Abstract
The realm of carbohydrate vaccines has expanded far beyond the capsular polysaccharides of bacterial pathogens to include a diverse collection of targets representing nearly every biological kingdom. Recent technological advances in glycobiology and glycochemistry are paving the way for a new era in carbohydrate vaccine design enabling greater efficiency in the identification, synthesis and evaluation of unique glycan epitopes found on a plethora of pathogens and malignant cells. This article reviews the progress being made in addressing challenges posed by targeting the surface carbohydrates of bacteria, protozoa, helminths, viruses, fungi and cancer for vaccine purposes.
Introduction
Since Edward Jenner’s seminal discovery in 1796 that inoculation with cowpox could protect against smallpox infection, vaccines have become a hugely important and successful counter measure to the threat of infectious disease 1. Vaccines provide protection by inducing humoral and/or cellular immunity to the pathogens causing disease. With respect to humoral immunity, the dense surface distribution of often unique glycan structures on diverse pathogens and on malignant cells makes carbohydrates attractive vaccine targets (Figure 1).
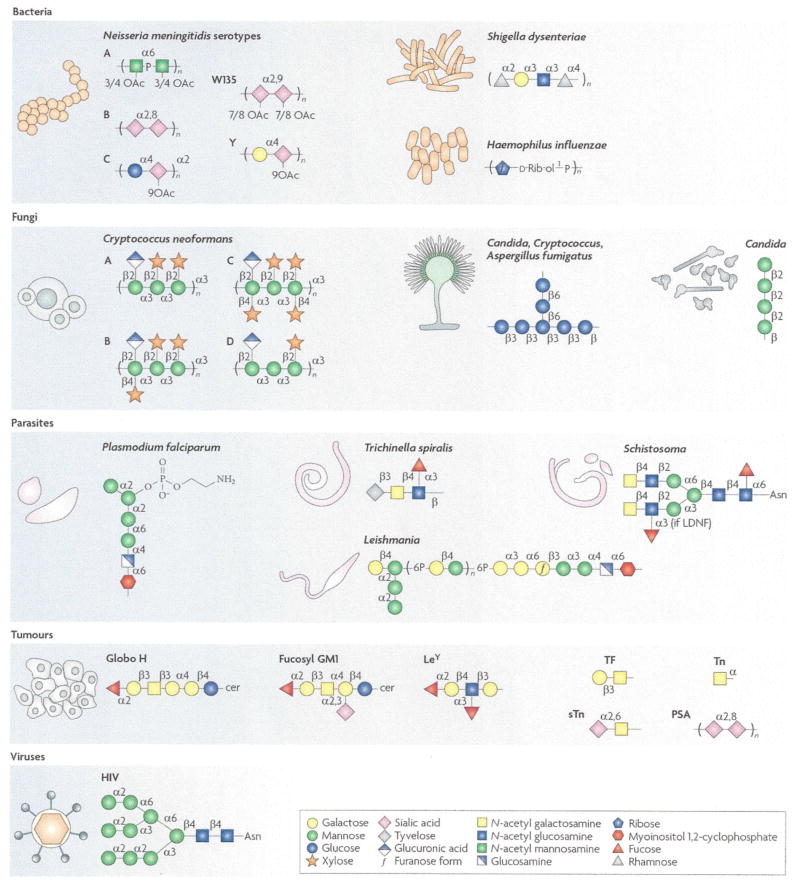
Figure 1. A diverse array of disease-causing agents and glycan antigens are targeted by existing and developmental carbohydrate vaccines.
Bacteria: capsular polysaccharide repeats associated with particular species (and serotypes). Fungi: β-glucan (Candida, Cryptococcus and Aspergillus), β-mannan (Candida), common GXM motifs for serotypes A-D (Cryptococcus). Parasites: synthetic GPI motif (Plasmodium falciparum), common tyvelose-containing Ag (Trichinella), LDN(F) (Schistosoma), common LPG (Leishmania). Viruses: high mannose, GlcNAc2Man9 (HIV). Tumours: common glycan antigens associated with glycolipids (Globo H, Fucosyl GM1, LeY) and glycoproteins (TF, LeY, Tn, sTn and PSA) found on various malignant tissues, see Table 3.
* Mannose residues may be 6O-acetylated.
Using carbohydrates to induce immunity is a relatively new strategy, even though Heidelberger and Avery made the connection between pneumococcal serotype and capsular polysaccharide as early as 1923 2. Francis and Tillet had then noted that intradermal immunization with type-specific polysaccharide elicited antibodies (Abs) against heterologous types of pneumococci species 3 and Heidelberger and coworkers had established that vaccination with pneumococcal capsular polysaccharide could be used to elicit persistent antibody-mediated immunity 4. Despite these key discoveries, the advent of chemotherapeutics and antibiotics during this same period dampened enthusiasm for developing carbohydrate vaccines. The steady increase in antibiotic resistance since then has catalyzed a renewed interest. In 1983, the first polysaccharide vaccine, PneumoVax™, was commercially launched. This vaccine was composed of unconjugated capsular polysaccharide isolated from 14 different pneumonia serotypes; the current incarnation includes 23 out of approximately 90 known serotypes 5, 6. In healthy adults, this vaccine induces protection against ~90% of infections caused by these pathogens. However, in high risk groups, i.e. neonates and children under 2 years of age, elderly and immunocompromised people, polysaccharides generally elicit poor antibody responses and do not induce adequate protection 7.
The poor quality of antibody responses to carbohydrates is but one of many obstacles (see below) associated with developing carbohydrate-based vaccines and is largely attributed to the T-cell independent immune responses typically generated by repetitive carbohydrate antigens (Ags) 8, 9. B-cell receptor crosslinking through binding repetitive motifs activates Ag-specific B-cells independent of CD4+ helper T cells. Such T-cell independent responses are less robust, short-lived and primarily consist of IgM Abs. In contrast, CD4+ T-cell help, as typically generated in response to proteins, enables the generation of high affinity, class-switched Abs and subsequently, long-lived Ab-mediated protection. Zwitterionic capsular polysaccharides from some bacteria are an exception as these carbohydrates, like proteins, can be processed and presented on MHC class II molecules for activation of CD4+ helper T-cells and the generation of T-cell dependent immune responses 10, 11. To recruit CD4+ T-cell help for Ab responses against the vast majority of glycans, exogenous CD4+ T cell epitopes must be provided, usually in the form of a carrier protein. As early as 1931, Avery and Goedel reported that conjugation of glycans to a suitable protein scaffold enhanced the immunogenicity of carbohydrates 12. It is now well known that immunization with neoglycoconjugates composed of capsular polysaccharide-derived glycans covalently coupled to an immunogenic protein carrier (conjugate vaccines) induces long lasting protection against encapsulated bacteria even amongst persons in high risk groups13, 14. The success of early conjugate vaccines was a key breakthrough that propelled the field forward15. Several conjugate versions of polysaccharide vaccines are now either commercially available or in development 16 (Table 1 and 2).
Table 1.
Licensed carbohydrate-based vaccines
| Indication | Vaccine | Manufacturer |
|---|---|---|
| Haemophilus influenzae type b (Hib) | Glycoconjugugate, polysaccharide with TT [ActHIB and Hiberix] | Sanofi Pasteur, SA (ActHIB); GlaxoSmithKline Biologicals, S.A. (Hiberix) |
| Diphtheria and tetanus toxoids and acellular pertussis adsorbed, inactivated poliovirus and Hib-TT conjugate vaccine [Pentacel] | Sanofi Pasteur, Ltd. | |
| Hib conjugate (meningococcal protein conjugate) [PedvaxHIB] | Merck & Co, Inc. | |
| Hib conjugate (meningococcal protein conjugate) and hepatitis B (recombinant) vaccine [Comvax] | Merck & Co, Inc. | |
|
| ||
| Neisseria meningitides A, C, Y and W-135 | Glycoconjugate, meningococcal polysaccharide with DT [Menactra] | Sanofi Pasteur, Inc. |
| Meningococcal polysaccharide [Menomune-A/C/Y/W-135 ] | Sanofi Pasteur, Inc. | |
|
| ||
| Salmonella typhi | Vi polysaccharide [TYPHIM Vi] | Sanofi Pasteur, SA |
|
| ||
| Streptococcus pneumoniae 4, 6B, 9V, 14, 18C, 19F, and 23F | Pneumococcal 7-valent CRM197 conjugate [Prevnar] | Wyeth Pharmaceuticals, Inc. |
| Streptococcus pneumoniae 1, 2, 3, 4, 5, 6B, 7F, 8, 9N, 9V, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19F, 19A, 20, 22F, 23F and 33F | Pneumococcal polysaccharide, 23-valent [Pneumovax 23] | Merck & Co, Inc. |
Note: This table was adapted from the complete list of vaccines licensed for immunization and distribution in the US, found at www.fda.gov.
Table 2.
Examples of carbohydrate-based vaccines in development
| Indication | Vaccine | Phase of development |
|---|---|---|
| Enterohemorrhagic Escherichia coli | O-specific polysaccharide conjugate | Phase I 179 |
|
| ||
| Group A streptococcus | Glycoconjugate, Group A polysaccharide with tetanus toxoid (TT) | Preclinical 180 |
|
| ||
| Group B streptococcus | Glycoconjugates, type Ia, Ib, II, III, and V polysaccharides linked to carrier proteins | Phase II 181 |
|
| ||
| Haemophilus influenzae (nontypeable) | Subunit detoxified lipooligosaccharide conjugate | Preclinical 182 |
|
| ||
| Pseudomonas aeruginosa | Octavalent glycoconjugate of O-polysaccharide with toxin A | Phase III 183 |
|
| ||
| Salmonella typhi | rPAE-Vi conjugate vaccine | Phase III 33, 184 |
|
| ||
| Shigella dysenteriae | O-specific polysaccharide-protein conjugate | PreclinicaI 42 |
|
| ||
| Shigella flexineri | O-specific polysaccharide-protein conjugate | Phase II 185 |
|
| ||
| Shigella sonnei | O-specific polysaccharide-protein conjugate | Phase III 186 |
|
| ||
| Streptococcus pneumoniae | Glycoconjugates of synthetic 6B polysaccharide motifs | Preclinical 43 |
|
| ||
| Vibrio cholerae | Lipopolysaccharide (LPS) protein conjugate | Phase I 187 |
|
| ||
| Aspergillus fumigatus | β-glucan CRM197 conjugate | Preclinical 65, 66 |
|
| ||
| Candida albicans | Cell surface oligomannosyl epitope (various conjugates) | Preclinical 63, 64 |
| β-glucan CRM197 conjugate | Preclinical 65, 66 | |
|
| ||
| Cryptococcus neoformans | Glycoconjugate of CPS with TT | Phase I 50 |
| β-glucan CRM197 conjugate | Preclinical 65, 66 | |
|
| ||
| Leishmania spp | lipophosphoglycan (LPG) | Preclinical 97 |
| LPG conjugates | Preclinical 103 | |
|
| ||
| Plasmodium falciparum | Glycosylphosphotidylinositol (GPI) conjugated to keyhole limpet haemocyanin (KLH) | Preclinical 93 |
|
| ||
| HIV-1 | Manα1–2Man oligomannosyl epitope (various conjugates, engineered yeast strains and modified glycoproteins) | Preclinical 17, 113–118, 165 |
|
| ||
| Breast cancer | Unimolecular hexavalent conjugates (Globo-H-GM2-Lewis-y-sTn- TF-Tn-R) | Preclinical 149 |
| sTn(c)-KLH, QS21 adjuvant | Phase I 137 | |
|
| ||
| Epithelial cancer | Globo-H-GM2-Lewis-y-MUC1–32(aa)-sTn(c)-TF(c)-Tn(c)-KLH conjugate vaccine, QS21 adjuvant | Phase I 147 |
|
| ||
| Melanoma | GM3NPhAc-KLH | preclinical 152 |
|
| ||
| Prostate cancer | Unimolecular hexavalent conjugates (Globo-H-GM2-Lewis-y-sTn- TF-Tn-R) | Preclinical 149 |
| TF(c)-KLH, QS21 adjuvant | Phase I 138 | |
| Tn(c)-KLH and Clustered Tn-palmitic acid (PAM) | Phase I 139 | |
| Globo H-GM2-Lewis-y-MUC1–32-mer-TF(c)-Tn(c)-KLH conjugate vaccine, QS21 adjuvant | Phase II 148 | |
Note: This table was adapted from “The Jordan Report Accelerated Development of Vaccines 2007” and updated with additional information from publically available resources. This table should not be considered inclusive of all ongoing carbohydrate vaccine research and development.
The field of carbohydrate vaccine design is now undergoing another quantum leap as a result of recent technological advances (Box 1). The explosion in glycomics research is opening doors for carbohydrate vaccine researchers to better tackle the challenges inherent to carbohydrate vaccine development, and to expand the field to encompass a broader spectrum of diseases, in addition to bacterial infections. While a number of reviews have described carbohydrate-based vaccines for specific indications 17–19, a broad survey is warranted to put in perspective the advances in the field as a whole (see also 20, 21). This Review will focus on the following questions: What are the shared and unique problems involved in creating carbohydrate vaccines for such diverse indications as bacterial, viral and parasitic infections and cancer? What solutions have been found and can they be applied across fields? What are the emerging challenges and future prospects for carbohydrate vaccine design and development?
Box 1. Glycomics and Glycovaccines.
Carbohydrate vaccine development is benefiting from the new glycomics technologies and the establishment of international glycomics consortia, such as the Consortium for Functional Glycomics (CFG) and EuroCarbDB 160. Advances in glycan analysis, synthesis, array fabrication and structure determination are especially valuable.
Glycan analysis
The identification of potential carbohydrate epitope targets is typically the first step in the development of a carbohydrate vaccine. Analysis and purification of naturally occurring glycans is complicated by the microheterogeneity of carbohydrates arising from non-template-driven biosynthesis. In addition, there is a trade-off between the ability to perform high-throughput analysis of glycan mixtures and the ability to do fine structure characterization. Biochemical and analytical methods such as nuclear magnetic resonance (NMR), electrospray ionization-mass spectrometry (ESI-MS), matrix-assisted laser desorption ionization MS (MALDI MS) and capillary electrophoresis have been developed to profile the repertoire of carbohydrate structures isolated from cells and tissues160. Each methodology is best suited for determining a certain set of attributes, e.g. chain length and mass composition relationships from MALDI-MS or monosaccharide composition and linkage information using NMR. To improve the utility of these methods and enable higher throughput analysis, informatics-based sequencing methodologies are being used to integrate information from multiple complementary techniques161, 162.
Glycan synthesis
A key bottleneck in carbohydrate vaccine research is access to the carbohydrate antigens themselves. Since natural sources are often limited and require complicated purification procedures, the onus has largely fallen on synthetic strategies to supply these ligands for vaccine research purposes. Advances in solution- and solid-phase glycan synthesis methods, as well as chemoenzymatic glycan assembly methods, have accelerated and simplified the production of complex carbohydrates such as β-glucan dodecasaccharide and GloboH163, 164, 165. Automated approaches are based on two versatile strategies: reactivity-based one-pot synthesis and solid support synthesis 163, 164. However, several issues remain to be addressed before these technologies are widely applicable, such as expansion of the repertoire of protected monosaccharide building blocks, glycosyltransferases and glycosidases. It should also be noted that each synthetic route must still be optimized and the required building blocks synthesized, which often requires several steps. Automated oligosaccharide synthesis often requires large, molar excesses of the building blocks, which also limits the appeal of this approach for many laboratories Together, the ongoing improvements in isolation and purification methods are expected to allow carbohydrate vaccine researchers easier and faster access to defined carbohydrates in the near future.
Glycan arrays
Improved access to a large assortment of purified carbohydrates has enabled the development of high-throughput glycan-binding assays. The low affinity of protein-glycan interactions and high dependency on multivalent interactions is addressed by displaying glycans in an arrayed chip-based format. Several glycan arrays have been fabricated that utilize either covalent or noncovalent attachment protocols 166. Fluorescent readouts are most common, though recently, surface plasmon resonance (SPR) and MALDI - time of flight (MALDI-TOF) MS have been used as label-free alternatives 167–170. From a vaccine perspective, these glycan arrays are invaluable tools for identifying relevant glycan targets and for evaluating candidate immunogens by revealing the fine specificity of relevant anti-carbohydrate Abs elicited during natural infection e.g. anti-HIV Ab 2G12117, 118, 171.
Glycan structure determination
Structural data of protein-glycan interactions may provide valuable insights into the appropriate presentation of glycan Ags. Unfortunately, these data are difficult to obtain and consequently, relatively rare. A significant challenge stems from the flexibility of glycans which assume multiple (defined) conformational states under physiological conditions. Currently, the combination of X-ray crystallography, NMR and molecular dynamics simulations and other computational methods, such as docking simulations and absolute and relative free energy calculations, are employed in structural glycobiology to elucidate the conformations of free carbohydrates and protein-carbohydrate complexes 172, 173. Emerging technologies include oxidative footprinting of carbohydrate binding surfaces, which complements epitope mapping techniques, such as saturation transfer difference (STD) NMR 174. Another promising approach for 3D carbohydrate-protein complex determination combines partially oriented NMR spectroscopy with computational simulations 174.
General challenges associated with carbohydrate vaccine development
For most vaccines, the induction of protective Abs is thought crucial to efficacy. The nature of glycans presents a number of challenges with respect to inducing protective Abs. As already discussed, carbohydrates are often poorly immunogenic. Furthermore, anti-carbohydrate Abs are typically low affinity (≥ μM), compared to anti-protein Abs (nM). Protein-carbohydrate binding is mediated by a high degree of hydrogen bonding, and van der Waal’s, hydrophobic and electrostatic interactions, i.e. the same sorts of interactions involved in protein-protein binding 22–26. Antibody binding to both carbohydrates and proteins involves a favorable enthalpy contribution to the free energy of interaction 22, 24. However, for carbohydrates, this is offset to a significant degree by an unfavorable entropy contribution, which has been attributed mainly to either the loss of conformational flexibility in the oligosaccharide upon complex formation 27 or to solvent rearrangement upon binding (as it has been suggested that water molecules hydrogen-bonded to amphiphilic surfaces of unbound oligosaccharides are more mobile and less strongly hydrogen-bonded than water molecules in bulk solution 28). These unfavorable solvent rearrangements may override favorable entropy contributions from hydrophobic effects when Abs bind to carbohydrates as opposed to proteins. Due to the inherent low affinity, glycan interactions rely upon avidity effects enabled through multivalent interactions. Glycan microheterogeneity on glycoprotein and glycolipids is yet another obstacle to overcome in the identification and targeting of specific Ags (see Box 1). Moreover, the heterogeneous display of glycans on target organisms (or cells) can dilute the efficacy of any specific anti-glycan Ab response.
Four important considerations are generally applicable to the design of modern carbohydrate-based glycoconjugate vaccines: Ag source, carrier, conjugation method and adjuvant (Figure 2). Glycan Ags are diverse and range from large elaborate capsular polysaccharides to small monosaccharide tumour Ags (Figure 1). In general, polysaccharides exist as a family of closely related species that vary in their degree of polymerization. Since the pertinent immunogenic epitopes comprise only part of the glycan, oligosaccharides are often adequate for vaccine preparation. These molecules may be derived from digestion of naturally derived polysaccharides or produced as a chemically homogenous species through synthetic methods. Carriers, most often proteins, e.g. toxoids, KLH, virus capsids, though other materials are possible (Figure 2), should be immunogenic and express multiple loci for conjugation as polyvalent display is crucial to generating anti-carbohydrate Ab responses. Coupling of oligosaccharide Ags to the carrier necessitates activation of the sugars and/or the carrier. Several procedures have been developed to activate polysaccharides, but most result in the creation of reactive groups that are randomly distributed throughout the polymer. This random array of conjugation points is not conducive to creating homogenous glycoconjugates. To generate well-defined conjugates, the linkage between sugar and carrier should be as specific as possible. One advantage of the synthetic route is that glycans can be produced with a readily-activatable linker so that a single conjugation chemistry can be used for a wide range of products (see Box 2). A number of synthetic linkers are available,29, 30 but one must be cautious of the immunogenicity of these linkers relative to the glycan Ag 31, 32. The immune response may be predominantly directed against the linker and away from the carbohydrate antigen. Also, steric issues may be addressed with bifunctional spacers to enhance the efficiency of loading. Finally, adjuvants are often included to improve the immunogenicity of the target carbohydrates antigens. Unfortunately, alum is still the only adjuvant approved for human use but several promising formulations are in clinical trials, e.g. QS-21.
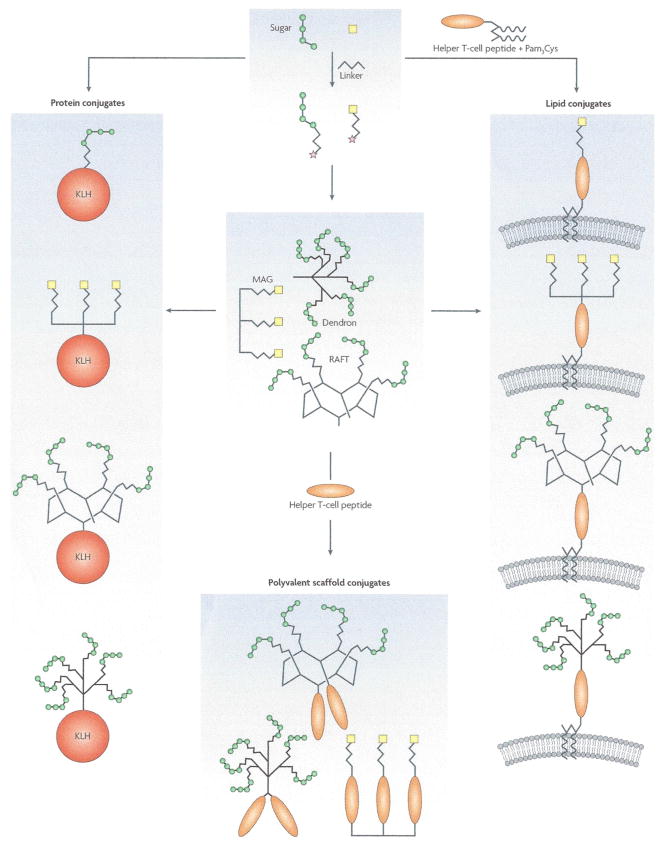
Figure 2. Schematic representation of glycoconjugate immunogen design.
Starting from activated glycans (* denotes activated group) from natural or synthetic sources, the production of three categories of glycoconjugate immunogens are shown: protein conjugates, lipid conjugates and polyvalent scaffold conjugates. The requirement for both polyvalent display and T helper epitopes, critical for achieving strong, long-lasting and class-switched Ab responses, are satisfied in each category. For protein conjugates, activated glycans are covalently attached to immunogenic protein carriers (e.g. KLH), which provide T helper epitopes and enable polyvalent display. Lipid conjugates, made by covalent linkage of activated glycans to T helper peptides attached to lipid moieties, allow polyvalency through formulation into lipid membranes. In addition, activated glycans may first be conjugated to synthetic polyvalent scaffolds (e.g. dendron, MAG and RAFT) which may then be used to make protein and lipid conjugates. Alternatively, polyvalent scaffold conjugates may be made through addition of T helper peptides alone. Adjuvants are usually included in the final glycoconjugate vaccine formulations (e.g. Alum and QS-21). Note: Pam3Cys also has adjuvant properties. MAG: multiple antigen glycopeptides. RAFT: regioselectively addressable functionalized templates
Box 2. First synthetic carbohydrate vaccine.
Haemophilus influenzae type b (Hib) is a pathogen that, prior to the introduction of Hib conjugate vaccines in 1988, was the leading cause of bacterial meningitis in children in the US. These vaccines, which contain capsular polysaccharide isolated from the pathogen, have reduced the incidence of bacterial meningitis and pneumonia in the developing world by more than 95% 175. However, introduction of the vaccine in developing countries has been very slow due to its high cost and limited availability176. The WHO estimates that, in the developing world, Hib is currently responsible for approximately three million serious illnesses and an estimated 386,000 deaths per year, almost all of which are children under the age of five 176.
The first commercial vaccine containing a synthetic carbohydrate antigen was developed in Cuba against Hib. This vaccine, QuimiHib® (Figure 3), exhibits several advantages associated with the synthetic route: i) potentially lower production costs compared to conventional vaccines using carbohydrates from natural sources, ii) controlled production of a homogeneous single compound including the linker, iii) minimal batch-to-batch variability during manufacturing process and iv) higher quality control standards are permitted, compared with the use of naturally derived agents. In addition, synthetic carbohydrates can be modified to increase their immunogenicity.
In 1989, a team from Cuba embarked on a project to produce a new, more economical conjugate anti-Hib vaccine from a fully synthetic capsular polysaccharide antigen (Figure 1). In collaboration with a Canadian chemist they spent 2 years developing a streamlined synthetic scheme that is amenable to large scale production40. The resulting synthetic pathway makes use of a one-step polycondensation reaction with the use of H-phosphonate chemistry and yields oligomers with on average 8 repeating units of polyribosylribitol with an 80% yield after purification by size exclusion chromatography 40. The antigen was first conjugated to human serum albumin (HSA) for antigenic evaluation and then to tetanus toxoid (TT) for immunogenicity studies in animals and finally clinical studies in adults, children and infants 40, 177, 178.
Fourteen years and seventeen clinical trials later, the result is a 99.7% success rate in children40. In 2004, QuimiHib® became part of Cuba’s national vaccination programme.
The remainder of this review will focus on carbohydrate vaccine research pertaining to specific pathogens and cancer.
Bacterial pathogens
The surface of bacterial pathogens is covered with a dense array of polysaccharide that is a unique feature of not only the particular species, but also the strain of bacteria considered. Anti-carbohydrate Abs are predominantly responsible for protection against bacteria with either a capsule or lipopolysaccharide on their surfaces. People lacking these Abs are at high risk, e.g. elderly and neonates. With the increasing prevalence of antibiotic-resistant bacterial strains, the proven track record of capsular polysaccharide-based vaccines has encouraged the development of carbohydrate-based vaccines against a broader array of pathogens. For many bacterial infections, glycoconjugate vaccines have been made based on fragments of their capsular polysaccharides, e.g. S. pneumoniae, N. meningitides, H. influenzae 16. With the advent of conjugate vaccines for several strains of the above three bacteria, the incidence of bacteraemia, meningitis and otitis media has almost been eliminated in countries where these vaccines are routinely used. Vaccines against several other clinically important bacterial pathogens that are based on their capsular polysaccharide (e.g. S. typhi, Vi)33 or lipopolysaccharide O-chains (e.g. S. dysenteriae) are currently under development 16 (Table 2). The Vi conjugate vaccine candidate was shown to be safe, immunogenic and 90% efficacious in children 2–5 years old, while the licensed vaccines confer approximately 70% immunity and do not protect young children 33.
The development of effective anti-carbohydrate vaccines against bacterial pathogens has historically been complicated by the considerable heterogeneity and complexity of capsular polysaccharides. For example, >90 different capsular types are described for S. pneumoniae 6, 34, 23 of which are included in the current polysaccharide vaccine. Generally, heterogeneity issues have been successfully addressed by isolating polysaccharide from the most clinically relevant serotypes (geographically) followed by degradation into smaller products for activation and conjugation to immunogenic carrier proteins to create multivalent conjugate vaccines e.g. Prevnar®, a heptavalent pneumococcal conjugate.
Another problem that hinders the development of some vaccines is the structural similarity between certain glycan Ags and host glycans, and therefore may be tolerated by the host’s immune system, resulting in vaccine formulations with poor immunogenicity. For example, similarities between the meningococcus Group B (MenB) capsular polysaccharide and ‘self’ sialic-acid containing glycans found during development and in the central nervous system likely make these bacterial glycans particularly poor immunogens 35, 36. One general strategy to overcome this immunotolerance is to immunize with a chemically modified version of the glycan, essentially rendering it more ‘foreign’ to the host. If the modification is sufficiently structurally conservative, the elicited antibodies may cross-react with the natural glycan on the pathogen. Efforts to increase their immunogenicity via N-propionylation of the polysialic acids resulted in moderately higher Ab titers that cross react with unmodified Group B capsular polysaccharide, but not with ‘self’ sugars 37. One general consideration in immunizing with glycans similar to those found on host tissue is that the benefit of the vaccine outweigh the risk of inducing autoimmunity. These risks may be less pronounced if the target structures are poorly expressed on host cells. The underlying risk of inducing autoimmune Ab responses combined with the identification of alternative MenB target Ags shifted the focus away from carbohydrate- based vaccine development for MenB38. Interestingly, a small increased risk of developing Guillain-Barre Syndrome may be associated with vaccination with Menactra®, the new multivalent meningococcal conjugate 39. The ability to break tolerance in a reliable, safe and effective manner against pathogen-associated carbohydrates that resemble ‘self’ is a hurdle that vaccinologists in other fields, especially in cancer and HIV (see below), must also overcome. Vaccine strategies that incorporate chemical modification of the target antigen show potential for addressing this issue.
Several conjugate vaccines composed of naturally-derived polysaccharides have excellent efficacy (usually ~90%) and safety profiles in the clinic 7. However, using naturally-derived polysaccharide to produce conjugate vaccines that meet FDA quality control and safety standards in a cost-effective manner is challenging as discussed above. Thus, a movement towards synthetic carbohydrate vaccines is underway 18. Synthetic glycans with uniform linkers can be used to manufacture well-characterized conjugates in a more economical, reproducible manner, free of bacterial contaminants ( see Box 2) 40.
Synthetic methods may also aid in addressing one of the main scientific challenges in this field: understanding the relationship between chain length (and/or saccharide density) and the potency of the protective Ab response. Synthetic methods enable the pursuit of empirical approaches to map the structural parameters that influence carbohydrate-specific immunogenicity such as chain length, saccharide composition and secondary structure. For instance, an investigation into a vaccine against Shigella dysenteriae included conjugates that displayed various densities of tetra-, octa-, dodeca- and hexadecasaccharides based on the tetrasaccharide repeat unit of the O-specific oligosaccharide 41. It was found that the octa-, dodeca- and hexadeca-, but not tetrasaccharide, conjugates were immunogenic and elicited protective Abs. Moreover, optimal loading densities were identified which were dependent on the chain length. The importance of the non-reducing terminal residue of oligosaccharides has also been demonstrated 42. The findings from these studies, however, cannot necessarily be extended to other systems. For example, an immunogenicity and protection study on S. pneumoniae 6B capsular polysaccharide showed that single repeats of either a di- or tetrasaccharide were sufficient to elicit protective Ab responses in rabbits and mice 43.
Fungal pathogens
Pathogenic fungi are significant infectious threats. Of particular concern are the opportunistic fungal agents, such as Candida, Cryptococcus and Aspergillus spp., which target the immunocompromised worldwide. The disease burden has not declined despite the availability of effective chemotherapeutics. Furthermore, current therapeutics are limited by toxicity concerns and the emergence of resistance. Interest in vaccine development was lacking until the 1990s as Abs were previously considered unimportant to host defence against mycoses 44. A major shift was catalyzed by the discovery that a monoclonal Ab against Cryptococcus neoformans polysaccharide protects against experimental cryptococcal infection in mice 45. Mounting evidence for anti-fungal Abs, against surface polysaccharides and other Ags, that mediate protection has since encouraged the development of vaccines to elicit such Abs 46. As the major component of the cell walls and capsules of fungi, polysaccharides have been the focus of considerable research.
Studies with glucuronoxylomannan (GXM), the major capsular polysaccharide of C. neoformans (Figure 1), illustrate some of the difficulties related to designing and developing carbohydrate vaccines against fungal pathogens. Immunization with a GXM-tetanus toxoid conjugate has been shown to elicit protective anti-GXM antibody responses and GXM-specific monoclonal Abs have been shown to protect mice against cryptococcosis 47. However, non-protective and even deleterious Abs which bind to different epitopes on GXM 48 have also been elicited by GXM conjugates 49, 50. In addition, Ab-mediated protection has been shown to be contingent, not only on the Ab specificity and titer, but also on the antibody isotype 51–53. Elegant studies using anti-GXM monoclonal Abs of the same specificity, but different isotype, showed that those that did not activate complement or opsonophagocytosis (human IgG2 and IgG4 and mouse IgG1) protected best against C. neoformans infection 51, 53. The potential use of GXM conjugate vaccines is further complicated by unwanted immunomodulatory effects exhibited by this polysaccharide, such as interference with leukocyte migration 54, 55. To circumvent some of these issues, alternative vaccine constructs containing defined Ags, including synthetic oligosaccharides 50 and peptide mimitopes 56, designed to represent the protective epitope(s) of GXM are being pursued.
A systematic approach that integrates immunogenicity studies with oligosaccharide synthesis and structure determination was used to identify a protective motif on the cell wall of Candida species and develop several carbohydrate vaccine candidates that are in various stages of testing. Early vaccine formulations based on mannan extracts elicited Abs capable of protecting mice against vaginal and disseminated C. albicans infection 57, 58. Extensive research on protective responses against Candida mannan components in experimental animals indicated that Abs against the unique minor β-mannan component – specifically, short β1–2 linked oligomannosides – but not the major α-mannan component, are protective against candidiasis 59. Abs against β1–2 mannotriose or mannobiose protect against systemic candidiasis caused by C. tropicalis and C. albicans and are also expected to show efficacy against other strains that synthesize these motifs (e.g. C. glabrata and C. lusitaniae strains) 57. Using the synthetic route, this motif has been methodically pursued as the basis of a defined glycoconjugate vaccine. In a synthetic panel of di- to hexa-mannosides, the small di- and trimannosides were the most effective inhibitors of protective monoclonal Abs that bind the β1–2 mannan motif, which was attributed to their well-ordered helical conformation 60, 61. Efficient synthetic strategies were devised to enable the preparation of gram quantities of β1–2 oligomannosides and several prototype oligomannoside conjugate vaccines 62–64. Immunization of experimental animals with clustered and unclustered di- and tri-mannoside tetanus toxoid conjugates elicited antibody titers that cross-react strongly with C. albicans β-mannan cell wall extract 63. In addition, synthetic glycopeptide vaccines that combine β1–2 trimannoside and peptide epitopes have recently been shown to induce protection against candidiasis 64.
Recently, β-glucan, a conserved structural component of many pathogenic fungi, has been described as a promising target for an effective vaccine against candidiasis – and potentially a wide range of other mycoses. An algal β1–3 glucan conjugated to a non-toxic mutant of diphtheria toxin, CRM197, was found to be immunogenic in mice and to provide Ab- mediated protection against infection by C. albicans and Aspergillus fumigatus 65, two phylogenetically distinct fungal species. Furthermore, the elicited Abs inhibited the growth of C. albicans, A. fumigatus and C. neoformans, in vitro in the absence of immune effector cells, which suggests that vaccine efficacy may not require cellular or other components of an intact immune system 65, 66. These findings have sparked considerable speculation about the possibility of developing a broad spectrum anti-fungal vaccine based on β-glucan that may be efficacious even in immunocompromised individuals 67.
Parasitic pathogens (protozoans and helminths)
It is disheartening to think that no vaccine is available for any of the major global parasitic infections – malaria, leishmaniasis, African trypanosomiasis, amebiasis, schistosomiasis and lymphatic filariasis. Several vaccines strategies are being pursued but, in general, progress in this field has been impeded by the complex biology of parasites, the immune evasion mechanisms employed by many parasites, and a poor understanding of the correlates of immunity 68. In fact, disease is often the result of the interplay between the host and the invading parasite, which involves complex immune responses 69–73.
Glycans make attractive vaccine targets on parasitic protozoans and helminths because unique glycan Ags are highly abundant and accessible on the surface of multiple developmental stages. These antigens also tend to be immunodominant, at least in helminth infections 74. The ability of Abs to protect against natural infection is not yet established for many parasites; however, there are some infections, such as malaria, filariasis and trypanosomiasis, where Abs have been shown to be important for host defense 71, 75–77. In addition, immunization with glycoprotein-rich materials has been shown to induce protective responses 74. For example, immunization studies with schistosome soluble egg antigens (SEA) and radiation attenuated (RA) cercariae have shown that protective immune responses correlate with strong anti-glycan Ab responses 78–80. Passive immunization with tyvelose-reactive Abs against a group of antigenic glycoproteins (termed Trichinella spiralis larvae group 1, TSL-1) provides protective immunity in rats by expelling invading larvae from the intestine 81, 82 and such Abs block epithelial invasion by the parasites in vitro 83, 84.
Identification of protective glycan epitopes in mixtures of glycan-rich material, such as SEA, RA cercariae or TSL-1, is a formidable task. For instance, immunization with a simple tyvelose-BSA conjugate failed to induce protective immunity against intestinal forms of T. spiralis despite the presence of tyvelose-specific Abs 85. Carbohydrate vaccine research has been limited due to difficulties in obtaining enough material to study as parasites are often notoriously difficult to culture, but recent technological advances (see Box 1) are allowing the investigation of potential vaccine Ags 86. For example, numerous surface glycans associated with Schistosoma species, e.g. LacdiNAc (LDN), fucosylated LacdiNAc (LDNF), Lewis X (LeX) and Leishmania species, e.g. lipophosphoglycan (LPG), have been identified and are being evaluated as immunogens 74, 87 (Figure 1).
Variation in size, antigenicity and accessibility to the immune system of the various developmental stages of parasitic pathogens complicates the selection of appropriate carbohydrate Ags for immune evaluation. For example, Leishmania avoid direct contact with immune effectors by invading host cells, while African trypanosomes continually genetically switch their highly immunogenic glycosylphosphatidylinositol (GPI)-anchored surface glycoprotein called variant surface glycoprotein (VSG) 88. Furthermore, complex carbohydrates have been shown to play key roles in the interaction of protozoan parasites with their hosts 69, 89–92.
Some of the considerations discussed above have led to the pursuit of non-traditional vaccines, including anti-pathogenesis and transmission-blocking vaccines, in addition to traditional prophylactics. The pathology of malaria is largely considered to have a toxic basis 69. The toxin was identified as the GPI anchor of the plasmodium species, which is invariant, abundant, and essential for anchoring several essential proteins involved in erythrocyte invasion 69. Pre-clinical studies with a synthetic version of GPI – conjugated to KLH (Figure 3) elicits high titers of IgG and shows promise in reducing the pathology of malaria, increasing survival rates in a mouse challenge model, without preventing infection 93. The LPG of leishmania, which is not normally immunogenic during natural infection 94, is the predominant glycoconjugate on the surface of promastigotes 89, 95. It is also an important virulence factor and is essential for survival and infectivity 89, 90. Development of parasites into non-infectious nectonomad forms was observed in the gut of sandflies that previously ingested anti-LPG Abs from LPG-immunized mice, suggesting the potential for an LPG-based transmission-blocking vaccine 96. Because of epitope heterogeneity and the observation of both protective and disease-promoting effects associated with LPG (depending on the immunization route) 97–100, several LPG-based constructs are being synthesized and evaluated as immunogens 101, 102. Early pre- clinical studies show that a synthetic glycoconjugate based on the LPG cap of L. donovans elicits primarily IgG and IgM responses that cross react with parasites from infected hamsters; protection experiments are anticipated 103.
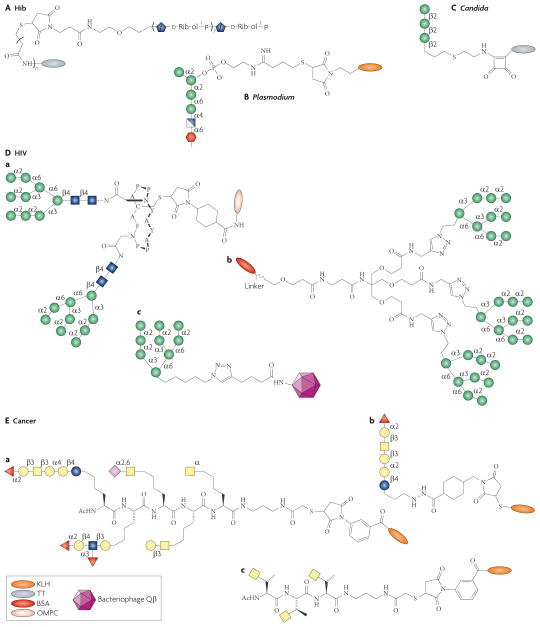
Figure 3. Synthetic glycoconjugate immunogens.
This figure shows examples of synthetic carbohydrate immunogens used in vaccine development. Hib: polyribosylribitol-TT (QuimiHib®); Candida: Man3-TT; Plasmodium: GPI-KLH. HIV: Man9 glycodendron BSA conjugate and Qβ-Man9 (overall representations also depicted), GlcNAc2Man9 divalent glycopeptide OMPC conjugate (outer membrane protein complex, derived from N. meningitidis); Cancer: KLH-Globo H, KLH-clustered Tn, unimolecular pentavalent KLH conjugate.
Viral pathogens
Several clinically important viruses express glycoproteins on their surfaces and the associated glycans have key roles in infectivity and immune evasion. In contrast to other pathogens, these viruses are decorated by ‘self’ glycans, which are expected to be tolerized, because they co-opt the glycosylation machinery of their host. However, a broadly neutralizing antibody against HIV-1, 2G12, isolated from a pool of B cells from infected individuals, neutralizes a wide range of HIV-1, and provides protection in animal models, by binding specifically to a conserved cluster of oligomannose glycans on the envelope glycoprotein, gp120 104–107. These observations provide the foundation for targeting conserved high mannose clusters on HIV.
A dense array of N-linked glycans, referred to as the ‘glycan shield’, covers much of the surface of gp120 in envelope spikes. The close spacing between carbohydrates on gp120, which is unusual for mammalian glycoproteins, is thought to impose conformational constraints on these glycans. It has been postulated that this dense and relatively rigid presentation of oligomannose on gp120, stabilized by a network of intermolecular hydrogen bonds, provides the basis for immunological discrimination by 2G12 108. Biochemical, glycan array, structural and modelling studies indicate that 2G12 binds with nM affinity to terminal Manα1→2Man residues of 3–4 high mannose glycans (e.g. GlcNAc2Man9) within a cluster on gp120 via a novel VH domain-exchanged structure that creates a multivalent binding surface 105, 106, 109. Two novel glycan binding sites within the VH-VH′ interface may also interact with gp120 25. Additional glycans within the cluster are also important, presumably for maintaining the conformation of this epitope 110, 111. Using 2G12 as a guide, it may be feasible to design an immunogen that elicits similar Abs provided the same immunological constraints that drove the development of 2G12 are replicated. Meeting these criteria is challenging owing to the involvement of ‘self’ glycans, the cluster-dependence for ‘non-self’ discrimination and the unique recognition mode of 2G12. Because the glycan shield is considered immunologically silent in the context of gp120, aside from 2G12, alternative presentations of clustered oligomannose (i.e. mimetics) have been sought.
Several Manα1→2Man containing oligomannoside ligands for 2G12 have been identified, in addition to GlcNAc2Man9, all of which bind 2G12 with similar affinities (Figure 3). Of these, the D1 arm (Manα1→2Manα1→ 2Manα1→3Man; Man4) represents the minimum recognition motif 105, 109. Several strategies to create glycoconjugate immunogens based on GlcNAc2Man9, Man8, Man9 and Man4 are being explored 17, 112–115. Though high mannose glycans are the natural ligands for 2G12, the use of synthetic derivatives (especially, Man4) may be advantageous for focusing the immune response and overcoming tolerance issues (e.g. short Manα1→2Man motifs are immunogenic in the context of Candida α-mannan). In addition, synthesis of Man4 is much easier than full length GlcNAc2Man9. Examples of synthetic strategies yielding high affinity multivalent mimetics include the display of oligomannose on Regioselectively Addressable Functionalized Templates (RAFTS), oligodendrons, and Qβ bacteriophage and the generation of cyclic glycopeptides (Figure 3) 17, 112–115. In addition, selective inhibition of glycosylation in mammalian and yeast cells using kifunensine 116, or deletion of glycosylation enzymes, have produced near homogeneous GlcNAc2Man8 or GlcNAc2Man9 glycoproteins 117, respectively, that bind 2G12. Candida species have also been identified that are recognized by 2G12 and these are being investigated as immunogens 116.
Limited progress thus far has been made in eliciting gp120 cross-reactive Abs and none of these constructs has elicited neutralizing Abs. A synthetic Man4 conjugate, BSA-(Man4)14, and a GlcNAc2Man9 cyclic glycopeptide conjugate both elicited reasonable IgG titers in laboratory animals against oligomannose, but these IgGs do not bind gp120 115, 118. In one rabbit, convincing gp120 cross-reactive anti-mannose Abs were elicited by a yeast mutant (exclusive GlcNAc2Man8 glycosylation); however, these Abs did not neutralize HIV 117. The current difficulties in generating the proper specificity of Abs may reflect inadequate mimicry of the glycan shield (e.g. flexible glycans), tolerance mechanisms and/or the inability to induce domain-exchange. Testing of these hypotheses is particularly challenging as the only model Ab, 2G12, has a unique architecture.
Cancer
The vaccines described thus far target exogenous causative agents of disease; such is not the case for cancer. The targets in this case are host cells that have undergone mutations allowing uncontrolled cell division and the ability to invade other tissues. Another defining feature of cancer is altered glycosylation, including increased expression of certain glycans, tumour associated carbohydrate antigens (TACAs) (Table 3) 119 relative to normal tissues (Figure 1). Commonly, changes in glycosyltransferase expression levels can lead to an increase in the size and branching of N-linked glycans, which creates additional sites for terminal sialic acid residues120. A corresponding increase in sialyltransferase expression ultimately leads to an overall increase in sialylation121. Overexpression of glycosyltransferases involved in linking terminal residues to glycans leads to the overexpression of certain terminal glycan epitopes on tumours, such as sLeX, sLea, sTn, LeY, Globo H and PSA (Table 3) 122–124. Certain glycoproteins, such as mucins, which can serve as a scaffold for several of the aforementioned TACAs, and glycolipids, such as gangliosides, a glycosphingolipid-containing sialic acid (e.g. GD2, GD3, GM2 and fucosyl GM1), are also overproduced125, 126. An expanding body of preclinical and clinical research show that Abs against TACA, can eliminate circulating tumour cells and micrometastases 127. Although TACAs are ‘self’ glycans, since they are generally poorly expressed or inaccessible on normal, healthy tissues, these glycans may serve as potential vaccine Ags. These observations form the rationale for carbohydrate-based therapeutic vaccines primarily for use in the adjuvant setting, that is, after completion of primary therapy (e.g. chemotherapy) 19.
Table 3.
Expression profiles of tumour associated carbohydrate antigens on malignant tissues.
| Tumour | Tumour associated carbohydrate antigens* | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| sLex | Lex | sLea | Lea | sTn | Tn | TF | Ley | Globo H | PSA | GD2 | GD3 | Fucosyl GM1 | GM2 | |
| B cell lymphoma | X | X | ||||||||||||
| Breast | X | X | X | X | X | X | X | |||||||
| Colon | X | X | X | X | X | |||||||||
| Lung | X | x | X | X | X | |||||||||
| Melanoma | X | X | X | |||||||||||
| Neuroblastoma | X | X | X | X | ||||||||||
| Ovary | X | X | X | X | X | |||||||||
| Prostate | X | X | X | X | X | |||||||||
| Sarcoma | X | X | X | |||||||||||
| Small cell lung cancer | X | X | X | X | X | |||||||||
| Stomach | X | X | X | X | X | X | X | X | X | |||||
The ability to elicit Abs against TACAs that effectively and selectively eliminate malignant cells leading to an improved clinical outcome is an ambitious goal. TACAs are poorly immunogenic and the heterogeneity of TACA expression (Table 3) and glycan microheterogeneity make it difficult to isolate unique glycoforms from natural sources (e.g. Globo H). Thus, synthetic methods play a large role in vaccine development. The general criteria for breaking tolerance of TACAs were delineated in the pursuit of the first generation monomeric vaccines: polyvalent display on an immune carrier (high sugar/carrier ratio), such as KLH, of TACAs closely resembling the natural presentation on target cells in the presence of a strong adjuvant like QS-21 128. Key studies on GD3-based conjugates established KLH and QS-21 as the most potent carrier/adjuvant pair for breaking tolerance to TACAs 129, 130. Chemical modification of the glycans is sometimes also necessary to increase immunogenicity, e.g. GD2- and GD3-lactone and N-propylated polysialic acid, which is reminiscent of the MenB capsular polysaccharide vaccines 131–133. Several KLH-monovalent vaccines, including those displaying synthetic carbohydrates (e.g. GloboH and LeY, Figure 3) are in various stages of clinical trials and they have generally been found to be safe and immunogenic 134. Nonetheless, alternative carrier/adjuvant strategies are in development. For instance, a fully synthetic three component vaccine, including a toll-like receptor 2 agonist, a promiscuous peptide T-helper epitope and a tumour-associated glycopeptide was recently shown to elicit robust Ab responses in mice that recognize tumour cells expressing the TACA 135.
Second generation vaccines rely heavily on synthetic methods to mimic the natural presentation of TACAs and the strategies developed have influenced other fields, especially HIV 136. For example, TACAs typically associated with mucins (e.g. sTn, Tn, TF) are found in clusters and mimicking this presentation is important for generating strong Ab responses that cross-react with these TACAs on mucins and tumour cells. Clinical studies with synthetic glycopeptide cluster KLH conjugates of Tn, sTn and TF have demonstrated their safety and the improved immunogenicity of these glycans 137–139. In addition, smaller fully synthetic cluster vaccines, based on presentation on the lipopeptide tripalmitoyl-S-glyceryl-cysteinylserine (Pam3CS) 140, multiple antigen glycopeptides (MAG) 141, 142 and RAFTs143, have shown potential in pre-clinical studies (Figure 2 and Figure 3). Synthetic glycopeptides vaccines based on MUC1, the membrane bound glycoprotein extensively overexpressed on epithelial tumour cells, are also being pursued. Interestingly, Abs against a sTn-MUC1 tandem-repeat glycopeptide conjugate specifically recognize not only the glycan but also the peptide backbone 144.
The heterogeneity of TACA expression on malignant cells (Table 3) has lead to the development of multivalent vaccines as either polyvalent monomeric or unimolecular multivalent formulations that can be tailored to a particular cancer (Table 3 and Figure 3). In pre-clinical studies, tetravalent and heptavalent monomeric vaccines were shown to induce similar Ab titers against individual TACAs compared with those achieved with the individual monovalent vaccines 145, 146; however, recent clinical studies show lower IgM and especially IgG titers against individual Ags in patients when immunized with multivalent vaccines 147, 148. Unimolecular pentavalent and hexavalent KLH and Pam3Cys conjugate vaccines have recently been synthesized, employing unnatural amino acids to link the carbohydrate Ags, and early immunogenicity studies are encouraging. The individual TACAs were more immunogenic in mice when delivered as a pentavalent unimolecular (on KLH or Pam3Cys) vaccine compared to the corresponding pool of monomeric KLH conjugates 149.
Recently, a novel immunotherapeutic strategy has been developed, which combines cell glycoengineering with vaccines made of unnatural TACA analogues, to tackle the problem of immunotolerance to TACAs 150. With GM3, for example, as a target, Guo et al have shown that tumor cells cells incubated with N-phenylacetyl-D-mannosamine efficiently expressed the unnatural GM3 analogue, GM3NPhAc, in place of the natural TACA151. They also showed that GM3NPhAc-KLH elicits strong T-cell dependent Ab responses150 and a GM3NPhAc-specific mAb mediated selective killing of melanoma cells glycoengineered to express the corresponding GM3 analogue 152.
Future perspectives and conclusions
In this review, we have presented an overview of the current state of carbohydrate vaccine research for a diverse set of diseases, discussing the challenges involved and progress made towards addressing them. Much has been done to broaden the scope of carbohydrate vaccinology to diseases outside of bacterial infections for which bespoke vaccines are currently in the clinic. However, significant issues remain to be addressed.
Of general importance, the mechanism(s) that control the relative immunotolerance of carbohydrate antigens is not fully understood; although, low level expression of the same antigens on self tissues, or during a developmental stage, and their structural similarity to self antigens is at least partially involved. The balance between exposure to an antigen on foreign organisms and the expression of the same, or very similar, antigen by the host, likely influences the level of immune tolerance to that structure. For example, the Galα1→3Galβ1→4GlcNAc-R (α-Gal) epitope is abundantly expressed on glycoconjugates of non-primate mammals, prosimians and New World monkeys but is not expressed on glycoconjugates of humans, apes and Old World monkeys due to the inactivation of a specific Gal transferase required for adding the α1–3 Gal cap on the epitope153. It has been postulated that the complete lack of α-Gal epitope expression and the continual exposure to α-Gal epitopes found on the intestinal microbial flora is largely responsible for the large abundance of anti-Gal Abs in the latter group153. In fact, a novel strategy takes advantage of these Abs to increase the immunogenicity of vaccines immunogens. By engineering in α-Gal epitopes into glycoprotein immunogens, such as influenza hemagglutinin, immune complexes can be made that help target the immunogen to antigen-presenting cells153.
There are also more specific challenges to be addressed within each area of carbohydrate vaccine design. The minimal protective epitopes for many bacterial pathogens have yet to be determined. Carbohydrate vaccine development for fungal and parasitic infections is comparatively new and, as such, the main issues involve the identification and validation of epitopes that elicit protective, rather than neutral or disease-enhancing, Abs and the elucidation of Ab-mediated mechanisms of protection. For HIV vaccinologists, the key difficulty lies in mimicking the presentation of the protective epitope, a dense cluster of glycans, in order to elicit neutralizing antibodies against HIV that discriminate against self glycoproteins. Cancer vaccinologists have addressed many issues similar to those currently facing researchers in the aforementioned fields, e.g. definition and presentation of several synthetic antigen candidates. These potential targets must still be validated in a clinical setting, which involves defining the populations that may benefit from these vaccines and how they should be used in combination with other available therapies or cytotoxic T cell immunogens 154, 155. Cancer-specific cytotoxic T cells may also be required in order to optimize immune-mediated tumour clearance.
As vaccine candidates approach clinical evaluation, more precise criteria for efficacy and clinical impact will need to be defined. As implied above, immune mechanisms associated with cancer and pathogen infections (especially viral and parasitic infections) are complex and often only partially defined. However, discussion of mechanisms outside of Ab-mediated protection is beyond the scope of this review. In general, low titers of antibodies that function in complement-mediated lysis and opsonization correlate with protection against bacterial infections/disease 1. For viruses, higher titers of neutralizing Abs and perhaps Ab-dependent cell-mediated cytotoxicity (ADCC) are important 1, 156. In cancer, Ab-mediated protection is thought to work mostly by ADCC, complement dependent cell lysis and opsonization 157. The situation is more complex for fungal vaccines because protective mechanisms appear to be specific for both the mycosis and antigen, e.g. the ability to bind complement and Fc receptors by anti-GXM IgG negatively correlates with protection against C. neoformans yet complement mediated-lysis is thought to be an important function of anti-β1–2 mannoside antibodies against Candida 46. Ab-mediated mechanisms of protection are poorly understood for parasitic infections; suggested mechanisms depend on the pathogen, life stage and antigen e.g. Ab-dependent cellular inhibition involving monocytes (Plasmodium blood stages) 158, toxin neutralization (Plasmodium) 93, and complement-mediated lysis (Trypanosoma brucei) 159. The evaluation of new glycoconjugate vaccines may be hindered by poorly defined clinical endpoint criteria, such as those based solely on ELISA titers rather than functional assays. Despite the immense diversity of carbohydrate vaccine indications, a number of common obstacles are apparent – poor immunogenicity, heterogeneity, antigenic mimicry of self glycans, identification and access to protective epitopes. Common solutions to these problems may be realized. For example, conjugation of carbohydrates to immunogenic protein carriers has become a universal method for increasing the immunogenicity of glycans and the quality of the resulting Ab response. Furthermore, the synthetic approaches developed for clustering TACAs are finding application in HIV vaccinology. While a number of significant challenges remain, the future looks bright as researchers continue to learn from the experiences of carbohydrate vaccinologists in other fields. In addition, advances in glycomics will continue to accelerate research and the development of new carbohydrate vaccines. However, it is too early to tell whether carbohydrate vaccines will provide sweet solutions to a variety of sticky situations aside from bacterial infections.
Acknowledgments
We are grateful to Dr. Robert Woods, Dr. James Paulson, Dr. Ian Wilson and Dr. Marianne Manchester for critical reading of the manuscript. We also appreciate the feedback and suggestions from Dr. Katherine Doores and Dr. Michael Huber during the writing process. We thank Christina Corbacci for the considerable time, effort and creativity she devoted to figure design for this review. The authors’ work is supported by the Natural Sciences and Engineering Research Council of Canada (to R.D.A.) the Neutralizing Antibody Consortium of the International AIDS Vaccine Initiative (to D.R.B.) and the National Institute of Allergy and Infectious Diseases.
Glossary
- serotypes
Groups of closely related microorganisms distinguished by a characteristic set of antigens
- conjugate vaccines
Carbohydrate-based vaccines in which the immunogens are glycan antigens covalently attached to a source of helper T-cell epitopes, often an immunogenic carrier protein, such as tetanus toxoid
- multivalent
Vaccines described as multivalent are composed of multiple, different glycan antigens (e.g. capsular polysaccharide fragments from different serotypes or different tumour associated antigens)
- microheterogeneity
Heterogeneity in glycan structures at a single glycosylation site due to differential enzymatic processing by local glycosyl transferases and glycosidases
- QS-21
Saponin derived from the tree bark of Quillaja saponaria that has been shown to augment anti-carbohydrate antibody responses
- Guillain-Barre Syndrome
Guillain-Barre syndrome is an uncommon, serious autoimmune disorder that attacks the peripheral nervous system, particularly myelin sheaths, which causes progressive muscle weakness and numbness that can eventually lead to full body paralysis. Some forms of this disease are mediated by anti-ganglioside antibodies that recognize antigens on peripheral nerves
- glucuronoxylomannan (GXM)
Linear polymer composed of repeating mannose trisaccharide motifs bearing a single β-1,2 glucuronic acid with variable xylose and O-acetyl substitutions to form 6 triads
- opsonophagocytosis
a specific mechanism by which the host protects against infection that involves the coating of a target (e.g. bacterium) with serum opsonins (antibodies and complement) which mediates engulfment and destruction of the target by phagocytes (e.g. macrophages)
- soluble egg antigens (SEA)
A variety of soluble products secreted by schistosome eggs in host tissues that promote endothelial cell attachment and induce T-cell mediated granuloma formation
- radiation attenuated (RA) cercariae
Irradiated schistosome larvae capable of infecting mammals but unable to develop into adult forms
- tyvelose
a monosaccharide found on some pathogenic bacteria and the parasitic nematode, T. spiralis
- broadly neutralizing antibody
An antibody that binds the native envelope of a broad range of isolates of a highly variable virus such as HIV, HCV or influenza and prevents infection of target cells
- promastigote
one of the morphological stages in the development of certain protozoa, characterized by a free anterior flagellum
- gp120
The highly glycosylated envelope surface glycoprotein responsible for receptor and co-receptor binding that together with gp41 comprise the heterodimeric envelope trimer spikes of HIV
- glycan shield of HIV
A dense array of N-linked glycans (high mannose and complex types) that covers much of the surface-accessible face of gp120 in the context of the HIV envelope spike. The shield may be roughly divided into two regions 190 : high mannose glycans tend to densely cluster together on the outer domain of gp120, while complex-type glycans are distributed around the more exposed receptor binding sites and variable loops. The high mannose glycans also tend to be more conserved than the complex glycans 190
- kifunensine
A mannose analogue of bacterial origin that competitively inhibits type-1 endoplasmic reticulum and, to a lesser extent, Golgi α-mannosidases, thus preventing the normal trimming of immature Man9GlcNAc2 glycans to Man5GlcNAc2 and subsequent glycan modifications that create hybrid and complex-type glycans
- therapeutic vaccines
In cancer, intended to elicit Abs against TACAs for the purpose of eliminating tumour cells that remain after primary therapy (e.g. chemotherapy), when the disease burden is minimal. Ultimate clinical goals are increased disease-free and overall survival
- monomeric vaccines
vaccine formulation containing a single carbohydrate antigen displayed in multiple copies on an immunogenic carrier (e.g. GloboH-KLH)
- polyvalent monomeric formulation
Mixture of two or more monomeric immunogens (e.g. sTn-KLH), each displaying the tumour antigen in multiple copies. Here, polyvalent refers to the inclusion of more than one type of antigen in the formulation rather than the display of multiple copies of an antigen
- unimolecular multivalent formulation
A construct containing different tumour antigens displayed on a single molecular backbone (often a peptide) that can then be conjugated to a carrier protein or other platform for immunization. Note: multivalent and polyvalent are usually used synonymously (as in the rest of this thesis) to denote the display of multiple copies of a single antigen
- reactivity-based one-pot synthesis
In the one-pot strategy, several glycosyl donors are allowed to react sequentially in the same vessel in solution, resulting in a single main oligosaccharide product. These protocols generally make use of three main concepts, namely “chemoselective principle”, “orthogonal glycosylation” and “pre-activation strategy”
- solid support synthesis
In solid support synthesis, an easily yet orthogonally cleavable linker tethers the first monosaccharide building block to a solid support. An activating agent then activates the donor for glycosylation with protected monosaccharide building blocks added in solution. Several cycles of activation, washing and deprotection ensue, followed by linker cleavage upon completion
References
- 1.Plotkin SA. Vaccines: correlates of vaccine-induced immunity. Clin Infect Dis. 2008;47:401–9. doi: 10.1086/589862. [DOI] [PubMed] [Google Scholar]
- 2.Heidelberger M, Avery OT. The soluble specific substance of pneumococcus. Journal of Experimental Medicine. 1923;38:0073–0079. doi: 10.1084/jem.38.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tillett WS, Francis T. Cutaneous reactions to the polysaccharides and proteins of pneumococcus in lobar pneumonia. Journal of Experimental Medicine. 1929;50:687–U152. doi: 10.1084/jem.50.5.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heidelberger M, Dilapi MM, Siegel M, Walter AW. Persistence of Antibodies in Human Subjects Injected with Pneumococcal Polysaccharides. Journal of Immunology. 1950;65:535–541. [PubMed] [Google Scholar]
- 5.Merck & Co., Inc. Pneumovax 23 (pneumococcal vaccine polyvalent) Whitehouse Station, NJ: Merck; 2009. website [online] [Google Scholar]
- 6.Robbins JB, et al. Considerations for formulating the second-generation pneumococcal capsular polysaccharide vaccine with emphasis on the cross-reactive types within groups. J Infect Dis. 1983;148:1136–59. doi: 10.1093/infdis/148.6.1136. [DOI] [PubMed] [Google Scholar]
- 7.Ada G, Isaacs D. Carbohydrate-protein conjugate vaccines. Clin Microbiol Infect. 2003;9:79–85. doi: 10.1046/j.1469-0691.2003.00530.x. [DOI] [PubMed] [Google Scholar]
- 8.Mond JJ, Lees A, Snapper CM. T cell-independent antigens type 2. Annu Rev Immunol. 1995;13:655–92. doi: 10.1146/annurev.iy.13.040195.003255. [DOI] [PubMed] [Google Scholar]
- 9.Snapper CM, Mond JJ. A model for induction of T cell-independent humoral immunity in response to polysaccharide antigens. J Immunol. 1996;157:2229–33. [PubMed] [Google Scholar]
- 10.Cobb BA, Wang Q, Tzianabos AO, Kasper DL. Polysaccharide processing and presentation by the MHCII pathway. Cell. 2004;117:677–87. doi: 10.016/j.cell.2004.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kalka-Moll WM, et al. Zwitterionic polysaccharides stimulate T cells by MHC class II-dependent interactions. J Immunol. 2002;169:6149–53. doi: 10.4049/jimmunol.169.11.6149. [DOI] [PubMed] [Google Scholar]
- 12.Avery OT, Goebel WF. Chemo-immunological studies on conjugated carbohydrate-proteins: V. The immunological specificity of an antigen prepared by combining the capsular polysaccharide of type III Pneumococcus with foreign protein. J Exp Med. 1931;54:437–447. doi: 10.1084/jem.54.3.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galiza EP, Heath PT. Pneumococcal conjugate vaccines. A review. Minerva Med. 2007;98:131–43. [PubMed] [Google Scholar]
- 14.Gessner BD, Adegbola RA. The impact of vaccines on pneumonia: key lessons from Haemophilus influenzae type b conjugate vaccines. Vaccine. 2008;26 (Suppl 2):B3–8. doi: 10.1016/j.vaccine.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 15.Robbins JB, Schneerson R, Anderson P, Smith DH. The 1996 Albert Lasker Medical Research Awards. Prevention of systemic infections, especially meningitis, caused by Haemophilus influenzae type b. Impact on public health and implications for other polysaccharide-based vaccines. JAMA. 1996;276:1181–5. doi: 10.1001/jama.276.14.1181. [DOI] [PubMed] [Google Scholar]
- 16.Jones C. Vaccines based on the cell surface carbohydrates of pathogenic bacteria. An Acad Bras Cienc. 2005;77:293–324. doi: 10.1590/s0001-37652005000200009. [DOI] [PubMed] [Google Scholar]
- 17.Wang LX. Toward oligosaccharide- and glycopeptide-based HIV vaccines. Curr Opin Drug Discov Devel. 2006;9:194–206. [PubMed] [Google Scholar]
- 18.Pozsgay V. Recent developments in synthetic oligosaccharide-based bacterial vaccines. Curr Top Med Chem. 2008;8:126–40. doi: 10.2174/156802608783378864. [DOI] [PubMed] [Google Scholar]
- 19.Cipolla L, Peri F, Airoldi C. Glycoconjugates in cancer therapy. Anticancer Agents Med Chem. 2008;8:92–121. doi: 10.2174/187152008783330815. [DOI] [PubMed] [Google Scholar]
- 20.Guo Z, Boons G-J. In: Carbohydrate-Based Vaccines and Immunotherapies. Want B, editor. John Wiley & Sons, Inc; Hoboken: 2009. [Google Scholar]
- 21.Roy R, editor. Carbohydrate-based Vaccines. Oxford University Press; Oxford: 2008. [Google Scholar]
- 22.Braden BC, Poljak RJ. Structural features of the reactions between antibodies and protein antigens. FASEB J. 1995;9:9–16. doi: 10.1096/fasebj.9.1.7821765. [DOI] [PubMed] [Google Scholar]
- 23.Brummell DA, et al. Probing the combining site of an anti-carbohydrate antibody by saturation-mutagenesis: role of the heavy-chain CDR3 residues. Biochemistry. 1993;32:1180–7. doi: 10.1021/bi00055a024. [DOI] [PubMed] [Google Scholar]
- 24.Bundle DR, Young NM. Carbohydrate-protein interactions in antibodies and lectins. Current Opinion in Structural Biology. 1992;2:666–673. [Google Scholar]
- 25.Calarese DA, et al. Antibody domain exchange is an immunological solution to carbohydrate cluster recognition. Science. 2003;300:2065–71. doi: 10.1126/science.1083182. [DOI] [PubMed] [Google Scholar]
- 26.Wilson IA, Stanfield RL. A Trojan horse with a sweet tooth. Nat Struct Biol. 1995;2:433–6. doi: 10.1038/nsb0695-433. [DOI] [PubMed] [Google Scholar]
- 27.Carver JP. Oligosaccharides - How Can Flexible Molecules Act as Signals. Pure and Applied Chemistry. 1993;65:763–770. [Google Scholar]
- 28.Lemieux RU, Delbaere LT, Beierbeck H, Spohr U. Involvement of water in host-guest interactions. Ciba Found Symp. 1991;158:231–45. doi: 10.1002/9780470514085.ch15. discussion 245–8. [DOI] [PubMed] [Google Scholar]
- 29.Pozsgay V, Kubler-Kielb J. In: Carbohydrate-based Vaccines. Roy R, editor. Oxford University Press; Oxford: 2008. pp. 36–70. [Google Scholar]
- 30.De Silva RA, Wang Q, Chidley T, Appulage DK, Andreana PR. Immunological response from an entirely carbohydrate antigen: design of synthetic vaccines based on Tn-PS A1 conjugates. J Am Chem Soc. 2009;131:9622–3. doi: 10.1021/ja902607a. [DOI] [PubMed] [Google Scholar]
- 31.Buskas T, Li Y, Boons GJ. The immunogenicity of the tumor-associated antigen Lewis(y) may be suppressed by a bifunctional cross-linker required for coupling to a carrier protein. Chemistry. 2004;10:3517–24. doi: 10.1002/chem.200400074. [DOI] [PubMed] [Google Scholar]
- 32.Ni J, Song H, Wang Y, Stamatos NM, Wang LX. Toward a carbohydrate-based HIV-1 vaccine: synthesis and immunological studies of oligomannose-containing glycoconjugates. Bioconjug Chem. 2006;17:493–500. doi: 10.1021/bc0502816. [DOI] [PubMed] [Google Scholar]
- 33.Lin FY, et al. The efficacy of a Salmonella typhi Vi conjugate vaccine in two-to-five-year-old children. N Engl J Med. 2001;344:1263–9. doi: 10.1056/NEJM200104263441701. [DOI] [PubMed] [Google Scholar]
- 34.Henrichsen J. Six newly recognized types of Streptococcus pneumoniae. J Clin Microbiol. 1995;33:2759–62. doi: 10.1128/jcm.33.10.2759-2762.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wyle FA, et al. Immunological Response of Man to Group B Meningococcal Polysaccharide Vaccines. Journal of Infectious Diseases. 1972;126:514-&. doi: 10.1093/infdis/126.5.514. [DOI] [PubMed] [Google Scholar]
- 36.Finne J, Leinonen M, Makela PH. Antigenic Similarities between Brain Components and Bacteria Causing Meningitis - Implications for Vaccine Development and Pathogenesis. Lancet. 1983;2:355–357. doi: 10.1016/s0140-6736(83)90340-9. [DOI] [PubMed] [Google Scholar]
- 37.Bruge J, Bouveret-Le Cam N, Danve B, Rougon G, Schulz D. Clinical evaluation of a group B meningococcal N-propionylated polysaccharide conjugate vaccine in adult, male volunteers. Vaccine. 2004;22:1087–96. doi: 10.1016/j.vaccine.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 38.Zimmer SM, Stephens DS. Serogroup B meningococcal vaccines. Curr Opin Investig Drugs. 2006;7:733–9. [PubMed] [Google Scholar]
- 39.Update: Guillain-Barre syndrome among recipients of Menactra meningococcal conjugate vaccine--United States June 2005-September 2006. MMWR Morb Mortal Wkly Rep. 2006;55:1120–4. [PubMed] [Google Scholar]
- 40.Verez-Bencomo V, et al. A synthetic conjugate polysaccharide vaccine against Haemophilus influenzae type b. Science. 2004;305:522–5. doi: 10.1126/science.1095209. [DOI] [PubMed] [Google Scholar]
- 41.Pozsgay V, et al. Protein conjugates of synthetic saccharides elicit higher levels of serum IgG lipopolysaccharide antibodies in mice than do those of the O-specific polysaccharide from Shigella dysenteriae type 1. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:5194–5197. doi: 10.1073/pnas.96.9.5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pozsgay V, Kubler-Kielb J, Schneerson R, Robbins JB. Effect of the nonreducing end of Shigella dysenteriae type 1 O-specific oligosaccharides on their immunogenicity as conjugates in mice. Proc Natl Acad Sci U S A. 2007;104:14478–82. doi: 10.1073/pnas.0706969104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jansen WTM, et al. Synthetic 6B di-, tri-, and tetrasaccharide-protein conjugates contain pneumococcal type 6A and 6B common and 6B-specific epitopes that elicit protective antibodies in mice. Infection and Immunity. 2001;69:787–793. doi: 10.1128/IAI.69.2.787-793.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Casadevall A. Antibody immunity and invasive fungal infections. Infect Immun. 1995;63:4211–8. doi: 10.1128/iai.63.11.4211-4218.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dromer F, Charreire J, Contrepois A, Carbon C, Yeni P. Protection of mice against experimental cryptococcosis by anti-Cryptococcus neoformans monoclonal antibody. Infect Immun. 1987;55:749–52. doi: 10.1128/iai.55.3.749-752.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cutler JE, Deepe GS, Jr, Klein BS. Advances in combating fungal diseases: vaccines on the threshold. Nat Rev Microbiol. 2007;5:13–28. doi: 10.1038/nrmicro1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mukherjee J, Casadevall A, Scharff MD. Molecular characterization of the humoral responses to Cryptococcus neoformans infection and glucuronoxylomannan-tetanus toxoid conjugate immunization. J Exp Med. 1993;177:1105–16. doi: 10.1084/jem.177.4.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mukherjee J, Nussbaum G, Scharff MD, Casadevall A. Protective and nonprotective monoclonal antibodies to Cryptococcus neoformans originating from one B cell. J Exp Med. 1995;181:405–9. doi: 10.1084/jem.181.1.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Devi SJ, et al. Cryptococcus neoformans serotype A glucuronoxylomannan-protein conjugate vaccines: synthesis, characterization, and immunogenicity. Infect Immun. 1991;59:3700–7. doi: 10.1128/iai.59.10.3700-3707.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oscarson S, Alpe M, Svahnberg P, Nakouzi A, Casadevall A. Synthesis and immunological studies of glycoconjugates of Cryptococcus neoformans capsular glucuronoxylomannan oligosaccharide structures. Vaccine. 2005;23:3961–72. doi: 10.1016/j.vaccine.2005.02.029. [DOI] [PubMed] [Google Scholar]
- 51.Beenhouwer DO, Yoo EM, Lai CW, Rocha MA, Morrison SL. Human immunoglobulin G2 (IgG2) and IgG4, but not IgG1 or IgG3, protect mice against Cryptococcus neoformans infection. Infect Immun. 2007;75:1424–35. doi: 10.1128/IAI.01161-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Taborda CP, Rivera J, Zaragoza O, Casadevall A. More is not necessarily better: prozone-like effects in passive immunization with IgG. J Immunol. 2003;170:3621–30. doi: 10.4049/jimmunol.170.7.3621. [DOI] [PubMed] [Google Scholar]
- 53.Yuan R, Casadevall A, Spira G, Scharff MD. Isotype switching from IgG3 to IgG1 converts a nonprotective murine antibody to Cryptococcus neoformans into a protective antibody. J Immunol. 1995;154:1810–6. [PubMed] [Google Scholar]
- 54.Ellerbroek PM, Walenkamp AM, Hoepelman AI, Coenjaerts FE. Effects of the capsular polysaccharides of Cryptococcus neoformans on phagocyte migration and inflammatory mediators. Curr Med Chem. 2004;11:253–66. doi: 10.2174/0929867043456188. [DOI] [PubMed] [Google Scholar]
- 55.Vecchiarelli A. Immunoregulation by capsular components of Cryptococcus neoformans. Med Mycol. 2000;38:407–17. doi: 10.1080/mmy.38.6.407.417. [DOI] [PubMed] [Google Scholar]
- 56.Fleuridor R, Lees A, Pirofski L. A cryptococcal capsular polysaccharide mimotope prolongs the survival of mice with Cryptococcus neoformans infection. J Immunol. 2001;166:1087–96. doi: 10.4049/jimmunol.166.2.1087. [DOI] [PubMed] [Google Scholar]
- 57.Han Y, Cutler JE. Antibody response that protects against disseminated candidiasis. Infect Immun. 1995;63:2714–9. doi: 10.1128/iai.63.7.2714-2719.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Han Y, Ulrich MA, Cutler JE. Candida albicans mannan extract-protein conjugates induce a protective immune response against experimental candidiasis. J Infect Dis. 1999;179:1477–84. doi: 10.1086/314779. [DOI] [PubMed] [Google Scholar]
- 59.Cutler JE. Defining criteria for anti-mannan antibodies to protect against candidiasis. Curr Mol Med. 2005;5:383–92. doi: 10.2174/1566524054022576. [DOI] [PubMed] [Google Scholar]
- 60.Nitz M, Bundle DR. Synthesis of di- to hexasaccharide 1,2-linked beta-mannopyranan oligomers, a terminal S-linked tetrasaccharide congener and the corresponding BSA glycoconjugates. J Org Chem. 2001;66:8411–23. doi: 10.1021/jo010570x. [DOI] [PubMed] [Google Scholar]
- 61.Nitz M, Ling CC, Otter A, Cutler JE, Bundle DR. The unique solution structure and immunochemistry of the Candida albicans beta -1,2-mannopyranan cell wall antigens. J Biol Chem. 2002;277:3440–6. doi: 10.1074/jbc.M109274200. [DOI] [PubMed] [Google Scholar]
- 62.Wu X, Bundle DR. Synthesis of glycoconjugate vaccines for Candida albicans using novel linker methodology. J Org Chem. 2005;70:7381–8. doi: 10.1021/jo051065t. [DOI] [PubMed] [Google Scholar]
- 63.Wu X, Lipinski T, Carrel FR, Bailey JJ, Bundle DR. Synthesis and immunochemical studies on a Candida albicans cluster glycoconjugate vaccine. Org Biomol Chem. 2007;5:3477–85. doi: 10.1039/b709912f. [DOI] [PubMed] [Google Scholar]
- 64.Xin H, Dziadek S, Bundle DR, Cutler JE. Synthetic glycopeptide vaccines combining {beta}-mannan and peptide epitopes induce protection against candidiasis. Proc Natl Acad Sci U S A. 2008 doi: 10.1073/pnas.0803195105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Torosantucci A, et al. A novel glyco-conjugate vaccine against fungal pathogens. J Exp Med. 2005;202:597–606. doi: 10.1084/jem.20050749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rachini A, et al. An anti-beta-glucan monoclonal antibody inhibits growth and capsule formation of Cryptococcus neoformans in vitro and exerts therapeutic, anticryptococcal activity in vivo. Infect Immun. 2007;75:5085–94. doi: 10.1128/IAI.00278-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Casadevall A, Pirofski LA. Polysaccharide-containing conjugate vaccines for fungal diseases. Trends Mol Med. 2006;12:6–9. doi: 10.1016/j.molmed.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 68.Price VL, Kieny MP. Vaccines for parasitic diseases. Curr Drug Targets Infect Disord. 2001;1:315–24. doi: 10.2174/1568005014606026. [DOI] [PubMed] [Google Scholar]
- 69.Schofield L, Grau GE. Immunological processes in malaria pathogenesis. Nat Rev Immunol. 2005;5:722–35. doi: 10.1038/nri1686. [DOI] [PubMed] [Google Scholar]
- 70.Murray HW, Berman JD, Davies CR, Saravia NG. Advances in leishmaniasis. Lancet. 2005;366:1561–77. doi: 10.1016/S0140-6736(05)67629-5. [DOI] [PubMed] [Google Scholar]
- 71.Anthony RM, Rutitzky LI, Urban JF, Jr, Stadecker MJ, Gause WC. Protective immune mechanisms in helminth infection. Nat Rev Immunol. 2007;7:975–87. doi: 10.1038/nri2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Diaz A, Allen JE. Mapping immune response profiles: the emerging scenario from helminth immunology. Eur J Immunol. 2007;37:3319–26. doi: 10.1002/eji.200737765. [DOI] [PubMed] [Google Scholar]
- 73.Maizels RM, Holland MJ, Falcone FH, Zang XX, Yazdanbakhsh M. Vaccination against helminth parasites--the ultimate challenge for vaccinologists? Immunol Rev. 1999;171:125–47. doi: 10.1111/j.1600-065x.1999.tb01345.x. [DOI] [PubMed] [Google Scholar]
- 74.Nyame AK, Kawar ZS, Cummings RD. Antigenic glycans in parasitic infections: implications for vaccines and diagnostics. Arch Biochem Biophys. 2004;426:182–200. doi: 10.1016/j.abb.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 75.Cohen S, Mc GI, Carrington S. Gamma-globulin and acquired immunity to human malaria. Nature. 1961;192:733–7. doi: 10.1038/192733a0. [DOI] [PubMed] [Google Scholar]
- 76.Krettli AU, Brener Z. Protective effects of specific antibodies in Trypanosoma cruzi infections. J Immunol. 1976;116:755–60. [PubMed] [Google Scholar]
- 77.Rajan B, Ramalingam T, Rajan TV. Critical role for IgM in host protection in experimental filarial infection. J Immunol. 2005;175:1827–33. doi: 10.4049/jimmunol.175.3.1827. [DOI] [PubMed] [Google Scholar]
- 78.Eberl M, et al. Cellular and humoral immune responses and protection against schistosomes induced by a radiation-attenuated vaccine in chimpanzees. Infect Immun. 2001;69:5352–62. doi: 10.1128/IAI.69.9.5352-5362.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Eberl M, et al. Antibodies to glycans dominate the host response to schistosome larvae and eggs: is their role protective or subversive? J Infect Dis. 2001;183:1238–47. doi: 10.1086/319691. [DOI] [PubMed] [Google Scholar]
- 80.Sun JB, et al. Nasal administration of Schistosoma mansoni egg antigen-cholera B subunit conjugate suppresses hepatic granuloma formation and reduces mortality in S. mansoni-infected mice. Scand J Immunol. 2001;54:440–7. doi: 10.1046/j.1365-3083.2001.00977.x. [DOI] [PubMed] [Google Scholar]
- 81.Bell RG, Appleton JA, Negrao-Correa DA, Adams LS. Rapid expulsion of Trichinella spiralis in adult rats mediated by monoclonal antibodies of distinct IgG isotypes. Immunology. 1992;75:520–7. [PMC free article] [PubMed] [Google Scholar]
- 82.Ellis LA, et al. Glycans as targets for monoclonal antibodies that protect rats against Trichinella spiralis. Glycobiology. 1994;4:585–92. doi: 10.1093/glycob/4.5.585. [DOI] [PubMed] [Google Scholar]
- 83.McVay CS, Bracken P, Gagliardo LF, Appleton J. Antibodies to tyvelose exhibit multiple modes of interference with the epithelial niche of Trichinella spiralis. Infect Immun. 2000;68:1912–8. doi: 10.1128/iai.68.4.1912-1918.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.McVay CS, Tsung A, Appleton J. Participation of parasite surface glycoproteins in antibody-mediated protection of epithelial cells against Trichinella spiralis. Infect Immun. 1998;66:1941–5. doi: 10.1128/iai.66.5.1941-1945.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Goyal PK, Wheatcroft J, Wakelin D. Tyvelose and protective responses to the intestinal stages of Trichinella spiralis. Parasitol Int. 2002;51:91–8. doi: 10.1016/s1383-5769(02)00002-8. [DOI] [PubMed] [Google Scholar]
- 86.Seeberger PH, Werz DB. Synthesis and medical applications of oligosaccharides. Nature. 2007;446:1046–51. doi: 10.1038/nature05819. [DOI] [PubMed] [Google Scholar]
- 87.Guha-Niyogi A, Sullivan DR, Turco SJ. Glycoconjugate structures of parasitic protozoa. Glycobiology. 2001;11:45R–59R. doi: 10.1093/glycob/11.4.45r. [DOI] [PubMed] [Google Scholar]
- 88.Ferguson MA. The structure, biosynthesis and functions of glycosylphosphatidylinositol anchors, and the contributions of trypanosome research. J Cell Sci. 1999;112 ( Pt 17):2799–809. doi: 10.1242/jcs.112.17.2799. [DOI] [PubMed] [Google Scholar]
- 89.Descoteaux A, Turco SJ. Glycoconjugates in Leishmania infectivity. Biochim Biophys Acta. 1999;1455:341–52. doi: 10.1016/s0925-4439(99)00065-4. [DOI] [PubMed] [Google Scholar]
- 90.Descoteaux A, Turco SJ. Functional aspects of the Leishmania donovani lipophosphoglycan during macrophage infection. Microbes Infect. 2002;4:975–81. doi: 10.1016/s1286-4579(02)01624-6. [DOI] [PubMed] [Google Scholar]
- 91.Dinglasan RR, Fields I, Shahabuddin M, Azad AF, Sacci JB., Jr Monoclonal antibody MG96 completely blocks Plasmodium yoelii development in Anopheles stephensi. Infect Immun. 2003;71:6995–7001. doi: 10.1128/IAI.71.12.6995-7001.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.O’Connor RM, Kim K, Khan F, Ward HD. Expression of Cpgp40/15 in Toxoplasma gondii: a surrogate system for the study of Cryptosporidium glycoprotein antigens. Infect Immun. 2003;71:6027–34. doi: 10.1128/IAI.71.10.6027-6034.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Schofield L, Hewitt MC, Evans K, Siomos MA, Seeberger PH. Synthetic GPI as a candidate anti-toxic vaccine in a model of malaria. Nature. 2002;418:785–9. doi: 10.1038/nature00937. [DOI] [PubMed] [Google Scholar]
- 94.Goel A, Vohra H, Varshney GC. Strain-specific recognition of live Leishmania donovani promastigotes by homologous antiserum raised against a crude membrane fraction of infected macrophages. Parasitol Res. 1999;85:19–24. doi: 10.1007/s004360050501. [DOI] [PubMed] [Google Scholar]
- 95.Turco SJ, Descoteaux A. The lipophosphoglycan of Leishmania parasites. Annu Rev Microbiol. 1992;46:65–94. doi: 10.1146/annurev.mi.46.100192.000433. [DOI] [PubMed] [Google Scholar]
- 96.Tonui WK, et al. Transmission blocking vaccine studies in leishmaniasis: I. Lipophosphoglycan is a promising transmission blocking vaccine molecule against cutaneous leishmaniasis. East Afr Med J. 2001;78:84–9. doi: 10.4314/eamj.v78i2.9094. [DOI] [PubMed] [Google Scholar]
- 97.Pinheiro RO, et al. Protection against cutaneous leishmaniasis by intranasal vaccination with lipophosphoglycan. Vaccine. 2007;25:2716–22. doi: 10.1016/j.vaccine.2006.05.093. [DOI] [PubMed] [Google Scholar]
- 98.McConville MJ, Bacic A, Mitchell GF, Handman E. Lipophosphoglycan of Leishmania major that vaccinates against cutaneous leishmaniasis contains an alkylglycerophosphoinositol lipid anchor. Proc Natl Acad Sci U S A. 1987;84:8941–5. doi: 10.1073/pnas.84.24.8941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Moll H, Mitchell GF, McConville MJ, Handman E. Evidence of T-cell recognition in mice of a purified lipophosphoglycan from Leishmania major. Infect Immun. 1989;57:3349–56. doi: 10.1128/iai.57.11.3349-3356.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Russell DG, Alexander J. Effective immunization against cutaneous leishmaniasis with defined membrane antigens reconstituted into liposomes. J Immunol. 1988;140:1274–9. [PubMed] [Google Scholar]
- 101.Hewitt MC, Seeberger PH. Solution and solid-support synthesis of a potential leishmaniasis carbohydrate vaccine. J Org Chem. 2001;66:4233–43. doi: 10.1021/jo015521z. [DOI] [PubMed] [Google Scholar]
- 102.Routier FH, Nikolaev AV, Ferguson MA. The preparation of neoglycoconjugates containing inter-saccharide phosphodiester linkages as potential anti-Leishmania vaccines. Glycoconj J. 1999;16:773–80. doi: 10.1023/a:1007171613195. [DOI] [PubMed] [Google Scholar]
- 103.Liu X, et al. Enhancement of the immunogenicity of synthetic carbohydrates by conjugation to virosomes: a leishmaniasis vaccine candidate. ACS Chem Biol. 2006;1:161–4. doi: 10.1021/cb600086b. [DOI] [PubMed] [Google Scholar]
- 104.Mascola JR, et al. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat Med. 2000;6:207– 10. doi: 10.1038/72318. [DOI] [PubMed] [Google Scholar]
- 105.Calarese DA, et al. Dissection of the carbohydrate specificity of the broadly neutralizing anti-HIV-1 antibody 2G12. Proc Natl Acad Sci U S A. 2005;102:13372–7. doi: 10.1073/pnas.0505763102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Scanlan C, et al. Antibody recognition of a carbohydrate epitope: a template for HIV vaccine design. Adv Exp Med Biol. 2005;564:7–8. doi: 10.1007/0-387-25515-X_4. [DOI] [PubMed] [Google Scholar]
- 107.Binley JM, et al. Comprehensive cross-clade neutralization analysis of a panel of anti- human immunodeficiency virus type 1 monoclonal antibodies. J Virol. 2004;78:13232–52. doi: 10.1128/JVI.78.23.13232-13252.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Scanlan CN, Offer J, Zitzmann N, Dwek RA. Exploiting the defensive sugars of HIV-1 for drug and vaccine design. Nature. 2007;446:1038–45. doi: 10.1038/nature05818. [DOI] [PubMed] [Google Scholar]
- 109.Ratner DM, Seeberger PH. Carbohydrate microarrays as tools in HIV glycobiology. Curr Pharm Des. 2007;13:173–83. doi: 10.2174/138161207779313650. [DOI] [PubMed] [Google Scholar]
- 110.Scanlan CN, et al. The broadly neutralizing anti-human immunodeficiency virus type 1 antibody 2G12 recognizes a cluster of alpha1-->2 mannose residues on the outer face of gp120. J Virol. 2002;76:7306–21. doi: 10.1128/JVI.76.14.7306-7321.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sanders RW, et al. The mannose-dependent epitope for neutralizing antibody 2G12 on human immunodeficiency virus type 1 glycoprotein gp120. J Virol. 2002;76:7293–305. doi: 10.1128/JVI.76.14.7293-7305.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wang J, Li H, Zou G, Wang LX. Novel template-assembled oligosaccharide clusters as epitope mimics for HIV-neutralizing antibody 2G12. Design, synthesis, and antibody binding study. Org Biomol Chem. 2007;5:1529–40. doi: 10.1039/b702961f. [DOI] [PubMed] [Google Scholar]
- 113.Wang SK, et al. Targeting the carbohydrates on HIV-1: Interaction of oligomannose dendrons with human monoclonal antibody 2G12 and DC-SIGN. Proc Natl Acad Sci U S A. 2008;105:3690–5. doi: 10.1073/pnas.0712326105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Astronomo RD, et al. Defining criteria for oligomannose immunogens for HIV using icosahedral virus capsid scaffolds. Chemistry and Biology. 2009 doi: 10.1016/j.chembiol.2010.03.012. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Joyce JG, et al. An oligosaccharide-based HIV-1 2G12 mimotope vaccine induces carbohydrate-specific antibodies that fail to neutralize HIV-1 virions. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:15684–15689. doi: 10.1073/pnas.0807837105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dunlop DC, et al. Antigenic mimicry of the HIV envelope by AIDS-associated pathogens. AIDS. 2008;22:2214–7. doi: 10.1097/QAD.0b013e328314b5df. [DOI] [PubMed] [Google Scholar]
- 117.Luallen RJ, et al. An engineered Saccharomyces cerevisiae strain binds the broadly neutralizing human immunodeficiency virus type 1 antibody 2G12 and elicits mannose- specific gp120-binding antibodies. J Virol. 2008;82:6447–57. doi: 10.1128/JVI.00412-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Astronomo RD, et al. A glycoconjugate antigen based on the recognition motif of a broadly neutralizing human immunodeficiency virus antibody, 2G12, is immunogenic but elicits antibodies unable to bind to the self glycans of gp120. J Virol. 2008;82:6359–68. doi: 10.1128/JVI.00293-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Meezan E, Wu HC, Black PH, Robbins PW. Comparative studies on the carbohydrate-containing membrane components of normal and virus-transformed mouse fibroblasts. II. Separation of glycoproteins and glycopeptides by sephadex chromatography. Biochemistry. 1969;8:2518–24. doi: 10.1021/bi00834a039. [DOI] [PubMed] [Google Scholar]
- 120.Dennis JW, Laferte S, Waghorne C, Breitman ML, Kerbel RS. Beta 1–6 branching of Asn-linked oligosaccharides is directly associated with metastasis. Science. 1987;236:582–5. doi: 10.1126/science.2953071. [DOI] [PubMed] [Google Scholar]
- 121.Kim YJ, Varki A. Perspectives on the significance of altered glycosylation of glycoproteins in cancer. Glycoconj J. 1997;14:569–76. doi: 10.1023/a:1018580324971. [DOI] [PubMed] [Google Scholar]
- 122.Gabius HJ. Biological and clinical implications of tumor lectins: present status and future prospects. Acta Histochem Suppl. 1988;36:209–16. [PubMed] [Google Scholar]
- 123.Sell S. Cancer-associated carbohydrates identified by monoclonal antibodies. Hum Pathol. 1990;21:1003–19. doi: 10.1016/0046-8177(90)90250-9. [DOI] [PubMed] [Google Scholar]
- 124.Taylor-Papadimitriou J, Epenetos AA. Exploiting altered glycosylation patterns in cancer: progress and challenges in diagnosis and therapy. Trends Biotechnol. 1994;12:227–33. doi: 10.1016/0167-7799(94)90121-X. [DOI] [PubMed] [Google Scholar]
- 125.Hakomori S, Zhang Y. Glycosphingolipid antigens and cancer therapy. Chem Biol. 1997;4:97–104. doi: 10.1016/s1074-5521(97)90253-2. [DOI] [PubMed] [Google Scholar]
- 126.Hollingsworth MA, Swanson BJ. Mucins in cancer: protection and control of the cell surface. Nat Rev Cancer. 2004;4:45–60. doi: 10.1038/nrc1251. [DOI] [PubMed] [Google Scholar]
- 127.Slovin SF, Keding SJ, Ragupathi G. Carbohydrate vaccines as immunotherapy for cancer. Immunol Cell Biol. 2005;83:418–28. doi: 10.1111/j.1440-1711.2005.01350.x. [DOI] [PubMed] [Google Scholar]
- 128.Kensil CR, Patel U, Lennick M, Marciani D. Separation and characterization of saponins with adjuvant activity from Quillaja saponaria Molina cortex. J Immunol. 1991;146:431–7. [PubMed] [Google Scholar]
- 129.Helling F, et al. GM2-KLH conjugate vaccine: increased immunogenicity in melanoma patients after administration with immunological adjuvant QS-21. Cancer Res. 1995;55:2783–8. [PubMed] [Google Scholar]
- 130.Helling F, et al. GD3 vaccines for melanoma: superior immunogenicity of keyhole limpet hemocyanin conjugate vaccines. Cancer Res. 1994;54:197–203. [PubMed] [Google Scholar]
- 131.Ritter G, et al. Antibody response to immunization with ganglioside GD3 and GD3 congeners (lactones, amide and gangliosidol) in patients with malignant melanoma. Int J Cancer. 1991;48:379–85. doi: 10.1002/ijc.2910480312. [DOI] [PubMed] [Google Scholar]
- 132.Ritter G, et al. Antibody response to immunization with purified GD3 ganglioside and GD3 derivatives (lactones, amide and gangliosidol) in the mouse. Immunobiology. 1990;182:32–43. doi: 10.1016/S0171-2985(11)80581-4. [DOI] [PubMed] [Google Scholar]
- 133.Krug LM, et al. Vaccination of small cell lung cancer patients with polysialic acid or N- propionylated polysialic acid conjugated to keyhole limpet hemocyanin. Clin Cancer Res. 2004;10:916–23. doi: 10.1158/1078-0432.ccr-03-0101. [DOI] [PubMed] [Google Scholar]
- 134.Musselli C, Livingston PO, Ragupathi G. Keyhole limpet hemocyanin conjugate vaccines against cancer: the Memorial Sloan Kettering experience. J Cancer Res Clin Oncol. 2001;127 (Suppl 2):R20–6. doi: 10.1007/BF01470995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ingale S, Wolfert MA, Gaekwad J, Buskas T, Boons GJ. Robust immune responses elicited by a fully synthetic three-component vaccine. Nat Chem Biol. 2007;3:663–7. doi: 10.1038/nchembio.2007.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Keding SJ, Danishefsky SJ. Prospects for total synthesis: a vision for a totally synthetic vaccine targeting epithelial tumors. Proc Natl Acad Sci U S A. 2004;101:11937–42. doi: 10.1073/pnas.0401894101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gilewski TA, et al. Immunization of high-risk breast cancer patients with clustered sTn- KLH conjugate plus the immunologic adjuvant QS-21. Clin Cancer Res. 2007;13:2977–85. doi: 10.1158/1078-0432.CCR-06-2189. [DOI] [PubMed] [Google Scholar]
- 138.Slovin SF, et al. Thomsen-Friedenreich (TF) antigen as a target for prostate cancer vaccine: clinical trial results with TF cluster (c)-KLH plus QS21 conjugate vaccine in patients with biochemically relapsed prostate cancer. Cancer Immunol Immunother. 2005;54:694–702. doi: 10.1007/s00262-004-0598-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Slovin SF, et al. Fully synthetic carbohydrate-based vaccines in biochemically relapsed prostate cancer: clinical trial results with alpha-N-acetylgalactosamine-O-serine/threonine conjugate vaccine. J Clin Oncol. 2003;21:4292–8. doi: 10.1200/JCO.2003.04.112. [DOI] [PubMed] [Google Scholar]
- 140.Toyokuni T, Hakomori S, Singhal AK. Synthetic carbohydrate vaccines: synthesis and immunogenicity of Tn antigen conjugates. Bioorg Med Chem. 1994;2:1119–32. doi: 10.1016/s0968-0896(00)82064-7. [DOI] [PubMed] [Google Scholar]
- 141.Lo-Man R, et al. Anti-tumor immunity provided by a synthetic multiple antigenic glycopeptide displaying a tri-Tn glycotope. J Immunol. 2001;166:2849–54. doi: 10.4049/jimmunol.166.4.2849. [DOI] [PubMed] [Google Scholar]
- 142.Lo-Man R, et al. A fully synthetic therapeutic vaccine candidate targeting carcinoma- associated Tn carbohydrate antigen induces tumor-specific antibodies in nonhuman primates. Cancer Res. 2004;64:4987–94. doi: 10.1158/0008-5472.CAN-04-0252. [DOI] [PubMed] [Google Scholar]
- 143.Grigalevicius S, et al. Chemoselective assembly and immunological evaluation of multiepitopic glycoconjugates bearing clustered Tn antigen as synthetic anticancer vaccines. Bioconjug Chem. 2005;16:1149–59. doi: 10.1021/bc050010v. [DOI] [PubMed] [Google Scholar]
- 144.Kaiser A, et al. A synthetic vaccine consisting of a tumor-associated sialyl-T(N)-MUC1 tandem-repeat glycopeptide and tetanus toxoid: induction of a strong and highly selective immune response. Angew Chem Int Ed Engl. 2009;48:7551–5. doi: 10.1002/anie.200902564. [DOI] [PubMed] [Google Scholar]
- 145.Ragupathi G, et al. Comparison of antibody titers after immunization with monovalent or tetravalent KLH conjugate vaccines. Vaccine. 2002;20:1030–8. doi: 10.1016/s0264-410x(01)00451-0. [DOI] [PubMed] [Google Scholar]
- 146.Ragupathi G, et al. A preclinical study comparing approaches for augmenting the immunogenicity of a heptavalent KLH-conjugate vaccine against epithelial cancers. Cancer Immunol Immunother. 2003;52:608–16. doi: 10.1007/s00262-003-0399-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sabbatini PJ, et al. Pilot study of a heptavalent vaccine-keyhole limpet hemocyanin conjugate plus QS21 in patients with epithelial ovarian, fallopian tube, or peritoneal cancer. Clin Cancer Res. 2007;13:4170–7. doi: 10.1158/1078-0432.CCR-06-2949. [DOI] [PubMed] [Google Scholar]
- 148.Slovin SF, et al. A polyvalent vaccine for high-risk prostate patients: “are more antigens better?”. Cancer Immunol Immunother. 2007;56:1921–30. doi: 10.1007/s00262-007-0335-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ragupathi G, et al. Preparation and evaluation of unimolecular pentavalent and hexavalent antigenic constructs targeting prostate and breast cancer: a synthetic route to anticancer vaccine candidates. J Am Chem Soc. 2006;128:2715–25. doi: 10.1021/ja057244+. [DOI] [PubMed] [Google Scholar]
- 150.Pan Y, Chefalo P, Nagy N, Harding C, Guo Z. Synthesis and immunological properties of N-modified GM3 antigens as therapeutic cancer vaccines. J Med Chem. 2005;48:875–83. doi: 10.1021/jm0494422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Chefalo P, Pan Y, Nagy N, Guo Z, Harding CV. Efficient metabolic engineering of GM3 on tumor cells by N-phenylacetyl-D-mannosamine. Biochemistry. 2006;45:3733–9. doi: 10.1021/bi052161r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wang Q, Zhang J, Guo Z. Efficient glycoengineering of GM3 on melanoma cell and monoclonal antibody-mediated selective killing of the glycoengineered cancer cell. Bioorg Med Chem. 2007;15:7561–7. doi: 10.1016/j.bmc.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Macher BA, Galili U. The Galalpha1,3Galbeta1,4GlcNAc-R (alpha-Gal) epitope: a carbohydrate of unique evolution and clinical relevance. Biochim Biophys Acta. 2008;1780:75–88. doi: 10.1016/j.bbagen.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Braun DP, et al. Aromatase inhibitors increase the sensitivity of human tumor cells to monocyte-mediated, antibody-dependent cellular cytotoxicity. Am J Surg. 2005;190:570–1. doi: 10.1016/j.amjsurg.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 155.Eggermont AM, et al. EORTC 18961: Post-operative adjuvant ganglioside GM2-KLH21 vaccination treatment vs observation in stage II (T3-T4N0M0) melanoma: 2nd interim analysis led to an early disclosure of the results. Journal of Clinical Oncology. 2008;26:Abstract 9004. [Google Scholar]
- 156.Huber M, Trkola A. Humoral immunity to HIV-1: neutralization and beyond. J Intern Med. 2007;262:5–25. doi: 10.1111/j.1365-2796.2007.01819.x. [DOI] [PubMed] [Google Scholar]
- 157.Livingston PO, Ragupathi G. Cancer vaccines targeting carbohydrate antigens. Hum Vaccin. 2006;2:137–43. doi: 10.4161/hv.2941. [DOI] [PubMed] [Google Scholar]
- 158.Bouharoun-Tayoun H, Attanath P, Sabchareon A, Chongsuphajaisiddhi T, Druilhe P. Antibodies that protect humans against Plasmodium falciparum blood stages do not on their own inhibit parasite growth and invasion in vitro, but act in cooperation with monocytes. J Exp Med. 1990;172:1633–41. doi: 10.1084/jem.172.6.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Crowe JS, Lamont AG, Barry JD, Vickerman K. Cytotoxicity of monoclonal antibodies to Trypanosoma brucei. Trans R Soc Trop Med Hyg. 1984;78:508–13. doi: 10.1016/0035-9203(84)90073-7. [DOI] [PubMed] [Google Scholar]
- 160.Raman R, Raguram S, Venkataraman G, Paulson JC, Sasisekharan R. Glycomics: an integrated systems approach to structure-function relationships of glycans. Nature Methods. 2005;2:817–824. doi: 10.1038/nmeth807. [DOI] [PubMed] [Google Scholar]
- 161.Guerrini M, et al. A novel computational approach to integrate NMR spectroscopy and capillary electrophoresis for structure assignment of heparin and heparan sulfate oligosaccharides. Glycobiology. 2002;12:713–9. doi: 10.1093/glycob/cwf084. [DOI] [PubMed] [Google Scholar]
- 162.Venkataraman G, Shriver Z, Raman R, Sasisekharan R. Sequencing complex polysaccharides. Science. 1999;286:537–542. doi: 10.1126/science.286.5439.537. [DOI] [PubMed] [Google Scholar]
- 163.Sears P, Wong CH. Toward automated synthesis of oligosaccharides and glycoproteins. Science. 2001;291:2344–50. doi: 10.1126/science.1058899. [DOI] [PubMed] [Google Scholar]
- 164.Seeberger PH. Automated oligosaccharide synthesis. Chem Soc Rev. 2008;37:19–28. doi: 10.1039/b511197h. [DOI] [PubMed] [Google Scholar]
- 165.Wang Y, Ye XS, Zhang LH. Oligosaccharide assembly by one-pot multi-step strategy. Org Biomol Chem. 2007;5:2189–200. doi: 10.1039/b704586g. [DOI] [PubMed] [Google Scholar]
- 166.Paulson JC, Blixt O, Collins BE. Sweet spots in functional glycomics. Nat Chem Biol. 2006;2:238–48. doi: 10.1038/nchembio785. [DOI] [PubMed] [Google Scholar]
- 167.Blixt O, et al. Printed covalent glycan array for ligand profiling of diverse glycan binding proteins. Glycobiology. 2004;14:1072–1072. doi: 10.1073/pnas.0407902101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Karamanska R, et al. Surface plasmon resonance imaging for real-time, label-free analysis of protein interactions with carbohydrate microarrays. Glycoconj J. 2008;25:69–74. doi: 10.1007/s10719-007-9047-y. [DOI] [PubMed] [Google Scholar]
- 169.Su J, Mrksich M. Using mass spectrometry to characterize self-assembled monolayers presenting peptides, proteins, and carbohydrates. Angew Chem Int Ed Engl. 2002;41:4715–8. doi: 10.1002/anie.200290026. [DOI] [PubMed] [Google Scholar]
- 170.Zhi ZL, et al. A versatile gold surface approach for fabrication and interrogation of glycoarrays. Chembiochem. 2008;9:1568–75. doi: 10.1002/cbic.200700788. [DOI] [PubMed] [Google Scholar]
- 171.Taylor ME, Drickamer K. Structural insights into what glycan arrays tell us about how glycan-binding proteins interact with their ligands. Glycobiology. 2009 doi: 10.1093/glycob/cwp076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Wormald MR, et al. Conformational studies of oligosaccharides and glycopeptides: complementarity of NMR, X-ray crystallography, and molecular modelling. Chem Rev. 2002;102:371–86. doi: 10.1021/cr990368i. [DOI] [PubMed] [Google Scholar]
- 173.Jimenez-Barbero J, Asensio JL, Canada FJ, Poveda A. Free and protein-bound carbohydrate structures. Current Opinion in Structural Biology. 1999;9:549–555. doi: 10.1016/s0959-440x(99)00004-4. [DOI] [PubMed] [Google Scholar]
- 174.DeMarco ML, Woods RJ. Structural glycobiology: A game of snakes and ladders. Glycobiology. 2008;18:426–440. doi: 10.1093/glycob/cwn026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Peltola H. Worldwide Haemophilus influenzae type b disease at the beginning of the 21st century: global analysis of the disease burden 25 years after the use of the polysaccharide vaccine and a decade after the advent of conjugates. Clin Microbiol Rev. 2000;13:302–17. doi: 10.1128/cmr.13.2.302-317.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.World Health Organization. Haemophilus influenzae type B (HiB) Geneva, Switzerland: World Health Organization; 2005. website [online] [Google Scholar]
- 177.Torano G, et al. Phase I clinical evaluation of a synthetic oligosaccharide-protein conjugate vaccine against Haemophilus influenzae type b in human adult volunteers. Clinical and Vaccine Immunology. 2006;13:1052–1056. doi: 10.1128/CVI.00144-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Fernandez-Santana V, et al. Antigenicity and immunogenicity of a synthetic oligosaccharide-protein conjugate vaccine against Haemophilus influenzae type b. Infection and Immunity. 2004;72:7115–7123. doi: 10.1128/IAI.72.12.7115-7123.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Ahmed A, Li J, Shiloach Y, Robbins JB, Szu SC. Safety and immunogenicity of Escherichia coli O157 O-specific polysaccharide conjugate vaccine in 2–5-year-old children. J Infect Dis. 2006;193:515–21. doi: 10.1086/499821. [DOI] [PubMed] [Google Scholar]
- 180.Sabharwal H, et al. Group A streptococcus (GAS) carbohydrate as an immunogen for protection against GAS infection. J Infect Dis. 2006;193:129–35. doi: 10.1086/498618. [DOI] [PubMed] [Google Scholar]
- 181.Paoletti LC, Kasper DL. Glycoconjugate vaccines to prevent group B streptococcal infections. Expert Opin Biol Ther. 2003;3:975–84. doi: 10.1517/14712598.3.6.975. [DOI] [PubMed] [Google Scholar]
- 182.Hong W, Peng D, Rivera M, Gu XX. Protection against nontypeable Haemophilus influenzae challenges by mucosal vaccination with a detoxified lipooligosaccharide conjugate in two chinchilla models. Microbes Infect. 2009 doi: 10.1016/j.micinf.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Zuercher AW, et al. Antibody responses induced by long-term vaccination with an octovalent conjugate Pseudomonas aeruginosa vaccine in children with cystic fibrosis. FEMS Immunol Med Microbiol. 2006;47:302–8. doi: 10.1111/j.1574-695X.2006.00103.x. [DOI] [PubMed] [Google Scholar]
- 184.Canh DG, et al. Effect of dosage on immunogenicity of a Vi conjugate vaccine injected twice into 2- to 5-year-old Vietnamese children. Infect Immun. 2004;72:6586–8. doi: 10.1128/IAI.72.11.6586-6588.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Passwell JH, et al. Safety and immunogenicity of Shigella sonnei-CRM9 and Shigella flexneri type 2a-rEPAsucc conjugate vaccines in one- to four-year-old children. Pediatr Infect Dis J. 2003;22:701–6. doi: 10.1097/01.inf.0000078156.03697.a5. [DOI] [PubMed] [Google Scholar]
- 186.Cohen D, et al. Double-blind vaccine-controlled randomised efficacy trial of an investigational Shigella sonnei conjugate vaccine in young adults. Lancet. 1997;349:155–9. doi: 10.1016/S0140-6736(96)06255-1. [DOI] [PubMed] [Google Scholar]
- 187.Gupta RK, Taylor DN, Bryla DA, Robbins JB, Szu SC. Phase 1 evaluation of Vibrio cholerae O1, serotype Inaba, polysaccharide-cholera toxin conjugates in adult volunteers. Infect Immun. 1998;66:3095–9. doi: 10.1128/iai.66.7.3095-3099.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Zhang S, et al. Selection of tumor antigens as targets for immune attack using immunohistochemistry: I. Focus on gangliosides. Int J Cancer. 1997;73:42–9. doi: 10.1002/(sici)1097-0215(19970926)73:1<42::aid-ijc8>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 189.Zhang S, et al. Selection of tumor antigens as targets for immune attack using immunohistochemistry: II. Blood group-related antigens. Int J Cancer. 1997;73:50–6. doi: 10.1002/(sici)1097-0215(19970926)73:1<50::aid-ijc9>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 190.Zhu X, Borchers C, Bienstock RJ, Tomer KB. Mass spectrometric characterization of the glycosylation pattern of HIV-gp120 expressed in CHO cells. Biochemistry. 2000;39:11194–204. doi: 10.1021/bi000432m. [DOI] [PubMed] [Google Scholar]