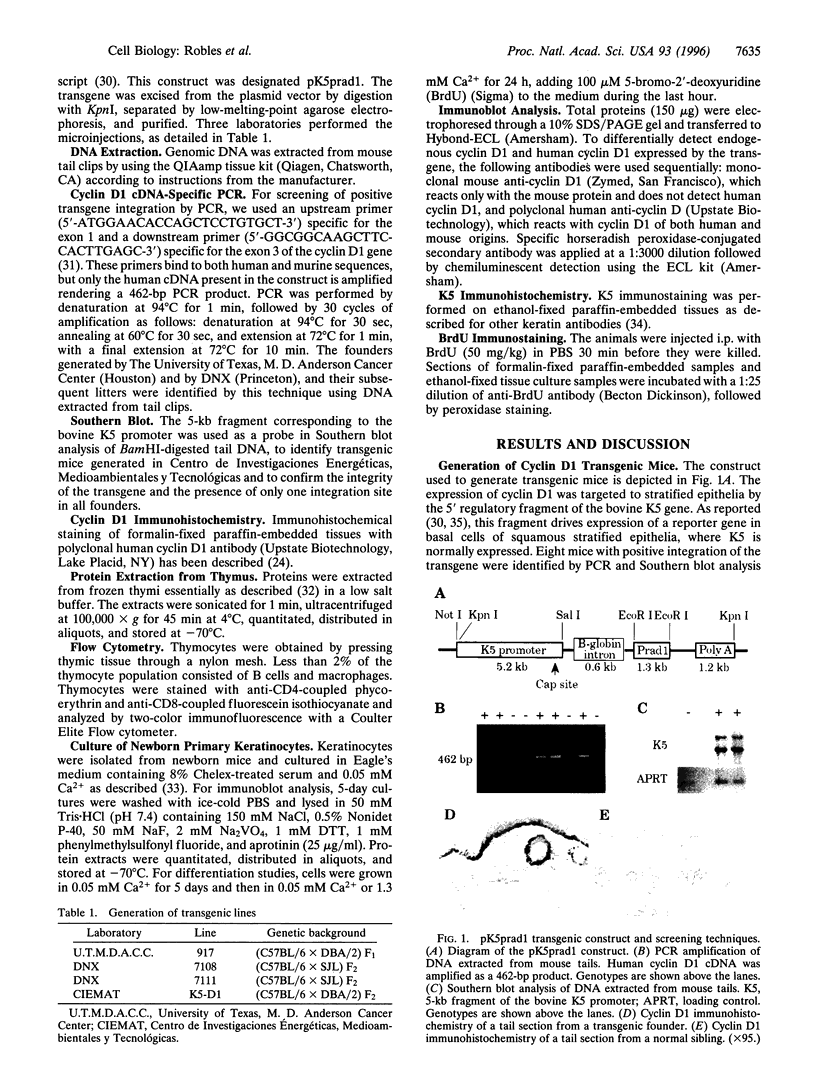
Abstract
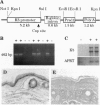
To study the involvement of cyclin D1 in epithelial growth and differentiation and its putative role as an oncogene in skin, transgenic mice were developed carrying the human cyclin D1 gene driven by a bovine keratin 5 promoter. As expected, all squamous epithelia including skin, oral mucosa, trachea, vaginal epithelium, and the epithelial compartment of the thymus expressed aberrant levels of cyclin D1. The rate of epidermal proliferation increased dramatically in transgenic mice, which also showed basal cell hyperplasia. However, epidermal differentiation was unaffected, as shown by normal growth arrest of newborn primary keratinocytes in response to high extracellular calcium. Moreover, an unexpected phenotype was observed in the thymus. Transgenic mice developed a severe thymic hyperplasia that caused premature death due to cardio-respiratory failure within 4 months of age. By 14 weeks, the thymi of transgenic mice increased in weight up to 40-fold, representing 10% of total body weight. The hyperplastic thymi had normal histology revealing a well-differentiated cortex and medulla, which supported an apparently normal T-cell developmental program based on the distribution of thymocyte subsets. These results suggest that proliferation and differentiation of epithelial cells are under independent genetic controls in these organs and that cyclin D1 can modulate epithelial proliferation without altering the initiation of differentiation programs. No spontaneous development of epithelial tumors or thymic lymphomas was perceived in transgenic mice during their first 8 months of life, although they continue under observation. This model provides in vivo evidence of the action of cyclin D1 as a pure mediator of proliferation in epithelial cells.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold A., Kim H. G., Gaz R. D., Eddy R. L., Fukushima Y., Byers M. G., Shows T. B., Kronenberg H. M. Molecular cloning and chromosomal mapping of DNA rearranged with the parathyroid hormone gene in a parathyroid adenoma. J Clin Invest. 1989 Jun;83(6):2034–2040. doi: 10.1172/JCI114114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartkova J., Lukas J., Strauss M., Bartek J. The PRAD-1/cyclin D1 oncogene product accumulates aberrantly in a subset of colorectal carcinomas. Int J Cancer. 1994 Aug 15;58(4):568–573. doi: 10.1002/ijc.2910580420. [DOI] [PubMed] [Google Scholar]
- Bianchi A. B., Fischer S. M., Robles A. I., Rinchik E. M., Conti C. J. Overexpression of cyclin D1 in mouse skin carcinogenesis. Oncogene. 1993 May;8(5):1127–1133. [PubMed] [Google Scholar]
- Bodrug S. E., Warner B. J., Bath M. L., Lindeman G. J., Harris A. W., Adams J. M. Cyclin D1 transgene impedes lymphocyte maturation and collaborates in lymphomagenesis with the myc gene. EMBO J. 1994 May 1;13(9):2124–2130. doi: 10.1002/j.1460-2075.1994.tb06488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botteri F. M., van der Putten H., Wong D. F., Sauvage C. A., Evans R. M. Unexpected thymic hyperplasia in transgenic mice harboring a neuronal promoter fused with simian virus 40 large T antigen. Mol Cell Biol. 1987 Sep;7(9):3178–3184. doi: 10.1128/mcb.7.9.3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filmus J., Robles A. I., Shi W., Wong M. J., Colombo L. L., Conti C. J. Induction of cyclin D1 overexpression by activated ras. Oncogene. 1994 Dec;9(12):3627–3633. [PubMed] [Google Scholar]
- Gimenez-Conti I. B., Lynch M., Roop D., Bhowmik S., Majeski P., Conti C. J. Expression of keratins in mouse vaginal epithelium. Differentiation. 1994 May;56(3):143–151. [PubMed] [Google Scholar]
- Hennings H., Michael D., Cheng C., Steinert P., Holbrook K., Yuspa S. H. Calcium regulation of growth and differentiation of mouse epidermal cells in culture. Cell. 1980 Jan;19(1):245–254. doi: 10.1016/0092-8674(80)90406-7. [DOI] [PubMed] [Google Scholar]
- Jiang W., Kahn S. M., Tomita N., Zhang Y. J., Lu S. H., Weinstein I. B. Amplification and expression of the human cyclin D gene in esophageal cancer. Cancer Res. 1992 May 15;52(10):2980–2983. [PubMed] [Google Scholar]
- Jiang W., Kahn S. M., Zhou P., Zhang Y. J., Cacace A. M., Infante A. S., Doi S., Santella R. M., Weinstein I. B. Overexpression of cyclin D1 in rat fibroblasts causes abnormalities in growth control, cell cycle progression and gene expression. Oncogene. 1993 Dec;8(12):3447–3457. [PubMed] [Google Scholar]
- Jiang W., Zhang Y. J., Kahn S. M., Hollstein M. C., Santella R. M., Lu S. H., Harris C. C., Montesano R., Weinstein I. B. Altered expression of the cyclin D1 and retinoblastoma genes in human esophageal cancer. Proc Natl Acad Sci U S A. 1993 Oct 1;90(19):9026–9030. doi: 10.1073/pnas.90.19.9026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyomarsi K., O'Leary N., Molnar G., Lees E., Fingert H. J., Pardee A. B. Cyclin E, a potential prognostic marker for breast cancer. Cancer Res. 1994 Jan 15;54(2):380–385. [PubMed] [Google Scholar]
- Kivinen L., Pitkänen K., Laiho M. Human retinoblastoma gene product prevents c-Ha-ras oncogene mediated cellular transformation of mouse fibroblasts. Oncogene. 1993 Oct;8(10):2703–2711. [PubMed] [Google Scholar]
- Lammie G. A., Fantl V., Smith R., Schuuring E., Brookes S., Michalides R., Dickson C., Arnold A., Peters G. D11S287, a putative oncogene on chromosome 11q13, is amplified and expressed in squamous cell and mammary carcinomas and linked to BCL-1. Oncogene. 1991 Mar;6(3):439–444. [PubMed] [Google Scholar]
- Liu J. J., Chao J. R., Jiang M. C., Ng S. Y., Yen J. J., Yang-Yen H. F. Ras transformation results in an elevated level of cyclin D1 and acceleration of G1 progression in NIH 3T3 cells. Mol Cell Biol. 1995 Jul;15(7):3654–3663. doi: 10.1128/mcb.15.7.3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovec H., Grzeschiczek A., Kowalski M. B., Möröy T. Cyclin D1/bcl-1 cooperates with myc genes in the generation of B-cell lymphoma in transgenic mice. EMBO J. 1994 Aug 1;13(15):3487–3495. doi: 10.1002/j.1460-2075.1994.tb06655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meilin A., Shoham J., Schreiber L., Sharabi Y. The role of thymocytes in regulating thymic epithelial cell growth and function. Scand J Immunol. 1995 Aug;42(2):185–190. doi: 10.1111/j.1365-3083.1995.tb03644.x. [DOI] [PubMed] [Google Scholar]
- Missero C., Serra C., Stenn K., Dotto G. P. Skin-specific expression of a truncated E1a oncoprotein binding to p105-Rb leads to abnormal hair follicle maturation without increased epidermal proliferation. J Cell Biol. 1993 Jun;121(5):1109–1120. doi: 10.1083/jcb.121.5.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll J., Eibel H., Schmid P., Sansig G., Botteri F., Palacios R., Van der Putten H. Thymic hyperplasia in transgenic mice caused by immortal epithelial cells expressing c-kit ligand. Eur J Immunol. 1992 Jun;22(6):1587–1594. doi: 10.1002/eji.1830220636. [DOI] [PubMed] [Google Scholar]
- Motokura T., Bloom T., Kim H. G., Jüppner H., Ruderman J. V., Kronenberg H. M., Arnold A. A novel cyclin encoded by a bcl1-linked candidate oncogene. Nature. 1991 Apr 11;350(6318):512–515. doi: 10.1038/350512a0. [DOI] [PubMed] [Google Scholar]
- Murillas R., Larcher F., Conti C. J., Santos M., Ullrich A., Jorcano J. L. Expression of a dominant negative mutant of epidermal growth factor receptor in the epidermis of transgenic mice elicits striking alterations in hair follicle development and skin structure. EMBO J. 1995 Nov 1;14(21):5216–5223. doi: 10.1002/j.1460-2075.1995.tb00206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto A., Jiang W., Kim S. J., Spillare E. A., Stoner G. D., Weinstein I. B., Harris C. C. Overexpression of human cyclin D1 reduces the transforming growth factor beta (TGF-beta) type II receptor and growth inhibition by TGF-beta 1 in an immortalized human esophageal epithelial cell line. Proc Natl Acad Sci U S A. 1994 Nov 22;91(24):11576–11580. doi: 10.1073/pnas.91.24.11576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quelle D. E., Ashmun R. A., Shurtleff S. A., Kato J. Y., Bar-Sagi D., Roussel M. F., Sherr C. J. Overexpression of mouse D-type cyclins accelerates G1 phase in rodent fibroblasts. Genes Dev. 1993 Aug;7(8):1559–1571. doi: 10.1101/gad.7.8.1559. [DOI] [PubMed] [Google Scholar]
- Ramírez A., Bravo A., Jorcano J. L., Vidal M. Sequences 5' of the bovine keratin 5 gene direct tissue- and cell-type-specific expression of a lacZ gene in the adult and during development. Differentiation. 1994 Nov;58(1):53–64. doi: 10.1046/j.1432-0436.1994.5810053.x. [DOI] [PubMed] [Google Scholar]
- Resnitzky D., Reed S. I. Different roles for cyclins D1 and E in regulation of the G1-to-S transition. Mol Cell Biol. 1995 Jul;15(7):3463–3469. doi: 10.1128/mcb.15.7.3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richie E. R. N-methyl-N-nitrosourea-induced and spontaneous AKR/J thymic lymphomas express distinct differentiation antigen phenotypes. Leuk Res. 1988;12(3):233–242. doi: 10.1016/0145-2126(88)90141-5. [DOI] [PubMed] [Google Scholar]
- Robles A. I., Conti C. J. Early overexpression of cyclin D1 protein in mouse skin carcinogenesis. Carcinogenesis. 1995 Apr;16(4):781–786. doi: 10.1093/carcin/16.4.781. [DOI] [PubMed] [Google Scholar]
- Rosenberg C. L., Kim H. G., Shows T. B., Kronenberg H. M., Arnold A. Rearrangement and overexpression of D11S287E, a candidate oncogene on chromosome 11q13 in benign parathyroid tumors. Oncogene. 1991 Mar;6(3):449–453. [PubMed] [Google Scholar]
- Schauer I. E., Siriwardana S., Langan T. A., Sclafani R. A. Cyclin D1 overexpression vs. retinoblastoma inactivation: implications for growth control evasion in non-small cell and small cell lung cancer. Proc Natl Acad Sci U S A. 1994 Aug 2;91(16):7827–7831. doi: 10.1073/pnas.91.16.7827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano M., Gómez-Lahoz E., DePinho R. A., Beach D., Bar-Sagi D. Inhibition of ras-induced proliferation and cellular transformation by p16INK4. Science. 1995 Jan 13;267(5195):249–252. doi: 10.1126/science.7809631. [DOI] [PubMed] [Google Scholar]
- Serrano M., Hannon G. J., Beach D. A new regulatory motif in cell-cycle control causing specific inhibition of cyclin D/CDK4. Nature. 1993 Dec 16;366(6456):704–707. doi: 10.1038/366704a0. [DOI] [PubMed] [Google Scholar]
- Seto M., Yamamoto K., Iida S., Akao Y., Utsumi K. R., Kubonishi I., Miyoshi I., Ohtsuki T., Yawata Y., Namba M. Gene rearrangement and overexpression of PRAD1 in lymphoid malignancy with t(11;14)(q13;q32) translocation. Oncogene. 1992 Jul;7(7):1401–1406. [PubMed] [Google Scholar]
- Sherr C. J. Mammalian G1 cyclins. Cell. 1993 Jun 18;73(6):1059–1065. doi: 10.1016/0092-8674(93)90636-5. [DOI] [PubMed] [Google Scholar]
- Sicinski P., Donaher J. L., Parker S. B., Li T., Fazeli A., Gardner H., Haslam S. Z., Bronson R. T., Elledge S. J., Weinberg R. A. Cyclin D1 provides a link between development and oncogenesis in the retina and breast. Cell. 1995 Aug 25;82(4):621–630. doi: 10.1016/0092-8674(95)90034-9. [DOI] [PubMed] [Google Scholar]
- Smith R., Peters G., Dickson C. Genomic organization of the mouse cyclin D1 gene (Cyl-1). Genomics. 1995 Jan 1;25(1):85–92. doi: 10.1016/0888-7543(95)80112-y. [DOI] [PubMed] [Google Scholar]
- Theillet C., Adnane J., Szepetowski P., Simon M. P., Jeanteur P., Birnbaum D., Gaudray P. BCL-1 participates in the 11q13 amplification found in breast cancer. Oncogene. 1990 Jan;5(1):147–149. [PubMed] [Google Scholar]
- Wang T. C., Cardiff R. D., Zukerberg L., Lees E., Arnold A., Schmidt E. V. Mammary hyperplasia and carcinoma in MMTV-cyclin D1 transgenic mice. Nature. 1994 Jun 23;369(6482):669–671. doi: 10.1038/369669a0. [DOI] [PubMed] [Google Scholar]
- Weinberg R. A. The retinoblastoma protein and cell cycle control. Cell. 1995 May 5;81(3):323–330. doi: 10.1016/0092-8674(95)90385-2. [DOI] [PubMed] [Google Scholar]
- Withers D. A., Harvey R. C., Faust J. B., Melnyk O., Carey K., Meeker T. C. Characterization of a candidate bcl-1 gene. Mol Cell Biol. 1991 Oct;11(10):4846–4853. doi: 10.1128/mcb.11.10.4846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodroofe C., Müller W., Rüther U. Long-term consequences of interleukin-6 overexpression in transgenic mice. DNA Cell Biol. 1992 Oct;11(8):587–592. doi: 10.1089/dna.1992.11.587. [DOI] [PubMed] [Google Scholar]
- de Boer C. J., Loyson S., Kluin P. M., Kluin-Nelemans H. C., Schuuring E., van Krieken J. H. Multiple breakpoints within the BCL-1 locus in B-cell lymphoma: rearrangements of the cyclin D1 gene. Cancer Res. 1993 Sep 15;53(18):4148–4152. [PubMed] [Google Scholar]