Abstract
Effective communication between pre- and postsynaptic compartments is required for proper synapse development and function. At the Drosophila neuromuscular junction (NMJ), a retrograde BMP signal functions to promote synapse growth, stability and homeostasis and coordinates the growth of synaptic structures. Retrograde BMP signaling triggers accumulation of the pathway effector pMad in motoneuron nuclei and at synaptic termini. Nuclear pMad, in conjunction with transcription factors, modulates the expression of target genes and instructs synaptic growth; a role for synaptic pMad remains to be determined. Here, we report that pMad signals are selectively lost at NMJ synapses with reduced postsynaptic sensitivities. Despite this loss of synaptic pMad, nuclear pMad persisted in motoneuron nuclei, and expression of BMP target genes was unaffected, indicating a specific impairment in pMad production/maintenance at synaptic termini. During development, synaptic pMad accumulation followed the arrival and clustering of ionotropic glutamate receptors (iGluRs) at NMJ synapses. Synaptic pMad was lost at NMJ synapses developing at suboptimal levels of iGluRs and Neto, an auxiliary subunit required for functional iGluRs. Genetic manipulations of non-essential iGluR subunits revealed that synaptic pMad signals specifically correlated with the postsynaptic type-A glutamate receptors. Altering type-A receptor activities via protein kinase A (PKA) revealed that synaptic pMad depends on the activity and not the net levels of postsynaptic type-A receptors. Thus, synaptic pMad functions as a local sensor for NMJ synapse activity and has the potential to coordinate synaptic activity with a BMP retrograde signal required for synapse growth and homeostasis.
Keywords: BMP signaling, Glutamatergic synapses, Glutamate receptor, Drosophila, Neuromuscular junction
INTRODUCTION
Synapse development is initiated by genetic programs, but is coordinated by intercellular communications between the pre- and postsynaptic compartments, and by neuronal activity itself. Neurons exert both instantaneous and long-lasting effects on the postsynaptic cell through synaptic transmission (reviewed by Malenka and Nicoll, 1999), and postsynaptic cells influence the growth, maturation and function of the presynaptic neurons through retrograde signals (Tao and Poo, 2001; Marqués and Zhang, 2006). Retrograde signals have been identified at both neuromuscular junctions (NMJs) and central synapses (Sanes and Lichtman, 1999; Tao and Poo, 2001; Davis, 2006; Turrigiano, 2007; Turrigiano, 2012), but little is known about how such signals detect the status of synaptic activity and relay this information to presynaptic neurons.
The Drosophila NMJ is an extremely useful model to study synapse development and plasticity. Drosophila NMJ synapses are glutamatergic, similar in composition and function to the mammalian central AMPA/kainate synapses (Littleton and Ganetzky, 2000). The fly NMJ ionotropic glutamate receptors (iGluRs) are heterotetrameric complexes composed of three essential subunits - GluRIIC, GluRIID and GluRIIE - and either GluRIIA or GluRIIB (DiAntonio, 2006). Mutations that delete any of the shared subunits, or GluRIIA and GluRIIB together, abolish the NMJ synaptic transmission and limit the localization of iGluRs at synaptic locations (DiAntonio et al., 1999; Marrus et al., 2004; Featherstone et al., 2005; Qin et al., 2005). Type-A and type-B receptors differ in their single-channel properties, synaptic currents and regulation by second messengers (DiAntonio, 2006). Mechanisms that differentially regulate the synaptic levels and activity of these two channels have profound effects on synapse strength and plasticity. Manipulations that decrease the activity of type-A receptors produce large decreases in quantal size (Petersen et al., 1997; Davis et al., 1998), yet the evoked transmission remains normal due to a compensatory increase in presynaptic release. Several factors have been shown to trigger the retrograde signal and control synaptic homeostasis (Haghighi et al., 2003; Frank et al., 2006; Goold and Davis, 2007; Dickman and Davis, 2009; Frank et al., 2009; Marie et al., 2010; Müller et al., 2011; Müller and Davis, 2012). However, the molecular nature of the retrograde signal remains a mystery.
At the Drosophila NMJ, Glass bottom boat (Gbb), a bone morphogenetic protein (BMP)-type ligand secreted by the muscle, provides a retrograde signal that promotes synaptic growth and confers synaptic homeostasis (Aberle et al., 2002; Marqués et al., 2002; Sweeney and Davis, 2002; McCabe et al., 2003; Goold and Davis, 2007). Gbb signals by binding to presynaptic heterotetrameric complex of type-I [Thickveins (Tkv) and Saxophone (Sax)] and type-II [Wishful thinking (Wit)] receptors. Activated receptors recruit and phosphorylate the BMP pathway effector Mad. Phosphorylated Mad (pMad) accumulates at two locations: in the motoneuron nuclei (nuclear pMad) and at the NMJ synapses (synaptic pMad) (McCabe et al., 2003; Dudu et al., 2006). Nuclear pMad in conjunction with other factors modulates expression of BMP target genes, including trio, which encodes for a Rac-activating protein important for cytoskeletal remodeling, and target of wit (twit), which encodes for a Ly-6 related molecule important for NMJ synapse activity (Ball et al., 2010; Kim and Marqués, 2012). The function of synaptic pMad remains less well understood. Recent evidence suggests that synaptic pMad does not translocate to the motoneuron nuclei and instead may engage in a local, unknown activity (Smith et al., 2012). Selective loss of presynaptic pMad in importin-β11 mutants causes developmental and functional defects at NMJ synapses (Higashi-Kovtun et al., 2010). Previous studies have placed synaptic pMad at the active zones, but also within the boundaries of endogenous iGluRs clusters at postsynaptic densities (PSDs) (Dudu et al., 2006). In the muscle, BMP signaling is triggered by glia-secreted TGFβ ligand Maverick (Mav), which activates Gbb transcription and modulates Gbb-dependent retrograde signaling and synaptic growth (Fuentes-Medel et al., 2012).
We have previously characterized Neto as an essential auxiliary subunit of glutamate receptor complexes required for iGluR synaptic clustering and formation of functional NMJs (Kim et al., 2012). Similar to disruptions in glutamate receptors, neto mutant embryos are completely paralyzed and have no detectable iGluR clusters at their NMJs. Synapses developing at suboptimal Neto levels have physiological and structural defects, but are also smaller in size, with reduced number of boutons, suggesting that Neto may influence one of the several signaling pathway known to modulate NMJ development. In particular, synapses with impairments in BMP signaling have fewer boutons and reduced excitatory junction potential amplitudes (reviewed by Marqués and Zhang, 2006) similar to neto mutants. As Neto contains two extracellular, putative BMP-binding complement CUB domains (Lee et al., 2009), we hypothesized that Neto modulates the BMP signaling at Drosophila NMJ.
Here, we report that Neto-deprived synapses exhibit selective loss of synaptic pMad. We found that neto interacts genetically with BMP pathway components and modulates synaptic pMad throughout development. Synaptic pMad follows the accumulation of postsynaptic Neto/iGluRs clusters and correlates exclusively with the type-A glutamate receptors. Furthermore, we found that synaptic pMad correlates with the activity of type-A receptors and constitutes an effective sensor of synaptic activity.
RESULTS
Synaptic but not nuclear pMad is diminished at suboptimal Neto levels
As Neto-deprived NMJs have phenotypes reminiscent of BMP signaling pathway mutants, we hypothesized that Neto influences the retrograde BMP signaling. We tested this possibility by examining the levels and distribution of pMad, the effector of the BMP signaling pathway. Whereas control animals had clear pMad puncta decorating synaptic boutons, the pMad signals were drastically reduced at synaptic locations in neto109 third instar larvae (Fig. 1A,B). This difference was not caused by genetic background, as control and neto109 animals are excisions of the same transposable element (Kim et al., 2012). The loss of synaptic pMad at suboptimal Neto levels was confirmed using several anti-pMad antibodies (supplementary material Fig. S1) and resembled the loss of pMad in mutants of the BMP pathway, such as wit (Fig. 1C). Downregulation of neto via RNAi uncovered a similar decrease of synaptic pMad following the levels of postsynaptic Neto (Fig. 1D,E). A duplication covering the neto locus effectively rescued the loss of pMad in neto109 boutons (Fig. 1F), indicating that synaptic pMad specifically depends on Neto. To test if Neto affects the levels or synaptic localization of BMP pathway components we compared the distribution of Mad and BMP receptors, Tkv and Wit, and the transcriptional response to the retrograde BMP signaling in neto109 larvae and controls (supplementary material Fig. S2; and see below). The loss of pMad signals at neto109 NMJs did not appear to be a consequence of reduced synaptic BMP pathway components. Also, the loss of synaptic pMad at Neto-deprived NMJs could not be attributed to defective PSD structures, as detailed below.
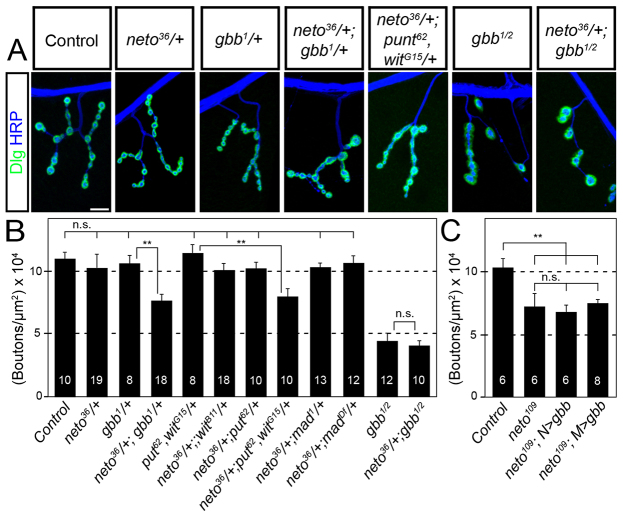
Fig. 1.
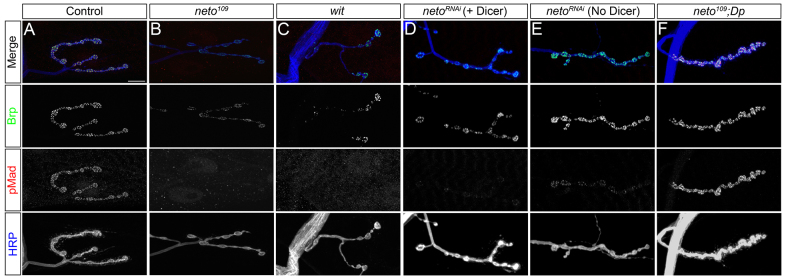
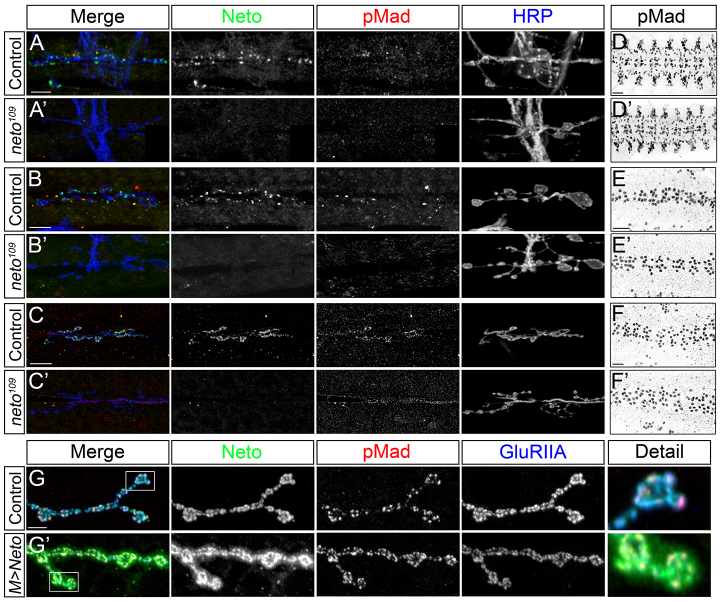
Suboptimal Neto prevents pMad accumulation at the NMJ. (A-F) Synaptic localization of Bruchpilot (green) and pMad (red) at third instar larvae NMJ (muscle 4, abdominal segment 3). The anti-horseradish peroxidase (HRP) antibody (blue) labels motoneuron arbors. pMad localizes at NMJ synapses in control animals (A), but is diminished in neto hypomorphs, neto109 (B), similar to wit null mutants (C). Synaptic pMad signals correlate with Neto levels (D,E). A duplication containing the neto gene restores synaptic pMad at neto109 NMJs (F). Scale bars: 10 μm. Genotypes: (A) control (precise excision); (B) neto109; (C) witB11/witA12; (D) UAS-Dicer/UAS-netoRNAi-6D; 24B-Gal4/+; (E) UAS-netoRNAi-6D/+; 24B-Gal4/+; (F) neto109;; Dp(1:3)DC270.
In contrast to synaptic terminals, nuclear pMad signals appeared normal in neto109 third instar ventral ganglia (Fig. 2A,B). Quantification revealed only mild reduction of nuclear pMad signals in neto109 compared with controls, whereas nuclear pMad was not detectable in wit mutants (Fig. 2C). A similar specific reduction in synaptic but not nuclear pMad was reported for importin-β11 mutants (Higashi-Kovtun et al., 2010). Furthermore, suboptimal Neto levels did not appear to impact transcription of BMP target genes in the motoneurons. Quantitative reverse transcription polymerase chain reaction (qRT-PCR) from third instar ventral ganglia indicated that twit expression was reduced by 16% in neto109 and by 83% in wit mutants compared with controls (Fig. 2D). Importantly, suboptimal Neto levels did not affect gbb expression in either ventral ganglia or muscle tissue (qPCR from larval muscle cDNA shown in Fig. 2E). At the Drosophila NMJ, multiple pathways control Gbb transcription and secretion and therefore influence the BMP retrograde signaling (Ellis et al., 2010; Nahm et al., 2010; Fuentes-Medel et al., 2012). Perturbations in any of these pathways impact Gbb extracellular distribution and lead to concerted variations of both synaptic and nuclear pMad. By contrast, selective loss of pMad at Neto-deprived synapses does not seem to originate from a general decrease in Gbb availability and appears to represent a more specific impairment.
Fig. 2.
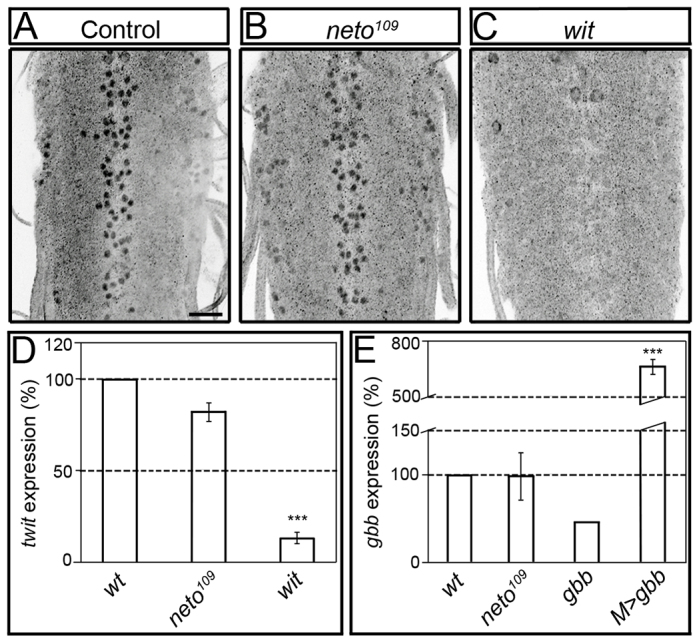
Nuclear pMad does not change in neto hypomorphs. (A-C) Confocal images of ventral ganglia of third instar larvae immunostained against pMad. Nuclear pMad was detected in both control (A) and neto109 mutant ventral ganglia (B), including motoneuron nuclei. Nuclear pMad is lost in wit mutants (C). (D,E) Quantitative analysis of gene expression. qRT-PCR analysis reveals a small decrease of twit expression in neto109 ventral ganglia compared with controls (D). wit mutants show significantly decreased in twit expression. The levels of gbb expression in larval striated muscles are similar in neto109 and control animals, but are reduced in gbb mutants, or increased when gbb is overexpressed in the muscle (E). Bars represent the mean relative expression of target gene from three independent experiments (n=3). Error bars represent s.e.m. (***P<0.001). Genotypes: (A-D) control (precise excision), neto109, and witB11/witA12; (E) control (precise excision), neto109, gbb1/gbb2, and M>gbb (UAS-gbb9.9/G14-Gal4). Scale bar: 20 μm.
Genetic interaction of neto with the BMP pathway
The similarities between NMJs deficient for neto and BMP pathway components, and the observed reduction of pMad at Neto-deprived synapses suggest that neto and BMP signaling pathway may interact to regulate synapse development. To test this hypothesis, we examined genetic interactions between neto and the BMP pathway components. Larvae with mutations in neto or BMP signaling components have smaller NMJs, but this phenotype is recessive; heterozygous larvae have normal numbers of synaptic boutons and are indistinguishable from controls (McCabe et al., 2003) (Fig. 3). However, animals with single copies of both neto and gbb (neto36/+; gbb1/+) had smaller NMJs with 30% fewer synaptic boutons. This significant decrease indicates a synergistic effect of mutations in neto and gbb. We did not observe a similar decrease in the synaptic arbors for neto and wit, put or mad trans-heterozygous combinations, but larvae heterozygous for neto, wit and put together had significantly smaller NMJs, with 25% fewer synaptic boutons (Fig. 3B). Taking advantage of the 50% lethality of neto109 hemizygotes we tested whether further reduction of BMP signaling pathway components affected the lethality. We found that neto109 lethality was increased from 50% to >80% when one copy of gbb or mad was removed (supplementary material Fig. S3). This synthetic lethality assay also revealed a modest interaction between neto and wit, but not put. Together, these genetic interactions indicated that neto and gbb formed trans-heterozygous combinations for which phenotypic threshold effects could be consistently observed.
Fig. 3.
neto interacts genetically with gbb. (A) Representative confocal images of muscle 4 NMJ, abdominal segment 3, in third instar larvae immunostained against Dlg (green) and HRP (blue). Scale bar: 10 μm. (B,C) Quantification of mean number of type-IB boutons on muscle 4 per μm2 of muscle surface area. Numbers of segments analyzed are indicated. Error bars represent s.e.m. ***P<0.005 compared with control. N.S. (not significant) denotes P>0.1. Genotypes: control (precise excision), gbb1/2 (gbb1/gbb2), neto109 N>gbb (neto109; UAS-gbb9.9/+; elav-Gal4/+), neto109 M>gbb (neto109; UAS-gbb9.9/G14-Gal4).
If neto is upstream of gbb in the BMP signaling pathway, then lowering Neto levels should not aggravate gbb null mutant phenotypes. Indeed, third instar gbb null larvae (gbb1/gbb2) showed a ∼50% reduction in synaptic boutons with either one or two copies of neto (Fig. 3B). However, overexpression of gbb did not rescue the synaptic arbors or local pMad in neto109 (Fig. 3C; supplementary material Fig. S4). In control experiments, overexpression of gbb in the muscle significantly rescued the NMJ growth phenotype of gbb null mutants (supplementary material Fig. S5). If neto is upstream gbb but cannot be rescued by genetic manipulations of Gbb levels, then how does Neto impact BMP signaling? One possibility could be that Neto controls localization of Gbb activities: in this scenario, overexpression of Gbb cannot suppress neto loss-of-function phenotypes, even though neto is upstream of gbb.
Synaptic pMad does not accumulate at Neto-deprived synapses throughout development
As reduced Neto levels efficiently disrupted synaptic pMad without affecting nuclear pMad levels, we wondered if our observations captured a cumulative effect on synaptic pMad. To rule out such a combined effect we performed developmental timecourse analyses and compared pMad in control and neto109 animals starting from late embryo stages.
In embryos 21 hours after egg laying (AEL), Neto forms distinct puncta that mark iGluR clusters at neuronal arbors (Kim et al., 2012) (Fig. 4A). Some of these puncta colocalized with a few pMad immunoreactivities present at this stage. The synaptic pMad puncta were very rare in control embryos and were never found in neto109 hemizygotes, which also lacked Neto-positive signals (Fig. 4A′). First and second instar larvae had well defined synapses with boutons decorated by Neto puncta (Fig. 4B,C). By second instar all Neto-positive puncta were accompanied by pMad signals in control larvae, but pMad immunoreactivities remained very small and scattered along the axonal arbor in neto109, mirroring the dim Neto signals (Fig. 4B′,C′, arrows). By contrast, nuclear pMad levels were similar in neto109 and control animals at all stages of development (Fig. 4D-F). Furthermore, although they die paralyzed, neto36 null embryos had normal nuclear pMad (not shown). The selective loss of pMad observed throughout development suggests that the loss of synaptic pMad is not a consequence of impaired development at Neto-deprived synapses; instead, neto is directly required for the accumulation of local pMad.
Fig. 4.
Synaptic pMad accumulation follows Neto clustering at the developing NMJ. (A-C′) Synaptic pMad is diminished throughout development at suboptimal Neto levels. Confocal images of body wall muscles 6/7 (abdominal segment 3) using anti-Neto antibodies (green), anti-pMad (red) and anti-HRP (blue) are shown. Synaptic pMad forms puncta that colocalize with Neto puncta in late control embryos (A) but are absent in neto109 (A′). First (B) and second instar (C) larvae also show Neto and pMad synaptic signals that are largely absent from neto hypomorphs (B′,C′). (D-F′) Nuclear pMad levels remain normal at suboptimal Neto levels throughout development, including late embryos, 21 hours AEL (D,D′), first instar (E,E′) and second instar larvae (F,F′). (G,G′) High levels of Neto in muscle do not affect synaptic pMad signals. Neto labels distinct puncta that colocalize with GluRIIA signals in control animals (muscle 4, abdominal segment 3) (G). When overexpressed, Neto positive fields expand beyond the GluRIIA signals to encompass the entire bouton (G′). pMad staining does not follow the pattern of elevated Neto signals and remains colocalized with GluRIIA and Neto-positive puncta. Scale bars: 10 μm (A-C′); 20 μm (D-F′); 5 μm (G,G′). Genotypes: (G) UAS-neto-A9/+; (G′) UAS-neto-A9/G14-Gal4.
As synaptic pMad followed the temporal and spatial accumulation of Neto at developing synapses, we examined whether increased Neto levels affect pMad accumulation. Synaptic Neto showed punctate as well as diffuse distribution when neto transgenes were overexpressed in the striated muscles (Fig. 4G). Neto puncta colocalized with the iGluR subunits, whereas diffuse Neto exceeded the receptor field. However, excess Neto did not affect the intensity and appearance of synaptic pMad signals, which remained organized in distinct puncta. The similarities between iGluRs and synaptic pMad labeling at various Neto levels raised the possibility that synaptic pMad correlates with clustered Neto/iGluRs.
Synaptic pMad correlates with glutamate receptor levels
To test whether synaptic pMad follows the Neto/iGluRs clusters we examined pMad at various iGluRs levels. We first targeted the essential subunits of iGluR complexes, GluRIIC, GluRIID and GluRIIE, via muscle specific RNAi. Staining and imaging conditions were kept identical to facilitate comparison of various genotypes. Mild reduction of iGluR levels, such as in GluRIIERNAi, induced attenuation of Neto and GluRIIC signals and a corresponding decrease in synaptic pMad (Fig. 5B; supplementary material Fig. S6). Strong reduction of iGluR levels (i.e. GluRIIDRNAi, GluRIICRNAi) disrupted larval development and reduced Neto and GluRIIC synaptic levels in a concentration-dependent manner (supplementary material Fig. S6). The levels of pMad closely followed the synaptic levels of Neto/iGluR clusters (Fig. 5C-E). A similar phenotype was found in GluRIIC ‘weakly’ rescued flies, carrying a leaky UAS-GluRIIC cDNA transgene (Marrus et al., 2004) (Fig. 5F). Similar to neto109, iGluR-deprived larvae had normal levels of nuclear pMad in ventral ganglia (Fig. 5G-I). Thus, synaptic pMad mirrors the levels of postsynaptic Neto/iGluRs, whereas nuclear pMad appears insensitive to the glutamate receptors status.
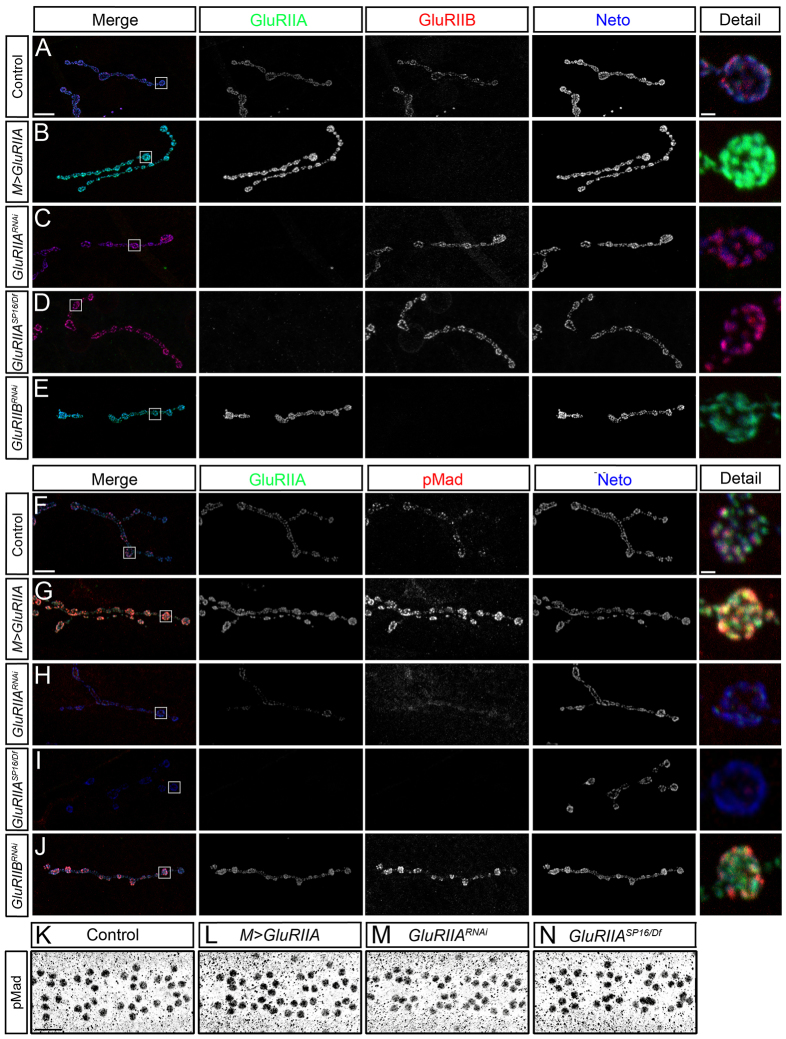
Fig. 5.
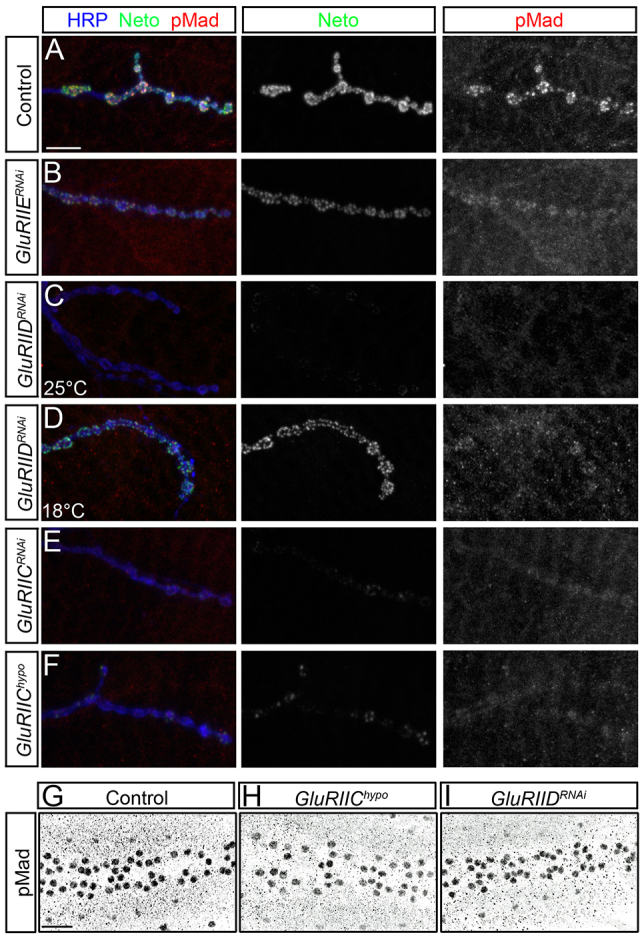
Neto/iGluR clusters control synaptic pMad accumulation. (A-F) Synaptic localization of Neto (green) and pMad (red) at third instar larvae NMJ (muscle 4, abdominal segment 3). Synaptic pMad signals are diminished when obligatory iGluR subunits are reduced via RNAi (B-E) or in hypomorphic combinations (F). Moderate reduction of synaptic Neto/iGluR complexes such as with GluRIIERNAi produce limited decrease in synaptic pMad (B). Strong loss of Neto/iGluR clusters in GluRIIDRNAi larvae induces severe reduction in the synaptic pMad signals (C); rearing GluRIIDRNAi animals at 18°C preserves some of the Neto/iGluR clusters as well as synaptic pMad (D). Reducing GluRIIC levels either by RNAi (E) or in strong hypomorphs (F) produces loss of Neto/iGluR clusters and synaptic pMad. (G-I) pMad accumulation in motoneuron nuclei remains unchanged when synaptic Neto/iGluR clusters are diminished. Nuclear pMad signals resemble controls (G) in GluRIIChypo (H) and GluRIIDRNAi (I). Scale bars: 10 μm (A-F); 20 μm (G-I). Genotypes: (A,G) control (precise excision); (B) GluRIIERNAi (G14-Gal4/+; UAS-GluRIIERNAi/+); (C,D,I) GluRIIDRNAi (G14-Gal4/+; UAS-GluRIIDRNAi/+); (E) GluRIICRNAi (G14-Gal4/+; UAS-GluRIICRNAi/+); (F,H) GluRIIEhypo (DGluRIII2/df(2L)ast1; P[UAS-cGluRIII]/+).
The Drosophila NMJ utilizes two types of iGluRs, type-A and type-B, which differ in their composition, channel properties and subcellular localization (DiAntonio, 2006). To test whether synaptic pMad differentiated between these channels, we examined the synapses at various GluRIIA and GluRIIB levels. Consistent with the previous demonstration that GluRIIA and GluRIIB compete for the essential subunits for synaptic targeting (Sigrist et al., 2002; Marrus et al., 2004), we found that GluRIIA overexpression produced strong reduction of GluRIIB synaptic levels (Fig. 6A,B). Also, lowering GluRIIA (or GluRIIB) levels produced a significant increase in synaptic accumulation of GluRIIB (or respectively, GluRIIA) (Fig. 6C-E). None of these genetic manipulations induced changes in the GluRIIC and Neto synaptic signals (not shown; Fig. 6). We found that synaptic pMad specifically followed synaptic GluRIIA levels over a wide range of concentrations, in RNAi experiments or heteroallelic combinations (Fig. 6F-J). These striking variations in synaptic pMad levels cannot be due to structural disruptions at postsynaptic specializations. Unlike neto109, GluRIIA mutant NMJs have normal distribution of postsynaptic scaffolds such as Discs-large (Dlg) (supplementary material Fig. S7) (Kim et al., 2012). Furthermore, nuclear pMad remained relatively constant over a wide range of GluRIIA levels (Fig. 6K-N). Thus, postsynaptic type-A receptors selectively modulate the accumulation of synaptic pMad.
Fig. 6.
Type-A receptors are required for synaptic pMad accumulation. (A-J) Confocal microscopy was performed on muscle 4 (abdominal segment 3) with anti-GluRIIA antibodies (green), anti-Neto (blue) and either anti-GluRIIB (red) (A-E) or anti-pMad (red) (F-J). Overexpression of GluRIIA in striated muscles reduces GluRIIB levels compared with controls (A-B). Reduction of GluRIIA levels via RNAi (C) or in strong hypomorphs (D) increases the GluRIIB levels, whereas reduction of GluRIIB via RNAi (E) increases the GluRIIA signals. Neto synaptic signals are largely unaffected by changes in GluRIIA/GluRIIB ratio. Overexpression of GluRIIA produces an increase in pMad signal intensities compared with controls (F,G). Reducing the GluRIIA levels leads to reduction of pMad signals (H,I), but reducing the GluRIIB levels has the opposite effect (J). (K-N) Confocal microscopy of third instar ventral ganglia using anti-pMad antibodies indicates that nuclear pMad signals were not affected by manipulations of GluRIIA levels. Scale bars: 10 μm (A-J); 20 μm (K-N). Genotypes: (A,F,K) control (precise excision); (B,G,L) M>GluRIIA (UAS-GluRIIA/+; G14-Gal4/+); (C,H,M) GluRIIARNAi (G14-Gal4/+; UAS-GluRIIARNAi/+); (D,I,N) GluRIIASP16/Df (GluRIIASP16/Df(2L)clh4); (E,J) GluRIIBRNAi (G14-Gal4/+; UAS-GluRIIBRNAi/+).
Activity-dependent accumulation of synaptic pMad
The relative ratio of synaptic type-A versus type-B glutamate receptors is also a key determinant of quantal size. Overexpression of GluRIIA induces a dose-dependent increase in quantal size, whereas overexpression of GluRIIB induces a dose-dependent decrease (Petersen et al., 1997; DiAntonio et al., 1999). Therefore, synaptic pMad may correlate with the net levels of type-A receptors or may reflect a change in synapse activity/quantal size. To distinguish between these two possibilities, we next manipulated the activity of postsynaptic glutamate receptors. Postsynaptic protein kinase A (PKA; PKA-C1 - FlyBase) controls quantal size through a mechanism that requires the presence of GluRIIA subunit; the current model is that PKA may phosphorylate and inhibit the activity of type-A receptors without changing the levels of synaptic receptors (Davis et al., 1998). If synaptic pMad depends on the activity of type-A receptors, then PKA activity should have profound effects on synaptic pMad accumulation. We tested this possibility by genetic manipulation of PKA activity in postsynaptic muscles using transgenes that express either a constitutively active catalytic subunit (PKA-act) or a mutant regulatory subunit of PKA with reduced affinity for cAMP that inhibits the release of catalytic subunits (PKA-inh) (Davis et al., 1998). Expression of these transgenic lines in selected muscles using BG487-Gal4 promoter produced mosaic animals suitable for comparative analyses within the same specimen (Budnik et al., 1996) (Fig. 7; supplementary material Fig. S8).
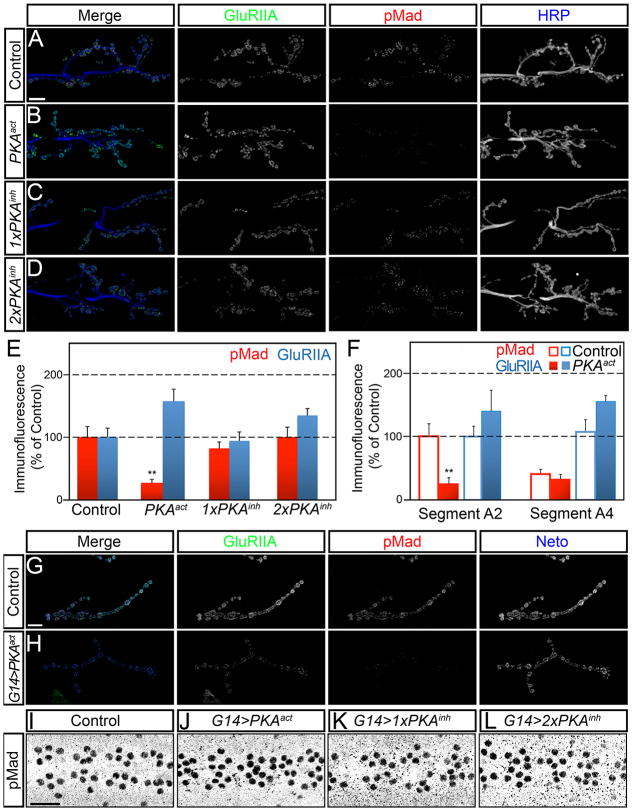
Fig. 7.
GluRIIA activity controls the accumulation of synaptic pMad. (A-D) Confocal microscopy was performed on muscle 6/7 (abdominal segment 3) with anti-GluRIIA antibodies (green), anti-pMad (red) and anti-HRP (blue). Compared with control (A), muscle expression of a constitutively active catalytic subunit (PKA-act) produces a significant reduction in synaptic pMad signals (B). Expression of one (C) or two (D) copies of a mutant regulatory subunit of PKA (PKA-inh) induces a small increase in synaptic pMad accumulation. (E,F) Quantification of synaptic pMad and GluRIIA signals at various levels of muscle PKA activity. (G,H) Confocal images of third instar larvae muscle 4 (abdominal segment 3) immunostained with anti-GluRIIA antibodies (green), anti-pMad (red) and anti-Neto (blue). Strong postsynaptic PKA activity throughout development leads to complete loss of synaptic pMad and significant reduction in GluRIIA synaptic levels compared with control. (I-L) pMad accumulation in motoneuron nuclei does not change at various levels of PKA activity in the muscle. Scale bars: 10 μm (A-D); 5 μm (G,H). Genotypes: (A) control (BG487-Gal4/UAS-PKAm-inh); (B) PKAact (BG487-Gal4/UAS-PKA.mC); (C) 1xPKAinh (BG487-Gal4/+; UAS-PKAinh(GDK33)/+;); (D) 2xPKAinh (BG487-Gal4/UAS-PKAinh(GDK22); UAS-PKAinh(BDK35)/+;); (G) G14 control (G14-Gal4/UAS-PKAm-inh); (H) G14>PKAact (G14-Gal4/UAS-PKA.mC).
We found that increased PKA activity in postsynaptic muscles produced a significant decrease in the levels of synaptic pMad, but not GluRIIA levels, compared with the control (Fig. 7A-E). We quantified this difference relative to HRP and found that pMad levels were reduced at synapses with normal or even slightly increased GluRIIA levels (Fig. 7E,F). The synaptic pMad levels were inversely proportional to the BG487-Gal4 expression pattern, including its anteroposterior gradient. For example, muscle 6/7 synapses in anterior segments had greatly increased PKA-act expression and showed decreased synaptic pMad, whereas posterior segments had mild expression of PKA-act and reduced change in synaptic pMad (supplementary material Fig. S8). By contrast, decreased PKA activity induced no significant change in synaptic pMad levels (Fig. 7C,D). Furthermore, high levels of PKA activity throughout development, such as driven by G14-Gal4, resulted in complete loss of synaptic pMad signals and larval lethality (Fig. 7E,F). In this case, escaper third instar larvae also showed a severe reduction of GluRIIA levels and diminished Neto signals. In addition, overexpression of a dominant-negative GluRIIA receptor with a mutation in the putative ion conduction pore (DiAntonio et al., 1999) induced a significant reduction in synaptic pMad indicating that synaptic pMad does not correlate with the net levels of GluRIIA (supplementary material Fig. S9). In spite of these drastic reductions in synaptic pMad, we found no changes in nuclear pMad levels in motoneurons (Fig. 7G-J). Together, these findings indicate that accumulation of synaptic pMad depends on the activity of type-A receptors and not the net receptor levels. Thus, synaptic pMad reflects the activity status of postsynaptic glutamate receptors.
DISCUSSION
We have previously described Neto as the first nonchannel subunit required for the clustering of iGluRs and formation of functional synapses at the Drosophila NMJ. Neto and iGluR complexes associate in the striated muscle and depend on each other for targeting and clustering at postsynaptic specializations. Here, we show that Neto/iGluR synaptic complexes induce accumulation of pMad at synaptic termini in an activity-dependent manner. The effect of Neto/iGluR clusters on BMP signaling is selective, and limited to synaptic pMad; nuclear accumulation of pMad appears largely independent of postsynaptic glutamate receptors. We demonstrate that synaptic pMad mirrors the activity of postsynaptic type-A receptors. As such, synaptic pMad may function as an acute sensor for postsynaptic sensitivity. Local fluctuations in synaptic pMad may provide a versatile means to relay changes in synapse activity to presynaptic neurons and coordinate synapse activity status with synapse growth and homeostasis.
Synaptic pMad as a sensor for synapse activity
Drosophila NMJs maintain their evoked potentials remarkably constant during development, from late embryo to the third instar larval stages (Keshisian and Kim, 2004). This coordination between motoneuron and muscle properties requires active trans-synaptic signaling, including a retrograde BMP signal, which promotes synaptic growth and confers synaptic homeostasis (Marqués, 2005; Goold and Davis, 2007). Nuclear pMad accumulates in motoneurons during late embryogenesis. However, embryos mutant for BMP pathway components hatch into the larval stages, indicating that BMP signaling is not required for the initial assembly of NMJ synapses and instead modulates NMJ growth and development (Aberle et al., 2002; Marqués et al., 2002). Here we demonstrate that synaptic accumulation of pMad follows GluRIIA arrival at nascent NMJs (Fig. 4) and depends on optimal levels of synaptic Neto and iGluRs (Figs 1, 5). As type-A receptors have been associated with nascent synapses, and type-B receptors mark mature NMJs, accumulation of synaptic pMad appears to correlate with a growing phase at NMJ synapses. Furthermore, synaptic pMad correlates with the activity and not the net levels of postsynaptic type-A receptors (Fig. 6). In fact, expression of a GluRIIA variant with a mutation in the putative ion conduction pore triggered reduction of synaptic pMad levels (supplementary material Fig. S9). Thus, synaptic pMad functions as a molecular sensor for synapse activity and may constitute an important element in synapse plasticity.
The synaptic pMad pool has been localized primarily to the presynaptic compartment (Marqués and Zhang, 2006; O’Connor-Giles et al., 2008; Higashi-Kovtun et al., 2010). However, a contribution for postsynaptic pMad to the pool of synaptic pMad is also possible. Postsynaptic pMad accumulates in response to glia-secreted Mav, which regulates gbb expression and indirectly modulates the Gbb-mediated retrograde signaling (Fuentes-Medel et al., 2012). RNAi experiments revealed that knockdown of mad in muscle induces a decrease in synaptic pMad, albeit much reduced in amplitude compared with knockdown of mad in motoneurons (supplementary material Fig. S10) (Fuentes-Medel et al., 2012). Also, knockdown of wit in motoneurons, but not in muscle, and knockdown of put in muscle, but not in motoneurons, triggers reduction of synaptic pMad (data not shown) (Fuentes-Medel et al., 2012). Intriguingly, the synaptic pMad is practically abolished in GluRIIA and neto109 mutants and cannot be further reduced by additional decrease in Mad levels (supplementary material Fig. S10). Whereas loss of postsynaptic pMad could be due to a Mav-dependent feedback mechanism that controls Gbb secretion from the muscle, the absence of presynaptic pMad demonstrates a role for GluRIIA and Neto in modulation of BMP retrograde signaling.
As BMP signals are generally short lived (O’Connor et al., 2006; Tucker et al., 2008; Ramel and Hill, 2012), synaptic pMad probably reflects accumulation of active BMP/receptor complexes at synaptic termini. Recent evidence suggests that BMP receptors traffic along the motoneuron axons, with Gbb/receptors complexes moving preferentially in a retrograde direction. By contrast, Mad does not appear to traffic (Smith et al., 2012). Thus, Mad is likely to be phosphorylated and maintained locally by a pool of active Gbb/BMP receptor complexes that remain at synaptic termini for the time postsynaptic type-A receptors are active.
How does postsynaptic glutamate receptor activity modulate local pMad?
The activity of type-A glutamate receptors may control synaptic pMad accumulation (1) indirectly via activity-dependent changes that are relayed to both pre- and postsynaptic cells, or (2) directly by influencing the production and signaling of varied Gbb ligand forms or by localizing Gbb activities. For example, inhibition of postsynaptic receptor activity induces trans-synaptic modulation of presynaptic Ca2+ influx (Müller and Davis, 2012). Such Ca2+ influx changes may trigger events that induce a local change in synaptic pMad accumulation. One possibility is that changes in Ca2+ influx may recruit Importin-β11 at presynaptic termini, which in turn mediate synaptic pMad accumulation.
At the Drosophila NMJ, Gbb is secreted in the synaptic cleft from both pre- and postsynaptic compartments. The secretion of Gbb is regulated at multiple levels, transcriptionally and post-translationally (Ellis et al., 2010; Nahm et al., 2010; James and Broihier, 2011; Fuentes-Medel et al., 2012; Nahm et al., 2013). Furthermore, the Gbb prodomain could be processed at several cleavage sites to generate Gbb ligands with varying activities (Akiyama et al., 2012). The longer, more active Gbb ligand retains a portion of the prodomain that could influence the formation of Gbb/BMP receptor complexes (Sengle et al., 2011). Synaptic pMad may result from signaling by selective forms of Gbb. Or type-A receptors could modulate secretion and processing of Gbb in an activity-dependent manner. Understanding the function of different pools and active forms of Gbb within the synaptic cleft will help explain the multiple roles for Gbb at Drosophila NMJs.
Alternatively, active postsynaptic type-A receptor complexes may directly engage and stabilize presynaptic Gbb/BMP receptor signaling complexes via trans-synaptic interactions. CUB domains can directly bind BMPs (Lee et al., 2009); thus Neto may utilize its extracellular CUB domains to engage Gbb and/or presynaptic BMP receptors. As synaptic pMad mirrors active type-A receptors, such trans-synaptic complexes will depend on Neto in complexes with active type-A receptors. We have been unable to capture a direct interaction between Gbb and Neto CUB domains in co-immunoprecipitation experiments (data not shown). Nonetheless, a trans-synaptic complex that depends on the activity of type-A receptors could offer a versatile means for relaying synapse activity status to the presynaptic neuron via fast assembly and disassembly.
Irrespective of the strategy that correlates synaptic pMad pool with the active type-A receptor/Neto complexes, further mechanisms must act to maintain the Gbb/BMP receptor complexes at synapses and protect them from endocytosis and retrograde transport. Such mechanisms must be specific, as general modulators of BMP receptors endocytosis impact both synaptic and nuclear pMad (Sweeney and Davis, 2002; Wang et al., 2007; O’Connor-Giles and Ganetzky, 2008; O’Connor-Giles et al., 2008; Nahm et al., 2013). A candidate for differential control of BMP/receptor complexes is Importin-β11. Loss of synaptic pMad in importin-β11 is rescued by neuronal expression of activated BMP receptors, by blocking retrograde transport, but not by neuronal expression of Mad (Higashi-Kovtun et al., 2010). As Mad does not appear to traffic (Smith et al., 2012), presynaptic Importin-β11 must act upstream of the BMP receptors, perhaps to stabilize active Gbb/BMP receptor complexes at the neuron membrane. By contrast, local pMad cannot be restored at Neto-deprived NMJs by overactivation of presynaptic BMP receptors or by blocking retrograde transport (supplementary material Fig. S4). As neto and gbb interact genetically, it is tempting to speculate that postsynaptic Neto/type-A complexes localize Gbb activities and stabilize Gbb/BMP receptor complexes from the extracellular side. Additional extracellular factors, for example heparan proteoglycans (Dani et al., 2012), or intracellular modulators, such as Nemo kinase (Merino et al., 2009), may control the distribution of sticky Gbb molecules within the synaptic cleft and their binding to BMP receptors, or may stabilize Gbb/BMP receptor complexes at synaptic termini.
Possible functions for synaptic pMad
Synaptic pMad may act locally and/or in coordination with the transcriptional control of BMP target genes to ensure proper growth and development of the synaptic structures. A presynaptic pool of pMad maintained by Importin-β11 neuronal activities ensures normal NMJ structure and function (Higashi-Kovtun et al., 2010). Like importin-β11, GluRIIA and Neto-deprived synapses show a significantly reduced number of boutons (Table 1) (Petersen et al., 1997; Higashi-Kovtun et al., 2010; Kim et al., 2012). Intriguingly, the absence of GluRIIA induces up to 20% reduction in bouton numbers, whereas knockdown of GluRIIB does not appear to affect NMJ growth (Table 1) (Petersen et al., 1997; Reiff et al., 2002). Although the amplitude of the growth phenotypes we observed in normal culturing conditions (25°C) was modest, this phenomenon may explain the requirement for GluRIIA reported for activity-dependent NMJ development (at 29°C) (Sigrist et al., 2002; Sigrist et al., 2003). Furthermore, knockdown of Neto or any iGluR essential subunit affect synaptic pMad and NMJ growth in a dose-dependent manner (Figs 1, 5; Table 1) (Kim et al., 2012). We could not find significant changes in nuclear pMad or expression of BMP target genes in GluRIIA or Neto-deprived animals, but the restoration of synaptic pMad by presynaptic constitutively active BMP receptors rescues the morphology and physiology of importin-β11 mutant NMJs (Higashi-Kovtun et al., 2010). The smaller NMJs observed in the absence of local pMad may reflect a direct contribution of synaptic pMad to retrograde BMP signaling, a pathway that provides an instructive signal for NMJ growth (O’Connor-Giles et al., 2008). Thus, BMP signaling may integrate synapse activity status with the control of synapse growth.
Table 1.
Genotypes and quantification of bouton numbers
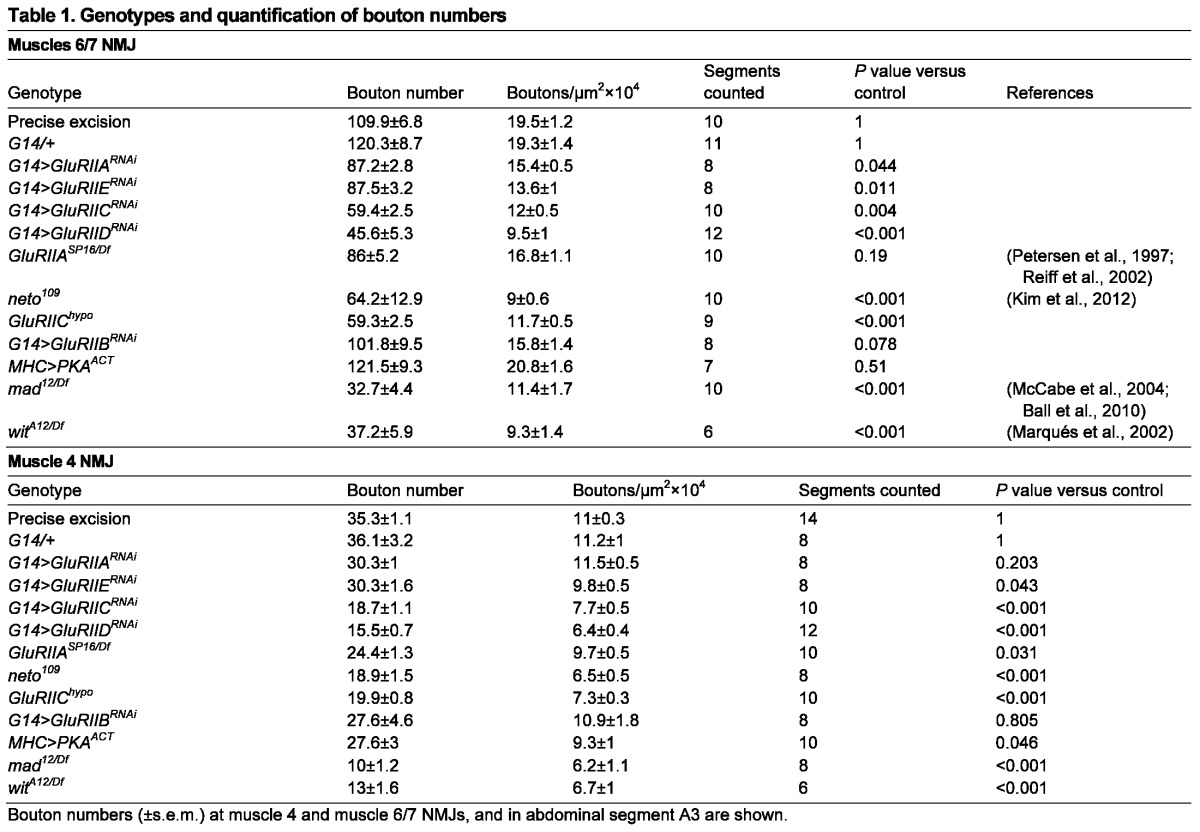
Synaptic pMad may also contribute to synapse stability. Mutants in BMP signaling pathway have an increased number of ‘synaptic footprints’: regions of the NMJ where the terminal nerve once resided and has retracted (Eaton and Davis, 2005). It has been proposed that Gbb binding to its receptors activates the Williams Syndrome-associated Kinase LIMK1 to stabilize the NMJ (Eaton and Davis, 2005). Synaptic pMad may further contribute to the stabilization of synapse contacts by engaging in interactions that anchor the Gbb/BMP receptor complexes at synaptic termini. During neural tube closure, local pSmad1/5/8 mediates stabilization of BMP signaling complexes at tight junction via binding to apical polarity complexes (Eom et al., 2011). Flies may utilize a similar anchor mechanism that relies on pMad-mediated interactions for stabilizing BMP signaling complexes and other components at synaptic junctions. Local active BMP signaling complexes are thought to function in this manner in the maintenance of stemness (Michel et al., 2011) and in epithelial-to-mesenchymal transition (Zeisberg et al., 2003).
Separate from its role in synapse growth and stability, BMP signaling is required presynaptically to maintain the competence of motoneurons to express homeostatic plasticity (Goold and Davis, 2007). The requirements for BMP signaling components for the rapid induction of presynaptic response may include a role for synaptic pMad in relaying acute perturbations of postsynaptic receptor function to the presynaptic compartment. At the very least, attenuation of local pMad signals, when postsynaptic type-A receptors are lost or inactive, may release local Gbb/BMP receptor complexes and allow them to traffic to neuron soma and increase the BMP transcriptional response, promoting expression of presynaptic components and neurotransmitter release. In addition, synaptic pMad-dependent complexes may influence the composition and/or activity of postsynaptic glutamate receptors. Although future experiments will be needed to address the nature and function of local pMad-containing complexes, our findings clearly demonstrate that synaptic pMad constitutes an exquisite monitor of synapse activity status, which has the potential to relay information about synapse activity to both pre- and postsynaptic compartments and contribute to synaptic plasticity. As BMP signaling plays a crucial role in synaptic growth and homeostasis at the Drosophila NMJ, the use of synaptic pMad as a sensor for synapse activity may enable the BMP signaling pathway to monitor synapse activity then function to adjust synaptic growth and stability during development and homeostasis.
MATERIALS AND METHODS
Fly stocks
The netoRNAi lines were generated by insertion of a neto cDNA PCR fragment in pUAST-R57 followed by germline transformation using standard protocols. The PCR primers utilized were: RNAi-F (5′-AAGGCCTACATGGCCGGACCGTTGTTTGTGTGACAGTGACAGTGC-3′) and RNAi-Rev (5′-AATCTAGAGGTACCTTTTGTGT GAGG - TTTGCCAGC-3′). Precise excisions of transposomal elements Mi(ET1)Neto[MB05569] and Mi(ET1)Neto[MB04917] were utilized to generate the neto109 and neto36 alleles were used as controls (Bellen et al., 2011; Kim et al., 2012). The duplication covering neto locus is Dp(1:3)DC270 (Venken et al., 2010).
Other stocks used in this study are as follows: witA12 and witB11 (Marqués et al., 2002); gbb1 (Khalsa et al., 1998); gbb2 (Wharton et al., 1999); UAS-gbb9.9 (McCabe et al., 2003) UAS-tkvQ253D (Nellen et al., 1996) and UAS-saxQ263D (Haerry et al., 1998) (from M. O’Connor, University of Minnesota); mad1 mutant (Takaesu et al., 2005); mad deficiency Df(2L)C28 (Adachi-Yamada et al., 1999); GluRIIChypo (DGluRIII2/df(2L)ast1; P[UAS-cDGluRIII]/+) (Marrus et al., 2004), UAS-GluRIIAM/R (DiAntonio et al., 1999), GluRIIASP16, Df(2L)clh4, and UAS-GluRIIA (Petersen et al., 1997) (all from A. DiAntonio, Washington University); impβ1124 and impβ1170 (Higashi-Kovtun et al., 2010); UAS-dynamitin (Duncan and Warrior, 2002); UAS-PKAinh, UAS-PKA.mC (Li et al., 1995), and control UAS-PKAm-inh (Kiger and O’Shea, 2001) (from B. White, NIH); BG380-Gal4 and BG487-Gal4 (Budnik et al., 1996); elav-Gal4 (BL-8760); 24B-Gal4 (BL-1716); G14-Gal4 and MHC-Gal4 (from C. Goodman, University of California at Berkeley). For RNAi-mediated knockout we used the following TRiP lines generated by the Transgenic RNAi Project, Harvard Medical School: GluRIIA (P[TRiP.JF02647]attP2), GluRIIB (P[TRiP.JF03145]attP2), GluRIIC (P[TRiP.JF01854]attP2), GluRIID (P[TRiP.JF02035]attP2) and GluRIIE (P[TRiP.JF01962]attP2).
Protein purification and antibody generation
A rabbit polyclonal anti-GluRIIC antibody was generated against the N-terminal domain of GluRIIC subunit. Drosophila S2 cells were used for producing recombinant proteins as described previously (Serpe and O’Connor, 2006). An AcPA-based construct containing the N-terminal domain of GluRIIC subunit (residues Q48-M446) C-tagged with RGS-Hx6 was transiently transfected into S2 cells, and the secreted fragment was purified from conditioned supernatant using a His-Trap column (Pharmacia). The antigen was further purified by SDS-PAGE and the gel pieces were used for immunization (Open Biosystems). Positive sera were cleaned by protein A/G affinity, eluted using 0.1 M Gly-HCl (pH 2.8) and quenched with 0.1 vol of 1 M Tris-HCl (pH 9.0).
Immunohistology
Embryos 18 hours AEL were dechorionated, genotyped and, after an additional incubation (2 hours at room temperature), dissected as described previously (Budnik et al., 2006). Larvae were dissected as described previously in ice-cooled Ca2+-free HL-3 solution (Stewart et al., 1994; Budnik et al., 2006). The samples were fixed in either 4% formaldehyde (Polysciences) for 30 minutes or in Bouin’s fixative (Bio-Rad) for 3 minutes and washed in phosphate-buffered saline (PBS) containing 0.5% Triton X-100. Primary antibodies from Developmental Studies Hybridoma Bank were used at the following dilutions: mouse anti-GluRIIA (MH2B), 1:200; mouse anti-Disc-large (Dlg) (4F3), 1:1000; mouse anti-Bruchpilot (Brp) (Nc82), 1:200; mouse anti-Wishful thinking (Wit) (23C7), 1:10. Other primary antibodies were as follows: rabbit anti-phosphorylated Mothers against decapentaplegic (pMad), 1:500 (a gift from Carl Heldin) (Persson et al., 1998); rabbit anti-pMad, 1:100 (Peluso et al., 2011); rabbit anti-pSmad3, 1:500 (Epitomics) (Smith et al., 2012); rabbit anti-GluRIIB, 1:2000 (a gift from Aaron DiAntonio) (Marrus et al., 2004); goat anti-Mad, 1:100 (Santa Cruz Biotechnology); FITC-, rhodamine- and Cy5-conjugated goat anti-HRP, 1:1000 (Jackson ImmunoResearch Laboratories); rabbit anti-GFP, 1:250 (Abcam); rat anti-Neto, 1:1000 (Kim et al., 2012). Alexa Fluor 488-, Alexa Fluor 568- and Alexa Fluor 647-conjugated secondary antibodies (Molecular Probes) were used at 1:400. All samples were mounted in ProLong Gold (Invitrogen). Samples of different genotypes were processed simultaneously and imaged under identical confocal settings using laser scanning confocal microscopes (Carl Zeiss 510 and LSM710). Boutons were counted using anti-HRP and anti-Dlg immunoreactivities. All quantifications were performed while blinded to genotype. The numbers of samples analyzed are indicated inside the bars.
Fluorescence intensity measurements
For nuclear pMad, confocal regions of interest (ROIs) were determined with Imaris software (Bitplane) by using the ‘spots’ feature to automatically identify pMad-positive motor nuclei. Spots were verified manually and mean center intensity for all nuclei in a given sample was recorded. This procedure was repeated for all samples of a given genotype and the mean was used for comparison between genotypes. For NMJ signal quantifications, mean signal intensity within the ROI encompassing the synaptic area was normalized to HRP signal. Student’s t-test was performed using Sigma Plot (Systat) to evaluate statistical significance. All graphs represent mean value of all samples of the given genotype ± s.e.m.
cDNA synthesis and quantitative real-time PCR
Ventral ganglia or carcasses were dissected in ice-cold HL-3, transferred in DEPC-treated PBS, and frozen on dry ice. Three independent samples (ten carcasses or 30 ventral ganglia per sample) were prepared for each genotype. Total RNA was isolated using Trizol (Invitrogen), purified with an RNeasy kit (Qiagen), and verified with Agilent Bioanalyzer. The RNA was reverse transcribed using AccuScript (Agilent), and qPCR reactions were prepared using Power Sybr Green (Invitrogen) and run on Applied Biosystems StepOnePlus system. Mean Ct of triplicate reactions was used to determine relative expression of target gene using 2-ΔΔCT method (Livak and Schmittgen, 2001). Amplification efficiency, determined by serial dilutions, was >98% for all primers. RP-49 was used as endogenous control. Primer pairs utilized were: RP-49-F: TGCACCAGGAACTTCTTGAATCCG; RP-49-R: ATGCTAAGCTGTCGCACAAATGGC; twit-F: GCCTGGCACATCACAGCCATAAAT; twit-R: TTAATGGTGCCGTTGCAGCTCCTT; gbb-F: TAATGTCGGGACTGCGAAACACCT; gbb-R: TCAGCACTCTGTGCATGATCGTCT.
Supplementary Material
Acknowledgments
We thank Bing Zhang, Ed Giniger, Mark Mayer, Chi-Hon Lee and Alan Hinnebusch for helpful discussions and suggestions. We are grateful to Aaron DiAntonio, Carl Heldin, Mike O’Connor and Ben White for antibodies and Drosophila stocks. We thank Peter Nguyen for technical assistance. We also thank the Bloomington Stock Center at the University of Indiana for fly stocks and the Developmental Studies Hybridoma Bank at the University of Iowa for antibodies.
Footnotes
Competing interests
The authors declare no competing financial interests.
Author contributions
M. Sulkowski, Y.-J.K. and M. Serpe designed and performed the experiments; M. Sulkowski and M. Serpe wrote the paper.
Funding
This research was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Child Health and Human Development [grants Z01 HD008869 and Z01 HD008914]. Deposited in PMC for release after 12 months.
Supplementary material
Supplementary material available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.097758/-/DC1
References
- Aberle H., Haghighi A. P., Fetter R. D., McCabe B. D., Magalhães T. R., Goodman C. S. (2002). wishful thinking encodes a BMP type II receptor that regulates synaptic growth in Drosophila. Neuron 33, 545–558 [DOI] [PubMed] [Google Scholar]
- Adachi-Yamada T., Nakamura M., Irie K., Tomoyasu Y., Sano Y., Mori E., Goto S., Ueno N., Nishida Y., Matsumoto K. (1999). p38 mitogen-activated protein kinase can be involved in transforming growth factor beta superfamily signal transduction in Drosophila wing morphogenesis. Mol. Cell. Biol. 19, 2322–2329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama T., Marqués G., Wharton K. A. (2012). A large bioactive BMP ligand with distinct signaling properties is produced by alternative proconvertase processing. Sci. Signal. 5, ra28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball R. W., Warren-Paquin M., Tsurudome K., Liao E. H., Elazzouzi F., Cavanagh C., An B. S., Wang T. T., White J. H., Haghighi A. P. (2010). Retrograde BMP signaling controls synaptic growth at the NMJ by regulating trio expression in motor neurons. Neuron 66, 536–549 [DOI] [PubMed] [Google Scholar]
- Bellen H. J., Levis R. W., He Y., Carlson J. W., Evans-Holm M., Bae E., Kim J., Metaxakis A., Savakis C., Schulze K. L., et al. (2011). The Drosophila gene disruption project: progress using transposons with distinctive site specificities. Genetics 188, 731–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budnik V., Koh Y. H., Guan B., Hartmann B., Hough C., Woods D., Gorczyca M. (1996). Regulation of synapse structure and function by the Drosophila tumor suppressor gene dlg. Neuron 17, 627–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budnik V., Gorczyca M., Prokop A. (2006). Selected methods for the anatomical study of Drosophila embryonic and larval neuromuscular junctions. Int. Rev. Neurobiol. 75, 323–365 [DOI] [PubMed] [Google Scholar]
- Dani N., Nahm M., Lee S., Broadie K. (2012). A targeted glycan-related gene screen reveals heparan sulfate proteoglycan sulfation regulates WNT and BMP trans-synaptic signaling. PLoS Genet. 8, e1003031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis G. W. (2006). Homeostatic control of neural activity: from phenomenology to molecular design. Annu. Rev. Neurosci. 29, 307–323 [DOI] [PubMed] [Google Scholar]
- Davis G. W., DiAntonio A., Petersen S. A., Goodman C. S. (1998). Postsynaptic PKA controls quantal size and reveals a retrograde signal that regulates presynaptic transmitter release in Drosophila. Neuron 20, 305–315 [DOI] [PubMed] [Google Scholar]
- DiAntonio A. (2006). Glutamate receptors at the Drosophila neuromuscular junction. Int. Rev. Neurobiol. 75, 165–179 [DOI] [PubMed] [Google Scholar]
- DiAntonio A., Petersen S. A., Heckmann M., Goodman C. S. (1999). Glutamate receptor expression regulates quantal size and quantal content at the Drosophila neuromuscular junction. J. Neurosci. 19, 3023–3032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickman D. K., Davis G. W. (2009). The schizophrenia susceptibility gene dysbindin controls synaptic homeostasis. Science 326, 1127–1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudu V., Bittig T., Entchev E., Kicheva A., Jülicher F., González-Gaitán M. (2006). Postsynaptic mad signaling at the Drosophila neuromuscular junction. Curr. Biol. 16, 625–635 [DOI] [PubMed] [Google Scholar]
- Duncan J. E., Warrior R. (2002). The cytoplasmic dynein and kinesin motors have interdependent roles in patterning the Drosophila oocyte. Curr. Biol. 12, 1982–1991 [DOI] [PubMed] [Google Scholar]
- Eaton B. A., Davis G. W. (2005). LIM Kinase1 controls synaptic stability downstream of the type II BMP receptor. Neuron 47, 695–708 [DOI] [PubMed] [Google Scholar]
- Ellis J. E., Parker L., Cho J., Arora K. (2010). Activin signaling functions upstream of Gbb to regulate synaptic growth at the Drosophila neuromuscular junction. Dev. Biol. 342, 121–133 [DOI] [PubMed] [Google Scholar]
- Eom D. S., Amarnath S., Fogel J. L., Agarwala S. (2011). Bone morphogenetic proteins regulate neural tube closure by interacting with the apicobasal polarity pathway. Development 138, 3179–3188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Featherstone D. E., Rushton E., Rohrbough J., Liebl F., Karr J., Sheng Q., Rodesch C. K., Broadie K. (2005). An essential Drosophila glutamate receptor subunit that functions in both central neuropil and neuromuscular junction. J. Neurosci. 25, 3199–3208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank C. A., Kennedy M. J., Goold C. P., Marek K. W., Davis G. W. (2006). Mechanisms underlying the rapid induction and sustained expression of synaptic homeostasis. Neuron 52, 663–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank C. A., Pielage J., Davis G. W. (2009). A presynaptic homeostatic signaling system composed of the Eph receptor, ephexin, Cdc42, and CaV2.1 calcium channels. Neuron 61, 556–569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentes-Medel Y., Ashley J., Barria R., Maloney R., Freeman M., Budnik V. (2012). Integration of a retrograde signal during synapse formation by glia-secreted TGF-β ligand. Curr. Biol. 22, 1831–1838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goold C. P., Davis G. W. (2007). The BMP ligand Gbb gates the expression of synaptic homeostasis independent of synaptic growth control. Neuron 56, 109–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haerry T. E., Khalsa O., O’Connor M. B., Wharton K. A. (1998). Synergistic signaling by two BMP ligands through the SAX and TKV receptors controls wing growth and patterning in Drosophila. Development 125, 3977–3987 [DOI] [PubMed] [Google Scholar]
- Haghighi A. P., McCabe B. D., Fetter R. D., Palmer J. E., Hom S., Goodman C. S. (2003). Retrograde control of synaptic transmission by postsynaptic CaMKII at the Drosophila neuromuscular junction. Neuron 39, 255–267 [DOI] [PubMed] [Google Scholar]
- Higashi-Kovtun M. E., Mosca T. J., Dickman D. K., Meinertzhagen I. A., Schwarz T. L. (2010). Importin-beta11 regulates synaptic phosphorylated mothers against decapentaplegic, and thereby influences synaptic development and function at the Drosophila neuromuscular junction. J. Neurosci. 30, 5253–5268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- James R. E., Broihier H. T. (2011). Crimpy inhibits the BMP homolog Gbb in motoneurons to enable proper growth control at the Drosophila neuromuscular junction. Development 138, 3273–3286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshishian H., Kim Y. S. (2004). Orchestrating development and function: retrograde BMP signaling in the Drosophila nervous system. Trends Neurosci. 27, 143–147 [DOI] [PubMed] [Google Scholar]
- Khalsa O., Yoon J. W., Torres-Schumann S., Wharton K. A. (1998). TGF-beta/BMP superfamily members, Gbb-60A and Dpp, cooperate to provide pattern information and establish cell identity in the Drosophila wing. Development 125, 2723–2734 [DOI] [PubMed] [Google Scholar]
- Kiger J. A., Jr, O’Shea C. (2001). Genetic evidence for a protein kinase A/cubitus interruptus complex that facilitates processing of cubitus interruptus in Drosophila. Genetics 158, 1157–1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim N. C., Marqués G. (2012). The Ly6 neurotoxin-like molecule target of wit regulates spontaneous neurotransmitter release at the developing neuromuscular junction in Drosophila. Dev. Neurobiol. 72, 1541–1558 [DOI] [PubMed] [Google Scholar]
- Kim Y. J., Bao H., Bonanno L., Zhang B., Serpe M. (2012). Drosophila Neto is essential for clustering glutamate receptors at the neuromuscular junction. Genes Dev. 26, 974–987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H. X., Mendes F. A., Plouhinec J. L., De Robertis E. M. (2009). Enzymatic regulation of pattern: BMP4 binds CUB domains of Tolloids and inhibits proteinase activity. Genes Dev. 23, 2551–2562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Ohlmeyer J. T., Lane M. E., Kalderon D. (1995). Function of protein kinase A in hedgehog signal transduction and Drosophila imaginal disc development. Cell 80, 553–562 [DOI] [PubMed] [Google Scholar]
- Littleton J. T., Ganetzky B. (2000). Ion channels and synaptic organization: analysis of the Drosophila genome. Neuron 26, 35–43 [DOI] [PubMed] [Google Scholar]
- Livak K. J., Schmittgen T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25, 402–408 [DOI] [PubMed] [Google Scholar]
- Malenka R. C., Nicoll R. A. (1999). Long-term potentiation - a decade of progress? Science 285, 1870–1874 [DOI] [PubMed] [Google Scholar]
- Marie B., Pym E., Bergquist S., Davis G. W. (2010). Synaptic homeostasis is consolidated by the cell fate gene gooseberry, a Drosophila pax3/7 homolog. J. Neurosci. 30, 8071–8082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marqués G. (2005). Morphogens and synaptogenesis in Drosophila. J. Neurobiol. 64, 417–434 [DOI] [PubMed] [Google Scholar]
- Marqués G., Zhang B. (2006). Retrograde signaling that regulates synaptic development and function at the Drosophila neuromuscular junction. Int. Rev. Neurobiol. 75, 267–285 [DOI] [PubMed] [Google Scholar]
- Marqués G., Bao H., Haerry T. E., Shimell M. J., Duchek P., Zhang B., O’Connor M. B. (2002). The Drosophila BMP type II receptor Wishful Thinking regulates neuromuscular synapse morphology and function. Neuron 33, 529–543 [DOI] [PubMed] [Google Scholar]
- Marrus S. B., Portman S. L., Allen M. J., Moffat K. G., DiAntonio A. (2004). Differential localization of glutamate receptor subunits at the Drosophila neuromuscular junction. J. Neurosci. 24, 1406–1415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe B. D., Marqués G., Haghighi A. P., Fetter R. D., Crotty M. L., Haerry T. E., Goodman C. S., O’Connor M. B. (2003). The BMP homolog Gbb provides a retrograde signal that regulates synaptic growth at the Drosophila neuromuscular junction. Neuron 39, 241–254 [DOI] [PubMed] [Google Scholar]
- McCabe B. D., Hom S., Aberle H., Fetter R. D., Marques G., Haerry T. E., Wan H., O’Connor M. B., Goodman C. S., Haghighi A. P. (2004). Highwire regulates presynaptic BMP signaling essential for synaptic growth. Neuron 41, 891–905 [DOI] [PubMed] [Google Scholar]
- Merino C., Penney J., González M., Tsurudome K., Moujahidine M., O’Connor M. B., Verheyen E. M., Haghighi P. (2009). Nemo kinase interacts with Mad to coordinate synaptic growth at the Drosophila neuromuscular junction. J. Cell Biol. 185, 713–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel M., Raabe I., Kupinski A. P., Pérez-Palencia R., Bökel C. (2011). Local BMP receptor activation at adherens junctions in the Drosophila germline stem cell niche. Nat. Commun. 2, 415 [DOI] [PubMed] [Google Scholar]
- Müller M., Davis G. W. (2012). Transsynaptic control of presynaptic Ca2□ influx achieves homeostatic potentiation of neurotransmitter release. Curr. Biol. 22, 1102–1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller M., Pym E. C., Tong A., Davis G. W. (2011). Rab3-GAP controls the progression of synaptic homeostasis at a late stage of vesicle release. Neuron 69, 749–762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahm M., Kim S., Paik S. K., Lee M., Lee S., Lee Z. H., Kim J., Lee D., Bae Y. C., Lee S. (2010). dCIP4 (Drosophila Cdc42-interacting protein 4) restrains synaptic growth by inhibiting the secretion of the retrograde Glass bottom boat signal. J. Neurosci. 30, 8138–8150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahm M., Lee M. J., Parkinson W., Lee M., Kim H., Kim Y. J., Kim S., Cho Y. S., Min B. M., Bae Y. C., et al. (2013). Spartin regulates synaptic growth and neuronal survival by inhibiting BMP-mediated microtubule stabilization. Neuron 77, 680–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nellen D., Burke R., Struhl G., Basler K. (1996). Direct and long-range action of a DPP morphogen gradient. Cell 85, 357–368 [DOI] [PubMed] [Google Scholar]
- O’Connor M. B., Umulis D., Othmer H. G., Blair S. S. (2006). Shaping BMP morphogen gradients in the Drosophila embryo and pupal wing. Development 133, 183–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor-Giles K. M., Ganetzky B. (2008). Satellite signaling at synapses. Fly (Austin) 2, 259–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor-Giles K. M., Ho L. L., Ganetzky B. (2008). Nervous wreck interacts with thickveins and the endocytic machinery to attenuate retrograde BMP signaling during synaptic growth. Neuron 58, 507–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peluso C. E., Umulis D., Kim Y. J., O’Connor M. B., Serpe M. (2011). Shaping BMP morphogen gradients through enzyme-substrate interactions. Dev. Cell 21, 375–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson U., Izumi H., Souchelnytskyi S., Itoh S., Grimsby S., Engström U., Heldin C. H., Funa K., ten Dijke P. (1998). The L45 loop in type I receptors for TGF-beta family members is a critical determinant in specifying Smad isoform activation. FEBS Lett. 434, 83–87 [DOI] [PubMed] [Google Scholar]
- Petersen S. A., Fetter R. D., Noordermeer J. N., Goodman C. S., DiAntonio A. (1997). Genetic analysis of glutamate receptors in Drosophila reveals a retrograde signal regulating presynaptic transmitter release. Neuron 19, 1237–1248 [DOI] [PubMed] [Google Scholar]
- Qin G., Schwarz T., Kittel R. J., Schmid A., Rasse T. M., Kappei D., Ponimaskin E., Heckmann M., Sigrist S. J. (2005). Four different subunits are essential for expressing the synaptic glutamate receptor at neuromuscular junctions of Drosophila. J. Neurosci. 25, 3209–3218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramel M. C., Hill C. S. (2012). Spatial regulation of BMP activity. FEBS Lett. 586, 1929–1941 [DOI] [PubMed] [Google Scholar]
- Reiff D. F., Thiel P. R., Schuster C. M. (2002). Differential regulation of active zone density during long-term strengthening of Drosophila neuromuscular junctions. J. Neurosci. 22, 9399–9409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes J. R., Lichtman J. W. (1999). Development of the vertebrate neuromuscular junction. Annu. Rev. Neurosci. 22, 389–442 [DOI] [PubMed] [Google Scholar]
- Sengle G., Ono R. N., Sasaki T., Sakai L. Y. (2011). Prodomains of transforming growth factor beta (TGFbeta) superfamily members specify different functions: extracellular matrix interactions and growth factor bioavailability. J. Biol. Chem. 286, 5087–5099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serpe M., O’Connor M. B. (2006). The metalloprotease tolloid-related and its TGF-beta-like substrate Dawdle regulate Drosophila motoneuron axon guidance. Development 133, 4969–4979 [DOI] [PubMed] [Google Scholar]
- Sigrist S. J., Thiel P. R., Reiff D. F., Schuster C. M. (2002). The postsynaptic glutamate receptor subunit DGluR-IIA mediates long-term plasticity in Drosophila. J. Neurosci. 22, 7362–7372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigrist S. J., Reiff D. F., Thiel P. R., Steinert J. R., Schuster C. M. (2003). Experience-dependent strengthening of Drosophila neuromuscular junctions. J. Neurosci. 23, 6546–6556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. B., Machamer J. B., Kim N. C., Hays T. S., Marqués G. (2012). Relay of retrograde synaptogenic signals through axonal transport of BMP receptors. J. Cell Sci. 125, 3752–3764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart B. A., Atwood H. L., Renger J. J., Wang J., Wu C. F. (1994). Improved stability of Drosophila larval neuromuscular preparations in haemolymph-like physiological solutions. J. Comp. Physiol. A 175, 179–191 [DOI] [PubMed] [Google Scholar]
- Sweeney S. T., Davis G. W. (2002). Unrestricted synaptic growth in spinster-a late endosomal protein implicated in TGF-beta-mediated synaptic growth regulation. Neuron 36, 403–416 [DOI] [PubMed] [Google Scholar]
- Takaesu N. T., Herbig E., Zhitomersky D., O’Connor M. B., Newfeld S. J. (2005). DNA-binding domain mutations in SMAD genes yield dominant-negative proteins or a neomorphic protein that can activate WG target genes in Drosophila. Development 132, 4883–4894 [DOI] [PubMed] [Google Scholar]
- Tao H. W., Poo M. (2001). Retrograde signaling at central synapses. Proc. Natl. Acad. Sci. USA 98, 11009–11015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker J. A., Mintzer K. A., Mullins M. C. (2008). The BMP signaling gradient patterns dorsoventral tissues in a temporally progressive manner along the anteroposterior axis. Dev. Cell 14, 108–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano G. (2007). Homeostatic signaling: the positive side of negative feedback. Curr. Opin. Neurobiol. 17, 318–324 [DOI] [PubMed] [Google Scholar]
- Turrigiano G. (2012). Homeostatic synaptic plasticity: local and global mechanisms for stabilizing neuronal function. Cold Spring Harb. Perspect. Biol. 4, a005736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venken K. J., Popodi E., Holtzman S. L., Schulze K. L., Park S., Carlson J. W., Hoskins R. A., Bellen H. J., Kaufman T. C. (2010). A molecularly defined duplication set for the X chromosome of Drosophila melanogaster. Genetics 186, 1111–1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Shaw W. R., Tsang H. T., Reid E., O’Kane C. J. (2007). Drosophila spichthyin inhibits BMP signaling and regulates synaptic growth and axonal microtubules. Nat. Neurosci. 10, 177–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wharton K. A., Cook J. M., Torres-Schumann S., de Castro K., Borod E., Phillips D. A. (1999). Genetic analysis of the bone morphogenetic protein-related gene, gbb, identifies multiple requirements during Drosophila development. Genetics 152, 629–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisberg M., Hanai J., Sugimoto H., Mammoto T., Charytan D., Strutz F., Kalluri R. (2003). BMP-7 counteracts TGF-beta1-induced epithelial-to-mesenchymal transition and reverses chronic renal injury. Nat. Med. 9, 964–968 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.