Abstract
Interleukin-10 (IL-10), a cytokine with anti-inflammatory and immunomodulatory functions, regulates the biology of B and T cells. The present review describes the role of IL-10 in normal renal physiology, during acute kidney injury and in the development of chronic renal failure. We further discuss IL-10-induced cellular and molecular pathways and their link to the progression of kidney injury.
Keywords: Transforming growth factor-β, Cystatin C, Interleukin-10 receptor, End-stage renal disease, Mesangial cell proliferation, Epithelial-to-mesenchymal transition, Allograft rejection
Core tip: Interleukin-10 (IL-10) gene expression and IL-10-induced signaling pathways have an important role in the regulation and maintenance of normal renal function. Accumulating evidence further demonstrates that abnormal IL-10 expression whether transient or prolonged, as well as interactions with other growth factors as a response to diverse stimuli is linked to the appearance and progression of a variety of kidney disorders. It has been thus suggested that selective targeting of IL-10 expression and IL-10-related pathways may provide the therapeutic features to many kidney diseases.
IMMUNOLOGICAL PROPERTIES OF INTERLEUKIN-10
The anti-inflammatory Th2 cytokine interleukin-10 (IL-10) was discovered by Fiorentino and colleagues in 1989 for its ability to inhibit the synthesis of IL-2 and interferon-γ (IFN-γ) by Th1 cells[1]. To date, the IL-10 cytokine family includes nine members produced by cells, IL-10, IL-19, IL-20, IL-22, IL-24, IL-26, IL-28A, IL-28B and IL-29, and four viral homologs. IL-10 is produced by several T-cell subpopulations, such as Th2 and T-regulatory cells (Tregs), NK cells, and a variety of cell types, including macrophages, dendritic cells and B cells. In the kidneys, IL-10 is secreted primarily by the mesangial and endothelial cells. The viral homologs of IL-10 can be produced by Epstein-Barr virus, cytomegalovirus, ORF virus and Herpes type 2 viruses[2-4].
The gene encoding human IL-10 (5.1 kb pairs) is located on chromosome 1 and comprises five exons. The IL-10 promoter region contains several single nucleotide polymorphisms (SNPs) that influence IL-10 expression and function[5,6] and are associated with a number of diseases. Indeed, the -1082G/A SNP of the IL-10 gene is more frequent in patients with IgA nephropathy and focal segmental glomerulosclerosis and is associated with a worse prognosis of the disease[7]. The -1082G/A, -819C/T, and -592C/A SNPs of the IL-10 promoter are consistently associated with type 2 diabetes[8], while the -1087G/A, -824C/T, -597C/A SNPs influence the prevalence of vascular-related damage in patients suffering from type 2 diabetes[9] and end-stage renal disease[10]. The -1082 SNP of the IL-10 gene affects the tumor development of renal cell carcinoma and shows a significant correlation with negative prognostic markers, such as tumor size, advanced disease stage and the presence of adenopathy[11,12].
Human IL-10 protein is a 35 kDa homodimer that is assembled from two non-covalently bound monomers. IL-10 acts through a specific receptor complex that consists of two subunits: IL-10R1 and IL-10R2. Binding of IL-10 to its receptor is a multistep process in which IL-10 initially binds to IL-10R1; the IL-10/IL-10R1 complex then binds to IL-10R2. Formation of the IL-10/IL-10R1 complex leads to modification of the cytokine’s conformation, enabling presentation of the binding site to IL-10R2[13]. While the IL-10R1 subunit is highly specific for initiating IL-10 effectors functions, the IL-10R2 subunit might bind other ligands, such as TNF-α and IFN-γ. Moreover, IL-10R2 is widely present in cells that do not express IL-10R1 and are thus unresponsive to IL-10[14-17].
Activation of the IL-10 receptor complex initiates a cascade of intracellular events. The first step involves activation of members of the Janus kinase family, Jak1 and Tyk2. Activation of Jak1 is related to IL-10R1, whereas Tyk2 binds to the IL-10R2 subunit. This step is followed by activation of members of the signal transducer and activator of transcription (STAT) family. STAT1, STAT3, and STAT5 molecules in their homo- or hetero-dimeric forms enter the nucleus and bind to STAT-binding elements (SBE) in the promoters of various IL-10-responsive genes. These events enhance the transcription of anti-apoptotic genes and genes associated with cell cycle-progression, such as Bcl, Cyclin D1, Cyclin D2, Cyclin D3, Cyclin A, c-Myc, p19Ink and others[18-20]. IL-10 also induces activation of phosphatidylinositol 3-kinase and its downstream targets: p70 S6-kinase and Akt/protein kinase B. This pathway is required for the proliferative effect of IL-10[21,22]. In addition, the IL-10 signaling cascade often interacts with other intracellular pathways.
For example, IL-10 modulates the translation of TNF-α mRNA via the activation of p38MAPK, thereby increasing TNF-α production by mononuclear cells[23].
In human monocytes, IL-10 up-regulates the expression and activity of the general cell protective stress protein heme oxygenase-1[24].
The complexity of IL-10 activities defines a broad spectrum of the properties of IL-10. The principal function of IL-10 is to control inflammation and instruct adaptive immune responses. IL-10 inhibits the activation and differentiation of antigen-presenting cells, such as dendritic cells and macrophages. IL-10 down-regulates the expression of major histocompatibility complex class II and co-stimulatory B7-1/B7-2 molecules and decreases the secretion of pro-inflammatory cytokines, such as TNF-α, IL-12, IL-1β, and others. IL-10 also regulates the growth and/or differentiation of B cells, NK cells, cytotoxic T and T helper cells, mast cells, keratinocytes, and endothelial and mesangial cells[2,5,25-28]. IL-10 protects the host from a variety of bacterial, parasitic, viral or fungal pathogens. Moreover, IL-10 has clear immunomodulatory properties[29-31].
IL-10 IN THE KIDNEY
IL-10 plays an important role in normal renal physiology, as well as during acute kidney injury, and in the progression of chronic renal failure.
Mesangial cells are the major local source of IL-10 in the normal adult kidney[32]. Mesangial cells are the key regulators of kidney function as they (1) provide structural support to the glomerulus by the secretion and maintenance of the extracellular matrix; (2) modulate the size of the glomerular capillary loops, thereby influencing the glomerular filtration rate; and (3) serve as both a source and target for many growth factors[33,34]. In the healthy adult kidney, mesangial cell turnover is always under tight control. Following a variety of initial insults, mesangial cells undergo activation and/or proliferation.
Activated/proliferating mesangial cells begin to secrete excessive amounts of vasoactive hormones, growth factors, cytokines, chemokines and extracellular matrix proteins. These factors in turn affect mesangial cells in an autocrine manner and mediate interactions with endothelial and epithelial tubular cells and blood-borne inflammatory cells[33,35,36]. IL-10 is an autocrine mesangial cell growth factor. In vitro, IL-10 induces dose-dependent proliferation of growth-arrested mesangial cells. In vivo, IL-10 administration to normal rats results in an increased number of intraglomerular cells and a transient reduction of creatinine clearance[28]. Several studies have demonstrated the association between the up-regulation of IL-10 and the pathophysiology of various kidney diseases, such as mesangioproliferative glomerulonephritis, IgA nephropathy, and the acute phase of microscopic polyangiitis, all of which are related to mesangial cell proliferation[37-39]. Abnormal production of growth factors by activated/proliferating mesangial cells contributes to the induction of renal structural intraglomerular and tubulointerstitial changes. These changes include glomerular and tubular cell hypertrophy, thickening of the glomerular basement membrane, and development of microalbuminuria, followed by accumulation of mesangial matrix and overt proteinuria. The degree of proteinuria correlates with the progression of glomerulosclerosis and tubulointerstitial fibrosis, pathological changes that lead to renal failure and end-stage renal disease[40]. In addition, IL-10 can promote mesangial deposition of immune complexes, thereby contributing to the progression of glomerular injury[41].
Elevated circulating IL-10 levels were found in diabetic patients. Moreover, increased IL-10 concentrations in serum predict albuminuria and correlate with the severity of diabetic nephropathy[42]. In vivo inhibition of IL-10 in rats with Thy1-induced glomerulonephritis greatly decreases glomerular mesangial cell expansion and protein excretion[43]. Anti-IL-10 treatment of mice that spontaneously develop systemic lupus erythematosus (SLE) or mice injected with peripheral blood mononuclear cells from human SLE patients delays the appearance of autoimmune manifestations. These benefits include a reduction of immune complex deposition in the glomeruli, the prevention of glomerular hypercellularity and mesangial expansion, and decreased proteinuria[44]. However, studies have shown that IL-10 is protective against SLE-induced renal damage due to the down-regulation of pathogenic Th1 responses[45]. IL-10 has a protective effect in anti-mouse glomerular basement membrane globulin-induced experimental crescentic glomerulonephritis, and the inhibition of IL-10 decreases renal function and is associated with worsening of histological features[46]. Experimental rats with chronically increased IL-10 levels after 5/6 nephrectomy show suppressed infiltration of inflammatory cells, decreased production of monocyte chemoattractant protein-1 and RANTES, and a significant reduction in mRNA for collagen type I and III in the remnant kidney. These phenomena result in a lower degree of proteinuria and a significant reduction in glomerulosclerosis and interstitial fibrosis[47]. Taken together, these findings demonstrate that under some conditions, IL-10 has a protective effect, reducing kidney injury, but in other cases, IL-10 aggravates defects in renal function. We suggest that the interdependence of the actions of IL-10 with those of other cytokines and growth factors is likely the reason for this phenomenon.
IL-10 controls the synthesis and secretion of Cystatin C (Cyst C), a cysteine protease inhibitor of great clinical importance[48]. Cyst C inhibits cathepsins and may thereby function as a tumor suppressor by inhibiting cathepsin-mediated tumor cell invasion. In addition, Cyst C regulates tissue inflammation, antigen presentation, and resistance to viral and bacterial infections[49-51]. In humans, Cyst C is produced by all nucleated cells. The blood concentrations of Cyst C are tightly correlated with the progression of autoimmune disease, inflammatory lung disorders and cardiovascular disease and may be used as a prognostic factor in cancer[51-54]. Serum Cyst C levels may be more accurate than the glomerular filtration rate as diagnostic value of renal function[55,56]. Today, the concentrations of Cyst C in serum and urine are used as reliable markers of acute kidney injury[57,58]. Similar to IL-10, Cyst C induces mesangial cell proliferation in an autocrine manner[59].
Another growth factor whose functions are closely related to IL-10 is transforming growth factor-β (TGF-β). The TGF-β-induced signaling network plays an important role in human diseases. TGF-β has an essential role in both normal kidney function and during the progression of renal injury. TGF-β executes its actions through activation of the Smad and mitogen-activated protein kinase intracellular signaling pathways. TGF-β isoforms are widely present and act on virtually every cell type. TGF-β regulates the proliferation, differentiation, migration, hypertrophy and apoptosis of intraglomerular and tubular cells, controls remodeling of the extracellular matrix, and promotes glomerular and interstitial fibrosis and the progression of glomerulosclerosis[60-64].
Furthermore, TGF-β induces the process of epithelial-to-mesenchymal transition (EMT) in normal mammary epithelial cells. During EMT, cells lose their epithelial identity, reflected in the loss of the expression of proteins associated with epithelial morphology, such as E-cadherin, α-, β-, and γ-catenins, and zonula occludens-1, and begin to synthesize de novo proteins associated with a mesenchymal phenotype, such as D-cadherin, fibronectin, vimentin, and α-smooth muscle actin. These events occur in parallel with a decrease in cell-cell adhesion and changes in the actin cytoskeleton[65,66]. TGF-β-induced EMT in podocytes is responsible for the appearance and progression of albuminuria and proteinuria. The severity of proteinuria correlates with the progression of glomerulosclerosis and tubulointerstitial fibrosis. Fibrosis is usually preceded by the infiltration of mononuclear inflammatory cells into the interstitium. These cells secrete cytokines and chemokines that stimulate resident tubular epithelial cells to differentiate into matrix-producing fibroblasts[64,67-69]. IL-10 and TGF-β may act synergistically to regulate the production of proinflammatory cytokines, chemokines and nitric oxide by mononuclear cells. Moreover, TGF-β induces IL-10 expression and vice versa in various cell types, including mesangial cells[7,32,70,71].
IL-10 acts on both TGF-β and Cyst C, and TGF-β and Cyst C separately influence IL-10 synthesis and activity[70,71-73]. It has also been shown that there is direct cross talk between TGF-β and Cyst C. TGF-β induces Cyst C expression[49,74], while Cyst C acts as a TGF-β antagonist that prevents the binding of TGF-β to its receptor and thereby inhibits its activity[49,75]. Cyst C is important in the acute phase of the kidney’s responses to injury, which are rapid and aggressive, whereas TGF-β promotes slower processes that lead to chronic renal failure and end-stage renal disease. It has been suggested that the major role of the dialogue between IL-10 and TGF-β, IL-10 and Cyst C, and Cyst C and TGF-β is to instruct and regulate the degree of the renal responses to primary injury. These responses include control of mesangial cell proliferation, accumulation of extracellular matrix, influx of mononuclear cells, glomerulosclerosis, and tubular fibrosis (Figure 1).
Figure 1.
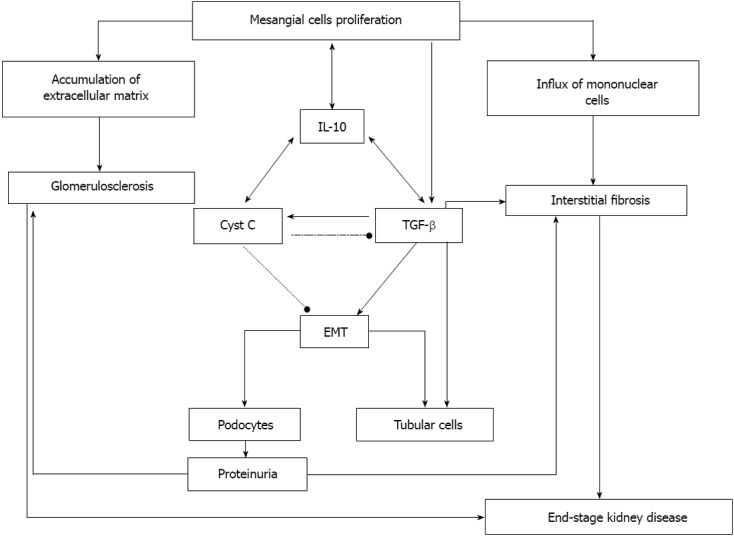
Interleukin-10 functions in the progression of renal failure. Interleukin-10 (IL-10) induces over-proliferation of mesangial cells that through an increased synthesis and secretion of a variety of growth factors, cytokines and chemokines, evoke several pathologic processes, leading to progression of renal failure. An increased secretion of components comprising the mesangial extracellular matrix results in its accumulation and is followed by the formation of fibrotic and sclerotic lesions in the glomeruli. IL-10 induces the synthesis and activity of Cystatin C (Cyst C) and transforming growth factor-β (TGF-β). Cystatin C regulates tissue inflammation and increases mesangial cell proliferation. Increased TGF-β levels act in parallel with IL-10 to promote fibrosis and glomerulosclerosis. In addition, the TGF-β-induced epithelial-to-mesenchymal transition in podocytes leads to the appearance of proteinuria. The development of proteinuria aggravates the processes of glomerulosclerosis and interstitial fibrosis and leads to end-stage kidney disease. EMT: Epithelial-to-mesenchymal transition.
IL-10 IN ALLOGRAFT SURVIVAL/REJECTION
Transplantation has become an accepted treatment for end-stage renal disease. The major barrier of transplantation from genetically disparate donors is the process of rejection, in which the recipient’s immune system recognizes the graft as foreign tissue and attacks it. Allograft rejection can occur through direct (cellular) or indirect (humoral) pathways and is a complex process involving both cell-mediated immunity and circulating antibodies. The role of cytokines and the particular role of IL-10 and the IL-10-induced signaling network in the development and progression of graft survival/rejection are subjects of intensive research[76-80]. Indeed, the IL-10-TGF-β pathway plays an important role in the progression of allograft fibrosis, while TGF-β is a potential therapeutic target for the prevention and therapy of fibrogenesis in kidney transplants[76,81]. However, whether IL-10 plays an overall helpful or detrimental role is not yet known. High intra-graft IL-10 expression was found in patients undergoing acute rejection[82]. In the case of chronic rejection, poorer graft survival, which is generally associated with evidence of interstitial fibrosis and tubular atrophy, is accompanied by the up-regulation of IL-10 gene expression[83]. IL-10 is also a stimulator of the immune system, inducing the differentiation and proliferation of B cells, thus leading the immune response toward the humoral pathway and enhancing antibody responses against the graft[83,84]. In contrast, IL-10 has a clear protective effect. It has been shown that the up-regulation of the IL-10 gene in a rat model of kidney allograft rejection improves renal function and prolongs allograft survival[85]. IL-10, when secreted by T-regulatory cells, suppresses antigen-specific effector cell responses via inhibition of pro-inflammatory cytokine production[86]. Additional findings show that an acute immune response during graft rejection is associated with an over-expression of pro-inflammatory Th1 cytokines, which appear in parallel with the accumulation of IL-10. It has been suggested that in this situation, the rise in IL-10 levels serves to regulate and limit the inflammatory responses[87].
IL-10 IN COMPENSATORY RENAL GROWTH
The discovery of the compensatory renal growth process is, without a doubt, the most important reason why the expansion of kidney transplantation from live donors has occurred in recent years. After removal of a single kidney, the remaining kidney becomes enlarged, mainly through the hypertrophy of tubular cells, and compensates for the loss of the contralateral organ within a short period of time. TGF-β has been suggested as the most important factor causing tubular cell hypertrophy and therefore has a pivotal role in compensatory renal growth[88]. Although the tubular cells are the main site at which compensatory renal growth takes place, studies from our group showed that mesangial cells initiate compensatory renal growth and control the degree of compensatory tubular cell hypertrophy by controlling IL-10 to TGF-β cross-talk[32,89].
Immediately after unilateral nephrectomy, the remaining kidney undergoes hyperfiltration. The changes in glomerular hemodynamics lead to a transient proliferation of mesangial cells, reaching a maximum at 24 h after surgery; proliferation is then arrested within 72 h. Proliferating mesangial cells secrete increased amounts of many growth factors, including IL-10. These growth factors affect mesangial cells in an autocrine manner as additional stimuli to over-proliferate, influence the conversion of TGF-β from the latent to the active form, and lead to increased TGF-β production. Among the resident renal cell types studied, only mesangial cells secrete and activate TGF-β[90,91]. A reduction in mesangial cell proliferation occurs in parallel with the appearance of renal tubular cell hypertrophy. When TGF-β accumulates to sufficient levels, it induces tubular cells to undergo hypertrophy themselves and, in parallel, acts on mesangial cells to inhibit their proliferation. Inhibition of mesangial cell proliferation, in turn, reduces TGF-β levels and inhibits compensatory tubular cell hypertrophy. TGF-β secretion may be affected by many growth factors, including angiotensin II, IGF-I, HGF, bFGF, TNF-α, EGF, PDGF, and others, all of which are produced by the mesangial cells[92-95]. The importance of IL-10 in this process may be underscored by the fact that the in vivo inhibition of IL-10 production by mesangial cells leads to a significant reduction in TGF-β expression in the remaining kidney; this is accompanied by an approximate 25% reduction in remaining kidney weight and a significant decrease in compensatory tubular cell hypertrophy[32,89]. Compensatory renal growth is regulated by a variety of growth factors and cytokines that initiate proliferative, hypertrophic, and apoptotic growth responses in the remaining kidneys. These growth factors may act in concert, and despite their apparent redundancy, they all must be present in sufficient concentrations to support maximal growth of the remaining kidney. Due to the interdependence between these cytokines, manipulation of the expression of one of these may affect the entire compensatory growth response in the remaining kidney.
In summary, IL-10 gene expression and IL-10-induced signaling pathways have an important role in the regulation and maintenance of normal renal function. Moreover, accumulating evidence further demonstrates that abnormal IL-10 expression, whether transient or prolonged, as well as interactions with other growth factors as a response to diverse stimuli, is linked to the appearance and progression of a variety of kidney disorders. It has thus been suggested that the selective targeting of IL-10 expression and IL-10-related pathways may provide therapeutic approaches for many kidney diseases.
Footnotes
P- Reviewer: Scherer A S- Editor: Wen LL L- Editor: A E- Editor: Yan JL
References
- 1.Fiorentino DF, Bond MW, Mosmann TR. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989;170:2081–2095. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 3.Sabat R. IL-10 family of cytokines. Cytokine Growth Factor Rev. 2010;21:315–324. doi: 10.1016/j.cytogfr.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 4.Zdanov A. Structural analysis of cytokines comprising the IL-10 family. Cytokine Growth Factor Rev. 2010;21:325–330. doi: 10.1016/j.cytogfr.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sabat R, Grütz G, Warszawska K, Kirsch S, Witte E, Wolk K, Geginat J. Biology of interleukin-10. Cytokine Growth Factor Rev. 2010;21:331–344. doi: 10.1016/j.cytogfr.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 6.Kim JM, Brannan CI, Copeland NG, Jenkins NA, Khan TA, Moore KW. Structure of the mouse IL-10 gene and chromosomal localization of the mouse and human genes. J Immunol. 1992;148:3618–3623. [PubMed] [Google Scholar]
- 7.Bantis C, Heering PJ, Aker S, Klein-Vehne N, Grabensee B, Ivens K. Association of interleukin-10 gene G-1082A polymorphism with the progression of primary glomerulonephritis. Kidney Int. 2004;66:288–294. doi: 10.1111/j.1523-1755.2004.00730.x. [DOI] [PubMed] [Google Scholar]
- 8.Mtiraoui N, Ezzidi I, Kacem M, Ben Hadj Mohamed M, Chaieb M, Haj Jilani AB, Mahjoub T, Almawi WY. Predictive value of interleukin-10 promoter genotypes and haplotypes in determining the susceptibility to nephropathy in type 2 diabetes patients. Diabetes Metab Res Rev. 2009;25:57–63. doi: 10.1002/dmrr.892. [DOI] [PubMed] [Google Scholar]
- 9.Forte GI, Pilato G, Vaccarino L, Sanacore M, Candore G, Romano GC, Testa R, Franceschi C, Capri M, Marra M, et al. Risk profiles in type 2 diabetes (metabolic syndrome): integration of IL-10 polymorphisms and laboratory parameters to identify vascular damages related complications. Curr Pharm Des. 2010;16:898–903. doi: 10.2174/138161210790883642. [DOI] [PubMed] [Google Scholar]
- 10.Nordfors L, Lindholm B, Stenvinkel P. End-stage renal disease--not an equal opportunity disease: the role of genetic polymorphisms. J Intern Med. 2005;258:1–12. doi: 10.1111/j.1365-2796.2005.01516.x. [DOI] [PubMed] [Google Scholar]
- 11.Romero JM, Sáenz-López P, Cózar JM, Carretero R, Canton J, Vazquez F, Concha A, Tallada M, Garrido F, Ruiz-Cabello F. A polymorphism in the interleukin-10 promoter affects the course of disease in patients with clear-cell renal carcinoma. Hum Immunol. 2009;70:60–64. doi: 10.1016/j.humimm.2008.10.020. [DOI] [PubMed] [Google Scholar]
- 12.Havranek E, Howell WM, Fussell HM, Whelan JA, Whelan MA, Pandha HS. An interleukin-10 promoter polymorphism may influence tumor development in renal cell carcinoma. J Urol. 2005;173:709–712. doi: 10.1097/01.ju.0000152493.86001.91. [DOI] [PubMed] [Google Scholar]
- 13.Yoon SI, Logsdon NJ, Sheikh F, Donnelly RP, Walter MR. Conformational changes mediate interleukin-10 receptor 2 (IL-10R2) binding to IL-10 and assembly of the signaling complex. J Biol Chem. 2006;281:35088–35096. doi: 10.1074/jbc.M606791200. [DOI] [PubMed] [Google Scholar]
- 14.Yoon SI, Jones BC, Logsdon NJ, Harris BD, Deshpande A, Radaeva S, Halloran BA, Gao B, Walter MR. Structure and mechanism of receptor sharing by the IL-10R2 common chain. Structure. 2010;18:638–648. doi: 10.1016/j.str.2010.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ding Y, Qin L, Zamarin D, Kotenko SV, Pestka S, Moore KW, Bromberg JS. Differential IL-10R1 expression plays a critical role in IL-10-mediated immune regulation. J Immunol. 2001;167:6884–6892. doi: 10.4049/jimmunol.167.12.6884. [DOI] [PubMed] [Google Scholar]
- 16.Kotenko SV, Gallagher G, Baurin VV, Lewis-Antes A, Shen M, Shah NK, Langer JA, Sheikh F, Dickensheets H, Donnelly RP. IFN-lambdas mediate antiviral protection through a distinct class II cytokine receptor complex. Nat Immunol. 2003;4:69–77. doi: 10.1038/ni875. [DOI] [PubMed] [Google Scholar]
- 17.Xie MH, Aggarwal S, Ho WH, Foster J, Zhang Z, Stinson J, Wood WI, Goddard AD, Gurney AL. Interleukin (IL)-22, a novel human cytokine that signals through the interferon receptor-related proteins CRF2-4 and IL-22R. J Biol Chem. 2000;275:31335–31339. doi: 10.1074/jbc.M005304200. [DOI] [PubMed] [Google Scholar]
- 18.Finbloom DS, Winestock KD. IL-10 induces the tyrosine phosphorylation of tyk2 and Jak1 and the differential assembly of STAT1 alpha and STAT3 complexes in human T cells and monocytes. J Immunol. 1995;155:1079–1090. [PubMed] [Google Scholar]
- 19.Wehinger J, Gouilleux F, Groner B, Finke J, Mertelsmann R, Weber-Nordt RM. IL-10 induces DNA binding activity of three STAT proteins (Stat1, Stat3, and Stat5) and their distinct combinatorial assembly in the promoters of selected genes. FEBS Lett. 1996;394:365–370. doi: 10.1016/0014-5793(96)00990-8. [DOI] [PubMed] [Google Scholar]
- 20.Donnelly RP, Dickensheets H, Finbloom DS. The interleukin-10 signal transduction pathway and regulation of gene expression in mononuclear phagocytes. J Interferon Cytokine Res. 1999;19:563–573. doi: 10.1089/107999099313695. [DOI] [PubMed] [Google Scholar]
- 21.Crawley JB, Williams LM, Mander T, Brennan FM, Foxwell BM. Interleukin-10 stimulation of phosphatidylinositol 3-kinase and p70 S6 kinase is required for the proliferative but not the antiinflammatory effects of the cytokine. J Biol Chem. 1996;271:16357–16362. doi: 10.1074/jbc.271.27.16357. [DOI] [PubMed] [Google Scholar]
- 22.Strle K, Zhou JH, Broussard SR, Venters HD, Johnson RW, Freund GG, Dantzer R, Kelley KW. IL-10 promotes survival of microglia without activating Akt. J Neuroimmunol. 2002;122:9–19. doi: 10.1016/s0165-5728(01)00444-1. [DOI] [PubMed] [Google Scholar]
- 23.Kontoyiannis D, Kotlyarov A, Carballo E, Alexopoulou L, Blackshear PJ, Gaestel M, Davis R, Flavell R, Kollias G. Interleukin-10 targets p38 MAPK to modulate ARE-dependent TNF mRNA translation and limit intestinal pathology. EMBO J. 2001;20:3760–3770. doi: 10.1093/emboj/20.14.3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jung M, Sabat R, Krätzschmar J, Seidel H, Wolk K, Schönbein C, Schütt S, Friedrich M, Döcke WD, Asadullah K, et al. Expression profiling of IL-10-regulated genes in human monocytes and peripheral blood mononuclear cells from psoriatic patients during IL-10 therapy. Eur J Immunol. 2004;34:481–493. doi: 10.1002/eji.200324323. [DOI] [PubMed] [Google Scholar]
- 25.Mosmann TR. Role of a new cytokine, interleukin-10, in the cross-regulation of T helper cells. Ann N Y Acad Sci. 1991;628:337–344. doi: 10.1111/j.1749-6632.1991.tb17266.x. [DOI] [PubMed] [Google Scholar]
- 26.Hedrich CM, Bream JH. Cell type-specific regulation of IL-10 expression in inflammation and disease. Immunol Res. 2010;47:185–206. doi: 10.1007/s12026-009-8150-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fiorentino DF, Zlotnik A, Mosmann TR, Howard M, O’Garra A. IL-10 inhibits cytokine production by activated macrophages. J Immunol. 1991;147:3815–3822. [PubMed] [Google Scholar]
- 28.Chadban SJ, Tesch GH, Foti R, Atkins RC, Nikolic-Paterson DJ. Interleukin-10 is a mesangial cell growth factor in vitro and in vivo. Lab Invest. 1997;76:619–627. [PubMed] [Google Scholar]
- 29.Saraiva M, O’Garra A. The regulation of IL-10 production by immune cells. Nat Rev Immunol. 2010;10:170–181. doi: 10.1038/nri2711. [DOI] [PubMed] [Google Scholar]
- 30.Couper KN, Blount DG, Riley EM. IL-10: the master regulator of immunity to infection. J Immunol. 2008;180:5771–5777. doi: 10.4049/jimmunol.180.9.5771. [DOI] [PubMed] [Google Scholar]
- 31.Strassmann G, Kambayashi T, Jacob CO, Sredni D. The immunomodulator AS-101 inhibits IL-10 release and augments TNF alpha and IL-1 alpha release by mouse and human mononuclear phagocytes. Cell Immunol. 1997;176:180–185. doi: 10.1006/cimm.1997.1087. [DOI] [PubMed] [Google Scholar]
- 32.Sinuani I, Averbukh Z, Gitelman I, Rapoport MJ, Sandbank J, Albeck M, Sredni B, Weissgarten J. Mesangial cells initiate compensatory renal tubular hypertrophy via IL-10-induced TGF-beta secretion: effect of the immunomodulator AS101 on this process. Am J Physiol Renal Physiol. 2006;291:F384–F394. doi: 10.1152/ajprenal.00418.2005. [DOI] [PubMed] [Google Scholar]
- 33.Cove-Smith A, Hendry BM. The regulation of mesangial cell proliferation. Nephron Exp Nephrol. 2008;108:e74–e79. doi: 10.1159/000127359. [DOI] [PubMed] [Google Scholar]
- 34.Stockand JD, Sansom SC. Glomerular mesangial cells: electrophysiology and regulation of contraction. Physiol Rev. 1998;78:723–744. doi: 10.1152/physrev.1998.78.3.723. [DOI] [PubMed] [Google Scholar]
- 35.Schena FP, Strippoli GF, Wankelmuth P. Renal growth factors: past, present and future. Am J Nephrol. 1999;19:308–312. doi: 10.1159/000013466. [DOI] [PubMed] [Google Scholar]
- 36.Schlöndorff D, Banas B. The mesangial cell revisited: no cell is an island. J Am Soc Nephrol. 2009;20:1179–1187. doi: 10.1681/ASN.2008050549. [DOI] [PubMed] [Google Scholar]
- 37.Yano N, Endoh M, Nomoto Y, Sakai H, Fadden K, Rifai A. Phenotypic characterization of cytokine expression in patients with IgA nephropathy. J Clin Immunol. 1997;17:396–403. doi: 10.1023/a:1027368308453. [DOI] [PubMed] [Google Scholar]
- 38.Niemir ZI, Ondracek M, Dworacki G, Stein H, Waldherr R, Ritz E, Otto HF. In situ upregulation of IL-10 reflects the activity of human glomerulonephritides. Am J Kidney Dis. 1998;32:80–92. doi: 10.1053/ajkd.1998.v32.pm9669428. [DOI] [PubMed] [Google Scholar]
- 39.Hirschberg R, Adler S. Insulin-like growth factor system and the kidney: physiology, pathophysiology, and therapeutic implications. Am J Kidney Dis. 1998;31:901–919. doi: 10.1053/ajkd.1998.v31.pm9631833. [DOI] [PubMed] [Google Scholar]
- 40.Makino H, Kashihara N, Sugiyama H, Kanao K, Sekikawa T, Okamoto K, Maeshima Y, Ota Z, Nagai R. Phenotypic modulation of the mesangium reflected by contractile proteins in diabetes. Diabetes. 1996;45:488–495. doi: 10.2337/diab.45.4.488. [DOI] [PubMed] [Google Scholar]
- 41.Lakkis FG, Baddoura FK, Cruet EN, Parekh KR, Fukunaga M, Munger KA. Anti-inflammatory lymphokine mRNA expression in antibody-induced glomerulonephritis. Kidney Int. 1996;49:117–126. doi: 10.1038/ki.1996.16. [DOI] [PubMed] [Google Scholar]
- 42.Myśliwska J, Zorena K, Semetkowska-Jurkiewicz E, Rachoń D, Suchanek H, Myśliwski A. High levels of circulating interleukin-10 in diabetic nephropathy patients. Eur Cytokine Netw. 2005;16:117–122. [PubMed] [Google Scholar]
- 43.Kalechman Y, Gafter U, Weinstein T, Chagnac A, Freidkin I, Tobar A, Albeck M, Sredni B. Inhibition of interleukin-10 by the immunomodulator AS101 reduces mesangial cell proliferation in experimental mesangioproliferative glomerulonephritis: association with dephosphorylation of STAT3. J Biol Chem. 2004;279:24724–24732. doi: 10.1074/jbc.M312006200. [DOI] [PubMed] [Google Scholar]
- 44.Kalechman Y, Gafter U, Gal R, Rushkin G, Yan D, Albeck M, Sredni B. Anti-IL-10 therapeutic strategy using the immunomodulator AS101 in protecting mice from sepsis-induced death: dependence on timing of immunomodulating intervention. J Immunol. 2002;169:384–392. doi: 10.4049/jimmunol.169.1.384. [DOI] [PubMed] [Google Scholar]
- 45.Ling GS, Cook HT, Botto M, Lau YL, Huang FP. An essential protective role of IL-10 in the immunological mechanism underlying resistance vs. susceptibility to lupus induction by dendritic cells and dying cells. Rheumatology (Oxford) 2011;50:1773–1784. doi: 10.1093/rheumatology/ker198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Summers SA, Phoon RK, Odobasic D, Dewage L, Kitching AR, Holdsworth SR. Signal transducer and activation of transcription 6 (STAT6) regulates T helper type 1 (Th1) and Th17 nephritogenic immunity in experimental crescentic glomerulonephritis. Clin Exp Immunol. 2011;166:227–234. doi: 10.1111/j.1365-2249.2011.04437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mu W, Ouyang X, Agarwal A, Zhang L, Long DA, Cruz PE, Roncal CA, Glushakova OY, Chiodo VA, Atkinson MA, et al. IL-10 suppresses chemokines, inflammation, and fibrosis in a model of chronic renal disease. J Am Soc Nephrol. 2005;16:3651–3660. doi: 10.1681/ASN.2005030297. [DOI] [PubMed] [Google Scholar]
- 48.Xu Y, Schnorrer P, Proietto A, Kowalski G, Febbraio MA, Acha-Orbea H, Dickins RA, Villadangos JA. IL-10 controls cystatin C synthesis and blood concentration in response to inflammation through regulation of IFN regulatory factor 8 expression. J Immunol. 2011;186:3666–3673. doi: 10.4049/jimmunol.1001934. [DOI] [PubMed] [Google Scholar]
- 49.Vray B, Hartmann S, Hoebeke J. Immunomodulatory properties of cystatins. Cell Mol Life Sci. 2002;59:1503–1512. doi: 10.1007/s00018-002-8525-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sokol JP, Schiemann WP. Cystatin C antagonizes transforming growth factor beta signaling in normal and cancer cells. Mol Cancer Res. 2004;2:183–195. [PubMed] [Google Scholar]
- 51.Kos J, Werle B, Lah T, Brunner N. Cysteine proteinases and their inhibitors in extracellular fluids: markers for diagnosis and prognosis in cancer. Int J Biol Markers. 2000;15:84–89. doi: 10.1177/172460080001500116. [DOI] [PubMed] [Google Scholar]
- 52.Hansen T, Petrow PK, Gaumann A, Keyszer G, Bräuer R, Kriegsmann J. Synovial giant cells in rheumatoid arthritis: expression of cystatin C, but not of cathepsin B. Exp Toxicol Pathol. 2000;52:312–316. doi: 10.1016/S0940-2993(00)80055-X. [DOI] [PubMed] [Google Scholar]
- 53.Kos J, Krasovec M, Cimerman N, Nielsen HJ, Christensen IJ, Brünner N. Cysteine proteinase inhibitors stefin A, stefin B, and cystatin C in sera from patients with colorectal cancer: relation to prognosis. Clin Cancer Res. 2000;6:505–511. [PubMed] [Google Scholar]
- 54.Koenig W, Twardella D, Brenner H, Rothenbacher D. Plasma concentrations of cystatin C in patients with coronary heart disease and risk for secondary cardiovascular events: more than simply a marker of glomerular filtration rate. Clin Chem. 2005;51:321–327. doi: 10.1373/clinchem.2004.041889. [DOI] [PubMed] [Google Scholar]
- 55.Shlipak MG. Cystatin C as a marker of glomerular filtration rate in chronic kidney disease: influence of body composition. Nat Clin Pract Nephrol. 2007;3:188–189. doi: 10.1038/ncpneph0404. [DOI] [PubMed] [Google Scholar]
- 56.Heilman RL, Mazur MJ. Cystatin C as a more sensitive indicator of diminished glomerular filtration rate. Liver Transpl. 2005;11:264–266. doi: 10.1002/lt.20361. [DOI] [PubMed] [Google Scholar]
- 57.Zhang Z, Lu B, Sheng X, Jin N. Cystatin C in prediction of acute kidney injury: a systemic review and meta-analysis. Am J Kidney Dis. 2011;58:356–365. doi: 10.1053/j.ajkd.2011.02.389. [DOI] [PubMed] [Google Scholar]
- 58.Koyner JL, Vaidya VS, Bennett MR, Ma Q, Worcester E, Akhter SA, Raman J, Jeevanandam V, O’Connor MF, Devarajan P, et al. Urinary biomarkers in the clinical prognosis and early detection of acute kidney injury. Clin J Am Soc Nephrol. 2010;5:2154–2165. doi: 10.2215/CJN.00740110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tavéra C, Leung-Tack J, Prévot D, Gensac MC, Martinez J, Fulcrand P, Collé A. Cystatin C secretion by rat glomerular mesangial cells: autocrine loop for in vitro growth-promoting activity. Biochem Biophys Res Commun. 1992;182:1082–1088. doi: 10.1016/0006-291x(92)91842-e. [DOI] [PubMed] [Google Scholar]
- 60.Blobe GC, Schiemann WP, Lodish HF. Role of transforming growth factor beta in human disease. N Engl J Med. 2000;342:1350–1358. doi: 10.1056/NEJM200005043421807. [DOI] [PubMed] [Google Scholar]
- 61.Böttinger EP, Bitzer M. TGF-beta signaling in renal disease. J Am Soc Nephrol. 2002;13:2600–2610. doi: 10.1097/01.asn.0000033611.79556.ae. [DOI] [PubMed] [Google Scholar]
- 62.Böttinger EP. TGF-beta in renal injury and disease. Semin Nephrol. 2007;27:309–320. doi: 10.1016/j.semnephrol.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 63.Marks DJ, Segal AW. Innate immunity in inflammatory bowel disease: a disease hypothesis. J Pathol. 2008;214:260–266. doi: 10.1002/path.2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu Y. Renal fibrosis: new insights into the pathogenesis and therapeutics. Kidney Int. 2006;69:213–217. doi: 10.1038/sj.ki.5000054. [DOI] [PubMed] [Google Scholar]
- 65.Miettinen PJ, Ebner R, Lopez AR, Derynck R. TGF-beta induced transdifferentiation of mammary epithelial cells to mesenchymal cells: involvement of type I receptors. J Cell Biol. 1994;127:2021–2036. doi: 10.1083/jcb.127.6.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zavadil J, Bitzer M, Liang D, Yang YC, Massimi A, Kneitz S, Piek E, Bottinger EP. Genetic programs of epithelial cell plasticity directed by transforming growth factor-beta. Proc Natl Acad Sci U S A. 2001;98:6686–6691. doi: 10.1073/pnas.111614398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Piek E, Moustakas A, Kurisaki A, Heldin CH, ten Dijke P. TGF-(beta) type I receptor/ALK-5 and Smad proteins mediate epithelial to mesenchymal transdifferentiation in NMuMG breast epithelial cells. J Cell Sci. 1999;112(Pt 24):4557–4568. doi: 10.1242/jcs.112.24.4557. [DOI] [PubMed] [Google Scholar]
- 68.Fan JM, Ng YY, Hill PA, Nikolic-Paterson DJ, Mu W, Atkins RC, Lan HY. Transforming growth factor-beta regulates tubular epithelial-myofibroblast transdifferentiation in vitro. Kidney Int. 1999;56:1455–1467. doi: 10.1046/j.1523-1755.1999.00656.x. [DOI] [PubMed] [Google Scholar]
- 69.Kang YS, Li Y, Dai C, Kiss LP, Wu C, Liu Y. Inhibition of integrin-linked kinase blocks podocyte epithelial-mesenchymal transition and ameliorates proteinuria. Kidney Int. 2010;78:363–373. doi: 10.1038/ki.2010.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bickerstaff AA, VanBuskirk AM, Wakely E, Orosz CG. Transforming growth factor-beta and interleukin-10 subvert alloreactive delayed type hypersensitivity in cardiac allograft acceptor mice. Transplantation. 2000;69:1517–1520. doi: 10.1097/00007890-200004150-00055. [DOI] [PubMed] [Google Scholar]
- 71.Maeda H, Kuwahara H, Ichimura Y, Ohtsuki M, Kurakata S, Shiraishi A. TGF-beta enhances macrophage ability to produce IL-10 in normal and tumor-bearing mice. J Immunol. 1995;155:4926–4932. [PubMed] [Google Scholar]
- 72.Fuss IJ, Boirivant M, Lacy B, Strober W. The interrelated roles of TGF-beta and IL-10 in the regulation of experimental colitis. J Immunol. 2002;168:900–908. doi: 10.4049/jimmunol.168.2.900. [DOI] [PubMed] [Google Scholar]
- 73.Frendéus KH, Wallin H, Janciauskiene S, Abrahamson M. Macrophage responses to interferon-gamma are dependent on cystatin C levels. Int J Biochem Cell Biol. 2009;41:2262–2269. doi: 10.1016/j.biocel.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 74.Afonso S, Tovar C, Romagnano L, Babiarz B. Control and expression of cystatin C by mouse decidual cultures. Mol Reprod Dev. 2002;61:155–163. doi: 10.1002/mrd.1142. [DOI] [PubMed] [Google Scholar]
- 75.Sokol JP, Neil JR, Schiemann BJ, Schiemann WP. The use of cystatin C to inhibit epithelial-mesenchymal transition and morphological transformation stimulated by transforming growth factor-beta. Breast Cancer Res. 2005;7:R844–R853. doi: 10.1186/bcr1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.VanBuskirk AM, Burlingham WJ, Jankowska-Gan E, Chin T, Kusaka S, Geissler F, Pelletier RP, Orosz CG. Human allograft acceptance is associated with immune regulation. J Clin Invest. 2000;106:145–155. doi: 10.1172/JCI9171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Waaga AM, Gasser M, Kist-van Holthe JE, Najafian N, Müller A, Vella JP, Womer KL, Chandraker A, Khoury SJ, Sayegh MH. Regulatory functions of self-restricted MHC class II allopeptide-specific Th2 clones in vivo. J Clin Invest. 2001;107:909–916. doi: 10.1172/JCI11427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Keller MR, Burlingham WJ. Loss of tolerance to self after transplant. Semin Immunopathol. 2011;33:105–110. doi: 10.1007/s00281-011-0252-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pöge U, Gerhardt T, Stoffel-Wagner B, Palmedo H, Klehr HU, Sauerbruch T, Woitas RP. Cystatin C-based calculation of glomerular filtration rate in kidney transplant recipients. Kidney Int. 2006;70:204–210. doi: 10.1038/sj.ki.5001502. [DOI] [PubMed] [Google Scholar]
- 80.Le Bricon T, Thervet E, Benlakehal M, Bousquet B, Legendre C, Erlich D. Changes in plasma cystatin C after renal transplantation and acute rejection in adults. Clin Chem. 1999;45:2243–2249. [PubMed] [Google Scholar]
- 81.Djamali A, Samaniego M. Fibrogenesis in kidney transplantation: potential targets for prevention and therapy. Transplantation. 2009;88:1149–1156. doi: 10.1097/TP.0b013e3181bcccea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Strehlau J, Pavlakis M, Lipman M, Shapiro M, Vasconcellos L, Harmon W, Strom TB. Quantitative detection of immune activation transcripts as a diagnostic tool in kidney transplantation. Proc Natl Acad Sci U S A. 1997;94:695–700. doi: 10.1073/pnas.94.2.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hueso M, Navarro E, Moreso F, O’Valle F, Pérez-Riba M, Del Moral RG, Grinyó JM, Serón D. Intragraft expression of the IL-10 gene is up-regulated in renal protocol biopsies with early interstitial fibrosis, tubular atrophy, and subclinical rejection. Am J Pathol. 2010;176:1696–1704. doi: 10.2353/ajpath.2010.090411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rousset F, Garcia E, Defrance T, Péronne C, Vezzio N, Hsu DH, Kastelein R, Moore KW, Banchereau J. Interleukin 10 is a potent growth and differentiation factor for activated human B lymphocytes. Proc Natl Acad Sci U S A. 1992;89:1890–1893. doi: 10.1073/pnas.89.5.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chen B, Kapturczak MH, Joseph R, George JF, Campbell-Thompson M, Wasserfall CH, Atkinson MA, Tisher CC, Flotte TR, Agarwal A, et al. Adeno-associated viral vector-mediated interleukin-10 prolongs allograft survival in a rat kidney transplantation model. Am J Transplant. 2007;7:1112–1120. doi: 10.1111/j.1600-6143.2007.01772.x. [DOI] [PubMed] [Google Scholar]
- 86.Roncarolo MG, Gregori S, Battaglia M, Bacchetta R, Fleischhauer K, Levings MK. Interleukin-10-secreting type 1 regulatory T cells in rodents and humans. Immunol Rev. 2006;212:28–50. doi: 10.1111/j.0105-2896.2006.00420.x. [DOI] [PubMed] [Google Scholar]
- 87.Edemir B, Kurian SM, Eisenacher M, Lang D, Müller-Tidow C, Gabriëls G, Salomon DR, Schlatter E. Activation of counter-regulatory mechanisms in a rat renal acute rejection model. BMC Genomics. 2008;9:71. doi: 10.1186/1471-2164-9-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu B, Preisig P. TGF-beta1-mediated hypertrophy involves inhibiting pRB phosphorylation by blocking activation of cyclin E kinase. Am J Physiol. 1999;277:F186–F194. doi: 10.1152/ajprenal.1999.277.2.F186. [DOI] [PubMed] [Google Scholar]
- 89.Sinuani I, Beberashvili I, Averbukh Z, Cohn M, Gitelman I, Weissgarten J. Mesangial cells initiate compensatory tubular cell hypertrophy. Am J Nephrol. 2010;31:326–331. doi: 10.1159/000287229. [DOI] [PubMed] [Google Scholar]
- 90.Kitamura M, Sütö T, Yokoo T, Shimizu F, Fine LG. Transforming growth factor-beta 1 is the predominant paracrine inhibitor of macrophage cytokine synthesis produced by glomerular mesangial cells. J Immunol. 1996;156:2964–2971. [PubMed] [Google Scholar]
- 91.Kaname S, Uchida S, Ogata E, Kurokawa K. Autocrine secretion of transforming growth factor-beta in cultured rat mesangial cells. Kidney Int. 1992;42:1319–1327. doi: 10.1038/ki.1992.423. [DOI] [PubMed] [Google Scholar]
- 92.Wolf G, Ziyadeh FN, Zahner G, Stahl RA. Angiotensin II is mitogenic for cultured rat glomerular endothelial cells. Hypertension. 1996;27:897–905. doi: 10.1161/01.hyp.27.4.897. [DOI] [PubMed] [Google Scholar]
- 93.Song K, Cornelius SC, Reiss M, Danielpour D. Insulin-like growth factor-I inhibits transcriptional responses of transforming growth factor-beta by phosphatidylinositol 3-kinase/Akt-dependent suppression of the activation of Smad3 but not Smad2. J Biol Chem. 2003;278:38342–38351. doi: 10.1074/jbc.M304583200. [DOI] [PubMed] [Google Scholar]
- 94.Sullivan DE, Ferris M, Pociask D, Brody AR. The latent form of TGFbeta(1) is induced by TNFalpha through an ERK specific pathway and is activated by asbestos-derived reactive oxygen species in vitro and in vivo. J Immunotoxicol. 2008;5:145–149. doi: 10.1080/15476910802085822. [DOI] [PubMed] [Google Scholar]
- 95.Hardie WD, Davidson C, Ikegami M, Leikauf GD, Le Cras TD, Prestridge A, Whitsett JA, Korfhagen TR. EGF receptor tyrosine kinase inhibitors diminish transforming growth factor-alpha-induced pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2008;294:L1217–L1225. doi: 10.1152/ajplung.00020.2008. [DOI] [PubMed] [Google Scholar]


