Summary
Resveratrol is a natural compound that affects energy metabolism and mitochondrial function and serves as a calorie restriction mimetic, at least in animal models of obesity. Here we treated 11 healthy, obese men with placebo and 150 mg/day resveratrol in a randomized double-blind cross-over study for 30 days. Resveratrol significantly reduced sleeping- and resting metabolic rate. In muscle, resveratrol activated AMPK, increased SIRT1 and PGC-1α protein levels, increased citrate synthase activity without change in mitochondrial content, and improved muscle mitochondrial respiration on a fatty acid-derived substrate. Furthermore, resveratrol elevated intramyocellular lipid levels, and decreased intrahepatic lipid content, circulating glucose, triglycerides, alanine-aminotransferase, and inflammation markers. Systolic blood pressure dropped and HOMA index improved after resveratrol. In the postprandial state, adipose tissue lipolysis and plasma fatty acid and glycerol decreased. In conclusion, we demonstrate that 30 days of resveratrol supplementation induces metabolic changes in obese humans, mimicking the effects of calorie restriction.
Introduction
In our western society, the number of age-related chronic diseases such as obesity, diabetes and cancer increases progressively (Crews, 2005).
The only non-pharmacological intervention known to date to alleviate these deleterious conditions is calorie restriction. Reduction of calorie intake to 30-50% below ad libitum levels, or every-other-day feeding, can delay the onset of age-related diseases, improve stress resistance, and decelerate functional decline (Barger et al., 2003; Goodrick et al., 1990; McCay et al., 1935). Although short-term dietary restriction has metabolic effects in humans such as lowering metabolic rate (Heilbronn et al., 2006), improving insulin sensitivity (Larson-Meyer et al., 2006; Lim et al., 2011) and reducing cardiovascular risk factors (Lefevre et al., 2009), eating less for the sake of creating a desirable metabolic profile is unlikely to gain widespread compliance. As such, the focus has been on the development of calorie restriction mimetics that evoke some of the benefits of calorie restriction without an actual reduction in calorie intake. In that respect, sirtuins are considered an important molecular target (Canto and Auwerx, 2009). Indeed, it was suggested that the yeast Sir2 gene (Lin et al., 2000) or its worm (Tissenbaum and Guarente, 2001) and fly (Rogina and Helfand, 2004) orthologues are required for the effects of calorie restriction, although the relevance of the role of Sir2/SIRT1 as a strictu sensu longevity regulator is debated (Burnett et al., 2011). What is clear, however, is that mammalian SIRT1 plays a context-dependent role in health span regulation, for instance by mediating effects in metabolic stress situations, such as high-fat diet-induced obesity (Baur et al., 2006; Lagouge et al., 2006; Pearson et al., 2008). As such, SIRT1 confers protection against ageing-associated metabolic diseases such as glucose intolerance and cancer (Herranz et al., 2010; Pearson et al., 2008; Rutanen et al., 2010). In light of the growing number of patients suffering from metabolic diseases, compounds that activate SIRT1 directly or indirectly might offer protection against the onset of metabolic damage and promote healthy ageing.
To this end, Howitz and colleagues performed an in vitro screen to identify small molecule activators of SIRT1 (Howitz et al., 2003). Resveratrol, a natural polyphenolic compound present in various dietary components such as mulberries, peanuts, grapes and red wine, was identified as the most potent activator of SIRT1 (Howitz et al., 2003). Recently, it was shown, however, that resveratrol may not activate SIRT1 directly (Beher et al., 2009; Pacholec et al., 2010), but rather exerts its effects on SIRT1 through activation of AMPK (Baur et al., 2006; Canto et al., 2009; Canto et al., 2010; Feige et al., 2008; Hawley et al., 2010; Um et al., 2010), although additional direct SIRT1 activation is not completely excluded (Dai et al., 2010). Regardless of the mode of activation, resveratrol treatment in mice fed a high calorie diet consistently improved various health parameters including glucose homeostasis, endurance and survival (Baur et al., 2006; Lagouge et al., 2006; Pearson et al., 2008; Sun et al., 2007), and has therefore been suggested to act as a calorie restriction mimetic. However, so far no human studies have been reported that have systematically examined the metabolic effects of resveratrol in vivo.
Here, we gave obese but otherwise healthy subjects a dietary supplement containing 99% pure trans-resveratrol (resVida™), during 30 days and examined whole-body energy expenditure, substrate utilization, ectopic lipid storage, mitochondrial function, and lipolysis in adipose tissue and skeletal muscle using a combination of in vivo and ex vivo measurements. Our data show that like calorie restriction, resveratrol supplementation lowers energy expenditure, improves metabolic profile, as well as global health parameters.
Results
Study design and plasma biochemistry
Eleven obese but otherwise healthy male volunteers without a family history of diabetes or any other endocrine disorder participated in this study. Baseline characteristics of the subjects are included in table 1. Subjects participated in two experimental trials: (1) a placebo and (2) a resveratrol (150 mg/day (99%); resVida™) (provided by DSM Nutritional Products, Ltd) condition, in a randomized double-blind crossover design with a four-week wash-out period. Subjects were instructed to take the first supplement on the day after the baseline measurements (day 1) and the last supplement in the evening on day 29.
Table 1. Subjects baseline characteristics.
Subject characteristics at the start of the intervention (day 0). Values are given as means ± SEM (n=11).
| Placebo | Resveratrol | p-value | |
|---|---|---|---|
| Age (years) | 52.5 ± 2.1 | 52.5 ± 2.1 | - |
| Body weight (kg) | 100.1 ± 3.5 | 99.6 ± 3.7 | 0.50 |
| BMI (kg/m2) | 31.59 ± 0.74 | 31.45 ± 0.82 | 0.48 |
| Fat percentage (%) | 26.44 ± 0.53 | 26.44 ± 0.53 | - |
| VO2max (ml.kg−1.min−1) | 24.96 ± 1.30 | 24.80 ± 1.00 | 0.83 |
| Systolic blood pressure (mmHg) | 131 ± 3.1 | 132 ± 3.0 | 0.22 |
| Diastolic blood pressure (mmHg) | 82 ± 2.5 | 83 ± 2.6 | 0.20 |
| Glucose (mmol/l) | 5.44 ± 0.10 | 5.44 ± 0.13 | 0.96 |
| Insulin (mU/l) | 16.37 ± 1.76 | 15.38 ± 2.05 | 0.67 |
| Triglycerides (mmol/l) | 1.86 ± 0.19 | 1.92 ± 0.21 | 0.80 |
| Non-esterified fatty acids (μmol/l) | 357 ± 69 | 320 ± 31 | 0.56 |
To ensure that the subjects adhered to the study protocol and to confirm systemic conversion of resveratrol to dihydroresveratrol (DHR), total (sum of conjugated and unconjugated resveratrol) and free plasma levels of both compounds were analyzed each week during the 30-day period of resveratrol or placebo supplementation. Whereas no resveratrol or DHR could be detected in the placebo group, both compounds were present in plasma of resveratrol-supplemented subjects (table 2, levels at day 30 are shown and representative for the whole intervention period). Whereas measurement of plasma resveratrol demonstrated compliance to the study protocol and indicated that the compound was well absorbed, measurement of DHR confirmed that resveratrol was efficiently metabolized, especially as the plasma levels of DHR exceeded those of resveratrol after an overnight fast. No detectable levels of free resveratrol and free DHR were observed in the plasma in both the resveratrol and placebo period, also confirming efficient metabolism. It is important to note that although the dosage of 150 mg of resveratrol per day is around 133-266 fold lower compared to the high doses of 200 to 400 mg/kg/day used to supplement mice (Baur et al., 2006; Lagouge et al., 2006), plasma resveratrol levels in our human intervention (231 ng/ml on average during the 30 days) were even higher than those obtained in mice (10-120 ng/ml) (Lagouge et al., 2006). These differences may be due to different metabolic rates of resveratrol between humans and mice, and therefore suggest that resveratrol may exert its effects at different concentration ranges in different mammals.
Table 2. Plasma biochemistry.
Plasma values after 30 days of resveratrol or placebo supplementation. Values are given as means ± SEM (n=11). See also table S1.
| Placebo | Resveratrol | p-value | |
|---|---|---|---|
| Resveratrol (ng/ml) | Not detectable | 182.59 ± 30.33 | - |
| Dihydroresveratrol (ng/ml) | Not detectable | 289.14 ± 93.57 | - |
| Glucose (mmol/l) | 5.28 ± 0.15 | 5.06 ± 0.13 | 0.05 |
| Insulin (mU/l) | 11.94 ± 1.11 | 10.31 ± 1.25 | 0.04 |
| HOMA index | 2.80 ± 0.20 | 2.43 ± 0.24 | 0.03 |
| Triglycerides (mmol/l) | 2.29 ± 0.23 | 2.16 ± 0.21 | 0.03 |
| Non-esterified fatty acids (μmol/l) | 572 ± 77 | 621 ± 38 | 0.59 |
| Leptin (ng/ml) | 14.28 ± 1.98 | 12.91 ± 1.84 | 0.04 |
| Adiponectin (μg/ml) | 6.47 ± 0.55 | 6.45 ± 0.56 | 0.95 |
| CRP (ng/ml) | 1.52 ± 0.35 | 1.33 ± 0.31 | 0.11 |
| IL-1β (pg/ml) | 1.33 ± 0.27 | 0.94 ± 0.15 | 0.20 |
| IL-6 (pg/ml) | 3.13 ± 0.67 | 2.42 ± 0.38 | 0.09 |
| IL-8 (pg/ml) | 4.94 ± 0.59 | 4.28 ± 0.25 | 0.19 |
| TNF-α (pg/ml) | 16.15 ± 2.27 | 15.14 ± 2.03 | 0.04 |
| Leukocytes (109/l) | 7.03 ± 0.44 | 6.48 ± 0.39 | 0.03 |
| ALAT (U/l) | 31.91 ± 2.21 | 28.09 ± 1.54 | 0.02 |
To ensure that resveratrol supplementation did not cause adverse effects, we screened plasma and blood of the subjects for several general health parameters. Clinical chemistry, hematology and coagulation analysis revealed no adverse effects after 30 days of resveratrol (see table S1 available online). Plasma ALAT concentrations were significantly lower after resveratrol compared to placebo (table 2). Also, leptin levels and leukocytes in the plasma were significantly lower in resveratrol-treated subjects (table 2). In line with the reduced leukocyte count, resveratrol also lowered some markers of systemic inflammation, for including IL-6 and TNF-α (table 2). ECGs were inconspicuous and did not raise clinical concern in any subject. No clinical adverse events were reported during the resveratrol supplementation.
Next, we assessed whether resveratrol also exerted positive effects on plasma markers that predict the metabolic risk for development of diabetes and shorter lifespan. After 30 days of resveratrol, plasma glucose and insulin concentrations were lower compared to placebo (table 2), resulting in a lower HOMA-index, indicative of improved insulin sensitivity (table 2). Plasma triglyceride concentrations were significantly lower after resveratrol compared to placebo. No differences were observed in plasma non-esterified fatty acids (table 2).
Clinical improvement after resveratrol supplementation
As calorie restriction was described to associate with reduced absolute and body weight-adjusted 24-hour energy expenditure and sleeping energy expenditure (Heilbronn et al., 2006), we sought to investigate whether resveratrol treatment induced similar metabolic adaptations. Indeed, resveratrol supplementation for a period of 30 days had a profound effect on resting energy expenditure (table 3). Subjects had a significantly lower sleeping metabolic rate (SMR) compared to placebo, even though 24-hour energy expenditure was similar between resveratrol and placebo (table 3). Although calorie restriction causes metabolic adaptations also partly through weight loss, we found no effect on body mass (99.9 ± 3.9 vs. 99.6 ± 2.5 kg, in resveratrol vs. placebo, p=0.43). Respiratory quotient (RQ) values over 24 hours tended to be higher after resveratrol supplementation, which was largely accounted for by higher RQ values during the daytime (table 3, the complete time-course for the RQ and the energy expenditure over 24 hours is shown in table S2). The higher RQ in the resveratrol group is especially apparent after periods of feeding and suggests improved metabolic flexibility, i.e. the ability to switch between energy substrates. No differences were observed in diet-induced thermogenesis or physical activity index (table 3).
Table 3. Clinical improvement after resveratrol.
Energy metabolism (n=10), and blood pressure (n=11) after 30 days of resveratrol or placebo supplementation. Values are given as means ± SEM. See also table S2.
| Placebo | Resveratrol | p-value | |
|---|---|---|---|
| 24h Respiratory quotient | 0.89 ± 0.007 | 0.91 ± 0.006 | 0.09 |
| Respiratory quotient daytime | 0.89 ± 0.004 | 0.91 ± 0.003 | 0.001 |
| Respiratory quotient nighttime | 0.87 ± 0.007 | 0.88 ± 0.009 | 0.18 |
| 24h Energy expenditure (MJ/d) | 11.86 ± 0.29 | 11.91 ± 0.29 | 0.64 |
| Sleeping metabolic rate first night (MJ/d) | 8.09 ± 0.24 | 7.75 ± 0.23 | 0.007 |
| Sleeping metabolic rate second night (MJ/d) | 8.06 ± 0.22 | 7.90 ± 0.18 | 0.06 |
| Diet-induced thermogenesis (MJ/d) | 1.02 ± 0.13 | 1.14 ± 0.17 | 0.33 |
| Physical activity index | 1.49 ± 0.02 | 1.50 ± 0.01 | 0.37 |
| Systolic blood pressure (mmHg) | 130.5 ± 2.7 | 124.7 ± 3.1 | 0.006 |
| Diastolic blood pressure (mmHg) | 81.6 ± 2.8 | 80.0 ± 2.9 | 0.18 |
| Mean arterial pressure (mmHg) | 97.9 ± 2.7 | 94.9 ± 2.9 | 0.02 |
Next, we investigated whether resveratrol also exerted favorable effects on other general health parameters. As long-term calorie restriction in humans (Lefevre et al., 2009; Meyer et al., 2006) and resveratrol treatment in mice (Lagouge et al., 2006) was shown to ameliorate cardiac function, we examined whether resveratrol also exerts beneficial effects on blood pressure. Resveratrol significantly lowered systolic blood pressure by ~5 mmHg, while resveratrol had no effect on diastolic blood pressure (table 3). Mean arterial pressure was significantly lower after resveratrol supplementation (table 3).
Postprandial substrate utilization and tissue lipolysis
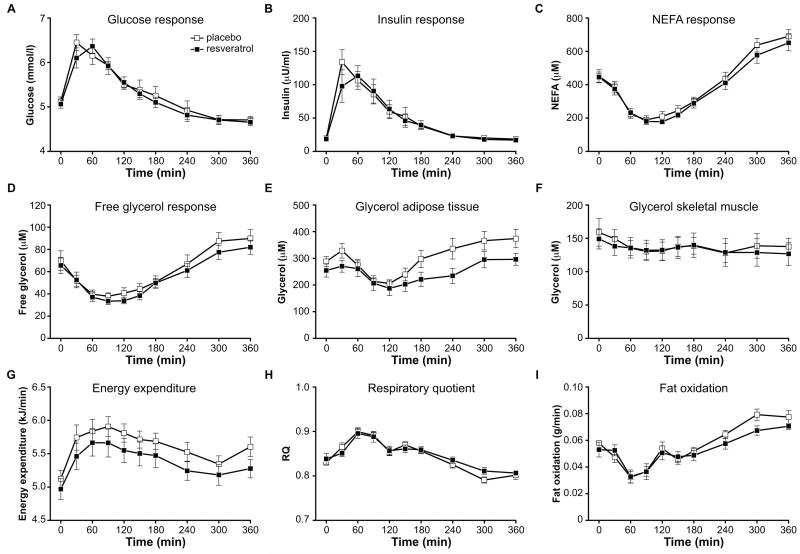
Mounting evidence implicates SIRT1 as a switch in substrate utilization, for instance from glucose to fatty acid oxidation under low-nutrient conditions (Canto and Auwerx, 2009; Feige et al., 2008). Therefore, we next investigated whether resveratrol is capable to increase fat oxidation in adipose tissue and skeletal muscle after a mixed meal, by stimulating tissue lipolysis. The peak glucose and insulin responses after consuming a liquid test meal were reached after 30 min for placebo and after 60 min for resveratrol (figure 1A,B). There was a tendency for non-esterified fatty acids (NEFA) (AUC 4h-6h, p=0.13) and free glycerol (AUC 4h-6h, p=0.06) to be lower in the late postprandial phase after resveratrol (figure 1C,D). Resveratrol did not alter postprandial triglyceride and lactate responses (data not shown). Using the microdialysis technique, ethanol out/in ratios were determined to measure blood flow. At baseline, ethanol out/in ratios were not different after resveratrol supplementation compared to placebo, both in adipose tissue (0.81 ± 0.02 vs. 0.81 ± 0.02, respectively) and skeletal muscle (0.46 ± 0.02 vs. 0.49 ± 0.02, respectively). Furthermore, resveratrol did not affect postprandial adipose tissue and skeletal muscle blood flow (data not shown). The interstitial glycerol concentration in adipose tissue was slightly lower in the resveratrol condition in the mid- and late postprandial phase, although it did not reach statistical significance (AUC 2h-4h, p=0.14; AUC 4h-6h, p=0.13) (figure 1E). There were no differences in interstitial glucose, pyruvate and lactate responses in adipose tissue between placebo and resveratrol (data not shown). For skeletal muscle, there were no differences in interstitial glucose, pyruvate, lactate and glycerol concentrations (glycerol data shown figure 1F). Interestingly, energy expenditure in the postprandial period was significantly lower after resveratrol supplementation (AUC, p=0.02) (figure 1G). During the late postprandial phase (4h-6h), RQ tended to be higher (AUC 4h-6h, p=0.07) (figure 1H) and fat oxidation was decreased in the resveratrol condition (AUC 4h-6h, p=0.007) (figure 1I).
Figure 1. Resveratrol decreases postprandial energy expenditure and lowers adipose tissue lipolysis.
Data are obtained on whole body (A-D), by microdialysis (E-F) and indirect calorimetry (G-I). Plasma metabolites during the postprandial microdialysis test after 30 days of placebo or resveratrol supplementation: (A) glucose response (B) insulin response (C) NEFA response (D) free glycerol response. Interstitial glycerol responses in (E) adipose tissue and (F) skeletal muscle during the postprandial microdialysis test. Indirect calorimetry results during the postprandial microdialysis test: (G) energy expenditure (H) respiratory quotient (I) fat oxidation. Values are given as means ± SEM (n=10).
Molecular mechanism of resveratrol treatment
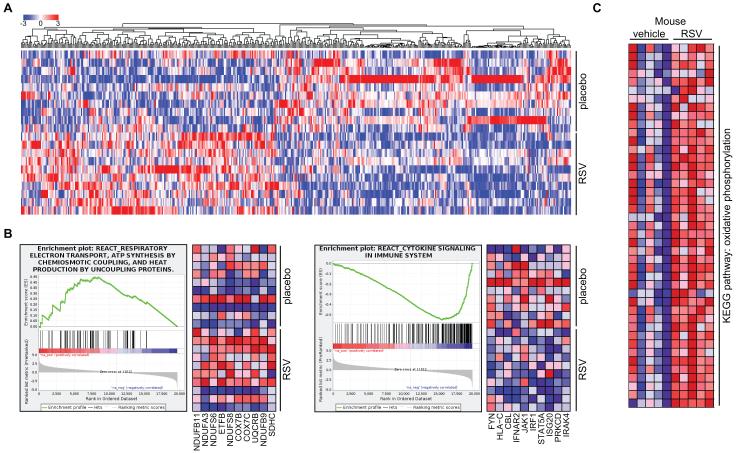
To clarify the mechanism of action of resveratrol, we performed microarray analysis on vastus lateralis muscle biopsies. We found 469 genes to be differentially expressed between placebo and resveratrol supplementation, 219 of which were increased and 250 decreased (figure 2A). We performed gene set enrichment analysis (GSEA) to define the pathways that were affected by resveratrol, and to compare the expression profile with previous studies in mouse (Lagouge et al., 2006). Several gene sets related to mitochondrial oxidative phosphorylation were upregulated, whereas pathways linked to inflammation were downregulated upon resveratrol supplementation (table S3). Indeed, when analyzing expression of the individual genes in the oxidative phosphorylation-related gene sets, we observed a marked increase (figure 2B, left panel), while for cytokine signaling, there was a clear decrease (figure 2B, right panel). Importantly, these effects are highly similar to the effects observed in mice treated with resveratrol (figure 2C), even though the effects in the latter are more pronounced, probably due to longer treatment time and their identical genetic and environmental background.
Figure 2. Resveratrol increases oxidative phosphorylation gene expression in man and mice.
(A) One-way hierarchical clustering of genes significantly changed in a microarray of vastus lateralis of subjects receiving resveratrol (RSV) or placebo. In muscle, 469 genes were differentially expressed between placebo and RSV (n=10, p<0.05). (B) Gene set enrichment analysis (GSEA) of muscle microarray revealed that gene sets related to mitochondrial oxidative phosphorylation were significantly increased (left panel) and cytokine signaling decreased (right panel) following resveratrol supplementation in humans. (C) Previously published microarray data of mice treated with 400 mpkd RSV (Lagouge et al., 2006) were reanalyzed by GSEA, showing, like the human data, a marked enrichment of oxidative phosphorylation genes. See also table S3.
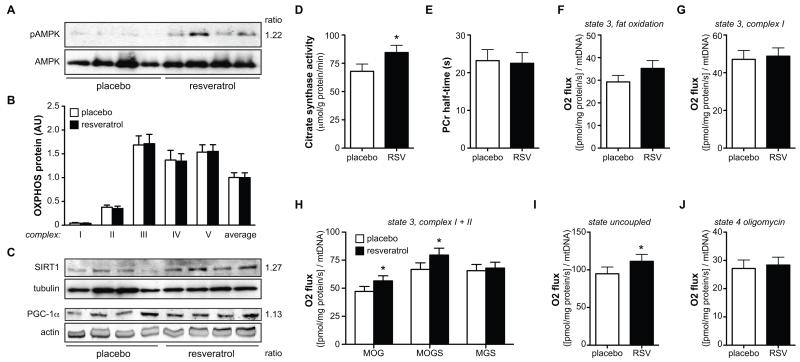
Since resveratrol was shown to activate AMPK, we measured levels of phosphorylated AMPK on the Thr172 residue of the α-subunit—a marker for its activation. Indeed, increased AMPK phosphorylation was observed in muscle biopsies of the resveratrol-treated subjects (figure 3A, S1A).
Figure 3. Resveratrol increases AMPK activity, increases mitochondrial efficiency and respiration on fatty acid substrates.
After 30 days of resveratrol or placebo, a muscle biopsy was obtained from the vastus lateralis muscle. Of total protein extracts from the vastus lateralis muscle, 50 μg of protein was used to check the expression levels of several proteins by Western blotting.
(A) The level of phosphorylation of residue Thr172 of the AMPKa subunit (top panel), indicative of AMPK activity, by Western blotting in a total of 9 subjects per group (representative subset is shown). Total level of the AMPKa protein is shown in the bottom panel. The ratio resveratrol/placebo is shown on the right of panel A. Relative quantification of the ratio of pAMPK/AMPK is shown in figure S1A.
(B) The protein content of the individual complexes of the electron transport chain are quantified by Western blotting in vastus lateralis muscle of 11 subjects per group. An antibody cocktail that detects all five complexes is used, and the data are represented as mean ± SEM for the individual complexes as well as for the average expression of all the complexes. A representative Western blot is included in figure S1B.
(C) SIRT1 and PGC-1α protein levels in vastus lateralis muscle by Western blotting (n=8 for SIRT1 and n=11 for PGC-1α, a representative subset is shown). The relative expression of both proteins is corrected for the loading control, tubulin for SIRT1 and actin for PGC-1α. The ratio of resveratrol/placebo is shown on the right of panel C. The relative quantification of the Western blots is shown in figure S1C.
(D) Citrate Synthase activity was significantly increased in vastus lateralis muscle of resveratrol (RSV)-treated subjects (n=11), * p<0.05. (E) In vivo mitochondrial function expressed as PCr half-time (n=11).
Evaluation of ex vivo mitochondrial function in vastus lateralis muscle: (F) ADP-stimulated respiration (state 3) upon a lipid substrate. (G) State 3 respiration fueled by Complex I-linked substrates. (H) State 3 respiration upon parallel electron input into Complex I and II, * p<0.05. (I) Maximally uncoupled respiration upon FCCP, * p<0.05. (J) Mitochondrial respiration uncoupled from ATP synthesis (state 4o). Values are given as means ± SEM (n=10). M, malate; O, octanoyl-carnitine; G, glutamate; S, succinate.
Mitochondrial metabolism
Our microarray data clearly suggested strong involvement of mitochondrial function in the beneficial effects of resveratrol. We hence performed some more detailed analyses of mitochondrial function. Mitochondrial DNA copy number in the resveratrol condition was similar to the values obtained after placebo (994.8 ± 101.4 vs. 1074.7 ± 109.9 AU in resveratrol vs. placebo, p=0.20). Also, neither the protein content of the individual structural components of the individual OXPHOS complexes nor the mean of all these complexes revealed differences in mitochondrial density in resveratrol compared to placebo supplementation (figure 3B, S1B). On the other hand, protein content of the AMPK downstream effector SIRT1 (Canto et al., 2009), as well as the mitochondrial master regulator PGC-1α were significantly increased after 30 days of resveratrol (figure 3C, S1C), as was citrate synthase activity (figure 3D), a common marker for mitochondrial activity.
Mitochondrial function can also be assessed in vivo by measurement of phosphocreatine (PCr) recovery rate after exercise (Schrauwen-Hinderling et al., 2007). To this end, single leg extension exercise was performed for 5 min with a weight corresponding to 60% of maximal capacity, which was determined with an incremental maximal test on the same ergometer on a different day. Low- to medium- intensity exercise was chosen to prevent acidification of the muscle, which is known to affect PCr recovery. Spectra were acquired 2 minutes before exercise, during the 5 minutes of knee extension exercise, and during the subsequent 5 minutes of recovery (rest). As targeted, PCr levels decreased during the knee-extension exercise until a steady state was reached. However, mean PCr recovery half time (PCr-t1/2) was unchanged by resveratrol compared to placebo (figure 3E).
To functionally characterize mitochondrial oxidative phosphorylation in more detail, we measured mitochondrial respiration using different substrate combinations. State 2 respiration (i.e., respiration in the presence of exogenous substrates alone) was not different between resveratrol versus placebo on any of the substrate combinations studied (data not shown). ADP-stimulated (state 3) respiration on a lipid substrate (malate + octanoyl-carnitine, MO) tended to be higher after resveratrol supplementation (p=0.075, figure 3F). State 3 respiration upon the Complex I-linked substrates malate + glutamate (MG) was unaffected (figure 3G). Respiration upon parallel electron input to both Complex I and II was ~10% higher upon resveratrol. Thus, state 3 respiration upon malate + octanoyl-carnitine + glutamate (MOG) was significantly higher in subjects receiving resveratrol (figure 3H). Similar differences were observed for state 3 respiration upon malate + octanoyl-carnitine + glutamate + succinate (MOGS) (figure 3H), but not in the absence of octanoyl-carnitine (malate + glutamate + succinate, MGS) (figure 3H). Maximal FCCP-induced uncoupled respiration, measured in the presence of octanoyl-carnitine, reflecting the maximal capacity of the electron transport chain, was also higher after 30 days of resveratrol (figure 3I). State 4o respiration (reflecting mitochondrial proton leak) was similar between resveratrol and placebo condition (figure 3J). Similar results were obtained if respiration rates were not corrected for mtDNA copy number.
Lipid accumulation in liver and skeletal muscle
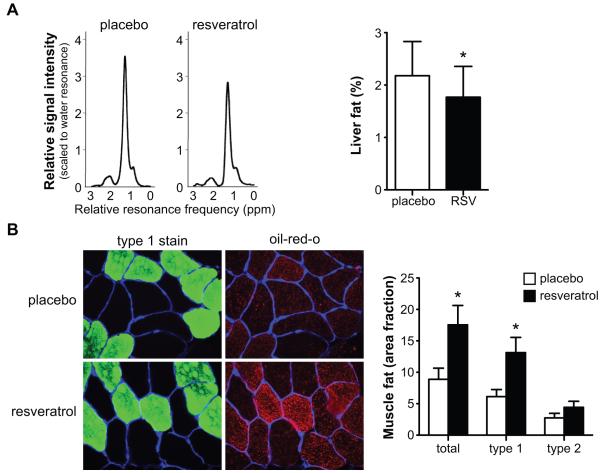
Since our data show that resveratrol clearly induced a shift in mitochondrial substrate selection, we tested whether this was also reflected in liver and skeletal muscle lipid accumulation. Intrahepatic lipid (IHL) content was lower after 30 days of resveratrol supplementation in comparison to placebo (figure 4A). This was paralleled by lower plasma ALAT values, as mentioned before, both indicating improved liver function. The mean intramyocellular lipid (IMCL) area fraction was ~2-fold higher in the resveratrol group (figure 4B). The increase in lipid accumulation was more pronounced in fibers identified as slow, oxidative (type 1) fibers than in type 2 muscle fibers (figure 4B).
Figure 4. Resveratrol decreases intrahepatic lipid content but increases intramyocellular lipid content.
Ectopic lipid deposition after 30 days of placebo or resveratrol supplementation. (A) Typical lipid regions of hepatic 1H-MR spectra from a subject receiving placebo (left) and resveratrol (right) at 3T, scaled to the water resonance (reference). The CH2 peak is used for quantification, the area under the curve is proportional to the lipid content in the liver. Quantification revealed that intrahepatic lipid content was reduced by resveratrol (n=9), * p<0.05. (B) Images of representative cross section of the vastus lateralis muscle from a subject receiving placebo (top) and resveratrol (bottom). Sections are stained for intramyocellular lipids with Oil Red O staining (in red), muscle laminin (in blue) and type 1 muscle fibers (in green) (400x magnification). IMCL content significantly increased by resveratrol treatment in muscle fibers, which was mostly accounted for by an increased lipid storage in type I muscle fibers (n=10), * p<0.05. Values are given as means ± SEM.
Discussion
Resveratrol, which was discovered in a small-molecule screen as a potent SIRT1 activator (Howitz et al., 2003), has been extensively studied in animal and cellular studies with promising results (Baur et al., 2006; Lagouge et al., 2006). Here, we show that resveratrol supplementation in humans exerted favorable metabolic adaptations that in many aspects mimic the effects of calorie restriction and/or endurance training (Civitarese et al., 2007; Heilbronn et al., 2006; Larson-Meyer et al., 2006; Larson-Meyer et al., 2008; Lefevre et al., 2009). These metabolic adaptations include a reduction in sleeping metabolic rate, blood pressure and hepatic lipid content, an improvement in skeletal muscle intrinsic mitochondrial function and several plasma markers of general health, an increase in intramyocellular lipid content, as well as an increase in skeletal muscle PGC-1α protein content. These data extend findings, which so far have only been observed in cell and rodent models, to the human situation, showing that resveratrol has promising beneficial metabolic effects, and suggests that resveratrol has the potential to improve metabolic health in subjects at risk for developing the metabolic syndrome.
Resveratrol exerts significant effects on energy metabolism. Sleeping metabolic rate (SMR) was significantly lower following 30 days of resveratrol supplementation, without changing 24-hour energy expenditure. It should be noted that SMR is the component of human energy metabolism that is most sensitive to metabolic changes—as it is not affected by physical activity—and small differences in sleeping energy expenditure can be detected with high accuracy. In line, we observed that basal and postprandial energy expenditure was also lower after 30 days of resveratrol supplementation. The 2-4% reduction in energy expenditure upon resveratrol treatment is consistent with the effects observed after calorie restriction (6% reduction) (Heilbronn et al., 2006; Martin et al., 2007).
It is important to note that basal and postprandial energy expenditure were reduced by resveratrol in our human study rather than increased, as was true for mice (Lagouge et al., 2006). Although our energy expenditure data are opposite to the effects seen in mice, a lowering of resting and sleeping metabolic rate is likely a reflection of improved metabolic efficiency, and completely in line with the endurance training or calorie restriction-like effects of resveratrol (Heilbronn et al., 2006; Martin et al., 2007). Although the dose used in our human study was ~200 fold lower than doses used in the mouse studies, we reached similar plasma resveratrol concentrations. We cannot exclude, however, that metabolism of resveratrol is different between mouse and man, and possibly more importantly that timing of treatment (30 days in our study vs 4-6 months in mice (Baur et al., 2006; Lagouge et al., 2006)) significantly impacts physiological outcome.
Prolonged calorie restriction (during six months) has also been suggested to increase the expression of genes encoding proteins involved in mitochondrial biogenesis and function (Civitarese et al., 2007). Similarly, our microarray data indicate increased mitochondrial gene expression in muscle following resveratrol supplementation. In fact, we showed that this induction is likely to be mediated by AMPK—which is activated by resveratrol—and resulted in increased SIRT1 and PGC-1α protein content, accompanied by increased citrate synthase activity, suggesting that mitochondrial activity was effectively improved. Moreover, detailed mitochondrial characterization revealed that resveratrol had beneficial effects on mitochondrial respiration when octanoyl-carnitine was used a substrate, but not when only glutamate was used. These data suggest that resveratrol specifically improves muscle fat oxidative mitochondrial capacity. Interestingly, we have recently found comparable effects in PGC-1α overexpressing mice, showing an improved intrinsic mitochondrial function (i.e. respiration per mitochondrion) but only when fatty acids are used as a substrate (Hoeks et al., 2011). The fact that mtDNA copy number, OXPHOS protein content, and PCr recovery were not changed in resveratrol-treated subjects suggests that 30 days resveratrol treatment mostly affects mitochondrial efficiency, not abundance. It is well possible that more long-term treatment would cause these parameters to change as well.
Interestingly, IMCL content was markedly increased after 30 days of resveratrol supplementation. Together with the improvement in muscle fat oxidative capacity and the other beneficial metabolic adaptations like lowering of circulating triglyceride and glucose levels, the present data hint towards endurance training-like effects of resveratrol (Dube et al., 2008; Meex et al., 2010). Consistent with data in rats, where resveratrol has been shown to reduce hepatic lipid synthesis (Ahn et al., 2008; Arichi et al., 1982), we observed reduced IHL content. Recent data suggest that endurance training is also able to lower IHL content (Kantartzis et al., 2009), as was calorie restriction, although this effect was mainly attributed to a reduction in body weight (Larson-Meyer et al., 2006; Lim et al., 2011). Taken together with the reduced plasma triglycerides and increased muscle fatty acid oxidation, we hypothesize that fat is liberated from peripheral depots to be metabolized by the muscle. Again, here our data suggest that resveratrol mimics the effect of calorie restriction and endurance training. Also, we have investigated postprandial metabolism, but only found a reduction in total energy expenditure that was reflected by a lower fat oxidation during the late postprandial phase—a change that is reminiscent of the role of SIRT1 in regulating the efficient switch in energy substrate utilization (Canto et al., 2009; Canto et al., 2010). Although postprandial lipolysis tended down, this does not refute our hypothesis of increased fat mobilization, but rather confirms the idea of improved metabolic flexibility.
A striking finding in our study was the resveratrol-induced reduction of systolic blood pressure by 5 mmHg. In rodents, resveratrol is also vasoactive and has been shown to cause vasodilatation and to improve aortic endothelial function in diabetic mice (Wang et al., 2011), to reduce heart rate (Lagouge et al., 2006) and even to lower blood pressure (Rivera et al., 2009). Also in human obese subjects endothelial function and cardiovascular health, dose-dependently improved after one week of resveratrol supplementation (Wong et al., 2010). Similarly, healthy non-obese individuals also improved their cardiovascular risk after six months of calorie restriction as evidenced by a reduction in blood pressure (Lefevre et al., 2009). Another indication that global health was improved upon resveratrol treatment was the decreased expression levels of genes of inflammatory pathways, as were plasma levels of several inflammatory markers, and leukocyte numbers.
Our data also point towards favorable effects on glucose homeostasis after 30 days of resveratrol supplementation in obese subjects. Indeed, HOMA-index was improved after resveratrol, suggesting favorable effects on insulin sensitivity. These beneficial effects of resveratrol on metabolism are in concordance with several findings of resveratrol supplementation in animals (Baur et al., 2006; Canto et al., 2009; Lagouge et al., 2006; Pearson et al., 2008; Sun et al., 2007). Unfortunately, we could not determine if these effects resulted in improved whole body insulin sensitivity.
In conclusion, we demonstrate beneficial effects of resveratrol supplementation for 30 days on the metabolic profile in healthy obese males, which seems to reflect effects observed during calorie restriction (table S4). Although most of the effects that we observed were modest, they were very consistently pointing towards beneficial metabolic adaptations. Furthermore, there were no effects on safety parameters and no adverse events were reported. Therefore, resVida was safe and well tolerated at the tested concentration. Future studies should investigate the long-term and dose-dependent metabolic effects of resveratrol supplementation in order to further establish whether resveratrol supplementation has the potential to overcome the metabolic aberrations that are associated with obesity in humans.
Experimental procedures
The study protocol was reviewed and approved by the Medical Ethical Committee of Maastricht University Medical Centre (MUMC+). All study participants gave written informed consent before initiation of the study.
Subject characteristics
Eleven healthy, obese, male volunteers without family history of diabetes or any other endocrine disorder participated in this study (Table 1). None of the subjects were on medication or were engaged in sports activities for more than two hours per week. Body composition was determined by a dual-energy X-ray absorptiometry scan (DXA, Discovery A; Hologic Corp, Bedford, MA) and maximal aerobic capacity was measured as described (Kuipers et al., 1985).
Clinical study design
Subjects participated in two experimental trials: a placebo and a resVida™ (150mg/day trans-resveratrol (99.9%) (provided by DSM Nutritional Products, Ltd) condition, in a randomized double-blind crossover design with a four-week wash-out period. Subjects were instructed to take the first supplement on the day after baseline measurements (d1) and the last supplement in the evening on day 29. The subjects were instructed to abstain from alcoholic beverages and foods containing substantial amounts of resveratrol (e.g. wine, red grapes, peanuts and berries) and were advised not to take any other food supplements during the study period. Compliance with these instructions was confirmed by verbal declaration of the subjects. Subjects were advised to maintain their normal living-, activity-, and sleeping-pattern during the intervention period. At the start (day 0) and end (day 30) of both intervention periods (resveratrol and placebo), blood samples were analyzed for general safety parameters including clinical chemistry, hematology and coagulation values. A twelve lead electrocardiogram (ECG) (Laméris, Veenendaal, The Netherlands) was performed at the beginning and end of both the resveratrol and placebo intervention. Each experimental trial lasted four weeks during which the subjects came on a weekly basis (day 0, 7, 14, 21, 30) to the university. The weekly check-up took place in the morning after an overnight fast, and included a measurement of body mass and withdrawal of a small blood sample for the analysis of resveratrol (original and metabolites) to confirm compliance to the protocol. On day 28 in the evening, subjects came to the university for 1H-MRS measurements of the liver, to quantify intrahepatic lipid content, and post-exercise PCr recovery rate was examined by 31P-MRS to estimate in vivo mitochondrial function (Schrauwen-Hinderling et al., 2007). To standardize food intake, subjects had lunch with the same food items in the two conditions and after lunch stayed fasted until the start of the measurement at 17 h.
After the MRS measurements on day 28, subjects stayed in the respiration chamber during 36 h to allow measurement of 24 h substrate oxidation and energy expenditure (Schoffelen et al., 1997). Before their stay in the respiration chamber, a standardized evening meal was provided. In the respiration chamber subjects were fed in energy balance (2332.5 kcal/day, 13.1 g of protein, 54.0 g of carbohydrate, 32.9 g of fat) and followed an activity protocol as described (Schrauwen et al., 1997). In the morning of day 30, after subjects left the respiration chamber, a muscle biopsy was taken to investigate ex vivo mitochondrial respiration, which was followed by the withdrawal of a fasting blood sample for the analysis of the effect of resveratrol on circulating substrates. Hereafter, adipose tissue and skeletal muscle lipolysis was examined by means of microdialysis.
Plasma biochemistry
To check compliance, resveratrol metabolites were measured by mass spectrometry in plasma on day 0, 7, 14, 21, 29 and 30 as described in the supplemental methods.
In the morning of day 30 - after a standardized overnight fast for 12 hours - and during the postprandial microdialysis test, blood samples were withdrawn for the determination of plasma metabolites according to standard procedures. Full experimental detail is described in the supplemental methods.
Blood pressure
On day 0 and day 30, blood pressure was measured after an overnight fast. By placing an automatic inflatable cuff (Omron Healthcare, Hamburg, Germany) on the non-dominant arm in the resting condition, systolic and diastolic blood pressure was measured in triplicate. Mean systolic and diastolic blood pressure values were used to calculate the mean arterial pressure.
Lipid accumulation in liver
On day 28, before subjects underwent the respiration chamber measurement, proton magnetic resonance spectroscopy (1H-MRS) was used to quantify hepatic lipid content (IHL) on a 3 T whole body scanner (Achieva; Philips Healthcare, Best, The Netherlands) using a five-element coil as described (Hamilton et al., 2011), however with a repetition time = 4000 msec, echo time = 37 msec, and number of averages = 64). To minimize motion artifacts, subjects were asked to breathe in the rhythm of the measurement and to be at end-expiration during acquisition of spectra. To determine the intensity of the lipid peak, the water signal was suppressed using frequency-selective prepulses. The unsuppressed water resonance was used as internal reference (number of averages=32) and spectra were fitted with AMARES (Vanhamme et al., 1997) in the jMURI software (Naressi et al., 2001). Values are given as T2-corrected ratios (according to (Hamilton et al., 2011)) of the CH2 peak, relative to the unsuppressed water resonance (as percentage).
31P-MRS-based measurement of mitochondrial function
On day 28, 31P-MRS measurements were performed in vastus lateralis muscle on a 1.5 T whole-body scanner (Intera; Philips Health Care, Best, The Netherlands) essentially according to an established methodology (Schrauwen-Hinderling et al., 2007).
Muscle biopsy
On day 30, when subjects left the respiration chamber, a muscle biopsy was taken from the vastus lateralis muscle under local anesthesia (2% lidocaïne), as previously described (Phielix et al., 2008). A portion of the muscle tissue was directly frozen in melting isopentane and stored at −80°C until assayed. Another portion (~30 mg) was immediately placed in ice-cold preservation medium for determination of ex vivo mitochondrial respiration (Phielix et al., 2008).
Molecular and protein expression
Mitochondrial DNA copy number, gene expression by microarray, and protein expression by Western blot, were performed according to standard procedures as described in the supplemental methods.
High resolution respirometry
Permeabilized muscle fibers were immediately prepared from the muscle tissue collected in the preservation medium, as described elsewhere (Boushel et al., 2007; Phielix et al., 2008). Subsequently, the permeabilized muscle fibers (~2.5 mg wet weight) were analyzed for mitochondrial function using an oxygraph (OROBOROS Instruments, Innsbruck, Austria) (Hoeks et al., 2010).
Intramyocellular lipids
Fresh cryosections (5 μm) were stained for intramyocellular lipids (IMCL) by Oil Red O staining combined with fibertyping and immunolabeling of the basal membrane marker laminin to allow quantification of IMCL, as described (Koopman et al., 2001).
Postprandial substrate utilization and tissue lipolysis
In 10 subjects, the lipolytic effects of resveratrol in adipose tissue and skeletal muscle were successfully determined by microdialysis, essentially according to (Goossens et al., 2004). A full description of the microdialysis method is provided as supplemental information.
Statistical analysis
Kolmogorov-Smirnov normality test was performed to evaluate normality distribution. Student’s paired t-test was used to compare placebo and resVida supplementation in normally distributed data, otherwise Wilcoxon signed-rank test was used. For the microdialysis test day, postprandial area under the curve (AUC) of plasma and interstitial metabolites and indirect calorimetry data were calculated using the trapezium rule. In addition to the total AUC (0h-6h after meal ingestion), also the early (0h-2h), mid (2h-4h) and late (4h-6h) AUCs were calculated to obtain more detailed information about the time course of postprandial responses. A P-Value < 0.05 was considered statistically significant. Data are reported as mean ± SEM. Statistical analyses were performed using the statistical program SPSS 16.0 for Mac OS X.
Accession number
Raw microarray datasets have been submitted to NBCI Gene Expression Omnibus (GSE32357).
Supplementary Material
Highlights.
Resveratrol reduced sleeping metabolic rate in human, mimicking calorie restriction
Resveratrol improved mitochondrial metabolism through the AMPK-SIRT1-PGC1α axis
Resveratrol decreased hepatic - and increased muscle lipid content
Resveratrol reduced inflammation markers in plasma and muscle
Acknowledgements
This study was funded by Top Institute Food and Nutrition. TI Food and Nutrition, formerly known as WCFS, is a unique public/private partnership that generates vision on scientific breakthroughs in food and nutrition, resulting in the development of innovative products and technologies that respond to consumer demands for safe, tasty and healthy foods. Partners are major Dutch food companies and research organizations. A VICI (grant 918.96.618) and a VENI (grant 916.11.136) for innovative research from the Netherlands Organization for Scientific Research (NWO) supports the work of P. Schrauwen and V. Schrauwen-Hinderling, respectively. The work in the Auwerx laboratory is supported by the European Research Council (Sirtuins; ERC-2008-AdG231-118), Swiss National Science Foundation, the Velux foundation, and Ecole Polytechnique Fédérale. JA is the Nestle Chair in Energy Metabolism. RHH is supported by a Rubicon fellowship of the Netherlands Organization for Scientific Research.
The authors would like to thank DSM Nutritional Products Ltd., Kaiseraugst, Switzerland for providing us with the resVida™ and placebo capsules and for performing the resveratrol and dihydroresveratrol analysis. The authors thank Jos Stegen for his excellent technical assistance with the biochemical analysis and Mark Boekschoten from the Netherlands Nutrigenomics Centre for the microarray analysis.
References
- Ahn J, Cho I, Kim S, Kwon D, Ha T. Dietary resveratrol alters lipid metabolism-related gene expression of mice on an atherogenic diet. J Hepatol. 2008;49:1019–1028. doi: 10.1016/j.jhep.2008.08.012. [DOI] [PubMed] [Google Scholar]
- Arichi H, Kimura Y, Okuda H, Baba K, Kozawa M, Arichi S. Effects of stilbene components of the roots of Polygonum cuspidatum Sieb. et Zucc. on lipid metabolism. Chem Pharm Bull. 1982;30:1766–1770. doi: 10.1248/cpb.30.1766. [DOI] [PubMed] [Google Scholar]
- Barger JL, Walford RL, Weindruch R. The retardation of aging by caloric restriction: its significance in the transgenic era. Exp Gerontol. 2003;38:1343–1351. doi: 10.1016/j.exger.2003.10.017. [DOI] [PubMed] [Google Scholar]
- Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beher D, Wu J, Cumine S, Kim KW, Lu SC, Atangan L, Wang M. Resveratrol is not a direct activator of SIRT1 enzyme activity. Chem Biol Drug Des. 2009;74:619–624. doi: 10.1111/j.1747-0285.2009.00901.x. [DOI] [PubMed] [Google Scholar]
- Boushel R, Gnaiger E, Schjerling P, Skovbro M, Kraunsoe R, Dela F. Patients with type 2 diabetes have normal mitochondrial function in skeletal muscle. Diabetologia. 2007;50:790–796. doi: 10.1007/s00125-007-0594-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett C, Valentini S, Cabreiro F, Goss M, Somogyvari M, Piper MD, Hoddinott M, Sutphin GL, Leko V, McElwee JJ, Vazquez R, Orfila A, Ackerman D, Au C, Vinti G, Riesen M, Howard K, Neri C, Bedalov A, Kaeberlein M, Soti C, Partridge L, Gems D. Absence of effects of Sir2 overexpression on lifespan in C. elegans and drosophila. Nature. 2011;477:482–485. doi: 10.1038/nature10296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canto C, Auwerx J. Caloric restriction, SIRT1 and longevity. Trends Endocrinol Metab. 2009;20:325–331. doi: 10.1016/j.tem.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canto C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, Elliott PJ, Puigserver P, Auwerx J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009;458:1056–1060. doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canto C, Jiang LQ, Deshmukh AS, Mataki C, Coste A, Lagouge M, Zierath JR, Auwerx J. Interdependence of AMPK and SIRT1 for metabolic adaptation to fasting and exercise in skeletal muscle. Cell Metab. 2010;11:213–219. doi: 10.1016/j.cmet.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civitarese AE, Carling S, Heilbronn LK, Hulver MH, Ukropcova B, Deutsch WA, Smith SR, Ravussin E. Calorie restriction increases muscle mitochondrial biogenesis in healthy humans. PLoS Med. 2007;4:e76. doi: 10.1371/journal.pmed.0040076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews DE. Artificial environments and an aging population: designing for age-related functional losses. J Physiol Anthropol Appl Human Sci. 2005;24:103–109. doi: 10.2114/jpa.24.103. [DOI] [PubMed] [Google Scholar]
- Dai H, Kustigian L, Carney D, Case A, Considine T, Hubbard BP, Perni RB, Riera TV, Szczepankiewicz B, Vlasuk GP, Stein RL. SIRT1 activation by small molecules: kinetic and biophysical evidence for direct interaction of enzyme and activator. J Biol Chem. 2010;285:32695–32703. doi: 10.1074/jbc.M110.133892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube JJ, Amati F, Stefanovic-Racic M, Toledo FG, Sauers SE, Goodpaster BH. Exercise-induced alterations in intramyocellular lipids and insulin resistance: the athlete’s paradox revisited. Am J Physiol Endocrinol Metab. 2008;294:E882–888. doi: 10.1152/ajpendo.00769.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feige JN, Lagouge M, Canto C, Strehle A, Houten SM, Milne JC, Lambert PD, Mataki C, Elliott PJ, Auwerx J. Specific SIRT1 activation mimics low energy levels and protects against diet-induced metabolic disorders by enhancing fat oxidation. Cell Metab. 2008;8:347–358. doi: 10.1016/j.cmet.2008.08.017. [DOI] [PubMed] [Google Scholar]
- Goodrick CL, Ingram DK, Reynolds MA, Freeman JR, Cider N. Effects of intermittent feeding upon body weight and lifespan in inbred mice: interaction of genotype and age. Mech Ageing Dev. 1990;55:69–87. doi: 10.1016/0047-6374(90)90107-q. [DOI] [PubMed] [Google Scholar]
- Goossens GH, Blaak EE, Saris WH, van Baak MA. Angiotensin II-induced effects on adipose and skeletal muscle tissue blood flow and lipolysis in normal-weight and obese subjects. J Clin Endocrinol Metab. 2004;89:2690–2696. doi: 10.1210/jc.2003-032053. [DOI] [PubMed] [Google Scholar]
- Hamilton G, Yokoo T, Bydder M, Cruite I, Schroeder ME, Sirlin CB, Middleton MS. In vivo characterization of the liver fat (1)H MR spectrum. NMR Biomed. 2011 doi: 10.1002/nbm.1622. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley SA, Ross FA, Chevtzoff C, Green KA, Evans A, Fogarty S, Towler MC, Brown LJ, Ogunbayo OA, Evans AM, Hardie DG. Use of cells expressing gamma subunit variants to identify diverse mechanisms of AMPK activation. Cell Metab. 2010;11:554–565. doi: 10.1016/j.cmet.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilbronn LK, de Jonge L, Frisard MI, DeLany JP, Larson-Meyer DE, Rood J, Nguyen T, Martin CK, Volaufova J, Most MM, Greenway FL, Smith SR, Deutsch WA, Williamson DA, Ravussin E. Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: a randomized controlled trial. JAMA. 2006;295:1539–1548. doi: 10.1001/jama.295.13.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herranz D, Munoz-Martin M, Canamero M, Mulero F, Martinez-Pastor B, Fernandez-Capetillo O, Serrano M. Sirt1 improves healthy ageing and protects from metabolic syndrome-associated cancer. Nat Commun. 2010;1:3. doi: 10.1038/ncomms1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeks J, Arany Z, Phielix E, Moonen-Kornips E, Hesselink MK, Schrauwen P. Enhanced lipid -but not carbohydrate- supported mitochondrial respiration in skeletal muscle of PGC-1alpha overexpressing mice. J Cell Physiol. 2011 doi: 10.1002/jcp.22812. (In press) [DOI] [PubMed] [Google Scholar]
- Hoeks J, van Herpen NA, Mensink M, Moonen-Kornips E, van Beurden D, Hesselink MK, Schrauwen P. Prolonged fasting identifies skeletal muscle mitochondrial dysfunction as consequence rather than cause of human insulin resistance. Diabetes. 2010;59:2117–2125. doi: 10.2337/db10-0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, Scherer B, Sinclair DA. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- Kantartzis K, Thamer C, Peter A, Machann J, Schick F, Schraml C, Konigsrainer A, Konigsrainer I, Krober S, Niess A, Fritsche A, Haring HU, Stefan N. High cardiorespiratory fitness is an independent predictor of the reduction in liver fat during a lifestyle intervention in non-alcoholic fatty liver disease. Gut. 2009;58:1281–1288. doi: 10.1136/gut.2008.151977. [DOI] [PubMed] [Google Scholar]
- Koopman R, Schaart G, Hesselink MK. Optimisation of oil red O staining permits combination with immunofluorescence and automated quantification of lipids. Histochem Cell Biol. 2001;116:63–68. doi: 10.1007/s004180100297. [DOI] [PubMed] [Google Scholar]
- Kuipers H, Verstappen FT, Keizer HA, Geurten P, van Kranenburg G. Variability of aerobic performance in the laboratory and its physiologic correlates. Int J Sports Med. 1985;6:197–201. doi: 10.1055/s-2008-1025839. [DOI] [PubMed] [Google Scholar]
- Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, Geny B, Laakso M, Puigserver P, Auwerx J. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Larson-Meyer DE, Heilbronn LK, Redman LM, Newcomer BR, Frisard MI, Anton S, Smith SR, Alfonso A, Ravussin E. Effect of calorie restriction with or without exercise on insulin sensitivity, beta-cell function, fat cell size, and ectopic lipid in overweight subjects. Diabetes Care. 2006;29:1337–1344. doi: 10.2337/dc05-2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson-Meyer DE, Newcomer BR, Heilbronn LK, Volaufova J, Smith SR, Alfonso AJ, Lefevre M, Rood JC, Williamson DA, Ravussin E. Effect of 6-month calorie restriction and exercise on serum and liver lipids and markers of liver function. Obesity. 2008;16:1355–1362. doi: 10.1038/oby.2008.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefevre M, Redman LM, Heilbronn LK, Smith JV, Martin CK, Rood JC, Greenway FL, Williamson DA, Smith SR, Ravussin E. Caloric restriction alone and with exercise improves CVD risk in healthy non-obese individuals. Atherosclerosis. 2009;203:206–213. doi: 10.1016/j.atherosclerosis.2008.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim EL, Hollingsworth KG, Aribisala BS, Chen MJ, Mathers JC, Taylor R. Reversal of type 2 diabetes: normalisation of beta cell function in association with decreased pancreas and liver triacylglycerol. Diabetologia. 2011 doi: 10.1007/s00125-011-2204-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SJ, Defossez PA, Guarente L. Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae. Science. 2000;289:2126–2128. doi: 10.1126/science.289.5487.2126. [DOI] [PubMed] [Google Scholar]
- Martin CK, Heilbronn LK, de Jonge L, DeLany JP, Volaufova J, Anton SD, Redman LM, Smith SR, Ravussin E. Effect of calorie restriction on resting metabolic rate and spontaneous physical activity. Obesity. 2007;15:2964–2973. doi: 10.1038/oby.2007.354. [DOI] [PubMed] [Google Scholar]
- McCay CM, Crowell MF, Maynard LA. The effect of retarded growth upon the length of life span and upon the ultimate body size. Journal of Nutrition. 1935;10:63–79. [PubMed] [Google Scholar]
- Meex RC, Schrauwen-Hinderling VB, Moonen-Kornips E, Schaart G, Mensink M, Phielix E, van de Weijer T, Sels JP, Schrauwen P, Hesselink MK. Restoration of muscle mitochondrial function and metabolic flexibility in type 2 diabetes by exercise training is paralleled by increased myocellular fat storage and improved insulin sensitivity. Diabetes. 2010;59:572–579. doi: 10.2337/db09-1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer TE, Kovacs SJ, Ehsani AA, Klein S, Holloszy JO, Fontana L. Long-term caloric restriction ameliorates the decline in diastolic function in humans. J Am Coll Cardiol. 2006;47:398–402. doi: 10.1016/j.jacc.2005.08.069. [DOI] [PubMed] [Google Scholar]
- Naressi A, Couturier C, Devos JM, Janssen M, Mangeat C, de Beer R, Graveron-Demilly D. Java-based graphical user interface for the MRUI quantitation package. MAGMA. 2001;12:141–152. doi: 10.1007/BF02668096. [DOI] [PubMed] [Google Scholar]
- Pacholec M, Bleasdale JE, Chrunyk B, Cunningham D, Flynn D, Garofalo RS, Griffith D, Griffor M, Loulakis P, Pabst B, Qiu X, Stockman B, Thanabal V, Varghese A, Ward J, Withka J, Ahn K. SRT1720, SRT2183, SRT1460, and resveratrol are not direct activators of SIRT1. J Biol Chem. 2010;285:8340–8351. doi: 10.1074/jbc.M109.088682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson KJ, Baur JA, Lewis KN, Peshkin L, Price NL, Labinskyy N, Swindell WR, Kamara D, Minor RK, Perez E, Jamieson HA, Zhang Y, Dunn SR, Sharma K, Pleshko N, Woollett LA, Csiszar A, Ikeno Y, Le Couteur D, Elliott PJ, Becker KG, Navas P, Ingram DK, Wolf NS, Ungvari Z, Sinclair DA, de Cabo R. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab. 2008;8:157–168. doi: 10.1016/j.cmet.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phielix E, Schrauwen-Hinderling VB, Mensink M, Lenaers E, Meex R, Hoeks J, Kooi ME, Moonen-Kornips E, Sels JP, Hesselink MK, Schrauwen P. Lower intrinsic ADP-stimulated mitochondrial respiration underlies in vivo mitochondrial dysfunction in muscle of male type 2 diabetic patients. Diabetes. 2008;57:2943–2949. doi: 10.2337/db08-0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera L, Moron R, Zarzuelo A, Galisteo M. Long-term resveratrol administration reduces metabolic disturbances and lowers blood pressure in obese Zucker rats. Biochem Pharmacol. 2009;77:1053–1063. doi: 10.1016/j.bcp.2008.11.027. [DOI] [PubMed] [Google Scholar]
- Rogina B, Helfand SL. Sir2 mediates longevity in the fly through a pathway related to calorie restriction. Proc Natl Acad Sci U S A. 2004;101:15998–16003. doi: 10.1073/pnas.0404184101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutanen J, Yaluri N, Modi S, Pihlajamaki J, Vanttinen M, Itkonen P, Kainulainen S, Yamamoto H, Lagouge M, Sinclair DA, Elliott P, Westphal C, Auwerx J, Laakso M. SIRT1 mRNA expression may be associated with energy expenditure and insulin sensitivity. Diabetes. 2010;59:829–835. doi: 10.2337/db09-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoffelen PF, Westerterp KR, Saris WH, Ten Hoor F. A dual-respiration chamber system with automated calibration. J Appl Physiol. 1997;83:2064–2072. doi: 10.1152/jappl.1997.83.6.2064. [DOI] [PubMed] [Google Scholar]
- Schrauwen P, van Marken Lichtenbelt WD, Saris WH, Westerterp KR. Changes in fat oxidation in response to a high-fat diet. Am J Clin Nutr. 1997;66:276–282. doi: 10.1093/ajcn/66.2.276. [DOI] [PubMed] [Google Scholar]
- Schrauwen-Hinderling VB, Kooi ME, Hesselink MK, Jeneson JA, Backes WH, van Echteld CJ, van Engelshoven JM, Mensink M, Schrauwen P. Impaired in vivo mitochondrial function but similar intramyocellular lipid content in patients with type 2 diabetes mellitus and BMI-matched control subjects. Diabetologia. 2007;50:113–120. doi: 10.1007/s00125-006-0475-1. [DOI] [PubMed] [Google Scholar]
- Sun C, Zhang F, Ge X, Yan T, Chen X, Shi X, Zhai Q. SIRT1 improves insulin sensitivity under insulin-resistant conditions by repressing PTP1B. Cell Metab. 2007;6:307–319. doi: 10.1016/j.cmet.2007.08.014. [DOI] [PubMed] [Google Scholar]
- Tissenbaum HA, Guarente L. Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature. 2001;410:227–230. doi: 10.1038/35065638. [DOI] [PubMed] [Google Scholar]
- Um JH, Park SJ, Kang H, Yang S, Foretz M, McBurney MW, Kim MK, Viollet B, Chung JH. AMP-activated protein kinase-deficient mice are resistant to the metabolic effects of resveratrol. Diabetes. 2010;59:554–563. doi: 10.2337/db09-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhamme L, van den Boogaart A, Van Huffel S. Improved method for accurate and efficient quantification of MRS data with use of prior knowledge. J Magn Reson. 1997;129:35–43. doi: 10.1006/jmre.1997.1244. [DOI] [PubMed] [Google Scholar]
- Wang N, Ko SH, Chai W, Li G, Barrett EJ, Tao L, Cao W, Liu Z. Resveratrol recruits rat muscle microvasculature via a nitric oxide-dependent mechanism that is blocked by TNFalpha. Am J Physiol Endocrinol Metab. 2011;300:E195–201. doi: 10.1152/ajpendo.00414.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong RH, Howe PR, Buckley JD, Coates AM, Kunz I, Berry NM. Acute resveratrol supplementation improves flow-mediated dilatation in overweight/obese individuals with mildly elevated blood pressure. Nutr Metab Cardiovasc Dis. 2010 doi: 10.1016/j.numecd.2010.03.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.