Abstract
Background
The optimal initial operative management of medullary thyroid cancer (MTC) and the use of biomarkers to guide the extent of operation remain controversial. We hypothesized that preoperative serum levels of calcitonin and carcinoembryonic antigen (CEA) correlate with extent of disease and postoperative levels reflect the extent of operation performed.
Methods
We assessed retrospectively clinical and pathologic factors among patients with MTC undergoing at least total thyroidectomy; these factors were correlated with biomarkers using regression analyses.
Results
Data were obtained from 104 patients, 28% with hereditary MTC. Preoperative calcitonin correlated with tumor size (P < .001) and postoperative serum calcitonin levels (P = .01) after multivariable adjustment for lymph node positivity, extent of operation, and hereditary MTC. No patient with a preoperative calcitonin level of <53 pg/mL (n = 20) had lymph node metastases. TNM stage (P = .001) and preoperative calcitonin levels (P = .04), but not extent of operation, independently correlated with the failure to normalize postoperative calcitonin. Postoperative CEA correlated with positive margins (adjusted P = 04). Neither preoperative nor postoperative CEA was correlated with lymph node positivity or extent of surgery.
Conclusion
Preoperative serum calcitonin and TMN stage, but not extent of operation, were independent predictors of postoperative normalization of serum calcitonin levels. Future studies should evaluate preoperative serum calcitonin levels as a determinate of the extent of initial operation.
Medullary thyroid carcinoma (MTC) is an uncommon malignancy arising from parafollicular C-cells and accounts for 2% of thyroid cancers.1 MTC may be more aggressive than well-differentiated thyroid cancer because it has a greater propensity to metastasize to regional lymph nodes and distant sites. Parafollicular cells do not concentrate iodine, hence 131-I is not a possible therapeutic option. Therefore, complete operative resection is the only modality able to cure patients with MTC.
Total thyroidectomy and central compartment neck dissection is recommended for patients with MTC without evidence of advanced local invasion or metastasis. For patients with evidence of regional disease on physical examination or cervical ultrasonography, compartment-oriented neck dissection in a systematic fashion is advised.2 Standard imaging with ultrasonography, however, can overlook lymph node metastasis in more than one third of MTC patients, complicating the decision regarding the appropriate extent of operation.3 Inadequate initial operative treatment results in persistent disease and disease recurrence and re-operation for recurrent or persistent disease is challenging technically and may result in increased morbidity. Moreover, even extensive reoperation results rarely in biochemical cure.4 A recent Surveillance, Epidemiology, and End Results database study found that 41% of MTC patients did not receive adequate operative therapy according to American Thyroid Association MTC guidelines, leading to significantly lesser survival.5 Some advocate a more aggressive initial treatment approach to maximize cure and minimize the need for reoperation in a scarred surgical field.
An increased level of serum calcitonin is the hallmark of MTC, and calcitonin has served as a biomarker for MTC since Melvin and Tashjian discovered that it was released in excessive quantities by MTC tissue in 1968. Preoperative serum calcitonin levels serve to confirm the diagnosis of MTC and detectable postoperative serum calcitonin levels confirm the presence of persistent or recurrent tumor, often before evident clinical disease. Disease progression and dissemination correlates with serum calcitonin and lesser doubling times of serum calcitonin correlate with increased mortality.6 Therefore, some have investigated the correlation of calcitonin and extent of disease and sought to utilize calcitonin levels to tailor the operative approach.7–9
The aim of the present study was to review our institutional experience for MTC patients undergoing initial operative management and identify clinicopathologic characteristics that correlate with serum biomarkers. Our hypothesis is that preoperative levels of calcitonin and carcinoembryonic antigen (CEA) correlate with extent of disease and that postoperative calcitonin and CEA reflect the extent of operation performed.
METHODS
Patients
A retrospective review was performed on patients with a diagnosis of MTC at three tertiary care centers from 1980 to 2009. Query into the surgical pathologic database for “medullary thyroid carcinoma” resulted in 312 patient records. Included in the study were all patients undergoing initial operative treatment at our institutions. Patients with recurrent or distant metastatic disease were excluded, leaving 104 patients. Metastatic workup for most patients with advanced locoregional disease consisted of computed tomography of the chest, abdomen, and pelvis or a whole body positron emission tomography/computed tomography. All data were obtained in accordance with the institutional internal review board.
Definitions
Patients were identified as having hereditary MTC if they carried a germline rearranged during transfection (RET) mutation or had a family history consistent with multiple endocrine neoplasia (MEN; before the discovery of the RET protooncogene in 1993). Patients with MEN2A may have pheochromocytoma and/or primary hyperparathyroidism with associated germline RET mutation. Patients with MEN2B have MTC, pheochromocytomas, marfanoid habitus with ganglio-neuromatosis, and associated germline RET mutations. Familial MTC is considered a phenotypic variant of MEN2A with MTC alone across ≥2 generations with associated germline RET mutations without pheochromocytomas or primary hyperparathyroidism.2
Biomarkers
The majority of patients had serum calcitonin measured using 1 of the following assays: radioimmunoassay (Quest Nichols Institute, CA; reference range <4 pg/mL for women, <8 pg/ mL for men); DPC Immulite 2000 Chemiluminescent Method (Quest Nichols Laboratories, VA; normal range, <10); chemiluminescent immunoassay (ARUP, UT; reference range, <4.6 pg/mL for women, <11.5 pg/mL for men); Automated Immunochemiluminometric Assay (Mayo Medical Laboratories, Rochester, MN; reference range, <16 pg/mL). Although the reference ranges differ slightly between the assays, chemiluminescent assays exhibit linearity. The CEA levels were processed using the following techniques: Abbott IMx CEA Microparticle Enzyme Immunoassay (reference range, <3.0 ng/mL); Roche Electro chemiluminescence Immunoassay (reference range, <3.4 ng/ mL); Seimens ADVIA centaur XP chemiluminescent immunoassay (reference range, <3.4 ng/mL before 2007; <2.5 ng/mL after 2007).
Operative procedures
The operative approach was left to the discretion of the attending surgeon. A subset of these patients who underwent lobectomy alone (either for misdiagnosis or concern for bilateral nerve injury) subsequently returned to the operating room for completion lobectomy with or without lymph node dissection. For these patients, the initial and second operations were viewed as a single sequence, because it comprised the initial management of the MTC with the intent to cure. If the patient underwent additional operative procedures owing to persistently increased levels of calcitonin, those later interventions were excluded from analysis. The extent of operation was ascertained retrospectively from the operative and pathology reports. At our institutions, compartment-oriented neck dissection is routinely practiced. Perithyroidal lymph nodes removed incidentally during thyroidectomy were not counted as patients receiving a formal central compartment nodal dissection; however, those lymph nodes were included for lymph node count and positivity analyses. Undissected lymph node basins were considered negative for analytic purposes.
Histopathology
Surgical pathology reports were reviewed for tumor size and lymph node involvement as well as the presence of multifocality, lymphovascular invasion, extrathyroidal extension, C-cell hyperplasia (CCH), and immunohistochemical (IHC) staining. If multiple foci of MTC were present, the largest tumor dimension was used for the analysis. Histopathologic features not mentioned in the pathology report were assumed to be negative. Tumor staging was based on the American Joint Committee on Cancer, 7th edition, TNM staging.
Data analysis
Univariate analyses included Fisher’s exact test, Wilcoxon rank sum test, Student t test, and Spearman correlation coefficients. Tumor size and serum biomarkers were considered continuous independent variables for regression analyses. Extent of operation was considered an ordinal variable increasing with each subsequent lymph node compartment dissection. Normalization of postoperative calcitonin levels (undetectable calcitonin) was considered on a per-patient basis, based on the specific assay. Linear regression was used for univariate analyses with serum bio-markers and logistic regression for lymph node positivity and postoperative calcitonin normalization. Candidates for multivariable model selection were chosen for clinical relevance and significance on univariate analysis.
RESULTS
Patient characteristics
A total of 104 patients met the inclusion criteria (61% female; mean age, 47 years; range, 5–86; Table I). Thirteen patients underwent prophylactic, preemptive thyroidectomy for known hereditary MTC. Twenty-nine patients had MTC associated with a hereditary syndrome: 28 with MEN2A and 1 with MEN2B. Of the 29 patients, 22 had RET testing performed and 21 carried germ-line RET mutations. Of the MEN2A patients with RET data available, 13 had V804M mutations in exon 14. The remaining 7 MEN2A patients had mutations involving codons 609 (n = 2), 618 (n = 3), and 620 (n = 2) in exon 10, and 634 in exon 11 (n = 1). The 1 patient with MEN2B had a mutation in codon 918 in exon 16. One patient with familial MTC tested negative for any RET mutation; this patient’s daughter with MTC was also RET negative. Of the 7 patients with hereditary MTC without RET mutation data, 5 were diagnosed before 1993; 1 had no record of RET testing; and 1 patient was not tested because she was a known V804M mutation kindred. All patients underwent total thyroidectomy.
Table I.
Patient, laboratory, and pathology variables
| Patient characteristic | Measure (n = 104) | Therapeutic (sporadic) (n = 74) | Therapeutic (hereditary) (n = 17) | Prophylactic (n = 13) | P value |
|---|---|---|---|---|---|
| Age, mean yrs (SD) | 47 (± 18.2) | 52 (± 14.8) | 41 (± 17.4) | 22 (± 12.9) | <.001 |
| Female, n (%) | 63 (61) | 46 (62) | 9 (53) | 8 (62) | .77 |
| Preoperative variables | |||||
| Preop serum calcitonin (pg/mL), median (IQR) | 643 (73–4,757) | 1,616 (315–5,914) | 834 (22–4,061) | 5 (5–9) | <.001 |
| Preop serum CEA (ng/mL), median (IQR) | 30 (2–108) | 36 (8–144) | 61 (1–139) | 0.7 (.5,1.4) | .002 |
| FNA calcitonin + stain* | 22/46 (48) | 18/40 (45) | 4/6 (67) | 0 (0) | .13 |
| FNA CEA + stain* | 6/46 (13) | 4/40 (10) | 2/6 (33) | 0 (0) | .39 |
| FNA chromogranin + stain* | 22/46 (48) | 18/40 (45) | 4/6 (67) | 0 (0) | .13 |
| Postoperative variables | |||||
| Median tumor size, cm (IQR) | 1.7 (0.9–3.0) | 1.9 (1.0–3.0) | 1.0 (0.4–2.5) | 0.1 (0.1–0.2) | .001 |
| C-cell hyperplasia, n (%) | 29 (28) | 6 (8) | 11 (65) | 12 (92) | <.001 |
| Lymphovascular invasion, n (%) | 20 (19) | 16 (22) | 4 (24) | 0 (0) | .14 |
| Extrathyroidal extension, n (%) | 12 (12) | 11 (15) | 1 (6) | 0 (0) | .34 |
| Multifocality, n (%) | 27 (26) | 15 (20) | 11 (65) | 1 (8) | <.001 |
| Margins positive, n (%) | 11 (11) | 9 (12) | 2 (12) | 0 (0) | .52 |
| Congo red (amyloid), n (%) | 35 (34) | 29 (39) | 6 (35) | 0 (0) | .01 |
| Calcitonin stain +, n (%) | 75 (72) | 54 (73) | 13 (77) | 8 (62) | .69 |
| CEA stain +, n (%) | 41 (39) | 31 (42) | 5 (29) | 5 (39) | .66 |
| Chromogranin A stain +, n (%) | 45 (43) | 30 (41) | 10 (59) | 5 (35) | .38 |
| Postop serum calcitonin, median (IQR) | 5 (2–145) | 5 (2–158) | 5 (2–716) | 2 (2–5) | .09 |
| Postop serum CEA, median (IQR) | 3 (1–17) | 3 (1–11) | 8.9 (1–45) | 2 (2–2) | .88 |
On the 46 patients with medullary thyroid cancer diagnosed on FNA.
CEA, Carcinoembryonic antigen; FNA, fine-needle aspiration; IQR, interquartile range; SD, standard deviation.
Extent of the operation
Of the 104 patients, 68 (65.4%) underwent completion lymph node dissection (CLND; 27/68 [40%] positive for meta-static cancer). Thirty-eight (36.5%) had ipsilateral modified radical neck dissection (MRND; 28/38 [74%] positive in the ipsilateral lateral compartment), 11 (10.6%) of whom underwent contralateral MRND (6/11 [55%] positive in the contralateral lateral compartment) in addition to ipsilateral MRND (Table II). None of the 10 patients with incidentally found perithyroidal lymph nodes identified on final pathology had lymph node metastases. All patients who underwent lateral neck dissections also had CLND, except for 2 patients who had ipsilateral MRND without CLND. Ten of the 38 patients who had MRND had biopsy-proven lateral neck disease preoperatively; 9 had radiographic imaging suspicious for lateral node metastasis. The remainder underwent MRND by surgeon preference or clinical suspicion. The average number of lymph nodes (±SD) harvested from the respective neck compartments was 7.3 ± 6.0 for CLND, 23.7 ± 13.4 for ipsilateral MRND, and 20.8 ± 10.6 for contralateral MRND. The mean number of positive nodes was 2.5 ± 4.4 for CLND, 6.1 ± 8.0 for ipsilateral MRND, and 1.5 ± 2.6 for contralateral MRND.
Table II.
Procedures performed
| Exploration | Total, n (%) | Therapeutic (sporadic), n (%) | Therapeutic (hereditary), n (%) | Prophylactic, n (%) |
|---|---|---|---|---|
| Total thyroidectomy | 104 (100) | 74 (100) | 17 (100) | 13 (100) |
| CLND | 68 (65) | 54 (73) | 12 (71) | 2 (15) |
| Ipsilateral MRND* | 38 (37) | 33 (45) | 5 (29) | 0 (0) |
| Bilateral MRND | 11 (11) | 9 (12) | 2 (12) | 0 (0) |
All MRND patients underwent CLND except for 2, who underwent ipsilateral MRND without CLND.
CLND, Completion lymph node dissection; MRND, modified radical neck dissection.
Pathology
Median tumor size was 1.5 cm. Excluding the 13 patients who had prophylactic thyroidectomies for known hereditary MTC, the median tumor size was 1.9 cm for sporadic MTC and 0.95 cm for hereditary patients. Where preoperative fine-needle aspiration (FNA) biopsy was available (n = 67), FNA diagnosed MTC accurately in 69% of patients. Other cytologic interpretations on FNA were as follows: Follicular neoplasm (9%), Hurthle cell neoplasm (6%), atypical cells (4.5%), nondiagnostic (4.5%), unclassified carcinoma (n = 2), neuroendocrine neoplasm (n = 1), anaplastic carcinoma (n = 1). There were no differences in calcitonin, CEA, or chromogranin-A IHC FNA staining between the hereditary and sporadic therapeutic groups with MTC diagnosed on FNA. Among the patients who underwent therapeutic thyroidectomy, the proportion of multifocality (P < .001) and CCH (P < .001) was greater in hereditary group. There was no difference in the percentage of patients with lymphovascular invasion, extrathyroidal extension, or positive margins between the sporadic and hereditary groups. Positivity on IHC staining for Congo red (amyloid), calcitonin, CEA, or chromogranin A were also similar among the therapeutic groups.
Extent of disease
All prophylactic patients were stage I. The stage distribution was similar between therapeutic sporadic and hereditary groups therapeutic groups (P = .59; Table III). Overall, 33 patients (32%) had metastases to cervical lymph nodes on final pathology. Five patients (4.8%) had positive nodes in the central compartment alone. Twenty-eight patients (27%) had lateral neck involvement; 22 with ipsilateral and 6 with both ipsilateral and contralateral disease. Twenty-two out of 27 patients (81%) with positive central (level VI) nodes also had ipsilateral lateral metastasis. Two of the 35 patients (6%) who underwent CLND and ipsilateral MRND had skip metastases. No patient had contralateral metastases without ipsilateral lateral node metastases.
Table III.
Stage distribution of cohort
| Stage* | Total, n (%) | Therapeutic (sporadic), n (%) | Therapeutic (hereditary), n (%) | Prophylactic, n (%) |
|---|---|---|---|---|
| I (stage 1: T1N0M0) | 52 (50) | 30 (40) | 9 (56) | 13 (100) |
| II (stage 2: T2/T3N0M0) | 19 (18) | 16 (21) | 3 (19) | 0 (0) |
| III (stage III: T1–T3N1aM0) | 5 (5) | 4 (5) | 1 (6) | 0 (0) |
| IV (stage IV: T4aN0/N1aM0 or T1–T4aN1bM0) | 28 (27) | 25 (33) | 3 (18) | 0 (0) |
According to the AJCC Cancer Staging Manual, 7th edition (2009).
Patients with known distant metastasis (M1) were excluded from the study.
Biomarkers
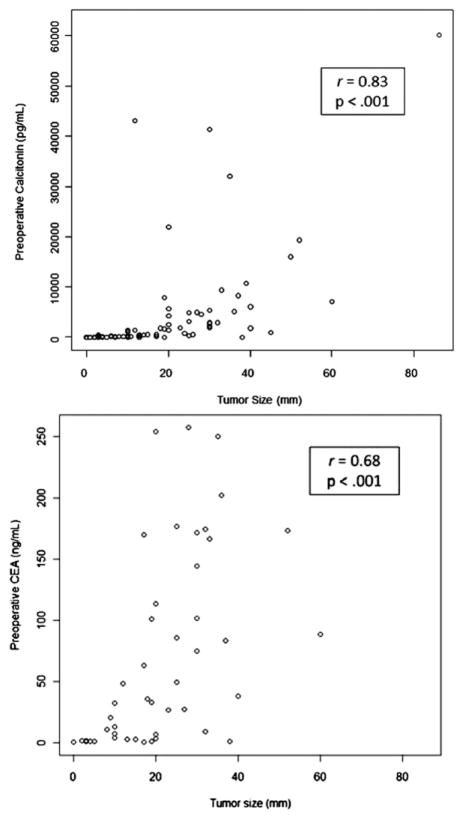
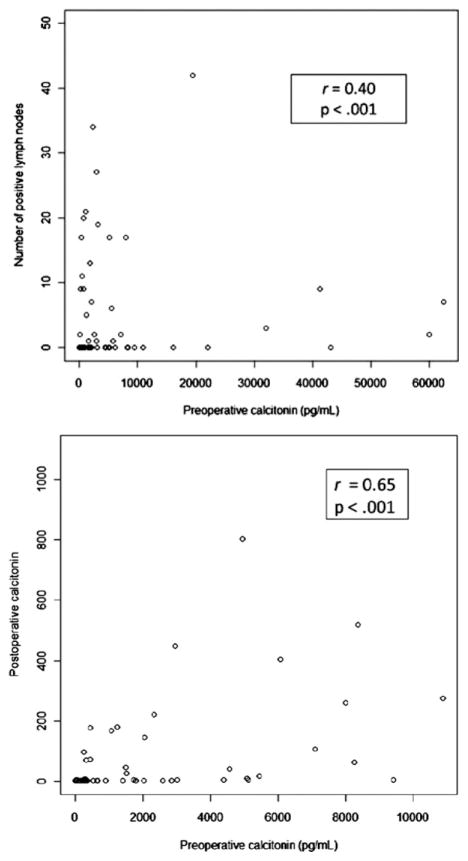
Preoperative calcitonin levels were available for 80 patients and preoperative CEA levels for 52. Postoperative calcitonin and CEA levels were available for 94 and 67 patients, respectively. Of those, there was no significant difference in preoperative calcitonin (P = .06), preoperative CEA (P = .68), postoperative calcitonin (P = .80), or postoperative CEA (P = .93) levels between the sporadic and therapeutic hereditary patients. Tumor size correlated strongly (r = 0.83; P <.001) and preoperative CEA correlated moderately (r = 0.68; P <.001) with preoperative calcitonin (Fig 1). Neither postoperative calcitonin (P = .11) nor postoperative CEA (P = .34) levels correlated with preoperative serum calcitonin levels. Tumor size was correlated with lymph node positivity after adjusting for preoperative calcitonin (odds ratio [OR], 1.6; 95% confidence interval [CI], 1.1–2.4; P = .03). On univariate analysis, preoperative calcitonin was correlated with tumor size (P <.001), TNM stage (P = .008) lymph node positivity (P = .01), postoperative serum calcitonin levels (P <.001), and CCH (P = .03), but not age, gender, extent of operation (P = .19), extrathyroidal extension (ETE), multifocality, positive margins, postoperative CEA, or number of metastatic nodes (P = .36). Preoperative calcitonin was not correlated with any of the FNA or surgical pathology IHC stains. Number of lymph node metastases correlated weakly (r = 0.40; P < .001) and postoperative calcitonin (r = 0.65; P < .001) correlated moderately with preoperative calcitonin (Fig 2). After adjusting for extent of operation, hereditary MTC, and lymph node positivity, tumor size (P <.001) and postoperative calcitonin (P = .01) were correlated independently with preoperative serum calcitonin levels. By linear regression analysis, preoperative CEA was correlated with preoperative calcitonin (P = .002). Preoperative CEA did not correlate with any of the FNA or surgical pathology IHC stains. On univariate analysis, postoperative calcitonin was significantly correlated with preoperative calcitonin (P < .001), lymph node positivity (P = .01), and postoperative CEA (P < .001), but not age, gender, tumor size (P = .11), extent of operation (P = .15), CCH, lympo-vascular invasion, ETE, multifocality, positive margins, or preoperative CEA. Postoperative calcitonin was correlated with CEA staining (P = .04), but not with any of the other FNA or surgical pathology IHC stains. Variables associated with failure to normalize postoperative calcitonin are shown in Table IV. TNM stage and preoperative calcitonin, but not extent of operation, correlated independently with biochemical cure (undetectable postoperative calcitonin). Of stage I patients, 88% had undetectable postoperative calcitonin vs 25% of stage IV patients. Of those that normalized postoperatively (n = 59, 63%), 49 (83%) were lymph node negative. Postoperative calcitonin normalized in 80% (49/61) of lymph node negative patients vs 30% (10/33) of lymph node positive patients (relative risk. 2.7; 95% CI, 1.6–4.5). A subgroup analysis was conducted excluding patients with stage IV disease (positive lateral nodes or extrathyroidal extension; n = 76). On univariate analysis, tumor size (OR, 2.0; 95% CI, 1.17–3.49) and TNM stage (OR, 2.8; 95% CI, 1.16–6.90) remained correlated with postoperative calcitonin normalization, whereas preoperative calcitonin approached significance (OR, 1.0; 95% CI, 0.99–1.08; P = .06). After multivariable adjustment, preoperative calcitonin neared significance (OR, 1.04; 95% CI, 1.00–1.08; P = .08), but stage no longer correlated; however, standard errors in the subgroup analysis were much greater. Seven of 28 (25%) patients with stage IV disease had postoperative calcitonin normalization. Postoperative CEA correlated with preoperative calcitonin (P = .002), postoperative calcitonin (P < .001), and independently with positive margins (adjusted P = .04), but not lymph node positivity, age, gender, tumor size, extent of surgery, CCH, lymphatic invasion, ETE, multifocality, or preoperative CEA. It correlated with positive staining of CEA on FNA (P = .01) and surgical specimen (P = .02), as well as chromogranin-A on surgical pathology (P = .03), but was not significantly associated with any of the other FNA or surgical pathology IHC stains. The only factor that correlated independently with postoperative CEA after multivariable adjustment was positive margins (P = .04).
Fig 1.
Preoperative biomarkers correlate with tumor size on univariate analysis.
Fig 2.
Number of lymph node metastasis and postoperative calcitonin correlate with preoperative calcitonin on univariate analysis.
Table IV.
Factors associated with failure to normalize postoperative calcitonin
| Variable | Odds ratio (CI) | Univariate P value | Adjusted OR (CI) | Adjusted P value |
|---|---|---|---|---|
| Lymph node positivity* | 9.4 (3.5–24.9) | <.0001 | ||
| Number of positive lymph nodes† | 2.0 (1.3–3.1) | .001 | ||
| Tumor size* (cm) | 1.9 (1.3–2.9) | <.001 | ||
| TNM stage | 2.7 (1.8–4.0) | <.0001 | 2.9 (1.4–6.2) | .005 |
| Extent of surgery | 2.1 (1.3–3.3) | .002 | .34 | |
| Preoperative calcitonin‡ | 1.05 (1.01–1.09) | .026 | 1.04 (1.00–1.08) | .04 |
Excluded on multivariable analysis because of colinearity with TNM stage.
Odds ratio quoted per increments of 5 lymph nodes.
Odds ratio quoted per increments of 500 pg/mL.
CI, Confidence interval.
DISCUSSION
MTC is an uncommon entity, comprising 2% of thyroid cancers in the United States.1 The quoted breakdown of hereditary MTC ranges from 20 to 30%, which is in keeping with the 28% hereditary cases found in our cohort.10,11 Similar to other studies, our mean age at diagnosis was greater in patients with sporadic MTC than in patients with hereditary MTC and there was a slight female predominance (61%).11–13 Of the 22 MEN patients tested for the RET germline mutation, 21 had positive results. Among those with MEN2A, the V804M mutation occurred most commonly. The remainder of the mutations affecting codons in exons 10 and 11 are also known to occur frequently among patients with MEN2A. The 1 patient with MEN2B carried a mutation in codon 918, a derangement found in 95% of MEN2B patients.2 One patient tested negative for a RET mutation. Negative RET testing is rare among families with hereditary MTC, with a rate of occurrence ranging between 2.4 and 8%.14
MTC is challenging to treat and has limited adjuvant treatment options. Optimal initial operative management of MTC remains controversial. Although it is standard practice to excise the primary tumor and all diseased lymph node basins, the identification of which neck compartments are involved with MTC remains problematic. Palpable or enlarged lymph nodes may be detected by physical examination or on imaging with reported wide sensitivity ranges of 28–78% for ultrasonography, 38–70% for serum calcitonin, and 32–47% for positron emission tomography/computed tomography.3,15,16 Micrometastases cannot be ascertained readily by current imaging techniques. Furthermore, the presence of subclinical MTC in regional lymph nodes is a predicament, because it cannot be eradicated by radioiodine as in differentiated thyroid cancer, but rather predisposes the patient to risk for local recurrence and potentially disseminated disease. Operative resection remains the mainstay treatment for MTC. Although aggressive attempts to clear all nodal compartments of microscopic or undetected disease may afford the best chance for cure, the approach also exposes patients to the associated risks of more extensive operation. Differences were observed among the surgeons at our institutions in regard to extent of operation. The average number of lymph nodes harvested from the respective neck compartments was 7.3 for CLND, 23.7 for ipsilateral MRND, and 20.8 ± 10.6 for contralateral neck dissection. Compared with the nodal yields cited in reports published previously of patients with therapeutic nodal dissections, the mean number of lymph nodes harvested in this study is lower for central neck dissection, but comparable for ipsilateral and contralateral neck dissections.17,18 The total mean yield from any lymphadenectomy in our study is similar to that reported in a Surveillance, Epidemiology, and End Results database study by Leggett et al19 looking at 534 patients with MTC and any lymphadenectomy (mean total yield, 18.3).
The biomarkers calcitonin and CEA play a central role in the diagnosis and postoperative surveillance of MTC. Recently, some groups have attempted to utilize these biomarkers to predict the extent of disease and guide the extent of operation. To be effective, preoperative biomarker levels must predict reliably the extent of disease. Also, biomarkers obtained postoperatively ideally would not only indicate the presence of residual disease, but also reflect the extent of operation performed. In the present study, we retrospectively assessed patients undergoing initial operative management for MTC to identify clinicopathologic characteristics that correlate significantly with preoperative and postoperative biomarker levels. We found the preoperative calcitonin, but not CEA, reflected extent of disease. Specifically, our findings agree with previously reported observations that preoperative basal serum calcitonin levels correlate with tumor size and degree of lymph node metastasis.
In the present work, tumor size was correlated independently with preoperative calcitonin. Similarly, Cohen et al7 reviewed retrospectively 226 patients and found that a calcitonin level >1,000 pg/mL corresponded with a median tumor size of 2.5 cm, whereas a serum calcitonin level <1,000 pg/mL corresponded with a median tumor size of 0.7 cm. A calcitonin level below a threshold calcitonin level of 100 pg/mL was associated with a median tumor size of 0.3 cm, vs 2.0 cm for a calcitonin level >100 pg/mL.7 Machens et al also confirmed that greater levels of biomarker reflect larger primary neoplasms. In their study of 300 patients, the mean preoperative calcitonin levels increased from 69 pg/mL for tumors <0.5 cm to 9,918 pg/mL for tumors >2.0 cm. They also found that the number of positive lymph nodes also increased with tumor size, from 0.8 nodes for tumors <0.5 cm to 16.8 for tumors >2.0 cm.20 A similar pattern was also observed in our study: Neoplasms <0.8 cm had 1.5 mean positive nodes, whereas neoplasms >3.0 cm had 7.9 mean positive nodes. The greater number of lymph node metastases from the Machens group likely reflects the more aggressive operations performed.
Machens and Dralle20 also identified a correlation between preoperative basal calcitonin levels and the progression of lymph node involvement by region. Lymph node metastases were found in the ipsilateral central and lateral neck, contralateral central neck, contralateral lateral neck, and upper mediastinum with incremental increases in basal calcitonin thresholds of 20, 50, 200, and 500 pg/mL, respectively. The authors concluded that bilateral central and lateral neck dissections may be the best therapeutic option for patients with preoperative basal calcitonin levels >200 pg/mL.20 Our data cannot replicate or corroborate the regional progression of lymph node metastasis by preoperative calcitonin level, because bilateral MRNDs are not performed routinely at our institution; however, our findings suggest that, the majority of the time, MTC does spread first to the central compartment, followed by the ipsilateral lateral and then contralateral lateral neck. Preoperative calcitonin did correlate with TNM stage, which, excluding extrathyroidal extension, stratifies nodal metastasis roughly by compartment (central [stage III] vs lateral compartments [stage IV]). Once the central compartment lymph nodes are positive, there is a high likelihood (81%) of the ipsilateral neck involvement as well. Very few (n = 2; 6%) who underwent CLND and ipsilateral MRND had skip metastasis, and every patient with contralateral metastasis also had ipsilateral lateral node disease. Although Machen’s group found central compartment lymph node metastases were excluded at a threshold of <20 pg/mL,20 no patient in our series with a preoperative calcitonin <53 pg/mL had lymph node metastases (although only 5 of the 20 patients in this subgroup underwent formal CLND). These findings suggest that there may be a threshold below which CLND and MRLND may be safely omitted from the operative plan; however, this possibility needs to be assessed prospectively. Moreover, the extent of operation should be based on the assimilation of clinical data, including preoperative serum calcitonin levels, as well as lymph node status, as demonstrated by radiography or FNA.
Biochemical normalization of preoperative calcitonin is highly desired with a survival rate of 97.7% at 10 years.21 After multivariable adjustment, factors that correlated with the failure to normalize postoperative calcitonin were greater preoperative calcitonin levels and TNM stage. Postoperative calcitonin levels normalized in lymph node-negative patients 83% of the time vs 30% in lymph node-positive patients. These values are similar to rates published previously of biochemical remission, which range from 62 to 95% for patients with node-negative MTC and 10 to 32% for those with node-positive tumors.9,22 Cohen et al7 also recognized preoperative calcitonin levels as a predictor of postoperative calcitonin normalization, with a preoperative levels <50 pg/mL leading to normalization 97% of the time. A separate study by Machens et al determined that preoperative basal calcitonin level of >500 pg/mL best predicted failure to achieve biochemical remission, followed by nodal status and reoperative status and that involvement of ≥10 lymph nodes or >2 lymph node compartments precluded normalization of calcitonin.9 In our study, preoperative calcitonin levels as low as 240 pg/mL were associated with failure of normalization. Modigliani’s GETC study of 899 patients with MTC found after multivariable analysis of factors including stage, tumor size, and lymph node involvement, that the only significant predictor of biochemical cure was stage at the time of operation.21
Our data suggest that extent of operation is less important than extent of disease at presentation in predicting postoperative calcitonin normalization, confirming what has been observed in other reports.9 Practically, it is likely that the extent of operation was influenced by the more advanced stage identified preoperatively. Although some colinearity may exist between stage and extent of operation, the observation that stage remains significantly associated with postoperative calcitonin normalization after adjustment for both extent of operation and stage suggests that stage is a better predictor of failure of calcitonin normalization. This finding implies that stage at the time of diagnosis of MTC is the most critical factor influencing prognosis. Although removal of as much disease as possible is desirable, it may be impossible to clear all involved nodal compartments at operation once serum calcitonin concentration exceeds an arbitrary threshold. Thus, early detection is essential in offering patients the greatest possible chance for cure.
In light of these findings, the effort to incorporate calcitonin measurement into the routine clinical workup for thyroid nodules may be justified.23–25 Screening calcitonin is recommended by the European Thyroid Association and cost-effectiveness analysis on US populations support this practice.23,26 Other European experts feel that the benefits of calcitonin screening are unresolved.27 The American Thyroid Association remains ambivalent concerning this practice.2 In their retrospective analysis of >10,000 patients, Elisei et al24 concluded that screening calcitonin has a greater sensitivity and specificity than FNA, leads to diagnosis of MTC at an earlier stage, results in a greater rate of postoperative calcitonin normalization and ultimately, improved survival. Indeed, in our study, 7 of 8 of patients with a false-negative FNA and available basal calcitonin levels had preoperative calcitonin of >200 pg/mL.
This study is limited primarily by the retrospective design and inherent treatment selection bias. Furthermore, the sample size limits the statistical power; however, we are constrained by the low prevalence of the disease. There is also potential misclassification of data; however, we expect this to be nondifferential. Nondissected lymph node basins were assumed to be negative, which may have lead to underestimation of the extent of disease in some patients.
This work indicates that preoperative calcitonin, but not CEA levels, reflect the extent of disease in patients undergoing a first operation for MTC. Postoperative calcitonin and CEA biomarkers do not correlate with the extent of operation performed. Moreover, preoperative calcitonin and stage were correlated with postoperative calcitonin and failure of calcitonin normalization. These findings underscore the importance of early detection in patients with MTC, especially because few adjuvant treatments exist. Prospective studies analyzing the utility of preoperative basal calcitonin levels for screening and as an adjunct to determining the extent of surgery as it relates to outcome are warranted.
References
- 1.Enewold L, Zhu K, Ron E, et al. Rising thyroid cancer incidence in the United States by demographic and tumor characteristics, 1980–2005. Cancer Epidemiol Biomarkers Prev. 2009;18:784–91. doi: 10.1158/1055-9965.EPI-08-0960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kloos RT, Eng C, Evans DB, et al. Medullary thyroid cancer: management guidelines of the American Thyroid Association. Thyroid. 2009;19:565–612. doi: 10.1089/thy.2008.0403. [DOI] [PubMed] [Google Scholar]
- 3.Kouvaraki MA, Shapiro SE, Fornage BD, et al. Role of pre-operative ultrasonography in the surgical management of patients with thyroid cancer. Surgery. 2003;134:946–54. doi: 10.1016/s0039-6060(03)00424-0. [DOI] [PubMed] [Google Scholar]
- 4.Kebebew E, Kikuchi S, Duh QY, Clark OH. Long-term results of reoperation and localizing studies in patients with persistent or recurrent medullary thyroid cancer. Arch Surg. 2000;135:895–901. doi: 10.1001/archsurg.135.8.895. [DOI] [PubMed] [Google Scholar]
- 5.Panigrahi B, Roman SA, Sosa JA. Medullary thyroid cancer: are practice patterns in the United States discordant from American Thyroid Association guidelines? Ann Surg Oncol. 2010;17:1490–8. doi: 10.1245/s10434-010-1017-0. [DOI] [PubMed] [Google Scholar]
- 6.Barbet J, Campion L, Kraeber-Bodere F, Chatal JF. Prognostic impact of serum calcitonin and carcinoembryonic antigen doubling-times in patients with medullary thyroid carcinoma. J Clin Endocrinol Metab. 2005;90:6077–84. doi: 10.1210/jc.2005-0044. [DOI] [PubMed] [Google Scholar]
- 7.Cohen R, Campos JM, Salaun C, et al. Preoperative calcitonin levels are predictive of tumor size and postoperative calcitonin normalization in medullary thyroid carcinoma. Groupe d’Etudes des Tumeurs a Calcitonine (GETC) J Clin Endocrinol Metab. 2000;85:919–22. doi: 10.1210/jcem.85.2.6556. [DOI] [PubMed] [Google Scholar]
- 8.Weber T, Schilling T, Frank-Raue K, et al. Impact of modified radical neck dissection on biochemical cure in medullary thyroid carcinomas. Surgery. 2001;130:1044–9. doi: 10.1067/msy.2001.118380a. [DOI] [PubMed] [Google Scholar]
- 9.Machens A, Schneyer U, Holzhausen HJ, Dralle H. Prospects of remission in medullary thyroid carcinoma according to basal calcitonin level. J Clin Endocrinol Metab. 2005;90:2029–34. doi: 10.1210/jc.2004-1836. [DOI] [PubMed] [Google Scholar]
- 10.Sippel RS, Kunnimalaiyaan M, Chen H. Current management of medullary thyroid cancer. Oncologist. 2008;13:539–47. doi: 10.1634/theoncologist.2007-0239. [DOI] [PubMed] [Google Scholar]
- 11.Pelizzo MR, Boschin IM, Bernante P, et al. Natural history, diagnosis, treatment and outcome of medullary thyroid cancer: 37 years experience on 157 patients. Eur J Surg Oncol. 2007;33:493–7. doi: 10.1016/j.ejso.2006.10.021. [DOI] [PubMed] [Google Scholar]
- 12.Modigliani E. Type 2 endocrine neoplasms. Clinical aspects. Presse Med. 1998;27:628–32. [PubMed] [Google Scholar]
- 13.Grozinsky-Glasberg S, Benbassat CA, Tsvetov G, et al. Medullary thyroid cancer: a retrospective analysis of a cohort treated at a single tertiary care center between 1970 and 2005. Thyroid. 2007;17:549–56. doi: 10.1089/thy.2006.0229. [DOI] [PubMed] [Google Scholar]
- 14.Romei C, Mariotti S, Fugazzola L, et al. Multiple endocrine neoplasia type 2 syndromes (MEN 2): results from the ItaMEN network analysis on the prevalence of different genotypes and phenotypes. Eur J Endocrinol. 2010;163:301–8. doi: 10.1530/EJE-10-0333. [DOI] [PubMed] [Google Scholar]
- 15.Skoura E, Rondogianni P, Alevizaki M, et al. Role of [(18)F] FDG-PET/CT in the detection of occult recurrent medullary thyroid cancer. Nucl Med Commun. 2010;31:567–75. doi: 10.1097/MNM.0b013e3283384587. [DOI] [PubMed] [Google Scholar]
- 16.Giraudet AL, Vanel D, Leboulleux S, et al. Imaging medullary thyroid carcinoma with persistent elevated calcitonin levels. J Clin Endocrinol Metab. 2007;92:4185–90. doi: 10.1210/jc.2007-1211. [DOI] [PubMed] [Google Scholar]
- 17.de Groot JW, Links TP, Sluiter WJ, Wolffenbuttel BH, Wiggers T, Plukker JT. Locoregional control in patients with palpable medullary thyroid cancer: results of standardized compartment-oriented surgery. Head Neck. 2007;29:857–63. doi: 10.1002/hed.20609. [DOI] [PubMed] [Google Scholar]
- 18.Moley JF, DeBenedetti MK. Patterns of nodal metastases in palpable medullary thyroid carcinoma: recommendations for extent of node dissection. Ann Surg. 1999;229:880–7. doi: 10.1097/00000658-199906000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leggett MD, Chen SL, Schneider PD, Martinez SR. Prognostic value of lymph node yield and metastatic lymph node ratio in medullary thyroid carcinoma. Ann Surg Oncol. 2008;15:2493–9. doi: 10.1245/s10434-008-0022-z. [DOI] [PubMed] [Google Scholar]
- 20.Machens A, Dralle H. Biomarker-based risk stratification for previously untreated medullary thyroid cancer. J Clin Endocrinol Metab. 2010;95:2655–63. doi: 10.1210/jc.2009-2368. [DOI] [PubMed] [Google Scholar]
- 21.Modigliani E, Cohen R, Campos JM, et al. Prognostic factors for survival and for biochemical cure in medullary thyroid carcinoma: results in 899 patients. The GETC Study Group. Groupe d’etude des tumeurs a calcitonine. Clin Endocrinol (Oxf) 1998;48:265–73. doi: 10.1046/j.1365-2265.1998.00392.x. [DOI] [PubMed] [Google Scholar]
- 22.Scollo C, Baudin E, Travagli JP, et al. Rationale for central and bilateral lymph node dissection in sporadic and hereditary medullary thyroid cancer. J Clin Endocrinol Metab. 2003;88:2070–5. doi: 10.1210/jc.2002-021713. [DOI] [PubMed] [Google Scholar]
- 23.Pacini F, Schlumberger M, Dralle H, Elisei R, Smit JW, Wiersinga W. European consensus for the management of patients with differentiated thyroid carcinoma of the follicular epithelium. Eur J Endocrinol. 2006;154:787–803. doi: 10.1530/eje.1.02158. [DOI] [PubMed] [Google Scholar]
- 24.Elisei R, Bottici V, Luchetti F, et al. Impact of routine measurement of serum calcitonin on the diagnosis and outcome of medullary thyroid cancer: experience in 10,864 patients with nodular thyroid disorders. J Clin Endocrinol Metab. 2004;89:163–8. doi: 10.1210/jc.2003-030550. [DOI] [PubMed] [Google Scholar]
- 25.Pacini F, Fontanelli M, Fugazzola L, et al. Routine measurement of serum calcitonin in nodular thyroid diseases allows the preoperative diagnosis of unsuspected sporadic medullary thyroid carcinoma. J Clin Endocrinol Metab. 1994;78:826–9. doi: 10.1210/jcem.78.4.8157706. [DOI] [PubMed] [Google Scholar]
- 26.Cheung K, Roman SA, Wang TS, Walker HD, Sosa JA. Calcitonin measurement in the evaluation of thyroid nodules in the United States: a cost-effectiveness and decision analysis. J Clin Endocrinol Metab. 2008;93:2173–80. doi: 10.1210/jc.2007-2496. [DOI] [PubMed] [Google Scholar]
- 27.Costante G, Durante C, Francis Z, Schlumberger M, Filetti S. Determination of calcitonin levels in C-cell disease: clinical interest and potential pitfalls. Nat Clin Pract Endocrinol Metab. 2009;5:35–44. doi: 10.1038/ncpendmet1023. [DOI] [PubMed] [Google Scholar]