Abstract
Individual Neisseria gonorrhoeae colony opacity-associated (Opa) protein variants can bind up to four different carcinoembryonic antigen-related cellular adhesion molecule (CEACAM) receptors. Most human cells encountered by gonococci express a combination of CEACAM receptors, thereby complicating the elucidation of intracellular signaling pathways triggered by individual receptors. Here, we compare the process of bacterial engulfment by a panel of stably transfected HeLa epithelial cell lines expressing each CEACAM receptor in isolation. CEACAM1 and CEACAM3 each contain proteinaceous transmembrane and cytoplasmic domains; however, the processes of neisserial uptake mediated by these receptors differ with respect to their susceptibilities to both tyrosine kinase inhibitors and the actin microfilament-disrupting agent cytochalasin D. Neisserial uptake mediated by glycosylphosphatidylinositol (GPI)-anchored CEACAM5 and CEACAM6 was not significantly affected by any of a broad spectrum of inhibitors tested. However, cleavage of the GPI anchor by phosphatidylinositol-specific phospholipase C reduced bacterial uptake by HeLa cells expressing CEACAM5, consistent with a single zipper-like mechanism of uptake mediated by this receptor. Regardless of the CEACAM receptor expressed, internalized gonococci were effectively killed by a microtubule-dependent process that required acidification of the bacterium-containing phagosome. Given the phase-variable nature of neisserial Opa proteins, these results indicate that the mechanism of bacterial engulfment and the cellular response to gonococcal infection depend on both the receptor specificities of the neisserial Opa protein variants expressed and the spectrum of CEACAM receptors present on target cells, each of which determines the combination of receptors ultimately engaged.
Despite the existence of effective antibiotic therapy to clear the bacterium, recent estimates indicate that ∼62 million new Neisseria gonorrhoeae infections occur each year (46). Infection likely begins with attachment of the gonococcal type IV pilus to the apical surface of mucosal epithelial cells (26, 43). Pilus retraction (27) then allows the colony opacity-associated (Opa) adhesins to confer a tight association between the bacteria and apically oriented host cellular receptors. These interactions facilitate bacterial entry into and transcellular transcytosis through epithelial cells and entry into the subepithelial compartment (11, 21, 26, 45), presumably allowing the establishment of localized and/or disseminated infection (11, 19).
Neisserial Opa proteins are integral outer membrane proteins that are predicted to span the lipid bilayer eight times with four surface-exposed loops (24). Individual gonococcal strains possess ∼11 different opa alleles, each of which may encode functionally and/or antigenically distinct variants (reviewed in reference 13). The expression of each allele is phase variable due to frequent RecA-independent DNA rearrangements occurring within a pentanucleotide repeat sequence present within the region that encodes the leader peptide of each Opa protein variant (28). These changes cause the downstream reading frame, which encodes the mature Opa protein, to be shifted in or out of register and thereby effectively maintain a heterogeneous population of bacteria that express either zero, one, or multiple Opa protein variants.
One of the 11 Opa protein variants expressed by N. gonorrhoeae MS11, known as Opa50, binds cell surface-associated heparan sulfate proteoglycans (HSPG) (7, 41). This interaction is sufficient to mediate bacterial entry into some cell lines via a pathway involving protein kinase C (PKC) (16), phosphatidylcholine-specific phospholipase C, and acidic sphingomyelinase (17). In other cell lines, efficient entry depends on the ability of Opa50 to bind a combination of an HSPG receptor(s) and the serum-derived extracellular matrix proteins fibronectin and vitronectin (14, 15, 40). In such instances, the extracellular matrix proteins appear to function as a molecular bridge, allowing gonococci to ligate HSPG-containing syndecan receptors with Fn/Vn-specific integrin receptors, thereby triggering their engulfment by host cells.
Most other Opa protein variants of N. gonorrhoeae and the closely related pathogen N. meningitidis instead recognize receptors of the carcinoembryonic antigen-related cellular adhesion molecule (CEACAM; previously termed CD66) family of receptors (reviewed in reference 13). CEACAMs represent a subgroup of the immunoglobulin (Ig) superfamily. Each member consists of an Ig variable region-like amino-terminal domain followed by up to six Ig constant region-like domains (1), and each receptor is anchored to the cell membrane via either a glycosylphosphatidylinositol (GPI) moiety (CEACAM5, CEACAM6, CEACAM7, and CEACAM8) or a proteinaceous transmembrane and cytoplasmic domain (CEACAM1, CEACAM3, and CEACAM4). Individual Opa protein variants bind various combinations of CEACAM1, CEACAM3, CEACAM5, and/or CEACAM6, while no variant has yet been observed to bind other CEACAM family members. In each instance, residues within the CEACAM Ig variable region-like domain mediate the interaction, with up to three different noncontiguous sequences contributing to Opa protein binding (reviewed in reference 4). Binding to any of these receptors appears to be sufficient to mediate bacterial entry, as recombinant Escherichia coli expressing neisserial Opa proteins is efficiently internalized by stably transfected cell lines expressing individual CEACAM receptors (6, 9, 10, 20, 29). However, the mechanism by which each CEACAM mediates neisserial uptake remains poorly characterized.
Engulfment of Opa protein-expressing gonococci by professional phagocytes requires a Src family tyrosine kinase(s) and the GTPase Rac1 (22); however, the contribution of individual CEACAMs to this process remains unclear due to the expression of multiple CEACAM family receptors by both neutrophils and the JOSK-M promonocytic cell line used in that study. Gonococcal uptake mediated by CEACAM3, which is restricted to neutrophils, involves tyrosine residues within the immunoreceptor tyrosine-based activation motif (ITAM) of the receptor (8, 25). These residues are phosphorylated upon binding by CEACAM3-specific Opa proteins, triggering the highly efficient engulfment of N. gonorrhoeae (25). Neisserial uptake mediated by CEACAM3 is not strictly dependent on tyrosine phosphorylation, as a less efficient mechanism of bacterial uptake becomes apparent when tyrosine phosphorylation is inhibited by tyrosine kinase inhibitors or ITAM mutagenesis (25, 34). However, the efficient CEACAM3-mediated engulfment of N. gonorrhoeae requires a Src family kinase(s) (25, 34) and the Rho family GTPases Rac1 and Cdc42 (3, 34), which trigger the assembly of dense actin-containing phagocytic cup-like structures below the bound bacteria (3, 25). The CEACAM3-dependent activation of class I and class III phosphatidylinositol 3-kinases (PI3-Ks) then appears to facilitate bacterial engulfment and maturation of bacterium-containing phagosomes, respectively (5).
The mechanism of bacterial uptake following ligation by receptors other than CEACAM3 has not yet been explored. However, given that CEACAM1 and CEACAM3 have different transmembrane and cytoplasmic domains (i.e., there is no ITAM in the CEACAM1 cytoplasmic domain) and that CEACAM5 and CEACAM6 are GPI anchored, it is likely that distinct processes mediate neisserial uptake following Opa protein binding to each of these receptors. In this study, we used an inhibitor-based approach to directly compare the processes of neisserial uptake by stably transfected HeLa epithelial cell lines constitutively expressing CEACAM1, CEACAM3, CEACAM5, or CEACAM6 (HeLa-CEACAM1, HeLa-CEACAM3, HeLa-CEACAM5, or HeLa-CEACAM6 cells, respectively) in isolation. We found that these closely related receptors provide distinct routes of gonococcal entry.
MATERIALS AND METHODS
Cell lines and bacterial strains.
Stably transfected HeLa cell lines expressing defined recombinant CEACAM1 (BGP), CEACAM3 (CGM1), CEACAM5 (CEA), and CEACAM6 (NCA) proteins were described previously (2, 18, 30). All HeLa cell lines were grown in RPMI 1640 medium with l-glutamine (Invitrogen, Burlington, Ontario, Canada) and 10% heat-inactivated fetal calf serum (Invitrogen). All cells and bacteria were incubated at 37°C in a humidified atmosphere with 5% CO2.
N. gonorrhoeae was grown from frozen stocks on Difco GC agar supplemented with 1% (vol/vol) IsoVitaleX enrichment (BD Bioscience, Mississauga, Ontario, Canada). Isogenic gonococcal strains N302 (pilus-negative Opa− control), N303 (expressing HSPG-specific Opa50), N309 (expressing CEACAM receptor-specific Opa52), and N313 (expressing CEACAM receptor-specific Opa57) were described previously (20, 23) and were generously provided by Thomas Meyer (Max-Planck-Institut für Infektionsbiologie, Berlin, Germany). These derivatives of N. gonorrhoeae MS11 are unable to make pili and contain a deletion of the opaC30 chromosomal locus encoding the only Opa protein variant that mediates HSPG-dependent host cellular invasion by this strain (23). While recombinant Opa protein variants are constitutively expressed, the remaining chromosomally encoded Opa protein variants are phase variable. Therefore, the gonococcal strains were subcultured daily by using a binocular microscope to select colonies that maintained the expression of the recombinant Opa proteins in the absence of the chromosomally encoded Opa protein variants. Confirmation that the expected Opa protein variants were expressed by cultures used for infection experiments was obtained by immunoblot analysis of total bacterial extracts with monoclonal antibody (MAb) 4B12/C11, which recognizes all Opa protein variants described and which was generously provided by Mark Achtman (Max-Planck-Institut für Infektionsbiologie).
Reagents.
Staurosporine, genistein, cytochalasin D, concanamycin A, and phosphatidylinositol-specific phospholipase C (PI-PLC) were obtained from Sigma (Oakville, Ontario, Canada). 4-Amino-5-(4-chlorophenyl)-7-(t-butyl)-pyrazolo(3,4-d)pyrimidine (PP2), nocodazole, and bisindolylmaleimide I were obtained from Calbiochem (La Jolla, Calif.). Antigonococcal polyclonal antibody UTR01 was generated by three subcutaneous immunizations with killed N. gonorrhoeae N302 (Opa−) as previously described (25). MAb D14HD11, which recognizes CEACAM1, CEACAM3, CEACAM5, and CEACAM6, was generously provided by Fritz Grunert (Genovac AG, Freiburg, Germany). Cy5-conjugated secondary antibodies were obtained from Jackson ImmunoResearch Laboratories (Mississauga, Ontario, Canada). Texas red-X-succinimidyl ester (TRXSE), Bodipy FL-conjugated secondary antibodies, and Texas red-conjugated phalloidin were purchased from Molecular Probes (Eugene, Oreg.).
Infection of cell lines.
Except as otherwise indicated, gentamicin assays to quantify viable intracellular and total associated bacteria were performed as previously described (18). For some assays, the HeLa cell lines were pretreated with inhibitors for 30 min at 37°C prior to infection, and the inhibitors were maintained throughout the infection experiment. When nocodazole was used, the cell lines were incubated for 1 h on ice in the presence of this inhibitor prior to infection. For experiments involving PI-PLC, the enzyme was not added prior to infection. Instead, the HeLa cell lines were infected, centrifuged and then immediately washed three times to remove nonadherent bacteria. PI-PLC then was added to selected samples at a final concentration of 0.5 U/well, and the samples were incubated for 30 min at room temperature. The infected samples were incubated at 37°C for 3 h, and viable adherent and intracellular bacteria were quantified by dilution plating according to a standard protocol (18).
Immunofluorescence microscopy to analyze infected samples was conducted with HeLa cells grown on 12-mm acid-washed glass coverslips; cultures showed less than 70% confluence at the time of infection. For some experiments, gonococci were prelabeled with TXRSE dye in phosphate-buffered saline (PBS) (pH 8.5) for 1 h at room temperature. Stained gonococci were washed extensively with RPMI 1640 medium to quench and remove reactive TXRSE and then were used to infect HeLa cells at a multiplicity of infection of 50 bacteria/cell. The samples were centrifuged onto the cell monolayer for 5 min at 67 × g to synchronize infection. For kinetic experiments, nonadherent bacteria were removed by washing the samples immediately following centrifugation. Infection of cells was arrested by washing the samples three times with PBS-Mg-Ca (0.5 mM MgCl2 and 1 mM CaCl2 in PBS) and then fixing the samples for 30 min at room temperature with 3.7% paraformaldehyde in PBS. Protocols for staining cells with fluorescent antibodies were previously described (18, 25). Stained samples were viewed by using a Leica DMIRB/E inverted fluorescence microscope equipped with a Hamamatsu Orca charge-coupled device camera, both of which were controlled by Improvisions OpenLab version 2.2.5 digital imaging software.
Infection of HeLa-CEACAM3 cells to assess tyrosine phosphorylation.
Tyrosine phosphorylation of CEACAM receptors was performed as previously outlined (25). Briefly, cytochalasin D-treated HeLa cell lines were infected with gonococcal strains for 3 h at 37°C and washed with RPMI 1640 medium. Host cell membranes were lysed with PBS-Mg-Ca containing 1% Triton X-100, 20 mM EDTA, 100 μM sodium vanadate, 10 mM H2O2, 10 mM NaF, 5 U of benzonase/ml, 1 μg of pepstatin/ml, 100 μg of phenylmethylsulfonyl fluoride/ml, 10 μg of leupeptin/ml, and 10 μg of aprotinin/ml. Recovered lysates were microcentrifuged at 13,000 × g and recovered supernatants were then immunoprecipitated with either phosphotyrosine-specific MAb 4G10 or CEACAM1-, CEACAM3-, CEACAM5-, and CEACAM6-cross-specific MAb D14HD11. Recovered proteins were assessed by immunoblot analysis (see the figure legends).
pH survival time analyses.
Gonococci were harvested from overnight cultures, and a standard inoculum (final optical density at 550 nm, ≅0.05) was used to inoculate Difco liquid brain heart infusion growth medium supplemented with BBL IsoVitaleX enrichment (BD Biosciences, Mississauga, Ontario) and buffered with citrate-phosphate buffer to pHs of 4 to 7. Cultures then were grown in sidearm flasks with shaking at 37°C. Culture densities were measured at regular time intervals with a Klett reader, and bacterial viability was quantified by dilution plating at various time points. In some experiments, the overnight cultures were grown as liquid cultures before their pHs were reduced; however, similar results were obtained (data not shown).
RESULTS
Kinetics of N. gonorrhoeae engulfment by CEACAM-expressing cell lines.
Previous work established that CEACAM receptor binding correlates with Opa protein-mediated bacterial uptake by both primary and immortalized cell lines (13); however, differences in the efficiency of bacterial uptake are evident when individual CEACAM receptors are compared by using stably transfected cell lines (6, 20, 42). The time course of experiments used in previous studies, including ≤2 h of treatment with gentamicin to kill extracellular associated bacteria, did not allow assessment of the kinetics of uptake by these cell lines.
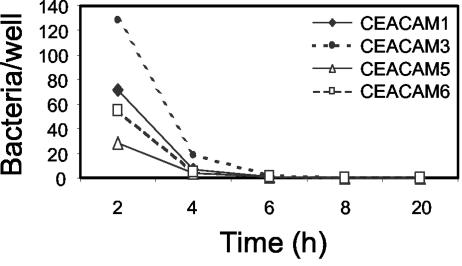
To monitor the rate of bacterial engulfment, we centrifuged N. gonorrhoeae expressing the CEACAM-specific Opa57 protein onto HeLa cell lines expressing individual CEACAM receptors, washed the samples to remove nonadherent bacteria, incubated the samples at 37°C for various times, and then arrested the infection by fixation. Clear differences in bacterial association and the efficiency of bacterial uptake were evident. Immediately following centrifugation, bacterial association ranged from less than 1.9 ± 1.3 (mean and standard deviation) bacteria per cell for HeLa-CEACAM3 cells to 26.3 ± 10.6 bacteria per cell for HeLa-CEACAM5 cells (Fig. 1A). The rate of uptake and the total number of internalized bacteria also differed considerably depending on the CEACAM receptor expressed. Similar numbers of gonococci were internalized by HeLa-CEACAM1, HeLa-CEACAM3, and HeLa-CEACAM6 cells during the 3-h infection, yet bound bacteria were engulfed much more rapidly by CEACAM3-expressing cells (Fig. 1A). The proportion of associated bacteria internalized by HeLa-CEACAM3 cells was also significantly greater than that apparent with the other cell lines (Fig. 1B). Despite the fact that CEACAM5-expressing cells bind significantly more gonococci per cell than do the other cell lines, bacterial internalization by HeLa-CEACAM5 cells was extremely inefficient (Fig. 1). Given that flow cytometry analyses indicated that CEACAM3 and CEACAM5 are expressed at similar levels (unpublished observations), the differences in the kinetics and magnitude of neisserial engulfment appear to be related to the specific receptor expressed rather than being a function of differences in receptor density.
FIG. 1.
Kinetics of neisserial uptake mediated by CEACAM family receptors. Stably transfected HeLa cell lines expressing the indicated CEACAM receptors were infected by centrifuging Opa57-expressing N. gonorrhoeae N313 onto HeLa cell monolayers, washed to remove nonadherent bacteria, incubated at 37°C for the indicated times, and then fixed to arrest the infection process. Bacterial association was quantified by fluorescence microscopy following differential staining of intracellular and extracellular bacteria. (A) Mean intracellular (black bars) and total associated (intracellular plus extracellular; white bars) numbers of bacteria per cell, calculated based on a total of 100 HeLa cells analyzed per sample. Standard deviations indicate variations among 12 to 22 microscopic fields counted per sample, depending on the density of the infected monolayer. Note different y-axis scale for CEACAM5. (B) Proportions of associated bacteria that were intracellular (mean and standard deviation per field).
Receptor phosphorylation during CEACAM-mediated uptake of N. gonorrhoeae.
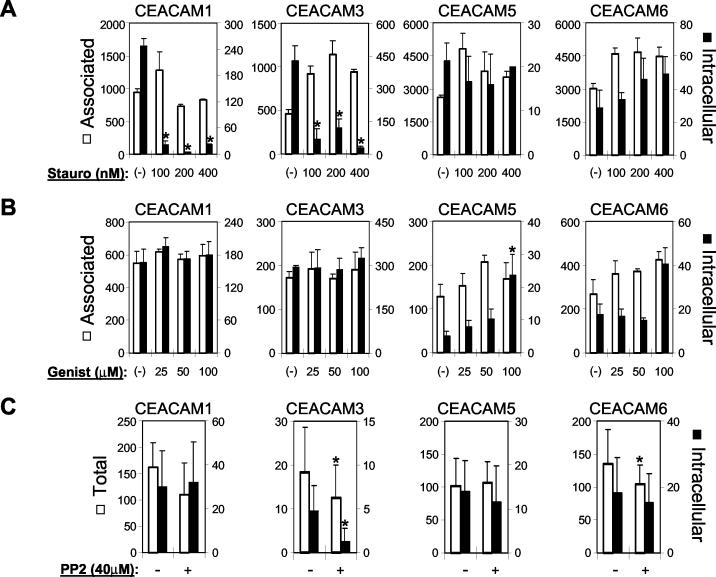
It was recently established that the Src family kinase-dependent phosphorylation of tyrosine residues within the CEACAM3 ITAM facilitates gonococcal uptake by HeLa-CEACAM3 cells (25). The role of tyrosine phosphorylation in uptake by the other CEACAM-expressing cell lines remains uncharacterized. We initially tested whether various agents that inhibit kinase function influence Opa protein-dependent uptake by the HeLa cell lines (18). The broad-spectrum kinase inhibitor staurosporine potently inhibited the internalization of gonococci by HeLa-CEACAM1 and HeLa-CEACAM3 cells but did not reduce gonococcal uptake by the other cell lines (Fig. 2A). Consistent with previous results (25), the Src family kinase-specific inhibitor PP2 reduced CEACAM3-mediated uptake (Fig. 2C). Critically, this effect was apparent when bacterial uptake was monitored by fluorescence microscopy (Fig. 2C) but was observed only rarely when uptake was tested by gentamicin assays (data not shown). Since gentamicin assays quantify the recovery of viable intracellular bacteria rather than the total number of intracellular bacteria that are visualized by microscopy, we interpret these results to indicate that the inhibition of Src family kinases both reduces CEACAM3-mediated bacterial uptake and blocks maturation of the bacterium-containing phagosome, thereby allowing increased survival of any bacteria that do become internalized. Such effects are reminiscent of observations obtained with inhibitors of PI3-Ks (5). PP2 did not significantly affect neisserial uptake by CEACAM1-, CEACAM5-, or CEACAM6-expressing cells, as determined by either microscopy (Fig. 2C) or gentamicin assays (data not shown).
FIG. 2.
Effects of kinase inhibitors on CEACAM receptor-mediated uptake of N. gonorrhoeae. HeLa cell lines stably expressing the indicated CEACAM receptors were infected in the presence of the indicated concentrations of the broad-spectrum serine kinase inhibitor staurosporine (Stauro) (A), the broad-spectrum tyrosine kinase inhibitor genistein (Genist) (B), or the Src family kinase-specific inhibitor PP2 (C). In each experiment, the effects of several inhibitor concentrations were tested by gentamicin assays, and the highest concentrations of inhibitors used were also assessed by differential staining of intracellular and extracellular bacteria for fluorescence microscopy. For staurosporine and genistein, results obtained by microscopy (data not shown) reflected those obtained with the gentamicin assays (A and B). For PP2, the inhibition of neisserial uptake mediated by CEACAM3 was apparent by microscopy (C) but was observed only inconsistently in gentamicin assays (data not shown). For the gentamicin assays (A and B), values indicate 104 (for associated) and 102 (for intracellular) means and standard deviations of triplicate samples and are representative of at least three independent experiments. For microscopy experiments (C), values indicate the mean and standard deviation per cell calculated from three groups of 15 cells and are representative of at least two independent experiments. In each panel, asterisks indicate samples that were significantly different (P < 0.01) from parallel untreated samples.
Somewhat unexpectedly, given the role of a Src family tyrosine kinase(s) in CEACAM3-mediated uptake (25), gonococcal uptake by HeLa-CEACAM3 cells was unaffected by the broad-spectrum tyrosine kinase inhibitor genistein (Fig. 2B). However, this result is consistent with previous observations regarding the internalization of the ITAM-containing B-cell receptor, which also involves Src family kinases but is unaffected by genistein (44). Genistein had no effect on gonococcal uptake by CEACAM1-expressing cells but tended to increase bacterial internalization by CEACAM5- and CEACAM6-expressing cells (Fig. 2B).
Given the effect of the tyrosine kinase inhibitors on gonococcal uptake by the various HeLa cell lines, we next assessed whether Opa protein binding affects tyrosine phosphorylation of the CEACAM receptors. Consistent with previous observations (25), CEACAM3 is specifically phosphorylated in response to infection with bacteria that express CEACAM-specific Opa proteins but not during infection with gonococci expressing the HSPG-specific Opa50 protein (Fig. 3). It was previously observed that the Opa protein-dependent tyrosine phosphorylation of CEACAM3 is inhibited by either staurosporine or PP2 (25). Consistent with its lack of effect on bacterial uptake by HeLa-CEACAM3 cells (Fig. 2B), genistein does not inhibit the tyrosine phosphorylation of CEACAM3 in response to gonococcal infection (data not shown). CEACAM1 was found to contain phosphotyrosine residues in uninfected HeLa-CEACAM1 cells, and the level of phosphorylation was not obviously different after infection with N. gonorrhoeae (Fig. 3). In contrast, no phosphotyrosine residues were apparent in infected or uninfected cell lines expressing GPI-anchored CEACAM5 or CEACAM6 at the times tested.
FIG. 3.
Tyrosine phosphorylation of CEACAM receptors following neisserial infection. HeLa cell lines stably expressing the indicated CEACAM receptors were either infected with N. gonorrhoeae expressing the HSPG-specific Opa50 or CEACAM-specific Opa52 proteins or left uninfected (uninf) as outlined in Materials and Methods. Phosphotyrosine-containing proteins (P-Tyr) and CEACAM receptors were immunoprecipitated from cellular lysates, and recovered CEACAM receptors were detected by immunoblot analysis. Schematic illustrations of individual CEACAM receptors reflect the relative sizes and presence of transmembrane and cytoplasmic domains (CEACAM1 and CEACAM3) or GPI anchors (CEACAM5 and CEACAM6); potential tyrosine phosphorylation sites are indicated by stars. The molecular mass estimates listed are based on the electrophoretic mobilities of proteins isolated from stably transfected HeLa cell lines used throughout this study and are consistent with the sizes of proteins displayed in the lower panels.
Role of cytoskeletal components in CEACAM-mediated uptake.
Cups of polymerized actin are clearly apparent below Opa52-expressing gonococci bound to HeLa-CEACAM3 cells (3, 25), and CEACAM3-mediated uptake of N. gonorrhoeae is highly sensitive to cytochalasin D (3, 25), an agent that blocks actin polymerization. Neisserial uptake mediated by other CEACAM receptors was largely unaffected by cytochalasin D until much (∼16-fold) higher concentrations of this toxin were applied (Fig. 4A), and immunofluorescence microscopy revealed no significant actin-containing structures associated with gonococci bound by CEACAM1, CEACAM5, or CEACAM6 (data not shown).
FIG. 4.
Role of the cytoskeleton in neisserial uptake mediated by CEACAM family receptors. Stably transfected HeLa cell lines expressing various CEACAM receptors were infected with N. gonorrhoeae expressing the CEACAM-specific Opa57 protein in the presence of the indicated concentrations of the actin microfilament-disrupting agent cytochalasin D (CytoD) or the tubulin-depolymerizing agent nocodazole. Internalized bacteria were detected by either gentamicin assays (A and B) or microscopy of samples following differential staining of intracellular (Intra) and extracellular bacteria (C). Cytochalasin D had similar effects on neisserial uptake regardless of whether gentamicin assays (A) or microscopy (data not shown) was used. In contrast, nocodazole increased the recovery of bacteria following gentamicin treatment (B) but tended either to have little effect on or to reduce the number of intracellular bacteria apparent by fluorescence microscopy (C). For gentamicin assays, values indicate 104 (for associated) and 102 (for intracellular) means and standard deviations of triplicate samples and are representative of at least three independent experiments. For microscopy experiments, values indicate the mean and standard deviation calculated from four independent experiments; the proportion of bacteria that were internalized in the absence (−) or presence (+) of nocodazole is listed below. In each panel, asterisks indicate samples that were significantly different (P < 0.01) from parallel untreated samples.
To assess the role of microtubules in neisserial uptake, we tested the effect of the highly specific tubulin-depolymerizing agent nocodazole. When used in gentamicin assays, nocodazole tended to increase the recovery of intracellular (gentamicin-resistant) bacteria in each of the HeLa cell lines (Fig. 4B), whereas it tended to have no effect or to reduce uptake slightly when the internalization of bacteria was quantified by fluorescence microscopy. The nocodazole-dependent reduction in bacterial uptake evident by microscopic analysis of infected HeLa-CEACAM3 cells was significantly different from that in untreated cells (∼40% reduction), while the effect of nocodazole on uptake mediated by the other CEACAM receptors was consistently evident but tended not to be statistically significant (Fig. 4C). Microscopic analysis of infected HeLa cell lines stained with a MAb specific for tubulin did not reveal any obvious alterations in microtubule organization associated with either infection or bacterial binding (data not shown). We interpret the apparent difference between the results of gentamicin assays, which depend on bacterial viability, and the results of microscopic analysis to indicate that nocodazole slightly reduces uptake efficiency but increases the survival of any bacteria that are internalized, regardless of the CEACAM receptor expressed.
Zipper-like engulfment of N. gonorrhoeae mediated by GPI-anchored CEACAM receptors.
A small proportion of N. gonorrhoeae bacteria adhering to primary epithelial cells have been observed to be engulfed through macropinocytosis (47); however, neither the bacterial adhesin nor the cellular receptor used for this process has been identified. Given the typical observation of a tight association between bacteria and phagosomal membranes during CEACAM-mediated entry, we postulated that the sequential recruitment of adjacent GPI-anchored receptors by the high density of Opa proteins on the bacterial surface may instead result in bacterial uptake through a zipper-like process (39). To test this notion, we took advantage of the fact that the GPI anchor of CEACAM5 is cleaved by PI-PLC while that of CEACAM6 is relatively resistant (F. Grunert, personal communication). Opa57-expressing gonococci were centrifuged onto each of the HeLa cell lines, and nonadherent bacteria were removed by washing. The infected cells were treated with PI-PLC before transfer to 37°C. This treatment caused an ∼50% reduction in neisserial entry into HeLa-CEACAM5 cells but did not impede bacterial engulfment by the other CEACAM-expressing cell lines (Fig. 5). In fact, the increased gonococcal binding to HeLa-CEACAM3 cells apparent following PI-PLC treatment suggested that a GPI-anchored protein(s) (unrelated to the CEACAMs) may, in some instances, hamper neisserial association with the short extracellular domain of CEACAM3. Nonetheless, these results indicate that the initial binding of CEACAM5 is not sufficient to trigger neisserial uptake, suggesting rather that continual ligation (“zippering”) of adjacent CEACAM5 is required.
FIG. 5.
Effect of PI-PLC on CEACAM receptor-mediated uptake of N. gonorrhoeae. Opa57-expressing gonococci were centrifuged onto CEACAM-expressing HeLa cell lines to promote binding; the samples were immediately washed to remove nonadherent bacteria. The samples then were treated with PI-PLC, which cleaves the GPI anchor of surface-expressed CEACAM5 without significant effects on other CEACAMs, prior to being incubated at 37°C. Values indicate 104 (for associated) and 102 (for intracellular) means and standard deviations of triplicate samples and are representative of at least three independent experiments. The asterisk indicates a sample that was significantly different (P < 0.06) from parallel untreated samples.
Intracellular survival of N. gonorrhoeae following CEACAM-mediated engulfment.
Throughout our studies, we consistently observed that gentamicin assays greatly underestimated the number of gonococci internalized by the CEACAM-expressing cell lines. Except as indicated above, the pattern of bacterial internalization was similar, but the number of bacteria that survived gentamicin treatment often was up to 100-fold smaller than that apparent by fluorescence microscopy. Such a discrepancy between the two techniques is not evident when the total numbers of associated bacteria are compared, suggesting that extracellular bacteria are effectively recovered but that the vast majority of intracellular bacteria have been killed. Consistent with this notion, the number of intracellular gonococci detected following gentamicin treatment rapidly decreased over time (Fig. 6). This effect was evident when gentamicin was added after 3 h of infection, maintained for 2 h to kill extracellular bacteria, washed away, and then reapplied for the final 2 h before plating. Similar results were obtained when the antibiotic was not washed away (i.e., was applied after 3 h of infection and was maintained until dilution plating; data not shown). Importantly, when the antibiotic was instead added after 3 h of infection, maintained for 2 h, washed away, and not reapplied before dilution plating, the number of viable bacteria recovered increased throughout the remainder of the experiment (data not shown). We interpret these results to indicate a proliferation of extracellular bacteria after removal of the antibiotic. Whether these organisms represent bacteria that survived gentamicin treatment in the extracellular compartment and/or indicate that a small proportion of internalized bacteria were recycled back to the cell surface after gentamicin treatment remains to be determined.
FIG. 6.
Intracellular survival of N. gonorrhoeae following CEACAM receptor-mediated uptake. HeLa cell lines expressing the indicated receptors were infected for 3 h, the samples were treated with gentamicin for 2 h to kill extracellular gonococci, the antibiotic was removed by washing, and then the antibiotic was reapplied for the final 2 h before eukaryotic cell lysis with saponin to recover and quantify viable intracellular bacteria by dilution plating. Similar results were obtained when the antibiotic was applied after 3 h of infection and maintained until saponin treatment (data not shown).
It was observed previously that the viability of gonococci internalized by HeLa-CEACAM3 cells increased when phagosomal acidification was inhibited (5). To test whether the acidification of bacterium-containing phagosomes is also required for N. gonorrhoeae killing following uptake mediated by other CEACAMs, we measured the effect of concanamycin A, an inhibitor of vacuolar (H+) ATPases. Concanamycin A treatment resulted in a dramatic increase in the recovery of intracellular bacteria during gentamicin assays (Fig. 7A) but had no obvious effect on bacterial engulfment evident by fluorescence microscopy (data not shown). These results are consistent with active killing of intracellular gonococci by transfected HeLa cells and could have resulted from a direct effect of an acidic pH on gonococcal viability or susceptibility to degradative enzymes active under acidic conditions. To determine the sensitivity of N. gonorrhoeae strains used in this study to low pHs, Opa57-expressing and Opa− strains were cultured in growth media buffered to various pHs. As shown in Fig. 7B, growth was not evident at pH 6 or below, indicating extreme sensitivity of these strains to acidic conditions. Together, these results indicate that phagosomal acidification is sufficient to kill internalized gonococci.
FIG. 7.
Influence of phagosomal acidification on the intracellular survival of N. gonorrhoeae. (A) Stably transfected HeLa cell lines expressing the indicated CEACAM receptors were infected in the presence or in the absence of concanamycin A (conA), which prevents phagosomal acidification by inhibiting vacuolar (H+) ATPases. After 3 h of infection, viable associated and intracellular bacteria were recovered by gentamicin assays. Values indicate the means and standard deviations of triplicate samples; the values obtained for each cell line in the absence of concanamycin A are shown as 100% recovery of total associated or intracellular bacteria. Results are representative of at least three independent experiments. Concanamycin A treatment had no obvious effect on the rate of neisserial uptake, as observed by differential staining of intracellular and extracellular bacteria for fluorescence microscopy (data not shown); these results indicate that the increased recovery of intracellular bacteria in gentamicin assays reflects the increased survival of internalized gonococci. Asterisks indicate samples that were significantly different (P < 0.01) from parallel untreated samples. (B) Growth of N. gonorrhoeae in culture media adjusted to the indicated pHs. At the indicated times, samples were removed and bacterial culture densities were assessed by dilution plating. The results presented are those obtained with Opa57-expressing N. gonorrhoeae N313; however, no significant difference was apparent when Opa− N. gonorrhoeae N302 or Opa52-expressing N. gonorrhoeae N309 was used in parallel (data not shown). Similar results were obtained when neisserial culture density was monitored by absorbance rather than dilution plating (data not shown).
DISCUSSION
Distinct combinations of CEACAM receptors are expressed by various cells and tissues exposed to Neisseria spp. during infection of humans. For example, of the CEACAM receptors recognized by Opa proteins, epithelial cells express CEACAM1, CEACAM5, and CEACAM6, granulocytes express CEACAM1, CEACAM3, and CEACAM6, while lymphocytes, monocytes/macrophage, and endothelial cells express only CEACAM1. The levels of expression of these receptors may also vary dramatically depending on cellular differentiation and/or activation state. Since individual Opa protein variants can bind different combinations of CEACAM receptors (reviewed in reference 13), the cellular response to infection is dependent on both the CEACAM(s) present and the Opa protein variant(s) expressed. The fact that most cell lines coexpress multiple different CEACAM receptors complicates the attribution of bacterial uptake and/or downstream events to any one of these proteins. This situation has prompted the use of stably transfected epithelial cell lines expressing each receptor in isolation and recombinant gonococcal strains expressing defined Opa protein variants in order to characterize Opa protein-CEACAM receptor interactions.
Here, we have used HeLa epithelial cells stably expressing single CEACAM receptors to reveal clear differences in the mechanisms of bacterial uptake mediated by these receptors; Fig. 8 shows a schematic illustration of known effectors. Consistent with previous observations (25), CEACAM3 binding triggers downstream events highly reminiscent of those seen in response to ligation of the ITAM-containing Ig Fc domain-specific receptors (e.g., FcγRIIA [12]). Specifically, CEACAM3 binding causes Src family kinase-dependent phosphorylation of tyrosine residues within the cytoplasmic ITAM of the receptor (25, 34); this event appears to allow the recruitment of phospholipase Cγ and Syk tyrosine kinase (8, 25), the localized accumulation of activated class I PI3-K (5), and the Rac- and Cdc42-dependent reorganization of actin microfilaments (3, 25, 34). Together, these events lead to the rapid engulfment of CEACAM3-bound bacteria by a process reminiscent of traditional phagocytosis by the Ig Fc domain-specific receptors (25).
FIG. 8.
Schematic model summarizing CEACAM receptor-mediated uptake by transfected HeLa cell lines. Events apparent during the engulfment of N. gonorrhoeae expressing CEACAM-specific Opa proteins by HeLa-CEACAM1, HeLa-CEACAM3, HeLa-CEACAM5, and HeLa-CEACAM6 cells are described in Discussion. Broken arrows indicate that a Syk tyrosine kinase is involved in CEACAM3 receptor-mediated uptake of N. gonorrhoeae by CEACAM3-expressing DT40 B cells (8) and, presumably, neutrophils, but is not expressed by HeLa cells (5). Note that CEACAM1 and CEACAM3 possess transmembrane and cytoplasmic domains containing tyrosine residues that are phosphorylated (indicated by “-P” adjacent to the receptor), either constitutively (CEACAM1) or in response to neisserial binding (CEACAM3), while CEACAM5 and CEACAM6 are anchored via GPI moieties. Once engulfed, maturation of the gonococcus-containing phagosome appears to be similar in the HeLa cell lines. PI, phosphatidylinositol; PLCγ, phospholipase Cγ.
Neisserial uptake by CEACAM1, which also has proteinaceous transmembrane and cytoplasmic domains, clearly occurs through a different process. CEACAM1 has been observed to associate with actin in some systems (33, 35); however, we observed no actin recruitment by bacteria adhering to HeLa-CEACAM1 cells, and the potent active depolymerizing agent cytochalasin D had little impact on neisserial entry into these cells. CEACAM1 is tyrosine phosphorylated (38; this study); however, we observed no obvious change in tyrosine phosphorylation levels coincident with bacterial binding at the times tested here, and gonococcal uptake was not affected by tyrosine kinase inhibitors. We did find that the broad-spectrum serine/threonine kinase inhibitor staurosporine effectively blocked neisserial uptake by HeLa-CEACAM1 cells. PKC and protein kinase A have both been reported to phosphorylate serine residues within the CEACAM1 cytoplasmic domain (31, 37), and PKC activity is required for CEACAM1-dependent bile transport (37), making it tempting to speculate that these kinases may also affect neisserial uptake by HeLa-CEACAM1 cells.
Despite the fact that CEACAM5 and CEACAM6 are anchored to the membrane via GPI moieties, the expression of CEACAM5 or CEACAM6 confers profound effects on mammalian cells. Homotypic binding of CEACAM5 or CEACAM6 by adjacent cells blocks myoblastic, adipogenic, and neurogenic differentiation of cells in vitro (36) and inhibits the normal apoptotic death (anoikis) of cells that lose anchorage to the substrate (32). Remarkably, the ability of these proteins to inhibit cellular differentiation is conferred by their specific GPI anchor, as protein or GPI anchors derived from other receptor proteins cannot replace this effect (36). While the internalization of CEACAM5 and/or CEACAM6 has not been described in these other systems, Opa protein binding to either of these receptors confers neisserial engulfment. Consistent with the putative association between GPI-anchored proteins and cholesterol- and sphingolipid-rich lipid rafts, ligation of CEACAM5 expressed by transfected rat basophilic leukemia cells triggers Src family kinase-dependent tyrosine phosphorylation of cellular proteins (36). If similar effects occur in HeLa-CEACAM5 cells, then the kinase activity is not required for neisserial uptake by these cells, as the Src family kinase-specific inhibitor PP2 did not influence bacterial entry. It is interesting in this regard that the broad-spectrum tyrosine kinase inhibitor genistein consistently increased the internalization of gonococci by both CEACAM5- and CEACAM6-expressing HeLa cells, suggesting that some phosphorylation events actually impede neisserial engulfment by these receptors. However, we observed that PI-PLC-dependent cleavage of the CEACAM5 GPI anchor after neisserial binding inhibits gonococcal uptake by HeLa-CEACAM5 cells. This finding is consistent with the progressive recruitment of adjacent receptors eventually causing the bacterium to become fully enveloped by the host cell membrane through a zipper-like process (39).
Different HeLa cell lines internalize gonococci through different processes, yet we did not observe obvious differences in the processing of intracellular bacteria. In each instance, the bacterium-containing phagosomes quickly acquired the late endosome-early lysosome-associated membrane protein LAMP-2 (unpublished observations), and the engulfed gonococci were effectively killed. An important observation concerns the fact that apparently contradictory results were obtained when the effects of some inhibitors were assessed by standard gentamicin assays versus microscopy techniques. For example, nocodazole, which depolymerizes microtubules, significantly increased the recovery of viable intracellular bacteria in gentamicin assays but tended to reduce bacterial uptake when assessed by fluorescence microscopy. We interpret these results to mean that microtubules are not essential for bacterial uptake but have important roles in the maturation of gonococcus-containing phagosomes and, ultimately, in the killing of the engulfed bacteria.
Inhibitors of PI3-Ks block phagosomal maturation and increase the survival of gonococci engulfed by HeLa-CEACAM1 and HeLa-CEACAM3 cells (5). The PI3-K inhibitors wortmannin and LY294002 also increased our recovery of viable intracellular bacteria following infection of HeLa-CEACAM5 and HeLa-CEACAM6 cells (unpublished observations), suggesting that PI3-Ks also facilitate the maturation of gonococcus-containing phagosomes in these cell lines. Following gonococcal engulfment by HeLa-CEACAM3 cells, the bacterium-containing phagosomes attain an average pH of 5.85 (5). This is clearly sufficient to kill the strains used here, as indicated by our inability to recover gonococci cultured at pH 6 or below. Indeed, phagosomal acidification appears to be necessary for gonococcal killing by each of the transfected HeLa cell lines, as concanamycin A, which inhibits vacuolar (H+) ATPases, significantly (four- to sixfold) increased the recovery of viable intracellular gonococci regardless of the CEACAM receptor expressed.
While the studies described here were performed with model cell lines to allow direct comparison of processes involved in uptake mediated by individual CEACAMs, it is important to consider that different effects may be apparent in different cell types. For example, it was previously observed that N. gonorrhoeae expressing CEACAM-specific Opa proteins effectively transcytoses across polarized monolayers of the T84 colonic carcinoma cell line, which expresses CEACAM1, CEACAM5, and CEACAM6 (45). Since these bacteria pass through (not between) these cells, at least some bacteria clearly survive in the intracellular niche for the duration of transit. Whether the trafficking and/or processing of bacterium-containing phagosomes within these cells are different from those observed in nonpolarized cell systems, such as those used here, remains to be determined. However, it is evident from this study that different processes are involved in neisserial uptake mediated by different CEACAM receptors. It also seems likely that the host cellular response to infection is affected by each of the various intracellular signals that emanate from Opa protein binding to these receptors. Considering that a population of gonococci contains a mixture of bacteria expressing different combinations of Opa protein variants and that each Opa protein variant may bind one or more CEACAM and/or HSPG receptors, it becomes clear that the phase variation of Opa proteins affords these pathogens a staggering array of different schemes by which to confront and persist within the human host.
Acknowledgments
This work was supported by Canadian Institutes for Health Research grant MOP-15499. S.D.G.-O. is supported by a new investigator award from the Canadian Institutes of Health Research.
We thank Christoph Dehio and Thomas F. Meyer for valuable discussions during the early stages of this work. We are also grateful to the members of our research group and to Keith Ireton for constructive comments throughout this study and to Ian Boulton for critical reading of the manuscript.
Editor: B. B. Finlay
REFERENCES
- 1.Beauchemin, N., P. Draber, G. Dveksler, P. Gold, S. Gray-Owen, F. Grunert, S. Hammarstrom, K. V. Holmes, A. Karlsson, M. Kuroki, S.-H. Lin, L. Lucka, S. M. Najjar, M. Neumaier, B. Obrink, J. E. Shively, K. M. Skubitz, C. P. Stanners, P. Thomas, J. A. Thompson, M. Virji, S. von Kleist, C. Wagener, S. Watt, and W. Zimmermann. 1999. Redefined nomenclature for members of the carcinoembryonic antigen family. Exp. Cell Res. 252:243-249. [DOI] [PubMed] [Google Scholar]
- 2.Berling, B., F. Kolbinger, F. Grunert, J. A. Thompson, F. Brombacher, F. Buchegger, S. von Kleist, and W. Zimmermann. 1990. Cloning of a carcinoembryonic antigen gene family member expressed in leukocyte of chronic myeoid leukemia patients and bone marrow. Cancer Res. 50:6534-6539. [PubMed] [Google Scholar]
- 3.Billker, O., A. Popp, V. Brinkmann, G. Wenig, J. Schneider, E. Caron, and T. F. Meyer. 2002. Distinct mechanisms of internalization of Neisseria gonorrhoeae by members of the CEACAM receptor family involving Rac1- and Cdc42-dependent and -independent pathways. EMBO J. 21:560-571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Billker, O., A. Popp, S. D. Gray-Owen, and T. F. Meyer. 2000. The structural basis of CEACAM-receptor targeting by neisserial Opa proteins. Trends Microbiol. 8:258-260. [DOI] [PubMed] [Google Scholar]
- 5.Booth, J. W., D. Telio, E. H. Liao, S. E. McCaw, T. Matsuo, S. Grinstein, and S. D. Gray-Owen. 2003. Phosphatidylinositol 3-kinases in CEACAM-mediated internalization of Neisseria gonorrhoeae. J. Biol. Chem. 278:14037-14045. [DOI] [PubMed] [Google Scholar]
- 6.Bos, M. P., F. Grunert, and R. J. Belland. 1997. Differential recognition of members of the carcinoembryonic antigen family by Opa variants of Neisseria gonorrhoeae. Infect. Immun. 65:2353-2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen, T., R. Belland, J. Wilson, and J. Swanson. 1995. Adherence of pilus− Opa+ gonococci to epithelial cells in vitro involves heparan sulfate. J. Exp. Med. 182:511-517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen, T., S. Bolland, I. Chen, J. Parker, M. Pantelic, F. Grunert, and W. Zimmermann. 2001. The CGM1a (CEACAM3/CD66d)-mediated phagocytic pathway of Neisseria gonorrhoeae expressing opacity proteins is also the pathway to cell death. J. Biol. Chem. 276:17413-17419. [DOI] [PubMed] [Google Scholar]
- 9.Chen, T., and E. C. Gotschlich. 1996. CGM1a antigen of neutrophils, a receptor of gonococcal opacity proteins. Proc. Natl. Acad. Sci. USA 93:14851-14856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen, T., F. Grunert, A. Medina-Marino, and E. C. Gotschlich. 1997. Several carcinoembryonic antigens (CD66) serve as receptors for gonococcal opacity proteins. J. Exp. Med. 185:1557-1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cooper, M. D., Z. A. McGee, M. H. Mulks, J. M. Koomey, and T. L. Hindman. 1984. Attachment to and invasion of human fallopian tube mucosa by an IgA1 protease-deficient mutant of Neisseria gonorrhoeae and its wild-type parent. J. Infect. Dis. 150:737-744. [DOI] [PubMed] [Google Scholar]
- 12.Daeron, M. 1997. Fc receptor biology. Annu. Rev. Immunol. 15:203-234. [DOI] [PubMed] [Google Scholar]
- 13.Dehio, C., S. D. Gray-Owen, and T. F. Meyer. 1998. The role of neisserial Opa proteins in interactions with host cells. Trends Microbiol. 6:489-495. [DOI] [PubMed] [Google Scholar]
- 14.Dehio, M., O. G. Gomez-Duarte, C. Dehio, and T. F. Meyer. 1998. Vitronectin-dependent invasion of epithelial cells by Neisseria gonorrhoeae involves αv integrin receptors. FEBS Lett. 424:84-88. [DOI] [PubMed] [Google Scholar]
- 15.Duensing, T. D., and J. P. van Putten. 1997. Vitronectin mediates internalization of Neisseria gonorrhoeae by Chinese hamster ovary cells. Infect. Immun. 65:964-970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Freissler, E., A. Meyer auf der Heyde, G. David, T. F. Meyer, and C. Dehio. 2000. Syndecan-1 and syndecan-4 can mediate the invasion of OpaHSPG-expressing Neisseria gonorrhoeae into epithelial cells. Cell. Microbiol. 2:69-82. [DOI] [PubMed] [Google Scholar]
- 17.Grassme, H., E. Gulbins, B. Brenner, K. Ferlinz, K. Sandhoff, K. Harzer, F. Lang, and T. F. Meyer. 1997. Acidic sphingomyelinase mediates entry of N. gonorrhoeae into nonphagocytic cells. Cell 91:605-615. [DOI] [PubMed] [Google Scholar]
- 18.Gray-Owen, S. D., C. Dehio, A. Haude, F. Grunert, and T. F. Meyer. 1997. CD66 carcinoembryonic antigens mediate interactions between Opa-expressing Neisseria gonorrhoeae and human polymorphonuclear phagocytes. EMBO J. 16:3435-3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gray-Owen, S. D., C. Dehio, T. Rudel, M. Naumann, and T. F. Meyer. 2001. Neisseria, p. 559-618. In E. A. Groisman (ed.), Principles of bacterial pathogenesis. Academic Press, Inc., San Diego, Calif.
- 20.Gray-Owen, S. D., D. R. Lorenzen, A. Haude, T. F. Meyer, and C. Dehio. 1997. Differential Opa specificities for CD66 receptors influence tissue interactions and cellular response to Neisseria gonorrhoeae. Mol. Microbiol. 26:971-980. [DOI] [PubMed] [Google Scholar]
- 21.Harkness, A. H. 1948. The pathology of gonorrhea. Br. J. Vener. Dis. 24:137-147. [PMC free article] [PubMed] [Google Scholar]
- 22.Hauck, C. R., T. F. Meyer, F. Lang, and E. Gulbins. 1998. CD66-mediated phagocytosis of Opa52 Neisseria gonorrhoeae requires a Src-like tyrosine kinase- and Rac1-dependent signalling pathway. EMBO J. 17:443-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kupsch, E.-M., B. Knepper, T. Kuroki, I. Heuer, and T. F. Meyer. 1993. Variable opacity (Opa) outer membrane proteins account for the cell tropisms displayed by Neisseria gonorrhoeae for human leukocytes and epithelial cells. EMBO J. 12:641-650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malorny, B., G. Morelli, B. Kusecek, J. Kolberg, and M. Achtman. 1998. Sequence diversity, predicted two-dimensional protein structure, and epitope mapping of neisserial Opa proteins. J. Bacteriol. 180:1323-1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCaw, S. E., J. Schneider, E. H. Liao, W. Zimmermann, and S. D. Gray-Owen. 2003. Immunoreceptor tyrosine-based activation motif (ITAM) phosphorylation during engulfment of Neisseria gonorrhoeae by the neutrophil-restricted CEACAM3 (CD66d) receptor. Mol. Microbiol. 49:623-637. [DOI] [PubMed] [Google Scholar]
- 26.Merz, A. J., D. B. Rifenbery, C. G. Arvidson, and M. So. 1996. Traversal of a polarized epithelium by pathogenic Neisseriae: facilitation by type IV pili and maintenance of epithelial barrier function. Mol. Med. 2:745-754. [PMC free article] [PubMed] [Google Scholar]
- 27.Merz, A. J., M. So, and M. P. Sheetz. 2000. Pilus retraction powers bacterial twitching motility. Nature 47:98-102. [DOI] [PubMed] [Google Scholar]
- 28.Meyer, T. F., C. P. Gibbs, and R. Haas. 1990. Variation and control of protein expression in Neisseria. Annu. Rev. Microbiol. 44:451-477. [DOI] [PubMed] [Google Scholar]
- 29.Muenzner, P., C. Dehio, T. Fujiwara, M. Achtman, T. F. Meyer, and S. D. Gray-Owen. 2000. Carcinoembryonic antigen family receptor specificity of Neisseria meningitidis Opa variants influences adherence to and invasion of proinflammatory cytokine-activated endothelial cells. Infect. Immun. 68:3601-3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nagel, G., F. Grunert, T. W. Kuijpers, S. M. Watt, J. Thompson, and W. Zimmermann. 1993. Genomic organization, splice variants and expression of CGM1, a CD66-related member of the carcinoembryonic antigen gene family. Eur. J. Biochem. 214:27-35. [DOI] [PubMed] [Google Scholar]
- 31.Najjar, S. M., N. Philippe, Y. Suzuki, G. A. Ignacio, P. Formisano, D. Accili, and S. I. Taylor. 1995. Insulin-stimulated phosphorylation of recombinant pp120/HA4, an endogenous substrate of the insulin receptor tyrosine kinase. Biochemistry 34:9341-9349. [DOI] [PubMed] [Google Scholar]
- 32.Ordonez, C., R. A. Screaton, C. Ilantzis, and C. P. Stanners. 2000. Human carcinoembryonic antigen functions as a general inhibitor of anoikis. Cancer Res. 60:3419-3424. [PubMed] [Google Scholar]
- 33.Sadekova, S., N. Lamarche-Vane, X. Li, and N. Beauchemin. 2000. The CEACAM1-L glycoprotein associates with the actin cytoskeleton and localizes to cell-cell contact through activation of Rho-like GTPases. Mol. Biol. Cell 11:65-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schmitter, T., F. Agerer, L. Peterson, P. Muenzner, and C. R. Hauck. 2004. Granulocyte CEACAM3 is a phagocytic receptor of the innate immune system that mediates recognition and elimination of human-specific pathogens. J. Exp. Med. 199:35-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schumann, D., C. J. Chen, B. Kaplan, and J. E. Shively. 2001. Carcinoembryonic antigen cell adhesion molecule 1 directly associates with cytoskeleton proteins actin and tropomyosin. J. Biol. Chem. 276:47421-47433. [DOI] [PubMed] [Google Scholar]
- 36.Screaton, R. A., L. DeMarte, P. Draber, and C. P. Stanners. 2000. The specificity for the differentiation blocking activity of carcinoembryonic antigen resides in its glycophosphatidyl-inositol anchor. J. Cell Biol. 150:613-626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sippel, C. J., R. J. Fallon, and D. H. Perlmutter. 1994. Bile acid efflux mediated by the rat liver canalicular bile acid transport/ecto-ATPase protein requires serine 503 phosphorylation and is regulated by tyrosine 488 phosphorylation. J. Biol. Chem. 269:19539-19545. [PubMed] [Google Scholar]
- 38.Skubitz, K. M., T. P. Ducker, and S. A. Goueli. 1992. CD66 monoclonal antibodies recognize a phosphotyrosine-containing protein bearing a carcinoembryonic antigen cross-reacting antigen on the surface of human neutrophils. J. Immunol. 148:852-860. [PubMed] [Google Scholar]
- 39.Swanson, J. A., and S. C. Baer. 1995. Phagocytosis by zippers and triggers. Trends Cell Biol. 5:89-93. [DOI] [PubMed] [Google Scholar]
- 40.van Putten, J., T. D. Duensing, and R. L. Cole. 1998. Entry of Opa+ gonococci into HEp-2 cells requires concerted action of glycosaminoglycans, fibronectin and integrin receptors. Mol. Microbiol. 29:369-379. [DOI] [PubMed] [Google Scholar]
- 41.van Putten, J. P., and S. M. Paul. 1995. Binding of syndecan-like cell surface proteoglycan receptors is required for Neisseria gonorrhoeae entry into human mucosal cells. EMBO J. 14:2144-2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Virji, M., D. Evans, A. Hadfield, F. Grunert, A. M. Teixeira, and S. M. Watt. 1999. Critical determinants of host receptor targeting by Neisseria meningitidis and Neisseria gonorrhoeae: identification of Opa adhesiotopes on the N-domain of CD66 molecules. Mol. Microbiol. 34:538-551. [DOI] [PubMed] [Google Scholar]
- 43.Virji, M., and J. E. Heckels. 1984. The role of common and type-specific pilus antigenic domains in adhesion and virulence of gonococci for human epithelial cells. J. Gen. Microbiol. 130:1089-1095. [DOI] [PubMed] [Google Scholar]
- 44.Wagle, N. M., J. H. Kim, and S. K. Pierce. 1998. Signaling through the B cell antigen receptor regulates discrete steps in the antigen processing pathway. Cell. Immunol. 184:1-11. [DOI] [PubMed] [Google Scholar]
- 45.Wang, J., S. D. Gray-Owen, A. Knorre, T. F. Meyer, and C. Dehio. 1998. Opa binding to cellular CD66 receptors mediates the transcellular traversal of Neisseria gonorrhoeae across polarised T84 epithelial cell monolayers. Mol. Microbiol. 30:657-671. [DOI] [PubMed] [Google Scholar]
- 46.World Health Organization. 2001. Global prevalence and incidence of selected curable sexually transmitted infections: overview and estimates. World Health Organization, Geneva, Switzerland.
- 47.Zenni, M. K., P. C. Giardina, H. A. Harvey, J. Shao, M. R. Ketterer, D. M. Lubaroff, R. D. Williams, and M. A. Apicella. 2000. Macropinocytosis as a mechanism of entry into primary human urethral epithelial cells by Neisseria gonorrhoeae. Infect. Immun. 68:1696-1699. [DOI] [PMC free article] [PubMed] [Google Scholar]