the earliest terrestrial plants, has a cuticle that is analogous in both structure and chemical composition to those of angiosperms. To test whether the underlying cuticle biosynthetic pathways were also shared among distant plant lineages, we generated a genetic knockout of the moss ATP binding cassette subfamily G (ABCG) transporter Pp-ABCG7, a putative ortholog of Arabidopsis thaliana ABCG transporters involved in cuticle precursor trafficking. We show that this mutant is severely deficient in cuticular wax accumulation and has a reduced tolerance of desiccation stress compared with the wild type. This work provides evidence that the cuticle was an adaptive feature present in the first terrestrial plants and that the genes involved in their formation have been functionally conserved for over 450 million years.
INTRODUCTION
The primary interface between a land plant and its aerial environment is provided by the cuticle, a specialized cell wall component produced by epidermal cells that consists of a matrix of the polyester cutin, coated and infiltrated with waxes. Cutin is comprised mainly of hydroxy fatty acids, diacids, and glycerol, whereas waxes can include very-long-chain fatty acids (VLCFAs), primary and secondary fatty alcohols, fatty ketones and aldehydes, alkanes, and wax esters, as well as other nonaliphatic components, such as triterpenoids and flavonoids (Samuels et al., 2008; Javelle et al., 2011). Cutin and waxes are integrated into the outer portion of the polysaccharide cell wall, forming a lipid-impregnated zone known as the cuticular layer (CL), and are also layered on top of the CL to create a hydrophobic surface devoid of polysaccharides called the cuticle proper (CP; Jeffree, 2006).
The cuticle has many functions, perhaps the most critical of which is limiting plant desiccation and regulating water exchange with the surrounding environment (Javelle et al., 2011; Yeats and Rose, 2013). In addition, it serves to separate adjacent organs during their development, acts as a defensive barrier against pests and pathogens (Barthlott and Neinhuis, 1997; Reina-Pinto and Yephremov, 2009), and provides protection from potentially damaging UV radiation (Shepherd and Wynne Griffiths, 2006).
Collectively, these protective roles have led to the suggestion that the development of a cuticle was a key evolutionary adaptation that allowed the first land plants to colonize terrestrial habitats, ∼450 million years ago (Wood, 2005; Rensing et al., 2008). The transition to life on land would have necessitated the ability to tolerate a range of abiotic and biotic stresses that would not have been present in exclusively aquatic environments, and the development of a cuticle was likely instrumental in this regard. Numerous reports have described a variety of wax and cutin compositions and cuticle architectural designs across diverse plant taxa (Holloway, 1982; Jetter et al., 2006), and, more recently, many of the genes involved in cuticle precursor biosynthesis, export and assembly have been identified (Javelle et al., 2011; Beisson et al., 2012; Yeats et al., 2012; Yeats and Rose, 2013). However, detailed studies of cuticle biology linking the underlying molecular genetic pathways with composition, structure, and physiological function have mostly focused on experimental model flowering plants, such as Arabidopsis thaliana (Javelle et al., 2011), maize (Zea mays; Javelle et al., 2011; Post-Beittenmiller, 1998), and tomato (Solanum lycopersicum; Leide et al., 2007; Isaacson et al., 2009; Matas et al., 2011). Consequently, there are many unanswered questions that are key to understanding the similarities and differences between cuticles of diverse and evolutionarily distant plant lineages. For example, is there a common ancestral form of the cuticle in earlier diverging taxa and, if so, are the molecular pathways underlying its biosynthesis and function similar to those of flowering plants? These questions are important for understanding the evolutionary origins of plant cuticles and represent the major focus of this current study.
The bryophytes, comprising the mosses, liverworts, and hornworts, are the extant relatives of the earliest diverging land plants (Rensing et al., 2008). They typically colonize moist habitats and collectively exhibit a range of adaptive strategies to resist or tolerate desiccating conditions, including an abscisic acid–responsive pathway to counter dehydration, accumulation of compatible solutes to retain water, production of rehydrins to prevent cellular damage following rehydration of dry tissues (Charron and Quatrano, 2009), and late embryogenesis abundant protein synthesis (Minami et al., 2005). In addition, there are many reports of cuticles both in early land plants from the fossil record (Jeffree, 2006) and in extant bryophytes (Schönherr and Ziegler, 1975; Caldicott and Eglinton, 1976; Proctor, 1979; Haas, 1982; Sack and Paolillo, 1983; Clayton-Greene et al., 1985; Nissinen and Sewón, 1994; Neinhuis and Jetter, 1995; Budke et al., 2011), though studies of the latter have highlighted chemical composition or architecture, but rarely both.
The moss Physcomitrella patens has emerged as a valuable model system to address questions related to bryophyte biology, plant development, and evolution. In addition, P. patens has been used extensively for bryophyte genetic studies due to its amenability to genetic modification via homologous recombination (Hohe et al., 2004; Cove et al., 2009). Moreover, following the publication of its genome sequence and the creation of associated functional genomics tools (Rensing et al., 2008), its utility has grown rapidly as a system in which to investigate the evolution of plant gene pathways. For these reasons, this moss represents a potentially excellent system in which to conduct an investigation of the structure, biosynthesis, and function of a putative bryophyte cuticle or equivalent structure. Wyatt et al. (2008) provided preliminary evidence that P. patens has a hydrophobic surface layer, but no additional characterization has been described to date. We report here that P. patens indeed has a cuticle that is similar in both composition and structure to those of flowering plants and that it shares at least one important cuticle biosynthetic molecular pathway with later diverging plant lineages. In addition, we address the hypothesis that such pathways were likely critical for the early colonization of land through studies of the growth and development of a transgenic P. patens line with altered cuticle composition, with particular focus on the effects of exposure to desiccating conditions.
RESULTS
The Phyllids of P. patens Are Covered by a Thin and Structurally Simple Cuticle
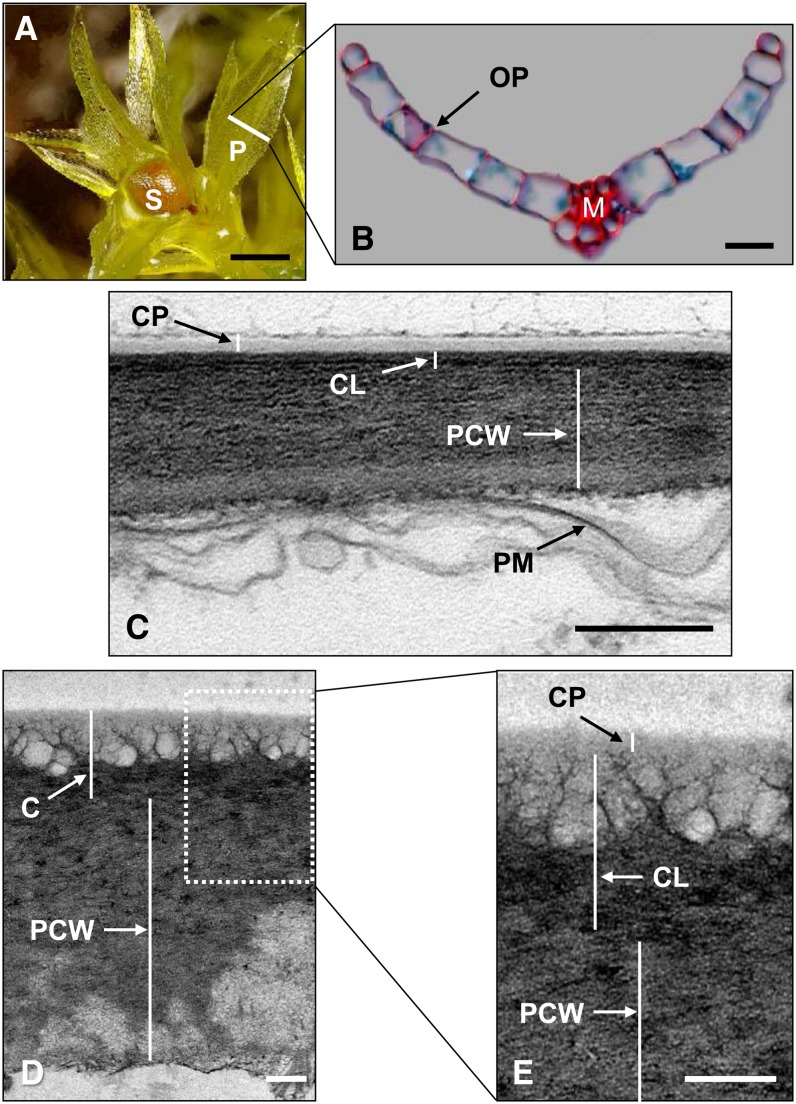
The dominant phase of the moss life cycle is haploid, unlike flowering plants, and development begins with the germination of a single haploid spore into a network of filamentous protonema (Cove et al., 2006). Protonemal cells can subsequently differentiate to form buds that develop into leafy gametophores. The gametophores of P. patens are comprised of leaf-like phyllids, a single cell layer thick, emanating from a central axis (Figures 1A and 1B). After a fertilization event, a sporophyte develops at the apex of a female gametophore shoot, anchored within the gametophyte tissue (Figure 1A). To study the cuticle architecture of P. patens, both gametophores and sporophytes were prepared for transmission electron microscopy (TEM) using osmium tetraoxide postfixation to generate image contrast and highlight the lipid components of the cells. A thin cuticle (∼50 nm) was observed on both abaxial and adaxial surfaces of the phyllids (Figure 1C) that exhibited similar structural features to those recently described in the moss Funaria hygrometrica (Budke et al., 2011). This cuticle can be classified as structural type six, defined by an amorphous CP consisting of an outer osmiophilic layer and an inner electron-lucent layer (Holloway, 1982). The CL is slightly more osmiophilic than the underlying polysaccharide cell wall, with which it is intercalated at the interface (Figure 1C). No apparent structural differences were observed between the abaxial and adaxial phyllid cuticles. Particular attention was paid in preparing the sample to prevent the detachment of the outermost osmiophilic layer of the CP, and only regions of the cell wall where this layer was still attached were selected for imaging. The cuticle of the sporophyte capsule was more substantial (∼400 nm), with a highly expanded reticulate region underlying the amorphous and electron-lucent CP (Figures 1D and 1E). The outer periclinal cell walls of the capsule epidermal layer were also much thicker than those of the phyllid epidermis (Figure 1D).
Figure 1.
P. patens Morphology and Cuticle Structure.
(A) P. patens gametophore with several phyllids (P) and an immature sporophyte (S) growing from the apex.
(B) P. patens phyllid shown in transverse section, corresponding to the white line in (A), consisting of a single-cell-layer-thick lamina attached to a central midrib (M). Cell walls are stained red and intracellular contents blue. OP, outer periclinal wall.
(C) Transmission electron micrograph of the phyllid outer periclinal cell wall, showing the layers of the cuticle (CL), polysaccharide cell wall (PCW), and plasma membrane (PM).
(D) Transmission electron micrograph of the sporophyte capsule outer periclinal cell wall (cuticle [C]).
(E) Expanded view of the box in (D).
Bars = 750 μm in (A), 20 μm in (B), and 200 nm in (C) to (E).
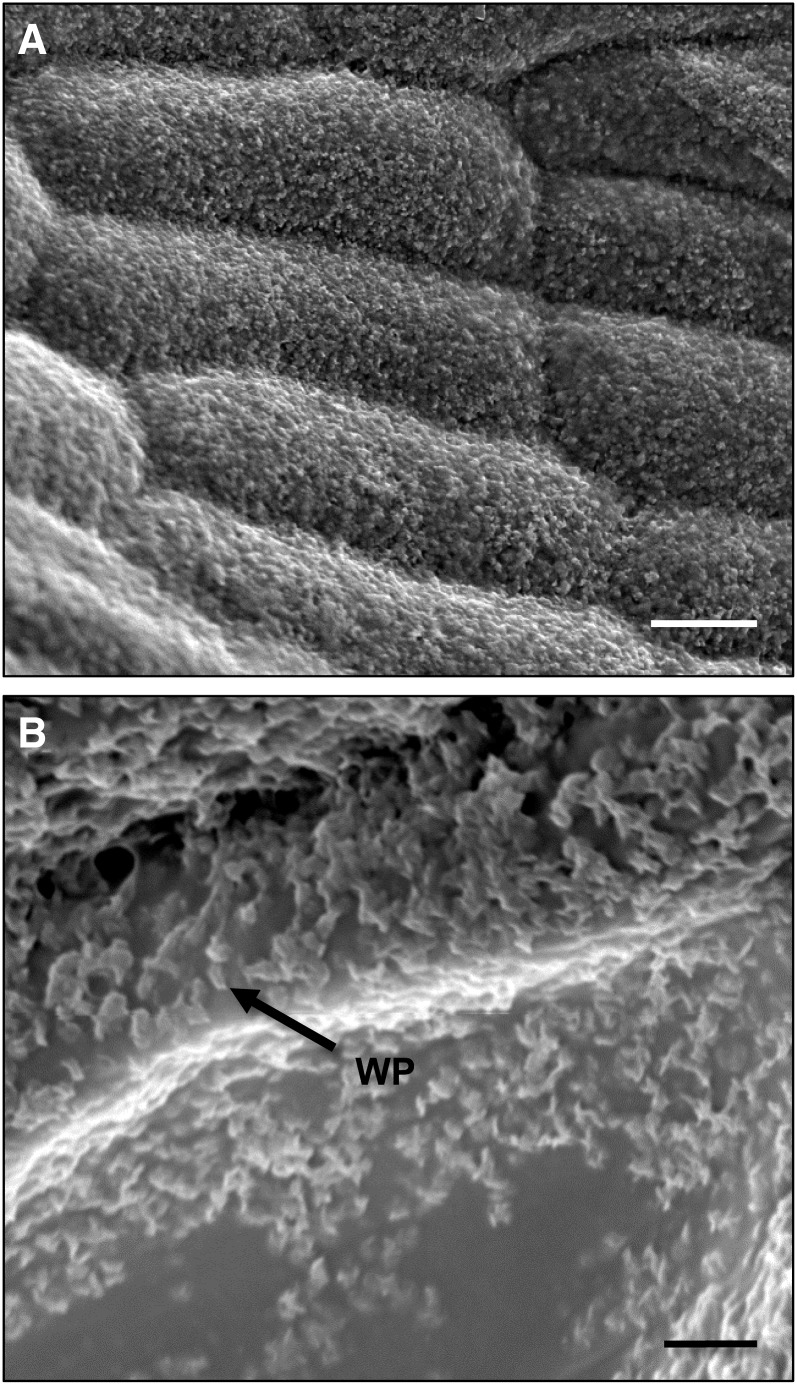
After analyzing the lamellar structure of the cuticle in transverse sections, phyllids were prepared for scanning electron microscopy to determine whether epicuticular wax crystals decorated the surface of the CP, as can often been seen in flowering plants. The gametophores were air dried for several hours prior to sputter coating as this decreased the number of cells that ruptured when the vacuum was applied. Areas with dense, irregularly shaped wax platelets were observed on many phyllids (Figures 2A and 2B), but the presence and density of wax crystals were highly variable between gametophores and phyllids, and even within regions of a single phyllid. Most phyllid epidermal cells exhibited a smooth surface, suggesting an amorphous epicuticular wax film, but when observed, the wax crystals were predominantly in areas over the anticlinal cell walls, as opposed to the center of the cell. Developmental differences in wax accumulation were also noted as wax crystals were more prevalent in older phyllids taken from the base of the gametophores and were not observed on younger phyllids close to the shoot apex.
Figure 2.
P. patens Epicuticular Wax.
(A) Scanning electron micrograph of phyllid epidermal cells densely covered in wax platelets.
(B) Higher magnification scanning electron micrograph showing morphology of wax platelets (WP; arrow).
Bars = 10 μm in (A) and 2 μm in (B).
P. patens Cutin Analysis
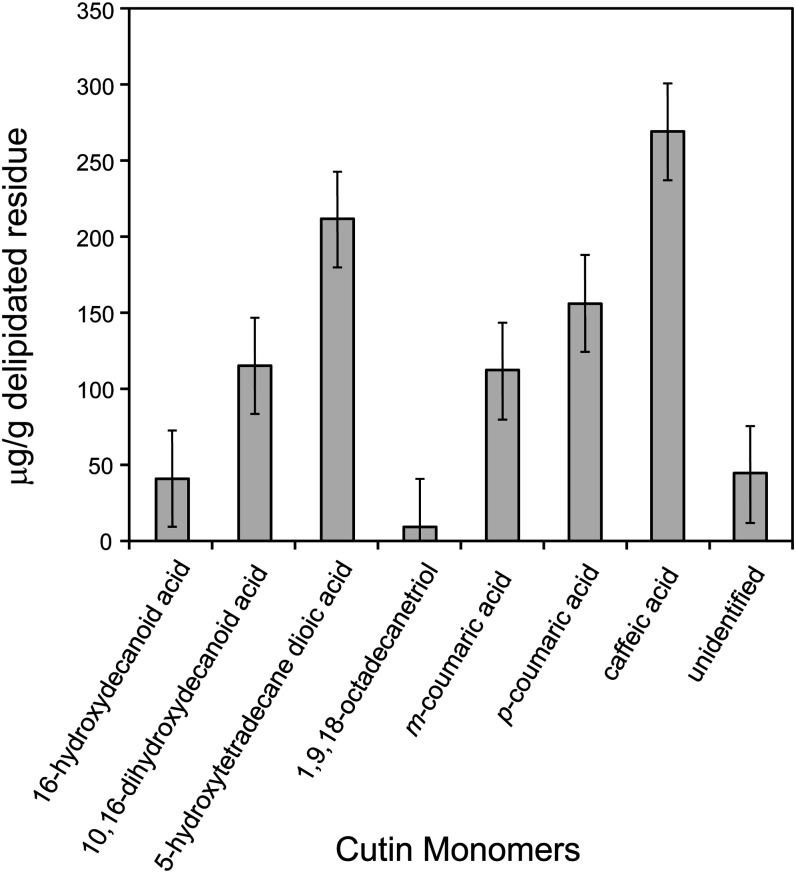
Protocols used to evaluate the cuticle composition of Arabidopsis (Li-Beisson et al., 2010) were adapted to investigate the chemical constituents of the P. patens cuticle. Ground moss colonies were extensively delipidated with a series of organic solvents, and the remaining plant residue was depolymerized by methanolysis to release methyl esters of any cutin monomers prior to analysis using gas chromatography–mass spectrometry (GC-MS). Cutin monomer identity was assigned based on reference to known mass pectra and retention index (Figure 3). The most abundant aliphatic monomer was 5-hydroxytetradecane dioic acid, and substantial amounts of 10,16-dihydroxyhexadecanoic acid were detected, together with lesser amounts of 16-hydroxyhexadecanoic acid. No substituted C18 fatty acids or diacids were detected, although a C18 triol was identified in minor quantities. Large amounts of phenolic cutin monomers, specifically m- and p-coumaric acid and caffeic acid, were also identified, although no ferulic acid appeared to be present. Several peaks corresponded to ions resulting from presumed α-cleavage of cutin monomers containing midchain hydroxyl groups. However, the structures from which these were derived could not be conclusively identified, and they were therefore designated as unidentified. Methyl esters of fatty acids were not considered further as they were likely derived from wall-bound lipids, a term that is commonly used to refer to noncuticular lipids that remain in cell wall extracts following cutin depolymerization treatments. Because of the complexity of the sample, we cannot exclude the possibility that other monomers comprising a small percentage of the cutin matrix were present but were not identified or detected due to their low abundance and/or unfamiliar fragmentation patterns.
Figure 3.
P. patens Cutin Monomer Composition and Amount, as Determined by GC-MS.
Error bars indicate se; n = 4.
P. patens Cuticular Wax Analysis
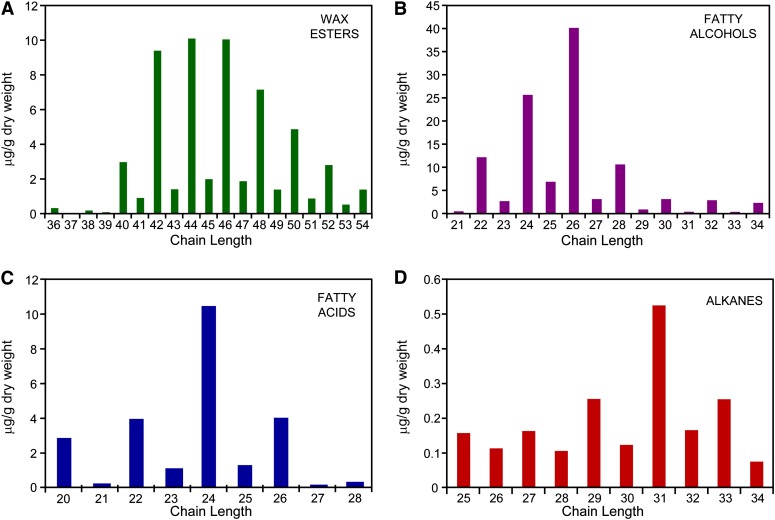
The composition of waxes was determined by chloroform extraction using a single large biological pool containing many thousands of individual moss plants, since the less abundant waxes were consistently below the detection limit in smaller sample sizes. This ensured that the extraction was consistent across all plants but meant that any minor compositional differences between moss colonies or gametophores were not considered. A prefractionation step using thin-layer chromatography (TLC) was included in the analysis to improve the identification and quantification of wax compounds and to reduce deterioration of peak quality during the subsequent GC-MS analysis that occurred when attempting to analyze derivatized crude wax extracts. The major compound classes present were primary fatty alcohols and long-chain wax esters, with minor amounts of VLCFAs and alkanes (Figures 4A to 4D), while secondary fatty alcohols, ketones, or fatty aldehydes were not detected. The additional TLC bands most likely resulted from intracellular lipid contamination and corresponded to triacyl glycerols and steryl esters, which are present in large amounts in gametophyte tissues (Huang et al., 2009).
Figure 4.
P. patens Wax Composition and Amount, as Determined by GC-MS.
Homolog series for wax esters (A), fatty alcohols (B), fatty acids (C), and alkanes (D).
The distribution of carbon chain length within each wax compound class followed the expected pattern with long-chain wax esters, primary fatty alcohols, and VLCFAs exhibiting a strong bias toward chain lengths with an even number of carbons (Figures 4A to 4C). Conversely, the alkanes, arising from decarbonylation of VLCFAs during their biosynthesis, exhibited an odd chain length bias (Figure 4D). Alkanes shorter than C25 were not considered in the survey as they did not exhibit the odd chain length preference characteristic of cuticular alkanes and likely corresponded to contaminants introduced during prefractionation from sources such as plasticware.
Functional Analysis of ABCG7, a Putative Moss Wax Transporter Gene
After determining that P. patens has a cuticle that is both structurally and compositionally similar to those of flowering plants, we addressed the hypothesis that the basic underlying molecular genetic pathways involved in cuticle formation have also been closely conserved throughout plant evolution. Since Arabidopsis has the most comprehensive set of characterized cuticle-associated genes, we used an extensive selection of these to search the P. patens genome sequence for putative orthologs. Many candidate genes were identified with a high degree of predicted protein sequence similarity to those known in Arabidopsis to be involved in various aspects of wax and cutin biosynthesis and cuticle formation.
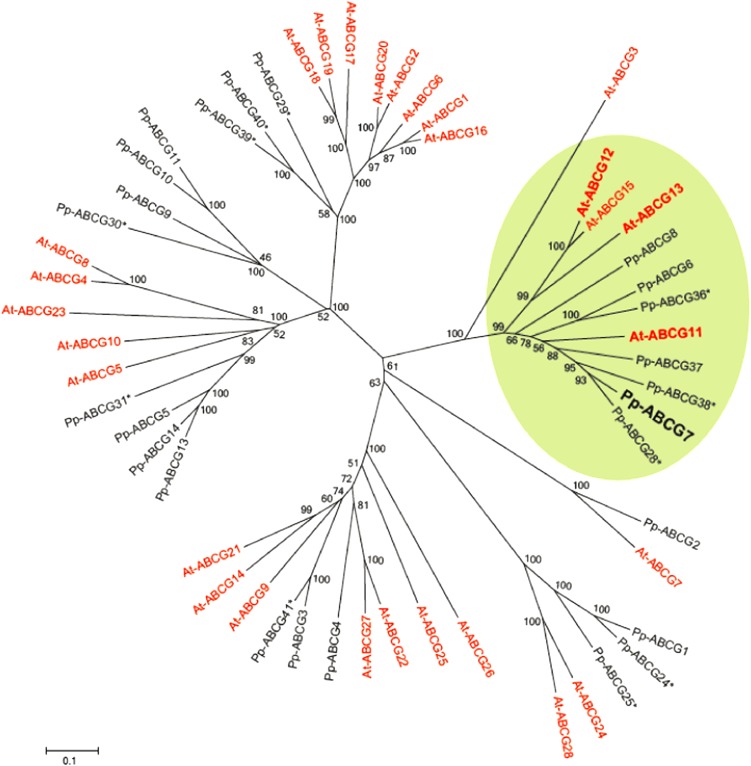
One of these, ABCG7 (Rensing et al., 2008), is predicted to be a 10-exon gene, spanning 3544 bp, and encoding an ABC half transporter in the G subfamily, with 63% amino acid identity and 78% similarity with the Arabidopsis protein At-ABCG11 (also named White Brown Complex11 [WBC11]) and slightly lower sequence homology to At-ABCG12 (also named WBC12 and Eceriferum 5; 52% identity and 72% similarity). A phylogenetic analysis of all predicted ATP binding cassette subfamily G (ABCG) half transporters from Arabidopsis and P. patens revealed that Pp-ABCG7 falls into a well-supported clade containing all of the Arabidopsis ABCG half transporters that have previously been shown to be involved in cuticle formation (Figure 5; see Supplemental Data Set 1 online). Of these, At-ABCG11 and At-ABCG12 are two well characterized members that are required for the trafficking of wax and cutin precursors through the plasma membrane and whose loss-of-function mutants show severe abnormal cuticle phenotypes (Pighin et al., 2004; Bird et al., 2007; Luo et al., 2007).
Figure 5.
Unrooted Neighbor Joining Protein Phylogeny of Predicted ABCG Half Transporters from Arabidopsis and P. patens.
Arabidopsis subfamily members are shown in red and P. patens subfamily members in black. Those marked by an asterisk are not supported by EST evidence. The clade highlighted by a green background contains all of the Arabidopsis ABCG half transporters known to be involved in cuticle precursor export (bold), as well as the putative moss wax transporter Pp-ABCG7. Bootstrap values (1000 replicates) are given for each node. Bar indicates 0.1 amino acid substitutions per site.
The subcellular localization of Pp-ABCG7 was evaluated by transient coexpression in onion (Allium cepa) epidermal cells of full-length Pp-ABCG7 fused to red fluorescent protein (RFP) at the C terminus, together with the plasma membrane resident Arabidopsis aquaporin At-PIP2a fused to green fluorescent protein (GFP). Confocal imaging revealed that PpABCG7-RFP and AtPIP2a-GFP showed overlapping expression in plasmolyzed transformed onion cells (see Supplemental Figure 1 online), indicating that Pp-ABCG7 localizes to the plasma membrane, consistent with its putative function as an ABCG half transporter.
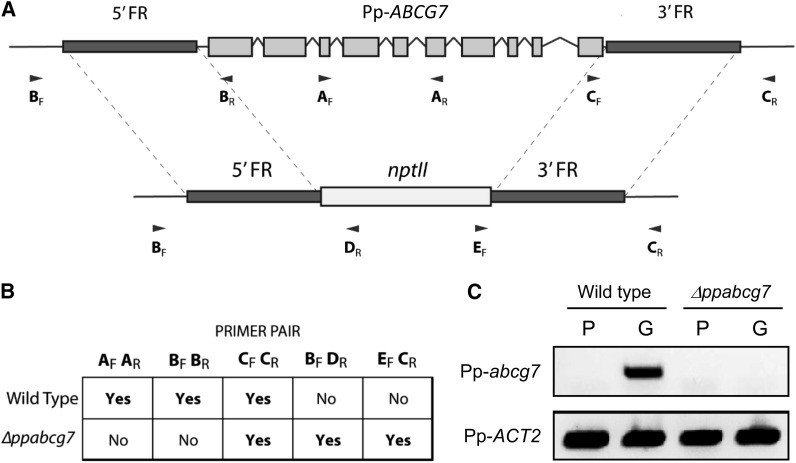
To establish whether Pp-ABCG7 has similar functions in trafficking cuticle components, a targeted ABCG7 knockout mutant was generated. Approximately 1.1 kb of flanking sequence both upstream and downstream of the gene was cloned into the pTN80 vector on either side of the nptII G418 resistance marker (Figure 6A). These 5′ and 3′ flanking regions served to target the knockout cassette to the Pp-ABCG7 gene and allowed replacement with the selectable marker. Wild-type moss protoplasts were transformed as previously described (Cove et al., 2009; Saavedra et al., 2011) via polyethylene glycol–mediated transformation. Transformed protoplasts were regenerated and the resulting moss colonies were subjected to two rounds of selection with the antibiotic G418 to yield a collection of potential stable knockout lines. Each of these lines was screened using appropriate PCR primers for the absence of Pp-ABCG7, presence of the nptII selectable marker, and for correct targeting of the selectable marker within the genome (Figures 6A and 6B; see Supplemental Table 1 online). One line was identified as being a correctly targeted, stable knockout of Pp-ABCG7 and was designated Δppabcg7. To verify that Pp-ABCG7 expression was completely abolished in Δppabcg7, mRNA was extracted from both wild-type and Δppabcg7 protonema and gametophores and used to generate cDNA libraries, which were then screened using PCR primers designed to amplify a 221-bp fragment in the 3′ untranslated region of Pp-ABCG7 (Figure 6C). The ABCG7 transcript was detected in the wild-type leafy gametophore tissue but not in the protonemal tissue, which is consistent with the expected expression pattern for a gene involved in cuticle formation, since the P. patens protonema lacks a cuticle (Wyatt et al., 2008). However, no expression was detected in either tissue from Δppabcg7, confirming the absence of ABCG7 expression in this line.
Figure 6.
Generation of the Δppabcg7 Knockout Mutant.
(A) Schematic diagram depicting the targeted replacement of Pp-ABCG7 with the nptII selectable marker by homologous recombination to yield a stable P. patens knockout mutant. (FR, flanking region).
(B) PCR screening of wild-type and Δppabcg7 genomic DNA with the PCR primer pairs shown in (A) showing the presence (Yes) or absence (No) of an amplified product. Primer pairs specific to Pp-ABCG7 generate a PCR product in the wild type but not in Δppabcg7.
(C) PCR screening of wild-type and Δppabcg7 cDNA with Pp-ABCG7 transcript-specific primers, showing that Pp-ABCG7 expression is abolished in Δppabcg7 (P, protonema; G, gametophore). Primers specific to P. patens actin (Pp-ACT2) were used as a control for cDNA quality.
Δppabcg7 Exhibits Stunted Growth and Altered Spore Wall Architecture
Development of protonemal colonies and initiation of gametophore shoots were similar in Δppabcg7 and the wild type (Figure 7A); however, Δppabcg7 gametophores were ∼50 to 75% smaller than the wild type, exhibiting a reduction in both gametophore height and phyllid size (Figures 7A and 7B). In addition, Δppabcg7 colonies had noticeably more rhizoids than wild-type plants (Figure 7B), and sporophytes showing stunted, shriveled, and abnormal development (see Supplemental Figure 2 online). The spores of the wild type and Δppabcg7 also showed distinct differences in their surface decorations: The protrusions of the wild-type spore wall were elongated, evenly spaced, and pointed (Figures 8A and 8B), while those of the Δppabcg7 spore wall were smaller, nonuniform in size, rounded, and more densely packed over the entire surface (Figure 8C). However, despite these structural abnormalities, Δppabcg7 spores were viable.
Figure 7.
Stunted Growth Phenotype of Δppabcg7.
(A) Development of wild-type and Δppabcg7 colonies over a 21-d time period showing stunted growth of the Δppabcg7 gametophores.
(B) Close-up view of wild-type and Δppabcg7 colonies, showing stunted growth of gametophores in the mutant line.
Bars = 5 mm in (A) and 2 mm in (B).
Figure 8.
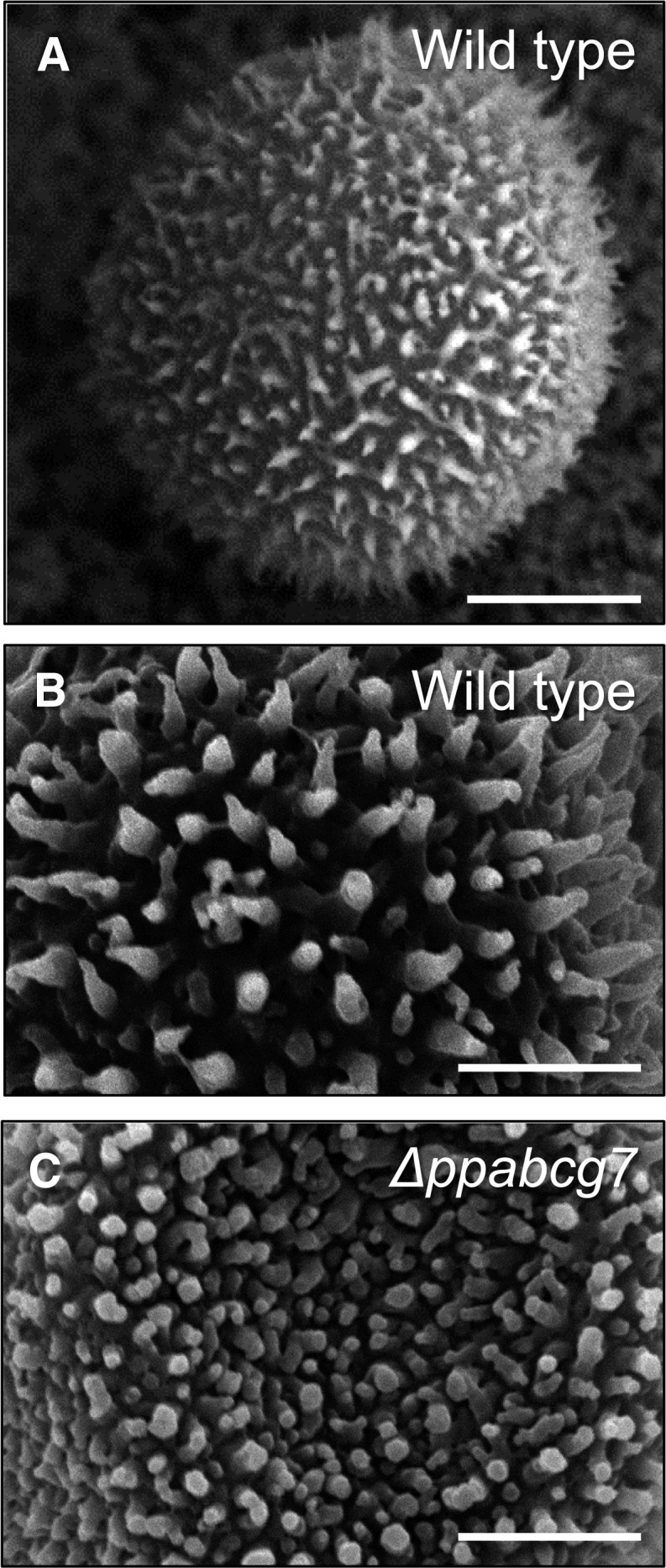
P. patens Spore Phenotypic Analysis.
Scanning electron micrographs of spore surface decorations in the wild type ([A] and [B]) and Δppabcg7 (C), showing irregular and rounded protrusions in the mutant. Bars = 10 μm in (A) and 5 μm in (B) and (C).
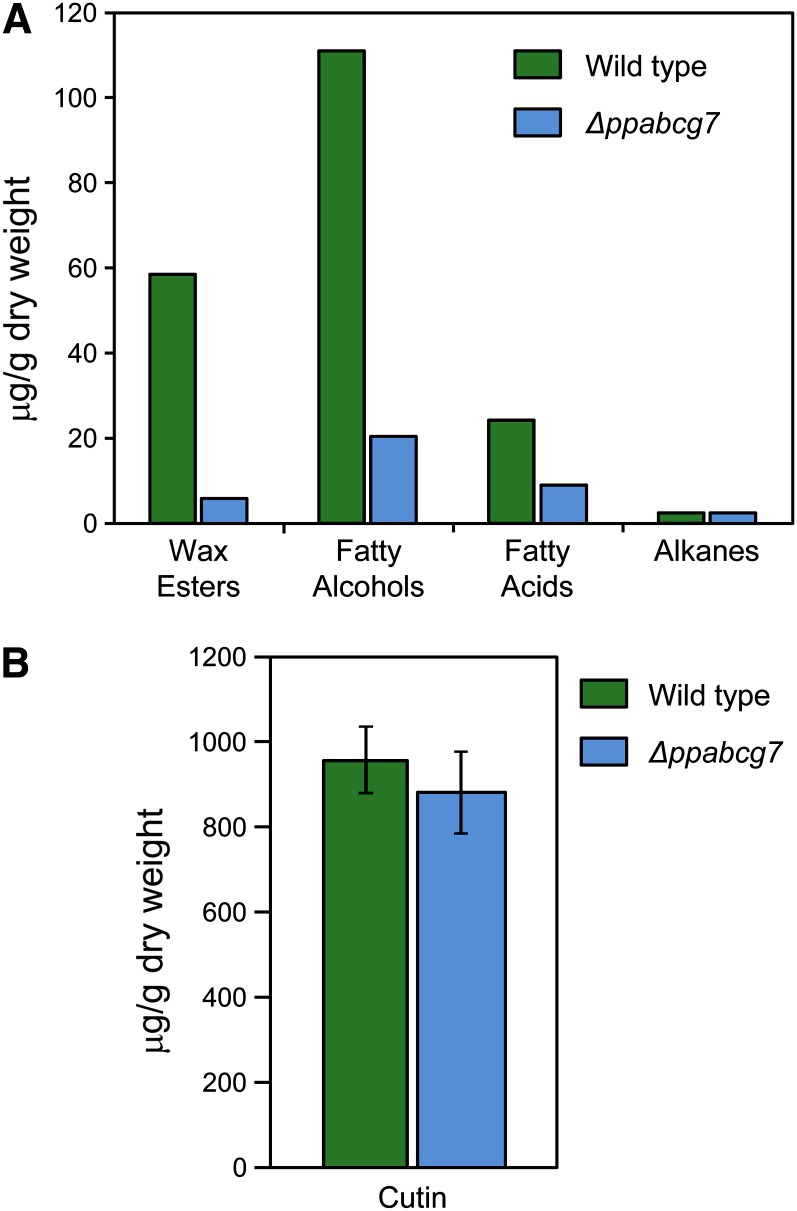
Δppabcg7 Has a Substantial Reduction in the Levels of Cuticular Wax but Not of Cutin
To determine whether cuticle biosynthesis in Δppabcg7 is perturbed, we evaluated wax and cutin content. Waxes were extracted from large biological pools of either wild-type or Δppabcg7 colonies. The latter showed a dramatic reduction in wax load for all compound classes of surface waxes except alkanes, which were only slightly reduced relative to the wild type (Figure 9A). By contrast, total cutin load was not significantly different compared with the wild type (Figure 9B), and the slight reduction observed can be attributed to lower levels of m-coumaric acid and caffeic acid in Δppabcg7. We note that replicate measurements of surface wax load reduction were not feasible in this study due to the extremely large amounts of moss material needed. However, the lack of a statistically significant difference in levels of cutin extracted from the same pooled biological samples, for which replicate analyses were performed (Figure 9B), indicates that the differences in waxes were specific and not due to a general reduction in cuticle material or some other artifact induced during sample preparation.
Figure 9.
Cuticle Composition Analysis.
(A) Cuticular wax levels in wild-type and Δppabcg7 moss.
(B) Cutin monomer levels in the wild type and Δppabcg7. Error bars indicate se; n = 4.
We prepared samples of Δppabcg7 gametophores to examine cuticle structure using TEM and scanning electron microscopy. No noticeable phenotypic differences were observed in the Δppabcg7 cuticle, either in the phyllid or in the sporophyte. Scanning electron microscopy analysis suggested a decrease in the abundance of epicuticular wax crystals on the phyllids, but because their presence was so variable in wild-type phyllids, it was not possible to conclude that there was a reduction in Δppabcg7. However, while areas containing small crystals were observed in Δppabcg7, regions with wax crystals as large or populous as those seen in the wild type (Figures 2A and 2B) were not apparent.
Pp-ABCG7 Does Not Functionally Complement a Mutation in the Homologous Arabidopsis Gene ABCG12
The substantial reduction in wax levels in Δppabcg7 is reminiscent of a similarly large reduction in waxes reported in the Arabidopsis abcg12 (wbc12) mutant (Pighin et al., 2004), which has perturbed expression of ABCG12, a closely related homolog of Pp-ABCG7 (Figure 5). To investigate whether Pp-ABCG7 is a functional ortholog of At-ABCG12, as the mutant phenotype suggests, transformants of the At-abcg12 mutant expressing Pp-ABCG7 driven by the constitutive 35S promoter were generated (see Supplemental Figure 3A online) and the cuticles of the transgenic plants evaluated to determine whether the mutant had been rescued. In wild-type Arabidopsis, the 29C alkane accounts for over 50% of the wax load in mature stems (Li-Beisson et al., 2010). This was used as a diagnostic compound to determine whether the complementation was successful. Wax was extracted from the inflorescence stems of T2 plants, some of which carried the transgene and some of which did not, as well as from wild-type Columbia-0 plants and the amount of 29C alkane in each line was determined (see Supplemental Figure 3B online)). The amount of 29C alkane in At-abcg12 mutant plants was substantially reduced relative to the wild type but was equally low in the Pro35S:PpABCG7 lines, indicating that complementation of the mutation did not occur.
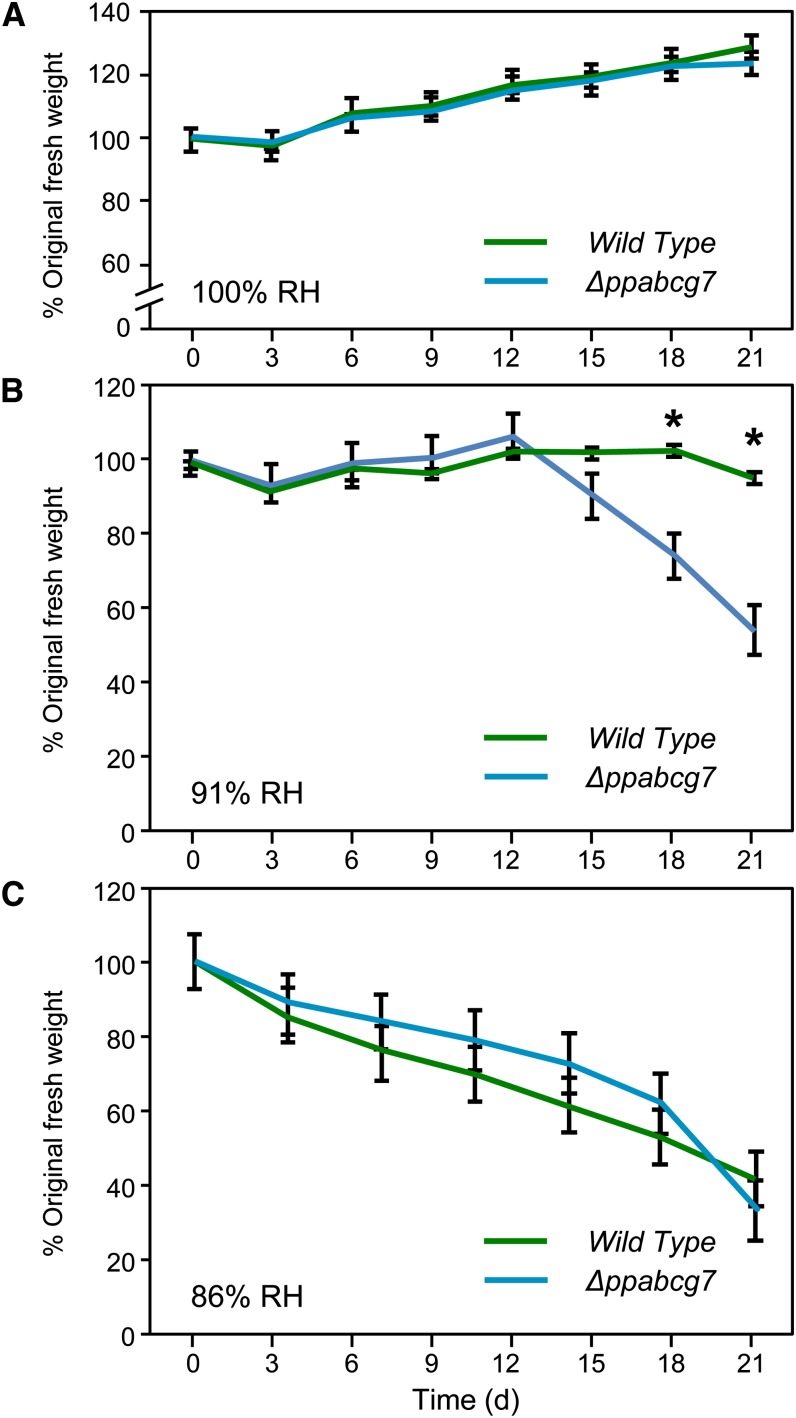
Δppabcg7 Has a Reduced Tolerance of Desiccation Stress
Given the substantially reduced wax load in Δppabcg7, we hypothesized that this might result in reduced vitality under desiccating conditions. Six-week-old colonies of both the wild type and Δppabcg7 were exposed to atmospheres of different RH and their growth monitored by measuring the fresh weight of the moss on each plate every 3 d over a 21-d period. At 100% RH, both the wild type and Δppabcg7 grew at the same rate (Figure 10A), while at 91% RH, wild-type colonies maintained their original fresh weight for the duration of the experiment but showed no significant growth. However, Δppabcg7 colonies exposed to 91% RH maintained their original fresh weight for only 12 d, before rapidly losing weight and eventually dying (Figure 10B). At 86% RH treatment, both lines steadily lost weight until they were highly desiccated and necrotic (Figure 10C).
Figure 10.
Response of Wild-Type and Mutant Moss Colonies to Desiccation Stress.
Fresh weight of wild-type and Δppabcg7 moss colonies at 100% RH (A), 91% RH (B), and 86% RH (C) was measured over a 21-d period. Asterisks indicate statistically significant differences between wild-type and transgenic lines (P < 0.05, Student’s t test). Error bars indicate se; 3 ≤ n ≤ 5.
DISCUSSION
P. patens Has a Cuticle That Is Architecturally and Compositionally Similar to Those of Later Diverging Land Plants
TEM imaging of the P. patens phyllid cell walls revealed a thin cuticle evenly coating the whole phyllid surface, approximately the same thickness as that of the Arabidopsis leaf cuticle (Figure 1C). Flowering plants are covered by a waterproof cuticle, and gas exchange for photosynthesis is facilitated by stomata that connect the outside environment with internal air spaces. However, bryophytes, other than a few select examples (Sack and Paolillo, 1983; Beerling and Franks, 2009), do not have stomata, and in the case of P. patens, the photosynthetic phyllids are only a single cell layer thick (Figures 1A and 1B). Therefore, a cuticle is likely necessary in preventing rapid water loss from phyllid cells, but it must at the same time be sufficiently permeable to allow gas exchange, as no alternate internal routes exist.
Despite the variability of epicuticular wax crystal density (Figure 2) on the phyllids, the predominance of crystals on older phyllids near the base of the gametophores suggests a developmental program for wax synthesis. The substantially thicker cuticle of the sporophyte capsule (Figures 1D and 1E) may reflect the fact that the moss sporophyte is nutritionally depended on the female gametophores to which it is anchored, so a cuticle that is sufficiently permeable to allow gas exchange for photosynthesis is presumably not as important. Furthermore, the water status of the sporophyte capsule must be carefully controlled to ensure spore dispersal under the appropriate conditions (Duckett et al., 2009), which would be facilitated by the presence of a more robust cuticle. Taken together, these results suggest that the elaboration of the cuticle biosynthetic program in the sporophyte generation may have served as the foundation for producing a larger and more complex cuticle in the sporophyte-dominant, later-diverging land plants.
Biochemical analysis resulted in the identification of the major P. patens cutin aliphatic monomers as 5-hydroxytetradecanedioic acid (C14), 10,16-dihydroxyhexadecanoic acid (C16), and 16-hydroxyhexadecanoic acid (C16) (Figure 3), revealing clear similarities with the cutin components of diverse plant taxa. Shorter chain length hydroxyalkane diacids (C14-C16) are substantial components of the cutin matrix in a variety of plant species, including Gnetum gnemon (Hunneman and Eglinton, 1972) and Limonia acidissima (Das and Thakur, 1989), as well as minor components of Sphagnum moss (Caldicott and Eglinton, 1976), Ginkgo (Hunneman and Eglinton, 1972), and coffee (Coffea arabica; Holloway et al., 1972). In some cases, a particular monomer can comprise >80% of the cutin precursor population, such as 10,16-dihydroxyhexadecanoic acid in tomato fruit cutin (Isaacson et al., 2009). We also found minor amounts of 1,9,18-octadecanetriol (C18). Alkanetriols have been shown to be present in large amounts in the cutins of earlier diverging land plants, such as Psilotum (Caldicott et al., 1975), suggesting that the incorporation of these monomers into cutins is ubiquitous in land plants.
A particularly notable result was the high levels of aromatic cutin monomers in P. patens cutin, with m- and p-coumaric acid and caffeic acid (Figure 3), which together account for >50% of the cutin monomers. There has been considerable debate as to whether these aromatic acids are actually incorporated into the cutin matrix (Holloway, 1982), but an Arabidopsis acyl-transferase was recently identified that esterifies ferulic acid to ω-hydroxy fatty acids and a loss of function of the corresponding gene results in the absence of ferulate in leaf cutin (Rautengarten et al., 2012). However, this deficiency does not apparently affect permeability of the cuticle to water, suggesting that these aromatic monomers do not significantly contribute to reducing transpirational water loss. The presence of such high amounts in moss cutin might therefore be taken to indicate other functions, such as defense against pathogens or protection from UV radiation, as suggested by Clarke and Robinson (2008), and warrants further investigation. We also note that the polymer suberin typically contains high amounts of aromatic monomers derived from cinnamic acid (Beisson et al., 2012) and suggest that the first pathways responsible for cutin synthesis in early diverging plants may have been the progenitors of suberin biosynthetic pathways in later diverging lineages.
The cutin polymer provides the major structural framework of the cuticle, but the wax that fills and covers this matrix is thought to contribute greatly to its sealing and protective properties, depending on both amount and composition (Yeats and Rose, 2013). Nonuniform deposition of waxes within the intra- and epicuticular regions of the cuticle is often observed and, together with the overall wax composition, leads to a diversity of epicuticular wax crystals and films (Jeffree, 2006). Analysis of P. patens waxes revealed four major compound classes: fatty alcohols, long-chain wax esters, VLCFAs, and alkanes (Figures 4A to 4D). In some plants, a particular compound class can account for most of the cuticular wax, as with the fern Pteridium aquilinum, where wax esters comprise >90% of the surface wax load (Baker and Gaskin, 1987). In other species, such as Arabidopsis, the surface wax is composed of comparable amounts of several compound classes (Li-Beisson et al., 2010). It is clear that the bryophytes have the capacity to synthesize the full complement of wax compound classes mentioned, even though not all of them were found in P. patens. For example, the cuticular wax of the moss Pogonatum urnigerum has substantial amounts of secondary alcohols and related species produce long-chain aldehydes (Neinhuis and Jetter, 1995). Taken together, we suggest that the biosynthetic pathways needed to synthesize the diverse suite of cuticular waxes that have been identified in land plant lineages were likely present in the first land plant taxa, prior to the divergence of vascular plants. Moreover, specific evolutionary pressures have refined cuticular wax compositions resulting in the observed compositional and structural diversity.
The Cuticle Phenotype of Δppabcg7 Suggests Deep Conservation of Cuticle-Associated Gene Pathways in Land Plants
Having establishing that the P. patens cuticle is architecturally and compositionally comparable to those of many later diverging plant lineages, we hypothesized that the underlying genetic pathways that regulate cuticle synthesis and assembly are likely also shared among land plants. To test this, we used the sequences of well-studied cuticle-associated genes in flowering plants to identify putative orthologs in the P. patens genome and indeed found that most of the gene families known to be involved in cuticle synthesis, trafficking, and assembly were represented. Among these genes was a putative ortholog (Pp-ABCG7) of the Arabidopsis ABCG transporters ABCG11 (WBC11) and ABCG12 (WBC12/CER5), which encode half transporters that form homo- or heterodimers and mediate the transport of waxes and cutin precursors across the plasma membrane (Kang et al., 2011). The Arabidopsis abcg12 (wbc12) mutant has a 50% decrease in surface wax load and lipid inclusions in the vacuoles of the epidermal cells (Pighin et al., 2004), while the abcg11 (wbc11) mutant phenotype is more severe, with a substantial reduction in levels of both cutin and wax, as well as severe dwarfing, a permeable cuticle, and postgenital organ fusions (Bird et al., 2007; Luo et al., 2007). A targeted knockout mutant of Pp-ABCG7 (Δppabcg7) also showed a dwarf phenotype (Figure 7), accompanied by a substantial reduction in surface wax, while cutin levels were not affected (Figure 9). Furthermore, Δppabcg7 had a reduced ability to tolerate low humidity conditions compared with the wild type (Figure 10B). The 91% RH conditions at which the reduced growth phenotype was apparent was selected because a study by Koster et al. (2010) indicated that wild-type P. patens is able to survive at this RH, but not lower. These conditions were therefore chosen as a tipping point at which impaired viability resulting from a defective cuticle might be more evident, as indeed proved to be the case.
The fact that no organ fusion phenotype or altered cuticle architecture was observed in Δppabcg7 further suggests that ABCG7 is responsible for wax transport, as such phenotypes are more typically associated with cutin deficiency (Javelle et al., 2011). In addition, water loss through the cuticle is thought to be controlled mainly by wax composition and amount and is not correlated with cutin content (Burghardt and Riederer, 2006; Isaacson et al., 2009). We conclude that the physiological phenotypes of Δppabcg7 are a consequence of its decreased cuticular wax content. The At-abcg12 (At-wbc12) mutant shows lipidic inclusion bodies within the epidermal cells, presumably comprised of cuticular waxes that are unable to be transported out of the cell (Pighin et al., 2004). These were not observed in the epidermal cells of the Pp-abcg7 mutant. It is possible that the moss cells have a feedback mechanism that prevents such waxes from accumulating in the cytoplasm or vacuole. Alternatively, the fact that Pp-abcg7 was cultured under conditions approaching 100% humidity may have reduced the need for it to produce copious amounts of wax.
The reduction in the amount of cuticular wax, but not cutin, in Δppabcg7, together with the length of the predicted gene sequence, suggests that Pp-ABCG7, like At-ABCG12 (At-WBC12), encodes a wax half transporter. In Arabidopsis, cuticle-associated half transporters dimerize in differing combinations to traffic different compound classes, and it is therefore likely that Pp-ABCG7 also forms either a homodimer or a heterodimer with another half transporter to traffic cuticular waxes. Due to these and other protein–protein interactions that may be necessary for the activity of this class of transporter, it is perhaps not surprising that Pp-ABCG7 does not complement the At-abcg12 mutant (see Supplemental Figure 3 online), since the precise protein conformations necessary for such interactions would likely not be conserved over the ∼450 million years since the divergence of these species. Alternatively, constitutive expression of Pp-ABCG7 in the At-abcg12 mutant driven by the 35S promoter may not be appropriate for its cell type–specific function, and use of an Arabidopsis epidermal-specific promoter might be sufficient for complementation. Another notable phenotype of Δppabcg7 was the abnormal surface decorations of the spore walls (Figure 8). The Arabidopsis half transporter ABCG26 is required for exine formation in pollen and is believed to be responsible for the export of sporopollenin precursors in tapetal cells (Kang et al., 2011). Our results suggest that ABCG7 may serve multiple functions in moss, depending on where it is expressed, or possibly with which other half transporter it dimerizes, and could play a role in both cuticle and spore wall formation.
Conclusions
The evolutionary origins of the molecular pathways that mediate cuticle biosynthesis and formation remain unclear, but it is likely that they were derived from those responsible for membrane lipid biosynthesis in the algal ancestors of the first land plants. An expansion of these new pathways in the first terrestrial plants into the complex cuticle biosynthetic machinery seen in angiosperms has been hypothesized for many years, but, until now, no concrete evidence has been presented. This study links cuticle formation in a bryophyte with one of its corresponding genetic pathways. The substantial reduction in cuticular wax in the Δppabcg7 moss line suggests that the role of ABCG transporters in trafficking cuticle components has been conserved since the earliest divergence of terrestrial plants. Taken together with the strong similarities in the chemical composition and structure between the P. patens cuticle and those of other diverse plant taxa, we conclude that at least some of the mechanisms required to synthesize a specialized lipidic cell wall have been conserved through plant evolution and were likely instrumental in allowing the first terrestrial plants to survive in desiccating environments.
METHODS
Plant Material and Cultivation
Wild-type Physcomitrella patens (Gransden 2008 strain, obtained from the Cold Spring Harbor Laboratory, NY) and mutant strains were cultured on BCDAT or BCD media (Nishiyama et al., 2000) supplemented with vancomycin (50 µg/mL) in 9-mm Petri plates overlaid with 9-mm-diameter cellophane discs (AA Packaging). Subcultures were performed by homogenizing protonemal material in water using a Power Gen 125 homogenizer (Fischer Scientific) or by placing small pieces of young protonemal tissue (<1 mm in diameter) on BCDAT plates to induce protonemal growth. To induce gametophore development, cellophane discs containing protonemal colonies were transferred to BCD medium without ammonium tartrate. Cultures were grown at 23°C under 120 µmol m−2 s−1 light with a long-day cycle (16 h light/8 h dark). Sporophytes were induced from gametophytes cultured in jiffy pots as described by Cove et al. (2009).
Light Microscopy
The phyllid transverse section depicted in Figure 1B was obtained from wild-type moss material fixed, embedded, and cryosectioned as described by Buda et al. (2009). A 6-µm cryosection was melted onto a VistaVision Histobond (VWR) slide, dried, and stained using safranin O (0.1% in 50% ethanol) with an alcian blue counterstain (0.1% in water). The slide was mounted in water and viewed using an AxioImager A1 microscope with an Achroplan ×20/0.45 objective and AxioCam Mrc color video camera (Zeiss) using differential interference contrast optics.
TEM
P. patens gametophores and sporophytes were fixed using conventional chemical protocols. Samples were fixed with 1% glutaraldehyde in 0.1 M Sorensen’s phosphate buffer, postfixed in 1% osmium tetroxide, dehydrated in acetone, and embedded in Spurr’s Low Viscosity Resin (EMS). Sections (60 to 80 nm) were cut using a diamond knife on a Reichert Om-U2 ultramicrotome (MOC), stained with uranyl acetate (1% in water) and lead citrate (0.1% in water), and viewed on a Libra 120 transmission electron microscope (Zeiss) at 120 kV, equipped with a Cantega G-2 camera (Olympus).
Scanning Electron Microscopy
Wild-type and Δppabcg7 gametophores (at least 8 weeks old) were air dried overnight in a laminar flow hood, and individual phyllids were carefully dissected. These were sputter coated with gold-palladium for 90 s at 2.2 kV, and epicuticular wax crystals were imaged on a JEOL 6480 variable-pressure scanning electron microscope (JEOL) with secondary electrons at 15 kV with a spot size of 55. The wild-type moss spores depicted in Figure 8A were placed on nitrocellulose, plunge frozen in liquid nitrogen, and viewed on a JEOL 6480 variable-pressure scanning electron microscope with backscattered electrons under variable-pressure mode with 30 Pa of vacuum and a spot size of 60. Spores shown in Figure 8B and 8C were sputter coated for 50 s and visualized with secondary electrons at 10 to 12 kV and a spot size of 60.
Wax Analysis
Plates containing colonies of wild-type and Δppabcg7 moss grown for 3 weeks on BCDAT medium, followed by 7 to 10 weeks on BCD medium, were opened for 2 h to reduce surface moisture. Colonies were then submerged for 30 s in a beaker of chloroform containing 150 µg each of methyl heptadecanoate, nonadecane, and heptadecanoic acid as internal standards. The extract was dried over anhydrous sodium sulfate, passed through filter paper, and concentrated using a rotary evaporator. Moss wax was prefractionated on preparative 20 × 20-cm TLC plates (Whatman; 250-µm coating, silica gel 60), using a mobile phase of 80:18:1 hexane:diethyl ether:glacial acetic acid and employing the sandwich technique, which consisted of a glass plate fastened to the TLC plate with a 1- to 2-mm space between it and the silica gel to allow for increased saturation of the atmosphere adjacent to the sample. Bands were visualized under a UV lamp after misting with primuline (0.005% in 80:20 acetone:water), scraped from the plate, and eluted from the silica with chloroform. The band extract corresponding to fatty acids and primary alcohols was derivatized using equal parts of bis-N,O-(trimethylsilyl)trifluoroacetamide and pyridine for 15 min at 100°C. All extracts were dried under a gentle stream of nitrogen and resuspended in chloroform. Wax compound identification was performed by GC-MS as described by Wang et al. (2011), using a 6890 gas chromatograph (Agilent) coupled to a GC Mate II mass spectrometer (JEOL). Individual waxes were quantified based on peak integration of the total ion chromatogram, except for wax esters, which were quantified using gas chromatography–flame ionization detection on a model 6850 gas chromatograph (Agilent) due to discrimination of the GC-MS against compounds in this molecular mass range. All waxes were quantified based on their corresponding internal standard, with the exception of primary alcohols, which were quantified using the heptadecanoic acid standard. AMDIS 32 GC-MS Analysis software (v 2.69) was used for peak identification and TSSPro GC-MS data reduction software (v 3.0) for manual peak integration.
Cutin Analysis
The same wild-type and Δppabcg7 tissue that was extracted for wax analysis (see above) was oven-dried at 50°C for several days, and 4 g of dried tissue from each line was powdered in a pestle and mortar with liquid nitrogen and distributed among four tubes. The material was exhaustively delipidated as outlined by Li-Beisson et al. (2010) with the following modifications. All extraction steps were performed twice for 1 h each using a rotary shaker set to 300 rpm, and an additional two extractions with acetone were included after the last step. Base-catalyzed depolymerization, extraction, and subsequent trimethylsilyl derivatization of the samples were performed as described above. Extracts were resuspended in chloroform, and cutin monomers were identified by GC-MS. Individual monomers were quantified using ω-pentadecalactone as an internal standard with the software listed above.
Transient Expression and Subcellular Localization of Pp-ABCG7 in Onion Cells
The full-length coding sequence Pp-ABCG7 containing the att recombination sites (forward, 5′-GGGGACAAGTTTGTACAAAAAAGCAGGCTTCATGGCTTCTTCTAATTGTTTACATGC-3′, and reverse, 5′-GGGGACCACTTTGTACAAGAAAGCTGGGTCATCAATTGAAGTGAAATTAAAATTGTCA-3′) was synthesized (Life Technologies) and cloned into the pDONR221 vector by overnight incubation in the presence of Gateway BP clonase (Invitrogen) at 25°C, according to the manufacturer’s instructions. The positive entry clones were then introduced into the pSITE-4NA (RFP tag in the C terminus) destination vector (Chakrabarty et al., 2007). Plasma membrane labeling was based on the full-length coding region of At-PIP2A, a plasma membrane aquaporin, acquired from The Arabidopsis Information Resource Stock Center (ABRC stock numbers: CD3-1003; Cutler et al., 2000; Nelson et al., 2007) fused to the N terminus of GFP. Approximately 1 μg each of the PpABCG7-RFP and AtPIP2A-GFP plasmids was mixed and used to bombard onion (Allium cepa) epidermal cells as described by Yamane et al. (2005). The tissue was incubated for 16 to 24 h at 24°C in the dark, the epidermal layer was peeled, transferred to glass slides, and mounted in a 30% Suc solution to induce plasmolysis, and the cells were observed using a Leica TCS-SP5 confocal scanning laser microscope (Leica Microsystems).
ABCG Half Transporter Phylogenetic Analysis
Amino acid sequences of all known ABCG half transporters from Arabidopsis thaliana and P. patens were obtained from GenBank (http://www.ncbi.nlm.nih.gov) and Cosmoss (http://cosmoss.org) databases, respectively. Sequences were aligned using ClustalW (Thompson et al., 1994), and an unrooted neighbor joining phylogenetic tree was generated using MEGA5 software (Tamura et al., 2007) with bootstrap values assigned to each node (1000 replicates).
Δppabcg7 Knockout Cassette Construction
To selectively disrupt ABCG7, the annotated gene sequence and surrounding genomic sequence were obtained from the latest version of the P. patens genome using the BLAST tool in the Cosmoss database. Primer pair PpABCG7-5FR (see Supplemental Table 1 online) incorporating the HindIII and EcoRI restriction sites was used to amplify the 5′ flanking region (1157 bp) of ABCG7. Similarly, primer pair PpABCG7-3FR (see Supplemental Table 1 online) incorporating the BamHI and SacI restriction sites was used to amplify the 3′ flanking region (1143 bp) of the target gene using ExTaq PCR (Takara). These flanking regions were subsequently cloned into the pTN80 vector (obtained from Mitsuyasu Hasebe at the National Institute for Basic Biology, Okazaki, Japan) on either side of the nptII selectable marker. The Pp-ABCG7 knockout cassette was then replicated in Escherichia coli, isolated, and linearized using the BssHII restriction enzyme prior to moss transformation.
Transformation to Generate Δppabcg7
Polyethylene glycol–mediated protoplast transformation was performed as outlined by Cove et al. (2009) and Saavedra et al. (2011). Colonies surviving the first round of selection on BCDAT medium supplemented with G418 were maintained for 1 week on BCDAT without antibiotics and then subjected to a second round of selection on BCDAT with G418 for 1 week. Several surviving colonies were propagated and screened using PCR primers specific to both Pp-ABCG7 and the nptII resistance marker (Figures 6A and 6B; see Supplemental Table 1 online) to verify correct targeting of the knockout cassette.
Expression Analysis
Total mRNA was isolated from moss protonema (2 weeks old) and gametophores (8 weeks old) using the Dynabead mRNA direct kit (Invitrogen Dynal) following the manufacturer’s instructions. One hundred micrograms of purified mRNA was used for subsequent cDNA synthesis using Superscript II (Invitrogen) and according to the manufacturer’s instructions. The gene-specific PCR primer pair PpABCG7-3FR (see Supplemental Table 1 online) was used to amplify a 221-bp fragment at the 3′ end of the mature mRNA of ABCG7. The protonema and gametophore cDNA libraries of the wild-type and Δppabcg7 lines were screened with these primers via PCR (94°C for 5 min; 94°C for 30 s, 54°C for 45 s, and 72°C for 30 s for 38 cycles) to determine presence or absence of gene expression. PCR primers designed to the 3′ end of Act2 mRNA (primer pair PpACT2; see Supplemental Table 1 online) were used as a positive control for gene expression between tissue types and genetic lines (Huang et al., 2009).
Physiological Assays
For the physiological studies of wild-type and Δppabcg7 moss grown under different RHs, cultures were initiated using three pieces of protonemal tissue (<1 mm in diameter) placed on a plate of BCDAT media supplemented with vancomycin (50 µg/µL) and overlaid with cellophane (AA Packaging). After 3 weeks, the 9-mm cellophane discs were transferred to BCD media for an additional 3 weeks to induce robust gametophore growth. Wild-type and Δppabcg7 cultures were weighed (cellophane and moss colonies only), placed onto plates containing BCD media, and exposed to different RH conditions. The plates were left open inside sealed chambers (Pyrex dishes) floating on solutions of saturated magnesium sulfate or potassium chloride to obtain RHs of 91 or 86%, respectively (Koster et al., 2010). Other moss cultures were sealed in plates containing BCD media supplemented with vancomycin (50 µg/µL) to generate close to 100% RH. The fresh weight of each sample (cellophane and moss colonies) was recorded every 3 d for 3 weeks. After 3 weeks, the final weight was recorded and the mass of the cellophane was subtracted from each previous measurement of the sample to obtain fresh weight values. Any plates with substantial microbial contamination were discarded and were not included in subsequent statistical analyses.
Complementation of At-abcg12 with PPABCG7
To construct the plasmid vector for complementation analysis, the Pp-ABCG7 coding sequence (as described above) was cloned into the pDONR Gateway shuttle vector and then subcloned in frame with the Pro35S:CPMV 5′-UTR in the destination vector pEAQ-HT-DEST1. The vector construct was transformed into 3-week-old abcg12 T-DNA insertional mutant Arabidopsis plants (Salk 036776) by Agrobacterium tumefaciens–mediated transformation (GV2260 strain) as previously described (Clough and Bent, 1998). To identify transformants, the T1 seeds were sown on one-quarter Murashige and Skoog plates containing 100 mg/L kanamycin and screened by PCR using Pp-ABCG7–specific primers (primer pair PpABCG7-S; see Supplemental Table 1 online). Offspring from a transformed line (a segregating T2 population) were grown and genotyped by PCR for the presence of the ABCG7 transgene. Inflorescence stems were collected from five transgenic and three nontransgenic T2 plants, as well as from three wild-type plants, grown concurrently. The stems, from which leaves, flowers, and siliques were removed, were cut into 3-cm sections, with eight to 15 sections collected per plant, and sections from each plant were swirled in 30 mL of chloroform, containing 100 μL of tetracosane as an internal standard, for 30 s. The diameters of the stem sections were measured at their center to calculate surface area. Wax extracts were dried over anhydrous sodium sulfate, filtered, and then concentrated to a volume of 4 mL. The level of C29 alkane in these samples was quantified as described above.
Accession Numbers
Sequence data from this article can be found in the GenBank/EMBL data libraries under the following accession numbers: XP_001775949 (Pp-ABCG7 genomic sequence), XP_001775897 (Pp-ABCG7 mRNA sequence), and XM_001775900 (Pp-ACT2 mRNA sequence).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. Localization of Pp-ABCG7 Fused to an RFP Marker Protein Using Confocal Scanning Laser Microscopy, following Transient Expression in Onion Epidermal Cells.
Supplemental Figure 2. Abnormal Sporophyte Phenotype of Δppabcg7.
Supplemental Figure 3. Overexpression of Pp-ABCG7 in the Arabidopsis abcg12 Mutant.
Supplemental Table 1. List of PCR Primers, Referenced in Methods.
Supplemental Data Set 1. Protein Alignment of All Known ABCG Half Transporters from Arabidopsis and P. patens.
Supplementary Material
Acknowledgments
We thank Antonio Matas for helpful discussion and for assisting with the statistical analysis, Eliel Ruiz-May for assistance with confocal microscopy, and Iben Sørensen for careful reading of the article. This work was supported by grants from the National Science Foundation (Plant Genome Program, DBI-0606595), by the U.S.–Israel Binational Agricultural Research and Development Fund (IS-4234-09), and by the Agriculture and Food Research Initiative Competitive Grants Program (2011-67013-19399) from the USDA National Institute of Food and Agriculture. G.J.B. and T.H.Y. were partly supported by a National Institutes of Health chemistry/biology interface training grant (T32 GM008500).
AUTHOR CONTRIBUTIONS
G.J.B. designed and performed the research, analyzed data, and cowrote the article. W.J.B., E.A.F., S.P., T.H.Y., L.Z., and D.S.D. performed the research and analyzed data. J.K.C.R. designed the research, analyzed data, and cowrote the article.
Glossary
- VLCFA
very-long-chain fatty acid
- CP
cuticle proper
- TEM
transmission electron microscopy
- GC-MS
gas chromatography–mass spectrometry
- TLC
thin layer chromatography
- ABCG
ATP binding cassette subfamily G
- RFP
red fluorescent protein
- GFP
green fluorescent protein
References
- Baker E.A., Gaskin R.E. (1987). Composition of leaf epicuticular waxes of Pteridium subspecies. Phytochemistry 26: 2847–2848 [Google Scholar]
- Barthlott W., Neinhuis C. (1997). Purity of the sacred lotus, or escape from contamination in biological surfaces. Planta 202: 1–8 [Google Scholar]
- Beerling D.J., Franks P.J. (2009). Evolution of stomatal function in ‘lower’ land plants. New Phytol. 183: 921–925 [DOI] [PubMed] [Google Scholar]
- Beisson F., Li-Beisson Y., Pollard M. (2012). Solving the puzzles of cutin and suberin polymer biosynthesis. Curr. Opin. Plant Biol. 15: 329–337 [DOI] [PubMed] [Google Scholar]
- Bird D., Beisson F., Brigham A., Shin J., Greer S., Jetter R., Kunst L., Wu X., Yephremov A., Samuels L. (2007). Characterization of Arabidopsis ABCG11/WBC11, an ATP binding cassette (ABC) transporter that is required for cuticular lipid secretion. Plant J. 52: 485–498 [DOI] [PubMed] [Google Scholar]
- Buda G.J., Isaacson T., Matas A.J., Paolillo D.J., Rose J.K.C. (2009). Three-dimensional imaging of plant cuticle architecture using confocal scanning laser microscopy. Plant J. 60: 378–385 [DOI] [PubMed] [Google Scholar]
- Budke J.M., Goffinet B., Jones C.S. (2011). A hundred-year-old question: Is the moss calyptra covered by a cuticle? A case study of Funaria hygrometrica. Ann. Bot. (Lond.) 107: 1279–1286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burghardt, M., and Riederer, M. (2006). Cuticular transpiration. In Biology of the Plant Cuticle, M. Riederer and C. Muller, eds (Oxford, UK: Blackwell Publishing), pp. 292–311. [Google Scholar]
- Caldicott A.B., Eglinton G. (1976). Cutin acids from bryophytes: An ω-1 hydroxy alkanoic acid in two liverwort species. Phytochemistry 15: 1139–1143 [Google Scholar]
- Caldicott A.B., Simoneit B.R.T., Eglinton G. (1975). Alkanetriols in psilotophyte cutins. Phytochemistry 14: 2223–2228 [Google Scholar]
- Chakrabarty R., Banerjee R., Chung S.M., Farman M., Citovsky V., Hogenhout S.A., Tzfira T., Goodin M. (2007). PSITE vectors for stable integration or transient expression of autofluorescent protein fusions in plants: Probing Nicotiana benthamiana-virus interactions. Mol. Plant Microbe Interact. 20: 740–750 [DOI] [PubMed] [Google Scholar]
- Charron A.J., Quatrano R.S. (2009). Between a rock and a dry place: The water-stressed moss. Mol. Plant 2: 478–486 [DOI] [PubMed] [Google Scholar]
- Clarke L.J., Robinson S.A. (2008). Cell wall-bound ultraviolet-screening compounds explain the high ultraviolet tolerance of the Antarctic moss, Ceratodon purpureus. New Phytol. 179: 776–783 [DOI] [PubMed] [Google Scholar]
- Clayton-Greene K.A., Collins N.J., Green T.G.A., Proctor M.C.F. (1985). Surface wax, structure and function in leaves of Polytrichaceae. J. Bryol. 13: 549–562 [Google Scholar]
- Clough S.J., Bent A.F. (1998). Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Cove D., Bezanilla M., Harries P., Quatrano R. (2006). Mosses as model systems for the study of metabolism and development. Annu. Rev. Plant Biol. 57: 497–520 [DOI] [PubMed] [Google Scholar]
- Cove, D.J., Perroud, P., Charron, A.J., McDaniel, S.F., Khandelwal, A., and Quatrano, R.S. (2009). The moss Physcomitrella patens: A novel model system for plant development and genomic studies. In Emerging Model Organisms, Vol. 1, (Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press), pp. 69–104. [DOI] [PubMed] [Google Scholar]
- Cutler S.R., Ehrhardt D.W., Griffitts J.S., Somerville C.R. (2000). Random GFP:cDNA fusions enable visualization of subcellular structures in cells of Arabidopsis at a high frequency. Proc. Natl. Acad. Sci. USA 97: 3718–3723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S., Thakur S. (1989). Constituent acids of Limonia acidissima leaf cutin. Phytochemistry 28: 509–511 [Google Scholar]
- Duckett J.G., Pressel S., P’ng K.M.Y., Renzaglia K.S. (2009). Exploding a myth: The capsule dehiscence mechanism and the function of pseudostomata in Sphagnum. New Phytol. 183: 1053–1063 [DOI] [PubMed] [Google Scholar]
- Haas K. (1982). Surface wax of Andreaea and Pogonatum species. Phytochemistry 21: 657–659 [Google Scholar]
- Hohe A., Egener T., Lucht J.M., Holtorf H., Reinhard C., Schween G., Reski R. (2004). An improved and highly standardised transformation procedure allows efficient production of single and multiple targeted gene-knockouts in a moss, Physcomitrella patens. Curr. Genet. 44: 339–347 [DOI] [PubMed] [Google Scholar]
- Holloway, P.J. (1982). The chemical constitution of plant cutins. In The Plant Cuticle, D.F. Cutler, K.L. Alvin, and C.E. Price, eds (London, UK: Academic Press), pp. 45–85. [Google Scholar]
- Holloway P.J., Deas A.H.B., Kabaara A.M. (1972). Composition of cutin from coffee leaves. Phytochemistry 11: 1443–1447 [Google Scholar]
- Hunneman D.H., Eglinton G. (1972). The constituent acids of gymnosperm cutins. Phytochemistry 11: 1989–2001 [Google Scholar]
- Huang C.Y., Chung C.I., Lin Y.C., Hsing Y.I., Huang A.H.C. (2009). Oil bodies and oleosins in Physcomitrella possess characteristics representative of early trends in evolution. Plant Physiol. 150: 1192–1203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson T., Kosma D.K., Matas A.J., Buda G.J., He Y., Yu B., Pravitasari A., Batteas J.D., Stark R.E., Jenks M.A., Rose J.K.C. (2009). Cutin deficiency in the tomato fruit cuticle consistently affects resistance to microbial infection and biomechanical properties, but not transpirational water loss. Plant J. 60: 363–377 [DOI] [PubMed] [Google Scholar]
- Javelle M., Vernoud V., Rogowsky P.M., Ingram G.C. (2011). Epidermis: The formation and functions of a fundamental plant tissue. New Phytol. 189: 17–39 [DOI] [PubMed] [Google Scholar]
- Jeffree, C.E. (2006). The fine structure of the plant cuticle. In Biology of the Plant Cuticle, M. Riederer and C. Muller, eds (Oxford, UK: Blackwell Publishing), pp. 11–125. [Google Scholar]
- Jetter, R., Kunst, L., and Samuels, A.L. (2006). Composition of plant cuticular waxes. In Biology of the Plant cuticle, M. Riederer and C. Muller, eds (Oxford, UK: Blackwell Publishing), pp. 145–181. [Google Scholar]
- Kang, J., Park, J., Choi, H., Burla, B., Kretzschmar, T., Lee, Y., and Martinoia, E. (2011). Plant ABC transporters. The Arabidopsis Book 9:e0153, /10.1199/tab.0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster K.L., Balsamo R.A., Espinoza C., Oliver M.J. (2010). Desiccation sensitivity and tolerance in the moss Physcomitrella patens: Assessing limits and damage. Plant Growth Regul. 62: 293–302 [Google Scholar]
- Leide J., Hildebrandt U., Reussing K., Riederer M., Vogg G. (2007). The developmental pattern of tomato fruit wax accumulation and its impact on cuticular transpiration barrier properties: Effects of a deficiency in a β-ketoacyl-coenzyme A synthase (LeCER6). Plant Physiol. 144: 1667–1679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li-Beisson, Y., et al. (2010). Acyl-lipid metabolism. The Arabidopsis Book 8:e0133, /10.1199/tab.0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo B., Xue X.Y., Hu W.L., Wang L.J., Chen X.Y. (2007). An ABC transporter gene of Arabidopsis thaliana, AtWBC11, is involved in cuticle development and prevention of organ fusion. Plant Cell Physiol. 48: 1790–1802 [DOI] [PubMed] [Google Scholar]
- Matas A.J., et al. (2011). Tissue- and cell-type specific transcriptome profiling of expanding tomato fruit provides insights into metabolic and regulatory specialization and cuticle formation. Plant Cell 23: 3893–3910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minami A., Nagao M., Ikegami K., Koshiba T., Arakawa K., Fujikawa S., Takezawa D. (2005). Cold acclimation in bryophytes: low-temperature-induced freezing tolerance in Physcomitrella patens is associated with increases in expression levels of stress-related genes but not with increase in level of endogenous abscisic acid. Planta 220: 414–423 [DOI] [PubMed] [Google Scholar]
- Neinhuis C., Jetter R. (1995). Ultrastructure and chemistry of epicuticular wax crystals in Polytrichales sporophytes. J. Bryol. 18: 399–406 [Google Scholar]
- Nelson B.K., Cai X., Nebenführ A. (2007). A multicolored set of in vivo organelle markers for co-localization studies in Arabidopsis and other plants. Plant J. 51: 1126–1136 [DOI] [PubMed] [Google Scholar]
- Nishiyama T., Hiwatashi Y., Sakakibara I., Kato M., Hasebe M. (2000). Tagged mutagenesis and gene-trap in the moss, Physcomitrella patens by shuttle mutagenesis. DNA Res. 7: 9–17 [DOI] [PubMed] [Google Scholar]
- Nissinen R., Sewón P. (1994). Hydrocarbons of Polytrichum commune. Phytochemistry 37: 179–182 [Google Scholar]
- Pighin J.A., Zheng H., Balakshin L.J., Goodman I.P., Western T.L., Jetter R., Kunst L., Samuels A.L. (2004). Plant cuticular lipid export requires an ABC transporter. Science 306: 702–704 [DOI] [PubMed] [Google Scholar]
- Post-Beittenmiller D. (1998). The cloned Eceriferum genes of Arabidopsis and the corresponding Glossy genes in maize. Plant Physiol. Biochem. 36: 157–166 [Google Scholar]
- Proctor M.C.F. (1979). Surface wax on the leaves of some mosses. J. Bryol. 10: 531–538 [Google Scholar]
- Rautengarten C., Ebert B., Ouellet M., Nafisi M., Baidoo E.E.K., Benke P., Stranne M., Mukhopadhyay A., Keasling J.D., Sakuragi Y., Scheller H.V. (2012). Arabidopsis Deficient in Cutin Ferulate encodes a transferase required for feruloylation of ω-hydroxy fatty acids in cutin polyester. Plant Physiol. 158: 654–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reina-Pinto J.J., Yephremov A. (2009). Surface lipids and plant defenses. Plant Physiol. Biochem. 47: 540–549 [DOI] [PubMed] [Google Scholar]
- Rensing S.A., et al. (2008). The Physcomitrella genome reveals evolutionary insights into the conquest of land by plants. Science 319: 64–69 [DOI] [PubMed] [Google Scholar]
- Saavedra L., Balbi V., Lerche J., Mikami K., Heilmann I., Sommarin M. (2011). PIPKs are essential for rhizoid elongation and caulonemal cell development in the moss Physcomitrella patens. Plant J. 67: 635–647 [DOI] [PubMed] [Google Scholar]
- Sack F.D., Paolillo D.J. (1983). Stomatal pore and cuticle formation in Funaria. Protoplasma 116: 1–13 [Google Scholar]
- Samuels L., Kunst L., Jetter R. (2008). Sealing plant surfaces: Cuticular wax formation by epidermal cells. Annu. Rev. Plant Biol. 59: 683–707 [DOI] [PubMed] [Google Scholar]
- Schönherr J., Ziegler H. (1975). Hydrophobic cuticular ledges prevent water entering the air pores of liverwort thalli. Planta 124: 51–60 [DOI] [PubMed] [Google Scholar]
- Shepherd T., Wynne Griffiths D. (2006). The effects of stress on plant cuticular waxes. New Phytol. 171: 469–499 [DOI] [PubMed] [Google Scholar]
- Tamura K., Dudley J., Nei M., Kumar S. (2007). MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol. Biol. Evol. 24: 1596–1599 [DOI] [PubMed] [Google Scholar]
- Thompson J.D., Higgins D.G., Gibson T.J. (1994). CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22: 4673–4680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Guhling O., Yao R., Li F., Yeats T.H., Rose J.K.C., Jetter R. (2011). Two oxidosqualene cyclases responsible for biosynthesis of tomato fruit cuticular triterpenoids. Plant Physiol. 155: 540–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood, A.J. (2005). Eco-physiological adaptations to limited water environments. In Plant Abiotic Stress, M.A. Jenks and P.M. Hasegawa, eds (Oxford, UK: Blackwell Publishing), pp. 1–13. [Google Scholar]
- Wyatt H.D.M., Ashton N.W., Dahms T.E.S. (2008). Cell wall architecture of Physcomitrella patens is revealed by atomic force microscopy. Botany 86: 385–397 [Google Scholar]
- Yamane H., Lee S.-J., Kim B.-D., Tao R., Rose J.K.C. (2005). A coupled yeast signal sequence trap and transient plant expression strategy to identify genes encoding secreted proteins from peach pistils. J. Exp. Bot. 56: 2229–2238 [DOI] [PubMed] [Google Scholar]
- Yeats T.H., Buda G.J., Wang Z., Chehanovsky N., Moyle L.C., Jetter R., Schaffer A.A., Rose J.K.C. (2012). The fruit cuticles of wild tomato species exhibit architectural and chemical diversity, providing a new model for studying the evolution of cuticle function. Plant J. 69: 655–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeats T.H., Rose J.K.C. (2013). The formation and function of plant cuticles. Plant Physiol. 163: 5–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.