Abstract
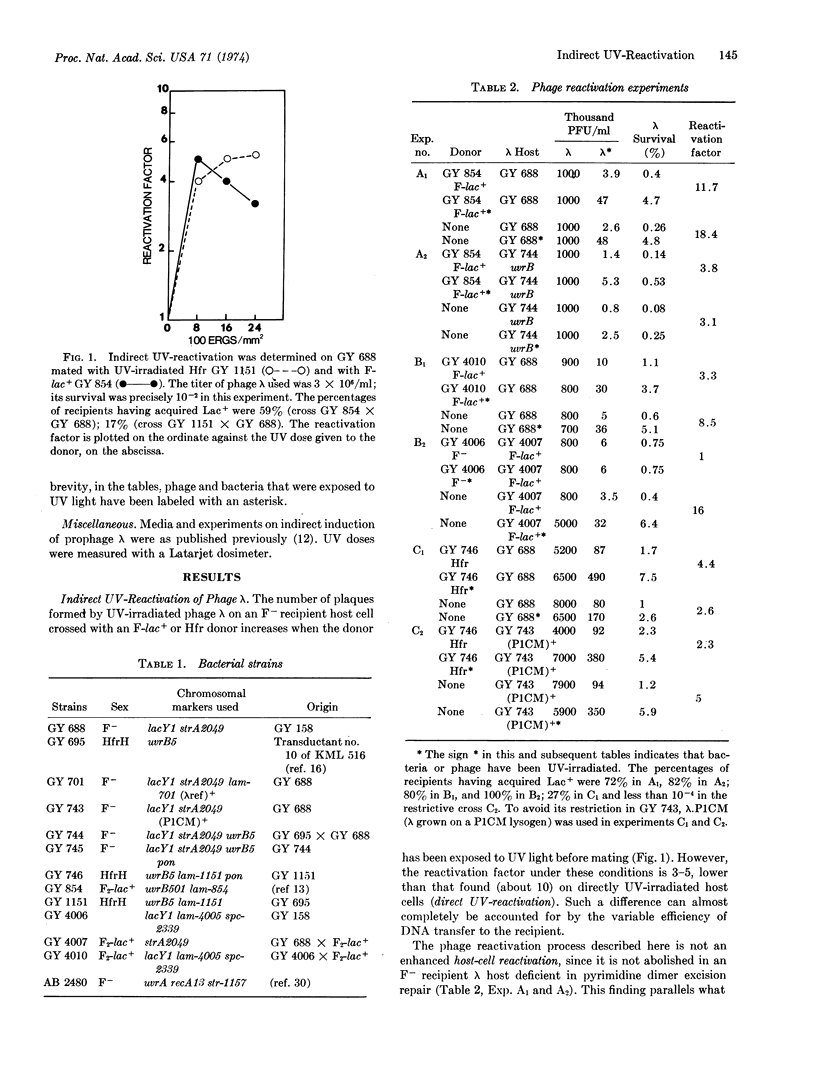
When an F- recipient Escherichia coli K12 bacterium receives Hfr or F-lac+ DNA from an ultraviolet-irradiated donor, its capacity to promote DNA repair and mutagenesis of ultraviolet-damaged phage λ is substantially increased.
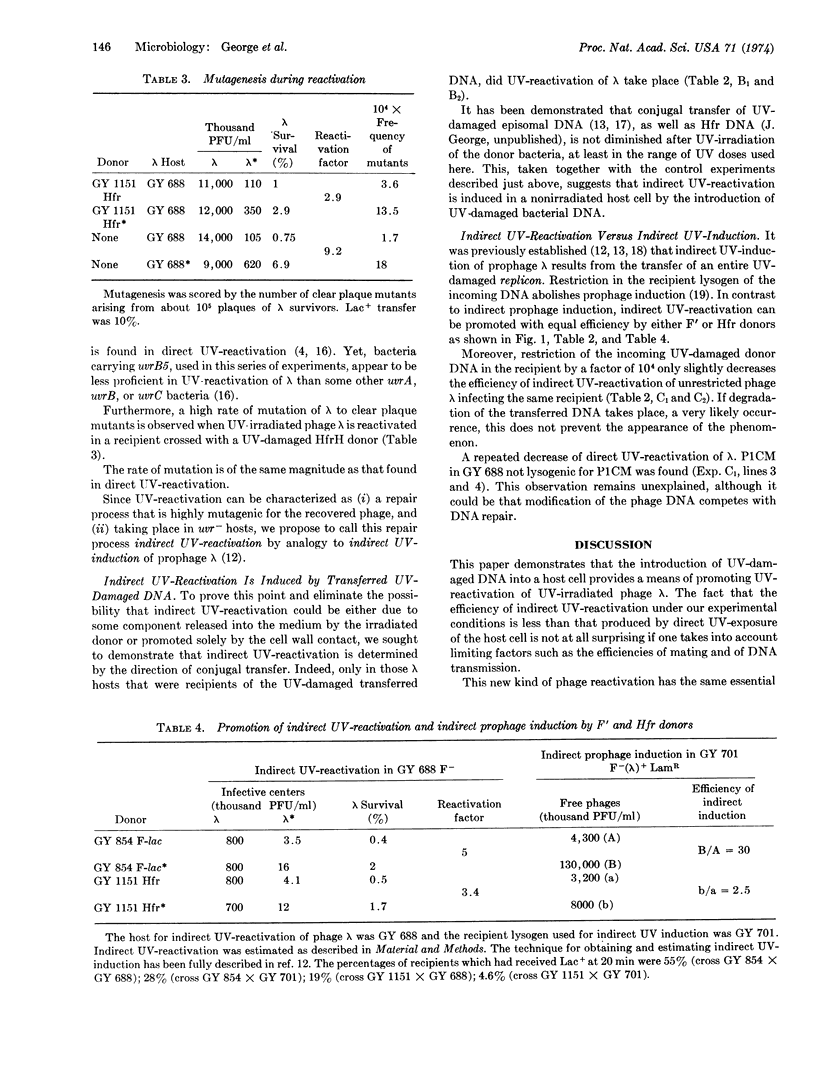
We call this phenomenon indirect ultraviolet-reactivation, since its features are essentially the same as those of ultraviolet-reactivation; this repair process occurs in pyrimidine dimer excision-deficient strains and produces clear plaque mutations of the restored phage. Moreover, this process is similar to indirect ultraviolet-induction of prophage λ, since it is promoted by conjugation. However, contrarily to indirect induction, it is produced by Hfr donors and occurs in recipients restricting the incoming ultraviolet-damaged donor DNA.
The occurrence of indirect ultraviolet-reactivation provides evidence for the existence in E. coli of an inducible error-prone mechanism for the repair of DNA.
Keywords: mutagenesis, repair of DNA, bacterial conjugation, Escherichia coli
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blanco M., Devoret R. Repair mechanisms involved in prophage reactivation and UV reactivation of UV-irradiated phage lambda. Mutat Res. 1973 Mar;17(3):293–305. doi: 10.1016/0027-5107(73)90001-8. [DOI] [PubMed] [Google Scholar]
- Borek E., Ryan A. THE TRANSFER OF IRRADIATION-ELICITED INDUCTION IN A LYSOGENIC ORGANISM. Proc Natl Acad Sci U S A. 1958 May;44(5):374–377. doi: 10.1073/pnas.44.5.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle J. M., Setlow R. B. Correlations between host-cell reactivation, ultraviolet reactivation and pyrimidine dimer excision in the DNA of bacteriophage lambda. J Mol Biol. 1970 Jul 14;51(1):131–144. doi: 10.1016/0022-2836(70)90275-5. [DOI] [PubMed] [Google Scholar]
- Brooks K., Clark A. J. Behavior of lambda bacteriophage in a recombination deficienct strain of Escherichia coli. J Virol. 1967 Apr;1(2):283–293. doi: 10.1128/jvi.1.2.283-293.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellazzi M., George J., Buttin G. Prophage induction and cell division in E. coli. I. Further characterization of the thermosensitive mutation tif-1 whose expression mimics the effect of UV irradiation. Mol Gen Genet. 1972;119(2):139–152. doi: 10.1007/BF00269133. [DOI] [PubMed] [Google Scholar]
- Defais M., Fauquet P., Radman M., Errera M. Ultraviolet reactivation and ultraviolet mutagenesis of lambda in different genetic systems. Virology. 1971 Feb;43(2):495–503. doi: 10.1016/0042-6822(71)90321-7. [DOI] [PubMed] [Google Scholar]
- Demerec M., Adelberg E. A., Clark A. J., Hartman P. E. A proposal for a uniform nomenclature in bacterial genetics. Genetics. 1966 Jul;54(1):61–76. doi: 10.1093/genetics/54.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devoret R., George J. Induction indirecte du prophage lambda par le rayonnement ultraviolet. Mutat Res. 1967 Nov-Dec;4(6):713–734. doi: 10.1016/0027-5107(67)90081-4. [DOI] [PubMed] [Google Scholar]
- Donch J., Greenberg J., Green M. H. Repression of induction by U.V. of lambda phage by exrA mutations in Escherichia coli. Genet Res. 1970 Feb;15(1):87–97. doi: 10.1017/s0016672300001397. [DOI] [PubMed] [Google Scholar]
- George J. Corrélation entre la disparition de l'induction indirecte du prophage lambda et la restriction du DNA transmis à la bactérie réceptrice inductible dans un croisement F+ XFhétérospécifique. C R Acad Sci Hebd Seances Acad Sci D. 1966 Apr 18;262(16):1805–1808. [PubMed] [Google Scholar]
- George J., Devoret R. Conjugal transfer of UV-damaged F-prime sex factors and indirect induction of prophage- . Mol Gen Genet. 1971;111(2):103–119. doi: 10.1007/BF00267786. [DOI] [PubMed] [Google Scholar]
- Harm W. Comment on the relationship between UV reactivation and host-cell reactivation in phage. Virology. 1966 Jul;29(3):494–494. doi: 10.1016/0042-6822(66)90226-1. [DOI] [PubMed] [Google Scholar]
- Hart M. G., Ellison J. Ultraviolet reactivation in bacteriophage lambda. J Gen Virol. 1970 Sep;8(3):197–208. doi: 10.1099/0022-1317-8-3-197. [DOI] [PubMed] [Google Scholar]
- Hertman I., Luria S. E. Transduction studies on the role of a rec+ gene in the ultraviolet induction of prophage lambda. J Mol Biol. 1967 Jan 28;23(2):117–133. doi: 10.1016/s0022-2836(67)80021-4. [DOI] [PubMed] [Google Scholar]
- Howard-Flanders P., Boyce R. P. DNA repair and genetic recombination: studies on mutants of Escherichia coli defective in these processes. Radiat Res. 1966;(Suppl):156+–156+. [PubMed] [Google Scholar]
- KELLENBERGER G., WEIGLE J. Etude au moyen des rayons ultraviolets de l'interaction entre bactériophage tempéré et bactérie hote. Biochim Biophys Acta. 1958 Oct;30(1):112–124. doi: 10.1016/0006-3002(58)90247-6. [DOI] [PubMed] [Google Scholar]
- KONDO E., MITSUHASHI S. DRUG RESISTANCE OF ENTERIC BACTERIA. IV. ACTIVE TRANSDUCING BACTERIOPHAGE P1 CM PRODUCED BY THE COMBINATION OF R FACTOR WITH BACTERIOPHAGE P1. J Bacteriol. 1964 Nov;88:1266–1276. doi: 10.1128/jb.88.5.1266-1276.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby E. P., Jacob F., Goldthwait D. A. Prophage induction and filament formation in a mutant strain of Escherichia coli. Proc Natl Acad Sci U S A. 1967 Nov;58(5):1903–1910. doi: 10.1073/pnas.58.5.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kneser H. Relationship between K-reactivation and UV-reactivation of bacteriophage lambda. Virology. 1968 Oct;36(2):303–305. doi: 10.1016/0042-6822(68)90148-7. [DOI] [PubMed] [Google Scholar]
- Miura A., Tomizawa J. I. Studies on radiation-sensitive mutants of E. coli. 3. Participation of the rec system in induction of mutation by ultraviolet irradiation. Mol Gen Genet. 1968;103(1):1–10. doi: 10.1007/BF00271151. [DOI] [PubMed] [Google Scholar]
- Monk M. Induction of phage lambda by transferred irradiated colI DNA. Mol Gen Genet. 1969;106(1):14–24. doi: 10.1007/BF00332817. [DOI] [PubMed] [Google Scholar]
- Ogawa H., Tomizawa J. I. Ultraviolet reactivation of lambda phage: assay of infectivity of DNA molecules by spheroplast transfection. J Mol Biol. 1973 Feb 5;73(4):397–406. doi: 10.1016/0022-2836(73)90089-2. [DOI] [PubMed] [Google Scholar]
- Radman M., Devoret R. UV-reactivation of bacteriophage lambda in excision repair-deficient hosts: independence of red functions and attachment regions. Virology. 1971 Feb;43(2):504–506. doi: 10.1016/0042-6822(71)90322-9. [DOI] [PubMed] [Google Scholar]
- Rosner J. L., Kass L. R., Yarmolinsky M. B. Parallel behavior of F and Pl in causing indirect induction of lysogenic bacteria. Cold Spring Harb Symp Quant Biol. 1968;33:785–789. doi: 10.1101/sqb.1968.033.01.090. [DOI] [PubMed] [Google Scholar]
- Rupp W. D., Wilde C. E., 3rd, Reno D. L., Howard-Flanders P. Exchanges between DNA strands in ultraviolet-irradiated Escherichia coli. J Mol Biol. 1971 Oct 14;61(1):25–44. doi: 10.1016/0022-2836(71)90204-x. [DOI] [PubMed] [Google Scholar]
- Weigle J. J. Induction of Mutations in a Bacterial Virus. Proc Natl Acad Sci U S A. 1953 Jul;39(7):628–636. doi: 10.1073/pnas.39.7.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin E. M. The mutability toward ultraviolet light of recombination-deficient strains of Escherichia coli. Mutat Res. 1969 Jul-Aug;8(1):9–14. doi: 10.1016/0027-5107(69)90135-3. [DOI] [PubMed] [Google Scholar]


