Abstract
Cranial irradiation is a critical and effective treatment for primary brain tumors and metastases. Unfortunately, most patients who are treated and survive for more than a few months develop neural and cognitive problems as the result of radiation-induced normal tissue injury. The neurobiological mechanisms underlying these cognitive deficits remain largely unknown and there are no validated treatments to prevent or ameliorate them; thus, there is a significant and continuing need for preclinical studies in animal models. Investigations from several laboratories have demonstrated neurobiological changes after cranial irradiation in rodents. To date, however, experimental studies in animal models have included little assessment of the systemic effects of cranial irradiation, despite evidence from the clinic that cranial irradiation results in changes throughout the body and recognition that systemic responses may influence the development of neural and cognitive deficits. This study evaluated systemic effects of clinically relevant, fractionated whole-brain irradiation in adult rats and demonstrates effects on the growth hormone/insulin-like growth factor-I axis, which may contribute to the development of neural changes. These and other systemic responses are important to consider in ongoing efforts to understand the mechanisms of radiation-induced normal tissue injury.
INTRODUCTION
Cranial irradiation is an effective treatment for brain tumors and metastases but doses are limited by toxicity to normal tissue. Each year ~100,000 patients in the U.S. receive partial- or whole-brain irradiation and survive long enough to develop radiation-induced neural injury and cognitive deficits (1), which are significant detriments to quality of life (2). Understanding the neurobiological mechanisms and developing treatments to ameliorate or prevent such dysfunction are important goals.
Animal studies are critical for mechanistic and other preclinical investigations. Studies of the effects of whole-brain irradiation (WBI) in animals have concentrated primarily on hippocampal dependent learning and memory as well as neurobiological mechanisms thought to be directly modulated by irradiation such as neurogenesis, oxidative stress and neuroinflammation. Systemic effects of WBI rarely are assessed, despite recognition in the clinic that radiation therapy limited to the cranium impacts the entire body, often through treatment-induced endocrinopathies (3–5). Such as systemic changes may impact neural function and neural responses to brain irradiation in animal models, and these neurobiological changes should be considered within the broader context of somatic effects, which remain largely unknown.
In ongoing studies of the effects of normal-tissue injury and cognitive dysfunction after WBI in young adult and aging rats, we observed that rats receiving fractionated WBI (fWBI) gain less weight than do sham-irradiated control animals. Others have reported a similar effect after fractionated (6) and even single-dose WBI (7, 8). Prompted by this clear somatic response to fWBI, we assessed changes in several systemic measures for more than one year after clinically relevant fWBI in young adult and middle-aged rats. The data demonstrated significant and sustained radiation-induced deficits in body weight, brain weight, pituitary weight, pituitary growth hormone (GH) and plasma insulin-like growth factor-I (IGF-I). These changes were greater in rats irradiated as young adults than in rats irradiated in middle age. It also appeared that the serum level of brain-derived neurotrophic factor (BDNF) was modestly decreased by fWBI in young adult rats. Given the many ways in which these and other systemic factors may influence cognitive and other neural functions, they may be important co-variables that contribute to the mechanisms of dysfunction after cranial radiation therapy.
MATERIALS AND METHODS
Animal Husbandry
Male Fischer 344/Brown Norway F1 hybrid rats (F344BNF1) were obtained from the colony maintained by Harlan for the National Institutes on Aging at 2 and 17 months of age. The F344BNF1 hybrid rat is often utilized in studies of cognitive aging (9) because of its long lifespan and lower levels of aging-related pathologies (10). Rats were housed in pairs on a reverse light cycle (off 9:00 am/on 9:00 pm) with ad libitum access to food and water. All animals were maintained on standard rat chow throughout the experiment and no eating-related morbidity was observed.
Irradiation Procedure
Irradiation/sham irradiation was begun when rats were 3 or 18 months old (N = 24 irradiated and 24 sham-irradiated controls at each age). Anesthesia was by i.p. injection of 80 mg/kg ketamine and 6 mg/ kg xylazine; irradiations were performed 6–8 h into the rats’ dark cycle. Sham-irradiated (control) rats remained in their home cages while irradiated rats (four at a time) were positioned in lateral recumbency in a custom jig for X-ray irradiation using a GE Isovolt Titan orthovoltage X-ray unit (300 kV, 10 mA, 1.25 Gy/min) with a Panflex variable collimator (12 × 14 cm2 field size). A 2 cm thick, 20 × 20 cm2 cover of high-Z fusible alloy (radiation shielding alloy) shielded the rats’ bodies with a 30 × 30 mm opening over the lateral surface of each rat’s head. Additional, moveable alloy shields with 11 × 16 mm apertures were positioned using the eye and ear as landmarks to allow irradiation of the brain alone through the right and left lateral surfaces of the head. Dosimetry analysis using tissue equivalent radiochromic film positioned within agarose phantoms representing the sagittal midline target and exposed to 5 Gy irradiation demonstrated a sharp dose gradient at the field edge, to approximately 17% of dose by 0.5 mm beyond the field edge and to less than 10% by 2 mm beyond the field edge.
A dose of 40 Gy fWBI was achieved by 2 fractions of 5 Gy per week over a four-week period. Each 5 Gy fraction was given by consecutive doses of 2.5 Gy per lateral field, one from each side and separated by <10 min. The biologically effective dose (BED) calculated for 8×5 Gy is 106.7 Gy based on the linear-quadratic model (11) and assuming an α/β ratio of 3 Gy for late delayed effects in the brain. The corresponding BED for a typical regimen in the clinic (e.g., 30 fractions of 2 Gy in 6 weeks) is 100.2 Gy. Thus, biological effects of this prolonged fractionation regimen may be more relevant to the clinical situation than those observed after large single doses or fractionated doses given over a short period. Rats were maintained for 34 or 62 weeks after completion of fWBI.
Tissue Collection
Cohorts of rats were euthanized by sodium pentobarbital overdose (34 weeks) or carbon dioxide overdose (62 weeks; the change in euthanasia method was necessitated by a temporary drug unavailability) and then decapitated. Brains were removed, weighed, bisected sagittally at the midline, and the right hemisphere dissected. Sampling included olfactory bulb, prefrontal cortex (bregma 2.0 to anterior extent), dorsal striatum, medial frontal- and lateral frontal-dorsal cortex (all bregma 2.0 to −0.5, dorsal to rhinal fissure), hippocampus, dorsal- and ventral-posterior cortex (bregma −0.5 to posterior extent divided 1 mm above the rhinal fissure) (12) and cerebellum. The pituitary and brain samples were weighed, frozen on dry ice and stored at −80°C. Trunk blood was collected for plasma (plus 40 units/ml heparin, no incubation) and serum (no heparin, clotted 40 min at 25°C) and centrifuged 15 min at 1300g. Plasma (34 and 62 weeks survival groups) and serum (62 weeks survival group) were stored at −80°C.
Two additional rats at each age received a single 5 Gy dose and were euthanized 40–80 min later to verify the anatomical extent of irradiation using immunohistochemical labeling of γ-H2AX positive DNA double-strand breaks (DSB). Paraformaldehyde-fixed sections were cut using a cryostat and stored at −20°C until immunolabeled.
γ-H2AX Labeling
Sections were labeled using a γ-H2A.X mouse monoclonal antibody (Millipore, no. 05–636; 2 µg/ml final concentration), visualized using a biotinylated secondary antibody, Vectastain Elite ABC and diaminobenzidine (Vector Laboratories, Burlingame, CA).
Pituitary Growth Hormone Assay
Frozen pituitaries were homogenized on ice using a Dounce tissue grinder at a ratio of 50 ul homogenization buffer (20 mM Hepes at pH 7.5, 300 mM NaCl, 2 mM EDTA plus 1:250 Sigma-Aldrich P8340 protease inhibitor cocktail) for each mg tissue. Homogenates were centrifuged at 15,000g for 10 min and supernatants collected and stored at −80°C. Growth hormone was measured using a rat/mouse GH ELISA (EZRMGH-45K, Millipore) in supernatants serially diluted 1:40,471 in assay buffer. Supernatant soluble protein was measured using a BCA microassay procedure (Thermo Scientific Pierce).
Plasma IGF-I Assay
IGF-I in plasma was determined using a mouse/rat IGF-I ELISA (MG100, R&D Systems) on plasma samples serially diluted 1:2091 in kit calibrator diluent.
Serum BDNF Assay
BDNF in serum was determined using a BDNF ELISA (CYT306, Millipore) on serum samples diluted 1:76 in a 1:1 mixture of kit sample diluent and phosphate-buffered saline, pH 7.4, containing 1% BSA (A7030, Sigma-Aldrich).
RESULTS
γ-H2AX Immunolabeling
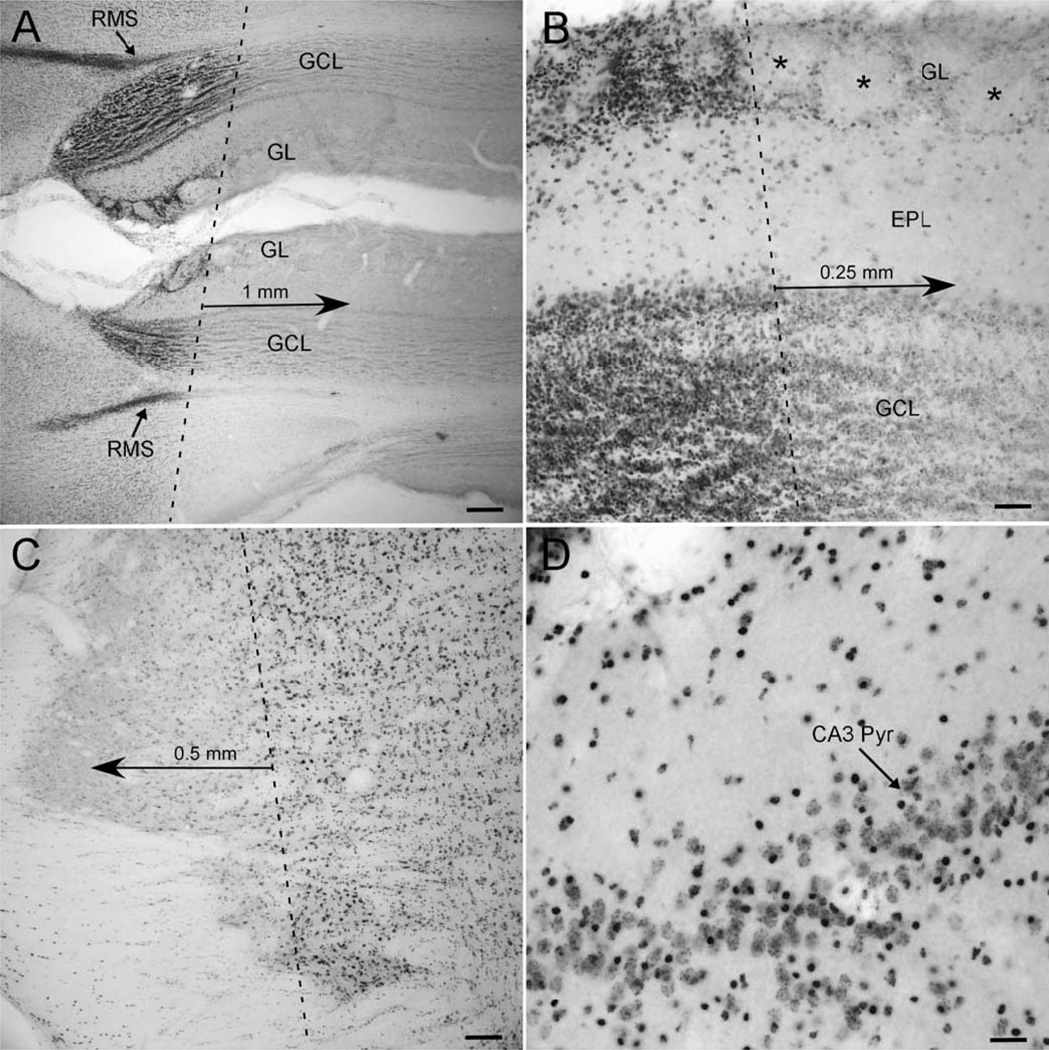
Visualizing DSB acutely after WBI demonstrated an irradiation field with sharply delineated edges that extended (1) anteriorly to include the frontal pole of the cortex but excluded the eyes and all but the most posterior portion of the olfactory bulb, and (2) posteriorly to include the cerebellum and rostral brain stem (Fig. 1). It was not possible to assess the irradiation field in each animal after each fraction, but the same pattern of DSB was evident in four rats, two of each age irradiated on each of two different days, indicating a high level of consistency across animals and fractions.
FIG. 1.
γ-H2AX immunolabeling of DNA double-strand breaks. Panel A: At low magnification the anterior margin of the irradiation field is clearest in regions of high cell density, the rostral migratory stream (RMS) and the glomerular (GL) and granule cell (GCL) layers of the olfactory bulb. Panel B: In the GL, glomeruli with almost no γ-H2AX-positive cells (asterisks) are immediately anterior to glomeruli in which every periglomerular cell appears labeled. Panel C: A similar edge of the irradiation field is evident in the brain stem. The dashed lines in panels A, B and C indicate the approximate edge of the radiation field; note the diminution in γ-H2AX immunolabeling over just a few hundred micrometers from that edge. Panel D: Every cell within the irradiated field appears γ-H2AX positive, but labeling of cell types is heterogeneous, as demonstrated in the pyramidal cell layer of CA3 in the hippocampus (CA3 Pyr). Scale bars = 250 µm (panel A), 50 µm (panel B), 100 µm (panel C), 25 µm (panel D).
Body Weight
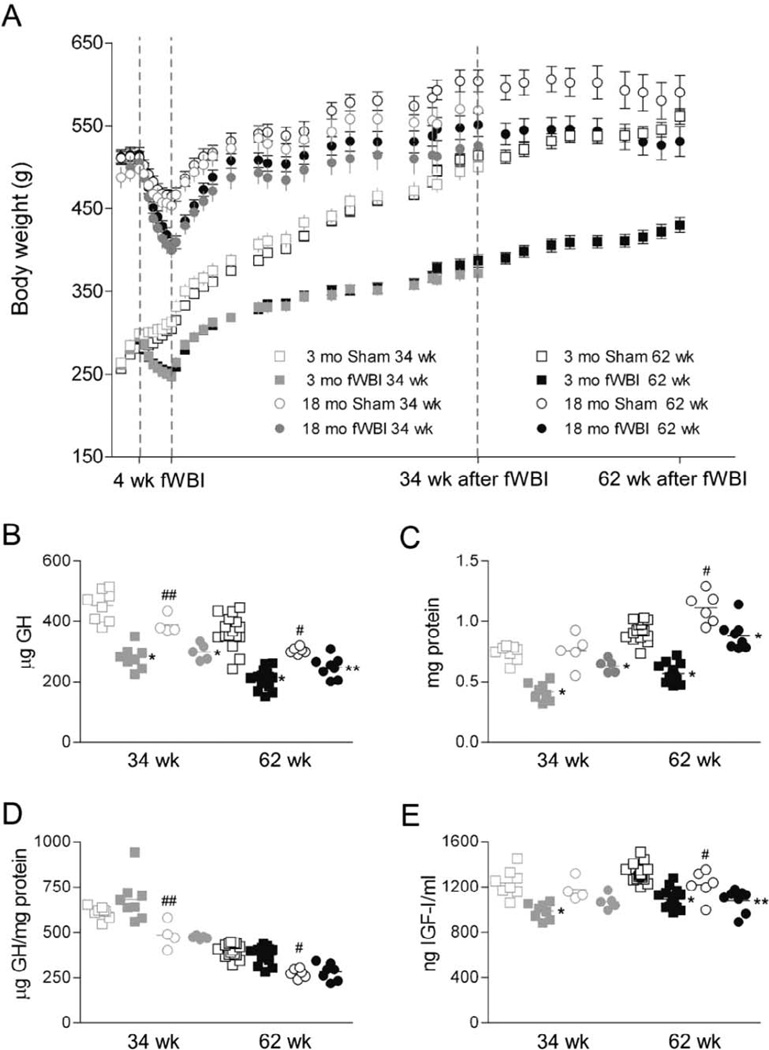
Anesthesia alone slowed growth in 3-month-old control rats and produced modest (~10%) and transient weight loss in 18-month-old control rats (Fig. 2A). The effect of anesthesia on body weight was exacerbated by irradiation, such that average weight declined ~15% in young adult rats and ~20% in middle aged rats during the 4 weeks of fWBI. Rats began to regain weight immediately after treatment, but irradiated rats remained smaller than control rats. For rats treated at 3 months of age, the difference between control and fWBI rats increased over the first 34 weeks, by which time fWBI rats weighed ~25% less than controls, and then stabilized or decreased slightly by 62 weeks after fWBI (~23% difference). For rats treated at 18 months of age, changes in the weight of irradiated rats after completion of fWBI paralleled those in age-matched control rats but irradiated rats remained 8.5% smaller than controls. Two-way repeated measures ANOVA testing for effects of condition and time after fWBI demonstrated that body weights differed significantly (P < 0.05) between irradiated and sham-irradiated control rats starting in the second week of irradiation for young adult rats and in the third week for middle aged rats. Weights remained significantly lower in fWBI rats than in age-matched controls until the rats were euthanized.
FIG. 2.
Effects of fWBI on body weight, growth hormone (GH) and IGF-I. Panel A: The mean (±SD) body weights of cohorts of rats surviving for 34 and 62 weeks are plotted. Panels B–E: Individual and mean values are plotted for sham and fWBI rats treated at 3 months of age (open and closed squares, respectively) or at 18 months of age (open and closed circles, respectively) and analyzed at 34 (gray) or 62 weeks (black) after completion of fWBI. Panel B: Total GH isolated from the pituitary. Panel C: Total pituitary protein. Panel D: Total GH normalized to total protein. Panel E: Plasma levels of IGF-I. *P < 0.001 and **P < 0.05 vs. sham control; #P < 0.001 and ##P < 0.05 comparing sham-irradiated control rats treated at 3 and 18 months of age (aging related change).
Total Brain, Regional and Pituitary Weights
Brain weight was stable in control rats across the ages represented, with no evidence of aging-related atrophy. However, at both the 34 and 62 week survival times, brain weight was significantly lower in irradiated rats (Tables 1 and 2), averaging ;8% lower in rats irradiated at 3 months and ~3% lower in rats irradiated at 18 months compared to age-matched controls.
TABLE 1.
Results of ANOVAs Testing for Main Effects of Age and fWBI and for Interactions for the Weights of the Brain, Brain Region that Showed Changes, and Pituitary and for Total Pituitary Growth Hormone, Total Pituitary Protein, the Ratio of Growth Hormone to Pituitary Protein and Plasma IGF-I
| Brain | Olfactory bulb |
Hippocampus | Dorsal cortex |
Cerebellum | Pituitary | Growth hormone |
Total pituitary protein |
Ratio | IGF-I | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 34 Weeks after fWBI | |||||||||||
| fWBI | F(1, 21) | 51.215 | 12.317 | 4.336 | 3.359 | 5.463 | 73.669 | 60.701 | 82.398 | 0.803 | 51.516 |
| P | <0.001 | 0.002 | 0.049 | 0.08 | 0.03 | <0.001 | <0.001 | <0.001 | 0.38 | <0.001 | |
| Age | F(1, 21) | 14.677 | 0.245 | 3.396 | 0.191 | 0.065 | 46.396 | 1.704 | 23.389 | 27.192 | 0.114 |
| p | <0.001 | 0.626 | 0.079 | 0.666 | 0.801 | <0.001 | 0.21 | <0.001 | <0.001 | 0.074 | |
| Interaction | F(1, 21) | 7.180 | 0.000 | 2.019 | 1.310 | 0.230 | 1.396 | 6.006 | 5.415 | 1.691 | 3.349 |
| P | 0.01 | 0.98 | 0.17 | 0.27 | 0.64 | 0.048 | 0.02 | 0.03 | 0.21 | 0.08 | |
| 62 Weeks after fWBI | |||||||||||
| fWBI | F(1, 45) | 48.415 | 6.525 | 3.886 | 21.177 | 21.610 | 72.515 | 61.817 | 95.915 | 0.449 | 3.855 |
| P | <0.001 | 0.01 | 0.06 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.51 | <0.001 | |
| Age | F(1, 45) | 0.890 | 0.657 | 1.706 | 0.084 | 0.101 | 93.501 | 0.879 | 73.390 | 61.066 | 38.160 |
| P | 0.35 | 0.42 | 0.20 | 0.77 | 0.75 | <0.001 | 0.35 | <0.001 | <0.001 | 0.06 | |
| Interaction | F(1, 45) | 9.231 | 8.690 | 4.603 | 0.540 | 1.207 | 2.177 | 13.390 | 4.603 | 1.337 | 2.390 |
| P | 0.004 | 0.005 | 0.04 | 0.47 | 0.28 | 0.15 | <0.001 | 0.04 | 0.26 | 0.13 | |
Note. Comparisons indicating significant effects of fWBI and/or age and significant interactions are highlighted in bold type.
TABLE 2.
Summary Values and Results of Post Hoc Comparisons of Brain, Region and Pituitary Weights (In Grams)
| Brain | Olfactory bulb | Hippocampus | Dorsal cortex | Cerebellum | Pituitary | |||
|---|---|---|---|---|---|---|---|---|
| 34 Weeks after | fWBI | |||||||
| 11 months | Sham | Mean | 2.255 | 0.050 | 0.068 | 0.187 | 0.147 | 0.011 |
| (N = 8) | Standard deviation | 0.045 | 0.005 | 0.005 | 0.012 | 0.009 | 0.001 | |
| fWBI | Mean | 2.067 | 0.045 | 0.062 | 0.176 | 0.135 | 0.007 | |
| (N = 8) | Standard deviation | 0.042 | 0.003 | 0.002 | 0.008 | 0.008 | 0.001 | |
| P (fWBI 3 months) | <0.001 | 0.01 | 0.01 | 0.03 | <0.001 | |||
| 26 months | Sham | Mean | 2.277 | 0.049 | 0.068 | 0.181 | 0.146 | 0.012 |
| (N = 5) | Standard deviation | 0.057 | 0.003 | 0.004 | 0.008 | 0.018 | 0.002 | |
| fWBI | Mean | 2.192 | 0.044 | 0.067 | 0.178 | 0.138 | 0.010 | |
| (N = 5) | Standard deviation | 0.050 | 0.005 | 0.006 | 0.007 | 0.007 | 0.000 | |
| P (fWBI 18 months) | 0.009 | 0.04 | 0.001 | |||||
| P (age in sham) | 0.004 | |||||||
| 62 Weeks after | fWBI | |||||||
| 17 months | Sham | Mean | 2.291 | 0.051 | 0.069 | 0.200 | 0.150 | 0.011 |
| (N = 16) | Standard deviation | 0.052 | 0.004 | 0.004 | 0.010 | 0.006 | 0.001 | |
| fWBI | Mean | 2.120 | 0.045 | 0.064 | 0.182 | 0.134 | 0.007 | |
| (N = 16) | Standard deviation | 0.051 | 0.003 | 0.005 | 0.011 | 0.011 | 0.001 | |
| P (fWBI 3 months) | <0.001 | <0.001 | 0.001 | <0.001 | <0.001 | <0.001 | ||
| 32 months | Sham | Mean | 2.255 | 0.049 | 0.068 | 0.196 | 0.146 | 0.014 |
| (N = 9) | Standard deviation | 0.045 | 0.003 | 0.002 | 0.012 | 0.010 | 0.002 | |
| fWBI | Mean | 2.188 | 0.049 | 0.068 | 0.183 | 0.137 | 0.011 | |
| (N = 9) | Standard deviation | 0.084 | 0.003 | 0.005 | 0.015 | 0.011 | 0.002 | |
| P (fWBI 18 months) | 0.02 | 0.02 | 0.03 | <0.001 | ||||
| P (age in sham) | <0.001 |
The weights of neural regions dissected from the brains of control rats were constant across the ages represented (~11–32 months). There were, however, region- and age-dependent effects of fWBI on the weights of 4 of the 9 neural regions collected (Tables 1 and 2). In rats treated at 3 months of age, the olfactory bulb, hippocampus and cerebellum weighed less in irradiated rats than in control rats at both 34 and 62 weeks after fWBI. The dorsal cortex also weighed less in irradiated rats at 62 weeks postirradiation. In rats treated at 18 months of age, the olfactory bulb was reduced ~10% in irradiated rats compared to control rats at 34 weeks after fWBI and the dorsal cortex was reduced ~7% at 62 weeks.
Pituitary weight in control rats increased across the ages examined (~35% greater in ~32-month-old rats than in ~11-month-old rats, Table 1), even after excluding pituitaries that showed gross hypertrophy (3 of 9 sham irradiated and 1 of 9 fWBI among rats irradiated at 18 months of age and examined at 62 weeks after fWBI). The effect of fWBI on the weight of the pituitary exceeded effects on the weight of the brain or any neural region (Table 1). In rats treated at 3 months of age, the pituitary was ~35% smaller in irradiated rats than in control rats at both 34 and 62 weeks after fWBI. The pituitary also was significantly smaller (15–20%) in irradiated rats than in control rats in the groups treated at 18 months of age.
Pituitary Growth Hormone
There was a significant aging related decline in the total amount of growth hormone isolated from the pituitary (Fig. 2B), despite an increase in total pituitary protein (Fig. 2C). Thus, the ratio of growth hormone to total protein declined progressively and significantly with age (Fig. 2D). Irradiation reduced total pituitary growth hormone at each age and survival period (Fig. 2B, effect similar in magnitude to the effect on pituitary weight) and also reduced total pituitary protein (Fig. 2C). Thus, the ratio of growth hormone to total protein was not affected by fWBI (Fig. 2D).
Plasma IGF-I
IGF-I levels in the 62 week survival group IGF-I were significantly lower in older (~32 months) than in younger (~17 months) control rats, indicating a decline late in life (Fig. 2E). Cranial irradiation produced a sustained, ~20% decline in plasma IGF-I in rats irradiated at 3 months of age and an ~10% decline in rats irradiated at 18 months of age (Fig. 2E).
Serum BDNF
In the 62 week survival group, mean (SD) serum BDNF levels (pg/ml) were: sham treated at 3 months 3261 (745), fWBI at 3 months 2836 (496), sham treated at 18 months 2603 (644), fWBI at 18 months 3081 (864). There was no overall effect of age [F(1,44) = 0.970, P = 0.33] or fWBI [F(1,44) = 0.015, p = 0.90] but there was a significant interaction [F(1,44) = 4.628, P = 0.04] with the mean BDNF level higher in young adult rats than in middle-age-sham-irradiated control rats (P = 0.04), indicating a decline associated with normal aging and a trend toward reduced BDNF levels in rats irradiated as young adults (P = 0.08).
DISCUSSION
Taken together, the results of this study indicate that the systemic effects of fWBI were caused by neural and subsequent endocrine changes, not by damage to extracranial structures. Visualizing DSB by immunolabeling for γ-H2AX provided an in vivo demonstration of the spatial extent of irradiation in the brain, demonstrating the anterior and posterior margins of the field and confirming that the entire dorsal-ventral extent was irradiated. Irradiating from the lateral aspect (rather than along the dorsal-ventral axis) spared the esophagus and the dimensions and positioning of the aperture were such that the sublingual, submaxillary and parotid glands, as well as the oral cavity and pharynx were shielded. Given its proximity to the ventral cranium, the dorsal-most component of the posterior nasopharynx may have received some dose (due to scatter) but less than 10% of the target dose, given the sharp drop off demonstrated using radiochromic film. Significantly, the systemic effects of fWBI in the present study differed greatly from those reported after wider head and neck irradiation in rats and attributed to extracranial damage (13). In that study, irradiated rats were emaciated (body weight reduced ≥50%) and clearly had increased morbidity and mortality, including malocclusion and associated dysphagia. In contrast, all of the irradiated rats in the current study: appeared healthy and behaved normally, remained on standard chow, did not develop malocclusion, and showed weight changes more or less in parallel with control rats but with irradiated rats just remaining smaller. Moreover, if radiation-induced weight changes were due to damage to salivary glands or the pharynx, one might expect the effects to be as great, if not greater, in older animals. In contrast, weight changes were much smaller in the rats irradiated in middle age, consistent with an age-dependent neuroendocrine mechanism.
It is clear in the clinic that brain irradiation changes the body and that systemic effects such as changes in endocrine pathways are prominent. Hypopituitarism is common after cranial irradiation in children and adults (3–5) and may impact growth, body composition, cardiovascular function, bone density and other factors that influence quality of life (4). To date, however, systemic effects of cranial irradiation have been little considered in experimental animal studies. Robinson and colleagues (14) reported that one dose of cranial irradiation (20–24 Gy) to eight-week-old rats induced dose- and time-dependent changes in pituitary hormones up to 20 weeks postirradiation, but longer-term effects and effects of clinically relevant fWBI have not been assessed previously. The influence of age at time of irradiation had not been evaluated previously, despite clinical evidence that systemic effects of WBI are age dependent. The goal of the current study was an initial assessment of the impact of fWBI on systems that are suggested by clinical observations to be sensitive to cranial irradiation.
Our findings demonstrate significant systemic changes, particularly changes in the GH/IGF-I axis, in a rodent model of radiation-induced brain injury. The magnitude of the observed changes was diminished in rats irradiated in middle age compared to those irradiated as young adults, indicating decreased vulnerability to these systemic effects with increasing age. This did not, however, represent a difference between a vulnerable “pediatric” brain and a radioresistant old brain. The younger animals in this study were as old or older than the rodents typically used in experimental studies of radiation-induced brain injury in adults and the older, middle aged rats clearly still showed systemic side effects, just like many middle aged and older patients in the clinic.
Radiation-induced changes in GH/IGF-I likely contributed to decreased body weight (presumably reflecting metabolic changes) and also may have contributed to decreased brain weight. The latter is consistent with our previous demonstration that adult-onset deficiency in growth hormone produces a significant decrease in brain weight (15). It is significant, as one considers possible mechanisms of radiation-induced cognitive dysfunction, that the many neural functions known to be influenced by growth hormone and IGF-I include each of the primary mechanisms currently proposed to underlie radiation-induced cognitive dysfunction: neurogenesis, neuroinflammation and oxidative stress. Neural precursor cells within the adult brain are directly responsive to growth hormone (16) as well as to IGF-I (17), and we and others have shown that the GH/IGF-I axis modulates hippocampal neurogenesis (15, 18, 19) and glial turnover (20, 21). IGF-I and its analogs also can modulate the response to neuroinflammatory stimuli (22), and alterations in growth hormone and/or IGF-I signaling influence oxidative stress defenses and responses (23, 24).
The indication here that fWBI resulted in decreases in the weight of some, but not all, neural regions raises an important caution as we and others continue to investigate the mechanisms of radiation-induced brain injury. It is widely thought that different neural regions may be differentially affected by brain irradiation, due to intrinsic differences in vulnerability (arising from heterogeneity in cell types, myelination, vascularization, local anti-inflammatory/antioxidant mechanisms, etc.) and/or differences in repair mechanisms. It may be necessary to consider an additional level of complexity–regional differences in the responses of the brain to systemic alterations in response to cranial irradiation.
The data presented here argue for assessment of systemic effects of WBI in experimental studies of the mechanisms and treatment of normal tissue injury following cranial irradiation, since such effects may, in turn and over time, significantly influence neural responses and development of cognitive deficits.
ACKNOWLEDGMENTS
This work was supported in part by NIH grant R01CA133483. We thank Jennifer Dorand for assistance with dosimetry studies. We also thank the late Dr. Mike Robbins for his insight and many contributions to this and other studies; he is missed greatly as a colleague and friend.
REFERENCES
- 1.Greene-Schloesser D, Robbins ME. Radiation-induced cognitive impairment—from bench to bedside. Neuro Oncol. 2012;4(Suppl):iv37–iv44. doi: 10.1093/neuonc/nos196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Butler JM, Rapp SR, Shaw EG. Managing the cognitive effects of brain tumor radiation therapy. Curr Treat Options Oncol. 2006;7:517–523. doi: 10.1007/s11864-006-0026-5. [DOI] [PubMed] [Google Scholar]
- 3.Darzy KH, Shalet SM. Hypopituitarism following radiotherapy revisited. Endocr Dev. 2009;15:1–24. doi: 10.1159/000207607. [DOI] [PubMed] [Google Scholar]
- 4.Thomas JD, Monson JP. Adult GH deficiency throughout lifetime. Eur J Endocrinol. 2009;161(Suppl 1):S97–S106. doi: 10.1530/EJE-09-0258. [DOI] [PubMed] [Google Scholar]
- 5.Madaschi S, Fiorino C, Losa M, Lanzi R, Mazza E, Motta M, et al. Time course of hypothalamic-pituitary deficiency in adults receiving cranial radiotherapy for primary extrasellar brain tumors. Radiother Oncol. 2011;99:23–28. doi: 10.1016/j.radonc.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 6.Yuan H, Gaber MW, Boyd K, Wilson CM, Kiani MF, Merchant TE. Effects of fractionated radiation on the brain vasculature in a murine model: blood-brain barrier permeability, astrocyte proliferation, and ultrastructural changes. Int J Radiat Oncol Biol Phys. 2006;66:860–866. doi: 10.1016/j.ijrobp.2006.06.043. [DOI] [PubMed] [Google Scholar]
- 7.Barlind A, Karlsson N, Bjo¨rk-Eriksson T, Isgaard J, Blomgren K. Decreased cytogenesis in the granule cell layer of the hippocampus and impaired place learning after irradiation of the young mouse brain evaluated using the IntelliCage platform. Exp Brain Res. 2010;201:781–787. doi: 10.1007/s00221-009-2095-8. [DOI] [PubMed] [Google Scholar]
- 8.Moravan MJ, Olschowka JA, Williams JP, O’Banion MK. Cranial irradiation leads to acute and persistent neuroinflammation with delayed increases in T-cell infiltration and CD11c expression in C57BL/6 mouse brain. Radiat Res. 2011;176:459–473. doi: 10.1667/rr2587.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bizon JL, Nicolle MM. Rat models of age-related cognitive decline. In: Conn PM, editor. Handbook of models for human aging. Burlington, MA: Elsevier; 2006. pp. 379–391. [Google Scholar]
- 10.Lipman RD, Chrisp CE, Hazzard DG, Bronson RT. Pathologic characterization of brown Norway, brown Norway x Fischer 344, and Fischer 344 x brown Norway rats with relation to age. J Gerontol A Biol Sci Med Sci. 1996;51:B54–B59. doi: 10.1093/gerona/51A.1.B54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fowler FJ. The linear quadratic formula and progress in fractionated radiotherapy. Br J Radiol. 1989;62:679–694. doi: 10.1259/0007-1285-62-740-679. [DOI] [PubMed] [Google Scholar]
- 12.Paxinos G, Watson C. The Rat brain in stereotaxic coordinates. 4th ed. San Diego, CA: Academic Press; 1998. [Google Scholar]
- 13.Nagler RM. Extended-term effects of head and neck irradiation in a rodent. Eur J Cancer. 2001;37:1938–1945. doi: 10.1016/s0959-8049(01)00213-1. [DOI] [PubMed] [Google Scholar]
- 14.Robinson IC, Fairhall KM, Hendry JH, Shalet SM. Differential radiosensitivity of hypothalamo-pituitary function in the young adult rat. J Endocrinol. 2001;169:519–526. doi: 10.1677/joe.0.1690519. [DOI] [PubMed] [Google Scholar]
- 15.Lichtenwalner RJ, Forbes ME, Sonntag WE, Riddle DR. Adultonset deficiency in growth hormone and insulin-like growth factor-I decreases survival of dentate granule neurons: Insights into the regulation of adult hippocampal neurogenesis. J Neurosci Res. 2006;83:199–210. doi: 10.1002/jnr.20719. [DOI] [PubMed] [Google Scholar]
- 16.Blackmore DG, Reynolds BA, Golmohammadi MG, Large B, Aguilar RM, Haro L, et al. Growth hormone responsive neural precursor cells reside within the adult mammalian brain. Sci Rep. 2012;2:250. doi: 10.1038/srep00250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anderson MF, Aberg MA, Nilsson M, Eriksson PS. Insulin-like growth factor-I and neurogenesis in the adult mammalian brain. Brain Res Dev Brain Res. 2002;134:115–122. doi: 10.1016/s0165-3806(02)00277-8. [DOI] [PubMed] [Google Scholar]
- 18.Lichtenwalner RJ, Forbes ME, Bennett SA, Lynch CD, Sonntag WE, Riddle DR. Intracerebroventricular infusion of insulin-like growth factor-1 ameliorates the age-related decline in hippocampal neurogenesis. Neuroscience. 2001;107:603–613. doi: 10.1016/s0306-4522(01)00378-5. [DOI] [PubMed] [Google Scholar]
- 19.Llorens-Martín M, Torres-Alemán I, Trejo JL. Mechanisms mediating brain plasticity: IGF1 and adult hippocampal neurogenesis. Neuroscientist. 2009;15:134–148. doi: 10.1177/1073858408331371. [DOI] [PubMed] [Google Scholar]
- 20.Aberg ND, Johansson UE, Aberg MA, Hellström NA, Lind J, Bull C, et al. Peripheral infusion of insulin-like growth factor-I increases the number of newborn oligodendrocytes in the cerebral cortex of adult hypophysectomized rats. Endocrin. 2007;148:3765–3772. doi: 10.1210/en.2006-1556. [DOI] [PubMed] [Google Scholar]
- 21.Hua K, Forbes ME, Lichtenwalner RJ, Sonntag WE, Riddle DR. Adult-onset deficiency in growth hormone and insulin-like growth factor-I alters oligodendrocyte turnover in the corpus callosum. Glia. 2009;57:1062–1071. doi: 10.1002/glia.20829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guan J, Gluckman PD. IGF-1 derived small neuropeptides and analogues: a novel strategy for the development of pharmaceuticals for neurological conditions. Br J Pharmacol. 2009;157:881–891. doi: 10.1111/j.1476-5381.2009.00256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Donahue AN, Aschner M, Lash LH, Syversen T, Sonntag WE. Growth hormone administration to aged animals reduces disulfide glutathione levels in hippocampus. Mech Ageing Dev. 2006;127:57–63. doi: 10.1016/j.mad.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 24.Brown-Borg HM, Rakoczy SG, Sharma S, Bartke A. Long-living growth hormone receptor knockout mice: potential mechanisms of altered stress resistance. Exp Gerontol. 2009;44:10–9. doi: 10.1016/j.exger.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]