Abstract
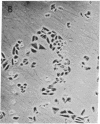
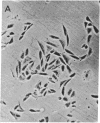
Ricin, a galactose-binding lectin with potent cytotoxic activity, was used to select a clone of Chinese hamster ovary cells with altered plant lectin-binding properties. The clone (15B) is 80-fold less sensitive to the toxic action of ricin than the parent line. In the absence of ricin, it grows both in monolayer and suspension culture with a normal generation time. Plating efficiency, however, is significantly reduced. Relative to the parent cells, its binding of the Ricinus communis lectins, Phaseolus vulgaris erythroagglutinating phytohemagglutinin, Abrus precatorius phytohemagglutinin, and soybean phytohemagglutinin is less than 7%, while binding of lentil phytohemagglutinin, wheat-germ agglutinin, and mushroom phytohemagglutinin is 17%, 40%, and 109%, respectively. In contrast, its concanavalin A binding is increased by 70%. Consistent with these alterations, crude membrane preparations of the 15B cells were found to contain the same sugars as the parent-cell membranes but in different proportions. The 15B membranes have 28% less sialic acid, 38% less N-acetylglucosamine, 49% less galactose, the same amount of N-acetylgalactosamine, and 53% more mannose than the membranes of the parent cells.
Keywords: ricin, glycoproteins, membranes
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cuatrecasas P. Protein purification by affinity chromatography. Derivatizations of agarose and polyacrylamide beads. J Biol Chem. 1970 Jun;245(12):3059–3065. [PubMed] [Google Scholar]
- Drysdale R. G., Herrick P. R., Franks D. The specificity of the haemagglutinin of the Castor bean, Ricinus communis. Vox Sang. 1968;15(3):194–202. doi: 10.1111/j.1423-0410.1968.tb01749.x. [DOI] [PubMed] [Google Scholar]
- Gürtler L. G., Horstmann H. J. Subunits of toxin and agglutinin of Ricinus communis. Biochim Biophys Acta. 1973 Feb 21;295(2):582–594. doi: 10.1016/0005-2795(73)90056-1. [DOI] [PubMed] [Google Scholar]
- Harris M. Mutation rates in cells at different ploidy levels. J Cell Physiol. 1971 Oct;78(2):177–184. doi: 10.1002/jcp.1040780204. [DOI] [PubMed] [Google Scholar]
- ISHIGURO M., TAKAHASHI T., FUNATSU G., HAYASHI K., FUNATSU M. BIOCHEMICAL STUDIES ON RICIN. I. PURIFICATION OF RICIN. J Biochem. 1964 Jun;55:587–592. doi: 10.1093/oxfordjournals.jbchem.a127930. [DOI] [PubMed] [Google Scholar]
- Kornfeld R., Keller J., Baenziger J., Kornfeld S. The structure of the glycopeptide of human gamma G myeloma proteins. J Biol Chem. 1971 May 25;246(10):3259–3268. [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. The structure of a phytohemagglutinin receptor site from human erythrocytes. J Biol Chem. 1970 May 25;245(10):2536–2545. [PubMed] [Google Scholar]
- Kornfeld S., Rogers J., Gregory W. The nature of the cell surface receptor site for Lens culinaris phytohemagglutinin. J Biol Chem. 1971 Nov;246(21):6581–6586. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lin J. Y., Liu K., Chen C. C., Tung T. C. Effect of crystalline ricin on the biosynthesis of protein, RNA, and DNA in experimental tumor cells. Cancer Res. 1971 Jul;31(7):921–924. [PubMed] [Google Scholar]
- Mezger-Freed L. Puromycin resistance in haploid and heteroploid frog cells: gene or membrane determined? J Cell Biol. 1971 Dec;51(3):742–751. doi: 10.1083/jcb.51.3.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolson G. L., Blaustein J. The interaction of Ricinus communis agglutinin with normal and tumor cell surfaces. Biochim Biophys Acta. 1972 May 9;266(2):543–547. doi: 10.1016/0005-2736(72)90109-5. [DOI] [PubMed] [Google Scholar]
- Olsnes S., Pihl A. Ricin - a potent inhibitor of protein synthesis. FEBS Lett. 1972 Feb 15;20(3):327–329. doi: 10.1016/0014-5793(72)80098-x. [DOI] [PubMed] [Google Scholar]
- Onozaki K., Tomita M., Sakurai Y., Ukita T. The mechanism of the cytotoxicity of Ricinus communis phytoagglutinin toward rat ascites tumor cells. Biochem Biophys Res Commun. 1972 Aug 21;48(4):783–788. doi: 10.1016/0006-291x(72)90675-4. [DOI] [PubMed] [Google Scholar]
- Poretz R. D., Goldstein I. J. An examination of the topography of the saccharide binding sites of concanavalin A and of the forces involved in complexation. Biochemistry. 1970 Jul 7;9(14):2890–2896. doi: 10.1021/bi00816a021. [DOI] [PubMed] [Google Scholar]
- Presant C. A., Kornfeld S. Characterization of the cell surface receptor for the Agaricus bisporus hemagglutinin. J Biol Chem. 1972 Nov 10;247(21):6937–6945. [PubMed] [Google Scholar]
- STECK T. L., HOELZLWALLACH D. F. THE BINDING OF KIDNEY-BEAN PHYTOHEMAGGLUTININ BY EHRLICH ASCITES CARCINOMA. Biochim Biophys Acta. 1965 Mar 8;97:510–522. [PubMed] [Google Scholar]
- Stewart C. C. Nutrient utilization by peritoneal exudate cells. J Reticuloendothel Soc. 1973 Oct;14(4):332–349. [PubMed] [Google Scholar]
- Tomita M., Kurokawa T., Onozaki K., Ichiki N., Osawa T., Ukita T. Purification of galactose-binding phytoagglutinins and phytotoxin by affinity column chromatography using sepharose. Experientia. 1972 Jan 15;28(1):84–85. doi: 10.1007/BF01928278. [DOI] [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]
- Waldschmidt-Leitz E., Keller L. XVIII. Uber Samenproteine. Uber Ricin; Reinigung und Differenzierung der Wirkungen. Hoppe Seylers Z Physiol Chem. 1969 Apr;350(4):503–509. [PubMed] [Google Scholar]
- Wright J. A. Evidence for pleiotropic changes in lines of Chinese hamster ovary cells resistant to concanavalin A and phytohemagglutinin-P. J Cell Biol. 1973 Mar;56(3):666–675. doi: 10.1083/jcb.56.3.666. [DOI] [PMC free article] [PubMed] [Google Scholar]