Abstract
Lung function measures are heritable, predict mortality and are relevant in diagnosis of chronic obstructive pulmonary disease (COPD). COPD and asthma are diseases of the airways with major public health impacts and each have a heritable component. Genome-wide association studies of SNPs have revealed novel genetic associations with both diseases but only account for a small proportion of the heritability. Complex copy number variation may account for some of the missing heritability. A well-characterised genomic region of complex copy number variation contains beta-defensin genes (DEFB103, DEFB104 and DEFB4), which have a role in the innate immune response. Previous studies have implicated these and related genes as being associated with asthma or COPD. We hypothesised that copy number variation of these genes may play a role in lung function in the general population and in COPD and asthma risk. We undertook copy number typing of this locus in 1149 adult and 689 children using a paralogue ratio test and investigated association with COPD, asthma and lung function. Replication of findings was assessed in a larger independent sample of COPD cases and smoking controls. We found evidence for an association of beta-defensin copy number with COPD in the adult cohort (OR = 1.4, 95%CI:1.02–1.92, P = 0.039) but this finding, and findings from a previous study, were not replicated in a larger follow-up sample(OR = 0.89, 95%CI:0.72–1.07, P = 0.217). No robust evidence of association with asthma in children was observed. We found no evidence for association between beta-defensin copy number and lung function in the general populations. Our findings suggest that previous reports of association of beta-defensin copy number with COPD should be viewed with caution. Suboptimal measurement of copy number can lead to spurious associations. Further beta-defensin copy number measurement in larger sample sizes of COPD cases and children with asthma are needed.
Introduction
Lung function measures are heritable traits that predict morbidity and mortality in the general population [1]–[4]. The ratio of forced expired volume in 1 second (FEV1) to forced vital capacity (FVC) is used in diagnostic criteria for Chronic obstructive pulmonary disease (COPD), whilst the FEV1 (expressed as % predicted FEV1) contributes to measures of COPD severity. Genome-wide association studies (GWAS) of single nucleotide polymorphisms (SNPs) have identified 26 regions of the genome showing association with FEV1 and/or FEV1/FVC [5]–[7]. Collectively these 26 variants only account for approximately 3.2% of the additive polygenic variance in FEV1/FVC [7]. COPD, characterised by irreversible airway obstruction, is a leading cause of global mortality and morbidity predicted to be the third leading cause of death by 2020 [8]. Although the major risk factor for COPD is smoking, there is a heritable component [9]. We previously hypothesised that some the genetic determinants of lung function would be genetic causes of COPD. Of the 26 genomic regions associated with lung function, 8 have so far been reported to be associated with airway obstruction or COPD [10]–[14].
Asthma, a form of reversible airway obstruction, has increased in prevalence and more than 300 million people worldwide are affected [15]. Whilst GWAS for asthma have shown association with at least 10 genomic regions, including those encoding proteins involved in the immune response [16]–[19], these variants explain only around 4% of asthma heritability [20]. Thus, most of the genetic determinants of lung function, COPD and asthma are yet to be identified. Copy number variation (CNV) is a type of genetic variation where sections of the genome are duplicated or deleted. It is currently estimated that up to 4% of the genome may be copy number variable [21]. Copy number variation, particularly complex copy number variation which is poorly tagged by the variants commonly studied in GWAS, may explain some of the remaining unexplained heritability of these clinically important traits.
One of the best-characterised regions of complex copy number variation is the beta-defensin locus; the repeat unit (referred to hereafter as DEFB) contains several beta defensin genes; DEFB107, DEFB106, DEFB105, DEFB104, DEFB103 and DEFB4 and also SPAG11, which vary in copy number as a block. In addition to their direct antimicrobial function, these beta-defensins can act as chemokines having an immunomodulatory role (for review see [22]). Both DEFB4 and DEFB103 are expressed in the lung and trachea [23], [24], and the mature peptides hbd2 (encoded by the gene DEFB4) and hbd3 (encoded by the gene DEFB103) have antimicrobial activity against a range of pathogens that are known to infect the airway [25]. The mature peptide hbd4 (encoded by the gene DEFB104) is also present in appreciable quantities in the lung [26], and expression of these three beta-defensins are modulated by pro-inflammatory molecules or bacteria [27], [28]. In the UK population, DEFB copy numbers of between 2 and 10 are observed at an appreciable frequency [29], [30]. Thus there has been considerable interest in relating DEFB copy number to immune-related diseases including COPD and asthma, as well as psoriasis [31], [32]. However, the findings from association studies of other diseases have not always been consistent and replicable [33]–[36].
We determined DEFB diploid copy number using a Paralogue Ratio Test (PRT), a method shown to have higher accuracy than previously employed methods [29], in a total of 2343 individuals from two UK-based general population cohorts of adults and children (Table 1 and Table 2). We tested association firstly with quantitative measures of lung function in all individuals (FEV1, FVC and FEV1/FVC) and with risk of asthma and risk of COPD in subsets of each cohort.
Table 1. Characteristics of Gedling and LRC individuals included in the study.
| LRC (n = 689) | Gedling (n = 1149) | |
| Age (mean[range]) | 12.8 [7.8–14.2] | 56.1 [27.5–80.1] |
| Sex (males:females) | 354∶335 | 569∶580 |
| Height cm (mean[range]) | 156.4 [122.6–187.7] | 168.0 [145.0–204.0] |
| FEV1 litres (mean[range]) | 2.70 [1.17–4.71] | 2.86 [0.51–5.81] |
| FVC litres (mean[range]) | 3.08 [1.43–5.84] | 3.68 [0.80–7.13] |
| FEV1/FVC (mean[range]) | 0.88 [0.64–0.997] | 0.77 [0.35–0.96] |
| Doctor diagnosed asthma (yes:no) | 255∶434 | 45∶629* |
| Asthma-ICS | 82∶441 | na |
| COPD GOLD Stage 2 | na | 42∶189# |
Individuals with % predicted FEV1<80% and FEV1/FVC<0.7 (GOLD stage 2 cases) excluded from cases and controls. #Characteristics of cases and controls are shown in Table 2.
Table 2. Characteristics of Gedling and replication COPD cases and controls.
| Gedling | Replication | |||
| Cases (n = 42) | Controls (n = 189) | Cases (n = 251) | Controls (n = 227) | |
| Age (mean[range]) | 67.4[45–80] | 59.4[41–80] | 66.8[35–90] | 57.0[38–88] |
| Sex (Males:Females) | 31∶11 | 119∶70 | 142∶109 | 95∶132 |
| Height (mean[range]) | 168.3[145–182] | 168.8[146–190] | 166.1[148–188] | 167.1[146–190] |
| FEV1 (mean[range]) | 1.72[0.7–2.93] | 2.97[1.65–5.14] | 1.01[0.25–2.53] | 2.80[1.38–4.83]] |
| % predicted FEV1 (mean[range]) | 60.9[24.0–79.9] | 97.3[80.2–151.7] | 37.8[11.2–77.2] | 93.5[80.0–127.7] |
| FVC (mean[range]) | 2.79[1.36–4.32] | 3.78[2.15–9.65] | 2.20[0.82–4.17] | 3.52[1.64–6.61] |
| FEV1/FVC (mean[range]) | 0.61[0.35–0.70] | 0.79[0.70–0.92] | 0.46[0.13–0.69] | 0.79[0.71–0.97] |
| Smoking pack years (mean[range]) | 42.7[6–150] | 22.5[5–90] | 46.6[20–150] | 35.6[20–145] |
Materials and Methods
Ethics Statement
The Gedling study was approved by the Nottingham City Hospital and Nottingham University Ethics committees (MREC/99/4/01) and written informed consent for genetic study was obtained from participants. The Leicester Respiratory Cohort was approved by the Leicestershire Health Authority Research Ethics Committee, Leicester, UK (07/H0407/70) and written informed consent for genetic study was obtained from the subjects and their parents. For the replication COPD cases and controls, approval was obtained from Southmead Local Research Ethics Committee, Bristol (134/01), and Lothian Research Ethics Committee, Edinburgh (LREC/2001/4/62) and written informed consent for genetic study was obtained from participants.
Sample selection
Gedling
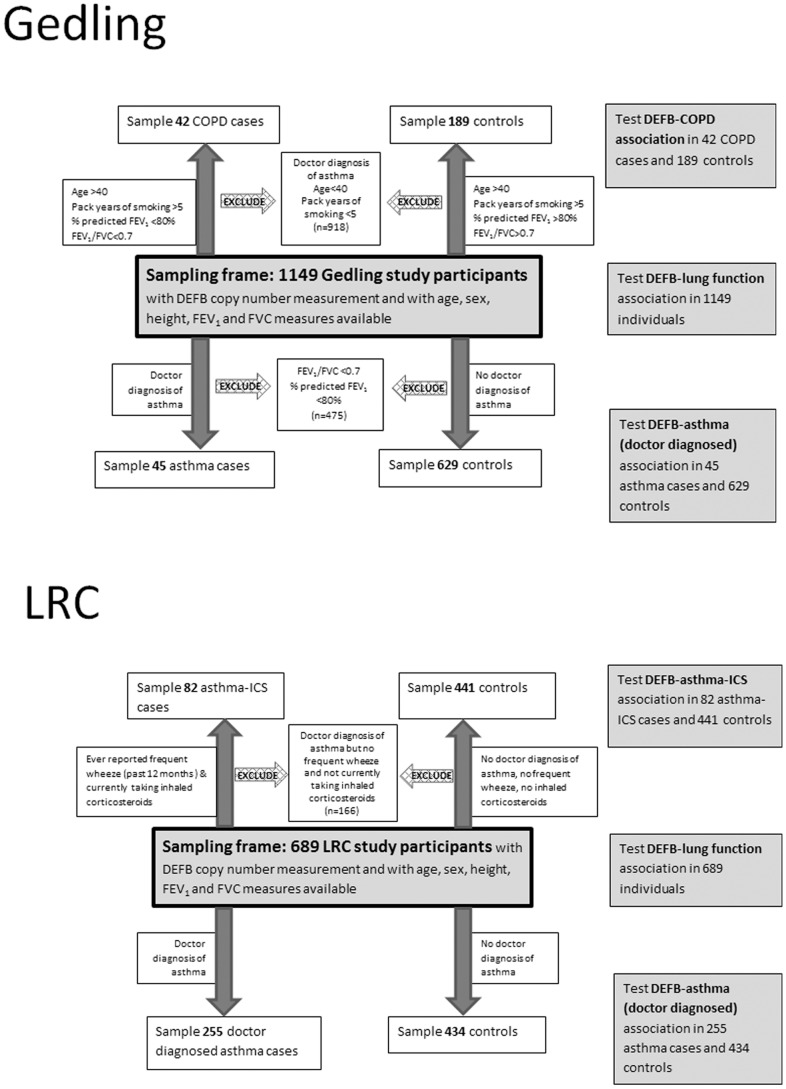
The Gedling cohort is a general population sample recruited in Nottingham in 1991 (18 to 70 years of age, n = 2,633) [37] and then followed-up in 2000 (n = 1346) when blood samples were taken for DNA extraction and FEV1 and FVC were measured using a calibrated dry bellows spirometer (Vitalograph, Buckingham, UK), recording the best of three satisfactory attempts [38]. DNA was extracted by Source Biosciences, UK. A total of 1176 European ancestry individuals for whom DNA and non-missing relevant phenotype data (age, sex, height, FEV1 (pre-bronchodilator), FVC (pre-bronchodilator), smoking status, asthma diagnosis) were available and were typed for DEFB copy number using PRT. Doctor diagnosed asthma cases in Gedling were defined as individuals for whom an asthma diagnosis had been confirmed by a doctor [37] (however, individuals with % predicted FEV1<80% and FEV1/FVC<0.7 (COPD GOLD stage 2 [39]) were excluded from both doctor diagnosed asthma case and control sets. Percent predicted FEV1 was calculated according to previously described equations [40], [41]). COPD cases were defined in Gedling as individuals age>40, smoking pack years>5, % predicted FEV1<80% and FEV1/FVC<0.7 (COPD GOLD stage 2 and above [39]). Controls for the COPD analysis were defined as age>40, pack years >5, %predicted FEV1>80% and FEV1/FVC>0.7. Individuals with a doctor diagnosis of asthma were excluded from the COPD case and control sets. Characteristics of these samples are given in Table 1 and Table 2. Figure 1 (top) illustrates how samples were selected for each analysis.
Figure 1. Sample selection for association analyses in Gedling and LRC.
The sampling frame for each cohort is shown centrally with the COPD and asthma association analysis sample subsets indicated above and below for each cohort.
Leicester Respiratory Cohort (LRC)
The paediatric dataset comes from a population-based respiratory cohort in Leicestershire, UK, described in detail elsewhere [42]. In short, perinatal data were collected at birth, and data on growth and development were acquired prospectively during childhood. Respiratory morbidity and individual and family-related exposures were assessed by repeated questionnaires (1998, 2001, 2003, 2006, 2010). In 2006 to 2010, families who had returned two or more questionnaires (n = 4,125) were invited to the laboratory for assessment of lung function by spirometry [43], [44]. We recorded FVC and FEV1 before and 15 minutes after administration of salbutamol (400 µg) by spacer. Pre-bronchodilator measurements were used in this study. Of 4,177 children who attended the lab, 903 individuals had saliva samples available for DNA extraction. DNA was extracted by LGC Genomics Ltd (previously KBiosciences Ltd). Of these, 689 were of European ancestry (self-reported), aged under 16 years and had non-missing relevant phenotype data (age, sex, height, FEV1, FVC, and asthma variables). The LRC is an unselected population-based cohort and most children reporting a doctor diagnosis of asthma had mild disease. Therefore, a potentially more clinically relevant subpopulation with moderately severe asthma was also defined as individuals who had ever reported frequent wheeze during the past 12 months (over four attacks or always accompanied by shortness of breath) and were taking inhaled corticosteroids at the time of questionnaire (controls for this analysis were all individuals who had never reported frequent wheeze during the past 12 months, were not taking inhaled corticosteroids at the time of questionnaire and did not report a doctor diagnosis of asthma). This phenotype is referred to hereafter as “asthma-ICS”. Characteristics of LRC subjects are given in Table 1. Figure 1 (bottom) illustrates how samples were selected for each analysis.
COPD replication collection
The criteria for recruitment of cases and controls in the replication collection are described elsewhere [45]. In brief, the criteria for cases was % predicted FEV1< = 70%, FEV1/FVC<70%, no significant reversibility on bronchodilation, a smoking history of > = 20 pack years, and no diagnosis of asthma or other alternative diagnosis for airway obstruction (including severe SERPINA1 deficiency). Controls were selected who had no history of airway obstruction or recent pulmonary infection, and had % predicted FEV1> = 80% and FEV1/FVC>70% and were selected to match the cases for age and sex. Controls were current or ex-smokers with smoking history similar to that of the cases. A total of 251 UK cases (112 from Bristol and 139 from Edinburgh) and 227 UK controls (136 from Bristol and 91 from Edinburgh) were copy number genotyped for DEFB copy number using PRT in the same laboratory as the Gedling and LRC samples. Cases and controls were randomised and the individual undertaking the assays was blind to COPD status. All individuals were of European ancestry. Characteristics of these samples are given in Table 2.
DEFB copy number assay
The DEFB copy number was determined, blind to clinical information, using a triplex PRT described previously [29], [46]. Every experiment included the same six positive controls of known copy number, the same samples as used in previous studies [29], [30], which were used to normalize the experimental results and minimize interexperimental technical variation in typing copy number. Maximum likelihood integer copy number was called by combining information from all three assays and estimating the most likely copy number given the data. In addition, non-integer copy number values were calculated by averaging the estimates from the three assays.
Statistical analysis
Association of the average raw PRT DEFB copy numbers with lung function in all individuals (FEV1, FVC and FEV1/FVC), COPD status (Gedling only) and asthma status (doctor diagnosed asthma in both cohorts and asthma-ICS in LRC) was tested using linear and logistic regression. Association of the maximum likelihood integer copy numbers with lung function, COPD status and asthma status was also undertaken. Age, age2, sex and height were included as covariates. Inverse normal transformation was applied to FEV1 and FEV1/FVC. R v2.15.2 was used to run all association analyses. Power was estimated by a simulated study of equivalent size to the Gedling study (n = 1149) with a similar distribution of DEFB copy number to that observed in our data. At a significance level of 0.05, power was 95% to detect an effect size of 0.1 SD change in lung function per one unit change in DEFB copy number. In 42 COPD cases and 189 controls, simulations showed, for a significance level of 0.05, power of 88% to detect an OR of 1.7 per unit change in DEFB copy number. Power simulations were run using Stata version 13.
Replication analysis
A subset of a larger European ancestry COPD case-control study was used to test for replication of an association with COPD observed in the Gedling cohort. Logistic regression was used to test for association with COPD using age, age2, sex and height as covariates in the model. Comparison of distributions of copy numbers was undertaken using a Mann-Whitney-Wilcoxon test and comparison of frequencies of samples with copy number > = 5 in cases and controls was undertaken using a Pearson's chi-squared test (undertaken using R v2.15.2).
Results
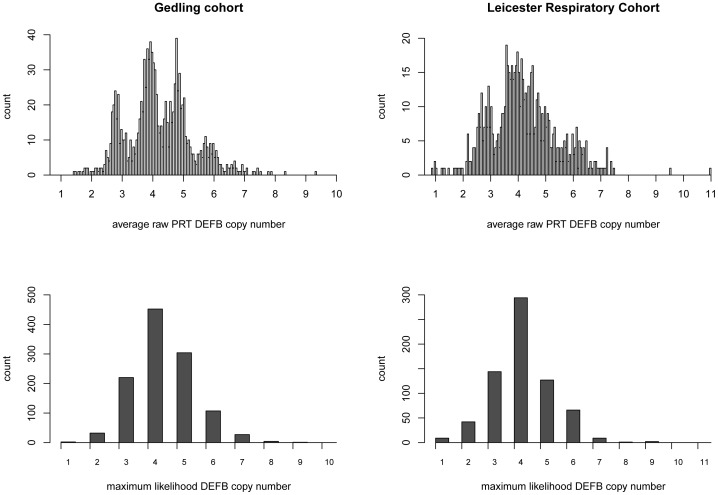
DEFB copy number was successfully measured using PRT in 689 LRC children and 1149 Gedling adults (Table 1). The distributions of raw PRT copy numbers and maximum likelihood integer copy numbers for each cohort are shown in Figure 2. In LRC, maximum likelihood integer copy numbers ranged from 1 to 9 with a mean copy number of 4.06. In the Gedling population, copy numbers ranged from 1 to 9 with a mean of 4.3. Association of DEFB raw PRT copy number and lung function (FEV1, FVC and FEV1/FVC), COPD (Gedling only) and asthma was tested separately in each cohort and the results are presented in Table 3. Association of maximum likelihood integer copy numbers of DEFB with all traits were also undertaken and the results were consistent with those obtained using the raw PRT copy numbers (results not shown).
Figure 2. Distributions of raw PRT copy number (top) and maximum likelihod integer copy number (bottom) for Gedling (right) and LRC (left) cohorts.
Table 3. Association of DEFB with lung function, asthma and COPD in LRC and Gedling.
| LRC | Gedling | |||||||
| Lung function (inverse normal transformed) | N | Beta | SE | P | N | Beta | SE | P |
| FEV1 | 689 | 0.019 | 0.020 | 0.333 | 1149 | −0.026 | 0.016 | 0.114 |
| FVC | 689 | 0.014 | 0.020 | 0.490 | 1149 | −0.008 | 0.015 | 0.581 |
| FEV1/FVC | 689 | 0.019 | 0.030 | 0.525 | 1149 | −0.043 | 0.025 | 0.090 |
| FEV1/FVC - never smokers only | 647 | −0.036 | 0.031 | 0.252 | ||||
| FEV1/FVC - ever smokers only | 502 | −0.067 | 0.0417 | 0.106 | ||||
| Asthma | N (case:control)# | OR | CI | P | N (case:control)# | OR | CI | P |
| Doctor diagnosed asthma | 255∶434 | 1.08 | 0.95–1.23 | 0.244 | 45∶629 | 1.03 | 0.79–1.37 | 0.784 |
| Asthma-ICS | 82∶441 | 1.26 | 1.07–1.54 | 0.016* | ||||
| COPD | N (case:control)# | OR | CI | P | ||||
| COPD GOLD Stage 2 | 42∶189 | 1.40 | 1.02–1.92 | 0.039 | ||||
P = 0.043 if maximum likelihood DEFB integer copy numbers used #controls for each analysis as defined in the method.
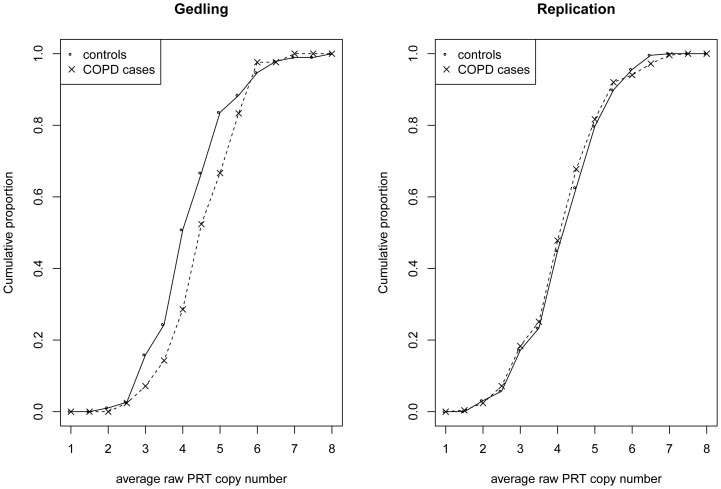
Although there was no evidence for association of DEFB copy number with lung function in all individuals in either cohort (even when the data for adults were stratified by smoking status), a significant (P<0.05) signal of association of DEFB copy number with COPD was observed within the adult Gedling population (OR = 1.34, 95% CI:1.02–1.77, P = 0.039) (Figure 3). When we sought replication in a sample of 251 UK COPD cases and 227 UK controls [45], there was no evidence for association of DEFB copy number and COPD (OR = 0.89, 95% CI:0.72–1.07, P = 0.217) (Figure 3). In fact, we found a trend for an association in the opposite direction (increased copy number associated with decreased risk of COPD) to that observed in the Gedling population.
Figure 3. Cumulative frequency distribution of average raw PRT DEFB copy number in Gedling COPD cases and controls (left) and replication COPD cases and controls (right).
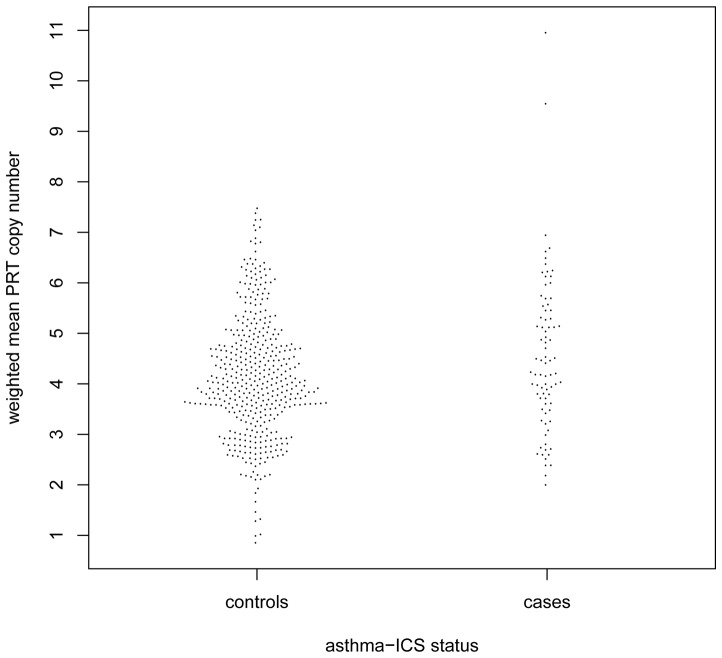
We did not observe evidence for association of DEFB copy number with doctor-diagnosed asthma for either LRC or Gedling. Because the majority of the children with doctor-diagnosed asthma in LRC had very mild disease, we also used a stricter definition of clinically relevant asthma (asthma-ICS) in LRC. We found a significant association of DEFB copy number with asthma-ICS for LRC (OR = 1.26, 95% CI:1.07–1.54, P = 0.016). However, a comparison of the distributions of DEFB copy number in the asthma-ICS cases and controls showed that the signal was driven by 2 cases with high copy numbers (Figure 4). After exclusion of these two individuals the strength of the association decreased considerably (OR = 1.18, 95% CI: 0.96–1.44, P = 0.12).
Figure 4. DEFB copy number in LRC asthma-ICS cases and controls.
Discussion
This study determined DEFB diploid copy number in a cohort of 1149 adults and in a separate cohort of 689 children using PRT, which is currently the most accurate method for measuring copy number variation at this locus. The distributions of copy number observed in each cohort were in good agreement with previously published distributions from the UK population [29], [47], although in the LRC cohort we observed nine children (>1%) with a copy number of 1, which in other cohorts is rare. We tested for association with lung function as a quantitative measurement (FEV1 and FEV1/FVC) in all individuals in each cohort as well as for association with COPD and asthma using appropriate case and control subsets of individuals within each cohort. Previous studies have reported some evidence for association of other beta-defensins with COPD [48], [49] and asthma [50]. However, our study, using a reliable measure of DEFB (comprising DEFB4, DEFB103 and DEFB104 genes) copy number, does not show a strong effect of DEFB copy number on these traits. In addition, we found no evidence for association with lung function in the general population.
A previous study presented evidence of an association of higher DEFB copy number with increased expression levels in nasal epithelial cells and with a higher risk of COPD [51]. Janssens et al [51] used real time quantitative PCR to measure diploid DEFB copy number and test for association with COPD in 204 COPD cases and 154 healthy smoker controls. A significant association was observed with the cases having a significantly higher median copy number than the controls (median of 5 in cases, 4 in controls, P<0.0001). In our replication collection of 251 cases and 227 controls, the median copy number was not significantly different in cases and controls (median of 4.05 in cases, 4.12 in controls, P = 0.316). Janssens et al showed a significant difference in the proportion of individuals with a copy number > = 5 in the COPD cases compared to the control group (52% and 38%, respectively, P = 0.008). However, in our replication collection, an opposite effect was seen with 18% of cases having copy number > = 5 and 20% of controls having copy number > = 5 (P = 0.67). We found no significant association between high copy number (> = 5 copies) and COPD risk (OR = 0.88, 95% CI: 0.6–1.4).
We recognise that the small case and control sample subsets within the Gedling cohort would limit statistical power to detect association with COPD risk and that diagnosis of COPD in this cohort was not based on reversibility. Previous genome-wide significant SNP associations with COPD have given ORs in the order of 1.1 to 1.3 per copy of the risk allele [10], [13]. In our discovery sample set (42 cases and 189 controls) we would have had 88% power to detect an OR of 1.7 per unit change in DEFB4 copy number. Power would have been higher in the larger replication set. However, we were not able to replicate the findings of Janssens et al [51]. Non-replication of the COPD association identified in the Gedling cohort by our replication collection may have been due to differences in the distributions of lung function measures between the discovery and replication case collections. This may be explained by differences in the ascertainment strategy of cases in each collection (diagnosis was based on reversibility in the replication collection). Given that SNPs reported in genome-wide association studies as associated with COPD also show association with lung function in general population samples [10]–[14] one might have expected any CNV showing association with COPD to be associated with lung function. Genome-wide significant associations with effect sizes of 0.03 to 0.09 standard deviations from the mean per allele have been observed for GWAS of SNPs including tens of thousands of samples [6], [7]. We had 75% power to detect an effect size of 0.075 per one unit change in DEFB4 copy number in the Gedling cohort (n = 1149).
A significant signal of association of DEFB copy number with a clinically relevant definition of asthma (asthma-ICS) in LRC was found to be driven by two cases with high copy number. The raw results from each independent component of the triplex PRT assay (data not shown) were consistent suggesting that the high copy numbers were real for these two cases. Although exclusion of these two individuals weakened the signal, we cannot rule out that rare instances of very high copy number could be causative for asthma.
LRC recruitment included children of European and South Asian ancestry; we limited this study to individuals of European ancestry based on questionnaire data (for ease of comparison with Gedling). Although there are no large differences in DEFB copy number variation across the world, some subpopulations, including two from South Asia, have been found to have outlying distributions of DEFB copy number [30].
The high mutation rate and multiallelic nature of the DEFB copy number variation means it is poorly tagged by nearby SNP alleles and measurement based on SNP probe intensity data is inadequate [30], [52], [53]. In this study, we measured DEFB copy number using PRT which has been shown to have higher accuracy for calling copy number than real time qPCR [46]. PRT is a form of qPCR which can more reliably measure copy number, particularly higher diploid copy numbers, through the use of identical primers to target the copy number variable region and a two-copy reference region. This avoids the issue of differential primer binding which can be a feature of real-time qPCR copy number measurement. Inaccurate copy number measurement has resulted in erroneous association of copy number with disease from this and other complex copy number variable regions. The previously reported association of DEFB copy number with COPD, described in detail above and not replicated in this study, was based on real-time qPCR measurements [51].
In conclusion, this study does not support previous findings of an association of DEFB copy number variation with COPD. We suggest that previous associations may have been due to inaccurate copy number measurement. A strength of our study was the accurate method of measurement of copy number. Prior to 2008, candidate SNP-based association studies often reported inconsistent findings, however these problems have diminished with the very large sample sizes now commonly employed in genome-wide association studies. Accurate copy number typing of very large studies will be needed to confidently rule out any modest effect of DEFB copy number variation on lung function, COPD or asthma.
Acknowledgments
We would like to acknowledge Theresa McNally and Ketna Parmar for lung function testing in LRC and Ann Millar (Bristol) and W MacNee (Edinburgh) of the EU COPD Gene Scan consortium for provision of the replication COPD cases and control samples.
Funding Statement
DNA extraction from LRC samples and PRT DEFB genotyping of LRC and Gedling samples was funded by a Higher Education Innovation Fund (HEIF) funded Impact Award to Louise V. Wain. The Nottingham Gedling cohort collection was funded by Asthma UK and the British Lung Foundation. Leicester Respiratory Cohort (LRC) was funded by Swiss National Science Foundation grant 3200B0-122341 and Asthma UK grant 07/048. Martin D. Tobin holds a Medical Research Council Senior Clinical Fellowship (G0902313). The research was part-funded by the National Institute for Health Research (NIHR). The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health. Ian Hall and Ian Sayers hold a Medical Research Council programme grant (G1000861). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Myint PK, Luben RN, Surtees PG, Wainwright NW, Welch AA, et al. (2005) Respiratory function and self-reported functional health: EPIC-Norfolk population study. Eur Respir J 26: 494–502. [DOI] [PubMed] [Google Scholar]
- 2. Schunemann HJ, Dorn J, Grant BJ, Winkelstein W Jr, Trevisan M (2000) Pulmonary function is a long-term predictor of mortality in the general population: 29-year follow-up of the Buffalo Health Study. Chest 118: 656–664. [DOI] [PubMed] [Google Scholar]
- 3. Strachan DP (1992) Ventilatory function, height, and mortality among lifelong non-smokers. J Epidemiol Community Health 46: 66–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Young RP, Hopkins R, Eaton TE (2007) Forced expiratory volume in one second: not just a lung function test but a marker of premature death from all causes. Eur Respir J 30: 616–622. [DOI] [PubMed] [Google Scholar]
- 5. Hancock DB, Eijgelsheim M, Wilk JB, Gharib SA, Loehr LR, et al. (2010) Meta-analyses of genome-wide association studies identify multiple loci associated with pulmonary function. Nat Genet 42: 45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Repapi E, Sayers I, Wain LV, Burton PR, Johnson T, et al. (2010) Genome-wide association study identifies five loci associated with lung function. Nat Genet 42: 36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Soler Artigas M, Loth DW, Wain LV, Gharib SA, Obeidat M, et al. (2011) Genome-wide association and large-scale follow up identifies 16 new loci influencing lung function. Nat Genet 43: 1082–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lopez AD, Shibuya K, Rao C, Mathers CD, Hansell AL, et al. (2006) Chronic obstructive pulmonary disease: current burden and future projections. Eur Respir J 27: 397–412. [DOI] [PubMed] [Google Scholar]
- 9. Silverman EK, Chapman HA, Drazen JM, Weiss ST, Rosner B, et al. (1998) Genetic epidemiology of severe, early-onset chronic obstructive pulmonary disease. Risk to relatives for airflow obstruction and chronic bronchitis. Am J Respir Crit Care Med 157: 1770–1778. [DOI] [PubMed] [Google Scholar]
- 10. Castaldi PJ, Cho MH, Litonjua AA, Bakke P, Gulsvik A, et al. (2011) The association of genome-wide significant spirometric loci with chronic obstructive pulmonary disease susceptibility. Am J Respir Cell Mol Biol 45: 1147–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cho MH, Boutaoui N, Klanderman BJ, Sylvia JS, Ziniti JP, et al. (2010) Variants in FAM13A are associated with chronic obstructive pulmonary disease. Nat Genet 42: 200–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pillai SG, Ge D, Zhu G, Kong X, Shianna KV, et al. (2009) A genome-wide association study in chronic obstructive pulmonary disease (COPD): identification of two major susceptibility loci. PLoS Genet 5: e1000421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Soler Artigas M, Wain LV, Repapi E, Obeidat M, Sayers I, et al. (2011) Effect of five genetic variants associated with lung function on the risk of chronic obstructive lung disease, and their joint effects on lung function. Am J Respir Crit Care Med 184: 786–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wilk JB, Shrine NR, Loehr LR, Zhao JH, Manichaikul A, et al. (2012) Genome-wide association studies identify CHRNA5/3 and HTR4 in the development of airflow obstruction. Am J Respir Crit Care Med 186: 622–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Braman SS (2006) The global burden of asthma. Chest 130: 4S–12S. [DOI] [PubMed] [Google Scholar]
- 16. Himes BE, Hunninghake GM, Baurley JW, Rafaels NM, Sleiman P, et al. (2009) Genome-wide association analysis identifies PDE4D as an asthma-susceptibility gene. Am J Hum Genet 84: 581–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Moffatt MF, Gut IG, Demenais F, Strachan DP, Bouzigon E, et al. (2010) A large-scale, consortium-based genomewide association study of asthma. N Engl J Med 363: 1211–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wan YI, Shrine NR, Soler Artigas M, Wain LV, Blakey JD, et al. (2012) Genome-wide association study to identify genetic determinants of severe asthma. Thorax 67: 762–768. [DOI] [PubMed] [Google Scholar]
- 19. Myers RA, Himes BE, Gignoux CR, Yang JJ, Gauderman WJ, et al. (2012) Further replication studies of the EVE Consortium meta-analysis identifies 2 asthma risk loci in European Americans. J Allergy Clin Immunol 130: 1294–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cookson WO, Moffatt MF (2011) Genetics of complex airway disease. Proc Am Thorac Soc 8: 149–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Conrad DF, Pinto D, Redon R, Feuk L, Gokcumen O, et al. (2010) Origins and functional impact of copy number variation in the human genome. Nature 464: 704–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Semple F, Dorin JR (2012) beta-Defensins: multifunctional modulators of infection, inflammation and more? J Innate Immun 4: 337–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bals R, Wang X, Wu Z, Freeman T, Bafna V, et al. (1998) Human beta-defensin 2 is a salt-sensitive peptide antibiotic expressed in human lung. J Clin Invest 102: 874–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ishimoto H, Mukae H, Date Y, Shimbara T, Mondal MS, et al. (2006) Identification of hBD-3 in respiratory tract and serum: the increase in pneumonia. Eur Respir J 27: 253–260. [DOI] [PubMed] [Google Scholar]
- 25. Tecle T, Tripathi S, Hartshorn KL (2010) Review: Defensins and cathelicidins in lung immunity. Innate Immun 16: 151–159. [DOI] [PubMed] [Google Scholar]
- 26. Yanagi S, Ashitani J, Ishimoto H, Date Y, Mukae H, et al. (2005) Isolation of human beta-defensin-4 in lung tissue and its increase in lower respiratory tract infection. Respir Res 6: 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Moranta D, Regueiro V, March C, Llobet E, Margareto J, et al. (2010) Klebsiella pneumoniae capsule polysaccharide impedes the expression of beta-defensins by airway epithelial cells. Infect Immun 78: 1135–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tsutsumi-Ishii Y, Nagaoka I (2003) Modulation of human beta-defensin-2 transcription in pulmonary epithelial cells by lipopolysaccharide-stimulated mononuclear phagocytes via proinflammatory cytokine production. J Immunol 170: 4226–4236. [DOI] [PubMed] [Google Scholar]
- 29. Fode P, Jespersgaard C, Hardwick RJ, Bogle H, Theisen M, et al. (2011) Determination of beta-defensin genomic copy number in different populations: a comparison of three methods. PLoS One 6: e16768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hardwick RJ, Machado LR, Zuccherato LW, Antolinos S, Xue Y, et al. (2011) A worldwide analysis of beta-defensin copy number variation suggests recent selection of a high-expressing DEFB103 gene copy in East Asia. Hum Mutat 32: 743–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hollox EJ, Huffmeier U, Zeeuwen PL, Palla R, Lascorz J, et al. (2008) Psoriasis is associated with increased beta-defensin genomic copy number. Nat Genet 40: 23–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stuart PE, Huffmeier U, Nair RP, Palla R, Tejasvi T, et al. (2012) Association of beta-defensin copy number and psoriasis in three cohorts of European origin. J Invest Dermatol 132: 2407–2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bentley RW, Pearson J, Gearry RB, Barclay ML, McKinney C, et al. (2010) Association of higher DEFB4 genomic copy number with Crohn's disease. Am J Gastroenterol 105: 354–359. [DOI] [PubMed] [Google Scholar]
- 34. Fellermann K, Stange DE, Schaeffeler E, Schmalzl H, Wehkamp J, et al. (2006) A chromosome 8 gene-cluster polymorphism with low human beta-defensin 2 gene copy number predisposes to Crohn disease of the colon. Am J Hum Genet 79: 439–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hardwick RJ, Amogne W, Mugusi S, Yimer G, Ngaimisi E, et al. (2012) beta-defensin genomic copy number is associated with HIV load and immune reconstitution in sub-saharan Africans. J Infect Dis 206: 1012–1019. [DOI] [PubMed] [Google Scholar]
- 36. Hollox EJ (2010) Beta-defensins and Crohn's disease: confusion from counting copies. Am J Gastroenterol 105: 360–362. [DOI] [PubMed] [Google Scholar]
- 37. Britton JR, Pavord ID, Richards KA, Knox AJ, Wisniewski AF, et al. (1995) Dietary antioxidant vitamin intake and lung function in the general population. Am J Respir Crit Care Med 151: 1383–1387. [DOI] [PubMed] [Google Scholar]
- 38. McKeever TM, Scrivener S, Broadfield E, Jones Z, Britton J, et al. (2002) Prospective study of diet and decline in lung function in a general population. Am J Respir Crit Care Med 165: 1299–1303. [DOI] [PubMed] [Google Scholar]
- 39.Global Initiative for Chronic Obstructive Lung Disease (GOLD) (2006) Global Strategy for the Diagnosis, Management and Prevention of COPD.
- 40. Hankinson JL, Crapo RO, Jensen RL (2003) Spirometric reference values for the 6-s FVC maneuver. Chest 124: 1805–1811. [DOI] [PubMed] [Google Scholar]
- 41. Hankinson JL, Kawut SM, Shahar E, Smith LJ, Stukovsky KH, et al. (2010) Performance of American Thoracic Society-recommended spirometry reference values in a multiethnic sample of adults: the multi-ethnic study of atherosclerosis (MESA) lung study. Chest 137: 138–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kuehni CE, Brooke AM, Strippoli MP, Spycher BD, Davis A, et al. (2007) Cohort profile: the Leicester respiratory cohorts. Int J Epidemiol 36: 977–985. [DOI] [PubMed] [Google Scholar]
- 43. Dogaru CM, Strippoli MP, Spycher BD, Frey U, Beardsmore CS, et al. (2012) Breastfeeding and lung function at school age: does maternal asthma modify the effect? Am J Respir Crit Care Med 185: 874–880. [DOI] [PubMed] [Google Scholar]
- 44.Strippoli MP, Kuehni CE, Dogaru CM, Spycher BD, McNally T, et al.. (2013) Etiology of Ethnic Differences in Childhood Spirometry. Pediatrics. [DOI] [PubMed]
- 45. Chappell S, Daly L, Morgan K, Guetta Baranes T, Roca J, et al. (2006) Cryptic haplotypes of SERPINA1 confer susceptibility to chronic obstructive pulmonary disease. Hum Mutat 27: 103–109. [DOI] [PubMed] [Google Scholar]
- 46. Aldhous MC, Abu Bakar S, Prescott NJ, Palla R, Soo K, et al. (2010) Measurement methods and accuracy in copy number variation: failure to replicate associations of beta-defensin copy number with Crohn's disease. Hum Mol Genet 19: 4930–4938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hollox EJ, Armour JA, Barber JC (2003) Extensive normal copy number variation of a beta-defensin antimicrobial-gene cluster. Am J Hum Genet 73: 591–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Andresen E, Gunther G, Bullwinkel J, Lange C, Heine H (2011) Increased expression of beta-defensin 1 (DEFB1) in chronic obstructive pulmonary disease. PLoS One 6: e21898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Liao Z, Dong J, Hu X, Wang T, Wan C, et al. (2012) Enhanced expression of human beta-defensin 2 in peripheral lungs of patients with chronic obstructive pulmonary disease. Peptides 38: 350–356. [DOI] [PubMed] [Google Scholar]
- 50. Levy H, Raby BA, Lake S, Tantisira KG, Kwiatkowski D, et al. (2005) Association of defensin beta-1 gene polymorphisms with asthma. J Allergy Clin Immunol 115: 252–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Janssens W, Nuytten H, Dupont LJ, Van Eldere J, Vermeire S, et al. (2010) Genomic copy number determines functional expression of {beta}-defensin 2 in airway epithelial cells and associates with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 182: 163–169. [DOI] [PubMed] [Google Scholar]
- 52. Abu Bakar S, Hollox EJ, Armour JA (2009) Allelic recombination between distinct genomic locations generates copy number diversity in human beta-defensins. Proc Natl Acad Sci U S A 106: 853–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wain LV, Armour JA, Tobin MD (2009) Genomic copy number variation, human health, and disease. Lancet 374: 340–350. [DOI] [PubMed] [Google Scholar]