Abstract
Pseudomonas aeruginosa is a leading cause of hospital-acquired pneumonia, and approximately 80% of patients with cystic fibrosis are infected with this bacterium. To investigate the overall role of complement and the complement activation pathways in the host defense against P. aeruginosa pulmonary infection, we challenged C3-, C4-, and factor B-deficient mice with P. aeruginosa via intranasal inoculation. In these studies, C3−/− mice had a higher mortality rate than C3+/+ mice. Factor B−/− mice, but not C4−/− mice, infected with P. aeruginosa had a mortality rate similar to that of C3−/− mice, indicating that in this model the alternative pathway of complement activation is required for the host defense against Pseudomonas infection. C3−/− mice had 6- to 7-fold more bacteria in the lungs and 48-fold more bacteria in the blood than did C3+/+ mice at 24 h postinfection. In vitro, phagocytic cells from C3+/+ or C3−/− mice exhibited a decreased ability to bind and/or ingest P. aeruginosa in the presence of C3-deficient serum compared to phagocytic cells in the presence of serum with sufficient C3. C3−/− mice displayed a significant increase in neutrophils in the lungs and had higher levels of interleukin-1β (IL-1β), IL-6, IL-10, KC, and MIP-2 in the lungs at 24 h postinfection than did C3+/+ mice. Collectively, these results indicate that complement activation by the alternative pathway is critical for the survival of mice infected with P. aeruginosa and that the protection provided by complement is at least in part due to C3-mediated opsonization and phagocytosis of P. aeruginosa.
Pseudomonas aeruginosa is a gram-negative environmental bacterium and an opportunistic pathogen that can cause pneumonia, particularly in people whose immune systems are suppressed, such as AIDS patients and cancer patients, and is a major source of hospital-acquired pneumonia (23). In addition, this pathogen causes chronic pulmonary infections in people with cystic fibrosis, leading to significant morbidity and mortality among these patients (23). A pulmonary infection with P. aeruginosa is characterized by a strong recruitment of neutrophils and significant inflammation in the lung parenchyma, which results in extensive damage to the lung tissue through the action of neutrophil enzymes and oxidants (23).
The complement system, which is made up of >30 serum and cell surface proteins, plays a crucial role in the host defense against infection and in the inflammatory response (17, 25). The complement system has three pathways of activation, the classical, alternative, and lectin pathways; all three pathways result in C3 activation and merge into a common pathway that results in the formation of the membrane attack complex (MAC), or C5b-9 (17). The classical pathway is primarily activated through the binding of C1q to antibody-antigen complexes. The lectin pathway is initiated when the mannose-binding lectin binds to mannose residues on bacteria or viruses, and the alternative pathway is initiated when spontaneously activated C3 binds to activating surfaces such as those of certain bacteria or viruses (17). In a complete deficiency of C3, almost all of the biological properties mediated by complement are absent, including opsonization and phagocytosis of bacteria (mediated by C3b, C3bi, CR1, CR3, and CR4), directed migration of inflammatory cells (mediated by C3a and C5a), and amplification of the immune response (mediated by C3dg) (17, 18, 25, 41).
Although there are numerous immune and inflammatory responses that occur in an acutely infected lung, recent in vivo experiments have indicated that complement plays a particularly critical role in providing a protective immune response against P. aeruginosa lung infection. For example, antibodies against CR3 (CD11b/CD18) reduce neutrophil emigration into the lungs of P. aeruginosa-infected mice (12, 28); C5- and C5a receptor (C5aR)-deficient mice succumb more readily to P. aeruginosa infection, despite the robust recruitment of neutrophils to the infected lungs (3, 16, 19); and mice pretreated with cobra venom factor have increased numbers of P. aeruginosa in the lungs 4 h after P. aeruginosa pulmonary infection (10, 13).
To more fully understand the overall role of complement and its activation pathways in the host defense against an acute primary P. aeruginosa pulmonary infection, we subjected mice that were completely deficient in C3, factor B, or C4 to P. aeruginosa infection as a model of P. aeruginosa-induced pneumonia. The results from these studies demonstrate that an intact alternative pathway is critical to host survival of an acute primary P. aeruginosa pulmonary infection. In addition, host clearance of P. aeruginosa was greatly impaired in C3−/− mice, which appeared to be due, at least in part, to the absence of C3-mediated opsonization and phagocytosis.
MATERIALS AND METHODS
Mice.
C57BL/6 mice are commonly used for animal disease models, including those for P. aeruginosa infection. Accordingly, complement-deficient mice that were backcrossed onto the C57BL/6 genetic background were used in all experimental procedures. The C3-deficient (C3−/−) mice used in these studies were described previously (4). Sera from C3−/− mice lack detectable C3 and complement activity (4). The C3−/− mice were backcrossed for 12 generations onto the C57BL/6 background, and wild-type littermates (C3+/+) were used as controls. Factor B-deficient (FB−/−) mice have been described elsewhere (24), and alternative pathway activity is not detectable in sera from these mice (24). The FB−/− mice were backcrossed for 11 generations onto the C57BL/6 background, and wild-type littermates (FB+/+) were used as controls. The C4-deficient (C4−/−) mice were generated as described previously (8) and were found to have no detectable C4 protein in their sera (8). The C4−/− mice were backcrossed for at least 10 generations onto the C57BL/6 background, and their wild-type littermates (C4+/+) were used as controls. Both male and female mice of 6 to 9 weeks of age were used for these studies, and institutional and National Institutes of Health guidelines for animal care and welfare were followed.
i.n. infection.
P. aeruginosa strain PA103 (22), kindly provided by Barbara Iglewski, University of Rochester Medical Center, was used for most of the studies to induce pneumonia in mice via intranasal (i.n.) inoculations. A clinical strain of P. aeruginosa (designated PA243) that was isolated from the sputum of an intensive care unit patient with pneumonia was kindly provided by Audrey Wanger, University of Texas Medical School—Houston, and was used for mortality studies. Bacteria were cultured in tryptic soy broth (TSB) at 37°C to mid-logarithmic phase, harvested by centrifugation, washed twice in sterile, nonpyrogenic 0.9% NaCl, and resuspended in 0.9% NaCl. Mice that had been anesthetized with an intraperitoneal injection of 2.5% tribromoethanol (0.016 ml/g of body weight) (Sigma-Aldrich, St. Louis, Mo.) (15) were given either 1 × 105 PA103 cells or 3 × 106 PA243 cells in a volume of 20 μl i.n. Control mice received 20 μl of sterile, nonpyrogenic 0.9% NaCl i.n. The number of bacteria present in the inoculum was verified by culturing serial dilutions of the inoculum on tryptic soy agar (TSA) plates.
Mortality studies.
C3−/− mice, FB−/− mice, C4−/− mice, and their respective wild-type littermates were infected via i.n. inoculation with either PA103, PA243, or 0.9% NaCl and were observed for mortality every 12 h for 7 days. Survival curves were graphed with the GraphPad Prism (San Diego, Calif.) software package, and statistical significance was assessed by using the log rank test. All statistical analyses for these studies were performed with GraphPad Prism software.
Bacterial clearance from the lungs and blood.
C3−/− mice and C3+/+ mice were infected i.n. with PA103 or 0.9% NaCl, and the lungs were collected either immediately or at 24 h postinfection. For determination of the amount of the initial inoculum that was received into the lungs of the mice, six to seven mice per group were infected and then immediately euthanized. Either immediately or 24 h after infection, lungs were collected and placed in sterile 0.9% NaCl on ice. The lungs were homogenized on ice in 2 ml of sterile 0.9% NaCl by pressing the lungs through 100-μm-pore-size cell strainers (BD Biosciences, San Diego, Calif.). Serial 10-fold dilutions of the lung homogenates were plated on TSA plates to determine the numbers of CFU. Bronchoalveolar lavage (BAL) was performed on some mice 24 h after infection in order to quantitate the number of bacteria present in BAL fluid. The lungs were subjected to lavage three times with 0.5 ml of phosphate-buffered saline (PBS), and the lavage fluid was placed on ice. Serial 10-fold dilutions of the BAL fluid were plated on TSA plates to determine the numbers of CFU. Prior to BAL or harvesting of the lungs, blood was drawn from the mice at 24 h postinfection, and 100 μl of blood from the C3+/+ mice and serial 10-fold dilutions of blood from the C3−/− mice were plated on TSA plates. All plates were incubated overnight at 37°C. The results are expressed as mean CFU per total lungs, mean CFU per milliliter of BAL fluid, or mean CFU per milliliter of blood ± standard errors of the means (SEM). Statistical significance between C3+/+ and C3−/− mice was assessed by using the two-tailed, unpaired Student t test.
Bactericidal assay.
Washed P. aeruginosa PA103 (2 × 108 cells/ml) or Escherichia coli HB101 (9 × 107 cells/ml) cells were incubated with either normal serum from C3+/+ or C3−/− mice or heat-inactivated serum (56°C for 30 min) from C3+/+ or C3−/− mice for 1 h at 37°C with gentle shaking (125 rpm). The sera used were pooled from six C3+/+ or six C3−/− mice. After 1 h, serial 10-fold dilutions of each treatment were made and were plated on TSA plates to determine the numbers of viable bacteria. The plates were incubated overnight at 37°C. The results are expressed as mean CFU per milliliter ± SEM. Statistical significance was assessed by using the two-tailed, unpaired Student t test.
In vitro assay for adherence and uptake of P. aeruginosa by mouse blood leukocytes.
Total blood leukocytes were obtained from C3+/+ and C3−/− mice as follows: 1-ml syringes containing 23-gauge needles were filled with 0.23 ml of dextran citrate solution (3.5% dextran T500, 70 mM sodium citrate, 34 mM citric acid in PBS), C3+/+ and C3−/− mice were bled to 1 ml via cardiac puncture, the red blood cells (RBCs) were allowed to settle to the bottom of the tube for 30 min, the layer above the RBCs was placed in a new tube and centrifuged at 200 × g for 8 min, the supernatants were discarded, the residual RBCs were lysed with ACK lysing buffer (BioWhittaker, Rockland, Maine), and the cells were washed one time with PBS. The washed leukocytes were resuspended in RPMI 1640 medium containing 2.05 mM l-glutamine (VWR, West Chester, Pa.) and 10% C3+/+ or C3−/− mouse serum, and the leukocytes were counted by using a hemacytometer. P. aeruginosa PA01, also kindly provided by Barbara Iglewski, and P. aeruginosa PA01 constitutively expressing green fluorescent protein (GFP) (30), kindly provided by Anne Camper, Montana State University, were opsonized in either 50% C3+/+ or C3−/− mouse serum for 30 min at 37°C. The leukocytes, at a concentration of 2 × 106 cells/ml, were incubated with 1 × 108 cells of preopsonized P. aeruginosa/ml for 1 h at 37°C with gentle shaking (100 rpm). The cells were centrifuged as described above at 4°C, and then the cells were resuspended in PBS and kept on ice for flow cytometry analysis. Flow cytometry using a FACSCalibur apparatus (BD Biosciences) was employed to quantify the adherence and phagocytosis of bacteria by analyzing cells that were positive for GFP. The forward scatter threshold was set so as to exclude free bacteria from the analysis. Leukocytes incubated with opsonized non-GFP-expressing P. aeruginosa PA01 were used as negative controls, and at least 10,000 cells in all samples were analyzed. The results are expressed as percentages of positive cells ± SEM. Statistical significance was assessed by using the two-tailed, unpaired Student t test.
Lung histology.
Mouse lungs were perfused with 0.3 ml of formalin 24 h after infection, and the tracheas were tied off with suture material. The lungs were removed from the mice and placed in formalin overnight at 4°C. The lungs were dehydrated with increasing concentrations of ethanol, embedded in paraffin, cut into 5-μm-thick sections, and stained with hematoxylin and eosin. The stained sections were examined by light microscopy to assess the level of inflammation.
BAL.
The types and numbers of inflammatory cells present in the lungs 24 h after infection were assessed by BAL, which was performed as described above. The total number of cells present in the lavage fluid was determined by using a hemacytometer. BAL fluid was centrifuged onto slides in a cytocentrifuge (Shandon Lipshaw, Pittsburgh, Pa.), and the slides were stained with Wright-Giemsa stain (Fisher Scientific, Pittsburgh, Pa.). Two hundred cells per slide were classified according to cell type (either polymorphonuclear [PMN] cell, macrophage, or lymphocyte) based on their characteristic morphologies under a light microscope. Absolute numbers of specific cell types were calculated from the recovered BAL volume, total cell count, and percent abundance of specific cells. Statistical significance between C3+/+ and C3−/− mice was assessed by analysis of variance followed by posthoc pairwise comparisons by the Tukey method.
Cytokine and chemokine analysis.
The levels of the proinflammatory cytokines interleukin-1β (IL-1β), IL-6, and tumor necrosis factor alpha (TNF-α), the anti-inflammatory cytokine IL-10, and the chemokines JE, KC, and MIP-2 were examined in mice at 24 h postinfection. BAL fluid was collected as described above and was centrifuged to remove cells. The BAL fluid supernatants were then assayed for the presence of these cytokines and chemokines by enzyme-linked immunosorbent assays (R & D Systems Inc., Minneapolis, Minn.) performed according to the manufacturer's instructions. Statistical significance between C3+/+ and C3−/− mice for each individual cytokine or chemokine was assessed by using the two-tailed, unpaired Student t test.
RESULTS
C3−/− mice exhibit increased mortality after P. aeruginosa infection.
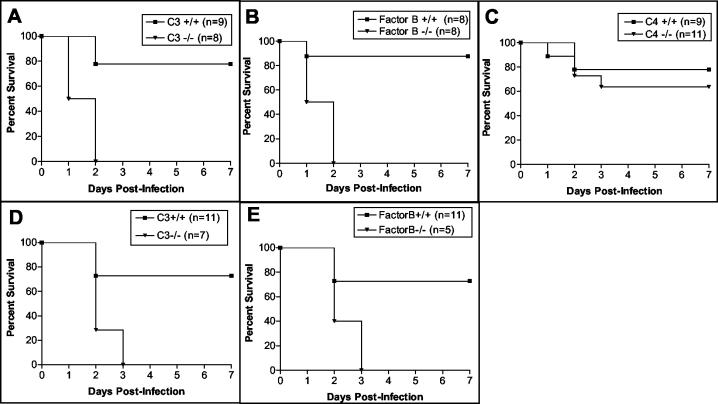
Mice deficient in C3 lack a functional complement system and are devoid of numerous activation fragments that mediate potent biological activities in inflammation and immunity (17, 18, 25, 41). Accordingly, to assess the overall impact of complement in an in vivo model of acute primary pneumonia, we challenged C3−/− and C3+/+ mice i.n. with P. aeruginosa PA103 (105 CFU) and monitored them for mortality. Within 48 h of infection, all of the C3−/− mice died, compared to only 22% of the C3+/+ mice (P = 0.001) (Fig. 1A). To show that the host protection provided by C3 is not unique to the PA103 strain, we also challenged C3−/− and C3+/+ mice i.n. with the clinical PA243 strain (3 × 106 CFU) and monitored them for mortality. Within 72 h of infection, all of the C3−/− mice died, compared to only 27% of the C3+/+ mice (P = 0.003) (Fig. 1D), demonstrating that complement plays a vital role in protecting the host from the lethal effects of an acute primary P. aeruginosa pulmonary infection.
FIG. 1.
Survival curves for C3−/−, FB−/−, and C4−/− mice infected via i.n. inoculation with 1 × 105 P. aeruginosa PA103 cells (A to C) or 3 × 106 P. aeruginosa PA243 cells (D and E). Data for the C3−/− mice (A and D), FB−/− mice (B and E), and C4−/− mice (C) are shown with those for their respective wild-type littermates. Mice were monitored for mortality every 12 h for 7 days.
FB−/− mice exhibit increased mortality after infection with P. aeruginosa.
P. aeruginosa infection could potentially result in the activation of all three complement pathways, the classical, lectin, and alternative pathways. To examine the significance of the complement activation pathways in providing a protective immune response against P. aeruginosa, we infected FB−/− mice (lacking a functional alternative pathway), C4−/− mice (lacking functional classical and lectin pathways), and their respective wild-type littermates with P. aeruginosa PA103 i.n. and monitored them for mortality. Similar to the C3−/− mice, all of the FB−/− mice died within 48 h of infection, compared to only 13% of the FB+/+ mice (P = 0.001) (Fig. 1B). In contrast, the C4−/− mice and the C4+/+ mice displayed similar survival rates (P > 0.05) (Fig. 1C). To confirm the importance of the alternative pathway in this model, we also infected FB+/+ and FB−/− mice with the clinical PA243 strain. Within 72 h of infection, all of the FB−/− mice died, compared to only 27% of the FB+/+ mice (P = 0.01) (Fig. 1E). These results indicate that in this model the classical and lectin pathways are less important than the alternative pathway for a protective immune response against primary P. aeruginosa pulmonary infections.
C3−/− mice are deficient in clearance of P. aeruginosa from the lungs and blood.
To determine if the increased mortality seen for the C3−/− mice correlated with an increase in bacterial numbers in the lungs and bloodstream, we infected both C3−/− and C3+/+ mice i.n. with P. aeruginosa. Twenty-four hours after infection, lungs were removed from one group of mice, homogenized, and plated on TSA plates, and for the other group of mice, BAL was performed and BAL fluid was plated on TSA plates. Blood was also drawn from all groups of mice at 24 h postinfection and was plated on TSA plates. Lungs from infected C3−/− mice contained six times more P. aeruginosa than lungs from infected C3+/+ mice (P = 0.003) (Table 1), and BAL fluid from infected C3−/− mice contained seven times more P. aeruginosa than BAL fluid from infected C3+/+ mice (P = 0.02) (Table 1). The C3−/− mice also had 48 times more P. aeruginosa present in the bloodstream 24 h after infection than did the C3+/+ mice (P = 0.001) (Table 1).
TABLE 1.
Clearance of P. aeruginosa from the lungs and dissemination into the bloodstream after i.n. infectiona
| Mouse genotype | CFU/total lungs
|
CFU/ml of BAL fluid at 24 h | CFU/ml of blood at 24 h | |
|---|---|---|---|---|
| 0 h | 24 h | |||
| C3+/+ | (6.02 ± 0.57) × 104 (n = 6) | (2.61 ± 0.96) × 106 (n = 12) | (4.45 ± 1.63) × 105 (n = 8) | (6.15 ± 2.15) × 102 (n = 13) |
| C3−/− | (7.05 ± 0.76) × 104 (n = 7) | (1.53 ± 0.46) × 107** (n = 7) | (3.06 ± 0.76) × 106* (n = 13) | (2.97 ± 0.81) × 104** (n = 13) |
Lungs were removed from the mice either immediately after infection (0 h) or 24 h postinfection, the lungs were homogenized in saline, serial 10-fold dilutions were made, and the dilutions were plated on TSA plates. In other experiments, the lungs were lavaged three times with 0.5 ml of PBS, serial 10-fold dilutions of the BAL fluid were made, and the dilutions were plated on TSA plates. Blood was also plated on TSA plates. Results are expressed as means ± SEM. Significant differences between C3+/+ and C3−/− mice are indicated as follows: *, P = 0.02; **, P ≤ 0.003.
Mouse serum does not affect viability of P. aeruginosa.
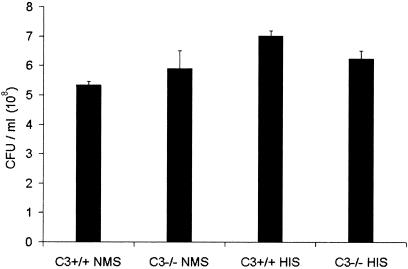
In the absence of C3, complement-mediated formation of the C5b-9 MAC can no longer occur (17, 25). Hence, the increased numbers of P. aeruginosa in the lungs and blood of the C3−/− animals could potentially be due to the absence of C5b-9 lytic killing of the bacteria. To examine this possibility, we incubated P. aeruginosa PA103 with C3+/+ or C3−/− mouse serum as well as heat-inactivated serum from these animals for 1 h at 37°C. Any remaining viable bacteria were subsequently quantitated as described in Materials and Methods. As shown in Fig. 2, P. aeruginosa was resistant to killing by mouse serum independent of whether C3+/+, C3−/−, or heat-inactivated serum was used. This finding indicates that mouse serum does not affect the viability of P. aeruginosa PA103, and hence the increased numbers of bacteria in the infected C3−/− mice were not due to an impairment in C5b-9-mediated lysis.
FIG. 2.
Serum bactericidal assay with P. aeruginosa and C3+/+ or C3−/− mouse serum. P. aeruginosa PA103 (2 × 108 CFU/ml) was incubated with either normal mouse serum (NMS) from C3+/+ or C3−/− mice or heat-inactivated serum (HIS) from C3+/+ or C3−/− mice for 1 h at 37°C. After 1 h, serial 10-fold dilutions of each treatment were made and plated on TSA plates to determine the number of viable bacteria. Results are expressed as CFU per ml ± SEM. E. coli HB101 was used as a positive control for this experiment and was unable to grow in the presence of C3+/+ mouse serum but able to grow in the presence of C3−/− mouse serum and heat-inactivated C3+/+ and C3−/− mouse sera (P < 0.03) (data not shown). This experiment was performed three times, and the data shown are from one representative experiment.
C3−/− mice are defective in adherence and uptake of P. aeruginosa.
In the absence of C3, complement activation will not generate the C3-derived polypeptide fragments C3b and C3bi. These fragments of C3 act as opsonins and facilitate phagocytosis by adhering C3b- and C3bi-coated bacteria to macrophages and neutrophils via binding to their receptors, named CR1 and CR3/CR4, respectively (7, 31). To determine if the deficiency in clearance of P. aeruginosa from the lungs and blood of C3−/− mice was due to a defect in adherence and phagocytosis, we conducted in vitro uptake studies using GFP-expressing P. aeruginosa and flow cytometry. Total blood leukocytes were harvested from C3+/+ and C3−/− mice, and these leukocytes were incubated with P. aeruginosa that had been preopsonized with either C3+/+ or C3−/− mouse serum. Leukocytes isolated from C3+/+ or C3−/− mice incubated with P. aeruginosa opsonized with serum with sufficient C3 had more bacteria associated with them than leukocytes incubated with bacteria opsonized in C3-deficient serum (Table 2). Our results indicate that in the absence of C3-mediated opsonization, the adherence of P. aeruginosa to phagocytic cells is impaired. This impairment likely results in ineffective phagocytosis, which may explain, in part, the higher numbers of P. aeruginosa found in the lungs and blood of C3−/− mice.
TABLE 2.
Adherence and phagocytosis of preopsonized P. aeruginosa by C3+/+ or C3−/− phagocytes
| Type of leukocytes | Type of serum used for GFP-PA01 incubation | % positive cellsa (mean ± SEM) |
|---|---|---|
| C3+/+ | C3+/+ | 55.14 ± 3.09 |
| C3+/+ | C3−/− | 42.01 ± 0.28* |
| C3−/− | C3+/+ | 48.37 ± 1.31 |
| C3−/− | C3−/− | 37.09 ± 3.68* |
The percent positive cells represents the number of leukocytes that were positive for GFP by flow cytometry analysis. The negative control, which was non-GFP-PA01 incubated with C3+/+ serum and C3+/+ leukocytes, had 0.12% positive cells. *, significant difference (P ≤ 0.04) between C3-sufficient and C3-deficient sera.
C3−/− mice display an increase in pulmonary inflammatory infiltrates after infection.
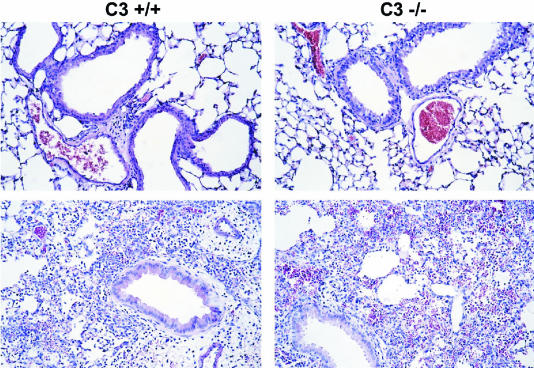
The attenuated bacterial clearance observed for C3−/− mice could be due to a reduced pulmonary cellular inflammatory response since the anaphylatoxins C3a and C5a will not be produced by complement activation in C3−/− mice. To compare the level of pulmonary inflammation in C3−/− mice to that in C3+/+ mice, we removed the lungs from both groups of mice for histology 24 h after P. aeruginosa infection. Lung sections that were stained with hematoxylin and eosin revealed the presence of inflammatory infiltrates in both the C3+/+ and C3−/− mice that had been infected with P. aeruginosa (Fig. 3, bottom panels). A normal lung histology was evident in lung sections from control C3+/+ and C3−/− mice infected with only saline (Fig. 3, top panels). Inflammatory cells were quantitated in BAL fluid taken from the mice at 24 h postinfection. The C3−/− mice had two times more total leukocytes and PMNs than the C3+/+ mice (P < 0.001) (Table 3), demonstrating that the C3−/− mice had elevated levels of inflammatory cells in the lungs after P. aeruginosa pulmonary infection. These results indicate that the increased numbers of P. aeruginosa in the C3−/− mice were not due to reduced pulmonary cellular recruitment.
FIG. 3.
Lung histology of C3+/+ and C3−/− mice 24 h after i.n. infection with either P. aeruginosa (bottom panels) or saline (top panels). The lungs were fixed in formalin, embedded in paraffin, sectioned into 5-μm-thick sections, and stained with hematoxylin and eosin. The sections shown are representative of three separate experiments. Magnification, ×20.
TABLE 3.
Leukocyte counts and differentials in BAL fluid 24 h after i.n. infection with P. aeruginosaa
| Mouse genotype | Treatment | Total leukocyte count (106 cells) | No. of PMNs (106 cells) | No. of macrophages (106 cells) | No. of lymphocytes (106 cells) |
|---|---|---|---|---|---|
| C3+/+ | Saline | 0.18 ± 0.01 | 0 | 0.18 ± 0.01 | 0 |
| C3−/− | Saline | 0.17 ± 0.01 | 0 | 0.17 ± 0.01 | 0 |
| C3+/+ | PA103 | 32.47 ± 1.50 | 30.88 ± 1.39 | 1.05 ± 0.10 | 0.54 ± 0.10 |
| C3−/− | PA103 | 67.07 ± 6.34*** | 63.91 ± 5.98*** | 1.28 ± 0.35 | 1.88 ± 0.22 |
BAL was performed on the mice 24 h after infection with either saline or PA103. Absolute numbers of specific cell types were calculated from the recovered BAL volume, total cell count, and percent abundance of specific cells. Results are expressed as means ± SEM. For the saline-treated groups, n = 5. For the PA103- treated groups, n = 8. ***, significant differences between C3+/+ and C3−/− mice infected with PA103 (P < 0.001).
C3−/− mice have higher levels of cytokines and chemokines after infection.
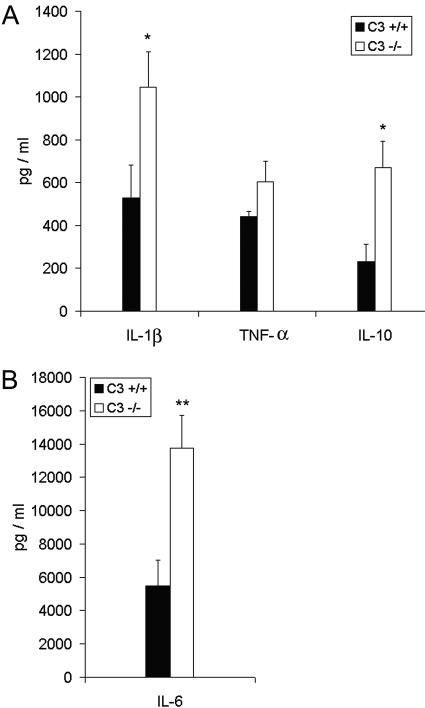
To further evaluate the inflammatory response in C3−/− mice and C3+/+ mice, we measured the levels of the proinflammatory cytokines IL-1β, IL-6, and TNF-α and the anti-inflammatory cytokine IL-10 in BAL fluid taken from mice at 24 h postinfection. The C3−/− mice had 2 times more IL-1β (P = 0.04), 2.9 times more IL-10 (P = 0.02), and 2.5 times more IL-6 (P = 0.005) than the C3+/+ mice (Fig. 4). The C3−/− mice and the C3+/+ mice had similar levels of TNF-α (P > 0.05) (Fig. 4A).
FIG. 4.
Cytokine levels in BAL fluid 24 h after infection with P. aeruginosa. The levels of IL-1β, TNF-α, and IL-10 (A) and the level of IL-6 (B) in BAL fluid were measured 24 h after infection with P. aeruginosa. BAL fluid from saline-treated control mice had no detectable levels of these cytokines. Results are expressed as means ± SEM for n values of 9 to 18. Significant differences between C3+/+ and C3−/− mice are indicated as follows: *, P ≤ 0.04; **, P = 0.005.
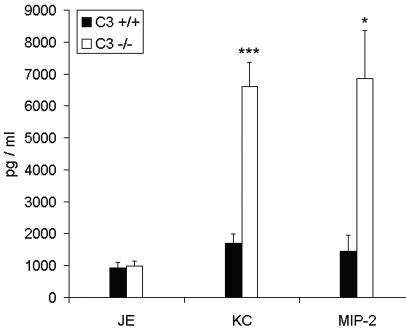
To determine if the C3−/− mice had more production of chemokines than the C3+/+ mice after infection, we measured the levels of KC, MIP-2, and JE in BAL fluid taken 24 h after infection with P. aeruginosa. The mouse chemokine JE is chemotactic for monocytes, macrophages, and lymphocytes (2, 11), and the mouse chemokines KC and MIP-2 are chemotactic for neutrophils (6, 37). The C3−/− mice had 3.9 times more KC (P < 0.0001) and 4.7 times more MIP-2 (P = 0.01) than the C3+/+ mice (Fig. 5), while JE levels were equivalent between the two groups of mice (P > 0.05) (Fig. 5). The increased expression of KC and MIP-2 may account for the high quantity of neutrophils present in the lungs of the C3−/− mice.
FIG. 5.
Chemokine levels in BAL fluid 24 h after infection with P. aeruginosa. The levels of JE, KC, and MIP-2 were measured in BAL fluid 24 h after infection with P. aeruginosa. BAL fluid from saline-treated control mice had no detectable levels of these chemokines. Results are expressed as means ± SEM for n values of 10 to 16. Significant differences between C3+/+ and C3−/− mice are indicated as follows: *, P = 0.01; ***, P < 0.0001.
DISCUSSION
With this study, we demonstrated for the first time with a mouse model of acute primary pneumonia the overall importance of complement activation by the alternative pathway in the immune response to primary P. aeruginosa pulmonary infection. Both C3−/− and FB−/− mice had much higher mortality rates than their respective wild-type littermates or C4−/− mice after i.n. infection with P. aeruginosa. The higher mortality rates of FB−/− mice than of C4−/− animals indicate that in this model of acute primary lung infection the alternative pathway of complement activation is more important than the classical or lectin pathway for protecting the host from a P. aeruginosa challenge. The lack of protection from the lectin pathway agrees with recent in vitro investigations which have indicated that P. aeruginosa does not bind human mannose-binding lectin (5, 26). Although our studies demonstrate the dominance of the alternative pathway of complement activation in providing host protection against a primary acute P. aeruginosa infection, they do not address the significance of the alternative pathway compared to the classical pathway once an antibody response to this organism is generated. Recent studies have indicated that human monoclonal antibodies against P. aeruginosa lipopolysaccharide (LPS) (14) and both mouse monoclonal (9) and rabbit polyclonal (32) antibodies against P. aeruginosa PcrV protect mice (9, 14, 32) and rabbits (32) from fatal P. aeruginosa sepsis, suggesting that once an antibody response is mounted against P. aeruginosa, the classical pathway may provide significant host protection. However, whether the classical or alternative pathway will dominate upon a subsequent host exposure to P. aeruginosa is likely dependent on the immunocompetence of the host, which will affect the level of antibody response to this opportunistic pathogen. The model used in our studies is likely to represent what happens in an individual who is infected with P. aeruginosa for the first time or who has previously been exposed to P. aeruginosa but due to a compromised immune system is unable to mount a protective antibody response.
C3−/− mice harbored 6 to 7 times more P. aeruginosa in the lungs and 48 times more P. aeruginosa in the bloodstream than C3+/+ mice at 24 h postinfection, indicating that complement significantly impacts the host clearance of P. aeruginosa. Although the bactericidal activity of the C5b-9 complex is important for host defense against many gram-negative bacteria (17), the results reported here demonstrate that P. aeruginosa PA103 is resistant to killing by mouse serum. Therefore, the increased numbers of P. aeruginosa in the C3−/− mice are not due to an impairment in MAC-mediated killing of P. aeruginosa. In addition, the reduced ability of the C3−/− mice to control the P. aeruginosa infection was not due to an inability of these mice to mount a neutrophil chemotactic response. In fact, a significantly elevated number of neutrophils was present in the lungs of infected C3−/− animals compared to those of their wild-type littermates. Similar increases in inflammatory cells have been reported for the lungs of C5- and C5aR-deficient mice after P. aeruginosa pulmonary infections (3, 16, 19). Collectively, our studies with C3−/− mice and the studies of others with C5- and C5aR-deficient mice indicate that C5a does not play an essential role in the recruitment of neutrophils to the P. aeruginosa-infected lung. In the absence of an intact complement system, multiple compensatory mechanisms exist for the recruitment of neutrophils into the lungs after P. aeruginosa infection. P. aeruginosa is itself chemotactic for neutrophils in vitro in the absence of serum (34, 39). The mouse chemokines MIP-2 and KC are CXC chemokines that are chemotactic for neutrophils (6, 37), and elevated levels of these chemokines are observed after P. aeruginosa pulmonary infection (21, 33, 36, 38, 44). The mouse receptor for these chemokines is CXCR2 (20, 36). Antibody neutralization of CXCR2 prior to the intratracheal inoculation of mice with P. aeruginosa resulted in a diminished recruitment of neutrophils into the lungs, increased mortality, and high P. aeruginosa numbers in the lungs and bloodstream compared to those in mice that received a control antibody (36). In our studies, the infected C3−/− mice had significantly higher levels of KC and MIP-2 in their BAL fluid than did the infected C3+/+ mice. Neutrophils and mononuclear phagocytes produce MIP-2 and KC in response to LPS and IL-1 (35), so the increased levels of LPS (due to increased numbers of P. aeruginosa) and IL-1β in the C3−/− mice could account for the high levels of MIP-2 and KC in these mice. The elevated levels of these potent chemokines in the lungs of infected C3−/− mice may explain why C3-deficient animals exhibit a more robust recruitment of neutrophils in response to pulmonary infections than do mice with sufficient complement.
Previous studies have shown that one of the defenses of the host against P. aeruginosa infection is the phagocytic clearance of bacteria by PMNs. For example, BALB/c mice that were made to be granulocytopenic by the injection of an antigranulocyte globulin prior to infection had a significant impairment in the clearance of P. aeruginosa from the lungs compared to control mice (29). C57BL/6 mice injected with an antineutrophil antibody prior to infection displayed 100% mortality within 2 days of P. aeruginosa lung infection compared to zero mortality in 7 days for mice treated with a control antibody (36). C3b and C3bi function as opsonins by coating bacteria and then binding to CR1 and CR3/CR4, respectively, which are present on macrophages and PMNs (7, 31). Several in vitro studies have demonstrated that normal human serum increases the phagocytosis of P. aeruginosa by PMNs in culture (1, 27, 43), and this increase in phagocytosis was abolished when the serum was heated to inactivate complement components. Our data support the importance of complement-mediated opsonization and phagocytosis in the clearance of P. aeruginosa. We showed that in the absence of C3, and hence in the absence of the C3b and C3bi opsonins, the adherence of P. aeruginosa to phagocytic cells is impaired. This finding suggests that the exacerbated P. aeruginosa infections seen for C3−/− mice may be due at least in part to inefficient phagocytosis of the bacteria in the absence of opsonization by C3-derived polypeptide fragments. Since C5a has been shown to cause increased expression of CR1, CR3, and CR4 on phagocytic cells (16, 40, 42), a lack of efficient phagocytosis could also be involved in the elevated numbers of P. aeruginosa found in the lungs of C5- and C5aR-deficient mice (3, 16, 19).
In summary, the studies presented here indicate that (i) complement activation by an intact alternative pathway is essential for host survival of acute pneumonia caused by a primary P. aeruginosa infection, (ii) C3-mediated opsonization and phagocytosis are important mechanisms by which complement provides the host protection against P. aeruginosa lung infection, and (iii) a complete deficiency of C3 in mice challenged i.n. with P. aeruginosa results in significantly increased lung levels of the chemokines KC and MIP-2, which may cause paradoxical elevated numbers of neutrophils observed in the lungs of infected C3−/− mice.
Acknowledgments
We thank Irma Gigli for critical evaluation of the data and the manuscript. We also thank Barbara Iglewski, Audrey Wanger, and Anne Camper for providing the P. aeruginosa strains used in these studies, David Haviland for assistance with the flow cytometer, and Patricia Dillard for technical support.
This work was supported by U.S. Public Health Service grants (AI025011 and HL074333) awarded to R.A.W.
Editor: J. N. Weiser
REFERENCES
- 1.Bjornson, A. B., and J. G. Michael. 1974. Factors in human serum promoting phagocytosis of Pseudomonas aeruginosa. I. Interaction of opsonins with the bacterium. J. Infect. Dis. 130(Suppl.):S119-S126. [DOI] [PubMed] [Google Scholar]
- 2.Bottazzi, B., S. Walter, D. Govoni, F. Colotta, and A. Mantovani. 1992. Monocyte chemotactic cytokine gene transfer modulates macrophage infiltration, growth, and susceptibility to IL-2 therapy of a murine melanoma. J. Immunol. 148:1280-1285. [PubMed] [Google Scholar]
- 3.Cerquetti, M. C., D. O. Sordelli, J. A. Bellanti, and A. M. Hooke. 1986. Lung defenses against Pseudomonas aeruginosa in C5-deficient mice with different genetic backgrounds. Infect. Immun. 52:853-857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Circolo, A., G. Garnier, W. Fukuda, X. Wang, T. Hidvegi, A. J. Szalai, D. E. Briles, J. E. Volanakis, R. A. Wetsel, and H. R. Colten. 1999. Genetic disruption of the murine complement C3 promoter region generates deficient mice with extrahepatic expression of C3 mRNA. Immunopharmacology 42:135-149. [DOI] [PubMed] [Google Scholar]
- 5.Davies, J., O. Neth, E. Alton, N. Klein, and M. Turner. 2000. Differential binding of mannose-binding lectin to respiratory pathogens in cystic fibrosis. Lancet 355:1885-1886. [DOI] [PubMed] [Google Scholar]
- 6.Driscoll, K. E. 1994. Macrophage inflammatory proteins: biology and role in pulmonary inflammation. Exp. Lung Res. 20:473-490. [DOI] [PubMed] [Google Scholar]
- 7.Ehlers, M. R. 2000. CR3: a general purpose adhesion-recognition receptor essential for innate immunity. Microbes Infect. 2:289-294. [DOI] [PubMed] [Google Scholar]
- 8.Fischer, M. B., M. Ma, S. Goerg, X. Zhou, J. Xia, O. Finco, S. Han, G. Kelsoe, R. G. Howard, T. L. Rothstein, E. Kremmer, F. S. Rosen, and M. C. Carroll. 1996. Regulation of the B cell response to T-dependent antigens by classical pathway complement. J. Immunol. 157:549-556. [PubMed] [Google Scholar]
- 9.Frank, D. W., A. Vallis, J. P. Wiener-Kronish, A. Roy-Burman, E. G. Spack, B. P. Mullaney, M. Megdoud, J. D. Marks, R. Fritz, and T. Sawa. 2002. Generation and characterization of a protective monoclonal antibody to Pseudomonas aeruginosa PcrV. J. Infect. Dis. 186:64-73. [DOI] [PubMed] [Google Scholar]
- 10.Gross, G. N., S. R. Rehm, and A. K. Pierce. 1978. The effect of complement depletion on lung clearance of bacteria. J. Clin. Investig. 62:373-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gu, L., B. Rutledge, J. Fiorillo, C. Ernst, I. Grewal, R. Flavell, R. Gladue, and B. Rollins. 1997. In vivo properties of monocyte chemoattractant protein-1. J. Leukoc. Biol. 62:577-580. [DOI] [PubMed] [Google Scholar]
- 12.Gyetko, M. R., S. Sud, T. Kendall, J. A. Fuller, M. W. Newstead, and T. J. Standiford. 2000. Urokinase receptor-deficient mice have impaired neutrophil recruitment in response to pulmonary Pseudomonas aeruginosa infection. J. Immunol. 165:1513-1519. [DOI] [PubMed] [Google Scholar]
- 13.Heidbrink, P. J., G. B. Toews, G. N. Gross, and A. K. Pierce. 1982. Mechanisms of complement-mediated clearance of bacteria from the murine lung. Am. Rev. Respir. Dis. 125:517-520. [DOI] [PubMed] [Google Scholar]
- 14.Hemachandra, S., K. Kamboj, J. Copfer, G. Pier, L. L. Green, and J. R. Schreiber. 2001. Human monoclonal antibodies against Pseudomonas aeruginosa lipopolysaccharide derived from transgenic mice containing megabase human immunoglobulin loci are opsonic and protective against fatal Pseudomonas sepsis. Infect. Immun. 69:2223-2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hogan, B., F. Costantini, and E. Lacy. 1986. Manipulating the mouse embryo. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 16.Hopken, U. E., B. Lu, N. P. Gerard, and C. Gerard. 1996. The C5a chemoattractant receptor mediates mucosal defence to infection. Nature 383:86-89. [DOI] [PubMed] [Google Scholar]
- 17.Janeway, C. A., P. Travers, M. Walport, and M. Shlomchik. 2001. Innate immunity, immunobiology, 5th ed. Garland Publishing, New York, N.Y.
- 18.Kohl, J. 2001. Anaphylatoxins and infectious and non-infectious inflammatory diseases. Mol. Immunol. 38:175-187. [DOI] [PubMed] [Google Scholar]
- 19.Larsen, G. L., B. C. Mitchell, T. B. Harper, and P. M. Henson. 1982. The pulmonary response of C5 sufficient and deficient mice to Pseudomonas aeruginosa. Am. Rev. Respir. Dis. 126:306-311. [DOI] [PubMed] [Google Scholar]
- 20.Lee, J., G. Cacalano, T. Camerato, K. Toy, M. W. Moore, and W. I. Wood. 1995. Chemokine binding and activities mediated by the mouse IL-8 receptor. J. Immunol. 155:2158-2164. [PubMed] [Google Scholar]
- 21.LeVine, A. M., K. E. Kurak, M. D. Bruno, J. M. Stark, J. A. Whitsett, and T. R. Korfhagen. 1998. Surfactant protein-A-deficient mice are susceptible to Pseudomonas aeruginosa infection. Am. J. Respir. Cell. Mol. Biol. 19:700-708. [DOI] [PubMed] [Google Scholar]
- 22.Liu, P. V. 1966. The roles of various fractions of Pseudomonas aeruginosa in its pathogenesis. III. Identity of the lethal toxins produced in vitro and in vivo. J. Infect. Dis. 116:481-489. [DOI] [PubMed] [Google Scholar]
- 23.Lyczak, J. B., C. L. Cannon, and G. B. Pier. 2002. Lung infections associated with cystic fibrosis. Clin. Microbiol. Rev. 15:194-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsumoto, M., W. Fukuda, A. Circolo, J. Goellner, J. Strauss-Schoenberger, X. Wang, S. Fujita, T. Hidvegi, D. D. Chaplin, and H. R. Colten. 1997. Abrogation of the alternative complement pathway by targeted deletion of murine factor B. Proc. Natl. Acad. Sci. USA 94:8720-8725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muller-Eberhard, H. J. 1988. Molecular organization and function of the complement system. Annu. Rev. Biochem. 57:321-347. [DOI] [PubMed] [Google Scholar]
- 26.Neth, O., D. L. Jack, A. W. Dodds, H. Holzel, N. J. Klein, and M. W. Turner. 2000. Mannose-binding lectin binds to a range of clinically relevant microorganisms and promotes complement deposition. Infect. Immun. 68:688-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peterson, P. K., Y. Kim, D. Schmeling, M. Lindemann, J. Verhoef, and P. G. Quie. 1978. Complement-mediated phagocytosis of Pseudomonas aeruginosa. J. Lab. Clin. Med. 92:883-894. [PubMed] [Google Scholar]
- 28.Qin, L., W. M. Quinlan, N. A. Doyle, L. Graham, J. E. Sligh, F. Takei, A. L. Beaudet, and C. M. Doerschuk. 1996. The roles of CD11/CD18 and ICAM-1 in acute Pseudomonas aeruginosa- induced pneumonia in mice. J. Immunol. 157:5016-5021. [PubMed] [Google Scholar]
- 29.Rehm, S. R., G. N. Gross, and A. K. Pierce. 1980. Early bacterial clearance from murine lungs. Species-dependent phagocyte response. J. Clin. Investig. 66:194-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rice, A. R., M. A. Hamilton, and A. K. Camper. 2000. Apparent surface associated lag time in growth of primary biofilm cells. Microb. Ecol. 40:8-15. [DOI] [PubMed] [Google Scholar]
- 31.Sengelov, H. 1995. Complement receptors in neutrophils. Crit. Rev. Immunol. 15:107-131. [PubMed] [Google Scholar]
- 32.Shime, N., T. Sawa, J. Fujimoto, K. Faure, L. R. Allmond, T. Karaca, B. L. Swanson, E. G. Spack, and J. P. Wiener-Kronish. 2001. Therapeutic administration of anti-PcrV F(ab′)(2) in sepsis associated with Pseudomonas aeruginosa. J. Immunol. 167:5880-5886. [DOI] [PubMed] [Google Scholar]
- 33.Skerrett, S. J., T. R. Martin, E. Y. Chi, J. J. Peschon, K. M. Mohler, and C. B. Wilson. 1999. Role of the type 1 TNF receptor in lung inflammation after inhalation of endotoxin or Pseudomonas aeruginosa. Am. J. Physiol. 276:L715-L727. [DOI] [PubMed] [Google Scholar]
- 34.Sordelli, D. O., M. C. Cerquetti, A. Morris Hooke, and J. A. Bellanti. 1985. Effect of chemotactins released by Staphylococcus aureus and Pseudomonas aeruginosa on the murine respiratory tract. Infect. Immun. 49:265-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Strieter, R. M., S. L. Kunkel, M. P. Keane, and T. J. Standiford. 1999. Chemokines in lung injury: Thomas A. Neff lecture. Chest 116:103S-110S. [DOI] [PubMed] [Google Scholar]
- 36.Tsai, W. C., R. M. Strieter, B. Mehrad, M. W. Newstead, X. Zeng, and T. J. Standiford. 2000. CXC chemokine receptor CXCR2 is essential for protective innate host response in murine Pseudomonas aeruginosa pneumonia. Infect. Immun. 68:4289-4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van Damme, J., A. Wuyts, G. Froyen, E. Van Coillie, S. Struyf, A. Billiau, P. Proost, J. M. Wang, and G. Opdenakker. 1997. Granulocyte chemotactic protein-2 and related CXC chemokines: from gene regulation to receptor usage. J. Leukoc. Biol. 62:563-569. [DOI] [PubMed] [Google Scholar]
- 38.van Heeckeren, A. M., J. Tscheikuna, R. W. Walenga, M. W. Konstan, P. B. Davis, B. Erokwu, M. A. Haxhiu, and T. W. Ferkol. 2000. Effect of Pseudomonas infection on weight loss, lung mechanics, and cytokines in mice. Am. J. Respir. Crit. Care Med. 161:271-279. [DOI] [PubMed] [Google Scholar]
- 39.Ward, P. A., I. H. Lepow, and L. J. Newman. 1968. Bacterial factors chemotactic for polymorphonuclear leukocytes. Am. J. Pathol. 52:725-736. [PMC free article] [PubMed] [Google Scholar]
- 40.Werfel, T., M. Oppermann, M. Schulze, G. Krieger, M. Weber, and O. Gotze. 1992. Binding of fluorescein-labeled anaphylatoxin C5a to human peripheral blood, spleen, and bone marrow leukocytes. Blood 79:152-160. [PubMed] [Google Scholar]
- 41.Wetsel, R. A., J. Kildsgaard, and D. L. Haviland. 2000. Complement anaphylatoxins (C3a, C4a, C5a) and their receptors (C3aR, C5aR/CD88) as therapeutic targets in inflammation, p. 113. In J. D. Lambris and V. M. Holers (ed.), Contemporary immunology: therapeutic interventions in the complement system. Humana Press, Totowa, N.J.
- 42.Yancey, K. B., J. O'Shea, T. Chused, E. Brown, T. Takahashi, M. M. Frank, and T. J. Lawley. 1985. Human C5a modulates monocyte Fc and C3 receptor expression. J. Immunol. 135:465-470. [PubMed] [Google Scholar]
- 43.Young, L. S., and D. Armstrong. 1972. Human immunity to Pseudomonas aeruginosa. I. In-vitro interaction of bacteria, polymorphonuclear leukocytes, and serum factors. J. Infect. Dis. 126:257-276. [DOI] [PubMed] [Google Scholar]
- 44.Yu, H., S. Z. Nasr, and V. Deretic. 2000. Innate lung defenses and compromised Pseudomonas aeruginosa clearance in the malnourished mouse model of respiratory infections in cystic fibrosis. Infect. Immun. 68:2142-2147. [DOI] [PMC free article] [PubMed] [Google Scholar]