Abstract
Tularemia outbreaks in humans have recently been reported in many European countries, but data on the occurrence in the animal population are scarce. In North America, seroconversion of omnivores and carnivores was used as indicator for the presence of tularemia, for the European fauna, however, data are barely available. Therefore, the suitability of wild boars (Sus scrofa) and red foxes (Vulpes vulpes) as indicators for the circulation of F. tularensis in Germany was evaluated. Serum samples from 566 wild boars and 457 red foxes were collected between 1995 and 2012 in three federal states in Central Germany (Hesse, Saxony-Anhalt, and Thuringia). The overall rate of seropositive animals was 1.1% in wild boars and 7.4% in red foxes. In conclusion, serological examination of red foxes is recommended, because they can be reliably used as indicator animals for the presence of F. tularensis in the environment.
Key Words: : Francisella tularensis, Fox, Tularemia, Wild boar, Wildlife, Zoonoses
Introduction
Francisella tularensis is a Gram-negative highly contagious bacterium. This zoonotic pathogen has been isolated from a wide range of animals that can be affected or play a role as reservoir (Ellis et al. 2002). Small rodents and lagomorphs are highly susceptible to F. tularensis, whereas omnivores and carnivores usually only seroconvert and remain healthy (Gese et al. 2004, Bischof and Rogers 2005). Various ticks, hematophagous insects, amoeba, and protozoa may be reservoirs and vectors (Gordon et al. 1983, Wicki et al. 2000, Gurycova et al. 2001, Keim et al. 2007, Zhan et al. 2009, Goethert and Telfort 2010, Kreizinger et al. 2013). In Europe, tularemia is exclusively caused by F. tularensis subsp. holarctica and mainly transmitted by contact with infected brown hares (Lepus europaeus) (Mörner et al. 1988, Anonymous 2000, Strauss and Pohlmeyer 2001, Anonymous 2002, Pikula et al. 2004, Keim et al. 2007, Splettstoesser et al. 2009, Gyuranecz et al. 2010). Transmission occurs by dermal and mucosal contact, orally, through contaminated aerosols, or by arthropod bites. In humans, the disease occurs in various clinical forms depending on the route of infection and the infectious dose. Systemic disease can have a fatal outcome. Although tularemia is a rare disease in Europe, outbreaks in humans have been reported in many countries, and individuals undertaking outdoor activities are particularly at risk (Tärnvik et al. 2004, Hofstetter et al. 2006, Larssen et al. 2006, Wik 2006, Komitova et al. 2010, Grunow et al. 2012). The often uncharacteristic signs and symptoms of tularemia in combination with low disease awareness can result in delayed or incorrect therapy (Komitova et al. 2010). In Germany, between 2005 and 2012, 130 human cases were reported to the Robert Koch Institute (Berlin, Germany) (SurvStat, http://www3.rki.de/SurvStat, 10.07.2013) with increasing numbers of cases per year. Tularemia is an autochthonous zoonosis in Germany, but imported cases have also been described (Schubert et al. 2011). However, studies on the natural reservoir and vectors and the resulting potential exposure of the human population are only fragmentary and practically incomparable (Kaysser et al. 2008, Splettstoesser et al. 2009, Decors et al. 2011, Kuehn et al. 2013).
Cultivation of F. tularensis and detection of Francisella-specific antigens or DNA by enzyme-linked immunosorbent assay (ELISA) or PCR assay are standard procedures to diagnose tularemia in infected animals. Many animal species do not develop clinical signs of disease, and bacteremia lasts only for a short period. Thus, there is little chance to obtain a positive result by antigen or DNA detection. Accordingly, testing for seroconversion is more adequate to ascertain previous exposure to the pathogen. Several serological methods can be used to detect antibodies against F. tularensis, such as ELISA (Porsch-Özcürümez et al. 2004, Schmitt et al. 2005), micro- or tube agglutination assay (Dedek et al. 1986, Dedek et al. 1990, Sato et al. 1990), and western blot assay (Schmitt et al. 2005). These procedures are established for human (Anonymous 2007) and animal samples (Mörner 2012). Schmitt et al. (2005) and Porsch-Özcürümez et al. (2004) demonstrated that the combination of ELISA and western blot assay is suitable for screening in humans.
Carnivores and omnivores have been shown to be adequate indicator animals for several zoonotic diseases, including plague and tularemia (McKeever et al. 1958, Vest et al. 1965, McCue and O'Farrell 1988, Miller et al. 2000, Ellis et al. 2002, Bischof and Rogers 2005). In northwestern Germany, antibodies to F. tularensis could be detected in only one out of 1061 sera of wild boars (Sus scrofa) collected between 1977 and 1984 (Dedek et al. 1986), whereas in another study, 3.1% of sera (n=763) were positive between 1995 and 1996 (Al Dahouk et al. 2005). Red foxes (Vulpes vulpes) may also become infected orally through consumption of infected animals, contaminated water sources, and ectoparasites. In the federal state of Brandenburg, Germany, 6.4–7.9% of wild animals (red foxes, raccoon dogs, and wild boars) were found seropositive between 2005 and 2009 (Kuehn et al. 2013).
Wild boars are routinely sampled in the frame of mandatory screening programs (e.g., classical swine fever), and red foxes are tested for rabies. Therefore, these animals are also ideal candidates for use as indicator animals for the circulation of F. tularensis in Germany. The present study was performed in central Germany in the two federal states of Hesse and Thuringia, where several cases of tularemia in hares have been documented each year, and in Saxony-Anhalt, where tularemia seems to be extremely rare (Müller et al. 2013).
Material and Methods
Wild boar and red fox samples
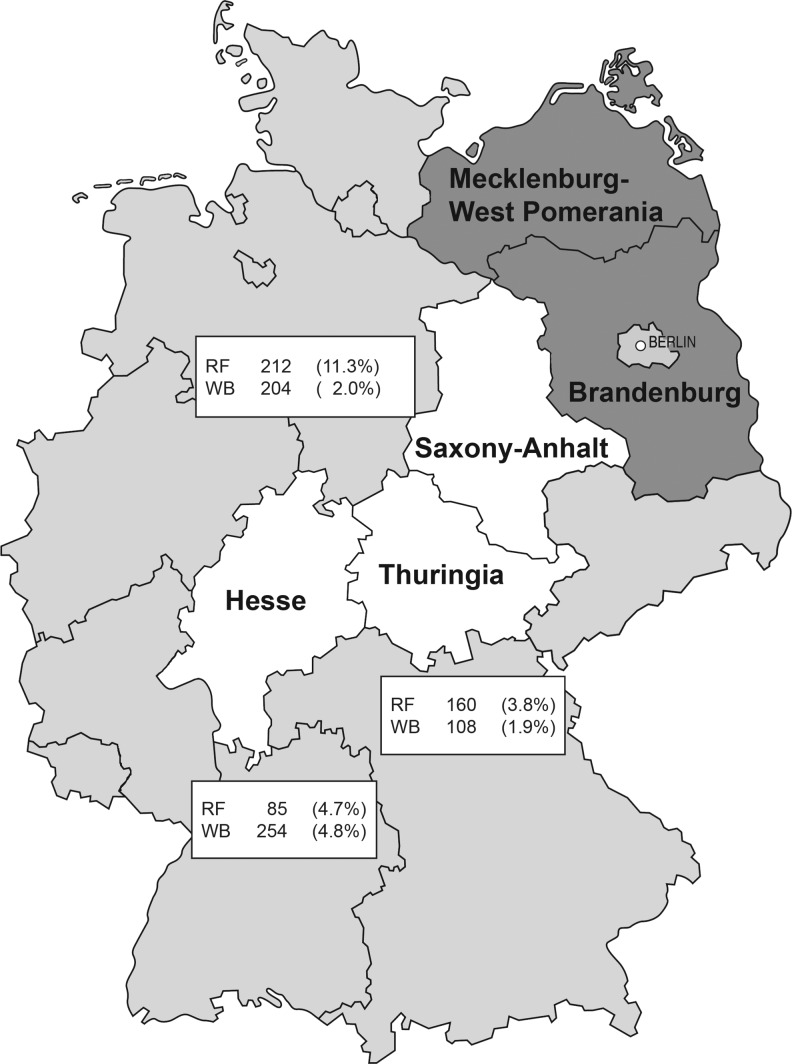
A percentage of animals with anti-Francisella antibodies above 2% (p=0.02) was considered typical for an endemic area in which human cases can potentially occur. Fosgate calculated the number needed to detect the disease with an expected prevalence of 2% and 95% confidence to be 149 samples (Fosgate 2009). Serum samples obtained from wild boars and red foxes were collected during the hunting seasons 2009–2012. A total of 407 fox samples were tested: 85 from Hesse, 212 from Saxony-Anhalt, and 110 from Thuringia. In total, 566 sera of wild boars were tested: 254 sera from Hesse, 204 from Saxony-Anhalt, and 108 from Thuringia. Furthermore, 50 red fox samples collected in Thuringia in 1995 were also included in this study.
A two-fold serial dilution of a positive sample was tested in the in-house ELISA and western blot assay to compare the analytical sensitivity of both assays.
Brucella spp. and Yersinia enterocolitica–positive swine samples
Cross-reactivity with Yersinia spp. and Brucella spp. was evaluated with a Y. enterocolitica–positive swine sample obtained by pooling sera from four experimentally infected animals and 11 Brucella suis biovar 2 swine samples.
F. tularensis subsp. holarctica–positive swine samples
The sensitivity of the assays was assessed with serum samples collected from four pigs immunized with whole-cell antigens of F. tularensis subsp. holarctica. The isolates 06T0001 and 10T0014 were cultivated on cysteine heart agar and harvested from plates with phosphate-buffered saline (PBS). The suspensions revealed optical densities of 0.51 and 0.53 at 600 (OD600=1.0), corresponding to an average of 8.0×107 colony-forming units/mL. After inactivation for 10 min at 90°C, the suspensions were mixed with aluminum hydroxide and injected intramuscularly in two 3-week-old pigs. Three weeks later, another four immunizations were performed at weekly intervals. Blood samples were taken before immunization and 1 week after the last immunization.
Assays
The in-house ELISA was performed as published by Porsch-Özcürümez et al. (2004) with some modifications. Microplates (96-well; Polysorb; Nunc, Roskilde, Denmark) were coated with 50 μL of a highly purified lipopolysaccharide (LPS) fraction (micromun, Greifswald, Germany) from the F. tularensis subsp. holarctica strain live vaccine strain (LVS) with a concentration of 5 μg LPS/mL in carbonate buffer (pH 9.0) overnight. The excess antigen was then removed, and the wells were washed three times with washing buffer (0.01 M PBS, pH 7.3; 0.05% Tween 20). The washing buffer was removed and 75 μL of blocking buffer (washing buffer with 4% skimmed milk powder) were added to the wells for 1 h at room temperature. The blocking buffer was removed, and the plates were washed with washing buffer, as described above. Serum samples were diluted 1:50 in dilution buffer (washing buffer with 1% skimmed milk powder). A total of 100 μL of each diluted serum sample was added to two antigen-coated wells. After 1 h of incubation at 4°C, the samples were removed and the plates were washed with washing buffer. After that, Protein G conjugated with horseradish peroxidase (Millipore, Temecula, CA), diluted 1:5,000 in dilution buffer, was added to the wells. The plates were incubated for 1 h at 37°C and then washed with washing buffer. After removal of the buffer, 100 μL of o-phenyl-N-diamine (Sigma-Aldrich Chemie, Taufkirchen, Germany) was added to the wells. After 10 min, 50 μL of 2 M sulfuric acid was used to stop the reaction. The OD was measured at 492 nm using a TECAN Spectra Rainbow Thermo Microtiter Plate Reader (Tecan Deutschland, Crailsheim, Germany). On the basis of the distribution of values of OD, the cutoff values were calculated for samples as follows: Mean OD (Delta;E)±3×standard deviation (SD). Samples with a mean OD492 value above 0.1 were considered as positive, and those below this value as negative.
The western blot assay published by Schmitt et al. (2005) was performed with slight modifications. Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) was performed using 4–12% Novex Tris-glycine gel (Invitrogen, Karlsruhe, Germany). A 180-μL stock solution of LPS (micromun) was mixed with 180 μL of Laemmli buffer (Sigma-Aldrich-Chemie). After boiling for 10 min, the mixture was subjected to SDS-PAGE at 200 V for 1 h. The gel was soaked for 10 min in Novex Tris-glycine transfer buffer (Invitrogen), and the separated LPS was transferred to a 0.45-μm nitrocellulose membrane (Invitrogen) using Novex Tris-glycine transfer buffer at 30 V for 1 h. The remaining binding sites on the membrane were blocked with blocking buffer (CANDOR, Wangen, Germany) overnight at 4°C. The cut membrane strips were incubated for 1.5 h at room temperature with serum dilution at 1:25, but the positive serum was diluted at 1:200. After three rinses with washing buffer, the strips were incubated for 1.5 h at room temperature with purified recomb® Protein A/G labeled with alkaline phosphatase (Pierce Biotechnology, Rockford, IL) in a dilution of 1:5000. A nitro-blue tetrazolium chloride/5-bromo-4-chloro-3-indolylphosphate solution induced the color reaction.
Samples that reacted positive in ELISA were subsequently tested in the western blot assay. Sera that demonstrated divergent results in tularemia assays were tested for the presence of antibodies against Brucella spp. and Yersinia spp. The presence of Brucella spp.–specific antibodies was determined using the Svanovir® Brucella-Ab C-ELISA kit (Svanova Biotech AB, Uppsala, Sweden). The recomLine Yersinia IgG 2.0 kit (Mikrogen, Neuried, Germany) was used to detect the presence of antibodies against Yersinia spp. Both tests were performed according to the manufacturer's instructions.
Results
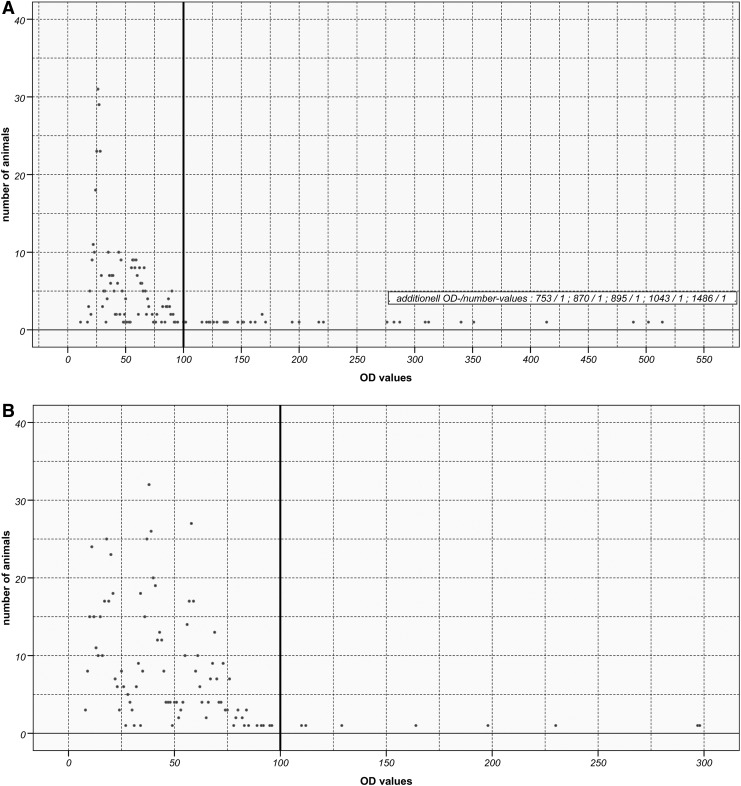
In Hesse, 4.8% of wild boars and 4.7% of red foxes were positive for antibodies to F. tularensis; in Saxony-Anhalt, 2.0% of wild boars and 11.3% of red foxes and in Thuringia, 1.9% of wild boars and 3.8% of red foxes were positive (Fig. 1). The 50 fox samples from Thuringia collected in 1995 were all negative. The distribution of the OD values obtained in the in-house ELISA for red foxes and wild boars shown in Figure 2, A and B, revealed that for both wild boars and foxes the respective frequency distributions of OD values above 100 showed only single events. For foxes and wild boars, the mean value (ΔE) of all OD values below or equal to 100 was calculated to be 42.7 (SD=20.1) and 38.7 (SD=20.1), respectively. For seven red fox samples, the western blot assay results were not confirmed in the ELISA. One of them was positive for Brucella spp. and three samples reacted with Yersinia spp. antigen.
FIG. 1.
The number of examined (absolutely) and Francisella-positive (relatively) red foxes (RF) and wild boars (WB) in the central states Hesse, Saxony-Anhalt, and Thuringia, Germany.
FIG. 2.
Optical density (OD) values obtained in in-house enzyme-linked immunosorbent assay (ELISA) for red foxes (A) and wild boars (B). Cutoff is marked with a bold line.
Prior to immunization, the pigs were negative for Brucella, Francisella, and Yersinia antibodies as assessed with ELISA. One week after the last Francisella vaccination, specific antibody titers of 1:100, 1:200, or 1:500 were found in three animals, respectively. The ELISA displayed a higher analytical sensitivity and detected one dilution step more than the western blot assay (data not shown). The specificity and the diagnostic sensitivity of the in-house ELISA were determined to be 90.0% and 83.3% when compared with the western blot assay. Brucella spp.– and Y. enterocolitca–positive swine samples did not react positively in the tularemia ELISA.
Discussion
Tularemia is a zoonotic disease in Europe, which is notifiable in Germany, because monitoring is considered necessary for prevention and control of the disease. European brown hares are the most affected animals, and contact with infected animals is the main source of infection in humans. Hares are highly susceptible to F. tularensis and usually die rapidly after infection (Mörner and Sandstedt 1983, Mörner et al. 1988, Decors et al. 2011), although a few chronic cases have been reported (Runge et al. 2011, Hofer 2012). No seropositive hares could be found in the federal state of Schleswig-Holstein, Germany (Frölich et al. 2003). However, in Lower Saxony, F. tularensis subsp. holarctica was detected by PCR and cultivation in 1.1% of the examined hares (Runge et al. 2011). Higher infection rates of 5.1% and 10.0% were found in two serological studies in hares in Hungary (Gyuranecz et al. 2011) and in Mecklenburg-Western Pomerania, Germany, respectively (Dedek et al. 1990).
In contrast to hares and rabbits, foxes and wild boars are screened in monitoring programs for rabies and classical swine fever, respectively. Therefore, serological samples are readily available, and we investigated red foxes and wild boars as indicators for the presence and circulation of F. tularensis in three German federal states (Hesse, Saxony-Anhalt, Thuringia; Fig. 1). In this study, the overall seropositivity was determined to be 1.1% in wild boars and 7.4% in red foxes. Looking at the results separately for the three regions, it is notable that the results of wild boars differ only slightly (1.9–4.8%), whereas strong differences are observed in foxes. In Saxony-Anhalt, the highest detection rate in foxes was found to be 11.3%. In this federal state, five human tularemia cases were reported in the period 2005–2012, whereas in Hesse and Thuringia only one case was reported in each federal state (SurvStat, http://www3.rki.de/SurvStat, 10.07.2013). However, due to the small number of human cases, no conclusion can be drawn with regard to a correlation between seropositive animals and the number of human cases.
A reason for the higher seropositivity of foxes in comparison to wild boars could be that foxes eat more voles and mice. These rodents are highly susceptible to F. tularensis and play a role as a natural reservoir of tularemia. The lack of detection of Francisella antibodies in the Thuringian foxes from 1995 does not change this assessment.
Al Dahouk et al. (2005) found a rate of 3.1% positive animals in wild boars in the federal state of Mecklenburg-Western Pomerania, and Kuehn et al. (2013) found 7.9% seropositive foxes and 7.5% wild boars in the federal state of Brandenburg. Conflicting results were reported for the time from 1977 to 1984 by Dedek et al. (1986), with only one positive animal among 1061 wild boars. This may be explained by the use in this older study of a microagglutination test, which has a much lower sensitivity than the ELISA used in the more recent studies.
In all three studies, the occurrence of tularemia in the animal population was higher than the small number of reported human cases might suggest. Between 1974 and 2005, 14, five, and one human cases were reported in Hesse, Saxony-Anhalt, and Thuringia, respectively (Grunow and Priebe 2007). Accordingly, the number of human cases is not necessarily representative of the presence of tularemia in the natural reservoir. Natural foci can be small, and Francisella cells are also known to survive in the environment without leading to outbreaks in human or animal populations (Sjöstedt 2007). Runge et al. (2011) observed this phenomenon in a German region with a high prevalence of tularemia in hares where no human cases were reported. The results of the present study suggest a similar situation in other German regions. However, tularemia is a rare disease with often unspecific symptoms, which may lead to underreporting in the human population (Pohanka et al. 2011). The western blot assay used as confirmatory assay was slightly less sensitive than the in-house ELISA, and therefore some weak positive samples could not be confirmed. However, the specificity of the in-house ELISA was sufficient: No cross-reaction with Yersinia spp.– and Brucella spp.–positive animal samples was observed. In contrast, some cross-reactions were observed in the western blot assay. Of the seven samples displaying incongruent results in tularemia tests, one was positive for Brucella spp. and three were positive for Yersinia spp.
This study demonstrates that red foxes are more suitable indicators for the presence of tularemia than wild boars, and we recommend systematic screening to monitor the geographic distribution and the activity of natural foci that may lead to human infections. These data may stimulate the awareness of physicians for tularemia in potentially affected regions and may thus result in better diagnosis and treatment.
Acknowledgments
The authors are thankful for the excellent technical work done by Renate Danner, Wolfram Maginot, and Carola Zmuda. We are grateful to Tonia Eckert, Tobias Eisengarten, Bernd Gehrmann, Lothar Hoffmann, Annette Schliephake, and Claudia Sauerwald for providing samples of foxes and wild boars. We thank Peter Valentin-Weigand and Bruno Garin-Bastuji for a Y. enterocolitica–positive swine sample and 11 Brucella suis biovar 2 swine samples, respectively.
Valérie Chaignat was financially supported by the European Community's Seventh Framework Programme (FP7/2007-2013) under grant agreement no. 261810.
Author Disclosure Statement
No competing financial interests exist.
References
- Al Dahouk S, Nöckler K, Tomaso H, Splettstoesser WD, et al. Seroprevalence of brucellosis, tularemia, and yersiniosis in wild boars (Sus scrofa) from north-eastern Germany. J Vet Med B Infect Dis Vet Public Health 2005; 52:444–455 [DOI] [PubMed] [Google Scholar]
- Anonymous Case report: Tularemia after consumption of hare meat (in German). Epidemiol Bull 2000; Nr. 18:146 [Google Scholar]
- Anonymous Tularemia—two cases of disease after processing and consumption of a hare (in German). Epidemiol Bull 2002; No. 9:71–72 [Google Scholar]
- Anonymous WHO Guidelines on Tularaemia. World Health Organization, Epidemic and Pandemic Alert and Response. Geneva: WHO Press, 2007 [Google Scholar]
- Bischof R, Rogers DG.Serologic survey of select infectious diseases in coyotes and raccoons in Nebraska. J Wildl Dis 2005; 41:787–791 [DOI] [PubMed] [Google Scholar]
- Decors A, Lesage C, Jourdain E, Giraud P, et al. Outbreak of tularaemia in brown hares (Lepus europaeus) in France, January to March 2011. Euro Surveill 2011; 16:pii= [PubMed] [Google Scholar]
- Dedek J, Loepelmann H, Nattermann H.Serological examinations of black game for brucellosis and tularaemia. Mh Vet Med 1986; 41:150–153 [Google Scholar]
- Dedek J, Nattermann H, Loepelmann H, Rinka E, et al. Results obtained from serological investigations of hare (Lepus europaeus Pallan, 1778) for selected infections (in German). Mh Vet Med 1990; 45:833–836 [Google Scholar]
- Ellis J, Oyston PCF, Green M, Titball RW, et al. Tularemia. Clin Microbiol Rev 2002; 15:631–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fosgate GT.Practical sample size calculations for surveillance and diagnostic investigations. J Vet Diagn Invest 2009; 21:3–14 [DOI] [PubMed] [Google Scholar]
- Frölich K, Wisser J, Schmuser H, Fehlberg U, et al. Epizootiologic and ecologic investigations of European brown hares (Lepus europaeus) in selected populations from Schleswig-Holstein, Germany. J Wildl Dis 2003; 39:751–761 [DOI] [PubMed] [Google Scholar]
- Gese EM, Karki SM, Klavetter ML, Schauster ER, et al. Serologic survey for canine infectious diseases among sympatric swift foxes (Vulpes velox) and coyotes (Canis latrans) in southeastern Colorado. J Wildl Dis 2004; 40:741–748 [DOI] [PubMed] [Google Scholar]
- Goethert HK, Telfort SR., 3rd Quantum of infection of Francisella tularensis tularensis in host-seeking Dermacentor variabilis. Ticks Tick-Borne Dis 2010; 1:66–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon JR, Mc Laughlin BG, Nutiuthai S.Tularaemia transmitted by ticks (Dermacentor andersoni) in Saskatchewan. Can J Comp Med 1983; 47:408–411 [PMC free article] [PubMed] [Google Scholar]
- Grunow R, Priebe H.-S.Tularämie—Zum Vorkommen in Deutschland, Analyse auf der Basis der Meldedaten von 1949 bis 2006. Epidemiol Bull 2007; 7:51–56 [Google Scholar]
- Grunow R, Kalaveshi A, Kühn A, Mulliqi-Osmani G, et al. Surveilance of tularaemia in Kosovo, 2001 to 2010. Euro Surveill 2012; 17:pii= [DOI] [PubMed] [Google Scholar]
- Gyuranecz M, Szeredi L, Makrai L, Fodor L, et al. Tularemia of European brown hare (Lepus europaeus). A pathological, histopathological, and immunohistological study. Vet Pathol 2010; 47:958–963 [DOI] [PubMed] [Google Scholar]
- Gyuranecz M, Rigo K, Dan A, Földvári G, et al. Investigation of the Ecology of Francisella tularensis during an Inter-Epizootic Period. Vector-Borne Zoonot Dis 2011; 11:1031–1037 [DOI] [PubMed] [Google Scholar]
- Gurycova D, Vyrostekova V, Khanakah G, Kocianova E, et al. Importance of surveillance of tularemia natural foci in the known endemic area of Central Europe, 1991–1997. Wien Klin Wochenschr 2001; 113:433–438 [PubMed] [Google Scholar]
- Hofer E. Occurrence and diagnosis of tularemia in Austria. Workshop of National Reference Laboratory for Tularemia of Friedrich Loeffler Institut, Jena, May10–11, 2012 [Google Scholar]
- Hofstetter I, Eckert J, Splettstoesser W, Hauri AM, et al. Tularaemia outbreak in hare hunters in the Darmstadt-Dieburg district, Germany. Euro Surveill 2006; 11:pii: [DOI] [PubMed] [Google Scholar]
- Kaysser P, Seibold E, Mätz-Rensing K, Pfeffer M, et al. Re-emergence of tularemia in Germany: Presence of Francisella tularensis in different rodent species in endemic areas. BMC Infect Dis 2008; 8:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keim P, Johansson A, Wagner DM.Molecular epidemiology, evolution, and ecology of Francisella. Ann NY Acad Sci 2007; 1105:30–66 [DOI] [PubMed] [Google Scholar]
- Komitova R, Nenova R, Padeshki P, Ivanov I, et al. Tularemia in Bulgaria 2003–2004. J Infect Dev Countries 2010; 4:689–694 [DOI] [PubMed] [Google Scholar]
- Kreizinger Z, Hornok S, Dán A, Hresko S, et al. Prevalence of Francisella tularensis and Francisella-like endosymbionts in the tick population of Hungary and the genetic variability of Francisella-like agents. Vector Borne Zoonotic Dis 2013; 13:160–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehn A, Schulze C, Kutzer P, Probst C, et al. Tularaemia seroprevalence of captured and wild animals in Germany: The fox (Vulpes vulpes) as a biological indicator. Epidemiol Infect 2013; 141:833–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larssen KW, Afset JE, Heier BT, Krogh T, et al. Outbreak of tularaemia in central Norway, January to March 2011. Euro Surveill 2011; 16:pii= [PubMed] [Google Scholar]
- McCue PM, O'Farrell TP.Serological survey for selected diseases in the endangered San Joaquin kit fox (Vulpes macrotis mutica). J Wildl Dis 1988; 24:274–281 [DOI] [PubMed] [Google Scholar]
- McKeever SJ, Schubert MD, Moody MD, Gorman GW, et al. Natural occurrence of tularemia in marsupials, carnivores, lagomorphs, and large rodents in south-western Gorgia and nortwestern Florida. J Infect Dis 1958; 103:120–126 [DOI] [PubMed] [Google Scholar]
- Miller DS, Covell DF, McLean RG, Adrian WJ, et al. Serologic survey for selected infectious disease agents in swift and kit foxes from the western United States. J Wildl Dis 2000; 36:798–805 [DOI] [PubMed] [Google Scholar]
- Mörner T, Sandstedt K. A serological survey of antibodies against Francisella tularensis in some Swedish mammals. Nord Vet Med 1983; 35:82–85 [PubMed] [Google Scholar]
- Mörner T, Sandstrom G, Mattsson R, Nilsson PO.Infections with Francisella tularensis biovar palaearctica in hares (Lepus timidus, Lepus europaeus) from Sweden. J Wildl Dis 1988; 24:422–433 [DOI] [PubMed] [Google Scholar]
- Mörner T.Manual of Diagnostic Tests and Vaccines for Terrestrial Animals (Mammals, Birds and Bees) In: OIE–World Organisation for Animal Health, 17th ed. Chapter 2.1.18. Paris: OIE, 2012:329–334 [Google Scholar]
- Müller W, Hotzel H, Otto P, Karger A, et al. German Francisella tularensis isolates from European brown hares (Lepus europaeus) reveal genetic and phenotypic diversity. BMC Microbiology 2013; 10.1186/1471-2180-13-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pikula J, Beklova M, Holesovoska Z, Treml F, et al. Ecology of European brown hare and distribution of natural foci of tularemia in the Czech Republic. Acta Vet Brno 2004; 73:267–273 [Google Scholar]
- Pohanka M, Chlibek R, Kuca K, Banouchová H, et al. Diagnosis of tularemia using biochemical, immunochemical and molecular methods: A review. Vet Med Czech 2011; 56:453–461 [Google Scholar]
- Porsch-Özcürümez M, Kischel N, Priebe H, Splettstösser W, et al. Comparison of enzyme-linked immunosorbent assay, Western blotting, microagglutination, indirect immunofluorescence assay, and flow cytometry for serological diagnosis of tularemia. Clin Diagn Lab Immunol 2004; 11:1008–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runge M, von Keyserlingk M, Braune S, Voigt U, et al. Prevalence of Francisella tularensis in brown hare (Lepus europaeus) populations in Lower Saxony, Germany. Eur J Wildlife Res 2011; 57:1085–1089 [Google Scholar]
- Sato T, Fujita H, Ohara Y, et al. Microagglutination test for early and specific serodiagnosis of tularemia. J Clin Microbiol 1990; 28:2372–2374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt P, Splettstösser W, Porsch-Özcürümez M, Finke EJ, et al. A novel screening ELISA and a confirmatory Western blot useful for diagnosis and epidemiological studies of tularemia. Epidemiol Infect 2005; 133:759–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert A, Splettstoesser W, Bätzing-Feigenbaum J.Tularaemia in Berlin—two independent cases in travellers returning from central Anatolia, Turkey, February 2011. Euro Surveill 2011; 16:pii= [PubMed] [Google Scholar]
- Sjöstedt A.Tularemia: History, epidemiology, pathogen physiology, and clinical manifestations. Ann NY Acad Sci 2007; 1105:1–29 [DOI] [PubMed] [Google Scholar]
- Splettstoesser WD, Piechotowski I, Buckendahl A, Frangoulidis D, et al. Tularemia in Germany: The tip of the iceberg? Epidemiol Infect 2009; 137:736–743 [DOI] [PubMed] [Google Scholar]
- Strauss E, Pohlmeyer K.Population density of European hares (Lepus europaeus Pallas, 1778) and hunting activity in Lower Saxony. Z Jagdwiss 2001; 47:43–62 [Google Scholar]
- Tärnvik A, Priebe HS, Grunow R.Tularaemia in Europe: An epidemiological overview. Scand J Infect Dis 2004; 36:350–355 [DOI] [PubMed] [Google Scholar]
- Vest ED, Lundgren DL, Parker DD, Johnson DE, et al. Results of a five-year survey for certain enzootic diseases in the fauna of western Utah. Am J Trop Med Hyg 1965; 14:124–135 [DOI] [PubMed] [Google Scholar]
- Wicki R, Sauter P, Mettler C, Natsch A, et al. Swiss Army Survey in Switzerland to determine the prevalence of Francisella tularensis, members of the Ehrlichia phagocytophila genogroup, Borrelia burgdorferi sensu lato, and tick-borne encephalitis virus in ticks. Eur J Clin Microbiol Infect Dis 2000; 19:427–432 [DOI] [PubMed] [Google Scholar]
- Wik O.Large tularaemia outbreak in Värmland, central Sweden. Euro Surveill 2006; 11:3052. [DOI] [PubMed] [Google Scholar]
- Zhan L, Cao WC, Chu CY, Jiang BG, et al. Tick-borne agents in rodents, China, 2004–2006. Emerg Infect Dis 2009; 15:1904–1908 [DOI] [PMC free article] [PubMed] [Google Scholar]