Abstract
Background
Human laryngeal muscles are composed of fibers that express type I, IIA, and IIX myosin heavy chains (MyHC), but the presence and quantity of atypical myosins such as perinatal, extraocular, IIB, and α (cardiac) remain in question. These characteristics have been determined by biochemical or immunohistologic tissue sampling but with no complementary evidence of gene expression at the molecular level. The distribution of myosin, the main motor protein, in relation to structure-function relationships in this specialized muscle group will be important for understanding laryngeal function in both health and disease.
Objectives
We determined the quantity of MyHC genes expressed in human posterior cricoarytenoid (PCA) and thyroarytenoid (TA) muscle using real-time quantitative reverse-transcriptase polymerase chain reaction in a large number of samples taken from laryngectomy subjects. The PCA muscle was divided into vertical (V) and horizontal (H) portions for analysis.
Results and Conclusions
No extraocular or IIB myosin gene message is present in PCA or TA, but IIB is expressed in human extraocular muscle. Low but detectable amounts of perinatal and α gene message are present in both of the intrinsic laryngeal muscles. In H-and V-PCA, MyHC gene amounts were β greater than IIA greater than IIX, but amounts of fast myosin RNA were greater in V-PCA. In TA, the order was β greater than IIX greater than IIA. The profiles of RNA determined here indicate that, in humans, neither PCA nor TA intrinsic laryngeal muscles express unique very fast-contracting MyHCs but instead may rely on differential synthesis and use of β, IIA, and IIX isoforms to perform their specialized contractile functions.
Keywords: Human laryngeal muscle, myosin heavy chain RNA, quantitative reverse-transcription polymerase chain reaction, extraocular myosin, myosin IIB
INTRODUCTION
The intrinsic laryngeal muscles coordinate vocal fold movements during respiration, deglutition, and phonation. Of this muscle group, the thyroarytenoid (TA) is one of several adductors, whereas the posterior cricoarytenoid muscle (PCA) is the lone abductor of the vocal folds. In humans, the TA muscle is composed of two functional compartments, a medial portion whose fibers intertwine with connective tissue elements of the vocal ligament folds and a lateral portion whose structure more resembles a muscle belly. The medial portion of the TA is sometimes referred to as the “vocalis portion” because it alters vocal fold tension and relaxation during speaking or singing. The lateral portion of the TA is termed the “muscularis portion” because it produces gross movements in the vocal folds.1 The human PCA muscle is composed of distinct horizontal (H) and vertical (V) fan-shaped portions2,3 that, respectively, coordinate rotation and sliding of the arytenoid cartilage for opening the glottis during respiration and vocalization.
Finer descriptions of the functional specialization of intrinsic laryngeal muscles have been largely teleologic, based primarily on animal experiments, the results of which, given the differences in anatomy, innervation, and composition of myosin isoforms, may not directly apply to the condition in humans. One of the best approaches for understanding the function of these muscles has been tissue level investigations of fiber type composition and contractile protein expression found in the different fiber types. Both the TA and PCA contain typical slow (type I) and fast (type IIA and IIX) skeletal muscle fiber types, which may be identified by myofibrillar ATPase histochemical analysis and iinmunohistochemistry using myosin heavy chain (MyHC)-specific antibodies.4,5 The TA is more abundant in type II fibers given the need for rapid contractions that position the vocal folds for initiation and variation of speech and singing. Given the functional diversity of human PCA muscle, the fiber type composition differs significantly among its two principal portions. The H belly, which is more active during inspiration, contains predominately type I fibers, whereas the V belly, which is more active during voice production, has a relatively equal distribution of type I and type II fibers.4 The PCA, TA, and other intrinsic laryngeal muscles belong to a larger subset of skeletal muscle generally termed specialized cranial muscles.5 Cranial muscles develop from populations of mesodermal cells, which are separate from the somatic lateral plate mesoderm that forms muscle of the axial skeleton.6 Physiologic adaptation for performance of highly specialized motor tasks is associated with these developmental differences, as exemplified by the muscle-specific expression of MyHC genes that include the extraocular myosin (MyHC-EO) in muscles associated with eye movement7,8 and the type II masticatory myosin (MyHC-IIM) in jaw-closing muscles.9 Similar to other cranial muscles, laryngeal muscles may express atypical myosins such as perinatal and α-cardiac and contain fibers that co-express myosins. Given the need for rapid contraction, the possibility also exists that MyHC-IIB (the fastest contracting myosin typically found in type II fibers of the limb muscles of smaller mammals) might be expressed in the intrinsic laryngeal muscles.
Evidence of the expression of another MyHC specific to laryngeal muscle fibers was first suggested by biochemical isolation of an additional MyHC isoform.10 In the absence of an MyHC–EO-specific antibody at the time to verify its identity, the protein species in rat laryngeal muscle was preliminarily considered to be an atypical myosin expression and designated type “II-L” or type II laryngeal. Subsequently, molecular techniques have been used to demonstrate expression of the MyHC-EO gene in rabbit laryngeal muscle,11 which strongly suggests that the “II-L” myosin in rat laryngeal muscle is also the MyHC-EO isoform species. However, no MyHC-EO has yet been detected in human intrinsic laryngeal muscles.12 Furthermore, there currently exists no evidence for novel MyHC isoforms that are specific to this muscle group.
Because most studies have used protein-based methods to characterize myosin composition and fiber types in human laryngeal muscles, elucidation of corresponding RNA species is important to more fully demonstrate the extent of MyHC expression at the gene level in these muscles. We have conducted a genetic analysis of muscle myosins using probes for major myosin RNA species that include β, which encodes the (β-cardiac isoform that is predominate in slow type I fibers, and IIA and IIX, which determine the fast isoforms that comprise type II fibers. Additional probes for less abundantly expressed myosins were also used, including α, which specifies the α-cardiac isoform primarily found in “the atrium, perinatal,” which encodes the developmental perinatal (or neonatal) isoform, extraocular, which is usually specific to eye muscle, and IIB, a fast-contracting myosin that is only selectively expressed in humans.
MATERIALS AND METHODS
Subjects and Tissues
Laryngeal muscles were obtained with approval of the university internal review board (IRB# 991280) from 15 patients 45 to 81 years of age (average age, 65 yr) undergoing total laryngectomy. Only subjects with normal vocal fold mobility, assessed prior to surgery, were included. At surgery, right and left PCA and TA muscles that were free of gross pathology were dissected from excised larynges. Availability of full sets of the laryngeal muscles, which included TA and right and left sides of both bellies of the PCA, was limited because of harvesting for pathology. During collection, obtainable PCA muscles were routinely divided into V and H bellies, and TA muscles were sampled from any available area that did not interfere with pathologic evaluation. For this reason, the number of TA samples from the subject group was limited in comparison with PCA samples. Infrahyoid muscle (IFH) was also sampled during laryngectomy and used as a “standard muscle” control. Biopsies of limb vastus medialis and extraocular internal oblique already available from previous work were also used for comparative purposes.
RNA Isolation and Reverse-Transcription Polymerase Chain Reaction
The average weights for the frozen muscle biopsies were 190 ± 27.2 mg for H-PCA, 205 ± 28.7 mg for V-PCA, and 128 ± 31.0 mg for TA. RNA was isolated from the muscle biopsies with TRIzol reagent (Invitrogen, Carlsbad, CA) followed by digestion with 10 U DNase I and heating at 65°C to inactivate enzyme. RNAqueous reagent (Ambion, Inc., Foster City, CA) was added to the sample digests, and RNA was purified according to the manufacturer’s specifications and quantified by absorbance at A260. The average recoveries of total muscle RNA were 278 ng/mg for H-PCA, 272 ng/mg for V-PCA, and 224 ng/mg for TA. Human atrium RNA was obtained from Ambion, Inc. for authentication of α-myosin expression. MyHC RNA was quantified by reverse-transcription polymerase chain reaction (RT-PCR) using Titanium One-Step RT-PCR kits (Clontech Laboratories, Mountain View, CA) that were supplemented with 0.4 μmol/L oligo dT(18 mer), 20 pg/μL random hexamer primers, and SYBR Green (Molecular Probes, Inc., Carlsbad, CA). Oligonucleotide PCR primers for MyHC-β, α, IIA, IIX, IIB, perinatal, and MyHC-EO gene message (Table I) were selected from GenBank mRNA sequences using Oligo 6 software and synthesized by a commercial source (Invitrogen). Qualitative analyses were first performed to determine the presence and general amounts of myosin isoform RNA, including MyHC-EO and IIB, in the laryngeal muscles. Thereafter, myosin RNA from samples was quantified in duplicate, 30 ng aliquots of total muscle RNA using a standard protocol for reverse transcription and PCR in all assays (Table II). Input muscle RNA and amplified complementary DNA were quantified from standard curves in each assay using QuantumRNA (Ambion, Inc.) 18S RNA Universal Standards (315 bp) and a plasmid (323 bp) DNA internal standard. RNA and DNA values were calculated at maximum amplification efficiency, with a slope within the range of −3.30 to −3.32 plotted for each standard curve. With use of analyses of variance (ANOVA), no statistical significance was detected for values of 18S RNA between samples taken from the different muscle types used in RT-PCR assays (P > .734). Amplification products were routinely verified by electrophoresis in 3% NuSieve 3:1 agarose gels containing 0.5 μg/mL ethidium bromide. Differences in quantities of myosin message in extracts of the several muscle types were tested for significant mean differences using ANOVA for a between or between-within case design.
TABLE I.
Primers for Reverse-Transcription Polymerase Chain Reaction of Myosin Heavy Chain (MyHC) Genes.
| MyHC | Sense Primer | Antisense Primer | Product Length (bp) |
|---|---|---|---|
| α | 5′-AAGCTGCAACTGAAGGTCAAGG-3′ | 5′-CAGTGTCACTCCTCATCGTGCATT-3′ | 212 |
| β | 5′-AGACGGAGGAGGACAGGAAA-3′ | 5′-AGATCAAGATGTGGCAAAGCTACT-3′ | 270 |
| IIA | 5′-AAGGTCTCCATTTACAAGCTCACG-3′ | 5′-TTGGACACCTGTTCTACAGTCTGG-3′ | 238 |
| IIX | 5′-CTGCAAGCAAAGGTGAAATCCTA-3′ | 5′-CACATTTTGTGCATTTCTTTGGTC-3′ | 243 |
| IIB | 5′-CGGGAGGTTCACACAAAAGTCATA-3′ | 5′-CCTTGATATACA GGACAGTGACAA-3′ | 114 |
| Perinatal | 5′-GAAACATGACCGACGAGTAAAAG-3′ | 5′-CAGGTGTGTTTACTCTGCACTGAT-3′ | 292 |
| Extraocular | 5′-GAGTAACATAGAAAGAACGTGCCG-3′ | 5′-GTTTCTTCTTCCATTTGCCTCTTA-3′ | 243 |
TABLE II.
Protocol for Reverse-Transcription Polymerase Chain Reaction of Myosin Heavy Chains.
| Step | Function | Temperature (°C) | Time |
|---|---|---|---|
| 1 | Reverse transcription | 50 | 60 min |
| 2 | 1° denature | 95 | 5 min |
| 3 | Cycle denature | 94 | 10 sec |
| 4 | Anneal | 62* | 30 sec |
| 5 | Extend | 72 | 1 min |
| 6 | Read plate 1 | 78 | 1 sec |
| 7 | Read plate 2 | 80 | 1 sec |
| 8 | Go to line 3, 35× | — | — |
| 9 | Final extend | 72 | 10 min |
65°C was used for reverse-transcription polymerase chain reaction of myosin heavy chain-IIB.
RESULTS
MyHC Gene Expression in Human PCA and TA
RT-PCR analyses were first performed to qualitatively determine whether RNA for the MyHC-EO isoform is present in human intrinsic laryngeal muscles. Agarose gel electrophoresis of RT-PCR products showed that MyHC-EO was expressed in a control sample of human extraocular muscle but not in vastus medialis or laryngeal PCA and TA muscles (Fig. 1). A similar analysis for the presence of MyHC-IIB message showed amplification of product in human extraocular muscle but no amplification in either compartment of human PCA, TA, and sartorius muscles (Fig. 2).
Fig. 1.
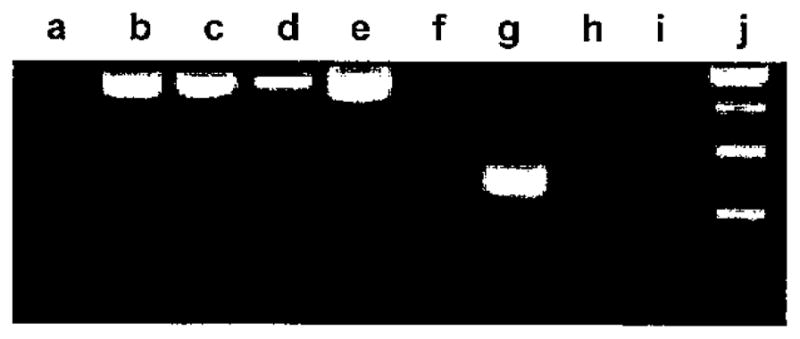
Muscle-specific expression of extraocular myosin heavy chains (MyHC). Human vastus medialis (b, f), extraocular (c, g), posterior cricoarytenoid (d, h), and thyroarytenoid (e, i). Classic 18S rRNA (489 bp; b to e). Extraocular MyHC RNA (243 bp; f to i). DNA standards: 25 bp (a) and 100 bp (j).
Fig. 2.
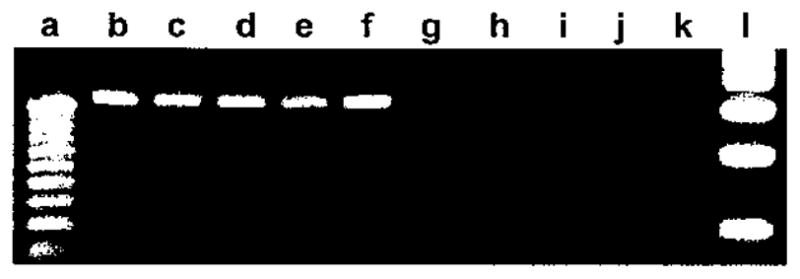
Muscle-specific expression of IIB myosin heavy chains (MyHC). Human extraocular (b, g), horizontal posterior cricoarytenoid (PCA) (c, h), vertical PCA (d, i), thyroarytenoid (e, j), and sartorius (f, k). Universal 18S rRNA (315 bp; b to f). IIB MyHC RNA (114 bp; g to k). DNA standards: 25 bp (a) and 100 bp (I).
Real-time RT-PCR analyses were performed to quantify the differential expression of message for β, IIA, IIX, α, and perinatal myosins in the laryngeal muscles and in the IFH neck muscle. Quantifiable amounts of RNA for all the myosins, including perinatal and α-myosin, were found in most all the muscle samples. Because myosin RNA content between the right and left sides of the H and V bellies of the PCA were not different in subjects, data from those lateral pairs of muscles were pooled for analyses of their MyHC distribution. Perinatal MyHC RNA was present in very low amounts, and its muscle-specific distribution was compared separately from the other myosins.
ANOVA on the data shown in Figure 3 indicated that levels of RNA for the classes of myosins differed significantly across the four muscle types (P = .002), with the highest total expression seen in the PCA muscles and the lowest in the IFH neck muscle. The pattern of RNA quantities was β greater than IIA greater than IIX greater than α in all of the muscles except in the IFH, which expressed equally low amounts of MyHC-IIX and α. Values for the three major isoforms β, IIA, and IIX in the TA muscle were close to one another, resulting in proportionately more fast MyHC expression. Overall, expression of β-MyHC was highest in the two bellies of PCA muscle and significantly greater than in TA (P < .010) and approached significance in IFH (P < .119). MyHC-IIA was most abundant in the V-PCA at levels that were close to significantly greater than IFH (P = .061) and were significant over H-PCA and TA (P < .004 and .019, respectively). Expression of MyHC-IIX was slightly greater in the TA than in either compartment of the PCA muscles and significantly greater than in the IFH (P < .034). α-MyHC RNA was detected at low levels in most samples but undetectable from a few specimens, with no apparent correlation between its presence and muscle type. Levels of α were higher, however, in the all of the laryngeal muscles in comparison with the IFH.
Fig. 3.
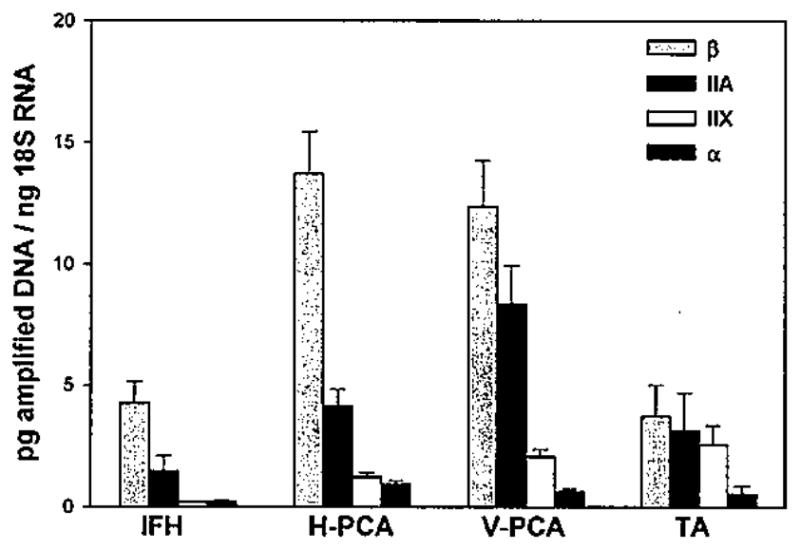
Myosin heavy chain (MyHC) RNA in infrahyold (IFH) and laryngeal muscles. Data are averages ± SE of MyHC-β, IIA, IIX, and α for IFH (n = 2), horizontal posterior cricoarytenoid (H-PCA) (n = 19), vertical (V)-PCA (n = 20), and thyroarytenoid (TA) (n = 6).
Although detected in very low amounts, the presence of perinatal MyHC RNA in all but a few of the muscle biopsies was considered of interest because of the atypical expression of this developmental myosin isoform in adult tissues. Specific quantities of perinatal myosin were all less than 0.1 pg amplified DNA/ng 18S RNA (Fig. 4) in the separate muscle types, with more amounts detected in the PCA and TA than in the IFH, but the differences between laryngeal muscles and the neck were not statistically significant (P = .371).
Fig. 4.
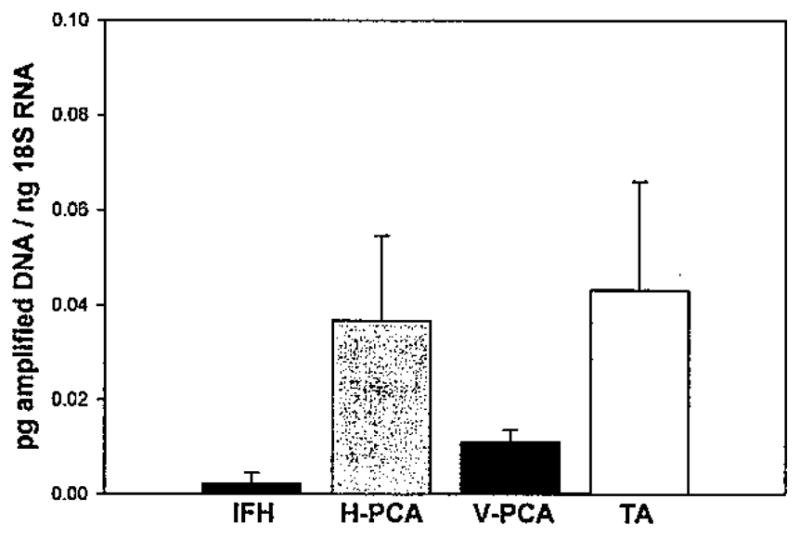
Perinatal MyHC RNA in infrahyoid (IFH) and laryngeal muscles. Data are averages of picograms of amplified DNA/ng 18S RNA ± SE for IFH (n = 2), horizontal posterior cricoarytenoid (H-PCA) (n = 19), vertical (V)-PCA (n = 20), and thyroarytenoid (TA) (n = 6).
When calculated as percentages of total MyHC RNA (Table III), β-MyHC comprised 71.6% of H-PCA, 55.4% of V-PCA, and 46.2% of TA. Fast MyHC gene expression, represented by the sum of RNA for IIA and IIX, accounted for 28.3% of H-PCA, 44.7% of V-PCA, and 53.8% of TA. In comparison, the IFH comprised 72.5% β and 27.5% IIA plus IIX myosin expression, of which only 3.8% was IIX.
DISCUSSION
The phenotypes and physical properties of laryngeal muscle fibers have been subjects of continuing interest and investigation.13 Many studies have described myosin protein isoform expression in human laryngeal muscles using immunohistochemical and biochemical techniques but often with limited sample size.14–16 This report is the first description of myosin gene expression in human laryngeal muscle from a relatively large group of subjects collected by the same laryngologist (C.R.). Understanding normal gene expression and modifications in expression is an important aspect of laryngeal skeletal muscle biology. We isolated muscle RNA to serve as a template for quantification of gene expression using an 18S RNA template as an internal standard between sets of muscle samples and different muscle types. A second set of standards using plasmid DNA was used to quantify amounts of amplified cDNA for the genes of interest. This served as a good approach given the need to determine expression values for five different genes in a large number of samples. The primers selected were specific for 3′ UTR regions of human MyHC genes that overlapped introns to verify that genomic DNA was not amplified during processing. Using these methods, we were able to determine at the molecular level that the extraocular muscle MyHC gene is not expressed in either PCA or TA muscle. Likewise, MyHC-IIB transcription is not found in the intrinsic laryngeal muscles. Notably, however, the IIB gene is expressed in human extraocular muscle similarly to that seen in specimens from rabbit,17,18 cow,19 and dog.20 In the latter two reports, both MyHC-IIB RNA and protein were detected in the vocalis portion of canine TA muscle, whereas no expression of IIB was found in bovine laryngeal muscles. We have previously reported that MyHC-IIB RNA is detectible by in situ hybridization in human masseter muscle, but its translated protein is not seen,21 so that expression of IIB myosin is complex and apparently regulated in a species- and muscle-specific manner that also may use posttranscriptional modification. Quantifiable small amounts of α and perinatal RNA were present in most all laryngeal muscle samples. Expression of perinatal myosin in the laryngeal muscles significantly exceeded that of control strap muscle of the neck. This overall comparative approach will be useful in future studies to determine variations in gene expression that occur in development or during disease or clinical interventions.
Typically, values of MyHC content in laryngeal muscle have been reported as a percent distribution of the total based on arbitrary units from densitometry of bands seen by gel electrophoresis22 or from Western blots.23 The real-time quantification of RNA by RT-PCR used here enables not only the comparison of the relative distribution in muscles but also the specific values for each MyHC expressed. We found the hierarchy of MyHC gene expression to be β greater than IIA greater than IIX in both H-PCA and V-PCA muscles, but the distribution of fast largest and strongest units, containing IIB fibres were recruited last (Burke et al., 1971). All fibre types belonging to the same motor neuron were found to be histochemically similar (i.e. units were composed of all type I, IIA, or IIB fibres and never a mixture of different fibre types; Burke et al., 1973). The same was true for human limb muscles in an interesting study conducted by (Garnett et al., 1979). Even in rapid voluntary contractions in man, fast motor units cannot be preferentially recruited (Desmedt and Godaux, 1977).
Another important finding from the glycogen depletion technique was an understanding of the physical location of motor units in the muscle organ. Motor unit territory is variable in species and specific muscles since these differences are performance related. It is also the case in healthy muscle that fibres of many different motor units occupy the same area of muscle and are intermixed, giving a typical moasic pattern of differing fibre types with histochemical staining (Figure 5). Fiber type clumping, adjacent fibres of the same type and from the same motor unit is indicative of pathological conditions, such as denervation or re-innervation (Karpati & Engel, 1968). Such conditions occur, for example, from sprouting of a single motor neuron, which establishes endplates on regenerating fibres in the same area.
Motor unit characteristics are described in several ways that include: the size of the fibres in the unit; the number of fibres in the unit; the motor unit territory (area and position the unit occupies relative to the total muscle); the force produced by motor unit contraction; and finally the speed of shortening of the fibres in the motor unit. Muscles that require fine, precise motor control usually have many motor units with a small number of fibres in the unit. The best example is the human extra-ocular muscles used in positioning the eye. They are estimated to have 2970 motor units with 9 fibres in each unit (Feinstein et al., 1955). The extra-ocular muscles also have a large representation in the motor cortex (Cushid, 1976). As an example of a relatively large human muscle which requires less precise movement the biceps brachii muscle is estimated to have 3552 motor units with 163 fibres in each unit (Christensen, 1959). The human temporalis is estimated to have 1331 motor units and 936 fibres in each unit and the masseter to have 1452 motor units and 640 fibres in each unit (Carlsöö, 1958). The masseter muscle does have a large representation in the motor cortex (Cushid, 1976) and is known to exhibit very fine movement in some of its functioning, so the size of its motor units were surprising. Since glycogen depletion experiments are not possible in living humans, investigators began applying bite force and electrophysiological measurements to human jaw-closing muscles to further describe motor units. Stalberg and Eriksson (1987) described some of the units in human masseter using these techniques and found most motor unit territory to be relatively small (from 0·6 to 4·5 mm. There were only a few motor units with very large territory in the range of 9·1–12·5 mm. The small motor units were confined to limited areas of masseter, but the few large motor units tended to span almost the whole muscle cross section. Large motor units spanning nearly the whole area of a limb muscle, to the best of our knowledge, have never been found in other studies (Buchthal and Schmall, 1980; Stalberg et al., 1976). It is possible that the small motor units in masseter are used for fine motor functions and the very large motor units to stabilize the entire masseter muscle for balance or when maximal force is necessary. The electrophysiological study by Stalberg and Eriksson (1987), however, is in sharp contrast to the general description given by Carlsöö, (1958), who produced his results from an anatomic study of the innervation ratio. Carlsöö attempted to estimate the total number of α-motor nerves and the total number of skeletal fibres in the entire temporalis and masseter muscles. Stalberg and Eriksson’s (1987) description of masseter motor units contained only those recruited to produce low bite forces and do not represent all of the units in the muscle. When similar EMG techniques were combined with magnetic resonance imaging of 162 motor units in human masseter, most of these units were found positioned between tendons and only a few (10 per cent) crossed tendons (Tonndorf and Hannam, 1994). This confirmed the idea that almost all masseter motor units are located in discrete compartments that may produce movement in a variety of directions, and some units cross tendons to stabilize compartments when necessary.
Animal experiments on motor unit territory and recruitment pattern do not necessarily provide answers for the way in which units work in human jaw-closing muscles given the very odd human phenotypes for masticatory skeletal muscle fibres. The only animal model which comes close to representing human jaw-closing muscle fibres is the rabbit, since its fibres express α-cardiac myosin heavy chain (Sciote and Kentish, 1996) and there is heterogeneous expression of myosin heavy chains in some individual fibres (Bredman et al., 1991). In glycogen depletion experiments, which show the three-dimensional relationship of the total motor unit area to the total masseter muscle area for rabbit (Weijs et al., 1993), the 11 motor units studied were restricted to small portions of masseter with total fibre number ranging from 40 to 424. The surprising finding in nearly half the motor unites investigated, however, was that not all fibres were of the same type. This observation directly contrasts Burke et al.’s (1973) finding in limb muscle that all motor units are homogeneous for fibre type. Those motor units found to be heterogeneous for fibre type contained a combination of fibre types, either α + IIA fibres or IIAB + IIA fibres. This data strongly suggests that in jaw-closing muscles of some mammals, fibres may be heterogeneous for myosin heavy chain isoforms and motor units may be heterogeneous for fibre type. However, the limited number of motor units examined in this study may not represent the whole muscle. Nevertheless, in general the α motor units had the smallest number of fibres and smallest territory, the IIA motor units had relatively much larger fibre number and territory, and the IIB units the largest fibre number and territory. Recruitment order of these unusual motor units was not described, but given anatomical description of unit size and myosin composition it is likely that rabbit masseter units are recruited in an orderly fashion from small to large and from slowest contracting to fastest contracting.
In human masseter, however, the recruitment of motor units has been investigated with electrophysiological techniques. The recruitment pattern is orderly for the size of the unit, but may not be orderly for the speed of shortening of the units. Yemm (1976, 1977) did find orderly recruitment for motor unit size and bite force, but found either no correlation between size and contraction speed, or a reverse correlation between size and contraction speed of some units. Yemm suggested that for contraction speed it is quite possible that faster motor units are recruited before slow motor units. Goldberg and Derfler (1977) produced results similar to that of Yemm in that there was an orderly recruitment of masseter motor units for size, but no correlation between size and speed of contraction. Both studies confirm the well documented results of fibre typing studies done on muscle sections which clearly show that the fast contracting fibres in human masseter are much smaller than the slow contraction type I fibres. It is also not surprising that a reverse correlation in motor unit recruitment for speed of shortening cannot always be found since myosin expression in masseter fibres is often very heterogeneous. Recent studies have used different EMG techniques to investigate human masseter motor unit recruitment (Scutter and Turker, 1998), but very little additional information regarding recruitment order and speed of contraction have been obtained.
The size principle was first proposed for gradually increasing isometric contraction, but muscle contraction in living animals is not always isometric. Even in muscles that can be studied for isometric contraction, the same motor unit may have different recruitment thresholds for flexion versus extension (ter Harr Romeny et al., 1982). Muscles that move bone in various directions do have consistently different motor unit recruitment based on the direction of force production. (Thomas et al., 1978; Desmit, 1977; Schmidt and Thomas, 1981) Such observations have often been termed ‘task specific behavior of motor units’. Ericksson et al., (1984) have described such behaviour in human masseter and English (1985) confirmed that not all the motor units in a muscle are active during contraction. The idea that there are subpopulations of motor neurons that will respond differentially to directional movement is an exception to the size principal for both jaw-closing and limb muscles, but for reasons different than fibre type composition of the muscle. Task specific behaviour is observed in multifasiculated muscles that do perform a variety of patterned movements (Freund, 1983). Such muscles also have very sophisticated afferent input to their motor neuron pools that help modify the excitability of motor neurons controlling the motor units (Kanda et al., 1977; Luescher et al., 1979). In the case of human masseter this afferent information is produced principally by highly complex muscle spindle arrangements (Rowlerson, 1990) and peridontal afferents from the tooth ligaments (Linden, 1990).
Acknowledgments
This work was supported by an RO1 grant to Dr. Sciote, NIDCD 5058-3.
References
- Aidley DJ. The Physiology of Excitable Cells. 4. Cambridge University Press; Cambridge: 1998. [Google Scholar]
- Barany M. ATPase activity of myosin correlated with speed of muscle shortening. Journal of Physiology. 1967;213:455–474. doi: 10.1085/jgp.50.6.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendall JR. Muscles, Molecules, and Movement. Elsevier; New York: 1969. [Google Scholar]
- Bloom W, Fawcett DW. Muscular tissue. In: Raviola E, editor. A Textbook of Histology. Saunders; Philadelphia: 1968. p. 273. [Google Scholar]
- Bottinelli R, Betto R, Schiaffino S, Reggiani C. Unloaded shortening velocity and myosin heavy chain and alkali light chain isoform composition in rat skeletal muscle fibres. Journal of Physiology. 1994;478:341–349. doi: 10.1113/jphysiol.1994.sp020254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brace CL. Occlusion to the anthropological eye. In: McNamara JA, editor. The Biology of Occlusal Development. Center for Human Growth & Development, University of Michigan; Ann Arbor: 1977. pp. 179–209. Monograph 7, Craniofacial Growth Series. [Google Scholar]
- Brace CL, Nagai M. Japanese tooth size: past and present. American Journal of Physical Anthropology. 1982;59:399–411. doi: 10.1002/ajpa.1330590410. [DOI] [PubMed] [Google Scholar]
- Bredman JJ, Wessels A, Weijs WA, Korfage JAM, Soffers CAS, Moorman AFM. Demonstration of ‘cardiac-specific’ myosin heavy chain in masticatory muscles of human and rabbit. Histochemical Journal. 1991;23:160–170. doi: 10.1007/BF01046587. [DOI] [PubMed] [Google Scholar]
- Brooke MH, Kaiser KK. Muscle fibres—how many and what kind? Archives of Neurology. 1970;23:369–379. doi: 10.1001/archneur.1970.00480280083010. [DOI] [PubMed] [Google Scholar]
- Buchthal F, Schmalbruch H. Motor unit of mammalian muscle. Physiological Reviews. 1980;60:90–142. doi: 10.1152/physrev.1980.60.1.90. [DOI] [PubMed] [Google Scholar]
- Burgess SA, Walker ML, White HD, Trinick J. Flexibility within myosin heads revealed by negative stain and single-particle analysis. Journal of Cell Biology. 1997;139:675–681. doi: 10.1083/jcb.139.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke RE, Levine DN, Zajac FE., III Mammalian motor units: physiological-histochemical correlation in three types in cat gastrocnemius. Science. 1971;174:709–712. doi: 10.1126/science.174.4010.709. [DOI] [PubMed] [Google Scholar]
- Burke RE, Levine DN, Tsairis P, Zajac FE., III Physiological types and histochemical profiles in motor units of the cat gastrocnemius. Journal of Physiology. 1973;234:723–748. doi: 10.1113/jphysiol.1973.sp010369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsöö S. Motor units and action potentials in masticatory muscles. An electromyographic study of the form of the action potentials and an anatomic study of the size of the motor units. Acta Morphology Neurology Scandinavia. 1958;2:13–19. [PubMed] [Google Scholar]
- Christensen E. Topography of terminal motor innervation in striated muscles from stillborn infants. American Journal Physical Medicine. 1959;38:65–78. [PubMed] [Google Scholar]
- Cushid JG. Correlative Neuroanatomy and Functional Neurology. Lange; Los Altos: 1976. [Google Scholar]
- DelGaudio JM, Sciote JJ, Carroll WR, Escalmado RM. Atypical myosin heavy chain in rat laryngeal muscle. Annals of Otology, Rhinology & Laryngology. 1995;104:237–245. doi: 10.1177/000348949510400310. [DOI] [PubMed] [Google Scholar]
- DeNardi C, Ausoni S, Moretti P, Gorza L, Velleca M, Buckingham M, Schiaffino S. Type 2X myosin heavy chain is coded by a muscle fibre type-specific and developmentally regulated gene. Journal of Cellular Biology. 1993;123:823–835. doi: 10.1083/jcb.123.4.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmedt JE, Godaux E. Fast motor units are not preferentially activated in rapid voluntary contractions in man. Journal of Physiology. 1977;264:673–694. doi: 10.1038/267717a0. [DOI] [PubMed] [Google Scholar]
- Desmedt JE, Godaux E. Recruitment patterns of single motor units in the human masseter muscle during brisk jaw clenching. Archives of Oral Biology. 1981;24:171–178. doi: 10.1016/0003-9969(79)90066-9. [DOI] [PubMed] [Google Scholar]
- Ebashi S, Nonomura Y, Kohama K, Kitazawa T, Mikawa T. Regulation of muscle contraction by Ca ion. Molecular Biology, Biochemistry & Biophysics. 1980;32:183–194. doi: 10.1007/978-3-642-81503-4_14. [DOI] [PubMed] [Google Scholar]
- Edström L, Kugelberg E. Histochemical composition, distribution of fibres and fatiguability of single motor units. Journal of Neurology, Neurosurgery and Psychiatry. 1968;31:424–433. doi: 10.1136/jnnp.31.5.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg E, Greene LE. The relation of muscle biochemistry to muscle physiology. Annual Review of Physiology. 1980;42:293–309. doi: 10.1146/annurev.ph.42.030180.001453. [DOI] [PubMed] [Google Scholar]
- English AW. Limbs vs. jaws: can they be compared? American Zoology. 1985;25:351–363. [Google Scholar]
- Eriksson PO. Muscle-fibre composition of the human mandibular locomotor system. Enzyme-histochemical and morphological characteristics of functionally different parts. Swedish Dental Journal. 1982;12 (Supplement):1–44. [PubMed] [Google Scholar]
- Eriksson PO, Eriksson A, Ringqvist A, Thornell LE. Histochemical fibre composition of the human digastric muscle. Archives Oral Biology. 1982;27:207–216. doi: 10.1016/0003-9969(82)90054-1. [DOI] [PubMed] [Google Scholar]
- Erriksson PO, Stalberg E, Antoni L. Flexibility in motor-unit firing pattern in the human temporal and masseter muscles related to type of activation and location. Archives Oral Biology. 1984;29:707–712. doi: 10.1016/0003-9969(84)90176-6. [DOI] [PubMed] [Google Scholar]
- Fawcett DW, Bloom W, Raviola E. Muscular tissue. In: Raviola E, editor. A Textbook of Histology. Chapman & Hall; London: 1994. pp. 260–308. [Google Scholar]
- Feinstein B, Lindegard B, Nyman E, Wholfart G. Morphologic studies of motor units in normal human muscles. Acta Anatomica Basel. 1955;23:127–142. doi: 10.1159/000140989. [DOI] [PubMed] [Google Scholar]
- Freund HJ. Motor unit and muscle activity in voluntary motor control. Physiology Reviews. 1983;63:387–435. doi: 10.1152/physrev.1983.63.2.387. [DOI] [PubMed] [Google Scholar]
- Garnett RAF, O’Donovan MJ, Stephens JA, Taylor A. Motor unit organization of human medial gastrocnemius. Journal of Physiology. 1979;287:33–43. doi: 10.1113/jphysiol.1979.sp012643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazith J, Himmelfarb S, Harrington WF. Studies on the subunit structure of myosin. Journal Biological Chemistry. 1970;245:15–22. [PubMed] [Google Scholar]
- Geeves MA. The dynamics of actin and myosin association and the crossbridge model of muscle contraction. Biochemical Journal. 1991;274:1–14. doi: 10.1042/bj2740001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg LJ, Derfler B. Relationship among recruitment order, spike amplitude, and twitch tension of single motor units in human masseter muscle. Journal of Neurophysiology. 1977;40:879–890. doi: 10.1152/jn.1977.40.4.879. [DOI] [PubMed] [Google Scholar]
- Gordon AM, Huxley AF, Julian FJ. The variation in isometric tension with sarcomere length in vertebrate muscle fibres. Journal of Physiology. 1966;184:170–192. doi: 10.1113/jphysiol.1966.sp007909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorza L. Identification of a novel type 2 fibre population in mammalian skeletal muscle by combined use of histochemical myosin ATPase and anti–myison monoclonal antibodies. Journal of Histochemistry and Cytochemistry. 1990;38:257–265. doi: 10.1177/38.2.2137154. [DOI] [PubMed] [Google Scholar]
- Hellam DC, Podolsky RJ. Force measurements in skinned muscle fibres. Journal of Physiology. 1969;200:807–819. doi: 10.1113/jphysiol.1969.sp008723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraiwa T. Histochemical properties of masticatory muscles of growing rat and of matured mammals. Comparative Biochemical Physiology A. 1978;59:231–238. [Google Scholar]
- Hoh JF, Hughes S, Walker ML, Kang LDH, Everett AW. Slow myosin heavy chains in cat jaw and limb muscles are phenotypically distinct: expression of jaw-specific slow myosin phenotype in regenerated and chronically stimulated jaw muscles. Basic and Applied Myology. 1991;1:285–294. [Google Scholar]
- Huxley AF. Muscle structure and theories of contraction. Progress in Biophysics. 1957;7:255–318. [PubMed] [Google Scholar]
- Huxley AF, Niedergerke R. Structural changes in muscle during contraction. Interference microscopy of living muscle fibres. Nature. 1954;173:971–973. doi: 10.1038/173971a0. [DOI] [PubMed] [Google Scholar]
- Huxley AF, Simmons RM. Proposed mechanism of force generation in striated muscle. Nature. 1971;233:533–538. doi: 10.1038/233533a0. [DOI] [PubMed] [Google Scholar]
- Huxley HE. The mechanism of muscular contraction. Science. 1969;164:1356–1365. [PubMed] [Google Scholar]
- Huxley HE, Hanson J. Changes in the cross-striations of muscle during contraction and stretch and their structural interpretation. Nature. 1954;173:973–976. doi: 10.1038/173973a0. [DOI] [PubMed] [Google Scholar]
- Karpati G, Engel WK. ‘Type grouping’ in skeletal muscles after experimental reinnervation. Neurology. 1968;18:447–455. doi: 10.1212/wnl.18.5.447. [DOI] [PubMed] [Google Scholar]
- Kanda K, Burke RE, Warmsley B. Differential control of fast and slow twitch motor units in the decerebrate cat. Experimental Brain Research. 1977;29:57–74. doi: 10.1007/BF00236875. [DOI] [PubMed] [Google Scholar]
- Kennedy JM, Kamel S, Tambone WW, Vrbova G, Zak R. The expression of myosin heavy chain isoforms in normal and hypertrophied chicken slow muscle. Journal of Cell Biology. 1986;103:977–983. doi: 10.1083/jcb.103.3.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larrson L, Moss RL. Maximum velocity of shortening in relation to myosin isoform composition in single fibres from human skeletal muscles. Journal of Physiology. 1993;473:595–614. doi: 10.1113/jphysiol.1993.sp019964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden RWA. Periodontal mechanoreceptors and their functions. In: Taylor A, editor. Neurophysiology of the Jaws and Teeth. Macmillan Press; London: 1990. pp. 52–95. [Google Scholar]
- Lowey S, Risby D. Light chains from fast and slow muscle myosins. Nature. 1971;234:81–85. doi: 10.1038/234081a0. [DOI] [PubMed] [Google Scholar]
- Luescher R, Ruenzel P, Henneman E. How the size of motoneurons determines their susceptibility to discharge. Nature. 1979;282:859–861. doi: 10.1038/282859a0. [DOI] [PubMed] [Google Scholar]
- Lynch GS, Frueh BR, Williams DA. Contractile properties of single skinned fibres from the extraocular muscles, the levator and superior rectus of the rabbit. Journal of Physiology. 1994;475:337–345. doi: 10.1113/jphysiol.1994.sp020074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda N, Kawasaki T, Osawa K, Yamamoto Y, Sumida H, Masuda T, Kumegawa M. Effects of long-term intake of a fine-grained diet on the mouse masseter muscle. Acta Anatomica. 1987;128:326–333. doi: 10.1159/000146360. [DOI] [PubMed] [Google Scholar]
- Mascarello F, Aureli G, Veggetti A. Muscoli masticatori. Determinazione istochimica dei tipi di fibre muscolari in mammiferi. Quaderno Anatomia Pratica. 1979;35:193–211. [Google Scholar]
- Matthews W, Jenkins RR, Gonyea WJ. Myosin isozyme expression in response to stretch-induced hyperthropy in the Japanese quail. Anatomical Record. 1990;228:225–261. doi: 10.1002/ar.1092280304. [DOI] [PubMed] [Google Scholar]
- Miyamoto K, Ishizuka Y, Tanne K. Changes in masseter muscle activity during orthodontic treatment evaluated by a 24-hour EMG system. Angle Orthodontist. 1996;66:223–228. doi: 10.1043/0003-3219(1996)066<0223:CIMMAD>2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- Moss RL, Reiser PJ, Greaser ML, Eddinger TJ. Varied expression of myosin alkali light chains is associated with altered speed of contraction in rabbit fast twitch skeletal muscles. In: Pette D, editor. The Dynamic State of Muscle Fibres. de Gruyter; Berlin: 1990. pp. 355–368. [Google Scholar]
- Perrie WT, Perry SV. An electrophoretic study of the molecular weight components of myosin. Biochemical Journal. 1970;119:31–38. doi: 10.1042/bj1190031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayment I, Holden HM, Whittaker M, Yohn CB, Lorenz M, Holmes KC, Milligan RA. Structure of the actin-myosin complex and its implications for muscle contraction. Science. 1993;261:58–65. doi: 10.1126/science.8316858. [DOI] [PubMed] [Google Scholar]
- Ringqvist M. Histochemical enzyme profiles of fibres in human masseter muscles with special regard to fibres with intermediate myofibrillar ATPase reaction. Journal of the Neurological Sciences. 1973;18:133–141. doi: 10.1016/0022-510x(73)90001-4. [DOI] [PubMed] [Google Scholar]
- Rokx JTM, Van-Willigen JD, Jansen HWB. Muscle fibre types and muscle spindles in the jaw musculature of the rat. Archives of Oral Biology. 1984;29:25–32. doi: 10.1016/0003-9969(84)90038-4. [DOI] [PubMed] [Google Scholar]
- Rosser BWC, Waldbillig DM, Lovo SD, Armstrong JD, Bandman E. Myosin heavy chain expression within the tapered ends of skeletal muscle fibres. Anatomical Record. 1995;242:462–470. doi: 10.1002/ar.1092420404. [DOI] [PubMed] [Google Scholar]
- Rowlerson AM. Acid myosin ATPases in mouse muscle. Journal of Histochemistry and Cytochemistry. 1991;39:383. doi: 10.1177/39.3.1825217. [DOI] [PubMed] [Google Scholar]
- Rowlerson AM. Specialization of mammalian jaw muscles: fibre type compositions and the distribution of muscle spindles. In: Taylor A, editor. Neurophysiology of the Jaws and Teeth. Macmillan Press; London: 1990. pp. 1–51. [Google Scholar]
- Rowlerson AM, Mascarello F, Veggetti A, Carpene E. The fibre type composition of the first brachial arch muscles in carnivora and primates. Journal of Muscle Research and Cell Motility. 1983;4:443–472. doi: 10.1007/BF00711949. [DOI] [PubMed] [Google Scholar]
- Rowlerson AM, Pope B, Murray J, Whalen RG, Weeds AG. A novel myosin present in cat jaw-closing muscles. Journal of Muscle Research and Cell Motility. 1981;2:415–438. [Google Scholar]
- Sant’Ana Pereira JA, Ennion S, Sargeant AJ, Moorman AF, Goldspink G. Comparison of the molecular, antigenic and ATPase determinants of fast myosin heavy chains in rat and human: a single-fibre study. Pflugers Archives – European Journal of Physiology. 1997;435:151–163. doi: 10.1007/s004240050495. [DOI] [PubMed] [Google Scholar]
- Sartore S, Mascarello F, Rowlerson A, Gorza L, Ausoni S, Vianello M, Schiaffino S. Fibre types in extraocular muscles: a new myosin isoform in the fast fibres. Journal of Muscle Research and Cell Motility. 1987;8:161–172. doi: 10.1007/BF01753992. [DOI] [PubMed] [Google Scholar]
- Scapolo PA, Mascarello F, Veggetti A, Carpene E. Caratterizzazione istoichimica ed immunoistochimica del muscolo digastrico. Atti Societa Italiana Scienze Veterinaire. 1981;35:331–332. [Google Scholar]
- Schiaffino S, Reggiani C. Molecular diversity of myofibrillar proteins: gene regulation and functional significance. Physiological Reviews. 1996;76:371–423. doi: 10.1152/physrev.1996.76.2.371. [DOI] [PubMed] [Google Scholar]
- Schmidt EM, Thomas JS. Motor unit recruitment order: modification under volitional control. In: Desmedt JE, editor. Progress in Clinical Neurophysiology. Vol. 9. Karger; Basel: 1981. pp. 145–148. [Google Scholar]
- Sciote JJ, Kentish JC. Unloaded shortening velocities of rabbit masseter muscle fibres expressing skeletal or α-cardiac myosin heavy chains. Journal of Physiology. 1996;492:659–667. doi: 10.1113/jphysiol.1996.sp021335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciote JJ, Rowlerson A. Skeletal fibre types and spindle distribution in limb and jaw muscles of the adult and neonatal opossum, monodelphis domestica. Anatomical Record. 1998;251:548–562. doi: 10.1002/(SICI)1097-0185(199808)251:4<548::AID-AR10>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Sciote JJ, Rowlerson AM, Hopper C, Hunt NP. Fibre type classification and myosin isoforms in the human masseter muscle. Journal of the Neurological Sciences. 1994;126:15–24. doi: 10.1016/0022-510x(94)90089-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scutter SD, Turker KS. Recruitment stability in masseter motor units during isometric voluntary contractions. Muscle and Nerve. 1998;21:1290–1298. doi: 10.1002/(sici)1097-4598(199810)21:10<1290::aid-mus7>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Serratrice G, Pellissier JF, Vignon C, Baret J. The histochemical profile of the human masseter. An autopsy and biopsy study. Journal of the Neurological Sciences. 1976;30:189–200. doi: 10.1016/0022-510x(76)90266-5. [DOI] [PubMed] [Google Scholar]
- Sherrington SC. On the anatomical constitutioin of nerves of skeletal muscles; with remarks on recurrent fibres in the ventral spinal nerve root. Journal of Physiology. 1894;17:211–258. doi: 10.1113/jphysiol.1894.sp000528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soussi-Yanicostas N, Barbet JP, Laurent-Winter C, Barton P, Butler-Browne GS. Transition of myosin isozymes during development of human masseter muscle. Persistence of developmental isoforms during postnatal stage. Development. 1990;108:239–249. doi: 10.1242/dev.108.2.239. [DOI] [PubMed] [Google Scholar]
- Stalberg E, Eriksson PO. A scanning electromyographic study of the topography of human masseter single motor units. Archives of Oral Biology. 1987;32:793–797. doi: 10.1016/0003-9969(87)90005-7. [DOI] [PubMed] [Google Scholar]
- Stalberg E, Schwartz MS, Thiele B, Schiller HH. The normal motor unit in man. A single fibre EMG multielectrode investigation. Journal of the Neurolgical Sciences. 1976;27:291–301. doi: 10.1016/0022-510x(76)90002-2. [DOI] [PubMed] [Google Scholar]
- Suzuki A. A comparative histochemical study of the masseter muscle of the cattle, sheep, swine, dog, guinea pig, and rat. Histochemistry. 1977;51:121–131. doi: 10.1007/BF00567218. [DOI] [PubMed] [Google Scholar]
- ter Haar Romeny BM, Denier van der Gon JJ, Gielen CCAM. Changes in recruitment order of motor units in the human biceps muscle. Experimental Neurology. 1982;78:360–368. doi: 10.1016/0014-4886(82)90054-1. [DOI] [PubMed] [Google Scholar]
- Thomas JS, Schmidt EM, Hambrecht FT. Facility of motor unit control during tasks defined directly in terms of unit behaviors. Experimental Neurology. 1978;59:384–395. doi: 10.1016/0014-4886(78)90230-3. [DOI] [PubMed] [Google Scholar]
- Thornell LE, Billeter R, Eriksson PO, Ringqvist M. Heterogenous distribution of myosin in human masticatory muscle fibres as shown by immunocytochemistry. Archives of Oral Biology. 1984;29:1–6. doi: 10.1016/0003-9969(84)90034-7. [DOI] [PubMed] [Google Scholar]
- Tonndorf ML, Hannam AG. Motor unit territory in relation to tendons in the human masseter muscle. Muscle and Nerve. 1994;17:436–443. doi: 10.1002/mus.880170412. [DOI] [PubMed] [Google Scholar]
- Vignon C, Pellissier JF, Serratrice G. Further histochemical studies on masticatory muscles. Journal of the Neurological Sciences. 1980;45:157–176. doi: 10.1016/0022-510x(80)90163-x. [DOI] [PubMed] [Google Scholar]
- Weijs WA, Jüch PJW, Kwa SHS, Korfage JAM. Motor unit territories and fibre types in rabbit masseter muscle. Journal of Dental Research. 1993;72:1491–1498. doi: 10.1177/00220345930720110601. [DOI] [PubMed] [Google Scholar]
- Weiss A, Leinwand LA. The mammalian myosin heavy chain gene family. Annual Reviews of Cell and Developmental Biology. 1996;12:417–439. doi: 10.1146/annurev.cellbio.12.1.417. [DOI] [PubMed] [Google Scholar]
- Yemm R. The properties of motor units, and length-tension relationships of the muscles. In: Anderson DJ, Matthews B, editors. Mastication. John Wright; Bristol: 1976. pp. 25–30. [Google Scholar]
- Yemm R. The orderly recruitment of motor units of the masseter and temporal muscles during voluntary isometric contraction in man. Journal of Physiology. 1977;265:163–174. doi: 10.1113/jphysiol.1977.sp011710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young WG. Anthropology, tooth wear, and occlusion aborigine. Journal of Dental Research. 1998;77:1860–1863. doi: 10.1177/00220345980770110101. [DOI] [PubMed] [Google Scholar]


