Abstract
Helicobacter pylori is a gastric bacterial pathogen that evades host immune responses in vivo and is associated with the development of gastritis, peptic ulcer disease, and gastric cancers. Induction of macrophage apoptosis is a method employed by multiple pathogens to escape host immune responses. Therefore, we hypothesized that H. pylori induces apoptosis of infected macrophages. RAW 264.7 cells were infected with H. pylori strain 60190, and apoptosis was assessed. Transmission electron microscopy and fluorescence microscopy showed that infected macrophages displayed morphological features characteristic of apoptosis. Quantification by acridine orange-ethidium bromide fluorescent-dye staining showed that apoptosis was dose and time dependent, and apoptosis was further confirmed by increased binding of annexin V-fluorescein isothiocyanate (FITC) to externalized phosphatidylserine of infected but not of control macrophages. Macrophages infected with isogenic mutants of H. pylori strain 60190 deficient in either cagA or vacA induced significantly less apoptosis than the parental strain, as assessed by increased binding of annexin V-FITC. Western blot analysis of whole-cell protein lysates revealed that infection with strain 60190 induced a time-dependent increase in cleavage of procaspase 8 and disappearance of full-length Bid compared with uninfected cells. Furthermore, pharmacological inhibition of caspase 8 caused a decrease in levels of apoptosis. Finally, infection caused a time-dependent increase in mitochondrial-membrane permeability and release of cytochrome c into the cytosol. These results suggest that H. pylori induces apoptosis of macrophages in association with alterations in the mitochondrial pathway. Elimination of this key immunomodulatory cell may represent a mechanism employed by the bacterium to evade host immune responses.
Helicobacter pylori is a gram-negative microaerophilic bacterium that causes a lifelong infection in over half of the world's human population (50). Without specific antimicrobial treatment, all individuals infected with H. pylori exhibit chronic gastric inflammation, and a small percentage will develop peptic ulcers and gastric adenocarcinoma or mucosa-associated lymphoid tissue lymphoma (14, 57). In response to infection, the host launches a vigorous immune response, including the mucosal infiltration of neutrophils, lymphocytes, and macrophages (18). However, this response is insufficient for clearance of the bacterium, suggesting that H. pylori is capable of evading host immune responses.
H. pylori strains are classified based on carriage of the cag pathogenicity island (PAI) and expression of the vacuolating cytotoxin (VacA) (9). Type I strains possess the cag PAI and secrete VacA; in contrast, type II strains lack the cag PAI and do not produce VacA. Controversy surrounds the role of cagA and vacA as bacterial virulence factors in vivo. For example, initial studies showed an increased risk of peptic-ulcer disease and gastric cancers in association with strains expressing CagA (4, 58); however, subsequent studies of both adults (24, 56) and children (43) provided conflicting results. Furthermore, infection with type I, but not type II, strains in Western populations is associated with increased risk for development of peptic-ulcer disease and gastric cancers (8, 9, 57), with contradictory data found in both Asian and Australian populations (47, 56).
Apoptosis can be signaled through a number of pathways, including the death receptor and mitochondrial pathways (2, 25). First, ligation of FasL to three Fas molecules activates the death receptor pathway, recruiting the Fas-associated death domain and the proenzyme form of caspase 8 to form the death-inducing signaling complex (53). Formation of the death-inducing signaling complex may result in cleavage of procaspase 3, leading to activation of downstream effectors and resulting in genomic fragmentation and other characteristic features of apoptosis (12, 17). Alternatively, apoptosis may be signaled through the mitochondrial pathway, where death signals increase mitochondrial-membrane permeability, allowing the subsequent release of proapoptotic factors, such as cytochrome c, into the cytosol (19), again leading to the induction of apoptosis (41). In some instances, both pathways may work synergistically when death receptor-dependent activation of procaspase 8 leads to cleavage and activation of Bid to damage mitochondrial-membrane permeability, release cytochrome c, and induce apoptosis involving both the mitochondrial and death receptor pathways.
Increasing evidence indicates that apoptosis of immune cells plays an important role in modulating the pathogenesis of a number of bacterially induced diseases (54). For example, the host response can include induction of macrophage apoptosis to purposefully eliminate intracellular bacteria, such as Mycobacterium tuberculosis and Mycobacterium bovis (49, 60), confirming the importance of this immune cell. Alternatively, Shigella flexneri and Salmonella enterica serovar Typhimurium trigger macrophage apoptosis to induce inflammation and persist within the host (54). Furthermore, macrophages play a central role in the inflammatory response to infection with H. pylori (20, 23). For example, increased numbers of macrophages are observed in the mucosa of gastric biopsy specimens obtained from H. pylori-infected children and correlate with the severity of gastritis (71). Although H. pylori is considered an extracellular pathogen, clinical isolates have been shown to invade epithelial monolayers (1, 66), and a recent study by Jhala et al. (32) showed the bacterium residing in the lamina propria in infected individuals, suggesting a possible point of interaction between the bacterium and mucosal macrophages. Alternatively, H. pylori secretes soluble bacterial products that traverse the epithelial barrier and interact with macrophages in the lamina propria. Indeed, H. pylori urease has been detected in the lamina propria in patients with H. pylori-associated gastritis but not in uninfected subjects (46). Moreover, H. pylori could interact with mucosal macrophages following the loss of surface epithelium during infection (1, 70), as H. pylori induces apoptosis of epithelial cells both in vitro (51) and in vivo (35).
H. pylori modulates apoptotic signaling pathways in a number of cell types in vivo and in vitro, including epithelial cells (33), polymorphonuclear leukocytes (28), and T lymphocytes (69). Recently, the ability of H. pylori to induce apoptosis in macrophages was demonstrated (23, 72). However, neither the apoptotic signaling pathways nor the bacterial factors involved have been fully delineated. Therefore, the aim of our study was to demonstrate that H. pylori induces apoptosis of macrophages and to delineate both the bacterial factors and the apoptotic signaling pathways involved.
MATERIALS AND METHODS
Bacterial growth conditions.
Wild-type H. pylori strain 60190 isolated from an adult with gastritis (ATCC 49503; cagA+ cagE+ VacA+) (36) and its isogenic cagA− and vacA− mutants (kindly provided by Richard Peek, Jr., Nashville, Tenn.) were grown under microaerophilic conditions (5% O2, 10% CO2, 85% N2) on Columbia blood agar plates containing 5% sheep blood for 72 h at 37°C or supplemented with 10% heat-inactivated fetal calf serum and 20 μg of kanamycin/ml (mutant strains) (36). The bacteria were then suspended in brucella broth (Difco Laboratories, Detroit, Mich.) supplemented with 10% heat-inactivated fetal bovine serum (Gibco BRL Life Technologies, Gaithersburg, Md.) and 20 μg of kanamycin/ml (mutant strains) (36) and shaken at 120 rpm overnight at 37°C under microaerophilic conditions (36). The cells were subsequently pelleted and resuspended in sterile phosphate-buffered saline (PBS), and optical density measurements were obtained by a spectrophotometric reading. Before the infection of eukaryotic cells, a droplet of culture was assessed for bacterial motility under bright-field microscopy, and after the infection of eukaryotic cells, the medium was cultured on blood agar plates to exclude contamination.
Eukaryotic-cell culture and conditions of infection.
The RAW 264.7 murine macrophage cell line, used as a model cell line to define H. pylori-induced signal transduction and its interaction with immune cells (23, 70, 72), was grown at 37°C in 5% CO2 in Dulbecco's modified Eagle's medium containing l-glutamine and glucose (Gibco BRL Life Technologies) and further supplemented with 10% heat-inactivated fetal bovine serum and 1% penicillin-streptomycin (Gibco BRL Life Technologies). Following the removal of antibiotics by rinsing the macrophages with PBS, the macrophages were infected with H. pylori at different multiplicities of infection (MOI) (from 10 bacteria to 1 macrophage to 100 bacteria to 1 macrophage) for 2 to 48 h. RAW 264.7 cells incubated with staurosporine (1 μM; Sigma Aldrich, Oakville, Ontario, Canada) for 8 to 24 h served as a positive control for the induction of apoptosis (6).
Assessment of apoptosis. (i) Transmission electron microscopy.
Macrophages were grown to 70% confluency and infected with H. pylori as described above, with uninfected cells serving as controls. After 24 h of infection, the adherent cells were scraped with a rubber policeman, pooled with cells in suspension, and pelleted by centrifugation. The cells were then fixed with 2% glutaraldehyde in 0.1 M phosphate buffer, postfixed in 2% osmium tetroxide, and dehydrated through a series of graded acetone washes (16). Samples were embedded in epoxy resin, sectioned, and placed onto 300-mesh copper grids. The grids were stained with uranyl acetate and lead salts, as previously described (16), and samples were examined for the presence of apoptotic cells using a Philips transmission electron microscope at the Electron Microscopy Facility of the Hospital for Sick Children.
(ii) Fluorescent-dye staining.
Staining cells with an acridine orange-ethidium bromide mixture allows the determination of apoptosis based on nuclear morphology and membrane integrity (34). Acridine orange is a cell-permeable dye that enters all cells and intercalates DNA to appear green (viable cells). Ethidium bromide, however, enters only nonviable cells that exhibit disrupted membrane integrity, overriding the acridine orange to fluoresce orange. Thus, viable, necrotic, and early and late apoptotic cells can be distinguished by the differential uptake and binding of these dyes (11): the nuclei in viable cells fluoresce green, while the nuclei in necrotic cells fluoresce orange. Early apoptotic cells are distinguished by the enhanced green fluorescence of their characteristic condensed chromatin, while late apoptotic cells that have lost membrane integrity display enhanced uniform orange fluorescence of their condensed chromatin.
Following infection, the suspended and scraped cells were pooled, pelleted by centrifugation, washed once in PBS, and resuspended in 1 ml of antibiotic-free medium at room temperature. Acridine orange-ethidium bromide (1 μM; Sigma Aldrich) in PBS was mixed with a 0.1-ml cell suspension, a drop was applied to a microscope slide, and viability was assessed by counting 500 cells in five randomly selected fields. The percentage of apoptotic cells was then calculated (11).
In some experiments, the specific caspase 8 inhibitor Z-IETD-FMK (R&D Systems, Minneapolis, Minn.) was resuspended in dimethyl sulfoxide and preincubated with RAW 264.7 cells for 2 h at a concentration of 100 μM (11). The cells were then infected with H. pylori for 24 h, and apoptosis was assessed by fluorescent-dye staining using acridine orange-ethidium bromide. Dimethyl sulfoxide alone served as a vehicle control.
(iii) Ann-V-PI flow cytometry.
The binding of annexin V-fluorescein isothiocyanate (Ann-V) to externalized phosphatidylserine was used as a measurement of apoptotic RAW 264.7 cells with an Ann-V-propidium iodide (PI) apoptosis detection kit (BD Biosciences, Montreal, Quebec, Canada) according to the manufacturer's instructions. Briefly, in viable cells, phosphatidylserine is strictly confined to the inner leaflet of the plasma membrane that faces the cytosol. The surface expression of this phospholipid is an early feature of apoptosis and occurs before the loss of membrane integrity (30). Thus, early apoptotic cells bind annexin V, a Ca2+-dependent phospholipid-binding protein with high affinity for externalized phosphatidylserine. Furthermore, living cells exclude PI, allowing specific detection and quantification of apoptosis by fluorescence-activated cell sorter (FACS) analysis (38).
Suspended and scraped cells were pooled, pelleted by centrifugation, washed once with ice-cold PBS, and resuspended in binding buffer (10 mM HEPES-NaOH [pH 7.4], 140 mM NaCl, 2.5 mM CaCl2) to a concentration of 106/ml. Next, 0.1 ml of this cell suspension was transferred to a 5-ml tube and incubated with 0.005 ml of Ann-V and 0.005 ml of PI for 15 min at 25°C in the dark. Finally, 0.4 ml of binding buffer was added, and samples were analyzed by flow cytometry within 1 h on a FACScan flow cytometer (BD Biosciences) at the Core Flow Cytometry Facility of the Hospital for Sick Children. Samples were gated on the basis of forward versus side scatter for size, and the results are presented as the percentage of cells that were viable (Ann-V− PI−), early apoptotic (Ann-V+ PI−), or nonviable (Ann-V+ PI+ or Ann-V− PI+).
Western blotting. (i) Whole-cell protein extraction.
Suspended and adherent macrophages were pooled, pelleted, washed once with cold PBS, and pelleted by centrifugation at 13,000 × g for 10 s. Subsequently, the cell pellet was resuspended in 150 μl of RIPA buffer (1% NP-40, 0.5% sodium deoxycholate, and 0.1% sodium dodecyl sulfate [SDS] in PBS) supplemented with 150 mM NaCl, 50 mM NaF, 1 mM Na3VO4, 20 μg of phenlymethylsulfonyl fluoride/ml, 10 μg of aprotinin/ml, 2 μg of pepstatin A/ml, and 2 μg of leupeptin/ml (all obtained from Sigma Aldrich) by being vortexed and was left at 4°C for 20 min. The lysates were then centrifuged at 11,200 × g for 10 min at 4°C, and the supernatant (whole-cell protein extract) was stored at −70°C until immunoblotting was performed (10).
(ii) Cytosolic protein extraction.
Cytosolic proteins were isolated as previously described (21). Briefly, suspended and adherent macrophages were pooled, pelleted, washed twice in cold PBS, and incubated for 30 min on ice in lysis buffer (68 mM sucrose, 200 mM mannitol, 50 mM KCl, 1 mM EDTA, 1 mM EGTA, 1 mM dithiothreitol, 10 μg of aprotinin/ml, 2 μg of pepstatin A/ml, 2 μg of leupeptin/ml, and 0.5 mM phenylmethylsulfonyl fluoride [all obtained from Sigma Aldrich]). The cells were then passed 40 times through a 25-gauge 5/8-in. needle and centrifuged at 1,500 × g for 10 min. The supernatant was transferred to a fresh Eppendorf tube and was further cleared by centrifugation at 22,000 × g for 15 min. The supernatant was stored at −70°C until the protein concentration was determined by the Bio-Rad assay for use in immunoblotting.
(iii) Immunoblotting.
Equal volumes of whole-cell protein extracts were added to 10 μl of 2× loading buffer, and samples were boiled for 3 min and then subjected to electrophoresis through an SDS-5% polyacrylamide gel electrophoresis (PAGE) stacking gel and an SDS-15% PAGE separating gel at 111 V for 1.25 h at room temperature (or 1.5 h for caspase 8 immunoblotting). For cytochrome c assays, 15 to 20 μg of cytosolic protein extract was loaded for SDS-PAGE. The proteins were subsequently transferred onto a nitrocellulose membrane (BioTrace NT; Pall Corp., Ann Arbor, Mich.) at 4°C and 85 V for 2 h, rinsed in distilled H2O, and then blocked with Tris-buffered saline containing 0.05% Tween 20 (TBST) supplemented with 5% low-fat milk for 30 min at room temperature. The membranes were probed overnight with shaking at 4°C with a polyclonal goat anti-mouse Bid antibody (1:4,000; R&D Systems), polyclonal rabbit anti-mouse caspase 8 antibody (1:1,000; Santa Cruz Biotechnology, Santa Cruz, Calif.), monoclonal mouse anti-cytochrome c antibody (1:250; BD Biosciences-Pharmingen), polyclonal goat anti-mouse actin antibody (1:1,000; Santa Cruz Biotechnology), or polyclonal rabbit anti-mouse actin antibody (1:1,000; Santa Cruz Biotechnology) in TBST-milk. The membranes were washed twice with distilled water and three times in TBST (10 min each time) and incubated with horseradish peroxidase-conjugated donkey anti-goat immunoglobulin G antibody (1:1,000; Santa Cruz Biotechnology) or horseradish peroxidase-conjugated donkey anti-rabbit immunoglobulin G antibody (1:1,000; Santa Cruz Biotechnology) for 1 h at room temperature. The membranes were then washed again with distilled water and TBST (three washes; 10 min each), and bands were visualized by chemiluminescence detection (Santa Cruz Biotechnology) using Kodak Biomax MR film.
Mitochondrial-membrane sensor flow cytometry.
A cationic dye that fluoresces differently in apoptotic and nonapoptotic cells (ApoAlert Mitochodrial Membrane Sensor Kit; BD Biosciences-Clontech) was utilized according to the manufacturer's instructions. Briefly, in living cells, the dye is taken up in the mitochondria to form red fluorescent aggregates. However, in apoptotic cells, increased mitochondrial-membrane permeability prevents the dye from accumulating inside mitochondria, and it remains in monomeric form in the cytosol, where it fluoresces green (13).
Uninfected and infected macrophages were pooled, pelleted by centrifugation, resuspended in 1 ml of diluted Mitosensor reagent/sample, and incubated at 37°C in 5% CO2 for 20 min. One milliliter of incubation buffer was added to the samples, followed by centrifugation and resuspension in incubation buffer according to the manufacturer's instructions. Samples were then analyzed by flow cytometry within 1 h of preparation. The results are presented as percent increase in membrane permeability over controls.
Statistical analysis.
Results are expressed as means ± standard errors. To test statistical significance among multiple groups, a one-way analysis of variance (ANOVA) was used, followed by post hoc comparisons with the Tukey-Kramer or Bonferronni multiple-comparison test, as indicated in the text. Alternatively, a two-tailed unpaired Student's t test was used where indicated.
RESULTS
H. pylori induces morphological features of apoptosis in RAW 264.7 cells.
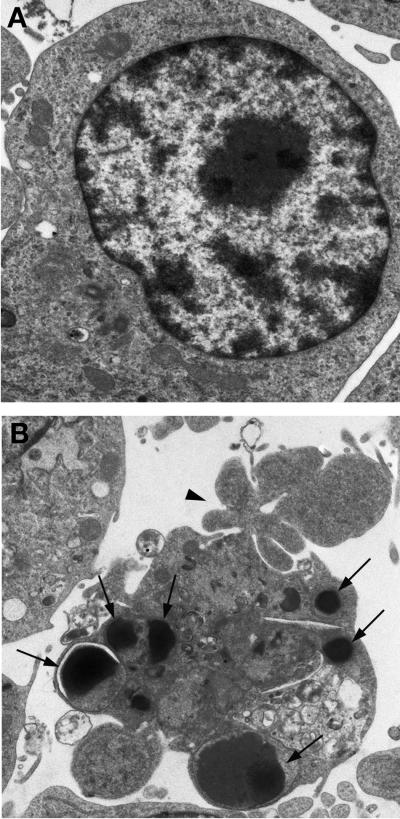
In comparison with uninfected controls (Fig. 1A), RAW 264.7 cells infected with H. pylori strain 60190 (24 h; MOI, 50:1) exhibited morphological features of apoptosis as assessed by transmission electron microscopy, including condensed and marginated nuclear chromatin, cellular blebbing, and cytoplasmic vacuolation (Fig. 1B).
FIG. 1.
Induction of apoptosis of RAW 264.7 cells by H. pylori as assessed by transmission electron microscopy. (A) Uninfected macrophages show normal cellular morphology, including intact plasma and nuclear membranes (magnification, ×20,000). (B) H. pylori-infected macrophages (24 h; MOI, 50:1) display characteristic features of apoptosis, including membrane blebbing (arrowhead), cytoplasmic vacuolation, and condensed and marginated nuclear chromatin (arrows) (magnification, approximately ×12,000).
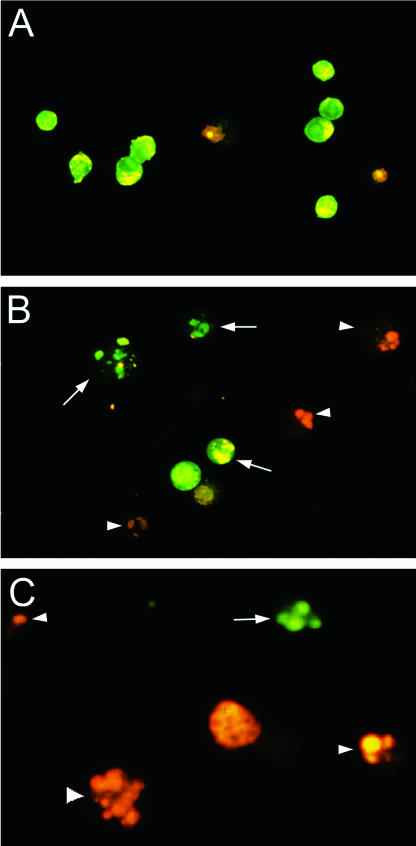
Induction of apoptosis was confirmed by utilizing a complementary, semiquantitative acridine orange-ethidium bromide fluorescent-dye staining assay. As shown in Fig. 2A, the majority of uninfected macrophages were viable and displayed normal cellular morphology and intact nuclear architecture, with nuclei that fluoresced green. In comparison with uninfected controls, macrophages treated with staurosporine (positive control) displayed both early (green) and late (orange) features of apoptosis, as determined by enhanced fluorescence of condensed chromatin (Fig. 2B). Similar findings were detected in H. pylori-infected RAW 264.7 cells, which displayed both early and late features of apoptosis (Fig. 2C).
FIG. 2.
Induction of apoptosis in RAW 264.7 cells by H. pylori as assessed by fluorescence microscopy using acridine orange and ethidium bromide staining. (A) Untreated RAW 264.7 cells show normal cellular morphology and display intact nuclear architecture, as demonstrated by the green fluorescence of their nuclei (magnification, ×250). (B) RAW 264.7 cells treated with staurosporine for 24 h (positive control for apoptosis) show morphological features characteristic of apoptosis, as demonstrated by the enhanced fluorescence of their condensed chromatin. Early apoptotic cells fluoresce green (arrows), and late apoptotic cells fluoresce orange (arrowheads) (magnification, ×250). (C) H. pylori-infected cells (MOI, 50:1; 24 h) show features of apoptosis similar to those of staurosporine-treated cells (magnification, ×400).
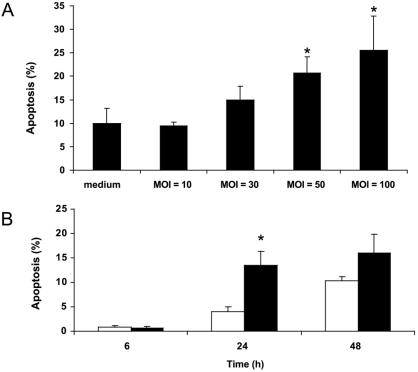
Quantification of the fluorescence microscopy data from five separate experiments demonstrated that RAW 264.7 cells infected with increasing MOI of H. pylori (24 h) displayed a dose-dependent increase in apoptosis, with statistically significant levels of apoptosis at MOIs of 50 and 100 bacteria to 1 macrophage compared to uninfected cells (23.1% ± 8.4% and 27.9% ± 12.9%, respectively, versus 9.9% ± 5.8%; ANOVA-Bonferroni multiple-comparisons test; P < 0.004; n = 5) (Fig. 3A). Furthermore, H. pylori-induced apoptosis of RAW 264.7 cells occurred in a time-dependent manner, with maximal programmed cell death occurring 24 h postinfection compared with uninfected controls (13.5% ± 2.9% versus 4.0% ± 1.0%; unpaired t test; P < 0.0004) (Fig. 3B).
FIG. 3.
Quantification of fluorescence microscopy data using acridine orange and ethidium bromide staining. (A) Infection with H. pylori at MOI of 10:1, 30:1, 50:1, and 100:1 for 24 h resulted in a dose-dependent induction of apoptosis, with increased levels of apoptosis observed at MOI of 50:1 and 100:1 compared with uninfected controls (23.1% ± 8.4% and 27.9% ± 12.9%, respectively, versus 9.9% ± 5.8%; ANOVA-Bonferroni multiple-comparison test; *P < 0.004; n = 5). (B) RAW 264.7 cells infected with H. pylori (solid bars) at an MOI of 50:1 for 6, 24, or 48 h showed a time-dependent increase in apoptosis at 24 h compared with time-matched uninfected controls (open bars) (13.5% ± 2.9% versus 4.0% ± 1.0%; unpaired t test; *, P < 0.0004; n = 4). The results are expressed as mean percentages of apoptotic cells per 500 cells enumerated, and the error bars represent standard errors of the mean.
H. pylori induces extracellular exposure of phosphatidylserine.
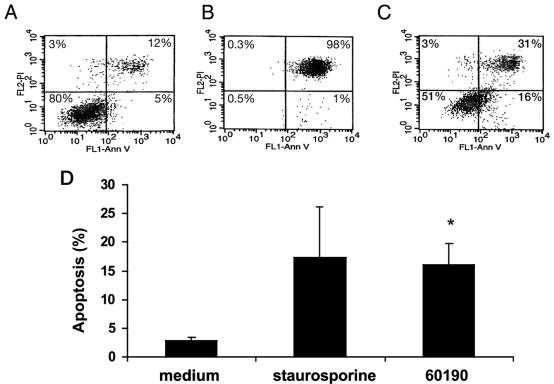
H. pylori-induced apoptosis of macrophages was further confirmed by assessing the surface expression of phosphatidylserine using Ann-V in conjunction with PI and FACS analysis. A FACS analysis representative of three individual experiments is depicted in Fig. 4. As shown in Fig. 4A, 80% of uninfected RAW 264.7 cells were viable (Annexin-V− PI−), while only 5% were apoptotic (Annexin-V+ PI−). In contrast, only 51% of H. pylori-infected RAW 264.7 cells were viable (Annexin-V− PI−), while 16% were apoptotic (Fig. 4C). Figure 4D displays the mean percentage of H. pylori-infected RAW 264.7 cells undergoing apoptosis (Annexin V+ PI−) from three separate experiments. In comparison with uninfected cells, H. pylori-infected macrophages displayed an increase in apoptosis similar to that detected in staurosporine-treated cells (16% ± 3.8% versus 2.8% ± 0.6%; ANOVA-Tukey-Kramer test; P < 0.05; n = 3) (Fig. 4D).
FIG. 4.
H. pylori induces apoptosis, as measured by annexin-V binding to externalized phosphatidylserine. RAW 264.7 cells were infected with H. pylori at an MOI of 50:1 for 24 h. Viable cells (annexin-V− PI−); nonviable, including late apoptotic or necrotic cells (annexin-V+ PI+ or annexin-V− PI+); and apoptotic cells (annexin-V+ PI−) were detected by the binding of Ann-V to externalized phospatidylserine in conjunction with PI, a dye excluded from viable cells. (A, B, and C) One FACS analysis representative of three individual experiments. (A) Eighty percent of uninfected RAW 264.7 cells were viable (annexin-V− PI−), while only 5% were apoptotic (annexin-V+ PI−). (B) One percent of RAW 264.7 cells treated with 1 μM staurosporine (positive control for apoptosis) were apoptotic (annexin-V+ PI−), and 98% of RAW 264.7 cells were nonviable (annexin-V+ PI+). (C) Sixteen percent of RAW 264.7 cells infected with H. pylori (60190) at an MOI of 50:1 for 24 h were apoptotic (annexin-V+ PI−), and 31% of the cells were nonviable (annexin-V+ PI+). FL1, flow cytometry channel 1 to detect PI stain; FL2, flow cytometry channel 2 to detect annexin-V. (D) Combined results of three separate FACS analyses depicting the mean levels of apoptotic cells (annexin-V+ PI−). Staurosporine-treated cells showed an increase in apoptosis over uninfected controls. RAW 264.7 cells infected with H. pylori (60190) at an MOI of 50:1 for 24 h showed an increase in apoptosis in comparison with uninfected controls (16.0% ± 3.7% versus 2.8% ± 0.6%; Tukey-Kramer test; *, P < 0.05).
cagA and vacA are involved in H. pylori-induced apoptosis of RAW 264.7 cells.
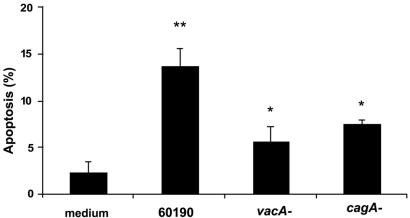
To delineate the bacterial virulence factors involved in H. pylori-induced apoptosis of macrophages, RAW 264.7 cells were infected with the parental strain 60190 (cagA+ VacA+) (24 h; MOI, 100:1) or its isogenic mutant strains (cagA or vacA mutant) (24 h; MOI, 100:1) and were subsequently analyzed by FACS for binding of annexin V to exteriorized phosphatidylserine. RAW 264.7 cells infected with the parental H. pylori strain 60190 displayed an ∼3-fold increase in apoptosis over uninfected controls (13.8% ± 1.8% versus 2.4% ± 1.1%; ANOVA-Tukey-Kramer test; P < 0.001; n = 3) (Fig. 5). In contrast, cells infected with isogenic mutant strains deficient in either cagA or vacA showed a reduction in apoptosis compared with cells infected with the wild-type strain (7.6% ± 0.35% and 5.7% ± 1.5%, respectively; ANOVA-Tukey-Kramer test; P < 0.002; n = 3).
FIG. 5.
vacA and cagA are involved in H. pylori-induced apoptosis of RAW 264.7 cells. RAW 264.7 cells infected with H. pylori strain 60190 (24 h; MOI, 100:1) showed an increase in apoptosis (annexin-V+ PI−) in comparison with uninfected controls (13.8% ± 1.8% versus 2.4% ± 1.1%; ANOVA; **, P < 0.001) as assessed by Ann-V-PI binding. However, isogenic mutant strains deficient in either cagA (24 h; MOI, 100:1) or vacA (24 h; MOI, 100:1) showed a reduction in apoptosis (annexin-V+ PI−) compared to RAW 264.7 cells infected with the wild-type strain (7.6% ± 0.35% and 5.7% ± 1.5%, respectively, versus 13.8% ± 1.8%; ANOVA-Tukey-Kramer test; *, P < 0.002; n = 3).
H. pylori induces cleavage of procaspase 8.
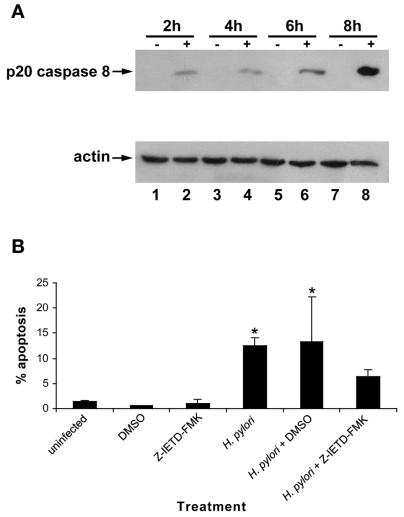
RAW 264.7 cells infected with H. pylori strain 60190 (MOI, 100:1; 2 to 8 h) displayed a time-dependent increase in the appearance of the p20 caspase 8 subunit, beginning as early as 2 h postinfection, compared with uninfected controls (Fig. 6A).
FIG. 6.
H. pylori infection of RAW 264.7 cells induces a time-dependent increase in cleavage of procaspase 8 (p20). (A) (Top) Immunoblot analysis of whole-cell protein lysates from H. pylori (60190)-infected RAW 264.7 cells probed with caspase 8 antibody. Uninfected RAW 264.7 cells (−) do not express the active caspase 8 fragment. However, H. pylori-infected RAW 264.7 cells (+) (2 to 8 h; MOI, 100:1) exhibit cleavage of procaspase 8 and appearance of caspase 8 (p20) beginning 2 h postinfection compared to time-matched uninfected controls (lanes 1 and 2). (Bottom) Actin levels were assayed to monitor protein-loading levels between samples (n = 3). (B) Preincubation with the specific caspase 8 inhibitor Z-IETD-FMK leads to a reduction in H. pylori-induced apoptosis of RAW 264.7 cells, as assessed by acridine orange-ethidium bromide fluorescent-dye staining. Similar to Fig. 2C, infection with H. pylori (24 h; MOI, 100:1) leads to an increase in levels of apoptosis compared to uninfected controls (12.5% ± 1.5% versus 1.4% ± 0.2%; ANOVA-Tukey-Kramer test; P < 0.001). However, preincubation with the caspase 8 inhibitor, but not the dimethyl sulfoxide vehicle control, caused a decrease in levels of apoptosis induced by H. pylori (6.4% ± 1.4% versus 12.5% ± 1.5%; ANOVA-Tukey-Kramer test; *, P < 0.05; n = 3). Error bars, standard errors.
In order to determine the importance of caspase 8 activation, RAW 264.7 cells were preincubated with the specific caspase 8 inhibitor Z-IETD-FMK for 2 h and infected with the bacterium (24 h; MOI, 100:1), and apoptosis was quantified by utilizing the acridine orange-ethidium bromide fluorescent-dye staining assay. As shown previously, H. pylori induced an increase in apoptosis compared to uninfected controls (12.5% ± 1.5% versus 1.4% ± 0.2%; ANOVA-Tukey Kramer test; P < 0.001) (Fig. 6B). Dimethyl sulfoxide vehicle alone neither induced nor prevented bacterially induced apoptosis. In contrast, preincubation with the specific caspase 8 inhibitor (Z-IETD-FMK) caused a reduction in apoptosis of H. pylori-infected RAW 264.7 cells (6.4% ± 1.4% versus 12.5% ± 1.5%; ANOVA-Tukey Kramer test; P < 0.05; n = 3).
H. pylori infection decreases expression levels of uncleaved Bid protein.
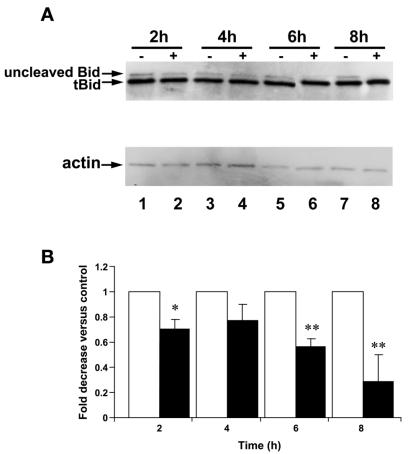
Since caspase 8 is the cysteine protease most commonly activated in association with the cleavage of Bid (42), we assayed protein expression levels of uncleaved Bid by immunoblotting. Truncated-Bid (tBid) levels were constant at 15 kDa among uninfected and H. pylori-infected cells. However, in comparison with uninfected controls, RAW 264.7 cells infected with H. pylori strain 60190 (MOI, 100:1; 2 to 8 h) showed a time-dependent decrease in expression levels of uncleaved Bid protein (22 kDa). The decrease in expression of the uncleaved Bid protein was detectable 6 h postinfection (Fig. 7A). Densitometry analysis of the immunoblotting results from three separate experiments where uncleaved Bid expression was normalized to actin levels demonstrates that there was a significant decrease in protein expression levels of uncleaved Bid at 2, 6, and 8 h postinfection (ANOVA-Tukey-Kramer test) (Fig. 7B).
FIG. 7.
Infection of RAW 264.7 cells with H. pylori leads to a time-dependent decrease in the expression of uncleaved Bid protein. (A) Whole-cell protein extracts from RAW 264.7 cells analyzed by immunoblotting. (Top) Uninfected RAW 264.7 cells (−) expressed both uncleaved (22-kDa) and cleaved (15-kDa) Bid protein. In contrast, uncleaved Bid protein expression decreased following infection with H. pylori (MOI, 100:1) (+) beginning at 6 h. (Bottom) Actin levels were assayed to monitor protein loading between samples (n = 3). (B) Densitometry corresponding to immunoblots from three separate experiments reflects decreased uncleaved Bid protein expression in H. pylori-infected samples (solid bars) when normalized to actin levels to control for protein loading (open bars) over time (*, P < 0.05; **, P < 0.001; ANOVA; n = 3). Error bars, standard errors.
H. pylori infection induces mitochondrial dysfunction and cytochrome c release.
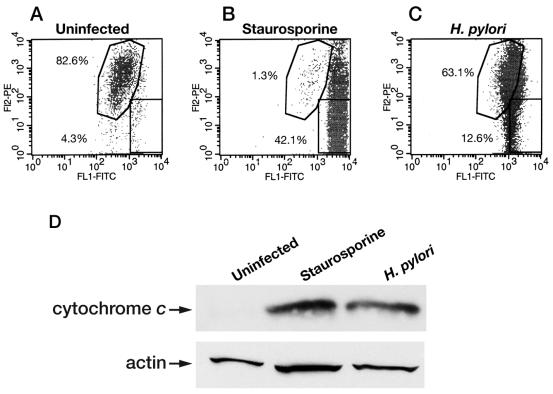
Since activation of Bid and caspase 8 can lead to increased mitochondrial-membrane permeability (67), we evaluated mitochondrial-membrane permeability after infection of RAW 264.7 cells. A time-dependent increase in mitochondrial-membrane permeability was observed in cells infected with H. pylori (60190) for 2 to 24 h (data not shown) (n = 1). A representative FACS analysis of three individual experiments is depicted in Fig. 8, showing uninfected (Fig. 8A), staurosporine-treated (24 h; 1 μM) (Fig. 8B), and H. pylori-infected (24 h; MOI, 100:1) (Fig. 8C) RAW 264.7 cells. As shown in Fig. 8A, 82.6% of uninfected RAW 264.7 cells displayed intact mitochondrial membranes, while only 4.3% showed an increase in membrane permeability. In contrast, 1.3% of staurosporine-treated macrophages displayed intact mitochondrial membranes, and 42.1% showed increased mitochondrial-membrane permeability (Fig. 8B). As depicted in Fig. 8C, 63.1% of RAW 264.7 cells infected with H. pylori strain 60190 (24 h; MOI, 100:1) displayed intact mitochondrial-membrane permeability, while 12.6% of the cells displayed increased mitochondrial-membrane permeability. Thus, an ∼3-fold increase in disrupted membrane integrity was detected in H. pylori-infected RAW 264.7 cells (Fig. 8C) versus uninfected controls (Fig. 8B) (n = 3).
FIG. 8.
H. pylori infection leads to increased mitochondrial-membrane permeability and increased cytosolic cytochrome c in RAW 264.7 cells. Macrophages were harvested after H. pylori (60190) infection (MOI, 100:1; 24 h), and mitochondrial-membrane permeability was assessed using FACS analysis. (A, B, and C) One representative FACS analysis from three separate experiments is shown, where the x axis represents fluorescence levels of monomeric dye remaining in the cytosol of cells with increased mitochondrial-membrane permeability (green emission). In contrast, the y axis represents the fluorescence levels of the aggregated dye taken up and retained in the mitochondria of cells with intact mitochondrial membranes (red emission). (A) A total of 4.3% of uninfected RAW 264.7 cells show increased mitochondrial-membrane permeability, while 82.6% show intact mitochondrial-membrane permeability. (B) A total of 42.1% of staurosporine-treated macrophages have increased mitochondrial-membrane permeability, while only 1.3% display intact mitochondrial-membrane permeability. (C) At 24 h postinfection, 12.6% of H. pylori-infected RAW 264.7 cells display increased mitochondrial-membrane permeability. FL1, flow cytometry channel 1 to detect FITC; FL2, flow cytometry channel 2 to detect PE. (D) Infection with H. pylori leads to an increase in cytosolic cytochrome c protein levels, as assessed by Western blot analysis. In comparison with cytosolic extracts from uninfected RAW 264.7 cells, H. pylori strain 60190-infected RAW 264.7 cells (H. pylori) (MOI, 100:1; 24 h) show increased protein levels of cytosolic cytochrome c, similar to staurosporine-treated cells (Staurosporine) (1 μM; 24 h; positive control; n = 3).
When mitochondrial-membrane integrity is compromised, cytochrome c may be released into the cytosol (41). Thus, we determined by Western blot analysis if there was an increase in cytosolic cytochrome c protein levels. As demonstrated in Fig. 8D, in comparison with cytosolic extracts obtained from uninfected RAW 264.7 cells, cells infected with H. pylori strain 60190 (MOI, 100:1; 24 h) showed increased levels of cytosolic cytochrome c, similar to that induced by staurosporine (positive control) (n = 3).
DISCUSSION
By utilizing several complementary techniques, including transmission electron microscopy, fluorescence microscopy, FACS analysis, and immunoblotting, this study supported (23, 72) and extended recent evidence that H. pylori induces apoptosis of murine macrophages in vitro. Mechanistically, we have established that H. pylori-induced apoptosis of macrophages is mediated by virulence factors encoded by vacA and cagA. Additionally, we showed that induction of macrophage apoptosis by H. pylori involves caspase 8 activation, disappearance of uncleaved Bid, increased mitochondrial-membrane permeability, and release of cytochrome c into the cytosol. Taken together, the results of this study indicate a role for the mitochondrial pathway in the apoptotic signaling cascade triggered by infection of macrophages by specific H. pylori factors.
Many investigators have focused on the ability of H. pylori to induce epithelial-cell apoptosis. Accelerated epithelial-cell apoptosis (33, 51) is hypothesized to result in peptic ulceration (37), while resistance to apoptosis has been observed and may lead to gastric malignancies (62). However, since infection causes the accumulation of immune cells in the stomach, understanding the interaction of H. pylori with cells of the immune system is also important in delineating the pathogenesis of infection (65). Indeed, H. pylori induces apoptosis of macrophages (23) and T lymphocytes (69), while H. pylori lipopolysaccharide impedes the apoptosis of polymorphonuclear leukocytes (28). Recent evidence demonstrates that apoptosis of macrophages is a common event in the pathogenesis of a number of microbial infections. Several bacterial pathogens, including S. flexneri (73), Legionella pneumophila (22), and Yersinia enterocolitica (15, 61), induce apoptosis in macrophages, in some cases decreasing the effectiveness of the immune response (74) or contributing to the inflammatory state by the release of preformed proinflammatory cytokines, such as interleukin-1 (29). In contrast, other intracellular bacteria, such as M. bovis (39), chlamydiae (19), and Brucella suis (26), inhibit macrophage apoptosis to maintain persistent infection within the host cell. Thus, both accelerating and inhibiting apoptosis may enable the bacteria to shield itself from the host immune response and protect its intracellular sanctuary (1). Therefore, we speculate that H. pylori induces apoptosis of macrophages to modulate host immune responses and establish chronic infection.
To delineate the bacterial factors involved in H. pylori-induced apoptosis of macrophages, we employed isogenic mutants deficient in CagA and VacA production. Since both mutants induced a reduction in apoptosis in comparison with the wild-type strain, our findings indicate roles for both the cag PAI and the vacuolating cytotoxin. It is known that CagA is injected into macrophages, undergoes tyrosine phosphorylation, and then binds and activates SRC homology 2 domain-containing tyrosine phosphatase (SHP-2) (48, 55). In epithelial cells, this leads to the induction of a growth factor-like morphological change called the “hummingbird” phenotype (5, 63). However, the mechanism through which it may contribute to the induction of apoptosis remains largely unclear. Recently, Tsutsumi et al. have implicated a CagA-induced SHP-2 signaling mechanism in the induction of epithelial-cell apoptosis (68), but this possibility is undefined in macrophages. Both S. flexneri and S. enterica serovar Typhimurium induce macrophage apoptosis by injecting invasion protein B (IpaB) and Salmonella invasion protein B (SipB), respectively, into host cells by a type III secretion system (27, 73). These bacterial proteins specifically bind to and activate caspase 1 to induce apoptosis (27, 73). As cag+ H. pylori strains are generally associated with the ability to induce increased apoptosis in epithelial cells (52), whether the type IV-secreted CagA protein acts in a similar fashion in macrophages remains to be determined.
In addition to CagA, we found that VacA was also involved in the induction of apoptosis. In epithelial cells, VacA has been shown to target the mitochondria, cause cytochrome c release, activate caspase 3, and lead to cleavage of downstream apoptosis-inducing substrates, such as poly(ADP-ribose) polymerase (21). Although H. pylori can induce distinct signal transduction pathways in macrophages versus epithelial cells (44), since we observed activation of the mitochondrial pathway, we speculate that VacA may also target mitochondria in macrophages.
Here, we have demonstrated that induction of macrophage apoptosis during H. pylori infection occurs in association with caspase 8 activation, decreased levels of uncleaved Bid, increased mitochondrial permeability, and cytochrome c release. Furthermore, preincubation with a caspase 8 inhibitor partially abrogated the apoptotic response to infection. Bid is a specific substrate of caspase 8 and is strongly dependent upon cleavage for its proapoptotic activity (42). Temporally, the disappearance of uncleaved Bid coincided with the largest increase in the cleaved caspase 8 fragment, and both occurred before apoptosis was observed. tBid is known to translocate from the cytosol to the mitochondrial membrane and to interact with the proapoptotic protein Bax to increase the mitochondrial-membrane potential and allow cytochrome c release (40). We consistently observed high levels of tBid in uninfected and infected RAW 264.7 cells. Thus, since the N terminus of Bid has an inhibitory effect on the proapoptotic activity of tBid (42), our observation of the disappearance of uncleaved Bid may be consistent with an apoptotic response. Finally, we observed an increase in mitochondrial-membrane permeability at 24 h by FACS analysis and cytochrome c immunoblotting, consistent with prior caspase 8 and Bid activation. Overall, this suggests a temporal sequence of events, beginning with activation of caspase 8 and Bid cleavage and followed by mitochondrial-membrane dysfunction and cytochrome c release, implicating Bid as a link between activated caspase 8 and the mitochondrial death pathway.
H. pylori induces epithelial-cell apoptosis through a type II pathway in vitro through activation of caspases 8, 9, 6, and 3, as well as a time-dependent activation of Bid and release of cytochrome c (3, 45, 59, 64). Furthermore, the cleavage of Bid by activated caspase 8 can also link the death receptor pathway to the mitochondrial pathway during infection of macrophages with other bacteria, such as Mycobacterium avium (7). Indirect evidence suggests that Bid also plays a role in the apoptosis of macrophages during infection with Y. enterocolitica (15). Increased Fas receptor expression during infection of epithelial cells in vitro (33) and the importance of the Fas death receptor pathway in the modulation of disease pathophysiology following infection with H. pylori in vivo have been demonstrated (31, 35). Whether the activation of caspase 8 that we observed is directly stimulated by bacterial activation of the Fas receptor remains to be elucidated.
In summary, this study showed that H. pylori infection induces apoptosis in macrophages through a caspase 8-, Bid-, and mitochondrion-dependent mechanism. This apoptotic signaling cascade is mediated in part by VacA and the cag PAI gene cagA. Thus, in addition to other strategies to evade host immune responses, such as disruption of phagosome maturation (72) and disruption of cytokine signaling (10), induction of macrophage apoptosis may represent a mechanism by which H. pylori usurps the host immune response to establish chronic infection in humans.
Acknowledgments
We thank Danny Aguilar from the Graphics Center at the Hospital for Sick Children for assistance in preparing the figures and Joyce Ching for helpful discussions regarding these experiments.
P.J.M.C. is supported by a Canadian Institutes of Health Research (CIHR)/Canadian Digestive Health Foundation Doctoral Award and is a CIHR Strategic Training fellow in Cell Signaling in Mucosal Inflammation and Pain (STP-53877). This work was funded by an operating CIHR grant to N.L.J.
Editor: B. B. Finlay
REFERENCES
- 1.Allen, L. A. 1999. Intracellular niches for extracellular bacteria: lessons from Helicobacter pylori. J. Leukoc. Biol. 66:753-756. [DOI] [PubMed] [Google Scholar]
- 2.Ashkenazi, A., and V. M. Dixit. 1998. Death receptors: signalling and modulation. Science 281:1305-1308. [DOI] [PubMed] [Google Scholar]
- 3.Ashktorab, H., M. Neapolitano, C. Bomma, C. Allen, A. Ahmed, A. Dubois, T. Naab, and D. T. Smoot. 2002. In vivo and in vitro activation of caspase-8 and -3 associated with Helicobacter pylori infection. Microb. Infect. 4:713-722. [DOI] [PubMed] [Google Scholar]
- 4.Atherton, J. C. 1997. The clinical relevance of strain types of Helicobacter pylori. Gut 40:701-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Backert, S., S. Moese, M. Selbach, V. Brinkmann, and T. F. Meyer. 2001. Phosphorylation of tyrosine 972 of the Helicobacter pylori CagA protein is essential for induction of a scattering phenotype in gastric epithelial cells. Mol. Microbiol. 42:631-644. [DOI] [PubMed] [Google Scholar]
- 6.Belmokhtar, C. A., J. Hillion, and E. Segal-Bendirdjian. 2001. Staurosporine induces apoptosis through both caspase-dependent and caspase-independent mechanisms. Oncogene 20:3354-3362. [DOI] [PubMed] [Google Scholar]
- 7.Bhattacharyya, A., S. Pathak, C. Basak, S. Law, M. Kundu, and J. Basu. 2003. Execution of macrophage apoptosis by Mycobacterium avium through apoptosis signal-regulating kinase 1/p38 mitogen-activated protein kinase signaling and caspase 8 activation. J. Biol. Chem. 278:26517-26525. [DOI] [PubMed] [Google Scholar]
- 8.Blaser, M. J., G. I. Perez-Perez, H. Kleanthous, T. L. Cover, R. M. Peek, P. H. Chyou, G. N. Stemmermann, and A. Nomura. 1995. Infection with Helicobacter pylori strains possessing cagA is associated with an increased risk of developing adenocarcinoma of the stomach. Cancer Res. 55:2111-2115. [PubMed] [Google Scholar]
- 9.Censini, S., C. Lange, Z. Xiang, J. E. Crabtree, P. Ghiara, M. Borodovsky, R. Rappuoli, and A. Covacci. 1996. Cag, a pathogenicity island of Helicobacter pylori, encodes type I-specific and disease-associated virulence factors. Proc. Natl. Acad. Sci. USA 93:14648-14653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ceponis, P. J. M., D. M. McKay, R. J. Menaker, E. Galindo-Mata, and N. L. Jones. 2003. Helicobacter pylori infection interferes with epithelial Stat6-mediated interleukin-4 signal transduction independent of cagA, cagE or vacA. J. Immunol. 171:2035-2041. [DOI] [PubMed] [Google Scholar]
- 11.Ching, J. C., N. L. Jones, P. J. Ceponis, M. A. Karmali, and P. M. Sherman. 2002. Escherichia coli Shiga-like toxins induce apoptosis and cleavage of poly(ADP-ribose) polymerase via in vitro activation of caspases. Infect. Immun. 70:4669-4677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen, G. M. 1997. Caspases: the executioners of apoptosis. Biochem. J. 326:1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Contreras, J. L., C. A. Smyth, G. Bilbao, C. T. Young, J. A. Thompson, and D. E. Eckhoff. 2002. 17β-Estradiol protects isolated human pancreatic islets against proinflammatory cytokine-induced cell death: molecular mechanisms and islet functionality. Transplantation 74:1252-1259. [DOI] [PubMed] [Google Scholar]
- 14.Covacci, A., J. L. Telford, G. D. Giudice, J. Parsonnet, and R. Rappuoli. 1999. Helicobacter pylori virulence and genetic geography. Science 284:1328-1333. [DOI] [PubMed] [Google Scholar]
- 15.Denecker, G., W. Declercq, C. A. Geuijen, A. Boland, R. Benabdillah, M. van Gurp, M. P. Sory, P. Vandenabeele, and G. R. Cornelis. 2001. Yersinia enterocolitica YopP-induced apoptosis of macrophages involves the apoptotic signaling cascade upstream of bid. J. Biol. Chem. 276:19706-19714. [DOI] [PubMed] [Google Scholar]
- 16.Dytoc, M. T., A. Ismaili, D. J. Philpott, R. Soni, J. L. Brunton, and P. M. Sherman. 1994. Distinct binding properties of eaeA-negative verocytotoxin-producing Escherichia coli of serotype O113:H21. Infect. Immun. 62:3494-3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Enari, M., H. Sakahira, H. Yokoyama, K. Okawa, A. Iwamatsu, and S. Nagata. 1998. A caspase-activated DNase that degrades DNA during apoptosis, and its inhibitor ICAD. Nature 391:43-50. [DOI] [PubMed] [Google Scholar]
- 18.Ernst, P. 1999. Review article: the role of inflammation in the pathogenesis of gastric cancer. Aliment. Pharmacol. Ther. Suppl. 1:13-18. [DOI] [PubMed] [Google Scholar]
- 19.Fan, T., H. Lu, H. Hu, L. Shi, G. A. McClarty, D. M. Nance, A. H. Greenberg, and G. Zhong. 1998. Inhibition of apoptosis in Chlamydia-infected cells: blockade of mitochondrial cytochrome c release and caspase activation. J. Exp. Med. 187:487-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fiocca, R., O. Luinetti, L. Villani, A. M. Chiaravalli, C. Capella, and E. Solcia.1994. Epithelial cytotoxicity, immune responses, and inflammatory components of Helicobacter pylori gastritis. Scand. J. Gastroenterol. Suppl. 205:11-21. [PubMed] [Google Scholar]
- 21.Galmiche, A., J. Rassow, A. Doye, S. Cagnol, J. C. Chambard, S. Contamin, V. de Thillot, I. Just, V. Ricci, E. Solcia, E. Van Obberghen, and P. Boquet. 2000. The N-terminal 34kDa fragment of Helicobacter pylori vacuolating cytotoxin targets mitochondria and induces cytochrome c release. EMBO J. 19:6361-6370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gao, L.-Y., and Y. Abu Kwaik. 1999. Apoptosis in macrophages and alveolar epithelial cells during early stages of infection by Legionella pneumophila and its role in cytopathogenicity. Infect. Immun. 67:862-870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gobert, A. P., C. Yulan, J.-Y. Wang, J.-L. Boucher, R. K. Iyer, S. D. Cederbaum, R. A. Casero, Jr., J. C. Newton, and K. T. Wilson. 2002. Helicobacter pylori induces macrophage apoptosis by activation of arginase II. J. Immunol. 168:4692-4700. [DOI] [PubMed] [Google Scholar]
- 24.Graham, D. Y., R. M. Genta, D. P. Graham, and J. E. Crabtree. 1996. Serum CagA antibodies in asymptomatic subjects and patients with peptic ulcer: lack of correlation of IgG antibody in patients with peptic ulcer or asymptomatic Helicobacter pylori gastritis. J. Clin. Pathol. 49:829-832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Green, D. R., and J. C. Reed. 1998. Mitochondria and apoptosis. Science 281:1309-1312. [DOI] [PubMed] [Google Scholar]
- 26.Gross, A., A. Terraza, S. Ouahrani-Bettache, J.-P. Liautard, and J. Dornand. 2000. In vitro Brucella suis infection prevents the programmed cell death of human monocytic cells. Infect. Immun. 68:342-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hersh, D., D. M. Monack, M. R. Smith, N. Ghori, S. Falkow, and A. Zychlinsky. 1999. The Salmonella invasin SipB induces macrophage apoptosis by binding to caspase 1. Proc. Natl. Acad. Sci. USA 96:2396-2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hofman, V., V. Ricci, B. Mograbi, P. Brest, F. Luciano, P. Boquet, B. Rossi, P. Auberger, and P. Hofman. 2001. Helicobacter pylori lipopolysaccharide hinders polymorphonuclear leukocyte apoptosis. Lab. Investig. 81:375-384. [DOI] [PubMed] [Google Scholar]
- 29.Hogquist, K. A., M. A. Nett, E. R. Unanue, and D. D. Chaplin. 1991. Interleukin 1 is processed and released during apoptosis. Proc. Natl. Acad. Sci. USA 88:8485-8489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Homburg, C. H. , M. de Haas, A. E. G. K. von dem Borne, A. J. Verhoeven, C. P. M. Reutelingsperger, and D. Roos. 1995. Human neutrophils lose their surface Fc-γ RIII and acquire annexin V binding sites during apoptosis in vitro. Blood 85:532-540. [PubMed] [Google Scholar]
- 31.Houghton, J., R. M. Korah, M. R. Condon, and K. H. Kim. 1999. Apoptosis in Helicobacter pylori-associated gastric and duodenal ulcer disease is mediated via the Fas antigen pathway. Dig. Dis. Sci. 44:465-478. [DOI] [PubMed] [Google Scholar]
- 32.Jhala, N. C., G. P. Siegal, K. Klemm, B. F. Atkinson, and D. N. Jhala. 2003. Infiltration of Helicobacter pylori in the gastric mucosa. Am. J. Clin. Pathol. 119:101-107. [DOI] [PubMed] [Google Scholar]
- 33.Jones, N. L., A. S. Day, H. A. Jennings, and P. M. Sherman. 1999. Helicobacter pylori induces gastric epithelial cell apoptosis in association with increased Fas receptor expression. Infect. Immun. 67:4237-4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jones, N. L., A. Islur, R. Haq, M. Mascarenhas, M. A. Karmali, M. H. Perdue, B. W. Zanke, and P. M. Sherman. 2000. Escherichia coli Shiga toxins induce apoptosis in epithelial cells that is regulated by the Bcl-2 family. Am. J. Physiol. Gastrointest. Liver Physiol. 278:G811-G819. [DOI] [PubMed] [Google Scholar]
- 35.Jones, N. L., A. S. Day, H. Jennings, P. T. Shannon, E. Galindo-Mata, and P. M. Sherman. 2002. Enhanced disease severity in Helicobacter pylori-infected mice deficient in Fas signaling. Infect. Immun. 70:2591-2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Keates, S., S. Sougioultzis, A. C. Keates, D. Zhao, R. M. Peek, Jr., L. M. Shaw, and C. P. Kelly. 2001. cag+ Helicobacter pylori induce transactivation of the epidermal growth factor receptor in AGS gastric epithelial cells. J. Biol. Chem. 276:48127-48134. [DOI] [PubMed] [Google Scholar]
- 37.Kohda, K., K. Tanaka, Y. Aiba, M. Yasuda, T. Miwa, and Y. Koga. 1999. Role of apoptosis induced by Helicobacter pylori infection in the development of duodenal ulcer. Gut 44:456-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koopman, G., C. P. M. Reutelingsperger, G. A. M. Kuijten, R. M. J. Keehnen, S. T. Pals, and M. H. J. van Oers. 1994. Annexin V for flow cytometric detection of phosphatidylserine expression on B cells undergoing apoptosis. Blood 84:1415-1420. [PubMed] [Google Scholar]
- 39.Kremer, L., J. Estaquier, E. Brandt, J. C. Ameisen, and C. Locht. 1997. Mycobacterium bovis Bacillus Calmette Guerin infection prevents apoptosis of resting human monocytes. Eur. J. Immunol. 27:2450-2456. [DOI] [PubMed] [Google Scholar]
- 40.Kuwana, T., M. R. Mackey, G. Perkins, M. H. Ellisman, M. Latterich, R. Schneiter, D. R. Green, and D. D. Newmeyer. 2002. Bid, Bax, and lipids cooperate to form supramolecular openings in the outer mitochondrial membrane. Cell 111:331-342. [DOI] [PubMed] [Google Scholar]
- 41.Li, P., D. Nijhawan, I. Budihardjo, S. M. Srinivasula, M. Ahmad, E. S. Alnemri, and X. Wang. 1997. Cytochrome c and dATP-dependent formation of Apaf-1/Caspase-9 complex initiates an apoptotic protease cascade. Cell 91:479-489. [DOI] [PubMed] [Google Scholar]
- 42.Li, H., H. Zhu, C. J. Xu, and J. Yuan. 1998. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell 94:491-501. [DOI] [PubMed] [Google Scholar]
- 43.Loeb, M., P. Jayaratne, N. Jones, A. Sihoe, and P. Sherman. 1998. Lack of correlation between vacuolating cytotoxin activity, cagA gene in Helicobacter pylori, and peptic ulcer disease in children. Eur. J. Clin. Microbiol. Infect. Dis. 17:653-656. [DOI] [PubMed] [Google Scholar]
- 44.Maeda, S., M. Akanuma, Y. Mitsuno, Y. Hirata, K. Ogura, H. Yoshida, Y. Shiratori, and M. Omata. 2001. Distinct mechanism of Helicobacter pylori-mediated NF-kappa β activation between gastric cancer cells and monocytic cells. J. Biol. Chem. 276:44856-44864. [DOI] [PubMed] [Google Scholar]
- 45.Maeda, S., H. Yoshida, Y. Mitsuno, Y. Hirata, K. Ogura, Y. Shiratori, and M. Omata. 2002. Analysis of apoptotic and antiapoptotic signalling pathways induced by Helicobacter pylori. Mol. Pathol. 55:286-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mai, U. E., G. I. Perez-Perez, J. B. Allen, S. M. Wahl, M. J. Blaser, and P. D. Smith. 1992. Surface proteins from Helicobacter pylori exhibit chemotactic activity for human leukocytes and are present in gastric mucosa. J. Exp. Med. 175:517-525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mitchell, H. M., S. L. Hazell, Y. Y. Li, and P. J. Hu. 1996. Serological response to specific Helicobacter pylori antigens: antibody against CagA antigen is not predictive of gastric cancer in a developing country. Am. J. Gastroenterol. 91:1785-1788. [PubMed] [Google Scholar]
- 48.Moese, S., M. Selbach, U. Zimny-Arndt, P. R. Jungblut, T. F. Meyer, and S. Backert. 2001. Identification of a tyrosine-phosphorylated 35 kDa carboxy-terminal fragment (p35CagA) of the Helicobacter pylori CagA protein in phagocytic cells: processing or breakage? Proteomics 1:618-629. [DOI] [PubMed] [Google Scholar]
- 49.Molloy, A., P. Laochumroonvorapong, and G. Kaplan. 1994. Apoptosis, but not necrosis, of infected monocytes is coupled with killing of intracellular bacillus Calmette-Guerin. J. Exp. Med. 180:1499-1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Montecucco, C., and R. Rappuoli. 2001. Living dangerously: how Helicobacter pylori survives in the human stomach. Nat. Rev. Mol. Cell Biol. 2:457-466. [DOI] [PubMed] [Google Scholar]
- 51.Moss, S. F., J. Calam, B. Agarwal, S. Wang, and P. R. Holt. 1996. Induction of gastric epithelial apoptosis by Helicobacter pylori. Gut 38:498-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moss, S. F., E. M. Sordillo, A. M. Abdalla, V. Makarov, Z. Hanzely, G. I. Perez-Perez, M. J. Blaser, and P. R. Holt. 2001. Increased gastric epithelial cell apoptosis associated with colonization with CagA+ Helicobacter pylori strains. Cancer Res. 61:1406-1411. [PubMed] [Google Scholar]
- 53.Nagata, S. 1997. Apoptosis by death factor. Cell 88:355-365. [DOI] [PubMed] [Google Scholar]
- 54.Navarre, W. W., and A. Zychlinsky. 2000. Pathogen-induced apoptosis of macrophages: a common end for different pathogenic strategies. Cell Microbiol. 2:265-273. [DOI] [PubMed] [Google Scholar]
- 55.Odenbreit, S., B. Gebert, J. Puls, W. Fischer, and R. Haas. 2001. Interaction of Helicobacter pylori with professional phagocytes: role of the cag pathogenicity island and translocation, phosphorylation and processing of CagA. Cell Microbiol. 3:21-31. [DOI] [PubMed] [Google Scholar]
- 56.Pan, Z. J., R. W. van der Hulst, M. Feller, S. D. Xiao, G. N. Tytgat, J. Dankert, and A. van der Ende. 1997. Equally high prevalences of infection with cagA-positive Helicobacter pylori in Chinese patients. J. Clin. Microbiol. 35:1344-1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Parsonnet, J., G. D. Friedman, N. Orentreich, and H. Vogelman. 1997. Risk for gastric cancer in people with CagA positive or CagA negative Helicobacter pylori infection. Gut 40:297-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Peek, R. M., Jr., M. F. Vaezi, G. W. Falk, J. R. Goldblum, G. I. Perez-Perez, J. E. Richter, and M. J. Blaser. 1999. Role of Helicobacter pylori cagA+ strains and specific host immune responses on the development of premalignant and malignant lesions in the gastric cardia. Int. J. Cancer 82:520-524. [DOI] [PubMed] [Google Scholar]
- 59.Potthoff, A., S. Ledig, J. Martin, O. Jandl, M. Cornberg, B. Obst, W. Beil, M. P. Manns, and S. Wagner. 2002. Significance of the caspase family in Helicobacter pylori induced gastric epithelial apoptosis. Helicobacter 7:367-377. [DOI] [PubMed] [Google Scholar]
- 60.Rojas, M., M. Olivier, P. Gros, L. F. Barrera, and L. F. Garcia. 1999. TNF-α and IL-10 modulate the induction of apoptosis by virulent Mycobacterium tuberculosis in murine macrophages. J. Immunol. 162:6122-6131. [PubMed] [Google Scholar]
- 61.Ruckdeschel, K., A. Roggenkamp, V. Lafont, P. Mangeat, J. Heesemann, and B. Rouot. 1997. Interaction of Yersinia enterocolitica with macrophages leads to macrophage cell death through apoptosis. Infect. Immun. 65:4813-4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Scotiniotis, I. A., T. Rokkas, E. E. Furth, B. Rigas, and S. J. Shiff. 2000. Altered gastric epithelial cell kinetics in Helicobacter pylori-associated intestinal metaplasia: implications for gastric carcinogenesis. Int. J. Cancer 85:192-200. [PubMed] [Google Scholar]
- 63.Segal, E. D., J. Cha, J. Lo, S. Falkow, and L. S. Tompkins. 1999. Altered states: involvement of phosphorylated CagA in the induction of host cellular growth changes by Helicobacter pylori. Proc. Natl. Acad. Sci. USA 96:14559-14564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shibayama, K., Y. Doi, N. Shibata, T. Yagi, T. Nada, Y. Iinuma, and Y. Arakawa. 2001. Apoptotic signaling pathway activated by Helicobacter pylori infection and increase of apoptosis-inducing activity under serum-starved conditions. Infect. Immun. 69:3181-3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shirin, H., and S. F. Moss. 1998. Helicobacter pylori induced apoptosis. Gut 43:592-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Su, B., S. Johansson, M. Fallman, M. Patarroyo, M. Granstrom, and S. Normark. 1999. Signal transduction-mediated adherence and entry of Helicobacter pylori into cultured cells. Gastroenterology 117:595-604. [DOI] [PubMed] [Google Scholar]
- 67.Tafani, M., N. O. Karpinich, K. A. Hurster, J. G. Pastorino, T. Schneider, M. A. Russo, and J. L. Farber. 2002. Cytochrome c release upon Fas receptor activation depends on translocation of full-length bid and the induction of the mitochondrial permeability transition. J. Biol. Chem. 277:10073-10082. [DOI] [PubMed] [Google Scholar]
- 68.Tsutsumi, R., H. Higashi, M. Higuchi, M. Okada, and M. Hatakeyama. 2003. Attenuation of Helicobacter pylori CagA x SHP-2 signaling by interaction between CagA and C-terminal Src kinase. J. Biol. Chem. 278:3664-3670. [DOI] [PubMed] [Google Scholar]
- 69.Wang, J., E. G. Brooks, K. B. Bamford, T. L. Denning, J. Pappo, and P. B. Ernst. 2001. Negative selection of T cells by Helicobacter pylori as a model for bacterial strain selection by immune evasion. J. Immunol. 167:926-934. [DOI] [PubMed] [Google Scholar]
- 70.Whitney, A. E., T. S. Emory, A. M. Marty, P. A. O'Shea, and G. W. Newman. 2000. Increased macrophage infiltration of gastric mucosa in Helicobacter pylori-infected children. Dig. Dis. Sci. 45:1337-1342. [DOI] [PubMed] [Google Scholar]
- 71.Wu, K. C., L. M. Jackson, A. M. Galvin, T. Gray, C. J. Hawkey, and Y. R. Mahida. 1999. Phenotypic and functional characterisation of myofibroblasts, macrophages, and lymphocytes migrating out of the human gastric lamina propria following the loss of epithelial cells. Gut 44:323-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zheng, P. Y., and N. L. Jones. 2003. Helicobacter pylori strains expressing the vacuolating cytotoxin interrupt phagosome maturation in macrophages by recruiting and retaining TACO (coronin 1) protein. Cell. Microbiol. 5:25-40. [DOI] [PubMed] [Google Scholar]
- 73.Zychlinsky, A., M. C. Prevost, and P. J. Sansonetti. 1992. Shigella flexneri induces apoptosis in infected macrophages. Nature 358:167-169. [DOI] [PubMed] [Google Scholar]
- 74.Zychlinsky, A., and P. J. Sansonetti. 1997. Apoptosis as a proinflammatory event: what can we learn from bacteria-induced cell death? Trends Microbiol. 5:201-204. [DOI] [PubMed] [Google Scholar]