Abstract
Significance: Reactive oxygen species (ROS) are signaling molecules that are important in physiological processes, including host defense, aging, and cellular homeostasis. Increased ROS bioavailability and altered redox signaling (oxidative stress) have been implicated in the onset and/or progression of chronic diseases, including hypertension. Recent Advances: Although oxidative stress may not be the only cause of hypertension, it amplifies blood pressure elevation in the presence of other pro-hypertensive factors, such as salt loading, activation of the renin-angiotensin-aldosterone system, and sympathetic hyperactivity, at least in experimental models. A major source for ROS in the cardiovascular-renal system is a family of nicotinamide adenine dinucleotide phosphate oxidases (Noxs), including the prototypic Nox2-based Nox, and Nox family members: Nox1, Nox4, and Nox5. Critical Issues: Although extensive experimental data support a role for increased ROS levels and altered redox signaling in the pathogenesis of hypertension, the role in clinical hypertension is unclear, as a direct causative role of ROS in blood pressure elevation has yet to be demonstrated in humans. Nevertheless, what is becoming increasingly evident is that abnormal ROS regulation and aberrant signaling through redox-sensitive pathways are important in the pathophysiological processes which is associated with vascular injury and target-organ damage in hypertension. Future Directions: There is a paucity of clinical information related to the mechanisms of oxidative stress and blood pressure elevation, and a few assays accurately measure ROS directly in patients. Such further ROS research is needed in humans and in the development of adequately validated analytical methods to accurately assess oxidative stress in the clinic. Antioxid. Redox Signal. 20, 164–182.
Introduction
Hypertension is a multifactorial, complex disorder, involving many organ systems (41, 215). Factors that are important in the development of hypertension include activation of the sympathetic nervous system, up-regulation of the renin-angiotensin-aldosterone system, altered G protein-coupled receptor signaling, and inflammation (81, 214). Recent studies also implicate a role of the immune system in hypertension (82, 175). Common to these processes is oxidative stress (excess levels of oxidants over antioxidants), due, mainly, to increased production of reactive oxygen species (ROS), decreased nitric oxide (NO) levels, and reduced antioxidant capacity in the cardiovascular, renal, and central nervous systems (Fig. 1) (208, 220).
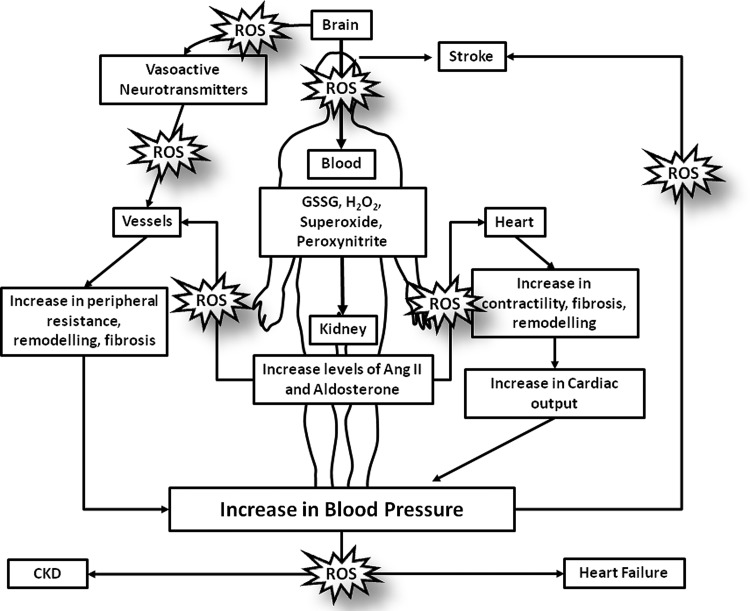
FIG. 1.
Systemic oxidative stress in humans: implications in hypertension. Pathophysiological levels of reactive oxygen species (ROS) will lead to a series of deleterious effects in the human body. In the brain, ROS increase the production and release of vasoactive neurotransmitters, which, in turn, will increase or decrease vascular resistance, an effect that influences blood pressure. In the kidney, ROS increases the production and the release of many vasoactive agents, including angiotensin II (Ang II) and aldosterone, which, in turn, influence cardiac, vascular, and renal function through redox-sensitive processes. ROS influence processes that are involved in vascular remodeling, endothelial dysfunction, fibrosis, and inflammation, which are characteristic features in hypertension. Moreover, in the heart, ROS have been linked to increased contractility, fibrosis and cardiac remodeling; reflecting in an increase in cardiac output and blood pressure. All of these ROS-related effects impact the development of hypertension and its target organ damage, predisposing to stroke, heart failure, and chronic kidney disease (CKD).
ROS, including superoxide (O2•−) and hydrogen peroxide (H2O2), were originally considered harmful metabolic byproducts of mitochondrial energetics and cell metabolism. However, ROS are now recognized to have important physiological functions through their modulation of the redox state of signaling molecules (52). ROS influence many signaling pathways, including mitogen-activated protein kinases (MAPK), tyrosine kinases, Rho kinase, transcription factors (NFκB, AP-1, and HIF-1), and protein tyrosine phosphatases that impact cardiovascular, renal, and neural cell function (26, 134, 158, 161, 189). ROS increase intracellular free Ca2+ concentration ([Ca2+]i) through ion channel activation and up-regulate protooncogene and proinflammatory gene expression and activity (40, 199). Uncontrolled generation of ROS promotes oxidative stress amd consequent damage to DNA, proteins, and lipids, leading to cell injury and cytotoxicity (96, 115, 118). Physiologically, ROS regulate cellular processes such as differentiation, proliferation, apoptosis, cell cycle, migration, secretion, cytoskeletal organization, activation of transcription factors, and gene expression (71, 98, 212). In the vascular system, ROS play a physiological role in controlling endothelial function and vascular tone and a pathophysiological role in processes underlying endothelial dysfunction, hyperreactivity, inflammation, and vascular remodeling in cardiovascular diseases, including hypertension (4, 19, 200, 218).
ROS are usually produced from cellular respiration and metabolic processes as byproducts via activation of enzymes such as xanthine oxidoreductase, uncoupled NO synthase (NOS), and mitochondrial respiratory enzymes (2, 65, 150, 186) (Fig. 2). In addition, ROS are produced by nicotinamide adenine dinucleotide phosphate (NADPH) oxidases (Nox). Noxs, of which there are seven isoforms, and that function primarily as ROS-generating enzymes, are an important source of O2•− and H2O2 in the cardiovascular system (209). When dysregulated Noxs play a role in increased ROS production, it leads to endothelial dysfunction and vascular remodeling in hypertension.
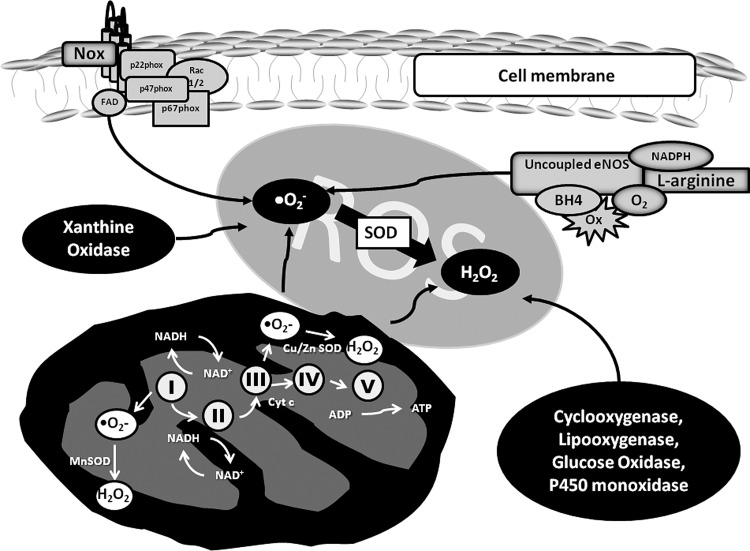
FIG. 2.
Sources of ROS in cells. ROS production is regulated by many tightly controlled systems. ROS are produced either as a byproduct of an enzyme or as the main product of nicotinamide adenine dinucleotide phosphate oxidase (Nox) activity. Except for Nox, which has as its main function the formation of ROS, the other enzymes generate ROS as byproducts of enzymatic activity. Uncoupled endothelial nitric oxide synthase (eNOS): In the absence of L-arginine or tetrahydrobiopterin, the electrons in the enzyme reduce molecular oxygen to superoxide, instead of NO. Damaged or dysfunctional mitochondria generate increased amounts of superoxide, creating a state of redox imbalance and consequent oxidative stress. Nox: The Nox family comprises seven isoforms, where the generation of ROS is the main and only function. For these enzymes to be biologically active, they require a number of subunits to assemble at the membrane in order to form ROS. Other enzymes that generate ROS are xanthine oxidase, cyclooxygenase, lipoxygenase, glucose oxidase, and P450 mono-oxidases.
The relationship between ROS and blood pressure was first suggested in the 1960s (170), but it was in the early 2000s that this association was explored in detail when it was shown that angiotensin II (Ang II)-induced hypertension in rats increases vascular ROS production via non-phagocytic NADPH oxidase activation (162). Most experimental models of hypertension exhibit some degree of oxidative stress (48, 67, 93, 95, 236). Moreover, mice with reduced antioxidant enzyme systems and those deficient in NADPH oxidase have higher blood pressures than those with intact systems. Based on extensive experimental data, it has been suggested that oxidative stress is causally associated with hypertension, at least in animal models.
In clinical medicine, the direct relationship between ROS and hypertension is not convincing, and there is still no definitive proof that oxidative stress is a direct cause of hypertension in humans. In fact, despite extensive data in the literature implicating a role for ROS and oxidative stress in many chronic diseases, such as cardiovascular disease, diabetes, cancer, and kidney disease, there are very few clinical conditions that are directly due to altered ROS levels. These include vitiligo, neurodegenerative diseases, and progeria (67, 99, 117, 145, 172, 216). With regard to clinical hypertension, most studies examining ROS are based on associations between plasma or urine markers of oxidative stress and blood pressure. Biomarkers of cell damage due to systemic oxidative stress, such as plasma thiobarbituric acid-reactive substances (TBARS) and 8-epi-isoprostanes, are elevated in patients with hypertension (45, 116). Antioxidant capacity and levels of antioxidant vitamins and enzymes have been shown to be reduced in patients with hypertension (66, 107). Such studies between hypertension and oxidative stress are purely correlative and are far from proving cause.
Hence, despite the notion that oxidative stress underlies hypertension, there is still little solid evidence for this at the clinical level. Possible reasons relate to a paucity of information on molecular mechanisms of ROS biology in human tissue, lack of adequate methods to evaluate ROS in the clinical setting, and inappropriately designed clinical trials to evaluate the effects of antioxidant therapy on hypertension. It is also possible that, although oxidative stress may be important in pathophysiological mechanisms that are associated with cardiac, vascular, renal, and neural dysfunction and remodeling, which could influence blood pressure (Fig. 1), it may not be an important primary causative factor in the pathogenesis of hypertension in humans. These themes will be developed and discussed in the present review. While it is appreciated that ROS have an impact on many systems that influence blood pressure regulation and development of hypertension, here, we will focus on the role of vascular ROS in hypertension, highlighting translational research and clinical studies.
Production of ROS in the Vascular System: Spotlight on Noxs
Noxs are transmembrane-associated proteins that transfer electrons across membranes, such that the final electron acceptor is O2 and O2•− is generated (113). The mammalian Nox family comprises seven isoforms: Nox1, Nox2, Nox3, Nox4, Nox5, Duox1, and Duox2 (16, 113, 182). Nox1, 2, 4, and 5 are expressed in multiple tissues; whereas Nox3 and the Duoxs are more tissue specific, with Nox3 expressed in the inner ear and Duox1/2 in the thyroid gland. All Noxs are transmembrane proteins that have a core catalytic subunit (Nox) and a number of regulatory subunits (Fig. 3).
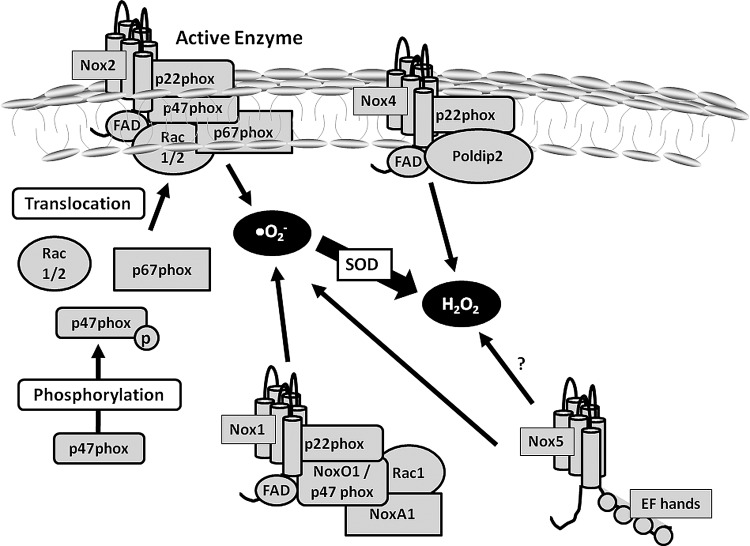
FIG. 3.
Nox activation in the cardiovascular system. Nox1, Nox2, Nox4, and Nox5 are members of the Nox family that are expressed in the cardiovascular system. Nox2 is the classical Nox that is primarily characterized in leukocytes and related to host defence responses. In order to be activated, cytosolic subunits (p47phox, p67phox, and Rac 1/2) should translocate from the cytosol to the membrane and bind to the other two membrane-bound subunits, Nox2 and p22phox. ROS is generated once the whole enzyme complex is formed. Nox1 activation is similar to Nox2, but it depends on the cytosolic subunits NoxO1 (a homologue of p47phox) and NoxA1 (a homologue of p67phox) in order to be active. Nox4 only depends on p22phox in order to be active, is constitutively activated, and ROS production is regulated by Poldip2. Nox5 does not depend on any other subunits to be activated. It has four EF hands, which are binding sites for calcium. Nox5 activity is regulated not only by calcium changes in the cells, but also by calmodulin and by phosphorylation of kinases.
The prototypical Nox is Nox2 (phagocytic NADPH oxidase) and comprises five subunits: p47phox, p67phox, p40phox, which are cytosolic regulatory proteins, p22phox, a membrane regulatory protein, and gp91phox, which is the catalytic subunit (12). Nox2 is activated when the regulatory subunit p47phox is phosphorylated, complexes with p67phox and p40phox, and translocates to the cell membrane to interact with p22phox and Nox2 to assemble the active oxidase, which then transfers electrons from the substrate (NADPH) to O2 forming O2•− (119).
Nox1, Nox2, Nox4, and Nox5 have been identified in the vascular system (24, 111). Hyperactivation of Noxs leads to excessive ROS production that disrupts redox networks, which is usually regulated by thiol-dependent antioxidant systems. This leads to oxidative stress, triggering molecular processes, which, in the vasculature, contributes to vascular injury and inflammation. The Noxs have been extensively reviewed (7, 16, 53, 82, 113, 160), and only an overview of recent developments is discussed here, focusing on the most recently characterized isoform, Nox5.
Nox1
Nox1 is abundant in colon epithelium, but is also found in endothelium, smooth muscle, fibroblasts, cardiomyocytes, and microglia (151). It requires p22phox, p47phox (or its homologue NoxO1 [Nox organizer 1]), and p67phox (or its homologue NoxA1 [Nox activator 1]) for its activity. Nox1-derived O2•− is increased in a stimulus-dependent manner, involving complex interactions between regulatory subunits and the redox chaperone protein disulfide isomerase (54, 58).
In cultured endothelial and vascular smooth muscle cells (VSMCs), Nox1 is up-regulated by mechanical factors (shear stress), vasoactive agents (Ang II, aldosterone), and growth factors (epidermal growth factor, platelet-derived growth factor [PDGF]) (128, 159). Induction of Nox1 by Ang II may involve mitochondria, possibly through a Ca2+-dependent mechanism (163). Nox1 is also activated by H2O2 in VSMCs. Exogenous H2O2, which may enter cells via aquaporins, increased Nox1-derived O2•− generation, leading to hypertrophy, a process that is mediated via Ask1 (3).
Nox1 plays a role in VSMC migration, proliferation, and extracellular matrix production, effects that are mediated by cofilin (219). It has also been implicated in blood flow regulation through a mechanism involving thrombospondin-1 (TSP1) and CD47 (46). Blockade of CD47 and Nox1 gene silencing in vivo in rats improved TSP1-induced impairment of tissue blood flow after ischemia reperfusion. These novel data suggest a highly regulated process of ROS stimulation and blood flow regulation promoted through direct TSP1/CD47-mediated activation of Nox1 and define a regulatory role for TSP1 via CD47, Nox1, and ROS in tissue injury and reperfusion.
Nox1 expression/activity is increased in the vasculature in models of cardiovascular disease, including hypertension, atherosclerosis, diabetes, and hypercholesterolemia (50, 190). Nox1-deficient mice have decreased expression of aortic AT1R (130), which may contribute to blunted pressor actions of Ang II infusion in these mice. Although there is extensive experimental data suggesting a role for Nox1 in cardiovascular disease, there is little information in humans, although expression of Nox1 and NoxA1 is increased in human atherosclerotic vessels (152).
Nox2
Nox2 is not only the catalytic subunit of the respiratory burst Nox in phagocytes, but it is also expressed in vascular cells (210), where it localizes in the cell membrane, as well as with the cytoskeleton, lipid rafts/caveolae, and the perinuclear compartment. The Nox2 gene is inducible and is highly regulated by Ang II and stretch (51, 149). Vascular Nox2, derived from resident macrophages or vascular cells, is up-regulated in experimental hypertension, atherosclerosis, ischemia-reperfusion injury, and neointimal formation (74, 211). Nox2 is also implicated in stroke in experimental models (34).
Nox4
Nox4 is found in vascular cells, fibroblasts, adipocytes, hepatocytes, and renal cells (64, 84, 180). In VSMCs, Nox4 co-localizes with p22phox and vinculin in focal adhesions and plays a role in cell migration, proliferation, and cell differentiation (35). Nox4 has been identified in the endoplasmic reticulum, mitochondria, and nucleus, and it is constitutively active and regulated mainly by its level of expression (8). Nox4 appears to generate H2O2, although the primary product is probably O2•−, which is rapidly dismutated to H2O2 (202, 232). Nox4 contributes to basal ROS production through its constitutive activity and to increased ROS generation when induced by Ang II, glucose, tumor necrosis factor α, and growth factors (90, 127). Recent studies have identified a 28-kDa Splice Variant of Nox4 located in the nucleus of vascular cells, which may be important in pathophysiologic effects through modulation of nuclear signaling and DNA damage (9). The pathological role of Nox4 is unclear, although it has been implicated in hypertension, atherosclerosis, and cardiovascular and renal complications of diabetes and in remodeling of pulmonary arteries in pulmonary hypertension (181, 206). Nox4-derivd ROS has also been suggested in cellular senescence and aging (104) and in insulin-mediated differentiation of adipocytes (177). Recent studies demonstrated that Nox4 may actually have protective effects, possibly through Nox4-derived H2O2, which may act as a vasodilator in some vascular beds (164, 238). This could explain why mice with targeted endothelial Nox4 overexpression have lower blood pressure and improved endothelium-dependent vasodilation versus wild-type controls (164).
Nox5
Nox5 is the most recently identified Nox and is unique: It is Ca2+ sensitive, possesses a calmodulin-like domain with Ca2+ binding sites, and does not require any Nox subunits for its activity (62, 92, 138, 185). Nox5 was first identified in testes and spleen and, more recently, in vascular cells. 5 splice variants have been identified: α, β, δ, γ, and ɛ (157). While the functional roles for each of these variants have yet to be discovered, all share a number of features common to all Noxs, including six transmembrane spanning domains, two groups of heme-spanning histidines, an NADPH-binding motif, and an FAD-binding domain. Vascular Nox5 is activated by PDGF, Ang II, and ET-1 and it involves ERK1/2, PI(4, 5)P2, PKC, and c-Abl (17, 137, 155). Hsp90 binds to and regulates Nox5 protein stability (56, 126, 33). In human endothelial cells (ECs), siRNA-mediated Nox5 knockdown reduced Ang II stimulated ERK1/2 activation, but not that of p38 MAPK or JNK (56). Nox5 generates ROS in response to increases in [Ca2+]i. Agonists signaling through increased [Ca2+]i (e.g., Ang II, ET-1) stimulate Nox5 via its Ca2+ binding hands (13, 33, 138, 156). Binding of calmodulin also enhances Nox5 Ca2+ affinity, while Ca2+/calmodulin-dependent kinase II phosphorylates Nox5β on Ser475 to increase ROS generation (156). The ability of Nox5 to respond to Ca2+-sensitive signaling may be especially important in vascular cells, where Ca2+ is critically involved in vascular function (contraction/dilation, growth). Of the many Ca2+ channels important in regulating vascular [Ca2+]i, transient receptor potential melastatin cation channel 2 (TRPM2) is interesting in the context of oxidative stress, as TRPM2 is highly redox sensitive (135, 203).
The significance of this novel isoform in the cardiovascular system is unknown, and to our knowledge, nothing is known about vascular Nox5 and hypertension. Since the Nox5 gene is present in humans but absent in rodents, a study of this isoform is challenging in the experimental setting.
Antioxidant Defense Mechanisms
In biological systems, the natural defense against ROS comprises enzymatic and nonenzymatic systems. Nuclear factor erythroid 2-related factor 2 (Nrf2), a transcriptional factor, is the master regulator of antioxidant genes and hence of antioxidant status (44). It may be possible, although still not proved, that Nrf2 is down-regulated in hypertension, which could contribute to decreased antioxidant status and consequent oxidative stress. Nrf-2 expression and activity are impaired in conditions associated with hypertension, such as kidney disease and diabetes (101), but the role in hypertension per se is unknown.
Major enzymatic antioxidants, which are regulated, in part by Nrf-2, include manganese superoxide dismutase (MnSOD), catalase, glutathione peroxidases, thioredoxin, and peroxiredoxin (31, 69, 89). SOD, of which there are three isoforms, and catalyze the dismutation of O2•− into H2O2 and O2. Of the three SOD isoforms eSOD is the main vascular SOD (69). Non-enzymatic antioxidants, which act as ROS scavengers, include vitamins A, C, and E; glutathione, billirubin, and uric acid.
Low antioxidant bioavailability promotes cellular oxidative stress and has been implicated in cardiovascular and renal oxidative damage that is associated with hypertension. Activity of SOD, catalase, and GSH peroxidase is lower, and the GSSG/GSH is higher in plasma and circulating cells from hypertensive patients than normotensive subjects (174). Moreover, a large population-based cross-sectional study conducted in more than 20,000 adults participating in the European Prospective Investigation Into Cancer-Norfolk demonstrated that individuals with high plasma vitamin C levels had lower blood pressure than those with low plasma vitamin C levels (144).
In mice deficient in EC-SOD and in rats in which GSH synthesis is inhibited, blood pressure is elevated, indicating that reduced antioxidant capacity is associated with elevated blood pressure (42). In angiotensinogen-overexpresing mice, which are hypertensive, catalase overexpression prevented blood pressure elevation and protected against kidney damage (68). In human studies, plasma vitamin C levels are inversely related to blood pressure levels, indicating a potential blood pressure-lowering effect of this antioxidant (94).
Oxidative Stress and Experimental Hypertension
There is now extensive experimental data showing that ROS play a role in the development of hypertension, with many models of hypertension exhibiting oxidative stress, including genetic forms (SHR, SHRSP), surgically induced (2K1C, aortic banding), hormone-induced (Ang II, ET-1, aldosterone, and DOCA), and diet-induced hypertension (salt, fat) (32, 87, 179). Oxidative stress and inflammation are common features in cardiac, vascular, renal, and retinal damage in hypertension, and especially in the context of diabetes (32) (Fig. 4). Mice deficient in ROS-generating enzymes (e.g., Nox2−/−, p47phox−/−, and Nox1−/−) have lower blood pressure compared with wild-type counterparts, and Ang II infusion fails to induce hypertension in these mice (15, 109). The exact mechanisms by which ROS influence blood pressure remain unclear, but many systems are involved, including the brain, heart, kidneys, and vessels. In addition, recent evidence indicates that the immune system may be involved. Oxidative stress precedes the development of hypertension in experimental models, and it may play a role in fetal programming and the development of hypertension in adult life (153). This may be related to alterations in antioxidant status, as impaired renal catalase and glutathione peroxide mRNA expression and activity were found to precede the development of hypertension in SHR (198). Markers of oxidative stress, such as TBARS, and F2α-isoprostanes, tissue concentrations of O2•− and H2O2, and activation of Nox and xanthine oxidase are increased; whereas levels of NO and antioxidant enzymes are reduced in experimental hypertension (61, 222). Moreover cross-talk between mitochondria and Noxs may amplify ROS generation (Fig. 5).
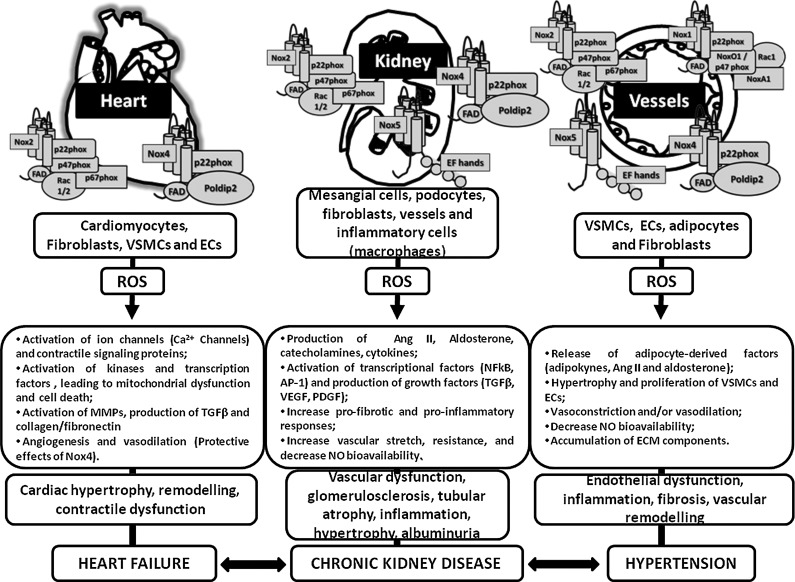
FIG. 4.
Nox distribution in the heart, kidney, and vessels. Nox homologues are differentially expressed in tissues from the cardiovascular-renal systems. As illustrated in the figure, Noxs not only play important roles in pathological conditions, but also regulate a series of responses that are important to the physiology of each cell type or tissue. An increase in ROS generation and a dysregulation of Nox expression/activity, followed by a decrease in ROS degradation, leads to an increase in ROS-induced injurious actions and oxidative damage. MMP, matrix metalloprotease; VSMC, vascular smooth muscle cells; EC, endothelial cells; ECM, extracellular matrix.
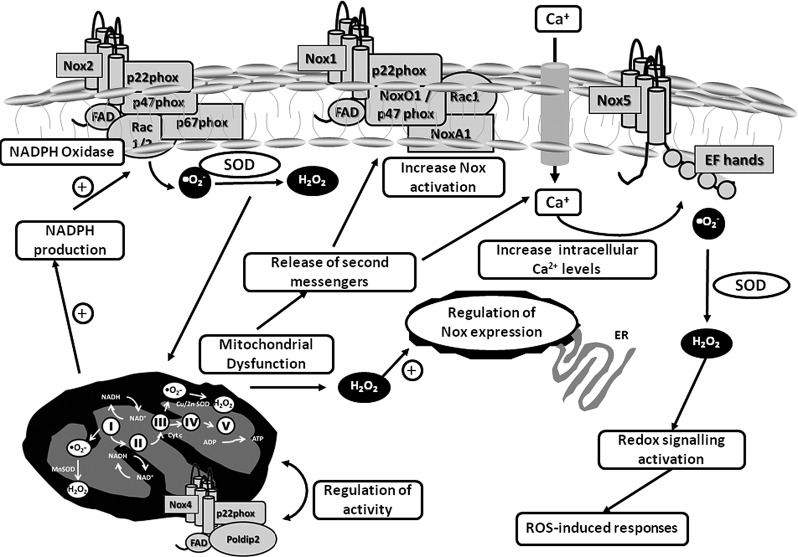
FIG. 5.
Interactions between Nox and mitochondria. Noxs can regulate mitochondrial function and vice versa. Through production of ROS, Noxs are able to induce mitochondrial dysfunction, leading to an increase in the production of ROS by the mitochondria. Either by ROS generation or by release of second messengers, the mitochondria will increase the expression and activation of Noxs, further increasing oxidative stress, activation of redox signaling, and ROS-induced effects. It has also been reported that Noxs and mitochondria colocalize and may regulate their respective activities.
Of the many models of hypertension in which oxidative stress has been shown to be important, Ang II-dependent hypertension, is the best characterized. In Ang II-infused rats and mice, expression of Noxs (Nox1, Nox2, and Nox4), Nox activity, and ROS generation are increased (27, 226). Ang II-induced hypertension is also associated with DNA double-strand breaks and the mutagenic DNA base modification 7,8-dihydro-8-oxo-guanine, effects that were blocked by the radical scavenger tempol (21). These data demonstrated oxidative stress-mediated genotoxic effects of Ang II in vivo, which may contribute to oxidative cardiovascular and kidney damage in hypertension (129). In p47phox knockout mice and in gp91phox (Nox2) knockout mice, Ang II infusion failed to induce hypertension, and these animals do not show the same augmentation in O2•− production, vascular hypertrophy, and endothelial dysfunction observed in wild-type counterparts (110). In Ang II-infused mice treated with siRNA targeted to renal p22phox, renal Nox activity was blunted; ROS formation was reduced; and blood pressure elevation was prevented (136). On the other hand, overexpression of vascular p22phox was associated with increased oxidative stress and vascular dysfunction, but no significant increase in blood pressure (114). Treatment with non-specific pharmacological inhibitors of Nox, such as apocynin or diphenylene iodinium, or gp91dstat, a novel-specific inhibitor of Nox, reduced vascular O2•− production, prevented cardiovascular remodeling, and attenuated development of hypertension in Ang II-dependent hypertension (73, 166). Nox1-deficient mice have reduced vascular O2•− production, and blood pressure elevation in response to Ang II is blunted (63); whereas in transgenic mice in which Nox1 is overxpressed in vascular smoothe muscle cells in the vascular wall, Ang II-mediated vascular hypertrophy and blood pressure elevation are enhanced (49). However, in a model of chronic Ang II-dependent hypertension, where transgenic mice expressing human renin (which exhibit an Ang II-sensitive hypertensive phenotype) were crossed with Nox2−/−or Nox1−/−mice, development of hypertension was not prevented even though oxidative stress was reduced, suggesting that Noxs may be more important in acute than in chronic hypertension (234). It should be stressed that in these Nox knockout or transgenic studies, baseline cardiovascular phenotypes of mice are surprisingly normal and it is only in the context of a challenge, such as with Ang II or salt, that mice exhibit vascular and blood pressure aberrations.
Oxidative stress in the brain plays an important role in the development of hypertension (103, 231, 239). Nox-induced ROS production in the rostral ventrolateral medulla causes sympathoexcitation in hypertensive rats, through mechanisms that involve NO and pro-inflammatory processes. Transgenic (mRen2)27 rats have increased medullary tissue Nox activity and increased ROS production in isolated mitochondria (146). Free radical signaling in the subfornical organ (SFO), an important forebrain circumventricular organ, is critical for sympathetic activation, driving the elevation in blood pressure in Ang II-infused mice (29). Suppression of ROS generation in the SFO by overexpression of CuZn-SOD prevented development of hypertension in these mice. The SFO mediates ROS-related effects through activation of the paraventricular hypothalamic nucleus, causing increased plasma vasopressin, up-regulation of endothelin-1 in cerebral resistance arterioles, and activation of endothelin type A receptors and through activation of cerebrovascular AT1 receptors by Ang II (29). Both pathways mediate vasomotor dysfunction by inducing vascular oxidative stress. The findings implicate the SFO and its efferent hypothalamic pathways in the cerebrovascular alterations induced by Ang II, and they identify vasopressin and endothelin-1 as potential therapeutic targets to counteract the damaging effects of hypertension on the brain.
Although it is becoming increasingly clear that ROS produced in the central nervous system promote sympathetic outflow, inflammation, and hypertension, the contribution of Noxs to these processes is unclear (38). In mice in which p22phox was deleted in the SFO, Ang II infusion failed to elicit a hypertensive response (120). These findings confirmed the importance of Noxs in the SFO as a critical determinant of the blood pressure and vascular inflammatory responses to Ang II (120). In addition to Nox-derived ROS, ER stress in the brain SFO is important in Ang II-induced hypertension (235).
Additional sources of Nox-generated ROS in Ang II-induced hypertension are cells of the immune system, specifically T cells. In mice which lack lymphocytes (RAG1−/− mice), hypertensive responses to Ang II are reduced, a response that is restored by adoptive transfer of T-lymphocytes, but not of B-lymphocytes (223). T cells influence blood pressure elevation by interacting with B7 ligands (CD80 and CD86) and the T-cell coreceptor CD28 (223) and through the dysegulation of T-regulatory and T-effector cells (14). Adoptive transfer of T-regulatory lymphocytes cells suppresses Ang II-mediated vascular injury and hypertension, in part, by reducing Nox-derived ROS generation (14).
Other atypical sources of ROS that may impact vascular redox status include perivascular adipose tissue (23, 80, 148). Adipose tissues possesses functionally active Nox4, which generates ROS, an important modulator of adipocyte biology and adipocytokine production. Perivascular adipose tissue influences vascular tone through adipocyte-derived vasoactive factors and ROS, effects that may be enhanced in hypertension, especially in the context of obesity, metabolic syndrome, and diabetes (148).
In order to further support a role for oxidative stress in experimental hypertension, treatment of hypertensive rats or mice with antioxidant vitamins, SOD mimetics (tempol [4-hydroxy-2,2,6,6-tetramethyl piperidinoxyl]), free radical scavengers, or tetrahydrobiopterin (BH4) attenuate or prevent development of hypertension and its associated target organ damage (37, 72).
ROS and Noxs in Human Vessels
Although vascular Noxs have been well characterized in experimental models of hypertension, with most studies demonstrating increased expression and activity of Nox1, Nox2, and Nox4 in a site- and cell-specific manner, little is known about vascular Noxs in human hypertension. Characterization of Noxs in human vessels has focused mainly on discarded surgical tissue from patients undergoing bypass surgery. Results from extensive human studies by the Channon group showed increased vascular Nox activity and expression of Nox2, Nox4, and p22phox, but not of Nox1, in patients with coronary artery disease or diabetes (10, 76–78). Others showed that vascular Nox5 expression is increased in patients with atherosclerosis and cardiac disease (18, 178, 195). Hahn et al. demonstrated the presence of Nox5 expression in human intramyocardial blood vessels and cardiomyocytes, with significant increases after myocardial infarction (79). In a recent comprehensive descriptive study, Pandey et al. (157) reported that Nox5 is present in human saphenous vein and internal mammary artery, cultured human VSMCs, and endothelium, but not in fibroblasts, with the α and β isoforms being most abundant in vascular cells. Adenovirus-mediated overexpression of Nox5 promoted phosphorylation of MAPK in ECs and VSMCs (195), which is similar to our studies in Ang II-stimulated Nox5-containing cells (151, 228). These studies, although informative of human Nox expression, have limitations, as they are essentially descriptive and do not reveal much insight into the mechanisms of oxidative stress. However, such studies consistently demonstrate up-regulation of vascular Noxs in cardiovascular disease, indicating their potential importance in such conditions.
ROS, Noxs, and Vascular Function: Clues from Studies in Patients with Hereditary Deficiency of Nox
Practical and ethical issues limit the direct study of Noxs and ROS in human cardiovascular disease. However, a series of studies by Viola and colleagues, in which patients with Nox deficiency (chronic granulomatous disease) were studied, demonstrated that Noxs and ROS are important in the regulation of vascular tone (122, 124, 225). This was evidenced by the following: patients with Nox 2 or p47phox deficiency had significantly higher forearm-mediated dilation and lower serum levels of soluble Nox2-derived peptide (marker of Nox2 activation) and 8-iso-PGF2α levels compared with healthy subjects; platelets from patients with Nox2 deficiency have reduced isoprostane formation; patients with CGD are protected from ischemia-reperfusion injury (121, 122, 124, 225). Moreover, women carriers of hereditary deficiency of Nox2 had higher flow-mediated dilation, lower intima-media thickness, reduced urinary isoprostanes and serum Nox2 activity, increased NO bioavailability, and higher serum nitrite/nitrate compared with controls, suggesting reduced vascular damage and atherosclerotic burden in carriers of Nox2 deficiency (224). Taken together, these studies implicate a role for Nox and ROS in the regulation of endotehlial function and vascular tone. However, it should be kept in mind that these findings may be influenced by the clinical conditions of these patients, as they have life-threatening infections, that are often treated prophylactically with antibiotics and antifungal agents.
ROS, Oxidative Stress and Human Hypertension
Hypertensive patients exhibit higher levels of plasma H2O2, increased plasma, and urine markers of oxidative stress such as TBARS, oxidized low-density lipoprotein (oxLDL), and 8 iso-prostane and reduced antioxidant capacity, compared with normotensive subjects (20, 171, 184, 205, 229). Isoprostanes are stereoisomers of prostaglandins, which are formed mainly by non-enzymatic peroxidation of arachidonic acid by ROS. Plasma levels of oxLDL are influenced primarily by the magnitude of oxidative stress within the vascular wall as well as by the susceptbility of LDL to oxidation. Treatment with antihypertensive drugs reduces oxidative stress biomarkers, in some cases independently of blood pressure lowering. In 2011, the European Food Safety Authority accepted urine isoprostanes as a biochemical marker of oxidative stress (55). However, testing of such biomarkers is not routine practice in the clinic, and standardized methodologies are not yet available at hospital laboratories.
Due to the potential predictive value of biomarkers, there is growing interest in identifying more specific and direct indices of oxidative stress. Recent advances in the field have focused on redox proteomics in which oxidative post-translational modifications can be identified in protein targets of oxidative or nitrosative stress (193). Redox proteomics technologies can identify oxidized proteins in serum, plasma, and urine. Advanced oxidation proteins are variants primarily of albumin and fibrinogen and have been identified in plasma and serum from patients with chronic kidney disease and hypertension (173, 183). Post-translational modifications of protein residues by ROS include thiol oxidation and carbonylation. In a large cohort of Chinese adults, plasma reactive carbonyl species was positively associated with blood pressure levels and was found to be an important risk factor for developing hypertension (36). Nitration of proteins, especially modification of tyrosine to 3-nitrotyrosine, is another form of redox modification and is increased in serum proteins in patients with hypertension, chronic kidney disease, and diabetes (25, 131, 217).
Normotensive subjects with a family history of hypertension have greater ROS production than blood pressure-matched subjects without a family history of hypertension, suggesting a genetic component that is associated with elevated production of free radicals (57, 105, 108). Racial differences in oxidative stress and inflammation have also been demonstrated. Human umbilical vein endothelial cells (HUVEC) from African Americans exhibited higher levels of NO, IL-6, p47phox, Nox2, and Nox4 and lower SOD activity than HUVECs from Caucasians (169).
ROS production is increased in VSMCs from resistance arteries of hypertensive patients, and this is associated with up-regulation of vascular Nox (75, 112, 142, 207, 213, 233). The importance of Nox in human cardiovascular disease is supported by studies showing that polymorphisms in Nox subunits are associated with increased atherosclerosis and hypertension. In particular, the −930(A/G) polymorphism in the p22(phox) promoter may be a novel genetic marker that is associated with hypertension. An association between the p22phox −930 G polymorphism has been associated with blood pressure in normotensive subjects (100). The C242T CYBA polymorphism is associated with essential hypertension, and hypertensive patients carrying the CC genotype of this polymorphism exhibit features of Nox-mediated oxidative stress and endothelial damage and are prone to cerebrovascular disease. The T allele of the p22-phox C242T polymorphism is also associated with higher left ventricular mass/height and increased Nox activity in Brazilian hypertensive patients, suggesting that genetic variation within Nox components may modulate left ventricular remodeling in subjects with hypertension (176). In a Japanese population, the G(−930)A polymorphism of CYBA was confirmed to be important in the pathogenesis of hypertension (141). Polymorphisms of the xanthine oxidase gene (−337GA and 565+64CT) have also been shown to be related to blood pressure and oxidative stress in hypertension (237).
In addition to essential hypertension, oxidative stress is found in other forms of hypertension. Patients with primary hyperaldosteronism exhibit increased levels of plasma ROS and markers of subclinical inflammation compared with essential hypertensive patients (60, 196). These findings were associated with increased cardiac fibrosis, phenomena that were independent of blood pressure elevation but related to proinflammatory and oxidative stress effects of aldosterone. Hypertension during pregnancy was also found to be associated with oxidative stress as evidenced by increased TBARS levels during labor (5). This was related to reduced plasma SOD activity and increased plasma GSH-Px with no change in GSH-Red activity (5). Alterations in antioxidant and pro-oxidant status during pregnancy may constitute an increased risk factor for hypertensive pregnant women. Elderly patients (∼75 years) who exhibit endothelial dysfunction and decreased antioxidant capacity responded positively to oral antioxidant therapy, with improved flow-mediated vasodilation, decreased plasma TBARS levels, and increased antioxidants (230). However, in young individuals (∼25 years), antioxidant therapy worsened endothelial function, suggesting that the age-related impairment is attributed, at least in part, to oxidative stress.
Decreased antioxidant defense mechanisms also contribute to oxidative stress in human hypertension. Patients with essential hypertension exhibit reduced activity and decreased plasma levels of antioxidant enzymes, including SOD, glutathione peroxidase, and catalase (192). Decreased levels of antioxidant vitamins A, C, and E have been shown in newly diagnosed, untreated hypertensive patients compared with normotensive controls (143). Nrf-2, the master transcription factor that regulates antioxidant genes, is protective in maternal diabetes-induced perinatal hypertension (30, 221). Antioxidant vitamins reduced blood pressure and arterial stiffness in patients with diabetes (240), but had no effect in postmenopausal women or in healthy subjects (140). Population studies have demonstrated an inverse association between plasma vitamin C levels and vitamin C consumption with blood pressure (28, 139), and a recent meta-analysis reported that vitamin C supplementation reduced systolic and diastolic blood pressure (147). In patients with white coat hypertension, serum protein carbonyl (indicating protein oxidation) was increased and endogenous antioxidant proteins (protein thiol, SOD, glutathione) were decreased compared with normotensive individuals, further supporting a relationship between low antioxidant capacity, increased oxidative stress, and hypertension (201).
ROS as Therapeutic Targets in Human Hypertension
Considering the possible pathophysiological role of oxidative stress in hypertension and other cardiovascular disorders, and the convincing experimental data, it is reasonable to imagine that reducing ROS bioavailability through antioxidants, ROS scavengers, and Nox inhibitors would have protective and blood pressure-lowering effects. Antioxidants that have been commonly studied, including vitamins A, C, and E, co-enzyme Q, beta carotene, polyphenols, and flavonoids. A recent meta-analysis evaluating the effects of vitamin C supplementation on blood pressure reported that vitamin C supplementation reduces blood pressure by 3.84/1.48 mm Hg (147). However, findings are inconsistent, and clinical trial data are inconclusive, with most large antioxidant clinical trials failing to demonstrate beneficial cardiovascular effects (129, 133, 187). Findings from the Physicians Health Study II, a randomized double-blind, placebo-controlled trial of a common daily multivitamin in which 14,641 men were studied for 11.2 years, revealed no significant effect of multivitamin supplementation on cardiovascular events (188). Similar negative results have been reported for many other large antioxidant trials, which have been recently reviewed (83, 85, 97, 129, 133, 187). In addition, clinical trials examining the effects of antioxidant vitamins (vitamins C and E) in the prevention of pre-eclampsia and gestational hypertension have been negative (132, 168). Reasons for these disappointing results are numerous, but as Brieger et al. (22) hypothesize, “antioxidant supplementation is too late, too little and too non-specific.”
Based on the lack of evidence proving antioxidant benefits in cardiovascular diseases, antioxidant supplementation is not recommended for the prevention or treatment of hypertension. However, most hypertension guidelines recommend that the general population consumes a diet rich in fruits, vegetables, and whole grains, which is a diet rich in antioxidants (123). The low sodium Dietary Approaches to Stop Hypertension (DASH) diet reduces oxidative stress and improves vascular function in salt-sensitive patients (6, 88).
Another important lifestyle modification that may have cardiovascular protective and blood pressure-lowering effects by reducing oxidative stress is exercise. In experimental models of hypertension and in patients with coronary artery disease, exercise reduced vascular Nox activity and ROS production, ameliorated vascular injury, and reduced blood pressure (1). Resistance training in men decreased circulating levels of matrix metalloprotease-9 and 8-isoprostane (43). However, in elderly patients, combining antioxidant therapy with exercise negated blood pressure-lowering beneficial effects of exercise (43).
Clinical studies examining the effects of xanthine oxidase inhibitors (70, 204), tetrahydrobiopterin (sapropterin dihydrochloride [6r-bh4]) (167), and N-acetylcysteine (165) have demonstrated improved vascular function and blood pressure lowering in patients with hypertension, chronic kidney disease, and pulmonary hypertension. However, a recent clinical trial demonstrated that in patients with CAD, oral tetrahydrobiopterin treatment failed to improve endothelial function or cardiovascular outcomes, possibly due to auto-oxidation of the compound (47).
Some of the beneficial effects of classical antihypertensive agents such as ß-adrenergic blockers, ACE inhibitors, AT1 receptor antagonists, and Ca2+ channel blockers may be mediated, in part, by decreasing vascular oxidative stress (59, 125, 154). These effects have been attributed to the direct inhibition of Nox activity and to intrinsic antioxidant properties of the drugs. However, some studies failed to show changes in oxidative stress despite significant blood pressure lowering by classical antihypertensive drugs (106, 191).
Other commonly used drugs have also been shown to reduce oxidative stress in patients with cardiovascular risk factors. Fenofibrate, a lipid-lowering agent with pleiotropic actions, improved endothelial function, measured by brachial flow-mediated dilation, in middle-aged and older normolipidemic adults by reducing oxidative stress and by increasing endothelial NOS expression and activity (227).
Noxs as Putative Targets in the Treatment of Hypertension
Antioxidants and radical scavengers increase rates of ROS degradation, whereas inhibitors of ROS-generating enzymes decrease rates of ROS formation. Of the many enzymes that are potential therapeutic targets are Noxs. Due to this, there has been enormous interest in the development of agents that inhibit Noxs in an isoform-specific manner (91, 102, 194, 197). Different strategies have been employed, including small-molecule inhibitors, peptide Nox inhibitors, and siRNAS. Several pharmacological compounds have been registered as Nox inhibitors in the patent literature (102). First-generation Nox inhibitors, including apocynin and diphenylene iodinium, are non specific, lack selectivity, and have multiple “off-target” side effects. Newer-generation NOX inhibitors are more specific and selective. To date, two different classes of compounds have been claimed as potent selective and orally active bioavailable Nox inhibitors: pyrazolopyridines (GKT136901 and GKT137831) and triazolopyrimidine derivatives (VAS2870 and VAS3947) (39, 91, 102, 194, 197). These agents target mainly Nox1 and Nox4, and, apparently, have a few “off-target” side effects (22, 217). The exact mechanisms by which GKT compounds inhibit Nox activity remain unclear, but they may act as competitive substrate inhibitors, as they structurally resemble NADPH. GKT137831 has already undergone safety studies in humans and was found to be safe and well tolerated (86). Its use will soon be tested in patients with diabetic nephropathy, and future studies may evaluate the effects on hypertension and other cardiovascular diseases (86). Although much research is still needed in the field, the clinical utility of Nox-specific inhibitors is promising.
Conclusions
Compelling findings from experimental and animal studies indicate a role for oxidative stress and Noxs in the etiology of hypertension. However, there is no direct clinical proof that oxidative stress causes hypertension in humans. What is clear from experimental and translational research is that dysregulation of Noxs increased ROS generation-altered redox signaling, and oxidative injury may be important in the pathophysiology of increased blood pressure, in large part through effects on endothelial function, vascular tone, arterial remodeling, and vascular inflammation. The recently identified Nox, Nox5, may be particularly important in vascular injury and cardiovascular disease in humans. More translational and clinical research in the field of oxidative stress and hypertension is needed, especially in the development of sensitive, specific, and reliable biomarkers and assays to assess the redox status of humans in health and disease. Clinical trials are designed to address the role of ROS specifically in the development of hypertension. With a better understanding of ROS (patho)biology in humans, it should be possible to target therapies more effectively so that damaging effects of ROS can be prevented or ameliorated. Such approaches may have potential benefit in the treatment of redox-sensitive pathologies that are associated with cardiovascular disease, including hypertension.
Abbreviations Used
- Ang II
angiotensin II
- BH4
tetrahydrobiopterin
- CKD
chronic kidney disease
- ECM
extracellular matrix
- ECs
endothelial cells
- eNOS
endothelial nitric oxide synthase
- H2O2
hydrogen peroxide
- HUVEC
human umbilical vein endothelial cells
- MAPK
mitogen-activated protein kinases
- MMP
matrix metalloprotease-9
- MnSOD
manganese superoxide dismutase
- NADPH
nicotinamide adenine dinucleotide phosphate
- NO
nitric oxide
- NOS
nitric oxide synthase
- Noxs
NADPH oxidase
- Nrf2
nuclear factor erythroid 2-related factor 2
- oxLDL
oxidized low-density lipoprotein
- PDGF
platelet-derived growth factor
- ROS
reactive oxygen species
- SFO
subfornical organ
- TBARS
thiobarbituric acid-reactive substances
- TRPM2
transient receptor potential melastatin cation channel 2
- TSP1
thrombospondin-1
- VSMC
vascular smooth muscle cells
Acknowledgments
Work from the author's laboratory was supported by grants 44018 and 57886 from the Canadian Institutes of Health Research (CIHR) and from the JDRF.
References
- 1.Adams V, Linke A, Kränkel N, Erbs S, Gielen S, Möbius-Winkler S, Gummert JF, Mohr FW, Schuler G, and Hambrecht R. Impact of regular physical activity on the NAD(P)H oxidase and angiotensin receptor system in patients with coronary artery disease. Circulation 111: 555–562, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Adlam D, Bendall JK, De Bono JP, Alp NJ, Khoo J, Nicoli T, Yokoyama M, Kawashima S, and Channon KM. Relationships between nitric oxide-mediated endothelial function, eNOS coupling and blood pressure revealed by eNOS-GTP cyclohydrolase 1 double transgenic mice. Exp Physiol 92: 119–126, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Al Ghouleh I, Frazziano G, Rodriguez AI, Csányi G, Maniar S, St Croix CM, Kelley EE, Egaña LA, Song GJ, Bisello A, Lee YJ, and Pagano PJ. Aquaporin 1, Nox1, and Ask1 mediate oxidant-induced smooth muscle cell hypertrophy. Cardiovasc Res 97: 134–142, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al Ghouleh I, Khoo NK, Knaus UG, Griendling KK, Touyz RM, Thannickal VJ, Barchowsky A, Nauseef WM, Kelley EE, Bauer PM, Darley-Usmar V, Shiva S, Cifuentes-Pagano E, Freeman BA, Gladwin MT, and Pagano PJ. Oxidases and peroxidases in cardiovascular and lung disease: new concepts in reactive oxygen species signaling. Free Radic Biol Med 51: 1271–1288, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al-Shebly MM. and Mansour MA. Evaluation of oxidative stress and antioxidant status in diabetic and hypertensive women during labor. Oxid Med Cell Longev 2012: 329743–32976, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al-Solaiman Y, Jesri A, Zhao Y, Morrow JD, and Egan BM. Low-sodium DASH reduces oxidative stress and improves vascular function in salt-sensitive humans. J Hum Hypertens 23: 826–830, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Altenhöfer S, Kleikers PW, Radermacher KA, Scheurer P, Rob Hermans JJ, Schiffers P, Ho H, Wingler K, and Schmidt HH. The NOX toolbox: validating the role of NADPH oxidases in physiology and disease. Cell Mol Life Sci 69: 2327–2343, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amanso AM, Debbas V, and Laurindo FR. Proteasome inhibition represses unfolded protein response and Nox4, sensitizing vascular cells to endoplasmic reticulum stress-induced death. PLoS One 6: e14591, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anilkumar N, Jose GS, Sawyer I, Santos CX, Sand C, Brewer AC, Warren D, and Shah AM. A 28-kDa splice variant of NADPH oxidase-4 is nuclear-localized and involved in redox signaling in vascular cells. Arterioscler Thromb Vasc Biol 33: e104–e112, 2013 [DOI] [PubMed] [Google Scholar]
- 10.Antoniades C, Bakogiannis C, Leeson P, Guzik TJ, Zhang MH, Tousoulis D, Antonopoulos AS, Demosthenous M, Marinou K, Hale A, Paschalis A, Psarros C, Triantafyllou C, Bendall J, Casadei B, Stefanadis C, and Channon KM. Rapid, direct effects of statin treatment on arterial redox state and nitric oxide bioavailability in human atherosclerosis via tetrahydrobiopterin-mediated endothelial nitric oxide synthase coupling. Circulation 124: 335–345, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aoyama T, Paik YH, Watanabe S, Laleu B, Gaggini F, Fioraso-Cartier L, Molango S, Heitz F, Merlot C, Szyndralewiez C, Page P, and Brenner DA. Nicotinamide adenine dinucleotide phosphate oxidase in experimental liver fibrosis: GKT137831 as a novel potential therapeutic agent. Hepatology 56: 2316–2327, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Babior BM. NADPH oxidase. Curr Opin Immunol 16: 42–47, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Bánfi B, Tirone F, Durussel I, Knisz J, Moskwa P, Molnár GZ, Krause KH, and Cox JA. Mechanism of Ca2+ activation of the NADPH oxidase 5 (NOX5). J Biol Chem 279: 18583–18591, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Barhoumi T, Kasal DA, Li MW, Shbat L, Laurant P, Neves MF, Paradis P, and Schiffrin EL. T regulatory lymphocytes prevent angiotensin II-induced hypertension and vascular injury. Hypertension 57: 469–476, 2011 [DOI] [PubMed] [Google Scholar]
- 15.Basset O, Deffert C, Foti M, Bedard K, Jaquet V, Ogier-Denis E, and Krause KH. NADPH oxidase 1 deficiency alters caveolin phosphorylation and angiotensin II-receptor localization in vascular smooth muscle. Antioxid Redox Signal 11: 2371–2384, 2009 [DOI] [PubMed] [Google Scholar]
- 16.Bedard K. and Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 87: 245–313, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Bedard K, Jaquet V, and Krause KH. NOX5: from basic biology to signaling and disease. Free Radic Biol Med 52: 725–734, 2012 [DOI] [PubMed] [Google Scholar]
- 18.BelAiba RS, Djordjevic T, Petry A, Diemer K, Bonello S, Banfi B, Hess J, Pogrebniak A, Bickel C, and Görlach A. NOX5 variants are functionally active in endothelial cells. Free Radic Biol Med 42: 446–459, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Bir SC, Kolluru GK, Fang K, and Kevil CG. Redox balance dynamically regulates vascular growth and remodeling. Semin Cell Dev Biol 23: 745–757, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bo S, Gambino R, Gentile L, Pagano G, Rosato R, Saracco GM, Cassader M, Durazzo M, and Cavallo-Perin P. High-normal blood pressure is associated with a cluster of cardiovascular and metabolic risk factors: a population-based study. J Hypertens 27: 102–108, 2009 [DOI] [PubMed] [Google Scholar]
- 21.Brand S, Amann K, and Schupp N. Angiotensin II-induced hypertension dose-dependently leads to oxidative stress and DNA damage in mouse kidneys and hearts. J Hypertens 31: 333–344, 2013 [DOI] [PubMed] [Google Scholar]
- 22.Brieger K, Schiavone S, Miller FJ, Jr, and Krause KH. Reactive oxygen species: from health to disease. Swiss Med Wkly 17: 142–147, 2012 [DOI] [PubMed] [Google Scholar]
- 23.Briones AM, Nguyen Dinh Cat A, Callera GE, Yogi A, Burger D, He Y, Correa J, Gagnon AM, Celso E. Gomez-Sanchez CE, Gomez-Sanchez EP, Sorisky A, Ooi TC, Ruzicka M, Burns KD, amd Touyz RM. Adipocytes produce aldosterone through calcineurin/NFAT-dependent signaling pathway - Implications in diabetes-associated obesity. Hypertension 59: 1069–1078, 2012 [DOI] [PubMed] [Google Scholar]
- 24.Briones AM, Tabet F, Callera GE, Montezano AC, Yogi A, He Y, Quinn MT, Salaices M, and Touyz RM. Differential regulation of Nox1, Nox2 and Nox4 in vascular smooth muscle cells from WKY and SHR. J Am Soc Hypertens 5: 137–153, 2011 [DOI] [PubMed] [Google Scholar]
- 25.Bruno RM, Daghini E, Landini L, Versari D, Salvati A, Santini E, Di Paco I, Magagna A, Taddei S, Ghiadoni L, and Solini A. Dynamic evaluation of renal resistive index in normoalbuminuric patients with newly diagnosed hypertension or type 2 diabetes. Diabetologia 54: 2430–2439, 2011 [DOI] [PubMed] [Google Scholar]
- 26.Burger D, Montezano AC, Nishigaki N, He Y, Carter A, and Touyz RM. Endothelial microparticle formation by angiotensin II is mediated via Ang II receptor type I/NADPH oxidase/Rho kinase pathways targeted to lipid rafts. Arterioscler Thromb Vasc Biol 31: 1898–1907, 2011 [DOI] [PubMed] [Google Scholar]
- 27.Byrne JA, Grieve DJ, Bendall JK, Li JM, Gove C, Lambeth JD, Cave AC, and Shah AM. Contrasting roles of NADPH oxidase isoforms in pressure-overload versus angiotensin II-induced cardiac hypertrophy. Circ Res 93: 802–805, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Caner M, Karter Y, Uzun H, Curgunlu A, Vehid S, Balci H, Yucel R, Güner I, Kutlu A, Yaldiran A, and Oztürk E. Oxidative stress in human sustained and white coat hypertension. Int J Clin Pract 60: 1565–1571, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Capone C, Faraco G, Peterson JR, Coleman C, Anrather J, Milner TA, Pickel VM, Davisson RL, and Iadecola C. Central cardiovascular circuits contribute to the neurovascular dysfunction in angiotensin II hypertension. J Neurosci 32: 4878–4886, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang SY, Chen YW, Zhao XP, Chenier I, Tran S, Sauvé A, Ingelfinger JR, and Zhang SL. Catalase prevents maternal diabetes-induced perinatal programming via the Nrf2-HO-1 defense system. Diabetes 61: 2565–2574, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chapple SJ, Siow RC, and Mann GE. Crosstalk between Nrf2 and the proteasome: therapeutic potential of Nrf2 inducers in vascular disease and aging. Int J Biochem Cell Biol 44: 1315–1320, 2012 [DOI] [PubMed] [Google Scholar]
- 32.Chen DD, Dong YG, Yuan H, and Chen AF. Endothelin 1 activation of endothelin A receptor/NADPH oxidase pathway and diminished antioxidants critically contribute to endothelial progenitor cell reduction and dysfunction in salt-sensitive hypertension. Hypertension 59: 1037–1043, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen F, Yu Y, Qian J, Wang Y, Cheng B, Dimitropoulou C, Patel V, Chadli A, Rudic RD, Stepp DW, Catravas JD, and Fulton DJ. Opposing actions of heat shock protein 90 and 70 regulate nicotinamide adenine dinucleotide phosphate oxidase stability and reactive oxygen species production. Arterioscler Thromb Vasc Biol 32: 2989–2999, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen H, Song YS, and Chan PH. Inhibition of NADPH oxidase is neuroprotective after ischemia-reperfusion. J Cereb Blood Flow Metab 29: 1262–1272, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen K, Kirber MT, Xiao H, Yang Y, and Keaney JF., , Jr.Regulation of ROS signal transduction by NAD(P)H oxidase 4 localization. J Cell Biol 181: 1129–1139, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen K, Xie F, Liu S, Li G, Chen Y, Shi W, Hu H, Liu L, and Yin D. Plasma reactive carbonyl species: potential risk factor for hypertension. Free Radic Res 45: 568–574, 2011 [DOI] [PubMed] [Google Scholar]
- 37.Chen X, Touyz RM, Park JB, and Schiffrin EL. Antioxidant effects of vitamins C and E are associated with altered activation of vascular NADPH oxidase and superoxide dismutase in stroke-prone SHR. Hypertension 38(3 Pt2): 606–611, 2001 [DOI] [PubMed] [Google Scholar]
- 38.Chrissobolis S, Banfi B, Sobey CG, and Faraci FM. Role of Nox isoforms in angiotensin II-induced oxidative stress and endothelial dysfunction in brain. J Appl Physiol 113: 184–191, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cifuentes-Pagano E, Csanyi G, and Pagano PJ. NADPH oxidase inhibitors: a decade of discovery from Nox2ds to HTS. Cell Mol Life Sci 69: 2315–2325, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cioffi DL. Redox regulation of endothelial canonical transient receptor potential channels. Antioxid Redox Signal 15: 1567–1582, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Coffman TM. Under pressure: the search for the essential mechanisms of hypertension. Nat Med 17: 1402–1409, 2010 [DOI] [PubMed] [Google Scholar]
- 42.Collins AR, Lyon CJ, Xia X, Liu JZ, Tangirala RK, Yin F, Boyadjian R, Bikineyeva A, Praticò D, Harrison DG, and Hsueh WA. Age-accelerated atherosclerosis correlates with failure to upregulate antioxidant genes. Circ Res 104: e42–e54, 2009 [DOI] [PubMed] [Google Scholar]
- 43.Cook MD, Heffernan KS, Ranadive S, Woods JA, and Fernhall B. Effect of resistance training on biomarkers of vascular function and oxidative stress in young African-American and Caucasian men. J Hum Hypertens 2012. [Epub ahead of print]; DOI: 10.1038/jhh.2012.48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Copple IM. The Keap1-Nrf2 cell defense pathway—a promising therapeutic target? Adv Pharmacol 63: 43–79, 2012 [DOI] [PubMed] [Google Scholar]
- 45.Cottone S, Mulè G, Guarneri M, Palermo A, Lorito MC, Riccobene R, Arsena R, Vaccaro F, Vadalà A, Nardi E, Cusimano P, and Cerasola G. Endothelin-1 and F2-isoprostane relate to and predict renal dysfunction in hypertensive patients. Nephrol Dial Transplant 24: 497–503, 2009 [DOI] [PubMed] [Google Scholar]
- 46.Csányi G, Yao M, Rodríguez AI, Ghouleh IA, Sharifi-Sanjani M, Frazziano G, Huang X, Kelley EE, Isenberg JS, and Pagano PJ. Thrombospondin-1 regulates blood flow via CD47 receptor-mediated activation of NADPH oxidase 1. Arterioscler Thromb Vasc Biol 32: 2966–2973, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cunnington C, Van Assche T, Shirodaria C, Kylintireas I, Lindsay AC, Lee JM, Antoniades C, Margaritis M, Lee R, Cerrato R, Crabtree MJ, Francis JM, Sayeed R, Ratnatunga C, Pillai R, Choudhury RP, Neubauer S, and Channon KM. Systemic and vascular oxidation limits the efficacy of oral tetrahydrobiopterin treatment in patients with coronary artery disease. Circulation 125: 1356–1366, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Di Castro S, Scarpino S, Marchitti S, Bianchi F, Stanzione R, Cotugno M, Sironi L, Gelosa P, Duranti E, Ruco L, Volpe M, and Rubattu S. Differential modulation of uncoupling protein 2 in kidneys of stroke-prone spontaneously hypertensive rats under high-salt/low-potassium diet. Hypertension 61: 534–541, 2013 [DOI] [PubMed] [Google Scholar]
- 49.Dikalova A, Clempus R, Lassegue B, Cheng G, McCoy J, and Dikalov S, et al Nox1 overexpression potentiates angiotensin II-induced hypertension and vascular smooth muscle hypertrophy in transgenic mice. Circulation 112: 2668–2676, 2005 [DOI] [PubMed] [Google Scholar]
- 50.Dikalova AE, Góngora MC, Harrison DG, Lambeth JD, Dikalov S, and Griendling KK. Upregulation of Nox1 in vascular smooth muscle leads to impaired endothelium-dependent relaxation via eNOS uncoupling. Am J Physiol Heart Circ Physiol 299: H673–H679, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Douglas G, Bendall JK, Crabtree MJ, Tatham AL, Carter EE, Hale AB, and Channon KM. Endothelial-specific Nox2 overexpression increases vascular superoxide and macrophage recruitment in ApoE?/? mice. Cardiovasc Res 94: 20–29, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Droge W. Free radicals in the physiological control of cell function. Physiol Rev 82: 47–95, 2002 [DOI] [PubMed] [Google Scholar]
- 53.Drummond GR, Selemidis S, Griendling KK, and Sobey CG. Combating oxidative stress in vascular disease: NADPH oxidases as therapeutic targets. Nat Rev Drug Discov 10: 453–471, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dutta S. and Rittinger K. Regulation of NOXO1 activity through reversible interactions with p22 and NOXA1. PLoS One 5: e10478, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA) Guidance on the scientific requirements for health claims related to antioxidants, oxidative damage and cardiovascular health. EFSA J 9: 2474–2478, 2011 [Google Scholar]
- 56.El Jamali A, Valente AJ, Lechleiter JD, Gamez MJ, Pearson DW, Nauseef WM, and Clark RA. Novel redox-dependent regulation of NOX5 by the tyrosine kinase c-Abl. Free Radic Biol Med 44: 868–881, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Feairheller DL, Park JY, Sturgeon KM, Williamson ST, Diaz KM, Veerabhadrappa P, and Brown MD. Racial differences in oxidative stress and inflammation: in vitro and in vivo. Clin Transl Sci 4: 32–37, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fernandes DC, Manoel AHO, Wosniak J, and Laurindo FR. Protein disulfide isomerise overexpression in vascular smooth muscle cells induces spontaneous preemptive NAD(P)H oxidase activation and Nox1 mRNA expression: effects of nitrosothiol exposure. Arch Biochem Biophys 484: 197–204, 2009 [DOI] [PubMed] [Google Scholar]
- 59.Fortuño A, Bidegain J, Robador PA, Hermida J, López-Sagaseta J, Beloqui O, Díez J, and Zalba G. Losartan metabolite EXP3179 blocks NADPH oxidase-mediated superoxide production by inhibiting protein kinase C: potential clinical implications in hypertension. Hypertension 54: 744–750, 2009 [DOI] [PubMed] [Google Scholar]
- 60.Freel EM, Mark PB, Weir RA, McQuarrie EP, Allan K, Dargie HJ, McClure JD, Jardine AG, Davies E, and Connell JM. Demonstration of blood pressure-independent noninfarct myocardial fibrosis in primary aldosteronism: a cardiac magnetic resonance imaging study. Circ Cardiovasc Imaging 5: 740–747, 2012 [DOI] [PubMed] [Google Scholar]
- 61.Fukai T, Ishizaka N, Rajagopalan S, Laursen JB, Capers QT, and Taylor WR, et al. p22phox mRNA expression and NAD(P)H oxidase activity are increased in aortas from hypertensive rats. Circ Res 80: 45–51, 1997 [DOI] [PubMed] [Google Scholar]
- 62.Fulton DJ. Nox5 and the regulation of cellular function. Antioxid Redox Signal 11: 2443–2452, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gavazzi G, Banfi B, Deffert C, Fiette L, Schappi M, Herrmann F, and Krause KH. Decreased blood pressure in NOX1-deficient mice. FEBS Lett 580: 497–504, 2006 [DOI] [PubMed] [Google Scholar]
- 64.Geiszt M, Kopp JB, Várnai P, and Leto TL. Identification of renox, an NAD(P)H oxidase in kidney. Proc Natl Acad Sci U S A 97: 8010–8014, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Geiszt M. NAD(P)H oxidases: new kids on the block. Cardiovasc Res 71: 289–299, 2006 [DOI] [PubMed] [Google Scholar]
- 66.Giustarini D, Dalle-Donne I, Tsikas D, and Rossi R. Oxidative stress and human diseases: origin, link, measurement, mechanisms, and biomarkers. Crit Rev Clin Lab Sci 46: 241–281, 2009 [DOI] [PubMed] [Google Scholar]
- 67.Glassman SJ. Vitiligo, reactive oxygen species and T-cells. Clin Sci 120: 99–120, 2011 [DOI] [PubMed] [Google Scholar]
- 68.Godin N, Liu F, Lau GJ, Brezniceanu ML, Chénier I, Filep JG, Ingelfinger JR, Zhang SL, and Chan JS. Catalase overexpression prevents hypertension and tubular apoptosis in angiotensinogen transgenic mice. Kidney Int 77: 1086–1097, 2010 [DOI] [PubMed] [Google Scholar]
- 69.Gongora MC, Qin Z, Laude K, Kim HW, McCann L, Folz JR, Dikalov S, Fukai T, and Harrison DG. Role of extracellular superoxide dismutase in hypertension. Hypertension 48: 473–481, 2006 [DOI] [PubMed] [Google Scholar]
- 70.Greig D, Alcaino H, Castro PF, Garcia L, Verdejo HE, Navarro M, López R, Mellado R, Tapia F, Gabrielli LA, Nogerol C, Chiong M, Godoy I, and Lavandero S. Xanthine-oxidase inhibitors and statins in chronic heart failure: effects on vascular and functional parameters. J Heart Lung Transplant 30: 408–413, 2011 [DOI] [PubMed] [Google Scholar]
- 71.Griendling KK, Sorescu D, Lassegue B, and Ushio-Fukai M. Modulation of protein kinase activity and gene expression by reactive oxygen species and their role in vascular physiology and pathophysiology. Arterioscler Thromb Vasc Biol 20: 2175–2183, 2000 [DOI] [PubMed] [Google Scholar]
- 72.Guerrero F, Thioub S, Goanvec C, Theunissen S, Feray A, Balestra C, and Mansourati J. Effect of tetrahydrobiopterin and exercise training on endothelium-dependent vasorelaxation in SHR. J Physiol Biochem 69: 277–287, 2013 [DOI] [PubMed] [Google Scholar]
- 73.Guimarães DD, Carvalho CC, and Braga VA. Scavenging of NADPH oxidase-derived superoxide anions improves depressed baroreflex sensitivity in spontaneously hypertensive rats. Clin Exp Pharmacol Physiol 39: 373–378, 2012 [DOI] [PubMed] [Google Scholar]
- 74.Gupte SA, Kaminski PM, George S, Kouznestova L, Olson SC, Mathew R, Hintze TH, and Wolin MS. Peroxide generation by p47phox-Src activation of Nox2 has a key role in protein kinase C-induced arterial smooth muscle contraction. Am J Physiol Heart Circ Physiol 296: H1048–H1057, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Guzik TJ, Chen W, Gongora MC, Guzik B, Lob HE, Mangalat D, Hoch N, Dikalov S, Rudzinski P, Kapelak B, Sadowski J, and Harrison DG. Calcium-dependent NOX5 nicotinamide adenine dinucleotide phosphate oxidase contributes to vascular oxidative stress in human coronary artery disease. J Am Coll Cardiol 52: 1803–1809, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Guzik TJ, Mussa S, Gastaldi D, Sadowski J, Ratnatunga C, Pillai R, and Channon KM. Mechanisms of increased vascular superoxide production in human diabetes mellitus: role of NAD(P)H oxidase and endothelial nitric oxide synthase. Circulation 105: 1656–1662, 2002 [DOI] [PubMed] [Google Scholar]
- 77.Guzik TJ, Sadowski J, Guzik B, Jopek A, Kapelak B, Przybylowski P, Wierzbicki K, Korbut R, Harrison DG, and Channon KM. Coronary artery superoxide production and nox isoform expression in human coronary artery disease. Arterioscler Thromb Vasc Biol 26: 333–339, 2006 [DOI] [PubMed] [Google Scholar]
- 78.Guzik TJ, Sadowski J, Kapelak B, Jopek A, Rudzinski P, Pillai R, Korbut R, and Channon KM. Systemic regulation of vascular NAD(P)H oxidase activity and nox isoform expression in human arteries and veins. Arterioscler Thromb Vasc Biol 24: 1614–1620, 2004 [DOI] [PubMed] [Google Scholar]
- 79.Hahn NE, Meischl C, Kawahara T, Musters RJ, Verhoef VM, van der Velden J, Vonk AB, Paulus WJ, van Rossum AC, Niessen HW, and Krijnen PA. NOX5 expression is increased in intramyocardial blood vessels and cardiomyocytes after acute myocardial infarction in humans. Am J Pathol 180: 2222–2229, 2012 [DOI] [PubMed] [Google Scholar]
- 80.Han CY, Umemoto T, Omer M, Den Hartigh LJ, Chiba T, LeBoeuf R, Buller CL, Sweet IR, Pennathur S, Abel ED, and Chait A. NADPH oxidase-derived reactive oxygen species increases expression of monocyte chemotactic factor genes in cultured adipocytes. J Biol Chem 287: 10379–10393, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Harris DM, Cohn HI, Pesant S, and Eckhart AD. GPCR signalling in hypertension: role of GRKs. Clin Sci (Lond) 115: 79–89, 2008 [DOI] [PubMed] [Google Scholar]
- 82.Harrison DG, Vinh A, Lob H, and Madhur MS. Role of the adaptive immune system in hypertension. Curr Opin Pharmacol 10: 203–207, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hasnain BA. and Mooradian AD. Recent trials of antioxidant therapy: what should we be telling our patients? Cleve Clin J Med 71: 327–334, 2004 [DOI] [PubMed] [Google Scholar]
- 84.Hilenski LL, Clempus RE, Quinn MT, Lambeth JD, and Griendling KK. Distinct subcellular localizations of Nox1 and Nox4 in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 24: 677–683, 2004 [DOI] [PubMed] [Google Scholar]
- 85.Houston MC. The role of cellular micronutrient analysis, nutraceuticals, vitamins, antioxidants and minerals in the prevention and treatment of hypertension and cardiovascular disease. Ther Adv Cardiovasc Dis 4: 165–183, 2010 [DOI] [PubMed] [Google Scholar]
- 86.http://news-medical.net/news/20121103/Genkyotex-announces-positive-results-from-GKT137831-Phase-I-studies-on-healthy-subjects.aspx
- 87.Huang BS, Zheng H, Tan J, Patel KP, and Leenen FH. Regulation of hypothalamic renin-angiotensin system and oxidative stress by aldosterone. Exp Physiol 96: 1028–1038, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hummel SL, Seymour EM, Brook RD, Kolias TJ, Sheth SS, and Rosenblum HR, Wells JM, Weder AB. Low-sodium dietary approaches to stop hypertension diet reduces blood pressure, arterial stiffness, and oxidative stress in hypertensive heart failure with preserved ejection fraction. Hypertension 60: 1200–1206, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hybertson BM, Gao B, Bose SK, and McCord JM. Oxidative stress in health and disease: the therapeutic potential of Nrf2 activation. Mol Aspects Med 32: 234–246, 2011 [DOI] [PubMed] [Google Scholar]
- 90.Ismail S, Sturrock A, Wu P, Cahill B, Norman K, Huecksteadt T, Sanders K, Kennedy T, and Hoidal J. NOX4 mediates hypoxia-induced proliferation of human pulmonary artery smooth muscle cells: the role of autocrine production of transforming growth factor-{beta}1 and insulin-like growth factor binding protein-3. Am J Physiol Lung Cell Mol Physiol 296: 489–499, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jaquet V, Scapozza L, Clark RA, Krause KH, and Lambeth JD. Small-molecule NOX inhibitors: ROS-generating NADPH oxidases as therapeutic targets. Antioxid Redox Signal 11: 2535–2552, 2009 [DOI] [PubMed] [Google Scholar]
- 92.Jay DB, Papaharalambus CA, Seidel-Rogol B, Dikalova AE, Lassègue B, and Griendling KK. Nox5 mediates PDGF-induced proliferation in human aortic smooth muscle cells. Free Radic Biol Med 45: 329–335, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jung O, Schreiber JG, Geiger H, Pedrazzini T, Busse R, and Brandes RP.gp91phox-containing NADPH oxidase mediates endothelial dysfunction in renovascular hypertension. Circulation 109: 1795–1801, 2004 [DOI] [PubMed] [Google Scholar]
- 94.Juraschek SP, Guallar E, Appel LJ, and Miller ER., 3rd.Effects of vitamin C supplementation on blood pressure: a meta-analysis of randomized controlled trials. Am J Clin Nutr 95: 1079–1088, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kagota S, Tada Y, Kubota Y, Nejime N, Yamaguchi Y, Nakamura K, Kunitomo M, and Shinozuka K. Peroxynitrite is involved in the dysfunction of vasorelaxation in SHR/NDmcr-cp rats, spontaneously hypertensive obese rats. J Cardiovasc Pharmacol 50: 677–685, 2007 [DOI] [PubMed] [Google Scholar]
- 96.Kakihana T, Nagata K, and Sitia R. Peroxides and peroxidases in the endoplasmic reticulum: integrating redox homeostasis and oxidative folding. Antioxid Redox Signal 16: 763–771, 2012 [DOI] [PubMed] [Google Scholar]
- 97.Kalpdev A, Saha SC, and Dhawan V. Vitamin C and E supplementation does not reduce the risk of superimposed PE in pregnancy. Hypertens Pregnancy 30: 447–456, 2011 [DOI] [PubMed] [Google Scholar]
- 98.Kalwa H, Sartoretto JL, Sartoretto SM, and Michel T. Angiotensin-II and MARCKS: a hydrogen peroxide- and RAC1-dependent signaling pathway in vascular endothelium. J Biol Chem 287: 29147–29158, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kamenisch Y. and Berneburg M. Progeroid syndromes and UV-induced oxidative DNA damage. J Investig Dermatol Symp Proc 14: 8–14, 2009 [DOI] [PubMed] [Google Scholar]
- 100.Kim CH. Association between the p22(phox) −930A/G polymorphism and blood pressure in normotensive subjects. Hypertens Res 33: 786–787, 2010 [DOI] [PubMed] [Google Scholar]
- 101.Kim HJ. and Vaziri ND. Contribution of impaired Nrf2-Keap1 pathway to oxidative stress and inflammation in chronic renal failure. Am J Physiol Renal Physiol 298: F662–F671, 2010 [DOI] [PubMed] [Google Scholar]
- 102.Kim JA, Neupane GP, Lee ES, Jeong BS, Park BC, and Thapa P. NADPH oxidase inhibitors: a patent review. Expert Opin Ther Pat 21: 1147–1158, 2011 [DOI] [PubMed] [Google Scholar]
- 103.Kishi T. and Hirooka Y. Oxidative stress in the brain causes hypertension via sympathoexcitation. Front Physiol 3: 335–340, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kodama R, Kato M, Furuta S, Ueno S, Zhang Y, Matsuno K, Yabe-Nishimura C, Tanaka E, and Kamata T. ROS-generating oxidases Nox1 and Nox4 contribute to oncogenic Ras-induced premature senescence. Genes Cells 18: 32–41, 2013 [DOI] [PubMed] [Google Scholar]
- 105.Kruger R, Schutte R, Huisman HW, Van Rooyen JM, Malan NT, Fourie CM, Louw R, van der Westhuizen FH, van Deventer CA, Malan L, and Schutte AE. Associations between reactive oxygen species, blood pressure and arterial stiffness in black South Africans: the SABPA study. J Hum Hypertens 26: 91–97, 2012 [DOI] [PubMed] [Google Scholar]
- 106.Kuklińska AM, Mroczko B, Musiał WJ, Sawicki R, Kozieradzka A, Usowicz-Szaryńska M, Kamiński K, Knapp M, Szmitkowski M. Hypotensive effect of atorvastatin is not related to changes in inflammation and oxidative stress. Pharmacol Rep 62: 883–890, 2010 [DOI] [PubMed] [Google Scholar]
- 107.Labiós M, Martínez M, Gabriel F, Guiral V, Dasi F, Beltrán B, and Muñoz A. Superoxide dismutase and catalase anti-oxidant activity in leucocyte lysates from hypertensive patients: effects of eprosartan treatment. J Renin Angiotensin Aldosterone Syst 10: 24–30, 2009 [DOI] [PubMed] [Google Scholar]
- 108.Lacy F, Kailasam MT, O'Connor DT, Schmid-Schonbein GW, and Parmer RJ. Plasma hydrogen peroxide production in human essential hypertension: role of heredity, gender, and ethnicity. Hypertension 36: 878–884, 2000 [DOI] [PubMed] [Google Scholar]
- 109.Lai EY, Solis G, Luo Z, Carlstrom M, Sandberg K, Holland S, Wellstein A, Welch WJ, and Wilcox CS. p47(phox) is required for afferent arteriolar contractile responses to angiotensin II and perfusion pressure in mice. Hypertension 59: 415–420, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Landmesser U, Cai H, Dikalov S, McCann L, Hwang J, and Jo H. Role of p47(phox) in vascular oxidative stress and hypertension caused by angiotensin II. Hypertension 40: 511–515, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lassegue B. and Clempus RE. Vascular NAD(P)H oxidases: specific features, expression, and regulation. Am J Physiol Regul Integr Comp Physiol 285: R277–R297, 2003 [DOI] [PubMed] [Google Scholar]
- 112.Lassègue B. and Griendling KK. NADPH oxidases: functions and pathologies in the vasculature. Arterioscler Thromb Vasc Biol 30: 653–661, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lassègue B, San Martín A, and Griendling KK. Biochemistry, physiology, and pathophysiology of NADPH oxidases in the cardiovascular system. Circ Res 110: 1364–1390, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Laude K, Cai H, Fink B, Hoch N, Weber DS, McCann L, Kojda G, Fukai T, Schmidt HH, Dikalov S, Ramasamy S, Gamez G, Griendling KK, and Harrison DG. Hemodynamic and biochemical adaptations to vascular smooth muscle overexpression of p22phox in mice. Am J Physiol Heart Circ Physiol 288: H7–H12, 2005 [DOI] [PubMed] [Google Scholar]
- 115.Laurindo FR, Pescatore LA, and Fernandes Dde C. Protein disulfide isomerase in redox cell signaling and homeostasis. Free Radic Biol Med 52: 1954–1969, 2012 [DOI] [PubMed] [Google Scholar]
- 116.Lavi S, Yang EH, Prasad A, Mathew V, Barsness GW, Rihal CS, Lerman LO, and Lerman A. The interaction between coronary endothelial dysfunction, local oxidative stress, and endogenous nitric oxide in humans. Hypertension 51: 127–133, 2008 [DOI] [PubMed] [Google Scholar]
- 117.Lee DH, Gold R, and Linker RA. Mechanisms of oxidative damage in multiple sclerosis and neurodegenerative diseases: therapeutic modulation via fumaric acid esters. Int J Mol Sci 13: 11783–11803, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lee J, Giordano S, and Zhang J. Autophagy, mitochondria and oxidative stress: cross-talk and redox signalling. Biochem J 441: 523–540, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Leto TL, Morand S, Hurt D, and Ueyama T. Targeting and regulation of reactive oxygen species generation by Nox family NADPH oxidases. Antioxid Redox Signal 11: 260–267, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lob HE, Schultz D, Marvar PJ, Davisson RL, and Harrison DG. Role of the NADPH oxidases in the subfornical organ in angiotensin II-induced hypertension. Hypertension 61: 382–387, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Loffredo L, Carnevale R, Cangemi R, Angelico F, Augelletti T, Di Santo S, Calabrese CM, Della Volpe L, Pignatelli P, Perri L, Basili S, and Violi F. NOX2 up-regulation is associated with artery dysfunction in patients with peripheral artery disease. Int J Cardiol 165: 184–192, 2013 [DOI] [PubMed] [Google Scholar]
- 122.Loffredo L, Carnevale R, Sanguigni V, Plebani A, Rossi P, Pignata C, De Mattia D, Finocchi A, Martire B, Pietrogrande MC, Martino S, Gambineri E, Giardino G, Soresina AR, Martino F, Pignatelli P, and Violi F. Does NADPH oxidase deficiency cause artery dilatation in humans? Antioxid Redox Signal 8: 1491–1496, 2013 [DOI] [PubMed] [Google Scholar]
- 123.Lopes HF, Martin KL, Nashar K, Morrow JD, Goodfriend TL, and Egan BM. DASH diet lowers blood pressure and lipid-induced oxidative stress in obesity. Hypertension 41: 422–430, 2003 [DOI] [PubMed] [Google Scholar]
- 124.Loukogeorgakis SP, van den Berg MJ, Sofat R, Nitsch D, Charakida M, Haiyee B, de Groot E, MacAllister RJ, Kuijpers TW, and Deanfield JE. Role of NADPH oxidase in endothelial ischemia/reperfusion injury in humans. Circulation 121: 2310–2316, 2010 [DOI] [PubMed] [Google Scholar]
- 125.Ma L, Gul R, Habibi J, Yang M, Pulakat L, Whaley-Connell A, Ferrario CM, and Sowers JR. Nebivolol improves diastolic dysfunction and myocardial remodeling through reductions in oxidative stress in the transgenic (mRen2) rat. Am J Physiol Heart Circ Physiol 302: H2341–H2351, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Manea A, Manea SA, Florea IC, Luca CM, and Raicu M. Positive regulation of NADPH oxidase 5 by proinflammatory-related mechanisms in human aortic smooth muscle cells. Free Radic Biol Med 52: 1497–1507, 2012 [DOI] [PubMed] [Google Scholar]
- 127.Manea A, Tanase LI, Raicu M, and Simionescu M. Jak/STAT signaling pathway regulates nox1 and nox4-based NADPH oxidase in human aortic smooth muscle cells. Arterioscler Thromb Vasc Biol 30: 105–112, 2010 [DOI] [PubMed] [Google Scholar]
- 128.Manea A, Tanase LI, Raicu M, and Simionescu M. Transcriptional regulation of NADPH oxidase isoforms Nox1 and Nox4, by nuclear factor-kappaB in human aortic smooth muscle cells. Biochem Biophys Res Commun 396: 901–907, 2010 [DOI] [PubMed] [Google Scholar]
- 129.Mann JF, Lonn EM, Yi Q, Gerstein HC, Hoogwerf BJ, Pogue J, Bosch J, Dagenais GR, and Yusuf S; HOPE Investigators. Effects of vitamin E on cardiovascular outcomes in people with mild-to-moderate renal insufficiency: results of the HOPE study. Kidney Int 65: 1375–1380, 2004 [DOI] [PubMed] [Google Scholar]
- 130.Matsuno K, Yamada H, Iwata K, Jin D, Katsuyama M, Matsuki M, Takai S, Yamanishi K, Miyazaki M, Matsubara H, and Yabe-Nishimura C. Nox1 is involved in angiotensin II-mediated hypertension: a study in Nox1-deficient mice. Circulation 112: 2677–2685, 2005 [DOI] [PubMed] [Google Scholar]
- 131.Mazzanti L, Raffaelli F, Vignini A, Nanetti L, Vitali P, Boscarato V, Giannubilo SR, and Tranquilli AL. Nitric oxide and peroxynitrite platelet levels in gestational hypertension and preeclampsia. Platelets 23: 26–35, 2012 [DOI] [PubMed] [Google Scholar]
- 132.McCance DR, Holmes VA, Maresh MJ, Patterson CC, Walker JD, Pearson DW, and Young IS; Diabetes and Pre-eclampsia Intervention Trial (DAPIT) Study Group Vitamins C and E for prevention of pre-eclampsia in women with type 1 diabetes (DAPIT): a randomised placebo-controlled trial. Lancet 376: 259–266, 2010. 20580423 [Google Scholar]
- 133.McQueen MJ, Lonn E, Gerstein HC, Bosch J, and Yusuf S. The HOPE (Heart Outcomes Prevention Evaluation) Study and its consequences. Scand J Clin Lab Invest Suppl 240: 143–156, 2005 [DOI] [PubMed] [Google Scholar]
- 134.Meng FG. and Zhang ZY. Redox regulation of protein tyrosine phosphatase activity by hydroxyl radical. Biochim Biophys Acta 1834: 464–469, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Miller BA. and Zhang W. TRP channels as mediators of oxidative stress. Adv Exp Med Biol 704: 531–544, 2011 [DOI] [PubMed] [Google Scholar]
- 136.Modlinger P, Chabrashvili T, Gill PS, Mendonca M, Harrison DG, Griendling KK, Li M, Raggio J, Wellstein A, Chen Y, Welch WJ, and Wilcox CS. RNA silencing in vivo reveals role of p22phox in rat angiotensin slow pressor response. Hypertension 47: 238–244, 2006 [DOI] [PubMed] [Google Scholar]
- 137.Montezano AC, Burger D, Ceravolo GS, Yusuf H, Montero M, and Touyz RM. Novel Nox homologues in the vasculature: focusing on Nox4 and Nox5. Clin Sci (Lond) 120: 131–141, 2011 [DOI] [PubMed] [Google Scholar]
- 138.Montezano AC, Paravicini TM, Chignalia AZ, Yusuf H, Almasri M, He Y, He G, Callera GE, Krause K-H, Lambeth D, and Touyz RM. Nicotinamide adenine dinucleotide phosphate reduced oxidase 5 (Nox5) regulation by angiotensin II and endothelin-1 is mediated via calcium/calmodulin-dependent pathways in human endothelial cells. Circ Res 106: 1363–1373, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Moran JP, Cohen L, Greene JM, Xu G, Feldman EB, Hames CG, and Feldman DS. Plasma ascorbic acid concentrations relate inversely to blood pressure in human subjects. Am J Clin Nutr 57: 213–217, 1993 [DOI] [PubMed] [Google Scholar]
- 140.Moreau KL, Gavin KM, Plum AE, and Seals DR. Ascorbic acid selectively improves large elastic artery compliance in postmenopausal women. Hypertension 45: 1107–1112, 2005 [DOI] [PubMed] [Google Scholar]
- 141.Moreno MU, Jose GS, Fortuno A, Beloqui O, Diez J, and Zalba G. The C242T CYBA polymorphism of NADPH oxidase is associated with essential hypertension. J Hypertens 24: 1299–1306, 2006 [DOI] [PubMed] [Google Scholar]
- 142.Morrow JD. Quantification of isoprostanes as indices of oxidant stress and the risk of atherosclerosis in humans. Arterioscler Thromb Vasc Biol 25: 279–286, 2005 [DOI] [PubMed] [Google Scholar]
- 143.Mullan BA, Young IS, Fee H, and McCance DR. Ascorbic acid reduces blood pressure and arterial stiffness in type 2 diabetes. Hypertension 40: 804–809, 2002 [DOI] [PubMed] [Google Scholar]
- 144.Myint PK, Luben RN, Wareham NJ, and Khaw KT. Association between plasma vitamin C concentrations and blood pressure in the European prospective investigation into cancer-Norfolk population-based study. Hypertension 58: 372–379, 2011 [DOI] [PubMed] [Google Scholar]
- 145.Nagasaka H, Takayanagi M, and Tsukahara H. Children's toxicology from bench to bed—liver injury: oxidative stress and anti-oxidant systems in liver of patients with Wilson disease. J Toxicol Sci 34: SP229–SP236, 2009 [DOI] [PubMed] [Google Scholar]
- 146.Nautiyal M, Katakam PV, Busija DW, Gallagher PE, Tallant EA, Chappell MC, and Diz DI. Differences in oxidative stress status and expression of MKP-1 in dorsal medulla of transgenic rats with altered brain renin-angiotensin system. Am J Physiol Regul Integr Comp Physiol 303: R799–R806, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Newberry SJ. What is the evidence that vitamin C supplements lower blood pressure? Am J Clin Nutr 95: 997–998, 2012 [DOI] [PubMed] [Google Scholar]
- 148.Nguyen Dinh Cat A, Briones AM, Callera GE, Yogi A, He Y, Montezano AC, and Touyz RM. Adipocyte-derived factors regulate vascular smooth muscle cells through mineralocorticoid and glucocorticoid receptors. Hypertension 58: 479–488, 2011 [DOI] [PubMed] [Google Scholar]
- 149.Nguyen Dinh Cat A, Montezano AC, Burger D, and Touyz RM. Angiotensin II, NADPH oxidase, and redox signaling in the vasculature. Antioxid Redox Signal 19: 1110–1120, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Nishino T, Okamoto K, Eger BT, Pai EF, and Nishino T. Mammalian xanthine oxidoreductase—mechanism of transition from xanthine dehydrogenase to xanthine oxidase. FEBS J 275: 3278–3289, 2008 [DOI] [PubMed] [Google Scholar]
- 151.Nisimoto Y, Tsubouchi R, Diebold BA, Qiao S, Ogawa H, Ohara T, and Tamura M. Activation of NAD(P)H oxidase 1 in tumour colon epithelial cells. Biochem J 415: 57–65, 2008 [DOI] [PubMed] [Google Scholar]
- 152.Niu XL, Madamanchi NR, Vendrov AE, Tchivilev I, Rojas M, Madamanchi C, Brandes RP, Krause KH, Humphries J, Smith A, Burnand KG, and Runge MS. Nox activator 1: a potential target for modulation of vascular reactive oxygen species in atherosclerotic arteries. Circulation 121: 549–559, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Nuyt AM. Mechanisms underlying developmental programming of elevated blood pressure and vascular dysfunction: evidence from human studies and experimental animal models. Clin Sci (Lond) 114: 1–17, 2008 [DOI] [PubMed] [Google Scholar]
- 154.Oliveira PJ, Goncalves L, Monteiro P, Providencia LA, and Moreno AJ. Are the antioxidant properties of carvedilol important for the protection of cardiac mitochondria? Curr Vasc Pharmacol 3: 147–158, 2005 [DOI] [PubMed] [Google Scholar]
- 155.Pandey D. and Fulton DJ. Molecular regulation of NADPH oxidase 5 via the MAPK pathway. Am J Physiol Heart Circ Physiol 300: H1336–H1344, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Pandey D, Gratton JP, Rafikov R, Black SM, and Fulton DJ. Calcium/calmodulin-dependent kinase II mediates the phosphorylation and activation of NADPH oxidase 5. Mol Pharmacol 80: 407–415, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Pandey D, Patel A, Patel V, Chen F, Qian J, Wang Y, Barman SA, Venema RC, Stepp DW, Rudic RD, and Fulton DJ. Expression and functional significance of NADPH oxidase 5 (Nox5) and its splice variants in human blood vessels. Am J Physiol Heart Circ Physiol 302: H1919–H1928, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Paravicini TM, Montezano AC, Yusuf H, and Touyz RM. Activation of vascular p38MAPK by mechanical stretch is independent of c-Src and NADPH oxidase: influence of hypertension and angiotensin II. J Am Soc Hypertens 6: 169–178, 2012 [DOI] [PubMed] [Google Scholar]
- 159.Pescatore LA, Bonatto D, Forti FL, Sadok A, Kovacic H, and Laurindo FR. Protein disulfide isomerase is required for platelet-derived growth factor-induced vascular smooth muscle cell migration, Nox1 NADPH oxidase expression, and RhoGTPase activation. J Biol Chem 287: 29290–29300, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Petry A, Weitnauer M, and Görlach A. Receptor activation of NADPH oxidases. Antioxid Redox Signal 13: 467–472, 2010 [DOI] [PubMed] [Google Scholar]
- 161.Knock GA. and Ward JP. Redox regulation of protein kinases as a modulator of vascular function. Antioxid Redox Signal 15: 1531–1547, 2011 [DOI] [PubMed] [Google Scholar]
- 162.Rajagopalan S, Kurz S, Munzel T, Tarpey M, Freeman BA, and Griendling KK. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NAD(P)H oxidase activation. Contribution to alterations of vasomotor tone. J Clin Invest 97: 1916–1923, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Rathore R, Zheng YM, Niu CF, Liu QH, Korde A, Ho YS, and Wang YX. Hypoxia activates NADPH oxidase to increase [ROS]i and [Ca2+]i through the mitochondrial ROS-PKCepsilon signaling axis in pulmonary artery smooth muscle cells. Free Radic Biol Med 45: 1223–1231, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ray R, Murdoch CE, Wang M, Santos CX, Zhang M, Alom-Ruiz S, Anilkumar N, Ouattara A, Cave AC, Walker SJ, Grieve DJ, Charles RL, Eaton P, Brewer AC, and Shah AM. Endothelial Nox4 NADPH oxidase enhances vasodilatation and reduces blood pressure in vivo. Arterioscler Thromb Vasc Biol 31: 1368–1376, 2011 [DOI] [PubMed] [Google Scholar]
- 165.Renke M, Tylicki L, Rutkowski P, Larczynski W, Neuwelt A, Aleksandrowicz E, Łysiak-Szydłowska W, Rutkowski B. The effect of N-acetylcysteine on blood pressure and markers of cardiovascular risk in non-diabetic patients with chronic kidney disease: a placebo-controlled, randomized, cross-over study. Med Sci Monit 16: PI13–PI18, 2010 [PubMed] [Google Scholar]
- 166.Rey FE, Cifuentes ME, Kiarash A, Quinn MT, and Pagano PJ. Novel competitive inhibitor of NAD(P)H oxidase assembly attenuates vascular O2- and systolic blood pressure in mice. Circ Res 89: 408–414, 2001 [DOI] [PubMed] [Google Scholar]
- 167.Robbins IM, Hemnes AR, Gibbs JS, Christman BW, Howard L, Meehan S, Cabrita I, Gonzalez R, Oyler T, Zhao L, Du RH, Mendes LA, and Wilkins MR. Safety of sapropterin dihydrochloride (6r-bh4) in patients with pulmonary hypertension. Exp Lung Res 37: 26–34, 2011 [DOI] [PubMed] [Google Scholar]
- 168.Roberts JM, Myatt L, Spong CY, Thom EA, Hauth JC, Leveno KJ, Pearson GD, Wapner RJ, Varner MW, Thorp JM, Jr, Mercer BM, Peaceman AM, Ramin SM, Carpenter MW, Samuels P, Sciscione A, Harper M, Smith WJ, Saade G, Sorokin Y, Anderson GB; and Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network Vitamins C and E to prevent complications of pregnancy-associated hypertension. N Engl J Med 362: 1282–1291, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Rock CL, Jahnke MG, Gorenflo DW, Swartz RD, and Messana JM. Racial group differences in plasma concentrations of antioxidant vitamins and carotenoids in hemodialysis patients. Am J Clin Nutr 65: 844–850, 1997 [DOI] [PubMed] [Google Scholar]
- 170.Romanowski A, Murray IR, and Huston MJ. Effects of hydrogen peroxide on normal and hypertensive rats. Pharm Acta Helv 35: 354–357, 1960 [PubMed] [Google Scholar]
- 171.Saraswathi R, Sankar D, Ali A, Uehara Y, Abe S, Sambandam G, and Rao MR. A pilot assessment of oxidative stress byproducts and antioxidant activities among Indian patients with various stages of hypertension. Clin Exp Hypertens 33: 437–443, 2011 [DOI] [PubMed] [Google Scholar]
- 172.Schallreuter KU, Moore J, Wood JM, Beazley WD, Gaze DC, Tobin DJ, Marshall HS, Panske A, Panzig E, and Hibberts NA. In vivo and in vitro evidence for hydrogen peroxide (H2O2) accumulation in the epidermis of patients with vitiligo and its successful removal by a UVB-activated pseudocatalase. J Invest Dermatol Symp Proc 4: 91–96, 1999 [DOI] [PubMed] [Google Scholar]
- 173.Schiffer E, Liabeuf S, Lacroix C, Temmar M, Renard C, Monsarrat B, Choukroun G, Lemke HD, Vanholder R, Mischak H, and Massy ZA. Markers of vascular disease in plasma from patients with chronic kidney disease identified by proteomic analysis. J Hypertens 29: 783–790, 2011 [DOI] [PubMed] [Google Scholar]
- 174.Schiffrin EL. Antioxidants in hypertension and cardiovascular disease. Mol Interv 10: 354–362, 2010 [DOI] [PubMed] [Google Scholar]
- 175.Schiffrin EL. T lymphocytes: a role in hypertension? Curr Opin Nephrol Hypertens 19: 181–186, 2010 [DOI] [PubMed] [Google Scholar]
- 176.Schreiber R, Ferreira-Sae MC, Ronchi JA, Pio-Magalhães JA, Cipolli JA, Matos-Souza JR, Mill JG, Vercesi AE, Krieger JE, Franchini KG, Pereira AC, and Nadruz Junior W. The C242T polymorphism of the p22-phox gene (CYBA) is associated with higher left ventricular mass in Brazilian hypertensive patients. BMC Med Genet 12: 114–118, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Schröder K, Wandzioch K, Helmcke I, and Brandes RP. Nox4 acts as a switch between differentiation and proliferation in preadipocytes. Arterioscler Thromb Vasc Biol 29: 239–245, 2009 [DOI] [PubMed] [Google Scholar]
- 178.Schulz E. and Münzel T. NOX5, a new “radical” player in human atherosclerosis? J Am Coll Cardiol 52: 1810–1813, 2008 [DOI] [PubMed] [Google Scholar]
- 179.Schupp N, Kolkhof P, Queisser N, Gärtner S, Schmid U, Kretschmer A, Hartmann E, Oli RG, Schäfer S, and Stopper H. Mineralocorticoid receptor-mediated DNA damage in kidneys of DOCA-salt hypertensive rats. FASEB J 25: 968–978, 2011 [DOI] [PubMed] [Google Scholar]
- 180.Sedeek M, Callera G, Montezano A, Gutsol A, Heitz F, Szyndralewiez C, Page P, Kennedy CR, Burns KD, Touyz RM, and Hébert RL. Critical role of Nox4-based NADPH oxidase in glucose-induced oxidative stress in the kidney: implications in type 2 diabetic nephropathy. Am J Physiol Renal Physiol 299: F1348–F1358, 2010 [DOI] [PubMed] [Google Scholar]
- 181.Sedeek M, Gutsol A, Montezano AC, Burger D, Nguyen Dinh Cat A, Kennedy CR, Burns KD, Cooper ME, Jandeleit-Dahm K, Page P, Szyndralewiez C, Heitz F, Hebert RL, and Touyz RM. Renoprotective effects of a novel Nox1/4 inhibitor in a mouse model of Type 2 diabetes. Clin Sci (Lond) 124: 191–202, 2013 [DOI] [PubMed] [Google Scholar]
- 182.Sedeek M, Montezano AC, Hebert RL, Gray SP, Di Marco E, Jha JC, Cooper ME, Jandeleit-Dahm K, Schiffrin EL, Wilkinson-Berka JL, and Touyz RM. Oxidative stress, Nox isoforms and complications of diabetes—potential targets for novel therapies. J Cardiovasc Transl Res 5: 509–518, 2012 [DOI] [PubMed] [Google Scholar]
- 183.Selmici L. Advanced oxidation protein products (AOPP): novel uremic toxins, or components of the non-enzymatic antioxidant system of the plasma proteome? Free Radic Res 45: 1115–1123, 2011 [DOI] [PubMed] [Google Scholar]
- 184.Serg M, Kampus P, Kals J, Zagura M, Zilmer M, Zilmer K, Kullisaar T, and Eha J. Nebivolol and metoprolol: long-term effects on inflammation and oxidative stress in essential hypertension. Scand J Clin Lab Invest 72: 427–432, 2012 [DOI] [PubMed] [Google Scholar]
- 185.Serrander L, Jaquet V, Bedard K, Plastre O, Hartley O, Arnaudeau S, Demaurex N, Schlegel W, and Krause KH. NOX5 is expressed at the plasma membrane and generates superoxide in response to protein kinase C activation. Biochimie 89: 1159–1167, 2007 [DOI] [PubMed] [Google Scholar]
- 186.Seshiah PN, Weber DS, Rocic P, Valppu L, Taniyama Y, and Griendling KK. Angiotensin II stimulation of NAD(P)H oxidase activity: upstream mediators. Circ Res 91: 406–413, 2002 [DOI] [PubMed] [Google Scholar]
- 187.Sesso HD, Buring JE, Christen WG, Kurth T, Belanger C, MacFadyen J, Bubes V, Manson JE, Glynn RJ, and Gaziano JM. Vitamins E and C in the prevention of cardiovascular disease in men: the Physicians' Health Study II randomized controlled trial. JAMA 300: 2123–2133, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Sesso HD, Christen WG, Bubes V, Smith JP, MacFadyen J, Schvartz M, Manson JE, Glynn RJ, Buring JE, and Gaziano JM. Multivitamins in the prevention of cardiovascular disease in men: the Physicians' Health Study II randomized controlled trial. JAMA 308: 1751–1760, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Shaw JH. and Lloyd PG. Post-transcriptional regulation of placenta growth factor mRNA by hydrogen peroxide. Microvasc Res 84: 155–160, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Sheehan AL, Carrell S, Johnson B, Stanic B, Banfi B, and Miller FJ., , Jr.Role for Nox1 NADPH oxidase in atherosclerosis. Atherosclerosis 216: 321–326, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Silva PS, Fontana V, Palei AC, Sertório JT, Biagi C, and Tanus-Santos JE. Antihypertensive effects exerted by enalapril in mild to moderate hypertension are not associated with changes in the circulating levels of nitric oxide-related markers. Eur J Clin Pharmacol 67: 365–370, 2011 [DOI] [PubMed] [Google Scholar]
- 192.Simic DV, Mimic-Oka J, Pljesa-Ercegovac M, Savic-Radojevic A, Opacic M, Matic D, Ivanovic B, and Simic T. Byproducts of oxidative protein damage and antioxidant enzyme activities in plasma of patients with different degrees of essential hypertension. J Hum Hypertens 20: 149–155, 2006 [DOI] [PubMed] [Google Scholar]
- 193.Smith MPW, Banks RE, Wood SL, Lewington AJP. and Selby PJ. Application of proteomic analysis to the study of renal diseases. Nat Rev Nephrol 5: 701–712, 2009 [DOI] [PubMed] [Google Scholar]
- 194.Spychalowicz A, Wilk G, Sliwa T, Ludew D, and Guzik TJ. Novel therapeutic approaches in limiting oxidative stress and inflammation. Curr Pharm Biotechnol 13: 2456–2466, 2012 [PubMed] [Google Scholar]
- 195.Stanic B, Pandey D, Fulton DJ, and Miller FJ., Jr.Increased epidermal growth factor-like ligands are associated with elevated vascular nicotinamide adenine dinucleotide phosphate oxidase in a primate model of atherosclerosis. Arterioscler Thromb Vasc Biol 32: 2452–2460, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Stehr CB, Mellado R, Ocaranza MP, Carvajal CA, Mosso L, Becerra E, Solis M, García L, Lavandero S, Jalil J, and Fardella CE. Increased levels of oxidative stress, subclinical inflammation, and myocardial fibrosis markers in primary aldosteronism patients. J Hypertens 28: 2120–2126, 2010 [DOI] [PubMed] [Google Scholar]
- 197.Streeter J, Thiel W, Brieger K, and Miller FJ., Jr.Opportunity Nox: the future of NADPH oxidases as therapeutic targets in cardiovascular disease. Cardiovasc Ther 31: 125–137, 2013 [DOI] [PubMed] [Google Scholar]
- 198.Sundaram A, Siew Keah L, Sirajudeen KN, and Singh HJ. Upregulation of catalase and downregulation of glutathione peroxidase activity in the kidney precede the development of hypertension in pre-hypertensive SHR. Hypertens Res 36: 213–218, 2013 [DOI] [PubMed] [Google Scholar]
- 199.Tabet F, Savoia C, Schiffrin EL, and Touyz RM. Differential calcium regulation by hydrogen peroxide and superoxide in vascular smooth muscle cells from spontaneously hypertensive rats. J Cardiovasc Pharmacol 44: 200–208, 2004 [DOI] [PubMed] [Google Scholar]
- 200.Tabet F, Schiffrin EL, Callera GE, He Y, Yao G, Ostman A, Kappert K, Tonks NK, and Touyz RM. Redox-sensitive signaling by angiotensin II involves oxidative inactivation and blunted phosphorylation of protein tyrosine phosphatase SHP-2 in vascular smooth muscle cells from SHR. Circ Res 103: 149–154, 2008 [DOI] [PubMed] [Google Scholar]
- 201.Takac I, Schröder K, and Brandes RP. The Nox family of NADPH oxidases: friend or foe of the vascular system? Curr Hypertens Rep 14: 70–78, 2012 [DOI] [PubMed] [Google Scholar]
- 202.Takac I, Schröder K, Zhang L, Lardy B, Anilkumar N, Lambeth JD, Shah AM, Morel F, and Brandes RP. The E-loop is involved in hydrogen peroxide formation by the NADPH oxidase Nox4. J Biol Chem 286: 13304–13313, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Takahashi N, Kozai D, Kobayashi R, Ebert M, and Mori Y. Roles of TRPM2 in oxidative stress. Cell Calcium 50: 279–287, 2011 [DOI] [PubMed] [Google Scholar]
- 204.Terawaki H, Nakayama M, Miyazawa E, Murata Y, Nakayama K, Matsushima M, Miyazaki M, Sato H, Sato M, Sato T, Taguma Y, and Ito S. Effect of allopurinol on cardiovascular incidence among hypertensive nephropathy patients: the Gonryo study. Clin Exp Nephrol 2012November29 [Epub ahead of print]; PMID: 23192770 [DOI] [PubMed] [Google Scholar]
- 205.Toba H, Nakagawa Y, Miki S, Shimizu T, Yoshimura A, Inoue R, Asayama J, Kobara M, and Nakata T. Calcium channel blockades exhibit anti-inflammatory and antioxidative effects by augmentation of endothelial nitric oxide synthase and the inhibition of angiotensin converting enzyme in the N(G)-nitro-L-arginine methyl ester-induced hypertensive rat aorta: vasoprotective effects beyond the blood pressure-lowering effects of amlodipine and manidipine. Hypertens Res 28: 689–700, 2005 [DOI] [PubMed] [Google Scholar]
- 206.Touyz RM. and Montezano AC. Vascular Nox4: a multifarious NADPH oxidase. Circ Res 110: 1159–1161, 2012 [DOI] [PubMed] [Google Scholar]
- 207.Touyz RM. and Schiffrin EL. Increased generation of superoxide by angiotensin II in smooth muscle cells from resistance arteries of hypertensive patients: role of phospholipase D-dependent NAD(P)H oxidase-sensitive pathways. J Hypertens 19: 1245–1254, 2001 [DOI] [PubMed] [Google Scholar]
- 208.Touyz RM. and Schiffrin EL. Reactive oxygen species in vascular biology: implications in hypertension. Histochem Cell Biol 122: 339–352, 2004 [DOI] [PubMed] [Google Scholar]
- 209.Touyz RM, Briones AM, Sedeek M, Burger D, and Montezano AC. NOX Isoforms and reactive oxygen species in vascular health. Mol Interv 11: 27–35, 2011 [DOI] [PubMed] [Google Scholar]
- 210.Touyz RM, Chen X, Tabet F, Yao G, He G, Quinn MT, Pagano PJ, and Schiffrin EL. Expression of a functionally active gp91phox-containing neutrophil-type NAD(P)H oxidase in smooth muscle cells from human resistance arteries: regulation by angiotensin II. Circ Res 90: 1205–1213, 2002 [DOI] [PubMed] [Google Scholar]
- 211.Touyz RM, Mercure C, He Y, Javeshghani D, Yao G, Callera GE, Yogi A, Lochard N, and Reudelhuber TL. Angiotensin II-dependent chronic hypertension and cardiac hypertrophy are unaffected by gp91phox-containing NAD(P)H oxidase. Hypertension 45: 530–537, 2005 [DOI] [PubMed] [Google Scholar]
- 212.Touyz RM, Tabet F, and Schiffrin EL. Redox-dependent signalling by angiotensin II and vascular remodelling in hypertension. Clin Exp Pharmacol Physiol 30: 860–866, 2003 [DOI] [PubMed] [Google Scholar]
- 213.Touyz RM, Yao G, Quinn MT, Pagano PJ, and Schiffrin EL. p47phox associates with the cytoskeleton through cortactin in human vascular smooth muscle cells: role in NAD(P)H oxidase regulation by angiotensin II. Arterioscler Thromb Vasc Biol 25: 512–518, 2005 [DOI] [PubMed] [Google Scholar]
- 214.Touyz RM. Molecular and cellular mechanisms in vascular injury in hypertension: role of angiotensin II. Curr Opin Nephrol Hypertens 14: 125–131, 2005 [DOI] [PubMed] [Google Scholar]
- 215.Touyz RM. New insights into mechanisms of hypertension. Curr Opin Nephrol Hypertens 21: 119–121, 2012 [DOI] [PubMed] [Google Scholar]
- 216.Trigueros-Motos L, Gonzalez JM, Rivera J, and Andres V. Hutchinson-Gilford progeria syndrome, cardiovascular disease and oxidative stress. Front Biosci (Schol Ed) 3: 1285–1297, 2011 [DOI] [PubMed] [Google Scholar]
- 217.Tyther R, Ahmeda A, Johns E, and Sheehan D. Protein carbonylation in kidney medulla of the spontaneously hypertensive rat. Proteomics Clin Appl 3: 338–346, 2009 [DOI] [PubMed] [Google Scholar]
- 218.Ushio-Fukai M, Alexander RW, Akers M, and Griendling KK. p38 Mitogen-activated protein kinase is a critical component of the redox-sensitive signaling pathways activated by angiotensin II. Role in vascular smooth muscle cell hypertrophy. J Biol Chem 273: 15022–15029, 1998 [DOI] [PubMed] [Google Scholar]
- 219.Valente AJ, Yoshida T, Murthy SN, Sakamuri SS, Katsuyama M, Clark RA, Delafontaine P, and Chandrasekar B. Angiotensin II enhances AT1-Nox1 binding and stimulates arterial smooth muscle cell migration and proliferation through AT1, Nox1, and interleukin-18. Am J Physiol Heart Circ Physiol 303: H282–H296, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Vaziri ND. and Rodriguez-Iturbe B. Mechanisms of disease: oxidative stress and inflammation in the pathogenesis of hypertension. Nat Clin Pract Nephrol 2: 582–593, 2006 [DOI] [PubMed] [Google Scholar]
- 221.Vaziri ND. Protective effect of Nrf2 and catalase in maternal diabetes-induced perinatal hypertension and kidney disease. Diabetes 61: 2400–2402, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Viel EC, Benkirane K, Javeshghani D, Touyz RM, and Schiffrin EL. Xanthine oxidase and mitochondria contribute to vascular superoxide anion generation in DOCA-salt hypertensive rats. Am J Physiol Heart Circ Physiol 295: H281–H288, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Vinh A, Chen W, Blinder Y, Weiss D, Taylor WR, Goronzy JJ, Weyand CM, Harrison DG, and Guzik TJ. Inhibition and genetic ablation of the B7/CD28 T-cell costimulation axis prevents experimental hypertension. Circulation 122: 2529–2537, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Violi F, Pignatelli P, Pignata C, Plebani A, Rossi P, Sanguigni V, Carnevale R, Soresina A, Finocchi A, Cirillo E, Catasca E, Angelico F, and Loffredo L. Reduced atherosclerotic burden in subjects with genetically determined low oxidative stress. Arterioscler Thromb Vasc Biol 33: 406–412, 2013 [DOI] [PubMed] [Google Scholar]
- 225.Violi F, Sanguigni V, Carnevale R, Plebani A, Rossi P, Finocchi A, Pignata C, De Mattia D, Martire B, Pietrogrande MC, Martino S, Gambineri E, Soresina AR, Pignatelli P, Martino F, Basili S, and Loffredo L. Hereditary deficiency of gp91(phox) is associated with enhanced arterial dilatation: results of a multicenter study. Circulation 120: 1616–1622, 2009 [DOI] [PubMed] [Google Scholar]
- 226.Virdis A, Neves MF, Amiri F, Touyz RM, and Schiffrin EL. Role of NAD(P)H oxidase on vascular alterations in angiotensin II-infused mice. J Hypertens 22: 535–542, 2004 [DOI] [PubMed] [Google Scholar]
- 227.Walker AE. and Kaplon RE, Lucking SM, Russell-Nowlan MJ, Eckel RH, Seals DR. Fenofibrate improves vascular endothelial function by reducing oxidative stress while increasing endothelial nitric oxide synthase in healthy normolipidemic older adults. Hypertension 60: 1517–1523, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.White SJ, Hayes EM, Lehoux S, Jeremy JY, Horrevoets AJ, and Newby AC. Characterization of the differential response of endothelial cells exposed to normal and elevated laminar shear stress. J Cell Physiol 226: 2841–2848, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Wray DW, Nishiyama SK, Harris RA, Zhao J, McDaniel J, Fjeldstad AS, Witman MA, Ives SJ, Barrett-O'Keefe Z, and Richardson RS. Acute reversal of endothelial dysfunction in the elderly after antioxidant consumption. Hypertension 59: 818–824, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Wray DW, Uberoi A, Lawrenson L, Bailey DM, and Richardson RS. Oral antioxidants and cardiovascular health in the exercise-trained and untrained elderly: a radically different outcome. Clin Sci (Lond) 116: 433–441, 2009 [DOI] [PubMed] [Google Scholar]
- 231.Wu KL, Chan SH, and Chan JY. Neuroinflammation and oxidative stress in rostral ventrolateral medulla contribute to neurogenic hypertension induced by systemic inflammation. J Neuroinflammation 9: 212–216, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Wu RF, Ma Z, Liu Z, and Terada LS. Nox4-derived H2O2 mediates endoplasmic reticulum signaling through local Ras activation. Mol Cell Biol 30: 3553–3568, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Xu S, He Y, Vokurkova M, and Touyz RM. Endothelial cells negatively modulate reactive oxygen species generation in vascular smooth muscle cells: role of thioredoxin. Hypertension 54: 427–433, 2009 [DOI] [PubMed] [Google Scholar]
- 234.Yogi A, Mercure C, Touyz J, Callera GE, Montezano AC, Aranha AB, Tostes RC, Reudelhuber T, and Touyz RM. Renal redox-sensitive signaling, but not blood pressure, is attenuated by Nox1 knockout in angiotensin II-dependent chronic hypertension. Hypertension 51: 500–506, 2008 [DOI] [PubMed] [Google Scholar]
- 235.Young CN, Cao X, Guruju MR, Pierce JP, Morgan DA, Wang G, Iadecola C, Mark AL, and Davisson RL. ER stress in the brain subfornical organ mediates angiotensin-dependent hypertension. J Clin Invest 122: 3960–3964, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Zalba G, Beaumont FJ, San Jose G, Fortuno A, Fortuno MA, and Etayo JC. Vascular NADH/NAD(P)H oxidase is involved in enhanced superoxide production in spontaneously hypertensive rats. Hypertension 35: 1055–1061, 2000 [DOI] [PubMed] [Google Scholar]
- 237.Zalba G, San Jose G, Moreno MU, Fortuno A, and Diez J. NADPH oxidase-mediated oxidative stress: genetic studies of the p22(phox) gene in hypertension. Antioxid Redox Signal 7: 1327–1336, 2005 [DOI] [PubMed] [Google Scholar]
- 238.Zhang M, Brewer AC, Schröder K, Santos CX, Grieve DJ, Wang M, Anilkumar N, Yu B, Dong X, Walker SJ, Brandes RP, and Shah AM. NADPH oxidase-4 mediates protection against chronic load-induced stress in mouse hearts by enhancing angiogenesis. Proc Natl Acad Sci U S A 107: 18121–18126, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Zimmerman MC, Sharma RV, and Davisson RL. Superoxide mediates angiotensin II-induced influx of extracellular calcium in neural cells. Hypertension 45: 717–723, 2005 [DOI] [PubMed] [Google Scholar]
- 240.Zureik M, Galan P, Bertrais S, Mennen L, Czernichow S, Blacher J, Ducimetière P, and Hercberg S. Effects of long-term daily low-dose supplementation with antioxidant vitamins and minerals on structure and function of large arteries. Arterioscler Thromb Vasc Biol 24: 1485–1491, 2004 [DOI] [PubMed] [Google Scholar]