Abstract
Improved therapies for cancer and other conditions have resulted in a growing population of long-term survivors. Infertility is an unfortunate side effect of some cancer therapies that impacts the quality of life of survivors who are in their reproductive or pre-reproductive years. Some of these patients have the opportunity to preserve their fertility using standard technologies that include sperm, egg or embryo banking, followed by in vitro fertilization and/or embryo transfer. However, these options are not available to all patients, especially the prepubertal patients who are not yet producing mature gametes. For these patients, there are several stem cell technologies in the research pipeline that may give rise to new fertility options and allow infertile patients to have their own biological children. We will review the role of stem cells in normal spermatogenesis as well as experimental stem cell based techniques that may have potential to generate or regenerate spermatogenesis and sperm. We will present these technologies in the context of the fertility preservation paradigm, but we anticipate that they will have broad implications for the assisted reproduction field.
Introduction
High dose chemotherapy, whole body radiation or radiation to the gonads can cause permanent infertility (1). This is a significant human health concern because over 75,000 people under the age of 40 in the United States are diagnosed with cancer each year and most are cured (2). Thus, cancer patients can look beyond their diagnosis and treatment to quality of life after cancer. Parenthood is important to cancer survivors and distress over infertility can have long-term psychological and relationship implications (3). Therefore, the American Society for Clinical Oncology (ASCO) (4) and the American Society for Reproductive Medicine (ASRM) Ethics Committee (5) recommend that the reproductive risks of gonadotoxic therapies and options for preserving fertility be discussed with patients before initiating treatment. While adoption and third-party reproduction provide alternative family building options, the available data indicate that most cancer survivors prefer to have their own biological children (4).
Post-pubertal adolescent and adult males have the option to cryopreserve sperm prior to oncologic treatment (Figure 1, Top). This is a simple and established method for preserving fertile potential and allows men to father their own genetic children. Nearly 17,000 men between the ages of 15 and 44 are diagnosed with cancer each year in the United States and nearly 2385 survivors will receive a treatment that puts them at high risk of azoospermia (SEER 2010)(2, 6). Unfortunately, only about 24% of men in this age range cryopreserved semen prior to their oncologic treatment (7). Therefore, we calculate that each year in the United States, over 1800 adult cancer survivors will be infertile with azoospermia and have limited options to have their own biological children because they did not save a semen sample. In some cases, sperm can be recovered surgically from small focal areas of spermatogenesis in the testes using the testicular sperm extraction (TESE) method and used to fertilize oocytes by intracytoplasmic sperm injection (ICSI)(8).
Figure 1.
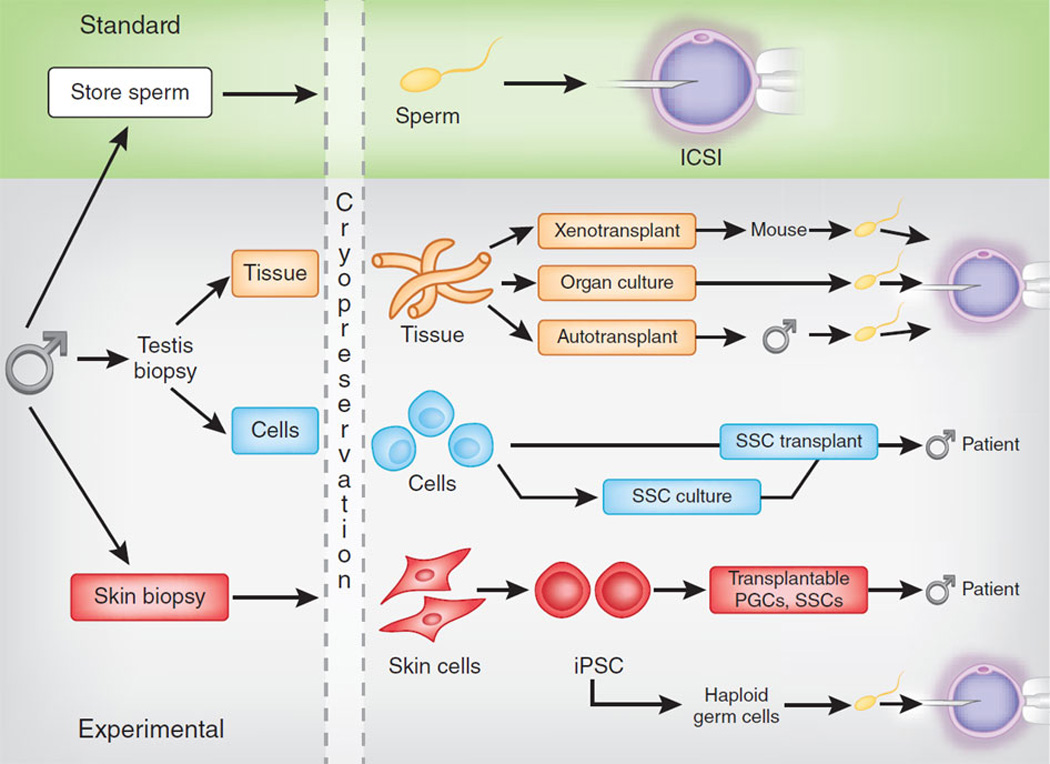
Standard and experimental options for preserving male fertility. Top, sperm obtained by ejaculation or surgical retrieval from the testes or epididymides are competent to fertilize oocytes using assisted reproductive techniques including intrauterine insemination (IUI), in vitro fertilization (IVF) or IVF with intracytoplasmic sperm injection (ICSI)) that are standard in most fertility clinics. These options are not available to prepubertal boys who are not producing sperm or to adult azoospermic men. Bottom, testis tissue obtained via biopsy from prepubertal boys contains SSCs that can produce sperm in the context of the intact tissue by xenotransplant, organ culture or autologous transplantation back into the individual (orange boxes). Sperm retrieved from cultured or transplanted tissue can be used for ICSI. Cells in suspension obtained from biopsied testicular tissue can be transplanted back into the endogenous seminiferous tubules of the patient (blue boxes). SSCs in the suspension can regenerate spermatogenesis and, in some cases, fertility. For infertile individuals who did not preserve germs cells before gonadotoxic therapy, induced pluripotent stem cells (IPSCs) may be produced from his somatic cells (e.g., skin or blood) to differentiate into transplantable germ cells (PGCs or SSCs) or haploid germ cells that can be used for ICSI (red boxes). Excerpted with permission from Clark AT, Phillips BT and Orwig KE 2011 NATURE MEDICINE 17:1564–1565.
There are no options to preserve the fertility of prepubertal boys, who are not yet making sperm. This is a significant problem because about 5131 boys under the age of 15 in the United States are expected to develop cancer each year and 83% are expected to survive (SEER, 2010)(2). A report from the Childhood Cancer Survivor Study indicates that the cytotoxic therapies for cancer reduce the number subsequently able to have children by 44% (6, 9). Based on these statistics, we calculate that each year in the United States, 1874 young male cancer patients will become sterile due to their treatment. In addition to cancer survivors, over 500 patients under the age of 20 receive hematopoietic stem cell (HSC) transplants each year in the United States for non-malignant conditions (e.g., bone marrow failure, blood and immune deficiencies, autoimmune disorders)(10). Myeloablative conditioning therapy prior to bone marrow transplantation is associated with a high risk of infertility (4, 9, 11, 12). The ASCO report notes that “Impaired future fertility is difficult for children to understand, but potentially traumatic to them as adults” (4). The available data indicate that greater than 80% of parents consented to fertility preservation procedures on behalf of their children prior to initiation of gonadotoxic therapies (13, 14).
The summed incidence of chemotherapy or radiation-induced male infertility that cannot be treated with existing reproductive therapies is approximately 4000 individuals each year in the United States. Therefore, responsible development of novel therapies to help these patients have biological children has a significant potential impact.
Promising results in animal models and human cell lines (Figure 1, Bottom) have generated enthusiasm that stem cells might be used or manipulated to preserve and/or restore the fertility of patients who are not producing sperm and have no other options to protect their future fertility before receiving gonadotoxic chemotherapy or radiation treatments (14–34). We will review the methods of spermatogonial stem cell transplantation (Figure 1, blue boxes), testicular tissue grafting, testicular tissue organ culture (Figure 1, orange boxes) and induced pluripotent stem cell differentiation into gametes or transplantable male germline stem cells (Figure 1, red boxes). Table 1 summarizes published reports detailing the progress of each method. Enthusiasm for these experimental stem cell technologies is tempered by concerns about feasibility and safety, particularly for the vulnerable prepubertal patient population (14, 35–37). Questions about feasibility stem from the early stage of technology development, uncertainty about optimal freezing conditions, concerns that small testicular biopsy will contain few stem cells and the lack of culture conditions to expand human germline stem cells. Questions about safety are associated with the risks of surgery to obtain testicular tissue and the potential for malignant contamination in transplanted cells or tissue. Nonetheless, academic centers around the world are already cryopreserving testicular tissues for prepubertal boys and men in anticipation that stem cells in that tissue can be used safely and effectively to restore future fertility (13, 16, 21, 38–41).
Table 1.
Literature reporting progress in stem cell technology development
| Stem Cell Technologies |
Experimental Endpointsa | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Histology/Immunological staining | Transplant | Functional readout | |||||||||
| (q)RT- PCR |
Histology | ICC | IHC | Flow/ FACS/ MACS |
Xeno | Homologous | Autologous | Spermb | Fertilization | Progeny | |
| SSC Transplant | |||||||||||
| Rodents | (30, 31, 48–51, 57, 58, 118, 119) | (71, 120) | (71, 118–120) | (30, 31, 48–51, 57, 58, 70, 121) | (30, 31, 48, 50, 51, 57, 58, 71, 118–121) | (31, 48, 50, 51, 57, 58, 71, 120, 121) | (31, 48, 50, 51, 57, 58, 71, 120, 121) | ||||
| Large Animalc | (54–56) | (56, 64, 72) | (64, 72) | (52–56) | (56) | (52–55) | (52, 55) | (52, 55) | |||
| Nonhuman Primate | (59, 60) | (122–125) | (122–125) | (29) | (29, 59–61) | (29, 59) | (29) | ||||
| Human | (21, 38, 78, 89, 109, 115, 117) | (21, 38, 78, 89, 109, 115, 117) | (67) | ||||||||
| SSC Culture | |||||||||||
| Rodents | (57, 58) | (70, 83) | (71) | (58, 70, 71, 121) | (71) | (57, 58, 70, 71, 121) | (57, 58, 71, 83, 121) | (57, 58, 71, 121) | (57, 58, 71, 121) | ||
| Large Animal | (73) | (72–75) | (72) | (72) | |||||||
| Nonhuman Primate | (76) | (76) | |||||||||
| Human | (21, 38, 79, 81) | (77, 79, 81, 82, 126) | (21, 38, 78, 80) | (77, 80–82, 126) | (21, 38, 79) | ||||||
| Testicular grafting | |||||||||||
| Rodents | (92, 93, 95) | (95) | (92, 93, 95) | (92, 93) | (92, 93) | (93) | |||||
| Large Animal | (92) | (92) | (92) | (92) | |||||||
| Nonhuman Primate | (94–98) | (97, 98) | (94–96) | (97, 98) | (94, 98) | (94) | |||||
| Human | (99, 101–104) | (100, 101, 103, 104) | (99–104) | ||||||||
| Testicular Organ Culture | |||||||||||
| Rodents | (27) | (27) | (27) | (27) | (27) | ||||||
| Large Animal | |||||||||||
| Nonhuman Primate | |||||||||||
| Human | |||||||||||
| Pluripotent-derived male germ cells | |||||||||||
| Rodents | (28) | (28) | (28) | (28) | (28) | (28) | |||||
| Large Animal | |||||||||||
| Nonhuman Primate | (32, 33) | (32, 33) | |||||||||
| Human | (22–24, 26) | (22–26) | (22, 23, 25, 26) | ||||||||
Abbreviations: RT-PCR, real time polymerase chain reaction; ICC, immunocytochemistry; IHC, immunohistochemistry; Flow, flow cytometry; FACS, fluorescence-activated cell sorting; MACS, magnetic cell sorting; Xeno, xenotransplantation
Sperm and spermatogenesis
Farm animals and companion animals
Spermatogonial stem cells and spermatogenesis
Spermatogonial stem cells (SSCs) are the adult tissue stem cells in the testes that balance self-renewing and differentiating divisions to maintain continuous sperm production throughout the postpubertal life of men. SSCs are rare cells located on the basement membrane of seminiferous tubules (Figure 2A and B). These stem cells give rise to undifferentiated progenitor spermatogonia that undergo several transit amplifying mitotic divisions, followed by two meiotic divisions and morphological differentiation to produce sperm. Spermatogenesis is an extraordinarily productive system that produces approximately 40 million sperm per gram of tissue per day in mice (42) and 5.5 million sperm per gram of tissue per day in men (43). The difference in sperm production in mice and men can be explained in part by differences in the number of transit amplifying mitotic divisions that precede meiosis (44). In humans, the spermatogonial stem cell pool is comprised of Adark and Apale spermatogonia. Adark are relatively quiescent, while Apale are mitotically active and undergo 1–2 transit amplifying divisions before giving rise to differentiating type B spermatogonia and then primary spermatocytes, which enter meiosis and migrate off the basement membrane of the seminiferous tubule (Figure 2B and C). Due to its highly proliferative nature, the spermatogenic system is an unintended target of chemo- and radiotherapies. The relative effects of various chemo- and radio-therapy regimens on male fertility are detailed in several previous reports (9, 11, 19, 45, 46). The extent that the fertility of a patient is affected, depends on the dose, type and frequency of treatment (1). There has been significant progress in minimizing the unwanted side-effects of the cancer treatment by adjusting and modifying the therapeutic regimens without compromising the efficiency of oncological treatments (47). Nonetheless, some cancer treatments, like whole body radiation, radiation to the gonads and alkylating chemotherapy, can be particularly gonadotoxic and result in prolonged or permanent azoospermia. In the following sections, we will review experimental stem cell-based approaches that may have application for preserving and/or restoring male fertility.
Figure 2.
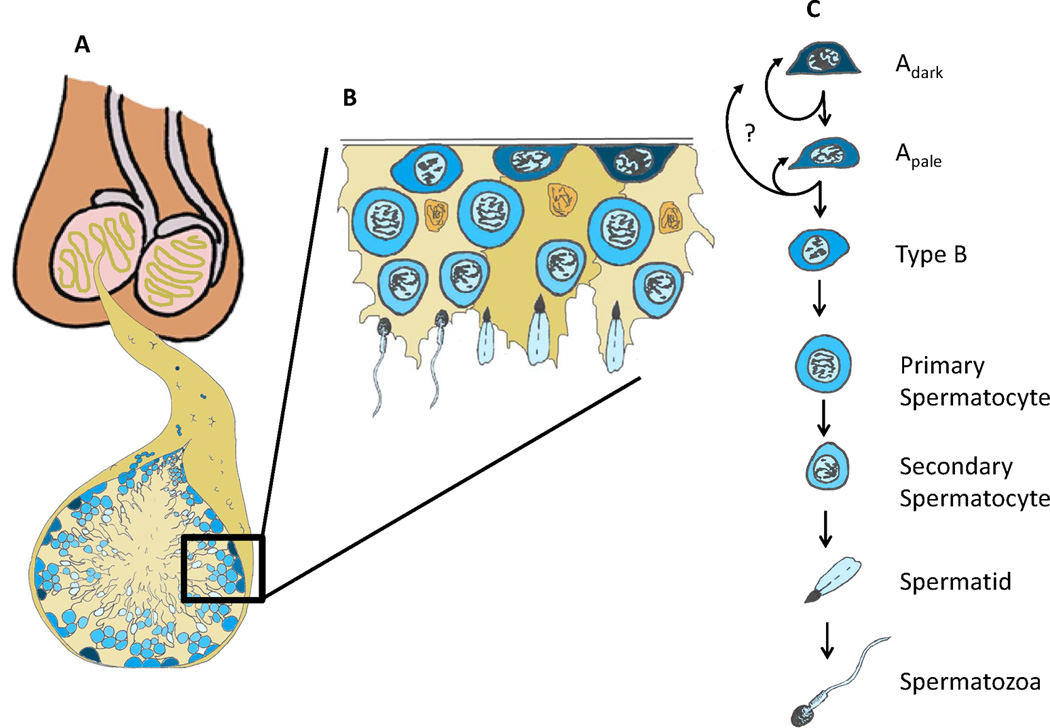
Human spermatogonial stem cells and spermatogenesis. (A) Testes are comprised of seminiferous tubules that start and end at the rete testis. (B) Cut-out of the basement membrane of the seminiferous epithelium. (B and C) The basement membrane of the seminiferous epithelium contains undifferentiated (Adark and Apale) spermatogonia and differentiating Type B spermatogonia. Type B spermatogonia give rise to primary spermatocytes that enter meiosis and migrate off the basement membrane. Subsequent meiotic divisions and morphogenesis give rise to secondary spermatocytes, spermatids and the terminally differentiated spermatozoa that are released into the lumen of the seminiferous tubules
Spermatogonial stem cell transplantation
Brinster and colleagues pioneered the technique for spermatogonial stem cell transplantation in mice in 1994, demonstrating that donor SSCs could engraft the seminiferous tubules of chemotherapy-treated recipient mice and regenerate spermatogenesis and fertility leading to the production of viable progeny through normal breeding (30, 31). The SSC transplantation technique has become the experimental gold standard for quantifying stem cell activity and may have application for treating male infertility. Homologous species SSC transplantation has now been reported in mice, rats, pigs, goats, bulls, sheep, dogs and monkeys, including the production of donor-derived progeny in mice, rats, goats and sheep (29, 48–61). SSCs from donors of all ages, newborn to adult, can regenerate spermatogenesis (49, 62) and SSCs can be cryopreserved and retain spermatogenic function upon thawing and transplantation (29, 63, 64). We recently demonstrated that SSCs from the testes of prepubertal and adult rhesus macaques could be frozen, thawed and transplanted to regenerate spermatogenesis and produce fertilization competent sperm (29, 59). Progress in these previous studies suggest that it should be feasible for prepubertal boys or adult men to cryopreserve testicular tissue containing SSCs prior to treatment and have these cells reintroduced into their testes at a later date to regenerate spermatogenesis.
Radford and colleagues initially introduced the autologous SSC transplantation technique to the human clinic in 1999 (65). In Manchester, the United Kingdom, testicular tissue from 12 male non-Hodgkin’s lymphoma patients was cryopreserved as a cell suspension prior to the initiation of chemotherapy. At later dates, seven of the patients had the cells injected back into their testes (66, 67). To our knowledge, there have been no follow up reports on the fertility status of those patients so the outcome of the experiment is unknown. Even if the men in that study fathered children, it would be difficult to demonstrate unequivocally (in the absence of a unique genetic marker) that those offspring resulted from sperm produced by transplanted stem cells rather than from surviving endogenous stem cells. There have been no subsequent reports of SSC transplantation in humans since the 1999 study. Nonetheless, this bold, pioneering study demonstrated that patients are willing to pursue experimental stem cell approaches to achieve fertility (65, 67). To date, published reports indicate that testicular tissue or cells have been cryopreserved for more than 150 prepubertal and adult male patients worldwide and this is likely an underestimate of actual cases (13, 14, 21, 38–41, 65, 67).
For SSC transplantation in rodents, the testes are typically accessed via a mid-ventral abdominal incision. Testicular cells (including SSCs) are injected using a pulled glass capillary pipet inserted via the efferent ducts into the rete testis space, which can be visualized on the surface of the testis and is contiguous with all seminiferous tubules (68). Testis anatomy in larger animals, including nonhuman primates and humans is different than rodents, with the rete testis being centrally located in the testes. Schlatt and colleagues (69) demonstrated that ultrasound can be used to visualize the rete testis and guide an injection needle into the rete testis space. Ultrasound-guided rete testis injection has now been employed for SSC transplantation in several large animals species, including nonhuman primates (29, 52–56, 60, 61). For this approach, an injection needle is simply inserted under ultrasound guidance through the scrotal skin and testicular parenchyma into the rete testis space (Figure 3 depicts an example from nonhuman primates)(29).
Figure 3.
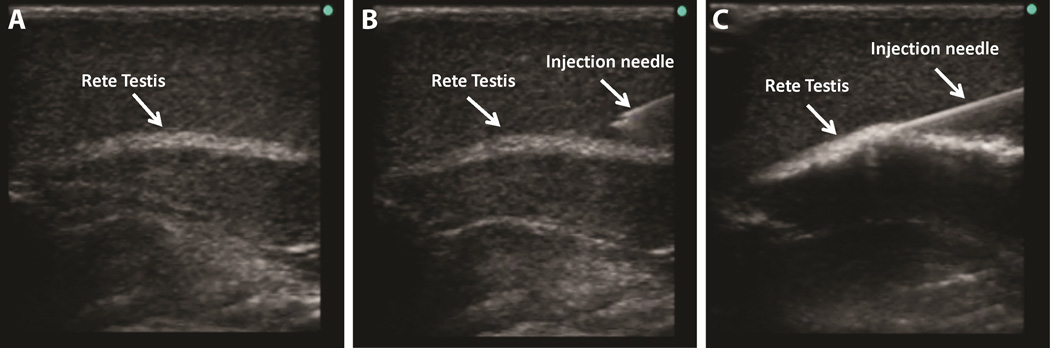
Ultrasound-guided rete testis injections. For SSC transplantation into larger animals, including nonhuman primates, (A) ultrasound is used to visualize the rete testis (echo-dense structure). (B and C) The injection needle is inserted under ultrasound guidance through the scrotal skin into the rete testis space, which is continuous with the seminiferous tubules. (C) Positive pressure is applied to the needle so the cells are slowly injected into the rete testis space and seminiferous tubules.
Clinical translation of the SSC transplantation technique appears eminent considering successes in several large animal models (Table 1) and that many patients have already cryopreserved testicular tissue or cells (detailed above). However, several questions and/or challenges remain. First, the small amount of tissue that can be obtained from testicular biopsies (especially from prepubertal boys) may contain a relatively small number of stem cells. Second, in the cancer survivor paradigm, it is essential to eliminate the risk of reintroducing malignant cells. The sections below describe approaches that may circumvent these two challenges. A third challenge for translating new stem cell technologies to the clinic is the limited availability of reagents and experimental tools for studying humans SSCs.
Spermatogonial stem cell culture
In rodents, SSCs can be greatly expanded in culture and maintain competence to produce spermatogenesis and restore fertility upon transplantation (57, 58, 70, 71). Several groups have reported extending SSC culture to large animal species (72–76). This approach could theoretically be employed to amplify the small number of stem cells that can be obtained from a biopsy and also to demonstrate the absence of malignant contamination. A number of laboratories have reported culturing human SSCs (21, 38, 77–82), including three from the testes of prepubertal patients (38, 78, 80). These human tissue studies are promising, but challenged by the inability to evaluate the full spermatogenic potential of cultured cells by homologous species transplantation into human testes. To date, each laboratory has used a different approach to culture human SSCs and different methods to assess outcomes. These published human SSC culture results need to be replicated in other laboratories and evaluated using robust markers of human spermatogonia and by xenotransplantation to nude mice (see section below on experimental methods to track human germline stem cells).
In a recent study, Elhija and colleagues reported that testicular cells from 7-day old immature mice could be expanded in a three-dimensional soft agar culture system and differentiated to produce postmeiotic germ cells, including morphologically normal sperm (83). Additional studies are needed to confirm that the resulting sperm are functionally competent to fertilize mouse oocytes. If a similar approach is successful in humans, this would eliminate the need to put cells back into the patient and thus the risk of reintroducing malignant cells into a cancer survivor.
Cell sorting strategies to isolate SSCs and remove malignant contamination
There are legitimate concerns about malignant contamination of preserved testicular tissue, especially from leukemia patients (84, 85). Several groups have demonstrated that it is feasible to isolate germ cells and remove malignant contamination from heterogeneous testicular cell suspensions using a fluorescence-activated cell sorter (FACS) and combinations of germ cell and/or cancer cell markers (86– 90). Despite these encouraging results, caution is warranted because even low levels of contamination can lead to cancer and current assays may lack the sensitivity to detect very rare malignant cells (85, 91). Therefore, alternative methods for decontamination and screening as well as methods that do not require transplantation should be considered in some cases.
Testicular tissue grafting
In contrast to SSC transplantation, which involves disaggregation of SSCs from their cognate niches, testicular tissue grafting and testicular tissue organ culture (next section) maintain the integrity of the stem cell/niche unit. Dobrinski and Schlatt demonstrated that testicular tissues obtained from newborn mice, pigs and goats could produce complete spermatogenesis when grafted under the skin of nude mice (92) and later reported the production of offspring from sperm obtained after ectopic (under the skin) grafting of mouse testicular tissue (93). Testis tissue from prepubertal rhesus macaques also produced complete spermatogenesis with fertilization competent sperm after ectopic xenografting into nude mice (94). Survival and spermatogenesis from adult testicular tissue grafts have been less successful than immature grafts (95).
Cryopreservation is an essential component of the fertility preservation paradigm. Jahnukainen and coworkers evaluated several cryopreservation strategies with immature monkey tissues and found that freezing in 1.4M DMSO provided good graft survival and spermatogenic induction up to the spermatocyte stage (96). However, haploid germ cells were not produced in any grafts from that study, fresh or frozen. Wistuba and colleagues performed autologous testicular grafting in two studies in marmoset monkeys (97, 98) and reported that complete spermatogenesis was obtained in orthotopic (in the scrotum), but not ectopic grafts. Frozen and thawed tissues were also grafted in that study, but these were only transplanted ectopically and did not produce spermatogenesis (98). Therefore, the question of whether frozen and thawed grafts can produce fertilization competent haploid germ cells still needs to be addressed.
Several groups have reported xenografting of human testicular tissue to nude mice and so far none have observed complete spermatogenesis or haploid gametes (99–104). In general, testicular tissues xenografted from prepubertal or adolescent boys survived long-term (4–12 months)(100, 101, 103, 104) with a calculated 3.7% spermatogonial recovery rate after six months in one study (104) and three studies reporting spermatocytes as the most advanced stage of germ cell development between 6 and 12 months after transplantation (101, 103, 104). Some of those tissues were already post-pubertal and contained meiotic or post-meiotic cells at the time of transplant, so it is difficult to exclude the possibility that spermatocytes or occasional spermatids that were observed had persisted from the time of transplant (101, 104). Importantly, the study by Sato and colleagues (103) observed primary spermatocytes one year after xenografting of testicular tissue from a 3 month old boy, which clearly did not contain spermatocytes prior to grafting. In contrast to prepubertal/adolescent tissues, xenografted adult testicular tissues regressed over time (99, 102). Although the human to mouse xenografting results are somewhat discouraging, the results of the monkey studies cited above suggest that autologous transplantation may be an option if suitable cryopreservation conditions are developed. Similar to SSC transplantation, autologous grafting will be problematic in cases where malignant contamination of the testicular tissue is suspected. Xenografting of human testicular tissue into animals could circumvent this problem, but is associated with additional concerns about xenobiotics and has been unsuccessful to date.
Testicular tissue organ culture
Sato and colleagues (27, 105) demonstrated that haploid male germ cells from mice could be generated in testicular tissue organ culture, using a gas-liquid interface method that was originally devised to keep differentiated organs alive in vitro (106). Testis tissue was obtained from 2.5 to 3.5 day old mice that contained only undifferentiated germ cells, similar to prepubertal patients. Testicular tissue was minced into fragments (1–3 mm diameter) that were placed on an agarose gel that was half soaked in medium, such that the tissue was exposed to air and absorbed nutrients through the agarose. Haploid round spermatids or sperm were retrieved after 23 or 42 days in culture, respectively, and used to successfully fertilize mouse oocytes in vitro by round spermatid injection (ROSI) and ICSI. The resulting embryos were transferred to recipient females and generated live offspring with normal development to adulthood and normal fertility (27). The authors also maintained frozen and thawed testicular tissue pieces in organ culture and were able to generate sperm but the fertilization potential of that sperm was not tested. If these results in mice can be translated to humans, testicular organ culture would circumvent the need to put tissues or cells back into the patient and may be a safe option for patients with malignancies that contaminate the testes.
Germ cells derived from pluripotent stem cells
For males who did not preserve sperm or SSCs prior to gonadotoxic treatment, generation of transplantable germ cells (Primordial germ cells or SSCs) or haploid gametes from embryonic stem cells (ESCs) or induced pluripotent stem cells (iPSCs) has been explored. The iPSC technology is especially exciting because it would theoretically allow patients with no germ cells in their testes to father genetically related children. The derivation of germ cells from pluripotent cells will be covered in more detail in other articles in this issue. Here we will summarize briefly that several groups have reported the generation of germ cells from nonhuman primate ESCs (32, 33) and human ESCs and/or iPSCs (22–26), including evidence of rare haploid cells in some cases. These results are exciting and revolutionary, but challenged by the inability to test the function of the resulting germ cells in the human system. Therefore, safety and feasibility will need to be established in animal models. In 2011 Hayashi et al. showed that mouse ESCs and iPSCs can be induced to an epiblast-like cell that then gives rise to primordial germ cells when BMP4 was added to a culture media (28). The resulting germ cells were transplanted into the testes of infertile mice and produced spermatogenesis with sperm that were used to fertilize oocytes using ICSI. The resulting embryos were transferred to recipient females and gave rise to viable offspring. These promising results need to be replicated in other laboratories and more research is needed before this technique can be translated to the clinic, as some of the offspring developed malignant tumors around their neck area (28). For iPSC-based technologies, it will be important to understand the effects of reprogramming, culture and differentiation on the genome and epigenome of in vitro-derived germ cells compared with endogenous germ cells (107).
Experimental methods to track and quantify human germline stem cells
Studies on human cells and/or tissues are a valuable stepping stone toward clinical translation. However, these studies are challenged by the limited experimental tools for quantifying human spermatogonia and testing their function. In animal studies, the most compelling experiments are those that demonstrate the ability of a test cell population to regenerate spermatogenesis and produce fertilization competent sperm, embryos and offspring. This is an unrealistic expectation for human studies because fertilization of human eggs and production of human embryos is not universally legal or fundable. Based on progress in animal models, human to human SSC transplantation is likely to be feasible in the clinical setting, but this is not an option for routine testing of spermatogenic potential in the laboratory setting. Therefore, experimental assessment of human germline stem cell potential must rely on descriptive endpoints and xenotransplantation for functional testing.
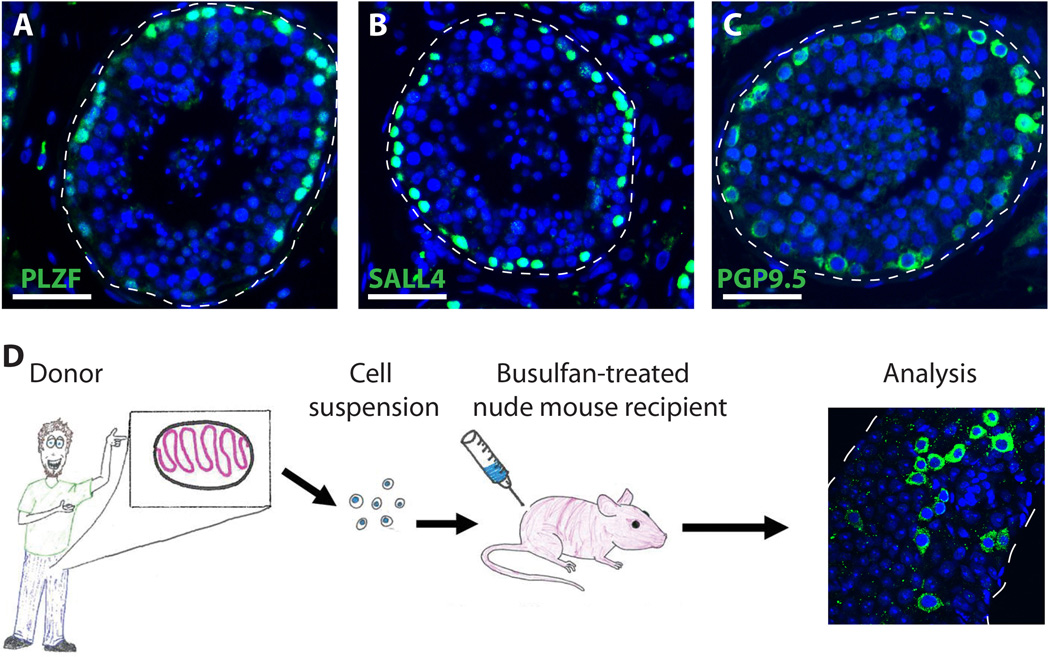
Here we propose that reliable markers of human spermatogonia are those with expression limited to germ cells located on the basement membrane of human seminiferous tubules. Proteins that meet these criteria, based on personal experience and review of the literature include PLZF, GFRα1, GPR125, SALL4, LIN28, UCHL1, UTF1, FGFR3, EXOSC10, DSG2, CBL, SSEA4, CD9, OCT2 and SSX (77, 78, 108–116). Examples of PLZF, SALL4 and PGP9.5 expression in the seminiferous tubules of adult human testes are shown in Figure 4A–C (Valli and Orwig, unpublished data).
Figure 4.
Experimental techniques to assay human spermatogonia. (A,B and C) Expression of spermatogonia markers PLZF (A), SALL4 (B) and PGP9.5 (C) is limited to germ cells located on the basement membrane of human seminiferous tubules. Thus, they are reliable markers to screen test cell populations for human spermatogonia. DAPI (blue) stains all cell nuclei. Scale bar = 50µm. (D) Human to nude mouse xenotransplantation assay. Human testicular tissue is made into a cell suspension and then transplanted into the testis of busulfan-treated infertile nude mice. Two months following the transplantation, the testes are recovered, the tunica is removed and the seminiferous tubules are gently dispersed to make a whole mount. The tubules are then stained with anti-primate antibody (122) to recognize the colonies of human spermatogonia (green).
In rodents, SSC transplantation is the gold standard that allows investigators to quantify germline stem cells by observing their biological potential to produce and maintain spermatogenesis in infertile recipient animals. At present, human to nude mouse xenotransplantation is the best functional assay to test the spermatogonial stem cell-like potential of a test cell population (21, 38, 78, 89, 109, 115, 117). This method does not recapitulate complete spermatogenesis from transplanted cells like mouse to mouse SSC transplantation, probably due to evolutionary distance between humans and mice. However, human to nude mouse xenotransplantation does assay the ability of transplanted cells to migrate to the basement membrane of seminiferous tubules, proliferate to produce characteristic colonies of spermatogonia and persist long term (78, 89, 109, 115, 117).
Conclusions
The assisted reproduction field is on the verge of a renaissance, which is fueled in part by exciting developments that merge new stem cell technologies with fertile outcomes. Thus, it is reasonable to expect in the next decade that the options to preserve and restore male fertility will expand from sperm freezing followed by IVF/ICSI to also include stem cell transplantation, tissue grafting and/or culture to produce fertilization competent gametes (Figure 1 and Table 1). As with any rapidly developing field that has potential to impact the clinic, it is essential to establish strict criteria to monitor progress and avoid sensationalism. Responsible technology development should ideally include: 1) Studies in lower mammals (e.g., rodents and other domestic species) where it is possible to examine functional readouts such as regeneration of spermatogenesis, fertilization, embryo development and generation of healthy offspring. 2) As technologies mature, preclinical studies in nonhuman primates that have anatomy and reproductive physiology similar to humans will demonstrate conservation of biological concepts and facilitate optimization of technical/surgical approaches (e.g., biopsy, cell processing, freezing and transplantation). Nonhuman primate studies are also amenable to functional assessments, although it is recognized that such studies will be limited to institutions with space, knowledge and technical expertise for research in primates and specifically primate assisted reproductive technology. 3) Direct investigations of human cells or tissues are particularly valuable on the road to clinical translation, but are challenged by limited availability of human samples, study to study variation in sample quantity and quality and the limited functional assays to assess experimental outcomes. Despite these challenges, human tissue studies to optimize tissue and cell processing procedures, cryopreservation methods and cell/tissue culture conditions will be the most relevant to the clinics that are already preserving testicular tissue for patients. Although it is not popular in the current era, descriptive studies of human germ lineage development in situ are essential to guide experimental design and interpretation of results of human stem cell studies.
Clinics worldwide are preserving testicular tissue for patients who do not have sperm in anticipation that new male fertility technologies will be available in the future. Each technology described in this review has merits and limitations that are detailed in the sections above. Due to uncertainty about which methods will ultimately be translated to the clinic, it seems reasonable to preserve intact testicular tissue fragments in a way that maximizes viability after freezing and thawing (an active focus of research in many laboratories) and will be amenable to tissue or cell based approaches in the future. Variability in patient circumstances and reproductive goals dictate the parallel development of multiple technologies for preserving and restoring male fertility.
Acknowledgments
Financial support: National Institutes of Health, Eunice Kennedy Shriver National Institute of Child Health & Human Development grants HD055475 and HD061289, Magee-Womens Research Institute and Foundation, Richard King Mellon Foundation (KEO) and the United States-Israel Binational Science Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclaimers: The authors have no potential conflicts of interest related to this manuscript.
References
- 1.Meistrich ML. Male gonadal toxicity. Pediatric Blood & Cancer. 2009;53:261–266. doi: 10.1002/pbc.22004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Howlader N, Noone AM, Krapcho M, Neyman N, Aminou R, Waldron W, et al. SEER Cancer Statistic Review 1975–2008. Bethesda, MD: National Cancer Institute; 2010. [Google Scholar]
- 3.Schover LR. Patient attitudes toward fertility preservation. Pediatr Blood Cancer. 2009;53:281–284. doi: 10.1002/pbc.22001. [DOI] [PubMed] [Google Scholar]
- 4.Lee SJ, Schover LR, Partridge AH, Patrizio P, Wallace WH, Hagerty K, et al. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J Clin Oncol. 2006;24:2917–2931. doi: 10.1200/JCO.2006.06.5888. [DOI] [PubMed] [Google Scholar]
- 5.Fertility preservation and reproduction in cancer patients. Fertility and Sterility. 2005;83:1622–1628. doi: 10.1016/j.fertnstert.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 6.Meistrich ML, Vassilopoulou-Sellin R, Lipshultz LI. Adverse effects of treatment: Gonadal dysfunction. In: DeVita VT, Hellman S, Rosenberg SA, editors. Principles and Practice of Oncology. 7th ed. Philadelphia: Lippincott Williams & Wilkins; 2004. pp. 2560–2574. [Google Scholar]
- 7.Schover LR, Brey K, Lichtin A, Lipshultz LI, Jeha S. Knowledge and Experience Regarding Cancer, Infertility, and Sperm Banking in Younger Male Survivors. Journal of Clinical Oncology. 2002;20:1880–1889. doi: 10.1200/JCO.2002.07.175. [DOI] [PubMed] [Google Scholar]
- 8.Hsiao W, Stahl PJ, Osterberg EC, Nejat E, Palermo GD, Rosenwaks Z, et al. Successful Treatment of Postchemotherapy Azoospermia With Microsurgical Testicular Sperm Extraction: The Weill Cornell Experience. Journal of Clinical Oncology. 2011;29:1607–1611. doi: 10.1200/JCO.2010.33.7808. [DOI] [PubMed] [Google Scholar]
- 9.Green DM, Kawashima T, Stovall M, Leisenring W, Sklar CA, Mertens AC, et al. Fertility of male survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2010;28:332–339. doi: 10.1200/JCO.2009.24.9037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pasquini MC, Wang Z. Current use and outcome of hematopoietic stem cell transplantation: CIBMTR Summary Slides. 2012 http://www.cibmtr.org. [Google Scholar]
- 11.Wallace WH, Anderson RA, Irvine DS. Fertility preservation for young patients with cancer: who is at risk and what can be offered? Lancet Oncol. 2005;6:209–218. doi: 10.1016/S1470-2045(05)70092-9. [DOI] [PubMed] [Google Scholar]
- 12.Mitchell RT, Saunders PT, Sharpe RM, Kelnar CJ, Wallace WH. Male fertility and strategies for fertility preservation following childhood cancer treatment. Endocr Dev. 2009;15:101–134. doi: 10.1159/000207612. [DOI] [PubMed] [Google Scholar]
- 13.Wyns C, Curaba M, Petit S, Vanabelle B, Laurent P, Wese JF, et al. Management of fertility preservation in prepubertal patients: 5 years' experience at the Catholic University of Louvain. Hum Reprod. 2011;26:737–747. doi: 10.1093/humrep/deq387. [DOI] [PubMed] [Google Scholar]
- 14.Ginsberg JP. New advances in fertility preservation for pediatric cancer patients. Current opinion in pediatrics. 2011;23:9–13. doi: 10.1097/MOP.0b013e3283420fb6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meistrich ML, Shetty G. Hormonal suppression for fertility preservation in males and females. Reproduction. 2008;136:691–701. doi: 10.1530/REP-08-0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ginsberg JP, Carlson CA, Lin K, Hobbie WL, Wigo E, Wu X, et al. An experimental protocol for fertility preservation in prepubertal boys recently diagnosed with cancer: a report of acceptability and safety. Hum Reprod. 2010;25:37–41. doi: 10.1093/humrep/dep371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ginsberg JP. Educational paper: the effect of cancer therapy on fertility, the assessment of fertility and fertility preservation options for pediatric patients. Eur J Pediatr. 2011;170:703–708. doi: 10.1007/s00431-010-1359-4. [DOI] [PubMed] [Google Scholar]
- 18.Orwig KE, Schlatt S. Cryopreservation and transplantation of spermatogonia and testicular tissue for preservation of male fertility. J Natl Cancer Inst Monogr. 2005;34:51–56. doi: 10.1093/jncimonographs/lgi029. [DOI] [PubMed] [Google Scholar]
- 19.Levine J, Canada A, Stern CJ. Fertility preservation in adolescents and young adults with cancer. J Clin Oncol. 2010;28:4831–4841. doi: 10.1200/JCO.2009.22.8312. [DOI] [PubMed] [Google Scholar]
- 20.Schlatt S, Ehmcke J, Jahnukainen K. Testicular stem cells for fertility preservation: preclinical studies on male germ cell transplantation and testicular grafting. Pediatr Blood Cancer. 2009;53:274–280. doi: 10.1002/pbc.22002. [DOI] [PubMed] [Google Scholar]
- 21.Sadri-Ardekani H, Mizrak SC, van Daalen SK, Korver CM, Roepers-Gajadien HL, Koruji M, et al. Propagation of human spermatogonial stem cells in vitro. JAMA. 2009;302:2127–2134. doi: 10.1001/jama.2009.1689. [DOI] [PubMed] [Google Scholar]
- 22.Park TS, Galic Z, Conway AE, Lindgren A, van Handel BJ, Magnusson M, et al. Derivation of primordial germ cells from human embryonic and induced pluripotent stem cells is significantly improved by coculture with human fetal gonadal cells. Stem Cells. 2009;27:783–795. doi: 10.1002/stem.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Easley CAt, Phillips BT, McGuire MM, Barringer JM, Valli H, Hermann BP, et al. Direct differentiation of human pluripotent stem cells into haploid spermatogenic cells. Cell reports. 2012;2:440–446. doi: 10.1016/j.celrep.2012.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kee K, Gonsalves JM, Clark AT, Pera RA. Bone morphogenetic proteins induce germ cell differentiation from human embryonic stem cells. Stem Cells Dev. 2006;15:831–837. doi: 10.1089/scd.2006.15.831. [DOI] [PubMed] [Google Scholar]
- 25.Kee K, Angeles VT, Flores M, Nguyen HN, Reijo Pera RA. Human DAZL, DAZ and BOULE genes modulate primordial germ-cell and haploid gamete formation. Nature. 2009;462:222–225. doi: 10.1038/nature08562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Panula S, Medrano JV, Kee K, Bergstrom R, Nguyen HN, Byers B, et al. Human germ cell differentiation from fetal- and adult-derived induced pluripotent stem cells. Hum Mol Genet. 2011;20:752–762. doi: 10.1093/hmg/ddq520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sato T, Katagiri K, Gohbara A, Inoue K, Ogonuki N, Ogura A, et al. In vitro production of functional sperm in cultured neonatal mouse testes. Nature. 2011;471:504–507. doi: 10.1038/nature09850. [DOI] [PubMed] [Google Scholar]
- 28.Hayashi K, Ohta H, Kurimoto K, Aramaki S, Saitou M. Reconstitution of the mouse germ cell specification pathway in culture by pluripotent stem cells. Cell. 2011;146:519–532. doi: 10.1016/j.cell.2011.06.052. [DOI] [PubMed] [Google Scholar]
- 29.Hermann BP, Sukhwani M, Winkler F, Pascarella JN, Peters KA, Sheng Y, et al. Spermatogonial stem cell transplantation into rhesus testes regenerates spermatogenesis producing functional sperm. Cell Stem Cell. 2012;11:715–726. doi: 10.1016/j.stem.2012.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brinster RL, Zimmermann JW. Spermatogenesis following male germ-cell transplantation. Proc Natl Acad Sci U S A. 1994;91:11298–11302. doi: 10.1073/pnas.91.24.11298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brinster RL, Avarbock MR. Germline transmission of donor haplotype following spermatogonial transplantation. Proc Natl Acad Sci U S A. 1994;91:11303–11307. doi: 10.1073/pnas.91.24.11303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Teramura T, Takehara T, Kawata N, Fujinami N, Mitani T, Takenoshita M, et al. Primate embryonic stem cells proceed to early gametogenesis in vitro. Cloning Stem Cells. 2007;9:144–156. doi: 10.1089/clo.2006.0070. [DOI] [PubMed] [Google Scholar]
- 33.Yamauchi K, Hasegawa K, Chuma S, Nakatsuji N, Suemori H. In vitro germ cell differentiation from cynomolgus monkey embryonic stem cells. PLoS ONE. 2009;4:e5338. doi: 10.1371/journal.pone.0005338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clark AT, Phillips BT, Orwig KE. Fruitful progress to fertility: male fertility in the test tube. Nat Med. 2011;17:1564–1565. doi: 10.1038/nm.2594. [DOI] [PubMed] [Google Scholar]
- 35.Holoch P, Wald M. Current options for preservation of fertility in the male. Fertility and Sterility. 2011;96:286–290. doi: 10.1016/j.fertnstert.2011.06.028. [DOI] [PubMed] [Google Scholar]
- 36.Wallace WH. Oncofertility and preservation of reproductive capacity in children and young adults. Cancer. 2011;117:2301–2310. doi: 10.1002/cncr.26045. [DOI] [PubMed] [Google Scholar]
- 37.Lamar CA, DeCherney AH. Fertility preservation: state of the science and future research directions. Fertility and Sterility. 2009;91:316–319. doi: 10.1016/j.fertnstert.2008.08.133. [DOI] [PubMed] [Google Scholar]
- 38.Sadri-Ardekani H, Akhondi MA, van der Veen F, Repping S, van Pelt AM. In vitro propagation of human prepubertal spermatogonial stem cells. JAMA. 2011;305:2416–2418. doi: 10.1001/jama.2011.791. [DOI] [PubMed] [Google Scholar]
- 39.Keros V, Hultenby K, Borgstrom B, Fridstrom M, Jahnukainen K, Hovatta O. Methods of cryopreservation of testicular tissue with viable spermatogonia in pre-pubertal boys undergoing gonadotoxic cancer treatment. Hum Reprod. 2007;22:1384–1395. doi: 10.1093/humrep/del508. [DOI] [PubMed] [Google Scholar]
- 40.Orwig KE, Shaw PH, Sanfilippo JS. Fertility Preservation in Pittsburgh. http://www.mwrif.org/220. [Google Scholar]
- 41.Goossens E, Van Saen D, Tournaye H. Spermatogonial stem cell preservation and transplantation: from research to clinic. Human Reproduction. 2013;28:897–907. doi: 10.1093/humrep/det039. [DOI] [PubMed] [Google Scholar]
- 42.Thayer KA, Ruhlen RL, Howdeshell KL, Buchanan DL, Cooke PS, Preziosi D, et al. Altered prostate growth and daily sperm production in male mice exposed prenatally to subclinical doses of 17α-ethinyl oestradiol. Human Reproduction. 2001;16:988–996. doi: 10.1093/humrep/16.5.988. [DOI] [PubMed] [Google Scholar]
- 43.Sharpe RM. Regulation of Spermatogenesis. In: Knobil E, Neill JD, editors. The Physiology of Reproduction. New York: Raven Press, Ltd.; 1994. pp. 1363–1434. [Google Scholar]
- 44.Hermann BP, Sukhwani M, Hansel MC, Orwig KE. Spermatogonial stem cells in higher primates: are there differences from those in rodents? Reproduction. 2010;139:479–493. doi: 10.1530/REP-09-0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meistrich M, Wilson G, Shetty G, Shuttlesworth G. Restoration of Spermatogenesis After Exposure to Toxicants: Genetic Implications. In: Robaire B, Hales B, editors. Advances in Male Mediated Developmental Toxicity. US: Springer; 2003. pp. 227–237. [DOI] [PubMed] [Google Scholar]
- 46.Meistrich ML. Effects of chemotherapy and radiotherapy on spermatogenesis. European urology. 1993;23:136–141. discussion 42. [PubMed] [Google Scholar]
- 47.van den Berg H, Furstner F, van den Bos C, Behrendt H. Decreasing the number of MOPP courses reduces gonadal damage in survivors of childhood hodgkin disease. Pediatric Blood & Cancer. 2004;42:210–215. doi: 10.1002/pbc.10422. [DOI] [PubMed] [Google Scholar]
- 48.Ogawa T, Dobrinski I, Avarbock MR, Brinster RL. Transplantation of male germ line stem cells restores fertility in infertile mice. Nat Med. 2000;6:29–34. doi: 10.1038/71496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shinohara T, Orwig KE, Avarbock MR, Brinster RL. Remodeling of the postnatal mouse testis is accompanied by dramatic changes in stem cell number and niche accessibility. Proceedings Of The National Academy Of Sciences Of The United States Of America. 2001;98:6186–6191. doi: 10.1073/pnas.111158198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nagano M, Brinster CJ, Orwig KE, Ryu BY, Avarbock MR, Brinster RL. Transgenic mice produced by retroviral transduction of male germ-line stem cells. Proc Natl Acad Sci U S A. 2001;98:13090–13095. doi: 10.1073/pnas.231473498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brinster CJ, Ryu BY, Avarbock MR, Karagenc L, Brinster RL, Orwig KE. Restoration of fertility by germ cell transplantation requires effective recipient preparation. Biol Reprod. 2003;69:412–420. doi: 10.1095/biolreprod.103.016519. [DOI] [PubMed] [Google Scholar]
- 52.Honaramooz A, Behboodi E, Megee SO, Overton SA, Galantino-Homer H, Echelard Y, et al. Fertility and germline transmission of donor haplotype following germ cell transplantation in immunocompetent goats. Biol Reprod. 2003;69:1260–1264. doi: 10.1095/biolreprod.103.018788. [DOI] [PubMed] [Google Scholar]
- 53.Mikkola M, Sironen A, Kopp C, Taponen J, Sukura A, Vilkki J, et al. Transplantation of normal boar testicular cells resulted in complete focal spermatogenesis in a boar affected by the immotile short-tail sperm defect. Reprod Domest Anim. 2006;41:124–128. doi: 10.1111/j.1439-0531.2006.00651.x. [DOI] [PubMed] [Google Scholar]
- 54.Kim Y, Turner D, Nelson J, Dobrinski I, McEntee M, Travis AJ. Production of donor-derived sperm after spermatogonial stem cell transplantation in the dog. Reproduction. 2008;136:823–831. doi: 10.1530/REP-08-0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Herrid M, Olejnik J, Jackson M, Suchowerska N, Stockwell S, Davey R, et al. Irradiation enhances the efficiency of testicular germ cell transplantation in sheep. Biol Reprod. 2009;81:898–905. doi: 10.1095/biolreprod.109.078279. [DOI] [PubMed] [Google Scholar]
- 56.Izadyar F, Den Ouden K, Stout TA, Stout J, Coret J, Lankveld DP, et al. Autologous and homologous transplantation of bovine spermatogonial stem cells. Reproduction. 2003;126:765–774. [PubMed] [Google Scholar]
- 57.Richardson TE, Chapman KM, Tenenhaus Dann C, Hammer RE, Hamra FK. Sterile testis complementation with spermatogonial lines restores fertility to DAZL-deficient rats and maximizes donor germline transmission. PLoS ONE. 2009;4:e6308. doi: 10.1371/journal.pone.0006308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kanatsu-Shinohara M, Ogonuki N, Inoue K, Miki H, Ogura A, Toyokuni S, et al. Long-term proliferation in culture and germline transmission of mouse male germline stem cells. Biol Reprod. 2003;69:612–616. doi: 10.1095/biolreprod.103.017012. [DOI] [PubMed] [Google Scholar]
- 59.Shetty G, Uthanmanthil RK, Zhou W, Shao SH, Weng CC, Tailor RC, et al. Hormone suppression with GnRH antagonist promotes spermatogenic recovery from transplanted spermatogonial stem cells in irradiated cynomolgus monkeys. Andrology. 2013 doi: 10.1111/j.2047-2927.2013.00126.x. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schlatt S, Foppiani L, Rolf C, Weinbauer GF, Nieschlag E. Germ cell transplantation into X-irradiated monkey testes. Hum Reprod. 2002;17:55–62. doi: 10.1093/humrep/17.1.55. [DOI] [PubMed] [Google Scholar]
- 61.Jahnukainen K, Ehmcke J, Quader MA, Saiful Huq M, Epperly MW, Hergenrother S, et al. Testicular recovery after irradiation differs in prepubertal and pubertal non-human primates, and can be enhanced by autologous germ cell transplantation. Hum Reprod. 2011;26:1945–1954. doi: 10.1093/humrep/der160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ryu BY, Orwig KE, Avarbock MR, Brinster RL. Stem cell and niche development in the postnatal rat testis. Dev Biol. 2003;263:253–263. doi: 10.1016/j.ydbio.2003.07.010. [DOI] [PubMed] [Google Scholar]
- 63.Dobrinski I, Avarbock MR, Brinster RL. Transplantation of germ cells from rabbits and dogs into mouse testes. Biol Reprod. 1999;61:1331–1339. doi: 10.1095/biolreprod61.5.1331. [DOI] [PubMed] [Google Scholar]
- 64.Dobrinski I, Avarbock MR, Brinster RL. Germ cell transplantation from large domestic animals into mouse testes. Mol Reprod Dev. 2000;57:270–279. doi: 10.1002/1098-2795(200011)57:3<270::AID-MRD9>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 65.Radford J, Shalet S, Lieberman B. Fertility after treatment for cancer. BMJ. 1999;319:935–936. doi: 10.1136/bmj.319.7215.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brook PF, Radford JA, Shalet SM, Joyce AD, Gosden RG. Isolation of germ cells from human testicular tissue for low temperature storage and autotransplantation. Fertility and sterility. 2001;75:269–274. doi: 10.1016/s0015-0282(00)01721-0. [DOI] [PubMed] [Google Scholar]
- 67.Radford J. Restoration of fertility after treatment for cancer. Horm Res. 2003;59(Suppl 1):21–23. doi: 10.1159/000067840. [DOI] [PubMed] [Google Scholar]
- 68.Ogawa T, Arechaga JM, Avarbock MR, Brinster RL. Transplantation of testis germinal cells into mouse seminiferous tubules. Int J Dev Biol. 1997;41:111–122. [PubMed] [Google Scholar]
- 69.Schlatt S, Rosiepen G, Weinbauer GF, Rolf C, Brook PF, Nieschlag E. Germ cell transfer into rat, bovine, monkey and human testes. Hum Reprod. 1999;14:144–150. doi: 10.1093/humrep/14.1.144. [DOI] [PubMed] [Google Scholar]
- 70.Kubota H, Avarbock MR, Brinster RL. Growth factors essential for self-renewal and expansion of mouse spermatogonial stem cells. Proc Natl Acad Sci U S A. 2004;101:16489–16494. doi: 10.1073/pnas.0407063101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ryu BY, Kubota H, Avarbock MR, Brinster RL. Conservation of spermatogonial stem cell self-renewal signaling between mouse and rat. Proc Natl Acad Sci U S A. 2005;102:14302–14307. doi: 10.1073/pnas.0506970102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Izadyar F, Den Ouden K, Creemers LB, Posthuma G, Parvinen M, De Rooij DG. Proliferation and differentiation of bovine type A spermatogonia during long-term culture. Biol Reprod. 2003;68:272–281. doi: 10.1095/biolreprod.102.004986. [DOI] [PubMed] [Google Scholar]
- 73.Kala S, Kaushik R, Singh KP, Kadam PH, Singh MK, Manik RS, et al. In vitro culture and morphological characterization of prepubertal buffalo (Bubalus bubalis) putative spermatogonial stem cell. Journal of Assisted Reproduction and Genetics. 2012;29:1335–1342. doi: 10.1007/s10815-012-9883-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Luo J, Megee S, Rathi R, Dobrinski I. Protein gene product 9.5 is a spermatogonia-specific marker in the pig testis: application to enrichment and culture of porcine spermatogonia. Molecular Reproduction and Development. 2006;73:1531–1540. doi: 10.1002/mrd.20529. [DOI] [PubMed] [Google Scholar]
- 75.Kuijk EW, Colenbrander B, Roelen BA. The effects of growth factors on in vitro-cultured porcine testicular cells. Reproduction. 2009;138:721–731. doi: 10.1530/REP-09-0138. [DOI] [PubMed] [Google Scholar]
- 76.Eildermann K, Gromoll J, Behr R. Misleading and reliable markers to differentiate between primate testis-derived multipotent stromal cells and spermatogonia in culture. Human Reproduction. 2012;27:1754–1767. doi: 10.1093/humrep/des091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.He Z, Kokkinaki M, Jiang J, Dobrinski I, Dym M. Isolation, characterization, and culture of human spermatogonia. Biol Reprod. 2010;82:363–372. doi: 10.1095/biolreprod.109.078550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wu X, Schmidt JA, Avarbock MR, Tobias JW, Carlson CA, Kolon TF, et al. Prepubertal human spermatogonia and mouse gonocytes share conserved gene expression of germline stem cell regulatory molecules. Proc Natl Acad Sci U S A. 2009;106:21672–21677. doi: 10.1073/pnas.0912432106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mirzapour T, Movahedin M, Tengku Ibrahim TA, Koruji M, Haron AW, Nowroozi MR, et al. Effects of basic fibroblast growth factor and leukaemia inhibitory factor on proliferation and short-term culture of human spermatogonial stem cells. Andrologia. 2012;44:41–55. doi: 10.1111/j.1439-0272.2010.01135.x. [DOI] [PubMed] [Google Scholar]
- 80.Chen B, Wang YB, Zhang ZL, Xia WL, Wang HX, Xiang ZQ, et al. Xeno-free culture of human spermatogonial stem cells supported by human embryonic stem cell-derived fibroblast-like cells. Asian J Androl. 2009;11:557–565. doi: 10.1038/aja.2009.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kokkinaki M, Djourabtchi A, Golestaneh N. Long-term culture of human SSEA-4 positive spermatogonial stem cells (SSCs) Journal of stem cell research and therapy. 2011;S2:003. doi: 10.4172/2157-7633.S2-003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu S, Tang Z, Xiong T, Tang W. Isolation and characterization of human spermatogonial stem cells. Reprod Biol Endocrinol. 2011;9:141. doi: 10.1186/1477-7827-9-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Abu Elhija M, Lunenfeld E, Schlatt S, Huleihel M. Differentiation of murine male germ cells to spermatozoa in a soft agar culture system. Asian J Androl. 2011;14:285–293. doi: 10.1038/aja.2011.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kim TH, Hargreaves HK, Brynes RK, Hawkins HK, Lui VK, Woodard J, et al. Pretreatment testicular biopsy in childhood acute lymphocytic leukaemia. Lancet. 1981;2:657–658. doi: 10.1016/s0140-6736(81)90996-x. [DOI] [PubMed] [Google Scholar]
- 85.Jahnukainen K, Hou M, Petersen C, Setchell B, Söder O. Intratesticular Transplantation of Testicular Cells from Leukemic Rats Causes Transmission of Leukemia. Cancer Research. 2001;61:706–710. [PubMed] [Google Scholar]
- 86.Fujita K, Ohta H, Tsujimura A, Takao T, Miyagawa Y, Takada S, et al. Transplantation of spermatogonial stem cells isolated from leukemic mice restores fertility without inducing leukemia. Journal of Clinical Investigation. 2005;115:1855–1861. doi: 10.1172/JCI24189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fujita K, Tsujimura A, Miyagawa Y, Kiuchi H, Matsuoka Y, Takao T, et al. Isolation of germ cells from leukemia and lymphoma cells in a human in vitro model: potential clinical application for restoring human fertility after anticancer therapy. Cancer Res. 2006;66:11166–11171. doi: 10.1158/0008-5472.CAN-06-2326. [DOI] [PubMed] [Google Scholar]
- 88.Hou M, Andersson M, Zheng C, Sundblad A, Soder O, Jahnukainen K. Decontamination of leukemic cells and enrichment of germ cells from testicular samples from rats with Roser's T-cell leukemia by flow cytometric sorting. Reproduction. 2007;134:767–779. doi: 10.1530/REP-07-0240. [DOI] [PubMed] [Google Scholar]
- 89.Dovey SL, Valli H, Hermann BP, Sukhwani M, Donohue J, Castro CA, et al. Eliminating malignant contamination from therapeutic human spermatogonial stem cells. J Clin Invest. 2013;123:1833–1843. doi: 10.1172/JCI65822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hermann BP, Sukhwani M, Salati J, Sheng Y, Chu T, Orwig KE. Separating spermatogonia from cancer cells in contaminated prepubertal primate testis cell suspensions. Human Reproduction. 2011;26:3222–3231. doi: 10.1093/humrep/der343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Geens M, Van de Velde H, De Block G, Goossens E, Van Steirteghem A, Tournaye H. The efficiency of magnetic-activated cell sorting and fluorescence-activated cell sorting in the decontamination of testicular cell suspensions in cancer patients. Human Reproduction. 2007;22:733–742. doi: 10.1093/humrep/del418. [DOI] [PubMed] [Google Scholar]
- 92.Honaramooz A, Snedaker A, Boiani M, Scholer H, Dobrinski I, Schlatt S. Sperm from neonatal mammalian testes grafted in mice. Nature. 2002;418:778–781. doi: 10.1038/nature00918. [DOI] [PubMed] [Google Scholar]
- 93.Schlatt S, Honaramooz A, Boiani M, Scholer HR, Dobrinski I. Progeny from sperm obtained after ectopic grafting of neonatal mouse testes. Biology of Reproduction. 2003;68:2331–2335. doi: 10.1095/biolreprod.102.014894. [DOI] [PubMed] [Google Scholar]
- 94.Honaramooz A, Li MW, Penedo MCT, Meyers S, Dobrinski I. Accelerated maturation of primate testis by xenografting into mice. Biology of Reproduction. 2004;70:1500–1503. doi: 10.1095/biolreprod.103.025536. [DOI] [PubMed] [Google Scholar]
- 95.Schlatt S, Kim SS, Gosden R. Spermatogenesis and steroidogenesis in mouse, hamster and monkey testicular tissue after cryopreservation and heterotopic grafting to castrated hosts. Reproduction. 2002;124:339–346. doi: 10.1530/rep.0.1240339. [DOI] [PubMed] [Google Scholar]
- 96.Jahnukainen K, Ehmcke J, Hergenrother SD, Schlatt S. Effect of cold storage and cryopreservation of immature non-human primate testicular tissue on spermatogonial stem cell potential in xenografts. Human Reproduction. 2007;22:1060–1067. doi: 10.1093/humrep/del471. [DOI] [PubMed] [Google Scholar]
- 97.Wistuba J, Luetjens CM, Wesselmann R, Nieschlag E, Simoni M, Schlatt S. Meiosis in autologous ectopic transplants of immature testicular tissue grafted to Callithrix jacchus. Biology of Reproduction. 2006;74:706–713. doi: 10.1095/biolreprod.105.048793. [DOI] [PubMed] [Google Scholar]
- 98.Luetjens CM, Stukenborg J-B, Nieschlag E, Simoni M, Wistuba J. Complete Spermatogenesis in Orthotopic But Not in Ectopic Transplants of Autologously Grafted Marmoset Testicular Tissue. Endocrinology. 2008;149:1736–1747. doi: 10.1210/en.2007-1325. [DOI] [PubMed] [Google Scholar]
- 99.Geens M, De Block G, Goossens E, Frederickx V, Van Steirteghem A, Tournaye H. Spermatogonial survival after grafting human testicular tissue to immunodeficient mice. Human Reproduction. 2006;21:390–396. doi: 10.1093/humrep/dei412. [DOI] [PubMed] [Google Scholar]
- 100.Goossens E, Geens M, De Block G, Tournaye H. Spermatogonial survival in long-term human prepubertal xenografts. Fertil Steril. 2008;90:2019–2022. doi: 10.1016/j.fertnstert.2007.09.044. [DOI] [PubMed] [Google Scholar]
- 101.Van Saen D, Goossens E, Bourgain C, Ferster A, Tournaye H. Meiotic activity in orthotopic xenografts derived from human postpubertal testicular tissue. Human Reproduction. 2011;26:282–293. doi: 10.1093/humrep/deq321. [DOI] [PubMed] [Google Scholar]
- 102.Schlatt S, Honaramooz A, Ehmcke J, Goebell PJ, Rubben H, Dhir R, et al. Limited survival of adult human testicular tissue as ectopic xenograft. Human Reproduction. 2006;21:384–389. doi: 10.1093/humrep/dei352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sato Y, Nozawa S, Yoshiike M, Arai M, Sasaki C, Iwamoto T. Xenografting of testicular tissue from an infant human donor results in accelerated testicular maturation. Human Reproduction. 2010;25:1113–1122. doi: 10.1093/humrep/deq001. [DOI] [PubMed] [Google Scholar]
- 104.Wyns C, Van Langendonckt A, Wese FX, Donnez J, Curaba M. Long-term spermatogonial survival in cryopreserved and xenografted immature human testicular tissue. Human Reproduction. 2008;23:2402–2414. doi: 10.1093/humrep/den272. [DOI] [PubMed] [Google Scholar]
- 105.Sato T, Katagiri K, Kubota Y, Ogawa T. In vitro sperm production from mouse spermatogonial stem cell lines using an organ culture method. Nat Protocols. 2013;8:2098–2104. doi: 10.1038/nprot.2013.138. [DOI] [PubMed] [Google Scholar]
- 106.Trowell OA. The culture of mature organs in a synthetic medium. Exp Cell Res. 1959;16:118–147. doi: 10.1016/0014-4827(59)90201-0. [DOI] [PubMed] [Google Scholar]
- 107.Byrne JA. Nuclear reprogramming and the current challenges in advancing personlized pluripotent stem cell-based therapies. Gene Therapy and Regulation. 2012;07:1230002. [Google Scholar]
- 108.Grisanti L, Falciatori I, Grasso M, Dovere L, Fera S, Muciaccia B, et al. Identification of spermatogonial stem cell subsets by morphological analysis and prospective isolation. Stem Cells. 2009;27:3043–3052. doi: 10.1002/stem.206. [DOI] [PubMed] [Google Scholar]
- 109.Zohni K, Zhang X, Tan SL, Chan P, Nagano M. CD9 Is Expressed on Human Male Germ Cells That Have a Long-Term Repopulation Potential after Transplantation into Mouse Testes. Biology of Reproduction. 2012 doi: 10.1095/biolreprod.112.098913. [DOI] [PubMed] [Google Scholar]
- 110.Eildermann K, Aeckerle N, Debowski K, Godmann M, Christiansen H, Heistermann M, et al. Developmental Expression of the Pluripotency Factor Sal-Like Protein 4 in the Monkey, Human and Mouse Testis: Restriction to Premeiotic Germ Cells. Cells, tissues, organs. 2012;196:206–220. doi: 10.1159/000335031. [DOI] [PubMed] [Google Scholar]
- 111.Aeckerle N, Eildermann K, Drummer C, Ehmcke J, Schweyer S, Lerchl A, et al. The pluripotency factor LIN28 in monkey and human testes: a marker for spermatogonial stem cells? Molecular Human Reproduction. 2012;18:477–488. doi: 10.1093/molehr/gas025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.von Kopylow K, Kirchhoff C, Jezek D, Schulze W, Feig C, Primig M, et al. Screening for biomarkers of spermatogonia within the human testis: a whole genome approach. Hum Reprod. 2010;25:1104–1112. doi: 10.1093/humrep/deq053. [DOI] [PubMed] [Google Scholar]
- 113.von Kopylow K, Staege H, Spiess AN, Schulze W, Will H, Primig M, et al. Differential marker protein expression specifies rarefaction zone-containing human Adark spermatogonia. Reproduction. 2012;143:45–57. doi: 10.1530/REP-11-0290. [DOI] [PubMed] [Google Scholar]
- 114.von Kopylow K, Staege H, Schulze W, Will H, Kirchhoff C. Fibroblast growth factor receptor 3 is highly expressed in rarely dividing human type A spermatogonia. Histochem Cell Biol. 2012;138:759–772. doi: 10.1007/s00418-012-0991-7. [DOI] [PubMed] [Google Scholar]
- 115.Izadyar F, Wong J, Maki C, Pacchiarotti J, Ramos T, Howerton K, et al. Identification and characterization of repopulating spermatogonial stem cells from the adult human testis. Hum Reprod. 2011;26:1296–1306. doi: 10.1093/humrep/der026. [DOI] [PubMed] [Google Scholar]
- 116.Lim J, Goriely A, Turner GD, Ewen KA, Jacobsen GK, Graem N, et al. OCT2, SSX and SAGE1 reveal the phenotypic heterogeneity of spermatocytic seminoma reflecting distinct subpopulations of spermatogonia. J Pathol. 2011;224:473–483. doi: 10.1002/path.2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nagano M, Patrizio P, Brinster RL. Long-term survival of human spermatogonial stem cells in mouse testes. Fertil Steril. 2002;78:1225–1233. doi: 10.1016/s0015-0282(02)04345-5. [DOI] [PubMed] [Google Scholar]
- 118.Clouthier DE, Avarbock MR, Maika SD, Hammer RE, Brinster RL. Rat spermatogenesis in mouse testis. Nature. 1996;381:418–421. doi: 10.1038/381418a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ogawa T, Dobrinski I, Avarbock MR, Brinster RL. Xenogeneic spermatogenesis following transplantation of hamster germ cells to mouse testes. Biol Reprod. 1999;60:515–521. doi: 10.1095/biolreprod60.2.515. [DOI] [PubMed] [Google Scholar]
- 120.Shinohara T, Kato M, Takehashi M, Lee J, Chuma S, Nakatsuji N, et al. Rats produced by interspecies spermatogonial transplantation in mice and in vitro microinsemination. Proceedings Of The National Academy Of Sciences Of The United States Of America. 2006;103:13624–13628. doi: 10.1073/pnas.0604205103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kubota H, Avarbock MR, Brinster RL. Culture conditions and single growth factors affect fate determination of mouse spermatogonial stem cells. Biol Reprod. 2004;71:722–731. doi: 10.1095/biolreprod.104.029207. [DOI] [PubMed] [Google Scholar]
- 122.Hermann BP, Sukhwani M, Lin CC, Sheng Y, Tomko J, Rodriguez M, et al. Characterization, cryopreservation, and ablation of spermatogonial stem cells in adult rhesus macaques. Stem Cells. 2007;25:2330–2338. doi: 10.1634/stemcells.2007-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hermann BP, Sukhwani M, Simorangkir DR, Chu T, Plant TM, Orwig KE. Molecular dissection of the male germ cell lineage identifies putative spermatogonial stem cells in rhesus macaques. Hum Reprod. 2009;24:1704–1716. doi: 10.1093/humrep/dep073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Nagano M, McCarrey JR, Brinster RL. Primate spermatogonial stem cells colonize mouse testes. Biol Reprod. 2001;64:1409–1416. doi: 10.1095/biolreprod64.5.1409. [DOI] [PubMed] [Google Scholar]
- 125.Maki CB, Pacchiarotti J, Ramos T, Pascual M, Pham J, Kinjo J, et al. Phenotypic and molecular characterization of spermatogonial stem cells in adult primate testes. Hum Reprod. 2009;24:1480–1491. doi: 10.1093/humrep/dep033. [DOI] [PubMed] [Google Scholar]
- 126.He Z, Kokkinaki M, Jiang J, Zeng W, Dobrinski I, Dym M. Isolation of human male germ-line stem cells using enzymatic digestion and magnetic-activated cell sorting. Methods Mol Biol. 2012;825:45–57. doi: 10.1007/978-1-61779-436-0_4. [DOI] [PMC free article] [PubMed] [Google Scholar]