Abstract
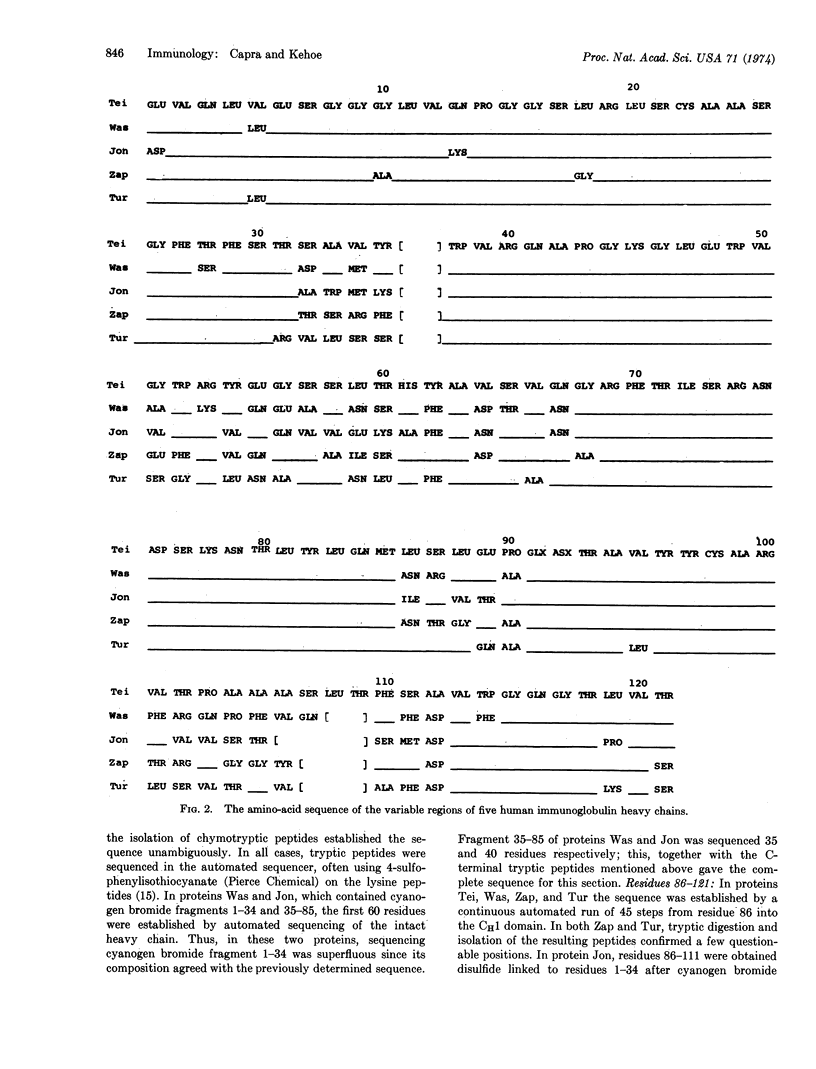
The variable regions of five human immunoglobulin heavy chains of the VHIII subgroup have been totally sequenced. Three of the heavy chains belonged to the IgG class and two to the IgA class. Examination of these sequences, and comparison with additional published heavy chain sequences, showed that a total of four hypervariable regions is characteristic of human heavy chain variable regions.
The relatively conserved character of large segments of the heavy chain variable region was very evident in these studies. The conserved segments, which are those sections located outside the hypervariable regions, comprise approximately 65% of the total heavy chain variable region. The following general structural pattern for antibody molecules emerges from this and related studies: an overall combining region superstructure is provided by the more conserved segments while the refinements of the active site specificity are a function of hypervariable regions.
Keywords: myeloma proteins, amino acid sequences, antibody combining site
Full text
PDF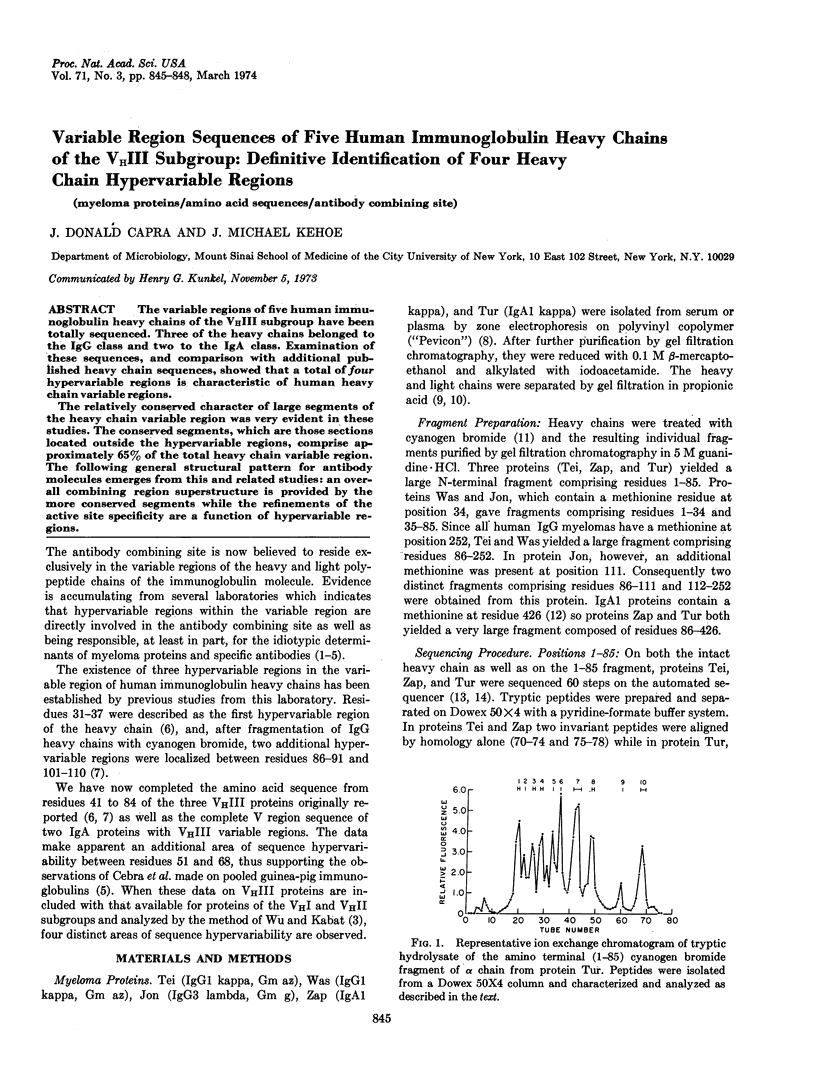


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bourgois A., Fougereau M., De Preval C. Sequence of amino acids of the NH 2 -terminal region of a mouse-clonal immunoglobulin heavy chain. Eur J Biochem. 1972 Jan 21;24(3):446–455. doi: 10.1111/j.1432-1033.1972.tb19705.x. [DOI] [PubMed] [Google Scholar]
- Capra J. D. Hypervariable region of human immunoglobulin heavy chains. Nat New Biol. 1971 Mar 10;230(10):61–63. doi: 10.1038/newbio230061a0. [DOI] [PubMed] [Google Scholar]
- Capra J. D., Kehoe J. M., Winchester R. J., Kunkel H. G. Structure-function relationships among anti-gamma globulin antibodies. Ann N Y Acad Sci. 1971 Dec 31;190:371–381. doi: 10.1111/j.1749-6632.1971.tb13549.x. [DOI] [PubMed] [Google Scholar]
- Capra J. D., Kunkel H. G. Aggregation of gamma-G3 proteins: relevance to the hyperviscosity syndrome. J Clin Invest. 1970 Mar;49(3):610–621. doi: 10.1172/JCI106272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capra J. D., Kunkel H. G. Amino acid sequence similarities in two human anti gamma globulin antibodies. Proc Natl Acad Sci U S A. 1970 Sep;67(1):87–92. doi: 10.1073/pnas.67.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capra J. D., Wasserman R. L., Kehoe J. M. Phylogenetically associated residues within the VH3 subgroup of several mammalian species. Evidence for a "pauci-gene" basis for antibody diversity. J Exp Med. 1973 Aug 1;138(2):410–427. doi: 10.1084/jem.138.2.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cebra J. J., Givol D., Porter R. R. Common peptides from the N-terminal half of heavy chain of immunoglobulin G from normal rabbit serum and a specific antibody. Biochem J. 1968 Mar;107(1):69–77. doi: 10.1042/bj1070069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesebro B., Metzger H. Affinity labeling of a phosphorylcholine binding mouse myeloma protein. Biochemistry. 1972 Feb 29;11(5):766–771. doi: 10.1021/bi00755a014. [DOI] [PubMed] [Google Scholar]
- Chuang C. Y., Capra J. D., Kehoe J. M. Evolutionary relationship between carboxyterminal region of a human alpha chain and other immunoglobulin heavy chain constant regions. Nature. 1973 Jul 20;244(5412):158–160. doi: 10.1038/244158a0. [DOI] [PubMed] [Google Scholar]
- Cunningham B. A., Pflumm M. N., Rutishauser U., Edelman G. M. Subgroups of amino acid sequences in the variable regions of immunoglobulin heavy chains. Proc Natl Acad Sci U S A. 1969 Nov;64(3):997–1003. doi: 10.1073/pnas.64.3.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman G. M., Cunningham B. A., Gall W. E., Gottlieb P. D., Rutishauser U., Waxdal M. J. The covalent structure of an entire gammaG immunoglobulin molecule. Proc Natl Acad Sci U S A. 1969 May;63(1):78–85. doi: 10.1073/pnas.63.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman P., Begg G. A protein sequenator. Eur J Biochem. 1967 Mar;1(1):80–91. doi: 10.1007/978-3-662-25813-2_14. [DOI] [PubMed] [Google Scholar]
- FLEISCHMAN J. B., PORTER R. R., PRESS E. M. THE ARRANGEMENT OF THE PEPTIDE CHAINS IN GAMMA-GLOBULIN. Biochem J. 1963 Aug;88:220–228. doi: 10.1042/bj0880220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischman J. B. Amino acid sequences in the Fd of a rabbit antibody heavy chain. Immunochemistry. 1973 Jun;10(6):401–407. doi: 10.1016/0019-2791(73)90147-x. [DOI] [PubMed] [Google Scholar]
- Franek F. Affinity labeling by m-nitrobenzenediazonium fluoroborate of porcine ANTI-dinitrophenyl antibodies. Position of labeled tyrosine in the lambda-chains. Eur J Biochem. 1971 Mar 11;19(2):176–183. doi: 10.1111/j.1432-1033.1971.tb01302.x. [DOI] [PubMed] [Google Scholar]
- GROSS E., WITKOP B. Nonenzymatic cleavage of peptide bonds: the methionine residues in bovine pancreatic ribonuclease. J Biol Chem. 1962 Jun;237:1856–1860. [PubMed] [Google Scholar]
- Goetzl E. J., Metzger H. Affinity labeling of a mouse myeloma protein which binds nitrophenyl ligands. Kinetics of labeling and isolation of a labeled peptide. Biochemistry. 1970 Mar 3;9(5):1267–1278. doi: 10.1021/bi00807a031. [DOI] [PubMed] [Google Scholar]
- Haimovich J., Eisen H. N., Hurwitz E., Givol D. Localization of affinity-labeled residues on the heavy and light chain of two myeloma proteins with anti-hapten activity. Biochemistry. 1972 Jun 20;11(13):2389–2398. doi: 10.1021/bi00763a001. [DOI] [PubMed] [Google Scholar]
- Inman J. K., Hannon J. E., Appella E. Demonstration of a simple method for reducing losses of tryptic peptides during automated sequencing. Biochem Biophys Res Commun. 1972 Mar 24;46(6):2075–2081. doi: 10.1016/0006-291x(72)90761-9. [DOI] [PubMed] [Google Scholar]
- KUNKEL H. G. Zone electrophoresis. Methods Biochem Anal. 1954;1:141–170. doi: 10.1002/9780470110171.ch6. [DOI] [PubMed] [Google Scholar]
- Kabat E. A., Wu T. T. Construction of a three-dimensional model of the polypeptide backbone of the variable region of kappa immunoglobulin light chains. Proc Natl Acad Sci U S A. 1972 Apr;69(4):960–964. doi: 10.1073/pnas.69.4.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehoe J. M., Capra J. D. Localization of two additional hypervariable regions in immunoglobulin heavy chains. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2019–2021. doi: 10.1073/pnas.68.9.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehoe J. M., Capra J. D. Sequence relationships among the variable regions of immunoglobulin heavy chains from various mammalian species. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2052–2055. doi: 10.1073/pnas.69.8.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel H. G., Agnello V., Joslin F. G., Winchester R. J., Capra J. D. Cross-idiotypic specificity among monoclonal IgM proteins with anti- -globulin activity. J Exp Med. 1973 Feb 1;137(2):331–342. doi: 10.1084/jem.137.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milstein C. Linked groups of residues in immunoglobulin k chains. Nature. 1967 Oct 28;216(5113):330–332. doi: 10.1038/216330a0. [DOI] [PubMed] [Google Scholar]
- Mole L. E., Jackson S. A., Porter R. R., Wilkinson J. M. Allotypically related sequences in the Fd fragment of rabbit immunoglobulin heavy chains. Biochem J. 1971 Sep;124(2):301–318. doi: 10.1042/bj1240301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponstingl H., Schwarz J., Reichel W., Hilschmann N. Die Primärstruktur eines monoklonalen gamma-1-Immunoglobulins (Myelomprotein NIE). I. Aminosäuresequenz des variablen Teils der H-Kette, Subgruppen variabler Teile. Hoppe Seylers Z Physiol Chem. 1970 Dec;351(12):1591–1594. [PubMed] [Google Scholar]
- Press E. M., Hogg N. M. The amino acid sequences of the Fd fragments of two human gamma-1 heavy chains. Biochem J. 1970 May;117(4):641–660. doi: 10.1042/bj1170641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray A., Cebra J. J. Localization of affinity-labeled residues in the primary structure of anti-dinitrophenyl antibody raised in strain 13 guinea pigs. Biochemistry. 1972 Sep 12;11(19):3647–3657. doi: 10.1021/bi00769a024. [DOI] [PubMed] [Google Scholar]
- Wikler M., Köhler H., Shinoda T., Putnam F. W. Macroglobulin structure: homology of mu and gamma heavy chains of human immunoglobulins. Science. 1969 Jan 3;163(3862):75–78. doi: 10.1126/science.163.3862.75. [DOI] [PubMed] [Google Scholar]
- Willims R. C., Jr, Kunkel H. G., Capra J. D. Antigenic specificities related to the cold agglutinin activity of gamma M globulins. Science. 1968 Jul 26;161(3839):379–381. doi: 10.1126/science.161.3839.379. [DOI] [PubMed] [Google Scholar]
- Wu T. T., Kabat E. A. An analysis of the sequences of the variable regions of Bence Jones proteins and myeloma light chains and their implications for antibody complementarity. J Exp Med. 1970 Aug 1;132(2):211–250. doi: 10.1084/jem.132.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]



