Abstract
We sought to review the epidemiology, international geographical distribution, and economic consequences of selected swine zoonoses. We performed literature searches in two stages. First, we identified the zoonotic pathogens associated with swine. Second, we identified specific swine-associated zoonotic pathogen reports for those pathogens from January 1980 to October 2012. Swine-associated emerging diseases were more prevalent in the countries of North America, South America, and Europe. Multiple factors were associated with the increase of swine zoonoses in humans including: the density of pigs, poor water sources and environmental conditions for swine husbandry, the transmissibility of the pathogen, occupational exposure to pigs, poor human sanitation, and personal hygiene. Swine zoonoses often lead to severe economic consequences related to the threat of novel pathogens to humans, drop in public demand for pork, forced culling of swine herds, and international trade sanctions. Due to the complexity of swine-associated pathogen ecology, designing effective interventions for early detection of disease, their prevention, and mitigation requires an interdisciplinary collaborative “One Health” approach from veterinarians, environmental and public health professionals, and the swine industry.
Keywords: swine, zoonoses, epidemiology, transmission, review
INTRODUCTION
The history of pig raising goes back as far as ∼9000 BC, likely with the domestication of wild boars in Eurasia.1 Since then, pork has served as a major source of human nutrition. In the last 50 years, the consumption of pork and the demands of products from pigs have increased, causing the global pig population to grow from 406 million to 966 million heads.2 Pigs are anatomically and physiologically similar to humans in terms of dentition, ocular, dermal, cardiovascular, renal, and digestive systems.3 While these have led to great advances in human and pig health, including substituting human organs with swine organs, these shared biological characteristics sometimes have the potential to permit pathogens to cross the species barrier.4,5 Although, pigs have been long known to serve as reservoirs for zoonotic pathogens, our understanding regarding zoonotic disease ecology in pigs is rather superficial.6,7 As such, although many swine pathogens are well-controlled, some zoonotic pathogens have become well-established in swine populations, imparting health and economic burdens. Some of these viruses, bacteria and parasites are emerging or re-emerging in nature, while others appear sporadically or transmit to man only under certain circumstances.8 Reducing these diseases in animals and humans often requires adopting primary or secondary prevention techniques, or a combination of both.9 However, doing so requires extensive understanding of husbandry practices, ecological preconditions, human risk behaviors, and the modes of transmission for swine-associated zoonoses. To facilitate a better understanding of their prevention and control, this review discusses the epidemiology, geographical distribution, and economic consequences of selected swine zoonoses from a global perspective.
LITERATURE REVIEW AND DATA SUMMARIZATION
We performed literature searches in two stages: first, to identify the zoonotic pathogens associated with swine and second, to identify the literature describing specific zoonotic pathogens. For the first stage, we performed a literature review in PubMed and in Google Scholar (English only) for articles published from January 1980 to October 2012, and searched by using the following terms: (swine or pig or boar or Sus scrofa) and (zoonoses or zoonosis or zoonotic). Additional relevant articles and books published between 1970 and 2012 were identified by reviewing the references from the collection of reports and through examining the authors' collections of publications. We included other swine-associated zoonotic diseases by reviewing lists compiled by the World Organization for Animal Health (www.oie.int) and the Merck Veterinary Manual (http://www.merckmanuals.com). Once the list of zoonoses was identified, we performed disease specific literature reviews to gather epidemiology and population level disease burden data from PubMed, Google Scholar, and in authors' personal files using the following terms: (disease name or pathogen name) and (swine or pig or boar or Sus scrofa).
We classified the swine-associated zoonoses in three major categories: emerging, endemic, and sporadic. An emerging zoonosis was defined when “the disease did not occur in humans before, or had occurred previously but affected only a small number of people in an isolated place, or had occurred in a population but was not recognized as a distinct disease”.10 Diseases were defined as endemic where they appeared to cluster geographically but not in time and as sporadic when they were clustered only in time.11 Zoonoses were sub-categorized into two groups: global occurrence and occurrence limited to a region(s) or geography. Additionally, we briefly reviewed the overall economic consequence data of swine zoonoses and swine-associated pathogens with zoonotic potential.
To demonstrate global distribution of the swine-associated zoonoses, we performed “geographically weighted regression”, an exploratory spatial analysis to develop a risk map for the emerging, endemic, and sporadic swine associated zoonoses after adjusting for population and swine density (2011) for each of the countries.12 We obtained human population density data from World Bank reports (www.worldbank.org), and pig density data from World Organization for Animal Health (www.oie.int).
We did not obtain formal ethical approval because this study reviewed data from already published literatures. For this body of research, the role of the funding agencies was to provide monetary support only. They did not have any role in the project's conception, design, analysis, or manuscript preparation. A detailed list of the primary data and their references are included as supplementary materials to the manuscript.
EMERGING SWINE ZOONOSES
Emerging swine-associated zoonoses occurring worldwide
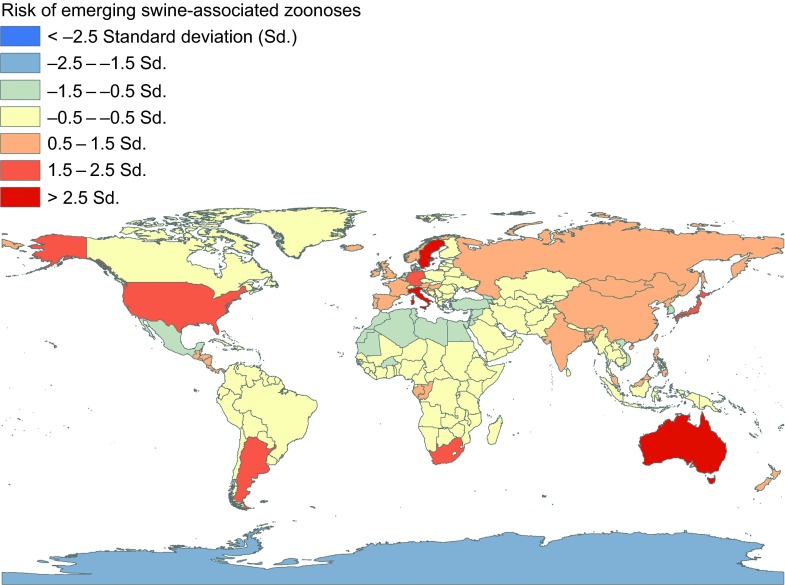
A number of emerging zoonotic swine pathogens are thought to have a worldwide distribution: hepatitis E virus (HEV), swine influenza viruses (SIV), livestock-associated methicillin-resistant Staphylococcus aureus (LA-MRSA), Streptococcus suis, Streptococcus porcinus, Clostridium difficile, Burkholderia pseudomallei, Cysticercus cellulosae (pork tapeworm), and Giardia intestinalis (Figure 1 and Supplementary Table S1).
Figure 1.
Global distribution of swine-associated emerging zoonoses, 1970 to 2012. These estimates are adjusted for 2011 human and pig population density of each of the countries.
Hepatitis E virus
First isolated in 1997 in the United States (US), swine HEV infections have since been identified in numerous countries.13 While data on human clinical infections with swine HEV is limited, experimental interspecies transmission of human and swine HEVs have been documented only between pigs and primates,14,15,16 demonstrating their zoonotic potential. In addition, seroepidemiological studies have presented evidence of swine HEV infections among swine veterinarians,17,18 indicating swine HEV may be causing asymptomatic infections in humans.
Influenza viruses
Since at least the 1918 influenza pandemic, public health professionals have been aware of cross-species influenza-like infections between man and pigs, but the connection was not evident until the 1920s when Dorset et. al. (1922) reported “hog flu”, later the experts began recalling similar illness in Iowa pigs five to six years before 1918 pandemic.7,19,20 Pigs' susceptibility to both human and avian influenza viruses permit them to be infected with both mammalian and avian origin viruses. This may result in reassortment of genetic materials between multiple subtype and species adapted influenza viruses, leading to new influenza A viruses.21 Beginning in 1958, serological studies started to report evidence of swine-origin influenza A virus in human and subsequently sporadic cases were intermittently detected.22,23 A 2007 review of SIV infections in man documented 50 human infections, with a 14% case-fatality rate.19 At that time such infections were generally perceived as rare and infrequent risk of human to human transmission. Since then, novel influenza virus detections have increased, and the reported numbers of swine-like influenza virus infections in man have tremendously escalated. Initial observations of high case fatality rates associated with human infection were likely biased in that novel influenza virus discovery was chiefly performed among those with serious illnesses. Later as molecular screening of influenza A strains became more widely available and surveillance increased more human SIV infections have been detected among persons with mild influenza disease. Recently, increased SIV detections among humans exposed to pigs at swine shows increased our awareness of SIV zoonoses, and it is clear that the influenza A viruses move both from pigs to man and from man to pigs.19,24,25,26 In particular, the 2009 swine-like influenza A [A(H1N1)pdm09] pandemic heightened our awareness. First detected in North America (early 2009), these novel H1N1 swine-like viruses spread between humans within months to 214 countries27 and by 2010, had caused an estimated 61 000 000 human infections; 274 000 hospitalizations; and 12 470 deaths.28 Within five months of the first human infections, A(H1N1)pdm09 virus was also identified in pigs,29,30 and now the virus is thought to be globally enzootic in many pig herds.31,32 Novel reassortant progeny from the A(H1N1)pdm09 virus are now a major concern. For example, as of November 2013, at least 309 humans in 10 US states have now been found to be infected with influenza A H3N2 variant virus; a virus that continues to spread in the US.33
Methicillin-resistant staphylococcus aureus
Discovered in the early 2000's, evidence suggests that LA-MRSA evolved as methicillin-susceptible Staphylococcus aureus (MSSA) in humans, and through genetic mutation moved into livestock, and later acquired methicillin resistance.34,35 Now identified in pigs in Asia, Europe and North America, LA-MRSA is often found colonizing noses and/or throats of pigs and may contribute to infection in persons occupationally exposed to pigs, as well as their household contacts.36,37,38,39,40 In addition, an environmental survey illustrated airborne transmission and deposition of LA-MRSA for up to 300 meters around swine barns with LA-MRSA infected pigs,41 further highlighting the public health risks for LA-MRSA exposure.
Emerging swine-associated zoonoses occurring in limited geographical locations
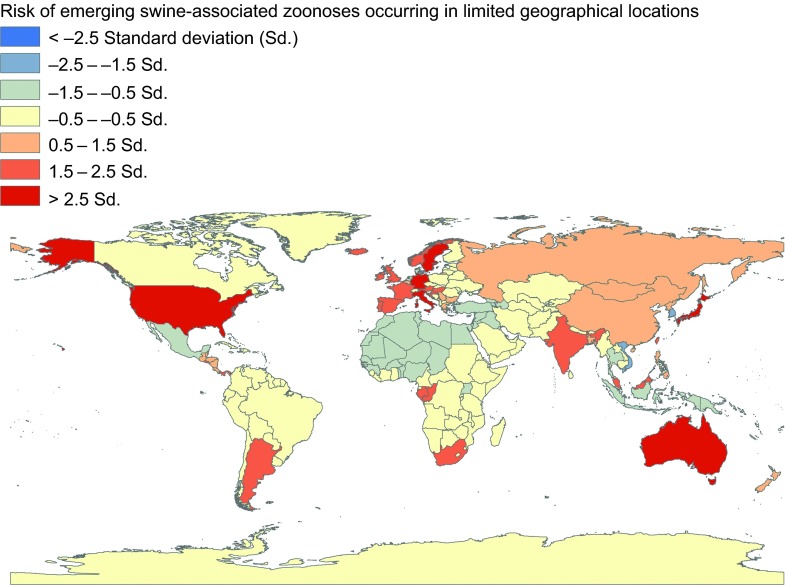
Emerging and re-emerging zoonotic swine pathogens with limited geographical distributions include Ebola Reston virus, Nipah virus, and Menangle viruses, which have the capability to cause severe diseases in humans and may have pandemic potential (Figure 2 and Supplementary Table S1).42,43,44,45
Figure 2.
Global distribution of emerging swine-associated zoonoses occurring in limited geographical locations, 1970 to 2012. These estimates are adjusted for 2011 human and pig population density of each of the countries.
Ebola virus
Since 1976, repeated outbreaks of Ebola virus-Zaire have been reported in Africa causing 47%–100% mortality in man.45,46 Pigs have shown the potential to transmit Ebola virus-Zaire to non-human primates.47 Ebola-Reston viruses were first reported in an imported non-human primate in the US and later were detected in pigs in the Philippines. There have now been at least three reports documenting human infections with Ebola-Reston virus although none have been associated with pigs.45,48,49 Experimentally, pigs are susceptible to both Ebola-Zaire and Ebola-Reston viruses,50,51 so there are concerns that pigs could play a role in future human outbreaks.
Nipah virus
There are several emerging and re-emerging zoonotic paramyxoviruses which have involved pigs in their transmission cycle. During 1998–1999, Nipah virus was identified in Malaysia and Singapore causing widespread zoonosis. Spillover from Pteropus bats triggered an outbreak in the pig population in Malaysia in 1998. A high proportion of pigs experience morbidity to Nipah virus infection, however most cases recover after several days of clinical illness. This illness, however, decreases the economic value of the commercially farmed pigs. During the outbreak the virus rapidly spread among swine farms, when the farmers attempted to take sick pigs to market to minimize economic loss. Trading sick pigs accelerated Nipah virus spread across the country (north-to-south and to Singapore).52 Overall human mortality due to Nipah viral infection was ∼40%. Having immediate contact with an infected pig was identified as a risk factor for Nipah virus infection,53,54 however, a similar virus caused more than 70% case fatality among humans in Bangladesh where pig's role in the ecology of the virus remains obscure.44,55,56
Menangle virus
Another swine pathogen, the Menangle virus, known to cause reproductive loss and death in pigs, has recently infected at least two humans in Australia who were exposed to clinically ill pigs.57,58 Although pig morbidity was as high as 90% in farms, the virus appears to have limited pig-to-human transmission capacity and seldom causes clinical illness in man.58 About 33% of the fruit bats sampled from outbreak areas had neutralizing antibodies against the virus, suggesting that they potentially are a natural reservoir for the virus.58
ENDEMIC (NON-EMERGING SWINE ZOONOSES)
Endemic swine-associated zoonoses occurring worldwide
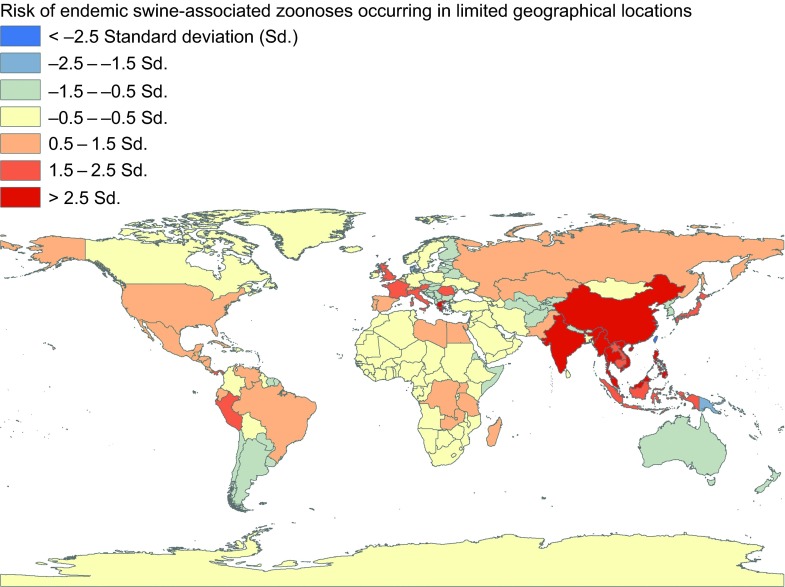
There exist numerous swine zoonoses that are distributed across multiple continents or at least several countries in a region (Figure 1). Human morbidity for these diseases is moderate to high with a low case fatality rate. This review for endemic swine zoonotic diseases highlights: brucellosis, Campylobacter enteritis, Escherichia coli infections, leptospiroses, listeriosis, pasteurellosis, salmonelloses, yersiniases, tuberculosis, erysipelas, West Nile virus infections, and echinococcosis (Supplementary Table S1).
Brucellosis
Each year, Brucella spp. cause more than 500 000 new cases of human brucellosis. Fortunately, the mortality remains low.59,60,61 B. suis, the organism responsible for swine brucellosis occurs in many countries throughout the world. Abundance of wild and domestic pigs is a major driver for B. suis occurrence.8,62 In South America, this organism has also adapted to cattle, resulting in more frequent disease outbreaks in those communities.8 A retrospective cohort study in Argentina conducted between 2008–2011 studied human brucellosis cases from clinical samples and isolated B. suis biovar 1 in 53%, B. abortus in 27% cases and the remaining isolates were not typed.63
Campylobacter
Campylobacter is one of the most common human pathogens occurring globally, causing frequent gastrointestinal illness in humans.64 It is estimated that approximately two million human cases of Campylobacter-related food-borne illness occurred in US in 1997; however, the number of cases has declined in recent years due to advances in food processing and chilling storage.65 Foodborne outbreak investigation report from 1998–2011 suggests the majority of the human illnesses are attributed to C. jejuni, followed by C. coli. 66 A nationwide survey in Denmark demonstrated that thermophilic Campylobacter strains (C. jejuni, C. coli and C. lari) were present in 46% pigs sampled, but the serotypes commonly infecting human also came from broiler poultry and cattle.67 However, C. coli is more commonly identified from pigs than C. jejuni.
Salmonellosis
Salmonella spp. are also a frequent cause of gastroenteritis in human.8 During 2009, the US Center for Disease Control and Prevention (CDC) reported approximately 15 cases per 100 000 people in the US.68 One US study identified approximately 3% of the pork products sold in supermarkets were contaminated with Salmonella.69 A Dutch study estimated that 450 new Salmonella cases (per 100 000 persons) occur each year and 5%–25% of all the cases were associated with pork consumption.70 However, it is estimated that only 5% of the Salmonella associated foodborne illnesses were attributed to pork.71
Parasitic zoonoses
Of the parasitic zoonoses, cystic echinococcosis (Echinococcus spp.) has multiple endemic foci with estimated annual human incidence rates of: 13–75 in European countries, 143 in South and Central America, 197 in East Asia, and 220 in Africa (per 100 000 population).72 The G1, G7, and Lion strain of E. granulosus and E. multilocularis (European, and Hokkaido isolates) cause swine-associated echinococcal zoonoses.72 Recent studies conducted in China and European countries suggested high variance in the echinococcosis prevalence [0.15%–66%] in pigs.73,74 In Lithuania echinococcosis was more common in family owned pig farms than the industrial pig farms (13.2% versus 4.1%).73 Other swine-associated parasitic zoonoses include cryptosporidiasis, trichinellosis, and toxoplasmosis which have a global distribution (Supplementary Table S1).
Endemic swine-associated zoonoses occurring in limited geographical locations
There are several swine-associated zoonoses endemic in specific regions of the world. This geographical isolation is due to the abundance of reservoirs and vectors, ecological factors, husbandry practices, and specific human behaviors facilitating zoonotic transmission of the diseases (Supplementary Table S1).
Yersiniosis
Food-borne bacterial enteritis caused by Yersinia enterocolitica are almost always associated with pigs or under-cooked pork products.75,76 This psychrophilic pathogen is mostly found in Canada, the western coast of South America, Europe, Australia, New Zealand, and South Africa.77,78,79 Yersinia pseudotuberculosis, has been frequently identified in Europe and parts of Asia, and occurs sporadically in the US. Pseudotuberculosis is commonly identified in rodents, and they are the probable source of infection among pigs. Humans often become infected via contaminated food and water.7
Tularemia
The zoonotic bacteria Francisella tularensis causes infections most prevalent in the US and Russia, and are sporadically reported in other Northern Hemisphere, including Scandinavia, the Czech Republics, Austria, Germany and Japan.8 However, recent reports suggest the pathogen is enzootic in Turkey, Yugoslavia, Spain, Kosovo, and Switzerland.80 More than 125 species of domestic and wild animals are reservoirs for this pathogen. Clinical and serological studies have identified F. tularensis infection both in wild and domestic pigs.81,82 Transmission of this pathogen occurs via all major routes and is remarkably efficient in transmitting itself from one host to another via all major transmission routes (Table 1).8
Table 1. Mode of exposure/transmission of the selected pathogens from pigs to man.
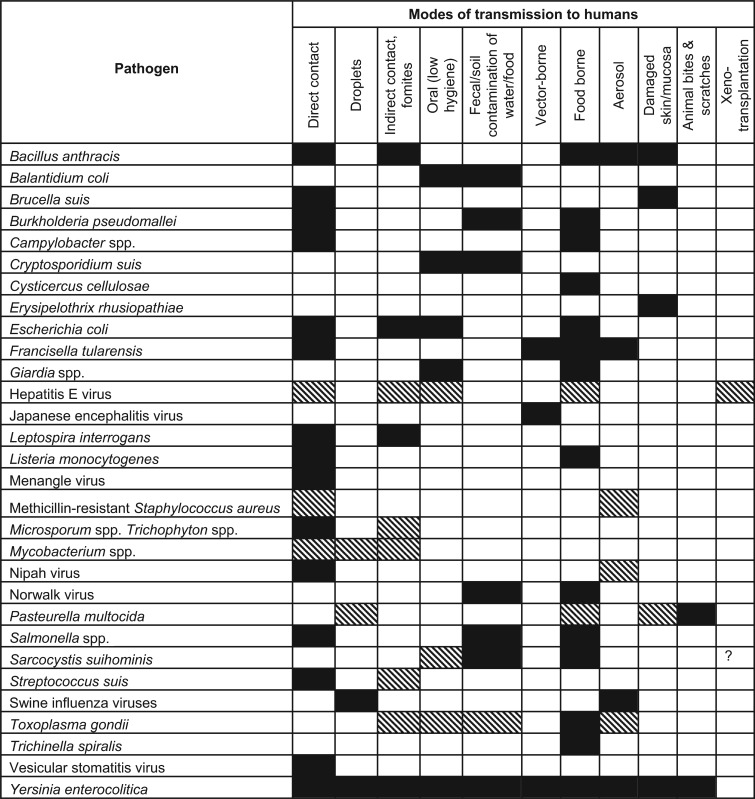 |
The dark shade indicates an established or primary route of transmission. The lighter shade indicates a suspected route of transmission.
? denotes limited data, findings remain suspected.
Japanese encephalitis
Japanese encephalitis virus is endemic in the southern and eastern part of Asia, and the Pacific.83 About one half of the global population are in the endemic region and about 30 000–50 000 new human cases occur annually in Asia, with 10 000 deaths, and about 15 000 cases develop permanent neurological and psychiatric sequelae.84,85 Factors, such as presence of abundant natural reservoirs (e.g. pigs and wading ardeid water birds) and vectors mosquitoes, that prefer to breed in the irrigated rice paddy fields in close proximity to humans, have contributed to the maintenance of the pathogen's transmission cycle.84
Vesicular stomatitis
Vesicular stomatitis virus infects pigs, cattle, horses, and human in the countries of North and South America, Africa, and Asia.7 Humans generally remain asymptomatic during infection, however, a small fraction of those infected may exhibit influenza-like-illness and hemorrhagic fever.8 This virus has been identified in multiple wild and domestic mammals, arthropod vectors (particularly Phlebotomus), and shown to infect humans through direct contact, transdermal, and transcutaneous routes.8,86
Parasitic zoonoses
Commonly occurring swine-associated parasitic diseases are predominantly seen in focal parts of Asia (Figure 3). These include giant intestinal fluke, Asian taeniasis, gastrodiscoidiasis, Chinese liver fluke, and schistosomiasis. These diseases are frequently seen in Eastern Asia, Southeast Asia, Kazakhstan, and Russia's Volga Delta region, and in Eastern Siberia.8,87,88,89,90,91,92,93 Multiple factors were related to elevated risk of human infection: the parasite is enzootic in animal reservoirs (including pigs) and in the environment; poor animal husbandry and particular risk behaviors like improper sanitation causing animal excreta to contaminate soil, water, aquatic plants, and other animals; ingestion of water plants, and animal products contaminated with infective states of the parasites; consuming raw or undercooked food; and occupational hazards such as agricultural workers and freshwater fishing.8,88,90,92,93 In summary, primitive pig production practices accompanied by poor sanitation and hygiene may lead to increased regional parasitic infection.
Figure 3.
Global distribution of endemic swine-associated zoonoses occurring in limited geographical locations, 1970 to 2012. These estimates are adjusted for 2011 human and pig population density of each of the countries.
Common routes of transmission for endemic swine-associated zoonoses
Globally enzootic swine-associated pathogens commonly transmit to man via direct contact, food and water contamination, fecal-oral transmission, and sometimes vector-borne routes (Table 1). Corresponding swine-associated zoonotic pathogens that are confined to specific geographic regions are more often influenced by factors affecting their ecological niches, such as vector-reservoir abundance, climatic factors, and human behaviors, particularly that of consuming undercooked food.7,8,92
SPORADIC SWINE ZOONOSES
Sporadic swine-associated zoonoses occurring worldwide
The majority of the swine-associated zoonoses that are sporadic in nature have a worldwide distribution. Influenza B and C viruses, clostridial infection, dermatophytosis (except Microsporum canis), sarcosporidiosis, and balantidiasis, all fall into this category (Supplementary Table S1). While the zoonosis due to influenza B virus is somewhat controversial, the influenza C virus has shown to infect both humans and pigs.94,95,96 Nevertheless, these viruses cause low morbidity and mortality in both species.97,98
Tetanus
Tetanus caused by Clostridium tetani occurs globally, but most often in developing countries among rural population with poor vaccination and public health infrastructure.99 According to World Health Organization (WHO), there were 14 132 reported cases worldwide in 2011 and 61 000 estimated deaths in children aged <5 years.100 Domestic animals such as cattle and horses are highly susceptible to clostridial infection and contaminate the environment through fecal shedding. In the high prevalence areas like New Guinea, pigs are reported to have contributed to the zoonotic transmission of C. tetani.99
Ringworm
The majority of the species of zoonotic ringworm causing fungi (Microsporum nanum, M. gypseum, Trichophyton mentagrophytes, T. rubrum, and T. verrucosum) occur worldwide,101 although M. canis seems limited to North and South America, Europe, and Africa.102 Pigs are the reservoir for M. nanum, but are also susceptible to the other species. This fungus has a broad spectrum of hosts including mammals and rodents.101 It is highly contagious among animal populations and often crosses the species barrier to infect humans via contaminated fomites. Although the mortality due to this ringworm is low, the cost of treatment puts this disease in the high economic burden category.7
Parasitic infections
Sarcosystis spp. cause zoonoses worldwide and pigs are the intermediate host for one of the causal organisms, S. suihominis. This is transmitted when humans consume undercooked pork.7 The protozoa is generally absent among swine herds that are raised under good hygienic conditions; however, a study in Germany showed that about 30%–40% of some swine herds may carry this zoonotic pathogen.8 Balantidium coli occurs worldwide, particularly in regions with a temperate or subtropical climate.8 Swine are the primary host for this ciliated protozoon. Disease prevalence in humans is less than 1%, but may be markedly higher in endemic regions.103 Most human infections are asymptomatic or limited to mild diarrhea and abdominal discomfort. However, in rare instances, the protozoa may lead to hemorrhagic lesions in the intestine, perforation, secondary bacterial infection, and generalized peritonitis.104
Sporadic swine-associated zoonoses occurring in limited geographical locations
The numbers of sporadically occurring zoonoses limited to particular regions are few. These zoonoses are primarily influenced by the abundance of reservoirs, and by particular human behaviors exposing them to the pathogen. One example is Pasteurella aerogenes infection, which is occasionally reported only from European countries.105 This organism is infrequently identified in swine as a normal oral and intestinal flora.106,107 In Europe, swine workers have acquired infection through bites from pigs.105
SWINE-ASSOCIATED ZOONOSES WITH LIMITED ZOONOTIC POTENTIAL
Rotavirus
Rotavirus frequently causes diarrhea in children under five years of age.108 It is most concerning in the less developed countries within Asia and sub-Saharan Africa.108 Rotavirus strains G3, G5, and G9, are predominantly found in pigs and other animal reservoirs. Recent evidences suggest that these viruses may exchange genetic materials with human viruses and cause increased human morbidity.109
West Nile virus
West Nile virus commonly occurs in Africa, Asia, Europe, and Australia, and it recently emerged and established itself in North America.110 The virus causes clinical signs of disease in only about 20%–30% the infected humans.111,112,113 Symptoms may range from uncomplicated fever to fatal encephalitis. Although laboratory studies suggest pigs develop enough viremia to play the role of a reservoir, the role of domestic pigs in the West Nile virus transmission remains obscure.7,113
Pseudorabies virus
Since 1914, there are several anecdotal reports of pseudorabies (Aujeszky's disease) in humans.114 Between 1983 and 1986, three suspected human cases of pseudorabies were identified in Europe. Each of these patients had a history of having direct contact with cats and other domestic animals. Researchers followed up the cases and identified pseudorabies antibodies through neutralization and immunoprecipitation assays, 5–15 months after clinical onset of illness.115 However, later serological studies were unable to detect pseudorabies antibodies in occupationally exposed populations.114 Pigs are the only reservoir for this virus.7
Norovirus
Typically, swine norovirus is only detected in fecal samples of apparently healthy adult pigs; however, experimental infections have resulted in mild gastroenteritis.116 Even though swine norovirus has not been found to cause illness in humans, antibodies against human norovirus strains have been detected in pigs.116 Because human norovirus strains are able to replicate in pigs, there is a potential for human and swine norovirus exchanging genetic material inside a swine host resulting in novel norovirus strains with zoonotic potential.117
Hendra virus
Hendra virus has caused recent sporadic equine and human outbreaks in Australia with a ∼40% case fatality rate in man.118 While Hendra virus infections have chiefly involved horses and man, laboratory studies show that pigs are susceptible to infection, which enables pigs to be a potential candidate to play a role in the disease ecology.119
Henipa-like virus
Recent studies identified evidence of henipa-like virus infections in pigs in Ghana and Bangladesh.56,120 Although evidence of human infection was not yet assessed in these studies, the report of this virus in pigs concerned public health experts as other henipa-like viruses may infect humans. Hendra and Nipah viruses (Henipaviruses) have caused zoonoses in Australia, Malaysia, Singapore, India, and Bangladesh.121 Nipah viruses caused 283 human cases and 109 deaths in Malaysia and Singapore during the 1998–1999 outbreaks.122 Laboratory studies also confirmed that pigs are capable of being infected with Hendra viruses which naturally infect fruit bats, horses, and have caused multiple human outbreaks in Australia.119
Xenotransplantation-associated zoonoses
Xenotransplantation, the process of using animal tissues or organs in man, has increased during last 100 years.5 Pig organs and tissues have become one of the most frequent transplant source in xenotransplantation. This additional pathway of transmission may enable certain pathogens to move from pigs to man. Retroviruses are a particular concern because of their history of crossing species barriers. It was hypothesized that the human immunodeficiency virus (HIV) and human T-cell leukemia virus have likely derived from simian immunodeficiency virus and simian T-cell leukemia virus, respectively.123,124 Studies suggest that porcine endogenous retroviruses may find its way to human hosts in the same manner.125 An in vitro study showed that human fibroblasts were susceptible to porcine lymphotropic herpesvirus and could be activated through xenotransplantation.125,126 Genotype 3 of HEV is most commonly identified in pigs in Europe and genotype 1 is common in humans.127,128 Recent studies suggest that HEV (particularly the genotype 3) infections are more commonly associated with organ allotransplant recipients.129,130 Emerging pathogens such as lymphocytic choriomeningitis virus and swine torque teno viruses have shown to infect humans through swine xenotransplantation deteriorating the immune systems of the HIV/AIDS patients and leading to death.131,132 Considering these pathogens as zoonotic should raise public health concerns and lead to defining pathogen-free swine stock for xenotransplantation.
ECONOMIC CONSEQUENCES OF SWINE ASSOCIATED ZOONOSES
Many of the emerging and re-emerging zoonoses causing diseases in humans and pigs have potential to cause severe economic consequences because of the mortality and production loss in pigs, trade sanctions on exporting animal products from an infected country or region, public health concerns leading to pig culling operations and reduced pork consumption, and public health burden of the diseases.43,58,133 Often the decisions to control a disease using drastic measures are influenced by cultural and community context.53,134,135 During the 1998–1999 Nipah outbreaks in Malaysia and Singapore, millions of pigs were culled to contain the outbreak that spread through the trade of sick pigs.53 This resulted in an estimated loss of USD $97 million and a drop in local pork consumption by 80%.136 Following the news on pandemic influenza H1N1 [A(H1N1)pdm09] outbreaks in April 2009, the reference to “swine flu” caused US pork prices to decline, reaching a low of USD $0.49 per lb in August 2009, which was about one half of the previous year's price (Figure 4). Twenty-seven countries imposed import restrictions for US pork products.137 Although there were several other reasons for the price drop, pandemic influenza H1N1 [A(H1N1)pdm09] likely contributed to the majority of the loss. The National Pork Board estimates US pork producers lost an estimated $13.64 per head from April 24 to May 2, 2009 and the industry accumulated some USD $7.2 million in losses daily (personal communication: National Pork Board, US 2009). An excessively cautious response was observed in Egypt during the 2009 pandemic when the country culled its entire swine population over concerns that the pandemic influenza virus in pigs would pose a major public health concern.138 In the US, swine brucellosis outbreaks caused considerable economic losses during the 1920–1950s. The country mostly eradicated the disease through changes in management and regulations; however, this disease continues to cause production losses in South America, most countries of Europe (except Britain and Scandinavia), Africa, and Southeast Asia.7,139 Cost effectiveness analyses of the swine-associated diseases' eradication programs may encourage a country or region to allocate sufficient resources to eradicate diseases posing public health threats.
Figure 4.
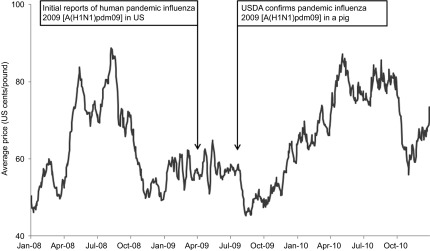
Emergence of pandemic influenza 2009 [A(H1N1)pdm09] in US human and pig population and the US pork price (negotiated carcass price) December 2007–2010.
In addition to the economic losses for the swine industry, swine zoonoses also cause human morbidity and mortality which have major economic consequences. However, for simplicity sake, we did not include the human economic consequences in these estimates.
DISCUSSION
In this report, we have described the epidemiology, geographical distributions, and economic consequences of major swine zoonoses. We have summarized the mechanisms of disease transmission along with the ecological and behavioral factors influencing the process. Our goal was to inform medical, veterinary, epidemiology, microbiology, and social science experts of the established and emerging threats common for humans and swine, as well as to shed light on some pathogens that may be potential future threats.
The majority of emerging human pathogens are zoonotic.140 Frequently changing husbandry practices and environmental factors (e.g. large scale domestic animal production, urbanization, interaction between wild and domestic swine populations with humans, population increases, etc.) may predispose humans and pigs to pathogens common to other species, or may allow for the adaptation of these organisms to humans or swine. Being omnivorous and having the anatomy and physiology similar to that of man, pigs are a good medium for the adaptation and increase in virulence of organisms that have so far not been identified as human pathogens.3 Moreover, with the increase in human populations, consumption of pork and pork products has increased markedly in the last one hundred years.2 Additionally, the scientific breakthrough that allowed xenotransplantation of porcine organs, tissues, and porcine hormones in human medicine, has opened a new pathway for future cross-species transmission of swine pathogens currently not common to humans.5
Pathogens that first emerged in wild species and in a particular geographical region and gained the ability to infect domesticated pigs may spread to a wider territory by the trade industry of food and livestock.52,141 Moreover, pathogens that adapt to and become established in swine have a much higher probability of spreading to humans, due to the intensity of swine farming worldwide and the close contact between pigs and humans. Swine workers are constantly exposed to bodily fluid secretions from a wide variety of swine products, and sometimes may become exposed to pathogens in ways that do not occur in natural conditions (e.g. respiratory spread of fecally-shed pathogens or aerosolization of organ fluids during slaughter house operations). Since their emergence, many of the swine-associated zoonoses have been infrequently considered as causes of human illness, especially among populations of humans that are occupationally and traditionally exposed to pigs or raw pig products. In several Asian countries, some parasitic swine zoonoses (e.g. trichinellosis, teniasis/cisticercosis) are quite common because of human food habits, particularly eating raw and undercooked food.7,8,87
Although pigs are one of the major sources of animal protein globally, and the industry represents a large portion of the economy for many countries, steps should be taken to minimize swine-associated zoonoses of public health concern. A solution to this requires uniform understanding and consensus between the swine industry, farmers, veterinarians, clinicians, public health professionals, and other stakeholders. Addressing these complex issues requires integrative and cross-disciplinary efforts to achieve optimum health for people, pigs and their environment through the “One Health” approach.142 Such an interdisciplinary, and inter-institutional collaborative approach provides a united platform upon which stakeholders can come together as collaborators, develop a more complete understanding regarding a complex problem, and tackle these problems with carefully designed, multiple interventions. Such a collaborative strategy has potential to gain much wider acceptability among swine farmers, the swine industry, as well as among public health professionals. Embracing the principles of “One Health” will improve swine zoonoses surveillance, raise stakeholders' awareness on swine-associated zoonoses, help reduce risky behaviors associated with swine production and pork consumption, encourage improved personal hygiene, and demonstrate the need for cost-benefit analyses of swine pathogen control efforts.
Acknowledgments
This study was supported by multiple grants from the US Department of Defense, Armed Forces Health Surveillance Center's Global Emerging Infections Surveillance and Response Program (Dr Gregory C Gray principal investigator) and grants from the National Institute of Allergy and Infectious Diseases (R01 AI068803-Dr Gregory C Gray).
Footnotes
Note: Supplementary information for this article can be found on Emerging Microbes & Infections' website (http://www.nature.com/emi/).
Supplementary Information
References
- Bökönyi S.History of domestic mammals in central and eastern Europe Akadémiai Kiadó; Budapest; 1974. Hungarian. [Google Scholar]
- Food and Agriculture Organization of the United Nations FAOSTAT Database Rome; FAO; 2012. Available at: http://faostat3.fao.org/faostat-gateway/go/to/home/E (accessed 26 November 2013). [Google Scholar]
- Pond WG, Pond H. The biology of the pig. Ithaca; Comstock Publishing Associates; 1978. [Google Scholar]
- Wolfe ND, Dunavan CP, Diamond J. Origins of major human infectious diseases. Nature. 2007;447:279–283. doi: 10.1038/nature05775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschamps JY, Roux FA, Sai P, Gouin E. History of xenotransplantation. Xenotransplantation. 2005;12:91–109. doi: 10.1111/j.1399-3089.2004.00199.x. [DOI] [PubMed] [Google Scholar]
- Morens DM, Taubenberger JK. Historical thoughts on influenza viral ecosystems, or behold a pale horse, dead dogs, failing fowl, and sick swine. Influenza Other Respir Viruses. 2010;4:327–337. doi: 10.1111/j.1750-2659.2010.00148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman J, Karriker L, Ramirez A, Schwartz A, Stevenson G.ed.). Diseases of Swine Ames; Wiley-Blackwell Publishing; 2012 [Google Scholar]
- Krauss H, Webber A, Appel M, et al. ed). Zoonoses: Infectious Diseases Transmissible from Animals to Humans Washington DC; ASM Press; 2003 [Google Scholar]
- Graham JD, Corso PS, Morris JM, Segui-Gomez M, Weinstein MC. Evaluating the cost-effectiveness of clinical and public health measures. Annu Rev Public Health. 1998;19:125–152. doi: 10.1146/annurev.publhealth.19.1.125. [DOI] [PubMed] [Google Scholar]
- Morse SS. Factors in the emergence of infectious diseases. Emerg Infect Dis. 1995;1:7–15. doi: 10.3201/eid0101.950102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordis L.ed). Epidemiology Philadelphia; W.B. Saunders Co; 2008 [Google Scholar]
- Getis A. Geographically weighted regression: The analysis of spatially varying relationships. J Regional Sci. 2003;43:794–796. [Google Scholar]
- Meng XJ, Purcell RH, Halbur PG, et al. A novel virus in swine is closely related to the human hepatitis E virus. Proc Natl Acad Sci USA. 1997;94:9860–9865. doi: 10.1073/pnas.94.18.9860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbur PG, Kasorndorkbua C, Gilbert C, et al. Comparative pathogenesis of infection of pigs with hepatitis E viruses recovered from a pig and a human. J Clin Microbiol. 2001;39:918–923. doi: 10.1128/JCM.39.3.918-923.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng XJ, Halbur PG, Shapiro MS, et al. Genetic and experimental evidence for cross-species infection by swine hepatitis E virus. J Virol. 1998;72:9714–9721. doi: 10.1128/jvi.72.12.9714-9721.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazaki Y, Mizuo H, Takahashi M, et al. Sporadic acute or fulminant hepatitis E in Hokkaido, Japan, may be food-borne, as suggested by the presence of hepatitis E virus in pig liver as food. J Gen Virol. 2003;84:2351–2357. doi: 10.1099/vir.0.19242-0. [DOI] [PubMed] [Google Scholar]
- Meng XJ, Wiseman B, Elvinger F, et al. Prevalence of antibodies to hepatitis E virus in veterinarians working with swine and in normal blood donors in the United States and other countries. J Clin Microbiol. 2002;40:117–122. doi: 10.1128/JCM.40.1.117-122.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouwknegt M, Engel B, Herremans MM, et al. Bayesian estimation of hepatitis E virus seroprevalence for populations with different exposure levels to swine in The Netherlands. Epidemiol Infect. 2008;136:567–576. doi: 10.1017/S0950268807008941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers KP, Olsen CW, Setterquist SF, et al. Are swine workers in the United States at increased risk of infection with zoonotic influenza virus. Clin Infect Dis. 2006;42:14–20. doi: 10.1086/498977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorset M, McBryde CN, WB N. Remarks on “hog flu”. J Am Vet Med Assoc. 1922;62:162–171. [Google Scholar]
- Ma W, Lager KM, Vincent AL, Janke BH, Gramer MR, Richt JA. The Role of Swine in the Generation of Novel Influenza Viruses. Zoonoses Public Health. 2009;56:326–337. doi: 10.1111/j.1863-2378.2008.01217.x. [DOI] [PubMed] [Google Scholar]
- Krueger WS, Gray GC. Swine Influenza Virus Infections in Man. Curr Top Microbiol Immunol. 2013;370:201–225. doi: 10.1007/82_2012_268. [DOI] [PubMed] [Google Scholar]
- Myers KP, Olsen CW, Gray GC. Cases of swine influenza in humans: a review of the literature. Clin Infect Dis. 2007;44:1084–1088. doi: 10.1086/512813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz-Cherry S, Olsen CW, Easterday BC. History of Swine Influenza. Curr Top Microbiol Immunol. 2013;370:21–28. doi: 10.1007/82_2011_197. [DOI] [PubMed] [Google Scholar]
- Shinde V, Bridges CB, Uyeki TM, et al. Triple-reassortant swine influenza A (H1) in humans in the United States, 2005–2009. N Engl J Med. 2009;360:2616–2625. doi: 10.1056/NEJMoa0903812. [DOI] [PubMed] [Google Scholar]
- Nelson MI, Gramer MR, Vincent AL, Holmes EC. Global transmission of influenza viruses from humans to swine. J Gen Virol. 2012;93:2195–2203. doi: 10.1099/vir.0.044974-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GJ, Vijaykrishna D, Bahl J, et al. Origins and evolutionary genomics of the 2009 swine-origin H1N1 influenza A epidemic. Nature. 2009;459:1122–1125. doi: 10.1038/nature08182. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention 2009 H1N1: Overview of a Pandemic Atlanta; CDC; 2010. Available at: http://www.cdc.gov/h1n1flu/yearinreview/yir5.htm (Accessed 12 October 2012). [Google Scholar]
- Pasma T, Joseph T. Pandemic (H1N1) 2009 infection in swine herds, Manitoba, Canada. Emerg Infect Dis. 2010;16:706–708. doi: 10.3201/eid1604.091636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray GC, Bender JB, Bridges CB, et al. Influenza A(H1N1) pdm09 Virus among Healthy Show Pigs, United States. Emerg Infect Dis. 2012;18:1519–1521. doi: 10.3201/eid1809.120431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereda A, Cappuccio J, Quiroga MA, et al. Pandemic (H1N1) 2009 outbreak on pig farm, Argentina. Emerg Infect Dis. 2010;16:304–307. doi: 10.3201/eid1602.091230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh MD, Baird PM, Guelbenzu-Gonzalo MP, et al. Initial incursion of pandemic (H1N1) 2009 influenza A virus into European pigs. Vet Rec. 2010;166:642–645. doi: 10.1136/vr.4851. [DOI] [PubMed] [Google Scholar]
- Jhung MA, Epperson S, Biggerstaff M, et al. Outbreak of Variant Influenza A(H3N2) Virus in the United States. Clin Infect Dis. 2013;57:1703–1712. doi: 10.1093/cid/cit649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price LB, Stegger M, Hasman H, et al. Staphylococcus aureus CC398: host adaptation and emergence of methicillin resistance in livestock MBio 20123pii: e00305–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald JR. Human origin for livestock-associated methicillin-resistant Staphylococcus aureus. MBio. 2012;3:e00082–00012. doi: 10.1128/mBio.00082-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui SH, Li JY, Hu CQ, et al. Isolation and characterization of methicillin-resistant Staphylococcus aureus from swine and workers in China. J Antimicrob Chemother. 2009;64:680–683. doi: 10.1093/jac/dkp275. [DOI] [PubMed] [Google Scholar]
- Roberts MC, Soge OO, No D, Beck NK, Meschke JS. Isolation and characterization of methicillin-resistant Staphylococcus aureus from fire stations in two northwest fire districts. Am J Infect Control. 2011;39:382–389. doi: 10.1016/j.ajic.2010.09.008. [DOI] [PubMed] [Google Scholar]
- Pu S, Han F, Ge B. Isolation and characterization of methicillin-resistant Staphylococcus aureus strains from Louisiana retail meats. Appl Environ Microbiol. 2009;75:265–267. doi: 10.1128/AEM.01110-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmeyer GN, Gahrn-Hansen B, Skov RL, Kolmos HJ. Pig-associated methicillin-resistant Staphylococcus aureus: Family transmission and severe pneumonia in a newborn. Scand J Infect Dis. 2010;42:318–320. doi: 10.3109/00365540903510708. [DOI] [PubMed] [Google Scholar]
- Frana TS, Beahm AR, Hanson BM, et al. Isolation and Characterization of Methicillin-Resistant Staphylococcus aureus from Pork Farms and Visiting Veterinary Students. PLoS One. 2013;8:e53738. doi: 10.1371/journal.pone.0053738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz J, Friese A, Klees S, et al. Longitudinal Study of the Contamination of Air and of Soil Surfaces in the Vicinity of Pig Barns by Livestock-Associated Methicillin-Resistant Staphylococcus aureus. Appl Environ Microbiol. 2012;78:5666–5671. doi: 10.1128/AEM.00550-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weingartl HM, Berhane Y, Czub M. Animal models of henipavirus infection: a review. Vet J. 2009;181:211–220. doi: 10.1016/j.tvjl.2008.10.016. [DOI] [PubMed] [Google Scholar]
- Chua KB. Nipah virus outbreak in Malaysia. J Clin Virol. 2003;26:265–275. doi: 10.1016/s1386-6532(02)00268-8. [DOI] [PubMed] [Google Scholar]
- Luby SP, Hossain MJ, Gurley ES, et al. Recurrent Zoonotic Transmission of Nipah Virus into Humans, Bangladesh, 2001–2007. Emerg Infect Dis. 2009;15:1229–1235. doi: 10.3201/eid1508.081237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention Ebola Hemorrhagic Fever: Known Cases and Outbreaks of Ebola Hemorrhagic Fever, in Chronological Order Atlanta; CDC; 2012. Available at: http://www.cdc.gov/ncidod/dvrd/spb/mnpages/dispages/ebola/ebolatable.htm (accessed 11 July 2012). [Google Scholar]
- World Health Organization Ebola haemorrhagic fever in Zaire, 1976. Bull World Health Organ. 1978;56:271–293. [PMC free article] [PubMed] [Google Scholar]
- Weingartl HM, Embury-Hyatt C, Nfon C, Leung A, Smith G, Kobinger G. Transmission of Ebola virus from pigs to non-human primates. Sci Rep. 2012;2:811. doi: 10.1038/srep00811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control (CDC) Update: filovirus infection in animal handlers. MMWR Morb Mortal Wkly Rep. 1990;39:221. [PubMed] [Google Scholar]
- Miranda ME, White ME, Dayrit MM, Hayes CG, Ksiazek TG, Burans JP. Seroepidemiological study of filovirus related to Ebola in the Philippines. Lancet. 1991;337:425–426. doi: 10.1016/0140-6736(91)91199-5. [DOI] [PubMed] [Google Scholar]
- Barrette RW, Metwally SA, Rowland JM, et al. Discovery of swine as a host for the Reston ebolavirus. Science. 2009;325:204–206. doi: 10.1126/science.1172705. [DOI] [PubMed] [Google Scholar]
- Kobinger GP, Leung A, Neufeld J, et al. Replication, Pathogenicity, Shedding, and Transmission of Zaire ebolavirus in Pigs. J Infect Dis. 2011;204:200–208. doi: 10.1093/infdis/jir077. [DOI] [PubMed] [Google Scholar]
- Chua KB, Goh KJ, Wong KT, et al. Fatal encephalitis due to Nipah virus among pig-farmers in Malaysia. Lancet. 1999;354:1257–1259. doi: 10.1016/S0140-6736(99)04299-3. [DOI] [PubMed] [Google Scholar]
- Chua KB, Bellini WJ, Rota PA, et al. Nipah virus: a recently emergent deadly paramyxovirus. Science. 2000;288:1432–1435. doi: 10.1126/science.288.5470.1432. [DOI] [PubMed] [Google Scholar]
- AbuBakar S, Chang LY, Ali AR, Sharifah SH, Yusoff K, Zamrod Z. Isolation and molecular identification of Nipah virus from pigs. Emerg Infect Dis. 2004;10:2228–2230. doi: 10.3201/eid1012.040452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu VP, Hossain MJ, Parashar UD, et al. Nipah virus encephalitis reemergence, Bangladesh. Emerg Infect Dis. 2004;10:2082–2087. doi: 10.3201/eid1012.040701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan MS, Crameri G, Gurley ES, Hossain MJ, Wang LF, Luby SP.Serological evidence of Nipah like viral infection in pigs in BangladeshASTMH 61st Annual Meeting; 11–15 November 2012; Atlanta, GAUSA. American Society of Tropical Medicine and Hygiene; Deerfield, ILUSA; 2012 [Google Scholar]
- Chant K, Chan R, Smith M, Dwyer DE, Kirkland P. Probable human infection with a newly described virus in the family Paramyxoviridae. The NSW Expert Group. Emerg Infect Dis. 1998;4:273–275. doi: 10.3201/eid0402.980215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philbey AW, Kirkland PD, Ross AD, et al. An apparently new virus (family Paramyxoviridae) infectious for pigs, humans, and fruit bats. Emerg Infect Dis. 1998;4:269–271. doi: 10.3201/eid0402.980214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappas G, Akritidis N, Bosilkovski M, Tsianos E. Brucellosis. N Engl J Med. 2005;352:2325–2336. doi: 10.1056/NEJMra050570. [DOI] [PubMed] [Google Scholar]
- Corbel MJ. Brucellosis: an overview. Emerg Infect Dis. 1997;3:213–221. doi: 10.3201/eid0302.970219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization Joint FAO/WHO expert committee on brucellosis. World Health Organ Tech Rep Ser. 1986;740:1–132. [PubMed] [Google Scholar]
- Meng XJ, Lindsay DS, Sriranganathan N. Wild boars as sources for infectious diseases in livestock and humans. Philos Trans R Soc Lond B Biol Sci. 2009;364:2697–2707. doi: 10.1098/rstb.2009.0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deodato BN, Mortarini M, Garro S, Wallach JC.Retrospective analysis of human brucelosis cases attended in an Infectious Diseases Hospital of Buenos Aires City between years 2008 and 2011Proceedings of the Brucellosis 2011 International Research Conference; September 21–23, 2011; Buenos Aires, Argentina. Argentine Association for Microbiology; Buenos Aires, Argentina; 2011 [Google Scholar]
- Nachamkin I, Szymanski CM, Blaster MJ.ed.). Campylobacter2nd ed. Washington DC; ASM Press; 2008 [Google Scholar]
- Tauxe RV. Emerging foodborne pathogens. Int J Food Microbiol. 2002;78:31–41. doi: 10.1016/s0168-1605(02)00232-5. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention Foodborne Outbreak Online Database (FOOD) Atlanta; CDC; 2013. Available at: http://wwwn.cdc.gov/foodborneoutbreaks/Default.aspx. (Accessed 13 August 2013). [Google Scholar]
- Nielsen EM, Engberg J, Madsen M. Distribution of serotypes of Campylobacter jejuni and C. coli from Danish patients, poultry, cattle and swine. FEMS Immunol Med Microbiol. 1997;19:47–56. doi: 10.1111/j.1574-695X.1997.tb01071.x. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Preliminary FoodNet Data on the Incidence of Infection with Pathogens Transmitted Commonly Through Food - 10 States, 2009. MMWR Morb Mortal Wkly Rep. 2010;59:418–422. [PubMed] [Google Scholar]
- Zhao CW, Ge BL, De Villena J, et al. Prevalence of Campylobacter spp., Escherichia coli, and Salmonella serovars in retail chicken, turkey, pork, and beef from the Greater Washington, DC, area. Appl Environ Microbiol. 2001;67:5431–5436. doi: 10.1128/AEM.67.12.5431-5436.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berends BR, Van Knapen F, Mossel DA, Burt SA, Snijders JM. Impact on human health of Salmonella spp. on pork in The Netherlands and the anticipated effects of some currently proposed control strategies. Int J Food Microbiol. 1998;44:219–229. doi: 10.1016/s0168-1605(98)00121-4. [DOI] [PubMed] [Google Scholar]
- Dreyfuss MS. Is Norovirus a Foodborne or Pandemic Pathogen? An Analysis of the Transmission of Norovirus-Associated Gastroenteritis and the Roles of Food and Food Handlers. Foodborne Pathog Dis. 2009;6:1219–1228. doi: 10.1089/fpd.2009.0320. [DOI] [PubMed] [Google Scholar]
- Schantz P, Chai J, Craig P.Epidemiology and control of hydatid diseaseIn: Thompson RCA, Lymbery A (eds). Echinococcus and Hydatid Disease Oxfordshire; CAB International; 1995223 [Google Scholar]
- Bruzinskaite R, Sarkunas M, Torgerson PR, Mathis A, Deplazes P. Echinococcosis in pigs and intestinal infection with Echinococcus spp. in dogs in southwestern Lithuania. Vet Parasitol. 2009;160:237–241. doi: 10.1016/j.vetpar.2008.11.011. [DOI] [PubMed] [Google Scholar]
- Wang Z, Wang X, Liu X. Echinococcosis in China, a review of the epidemiology of Echinococcus spp. Ecohealth. 2008;5:115–126. doi: 10.1007/s10393-008-0174-0. [DOI] [PubMed] [Google Scholar]
- Christensen SG. Yersinia enterocolitica in Danish pigs. J Appl Bacteriol. 1980;48:377–382. doi: 10.1111/j.1365-2672.1980.tb01025.x. [DOI] [PubMed] [Google Scholar]
- Drummond N, Murphy BP, Ringwood T, Prentice MB, Buckley JF, Fanning S. Yersinia enterocolitica: a brief review of the issues relating to the zoonotic pathogen, public health challenges, and the pork production chain. Foodborne Pathog Dis. 2012;9:179–189. doi: 10.1089/fpd.2011.0938. [DOI] [PubMed] [Google Scholar]
- Falcao JP, Brocchi M, Proenca-Modena JL, Acrani GO, Correa EF, Falcao DP. Virulence characteristics and epidemiology of Yersinia enterocolitica and Yersiniae other than Y. pseudotuberculosis and Y. pestis isolated from water and sewage. J Appl Microbiol. 2004;96:1230–1236. doi: 10.1111/j.1365-2672.2004.02268.x. [DOI] [PubMed] [Google Scholar]
- Fenwick SG, Wauters G, Ursing J, Gwozdz M. Unusual Yersinia enterocolitica strains recovered from domestic animals and people in New Zealand. FEMS Immunol Med Microbiol. 1996;16:241–245. doi: 10.1111/j.1574-695X.1996.tb00142.x. [DOI] [PubMed] [Google Scholar]
- Ellis J, Riemann HP, Cliver DO.eds). Foodborne Infections and Intoxications London; Elsevier Academic Press; 2006 [Google Scholar]
- Ellis J, Oyston PC, Green M, Titball RW. Tularemia. Clin Microbiol Rev. 2002;15:631–646. doi: 10.1128/CMR.15.4.631-646.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merck Veterinary Manual Overview of Tularemia Whitehouse Station; MVM; 2012. Available at: http://www.merckvetmanual.com/mvm/index.jsp?cfile=htm/bc/52400.htm (assessed 3 July 2012). [Google Scholar]
- Al Dahouk S, Nockler K, Tomaso H, et al. Seroprevalence of brucellosis, tularemia, and yersiniosis in wild boars (Sus scrofa) from north-eastern Germany. J Vet Med B Infect Dis Vet Public Health. 2005;52:444–455. doi: 10.1111/j.1439-0450.2005.00898.x. [DOI] [PubMed] [Google Scholar]
- Solomon T. Control of Japanese encephalitis—within our grasp. N Engl J Med. 2006;355:869–871. doi: 10.1056/NEJMp058263. [DOI] [PubMed] [Google Scholar]
- Erlanger TE, Weiss S, Keiser J, Utzinger J, Wiedenmayer K. Past, Present, and Future of Japanese Encephalitis. Emerg Infect Dis. 2009;15:1–7. doi: 10.3201/eid1501.080311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain MJ, Gurley ES, Montgomery S, et al. Hospital-Based Surveillance for Japanese Encephalitis at Four Sites in Bangladesh, 2003–2005. Am J Trop Med Hyg. 2010;82:344–349. doi: 10.4269/ajtmh.2010.09-0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Organisation for Animal Health Manual of diagnostic tests and vaccines for terrestrial animals 2010 - Vesicular Stomatitis Paris; OIE; 2012. Available at: http://web.oie.int/esp/normes/mmanual/2008/pdf/2.01.19_VESICULAR_STOMITIS.pdf (accessed 26 November 2013). [Google Scholar]
- Chai JY, Shin EH, Lee SH, Rim HJ. Foodborne intestinal flukes in Southeast Asia. Korean J Parasitol. 2009;47 Suppl:S69–S102. doi: 10.3347/kjp.2009.47.S.S69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eom KS, Jeon HK, Rim HJ. Geographical distribution of Taenia asiatica and related species. Korean J Parasitol. 2009;47 Suppl:S115–S124. doi: 10.3347/kjp.2009.47.S.S115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaver P, Jung R, Cupp E.eds). Clinical Parasitology. Philadelphia; Lea & Febiger; 1984 [Google Scholar]
- Mas-Coma S, Bargues MD, Valero MA. Fascioliasis and other plant-borne trematode zoonoses. Int J Parasitol. 2005;35:1255–1278. doi: 10.1016/j.ijpara.2005.07.010. [DOI] [PubMed] [Google Scholar]
- International Agency for Research on Cancer Schistosomes, liver flukes and Helicobacter pylori. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Lyon, 7–14 June 1994. IARC Monogr Eval Carcinog Risks Hum. 1994;61:1–241. [PMC free article] [PubMed] [Google Scholar]
- Conlan JV, Sripa B, Attwood S, Newton PN. A review of parasitic zoonoses in a changing Southeast Asia. Vet Parasitol. 2011;182:22–40. doi: 10.1016/j.vetpar.2011.07.013. [DOI] [PubMed] [Google Scholar]
- Zou FC, Dong GD, Yang JF, et al. Prevalences of Schistosoma japonicum infection in reservoir hosts in south-western China. Ann Trop Med Parasitol. 2010;104:181–185. doi: 10.1179/136485910X12607012374118. [DOI] [PubMed] [Google Scholar]
- Chang CP, New AE, Taylor JF, Chiang HS. Influenza virus isolations from dogs during a human epidemic in Taiwan. Int J Zoonoses. 1976;3:61–64. [PubMed] [Google Scholar]
- Osterhaus AD, Rimmelzwaan GF, Martina BE, Bestebroer TM, Fouchier RA. Influenza B virus in seals. Science. 288:1051–1053. doi: 10.1126/science.288.5468.1051. [DOI] [PubMed] [Google Scholar]
- Kimura H, Abiko C, Peng G, et al. Interspecies transmission of influenza C virus between humans and pigs. Virus Res. 1997;48:71–79. doi: 10.1016/s0168-1702(96)01427-x. [DOI] [PubMed] [Google Scholar]
- Merck Veterinary Manual Swine Influenza Whitehouse Station; MVM; 2012. Available at: http://www.merckmanuals.com/vet/respiratory_system/respiratory_diseases_of_pigs/swine_influenza.html?qt=influenza&alt=sh (accessed 26 November 2013). [Google Scholar]
- Neumann EJ, Ramirez A, Schwartz KJ.eds). Swine disease manual Perry; American Association of Swine Veterians; 2012 [Google Scholar]
- Beran GW. Handbook of Zoonoses, Second Edition, Section a: Bacterial, Rickettsial, Chlamydial, and Mycotic Zoonoses. Boca Raton; CRC Press; 1994. [Google Scholar]
- World Health Organization Immunization surveillance, assessment and monitoring- Tetanus Geneva; WHO; 2012. Available at: http://www.who.int/immunization_monitoring/diseases/tetanus/en/index.html (accessed 15 December 2012). [Google Scholar]
- Chermette R, Ferreiro L, Guillot J. Dermatophytoses in animals. Mycopathologia. 2008;166:385–405. doi: 10.1007/s11046-008-9102-7. [DOI] [PubMed] [Google Scholar]
- Aly R. Ecology and epidemiology of dermatophyte infections. J Am Acad Dermatol. 1994;31:S21–S25. doi: 10.1016/s0190-9622(08)81262-5. [DOI] [PubMed] [Google Scholar]
- Esteban JG, Aguirre C, Angles R, Ash LR, Mas-Coma S. Balantidiasis in Aymara children from the northern Bolivian Altiplano. Am J Trop Med Hyg. 1998;59:922–927. doi: 10.4269/ajtmh.1998.59.922. [DOI] [PubMed] [Google Scholar]
- Zaman V.Balantidium coliIn: Kreier JP, Baker JR (eds.)Parasitic Protozoa Salt Lake City; Academic Press; 199343–46. [Google Scholar]
- Ejlertsen T, Gahrn-Hansen B, Sogaard P, Heltberg O, Frederiksen W. Pasteurella aerogenes isolated from ulcers or wounds in humans with occupational exposure to pigs: a report of 7 Danish cases. Scand J Infect Dis. 1996;28:567–570. doi: 10.3109/00365549609037962. [DOI] [PubMed] [Google Scholar]
- McAllister HA, Carter GR. An aerogenic Pasteurella-like organism recovered from swine. Am J Vet Res. 1974;35:917–922. [PubMed] [Google Scholar]
- Hommez J, Devriese LA. Pasteurella aerogenes isolations from swine. Zentralbl Veterinarmed B. 1976;23:265–268. doi: 10.1111/j.1439-0450.1976.tb00681.x. [DOI] [PubMed] [Google Scholar]
- Parashar UD, Gibson CJ, Bresee JS, Glass RI. Rotavirus and severe childhood diarrhea. Emerg Infect Dis. 2006;12:304–306. doi: 10.3201/eid1202.050006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desselberger UM, Iturriza-Gomara M, Gray JJ.Rotavirus epidemiology and surveillance Novartis Found Symp 2001238125–147.discussion; 147–152. [DOI] [PubMed] [Google Scholar]
- Campbell GL, Marfin AA, Lanciotti RS, Gubler DJ. West Nile virus. Lancet Infect Dis. 2002;2:519–529. doi: 10.1016/s1473-3099(02)00368-7. [DOI] [PubMed] [Google Scholar]
- Mostashari F, Bunning ML, Kitsutani PT, et al. Epidemic West Nile encephalitis, New York, 1999: results of a household-based seroepidemiological survey. Lancet. 2001;358:261–264. doi: 10.1016/S0140-6736(01)05480-0. [DOI] [PubMed] [Google Scholar]
- Craven RB, Roehrig JT. West Nile virus. JAMA. 2001;286:651–653. doi: 10.1001/jama.286.6.651. [DOI] [PubMed] [Google Scholar]
- Murray KO, Walker C, Gould E. The virology, epidemiology, and clinical impact of West Nile virus: a decade of advancements in research since its introduction into the Western Hemisphere. Epidemiol Infect. 2011;139:807–817. doi: 10.1017/S0950268811000185. [DOI] [PubMed] [Google Scholar]
- Skinner GRB, Ahmad A, Davies JA. The infrequency of transmission of herpesviruses between humans and animals; postulation of an unrecognised protective host mechanism. Comp Immunol Microbiol Infect Dis. 2001;24:255–269. doi: 10.1016/s0147-9571(01)00014-5. [DOI] [PubMed] [Google Scholar]
- Mravak S, Bienzle U, Feldmeier H, Hampl H, Habermehl KO. Pseudorabies in man. Lancet. 1987;1:501–502. doi: 10.1016/s0140-6736(87)92105-2. [DOI] [PubMed] [Google Scholar]
- Farkas T, Nakajima S, Sugieda M, Deng X, Zhong W, Jiang X. Seroprevalence of noroviruses in swine. J Clin Microbiol. 2005;43:657–661. doi: 10.1128/JCM.43.2.657-661.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopmans M. Progress in understanding norovirus epidemiology. Curr Opin Infect Dis. 2008;21:544–552. doi: 10.1097/QCO.0b013e3283108965. [DOI] [PubMed] [Google Scholar]
- Field H, Young P, Yob JM, Mills J, Hall L, Mackenzie J. The natural history of Hendra and Nipah viruses. Microbes Infect. 2001;3:307–314. doi: 10.1016/s1286-4579(01)01384-3. [DOI] [PubMed] [Google Scholar]
- Li MY, Embury-Hyatt C, Weingartl HM. Experimental inoculation study indicates swine as a potential host for Hendra virus. Vet Res. 2010;41:33. doi: 10.1051/vetres/2010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayman DT, Wang LF, Barr J, et al. Antibodies to henipavirus or henipa-like viruses in domestic pigs in Ghana, West Africa. PLoS One. 2011;6:e25256. doi: 10.1371/journal.pone.0025256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ksiazek TG, Rota PA, Rollin PE. A review of Nipah and Hendra viruses with an historical aside. Virus Res. 2011;162:173–183. doi: 10.1016/j.virusres.2011.09.026. [DOI] [PubMed] [Google Scholar]
- Chua KB. Epidemiology, surveillance and control of Nipah virus infections in Malaysia. Malays J Pathol. 2010;32:69–73. [PubMed] [Google Scholar]
- Brown J, Matthews AL, Sandstrom PA, Chapman LE. Xenotransplantation and the risk of retroviral zoonosis. Trends Microbiol. 1998;6:411–415. doi: 10.1016/s0966-842x(98)01347-x. [DOI] [PubMed] [Google Scholar]
- Weiss RA, Wrangham RW. From Pan to pandemic. Nature. 1999;397:385–386. doi: 10.1038/17008. [DOI] [PubMed] [Google Scholar]
- Scobie L, Takeuchi Y. Porcine endogenous retrovirus and other viruses in xenotransplantation. Curr Opin Organ Transplant. 2009;14:175–179. doi: 10.1097/mot.0b013e328327984d. [DOI] [PubMed] [Google Scholar]
- Mueller NJ, Sulling K, Gollackner B, et al. Reduced efficacy of ganciclovir against porcine and baboon cytomegalovirus in pig-to-baboon xenotransplantation. Am J Transplant. 2003;3:1057–1064. doi: 10.1034/j.1600-6143.2003.00192.x. [DOI] [PubMed] [Google Scholar]
- Banks M, Bendall R, Grierson S, Heath G, Mitchell J, Dalton H. Human and porcine hepatitis E virus strains, United Kingdom. Emerg Infect Dis. 2004;10:953–955. doi: 10.3201/eid1005.030908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgen K, Herremans T, Duizer E, et al. Non-travel related Hepatitis E virus genotype 3 infections in the Netherlands; a case series 2004–2006. BMC Infect Dis. 2008;8:61. doi: 10.1186/1471-2334-8-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerolami R, Moal V, Colson P. Chronic hepatitis E with cirrhosis in a kidney-transplant recipient. N Engl J Med. 2008;358:859–860. doi: 10.1056/NEJMc0708687. [DOI] [PubMed] [Google Scholar]
- Kamar N, Mansuy JM, Cointault O, et al. Hepatitis E virus-related cirrhosis in kidney- and kidney-pancreas-transplant recipients. Am J Transplant. 2008;8:1744–1748. doi: 10.1111/j.1600-6143.2008.02286.x. [DOI] [PubMed] [Google Scholar]
- Fischer SA, Graham MB, Kuehnert MJ, et al. Transmission of lymphocytic choriomeningitis virus by organ transplantation. N Engl J Med. 2006;354:2235–2249. doi: 10.1056/NEJMoa053240. [DOI] [PubMed] [Google Scholar]
- Thom K, Petrik J. Progression towards AIDS leads to increased Torque teno virus and Torque teno minivirus titers in tissues of HIV infected individuals. J Med Virol. 2007;79:1–7. doi: 10.1002/jmv.20756. [DOI] [PubMed] [Google Scholar]
- Bruschke CJ, Pittman M, Laddomada A. International regulations and standards for avian influenza, including the vaccine standards of the World Organisation for Animal Health. Rev Sci Tech. 2009;28:379–389. doi: 10.20506/rst.28.1.1852. [DOI] [PubMed] [Google Scholar]
- Audi N.Culling Pigs in Flu Fight, Egypt Angers Herders and Dismays U.N New York; The New York Times; 2009. Available at: http://www.nytimes.com/2009/05/01/health/01egypt.html?_r=0 (accessed April 30, 2009). [Google Scholar]
- Abdelwhab EM, Hafez HM. An overview of the epidemic of highly pathogenic H5N1 avian influenza virus in Egypt: epidemiology and control challenges. Epidemiol Infect. 2011;139:647–657. doi: 10.1017/S0950268810003122. [DOI] [PubMed] [Google Scholar]
- Center for Food Security and Public Health Animal Disease Information - Nipah Virus Ames; ISU; 2011. Available at: http://www.cfsph.iastate.edu/DiseaseInfo/notes/Nipah.pdf (accessed 22 January 2013). [Google Scholar]
- United State House Committee on Agriculture Hearing to review the economic conditions facing the pork industry: hearing before the subcommittee on livestock, dairy, and poultry of the committee on Agriculture, House of Representatives, one hundred eleventh congress, first session, October 22, 2009 Washington DC; US House Committee on Agriculture; 2009. Available at: http://www.gpo.gov/fdsys/pkg/CHRG-111hhrg54577/html/CHRG-111hhrg54577.htm (accessed on 26 November 2013). [Google Scholar]
- World Organization for Animal Health The OIE advocates implementing international standards for humane killing of animals for disease prevention purposes Paris; OIE; 2012. Available at: http://www.oie.int/en/for-the-media/press-releases/detail/article/the-oie-advocates-implementing-international-standards-for-humane-killing-of-animals-for-disease-pre/ (accessed11 July 2012). [Google Scholar]
- Alton G.ed). Brucella suis Boca Raton; CRC Press; 1990 [Google Scholar]
- Taylor LH, Latham SM, Woolhouse ME. Risk factors for human disease emergence. Philos Trans R Soc Lond B Biol Sci. 2001;356:983–989. doi: 10.1098/rstb.2001.0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Editorial team Ebola Reston Virus Detected Pigs in the Philippines Euro surveill 200914Pii : 19105. [PubMed] [Google Scholar]
- American Veterinary Medical Association One Health: A New Professional Imperative Schaumburg; AVMA; 2008. Available at: https://www.avma.org/KB/Resources/Reports/Documents/onehealth_final.pdf (accessed 21 January 2013). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.