Abstract
Four new sesquiterpenes, sinularianins C–F (3–6), together with known sinularianins A (1) and B (2) were identified from a South China Sea soft coral Sinularia sp. Compounds 1–6 were evaluated for inhibition of NF-κB activation using the cell-based HEK293 NF-κB luciferase reporter gene assay. Compounds 1 and 4 were exhibited a potent effect with inhibitory rates of 41.3% and 43.0% at the concentration of 10 µg/mL, respectively.
Keywords: soft coral, Sinularia sp., sesquiterpenes, sinularianins, NF-κB
1. Introduction
The genus Sinularia is the most widely distributed soft coral, consisting of almost 90 species, of which more than 50 have been chemically examined [1,2,3,4,5]. Up to now, Sinularia has yielded many metabolites, including sesquiterpenes, diterpenes, alkaloids, and polyhydroxylated steroids [6,7,8,9,10,11,12]. These metabolites display a wide range of biological activities, such as antimicrobial, anti-inflammatory, and cytotoxic activities [13,14,15,16,17,18]. In our endeavor to explore the bioactive secondary metabolites from marine invertebrates, sinularianins A (1) and B (2) were reisolated along with four new sesquiterpenes, sinularianins C–F (3–6) from soft coral Sinularia sp., collected at Dongluo Island, Hainan province, China, at a depth of 10 m. Sinularianin A and B have been isolated from the Formosan coral Sinularia sp., but their anti-inflammatory activation were tested for the first time. Similar sesquiterpenes had been isolated mostly from the plant Valeriana officinalis, which was used as an anti-inflammatory remedy in Europe, and were active as inhibitors of NF-κB [19]. In this paper, we describe the isolation, structure elucidation, and the NF-κB inhibitory potential of these compounds.
2. Results and Discussion
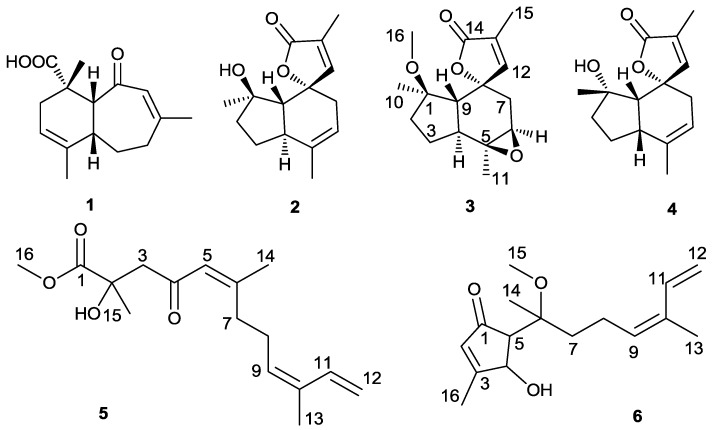
The soft coral Sinularia sp. was dissolved in 85% EtOH, and the extract separated by silica gel column chromatography, Sephadex LH-20, and semi-preparative HPLC to obtain new sesquiterpenes, sinularianins C–F (3–6), and two known compounds (1, 2) (Figure 1).
Figure 1.
Structures of the compounds 1–6.
Sinularianins A (1) and B (2) were previously isolated from the soft coral Sinularia sp., collected off the northeastern Taiwan coast, in May 2004, at a depth of 10 m. Sinularianin A (1) possesses an unprecedented bicyclic skeleton sinulariolane. Sinularinin B (2) was the only example of valerenane-related sesquiterpene with a spiro-butenolide moiety [10]. The valerenane-related sesquiterpenes had been firstly reported from the plant Valeriana officinalis [20,21], and several representatives have been reported from a marine alga [22] and a soft coral [23]. Sinularinin A (1) and B (2), were reisolated and identified by comparison of their 1H and 13C NMR data with those reported [10].
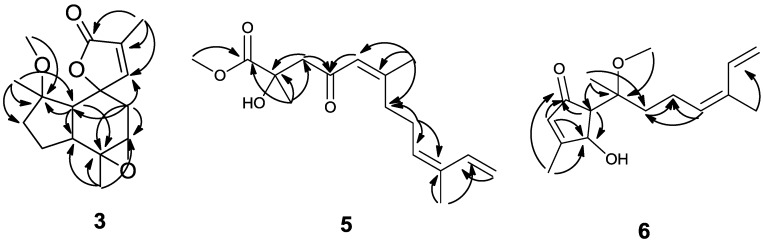
Sinularianin C (3) was isolated as a colorless oil. Its molecular formula was established as C16H22O4 on the basis of the positive HRESIMS at m/z 301.1416 (Calcd for C16H22NaO4, 301.1416), indicating six degrees of unsaturation (Supplementary Figure S1). The 1H NMR spectrum (Table 1) revealed the presence of four singlet methyls (δH 1.00, 1.41, 1.86, 3.17), three methylene signals (δH 1.95, 1H, m; 1.53, 1H, m; 1.96, 1H, m; 1.56, 1H, m; 2.26, 1H, d, J = 16.0 Hz; 1.76, 1H, dd, J = 16.0, 5.0 Hz), three methine signals (2.00, 1H, d, J = 13.0 Hz; 2.43, 1H, m; 3.11, 1H, d, J = 5.0 Hz), and one olefinic proton (δH 7.17, 1H, d, J = 1.5 Hz) (Supplementary Figure S2). The 13C NMR spectra, together with HSQC, showed 16 signals for four methyls (δC 10.3, 20.5, 21.3, 50.8), three sp3 methylenes (δC 23.6, 35.7, 38.7), three sp3 methines (δC 41.1, 50.4, 59.5), three sp3 oxygenated quaternary carbons (δC 61.8, 84.2, 86.5), one sp2 methine (δC 154.7), one sp2 quaternary carbon (δC 129.5), and one carbonyl carbon (δC 175.6) (Supplementary Figures S3 and S4). Both the 1H and 13C NMR spectra of 3 showed a close similarity to those of 2 [10]. However, the close comparison of the 13C NMR spectroscopic data of 2 and 3 revealed some differences: one trisubstituted double bond in 2 was changed to the epoxy three-menbered ring (δC 61.8, 59.5) in 3, and an additional methoxyl (δC 50.8, δH 3.17, 3H, s, H-16) was observed in 3. This assumption was supported by the correlation of H-11 to C-4, C-5, and C-6, H-6 to C-5, and C-7, H-7 to C-5 and C-6 in the HMBC spectrum (Figure 2). Furthermore, the methoxyl substituent was determined to be connected to position C-1 on the basis of the HMBC correlation from 16-OMe to C-1 (Supplementary Figure S5).
Table 1.
1H and 13CNMR spectroscopic data for compounds 3 (500/125 MHz, in MeOD, δ in ppm, J in Hz) and 4 (in CDCl3).
| Position | 3 | 4 | ||
|---|---|---|---|---|
| 1H | 13C | 1H | 13C | |
| 1 | 84.2 | 78.4 | ||
| 2 | 1.95 m | 35.7 | 1.92 m | 43.1 |
| 1.53 m | 1.79 m | |||
| 3 | 1.96 m | 23.6 | 2.01 m | 25.9 |
| 1.56 m | ||||
| 4 | 2.43 m | 41.1 | 2.90 m | 41.8 |
| 5 | 61.8 | 137.5 | ||
| 6 | 3.11 d (5.0) | 59.5 | 5.26 s | 117.2 |
| 7 | 2.26 d (16.0) | 38.7 | 2.53 m | 39.4 |
| 1.76 dd (16.0, 5.0) | 1.91 m | |||
| 8 | 86.5 | 85.6 | ||
| 9 | 2.00 d (13.0) | 50.4 | 1.55 d (12.5) | 55.5 |
| 10 | 1.00 s | 21.3 | 1.26 s | 28.5 |
| 11 | 1.41 s | 20.5 | 1.74 s | 20.5 |
| 12 | 7.17 d (1.5) | 154.7 | 7.03 d (1.5) | 150.9 |
| 13 | 129.5 | 129.7 | ||
| 14 | 175.6 | 174 | ||
| 15 | 1.86 s | 10.3 | 1.94 d (1.5) | 10.6 |
| 16 | 3.17 s | 50.8 | ||
Figure 2.
Selected HMBC correlations (H → C)of compounds 3, 5, and 6.
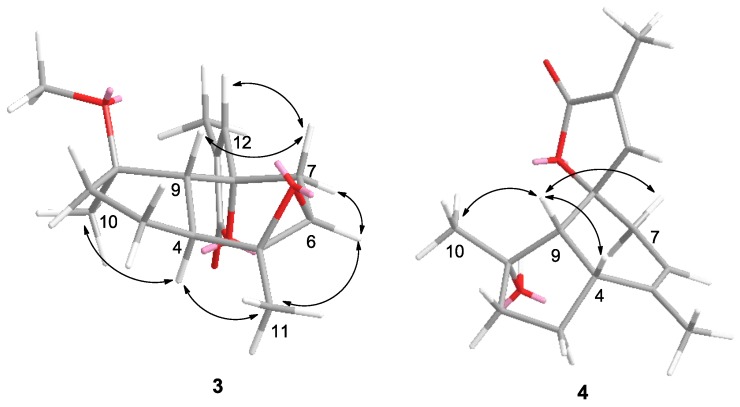
The relative stereochemistry of 3 was established by the detailed analysis of correlations observed in the NOESY spectrum (Figure 3). In the NOESY spectrum, H-9 showed correlation with H-7β (δH 2.26, d, J = 16.0 Hz), which in turn correlated with H-12, suggesting the β orientations of H-9 and H-12. Furthermore, NOE interactions were observed between H3-10/H-4, H3-11/H-4, H3-11/H-6, and H-6/H-7α (δH 1.76, dd, J = 16.0, 5.0 Hz), while both H3-10 and H-4 did not show correlations with H-9, suggesting the α orientation of H3-10, H3-11, H-4, and H-6 (Supplementary Figure S6).
Figure 3.
Selected NOE correlations of compounds 3 and 4.
Sinularianin D (4) was isolated as a colorless oil. The ESI-MS showed the [M + Na]+ ion at m/z: 271 (Supplementary Figure S7). Its 1H and 13C NMR spectroscopic data were also very similar to those of 2 (Supplementary Figures S8 and S9). However, a close inspection of their 1H NMR spectroscopic data revealed some difference: H-4 and H3-10 were shifted downfield from 2.57 to 2.90, and from 1.12 to 1.26 respectively, and H-9 was shifted upfield from 1.99 to 1.55. This suggested that the configuration at H-1 and H-4 in 4 should be β orientation compared to α orientation in 2, which was supported by the NOESY experiment (Figure 3). In the NOESY spectrum, H-9 showed correlation with H3-10, H-4, and H-7β, suggesting the β orientations of H-4, H-9, H-7β, and H3-10 (Supplementary Figure S10).
Sinularianin E (5) was isolated as a colorless oil, and assigned the molecular formula of C16H24O4 by the positive HRESIMS at m/z 303.1563 (Calcd for C16H24NaO4, 303.1572) (Supplementary Figure S11). The 1H and 13C NMR spectroscopic data of 5 indicated sixteen carbon signals: four singlet methyls, four methylenes, three olefinic methines, and five quaternary carbons (Supplementary Figures S12–S14). The 1H NMR spectrum showed signals of four olefinic protons (δH 5.42, 1H, t, J = 7.0 Hz; 6.36, 1H, dd, J = 17.5, 10.5 Hz; 5.13, 1H, d, J = 17.5 Hz; 4.96, 1H, d, J = 10.5 Hz), one methoxy group (δH 3.72), two vinyl methyls (δH 2.14, s; 1.74, s), and one other methyl (δH 1.40, s) (Table 2). The gross structure of 5 was established by the assistance of extensive 2D NMR analysis (Figure 2). The methoxycarbonyl was confirmed by HMBC correlations from 16-OMe to C-1. The methyl protons resonating at δH 1.40 and the quaternary carbon resonating at δC 72.9 indicated that this methyl and a hydroxyl group should be positioned at C-2 by the HMBC correlations from H-15 to C-1, C-2, and C-3 (Supplementary Figure S15). The olefinic methyls (δH 2.14, s; 1.74, s) attached at C-6 and C-10 were confirmed by the HMBC correlations from H-14 to C-5, C-6, and C-7 and H-13 to C-9, C-10, and C-11. Furthermore, the HMBC correlations from H-9 to C-8, and C-10, H-12 to C-10, and C-11 established the terminal diene unit. Other key informative HMBC correlations from H-3 to C-2, and C-4, H-5 to C-4, H-8 to C-7, C-9, and C-10, established the planar structure of 5. The double bond at C-5 was assigned the Z-geometry on the basis of the downfield chemical shifts of C-14 (δH 19.7) [24]. The geometry of the disubstituted double bond (C-9) was determined to be E by comparison of the spectral data with those reported in literature [24], whereas the configurations at C-2 remained to be determined. On the basis of above evidences, compound 5 was then identified, and named sinularianin E.
Table 2.
1H and 13C NMR spectroscopic data for compounds 5 and 6 (500/125 MHz, in CDCl3, δ in ppm, J in Hz).
| Position | 5 | 6 | ||
|---|---|---|---|---|
| 1H | 13C | 1H | 13C | |
| 1 | 176.5 | 203.2 | ||
| 2 | 72.9 | 5.86 s | 131.2 | |
| 3 | 3.15 d (17.5) | 52.9 | 174.9 | |
| 2.80 d (17.5) | ||||
| 4 | 199.3 | 4.79 s | 75.3 | |
| 5 | 6.01 s | 122.9 | 2.65 s | 61.5 |
| 6 | 160.3 | 78 | ||
| 7 | 2.22 m | 40.9 | 1.82 m | 34.1 |
| 2.12 m | ||||
| 8 | 2.34 m | 25.8 | 2.19 m | 22.1 |
| 2.20 m | ||||
| 9 | 5.42 t (7.0) | 130.8 | 5.52 m | 132.3 |
| 10 | 135 | 134.1 | ||
| 11 | 6.36 dd (17.5, 10.5) | 141 | 6.36 dd (17.0, 10.5) | 141.5 |
| 12 | 5.13 d (17.5) | 111.3 | 5.10 d (17.0) | 110.7 |
| 4.96 d (10.5) | 4.94 d (10.5) | |||
| 13 | 1.74 s | 11.7 | 1.76 s | 11.6 |
| 14 | 2.14 s | 19.7 | 1.03 s | 22.7 |
| 15 | 1.40 s | 26.2 | 3.21 s | 48.5 |
| 16 | 3.72 s | 52.7 | 2.16 s | 15.6 |
Sinularianin F (6) was isolated as a colorless oil. It was assigned a molecular formula of C16H24O3 by positive HR-ESI-MS at m/z 287.1613 (Calcd for C16H24NaO3, 287.1623) (Supplementary Figure S16). Analysis of 1H and 13C NMR data revealed the presence of four methyl groups, three methylene carbons, five methine carbons, and four quaternary carbons (Supplementary Figures S17–S19). The 1H NMR spectrum showed signals of five olefinic protons (δH 5.86, s; 5.52, m; 6.36, dd, J = 17.0, 10.5 Hz; 5.10, d, J = 17.0 Hz; 4.94, d, J = 10.5 Hz), one oxygenated methane (δH 4.79, s), one methoxy group (δH 3.21, s), two vinyl methyls (δH 1.76, s; 2.16, s), and one other methyl (δH 1.03, s) (Table 2). The HMBC correlations from H-9 to C-8, and C-10, H-12 to C-10, and C-11, H-13 to C-10, and C-11 established the terminal diene unit (Supplementary Figure S20). The key HMBC correlations of H3-16 to C-2, C-3, and C-4 and H-2 to C-1, C-3, C-4, and C-5 indicated the presence of a five-membered carbocycle containing a ketone carbonyl and a trisubstituted double bond (Figure 2), as well as by comparison of the data with that of in agreement with the data of cycloabiesesquine A [25]. The two fragments may be connected via the correlations of H-15 to C-5, C-6, and C-7, H-14 to C-6 and H-7 to C-6, C-7, and C-8 in the HMBC spectrum. Two double bonds in the molecule possessed 2Z and 9E configuration on the basis of the chemical shifts of C-16 and C-13 (δ 15.6 and 11.6, respectively) [24,25].
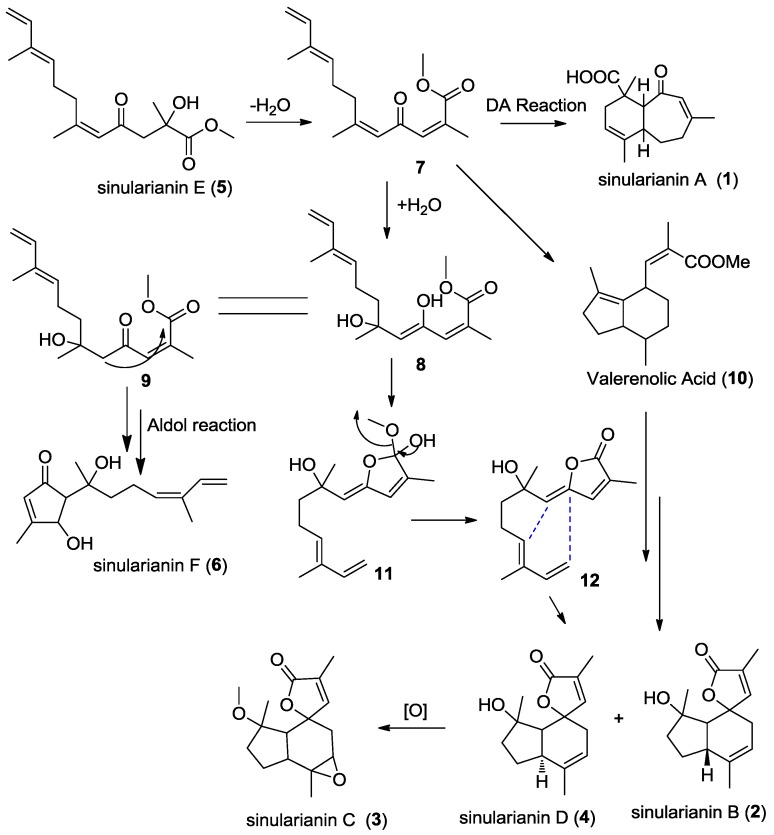
Although sinularianins E (5) and F (6) formally displayed a quite different skeleton from that of sinularianins A–D (1–4), however, they are actually related to each other. From a biosynthetic aspect, sinularinins A–D (1–4), and F (6) could be generated from sinularinin E (5), via different reaction cascades as illustrated in the hypothetical biosynthetic pathway (Scheme 1). As a precursor, sinularianin E (5) potentially could be transformed into the key intermediate A by dehydration reaction. Intermediate A could be through different intramolecular Diels Alder cyclization reaction to form sinularianin A (1) or valerenolic acid, respectively, and the latter was further modified to produce sinularianin B (2). Intermediate A could be also adapted by Michael addition under the H2O attack and then immediately lactonized, followed by a DA cyclization to yield sinularianin B (2), which after epoxidation and dehydration potentially could be produce epoxide sinularianin C (3). Intermediate A might form sinularianin F (6) by an aldol condensation.
Scheme 1.
Proposed biosynthetic pathway for 1–6.
Nuclear factor-κ B (NF-κB) plays a key role in regulating the immune response to infection. Incorrect regulation of NF-κB has been linked to cancer, inflammatory and autoimmune diseases, septic shock, viral infection, and improper immune development [26]. Compounds 1–6 were evaluated for inhibition of NF-κB activation using the cell-based HEK293 NF-κB luciferase reporter gene assay. At concentration of 10 µg/mL, sinularianin A and D exhibits a potent effect with inhibitory rates of 41.3%, and 43.0%, respectively. At the same concentration, other compounds showed moderate effects at the same (Table 3). The past studies have provided biochemical evidence of valerenane-related sesquiterpenes as anti-inflammatory agents acting via the NF-κB inhibitory potential. The valerenic acid (3) reduced NF-κB activity to 25% at concentration of 100 µg/mL [19].
Table 3.
Inhibitory rates of NF-κB activation of compounds 1–6.
| Concentration | IR (%) | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| 10 µg/mL | 41.3 | 29.6 | 24.3 | 43.0 | 30.0 | 36.1 |
3. Experimental Section
3.1. General Experimental Procedures
The NMR spectra were recorded on a Bruker AC 500 NMR spectrometer with TMS as an internal standard. IR spectra were recorded on a Nicolet 6700 FT-IR spectrometer. UV spectra were recorded on a Shimadzu UV-2600 UV-Vis spectrophotometer. Optical rotations were measured on a PerKin Elmer 341 polarimeter using a 1 dm path length cell. HR-ESI-MS data were measured on AQUITY UPLC/Q-TOF mass spectrometer. ESI-MS data were measured on Bruker’s amaZon SL ion trap LC/MS. Materials for column chromatography were silica gel (100–200, 200–300 mesh, Qingdao Marine Chemical Factory, Qingdao, China), Sephadex LH20 (40–70 µm, Amersham Pharmacia Biotech AB, Uppsala, Sweden), and YMC Gel ODS-A (12 nm, S-50 µm YMC, MA, USA). The silica gel GF254 (0.4–0.5 mm) used for TLC were supplied by the Qingdao Marine Chemical Factory, Qingdao, China. HPLC was carried on shimadzu LC-10ATvp with YMC ODS SERIES (YMC-Pack ODS-A, 250 × 10 mm I.D., S-5 µm, 12 nm).
3.2. Animal Material
The soft coral Sinularia sp. was collected from Dongluo Island, Hainan province of China in July 2009 (7–10 m depth) and identified by Professor Hui Huang, South China Sea Institute of Oceanology, Chinese Academy of Sciences. A voucher specimen (No. 0907010) was deposited in the CAS Key Laboratory of Tropical Marine Bio-resources and Ecology, South China Sea Institute of Oceanology, Chinese Academy of Sciences, Guangzhou, China.
3.3. Extraction and Isolation
The fresh soft coral (wet, 6 kg) was extracted three times with 95% EtOH (20 L). The extract was concentrated under reduced pressure, and partitioned between H2O (4 L) and CHCl3 (4 L); the CHCl3 layer (120 g) was further partitioned between 85% EtOH (4 L) and petroleum ether (PE; 4 L) to yield 85% EtOH (34 g) and PE (75.6 g) fractions. The 85% EtOH fraction was separated by silica gel column using CHCl3/MeOH to yield 11 portions (Frs. s1–s11). Fr. s3 was purified by silica gel column to yield 12 portions, and portion 10 was further purified with semi-preparative HPLC, eluting with MeOH/H2O = 65:35 at a flow rate of 2 mL/min, to afford 1 (6.0 mg) and 2 (7.2 mg). Fr. s5 was purified by Sephadex LH-20 using CHCl3/MeOH = 1:1 to yield 3 portions, and portion 1 was further purified with semi-preparative HPLC, eluting with MeOH/H2O = 57:43 at a flow rate of 2 mL/min, to afford 5 (2.2 mg) and 6 (2.6 mg). Fr. s6 was separated by silica gel column using PE/EtOAc to yield 7 portions, and portion 1 was further purified with semi-preparative HPLC, eluting with MeOH/H2O = 70:30 at a flow rate of 2 mL/min, to afford 3 (2.2 mg) and 4 (3.7 mg).
Sinularianin C (3): Colorless oil; 1H- and 13C-NMR (see Table 1); HR-ESI-MS m/z 301.1416 [M + Na]+, (Calcd for C16H22NaO4, 301.1416).
Sinularianin D (4): Colorless oil; 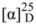 = −6.0 (c = 0.01, MeOH); UV (MeOH): λmax (log ε) = 204.2 (1.70); IR (KBr) νmax 3421, 2927, 2854, 1735, 1666 cm–1 (Supplementary Figure S21); 1H- and 13C-NMR (see Table 1); ESI-MS m/z 271 [M + Na]+, 519 [2M + Na]+.
= −6.0 (c = 0.01, MeOH); UV (MeOH): λmax (log ε) = 204.2 (1.70); IR (KBr) νmax 3421, 2927, 2854, 1735, 1666 cm–1 (Supplementary Figure S21); 1H- and 13C-NMR (see Table 1); ESI-MS m/z 271 [M + Na]+, 519 [2M + Na]+.
Sinularianin E (5): Colorless oil; 1H- and 13C-NMR (see Table 2); HR-ESI-MS m/z 303.1563 [M + Na]+, (Calcd for C16H24NaO4, 303.1572).
Sinularianin F (6): Colorless oil; 1H- and 13C-NMR (see Table 2); HR-ESI-MS m/z 287.1613 [M + Na]+, (Calcd for C16H24NaO3, 287.1623).
3.4. The Cell-Based HEK293 NF-κB Luciferase Reporter Gene Assay
All compounds were evaluated for inbibition of NF-κB activation using the cell-based HEK 293 NF-κB luciferase reporter gene assay according to the previously reported procedures [19].
4. Conclusions
The investigation of bioactive natural products from a Hainan soft coral, Sinularia sp., has led to the isolation of four new sesquiterpenes, sinularianins C–F (3–6), along with two other analogues, sinularianins A (1) and B (2). Compounds 1 and 4 were exhibited a potent inhibitory effect with inhibitory rates of 41.3% and 43.0% at the concentration of 10 µg/mL, respectively. The discovery of new compounds 3–6 has added to an extremely diverse and complex array of soft coral sesquiterpenes.
Acknowledgments
This study was supported by grants from the National Key Basic Research Program of China (973)’s Project (2010CB833800 and 2011CB915503), the National High Technology Research and Development Program (863 Program, 2012AA092104), National Natural Science Foundation of China (21302198, 31270402, 21172230, 30973679, 41376162 and 41176148), Knowledge Innovation Program of Chinese Academy of Science (SQ201117 and SQ201019), Guangdong Province-CAS Joint Research Program (2011B090300023 and 2012B091100264), and Guangdong Marine Economic Development and Innovation of Regional Demonstration Project (GD2012-D01-001 and GD2012-D01-002).
Supplementary Files
Supplementary Information (PDF, 1507 KB)
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Yang B., Zhou X.F., Huang H., Yang X.W., Liu J., Lin X.P., Li X.B., Peng Y., Liu Y.H. New cembrane diterpenoids from a Hainan soft coral Sinularia sp. Mar. Drugs. 2012;10:2023–2032. doi: 10.3390/md10092023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chao C.H., Chou K.J., Huang C.Y., Wen Z.H., Hsu C.H., Wu Y.C., Dai C.F., Sheu J.H. Steroids from the soft coral Sinularia crassa. Mar. Drugs. 2012;10:439–450. doi: 10.3390/md10020439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng S.Y., Huang K.J., Wang S.K., Duh C.Y. Capilloquinol: A novel farnesyl quinol from the Dongsha atoll soft coral Sinularia capillosa. Mar. Drugs. 2011;9:1469–1476. doi: 10.3390/md9091469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li R., Shao C.L., Qi X., Li X.B., Li J., Sun L.L., Wang C.Y. Polyoxygenated sterols from the South China Sea soft coral Sinularia sp. Mar. Drugs. 2012;10:1422–1432. doi: 10.3390/md10071422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kamel H.N., Slattery M. Terpenoids of Sinularia: Chemistry and biomedical applications. Pharm. Biol. 2005;43:253–269. doi: 10.1080/13880200590928852. [DOI] [Google Scholar]
- 6.Su J.H., Wen Z.H. Bioactive cembrane-based diterpenoids from the soft coral Sinularia triangular. Mar. Drugs. 2011;9:944–951. doi: 10.3390/md9060944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsai T.C., Wu Y.J., Su J.H., Lin W.T., Lin Y.S. A new spatane diterpenoid from the cultured soft coral Sinularia leptoclados. Mar. Drugs. 2013;11:114–123. doi: 10.3390/md11010114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tseng Y.J., Shen K.P., Lin H.L., Huang C.Y., Dai C.F., Sheu J.H. Lochmolins A–G, new sesquiterpenoids from the soft coral Sinularia lochmodes. Mar. Drugs. 2012;10:1572–1581. doi: 10.3390/md10071572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lai D.W., Li Y.X., Xu M.J., Deng Z.W., van Ofwegen L., Qian P.Y., Proksch P., Lin W.H. Sinulariols A–S, 19-oxygenated cembranoids from the Chinese soft coral Sinularia rigida. Tetrahedron. 2011;67:6018–6029. [Google Scholar]
- 10.Chao C.H., Hsieh C.H., Chen S.P., Lu C.K., Dai C.F., Sheu J.H. Sinularianins A and B, novel sesquiterpenoids from the Formosan soft coral Sinularia sp. Tetrahedron Lett. 2006;47:5889–5891. [Google Scholar]
- 11.Lu M.C., Lee N.L., Tseng S.W., Su J.H. Sinutriangulin A, a novel diterpenoid from the soft coral Sinularia triangula. Tetrahedron Lett. 2011;52:5869–5871. [Google Scholar]
- 12.Putra M.Y., Ianaro A., Panza E., Bavestrello G., Cerrano C., Fattorusso E., Taglialatela-Scafati O. Sinulasulfoxide and sinulasulfone, sulfur-containing alkaloids from the Indonesian soft coral Sinularia sp. Tetrahedron Lett. 2012;53:3937–3939. doi: 10.1016/j.tetlet.2012.05.095. [DOI] [Google Scholar]
- 13.Yamashita T., Nakao Y., Matsunaga S., Oikawa T., Imahara Y., Fusetani N. A new antiangiogenic C-24 oxylipin from the soft coral Sinularia numerosa. Bioorg. Med. Chem. 2009;17:2181–2184. doi: 10.1016/j.bmc.2008.10.083. [DOI] [PubMed] [Google Scholar]
- 14.Chai M.C., Wang S.K., Dai C.F., Duh C.Y. A cytotoxic lobane diterpene from the Formosan soft coral Sinularia inelegans. J. Nat. Prod. 2000;63:843–844. doi: 10.1021/np990539e. [DOI] [PubMed] [Google Scholar]
- 15.Sheu J.H., Chang K.C., Duh C.Y. A cytotoxic 5α,8α-epidioxysterol from a soft coral Sinularia species. J. Nat. Prod. 2000;63:149–151. doi: 10.1021/np9903954. [DOI] [PubMed] [Google Scholar]
- 16.Chao C.H., Chou K.J., Huang C.Y., Wen Z.H., Hsu C.H., Wu Y.C., Dai C.F., Sheu J.H. Bioactive cembranoids from the soft coral Sinularia crassa. Mar. Drugs. 2011;9:1955–1968. doi: 10.3390/md9101955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi H.Y., Yu S.J., Liu D., van Ofwegen L., Proksch P., Lin W.H. Sinularones A–I, new cyclopentenone and butenolide derivatives from a marine soft coral Sinularia sp. and their antifouling activity. Mar. Drugs. 2012;10:1331–1344. doi: 10.3390/md10061331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wright A.D., Nielson J.L., Tapiolas D.M., Liptrot C.H., Motti C.A. A great barrier reef Sinularia sp. yields two new cytotoxic diterpenes. Mar. Drugs. 2012;10:1619–1630. doi: 10.3390/md10081619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jacobo-Herrera N.J., Vartiainen N., Bremner P., Gibbons S., Koistinaho J., Heinrich M. NF-κB modulators from Valeriana officinalis. Phytother. Res. 2006;20:917–919. doi: 10.1002/ptr.1972. [DOI] [PubMed] [Google Scholar]
- 20.Bos R., Hendriks H., Bruins A.P., Kloosterman J., Sipma G. Isolation and identification of valerenane sesquiterpenoids from Valeriana officinalis. Phytochemistry. 1986;25:133–135. [Google Scholar]
- 21.Birnbaum G.I., Findlay J.A., Krepinsky J.J. Stereochemistry of valerenane sesquiterpenoids-crystal-structure of valerenolic acid. J. Org. Chem. 1978;43:272–276. doi: 10.1021/jo00396a020. [DOI] [Google Scholar]
- 22.Mao S.C., Guo Y.W., Shen X. Two novel aromatic valerenane-type sesquiterpenes from the Chinese green alga Caulerpa taxifolia. Bioorg. Med. Chem. Lett. 2006;16:2947–2950. doi: 10.1016/j.bmcl.2006.02.074. [DOI] [PubMed] [Google Scholar]
- 23.Kobayashi M., Yasuzawa T., Kyogoku Y., Kido M., Kitagawa I. Three new ent-valerenane sesquiterpenes from an Okinawan soft coral. Chem. Pharm. Bull. 1982;30:3431–3434. [Google Scholar]
- 24.Bowden B.F., Coll J.C., Desilva E.D., Decosta M.S.L., Djura P.J., Mahendran M., Tapiolas D.M. Studies of Australian soft corals. XXXI. Novel furanosesquiterpenes from several Sinularian soft corals (Coelenterata, Octocorallia, Alcyonacea) Aust. J. Chem. 1983;36:371–376. doi: 10.1071/CH9830371. [DOI] [Google Scholar]
- 25.Yang X.W., Ding Y.Q., Li X.C., Ferreira D., Shen Y.H., Li S.M., Wang N., Zhang W.D. Cycloabiesesquine A, a unique sesquiterpenoid from Abies delavayi. Chem. Commun. 2009;25:3771–3773. doi: 10.1039/b905710b. [DOI] [PubMed] [Google Scholar]
- 26.Peddibhotla S., Shi R.X., Khan P., Smith L.H., Mangravita-Novo A., Vicchiarelli M., Su Y., Okolotowicz K.J., Cashman J.R., Reed J.C., et al. Inhibition of protein kinase C-driven nuclear factor-kappa B activation: Synthesis, structure-activity relationship, and pharmacological profiling of pathway specific benzimidazole probe molecules. J. Med. Chem. 2010;53:4793–4797. doi: 10.1021/jm1000248. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information (PDF, 1507 KB)