Abstract
Sarcopenia corresponds to the loss of muscle mass occurring during aging, and is associated with a loss of muscle functionality. Proteomic links the muscle functional changes with protein expression pattern. To better understand the mechanisms involved in muscle aging, we performed a proteomic analysis of Vastus lateralis muscle in mature and older women. For this, a shotgun proteomic method was applied to identify soluble proteins in muscle, using a combination of high performance liquid chromatography and mass spectrometry. A label-free protein profiling was then conducted to quantify proteins and compare profiles from mature and older women. This analysis showed that 35 of the 366 identified proteins were linked to aging in muscle. Most of the proteins were under-represented in older compared with mature women. We built a functional interaction network linking the proteins differentially expressed between mature and older women. The results revealed that the main differences between mature and older women were defined by proteins involved in energy metabolism and proteins from the myofilament and cytoskeleton. This is the first time that label-free quantitative proteomics has been applied to study of aging mechanisms in human skeletal muscle. This approach highlights new elements for elucidating the alterations observed during aging and may lead to novel sarcopenia biomarkers.
A gradual degenerative loss of skeletal muscle mass and function is one of the most consistent hallmarks of normal aging. When it reaches defined thresholds, this condition is referred to as sarcopenia (1, 2), and can be associated with disability, poor quality of life, frailty, and increased mortality (3). Aging impacts the morphology, function and biochemical properties of skeletal muscle, but the mechanisms leading to the changes in muscle tissue remain unclear.
Proteomics links the muscle functional changes with the protein expression pattern. Several proteomic approaches have already been used to study sarcopenia. Protein profiling of whole tissue homogenates has been performed using two-dimensional gel electrophoresis (2DGE)1 and mass spectrometry to identify the proteins differentially expressed during aging in rat (4–6) and human muscle (7, 8). Other studies have focused on specific fractions such as mitochondrial proteins (9), phosphoproteins (10), glycoproteins (11), basic proteins (12), or calpain interacting proteins (13). The few proteomic studies available on human skeletal muscle are mostly based on the 2DGE approach, which implies focusing on a specific pH range (7, 8). Despite its power of high-resolution, 2DGE presents a limited dynamic range and scarcely resolves low abundance regulatory proteins, hydrophobic proteins, and proteins with extreme pI and/or Mr (14).
To circumvent these limitations, we propose in the present study to apply a label-free protein profiling based on a shotgun proteomics approach. This technique permits to identify proteins in a complex mixture after trypsin hydrolysis, using a combination of high performance liquid chromatography and mass spectrometry. In a shotgun analysis previously performed on whole muscle extracts, the major isoforms of myosin heavy-chain comprise ∼42% of the total spectra (15). Because these major isoforms may hamper identification of other proteins, we decided to precipitate myofibrils at low ionic strength (16, 17) and to focus on the soluble fraction. In this paper, we present the analytical steps of label-free quantitation, which resulted in the identification and quantitation of 255 muscle proteins common to all ten individuals. The comparison of protein profiling between mature and older women highlighted 35 differentially expressed proteins during aging, 25 proteins that have not previously been related to muscle aging. The functional interactions network linking these proteins showed that the two main biological processes were represented by proteins involved in energy metabolism and contractile proteins.
EXPERIMENTAL PROCEDURES
Subjects
Patients were included in the Rijnland Hospital (Leiderdorp, The Netherlands), and in the Leiden University Medical Center (Leiden, The Netherlands) between June 2010 to September 2012. Exclusion criteria consisted of previous knee or hip surgery (with the exception of arthroscopy), rheumatoid disease, diabetes mellitus, use of oral corticosteroids, and metastasized malignancy. Ten post-menopausal women undergoing hip surgery for hip arthrosis were selected in the present analysis. The mean age was 53.0 ± 3.5 years (n = 6) for the mature group, and 77.6 ± 2.0 years (n = 4) for the old group. As described in Table I, the mean height, body weight, and body mass index (BMI) were similar between the two groups of women. The study was approved by the medical ethical committees of Leiden University Medical Center and the Rijnland Hospital (P10.060 - HEALTH-2007–2.4.5–10: Understanding and combating age related muscle weakness “MYOAGE”). Written informed consent was obtained from all patients.
Table I. Physical characteristics of mature (n = 6) and old (n = 4) women. Age, weight (kg), height (cm), and Body Mass Index1 (BMI; kg/cm) are expressed as mean ± standard deviation and statistical results are indicated (***: p value < 0.01; NS: Non Significant).
| Mature women n = 6 | Old women n = 4 | Significance | |
|---|---|---|---|
| Age | 53.0 ± 3.5 | 77.6 ± 2.0 | *** |
| Weight, kg | 69.3 ± 14.7 | 74.3 ± 13.0 | NS |
| Height, cm | 166.5 ± 8.9 | 165.3 ± 13.0 | NS |
| BMI1, kg/cm | 25.0 ± 4.9 | 27.1 ± 3.6 | NS |
The Vastus lateralis muscle samples were obtained during surgery, immediately frozen in liquid nitrogen and stored at −80 °C until used.
Protein Extraction
Proteins soluble at low ionic strength (LIS) were extracted from muscle biopsies as described by Sayd et al. (18). Frozen samples were homogenized in 40 mm Tris (pH 7.0), 2 mm EDTA, and protease inhibitors mixture using a TissueRuptor (Qiagen, Courtaboeuf, France). After centrifugation at 4 °C for 10 min at 10,000 × g, the supernatant, referred to as the LIS extract, was stored at −80 °C.
Protein Separation
Samples were mixed with 1 volume 2% (w/v) SDS, 5% β-mercaptoethanol, 10% glycerol and 62 mm Tris-HCl, pH 6.8 (19), and heated at 95 °C for 5 min. SDS-PAGE (12% acrylamide) was performed using a Mini-Protean II electrophoresis unit (BioRad, Marnes-La-Coquette, France). Samples were loaded at 20 μg protein per lane. To concentrate the samples, gels were run at 100 V until the dye front reached the bottom of the concentration gel. Gels were stained overnight in Coomassie brilliant blue G-250. Excised lanes were reduced in 10 mm dithiotreitol in 50 mm acetonitrile, and alkylated in 55 mm iodoacetamide in 50 mm acetonitrile.
Protein Digestion
Each lane was incubated in 25 mm ammonium bicarbonate with acetonitrile 50/50 (v/v) until destaining. After incubation in 100% acetonitrile, gel pieces were dried in a vacuum SpeedVac. They were further rehydrated with 30 μl of a trypsin solution (10 ng/μl in 25 mm ammonium bicarbonate; V5111, Promega), and finally incubated overnight at 37 °C. Peptide extraction was optimized by adding 24 μl of acetonitrile 100% followed by 10 min of sonication. The trypsin digests were dried in a vacuum SpeedVac and stored at −20 °C in a solution of 2% acetonitrile, 0.05% trifluoroacetic acid before LC-MS/MS analysis.
Nano-LC-MS/MS Analysis
For Nano-LC-ESI-MS/MS analysis, peptides mixtures were analyzed in duplicate by online nanoflow liquid chromatography using the Ultimate 3000 RSLC (Dionex, Voisins le Bretonneux, France) with nanocapillary columns of 25 cm length × 75 μm I.D., 3 μm, 100Å (Acclaim PepMap100 C18, Dionex). The solvent gradient increased linearly from 4% to 50% acetonitrile in 0.5% formic acid at a flow rate of 200 nL/min for 100 min. The eluate was then electrosprayed in positive-ion mode at 2.7 kV in a LTQ-VELOS mass spectrometer (Thermo Fisher Scientific, Courtaboeuf, France) through a nanoelectrospray ion source that was operated in a CID top 10 mode (i.e. 1 full scan MS and the 10 major peaks in the full scan were selected for MS/MS). Full-enhanced-scan MS spectra were acquired with 1 microscan (m/z 400 - 1600). Dynamic exclusion was used with 2 repeat counts, 15 s repeat duration and 45 s exclusion duration. For MS/MS, isolation width for ion precursor was fixed at 2 m/z, single charged species were rejected; fragmentation used 37% normalized collision energy as the default activation of 0.25.
Database Search and Protein Identification
Thermo Proteome Discoverer v1.3 was used for raw data file processing, and MASCOT was used for database search (http://www.matrixscience.com). For protein identification, the Uniprot Taxonomy Human (01/10/2012, 84,843 seq) protein database was used. The following parameters were considered for the searches: peptide mass tolerance was set to 1.5 Da, fragment mass tolerance was set to 0.5 Da and a maximum of two missed cleavages was allowed. Variable modifications were methionine oxidation (M) and carbamidomethylation (C) of cysteine. Protein identification was considered valid if at least one peptide with a statistically significant Mascot score assigned it (with Mascot score ≥ 36 for p value < 0.05 with a False Discovery Rate (FDR) at 1%). Identification of proteins based on one peptide was accepted after checking the correct assignment of fragment ion matches (at least three consecutive fragments b/y, match peaks well above the background noise) (supplementary Data). Identifications not satisfying these defined criteria were rejected.
Label-free Quantification
The acquired spectra (Thermo raw files) were loaded into the Progenesis LC-MS software (version 4.1, Nonlinear Dynamics, Newcastle, UK) and label-free quantification was performed. Briefly, for each migration lane from the SDS-PAGE, the profile data of the MS scans as well as MS/MS spectra were transformed to peak lists with Progenesis LC-MS using a proprietary algorithm and then stored in peak lists comprising m/z and abundance. One sample was set as the reference, and the retention times of all other samples within the experiment were automatically aligned to create maximal overlay of the two-dimensional feature maps. At this point, features with only one charge, with retention time windows lower than 6 s or with retention time lower than 20 min and higher than 80 min were masked and excluded from further analyses. All remaining features were used to calculate a normalization factor for each sample that corrects for experimental variation. Samples were then allocated to their experimental group (mature versus older women). For quantification, all unique validated peptides (with Mascot score ≥ 36 for p value < 0.05) of an identified protein were included and the total cumulative abundance was calculated by summing the abundances of all peptides allocated to the respective protein. Statistical analysis was performed using the “between subject design” and p values were calculated by a repeated measures analysis of variance using the normalized abundances across all runs.
Immunoblot Analysis
Western-blot analyses were performed on whole muscle extracts. Muscle aliquots were lysed in ice-cold buffer containing 8.3 m urea, 2 m thiourea, 2% CHAPS, 1% dithiotreitol and extracts were clarified at 10,000 × g for 30 min. Whole muscle extracts (20 μg proteins) were resolved by SDS-PAGE and electrotransferred to HybonTM-P membranes (Dutscher, Brumath, France). Membranes were probed with anti-transgelin (1:8000; Euromedex, Souffelweyersheim, France), anti-medium-chain specific acyl-CoA dehydrogenase (1:2000; Euromedex), anti-alpha-2-HS glycoprotein (1:3000; Euromedex), anti-aspartate aminotransferase (1:2000; Euromedex), anti-ATP synthase subunit alpha (1:1000; Euromedex), and anti-carbonic anhydrase 3 (1:8000; Euromedex), diluted with TTBS (5% milk in 25 mm Tris (pH 7.8), 150 mm NaCl, 0.1% Tween 20). Primary antibodies were resolved with corresponding horseradish peroxidase-linked secondary antibodies (Luminata, Millipore, Molsheim, France), and immunoreactive proteins were detected using enhanced chemiluminescence and a Charge Coupled Device camera (GBOX, Syngene, Cambridge, UK). For normalization, membranes were dehybridized in 1× Re-blot Plus (Millipore) for 30 min, washed three times 5 min with TTBS, and probed overnight with anti-actin (1:10000) in TTBS. To determine the significance of aging, a Student's t test was used; results are expressed as the mean ± standard deviation.
Functional Correlation and Pathway Analysis
The differentially expressed proteins defined by label-free analysis were classified using the Gene Ontology categories “Cellular Component,” “Molecular Function,” and “Biological Process.” Pathway analysis was performed using the Search Tool for the Retrieval of INteracting Genes (String) 9.0 database (http://string-db.org, (20)). String analysis options were based on “evidence” mode, disconnected nodes were hidden, we did not add or remove any protein partners, and we used clustering by K means to reveal subgrouping within the network.
RESULTS AND DISCUSSION
The goals of this study were to identify and quantify muscle proteins during human aging. For that purpose, we used a proteomic approach combining shotgun methodology and label-free quantitation. We compared the protein profiling of muscle low ionic strength (LIS) extracts between two groups of mature and older women to determine the proteins differentially expressed during muscle aging.
Protein Identification
We performed protein extraction from human vastus lateralis muscle to collect the LIS fraction. The amount of proteins extracted was similar between the mature and old women (9.30 ± 2.02 mg/ml and 9.47 ± 1.51 mg/ml respectively), indicating that extractability of LIS fraction was not affected by aging. For each sample, we performed duplicates of LC-MS/MS mass spectrometry analysis. Following the protein digestion of duplicated samples, a total number of 366 proteins were identified (Supplemental Table S1).
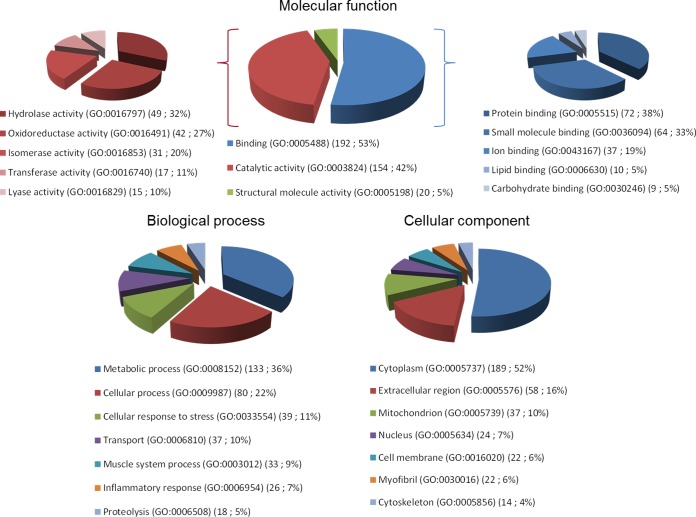
The quantitation analysis by LC-Progenesis was performed on the 255 proteins detected in all 10 samples. All the proteins identified in both mature and older women were classified using the Gene Ontology categories: “Molecular function,” “Biological process,” and “Cellular component” (Fig. 1). The classification of proteins according to their molecular function showed a repartition between binding activity (53%), catalytic activity (42%), and structural molecule activity (5%). The biological process repartition analysis showed that the proteins were mainly involved in metabolic (36%) and cellular (22%) processes. The metabolic processes include protein, lipid, and carbohydrate metabolisms. The cellular component analysis revealed that the main protein localization was cytoplasmic (52%), which is consistent with the extraction method we used. Moreover, a previous proteomic study performed on human vastus lateralis muscle (15), showed that cytoplasmic proteins represented 59% of whole muscle proteome.
Fig. 1.
Identified proteins from LIS fraction of vastus lateralis muscle of mature and old women (Supplemental Table 1) analyzed with the PANTHER bioinformatics tool (http://www.pantherdb.org) using the Gene Ontology categories “Molecular function,” “Biological Process,” and “Cellular Component.” The molecular functions “Catalytic activity” and “Binding” are detailed. For each category, the number of identified proteins and their percentage of the total number of proteins in the pie chart are indicated for each Gene Ontology term.
Label-free Quantitation of Proteins from LIS Fraction
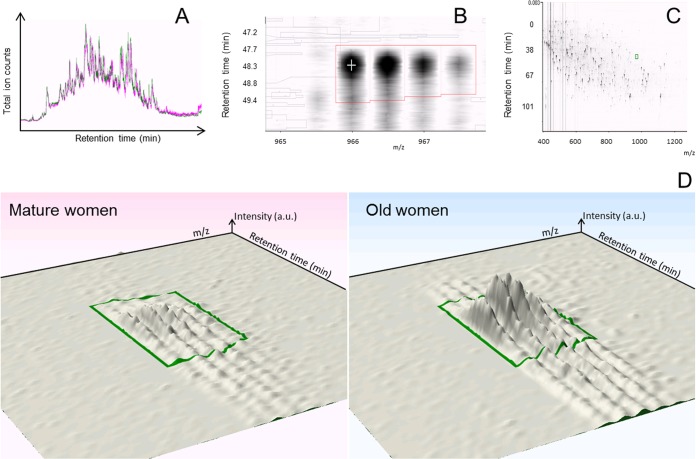
The label-free quantitation analysis of LIS protein fraction from mature and older women was performed with LC-Progenesis software, using an algorithm based on the pairwise features detection at the LC-MS level (21). The total ion chromatograms of LIS protein fraction from mature (pink) and older (green) women are shown in Fig. 2A. A regression analysis based on the liquid chromatography retention time and the m/z of all detected features (example in Fig. 2B) was calculated and resulted in an alignment grid that included all detected peptides (Fig. 2C). One sample was chosen as the reference and the other samples were aligned using on average 357 and 456 alignment vectors in samples from mature and older women respectively. The overall percentage score for the run alignment was on average 85% showing the quality of alignment. For each aligned peptide, the ion intensity was calculated, so that the relative abundances of the peptide among the mature and older women were determined. The fragmentation spectrum of each quantified peptide was introduced in the analysis, so that the protein identity and abundance were obtained and compared between mature and older women (example in Fig. 2D). For each differentially expressed protein quantified from one peptide, we plotted the normalized abundances for all the individuals (supplemental Table S2); for each differentially expressed protein quantified from more than one peptide, the normalized abundances of all the peptides are represented in supplemental Table S3.
Fig. 2.
Quantitative analysis with LCProgenesis software. A, Total ion chromatograms of LIS protein fraction from mature (pink) and old (green) women. B, Detected feature of carbonic anhydrase 3, according to the liquid chromatography retention time and the m/z. C, Alignment grid according to the liquid chromatography retention time and the m/z of all the detected peptides. D, Representation of the abundance of one peptide from carbonic anhydrase 3 protein within mature (pink) and old (blue) women representative samples.
The results of the label-free quantitation analysis performed on the 255 LIS proteins indicated that 35 were significantly differentially expressed between mature and older women (Table II). Principal component analysis (PCA) was applied to reveal variances or combination of variables among these differentially expressed proteins. The analysis resulted in a good separation between mature and older women according to the principal component one with 42.6% of explained variance (Fig. 3). Statistical analysis revealed that 29 proteins were under-represented and six proteins were over-represented in the older women (Table II).
Table II. Significantly differentially expressed proteins of LIS fraction from vastus lateralis muscle of mature and old women, quantified by label-free mass spectrometry.
| Gene | UniProt accession | Protein name | Ratio (Old/Mature women) | Quantitation analysis (p value) | Western-Blot (p value) | Peptides used for quantitation | Mascot Score | Main biological function |
|---|---|---|---|---|---|---|---|---|
| ACADM | P11310 | Medium-chain specific acyl-CoA dehydrogenase, mitochondrial | −1.16 | 0.01 | 0.05 | 2 | 119 | Energy metabolism |
| ATP5A1 | P25705 | ATP synthase subunit alpha, mitochondrial | −1.12 | 0.01 | 0.05 | 3 | 193 | Energy metabolism |
| ATP5B | P06576 | ATP synthase subunit beta, mitochondrial | −1.11 | 0.04 | 3 | 107 | Energy metabolism | |
| CRAT | P43155 | Carnitine O-acetyltransferase | −1.21 | 0.001 | 1 | 50 | Energy metabolism | |
| FH | P07954 | Fumarate hydratase, mitochondrial | −1.27 | 0.004 | 2 | 163 | Energy metabolism | |
| GOT2 | P00505 | Aspartate aminotransferase, mitochondrial | −1.22 | 0.02 | 0.05 | 9 | 575 | Energy metabolism |
| LDHB | P07195 | l-lactate dehydrogenase B chain | −1.20 | 0.046 | 11 | 831 | Energy metabolism | |
| SLC25A4 | P12235 | ADP/ATP translocase 1 | −1.19 | 0.03 | 1 | 38 | Energy metabolism | |
| TALDO1 | P37837 | Transaldolase | −1.16 | 0.03 | 1 | 75 | Energy metabolism | |
| FABP4 | P15090 | Fatty acid-binding protein, adipocyte | 1.69 | 0.002 | 2 | 75 | Energy metabolism | |
| PFKL | P17858 | 6-phosphofructokinase, liver type | 1.41 | 0.03 | 1 | 58 | Energy metabolism | |
| MYH1 | P12882 | Myosin-1 | −1.16 | 0.002 | 2 | 64 | Myofibrillar proteins | |
| MYL1 | P05976 | Myosin light chain 1/3, skeletal muscle isoform | −1.29 | 0.003 | 1 | 50 | Myofibrillar proteins | |
| TTN | Q8WZ42 | Titin | −1.19 | 0.01 | 8 | 401 | Myofibrillar proteins | |
| ANKRD2 | Q9GZV1 | Ankyrin repeat domain-containing protein 2 | 1.25 | 0.02 | 2 | 101 | Myofibrillar proteins | |
| ACTB | P60709 | Actin, cytoplasmic 1 | −1.16 | 0.01 | 7 | 342 | Actin microfilament | |
| FLNC | Q14315 | Filamin-C | −1.15 | 0.03 | 3 | 148 | Actin microfilament | |
| ANXA2 | P07355 | Annexin A2 | −1.18 | 0.03 | 2 | 132 | Actin microfilament | |
| CFL1 | P23528 | Cofilin-1 | 1.47 | 0.03 | 1 | 43 | Actin microfilament | |
| TAGLN | Q01995 | Transgelin | 1.33 | 0.03 | 0.001 | 1 | 91 | Actin microfilament |
| BLVRB | P30043 | Flavin reductase (NADPH) | −1.30 | 0.01 | 1 | 86 | Detoxification, Cytoprotection | |
| HSPA4 | P34932 | Heat shock 70 kDa protein 4 | −1.24 | 0.01 | 2 | 61 | Detoxification, Cytoprotection | |
| OAS2 | P29728 | 2′−5′-oligoadenylate synthase 2 | −1.14 | 0.04 | 1 | 42 | Detoxification, Cytoprotection | |
| CA3 | P07451 | Carbonic anhydrase 3 | 1.41 | 0.04 | 0.05 | 16 | 1464 | Detoxification, Cytoprotection |
| APEH | P13798 | Acylamino-acid-releasing enzyme | −1.11 | 0.03 | 1 | 41 | Protein turnover | |
| RPS27A | P62979 | Ubiquitin-40S ribosomal protein S27a | −1.20 | 0.0002 | 2 | 106 | Protein turnover | |
| EEF2 | P13639 | Elongation factor 2 | −1.14 | 0.048 | 4 | 159 | Protein turnover | |
| PRKAR2A | P13861 | cAMP-dependent protein kinase type II-alpha regulatory subunit | −1.18 | 0.03 | 1 | 62 | Signal transduction | |
| YWHAE | P62258 | 14–3-3 protein epsilon | −1.17 | 0.0003 | 7 | 220 | Signal transduction | |
| AHSG | P02765 | Alpha-2-HS-glycoprotein | −1.23 | 0.01 | 0.05 | 1 | 55 | Serum |
| FGA | P02671 | Fibrinogen alpha chain | −1.20 | 0.01 | 4 | 142 | Serum | |
| HV304 | P01765 | Ig heavy chain V-III region BRO | −1.19 | 0.046 | 1 | 76 | Serum | |
| KV402 | P01625 | Ig kappa chain V-IV region Len | −1.14 | 0.02 | 2 | 128 | Serum | |
| C4A | P0C0L4 | Complement C4-A | −1.22 | 0.002 | 2 | 115 | Serum | |
| HBG1 | P69891 | Hemoglobin subunit gamma-1 | −1.31 | 0.002 | 1 | 69 | Serum |
Fig. 3.
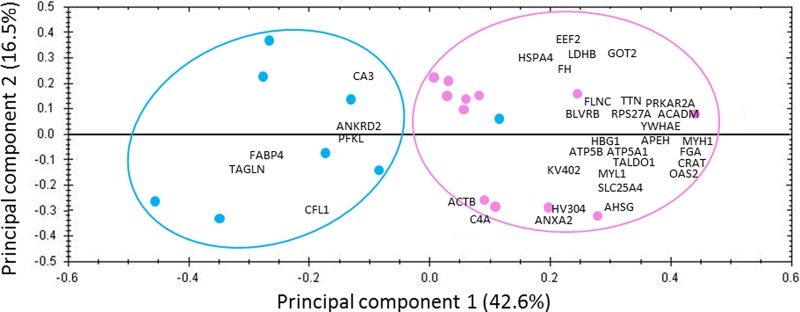
Score plot and loadings of principal component analysis from quantified proteins in LIS fraction of vastus lateralis from mature and old women. Discriminations are on principal component 1, and revealed the protein expressions related to muscle aging. The pink line delineates the mature women (pink circles) and the blue line delineates the old women (blue circles). The differentially expressed proteins are marked by abbreviations: ACADM, medium-chain specific acyl-CoA dehydrogenase, mitochondrial; ACTB, actin, cytoplasmic 1; AHSG, alpha-2-HS glycoprotein; ANKRD2, ankyrin repeat domain-containing protein 2; ANXA2, annexin 2; APEH, acylamino-acid-releasing enzyme; ATP5A1, ATP synthase subunit alpha, mitochondrial; ATP5B, ATP synthase subunit beta, mitochondrial; BLVRB, flavin reductase (NADPH); C4A, complement C4-A; CA3, carbonic anhydrase 3; CFL1, cofilin-1; CRAT, carnitine O-acetyltransferase; EEF2, elongation factor 2; FABP4, fatty acid-binding protein, adipocyte; FGA, fibrinogen alpha chain; FH, fumarate hydratase, mitochondrial; FLNC, filamin-C; GOT2, aspartate aminotransferase, mitochondrial; HBG1, hemoglobin subunit gamma-1; HSPA4, heat shock 70 kDa protein 4; HV304, Ig heavy chain V-III region TEI; KV402, Ig kappa chain V-IV region Len; LDHB, l-lactate dehydrogenase B chain; MYH1, myosin-1; MYL1, myosin light chain 1/3, skeletal muscle isoform; OAS2, 2′-5′-oligoadenylate synthase 2; PFKL, 6-phosphofructokinase, liver type; PRKAR2A, cAMP-dependent protein kinase type II-alpha regulatory subunit; RPS27A, ubiquitin-40S ribosomal protein 27A; SLC25A4, ADP/ATP translocase 1; TAGLN, transgelin; TALDO1, transaldolase; TTN, titin; YWHAE, 14–3-3 protein epsilon.
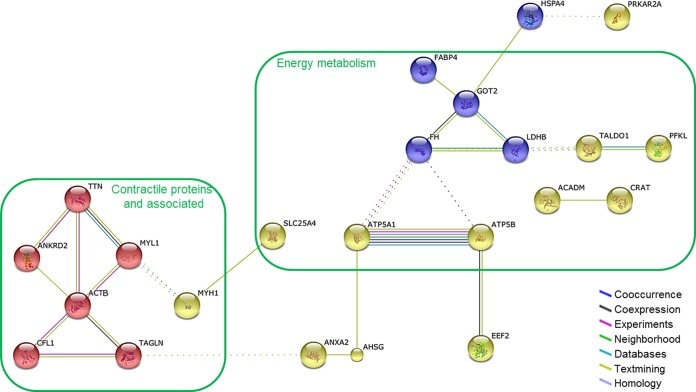
We performed pathway analysis to determine the functional interactions network linking the differentially expressed proteins between mature and older women. For this, we data mined these findings with the String database (20). This analysis showed that 23 out the 35 proteins were implicated in the resulting network (Fig. 4). To determine the substructure of the network, we applied a K-mean classification that revealed three groups of proteins. The first one (red cluster) was mainly composed by contractile proteins and associated: titin (TTN), ankyrin repeat domain-containing protein 2 (ANKRD2), myosin light chain 1/3 skeletal muscle isoform (MYL1), myosin-1 (MYH1), actin cytoplasmic 1 (ACTB), cofilin-1 (CFL1), and transgelin (TAGLN). The second group (blue cluster) included predominantly proteins involved in energy metabolism: fatty acid binding protein adipocyte (FABP4), aspartate aminotransferase mitochondrial (GOT2), fumarate hydratase mitochondrial (FH), and l-lactate dehydrogenase B chain (LDHB); one protein with a different biological function was related to this cluster: the heat shock protein 70 kDa protein 4 (HSPA4). The third group (yellow cluster) also contained proteins involved in energy metabolism: transaldolase (TALDO1), 6-phosphofructokinase liver type (PFKL), ATP synthase subunit alpha mitochondrial (ATP5A1), ATP synthase subunit beta mitochondrial (ATP5B), medium-chain specific acyl-CoA dehydrogenase mitochondrial (ACADM), carnitine O-acetyltransferase (CRAT), and ADP/ATP translocase 1 (SLC25A4); and other linked proteins: alpha-2-HS-glycoprotein (AHSG), elongation factor 2 (EEF2), and annexin A2 (ANXA2). One more protein was related to this cluster: cAMP-dependent protein kinase type II-alpha regulatory subunit (PRKAR2A). The functional interactions network we built through all the significantly differentially expressed proteins revealed that the main impact of aging on the skeletal muscle LIS proteome was on energy metabolism and contractile properties.
Fig. 4.
Functional interactions network linking the significantly differentially expressed proteins between mature and old women (String database) (20). K-mean classification revealed three substructures mainly represented by contractile and energy metabolism proteins. The proteins involved in the network are marked by abbreviations: ACADM, medium-chain specific acyl-CoA dehydrogenase, mitochondrial; ACTB, actin, cytoplasmic 1; AHSG, alpha-2-HS glycoprotein; ANKRD2, ankyrin repeat domain-containing protein 2; ANXA2, annexin 2; ATP5A1, ATP synthase subunit alpha, mitochondrial; ATP5B, ATP synthase subunit beta, mitochondrial; CFL1, cofilin-1; CRAT, carnitine O-acetyltransferase; EEF2, elongation factor 2; FABP4, fatty acid-binding protein, adipocyte; FH, fumarate hydratase, mitochondrial; GOT2, aspartate aminotransferase, mitochondrial; HSPA4, heat shock 70 kDa protein 4; LDHB, l-lactate dehydrogenase B chain; MYH1, myosin-1; MYL1, myosin light chain 1/3, skeletal muscle isoform; PFKL, 6-phosphofructokinase, liver type; PRKAR2A, cAMP-dependent protein kinase type II-alpha regulatory subunit; SLC25A4, ADP/ATP translocase 1; TAGLN, transgelin; TALDO1, transaldolase; TTN, titin.
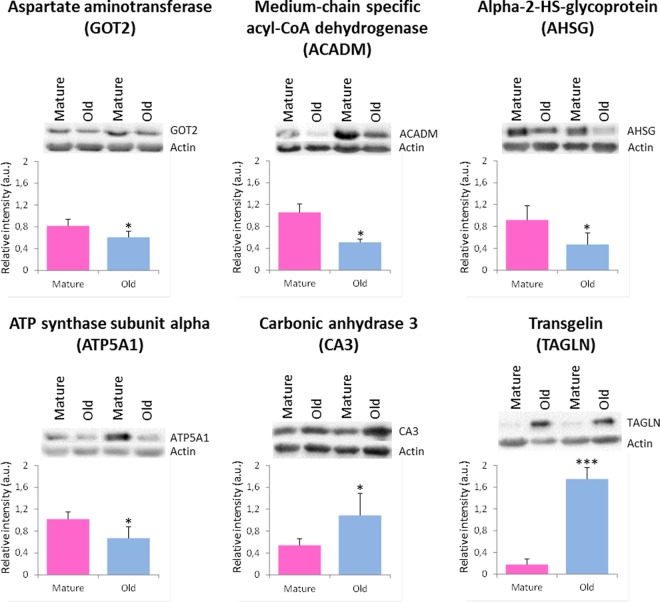
To select proteins as candidates to be validated by Western-blot analysis, we chose to focus on the main biological functions, i.e. “energy metabolism,” “actin myofilament,” “detoxification and cytoprotection,” and “serum proteins.” Thus, we studied transgelin abundance within mature and old women because it was identified and quantified by one peptide; transgelin belongs to the cytoskeleton. Then, we studied medium-chain specific acyl-CoA dehydrogenase, aspartate aminotransferase and ATP synthase subunit alpha abundances within mature and old women. We chose these proteins because their main biological function corresponds to energy metabolism and they were all less abundant in older women. We chose carbonic anhydrase 3, which belong to the detoxification and cytoprotection cluster, to confirm its higher abundance in old women because its expression was previously described in literature. We studied alpha-2-HS glycoprotein abundance within mature and old women because it was identified and quantified by one peptide, and it belongs to the serum proteins. Some of these proteins are identified for the first time to be involved in age related sarcopenia. Their validation by Western-blot would confirm their relevance in our biological system. To check that there was no difference in the overall protein expression pattern between individuals used in this study, we performed a control full length protein gel (supplementary Data 2). Statistical results revealed that all of these six candidates proteins were validated by Western-blot analysis (Fig. 5), because the abundances calculated within mature and old women groups were statistically different: aspartate aminotransferase (p value < 0.01), medium-chain specific acyl-CoA dehydrogenase (p value < 0.01), alpha-2-HS glycoprotein (p value < 0.01), ATP synthase subunit alpha (p value < 0.01), carbonic anhydrase 3 (p value < 0.01), and transgelin (p value < 0.001).
Fig. 5.
Representative Western blot of aspartate aminotransferase (GOT2), medium-chain specific acyl-CoA dehydrogenase (ACADM), alpha-2-HS glycoprotein (AHSG), ATP synthase subunit alpha (ATP5A1), carbonic anhydrase 3 (CA3), and transgelin (TAGLN), between mature (pink) and old (blue) women. Results are indicated as mean ± standard deviation. Significance: *: p value < 0.05 and ***: p value < 0.001.
All the differentially expressed proteins that we identified as differentially expressed by shotgun proteomics can be classified according to their main biological function: energy metabolism, myofibrillar proteins, actin microfilament, detoxification and cytoprotection, protein turnover, signal transduction, and serum proteins.
Energy Metabolism
Overall, proteins involved in energy metabolism showed a lower abundance in older women. Indeed, among the 11 proteins showing significant differences, nine were less abundant in older women. Of note, only four of those proteins were previously mentioned in proteomics studies of muscle aging.
l-lactate dehydrogenase B chain catalyzes the interconversion of pyruvate and lactate with concomitant interconversion of NADH and NAD+. The down-regulation during aging of l-lactate dehydrogenase is in agreement with a proteomic study performed on rat gastrocnemius muscle (22), although an up-regulation has also been reported by (23). The down-regulation of l-lactate dehydrogenase is in agreement with reduced lactate dehydrogenase activity previously observed in human muscle during aging (24). Mitochondrial aspartate aminotransferase takes part in amino acid metabolism and in the malate-aspartate shuttle, which is essential for mitochondrial oxidation of glycolytic NADH. In proteomic studies performed on human vastus lateralis muscle, mitochondrial aspartate aminotransferase was also found to be down-regulated during aging (9), whereas its cytoplasmic isoform was up-regulated (7). ATP synthase subunits alpha and beta form the catalytic core of mitochondrial membrane ATP synthase, which produces ATP from ADP in the presence of a proton gradient across the membrane. The down-regulation during aging of the ATP synthase chains agrees with previous studies on rat gastrocnemius muscle (22, 25), but disagrees with others that found higher ATP synthase contents in old compared with young muscles (7, 23). In agreement with our observation, Papa (26) reported decreased mitochondrial ATPase activity in old human muscle when compared with young muscle.
All other proteins involved in energy metabolism have not been previously identified in proteomics studies of muscle aging. Transaldolase is implicated in the nonoxidative phase of the pentose phosphate pathway, and is responsible for the generation of NADPH to protect cellular integrity from reactive oxygen species (27). Fumarate hydratase catalyzes the reversible hydration/dehydration of fumarate to malate in the Krebs cycle. ADP/ATP translocase 1 catalyzes the exchange of cytoplasmic ADP with mitochondrial ATP across the mitochondrial inner membrane. Medium-chain specific acyl-CoA dehydrogenase is involved in mitochondrial fatty acid β-oxidation and is specific for acyl chain lengths of 4 to 16. Carnitine O-acetyltransferase is important for short-chain fatty acid metabolism (28), and has recently been described as a modulator of whole body glucose homeostasis (29). Notably, the muscle-specific loss of carnitine O-acetyltransferase function has been reported to promote insulin-resistance (29), which may be central for the age-related development of metabolic syndrome in the elderly.
Two proteins were more abundant in older women: fatty acid binding protein (FABP), adipocyte; and 6-phosphofructokinase, liver type. The 6-phosphofructokinase protein catalyzes the third step of glycolysis, i.e. the phosphorylation of fructose-6-phosphate by ATP to generate fructose 1,6-biphosphate and ADP. Fatty acid binding protein is implicated in intracellular lipid transport (30); FABP adipocyte type was found to be expressed in skeletal muscle but to a lower extent than FABP heart type (31). The up-regulation during muscle aging of FABP4 may relate to an increased number of adipocytes and/or to an increased expression in skeletal muscle fibers. The over-expression of FABP adipocyte type during muscle aging agrees with previous studies (32, 33) indicating an age-related increase in FABP heart type, and this was interpreted as a shift of old fibers to aerobic oxidative metabolism and slower twitching activity.
Myofibrillar Proteins
Ankyrin repeat domain-containing protein 2 is localized in both the nucleus and the sarcomeric I-band, and is involved in a mechano-signaling pathway that links myofibrillar stress to gene expression (34). It is preferentially expressed in slow type I fibers of human muscle (35), and its up-regulation is in agreement with a fast-to-slow transition during aging. Ankyrin repeat-domain containing protein 2 is also induced by denervation (36). Its up-regulation during muscle aging is then consistent with the development of sarcopenia because an impaired capacity for axonal reinnervation of deinnervated muscle fibers has been shown in aged animals (37).
Although the bulk of the myofibrillar proteins precipitate, a small percentage of them is easily releasable and found in the LIS extract (17). The three myofibrillar proteins, which we identified in muscle LIS extract as differentially expressed during aging, were less abundant in older women: myosin-1; titin, and myosin light chain 1/3, skeletal muscle isoform. Myosin, the motor protein for muscular contraction, is a hexameric protein that consists of two heavy chain subunits (which myosin-1 belongs to), two alkali light chain subunits and two regulatory light chain subunits (which myosin light chain 1/3 belongs to). Titin is essential for myofibrillar assembly by connecting the Z line to the M line in the sarcomere, and represents also a regulatory node for various transduction pathways (38). The function of the easily releasable proteins remains unknown, although they were suggested to represent intermediate products in the turnover of myofibrillar proteins (39, 17). Therefore their reduced levels in old LIS extract may suggest a decreased myofibrillar protein turnover in the old muscle.
Actin Microfilament
Five cytoskeletal proteins were differentially expressed during muscle aging. Actin cytoplasmic 1 (or β-actin), annexin A2 and filamin-C were down-regulated between mature and older women. Only β-actin has been previously reported to be modified in a muscle aging study, and was similarly found to decline in old human vastus lateralis muscle (8). Polymerization of β-actin leads to the formation of cytoskeletal actin microfilaments. Annexin A2 can bind specifically to actin and this binding has been linked to the formation and/or stabilization of actin cytoskeleton at cellular membranes (40). Filamin-C is a muscle specific actin-binding protein, that interacts with both Z-disc proteins and sarcolemma-associated proteins and is involved in membrane anchorage of myofibrils. Notably, filamin C mutations are responsible for a myofibrillar myopathy, which preferentially occurs as a result of aging-related impairments in the proteolytic machinery (41).
Two cytoskeletal proteins were more abundant in older women: cofilin-1, and transgelin. Cofilin-1 binds to β-actin and regulates actin cytoskeleton organization (42, 43). Although we observed an age-related increase in the nonmuscle isoform, cofilin-1, previous studies in rats demonstrated an increase in the muscle isoform cofilin-2 (44). Transgelin is an actin stress fiber-associated protein that acts to stabilize actin polymers (45). Therefore, our results showed a global age-related deregulation of cytoskeletal proteins that are related to actin microfilaments. Previous studies in rat muscle (4, 46) also showed an altered expression of proteins of the intermediate filament and microtubule network. The differential expression of cytoskeletal proteins during aging may be important for cellular integrity and for the reorganization of contractile structures.
Detoxification and Cytoprotection
Four proteins with detoxification and cytoprotection functions are found to be differentially expressed during aging. Only carbonic anhydrase 3 was more abundant in older women and previously reported in muscle aging studies (44, 8). Carbonic anhydrase catalyzes the reversible hydration of carbon dioxide and this increase with aging is interpreted as a higher demand for efficient CO2 removal during muscle aging (8). Slow-twitch muscles present higher level of carbonic anhydrase 3, and this observation is in accordance with a fast-to-slow transition of the old skeletal muscle. The protein 2′-5′-oligoadenylate synthase 2 uses ATP in 2′-specific nucleotidyl transfer reactions to synthesize 2′-5′-oligoadenylates, which activates latent ribonuclease, resulting in degradation of viral RNA and inhibition of virus replication. NADPH-flavin reductase is the fetal form of biliverdine reductase, and has been found to be expressed in adult human skeletal muscle (47, 48). NADPH-flavin reductase catalyzes the NADPH-dependent reduction of FMN, FAD, riboflavin and biliverdin (49), which produces bilirubin, a recognized potent antioxidant (50, 51). Heat shock 70 kDa protein 4 is a chaperone not inducible by heat-shock and a component of the cytosolic protein folding machinery (52). The age-related decrease in NADPH-flavin reductase and heat shock 70 kDa protein 4 may promote ROS-induced oxidative damages and the accumulation of aggregated proteins.
Protein Turnover
Three proteins related to protein turnover were found to significantly decline during aging, elongation factor 2, ubiquitin-40S ribosomal protein S27a, and acylamino-acid-releasing enzyme; none has been previously reported in muscle aging studies. Elongation factor 2 determines the GTP-dependent ribosomal translocation step during translation elongation, and is essential for the regulation of protein synthesis (53). Ubiquitin-40S ribosomal protein S27a is a fusion protein that is post-translationally processed to generate ubiquitin and protein S27a, a component of the 40S ribosome. Ubiquitin-40S ribosomal protein S27a thus contributes not only to the cellular ubiquitin pool and to proteolysis, but also the 40S ribosome and to protein synthesis. In response to ribosomal stress, protein S27a is also important for p53 activation (54). Acylamino-acid-releasing enzyme catalyzes the hydrolysis of the N-terminal peptide bond of an N-acetylated protein to generate a protein with a free N terminus. Acylamino-acid-releasing enzyme acts cooperatively with the proteasome, and its inhibition triggers proteasome down-regulation (55). Acylamino-acid-releasing enzyme is also involved in the degradation of oxidatively damaged proteins (56). The age-related decline in elongation factor 2, ubiquitin-40S ribosomal protein S27a and acylamino-acid-releasing enzyme may then be important for the regulation of protein turnover and oxidative stress during muscle aging (14).
Signal Transduction
Two proteins with signaling functions were less abundant in older women than in mature women: cAMP-dependent protein kinase type II-alpha regulatory subunit, and14–3-3 protein epsilon. cAMP-dependent protein kinase type II-alpha regulatory subunit is involved in cAMP signaling in cells, and has not been previously reported in muscle aging studies. The 14–3-3 protein epsilon belongs to the 14–3-3 family of proteins, which mediate signal transduction by binding to phosphoserine-containing proteins. The 14–3-3 protein epsilon was similarly found to be down-regulated during aging in the gastrocnemius muscle of rats (22).
Serum Proteins
Six serum proteins were found to be less abundant in older women than in mature women: alpha-2-HS-glycoprotein, fibrinogen alpha chain, Ig heavy chain V-III region TEI, and Ig kappa chain V-IV chain Len, complement C4-A protein, and hemoglobin subunit gamma-1. Alpha-2-HS-glycoprotein is involved in endocytosis. Immunoglobulin proteins are involved in the immune response, and complement C4-A protein plays a central role in the activation of the classical pathway of the complement system. Fibrinogen has a double function: yielding monomers that polymerize into fibrin and acting as a cofactor in platelet activation. Hemoglobin subunit gamma-1 was down-regulated between mature and old women; this result agrees with previous studies (57, 33). The decrease in serum proteins during muscle aging is in agreement with an impaired blood-flow distribution in aged fibers (58).
CONCLUSION
The purpose of this study was to elucidate the implication of modifications in the LIS protein fraction in the mechanisms of muscle aging. The label-free analysis revealed that 35 proteins were differentially expressed during aging. Most of these proteins were down-regulated in the muscles of the older women, and were mainly proteins involved in energy metabolism and proteins from the myofilament and cytoskeleton. The functional interactions network linking the differentially expressed proteins between mature and old women provides new insight into the origin of both structural and functional changes induced by aging in skeletal muscle. To our knowledge, this study is the first to apply shotgun analysis to aging in human skeletal muscle. Thanks to this global approach, we identified new proteins linked to sarcopenia, involved in different biological processes such as energy metabolism, muscle process or proteolysis.
Supplementary Material
Acknowledgments
We thank Professor Philippe Courpron for his constant support, Franck Giacomoni and Christophe Duperier for informatics assistance.
Footnotes
Author contributions: L.T., A.B., G.B., A.M., D.B., and C. Chambon designed research; L.T., M.G., C. Coudy, D.V., and C. Chambon performed research; L.T., M.G., C. Coudy, D.V., D.B., and C. Chambon contributed new reagents or analytic tools; L.T., D.B., and C. Chambon analyzed data; L.T., D.B., and C. Chambon wrote the paper.
* This work was supported by grants from European Commission MyoAge (EC Fp7 CT-223756), Caisse d'Epargne Rhone Alpes (CERA), and Fonds Européens de Développement Régional (FEDER). LT was supported by a postdoctoral fellowship from FEDER (n°35380 T2a 2011 Prenusa), and MG by a postgraduate fellowship from Région Auvergne and FEDER (n°23000422).
 This article contains supplemental Tables S1 to S3 and Data Files S1 and S2.
This article contains supplemental Tables S1 to S3 and Data Files S1 and S2.
1 The abbreviations used are:
- 2DGE
- two-dimensional gel electrophoresis
- LIS
- low ionic strength
- FDR
- false discovery rate
- PCA
- principal component analysis.
REFERENCES
- 1. Bijlsma A. Y., Meskers C. G., Ling C. H., Narici M., Kurrle S. E., Cameron I. D., Westendorp R. G., Maier A. B. (2013) Defining sarcopenia: the impact of different diagnostic criteria on the prevalence of sarcopenia in a large middle aged cohort. Age 35(3), 871–881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cruz-Jentoft A. J., Baeyens J. P., Bauer J. M., Boirie Y., Cederholm T., Landi F., Martin F. C., Michel J. P., Rolland Y., Schneider S. M., Topinkova E., Vandewoude M., Zamboni M. (2010) Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 39, 412–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Szulc P., Munoz F., Marchand F., Chapurlat R., Delmas P. D. (2010) Rapid loss of appendicular skeletal muscle mass is associated with higher all-cause mortality in older men: the prospective MINOS study. American Journal of Clinical Nutrition 91(5), 1227–1236 [DOI] [PubMed] [Google Scholar]
- 4. Piec I., Listrat A., Alliot J., Chambon C., Taylor R. G., Bechet D. (2005) Differential proteome analysis of aging in rat skeletal muscle. FASEB J. 19, 1143–1145 [DOI] [PubMed] [Google Scholar]
- 5. Doran P., Gannon J., O'Connell K., Ohlendieck K. (2007) Aging skeletal muscle shows a drastic decrease in the small heat shock proteins αB-crystallin/HspB5 and cvHsp/HspB7. Eur. J. Cell Biol.. 86, 629–640 [DOI] [PubMed] [Google Scholar]
- 6. O'Connell K., Gannon J., Doran P., Ohlendieck K. (2007) Proteomic profiling reveals a severely perturbed protein expression pattern in aged skeletal muscle. Int. J. Mol. Med. 20, 145–153 [PubMed] [Google Scholar]
- 7. Gelfi C., Vigano A., Ripamonti M., Pontoglio A., Begum S., Pellegrino M. A., Grassi B., Bottinelli R., Wait R., Cerretelli P. (2006) The human muscle proteome in aging. J. Proteome Res. 5, 1344–1353 [DOI] [PubMed] [Google Scholar]
- 8. Staunton L., Zweyer M., Swandulla D., Ohlendieck K. (2012) Mass spectrometry-based proteomic analysis of middle-aged vs. aged vastus lateralis reveals increased levels of carbonic anhydrase isoform 3 in senescent human skeletal muscle. Int. J. Mol. Med. 30, 723–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Short K., Bigelow M. L., Kahl J., Singh R., Coenen-Schimke J. C., Raghavakaimal S., Nair S. (2005) Decline in skeletal muscle mitochondrial function with aging in humans. Proc. Natl. Acad. Sci. U.S.A. 102, 5618–5623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gannon J., Staunton L., O'Connell K., Doran P., Ohlendieck K. (2008) Phosphoproteomic analysis of aged skeletal muscle. Int. J. Mol. Med. 22, 33–42 [PubMed] [Google Scholar]
- 11. O'Connell K., Doran P., Gannon J., Ohlendieck K. (2008) Lectin-based proteomic profiling of aged skeletal muscle: Decreased pyruvate kinase isozyme M1 exhibits drastically increased levels of N-glycosylation. Eur. J. Cell Biol. 87, 793–805 [DOI] [PubMed] [Google Scholar]
- 12. Gannon J., Ohlendieck K. (2012) Subproteomic analysis of basic proteins in aged skeletal muscle following offgel pre-fractionation. Mol. Med. Reports 5, 993–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brule C., Dargelos E., Diallo R., Listrat A., Bechet D., Cottin P., Poussard S. (2010) Proteomic study of calpain interacting proteins during skeletal muscle aging. Biochimie 90, 359–368 [DOI] [PubMed] [Google Scholar]
- 14. Baraibar M. A., Gueugneau M., Duguez S., Butler-Browne G., Bechet D., Friguet B. (2013) Expression and modification proteomics during skeletal muscle ageing. Biogerontology 14(3), 339–352 [DOI] [PubMed] [Google Scholar]
- 15. Hojlund K., Yi Z., Hwang H., Bowen B., Lefort N., Flynn C. R., Langlais P., Weintraub S. T., Mandarino L. J. (2008) Characterization of the human skeletal muscle proteome by one-dimensional gel electrophoresis and HPLC-ESI-MS/MS. Mol. Cell.Proteomics 7, 257–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Martin A. F., Rabinowitz M., Blough R., Prior G., Zak R. (1977) Measurements of half-life of rat cardiac myosin heavy chain with leucyl-tRNA used as precursor pool. J. Biol. Chem.. 252, 3422–3429 [PubMed] [Google Scholar]
- 17. Neti G., Novak S. M., Thompson V. F., Goll D. E. (2009) Properties of easily releasable myofilaments: are they the first step in myofibrillar protein turnover? Am. J. Cell Physiol. 296, 1383–1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sayd T., Morzel M., Chambon C., Franck M., Figwer P., Larzul C., Le Roy P., Monin G., Cherel P., Laville E. (2006) Proteome analysis of the sarcoplasmic fraction of pig semimembranosus muscle: Implications on meat color development. J. Agricultural Food Chem.. 24, 2732–2737 [DOI] [PubMed] [Google Scholar]
- 19. Laemmli U. K. (1970) Cleavage structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685 [DOI] [PubMed] [Google Scholar]
- 20. Szklarczyk D., Franceschini A., Kuhn M., Simonovic M., Roth A., Minguez P., Doerks T., Stark M., Muller J., Bork P., Jensen L. J., von Mering C. (2011) The STRING database in 2011: functional interaction networks of proteins, globally integrated and scored. Nucleic Acid Res. 39, 561–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhu W., Smith J. W., Huang C. M. (2010) Mass spectrometry-based label-free quantitative proteomics. J. Med. Biotechnol. 840518, 1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Altun M., Edstrom E., Spooner E., Flores-Moralez A., Bergman E., Tollet-Egnell P., Norstedt G., Kessler B. M., Ulfhake B. (2007) Iron load and redox stress in skeletal muscle of aged rats. Muscle Nerve 36, 223–233 [DOI] [PubMed] [Google Scholar]
- 23. Donoghue P., Staunton L., Mullen E., Manning G., Ohlendieck K. (2010) DIGE analysis of rat skeletal muscle proteins using nonionic detergent phase extraction of young adult versus aged gastrocnemius tissue. J. Proteomics 73, 1441–1453 [DOI] [PubMed] [Google Scholar]
- 24. Pastoris O., Boschi F., Verri M., Baiardi P., Felzani G., Vecchiet J., Dossena M., Catapano M. (2000) The effects of aging on enzyme activities and metabolite concentrations in skeletal muscle from sedentary male and female subjects. Exp. Gerontol. 35, 95–104 [DOI] [PubMed] [Google Scholar]
- 25. Lombardi A., Silvestri E., Cioffi F., Senese R., Lanni A., Goglia F., De Lange P., Moreno M. (2009) Defining the transcriptomic and proteomic profiles of rat ageing skeletal muscle by the use of a cDNA array, 2D- and Blue native-PAGE approach. J. Proteomics 72, 708–721 [DOI] [PubMed] [Google Scholar]
- 26. Papa S. (1996) Mitochondrial oxidative phosphorylation changes in the life span. Molecular aspects and physiopathological implications. Biochim. Biophys. Acta 87–105 [DOI] [PubMed] [Google Scholar]
- 27. Samland A. K., Sprenger G. A. (2009) Transaldolase: From biochemistry to human disease. Int. J. Biochem. Cell Biol. 41, 1482–1494 [DOI] [PubMed] [Google Scholar]
- 28. Violante S., Ijlst L., Ruiter J., Koster J., van Lenthe H., Duran M., Tavares de Almeida I., Wanders R. J. A., Houten S. M., Ventura F. V. (2013) Substrate specificity of human carnitine acetyltransferase: Implications for fatty acid and branched-chain amino acid metabolism. Biochim. Biophys. Acta 1832, 773–779 [DOI] [PubMed] [Google Scholar]
- 29. Muoio D. M., Noland R. C., Kovalic J. P., Seiler S. E., Davies M. N., DeBalsi K. L., Ilkayezva O. R., Stevens R. D., Kheterpal I., Zhang J., Covington J. D., Bajpeyi S., Ravussin E., Kraus W., Koves T. R., Mynatt R. L. (2012) Muscle specific deletion of carnitine acetyltransferase compromises glucose tolerance and metabolic flexibility. Cell Metabolism 15, 764–777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Smathers R. L., Petersen D. R. (2011) The human fatty acid-binding protein family: Evolutionary divergences and functions. Human Genomics 5, 170–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fisher H., Gustafsson T., Sundberg C. J., Norrbom J., Ekman M., Johansson O., Jansson E. (2006) Fatty acid binding protein 4 in human skeletal muscle. Biochem. Biophys. Res. Commun. 346, 125–130 [DOI] [PubMed] [Google Scholar]
- 32. Vandervoort A. A. (2002) Aging of the human neuromuscular system. Muscle Nerve 25, 17–25 [DOI] [PubMed] [Google Scholar]
- 33. Doran P., O'Connell K., Gannon J., Kavanagh M., Ohlendieck K. (2008) Opposite pathobiochemical fate of pyruvate kinase and adenylate kinase in aged rat skeletal muscle as revealed by proteomic DIGE analysis. Proteomics 8, 364–377 [DOI] [PubMed] [Google Scholar]
- 34. Belgrano A., Rakicevic L., Mittempergher L., Campanaro S., Martinelli V. C., Mouly V., Valle G., Kojic S., Faulkner G. (2011) Multi-tasking role of the mechanosensing protein Ankrd2 in the signaling network of striated muscle. PlosOne 6, e25519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pallavicini A., Kojic S., Bean C., Vainzof M., Salamon M., Levolella C., Bortoletto G., Pacchioni B., Zatz M., Lanfranchi G., Faulkner G., Valle G. (2001) Characterization of human skeletal muscle Ankrd2. Biochem. Biophys. Res. Commun. 285, 378–386 [DOI] [PubMed] [Google Scholar]
- 36. Tsukamoto Y., Senda T., Nakano T., Nakada C., Hida T., Ishiguro N., Kondo G., Baba T., Sato K., Osaki M., Mori S., Ito H., Moriyama M. (2002) Arpp, a new homolog of carp, is preferentially expressed in type 1 skeletal muscle fibers and is markedly induced by denervation. Lab. Invest. 82, 645–655 [DOI] [PubMed] [Google Scholar]
- 37. Edstrom E., Altun M., Bergman E., Johnson H., Kullberg S., Ramirez-Leon V., Ulfhake B. (2007) Factors contributing to neuromuscular impairment and sarcopenia during aging. Physiol. Behav. 92, 129–135 [DOI] [PubMed] [Google Scholar]
- 38. Kruger M., Linke W. A. (2011) The giant protein titin: A regulatory node that integrates myocyte signaling pathways. J. Biol. Chem. 286, 9905–9912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dahlmann B., Rutschmann M., Reinauer H. (1986) Effect of starvation or treatment with corticosterone on the amount of easily releasable myofilaments in rat skeletal muscles. Biochem. J. 234, 659–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Grieve A. G., Moss S. E., Hayed M. J. (2012) Annexin A2 at the interface of actin and membrane dynamics: A focus on its roles in endocytosis and cell polarization. Int. J. Cell Biol. doi:10.1155/2012/852430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Furst D. O., Goldfarb L. G., Kley R. A., Vorgerd M., Olive M., van der Ven P. F. M. (2013) Filamin C-related myopathies: pathology and mechanisms. Acta Neuropathol. 125, 33–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ghosh M., Song X., Mouneimne G., Sidani M., Lawrence D. S., Condeelis J. S. (2004) Cofilin promotes actin polymerization and defines the direction of cell motility. Science 304, 743–746 [DOI] [PubMed] [Google Scholar]
- 43. Li J., Brieher W. M., Scimone M. L., Kang S. J., Zhu H., Yin H., von Andrian U. H., Mitchison T., Yuan J. (2007) Caspase-11 regulates cell migration by promoting Aip1-Cofilin-mediated actin depolymerization. Nat. Cell Biol. 9, 276–286 [DOI] [PubMed] [Google Scholar]
- 44. Capitanio D., Vasso M., Fania C., Moriggi M., Vigano A., Procacci P., Magnaghi V., Gelfi C. (2009) Comparative proteomic profile of rat sciatic nerve and gastrocnemius muscle tissues in ageing by 2-D DIGE. Proteomics 9, 2004–2020 [DOI] [PubMed] [Google Scholar]
- 45. Assinder S. J., Stanton J. A. L., Prasad P. D. (2009) Transgelin: An actin-binding protein and tumour suppressor. Int. J. Biochem. Cell Biol. 41, 482–486 [DOI] [PubMed] [Google Scholar]
- 46. Meunier B., Dumas E., Piec I., Bechet D., Hebraud M., Hocquette J. F. (2007) Assessment of hierarchical clustering methodologies for proteomic data mining. J. Proteome Res. 6, 358–366 [DOI] [PubMed] [Google Scholar]
- 47. Quandt K. S., Hultquist D. D. (1994) Flavin reductase: Sequence of cDNA from bovine liver and tissue distribution. Proc. Natl. Acad. Sci. U. S. A. 91, 9322–9326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Komuro A., Tobe T., Hashimoto K., Nakano Y., Yamaguchi T., Nakajima H., Tomita M. (1996) Molecular cloning and expression of human liver biliverdin-IX beta reductase. Biol. Pharmaceut. Bull. 19(6), 796–804 [DOI] [PubMed] [Google Scholar]
- 49. Cunningham O., Gore M. G., Mantle T. J. (2000) Initial-rate kinetics of the flavin reductase reaction catalyzed by human biliverdin-Ixβ reductase (BVR-B). Biochem. J. 345, 393–399 [PMC free article] [PubMed] [Google Scholar]
- 50. Stocker R., Ames B. N. (1987) Potential role of conjugatedbilirubin and copper in the metabolism of lipid peroxides in bile. Proc. Natl. Acad. Sci. U. S. A. 84, 8130–8134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Florczyk U. M., Jozkowicz A., Dulak J. (2008) Biliverdin reductase: new features of an old enzyme and its potential therapeutic significance. Pharmalog. Reports 60, 38–48 [PMC free article] [PubMed] [Google Scholar]
- 52. Gotoh K., Nonoguchi K., Higashitsuji H., Kaneko Y., Sakurai T., Sumitomo Y., Itoh K., Subjeck J. R., Fujita J. (2004) Apg-2 has a chaperone-like activity similar to Hsp110 and is overexpressed in hepatocellular carcinomas. FEBS Lett. 560, 19–24 [DOI] [PubMed] [Google Scholar]
- 53. Kaul G., Pattan G., Rafeequi T. (2011) Eukaryotic elongation factor-2 (eEF2): its regulation and peptide chain elongation. Cell Biochem. Function 29, 227–234 [DOI] [PubMed] [Google Scholar]
- 54. Sun X. X., Devine T., Challagundla K. B., Dai M. S. (2011) Interplay between ribosomal protein S27a and MDM2 protein in p53 activation in response to ribosomal stress. J. Biol. Chem. 286, 22730–22741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Palmieri G., Bergamo P., Luini A., Ruvo M., Gogliottino M., Langella E., Saviano M., Hegde R. N., Sandomenico A., Rossi M. (2011) Acylpeptide hydrolase inhibition as targeted strategy to induce proteasomal down-regulation. PlosOne 6, e25888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Shimizu K., Kiuchi Y., Ando K., Hayakawa M., Kikugawa K. (2004) Coordination of oxidized protein hydrolase and the proteasome in the clearance of cytotoxic denatured proteins. Biochem. Biophys. Res. Commun. 234, 140–146 [DOI] [PubMed] [Google Scholar]
- 57. Degens H. (1998) Age-related changes in the microcirculation of skeletal muscle. Adv. Exp. Med. Biol. 454, 343–348 [DOI] [PubMed] [Google Scholar]
- 58. Bearden S. E. (2006) Effect of aging on the structure and function of skeletal muscle microvascular networks. Microcirculation 13, 279–288 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.